Abbreviations
- BAT
basophil activation test
- FcRn
neonatal Fc receptors
- Oma
omalizumab
To the Editor,
The transport of maternal IgG to the fetus is mediated by neonatal Fc receptors (FcRn) expressed on the placenta, which provides passive immunity in utero. Humanized monoclonal antibodies such as omalizumab bind to FcRn and are transferred to the fetus. 1 Recently, Bundhoo et al. provided in vitro data of FcRn‐mediated anti‐IgE IgG/IgE immune complexes, suggesting a mechanism for IgE transfer across the placenta to the fetus. 2 As IgG‐bound allergens are also known to cross the placenta, 3 it is important to understand the impact of omalizumab, one of the most frequently prescribed monoclonal antibodies to treat severe asthma in reproductive age.
We report the use of a state‐of‐the‐art ex vivo human placental perfusion system (Table S1) 4 to investigate the impact of omalizumab on the transport of peanut allergen and IgE across the placenta (Figure 1A). We compared the transport of omalizumab (Oma), peanut extract (PE), PE/peanut‐allergic plasma (PA), and PE/PA/Oma. The kinetics and functionality of the transported allergen were examined in vitro (Table S2, see Online Repository for a detailed description of methods), by sampling perfusates collected at different time points ranging from 0 to 240 min. Ara h 2 as a proxy of peanut protein transfer was measured by ELISA (Indoor Biotechnologies, Virginia, USA; EPC‐AH2‐X). Basophil activation tests (BAT) were performed with sampled perfusates to determine the functionality of the transferred peanut allergens.
FIGURE 1.
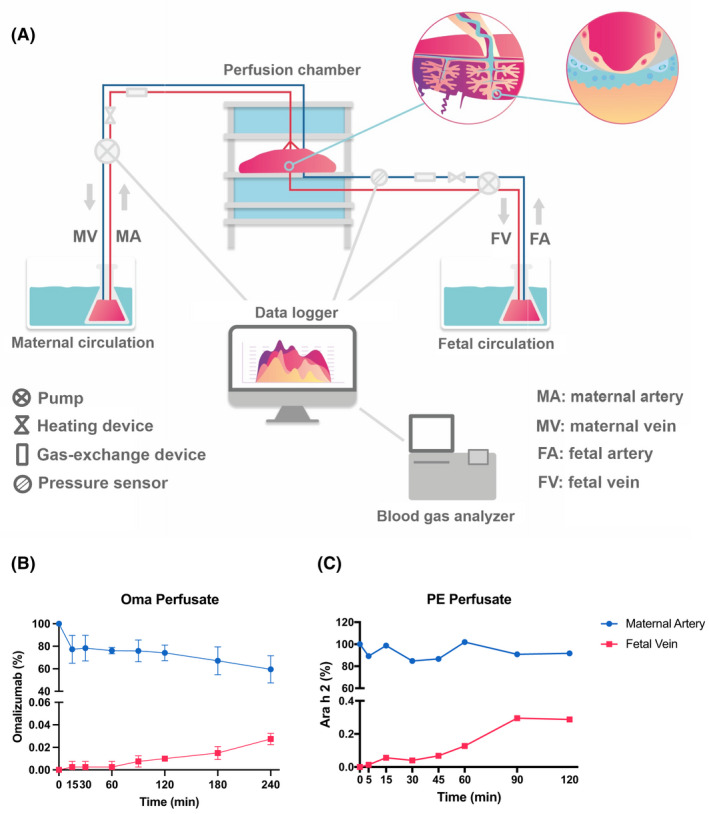
Transfer of Omalizumab and Peanut protein across the placental barrier. (A) In the human placental ex vivo model sample in‐flow (artery) and out‐flow (vein) are recorded to and from the placental tissue. (B) Omalizumab and (C) peanut extract transfer kinetics as percent change from maternal artery to fetal vein detected via ELISA.
Transported Oma was detected in the fetal compartment after 15 min and relative levels steadily increased until the end of the experiment (240 min, Figure 1B), consistent with previous studies reporting linear, concentration‐dependent maternal‐fetal antibody transfer. 5 In parallel, PE protein was detectable at the fetal side within the first 5 min of tissue perfusion (Figure 1C), reached the maximum concentration (0.3% of the maternal reservoir) after 90 min, and remained stable thereafter. Ara h 2 transport has been proposed to follow the endosomal/lysosomal pathway, independent of Fc receptors, with a synergistic uptake via caveolin‐mediated endocytosis and micropinocytosis. 6 Thus, FcRn‐mediated omalizumab transfer and uptake may be slower, attributable to recycling processes. However, endocytosis/micropinocytosis mediated uptake of Ara h 2 may be faster and further enhanced by additionally formed endosomes carrying IgG in the presence of omalizumab.
To compare the degree to which FcRn might facilitate the transfer of allergen‐IgG complexes across the placenta, we examined Ara h 2 transfer in the presence (PE/PA) or absence (PE) of plasma from peanut‐allergic individuals, with (PE/PA/Oma) and without omalizumab (Figure 2A). PE/PA crossed the placenta with a moderate rise of Ara h 2 levels at the fetal side after 120 min, comparable to PE alone (1.03 ng/ml). Extending the perfusion time to 240 min resulted in a near twofold increase (3.82 ng/ml) of PE via PE/PA/Oma vs. PE/PA (1.97 ng/ml).
FIGURE 2.
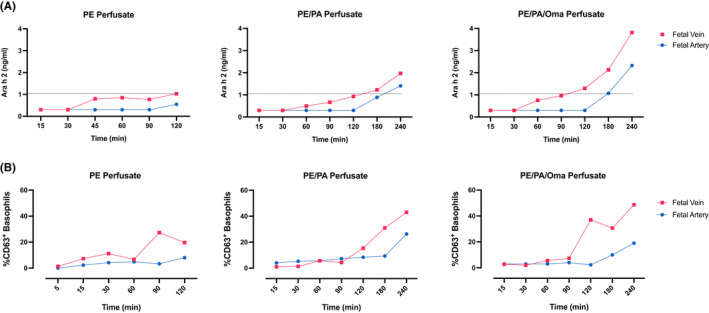
Placental peanut allergen transfer in the context of plasma from allergic individual and omalizumab. (A) Ara h 2 concentrations (ng/ml) in fetal vein and artery samples were determined by ELISA in PE alone (dashed line, 1.03 ng/ml) and PE/PA compared to PE/PA/Oma experiments. (B) Basophil activation tests were conducted by flow cytometry to assess the functionality of transferred allergen. Activation levels are expressed as %CD63+ basophils in PE, PE/PA, and PE/PA/Oma experiments.
The transferred peanut proteins were fully capable of crosslinking IgE as confirmed via BAT (Figure 2B). The higher degree of basophil activation matched the extent of Ara h 2 transfer. We confirmed that perfusion medium alone did not activate basophils (Figure S1). Furthermore, evidence for omalizumab‐driven IgE transfer resulting in free IgE with possible functionality was assessed via incubation of stripped basophils with perfusates. We could not find evidence for IgE binding to basophils from these eluates (Figure S2).
In conclusion, functional peanut allergen is actively transported across the human placenta and this process may be enhanced by omalizumab. Active transfer of free IgE to the fetal side due to FcRn‐mediated complex formation was not observed. Further studies are needed to better understand how allergen‐antibody complexes affect allergen‐specific priming in the fetus with and without biological usage during pregnancy.
FUNDING INFORMATION
This work was supported by The Hospital for Sick Children (Food Allergy and Anaphylaxis Program, start‐up funds by the SickKids Research Institute and the Department of Pediatrics, Restracomp Graduate Scholarship to AK), and an Ontario Graduate Scholarship from the Province of Ontario to AK.
CONFLICT OF INTEREST
AK, BH, JSL, BP, KS, and CW have nothing to disclose. ZS holds advisory board roles for Nutricia/Danone, Aimmune, and Sanofi. TE reports to act as local PI for company‐sponsored trials by DBV and sub‐investigator for Regeneron, holds grants from Innovation Fund Denmark, CIHR outside the submitted work. He is Co‐Investigator or scientific lead in three investigator‐initiated oral immunotherapy trials supported by the Food Allergy and Anaphylaxis Program SickKids and serves as an associate editor for Allergy. He/his laboratory received unconditional/in‐kind contributions from Macro Array Diagnostics and an unrestricted grant from ALK. He holds advisory board roles for ALK, Nutricia/Danone, and Aimmune.
Supporting information
Appendix S1
Kothari and Hirschmugl contributed equally.
Wadsack and Eiwegger contributed equally.
Contributor Information
Christian Wadsack, Email: christian.wadsack@medunigraz.at.
Thomas Eiwegger, Email: thomas.eiwegger@stpoelten.lknoe.at.
REFERENCES
- 1. Pfaller B, José Yepes‐Nuñez J, Agache I, et al. Biologicals in atopic disease in pregnancy: An EAACI position paper. Allergy. 2021;76(1):71‐89. doi: 10.1111/ALL.14282 [DOI] [PubMed] [Google Scholar]
- 2. Bundhoo A, Paveglio S, Rafti E, Dhongade A, Blumberg RS, Matson AP. Evidence that FcRn mediates the transplacental passage of maternal IgE in the form of IgG anti‐IgE/IgE immune complexes. Clin Exp Allergy. 2015;45(6):1085‐1098. doi: 10.1111/cea.12508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Szépfalusi Z, Loibichler C, Pichler J, Reisenberger K, Ebner C, Urbanek R. Direct evidence for transplacental allergen transfer. Pediatr Res. 2000;48(3):404‐407. doi: 10.1203/00006450-200009000-00024 [DOI] [PubMed] [Google Scholar]
- 4. Hirschmugl B, Wadsack C. Transplacental transfer of venlafaxine evaluated by ex vivo perfusion. Placenta. 2021;117:150‐153. doi: 10.1016/J.PLACENTA.2021.12.007 [DOI] [PubMed] [Google Scholar]
- 5. Urbaniak SJ, Duncan JI, Armstrong‐Fisher SS, Abramovich DR, Page KR. Transfer of anti‐D antibodies across the isolated perfused human placental lobule and inhibition by high‐dose intravenous immunoglobulin: a possible mechanism of action. Br J Haematol. 1997;96(1):186‐193. doi: 10.1046/J.1365-2141.1997.8762507.X [DOI] [PubMed] [Google Scholar]
- 6. Price D, Ackland ML, Suphioglu C. Identifying epithelial endocytotic mechanisms of the peanut allergens Ara h 1 and Ara h 2. Int Arch Allergy Immunol. 2017;172(2):106‐115. doi: 10.1159/000451085 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


