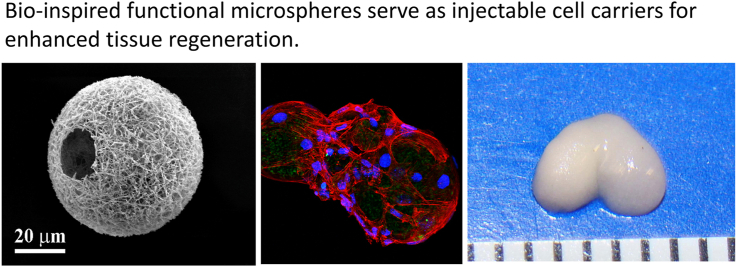
Abstract
As a new type of injectable biomaterials, functional microspheres have attracted increasing attention in tissue regeneration because they possess some advantageous properties compared to other biomaterials, including hydrogels. A variety of bio-inspired microspheres with unique structures and properties have been developed as cellular carriers and drug delivery vehicles in recent years. In this review, we provide a comprehensive summary of the progress of functional and biodegradable microspheres that have been used for tissue regeneration over the last two decades. First, we briefly introduce the biomaterials and general methods for microsphere fabrication. Next, we focus on the newly developed technologies for preparing functional microspheres, including macroporous microspheres, nanofibrous microspheres, hollow microspheres, core-shell structured microspheres, and surface-modified functional microspheres. After that, we discuss the application of functional microspheres for tissue regeneration, specifically for bone, cartilage, dental, neural, cardiac, and skin tissue regeneration. Last, we present our perspectives and future directions of functional microspheres as injectable carriers for the future advancement of tissue regeneration.
Keywords: Microsphere, Injectable, Biodegradable, Tissue engineering, Scaffold
Graphical abstract
Highlights
-
•
Focus on the recent advances of bio-inspired functional microspheres as new type of injectable cell carriers for tissue regeneration.
-
•
Five types of functional microspheres and their fabrication and applications are discussed.
-
•
Challenges and future perspectives are presented to provide inspiration for future advancement of cell-instructive functional microspheres.
1. Introduction
Tissue engineering strategy represents a promising approach to replacing damaged tissue structures and restoring their biological functions. In tissue engineering, a scaffold is an artificial extracellular matrix (ECM) and serves as a template for cell growth and tissue regeneration. A scaffold can be classified as a pre-formed or an injectable scaffold depending on when it is shaped [1]. A pre-formed scaffold has a defined shape prior to its application, whereas an injectable one forms the shape in situ. The administration of an injectable scaffold is performed in a minimally invasive manner; therefore, it decreases the risk of infection and improves patient comfort in clinic. In addition, an injectable scaffold can easily fill any irregularly shaped defects. Given the irregular sizes and complicated structures of defects, an injectable scaffold is more appealing than a pre-formed one in many clinical practices.
Microspheres, sometimes also called microcarriers, are a type of injectable scaffolds. Compared to hydrogels (the other type of injectable scaffolds), microspheres allow cell adhesion and growth on their surfaces prior to injecting the cell/microsphere construct to a defective area. Unlike hydrogels that do not allow cell migration until the outmost of the hydrogels is degraded, the interspaces among the microspheres facilitate cell migration and proliferation to accelerate new tissue formation. Microspheres have high surface area to volume ratios and provide sufficient spaces for cell growth. Furthermore, microspheres with functional structures (e.g., hollow, core-shell) can be readily tailored and fabricated to effectively enhance the delivery of cells and bioactive molecules. Therefore, functional microspheres as a new type of injectable scaffolds have attracted significant attention in recent years [[2], [3], [4]].
Generally, microspheres refer to the spheres with a diameter range between 1 and 1000 μm. However, small sized microspheres (<20 μm in diameter) are not suitable as cellular carriers since the diameter of most cells is larger than 20 μm when the cell adheres and spreads on a biomaterial [5]. Meanwhile, large sized microspheres (>200 μm in diameter) are difficult to be injected out of the needles of routine gauges (gauge 18 or above). Therefore, microspheres as an injectable biomaterial for tissue engineering should be in the range of 20–200 μm. While several review papers on microspheres for regenerative engineering were published [[6], [7], [8], [9]], they did not consider the size requirement of the microspheres when used as cell carriers. Consequently, a few microspheres and their fabrication methods discussed in those papers are not applicable for tissue regeneration. On the other hand, functional microspheres as cellular carriers have been developing rapidly in recent years, and there is a need to summarize the advancement of this new type of injectable biomaterials for tissue engineering. Given the above considerations, we focus on the discussion of functional microspheres with diameters in the range of 20–200 μm in this review. After a brief introduction of the biomaterials and methods for microsphere fabrication, the development and applications of functional microspheres are discussed, including macroporous microspheres, nanofibrous microspheres, hollow microspheres, core-shell structured microspheres, and surface-modified functional microspheres. Finally, the perspectives and future directions of functional microspheres as injectable cellular carriers for future advancement of tissue regeneration are provided.
2. Biomaterials for microspheres
2.1. Polymeric microspheres
Biodegradable polymers are the most widely used building biomaterials for microspheres. Biodegradable polymers can be classified into natural and synthetic polymers. Collagen, gelatin, chitosan, and alginate are the main natural biodegradable polymers, and polycaprolactone (PCL), poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and poly(lactic-co-glycolic acid) (PLGA) are the main synthetic polymers used in tissue engineering. As summarized in Table 1, polymeric microspheres are usually fabricated via emulsification and solvent evaluation, spray drying, phase separation, microfluidics, or electrospray methods.
Table 1.
Summary of methods for microsphere fabrication.
| Fabrication Methods | Materials | Advantages/Disadvantages | Size Range |
|---|---|---|---|
| Emulsification and solvent evaporation | PLGA [10], Chitosan [11], Gelatin [12], PCL/HA [13], Collagen [14], Collagen/chitosan [15] | Simple to operate Limited control over microsphere sizes |
A few hundred nanometers to several millimeters |
| Spray drying | PLA [16], Chitosan [17], Alginate/HA [18] | Simple to operate Convenient to encapsulate bioactive molecules in microspheres Uncontrollable particle size and morphology |
A few micrometers to several hundred micrometers |
| Phase separation | PLGA [19], Poly(l-glutamic acid)/chitosan [20], Chitosan [21] | Porous microspheres Effective for only a few polymers |
A few micrometers to several millimeters |
| Microfluidics | PLGA [22], Bacterial cellulose [23], PCL [24] | Well controlled microsphere sizes Convenient drug loading Low productivity Time consuming |
A few micrometers to several millimeters |
| Electrospray | PLGA [25], PLA [26], PCL/gelatin [27], | Simple to operate Uncontrollable particle size and morphology |
A few micrometers to several hundred micrometers |
Collagen is the major component of natural ECM in many tissues, including skin, bone, and cartilage [28]. Collagen is conducive for cell adhesion, proliferation, and differentiation. The conventional fabrication method of collagen microspheres is emulsification. During the fabrication process, a water-in-oil (w/o) emulsion is generated to form collagen droplets that are subsequently underwent gelation to form collagen microspheres [14]. The emulsion process generates microspheres with a wide range of size distributions, so it is necessary to sieve the microspheres to obtain the desired sizes. Modification of collagen crosslinking density in the collagen's network can adjust the degradation rate and therefore control the release of bioactive factors within the microspheres [29].
Gelatin is derived from collagen by breaking down the triple-helical structure of collagen. Gelatin contains the sequence of arginine-glycine-aspartic acid (RGD) that is the specific recognition site of integrins and is involved in regulating the interactions between cells and ECM [30]. Generally, gelatin microspheres are fabricated via emulsification [12], spray drying [31], or electrospray [32]. Among them, emulsification is the most commonly used method because of the simple fabrication process. The resulting gelatin microspheres usually have sizes ranged from 1 to 1000 μm, and a sieving step is required to obtain desired sizes. Crosslinking of gelatin strands is necessary to improve the thermal and mechanical stability under the physiological conditions. Studies have shown that drug and biomolecules can be readily loaded into gelatin microspheres via diffusion, which avoids the contact with harsh organic substances during the crosslinking reaction [33].
Chitosan is a partial deacetylated form of chitin obtained mainly from the exoskeleton of crustaceans. The degree of deacetylation represents the molar ratio of the d-glucosamine units to the sum of both N-acetyl-d-glucosamine and d-glucosamine units [34]. Depolymerization of chitosan occurs by enzymes like glucosaminidases, lipases, and lysozyme. Chitosan is depolymerized into bioactive chito-oligosaccharides with superior antimicrobial properties. Chitosan is biodegradable and has excellent biocompatibility because its degradation product (glucosamine) is neutral or slightly alkaline and can be metabolized or excreted from the body [35,36]. Chitosan microspheres are produced via an emulsion method [37,38], freeze-drying [39], phase separation [20], or spray drying [17,40]. Among those techniques, the emulsion method is simple and has high product yields, while freeze-drying and phase separation can create porous structure in chitosan microspheres, and spray drying is desirable for powder formulation.
Alginate originates from natural polysaccharides present in brown algae or the cell walls of brown seaweed. Alginate lacks cell adhesive ability, therefore, a covalent modification with a cell adhesion motif such as RGD is needed to promote cell attachment and growth [41]. Alginate microspheres can be formulated through techniques such as emulsification, spray drying, or microfluidics methods [42]. Among them, emulsification is the most widely used for the formulation of alginate microspheres. Divalent cations, such as calcium chloride, are used for alginate microsphere formation. The sizes of microspheres are controlled by a variety of parameters, including viscosity of alginate solutions, organic solvents in the oil phase, surfactant concentrations, and stirring speeds [43]. Cells encapsulated in alginate microspheres have high living/dead ratios owing to the mild processing condition.
Synthetic polymeric materials have minimum risks of disease transmission and have well-controlled degradation rates [44]. In addition, they exhibit excellent reproducible mechanical properties such as tensile strength and elastic modulus. As synthetic biodegradable polymers, PCL, PLA, PGA, and PLGA undergo hydrolytic degradation in the body. The degradation time varies from days to years by altering the type of polymers, the molecular weight, and the structure of microspheres [45]. Synthetic polymeric materials are widely used to fabricate microspheres [44,46]. An oil-in-water (o/w) emulsification process is the most common method for the preparation of polymer microspheres. Hydrophobic drugs can be readily encapsulated into microspheres due to the hydrophobicity of the polymers [47]. To efficiently encapsulate hydrophilic and amphiphilic drug/protein, the water-in-oil-in-water (w/o/w) double emulsification method is used [48].
Although possessing a wide array of advantages, polymeric materials have relatively weak mechanical strengths when they are used alone, which limits their wide application in tissue regeneration. To improve their mechanical properties, polymeric materials can be blended with inorganic components to form composite microspheres [[49], [50], [51]].
2.2. Bioceramic microspheres
Bioceramics have excellent biocompatibility and adequate mechanical properties [52,53]. Compared with polymers, bioceramics exhibit an osteoconductive property that facilitates mineralized tissue regeneration [54]. Selective inorganic bioceramic materials, such as hydroxyapatite (HA), has similar chemical composition to natural bone. Apatite microspheres can be formed via a wet precipitation reaction between orthophosphoric acid and calcium hydroxide. During the wet precipitation reaction, apatite powders were added to a polymer solution, and then dropped into a calcium chloride crosslinking solution. The beads were washed, dried, and sintered to produce apatite microspheres [55,56]. Beta-tricalcium phosphate (β-TCP) and biphasic calcium phosphate (BCP) are two other bioceramic materials used for microsphere fabrication. Compared with HA, β-TCP shows better biodegradability [57]. β-TCP microspheres can be fabricated via a solid-in-oil-in-water (s/o/w) emulsification method [58]. For the s/o/w approach, nanosized inorganic particles/powders are first added into an organic solvent to form a mixture. Under a constant stirring condition, the mixture is dispersed in water to form microsphere precursors. A sintering step of heating to over 1000 °C is crucial to form bioceramic microspheres. BCP ceramics, which are composed of poorly degradable HA and biodegradable β-TCP, have been considered as promising bone replacement materials due to their improved biocompatibility and osteoconductivity. BCP microspheres can be fabricated via a s/o/w emulsification method [59]. However, a major limitation of the s/o/w method is that microspheres with diameters less than 200 μm are difficult to be fabricated by this method. Overall, the methods to effectively fabricate bioceramic microspheres at the range of 20–200 μm are limited.
2.3. Composite microspheres
Composite microspheres are fabricated from two or more components that have a synergistic effect on providing a better mechanical and/or biochemical property compared to the individual components. For example, incorporation of HA into PLGA generated composite microspheres with increased hydrophilicity and osteoconductivity [60,61]. With the increase of HA content, the protein adsorption capacity of the composite microspheres significantly increased [61,62]. Composite microspheres are fabricated via emulsification [13,61,63], spray drying [18] or airflow shearing [64]. Emulsification is the most common method to fabricate composite microspheres [65]. Apart from the addition of inorganic nanoparticles in a polymeric solution (oil phase) at the first step, all other steps are the same as those described for the fabrication of polymeric microspheres. Due to the low solubility of inorganic nanoparticles in the oil phase, a large amount of the nanoparticles are migrated into the water phase during the stirring process, which leads to a low percentage of the nanoparticles encapsulated in the composite microspheres. Therefore, the ratios between different components within the microspheres deviated from their initial ratios in the solution. Airflow shearing method can fabricate composite microspheres with desired sizes depending on nozzle sizes. For example, HA/PLGA microspheres were prepared with various proportions of HA [64]. The HA/PLGA suspension extruded from the nozzle was cut into uniform droplets by a steady airflow. One limitation is that the nozzle was easily blocked because of the poor dispersion of HA in the PLGA solution.
3. Preparation of functional microspheres
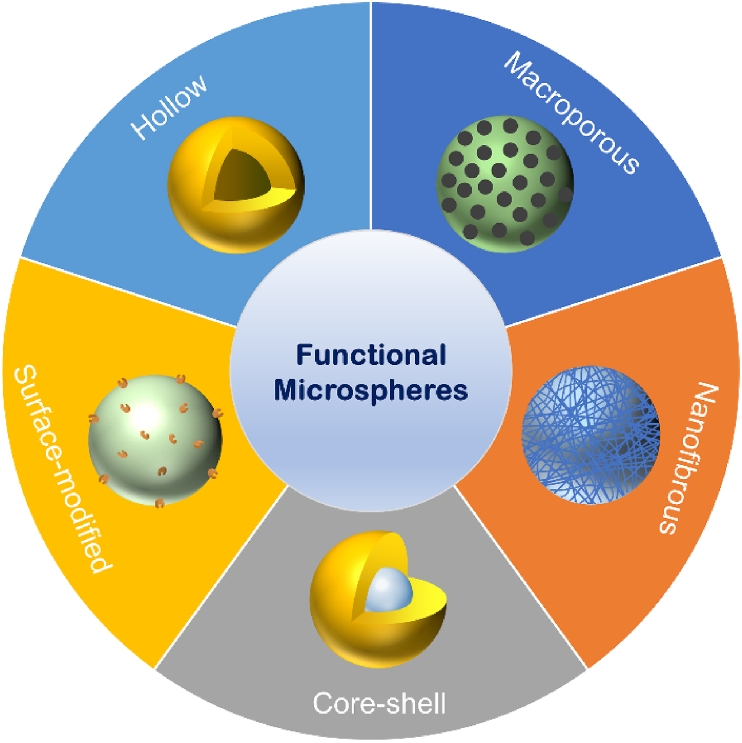
Microspheres fabricated from conventional methods usually form a thin and smooth layer on the surface, which is suboptimal for cell adhesion and migration. In addition, these microspheres have a solid-interior structure, therefore, possess limited surface areas for cell proliferation. Meanwhile, most of the microspheres do not present appropriate biophysical and biochemical cues to regulate cellular activity, neo tissue formation, and wound healing. Therefore, conventional microspheres cannot fulfil the increasingly complicated requirements for tissue regeneration. To address these challenges, functional microspheres that possess high surface areas and present unique biophysical and biochemical cues to targeted cells have been explored in recent years. In this section, we introduce five types of functional microspheres (Fig. 1), as well as the methods and technologies for the fabrication of these functional microspheres.
Fig. 1.
Illustration of five types of functional microspheres.
3.1. Macroporous microspheres
Introduction of macropores into microspheres increases the surface areas of microspheres and facilitates cell adhesion and migration. As cells adhere to the surfaces of microspheres, the porous structure of the microspheres facilitates the cells to extend their pseudopodia and lamellipodia into the pores and enhance cell-material interactions. The macropores allow cell migration inside the microspheres and therefore provide more spaces for cell growth and neo tissue formation. In addition, the porous structure of microspheres provides a variety of choices to load bioactive molecules into the microspheres, leading to a beneficial microenvironment for tissue regeneration.
The preparation of macroporous microspheres usually involves an emulsion/solvent evaporation process that includes a porogen. Many porogens, such as ammonium bicarbonate [66], ethanol [67], camphene [68], and paraffin [69] have been explored for the fabrication of macroporous microspheres. The porogens are removed after the microspheres are hardened, leaving the porous structure in the microspheres.
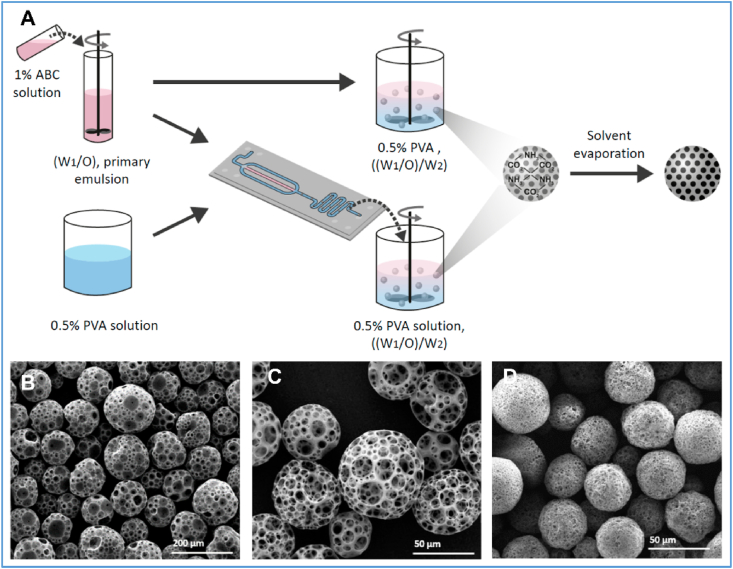
While emulsification and solvent evaporation is the most popular method to fabricate macroporous microspheres, microfluidics has attracted increasing attention because it can efficiently produce particles at the micrometer level with controlled microstructure and narrow size distribution [70]. For example, when the ammonium bicarbonate/PLGA emulsion was introduced to poly(vinyl alcohol) (PVA) solution via a microfluidic droplet generation chip, macroporous PLGA microspheres with a narrow size distribution were generated. Moreover, the particle size and porosity of the microspheres can be precisely controlled by tuning parameters such as flow rates (Fig. 2) [71].
Fig. 2.
Fabrication of macroporous PLA and PLGA microspheres using a conventional double emulsion process and a combination of double emulsion with microfluidic fabrication. (A) A schematic illustration of the fabrication of the macroporous microspheres. (B) Macroporous PLA microspheres fabricated under the condition of 0.3 mL/min 0.5% PVC solution. (B) Macroporous PLA microspheres fabricated under the condition of 0.6 mL/min 0.5% PVC solution. (D) Macroporous PLGA7525 microspheres fabricated under the condition of 0.6 mL/min 0.5% PVC solution. Adapted from Ref. [71], copyright 2019 MDPI.
Microspheres can also be used as porogens to form macroporous microspheres. In one study, chitosan microspheres were selected as a sacrificial template to generate macroporous microspheres of chitin [72]. After a w/o emulsification and hardening process, the entrapped chitosan microspheres were removed via immersing chitin microspheres in an acetic acid solution, leading to the formation of macroporous chitin microspheres. The macropores on the microspheres were at the range of 60–140 μm, which is too large and significantly affects the mechanical properties given the microsphere diameters are smaller than 200 μm. Further modification of the fabrication parameters is needed to obtain uniform chitin microspheres with better morphology and mechanical strengths.
3.2. Nanofibrous microspheres (NF-MS)
In tissue engineering, scaffolds act as a temporary artificial ECM for cell adhesion and growth. Many natural ECM components are organized in nanoscale fibrillar constructs. For example, type I collagen is the major protein of the ECM of many tissues, and the collagen is assembled into nanofibers ranging from 50 to 500 nm in diameter to regulate various cellular behaviors [73]. Thus, a scaffold (cell carrier) with an ECM-mimicking nanofibrous structure presents an advantageous microenvironment than its smooth (non-nanofibous) counterpart, as evidenced by the increased cytocompatibility and cell-matrix interaction from numerous studies [74].
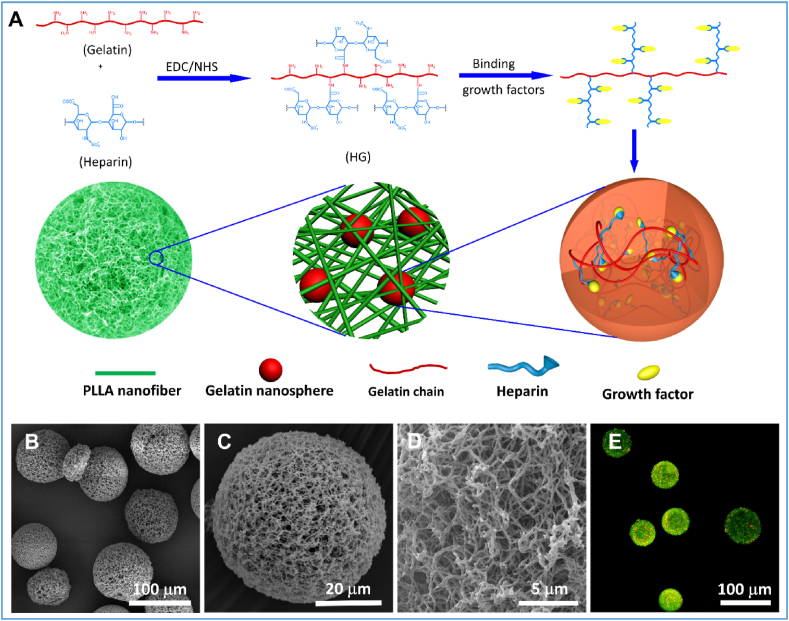
Electrospinning is the most widely used approach to prepare ECM-like nanofibers due to its simplicity and applicability to many biomaterials. Electrospinning is generally useful to generate nanofibrous polymeric sheets but has a significant challenge of forming injectable nanofibrous microspheres. An alternative method is the formation of short nanofibers by electrospinning and ultrasonic homogenization, followed by the electrospray of the short nanofibers [27]. The obtained microspheres are composed of loose short nanofibers, therefore, have low mechanical strengths and limited applications. A more useful approach to construct nanofibrous structure is the combination of emulsification with a thermally induced phase separation (TIPS) or self-assembly [[75], [76], [77], [78]]. For example, chitin-based nanofibrous microspheres (NF-MS) were generated in a NaOH/urea aqueous solution via thermally induced self-assembly [78]. The size of the chitin NF-MS was ranged from 3 to 130 μm and was controlled by varying the oil/water ratio, surfactant amount, and stirring speed. At a low temperature, the urea-NaOH-chitin chain complex and its nanofibrous aggregates co-existed in a dilute solution. As the temperature elevated, the urea-NaOH sheath around the chitin was destroyed, and the chitin chains self-aggregated into nanofibers to form NF-MS. Our group generated hierarchical nanofibrous microspheres via the combination of a water-in-oil-in-oil (w/o/o) double emulsion process and a TIPS process (Fig. 3) [75]. In the hierarchical microspheres, the growth factor binds with heparin and is encapsulated into heparin-conjugated gelatin (HG) nanospheres, which are further immobilized in the injectable nanofibrous microspheres. This system allows the integration of nanofibrous architecture with controlled growth factor delivery into one injectable microsphere. The hierarchical nanofibrous microspheres show great promise for bone tissue regeneration [75].
Fig. 3.
Fabrication growth factor-loaded nanofibrous microspheres. (A) Schematic illustration of the hierarchical nanofibrous microspheres. Growth factors bind with heparin and are encapsulated into heparin-conjugated nanospheres, which are further immobilized in a nanofibrous microsphere. (B) The SEM image of the hierarchical nanofibrous microspheres. (C) SEM image of a typical hierarchical nanofibrous microsphere, showing that the microsphere was self-assembled with nanofibers. (D) A high-magnification image of (C), showing the nanofibers on the surface of the microsphere. (E) A 2D cross-sectional confocal image of the growth-factor loaded nanofibrous microspheres, showing the growth factor-loaded nanospheres (red) encapsulated in nanofibrous microspheres (green). Adapted from Ref. [75] with minor modification. Copyright 2015 WILEY-VCH Verlag GmbH & Co.
The use of TIPS to generate nanofibers is technically demanding and needs to be performed under the proper conditions. Previously successful attempts of generating nanofibrous matrices using the TIPS method were empirical and lacked theoretical guidance. In a recent publication, our group reported a new methodology to generate polymer nanofibers through control of the polymer-solvent interaction parameters [79]. By integrating the Flory-Huggins classic lattice model and Hildebrand-Scott solubility parameter equation, we derived a critical polymer-solvent interaction parameter equation and applied it as a criterion to predict the formation of nanofibers from TIPS. Using this methodology, we successfully fabricated nanofibrous microspheres with 15 widely used biodegradable polymers. This approach is universally effective to fabricate nanofibers from polymeric materials, therefore, greatly expands the modality to design and fabricate nanofibrous microspheres.
3.3. Hollow microspheres
Hollow microparticles have attracted increasing attention because of the hierarchical 3D structure that allows to incorporate high dosages of drugs inside the microspheres and releases them at a controlled rate [80]. In such a case, the hollow microspheres act as both cell carriers and drug delivery vehicles. Polymers, ceramics, and composites have been tested for the fabrication of hollow microspheres using the template approaches, such as emulsion solvent extraction/evaporation [[81], [82], [83], [84]], microfluidics [85], and electrospraying [86]. Generally, inorganic materials, non-crosslinked polymeric microparticles, liquid droplet, or gas are employed as sacrificial templates to create the hollow structure. For example, borate glass microparticles were used as a template to fabricate hollow HA microspheres via ion exchange in a solution containing PO43− and OH−. As the glass core continued to dissolve under a weak basic condition, Ca2+ and other ions were dissolved and reacted with PO43− and OH− to form amorphous calcium phosphate (ACP) on the surface of the glass microparticles. Afterwards, ACP was converted to HA toward the formation of hollow ceramic microspheres. This approach, however, was time-consuming, and it took 2–7 days to complete the conversion process [87].
In one study, gas was directly used as a template to fabricate hollow microspheres in a microfluidic chip [85]. Microdroplets were composed of a gas core and an organic phase shell and were generated at a single junction on a silicon chip. By adjusting the ratio of the inner/middle/continuous phases and the flow rates, the diameters of the hollow microspheres can be controlled. However, the thickness of the shell of the microspheres was approximately 3 μm, leading to potential collapse of the spherical structure.
Electrospray is a technique to generate microparticles. This technique applies high voltage to the conductive liquid at the needle tip to form microdroplets. In contrast to electrospinning that uses high polymer concentrations to form nano- or microfibers, electrospray adopts low polymer concentrations to generate microparticles that usually do not possess spherical morphologies or hollow structures. Recently, PCL-based polyurethane hollow microspheres were fabricated under certain conditions [86]. The hollow microspheres exhibited spherical morphologies with an average diameter of 29 μm. While this is a one-step facile method, it is unclear whether this method can be extended for the fabrication of other polymeric hollow microspheres.
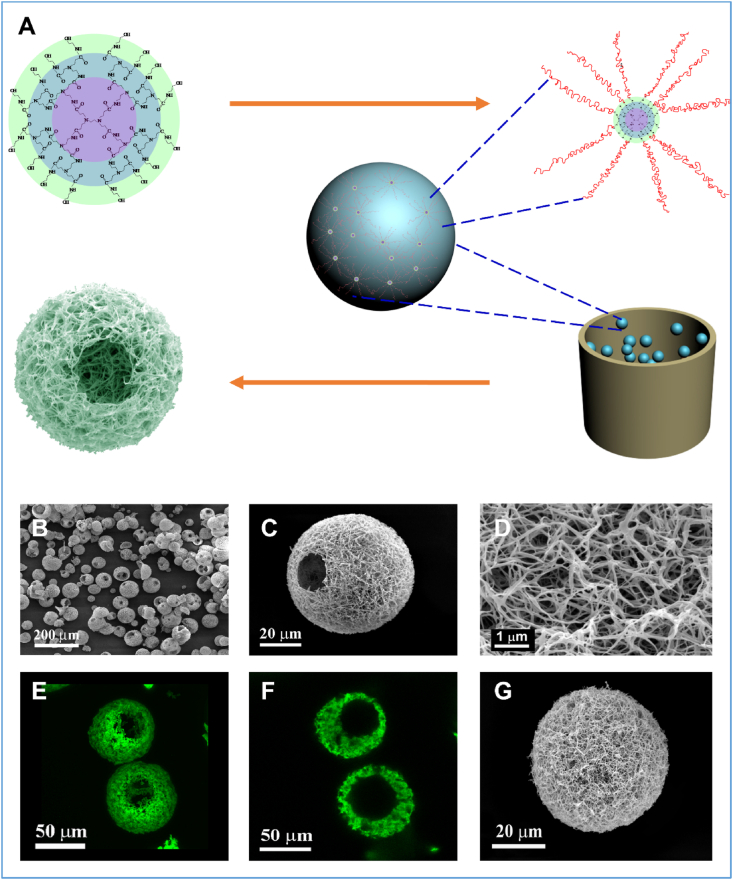
A unique approach was recently developed to introduce nanofibrous architecture into hollow microspheres (Fig. 4) [88]. The nanofibrous hollow microspheres were composed of nanofibers with an average diameter of 160 nm, which is at the same length scale as collagen fibers in natural ECM. The hollow structure is attributed to the removal of glycerol in the core of the microspheres after emulsification and phase separation. This method eliminates the potential complications associated with surfactant removal because no surfactant is added during the emulsification process. One striking feature of the nanofibrous hollow microspheres is the formation of open pores on the shell of the microspheres that facilitate cell migration inside the microspheres. The diameter of open pores ranged from 10 to 50 μm depending on the polymer concentration. The formation of open holes on the shells of the nanofibrous hollow microspheres were attributed to the star-shaped architecture of the polymer. Because of the nanofibrous and hollow architecture, those microspheres had an overall density of less than 1/30 of the density of the solid-interior microspheres. In addition, the surface area of the nanofibrous hollow microspheres was more than three orders of magnitude higher than that of the solid-interior counterparts. These advantageous features make the nanofibrous hollow microspheres an excellent injectable cell carrier for cartilage regeneration [82].
Fig. 4.
Fabrication of nanofibrous hollow microspheres. (A) Schematic illustration of the nanofibrous hollow microsphere formation. (B) The SEM image of nanofibrous hollow microspheres fabricated, showing open holes on the shell. (C) SEM image of a representative nanofibrous hollow microsphere, showing the nanofibrous architecture and a hole of approximately 20 μm on the microsphere shell. (D) A high-magnification image of the microsphere in (C), showing the nanofibers. (E) A 3D reconstruction of nanofibrous hollow microspheres from confocal image stacks. (F) A 2D cross-section confocal image of the nanofibrous hollow microspheres, confirming the hollow structure of the microsphere. (G), SEM image of a representative nanofibrous microsphere, showing the nanofibrous architecture on the microsphere surface. (H) SEM image of a representative solid-interior microsphere, showing the smooth surface of the microsphere. Adapted from Ref. [88], copyright 2011 Macmillan Publishers Limited.
3.4. Core-shell structured microspheres
Core-shell microspheres (CS-MSs) are composed of a core (inner material) and a shell (outer layer material). Like hollow microspheres, CS-MSs have a hierarchical structure and can act as a cell carrier and deliver bioactive molecules in a well-controlled manner. Both the components and the geometries of the core and shell regulate the release behaviors. Therefore, by adjusting the polymer component, mass ratio, and relative spatial location of the core-shell system, the release kinetics of the encapsulated molecules can be precisely controlled [89,90]. Generally, the shell serves as an effective diffusion barrier that slows down the diffusion of hydrophilic drugs from the core, thus alleviating the initial burst release. Apart from bioactive molecules, cells can also be loaded within the core on-demand and are protected for in vitro cell proliferation and in vivo tissue regeneration. Moreover, the cells are encapsulated in an ECM-mimicking microenvironment in which bioactive molecules are released to the surrounding tissues.
Fabrication of a core-shell structure relies on the immiscibility between the core and shell material since the wall-like structure is produced by phase separation of the immiscible polymers. Alginate is widely selected as the shell biomaterial because alginate readily forms a gel to cover the core surface when contacting with Ca2+ in aqueous solution. Traditionally, CS-MSs are fabricated via two-step or single-step double emulsion methods. For the two-step method, a polymeric core is first generated. Afterwards, the core is dispersed in another polymeric solution and re-emulsified in an extracting solvent to solidify the shell. For the single-step method, the drug-suspended polymer solution is emulsified in another polymer solution, followed by solvent extraction and evaporation in the external aqueous phase. Therefore, the single-step method is known as the w1/o1/o2/w2 or s/o1/o2/w emulsification method. Owing to the high shear employed in both approaches, the encapsulation efficiencies are far from being satisfactory with poor reproducibility and variable sizes and morphologies of the resulting CS-MSs [91].
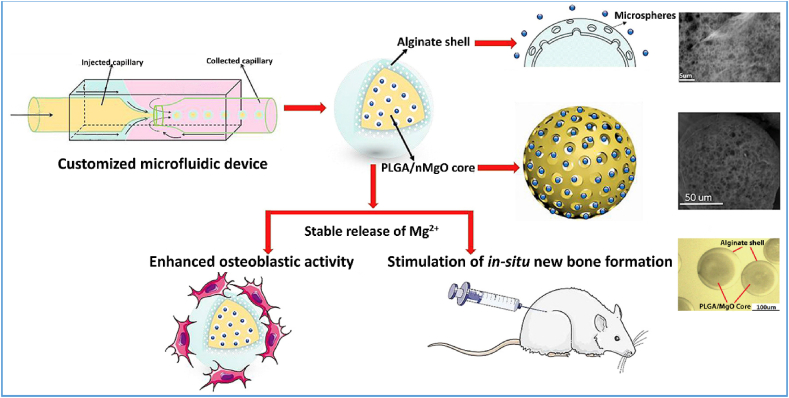
Microfluidics overcomes some of the above drawbacks and is used to fabricate CS-MSs with a narrow size distribution [92,93]. In one study, PLGA/MgO-alginate core-shell microspheres were prepared by the o/w/o emulsification in a microfluidic capillary device (Fig. 5) [92]. The obtained core-shell microspheres had an average size of 115 μm, while the PLGA/MgO core had an average size of 113 μm.
Fig. 5.
Schematic illustration of the fabrication of PLGA/MgO-Alginate core-shell structured microspheres for bone regeneration. Adapted from Ref. [92] with modification, copyright 2018 Elsevier Ltd.
Despite its elaborate structure, CS-MSs have some limitations when used as a cell carrier. First, it is difficult to incorporate functional groups on the surface, because the modification process is prone to destroy the core-shell configuration owing to the softness of the shell layer. Furthermore, the core-shell structure may impede efficient solvent evaporation during microsphere fabrication, leading to excessive residual organic solvents that are accumulated inside the CS-MS. More work is needed to address those problems.
3.5. Surface-modified microspheres
Many synthetic polymeric biomaterials have been explored as injectable microspheres for tissue regeneration. However, a major limitation of the synthetic polymeric microspheres lies in their lack of biological cues to regulate cell functions. A variety of strategies have been developed to modify the surface of the microspheres to improve their bioactivity. Surface coating is one of the most used methods to enhance protein adsorption and cell-material interaction. Surface coating is a physical process and can be achieved either from in situ microsphere synthesis or from a post-synthesis process. Collagen coating is a pragmatic approach for bioinert materials owing to the capability of collagen to efficiently interact with cell surface receptors and facilitate cell adhesion and proliferation. The surface of PLGA microspheres was modified by soaking the microspheres in a collagen solution overnight, and the resultant microspheres significantly promoted cell proliferation [66]. Apart from collagen, other natural protein-based biomacromolecules such as chitosan [94], gelatin [95] and polydopamine [96] were also used as surface coating regents for microspheres.
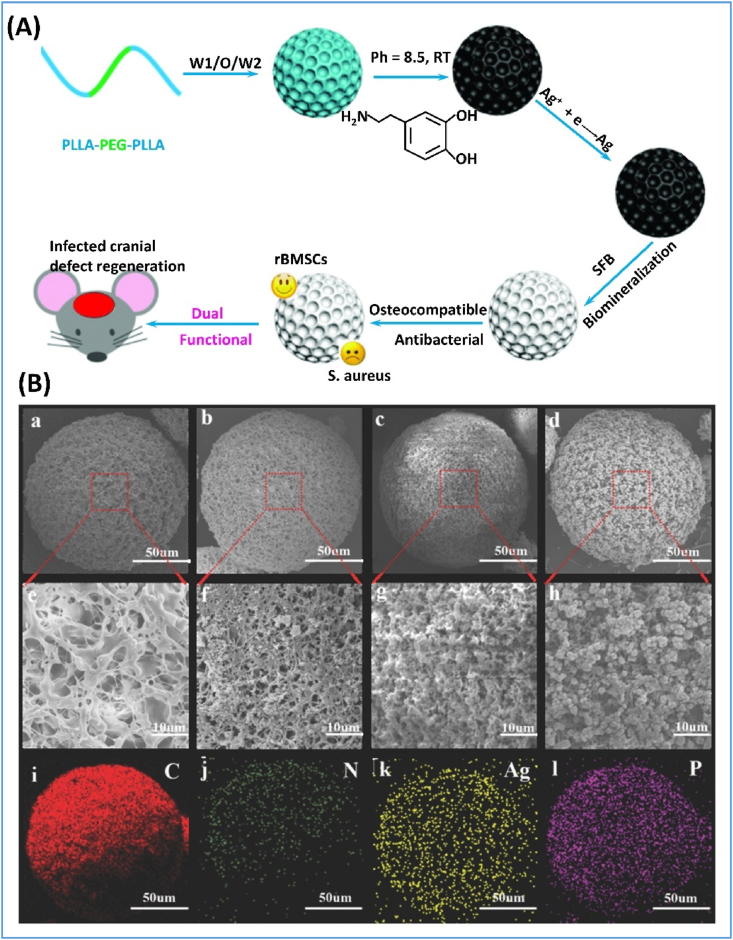
Microspheres were also modified with minerals on the surfaces when the microspheres were used for mineralized tissue regeneration. As an example of the biomineralization method, coating of apatite onto the microsphere surfaces is achieved through the immersion of the microspheres in a simulated body fluid (SBF). Poly(l-lactic acid)-polyethylene glycol (PLLA-PEG) microspheres were prepared using a double emulsion method, and the microspheres were subsequently coated with polydopamine via oxidization polymerization, which provided nucleation sites for apatite deposition from the SBF (Fig. 6) [97]. Furthermore, drugs and growth factors were immobilized in the coating layer for sustained release [95,98].
Fig. 6.
Fabrication of surface-modified microspheres. (A) Schematic illustration of dual-purpose porous microspheres modified with nanosilver and apatite for bone regeneration. (B) Characterization of the surface modified microspheres. (a–h) SEM images of microspheres prepared at different conditions and (i–l) the corresponding elemental mapping images. (a,e,i) PEG10%; (b,f,j) PEG10%+polydopamine coating; (c,g,k) PEG10%+polydopamine coating+Ag; (d,h,l) PEG10%+polydopamine coating+Ag+SFB coating. Adapted from Ref. [97] with modification, copyright 2018 WILEY-VCH Verlag GmbH & Co.
Chemical modification is another approach to introduce cell-adhesive peptides or functional groups onto the surfaces of microspheres [99]. Various techniques that facilitate cell bonding or bio-active grafting have been reported, including aminolysis or hydrolysis [[100], [101], [102]], plasma polymerization [103] and photopolymerization [104]. Surface hydrolysis or aminolysis is a simple and accessible method to increase the hydrophilicity of the surface and improve cell adhesion and proliferation on microspheres. For example, after PLA microspheres were incubated in NaOH or ethylenediamine aqueous solution for aminolysis and hydrolysis, the microsphere surfaces were modified with hydrophilic amine, hydroxyl and carboxyl groups, which improved cell attachment, adhesion, and proliferation [100]. Similarly, functional domains like RGD could be introduced to increase cell adhesion and other functions [105]. During the surface modification, a precise control of the hydrolysis or aminolysis is important since this process alters polymer chain structures and therefore the degradation rates.
4. Applications of functional microspheres for tissue regeneration
As injectable biomaterials, functional microspheres can be conveniently injected into damaged or dysfunctional tissues, and minimal trauma is expected during the transplantation process. More importantly, functional microspheres possess many other unique features, which make them excellent cell carriers and drug delivery vehicles for tissue repair and regeneration. Increasing efforts have been made to explore the applications of functional microspheres in the regeneration of different tissues [3,15,83,[106], [107], [108], [109]]. In this section, the applications of functional injectable microspheres in bone, cartilage, dentin-pulp complex, neural tissue, cardiac tissue, and skin regeneration are discussed.
4.1. Functional microspheres for bone tissue regeneration
As the mineral component of natural bone, calcium phosphate possesses inherent biocompatibility and osteogenic activity. Therefore, introduction of calcium phosphate into functional microspheres is an effective approach to promote bone regeneration. HA/chitosan microspheres using vanillin as a crosslinker accelerated MG63 cell growth [65]. Similarly, chitosan microspheres coated with apatite enhanced MC3T3-E1 cell attachment, proliferation, and differentiation [11]. PLGA microspheres modified with –NH2 groups promoted osteogenic differentiation of MSCs, while those modified with –OH groups induced chondrogenic differentiation [103].
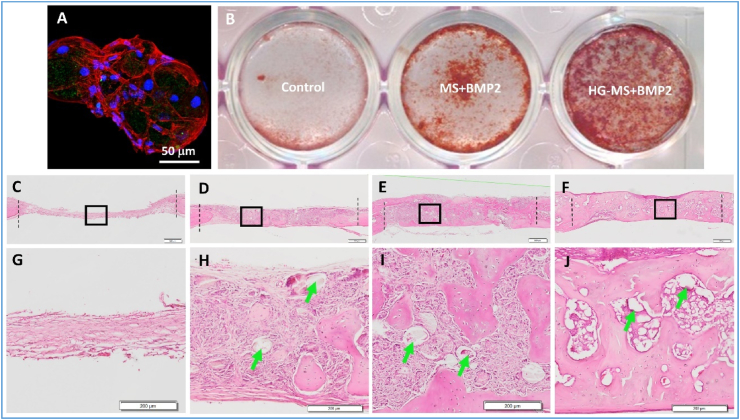
Generally, microspheres have limited effects in promoting osteogenesis, and the incorporation of osteo-inductive factors into microspheres is needed to enhance bone regeneration. Various bioactive reagents including growth factors (e.g., BMPs), drugs (e.g., simvastatin, icariin and FTY720), microRNAs, ions (e.g., Mg2+) and platelet rich plasma (PRP) were encapsulated in microspheres to accelerate bone regeneration [[110], [111], [112], [113], [114], [115]]. Among them, BMPs are the most important ones. The strategy of using exogenous BMPs to accelerate bone regeneration has been employed for decades. Physical adsorption of BMPs is a simple method to encapsulate the growth factor onto microspheres. However, physical adsorption led to a high initial burst release and the majority of the BMPs was released within a short period of time [15,116]. To overcome this barrier, researchers developed a method to bind BMP2 to heparin and encapsulated it into heparin-conjugated gelatin nanospheres [75]. Heparin binding stabilized BMP2 and protected it from denaturation and proteolytic degradation. The BMP2-loaded nanospheres were then immobilized in the injectable nanofibrous microspheres [75]. Using this method, the BMP2 was released in a multiple-controlled manner from the hierarchical microspheres. Specifically, the release of BMP2 from the hierarchical microspheres was regulated by 1) the association with heparin; 2) the degradation of the nanospheres, and 3) the physical adsorption of the nanofibers. Therefore, the hierarchical structures provided excellent control over the release of the BMP2 from the microspheres. The sizes of BMP2-loaded nanofibrous microspheres were ranged from 106 to 150 μm, which is appropriate for BMSCs to grab and spread on the microspheres to form BMSC/microsphere aggregates. The formation of BMSC/microsphere aggregates after injection prevent the migration of BMSCs and diffusion of microspheres to unintended sites, which is beneficial for defect repair and regeneration. Consequently, the BMP2-loaded nanofibrous microspheres greatly enhanced BMSC differentiation, mineralization, and new bone tissue formation (Fig. 7). This unique system was later expanded to treat diabetes-induced alveolar bone loss [77].
Fig. 7.
Bone regeneration from nanofibrous microspheres. (A) A confocal image of BMSCs adhering to heparin-modified nanofibrous microspheres (HG-MS). (B) The proliferation of BMSCs on the control (2D culture dish), BMP2-loaded MS, and BMP2-loaded HG-MS. (C–J) The H&E staining images of the calvarial bony defects 6 weeks after implantation. (C, G) the blank control group. (D, H) the MS group. (E, I) BMP2-loaded MS group. (F, J) BMP2-loaded HG-MS group. Green arrows indicate residual microspheres in the tissues. Adapted from Ref. [75] with minor modification. Copyright 2015 WILEY-VCH Verlag GmbH & Co.
Co-loading and sequential delivery of bioactive factors provided synergistic effects. In one study, osteogenic growth peptide (OGP) was first incorporated in porous PLGA microspheres by a simple solution-dipping method. Next, BMP2-containing multilayered polyelectrolyte coating was fabricated on the surface of the OGP-loaded PLGA microspheres using a layer-by-layer self-assembly process. This approach showed a sequential and prolonged release of both BMP2 and OGP, leading to accelerated BMSC proliferation and osteogenic differentiation [117]. Moreover, in a triple-delivery microsphere system, three bioactive molecules (IL-2, TGF-β, and miR-10a) was integrated into PLLA/PEG microspheres [110]. The mesoporous silica nanoparticles released IL-2/TGF-β to locally recruit T cells, while the PLGA microspheres are used to release miR-10a to stimulate T cells differentiation towards regulatory T cells (Tregs), and the PLLA served as a scaffold for Treg adhesion and proliferation. This system resulted in significant Treg enrichment and Treg-mediated immune therapy against bone loss in a mouse periodontitis model. A study based on a periodontal disease model showed that these molecules had a synergistic effect, mediated immune homeostasis, and rescued alveolar bone loss [110].
Since microspheres can serve as both a cell carrier and a bioactive factor delivery vehicle, loading cells onto functional microspheres is an effective approach to accelerate bone tissue formation and remodeling. For example, GelMA microspheres that co-delivered BMSCs and BMP2 accelerated the differentiation and mineral deposition of BMSCs, and promoted osseous tissue formation in a rabbit femoral defect model [118]. In another study, the surfaces of nanofibrous PLLA microspheres were modified with polydopamine (PDA) and heparin, which was capable of immobilizing and slowly releasing BMP2 [96]. Combination of the surface modified microspheres with human exfoliated deciduous teeth stem cells (SHED) showed a strong synergistic effect on bone tissue regeneration in a calvarial defect mouse model.
Angiogenesis plays a critical role in bone regeneration [119]. Newly formed blood vessels not only allow the infusion of oxygen and nutrients to the metabolically active forming bone callus, but also function as a route through which inflammatory cells and precursor cells reach the injury site [120]. Growth factors like vascular endothelial growth factor (VEGF) and fibroblast growth factor-2 (FGF2) are potent inducers of endothelial cells during angiogenesis, and microspheres loaded with these bioactive factors promoted angiogenesis and bone regeneration. In one study, FGF2 and BMP2 were loaded into the PLLA core and the PLGA shell, respectively, and the growth factors were sequentially released from the core-shell double-walled microsphere system. In vivo rat bone graft model suggested that this sequential delivery efficiently accelerated the bridging and remodeling of the critical-sized bone grafts [121]. Platelet-derived growth factor (PDGF) is another angiogenic factor. Released by degranulating platelets, PDGF upregulates VEGF expression, and is capable of recruiting osteoblasts and promoting cell proliferation, thus it has shown great potential in inducing bone regeneration [122]. Microspheres with a simvastatin-encapsulated PLLA core and a PDGF-loaded alginate shell were fabricated [3]. The system displayed sequential and controlled release of simvastatin and PDGF, and promoted neo bone formation with better mechanical strength and quality [3]. A study examined the effect of PRP-loaded alginate microspheres on the in vitro differentiation of BMP2-modified-MSC as well as on the ex vivo fracture defect [115]. Results showed that the combination of PRP and BMP2 significantly increased BMSC differentiation and bone formation, likely because BMP2 promoted BMSCs differentiation, while sustained release of cytokines from PRP accelerated MSCs proliferation and differentiation.
In clinical settings, bone loss often occurs along with infection, and excessive inflammation disturbs new bone formation. Therefore, the dual strategy comprising anti-bacterial and pro-osteogenesis therapies holds great promise for effective bone regeneration. Strontium ranelate-loaded PLGA microspheres assembled with silver nanoparticles and HA nanoparticles via a solid-in-oil nano-suspension approach displayed desirable osteo-induction and antibiosis functions [123]. Similarly, polydopamine-coated PEG/PLLA porous microspheres were loaded with nano-silver via reduction of silver nitrate along with loading of apatite via surface biomineralization [97]. Prominent anti-infection and pro-osteogenesis effects were observed. Besides silver components, antibacterial drugs and peptides [124] were also used for bone regeneration. For instance, vancomycin was adsorbed onto mesoporous silica nanoparticles and encapsulated into strontium-loaded microspheres. When the multifunctional microspheres were injected to the S. aureus-created infected rabbit femoral condyle defect, significant antibacterial activity and efficient new bone formation were observed after 12 weeks post-operation [125]. Clarithromycin is known to exhibit antibacterial property against a broad-spectrum of Gram-positive bacteria and some Gram-negative bacteria. The sustained release of clarithromycin from PLGA microspheres within β-TCP showed significantly high amount of new tissue formation [126].
4.2. Functional microspheres for cartilage tissue regeneration
Cartilage has limited capacity of self-repair or regeneration owing to its unique avascular microstructure. Functional microspheres were tested for cartilage regeneration because they offer advantages such as promoting chondrocyte-oriented differentiation and maintaining the differentiated chondrocyte-like cell phenotype. For example, macroporous poly(l-glutamic acid)/chitosan polyelectrolyte microspheres promoted chondrocyte attachment and proliferation. After subcutaneous injection for 8 weeks, the polyelectrolyte microspheres loaded with chondrocytes produced cartilaginous matrices that are significantly more than those that chitosan microspheres generated [20]. In addition, when synthetic polypeptides poly(γ-benzyl-ι-glutamate) was used as the raw material for microsphere fabrication and gelatin as the porogen, the resultant macroporous microspheres exhibited advantageous bioactivities that enhanced articular chondrocytes proliferation and cartilaginous extracellular matrix deposition [127].
Cell source is a critical factor that affects cartilage regeneration. In vitro chondrocyte expansion is often required to obtain sufficient cell number for implantation since chondrocytes are only available in limited quantities. Therefore, finding alternative cell sources are important for cartilage regeneration. MSCs isolated from autologous sources like bone marrow (for BMSCs) and adipose tissues (for ADSCs) are relatively accessible and hypoimmunogenic. Moreover, they possess multi-lineage differentiation potential and self-renewal capability [128]. BMSCs cultured within porous PLGA microspheres displayed enhanced cell proliferation, up-regulated expression of cartilage-related mRNAs and proteins, and improved cartilage regeneration [129], confirming the feasibility of using BMSCs in cartilage regeneration. Moreover, open-porous PLGA microspheres facilitated BMSC infiltration and migration into the inner space of the microspheres, and up-regulated chondrogenic markers like COL-II, aggrecan, and SOX-9. In addition, accelerated cartilage regeneration in a rat knee articular osteochondral defect model was detected [129].
Signaling molecules, such as TGF-β, BMP-4 and FGF-2, are widely explored to promote chondrogenesis. For example, combination of human ADSCs and TGF-β3-conjugated PLGA microspheres enhanced cartilage regeneration [107]. Moreover, cytomodulin-conjugated nanofibrous microspheres efficiently stimulated chondrocyte proliferation and prevented cartilage hypertrophy [83]. To further prolong the retention of encapsulated cells and microspheres in the defect site, researchers introduced hydrogels to the microsphere system and successfully facilitated the proliferation of chondrogenic progenitor cells [130] and the repair of the cartilage defect with a normal tissue structure [131]. Kartogenin (KGN) is a small molecule drug to induce chondrogenesis and reconstruct the subchondral bone. In one study, a controlled drug delivery system comprised of dimethyloxalyl glycine (DMOG)-loaded hydroxypropyl chitin hydrogel and KGN-conjugated chitosan microspheres was developed and showed effectively osteochondral regeneration [132].
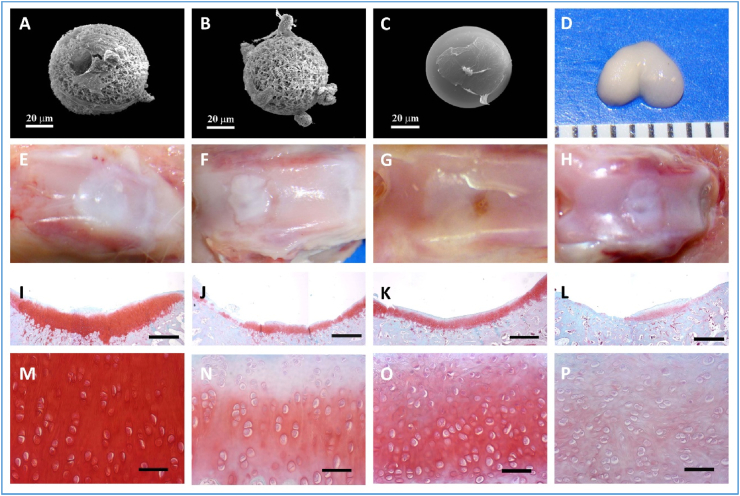
Our group fabricated nanofibrous hollow microspheres based on star-shaped PLLA polymers via a surfactant-free emulsification process, which significantly facilitated chondrocyte adhesion, migration, and the cartilage tissue formation in vitro. Moreover, in a critical-size rabbit osteochondral defect-repair model, the nanofibrous hollow microspheres achieved substantially better cartilage repair than other groups, including the chondrocyte group that simulates the clinically autologous chondrocyte implantation (ACI) procedure, indicating that the nanofibrous hollow microspheres are an excellent injectable cell carrier for cartilage regeneration [88] (Fig. 8). Large animal models are expected to test the effectiveness of the nanofibrous hollow microspheres for full-length hyaline cartilage regeneration.
Fig. 8.
Regenerative of hyaline cartilage using nanofibrous hollow microspheres. (A) The 3D morphology of chondrocytes on nanofibrous hollow microspheres, showing that some chondrocytes migrated inside the holes. (B) The 3D morphology of chondrocytes on nanofibrous microspheres. (C) The morphology of chondrocytes on solid-interior microspheres, showing that the chondrocytes were flat and spread over a large area. (D) De novo cartilage tissue formation with the anatomical shape of a rat femoral condyle after culture for four weeks, showing that the newly formed tissue from the nanofibrous hollow microspheres. (E–P) Critical-size rabbit osteochondral defect repair eight weeks after injection. (E, I, M) the nanofibrous hollow microspheres/chondrocytes group. (F, J, N) the PEG/chondrocytes group. (G, K, O) the chondrocytes-alone group. (H, K, P) the sham control group. (I–P) safranin-O staining. The scale bars represent 1 mm in (I–L), and 100 μm in (M − P). Adapted from Ref. [88], copyright 2011 Macmillan Publishers Limited.
4.3. Functional microspheres for dental tissue regeneration
Dentin-pulp complex regeneration is the core topic of regenerative endodontics. The hurdles of dentin-pulp regeneration lie in several aspects. First, the limited space of root canal prevents the utilization of traditional pre-set scaffolds. Second, dental pulp is a vascularized connective tissue with a small canal opening which limits nutrient diffusion and the ingrowth of blood vessels, therefore, full-length pulp regeneration with ample angiogenesis is difficult to achieve. Moreover, tubular dentin is difficult to reconstruct. Functionalized microspheres possess advantageous properties like controllable injectability, allowing rapid oxygen/nutrient diffusion, supporting cell viability and bioactivity, which make them attractive for endodontic regeneration [133]. Research has shown that nanofibrous PLLA-b-PLYS microspheres and surface-modified PLGA microspheres enhanced dental pulp stem cells (DPSCs) attachment, proliferation, and odontogenic differentiation in vitro [4,66]. Given the hypoxic microenvironment within the root canal system, hypoxia-primed hDPSCs-microsphere complexes were examined to enhance angiogenesis and form odontoblast-like cells lining along the dentin-pulp interface after implantation inside the pulp cavities of rabbit molars [108]. Those results, however, could not address the challenge of regeneration pulp-dentin complex in a full-length tooth root.
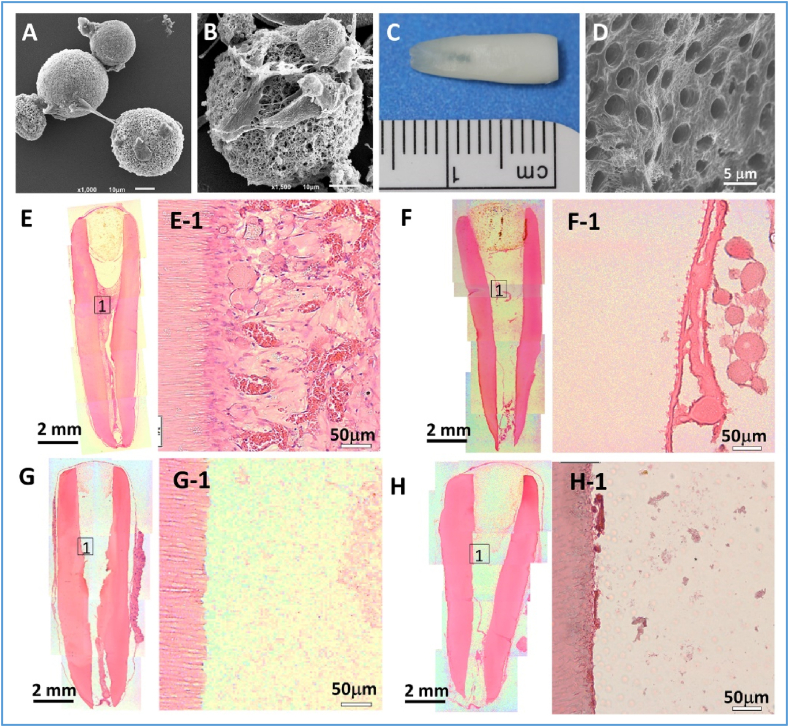
Our group recently reported a hierarchical nanofibrous microsphere system for pulp regeneration in a full-length human tooth root [76]. In that microsphere system, VEGF was first encapsulated into heparin-conjugated gelatin nanospheres, which was further immobilized into the PLLA nanofibrous microspheres. The VEGF-heparin binding protects VEGF from denaturation or proteolytic degradation, and the double immobilization prolongs its sustained release. The in vivo results showed that the pulp-like tissues which contained many neo blood vessels were successfully regenerated throughout the entire apical and middle thirds of the root canal and even reached the coronal third at some regions, indicating a promising future for the full-length pulp regeneration (Fig. 9). Moreover, polarized DPSCs aligned along the pulp-dentin interface and tubular dentin were also observed, showing the potential of functional dentin-pulp complex regeneration. An in situ large animal model is pivotal to test the effectiveness of the hierarchical microspheres for regenerative endodontics.
Fig. 9.
Pulp-dentin complex regeneration using a hierarchical growth factor-loaded nanofibrous microsphere scaffolding system. (A) An SEM image showing the DPSCs that adhere to the surfaces of the heparin-modified nanofibrous microspheres (NF-MS). (B) A typical SEM image of the DPSCs adhering to a NF-MS surface, showing the close interaction between the cell and the microsphere. (C) A photographic image of a full-length root. (D) A SEM image of the open dentin tubules of the full-length root. (E–H) Hematoxylin and eosin (H&E) staining images of regenerated pulp-like tissues in the full-length root canal after in vivo implantation for nine weeks. (E) VEGF-loaded NF-MS/DPSC group. (F) VEGF-loaded NF-MS group. (G) DPSC group. (H) Empty control group. Adapted from Ref. [76], 2016 Elsevier Ltd.
4.4. Functional microspheres for neural tissue regeneration
Neurotrophic factors, such as nerve growth factor (NGF), glial cell derived neurotrophic factor (GDNF), brain-derived neurotrophic factor (BDNF) and fibroblast growth factors (FGF), have been demonstrated to improve nerve regeneration both in vitro and in vivo [[134], [135], [136], [137], [138], [139]]. To provide sustained and controlled release of these factors, biocompatible and biodegradable microspheres have been prepared and tested in a series of studies. For example, BDNF-loaded PLGA microspheres promoted the migration and neural differentiation of adipose-derived stem cells (ADSCs), ameliorated nerve conduction velocity, and prevented neuralgic amyotrophy [138]. Moreover, the use of heparin as a mediator to bind β-FGF to poly(d,l-lactide-co-1,3-trimethylene carbonate) microparticles resulted in a sustained release of β-FGF for over 4 weeks [140]. Similar results were observed in core-shell microspheres [135]. In that work, NGF-loaded PLGA microspheres were embedded in chitosan microspheres and crosslinked with tripolyphosphate (TPP), and the resultant TPP/chitosan/PLGA-NGF microspheres protected the bioactivity of NGF, improved the quality of axon outgrowth, and enhanced functional recovery after nerve injury [135]. Besides growth factors, drugs like FK506 have been shown to enhance nerve regeneration following peripheral nerve injuries. One study indicated that FK506-encapsulated PLGA microspheres in fibrin gels regenerated more motor and sensory neurons compared to control groups [141].
Microspheres were also tested as cell carriers for neural tissue regeneration [14,142,143]. In one study, neural progenitors were encapsulated into chitosan-based microspheres and injected into the lesion cavity of rat brains with sustained cortical injury [142]. The cells exhibited mature neuronal and glial markers and a significant functional recovery was observed. In another study, neural stem cells and endothelial cells were co-encapsulated into polyethylene glycol microspheres and were transplanted into mouse brain, which led to enhanced stem cell viability and reduced inflammation [143].
4.5. Functional microspheres for cardiac tissue regeneration
Myocardial infarction that is initiated by coronary occlusion leads to tissue hypoxia and massive cardiomyocyte death at the site of injury. Stem cell-based therapy is considered as a promising approach for cardiac repair. However, inadequate cell retention and survival after cell injection dampens the overall effectiveness. Approaches like cell-laden microspheres were investigated to ameliorate the regenerative effect [[144], [145], [146]]. Studies showed that gelatin microspheres supported the attachment of cardiac progenitor cells and enhanced cell engraftment in a myocardial infarction mouse model [144]. The incorporation of poly(N-isopropyl acrylate) (PNIPA) into PLLA can be utilized to fabricate temperature-responsive nanofibrous microspheres that are transformed into a hydrogel at 37 °C. Loading cardiomyocytes to the PLLA-PEG-PNIPA microspheres reduced infarct size, enhanced integration of the transplanted cardiomyocytes, and led to an improved recovery of cardiac function [146]. The size of microspheres affected cardiac progenitor cell response. One study indicated a preferential cell adherence to microspheres with a smaller size [147]. Growth factors, such as hepatocyte growth factor (HGF) and insulin-like growth factor (IGF-1), were encapsulated in microspheres to improve the cardiac function [148,149]. A combinational approach of stem cells with growth factors stimulated the myocardial regeneration and improved engraftment. For example, PLGA microspheres loaded with HGF and IGF-1 induced the expressions of cardiac muscle markers [150].
4.6. Functional microspheres for skin tissue regeneration
Microspheres have also been explored for skin tissue regeneration. For example, hollow bacterial cellulose microspheres were fabricated to promote cell proliferation, leading to rapid wound-healing in a rat skin model [23]. Given the critical role of keratinocyte growth factor-2 (KGF-2) in regulating keratinocyte proliferation and differentiation, KGF-2 was encapsulated into polysaccharide hemostasis microspheres to accelerate wound healing process [151]. Gelatin microspheres were loaded with PRP to shorten wound healing time through decreasing inflammatory response and promoting angiogenesis [152]. Immobilization of polyvinylpyrrolidone-iodine in chitosan microspheres resulted in improved antimicrobial performance and accelerated wound closure in diabetic wounds [153]. Engineered skin constructs consisted of BMSCs-loaded epidermal growth factor (EGF) microspheres were developed to enhance cutaneous wound healing and sweat-gland repair in a mouse model [154].
5. Conclusions and future perspectives
Functional microspheres for tissue regeneration have been increasingly explored in recent years. In this paper, we have summarized the advancements of functional microspheres, including the types of biomaterials used for microsphere fabrication, the fabrication methods of functional microspheres, and the applications of functional microspheres for bone, cartilage, dentin-pulp complex, neural tissue, cardiac tissue, and skin regeneration. The introduction of new structures and functionalities into microspheres have greatly expanded their applications. For instance, the core-shell structure can be readily utilized to integrate controlled growth factor delivery with cells in one microsphere. The hollow structure and macropores on the surface of a microsphere provide abundant spaces for cell migration and growth within the microsphere. Moreover, the incorporation of nanofibrous architecture into microspheres mimics the structure of natural ECM and presents an ECM-like microenvironment for guiding tissue regeneration. However, much work remains to be done prior to the application of functional microspheres from bench to bedside. Some of the main challenges for the development of functional microspheres are listed below:
First, more innovative approaches are needed to introduce biomimetic features into functional microspheres. Currently, phase separation is the only effective method to prepare nanofibrous microspheres. Electrospinning and electrospray are used to fabricate nanofibrous microparticles but with poorly controlled sizes and geometries. Conventional layer-by-layer self-assembly process is limited in its capability of preparing micrometer-sized spheres. While microfluidics is one of the most effective methods to fabricate functional microspheres, the fabrication of nanofibrous microspheres using this technique is still a challenge. New fabrication approaches that integrate current microsphere techniques will promote the development of more bio-inspired functional microspheres.
Second, there is a need to control the release of bioactive molecules more precisely from microspheres. At present, bioactive molecules released from most microsphere systems range from several days to a week, which is not sufficient for the regeneration of many tissues. Moreover, initial burst release from microspheres is high and reducing the extent of the burst release is pivotal to improve drug efficacy. In addition to the conventional fabrication parameters, such as polymer concentration and crosslinking time, methods and techniques involving new chemical and physical interaction parameters are expected to tune and reduce the initial burst release from functional microspheres.
The third one is related to the mechanical strengths of microspheres. Since most of microspheres are prepared from polymeric materials and usually have porous structure, the microspheres have relatively low compressive moduli. While the incorporation of inorganic components, such as HA, increases the mechanical strength, the microspheres still cannot be used for load-bearing scenarios. Therefore, increasing mechanical properties of microspheres is a research direction to further expand the application of functional microspheres.
Fourth, the stability of microspheres in vivo needs to be further warranted. Microspheres are usually chemically crosslinked to prevent the microspheres from diffusion to the vicinity defective areas. Because the crosslinking of microspheres occurs at the interfaces of microspheres, which is different from a conventional homogenous crosslinking system, the in vivo stability of microspheres prior to their degradation should be thoroughly investigated.
Last but not least, there are still multiple challenges for scaling-up functional microspheres at the industrial-scale quantities for practical applications, in particular uniformity, size, stability, synthesis scale, and production cost of functional microspheres.
Despite all the above challenges, functional microsphere-based regenerative medicine therapy is a rapidly developing field. With the advances of nanofabrication and microfabrication technologies in bioengineering, it is expected that continuing efforts on cell-instructive functional microspheres will be developed to meet clinical requirements in the near future.
Ethics approval and consent to participate
Not Applicable.
Declaration of competing interest
The authors declare no conflict of interests.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Lima D.B., et al. Injectable bone substitute based on chitosan with polyethylene glycol polymeric solution and biphasic calcium phosphate microspheres. Carbohydr. Polym. 2020;245 doi: 10.1016/j.carbpol.2020.116575. [DOI] [PubMed] [Google Scholar]
- 2.Xiao W., et al. Hollow hydroxyapatite microspheres: a novel bioactive and osteoconductive carrier for controlled release of bone morphogenetic protein-2 in bone regeneration. Acta Biomater. 2013;9(9):8374–8383. doi: 10.1016/j.actbio.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yan M., et al. Local controlled release of simvastatin and PDGF from core/shell microspheres promotes bone regeneration in vivo. RSC Adv. 2017;7(32):19621–19629. [Google Scholar]
- 4.Kuang R., et al. Nanofibrous spongy microspheres enhance odontogenic differentiation of human dental pulp stem cells. Adv Healthc Mater. 2015;4(13):1993–2000. doi: 10.1002/adhm.201500308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ge J., et al. The size of mesenchymal stem cells is a significant cause of vascular obstructions and stroke. Stem Cell Reviews and Reports. 2014;10(2):295–303. doi: 10.1007/s12015-013-9492-x. [DOI] [PubMed] [Google Scholar]
- 6.Li W., et al. Microfluidic fabrication of microparticles for biomedical applications. Chem. Soc. Rev. 2018;47(15):5646–5683. doi: 10.1039/c7cs00263g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta V., et al. Microsphere-based scaffolds in regenerative engineering. Annu. Rev. Biomed. Eng. 2017;19:135–161. doi: 10.1146/annurev-bioeng-071516-044712. [DOI] [PubMed] [Google Scholar]
- 8.He Q., et al. Current advances in microsphere based cell culture and tissue engineering. Biotechnol. Adv. 2020;39 doi: 10.1016/j.biotechadv.2019.107459. [DOI] [PubMed] [Google Scholar]
- 9.Huang W., et al. Microsphere based scaffolds for bone regenerative applications. Biomater. Sci. 2014;2(9):1145–1153. doi: 10.1039/c4bm00161c. [DOI] [PubMed] [Google Scholar]
- 10.Andreas K., et al. Biodegradable insulin-loaded PLGA microspheres fabricated by three different emulsification techniques: investigation for cartilage tissue engineering. Acta Biomater. 2011;7(4):1485–1495. doi: 10.1016/j.actbio.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Shen S., et al. The design and features of apatite-coated chitosan microspheres as injectable scaffold for bone tissue engineering. Biomed Mater. 2013;8(2) doi: 10.1088/1748-6041/8/2/025007. [DOI] [PubMed] [Google Scholar]
- 12.Solorio L.D., et al. Spatiotemporal regulation of chondrogenic differentiation with controlled delivery of transforming growth factor-beta1 from gelatin microspheres in mesenchymal stem cell aggregates. Stem Cells Transl Med. 2012;1(8):632–639. doi: 10.5966/sctm.2012-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen G., et al. Functionalized polycaprolactone/hydroxyapatite composite microspheres for promoting bone consolidation in a rat distraction osteogenesis model. J. Orthop. Res. 2020;38(5):961–971. doi: 10.1002/jor.24542. [DOI] [PubMed] [Google Scholar]
- 14.Yao L., Phan F., Li Y. Collagen microsphere serving as a cell carrier supports oligodendrocyte progenitor cell growth and differentiation for neurite myelination in vitro. Stem Cell Res. Ther. 2013;4(5):1–8. doi: 10.1186/scrt320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou J., et al. Segmental bone regeneration using rhBMP-2-loaded collagen/chitosan microspheres composite scaffold in a rabbit model. Biomed Mater. 2012;7(3) doi: 10.1088/1748-6041/7/3/035002. [DOI] [PubMed] [Google Scholar]
- 16.Feng J., Huang Z., Dong Y. Preparation of ice microspheres and their application in the preparation of porous poly(l-lactic acid) (PLLA) scaffolds. J. Mater. Sci. 2018;54(4):3661–3670. [Google Scholar]
- 17.Custodio C.A., et al. Cell selective chitosan microparticles as injectable cell carriers for tissue regeneration. Biomaterials. 2015;43:23–31. doi: 10.1016/j.biomaterials.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 18.Bosch-Rue E., et al. Multiple ion scaffold-based delivery platform for potential application in early stages of bone regeneration. Materials. 2021;14(24) doi: 10.3390/ma14247676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeon B.J., et al. Fabrication of porous PLGA microspheres with BMP-2 releasing polyphosphate-functionalized nano-hydroxyapatite for enhanced bone regeneration. Macromol. Res. 2012;20(7):715–724. [Google Scholar]
- 20.Fang J., et al. Poly(L-glutamic acid)/chitosan polyelectrolyte complex porous microspheres as cell microcarriers for cartilage regeneration. Acta Biomater. 2014;10(1):276–288. doi: 10.1016/j.actbio.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Huang L., et al. Porous chitosan microspheres as microcarriers for 3D cell culture. Carbohydr. Polym. 2018;202:611–620. doi: 10.1016/j.carbpol.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Omidi M., Hashemi M., Tayebi L. Microfluidic synthesis of PLGA/carbon quantum dot microspheres for vascular endothelial growth factor delivery. RSC Adv. 2019;9(57):33246–33256. doi: 10.1039/c9ra06279c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J., et al. Production of hollow bacterial cellulose microspheres using microfluidics to form an injectable porous scaffold for wound healing. Adv Healthc Mater. 2016;5(23):2983–2992. doi: 10.1002/adhm.201600898. [DOI] [PubMed] [Google Scholar]
- 24.Park J.-H., et al. Preparation of highly monodispersed porous-channeled poly(caprolactone) microspheres by a microfluidic system. Mater. Lett. 2016;181:92–98. [Google Scholar]
- 25.Nath S.D., et al. Preparation and characterization of PLGA microspheres by the electrospraying method for delivering simvastatin for bone regeneration. Int. J. Pharm. 2013;443(1–2):87–94. doi: 10.1016/j.ijpharm.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 26.Liang C.Z., et al. Dual delivery for stem cell differentiation using dexamethasone and bFGF in/on polymeric microspheres as a cell carrier for nucleus pulposus regeneration. J. Mater. Sci. Mater. Med. 2012;23(4):1097–1107. doi: 10.1007/s10856-012-4563-0. [DOI] [PubMed] [Google Scholar]
- 27.Boda S.K., et al. Electrospraying electrospun nanofiber segments into injectable microspheres for potential cell delivery. ACS Appl. Mater. Interfaces. 2018;10(30):25069–25079. doi: 10.1021/acsami.8b06386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cen L., et al. Collagen tissue engineering: development of novel biomaterials and applications. Pediatr. Res. 2008;63(5):492–496. doi: 10.1203/PDR.0b013e31816c5bc3. [DOI] [PubMed] [Google Scholar]
- 29.Wang H., et al. The use of micro- and nanospheres as functional components for bone tissue regeneration. Tissue Eng. B Rev. 2012;18(1):24–39. doi: 10.1089/ten.teb.2011.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Echave M.C., et al. Progress of gelatin-based 3D approaches for bone regeneration. J. Drug Deliv. Sci. Technol. 2017;42:63–74. [Google Scholar]
- 31.Diba M., et al. Nanostructured raspberry-like gelatin microspheres for local delivery of multiple biomolecules. Acta Biomater. 2017;58:67–79. doi: 10.1016/j.actbio.2017.05.059. [DOI] [PubMed] [Google Scholar]
- 32.Gómez-Estaca J., Gavara R., Hernández-Muñoz P. Encapsulation of curcumin in electrosprayed gelatin microspheres enhances its bioaccessibility and widens its uses in food applications. Innovat. Food Sci. Emerg. Technol. 2015;29:302–307. [Google Scholar]
- 33.Forni F., Vandelli M.A., Cameroni R. Influence of drug loading level on drug release and dynamic swelling of crosslinked gelatin microspheres. J. Microencapsul. 1992;9(1):29–39. doi: 10.3109/02652049209021220. [DOI] [PubMed] [Google Scholar]
- 34.Croisier F., Jérôme C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013;49(4):780–792. [Google Scholar]
- 35.Xu F., et al. Effects of drying methods on the preparation of dexamethasone-loaded chitosan microspheres. Biomed Mater. 2014;9(5) doi: 10.1088/1748-6041/9/5/055003. [DOI] [PubMed] [Google Scholar]
- 36.LogithKumar R., et al. A review of chitosan and its derivatives in bone tissue engineering. Carbohydr. Polym. 2016;151:172–188. doi: 10.1016/j.carbpol.2016.05.049. [DOI] [PubMed] [Google Scholar]
- 37.Li J., et al. Porous chitosan microspheres for application as quick in vitro and in vivo hemostat. Mater Sci Eng C Mater Biol Appl. 2017;77:411–419. doi: 10.1016/j.msec.2017.03.276. [DOI] [PubMed] [Google Scholar]
- 38.Li Q., et al. Cross-linked chitosan microspheres entrapping silver chloride via the improved emulsion technology for iodide ion adsorption. Carbohydr. Polym. 2020;234 doi: 10.1016/j.carbpol.2020.115926. [DOI] [PubMed] [Google Scholar]
- 39.Lv B., Wang Y., Chen W. Preparation, characterization, and bioactivity of chitosan microspheres containing basic fibroblast growth factor. J. Nanomater. 2014;2014:1–7. [Google Scholar]
- 40.Skop N.B., et al. Heparin crosslinked chitosan microspheres for the delivery of neural stem cells and growth factors for central nervous system repair. Acta Biomater. 2013;9(6):6834–6843. doi: 10.1016/j.actbio.2013.02.043. [DOI] [PubMed] [Google Scholar]
- 41.Alsberg E., et al. Cell-interactive alginate hydrogels for bone tissue engineering. J. Dent. Res. 2001;80(11):2025–2029. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- 42.Dhamecha D., et al. Applications of alginate microspheres in therapeutics delivery and cell culture: past, present and future. Int. J. Pharm. 2019;569 doi: 10.1016/j.ijpharm.2019.118627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Piornos J.A., et al. Highly efficient encapsulation of linseed oil into alginate/lupin protein beads: optimization of the emulsion formulation. Food Hydrocolloids. 2017;63:139–148. [Google Scholar]
- 44.Puppi D., et al. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010;35(4):403–440. [Google Scholar]
- 45.Cui F., et al. Preparation and characterization of melittin-loaded poly (DL-lactic acid) or poly (DL-lactic-co-glycolic acid) microspheres made by the double emulsion method. J. Contr. Release. 2005;107(2):310–319. doi: 10.1016/j.jconrel.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Sabir M.I., Xu X., Li L. A review on biodegradable polymeric materials for bone tissue engineering applications. J. Mater. Sci. 2009;44(21):5713–5724. [Google Scholar]
- 47.Han F.Y., et al. Bioerodable PLGA-based microparticles for producing sustained-release drug formulations and strategies for improving drug loading. Front. Pharmacol. 2016;7:185. doi: 10.3389/fphar.2016.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park H., et al. Effect of stabilizers on encapsulation efficiency and release behavior of exenatide-loaded PLGA microsphere prepared by the W/O/W solvent evaporation method. Pharmaceutics. 2019;11(12) doi: 10.3390/pharmaceutics11120627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park J.H., et al. Preparation of in situ hardening composite microcarriers: calcium phosphate cement combined with alginate for bone regeneration. J. Biomater. Appl. 2014;28(7):1079–1084. doi: 10.1177/0885328213496486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao F., et al. Fabrication and characterization of bioactive glass/alginate composite scaffolds by a self-crosslinking processing for bone regeneration. RSC Adv. 2016;6(94):91201–91208. [Google Scholar]
- 51.Guo Y.H., et al. Effects of ginsenoside Rg1-loaded alginate-chitosan microspheres on human bone marrow stromal cells. Biosci. Rep. 2017;37(3) doi: 10.1042/BSR20160566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dorozhkin S. Medical application of calcium orthophosphate bioceramics. Bio. 2011;1(1):1–51. [Google Scholar]
- 53.Hong S.J., Yu H.S., Kim H.W. Preparation of porous bioactive ceramic microspheres and in vitro osteoblastic culturing for tissue engineering application. Acta Biomater. 2009;5(5):1725–1731. doi: 10.1016/j.actbio.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 54.Tahriri M., et al. Evaluation of the in vitro biodegradation and biological behavior of poly(lactic-co-glycolic acid)/nano-fluorhydroxyapatite composite microsphere-sintered scaffold for bone tissue engineering. J. Bioact. Compat Polym. 2017;33(2):146–159. [Google Scholar]
- 55.Lim P.N., et al. In-vivo evaluation of subcutaneously implanted cell-loaded apatite microcarriers for osteogenic potency. J. Mater. Sci. Mater. Med. 2017;28(6):86. doi: 10.1007/s10856-017-5897-4. [DOI] [PubMed] [Google Scholar]
- 56.Feng J., et al. A scalable approach to obtain mesenchymal stem cells with osteogenic potency on apatite microcarriers. J. Biomater. Appl. 2014;29(1):93–103. doi: 10.1177/0885328213515734. [DOI] [PubMed] [Google Scholar]
- 57.Yuan H., et al. Osteoinductive ceramics as a synthetic alternative to autologous bone grafting. Proc. Natl. Acad. Sci. U. S. A. 2010;107(31):13614–13619. doi: 10.1073/pnas.1003600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li B., et al. Preparation of bioactive β-tricalcium phosphate microspheres as bone graft substitute materials. Mater. Sci. Eng. C. 2017;70:1200–1205. doi: 10.1016/j.msec.2016.03.040. [DOI] [PubMed] [Google Scholar]
- 59.Chen Y., et al. Strontium-substituted biphasic calcium phosphate microspheres promoted degradation performance and enhanced bone regeneration. J. Biomed. Mater. Res. 2020;108(4):895–905. doi: 10.1002/jbm.a.36867. [DOI] [PubMed] [Google Scholar]
- 60.Perez R.A., et al. Micro- and nanostructured hydroxyapatite-collagen microcarriers for bone tissue-engineering applications. J Tissue Eng Regen Med. 2013;7(5):353–361. doi: 10.1002/term.530. [DOI] [PubMed] [Google Scholar]
- 61.Wijerathne H.M.C.S., et al. Effect of nano-hydroxyapatite on protein adsorption and cell adhesion of poly(lactic acid)/nano-hydroxyapatite composite microspheres. SN Appl. Sci. 2020;2(4) [Google Scholar]
- 62.Ding Q., et al. Preparation of bone marrow mesenchymal stem cells combined with hydroxyapatite/poly(d,l-lactide) porous microspheres for bone regeneration in calvarial defects. ACS Appl. Bio Mater. 2018;1(4):1084–1093. doi: 10.1021/acsabm.8b00312. [DOI] [PubMed] [Google Scholar]
- 63.Li L., et al. Synergistic anti-inflammatory and osteogenic n-HA/resveratrol/chitosan composite microspheres for osteoporotic bone regeneration. Bioact. Mater. 2021;6(5):1255–1266. doi: 10.1016/j.bioactmat.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wenzhi S., et al. Assessment of nano-hydroxyapatite and poly (lactide-co-glycolide) nanocomposite microspheres fabricated by novel airflow shearing technique for in vivo bone repair. Mater Sci Eng C Mater Biol Appl. 2021;128 doi: 10.1016/j.msec.2021.112299. [DOI] [PubMed] [Google Scholar]
- 65.Cai B., et al. Fabrication and cell viability of injectable n-HA/chitosan composite microspheres for bone tissue engineering. RSC Adv. 2016;6(89):85735–85744. [Google Scholar]
- 66.Zou H., et al. Investigation of human dental pulp cells on a potential injectable poly(lactic-co-glycolic acid) microsphere scaffold. J. Endod. 2017;43(5):745–750. doi: 10.1016/j.joen.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 67.Jiang H., et al. A green and facile strategy for the fabrication of all-natural porous proteinaceous microspheres. Mater. Chem. Front. 2021;5(10):3897–3902. [Google Scholar]
- 68.Hong S.J., Yu H.S., Kim H.W. Tissue engineering polymeric microcarriers with macroporous morphology and bone-bioactive surface. Macromol. Biosci. 2009;9(7):639–645. doi: 10.1002/mabi.200800304. [DOI] [PubMed] [Google Scholar]
- 69.Zhou A., et al. Bioactive poly(epsilon-caprolactone) microspheres with tunable open pores as microcarriers for tissue regeneration. J. Biomater. Appl. 2019;33(9):1242–1251. doi: 10.1177/0885328218825371. [DOI] [PubMed] [Google Scholar]
- 70.Ryu T.K., et al. Uniform tricalcium phosphate beads with an open porous structure for tissue engineering. Colloids Surf. B Biointerfaces. 2013;112:368–373. doi: 10.1016/j.colsurfb.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 71.Amoyav B., Benny O. Microfluidic based fabrication and characterization of highly porous polymeric microspheres. Polymers. 2019;11(3):419. doi: 10.3390/polym11030419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Su X., et al. Hierarchical microspheres with macropores fabricated from chitin as 3D cell culture. J. Mater. Chem. B. 2019;7(34):5190–5198. doi: 10.1039/c9tb01046g. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Z., et al. Simultaneous nano- and microscale control of nanofibrous microspheres self-assembled from star-shaped polymers. Adv Mater. 2015;27(26):3947–3952. doi: 10.1002/adma.201501329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garzon I., et al. Bioactive injectable aggregates with nanofibrous microspheres and human dental pulp stem cells: a translational strategy in dental endodontics. J Tissue Eng Regen Med. 2018;12(1):204–216. doi: 10.1002/term.2397. [DOI] [PubMed] [Google Scholar]
- 75.Ma C., et al. Hierarchical nanofibrous microspheres with controlled growth factor delivery for bone regeneration. Adv Healthc Mater. 2015;4(17):2699–2708. doi: 10.1002/adhm.201500531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X., et al. Pulp regeneration in a full-length human tooth root using a hierarchical nanofibrous microsphere system. Acta Biomater. 2016;35:57–67. doi: 10.1016/j.actbio.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 77.Hu Z., et al. Immunomodulatory ECM-like microspheres for accelerated bone regeneration in diabetes mellitus. ACS Appl. Mater. Interfaces. 2018;10(3):2377–2390. doi: 10.1021/acsami.7b18458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Duan B., et al. Highly biocompatible nanofibrous microspheres self-assembled from chitin in NaOH/urea aqueous solution as cell carriers. Angew Chem. Int. Ed. Engl. 2015;54(17):5152–5156. doi: 10.1002/anie.201412129. [DOI] [PubMed] [Google Scholar]
- 79.Ma C., Liu X. formation of nanofibrous matrices, three-dimensional scaffolds, and microspheres: from theory to practice. Tissue Eng. C Methods. 2017;23(1):50–59. doi: 10.1089/ten.tec.2016.0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Si Y., Chen M., Wu L. Syntheses and biomedical applications of hollow micro-/nano-spheres with large-through-holes. Chem. Soc. Rev. 2016;45(3):690–714. doi: 10.1039/c5cs00695c. [DOI] [PubMed] [Google Scholar]
- 81.Zhou W.-Q., et al. Synthesis of macroporous poly (glycidyl methacrylate) microspheres by surfactant reverse micelles swelling method. Eur. Polym. J. 2007;43(10):4493–4502. [Google Scholar]
- 82.Feng W., et al. Synthesis and characterization of nanofibrous hollow microspheres with tunable size and morphology via thermally induced phase separation technique. RSC Adv. 2015;5(76):61580–61585. [Google Scholar]
- 83.Zhang Z., et al. Injectable peptide decorated functional nanofibrous hollow microspheres to direct stem cell differentiation and tissue regeneration. Adv. Funct. Mater. 2015;25(3):350–360. doi: 10.1002/adfm.201402618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fan R., et al. Dual drug loaded biodegradable nanofibrous microsphere for improving anti-colon cancer activity. Sci. Rep. 2016;6 doi: 10.1038/srep28373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoon D., et al. formation of polymeric hollow microcapsules and microlenses using gas-in-organic-in-water droplets. Micromachines. 2015;6(5):622–633. [Google Scholar]
- 86.Pu W., et al. Preparation of hollow polyurethane microspheres with tunable surface structures via electrospraying technology. RSC Adv. 2017;7(79):49828–49837. [Google Scholar]
- 87.Fu H., et al. Evaluation of bone regeneration in implants composed of hollow HA microspheres loaded with transforming growth factor β1 in a rat calvarial defect model. Acta Biomater. 2013;9(3):5718–5727. doi: 10.1016/j.actbio.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu X.H., Jin X.B., Ma P.X. Nanofibrous hollow microspheres self-assembled from star-shaped polymers as injectable cell carriers for knee repair. Nat. Mater. 2011;10(5):398–406. doi: 10.1038/nmat2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Perez R.A., Kim H.W. Core-shell designed scaffolds for drug delivery and tissue engineering. Acta Biomater. 2015;21:2–19. doi: 10.1016/j.actbio.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 90.Kong T., et al. Microfluidic fabrication of polymeric core-shell microspheres for controlled release applications. Biomicrofluidics. 2013;7(4) doi: 10.1063/1.4819274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boanini E., Bigi A. Biomimetic gelatin-octacalcium phosphate core-shell microspheres. J. Colloid Interface Sci. 2011;362(2):594–599. doi: 10.1016/j.jcis.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 92.Lin Z., et al. Precisely controlled delivery of magnesium ions thru sponge-like monodisperse PLGA/nano-MgO-alginate core-shell microsphere device to enable in-situ bone regeneration. Biomaterials. 2018;174:1–16. doi: 10.1016/j.biomaterials.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 93.Wu J., et al. Fabrication and characterization of monodisperse PLGA-alginate core-shell microspheres with monodisperse size and homogeneous shells for controlled drug release. Acta Biomater. 2013;9(7):7410–7419. doi: 10.1016/j.actbio.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 94.Yu Y., et al. Enhancement of VEGF-mediated angiogenesis by 2-N,6-O-sulfated chitosan-coated hierarchical PLGA scaffolds. ACS Appl. Mater. Interfaces. 2015;7(18):9982–9990. doi: 10.1021/acsami.5b02324. [DOI] [PubMed] [Google Scholar]
- 95.Kim S.Y., Hwang J.Y., Shin U.S. Preparation of nano/macroporous polycaprolactone microspheres for an injectable cell delivery system using room temperature ionic liquid and camphene. J. Colloid Interface Sci. 2016;465:18–25. doi: 10.1016/j.jcis.2015.11.055. [DOI] [PubMed] [Google Scholar]
- 96.Fang T., et al. Synergistic effect of stem cells from human exfoliated deciduous teeth and rhBMP-2 delivered by injectable nanofibrous microspheres with different surface modifications on vascularized bone regeneration. Chem. Eng. J. 2019;370:573–586. [Google Scholar]
- 97.Wei P., et al. Bioresorbable microspheres with surface-loaded nanosilver and apatite as dual-functional injectable cell carriers for bone regeneration. Macromol. Rapid Commun. 2018;39(20):e1800062. doi: 10.1002/marc.201800062. [DOI] [PubMed] [Google Scholar]
- 98.Kim S.E., et al. Fabrication of a BMP-2-immobilized porous microsphere modified by heparin for bone tissue engineering. Colloids Surf. B Biointerfaces. 2015;134:453–460. doi: 10.1016/j.colsurfb.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 99.Tao C., et al. Development and characterization of GRGDSPC-modified poly(lactide-co-glycolide acid) porous microspheres incorporated with protein-loaded chitosan microspheres for bone tissue engineering. Colloids Surf. B Biointerfaces. 2014;122:439–446. doi: 10.1016/j.colsurfb.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 100.Shi X., et al. Promoting cell growth on porous PLA microspheres through simple degradation methods. Polym. Degrad. Stabil. 2019;161:319–325. [Google Scholar]
- 101.Mamat N., Jaafar M., Abdul Hamid Z.A. Surface modification of gentamicin-loaded polylactic acid (PLA) microsphere using double emulsion and solvent evaporation: effect on protein adsorption and drug release behaviour. J. Phys. Sci. 2019;30:109–124. Sup.1. [Google Scholar]
- 102.Vignali A., et al. Lightweight poly(epsilon-caprolactone) composites with surface modified hollow glass microspheres for use in rotational molding: thermal, rheological and mechanical properties. Polymers. 2019;11(4) doi: 10.3390/polym11040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Curran J.M., et al. The osteogenic response of mesenchymal stem cells to an injectable PLGA bone regeneration system. Biomaterials. 2013;34(37):9352–9364. doi: 10.1016/j.biomaterials.2013.08.044. [DOI] [PubMed] [Google Scholar]
- 104.Lima A.C., et al. Free and copolymerized gamma-cyclodextrins regulate the performance of dexamethasone-loaded dextran microspheres for bone regeneration. J. Mater. Chem. B. 2014;2(30):4943–4956. doi: 10.1039/c3tb21665a. [DOI] [PubMed] [Google Scholar]
- 105.Chen R., et al. The use of poly(l-lactide) and RGD modified microspheres as cell carriers in a flow intermittency bioreactor for tissue engineering cartilage. Biomaterials. 2006;27(25):4453–4460. doi: 10.1016/j.biomaterials.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 106.Xiong L., et al. BMP2-loaded hollow hydroxyapatite microspheres exhibit enhanced osteoinduction and osteogenicity in large bone defects. Int. J. Nanomed. 2015;10:517–526. doi: 10.2147/IJN.S74677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sun Q., et al. Combined use of adipose derived stem cells and TGF-beta3 microspheres promotes articular cartilage regeneration in vivo. Biotech. Histochem. 2018;93(3):168–176. doi: 10.1080/10520295.2017.1401663. [DOI] [PubMed] [Google Scholar]
- 108.Kuang R., et al. Nanofibrous spongy microspheres for the delivery of hypoxia-primed human dental pulp stem cells to regenerate vascularized dental pulp. Acta Biomater. 2016;33:225–234. doi: 10.1016/j.actbio.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang W., et al. Dentin regeneration by stem cells of apical papilla on injectable nanofibrous microspheres and stimulated by controlled BMP-2 release. Acta Biomater. 2016;36:63–72. doi: 10.1016/j.actbio.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu Z., et al. Nanofibrous spongy microspheres to distinctly release miRNA and growth factors to enrich regulatory T cells and rescue periodontal bone loss. ACS Nano. 2018;12(10):9785–9799. doi: 10.1021/acsnano.7b08976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yuan Z., et al. Injectable PLGA microspheres with tunable magnesium ion release for promoting bone regeneration. Acta Biomater. 2019;85:294–309. doi: 10.1016/j.actbio.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 112.Zhang H.X., et al. Biocompatibility and osteogenesis of calcium phosphate composite scaffolds containing simvastatin-loaded PLGA microspheres for bone tissue engineering. J. Biomed. Mater. Res. 2015;103(10):3250–3258. doi: 10.1002/jbm.a.35463. [DOI] [PubMed] [Google Scholar]
- 113.Chen J., et al. Preparation of chitosan/nano hydroxyapatite organic-inorganic hybrid microspheres for bone repair. Colloids Surf. B Biointerfaces. 2015;134:401–407. doi: 10.1016/j.colsurfb.2015.06.072. [DOI] [PubMed] [Google Scholar]
- 114.Das A., et al. Delivery of bioactive lipids from composite microgel-microsphere injectable scaffolds enhances stem cell recruitment and skeletal repair. PLoS One. 2014;9(7):e101276. doi: 10.1371/journal.pone.0101276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fernandes G., et al. Combination of controlled release platelet-rich plasma alginate beads and bone morphogenetic protein-2 genetically modified mesenchymal stem cells for bone regeneration. J. Periodontol. 2016;87(4):470–480. doi: 10.1902/jop.2016.150487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhou P., et al. Loading BMP-2 on nanostructured hydroxyapatite microspheres for rapid bone regeneration. Int. J. Nanomed. 2018;13:4083–4092. doi: 10.2147/IJN.S158280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang B.J., et al. Multilayered pore-closed PLGA microsphere delivering OGP and BMP-2 in sequential release patterns for the facilitation of BMSCs osteogenic differentiation. J. Biomed. Mater. Res. 2018;106(1):95–105. doi: 10.1002/jbm.a.36210. [DOI] [PubMed] [Google Scholar]
- 118.Zhao X., et al. Injectable stem cell-laden photocrosslinkable microspheres fabricated using microfluidics for rapid generation of osteogenic tissue constructs. Adv. Funct. Mater. 2016;26(17):2809–2819. [Google Scholar]
- 119.Gaihre B., Uswatta S., Jayasuriya A.C. Reconstruction of craniomaxillofacial bone defects using tissue-engineering strategies with injectable and non-injectable scaffolds. J. Funct. Biomater. 2017;8(4) doi: 10.3390/jfb8040049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hankenson K.D., et al. Angiogenesis in bone regeneration. Injury. 2011;42(6):556–561. doi: 10.1016/j.injury.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang S., et al. Core-shell microspheres delivering FGF-2 and BMP-2 in different release patterns for bone regeneration. J. Mater. Chem. B. 2015;3(9):1907–1920. doi: 10.1039/c4tb01876a. [DOI] [PubMed] [Google Scholar]
- 122.Shikada Y., et al. Platelet-derived growth factor-AA is an essential and autocrine regulator of vascular endothelial growth factor expression in non-small cell lung carcinomas. Cancer Res. 2005;65(16):7241–7248. doi: 10.1158/0008-5472.CAN-04-4171. [DOI] [PubMed] [Google Scholar]
- 123.Mao Z., et al. Osteoinductivity and antibacterial properties of strontium ranelate-loaded poly(lactic-co-glycolic acid) microspheres with assembled silver and hydroxyapatite nanoparticles. Front. Pharmacol. 2018;9:368. doi: 10.3389/fphar.2018.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li Y., et al. Fabrication of antimicrobial peptide-loaded PLGA/chitosan composite microspheres for long-acting bacterial resistance. Molecules. 2017;22(10) doi: 10.3390/molecules22101637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wei P., et al. Vancomycin- and strontium-loaded microspheres with multifunctional activities against bacteria, in angiogenesis, and in osteogenesis for enhancing infected bone regeneration. ACS Appl. Mater. Interfaces. 2019;11(34):30596–30609. doi: 10.1021/acsami.9b10219. [DOI] [PubMed] [Google Scholar]
- 126.Alenezi A., et al. Controlled release of clarithromycin from PLGA microspheres enhances bone regeneration in rabbit calvaria defects. J. Biomed. Mater. Res. B Appl. Biomater. 2018;106(1):201–208. doi: 10.1002/jbm.b.33844. [DOI] [PubMed] [Google Scholar]
- 127.Fang J., et al. Novel injectable porous poly(gamma-benzyl-l-glutamate) microspheres for cartilage tissue engineering: preparation and evaluation. J. Mater. Chem. B. 2015;3(6):1020–1031. doi: 10.1039/c4tb01333f. [DOI] [PubMed] [Google Scholar]
- 128.Bozkurt M., et al. Autologous stem cell-derived chondrocyte implantation with bio-targeted microspheres for the treatment of osteochondral defects. J. Orthop. Surg. Res. 2019;14(1):394. doi: 10.1186/s13018-019-1434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Qu M., et al. Injectable open-porous PLGA microspheres as cell carriers for cartilage regeneration. J. Biomed. Mater. Res. 2021;109:2091–2100. doi: 10.1002/jbm.a.37196. [DOI] [PubMed] [Google Scholar]
- 130.Ahearne M., Kelly D.J. A comparison of fibrin, agarose and gellan gum hydrogels as carriers of stem cells and growth factor delivery microspheres for cartilage regeneration. Biomed Mater. 2013;8(3) doi: 10.1088/1748-6041/8/3/035004. [DOI] [PubMed] [Google Scholar]
- 131.Liao J., et al. Injectable Alginate hydrogel cross-linked by calcium gluconate-loaded porous microspheres for cartilage tissue engineering. ACS Omega. 2017;2(2):443–454. doi: 10.1021/acsomega.6b00495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ji X., et al. Injectable immunomodulation-based porous chitosan microspheres/HPCH hydrogel composites as a controlled drug delivery system for osteochondral regeneration. Biomaterials. 2022;285 doi: 10.1016/j.biomaterials.2022.121530. [DOI] [PubMed] [Google Scholar]
- 133.Yang T., et al. hDPSC-laden GelMA microspheres fabricated using electrostatic microdroplet method for endodontic regeneration. Mater Sci Eng C Mater Biol Appl. 2021;121 doi: 10.1016/j.msec.2020.111850. [DOI] [PubMed] [Google Scholar]
- 134.Wood M.D., et al. Fibrin gels containing GDNF microspheres increase axonal regeneration after delayed peripheral nerve repair. Regen. Med. 2013;8(1):27–37. doi: 10.2217/rme.12.105. [DOI] [PubMed] [Google Scholar]
- 135.Zeng W., et al. TPP ionically cross-linked chitosan/PLGA microspheres for the delivery of NGF for peripheral nerve system repair. Carbohydr. Polym. 2021;258 doi: 10.1016/j.carbpol.2021.117684. [DOI] [PubMed] [Google Scholar]
- 136.Lan L., et al. Implantable porous gelatin microspheres sustained release of bFGF and improved its neuroprotective effect on rats after spinal cord injury. PLoS One. 2017;12(3):e0173814. doi: 10.1371/journal.pone.0173814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Skop N.B., et al. Optimizing a multifunctional microsphere scaffold to improve neural precursor cell transplantation for traumatic brain injury repair. J Tissue Eng Regen Med. 2016;10(10):E419–E432. doi: 10.1002/term.1832. [DOI] [PubMed] [Google Scholar]
- 138.Shi Z.L., et al. Localized delivery of brain-derived neurotrophic factor from PLGA microspheres promotes peripheral nerve regeneration in rats. J. Orthop. Surg. Res. 2022;17(1):172. doi: 10.1186/s13018-022-02985-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zeng W., et al. Development and characterization of cores-shell poly(lactide-co-glycolide)-chitosan microparticles for sustained release of GDNF. Colloids Surf. B Biointerfaces. 2017;159:791–799. doi: 10.1016/j.colsurfb.2017.08.052. [DOI] [PubMed] [Google Scholar]
- 140.Gao M., et al. Improved poly(d,l-lactide-co-1,3-trimethylene carbonate)6 copolymer microparticle vehicles for sustained and controlled delivery of bioactive basic fibroblast growth factor. J. Bioact. Compat Polym. 2015;30(4):381–396. [Google Scholar]
- 141.Tajdaran K., et al. Local delivery of FK506 to injured peripheral nerve enhances axon regeneration after surgical nerve repair in rats. Acta Biomater. 2019;96:211–221. doi: 10.1016/j.actbio.2019.05.058. [DOI] [PubMed] [Google Scholar]
- 142.Skop N.B., et al. Subacute transplantation of native and genetically engineered neural progenitors seeded on microsphere scaffolds promote repair and functional recovery after traumatic brain injury. ASN Neuro. 2019;11 doi: 10.1177/1759091419830186. p. 1759091419830186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Matta R., et al. Minimally invasive delivery of microbeads with encapsulated, viable and Quiescent neural stem cells to the adult subventricular zone. Sci. Rep. 2019;9(1) doi: 10.1038/s41598-019-54167-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Feyen D.A., et al. Gelatin microspheres as vehicle for cardiac progenitor cells delivery to the myocardium. Adv Healthc Mater. 2016;5(9):1071–1079. doi: 10.1002/adhm.201500861. [DOI] [PubMed] [Google Scholar]
- 145.Smit N.W., et al. Recombinant human collagen-based microspheres mitigate cardiac conduction slowing induced by adipose tissue-derived stromal cells. PLoS One. 2017;12(8):e0183481. doi: 10.1371/journal.pone.0183481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhao C., et al. Biodegradable nanofibrous temperature-responsive gelling microspheres for heart regeneration. Adv. Funct. Mater. 2020;30(21) doi: 10.1002/adfm.202000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rosellini E., et al. Influence of injectable microparticle size on cardiac progenitor cell response. J. Appl. Biomater. Funct. Mater. 2018;16(4):241–251. doi: 10.1177/2280800018782844. [DOI] [PubMed] [Google Scholar]
- 148.Rosellini E., et al. IGF-1 loaded injectable microspheres for potential repair of the infarcted myocardium. J. Biomater. Appl. 2021;35(7):762–775. doi: 10.1177/0885328220948501. [DOI] [PubMed] [Google Scholar]
- 149.Riaud M., et al. Pharmacology active microcarriers delivering HGF associated with extracellular vesicles for myocardial repair. Eur. J. Pharm. Biopharm. 2021;169:268–279. doi: 10.1016/j.ejpb.2021.10.018. [DOI] [PubMed] [Google Scholar]
- 150.Karam J.P., et al. Pharmacologically active microcarriers associated with thermosensitive hydrogel as a growth factor releasing biomimetic 3D scaffold for cardiac tissue-engineering. J. Contr. Release. 2014;192:82–94. doi: 10.1016/j.jconrel.2014.06.052. [DOI] [PubMed] [Google Scholar]
- 151.Pan H., et al. Controlled release of KGF-2 for regulation of wound healing by KGF-2 complexed with "lotus seedpod surface-like" porous microspheres. J. Mater. Chem. B. 2021;9(19):4039–4049. doi: 10.1039/d1tb00148e. [DOI] [PubMed] [Google Scholar]
- 152.Zhou S., et al. Injectable gelatin microspheres loaded with platelet rich plasma improve wound healing by regulating early inflammation. Int. J. Med. Sci. 2021;18(9):1910–1920. doi: 10.7150/ijms.51060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yu J., et al. Carboxymethyl chitosan-grafted polyvinylpyrrolidone-iodine microspheres for promoting the healing of chronic wounds. Bioengineered. 2022;13(4):8735–8746. doi: 10.1080/21655979.2022.2054911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Huang S., et al. Mesenchymal stem cells delivered in a microsphere-based engineered skin contribute to cutaneous wound healing and sweat gland repair. J. Dermatol. Sci. 2012;66(1):29–36. doi: 10.1016/j.jdermsci.2012.02.002. [DOI] [PubMed] [Google Scholar]