Abstract
Nanomaterials (NMs) have increasingly been used for the diagnosis and treatment of head and neck cancers (HNCs) over the past decade. HNCs can easily infiltrate surrounding tissues and form distant metastases, meaning that most patients with HNC are diagnosed at an advanced stage and often have a poor prognosis. Since NMs can be used to deliver various agents, including imaging agents, drugs, genes, vaccines, radiosensitisers, and photosensitisers, they play a crucial role in the development of novel technologies for the diagnosis and treatment of HNCs. Indeed, NMs have been reported to enhance delivery efficiency and improve the prognosis of patients with HNC by allowing targeted delivery, controlled release, responses to stimuli, and the delivery of multiple agents. In this review, we consider recent advances in NMs that could be used to improve the diagnosis, treatment, and prognosis of patients with HNC and the potential for future research.
Keywords: Nanomaterial, Head and neck cancer, Nanocarrier, Delivery, Targeting
Graphical abstract
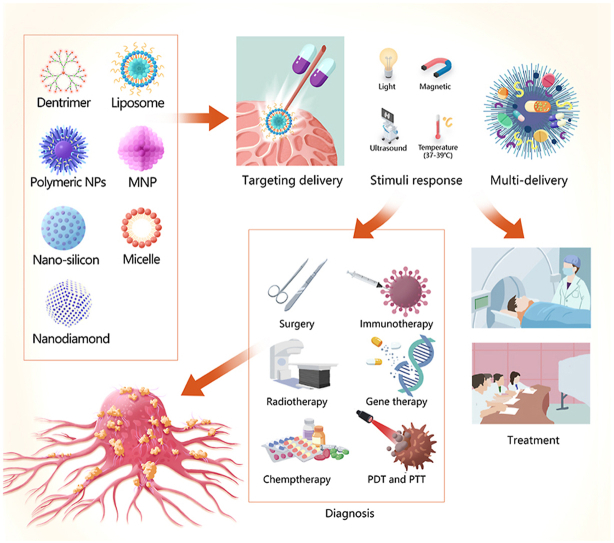
Application of nanobiomaterials for the diagnosis and treatment of head and neck cancers (HNCs). Nanomaterials (NMs) can be used as carriers to encapsulate agents, including imaging agents, drugs, genes, vaccines, radiosensitisers, and photosensitisers. Nanocarriers facilitate targeted delivery, stimuli response, and multi-delivery, which could improve the diagnosis and treatment efficiency of HNCs. Abbreviations: MNP, metallic nanoparticle; PDT, photodynamic therapy; PTT, photothermal therapy.
Highlights
-
•
Overview of nanomaterials(NMs) for head and neck cancers(HNCs) are presented.
-
•
Advances in NMs for the diagnosis and treatment of HNCs are summarized.
-
•
The existing problems and challenges of NMs for HNCs are discussed.
Abbreviations
- AIEgen
aggregation-induced emission luminogen
- AuNR
gold nanorod
- CDDP
cisplatin
- CoPoP
cobalt-porphyrin-phospholipid
- DTPA
diethylenetriaminepentaacetic acid
- EBV
Epstein–Barr virus
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EPR
enhanced permeability and retention
- FA
folic acid
- hEGF
human epidermal growth factor
- HNC
head and neck cancer
- HNSCC
head and neck squamous cell carcinoma
- HPV
human papillomavirus
- HSD
heparosan and deoxycholic acid conjugate
- ICG
indocyanine green
- iLIPICG
ICG-encapsulated liposome
- LPS
lipopolysaccharide
- MNP
metallic nanoparticle
- NM
nanomaterial
- ND
nanodiamond
- NM
nanomaterial
- NP
nanoparticle
- PDT
photodynamic therapy
- PLT
platelet
- PTT
photothermal therapy
- PEG
polyethylene glycol
- PLGA
poly(lactic-co-glycolic acid)
- SWCNT
single-walled carbon nanotube
1. Introduction
Head and neck cancers (HNCs), of which over 90% of are squamous cell carcinomas, include tumours arising in the oral cavity, lips, larynx, pharynx, paranasal sinuses, and salivary glands, and are the sixth most common type of malignant tumours worldwide, with approximately 800,000 new cases and 400,000 deaths per year [1]. Organs in the head and neck region have complex anatomies and, once HNC tumours occur, they can easily infiltrate the surrounding epithelial tissues of the aerodigestive tract and spread distantly [2]. Approximately one-third of HNC patients are diagnosed early, with a five-year survival rate of 70–90% after treatment; however, most patients are diagnosed at an advanced stage as they ignore common symptoms of HNCs, such as persistent painless neck masses, oral ulcers, and voice changes, and thus have a poor prognosis [3]. Most treatments for HNCs currently require multidisciplinary approaches that primarily involve surgery along with radiotherapy and chemotherapy. Since patients that are initially diagnosed during the middle and late stages of HNC often have large tumours, surgical treatment can result in large structural defects of the maxillofacial region that adversely affect the patients’ aesthetics, chewing and swallowing functions, and future quality of life [4]. Therefore, it is important to improve the efficiency of HNC diagnosis and treatment.
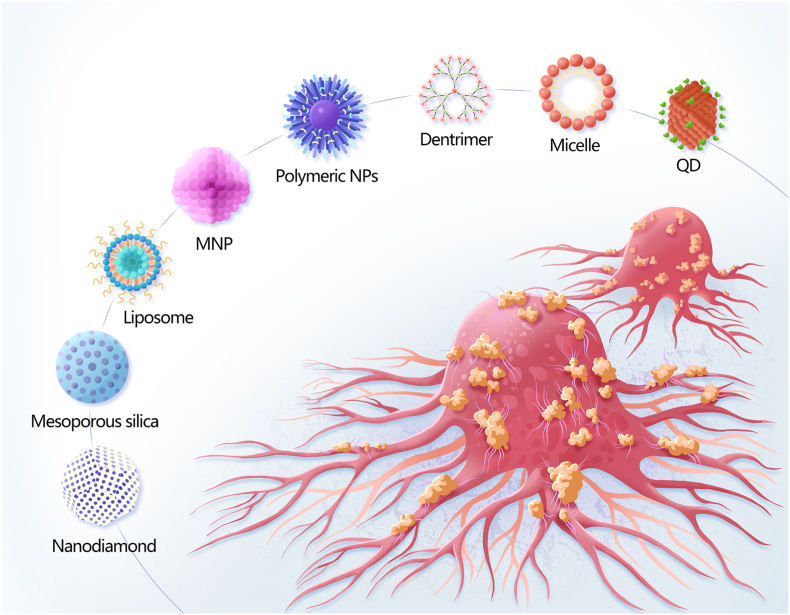
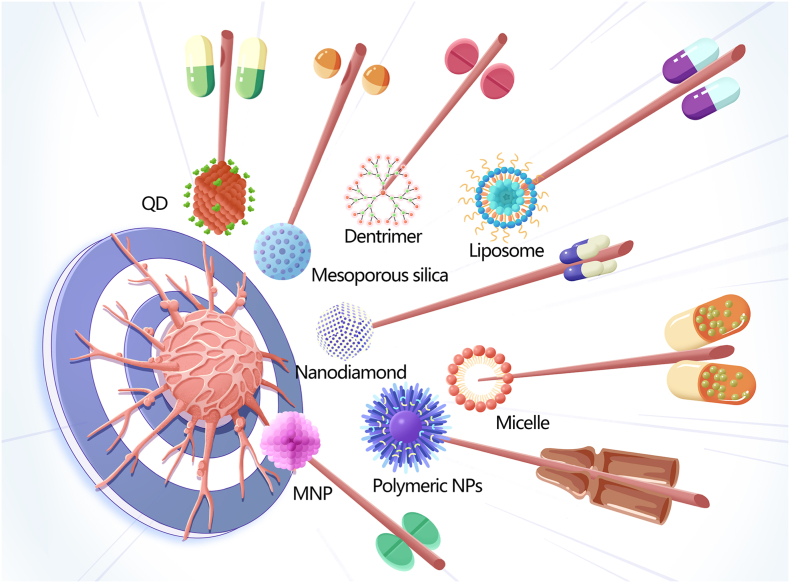
Nanomaterials (NMs) are materials in which at least 50% of the constituent particles have one or more dimensions of 1–100 nm [5]. Several distinct categories of NMs have been recognised based on their dimensionality (1D, 2D, or 3D), source (synthetic or natural), morphology (high or low aspect ratio), and composition (organic, inorganic, composite/hybrid, and carbon-based) [6]. The applications of NMs are closely related to their size. For instance, small NMs (<20 nm) are usually used for detection and imaging as they can cross the blood-brain barrier and be rapidly cleared through extravasation or the kidneys. Meanwhile, larger (>20 nm) NMs are often used as delivery carriers as they can avoid physiological barriers and circulate for prolonged periods. The structures, characteristics, and application of some representative NMs are summarised in Table 1. Since it is possible to control the size, shape, morphology, surface charge, and physicochemical properties of NMs, they are often used as carriers to deliver drugs, genes, vaccines, radiosensitisers, and photosensitisers. Further improving the pharmacokinetic properties and biodistribution of agents carried by NMs could allow their widespread use in biomedicine, including the diagnosis and treatment of HNC [7] (Fig. 1).
Table 1.
Structures, characteristics, and application of some representative nanomaterials (NMs).
| Type of nanomaterials | Structure | Characteristics | Application | Reference |
|---|---|---|---|---|
| Nanodiamonds (NDs) | Truncated octahedral nanocarbon with a diameter of 4–5 nm. | Tuneable surface structures Large surface areas Excellent mechanical and optical properties |
Imaging agents Drug carriers Scaffolds for tissue engineering |
[[8], [9], [10]] |
| Liposomes | Nano- to micrometre-sized lipid-based vesicles with a bilayer spherical structure comprising outer hydrophilic head groups and inner hydrophobic acyl chains. Mainly phospholipids and cholesterol. | Biodegradable Biocompatible Able to pass through cell membranes |
Imaging agents Drug carriers |
[[11], [12], [13], [14], [15], [16]] |
| Polymeric nanoparticles (NPs) | Polymer-based solid NPs, dendrimers, micelles, polyplexes, or dendrimers with diameters of less than 300 nm. | Biodegradable Biocompatible Poor stability Predictable pharmacokinetics High encapsulation efficiency Controlled drug release |
Imaging agents Drug carriers Stimuli response system Catalyst |
[[17], [18], [19], [20], [21], [22], [23]] |
| Hydrogels | Composite materials with covalently or physically embedded nanostructures or nanoparticles within a crosslinked polymer. | High solvent content High encapsulation efficiency Controlled drug release Highly permeable/porous Electrically conductive Magnetic Mechanical reinforcement Catalytic activity |
Drug carriers Stimuli response system |
[24,25] |
| Mesoporous silicas | Ordered mesoporous materials formed by combining surfactant molecules with silica precursors. | Tuneable shapes and sizes Extensive pore volume High specific surface area Abundant surface chemistry High dispersity Colloidal stability |
Imaging agents Sensors Tissue engineering Catalyst |
[26,27] |
| Quantum dots | Fluorescent semiconductor nanocrystals composed of II–IV or III–V group elements with physical sizes smaller than the Bohr's radius. | Good chemical and photo-stability High quantum yield Tuneable size Light emission |
Sensors Drug delivery Imaging agents |
[[28], [29], [30]] |
| Metal nanoparticles (MNPs) | Nanoparticles with metallic cores with sizes typically in the 10–200 nm range. Common metallic cores include metallic oxides (e.g. iron oxide, zinc oxide, cerium oxide, titanium dioxide) or pure metal (e.g. silver, gold). | Large surface area Tuneable size and morphology Surface modification Unique optical, mechanical, electromagnetic, and chemical properties |
Imaging agents Drug carriers Catalyst Photonics Electronics |
[[31], [32], [33], [34]] |
Fig. 1.
Schematic diagram of nanomaterials employed to diagnose and treat head and neck cancers, including nanodiamonds, mesoporous silica, metal nanoparticles (MNPs), liposomes, quantum dots (QDs), polymeric NPs, micelles, and dendrimers.
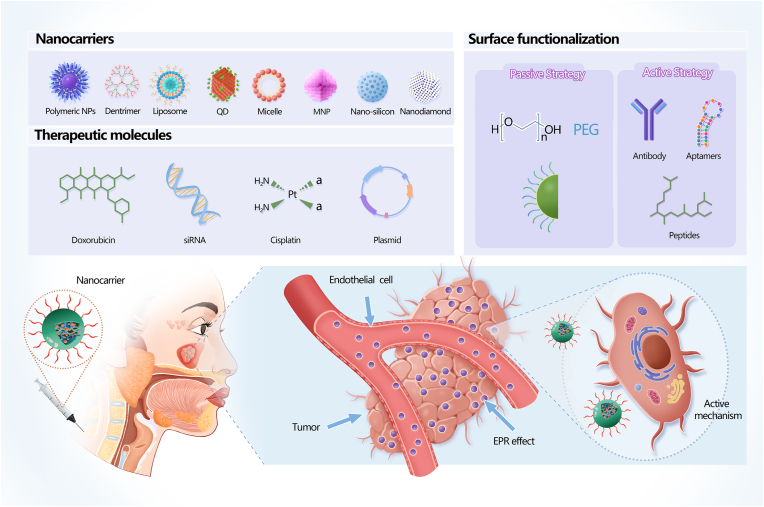
The accuracy and specificity of HNC diagnosis and treatment can be significantly improved by using NMs that increase delivery efficiency through passive and active targeted delivery mechanisms (Fig. 2). NMs can achieve passive targeted delivery through the enhanced permeability and retention (EPR) effect, while combining NMs with aptamers, receptor-specific peptides, and monoclonal antibodies can facilitate active targeted delivery. Since targeted delivery can maximise tumour death, minimise dose-dependent side effects, and reduce the frequency of serious complications, NMs can enhance the curative efficacy of traditional chemotherapy and radiotherapy, and reduce the rate of recurrence [11,35]. Furthermore, NMs can respond to multiple stimuli, allowing them to improve tumour diagnosis and be used in combination therapies like photodynamic therapy (PDT) and photothermal therapy (PTT) [36]. Thus, NMs can help to control the treatment range and intensity, effectively reduce adverse reactions, and improve the efficiency of oncotherapy.
Fig. 2.
Nanomaterials (NMs) used to treat HNC. Therapeutic molecules carried by NMs accumulate at the tumour site through active and passive mechanisms. One such passive mechanism is the enhanced permeability and retention (EPR) effect, whereby NMs preferentially deliver agents to tumour tissues owing to the increased vascular permeability of tumour tissues. Active mechanisms of targeted delivery can be achieved by combining NMs with agents such as aptamers, receptor-specific peptides, and monoclonal antibodies. Reproduced with permission [37]. Copyright 2016. SciELO.
Although the benefits of NMs have been proposed for decades, an increasing number of studies have been conducted to improve their targeting mechanisms and therapeutic diagnostic capabilities in the last decade. In this review, we evaluate recent progress in the use of NMs for the diagnosis and treatment of HNC in order to identify new approaches that could improve patient diagnosis, treatment, and prognosis. We mainly focus on high-quality articles published in the last 5 years by searching the Pubmed, Embase, and SCI electronic databases (last search updated on July 30, 2022). Furthermore, we suggest new clinical applications for NMs in HNC and discuss related challenges and future developments.
2. HNC characteristics
2.1. Aetiology
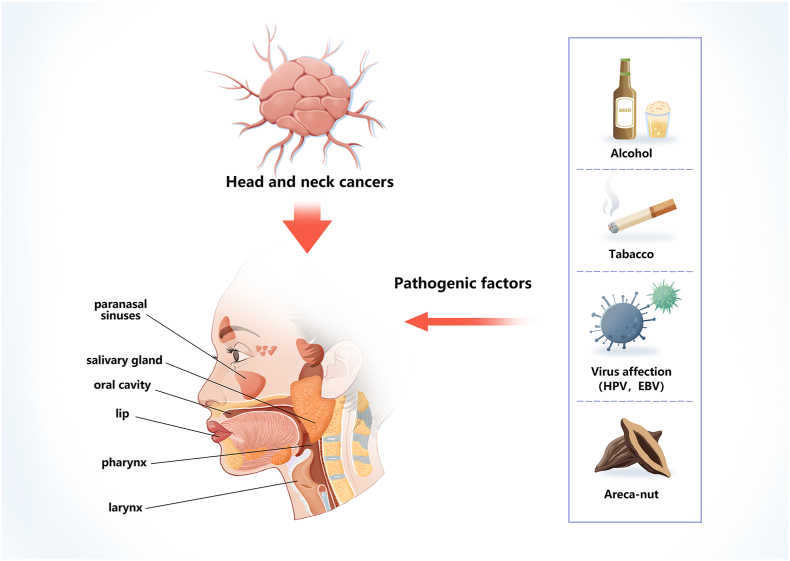
The main factors associated with the pathogenesis of HNCs are alcohol, tobacco, areca nuts, and infection with the Epstein-Barr virus (EBV) or high-risk strains of human papillomavirus (HPV) [38,39] (Fig. 3). Irrespective of their viral or environmental aetiology, men are generally at a higher risk than women for all forms of head and neck squamous cell carcinoma (HNSCC). The typical manifestations of HNSCC depend on the anatomical site of the primary tumour and the presence of pathogenic viruses, such as HPV and EBV [40]. According to their HPV status, patients with HNSCC can be divided into two distinct groups: HPV positive and HPV negative. In HPV-negative patients, environmental factors such as tobacco and alcohol use are the main pathogenic factors, whereas the primary pathogenic factor for HPV-positive patients is infection with HPV, mainly the HPV-16 and HPV-18 strains [40]. HNSCCs are adult cancers, with a median age at diagnosis of 53 years for HPV-positive patients, 66 years for HPV-negative patients, and 50 years for EBV-positive patients. HPV-positive HNSCC mainly occurs in young white men, who develop a local T-shaped tumour with a poorly differentiated histology. Patients with HPV-positive HNSCCs of the oropharynx, oral cavity, larynx, and hypopharynx have higher survival rates than HPV-negative patients; however, there is no statistical difference in survival between patients with HPV-positive and -negative HNSCCs in the paranasal sinuses and nasopharynx [41].
Fig. 3.
Pathogenic factors for head and neck cancers (HNCs). Alcohol, tobacco abuse, high-risk types of human papillomavirus (HPV), Epstein–Barr virus (EBV), and areca-nut abuse are the main pathogenic factors.
2.2. Current diagnosis and treatment
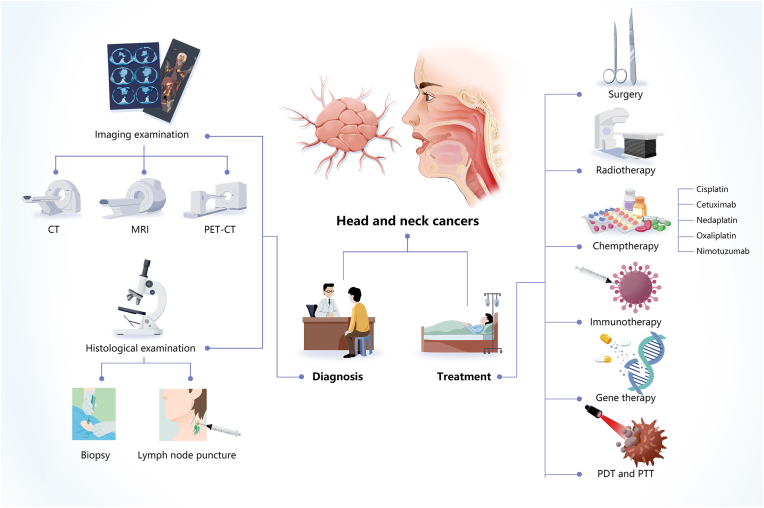
HNC diagnosis often relies on clinical manifestations and medical imaging techniques, such as enhanced computerised tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET)-CT. Histological examinations of the tumour, mainly based on biopsies, are also widely used for staging diagnosis and treatment selection, while lymph node punctures may also aid staging [42]. Since hypopharyngeal cancer may be accompanied by oesophageal tumours, oesophagogastroscopy is usually recommended [23,43] and complete upper gastrointestinal endoscopy may be used to identify second primary tumours.
Enhanced CT, MRI, and PET-CT all have advantages and disadvantages. Enhanced CT is simple, fast, popular, and provides good discrimination for characteristic lymph node necrosis, which improves staging at diagnosis since the lymph nodes in the neck are the most common area for HNC metastasis. However, enhanced CT uses radioactive particles and is therefore unsuitable for patients with an iodine allergy or severe renal insufficiency. Although MRI provides significantly higher resolution for soft tissue imaging than enhanced CT, it is relatively expensive, has a much longer operating time, and it is not suitable for patients with metal implants or claustrophobia. Meanwhile, PET-CT can detect cervical lymph nodes and distant metastases with greater sensitivity than either CT or MRI but has a lower resolution than enhanced CT and higher false-positive and false-negative rates [44,45]. Therefore, a multidisciplinary approach is necessary to diagnose and treat HNCs.
The main treatments for early-stage HNSCCs include surgery, radiotherapy, chemotherapy, immunotherapy, gene therapy, PDT, and PTT. The main treatment for oral cancer is surgery, whereas the main treatment for nasopharyngeal cancer is radiotherapy (Table 2).
Table 2.
| Oral cancer | Oropharynx cancer | Laryngeal cancer | Hypopharyngeal cancer | Nasopharynx cancer | ||
|---|---|---|---|---|---|---|
| T1-2N0 | Suited for surgery | Surgery | Surgery or radiotherapy | Surgery or radiotherapy | Surgery or radiotherapy | Radiotherapy |
| Not suited for surgery | Radiotherapy | Radiotherapy | Radiotherapy | Radiotherapy |
It is particularly important to use multidisciplinary treatments for patients with advanced HNSCC throughout the therapeutic process [42], including a combination of early treatment, chemotherapy, and chemoradiotherapy. The treatment plan should be continually adjusted according to changes in the patient's body and the reaction of the tumour in order to optimise the survival time, curative rate, and quality of life [46]. For instance, surgical HNC treatment can greatly affect the appearance of the patient, as well as their ability to chew or swallow, which can also be affected by mucosal fibrosis caused by radiotherapy. The chemotherapy drugs most commonly used to treat HNCs include cisplatin (CDDP) [48], cetuximab [44,49], nedaplatin [50], oxaliplatin [51], and nimotuzumab [52]. However, their applications are limited by issues related to toxicity and their utilisation rate. Chemotherapy can also cause various side effects including severe pain that prevents some patients from completing treatment. Therefore, it is necessary to improve the diagnosis and treatment of HNC (Fig. 4).
Fig. 4.
Diagnosis and treatment processes for head and neck cancer (HNC). The diagnosis of HNC often utilises clinical manifestations; imaging examinations such as computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET)-CT; and histological examinations such as biopsy and lymph node puncture. The main treatments for early HNC include surgery, radiotherapy, chemotherapy, immunotherapy, gene therapy, photodynamic therapy (PDT), and photothermal therapy (PTT).
3. Applications of NMs for the diagnosis and treatment of HNC
HNC is a complex disease that can easily infiltrate surrounding tissues and metastasise, thereby complicating the treatment of patients with HNC. However, NMs may provide new opportunities for improving the diagnosis and treatment of HNC (Table 3).
Table 3.
Representative examples of nanomaterial-based drug-delivery systems in head and neck cancer.
| Objective | Type of nanocarrier | Drug-delivery system | Nature of study | Study model | Reference |
|---|---|---|---|---|---|
| Imaging agents | MNPs | Cysteamine-linked folic acid AuNPs | In vivo | Nude male mice with KB tumours | [53] |
| Quantum dots | Uniformly sized N-rich mesoporous C nanospheres from pyrrole and aniline precursors | In vitro | Human oral cancer cells (FaDu) | [54] | |
| Graphene | GQD nanozymes to oxidise ABTS in the presence of H2O2 for use as a contrast agent for PAI | In vivo | Tumour-bearing mouse model/female BALB/c nude mice | [55] | |
| MNPs | AuNPs combined with AG1478 to enhance irradiation effects on HNSCC cells | In vitro | Human HNSCC cell line HSC-3 | [56] | |
| Radiotherapy | Polymeric NPs | PTX co-encapsulated with radio-luminescent CaWO4 NPs within protective capsules formed by biocompatible/biodegradable polymers (PEG-PLA) | In vivo | Immune-deficient non-obese diabetic (NOD) rag gamma (NRG) mice/HN31 xenograft-bearing NRG mice | [57] |
| Hydrogel | CDDP-loaded gellan NPs and PTX-loaded liposomes incorporated into hydrogels | In vivo and in vitro | NOD-SCID mice | [58] | |
| Polymeric NPs | Self-destructive PEG shell and different peptides designed as irinotecan and miR-200 nanovectors | In vivo | Male BALB/c nude mice | [59] | |
| Chemotherapy | Liposomes | Lipid-based mTHPC nanovesicles | In vitro | FaDu (human pharynx squamous cell carcinoma) cell line | [60] |
| MNPs | AuNPs coated with glucose and CDDP | In vivo | Nude mice | [61] | |
| Quantum dots | GE11‐modified GQDs used as drug carriers for clinical chemotherapeutics CDDP and DOX | In vivo | Balb/C nude mice | [62] | |
| Polymeric NPs | Self-destructive PEG shell and different peptides were designed as irinotecan and miR-200 nanovectors | In vivo | Male BALB/c nude mice | [59] | |
| Gene therapy | MNPs | T-cells loaded with citrate-coated superparamagnetic iron oxide nanoparticles | In vitro | T lymphocytes from mouse lymphoma | [63] |
| Immunotherapy | Polymeric NPs | ALA and MTHFD1L shRNA loaded CS-TPP NPs | In vivo | BALB/c nude mice | [64] |
| Photodynamic therapy (PDT) | Hydrogel | Nano DOX-ICG matrix MMP-responsive hydrogel | In vivo | BALB/c nude mice | [65] |
| Liposomes | PS-lipid conjugates (lipo-IRDye700DX and lipo-BPD-PC) | In vivo | Nude mice | [66] | |
| MNPs | Transformable NCs composed of ICG, Fe3O4, and PAA with pH-responsive PEG-b-PAEMA-PDMA | In vivo | Female BALB/c mice/HNE-1 cell line | [67] | |
| Quantum dots | PEG-conjugated GQDs | In vivo | Wild-type C3H mice | [68] | |
| Hydrogel | Protein hybrid hydrogel (E72-chitosan-Ag3AuS2) | In vivo | BALB/c nude mice | [69] | |
| Photothermal therapy (PTT) | Polymeric NPs | Hybrid NPs of organic compound encapsulated in PEG-PCL with ICG | In vivo | Nude mice | [70] |
| Liposomes | iRGD-PEG-DSPE lipid | In vivo | Immunodeficient (SCID) mice | [71] | |
| Mesoporous silica | Photo-triggered AuND-capped MSN loaded with Cap | In vivo | Immunodeficient (SCID) mice | [71] | |
| MNPs | t NAs-cisPt | In vitro | Human squamous cell carcinoma SCC-25 | [72] |
3.1. HNC diagnosis
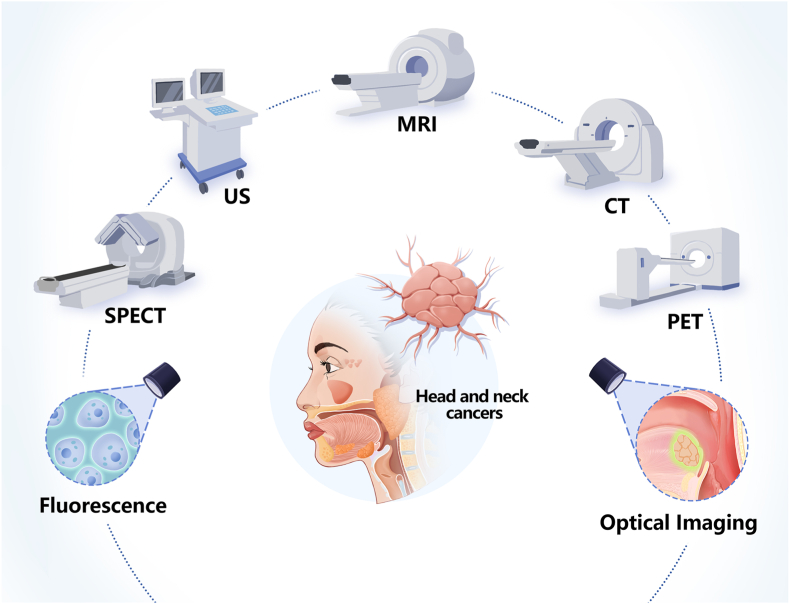
Contrast agents are an important aspect of the different imaging technologies that are used to diagnose tumours, including CT, MRI, PET, ultrasound, fluorescence imaging, and optical imaging (Fig. 5). However, most imaging agents are currently limited by poor in vivo stability, poor targeting, and explosive release in vivo [18]. Multiple studies have shown that NMs such as polymeric nanoparticles (NPs), dendrimers, metallic nanoparticles (MNPs), nanodiamonds (NDs), and core–shell NPs can be outstanding carriers for different imaging techniques, and could therefore be used to solve some of these problems.
Fig. 5.
Application of nanobiomaterials for imaging detection of head and neck cancers. Abbreviations: US, ultrasound; MRI, magnetic resonance imaging; CT, computed tomography; SPECT, single-photon emission computed tomography; PET, positron emission tomography.
Dyes can be loaded with polymeric NPs to produce fluorescent polymer NPs, which have important applications in optical sensing for diagnostic imaging, biomarker analysis, and immunoassays. Moreover, biodegradable and non-biodegradable polymeric NPs can be used as signal sensors as they can convert the presence of analytes into a quantifiable optical response. Notably, biodegradable polymeric NPs have better light stability, biocompatibility, loading capacity, adjustability, manufacturing, and surface functionalisation than non-biodegradable polymeric NPs [22]. For example, aggregation-induced emission (AIE) luminogens (AIEgens) are a type of polymeric NP that contain fluorogens or polymers conjugated with an AIE core. AIEgens do not emit light in a dissolved state but can produce substantial emissions when aggregated, meaning that they have great potential for biomedical applications. Furthermore, AIEgens can effectively overcome aggregation-induced quenching effects and exhibit excellent photosensitivity [73]. Polyethylene glycol (PEG) is the most common carrier used to prepare polymeric NPs since PEGylation can significantly increase their metabolic half-life and reduce their toxicity and immunogenicity. Moreover, the bioactive functional groups on PEG polymers can be modified to improve their biological effects, such as by adding hydrophilic substituents to the dye framework. Combining PEG polymers and AIEgens could therefore provide new clinical strategies for cancer imaging and treatment [22,74].
Dendrimers are also widely used in medical imaging technologies such as MRI, CT, and PET-CT. When used together with iodinated micromolecular contrast agents or fluorescent molecules, the surface of dendrimers can be functionalised with various targeting ligands to enhance cellular uptake and improve imaging specificity through multivalent bonding. Dendrimer-based contrast agents are nanosized, which helps to overcome the limitations of micromolecular iodinated contrast agents, allowing them to be employed for imaging multiple biological systems. Dendrimers can be used to deliver various agents and thereby improve the contrast of CT and MRI while reducing the required dose. Furthermore, dendrimers can be labelled with various radionuclides to produce agents with the functions required to enhance PET-CT images of specific pathological tissues [75]. Indeed, amphiphilic dendrimers bearing various PET-reporting units have been used for PET-CT. The superior imaging specificity and sensitivity of this nanosystem have been attributed to EPR-mediated passive tumour targeting and combined dendrimeric multivalence. Unlike conventional imaging strategies, dendrimer systems can also detect imaging-refractory low-glucose-uptake carcinomas and are safe with favourable pharmacokinetics, making them a promising strategy for cancer imaging and diagnosis [76].
As an alternative to traditional iodinated contrast agents, MNPs such as gold and bismuth have been reported to have good tumour-targeting abilities; however, they have a long circulation time and a long residence period, which may result in unexpected renal toxicity. Diethylenetriaminepentaacetic acid (DTPA) has been used as a chelating agent and a small-molecule bismuth chelate has been proposed as a CT contrast agent. Compared to iohexol, bismuth provides higher quality CT images and has excellent biocompatibility with rapid clearance from the body. Moreover, it has been discovered that ultrasmall and luminescent gold NPs can be cleared by the renal system as they display aggregation and pH-induced charge-reversal properties. In-situ aggregation and the increased tubular reabsorption of gold NPs in injured kidneys can enhance the contrast of ultrasound images. Consequently, bismuth agents and renal-clearable luminescent gold NPs have great potential as contrast agents for ultrasound and fluorescence imaging [77,78].
NDs are useful for labelling tumour images as they have nitrogen and silicon vacancy centres. Unlike conventional dyes, NDs do not undergo bleaching and can therefore be used for long-term measurements. In addition, NDs can be observed using several different imaging techniques, including fluorescence microscopy, fluorescence lifetime, near-infrared, magnetic resonance, photoacoustic, and cathodoluminescence imaging [79,80].
Core–shell NPs that combine X-ray fluorescence and optical characteristics have been designed for X-ray fluorescence CT. Due to their multimodal properties, NPs have also been used in confocal microscopy to study and track intracellular localisation. Indeed, in-situ X-ray fluorescence CT has demonstrated the potential of in vivo multimodal bioimaging for multi-target research using a minimal radiation dose. Thus, NPs could be used as microscopic and macroscopic imaging tools [81]. Single contrast agents are typically unsuitable for multiple imaging strategies; however, lanthanide-based core–shell–shell NPs have been designed as tri-modal contrast agents that can simultaneously improve MRI, photoluminescence, and CT imaging performance without adversely affecting single-modality performance [82].
3.2. HNC treatment
Multiple oncotherapies are used to treat HNC, including radiotherapy, chemotherapy, immunotherapy, gene therapy, PDT, and PTT, and their efficacy can be improved using NMs.
3.2.1. Radiotherapy
Radiotherapy is one of the most widely used and effective methods for treating HNCs. Radiation can damage DNA through direct physical ionisation or the creation of free radicals via indirect water ionisation. Ultimately, the purpose of radiotherapy is to supply a fatal dose of radiation to the tumour site whilst sparing the surrounding normal tissue; therefore, the maximum dose depends on the toxicity of the treatment to surrounding tissues [83]. Since radiosensitisers can produce free radicals and accelerate DNA damage, they can potentially increase damage to tumour tissues; however, NMs have emerged as promising radiosensitisers [84,85].
For instance, MNPs display favourable kinetic properties and flexible surface structures, as well as greater ionising radiation stopping power in soft tissue; allowing them to be used as radiosensitisers to improve the efficacy of radiotherapy [86] in many tumour models, including HNC [87]. The radiosensitising effects of MNPs have been demonstrated for silver, bismuth, gold, gadolinium, and various metal oxide NPs. Furthermore, it has been reported that the radiation dose increases when MNPs are irradiated due to the generation of secondary photoelectrons, X-rays, and Auger electrons [88].
Polymeric NPs can also be used as radiosensitisers. For instance, using a surface conjugated to DTPA–human epidermal growth factor (hEGF), poly(lactic-co-glycolic acid; PLGA) NPs were designed to encapsulate a ruthenium-based DNA replication radiosensitiser and inhibitor. These radiolabelled PLGA NPs are preferentially taken up by oesophageal cancer cells, which overexpress epidermal growth factor (EGF) receptors (EGFRs), where they cause DNA damage and radiotoxicity, suggesting that polymeric NPs with surface modification and radiosensitiser encapsulation can achieve targeted and combined efficacy in EGFR-overexpressing tumour cells [89].
3.2.2. Chemotherapy
Platinum-based drugs are the most common chemotherapeutic agents used to treat HNC but are limited by poor efficiency and off-target effects. Chemotherapy drugs have harsh side effects and can cause systemic toxicity and drug resistance; therefore, they are used at the lowest possible dose to improve antitumour activity. Controlled release can help to protect chemotherapy drugs prior to their delivery to the target tissue, cell, or intracellular location, and ensure that they are released in a sustained manner [17]. Some NMs, such as liposomes, polymeric NPs, dendrimers, MNPs, and NDs have been used to amplify the properties of bioactive agents, including their absorption, metabolism, distribution, and elimination. In addition, many NMs have good solubility and bioavailability, which can reduce drug resistance and facilitate controlled drug release.
Liposomes are a useful drug delivery system as they can carry hydrophilic drugs in their aqueous core, lipophilic drugs in their lipid bilayer, and amphiphilic drugs on the bilayer surface [13]. Liposomal delivery increases the drug circulation time because the cholesterol and saturated lipids in the liposomes increase their stability. In addition, liposomes can preferentially deliver drugs to tumour tissues via the EPR effect. For example, liposomes with long circulation times, such as PEG and stealth liposomes, have surface modifications that can resist recognition and uptake by reticuloendothelial system cells. This reduces the adsorption of blood plasma proteins by liposomes and allows them to slowly extravasate from newly formed tumour blood vessels, thus providing passive tumour targeting [14].
Polymeric NPs can conjugate agents such as drugs, proteins, aptamers, multicomponent non-viral vectors, and copolymer micelles [18], control the release of drugs from the matrix to the target site in the body, and their surfaces can be modified with ligands. Therefore, controlled release is usually achieved by adjusting the rate of polymeric NP biodegradation and drug diffusion through the matrix [18]. For example, PLGA has good biocompatibility and can release drugs continuously. In tumours, PLGA loaded with drugs can extravasate through tumour vasculature and is transferred to cells through the EPR effect, thus improving the therapeutic efficacy of the drugs [90]. In this way, polymeric NPs can reduce the toxicity of drugs to surrounding normal tissues and increase the water solubility of anticancer agents, thereby minimising adverse effects against healthy tissues [18]. Furthermore, polymeric NPs can be administered via gels, tablets, films, patches, and injectable systems for localised drug delivery to oral cancers [91].
Synthetic MNP strategies can support a vast number of therapeutic agents, such as nucleic acids, chemotherapeutic drugs, radioenhancers, photosensitisers, and peptides, via ligands, porous nanostructures, metal clusters, and their surfaces. Combining different therapeutic agents with MNPs allows them to function synergistically and improves the overall curative effect. Treatment strategies may include incorporating various chemotherapeutic drugs, therapeutic metallic cores, and ligands into one nanostructure, or co-transporting different therapeutic agents (e.g., radio-enhancers, chemotherapeutics, and biological agents) [32]. For example, CDDP-loaded magnetic iron oxide NPs were wrapped in surface-regulating folic acid (FA) and the intracellular aggregating peptide (Cys(StBu)-Lys-CBT) to form a new drug called FA-MNP-CDDP-CBT. Compared with CDDP alone, FA-MNP-CDDP-CBT is less vulnerable to drug resistance and has a reduced half-maximal inhibitory concentration in human nasal epithelial cells. Furthermore, FA-MNP-CDDP-CBT generates more reactive oxygen species (ROS) and provides better-targeted uptake. Hence, this nanosystem provides a flexible approach that can be used to improve antitumour activity, reduce systemic toxicity and the side effects of chemotherapy, and minimise CDDP treatment resistance in patients with nasopharyngeal carcinoma [92].
Owing to their high surface chemical interactivity, biocompatibility, and small size, NDs are commonly attached to small molecule proteins and chemotherapy drugs to provide long-term drug release, which is beneficial for drug delivery [9,93]. For example, protamine sulfate-NDs can deliver miRNA-203 to oesophageal cancer cells to suppress tumour proliferation and migration [94], while NDs can be used to deliver celecoxib, a common non-steroidal anti-inflammatory drug, to suppress tumourigenesis and cancer progression in oral squamous cell carcinomas [95,96].
3.2.3. Immunotherapy
NMs are widely used in immunotherapy and are a new and promising treatment for tumours [97,98]. Since liposomes are effective immune adjuvants for protein and peptide antigens, they can present antigens in a gradual and continuous manner to trigger immune responses in bodily fluids and cells [13]. During cancer treatment, the immune response to liposomes usually occurs in the tumour microenvironment and in circulating blood. By combining liposome delivery with antibody-mediated tumour recognition, immunoliposomes can induce intracellular drug release [99]. Encapsulated protein or peptide antigens will accumulate in macrophages as they phagocytise lysosomes. Degraded peptides are then presented to major histocompatibility complex II on the surface of the macrophages, which stimulates specific T-helper cells and then specific B cells to secrete antibodies and trigger the cytotoxic T-lymphocyte response. For example, HPV-16 E6 and E7 oncogene expression has been associated with multiple HNCs. Based on cobalt-porphyrin-phospholipids (CoPoPs), a liposomal vaccine adjuvant was mixed with synthetic 9-mer epitopes attached to three histidine residues, leading to the rapid formation of peptide-liposome particles. Vaccine monotherapy combined with CoPoPs was reported to reduce tumour development and destroy subcutaneous tumours up to 100 mm3 in size [100].
In addition, dendrimers are widely used in immunoassays as they have a highly branched architecture with multiple active external groups, which can increase the sensitivity of immunosensors by controlling the direction of antibodies on the surface [101].
3.2.4. Gene therapy
HNC cells have high proliferation and invasion potential, which also makes them resistant to treatment; however, the mechanisms of drug resistance are complicated and involve epithelial–mesenchymal transitions, drug efflux, autophagy, and DNA damage/repair. Determining the potential molecular pathways and mechanisms involved in the progression of these tumours could allow the development of gene therapies that can alter these molecular pathways to reduce chemical and radiation resistance. In particular, nano-delivery platforms are a compelling strategy for minimising drug resistance as they allow controlled release, tumour targeting, and have persistent pharmacokinetic profiles.
Clustered regularly interspaced short palindromic repeats (CRISPR) systems can be used to regulate the functions of genes in drug-resistant HNC via NPs [102]. CRISPR-associated protein 9 (Cas9) technology uses engineered single guide RNA for site recognition to correct gene mutations via non-homologous end joining or homology-directed repair, and thus represents a flexible approach for treating diseases. Multiple vehicles, such as black phosphorus, gold NPs, graphene oxide, and polymeric NPs have been used to deliver Cas9 [103].
The surfaces of polymeric NPs can be modified with ligands to encapsulate unstable molecules, such as RNA, DNA, and proteins, which prevents their degradation and provides excellent stability [18]. Dendrimers are often used as a drug and non-viral gene carriers in tumour research and can be combined with ligands such as biotin, FA, riboflavin, and N-acetyl-glucosamine to achieve targeted drug delivery. Dendrimers such as poly(amidoamine)-PEG, glycol, triazine, carbosilane, and phosphorus are widely used as anti-cancer drugs [75] and can be further optimised using surface engineering modification strategies. For example, multifunctional nanoparticles modified with pH-sensitive EGFR-targeting and nucleus-directed peptides were constructed to effectively deliver epirubicin and HuR CRISPR into pH-sensitive human tongue squamous carcinoma (SAS) cells, which respond to pH and act as a switch to release target peptides. The transfection efficiency and uptake by tumour cells were improved through ligand-mediated endocytosis, EGFR targeting, and endosomal escape via the EGFR/PI3K/mTOR/AKT axis. Once transported into the nucleus, CRISPR/Cas9 successfully knocks out HuR to inhibit the proliferation, resistance, and metastasis of SAS cells, while co-treatment with HuR CRISPR and epirubicin further facilitates apoptosis and necroptosis, leading to cancer cell death [103].
Carbon nanotube-based drug delivery systems are also a desirable approach for gene therapy. For instance, a drug–single-walled carbon nanotube (SWCNT) bioconjugate was designed to target and kill cancer cells with superior efficacy compared to non-targeted bioconjugates. EGF and CDDP were attached to the SWCNTs to allow them to specifically target squamous cancer cells, with confocal microscopy and QD luminescence revealing that SWCNT–QD–EGF bioconjugates were quickly internalised by EGFR-overexpressing HNSCCs. In addition, two-photon three-colour intravital video imaging showed that in vivo-injected SWCNT–QD–EGF was selectively absorbed by HNCs, affected cell proliferation, and induced tumour regression [104].
Cationic liposome complexes composed of cationic lipids and negatively charged nucleic acids, such as DNA, RNA, and antisense oligonucleotides have also been used for gene therapy. To prevent uptake by the reticuloendothelial system, the lipid surfaces are optimised with antibodies or ligands recognised by specific cells to enhance tissue targeting [13,105]. Stemness can be induced in cancerous oesophageal squamous cells by stimulating them with high lipopolysaccharide (LPS) concentrations. LPS stimulation can increase TET3 expression via the p38/ERK–MAPK pathway, which correlates negatively with patient survival. Thus, LPS can be regarded as a tumour promoter and may provide a novel strategy for treating oesophageal squamous cell cancer [106]. Together, these studies demonstrate that NMs display potential for the delivery of genes for HNC therapy.
3.2.5. PDT and PTT
NMs can be activated on-demand using an external light source whose spectral characteristics, illumination time, and intensity can be controlled. Thus, light-triggered NMs can be used for PDT and PTT [107,108].
During PDT, photosensitising agents are exposed to particular types of light of a specific wavelength, usually via a laser source, and produce ROS, which kills nearby cells. Functionalised dendrimers can help the photosensitising agents selectively attach to target tissues or cells, and can regulate the biological effects of metal particles, improve biocompatibility, and aid surface modification. Thus, dendrimers can be used as contrast agents and biomarkers in PDT [75]. Traditional photodynamic agents cause long-term phototoxicity and unwelcome singlet oxygen (1O2) quantum yields. In clinical trials, sulphur-doped carbon dots were used as nano-photosensitisers to increase the efficiency of PDT for oral cancer treatment but produced a high 1O2 yield. However, a low dose (nmol/L) of sulphur-doped carbon dots was found to be safe and could be used as a prospective photosensitiser for oral cancer treatment [109].
PTT has attracted attention as a minimally invasive method for treating tumours that uses photothermal agents combined with various light sources to send strong heat energy to tumour sites while causing limited damage to peripheral tissues. Many inorganic photoactive materials, such as gold NPs and carbon nanotubes, have limited applications as photothermal agents for cancer treatment due to their low biodegradability and poor biocompatibility. However, light-absorbing polymeric NPs have strong biocompatibility, photostability, and photothermal conversion efficiency [20]. For example, micelles can encapsulate and dissolve meso-tetra-hydroxyphenyl-porphyrin at high loading densities, have a uniform size distribution, and are cytotoxic to HNC cells in vitro when mediated by fluorescence and PDT [110]. Meanwhile, platelets (PLTs) loaded with gold nanorods (AuNRs) via electroporation can act as vectors for the targeted delivery of photosensitisers to tumour sites, thereby improving the efficacy of PTT in HNC. AuNR-loaded PLTs (PLT–AuNRs) inherit the PTT characteristics of MNPs and the tumour-targeting and prolonged blood circulation properties of PLTs, making PLT–AuNR–PTT a promising oncotherapy [111].
3.3. Limitations and modifications
Unlike conventional small-molecule-based drug carriers, NMs have several limitations [112]. For instance, drugs carried by NMs can be eliminated rapidly by the reticuloendothelial system, whereas the NMs themselves are not excreted efficiently and may be retained in the body, increasing their potential toxicity [113]. However, NMs can improve therapeutic efficacy by facilitating targeted delivery, controlled drug release, and combined therapies.
3.3.1. Targeting delivery
Treatment efficiency is largely restricted by the broad distribution of drugs throughout the body; however, targeted delivery can provide tumour tissues with a sufficient drug concentration while suppressing systemic side effects. This can be achieved through the controlled release and preferential uptake of drug-containing NMs (Fig. 6), which can be delivered to tissues of interest through active or passive targeting and show tremendous promise for drug delivery.
Fig. 6.
Examples of nanomaterials (NMs) for targeted drug delivery. The main components of NM-based drug delivery systems include a nanocarrier, targeting moiety (e.g. aptamers, receptor-specific peptides, or monoclonal antibodies), and cargo (the desired chemotherapeutic drugs).
Liposome preparations can enhance tumour-targeting characteristics when bound to functionalised peptides or aptamers. Functionalised peptides can mediate specific drug delivery, improve drug penetration, accumulate specifically at target sites, and enhance therapeutic effects [24,114]. Aptamers are short synthetic single-stranded DNA or RNA molecules that can bind to targets with high specificity and affinity. Since aptamers are small and lack the fragment crystallisable region of antibodies, they can be attached to liposome surfaces at higher densities [115]. For example, Lo et al. [59] designed liposomes with self-destructive pH-sensitive PEG shells and different peptides for use as miRNA and irinotecan carriers to improve their tumour-specific accumulation. These peptides included one cell-penetrating peptide, one mitochondrion-directed apoptosis-inducing peptide, and one ligand targeting tumour neovasculature, which enhanced overall selectivity and potency towards tumour cells. In addition, the modified liposomes displayed superior safety and therapeutic efficacy compared to the other tested formulations. Thus, PEG and peptides can enhance the targeting effect of liposomes and improve their therapeutic efficacy [59].
Drugs can be loaded onto polymeric NPs through adsorption onto the polymer surface, covalent bonding with the polymer backbone, or encapsulation within the polymer matrix during preparation [17]. Modifying the surfaces of polymeric NPs can control drug release from the matrix to the target site in the body [18]. For instance, Xu et al. [116] developed an FA-decorated polyamidoamine dendrimer G4 (G4-FA) as a carrier for the localised delivery of siRNA, which suppressed tumour growth in an HN12 xenograft tumour model by allowing prolonged NP retention and enhanced siRNA uptake in the tumour. In addition, decorating the surface with FA made G4-FA a better platform for local siRNA delivery to treat cancers such as HNSCC [116,117].
3.3.2. Stimulus-responsive systems
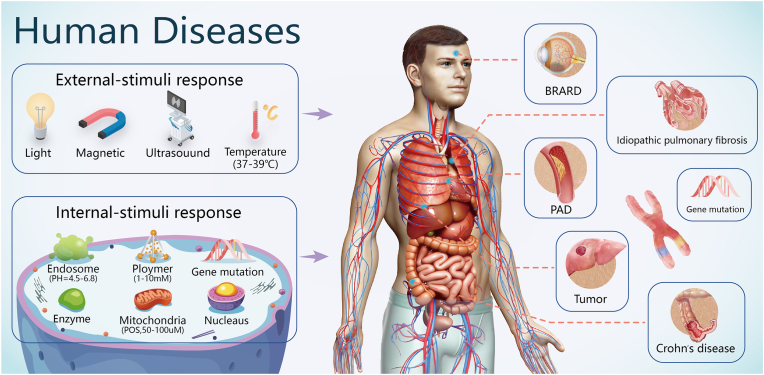
Stimulus-responsive systems are a promising means of delivering and releasing drugs in a site-specific manner (Fig. 7) to improve bioavailability and reduce toxic side effects. The discovery of responsive NMs has accelerated their application in site-specific delivery [118].
Fig. 7.
Stimulus-responsive nanocarriers for human diseases including solid tumours, cardiovascular diseases (e.g. atherosclerotic peripheral arterial disease (PAD)), single-gene diseases (e.g. biallelic RPE65-associated retinal dystrophy (BRARD)), and idiopathic diseases (e.g. Crohn's disease and idiopathic pulmonary fibrosis) Reproduced with permission [118]. Copyright 2021. Wiley-VCH.
In stimulus-responsive systems, polymer NPs can release drugs in response to one or more internal or external stimuli, such as pH, redox, temperature, magnetism, and light [19,20,119]. These stimuli alter the structural and chemical properties of the nanocarriers, prompting them to release their cargo in a specific biological environment [21]. This method has been used to achieve unprecedented control over drug delivery and release, with excellent antitumour effects.
Similarly, environment-specific stimuli (e.g., redox potential, pH, or enzyme activity) and external stimuli (e.g., heat or light) can alter the stability of liposome membranes to allow the cargo drug to be released in the target tissue at a controlled rate [14,120]. For example, the spectral characteristics, illumination time, and intensity of an external light source can be controlled to release liposomes on demand [107]. Light-triggered liposomes can be used for PDT, PTT, drug delivery in cancer immunotherapy, and CRISPR-based genome editing [121,122].
Sun et al. [123] used heparosan and deoxycholic acid conjugates (HSDs) to develop a redox-responsive drug delivery system to treat laryngopharyngeal carcinomas. They showed that nanoscale micelles can encapsulate amphiphilic HSDs and support a high concentration of doxorubicin. The HSD micelles were internalised by FaDu cancer cells (isolated from hypopharyngeal tumours) via endocytosis and, when triggered by high glutathione levels, the HSD micelles released the drug and inhibited cell growth.
3.3.3. Combination therapy
Compared to drug treatment alone, greater therapeutic efficacy can be achieved using combination therapies, which can include a variety of small-molecule therapies, free siRNAs, or antisense oligonucleotides that sensitise cells to small-molecule therapies, one or two liposome drugs targeting two or more different antigens, and particles targeting ligands with remote stimulation systems to increase transfection efficiency. By improving the pharmacokinetics of drugs, NMs can promote their therapeutic effects and reduce adverse side effects associated with high doses [124]. The main limitations of conventional liposomes are their thermodynamic instability, poor retention ability, uncontrolled release, and rapid elimination from the blood. However, lipid systems that combine therapies with different mechanisms of action and side effects that do not overlap are highly promising [125].
The quality of dual- or multimodal-images can be improved significantly by selecting dendrimers with appropriate structures alongside dual- or multimodal-contrast agents, such as MR/fluorescence or MR/CT contrast agents, which can improve diagnostic accuracy [75]. Using a surface conjugated to DTPA–hEGF, Gill et al. [89] designed PLGA NPs to encapsulate a ruthenium-based DNA replication radiosensitiser and inhibitor. The radiolabelled PLGA NPs were preferentially taken up by EGFR-overexpressing oesophageal cancer cells, where they caused cellular DNA damage and exhibited radiotoxicity. Thus, polymeric NPs with surface modification and radiosensitiser encapsulation can be used to achieve targeted and combined efficacy in EGFR-overexpressing tumour cells.
Wu et al. [126] developed multifunctional nanoparticles and internalised Arg-Gly-Asp-modified indocyanine green (ICG)-encapsulated liposomes (iLIPICG) for PDT and PTT to treat laryngeal carcinomas. The iLIPICG displayed good blood circulation, tumour penetration, tumour targeting, and tumour accumulation properties, and their spatial and temporal control could simultaneously generate ROS and hyperthermia. In addition, the iLIPICG achieved fluorescence-guided impact by using ICG to clear laryngeal carcinoma cells under laser irradiation, thus improving phototherapeutic efficacy against laryngeal carcinomas.
4. Conclusions and future perspectives
New methods are required to diagnose and treat HNC due to the increasing number of patients and the limitations of traditional treatment methods. Over the past decade, significant progress has been made in the design, synthesis, and manufacture of NMs that have unique advantages for tumour diagnosis and treatment, as indicated by the rapid increase in the number of articles related to ‘nanomaterials AND tumour/cancer/carcinoma’ in PubMed/Embase/SCI in the latest decade. Ongoing research in this field has provided new avenues for overcoming the limitations of traditional methods used for tumour diagnosis and treatment.
NMs are ideal carriers for imaging agents, drugs, genes, vaccines, radiosensitisers, and photosensitisers [27,127]. Thus, NMs could improve the diagnosis, treatment, and prognosis of HNC patients through targeted delivery, controlled release, stimuli responses, and the delivery of multiple agents [128,129]. In addition, NMs could improve the survival and aesthetic, chewing, and pronunciation abilities of patients with HNC. Ongoing and completed clinical trials related to the application of NMs in HNC are summarised in Table 4. Most nanocarrier systems treat tumours through intravenous injection and systemic delivery, and can preferentially accumulate on tumours through defective tumour microvessels [130]. However, EPR is not yet thoroughly understood and the biosafety of NMs remains a concern [131,132].
Table 4.
Typical nanomedicine in clinical trials for head and neck cancers (HNCs).
| Product | Drug delivered | Trail phase | Status | NCT number | References |
|---|---|---|---|---|---|
| Anti-EGFR immunoliposomes | Doxorubicin | Phase 1 | Completed | NCT01702129 | [133] |
| LiPlaCis | Cisplatin | Phase 1/2 | Completed | NCT01861496 | |
| NC-6004 | Cisplatin | Phase 1/2 | Completed | NCT03109158 | [134] |
| Phase 2 | Recruiting | NCT03771820 | |||
| TumoCure | Cisplatin | Phase 1 | Not yet recruiting | NCT05200650 | |
| NBTXR3 | HfO2-containing NPs | Phase 3 | Recruiting | NCT04892173 | [[135], [136], [137]] |
| Phase 2 | Recruiting | NCT04862455 | |||
| Phase 2 | Active, not recruiting | NCT04834349 | |||
| Phase 1 | Recruiting | NCT01946867 | |||
| Phase 1/2 | Terminated | NCT02901483 | |||
| SNB-101 | Irinotecan | Phase 1 | Recruiting | NCT04640480 | |
| OSI-211 | Lurtotecan | Phase 2 | Completed | NCT00022594 | [138] |
| PRECIOUS-01 | Threitolceramide-6; NY-ESO-1 peptides |
Phase 1 | Recruiting | NCT04751786 | [139] |
| Abraxane | Paclitaxel | Phase 1/2 | Completed | NCT00851877, NCT00833261, | [140,141] |
| Phase 2 | Recruiting | NCT01412229, NCT00736619 | |||
| Phase 2 | Active, not recruiting |
NCT04857164, NCT04922450 NCT02270814, NCT03174275 |
|||
| BIND014 | Docetaxel | Phase 1 | Completed | NCT01300533 | [142] |
| Docetaxel polymeric micelle | Docetaxel | Phase 2 | Unknown status | NCT02639858 | [143] |
| CMP-001 | TLR9 agonist | Phase 2 | Recruiting | NCT04633278 | [144] |
| AuroShell | Gold-Silica nanoshells | Not applicable | Completed | NCT00848042 | |
| Mitoxantrone hydrochloride liposome | Mitoxantrone hydrochloride | Phase 1 | Not yet recruiting | NCT04902027 | |
| DC-cholesterol liposome | EGFR antisense DNA | Phase 1 | Completed | NCT00009841 | |
| Doxil | Doxorubicin | Phase 1 | Completed | NCT00252889, NCT02262455, NCT04244552 | [145] |
| Albumin-bound rapamycin NPs | Rapamycin | Early phase 1 | Completed | NCT02646319 | [146] |
| ONM-100 | FDA-approved fluorophore | Phase 2 | Completed | NCT03735680 | [147] |
Although preclinical data in vitro have shown that nanocarriers display promising pharmacological parameters, including drug concentration, clearance rate, and half-life, it is impossible to fully monitor drugs from their entry into the human body to their clearance. In addition, basic preclinical research data differs from clinical research data, often due to a lack of tumour models that can simulate human tumours, resulting in a poor understanding of the interaction between NMs and complex organisms. Therefore, it is necessary to develop tumour models that can accurately simulate the tumour heterogeneity and microenvironment in the human body (e.g., humanised animal models) to allow the clinical transformation of nanotechnology. Another equally significant issue, which is often overlooked, is that NM loading decreases after systemic administration as they travel through the circulatory system to tumour tissues [7,14]. Therefore, drug release should also be considered during the clinical transformation of nanocarriers. Various stimulus-responsive NMs have been developed to control drug release [118]. Since the tumour microenvironment has lower pH and oxygen levels and higher glutathione and H2O2 levels than normal tissues [148,149], designing NMs to respond to these subtle environmental changes can enable the temporal and spatial control of drug release. NM-based methods allowing accurate drug release could therefore narrow the gap between basic studies and potential clinical applications; however, more in-depth research is required.
Future developments related to NMs in oncology, molecular biology, and pharmacy could overcome the obstacles related to the clinical transformation of NMs from various perspectives. Firstly, NM preparation methods should be improved to increase drug loading. In addition, drugs should be selected that can be released from nanocarriers under biological conditions while maintaining their functions and therapeutic properties. Moreover, NMs could be modified with appropriate functional ligands, peptides, and antibodies to allow active targeting to pathological sites and reduce passive accumulation in normal tissues. Furthermore, the mechanisms of NM uptake and retention in vivo should be clarified and the fundamental physiological discrepancies between humans and experimental animals should be addressed to improve the clinical translation process.
Overall, recent progress in the field of NMs has provided many new avenues for the diagnosis and treatment of HNC; however, further studies are required to transform raw materials into mature drugs for clinical use. Increased interdisciplinary cooperation has allowed rapid advances in tumour research and nanotechnology, as well as the development of NMs that integrate the benefits of nanotechnology with the biological characteristics of HNC. Therefore, we expect that the applications of NMs will improve the prognosis of patients with HNC.
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors. For this type of study, formal consent is not required.
CRediT authorship contribution statement
Cheng Yu: Investigation, Writing – original draft, Writing – review & editing. Long Li: Investigation, Writing – original draft. Shiwen Wang: Investigation, Writing – review & editing. Yuanhang Xu: Investigation. Lu Wang: Investigation. Yongbiao Huang: Writing – review & editing. Ahmed Hieawy: Writing – review & editing. He Liu: Conceptualization, Supervision, Writing – review & editing. Jingzhi Ma: Conceptualization, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare no competing financial interests in this work.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant numbers 62171193, 81802710).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
He Liu, Email: endoliuhe@gmail.com.
Jingzhi Ma, Email: majingzhi@hust.edu.cn.
References
- 1.WHO Global Cancer Observatory 2020. http://gco.iarc.fr/
- 2.Pfister D.G., Spencer S., Adelstein D., Adkins D., Anzai Y., Brizel D.M., Bruce J.Y., Busse P.M., Caudell J.J., Cmelak A.J., Colevas A.D., Eisele D.W., Fenton M., Foote R.L., Galloway T., Gillison M.L., Haddad R.I., Hicks W.L., Hitchcock Y.J., Jimeno A., Leizman D., Maghami E., Mell L.K., Mittal B.B., Pinto H.A., Ridge J.A., Rocco J.W., Rodriguez C.P., Shah J.P., Weber R.S., Weinstein G., Witek M., Worden F., Yom S.S., Zhen W., Burns J.L., Darlow S.D. Head and neck cancers, version 2.2020, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2020;18:873–898. doi: 10.6004/jnccn.2020.0031. [DOI] [PubMed] [Google Scholar]
- 3.Hashim D., Genden E., Posner M., Hashibe M., Boffetta P. Head and neck cancer prevention: from primary prevention to impact of clinicians on reducing burden. Ann. Oncol. 2019;30:744–756. doi: 10.1093/annonc/mdz084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Townsend M., Kallogjeri D., Scott-Wittenborn N., Gerull K., Jansen S., Nussenbaum B. Multidisciplinary clinic management of head and neck cancer. JAMA Otolaryngol. Head. Neck. Surg. 2017;143:1213–1219. doi: 10.1001/jamaoto.2017.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mech A., Wohlleben W., Ghanem A., Hodoroaba V.D., Weigel S., Babick F., Brungel R., Friedrich C.M., Rasmussen K., Rauscher H. Nano or not nano? A structured approach for identifying nanomaterials according to the European commission's definition. Small. 2020;16 doi: 10.1002/smll.202002228. [DOI] [PubMed] [Google Scholar]
- 6.Oksel Karakus C., Bilgi E., Winkler D.A. Biomedical nanomaterials: applications, toxicological concerns, and regulatory needs. Nanotoxicology. 2021;15:331–351. doi: 10.1080/17435390.2020.1860265. [DOI] [PubMed] [Google Scholar]
- 7.Banik B.L., Fattahi P., Brown J.L. Polymeric nanoparticles: the future of nanomedicine. Wiley. Interdiscip. Rev. Nanomed. Nanobiotechnol. 2016;8:271–299. doi: 10.1002/wnan.1364. [DOI] [PubMed] [Google Scholar]
- 8.Mochalin V.N., Shenderova O., Ho D., Gogotsi Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2011;7:11–23. doi: 10.1038/nnano.2011.209. [DOI] [PubMed] [Google Scholar]
- 9.Gao G., Guo Q., Zhi J. Nanodiamond-based theranostic platform for drug delivery and bioimaging. Small. 2019;15 doi: 10.1002/smll.201902238. [DOI] [PubMed] [Google Scholar]
- 10.Reina G., Zhao L., Bianco A., Komatsu N. Chemical functionalization of nanodiamonds: opportunities and challenges ahead. Angew. Chem. Int. Ed. Engl. 2019;58:17918–17929. doi: 10.1002/anie.201905997. [DOI] [PubMed] [Google Scholar]
- 11.Bu L.L., Yan J., Wang Z., Ruan H., Chen Q., Gunadhi V., Bell R.B., Gu Z. Advances in drug delivery for post-surgical cancer treatment. Biomaterials. 2019;219 doi: 10.1016/j.biomaterials.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 12.Ajeeshkumar K.K., Aneesh P.A., Raju N., Suseela M., Ravishankar C.N., Benjakul S. Advancements in liposome technology: preparation techniques and applications in food, functional foods, and bioactive delivery: a review. Compr. Rev. Food Sci. Food Saf. 2021;20:1280–1306. doi: 10.1111/1541-4337.12725. [DOI] [PubMed] [Google Scholar]
- 13.Hu M., Zhang J., Kong L., Yu Y., Hu Q., Yang T., Wang Y., Tu K., Qiao Q., Qin X., Zhang Z. Immunogenic hybrid nanovesicles of liposomes and tumor-derived nanovesicles for cancer immunochemotherapy. ACS Nano. 2021;15:3123–3138. doi: 10.1021/acsnano.0c09681. [DOI] [PubMed] [Google Scholar]
- 14.Large D.E., Abdelmessih R.G., Fink E.A., Auguste D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021;176 doi: 10.1016/j.addr.2021.113851. [DOI] [PubMed] [Google Scholar]
- 15.Kamaly N., Miller A.D. Paramagnetic liposome nanoparticles for cellular and tumour imaging. Int. J. Mol. Sci. 2010;11:1759–1776. doi: 10.3390/ijms11041759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao P., Hou X., Yan J., Du S., Xue Y., Li W., Xiang G., Dong Y. Long-term storage of lipid-like nanoparticles for mRNA delivery. Bioact. Mater. 2020;5:358–363. doi: 10.1016/j.bioactmat.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sousa de Almeida M., Susnik E., Drasler B., Taladriz-Blanco P., Petri-Fink A., Rothen-Rutishauser B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021;50:5397–5434. doi: 10.1039/d0cs01127d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z., Ho W., Bai X., Li F., Chen Y.J., Zhang X.Q., Xu X. Nanoparticle depots for controlled and sustained gene delivery. J. Contr. Release. 2020;322:622–631. doi: 10.1016/j.jconrel.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Hui Y., Yi X., Hou F., Wibowo D., Zhang F., Zhao D., Gao H., Zhao C.X. Role of nanoparticle mechanical properties in cancer drug delivery. ACS Nano. 2019;13:7410–7424. doi: 10.1021/acsnano.9b03924. [DOI] [PubMed] [Google Scholar]
- 20.Algar W.R., Massey M., Rees K., Higgins R., Krause K.D., Darwish G.H., Peveler W.J., Xiao Z., Tsai H.Y., Gupta R., Lix K., Tran M.V., Kim H. Photoluminescent nanoparticles for chemical and biological analysis and imaging. Chem. Rev. 2021;121:9243–9358. doi: 10.1021/acs.chemrev.0c01176. [DOI] [PubMed] [Google Scholar]
- 21.Grzelczak M., Liz-Marzán L.M., Klajn R. Stimuli-responsive self-assembly of nanoparticles. Chem. Soc. Rev. 2019;48:1342–1361. doi: 10.1039/c8cs00787j. [DOI] [PubMed] [Google Scholar]
- 22.Ong S.Y., Zhang C., Dong X., Yao S.Q. Recent advances in polymeric nanoparticles for enhanced fluorescence and photoacoustic imaging. Angew. Chem. Int. Ed. Engl. 2021;60:17797–17809. doi: 10.1002/anie.202101964. [DOI] [PubMed] [Google Scholar]
- 23.Pracy P., Loughran S., Good J., Parmar S., Goranova R. Hypopharyngeal cancer: United Kingdom national multidisciplinary guidelines. J. Laryngol. Otol. 2016;130:S104–S110. doi: 10.1017/S0022215116000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trac N.T., Chung E.J. Peptide-based targeting of immunosuppressive cells in cancer, Bioact. Mater. 2020;5:92–101. doi: 10.1016/j.bioactmat.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan W., Li Z., Xie X., Zhang Z.Y., Bian L. Bisphosphonate-based nanocomposite hydrogels for biomedical applications. Bioact. Mater. 2020;5:819–831. doi: 10.1016/j.bioactmat.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barkat A., Beg S., Panda S.K., S.A K., Rahman M., Ahmed F.J. Functionalized mesoporous silica nanoparticles in anticancer therapeutics. Semin. Cancer Biol. 2021;69:365–375. doi: 10.1016/j.semcancer.2019.08.022. [DOI] [PubMed] [Google Scholar]
- 27.Majumder J., Taratula O., Minko T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv. Drug Deliv. Rev. 2019;144:57–77. doi: 10.1016/j.addr.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun J., Liu F., Yu W., Fu D., Jiang Q., Mo F., Wang X., Shi T., Wang F., Pang D.W., Liu X. Visualization of vaccine dynamics with quantum dots for immunotherapy. Angew. Chem. Int. Ed. Engl. 2021;60:24275–24283. doi: 10.1002/anie.202111093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filali S., Pirot F., Miossec P. Biological applications and toxicity minimization of semiconductor quantum dots. Trends Biotechnol. 2020;38:163–177. doi: 10.1016/j.tibtech.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Ginsberg N.S., Tisdale W.A. Busting through quantum dot barriers. Nat. Mater. 2022;21:497–499. doi: 10.1038/s41563-022-01246-w. [DOI] [PubMed] [Google Scholar]
- 31.Boey A., Ho H.K. All roads lead to the liver: metal nanoparticles and their implications for liver health. Small. 2020;16 doi: 10.1002/smll.202000153. [DOI] [PubMed] [Google Scholar]
- 32.Jiang X., He C., Lin W. Supramolecular metal-based nanoparticles for drug delivery and cancer therapy. Curr. Opin. Chem. Biol. 2021;61:143–153. doi: 10.1016/j.cbpa.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y., Elechalawar C.K., Hossen M.N., Francek E.R., Dey A., Wilhelm S., Bhattacharya R., Mukherjee P. Gold nanoparticles inhibit activation of cancer-associated fibroblasts by disrupting communication from tumor and microenvironmental cells. Bioact. Mater. 2021;6:326–332. doi: 10.1016/j.bioactmat.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X., Yu L., Zhang C., Niu X., Sun M., Yan Z., Wang W., Yuan Z. Tumor acid microenvironment-activated self-targeting & splitting gold nanoassembly for tumor chemo-radiotherapy. Bioact. Mater. 2022;7:377–388. doi: 10.1016/j.bioactmat.2021.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang P., Zhang L., Qin Z., Hua S., Guo Z., Chu C., Lin H., Zhang Y., Li W., Zhang X., Chen X., Liu G. Genetically engineered liposome-like nanovesicles as active targeted transport platform. Adv. Mater. 2018;30 doi: 10.1002/adma.201705350. [DOI] [PubMed] [Google Scholar]
- 36.Gao Z., Li C., Shen J., Ding D. Organic optical agents for image-guided combined cancer therapy. Biomed. Mater. 2021;16 doi: 10.1088/1748-605X/abf980. [DOI] [PubMed] [Google Scholar]
- 37.Vieira D.B., Gamarra L.F. Advances in the use of nanocarriers for cancer diagnosis and treatment. Einstein (Sao Paulo) 2016;14:99–103. doi: 10.1590/S1679-45082016RB3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alsahafi E., Begg K., Amelio I., Raulf N., Lucarelli P., Sauter T., Tavassoli M. Clinical update on head and neck cancer: molecular biology and ongoing challenges. Cell Death Dis. 2019;10:540. doi: 10.1038/s41419-019-1769-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Witzleben A., Wang C., Laban S., Savelyeva N., Ottensmeier C.H. HNSCC: tumour antigens and their targeting by immunotherapy. Cells. 2020;9:2103. doi: 10.3390/cells9092103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson D.E., Burtness B., Leemans C.R., Lui V.W.Y., Bauman J.E., Grandis J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020;6:92. doi: 10.1038/s41572-020-00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H., Torabi S.J., Yarbrough W.G., Mehra S., Osborn H.A., Judson B. Association of human papillomavirus status at head and neck carcinoma subsites with overall survival. JAMA Otolaryngol. Head. Neck. Surg. 2018;144:519–525. doi: 10.1001/jamaoto.2018.0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shellenberger T.D., Weber R.S. Multidisciplinary team planning for patients with head and neck cancer. Oral Maxillofac. Surg. Clin. 2018;30:435–444. doi: 10.1016/j.coms.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 43.Lang J., Hu C., Lu T., Pan J., Lin T. Chinese expert consensus on diagnosis and treatment of nasopharyngeal carcinoma: evidence from current practice and future perspectives. Cancer Manag. Res. 2019;11:6365–6376. doi: 10.2147/CMAR.S197544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mody M.D., Rocco J.W., Yom S.S., Haddad R.I., Saba N.F. Head and neck cancer. Lancet. 2021;398:2289–2299. doi: 10.1016/S0140-6736(21)01550-6. [DOI] [PubMed] [Google Scholar]
- 45.Forastiere A.A., Ismaila N., Lewin J.S., Nathan C.A., Adelstein D.J., Eisbruch A., Fass G., Fisher S.G., Laurie S.A., Le Q.T., O'Malley B., Mendenhall W.M., Patel S., Pfister D.G., Provenzano A.F., Weber R., Weinstein G.S., Wolf G.T. Use of larynx-preservation strategies in the treatment of laryngeal cancer: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2018;36:1143–1169. doi: 10.1200/JCO.2017.75.7385. [DOI] [PubMed] [Google Scholar]
- 46.Sun L., Chin R.I., Gastman B., Thorstad W., Yom S.S., Reddy C.A., Nussenbaum B., Wang S.J., Knackstedt T., Vidimos A.T., Koyfman S.A., Manyam B.V. Association of disease recurrence with survival outcomes in patients with cutaneous squamous cell carcinoma of the head and neck treated with multimodality therapy. JAMA Dermatol. 2019;155:442–447. doi: 10.1001/jamadermatol.2018.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koyfman S.A., Ismaila N., Crook D., D'Cruz A., Rodriguez C.P., Sher D.J., Silbermins D., Sturgis E.M., Tsue T.T., Weiss J., Yom S.S., Holsinger F.C. Management of the neck in squamous cell carcinoma of the oral cavity and oropharynx: ASCO clinical practice guideline. J. Clin. Oncol. 2019;37:1753–1774. doi: 10.1200/JCO.18.01921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun X.S., Tao Y., Le Tourneau C., Pointreau Y., Sire C., Kaminsky M.C., Coutte A., Alfonsi M., Boisselier P., Martin L., Miroir J., Ramee J.F., Delord J.P., Clatot F., Rolland F., Villa J., Magne N., Elicin O., Gherga E., Nguyen F., Lafond C., Bera G., Calugaru V., Geoffrois L., Chauffert B., Zubel A., Zanna C., Brienza S., Crompton P., Rouits E., Gollmer K., Szyldergemajn S., Bourhis J., Debio 1143 and high-dose cisplatin chemoradiotherapy in high-risk locoregionally advanced squamous cell carcinoma of the head and neck: a double-blind, multicentre, randomised, phase 2 study. Lancet Oncol. 2020;21:1173–1187. doi: 10.1016/S1470-2045(20)30327-2. [DOI] [PubMed] [Google Scholar]
- 49.Burtness B., Harrington K.J., Greil R., Soulières D., Tahara M., de Castro G., Jr., Psyrri A., Basté N., Neupane P., Bratland Å., Fuereder T., Hughes B.G.M., Mesía R., Ngamphaiboon N., Rordorf T., Wan Ishak W.Z., Hong R.L., González Mendoza R., Roy A., Zhang Y., Gumuscu B., Cheng J.D., Jin F., Rischin D. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 50.Tang Q.N., Liu L.T., Qi B., Guo S.S., Luo D.H., Sun R., Sun X.S., Chen D.P., Guo L., Mo H.Y., Wang P., Liu S.L., Liang Y.J., Li X.Y., Yang Z.C., Chen Q.Y., Mai H.Q., Tang L.Q. Effect of concurrent chemoradiotherapy with nedaplatin vs cisplatin on the long-term outcomes of survival and toxic effects among patients with stage II to IVB nasopharyngeal carcinoma: a 5-year follow-up secondary analysis of a randomized clinical trial. JAMA Netw. Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.38470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X., Liang H., Li Z., Xue Y., Wang Y., Zhou Z., Yu J., Bu Z., Chen L., Du Y., Wang X., Wu A., Li G., Su X., Xiao G., Cui M., Wu D., Chen L., Wu X., Zhou Y., Zhang L., Dang C., He Y., Zhang Z., Sun Y., Li Y., Chen H., Bai Y., Qi C., Yu P., Zhu G., Suo J., Jia B., Li L., Huang C., Li F., Ye Y., Xu H., Wang X., Yuan Y., J.Y E., Ying X., Yao C., Shen L., Ji J. Perioperative or postoperative adjuvant oxaliplatin with S-1 versus adjuvant oxaliplatin with capecitabine in patients with locally advanced gastric or gastro-oesophageal junction adenocarcinoma undergoing D2 gastrectomy (RESOLVE): an open-label, superiority and non-inferiority, phase 3 randomised controlled trial. Lancet Oncol. 2021;22:1081–1092. doi: 10.1016/S1470-2045(21)00297-7. [DOI] [PubMed] [Google Scholar]
- 52.Patel U., Kannan S., Rane S.U., Mittal N., Gera P., Patil A., Manna S., Shejwal V., Noronha V., Joshi A., Patil V.M., Prabhash K., Mahimkar M.B. Prognostic and predictive roles of cancer stem cell markers in head and neck squamous cell carcinoma patients receiving chemoradiotherapy with or without nimotuzumab. Br. J. Cancer. 2022;126:1439–1449. doi: 10.1038/s41416-022-01730-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khademi S., Sarkar S., Shakeri-Zadeh A., Attaran N., Kharrazi S., Ay M.R., Azimian H., Ghadiri H. Targeted gold nanoparticles enable molecular CT imaging of head and neck cancer: an in vivo study. Int. J. Biochem. Cell Biol. 2019;114 doi: 10.1016/j.biocel.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 54.Das R.K., Panda S., Bhol C.S., Bhutia S.K., Mohapatra S. N-Doped carbon quantum dot (NCQD)-deposited carbon capsules for synergistic fluorescence imaging and photothermal therapy of oral cancer. Langmuir. 2019;35:15320–15329. doi: 10.1021/acs.langmuir.9b03001. [DOI] [PubMed] [Google Scholar]
- 55.Ding H., Cai Y., Gao L., Liang M., Miao B., Wu H., Liu Y., Xie N., Tang A., Fan K., Yan X., Nie G. Exosome-like nanozyme vesicles for H(2)O(2)-responsive catalytic photoacoustic imaging of xenograft nasopharyngeal carcinoma. Nano Lett. 2019;19:203–209. doi: 10.1021/acs.nanolett.8b03709. [DOI] [PubMed] [Google Scholar]
- 56.Kashin M., Kakei Y., Teraoka S., Hasegawa T., Yamaguchi A., Fukuoka T., Sasaki R., Akashi M. Gold nanoparticles enhance EGFR inhibition and irradiation effects in head and neck squamous carcinoma cells, Biomed. Res. Int. 2020;2020 doi: 10.1155/2020/1281645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Misra R., Sarkar K., Lee J., Pizzuti V.J., Lee D.S., Currie M.P., Torregrosa-Allen S.E., Long D.E., Durm G.A., Langer M.P., Elzey B.D., Won Y.Y. Radioluminescent nanoparticles for radiation-controlled release of drugs. J. Contr. Release. 2019;303:237–252. doi: 10.1016/j.jconrel.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 58.Bhardwaj P., Gota V., Vishwakarma K., Pai V., Chaudhari P., Mohanty B., Thorat R., Yadav S., Gurjar M., Goda J.S., Banerjee R. Loco-regional radiosensitizing nanoparticles-in-gel augments head and neck cancer chemoradiotherapy. J. Contr. Release. 2022;343:288–302. doi: 10.1016/j.jconrel.2022.01.040. [DOI] [PubMed] [Google Scholar]
- 59.Lo Y.L., Chang C.H., Wang C.S., Yang M.H., Lin A.M., Hong C.J., Tseng W.H. PEG-coated nanoparticles detachable in acidic microenvironments for the tumor-directed delivery of chemo- and gene therapies for head and neck cancer. Theranostics. 2020;10:6695–6714. doi: 10.7150/thno.45164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yakavets I., Francois A., Lamy L., Piffoux M., Gazeau F., Wilhelm C., Zorin V., Silva A.K.A., Bezdetnaya L. Effect of stroma on the behavior of temoporfin-loaded lipid nanovesicles inside the stroma-rich head and neck carcinoma spheroids. J. Nanobiotechnol. 2021;19:3. doi: 10.1186/s12951-020-00743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davidi E.S., Dreifuss T., Motiei M., Shai E., Bragilovski D., Lubimov L., Kindler M.J.J., Popovtzer A., Don J., Popovtzer R. Cisplatin-conjugated gold nanoparticles as a theranostic agent for head and neck cancer. Head Neck. 2018;40:70–78. doi: 10.1002/hed.24935. [DOI] [PubMed] [Google Scholar]
- 62.Yu C., Long Z., Qiu Q., Liu F., Xu Y., Zhang T., Guo R., Zhong W., Huang S., Chen S. Graphene quantum dots-based targeted nanoprobes detecting drug delivery, imaging, and enhanced chemotherapy of nasopharyngeal carcinoma. Bioeng. Transl. Med. 2022;7 doi: 10.1002/btm2.10270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muhlberger M., Janko C., Unterweger H., Friedrich R.P., Friedrich B., Band J., Cebulla N., Alexiou C., Dudziak D., Lee G., Tietze R. Functionalization of T lymphocytes with citrate-coated superparamagnetic iron oxide nanoparticles for magnetically controlled immune therapy. Int. J. Nanomed. 2019;14:8421–8432. doi: 10.2147/IJN.S218488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J., Wang K., Liang J., Jin J., Wang X., Yan S. Chitosan-tripolyphosphate nanoparticles-mediated co-delivery of MTHFD1L shRNA and 5-aminolevulinic acid for combination photodynamic-gene therapy in oral cancer. Photodiagnosis Photodyn. Ther. 2021;36 doi: 10.1016/j.pdpdt.2021.102581. [DOI] [PubMed] [Google Scholar]
- 65.Wang H.H., Fu Z.G., Li W., Li Y.X., Zhao L.S., Wen L., Zhang J.J., Wen N. The synthesis and application of nano doxorubicin- indocyanine green matrix metalloproteinase-responsive hydrogel in chemophototherapy for head and neck squamous cell carcinoma. Int. J. Nanomed. 2019;14:623–638. doi: 10.2147/IJN.S191069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guirguis M., Bhandari C., Li J., Eroy M., Prajapati S., Margolis R., Shrivastava N., Hoyt K., Hasan T., Obaid G. Membrane composition is a functional determinant of NIR-activable liposomes in orthotopic head and neck cancer. Nanophotonics. 2021;10:3169–3185. doi: 10.1515/nanoph-2021-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bejjanki N.K., Zhong Y., Liu J., Li Q., Xu H., Shen H., Xie M. Surface charge transition nano-theranostics based on ultra-small Fe3O4 nanoparticles for enhanced photodynamic and photothermal therapy against nasopharyngeal carcinoma. Biochem. Biophys. Res. Commun. 2021;557:240–246. doi: 10.1016/j.bbrc.2021.03.168. [DOI] [PubMed] [Google Scholar]
- 68.Zhang X., Li H., Yi C., Chen G., Li Y., Zhou Y., Chen G., Li Y., He Y., Yu D. Host immune response triggered by graphene quantum-dot-mediated photodynamic therapy for oral squamous cell carcinoma. Int. J. Nanomed. 2020;15:9627–9638. doi: 10.2147/IJN.S276153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Su J., Lu S., Jiang S., Li B., Liu B., Sun Q., Li J., Wang F., Wei Y. Engineered protein photo-thermal hydrogels for outstanding in situ tongue cancer therapy. Adv. Mater. 2021;33 doi: 10.1002/adma.202100619. [DOI] [PubMed] [Google Scholar]
- 70.Ren S., Cheng X., Chen M., Liu C., Zhao P., Huang W., He J., Zhou Z., Miao L. Hypotoxic and rapidly metabolic PEG-PCL-C3-ICG nanoparticles for fluorescence-guided photothermal/photodynamic therapy against OSCC. ACS Appl. Mater. Interfaces. 2017;9:31509–31518. doi: 10.1021/acsami.7b09522. [DOI] [PubMed] [Google Scholar]
- 71.Yu T., Tong L., Ao Y., Zhang G., Liu Y., Zhang H. Novel design of NIR-triggered plasmonic nanodots capped mesoporous silica nanoparticles loaded with natural capsaicin to inhibition of metastasis of human papillary thyroid carcinoma B-CPAP cells in thyroid cancer chemo-photothermal therapy. J. Photochem. Photobiol., B. 2019;197 doi: 10.1016/j.jphotobiol.2019.111534. [DOI] [PubMed] [Google Scholar]
- 72.Mapanao A.K., Santi M., Voliani V. Combined chemo-photothermal treatment of three-dimensional head and neck squamous cell carcinomas by gold nano-architectures. J. Colloid Interface Sci. 2021;582:1003–1011. doi: 10.1016/j.jcis.2020.08.059. [DOI] [PubMed] [Google Scholar]
- 73.Dong Y., Liu B., Yuan Y. AIEgen based drug delivery systems for cancer therapy. J. Contr. Release. 2018;290:129–137. doi: 10.1016/j.jconrel.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 74.Dai J., Dong X., Wang Q., Lou X., Xia F., Wang S. PEG-polymer encapsulated aggregation-induced emission nanoparticles for tumor theranostics. Adv. Healthc. Mater. 2021;10 doi: 10.1002/adhm.202101036. [DOI] [PubMed] [Google Scholar]
- 75.Sharma A.K., Gothwal A., Kesharwani P., Alsaab H., Iyer A.K., Gupta U. Dendrimer nanoarchitectures for cancer diagnosis and anticancer drug delivery. Drug Discov. Today. 2017;22:314–326. doi: 10.1016/j.drudis.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 76.Garrigue P., Tang J., Ding L., Bouhlel A., Tintaru A., Laurini E., Huang Y., Lyu Z., Zhang M., Fernandez S., Balasse L., Lan W., Mas E., Marson D., Weng Y., Liu X., Giorgio S., Iovanna J., Pricl S., Guillet B., Peng L. Self-assembling supramolecular dendrimer nanosystem for PET imaging of tumors. Proc. Natl. Acad. Sci. U S A. 2018;115:11454–11459. doi: 10.1073/pnas.1812938115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fu J.J., Guo J.J., Qin A.P., Yu X.Y., Zhang Q., Lei X.P., Huang Y.G., Chen M.Y., Li J.X., Zhang Y., Liu J.P., Dang Y.Y., Wu D., Zhao X.Y., Lin Z.X., Lin Y.L., Li S.P., Zhang L.Y. Bismuth chelate as a contrast agent for X-ray computed tomography. J. Nanobiotechnol. 2020;18:110. doi: 10.1186/s12951-020-00669-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tan Y., Chen M., Chen H., Wu J., Liu J. Enhanced ultrasound contrast of renal-clearable luminescent gold nanoparticles. Angew. Chem. Int. Ed. Engl. 2021;60:11713–11717. doi: 10.1002/anie.202017273. [DOI] [PubMed] [Google Scholar]
- 79.Torelli M.D., Nunn N.A., Shenderova O.A. A perspective on fluorescent nanodiamond bioimaging. Small. 2019;15 doi: 10.1002/smll.201902151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van der Laan K., Hasani M., Zheng T., Schirhagl R. Nanodiamonds for in vivo applications. Small. 2018;14 doi: 10.1002/smll.201703838. [DOI] [PubMed] [Google Scholar]
- 81.Saladino G.M., Vogt C., Li Y., Shaker K., Brodin B., Svenda M., Hertz H.M., Toprak M.S. Optical and X-ray fluorescent nanoparticles for dual mode bioimaging. ACS Nano. 2021;15:5077–5085. doi: 10.1021/acsnano.0c10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.He S., Johnson N.J.J., Nguyen Huu V.A., Cory E., Huang Y., Sah R.L., Jokerst J.V., Almutairi A. Simultaneous enhancement of photoluminescence, MRI relaxivity, and CT contrast by tuning the interfacial layer of lanthanide heteroepitaxial nanoparticles. Nano Lett. 2017;17:4873–4880. doi: 10.1021/acs.nanolett.7b01753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choi J., Kim G., Cho S.B., Im H.J. Radiosensitizing high-Z metal nanoparticles for enhanced radiotherapy of glioblastoma multiforme. J. Nanobiotechnol. 2020;18:122. doi: 10.1186/s12951-020-00684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xie J., Gong L., Zhu S., Yong Y., Gu Z., Zhao Y. Emerging strategies of nanomaterial-mediated tumor radiosensitization. Adv. Mater. 2019;31 doi: 10.1002/adma.201802244. [DOI] [PubMed] [Google Scholar]
- 85.Shi S., Vissapragada R., Abi Jaoude J., Huang C., Mittal A., Liu E., Zhong J., Kumar V. Evolving role of biomaterials in diagnostic and therapeutic radiation oncology. Bioact. Mater. 2020;5:233–240. doi: 10.1016/j.bioactmat.2020.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang H., Mu X., He H., Zhang X.D. Cancer radiosensitizers, trends. Pharmacol. Sci. 2018;39:24–48. doi: 10.1016/j.tips.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 87.Dubey P., Sertorio M., Takiar V. Therapeutic advancements in metal and metal oxide nanoparticle-based radiosensitization for head and neck cancer therapy. Cancers. 2022;14:514. doi: 10.3390/cancers14030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Y., Zhang P., Li F., Jin X., Li J., Chen W., Li Q. Metal-based nanoenhancers for future radiotherapy: radiosensitizing and synergistic effects on tumor cells. Theranostics. 2018;8:1824–1849. doi: 10.7150/thno.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gill M.R., Menon J.U., Jarman P.J., Owen J., Skaripa-Koukelli I., Able S., Thomas J.A., Carlisle R., Vallis K.A. 111In-labelled polymeric nanoparticles incorporating a ruthenium-based radiosensitizer for EGFR-targeted combination therapy in oesophageal cancer cells. Nanoscale. 2018;10:10596–10608. doi: 10.1039/c7nr09606b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chereddy K.K., Payen V.L., Préat V. PLGA: from a classic drug carrier to a novel therapeutic activity contributor. J. Contr. Release. 2018;289:10–13. doi: 10.1016/j.jconrel.2018.09.017. [DOI] [PubMed] [Google Scholar]
- 91.Desai K.G.H. Polymeric drug delivery systems for intraoral site-specific chemoprevention of oral cancer. J. Biomed. Mater. Res. B Appl. Biomater. 2018;106:1383–1413. doi: 10.1002/jbm.b.33943. [DOI] [PubMed] [Google Scholar]
- 92.Bejjanki N.K., Xu H., Xie M. GSH triggered intracellular aggregated-cisplatin-loaded iron oxide nanoparticles for overcoming cisplatin resistance in nasopharyngeal carcinoma. J. Biomater. Appl. 2021;36:45–54. doi: 10.1177/0885328220982151. [DOI] [PubMed] [Google Scholar]
- 93.Karami P., Salkhi Khasraghi S., Hashemi M., Rabiei S., Shojaei A. Polymer/nanodiamond composites - a comprehensive review from synthesis and fabrication to properties and applications. Adv. Colloid Interface Sci. 2019;269:122–151. doi: 10.1016/j.cis.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 94.Cao M., Deng X., Su S., Zhang F., Xiao X., Hu Q., Fu Y., Yang B.B., Wu Y., Sheng W., Zeng Y. Protamine sulfate-nanodiamond hybrid nanoparticles as a vector for MiR-203 restoration in esophageal carcinoma cells. Nanoscale. 2013;5:12120–12125. doi: 10.1039/c3nr04056a. [DOI] [PubMed] [Google Scholar]
- 95.Chipaux M., van der Laan K.J., Hemelaar S.R., Hasani M., Zheng T., Schirhagl R. Nanodiamonds and their applications in cells. Small. 2018;14 doi: 10.1002/smll.201704263. [DOI] [PubMed] [Google Scholar]
- 96.Zhang K. A chemopreventive nanodiamond platform for oral cancer treatment. J. Calif. Dent. Assoc. 2016;44:121–127. [PubMed] [Google Scholar]
- 97.Yang M., Li J., Gu P., Fan X. The application of nanoparticles in cancer immunotherapy: targeting tumor microenvironment. Bioact. Mater. 2021;6:1973–1987. doi: 10.1016/j.bioactmat.2020.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xie X., Feng Y., Zhang H., Su Q., Song T., Yang G., Li N., Wei X., Li T., Qin X., Li S., Wu C., Zhang X., Wang G., Liu Y., Yang H. Remodeling tumor immunosuppressive microenvironment via a novel bioactive nanovaccines potentiates the efficacy of cancer immunotherapy. Bioact. Mater. 2022;16:107–119. doi: 10.1016/j.bioactmat.2022.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Moosavian S.A., Bianconi V., Pirro M., Sahebkar A. Challenges and pitfalls in the development of liposomal delivery systems for cancer therapy. Semin. Cancer Biol. 2021;69:337–348. doi: 10.1016/j.semcancer.2019.09.025. [DOI] [PubMed] [Google Scholar]
- 100.He X., Zhou S., Quinn B., Jahagirdar D., Ortega J., Abrams S.I., Lovell J.F. HPV‐associated tumor eradication by vaccination with synthetic short peptides and particle‐forming liposomes. Small. 2021;17 doi: 10.1002/smll.202007165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thakare S., Shaikh A., Bodas D., Gajbhiye V. Application of dendrimer-based nanosensors in immunodiagnosis. Colloids Surf. B Biointerfaces. 2022;209 doi: 10.1016/j.colsurfb.2021.112174. [DOI] [PubMed] [Google Scholar]
- 102.Meng X., Lou Q.Y., Yang W.Y., Wang Y.R., Chen R., Wang L., Xu T., Zhang L. The role of non-coding RNAs in drug resistance of oral squamous cell carcinoma and therapeutic potential. Cancer Commun. 2021;41:981–1006. doi: 10.1002/cac2.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang C.S., Chang C.H., Tzeng T.Y., Lin A.M., Lo Y.L. Gene-editing by CRISPR-Cas9 in combination with anthracycline therapy via tumor microenvironment-switchable, EGFR-targeted, and nucleus-directed nanoparticles for head and neck cancer suppression. Nanoscale. Horiz. 2021;6:729–743. doi: 10.1039/d1nh00254f. [DOI] [PubMed] [Google Scholar]
- 104.Bhirde A.A., Patel V., Gavard J., Zhang G., Sousa A.A., Masedunskas A., Leapman R.D., Weigert R., Gutkind J.S., Rusling J.F. Targeted killing of cancer cells in vivo and in vitro with EGF-directed carbon nanotube-based drug delivery. ACS Nano. 2009;3:307–316. doi: 10.1021/nn800551s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu Y., Castro Bravo K.M., Liu J. Targeted liposomal drug delivery: a nanoscience and biophysical perspective. Nanoscale. Horiz. 2021;6:78–94. doi: 10.1039/d0nh00605j. [DOI] [PubMed] [Google Scholar]
- 106.Xu F., Liu Z., Liu R., Lu C., Wang L., Mao W., Zhu Q., Shou H., Zhang K., Li Y., Chu Y., Gu J., Ge D. Epigenetic induction of tumor stemness via the lipopolysaccharide-TET3-HOXB2 signaling axis in esophageal squamous cell carcinoma. Cell Commun. Signal. 2020;18:17. doi: 10.1186/s12964-020-0510-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ghosh S., Carter K.A., Lovell J.F. Liposomal formulations of photosensitizers. Biomaterials. 2019;218 doi: 10.1016/j.biomaterials.2019.119341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang X., Tang J., Li C., Lu Y., Cheng L., Liu J. A targeting black phosphorus nanoparticle based immune cells nano-regulator for photodynamic/photothermal and photo-immunotherapy. Bioact. Mater. 2021;6:472–489. doi: 10.1016/j.bioactmat.2020.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Li Q., Zhou R., Xie Y., Li Y., Chen Y., Cai X. Sulphur-doped carbon dots as a highly efficient nano-photodynamic agent against oral squamous cell carcinoma. Cell Prolif. 2020;53 doi: 10.1111/cpr.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cohen E.M., Ding H., Kessinger C.W., Khemtong C., Gao J., Sumer B.D. Polymeric micelle nanoparticles for photodynamic treatment of head and neck cancer cells. Otolaryngol. Head Neck Surg. 2010;143:109–115. doi: 10.1016/j.otohns.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 111.Rao L., Bu L.L., Ma L., Wang W., Liu H., Wan D., Liu J.F., Li A., Guo S.S., Zhang L., Zhang W.F., Zhao X.Z., Sun Z.J., Liu W. Platelet-facilitated photothermal therapy of head and neck squamous cell carcinoma. Angew. Chem. Int. Ed. Engl. 2018;57:986–991. doi: 10.1002/anie.201709457. [DOI] [PubMed] [Google Scholar]
- 112.Lee D., Kwon S., Jang S.Y., Park E., Lee Y., Koo H. Overcoming the obstacles of current photodynamic therapy in tumors using nanoparticles. Bioact. Mater. 2022;8:20–34. doi: 10.1016/j.bioactmat.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim E.E. Radionanomedicine: combined nuclear and nanomedicine. J. Nucl. Med. 2019;60:873.1–87873. [Google Scholar]
- 114.Sonju J.J., Dahal A., Singh S.S., Jois S.D. Peptide-functionalized liposomes as therapeutic and diagnostic tools for cancer treatment. J. Contr. Release. 2021;329:624–644. doi: 10.1016/j.jconrel.2020.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moosavian S.A., Sahebkar A. Aptamer-functionalized liposomes for targeted cancer therapy. Cancer. Lett. 2019;448:144–154. doi: 10.1016/j.canlet.2019.01.045. [DOI] [PubMed] [Google Scholar]
- 116.Tang J., Zhang X., Cheng L., Liu Y., Chen Y., Jiang Z., Liu J. Multiple stimuli-responsive nanosystem for potent, ROS-amplifying, chemo-sonodynamic antitumor therapy. Bioact. Mater. 2022;15:355–371. doi: 10.1016/j.bioactmat.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu L., Yeudall W.A., Yang H. Folic acid-decorated polyamidoamine dendrimer exhibits high tumor uptake and sustained highly localized retention in solid tumors: its utility for local siRNA delivery. Acta Biomater. 2017;57:251–261. doi: 10.1016/j.actbio.2017.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yu C., Li L., Hu P., Yang Y., Wei W., Deng X., Wang L., Tay F.R., Ma J. Recent advances in stimulus-responsive nanocarriers for gene therapy. Adv. Sci. 2021;8 doi: 10.1002/advs.202100540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Feng Q., Wilhelm J., Gao J. Transistor-like ultra-pH-sensitive polymeric nanoparticles. Acc. Chem. Res. 2019;52:1485–1495. doi: 10.1021/acs.accounts.9b00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lee Y., Thompson D.H. Interdiscip. Rev. Nanomed. Nanobiotechnol. vol. 9. Wiley; 2017. Stimuli-responsive liposomes for drug delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cheng X., Gao J., Ding Y., Lu Y., Wei Q., Cui D., Fan J., Li X., Zhu E., Lu Y., Wu Q., Li L., Huang W. Multi-functional liposome: a powerful theranostic nano-platform enhancing photodynamic therapy. Adv. Sci. 2021;8 doi: 10.1002/advs.202100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen W., Goldys E.M., Deng W. Light-induced liposomes for cancer therapeutics. Prog. Lipid Res. 2020;79 doi: 10.1016/j.plipres.2020.101052. [DOI] [PubMed] [Google Scholar]
- 123.Sun C., Li X., Du X., Wang T. Redox-responsive micelles for triggered drug delivery and effective laryngopharyngeal cancer therapy. Int. J. Biol. Macromol. 2018;112:65–73. doi: 10.1016/j.ijbiomac.2018.01.136. [DOI] [PubMed] [Google Scholar]
- 124.Zununi Vahed S., Salehi R., Davaran S., Sharifi S. Liposome-based drug co-delivery systems in cancer cells, Mater. Sci. Eng. C Mater. Biol. Appl. 2017;71:1327–1341. doi: 10.1016/j.msec.2016.11.073. [DOI] [PubMed] [Google Scholar]
- 125.Tan C., Wang J., Sun B. Biopolymer-liposome hybrid systems for controlled delivery of bioactive compounds: recent advances. Biotechnol. Adv. 2021;48 doi: 10.1016/j.biotechadv.2021.107727. [DOI] [PubMed] [Google Scholar]
- 126.Wu D., Zhao Z., Wang N., Zhang X., Yan H., Chen X., Fan Y., Liu W., Liu X. Fluorescence imaging-guided multifunctional liposomes for tumor-specific phototherapy for laryngeal carcinoma. Biomater. Sci. 2020;8:3443–3453. doi: 10.1039/d0bm00249f. [DOI] [PubMed] [Google Scholar]
- 127.Chariou P.L., Ortega-Rivera O.A., Steinmetz N.F. Nanocarriers for the delivery of medical, veterinary, and agricultural active ingredients. ACS Nano. 2020;14:2678–2701. doi: 10.1021/acsnano.0c00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Guo J., Yu Z., Sun D., Zou Y., Liu Y., Huang L. Two nanoformulations induce reactive oxygen species and immunogenetic cell death for synergistic chemo-immunotherapy eradicating colorectal cancer and hepatocellular carcinoma. Mol. Cancer. 2021;20:10. doi: 10.1186/s12943-020-01297-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guo J., Yu Z., Das M., Huang L. Nano codelivery of oxaliplatin and folinic acid achieves synergistic chemo-immunotherapy with 5-fluorouracil for colorectal cancer and liver metastasis. ACS Nano. 2020;14:5075–5089. doi: 10.1021/acsnano.0c01676. [DOI] [PubMed] [Google Scholar]
- 130.Fang J., Islam W., Maeda H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020;157:142–160. doi: 10.1016/j.addr.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 131.Mohammadpour R., Dobrovolskaia M.A., Cheney D.L., Greish K.F., Ghandehari H. Subchronic and chronic toxicity evaluation of inorganic nanoparticles for delivery applications. Adv. Drug Deliv. Rev. 2019;144:112–132. doi: 10.1016/j.addr.2019.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Luo Z., Li Z., Xie Z., Sokolova I.M., Song L., Peijnenburg W., Hu M., Wang Y. Rethinking nano-TiO(2) safety: Overview of toxic effects in humans and aquatic animals. Small. 2020;16 doi: 10.1002/smll.202002019. [DOI] [PubMed] [Google Scholar]
- 133.Mamot C., Ritschard R., Wicki A., Stehle G., Dieterle T., Bubendorf L., Hilker C., Deuster S., Herrmann R., Rochlitz C. Tolerability, safety, pharmacokinetics, and efficacy of doxorubicin-loaded anti-EGFR immunoliposomes in advanced solid tumours: a phase 1 dose-escalation study. Lancet Oncol. 2012;13:1234–1241. doi: 10.1016/S1470-2045(12)70476-X. [DOI] [PubMed] [Google Scholar]
- 134.Subbiah V., Grilley-Olson J.E., Combest A.J., Sharma N., Tran R.H., Bobe I., Osada A., Takahashi K., Balkissoon J., Camp A., Masada A., Reitsma D.J., Bazhenova L.A. Phase Ib/II trial of NC-6004 (nanoparticle cisplatin) plus gemcitabine in patients with advanced solid tumors. Clin. Cancer Res. 2018;24:43–51. doi: 10.1158/1078-0432.CCR-17-1114. [DOI] [PubMed] [Google Scholar]
- 135.Hu Y., Paris S., Barsoumian H., Abana C.O., He K., Wasley M., Younes A.I., Masrorpour F., Chen D., Yang L., Dunn J.D., Zhang J., Gandhi S., Nguyen Q.N., Cortez M.A., Welsh J. Radiation therapy enhanced by NBTXR3 nanoparticles overcomes Anti-PD1 resistance and evokes abscopal effects. Int. J. Radiat. Oncol. Biol. Phys. 2021;111:647–657. doi: 10.1016/j.ijrobp.2021.06.041. [DOI] [PubMed] [Google Scholar]
- 136.Zhang P., Darmon A., Marill J., Mohamed Anesary N., Paris S. Radiotherapy-activated hafnium oxide nanoparticles produce abscopal effect in a mouse colorectal cancer model. Int. J. Nanomed. 2020;15:3843–3850. doi: 10.2147/IJN.S250490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bonvalot S., Rutkowski P.L., Thariat J., Carrère S., Ducassou A., Sunyach M.P., Agoston P., Hong A., Mervoyer A., Rastrelli M., Moreno V., Li R.K., Tiangco B., Herraez A.C., Gronchi A., Mangel L., Sy-Ortin T., Hohenberger P., de Baère T., Le Cesne A., Helfre S., Saada-Bouzid E., Borkowska A., Anghel R., Co A., Gebhart M., Kantor G., Montero A., Loong H.H., Vergés R., Lapeire L., Dema S., Kacso G., Austen L., Moureau-Zabotto L., Servois V., Wardelmann E., Terrier P., Lazar A.J., Bovée J., Le Péchoux C., Papai Z. NBTXR3, a first-in-class radioenhancer hafnium oxide nanoparticle, plus radiotherapy versus radiotherapy alone in patients with locally advanced soft-tissue sarcoma (Act.In.Sarc): a multicentre, phase 2-3, randomized, controlled trial, Lancet. Oncol. 2019;20:1148–1159. doi: 10.1016/S1470-2045(19)30326-2. [DOI] [PubMed] [Google Scholar]
- 138.Duffaud F., Borner M., Chollet P., Vermorken J.B., Bloch J., Degardin M., Rolland F., Dittrich C., Baron B., Lacombe D., Fumoleau P. Phase II study of OSI-211 (liposomal lurtotecan) in patients with metastatic or loco-regional recurrent squamous cell carcinoma of the head and neck. An EORTC new drug development group study. Eur. J. Cancer. 2004;40:2748–2752. doi: 10.1016/j.ejca.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 139.Creemers J.H.A., Pawlitzky I., Grosios K., Gileadi U., Middleton M.R., Gerritsen W.R., Mehra N., Rivoltini L., Walters I., Figdor C.G., Ottevanger P.B., de Vries I.J.M. Assessing the safety, tolerability and efficacy of PLGA-based immunomodulatory nanoparticles in patients with advanced NY-ESO-1-positive cancers: a first-in-human phase I open-label dose-escalation study protocol. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2021-050725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Oppelt P., Ley J., Daly M., Rich J., Paniello R., Jackson R.S., Pipkorn P., Liu J., Gay H., Palka K., Neupane P., Powell S., Spanos W.C., Gitau M., Zevallos J., Thorstad W., Adkins D. nab-Paclitaxel and cisplatin followed by cisplatin and radiation (Arm 1) and nab-paclitaxel followed by cetuximab and radiation (Arm 2) for locally advanced head and neck squamous-cell carcinoma: a multicenter, non-randomized phase 2 trial. Med. Oncol. 2021;38:35. doi: 10.1007/s12032-021-01479-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Adkins D., Ley J., Atiq O., Powell S., Spanos W.C., Gitau M., Rigden C., Palka K., Liu J., Oppelt P. Nanoparticle albumin-bound paclitaxel with cetuximab and carboplatin as first-line therapy for recurrent or metastatic head and neck cancer: a single-arm, multicenter, phase 2 trial. Oral Oncol. 2021;115 doi: 10.1016/j.oraloncology.2020.105173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Von Hoff D.D., Mita M.M., Ramanathan R.K., Weiss G.J., Mita A.C., LoRusso P.M., Burris H.A., 3rd, Hart L.L., Low S.C., Parsons D.M., Zale S.E., Summa J.M., Youssoufian H., Sachdev J.C. Phase I study of PSMA-targeted docetaxel-containing nanoparticle BIND-014 in patients with advanced solid tumors. Clin. Cancer Res. 2016;22:3157–3163. doi: 10.1158/1078-0432.CCR-15. [DOI] [PubMed] [Google Scholar]
- 143.Zahednezhad F., Zakeri-Milani P., Shahbazi Mojarrad J., Valizadeh H. The latest advances of cisplatin liposomal formulations: essentials for preparation and analysis. Expet Opin. Drug Deliv. 2020;17:523–541. doi: 10.1080/17425247.2020.1737672. [DOI] [PubMed] [Google Scholar]
- 144.Cheng Y., Lemke-Miltner C.D., Wongpattaraworakul W., Wang Z., Chan C.H.F., Salem A.K., Weiner G.J., Simons A.L. In situ immunization of a TLR9 agonist virus-like particle enhances anti-PD1 therapy. J. Immunother. Cancer. 2020;8 doi: 10.1136/jitc-2020-000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rose P.G., Fu F., Chambers L.M., Mei L., De Bernardo R., Prendes B.L., Lamarre E. Incidence of squamous cell carcinomas of the head and neck following prolonged pegylated liposomal doxorubicin. Anti Cancer Drugs. 2020;31:747–750. doi: 10.1097/CAD.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 146.Abu-Khalaf M.M., Baumgart M.A., Gettinger S.N., Doddamane I., Tuck D.P., Hou S., Chen N., Sullivan C., Lezon-Geyda K., Zelterman D., Hatzis C., Deshpande H., Digiovanna M.P., Azodi M., Schwartz P.E., Harris L.N. Phase 1b study of the mammalian target of rapamycin inhibitor sirolimus in combination with nanoparticle albumin-bound paclitaxel in patients with advanced solid tumors. Cancer. 2015;121:1817–1826. doi: 10.1002/cncr.29254. [DOI] [PubMed] [Google Scholar]
- 147.Voskuil F.J., Steinkamp P.J., Zhao T., van der Vegt B., Koller M., Doff J.J., Jayalakshmi Y., Hartung J.P., Gao J., Sumer B.D., Witjes M.J.H., van Dam G.M. Exploiting metabolic acidosis in solid cancers using a tumor-agnostic pH-activatable nanoprobe for fluorescence-guided surgery. Nat. Commun. 2020;11:3257. doi: 10.1038/s41467-020-16814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Singleton D.C., Macann A., Wilson W.R. Therapeutic targeting of the hypoxic tumour microenvironment. Nat. Rev. Clin. Oncol. 2021;18:751–772. doi: 10.1038/s41571-021-00539-4. [DOI] [PubMed] [Google Scholar]
- 149.DeBerardinis R.J. Tumor microenvironment, metabolism, and immunotherapy. N. Engl. J. Med. 2020;382:869–871. doi: 10.1056/NEJMcibr1914890. [DOI] [PubMed] [Google Scholar]