Abstract
Background and aims
Treatment of de novo malignancies and recurrent hepatocellular carcinoma with immune checkpoint inhibitors (ICI) in liver transplant recipients (LT) is an attractive strategy that is infrequently pursued because of the lack of strong evidence regarding their safety and efficacy. In this systematic review with pooled analysis, we aimed to assess safety and efficacy of ICI therapy following LT.
Methods
We performed a systematic search of case reports and series published until January 2022. We included 31 publications reporting a total of 52 patients treated with ICIs after LT and assessed in a pooled analysis the risk of graft rejection and the outcome of ICI therapy.
Results
Acute graft rejection occurred in 15 patients (28.8%) and 7 patients (13.4% of the total cohort) died because of graft loss. Rejection was associated with shorter overall survival (OS) (17.2 months, confidence interval [CI] 12.1–22.2 vs. 3.5 months, CI 1.6–5.4, p < 0.001). Disease control rate was 44.2% (n = 23), and in these patients, OS was longer than in non‐responders (26.4 months, CI 20.8–32.0 vs. 3.4 months, CI 2.1–4.7, p < 0.001).
Conclusions
Observational, off‐label experience suggests that treatment with ICI for advanced malignancies in LT recipients might not be discarded a priori. This notwithstanding, ICI treatment in these patients is associated with a substantial risk of graft rejection and mortality. Prospective studies are needed to provide adequate safety and efficacy figures of ICI treatment in this fragile population.
Keywords: hepatocellular carcinoma, immunotherapy, predictors, rejection, survival
Abbreviations
- CI
confidence interval
- CTLA‐4
cytotoxic T‐lymphocyte‐associated antigen‐4
- DCR
disease control rate
- HCC
hepatocellular carcinoma
- ICI
immune checkpoint inhibitors
- IQR
inter quartile range
- LT
liver transplantation
- OS
overall survival
- PD‐1
programmed death 1
- PD‐L1
programmed death ligand 1
- PFS
progression‐free survival
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- RMST
restricted mean survival time
- ROC
receiver operating characteristic curve
Lay Summary.
Safety and efficacy of immune checkpoint inhibitors treatment following liver transplantation are still incompletely characterized as most of the information come from case reports or small series. In a pooled analysis of patients treated with immune checkpoint inhibitors following liver transplantation we observed that the risk of graft rejection is 28.8% and that in patients with rejection mortality because of graft loss is elevated (43%). However, disease control rate is substantial (44.2%) and is associated with longer overall survival than in non‐responders (26.4 vs. 3.4 months).
1. INTRODUCTION
The use of systemic therapy in de novo malignancies or recurrent hepatocellular carcinoma (HCC) in patients who have undergone liver transplantation (LT) represents a therapeutic challenge. In transplanted patients, the risk of developing cancer is not only significantly higher compared to the general population, but overall outcomes are worse. 1 , 2 Moreover, drug interactions, graft toxicity and rejection may represent obstacles to anticancer treatment, independently posing threats to the general outcome of these patients, irrespective of oncological determinants. 3 Thus, safe and effective therapies are needed to improve treatment outcomes and prognosis of this complex and difficult‐to‐treat population.
Outside the transplant setting, systemic treatment of advanced malignancies has been dramatically revolutionized by the advent of Immune checkpoint inhibitors (ICIs). These medications offer a new modality of cancer treatment that has demonstrated an ability to provide a complete oncologic response and significantly increase the life expectancy of patients as compared to traditional chemotherapy. 4
Immune checkpoint inhibitors are biological drugs able to promote T‐cell activation leading to immunopotentiation towards tumoral cells and include medications that target cytotoxic T‐lymphocyte‐associated antigen‐4 (CTLA‐4) receptors, and programmed death 1/programmed death ligand 1 (PD‐1/PD‐L1) axis receptors. 5 These treatments have become the standard of care in several cancers and are gaining a definite role in the treatment of HCC. 6 , 7 , 8 , 9 Recently, the combination therapy of atezolizumab and bevacizumab, a PD‐L1 and vascular endothelial growth factor inhibitor, showed superior overall (OS) and progression‐free survival (PFS) against sorafenib in patients with unresectable HCC, 6 and this combination is now the recommended first‐line systemic treatment in patients with HCC. However, ICIs are associated with the potential occurrence of immune‐related adverse effects, that may involve several systems and that are due to perturbation of the immune homeostasis, with immune activation directed against both tumoral cells and other organs; severe, immune‐related adverse events may occur in approximately 10% of patients and jeopardize further treatment with this class of drugs, besides being potentially life‐threatening. 10 , 11
The safety and efficacy of ICIs in the setting of LT have not been fully investigated, and a history of solid organ transplant is considered an absolute contraindication to their use due to concerns related to the increased risk of rejection and the potential lower efficacy because of pharmacological immunosuppression. 12 Given these concerns, transplanted patients were excluded from ICI trials, resulting in a paucity of data about their safety and efficacy in this setting and most of the evidence regarding ICI use in LT recipients is based upon case series and case reports. 13 However, because of the efficacy of ICIs in various malignancies, off‐label use among oncologic patients with a history of LT has accumulated, allowing further data on safety and efficacy to be extrapolated.
In this study, we systematically reviewed the available literature regarding the use of ICIs for the management of advanced cancer in patients who underwent LT. We carried out a pooled analysis of all the cases published for the treatment of HCC and non‐HCC cancer following LT with the aim to provide a more comprehensive overview of the safety and efficacy of these medications in this specific population.
2. MATERIALS AND METHODS
2.1. Protocol and eligibility criteria of included manuscripts
The present study is a systematic review with pooled analysis conducted following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) and a validated protocol for review and meta‐analyses of case reports and case series. 14 , 15 We selected all the publications that enrolled patients of any age who underwent LT and who experienced de novo or recurrent cancer that was treated with ICIs, regardless of the type of tumour.
We considered all the publications that exhaustively reported the treatment with ICIs including the occurrence of graft rejection, the response to treatment, duration of treatment, overall survival (OS) and progression‐free survival (PFS). The included publications reported in their dataset the type of malignancy, the ICI used, the other treatments administered and the lead‐time between LT and ICI use. Furthermore, we considered only the publications reporting the general characteristics of the patients (i.e., age, gender, comorbidities) and the immunosuppressant regimen used in the prevention of graft rejection.
2.2. Outcome measures
The primary outcome of the present analysis was to evaluate the influence of ICI administration on the risk of graft rejection and OS in patients who had previously undergone LT. The outcome measures considered are the incidence of rejection and the efficacy of ICIs in terms of OS and PFS.
Rejection was defined as the presence of acute or late acute rejection at any time after initiation of ICIs administration. Treatment‐related mortality because of graft rejection was also evaluated (i.e., grade 5 treatment‐related adverse events by Common Terminology Criteria for Adverse Events criteria). 16
OS was defined as the time from ICI initiation until death for any reason. We also reported the incidence of early mortality which we considered as the occurrence of death within the first month after ICI administration, as previously defined. 17
Response to ICIs was defined according to Response Evaluation Criteria in Solid Tumours 1.1, when available. 18 Since some publications did not define oncologic response criteria, we based the response to ICI treatment on authors' report while remaining aware of this limitation. In detail, a patient was considered responder to ICIs in the case of Complete Response, Partial Response and Stable Disease, and these latter types of response are summarized as disease control rate (DCR). Some publications did not report imaging results, and therefore response to ICIs was based on clinical improvement as assessed in the study.
2.3. Information sources, search strategy and data collection
We searched PubMed, Embase® and Medline, using search terms referring to LT, recurrent or new onset tumour and ICI use. The detailed web research can be found in the Supporting Information (Table S1). Non‐original research such as editorials and commentaries were excluded. Databases were last accessed on 10 January 2022. All titles and abstracts obtained by database search were screened by two independent authors (AP and SK) and duplicate reports and studies not meeting the eligibility criteria were excluded. Then, the full text of the included articles was obtained thanks to institutional access or open‐access licence and further revised to screen the manuscripts suitable for data extraction. Translation of non‐English full‐text articles was performed with Google Translate and questions were resolved with native speakers. Disagreements were solved by discussion with a third author (EGG). Data abstraction was performed independently by two authors (AP and SK), into a standardized datasheet (Table S2) and disagreements were solved by discussion. No contact with corresponding authors was needed to obtain further data.
2.4. Statistical analysis
A Kolmogorov–Smirnov analysis was performed to test the normality of variables. The results of continuous variables were expressed as median and interquartile range. For ordinal and nominal variables, contingency tables were used for indicating frequency and percentage in the population. For the comparison of continuous variables between different groups of patients, non‐parametric tests of Kruskal–Wallis or Mann–Whitney were used, when appropriate. Nominal variables were examined with the Pearson chi‐square (χ2) test and with Spearman's rank correlation index for the correlation with continuous variables. Intergroup comparisons were adjusted for multiple comparisons with the Bonferroni correction.
Area under the receiver operating characteristic curves (ROC) were used to determine the cut‐off value of the time elapsed from LT to ICI administration in predicting graft rejection and response to ICI therapy. OS was assessed by Kaplan–Meier curves, and the log‐rank test was used to compare the survival distribution in a subgroup of patients. The Restricted Mean Survival Time (RMST) was obtained by calculating the area under the Kaplan–Meier curve until a priori established times, which was set at 6, 12 and 18 months. The computation was obtained by integration of parts. The confidence interval for RMSTs and statistical significance of comparison among patients subgroups was established as previously described. 19
The Cox proportional hazards model was adjusted for death‐related risk factors identified by statistical analysis (the probability value for correction was p = 0.10). The data analysed were set as follows: type of cancer (0 = other malignancy, 1 = HCC) and the time between LT and ICI administration (+1 year). Statistical analysis was performed using IBM SPSS Statistics, Version 25.0 (SPSS Inc., www.spss.com).
3. RESULTS
3.1. General characteristics of publications and patients
The detailed publications sorting process is reported in Figure S1. All the publications included were represented by case reports or case series as there were no higher quality studies in the literature, and the median number of patients included was 1 (inter quartile range [IQR], 1–2; range, 1–8). Overall, the 31 publications included a total of 52 patients treated with ICIs after LT, with median age of 62 years (IQR, 53–66 years) and an overall prevalence of male gender of 76.9%. 17 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 The main indication for LT was the presence of HCC (37 patients, 71.3%) followed by decompensated cirrhosis (7 patients, 13.5%), biliary atresia (2 patients, 3.8%), cholangiocarcinoma (2 patients, 3.9%), Primary Sclerosing Cholangitis (2 patients, 3.8%), drug‐induced liver injury (1 patient, 1.9%) and metastasis from melanoma in experimental protocol (1 patient, 1.9%).
The target tumour of ICI treatment was HCC in most cases (recurrent in 27 patients, 51.9%; de novo in 1 patient), followed by melanoma (13 patients, 25.0%), lung cancer (6 patients [11.5%] and 3 patients [5.8%] for small and non‐small cell lung cancer, respectively), cerebral post‐transplant lymphoproliferative disorder and colorectal carcinoma (one patient, 1.9%, each). Nivolumab was the most widely used drug (29 patients, 55.8%) and its class (PD‐1 antagonist) was the most frequently used ICI (46 patients, 88.5%). Tacrolimus, alone or co‐administered with mofetil mycophenolate or everolimus, was the most frequently dispensed immunosuppressant.
Tables S2 and S3 report the characteristics of the reports included in the pooled analysis, tumour stage (i.e., locally advanced or metastatic disease), the diagnostic method of graft rejection (i.e., histology or clinical diagnosis) and the cause of death.
The median time from LT to ICI treatment was 4 years (IQR, 2–8 years), and ICI was mainly used as 3rd line treatment (median 3rd; IQR, 2nd–4th), with a median time elapsed from cancer diagnosis to ICI treatment of 12 months (IQR, 4–19 months). The median follow‐up period reported in the publications was 6 months (IQR, 1.8–13.5).
3.2. Safety: the risk of graft rejection
Acute graft rejection occurred in 15 out of 52 patients (28.8%) with a cumulative incidence (mean cumulative hazard rate) of 4% per month over 6 months, 3% per month over 12 months, and 2% per month over 2 years (Figure 1).
FIGURE 1.
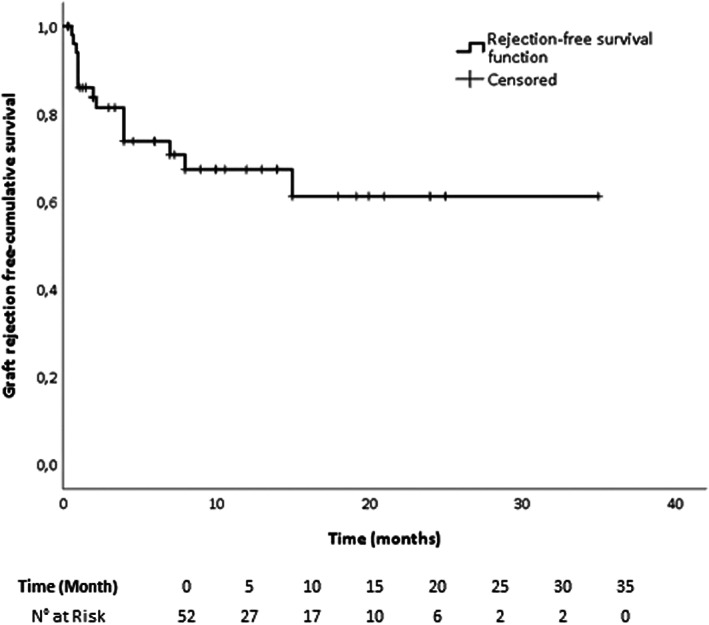
Kaplan–Meier curve of graft rejection‐free cumulative survival in all patients
Table 1 reports the main demographic and clinical characteristics, and the outcomes of patients treated with ICI after LT analysed according to the development of graft rejection. No significant differences were observed in terms of age, gender, line of anti‐tumoral therapy and time elapsed between LT and ICI therapy. AUROC analysis of time elapsed between LT and ICI treatment in predicting graft rejection showed that a statistically significant cut‐off was not identifiable (AUROC 0.373; CI, 0.201–0.546, p = 0.170). Patients who experienced graft rejection showed a statistically significant shorter PFS and OS than patients with tolerance. Graft rejection was significantly more frequent in non‐responders to ICI treatment, and the occurrence of overall and early mortality was significantly higher in patients who experienced graft rejection. More in detail, among the 15 patients who had acute graft rejection only 1 had a response to ICIs and 7 died because of liver failure related to graft rejection (i.e., grade 5 treatment‐related adverse events).
TABLE 1.
Characteristics and outcomes of patients are subdivided according to the development of graft rejection following treatment with immune checkpoint inhibitors.
| Parameters | Graft rejection | p | |
|---|---|---|---|
| Absence | Presence | ||
| (n = 37) | (n = 15) | ||
| Gender (male) | 29 (78.4) | 11 (73.3) | .694 |
| Age (years) | 60 (54–63) | 63 (48–69) | .302 |
| Previous lines of treatment (n) | 3 (2–4) | 2 (2–3) | .137 |
| Time from LT to ICI treatment (years) | 4 (2–8) | 3 (1–5) | .170 |
| Time from cancer diagnosis to ICI (months) | 12.0 (5.0–20.0) | 10.0 (3.0–18.0) | .378 |
| Death (n) | 20 (54.1) | 14 (93.3) | .007 |
| Early mortality (n) | 2 (5.4) | 7 (46.7) | <.001 |
| Overall survival (months) | 8.0 (3.0–15.0) | 2.0 (1.0–4.0) | .002 |
| Progression‐free survival (months) | 3.3 (0–12.2) | 0 (0–0) | .001 |
| Response to ICI (present) | 22 (59.5) | 1 (6.7) | .001 |
Note: Continuous data are shown as median and interquartile range, and nominal data are numbers (%).
Abbreviations: ICI, immune checkpoint inhibitors; LT, liver transplantation.
Nine patients underwent graft biopsy to assess the expression of PD‐L1 in liver tissue. All 4 patients that had positive PD‐L1 staining experienced graft rejection, whereas the remaining 5 with negative PD‐L1 staining on histology had no bouts of rejection (p = 0.008).
The presence of graft rejection was independent of both the class and the type of ICI used (Table S4). Additionally, no difference in rejection was observed based on tumour type (Table 2).
TABLE 2.
Characteristics and outcomes of patients suffering from recurrent HCC or other malignancy following liver transplantation and who were treated with immune checkpoint inhibitors.
| Other malignancies | HCC recurrence | p | |
|---|---|---|---|
| n = 24 | n = 28 | ||
| Gender (male) | 18 (75.0) | 22 (78.6) | .761 |
| Age (years) | 63 (55–67) | 60 (51–64) | .312 |
| Time from LT to ICI treatment (years) | 6 (4–12) | 3 (1–5) | .006 |
| Time from cancer diagnosis to ICI (months) | 10.5 (4.5–17.5) | 15.0 (4.0–23.5) | .422 |
| Death (n) | 12 (50.0) | 22 (78.6) | .031 |
| Early mortality (n) | 3 (12.5) | 6 (21.4) | .594 |
| Overall survival (month) | 7.5 (2.0–15.0) | 4.3 (1.2–10.0) | .177 |
| Progression‐free survival (months) | 3.0 (0–12.0) | 0.7 (0–6.0) | .650 |
| Graft rejection (n) | 8 (33.3) | 7 (25.0) | .473 |
| Response to ICI (present) | 13 (54.2) | 10 (35.7) | .182 |
Note: Continuous data are shown as median and interquartile range, and nominal data are numbers (%).
Abbreviations: HCC, hepatocellular carcinoma; ICI, immune checkpoint inhibitors; LT, liver transplantation.
The Kaplan–Meier survival curves reported in Figure S2 show a significantly longer OS in patients without (17.2 months, confidence interval [CI] 12.1–22.2) versus with graft rejection (3.5 months, CI 1.6–5.4, p < 0.001).
3.3. Efficacy
Median OS was 6 months (IQR, 1.8–13.5 months) with a PFS of 1 month (IQR, 0–8.4 months). The observed mortality was 65.4% (34 out of 52 patients), with 26.5% (14 out of 52 patients) experiencing early mortality. The estimated mean OS was significantly longer in responders (26.4 months, CI 20.8–32.0) than in non‐responders to ICI therapy (3.4 months, CI 2.1–4.7, Figure 2). Patients suffering from HCC recurrence showed a mean OS of 8.4 months (CI, 5.1–11.7) versus 18.6 months (CI, 12.2–25.1) in patients with other malignancies (Figure S3).
FIGURE 2.
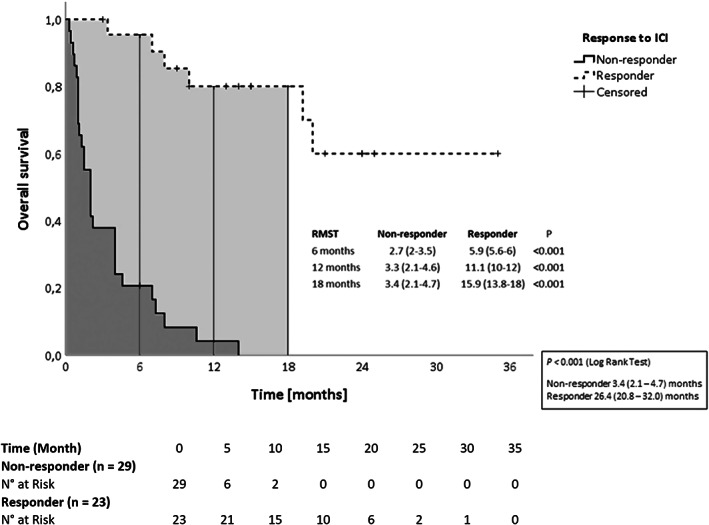
Kaplan–Meier curves of overall survival in patients are divided according to the presence of response to ICI therapy. Data in box are mean survival and confidence interval. ICI, immune checkpoint inhibitors; RMST, restricted mean survival time
Overall, ORR amounted to 34.6% (18 patients) and the DCR was 44.2% (23 patients) and these patients were considered responders to ICI treatment.
In these patients, the time elapsed between LT and tumour onset/recurrence was significantly longer in responders than in non‐responders. ROC curve analysis (accuracy 75.0%, 61.4%–88.6%) identified a cut‐off of 3 years and 10.2 months (sensitivity 72.7%, specificity 66.7%, PPV 63.4%, NPV 75.5%, +LR 2.18, −LR 0.41, p = 0.003) from LT to ICI administration as the best timing predictor of response to ICI therapy. Table 3 summarizes the characteristics and outcomes of patients subdivided according to the response (DCR) to ICI treatment. No differences were observed in gender, age or line of treatment. No differences in treatment outcome were observed among types and classes of ICI used (Table S4), or among target tumours (i.e., HCC and non‐HCC).
TABLE 3.
Characteristics and outcomes of patients are divided according to response to immune checkpoint inhibitors treatment following liver transplantation.
| Parameters | Non‐responders | Responders | p |
|---|---|---|---|
| n = 29 | n = 23 | ||
| Gender (male) | 22 (75.9) | 18 (78.3) | .838 |
| Age (years) | 63 (53–66) | 59 (54–65) | .599 |
| Therapeutic approach (n) | 3 (2–4) | 3 (2–4) | .674 |
| LT to ICI treatment (years) | 3 (1–5) | 6 (4–11) | .003 |
| Time from cancer diagnosis to ICI (months) | 15.0 (5.0–20.0) | 8.5 (4.0–18.0) | .584 |
| Death | 28 (96.6) | 6 (26.1) | <.0001 |
| Early mortality | 9 (31.0) | 0 (0) | .006 |
| Overall survival (months) | 2.0 (1.0–4.0) | 14.0 (8.0–21.0) | <.0001 |
| Progression‐free survival (months) | 0 (0–0) | 11.0 (4.0–18.0) | <.0001 |
| Graft rejection (present) | 14 (48.3) | 1 (4.3) | .001 |
Note: Continuous data are median and interquartile range, and nominal data are numbers (%).
Abbreviations: ICI, immune checkpoint inhibitors; LT, liver transplantation.
The Kaplan–Meier survival curves reported in Figure S4 show that the estimated OS is longer in patients who exhibited a response to ICI treatment, as compared to non‐responders regardless of the presence of graft rejection. Lastly, a multivariate Cox regression analysis showed that the type of tumour and the time elapsed from LT are not independent predictors of survival in patients undergoing ICI therapy (Figure 3). The adjusted survival curve obtained by the Cox regression analysis is reported in Figure S5.
FIGURE 3.
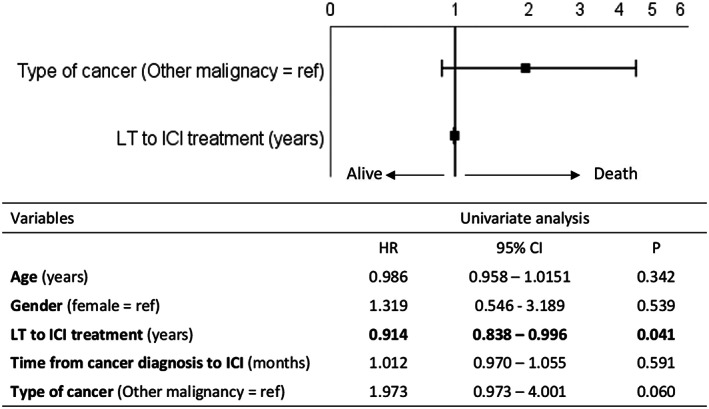
Forrest plot of multivariate Cox regression analysis of overall survival. Dependent variables that entered the models were the type of cancer (0 = other malignancy, 1 = recurrence of HCC) and the time between LT and ICI administration (+1 year). The hazard ratio is expressed as the log10 function. The table below reports the results of the univariate analysis. HCC, hepatocellular carcinoma; ICI, immune checkpoint inhibitors; LT, liver transplantation
4. DISCUSSION
The purpose of the present systematic review with pooled analysis was to assess the safety and efficacy of treatment with ICIs in LT recipients affected by metastatic or locally advanced malignancies. ICIs are increasingly being used in the post‐transplant setting despite a lack of evidence on their safety and efficacy. Indeed, the existing literature is exclusively represented by case reports or case series, as previous organ transplantation is considered an absolute contraindication to their use. Therefore, given the lack of evidence, we aimed to provide a formal analysis of the potential risks associated with the use of ICIs and of their efficacy, based on the available, albeit fragmented literature. We considered risk of rejection, oncological efficacy and their potential inter‐relationship, using a pooled analysis of systematically searched literature in the hope to provide useful information until controlled prospective trials are performed.
Our analysis shows that treatment of advanced cancer with ICIs might be considered only in selected LT recipients when other therapies have failed or are contraindicated, since the risk of graft rejection, a severe and potentially fatal event, is substantial. Indeed, acute graft rejection occurred in approximately one out of three patients (15/52 patients) treated with ICIs, resulting in early mortality secondary to liver failure (unrelated to the oncologic burden) in almost half of them (7/15 patients), resulting in overall treatment‐associated mortality of 13.5% (7/52 patients). In comparison, rejection was not reported in LT patients treated with sorafenib for recurrent HCC after 1 year of follow‐up, and the risk of graft rejection in patients suffering from malignancies other than HCC does not seem to be significantly modified by traditional chemotherapy. 50 , 51 Thus, we feel the substantial risk of fatal graft rejection associated with ICI therapy in this setting must be adequately weighed in the case this off‐label indication of ICIs should be pursued, and adequate assessment of any standard potential treatment option should be thoroughly evaluated before their use.
The increased risk of rejection attributed to ICI could be related to two factors. First, the proteins CTLA‐4, PD‐1 and its ligand PD‐L1 may play a pivotal role in graft tolerance, although the exact mechanism presiding this regulation has not been fully elucidated yet. 52 It has been demonstrated that CTLA‐4 plays a pivotal role in enhancing T‐regulatory activity and reducing T‐helper cell activity thus leading to graft/immune tolerance during the induction or acute post‐transplant phase. 52 The PD‐1/PD‐L1 axis inhibits alloreactive T‐cell activation, can also promote regulatory T‐cell development and is felt to contribute more substantially to immune tolerance during the maintenance phase. Second, both development of malignancy and treatment with ICIs is often preceded by a reduction in immunosuppressive therapy to increase the chances of a pharmacological response and enhance natural immunity. 53 Therefore, both these mechanisms may synergistically play a role in heightening the risk of rejection in patients treated with ICI following LT.
Recent studies showed a higher rate of graft rejection among patients treated with PD‐1 inhibitors than those who received anti‐CTLA‐4 therapy, although this difference was not statistically significant. 45 , 54 Our findings are consistent with this trend, in particular, graft rejection was observed in 14 out of 46 patients treated with PD‐1 inhibitors, while only 1 out of 5 patients treated with anti‐CTLA‐4 experienced graft rejection. We understand these results—though apparently consistent—should be interpreted with caution, because of the small number of patients treated with anti‐CTLA‐4. Furthermore, to the best of our knowledge, no data are available with the combined use of PD‐1/PD‐L1 inhibitors and anti‐CTLA‐4 treatment in this setting.
As patients with PD‐1/PD‐L1 positive histology appear to carry a higher risk of rejection, it has been proposed that a liver biopsy pre‐ICI administration may have a role in predicting the rejection risk. 36 In our analysis, positive PD‐L1 staining on histology was associated with an increased risk of graft rejection, but the results did not reach significance, likely because of the low number of cases with pre‐treatment liver biopsies. Quite recently, a case of recurrent HCC after LT treated with atezolizumab/bevacizumab underwent biopsy to assess PD‐L1 staining, that was negative, tolerated at least two cycles of treatment with subsequent progression. The lack of clear data regarding pre‐ICI treatment, its timing, immunosuppressive regimen and adequate assessment of response precluded its inclusion in our analysis and the fact that the patient had tumour progression after two cycles did not allow to assess the usefulness of PD‐L1 staining to predict harm. 55 We feel that histological assessment of PD‐1/PD‐L1 may represent a potentially useful tool that needs to be further explored in the future, prospective studies in this population, and concur with the suggestion that this tool should not be relied upon as a predictive biomarker unless solid evidence is provided.
It may be hypothesized that the shorter the time from LT the greater the risk of experiencing rejection after initiating ICI therapy, although there is no clear consensus on this issue. In our pooled analysis, patients who experienced graft rejection received ICI treatment earlier following LT compared to patients without rejection, although this difference was not statistically significant, and we were unable to identify a definite cut‐off to define a safe timing to predict a lower risk of graft rejection. We feel that this relevant issue represents an unmet need and needs to be further explored in future studies to provide a guide to treating physicians.
On the other hand, we observed that the time elapsed between LT and ICI administration was significantly associated with a higher rate of therapeutic response, comprehensively summarized in DCR. In fact, the median LT‐to‐ICI interval was 6 years in patients who showed a response to ICI treatment and 3 years in non‐responders. It is likely that the immune activity towards the graft might be responsible for this finding. In fact, immunosuppression following LT is generally reduced over time as the risk of rejection decreases and, consequently, patients with older and more immune‐tolerant grafts may have an immunity more focused against the tumour than against the organ.
Our data show that a cut‐off value of approximately 4 years predicts an increased chance of response to ICI with a sensitivity of 72.7% and a specificity of 66.7%. Further studies are needed to confirm this result. Unfortunately, the data reported did not allow us to reliably assess the impact of the type of immunosuppression. This is a potential confounder as calcineurin inhibitors and mammalian target of rapamycin, the most common classes of immunosuppressants used following LT, target different cellular pathways. 53 The potential effect of these different medications on both ICIs antitumor efficacy, graft protection and tumour properties, namely HCC, needs further evaluation.
The overall DCR to ICI in our pooled analysis was 44.2% (ORR 34.6%), and this result is higher than the one previously reported in a smaller series by Au et al. (i.e., 32%). 17 In our study, we considered evidence of disease control (i.e., DCR) as response to ICI treatment. A greater granularity in the assessment of response would have reduced too much the sample size. Lastly, a difference in DCR was observed between patients treated for HCC recurrence and those who received ICI for de novo malignancies. This latter group of patients showed a trend towards an incremental benefit from ICI therapy as compared to patients treated for recurrent HCC, although this difference in treatment benefit should be considered in the risk/benefit analysis.
In our analysis, the observed OS and PFS are in line with those previously reported in the literature, although our study presents a survival analysis stratified per patient response. 13 Further studies are needed to evaluate the optimal time to assess response to ICI and the duration of treatment despite the risk of graft rejection.
This review has several limitations. First, the sample was obtained by extraction from individual case reports and case series, and therefore homogeneity may be compromised. Second, the differential role played by immunosuppressive regimens could not be assessed due to lack of precise data in several reports. Third, presence and degree of comorbidities, such as presence of advanced liver disease in the graft were often not reported, thus we could not assess their role in treatment results and tolerance. Moreover, despite our analysis is comprehensive, we cannot exclude a publication bias, as only the cases of greatest impact might have been reported. Lastly, the definition of response to treatment we used was comprehensive (disease control), although we must emphasize that because of this potential limitation the main outcome of interest was considered OS. Notwithstanding these limitations, we believe that our contribution has several strengths such as the sample size that, to the best of our knowledge, is the largest analysed so far. Furthermore, we provide a detailed analysis of both the response ICI treatment and its potential harm of treatment in this frail population.
In conclusion, despite the paucity of data published in the literature, we observed that ICI treatment of advanced malignancy in LT patients is associated with a substantial risk of graft rejection and rejection‐related fatalities, but also with increased survival in patient that do not experience a rejection. This observation calls for properly designed prospective studies to identify biomarkers able to characterize safety and efficacy of the use of ICIs in LT patients able to stratify the risk of rejection and response.
CONFLICT OF INTEREST
Edoardo G. Giannini: participating in Advisory Boards for EISAI, MSD, Roche, Astra Zeneca. Mario Strazzabosco: participating in Advisory Board for Engitix.
Supporting information
Table S1.
ACKNOWLEDGMENT
Open Access Funding provided by Universita degli Studi di Genova within the CRUI‐CARE Agreement.
Kayali S, Pasta A, Plaz Torres MC, et al. Immune checkpoint inhibitors in malignancies after liver transplantation: A systematic review and pooled analysis. Liver Int. 2023;43:8‐17. doi: 10.1111/liv.15419
Handling Editor: Alejandro Forner
Stefano Kayali and Andrea Pasta contributed equally to the manuscript and share first authorship.
Mario Strazzabosco, Simona Marenco and Edoardo G. Giannini contributed equally to the manuscript and share last authorship.
REFERENCES
- 1. Huo Z, Li C, Xu X, et al. Cancer risks in solid organ transplant recipients: results from a comprehensive analysis of 72 cohort studies. Onco Targets Ther. 2020;9(1):1848068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Colmenero J, Tabrizian P, Bhangui P, et al. De novo malignancy after liver transplantation: risk assessment, prevention, and management‐guidelines from the ILTS‐SETH consensus conference. Transplantation. 2022;106(1):e30‐e45. [DOI] [PubMed] [Google Scholar]
- 3. Nishihori T, Strazzabosco M, Saif MW. Incidence and management of colorectal cancer in liver transplant recipients. Clin Colorectal Cancer. 2008;7:260‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Billan S, Kaidar‐Person O, Gil Z. Treatment after progression in the era of immunotherapy. Lancet Oncol. 2020;21:e463‐e476. [DOI] [PubMed] [Google Scholar]
- 5. Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894‐1905. [DOI] [PubMed] [Google Scholar]
- 7. Montenegro GB, Farid S, Liu SV. Immunotherapy in lung cancer. J Surg Oncol. 2021;123:718‐729. [DOI] [PubMed] [Google Scholar]
- 8. Frampton AE, Sivakumar S. A new combination immunotherapy in advanced melanoma. N Engl J Med. 2022;386:91‐92. [DOI] [PubMed] [Google Scholar]
- 9. Hasanov E, Gao J, Tannir NM. The immunotherapy revolution in kidney cancer treatment: scientific rationale and first‐generation results. Cancer J. 2020;26:419‐431. [DOI] [PubMed] [Google Scholar]
- 10. Sosa A, Lopez Cadena E, Simon Olive C, Karachaliou N, Rosell R. Clinical assessment of immune‐related adverse events. Ther Adv Med Oncol. 2018;10:1758835918764628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Postow MA, Sidlow R, Hellmann MD. Immune‐related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158‐168. [DOI] [PubMed] [Google Scholar]
- 12. Guba M, Graeb C, Jauch KW, Geissler EK. Pro‐ and anti‐cancer effects of immunosuppressive agents used in organ transplantation. Transplantation. 2004;77:1777‐1782. [DOI] [PubMed] [Google Scholar]
- 13. Kumar V, Shinagare AB, Rennke HG, et al. The safety and efficacy of checkpoint inhibitors in transplant recipients: a case series and systematic review of literature. Oncologist. 2020;25:505‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nambiema A, Sembajwe G, Lam J, et al. A protocol for the use of case reports/studies and case series in systematic reviews for clinical toxicology. Front Med. 2021;8:708380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Basch E, Reeve BB, Mitchell SA, et al. Development of the National Cancer Institute's patient‐reported outcomes version of the common terminology criteria for adverse events (PRO‐CTCAE). J Natl Cancer Inst. 2014;106:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Au KP, Chok KSH. Immunotherapy after liver transplantation: where are we now? World J Gastrointest Surg. 2021;13:1267‐1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 19. Uno H. Vignette for survRM2 Package: Comparing Two Survival Curves Using the Restricted Mean Survival Time Sample Data. Available at: https://cran.r‐project.org/web/packages/survRM2/vignettes/survRM2‐vignette3‐2.html [Google Scholar]
- 20. De Toni EN, Gerbes AL. Tapering of immunosuppression and sustained treatment with nivolumab in a liver transplant recipient. Gastroenterology. 2017;152:1631‐1633. [DOI] [PubMed] [Google Scholar]
- 21. Brumfiel CM, Patel MH, Aqel B, Lehrer M, Patel SH, Seetharam M. Immune checkpoint inhibitor therapy in a liver transplant recipient with autoimmune disease and metastatic cutaneous squamous cell carcinoma. JAAD Case Rep. 2021;14:78‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bittner A, Radke J, Eurich D, et al. Cerebral EBV‐positive PTLD controlled by PD‐1 checkpoint blockade in a liver transplant patient. Leuk Lymphoma. 2021;62:2026‐2029. [DOI] [PubMed] [Google Scholar]
- 23. Ben Khaled N, Roessler D, Reiter FP, Seidensticker M, Guba M, de Toni EN. Extending the use of atezolizumab and bevacizumab to a liver transplant recipient: need for a posttransplant registry. Liver Transpl. 2021;27:928‐929. [DOI] [PubMed] [Google Scholar]
- 24. Kondo T, Kawachi S, Nakatsugawa M, et al. Nivolumab for recurrent/metastatic hypopharyngeal squamous cell carcinoma in a liver transplant recipient. Auris Nasus Larynx. 2022;49:721‐726. [DOI] [PubMed] [Google Scholar]
- 25. Tsung I, Worden FP, Fontana RJ. A pilot study of checkpoint inhibitors in solid organ transplant recipients with metastatic cutaneous squamous cell carcinoma. Oncologist. 2021;26:133‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Owoyemi I, Vaughan LE, Costello CM, et al. Clinical outcomes of solid organ transplant recipients with metastatic cancers who are treated with immune checkpoint inhibitors: a single‐center analysis. Cancer. 2020;126:4780‐4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Al Jarroudi O, Ulusakarya A, Almohamad W, et al. Anti‐programmed cell death protein 1 (PD‐1) immunotherapy for metastatic hepatocellular carcinoma after liver transplantation: a report of three cases. Cureus. 2020;12:e11150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Braun M, Fuchs V, Kian W, et al. Nivolumab induced hepatocanalicular cholestasis and liver rejection in a patient with lung cancer and liver transplant. J Thorac Oncol. 2020;15:e149‐e150. [DOI] [PubMed] [Google Scholar]
- 29. Anugwom C, Leventhal T. Nivolumab‐induced autoimmune‐like cholestatic hepatitis in a liver transplant recipient. ACG Case Rep J. 2020;7:e00416.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pandey A, Cohen DJ. Ipilumumab for hepatocellular cancer in a liver transplant recipient, with durable response, tolerance and without allograft rejection. Immunotherapy. 2020;12:287‐292. [DOI] [PubMed] [Google Scholar]
- 31. Amjad W, Kotiah S, Gupta A, Morris M, Liu L, Thuluvath PJ. Successful treatment of disseminated hepatocellular carcinoma after liver transplantation with nivolumab. J Clin Exp Hepatol. 2020;10:185‐187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhuang L, Mou HB, Yu LF, et al. Immune checkpoint inhibitor for hepatocellular carcinoma recurrence after liver transplantation. Hepatobiliary Pancreat Dis Int. 2020;19:91‐93. [DOI] [PubMed] [Google Scholar]
- 33. Lee BT, Horwich BH, Chopra S, Ahearn A, Han HH. Checkpoint inhibitor‐induced rejection of a liver allograft: a combination of acute T cell‐mediated and antibody‐mediated rejection. Liver Transpl. 2019;25:1845‐1848. [DOI] [PubMed] [Google Scholar]
- 34. Chen JA, Esteghamat N, Kim EJ, et al. PD‐1 blockade in a liver transplant recipient with microsatellite unstable metastatic colorectal cancer and hepatic impairment. J Natl Compr Canc Netw. 2019;17:1026‐1030. [DOI] [PubMed] [Google Scholar]
- 35. Abdel‐Wahab N, Safa H, Abudayyeh A, et al. Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J Immunother Cancer. 2019;7:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. DeLeon TT, Salomao MA, Aqel BA, et al. Pilot evaluation of PD‐1 inhibition in metastatic cancer patients with a history of liver transplantation: the Mayo Clinic experience. J Gastrointest Oncol. 2018;9(6):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tio M, Rai R, Ezeoke OM, et al. Anti‐PD‐1/PD‐L1 immunotherapy in patients with solid organ transplant, HIV or hepatitis B/C infection. Eur J Cancer. 2018;104:137‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nasr F, AlGhoche A, Diab S, et al. Pembrolizumab monotherapy in relapsed hepatocellular carcinoma post living donor liver transplantation and sorafenib. Int J Oncol Res. 2018;1:9. [Google Scholar]
- 39. Guoying W, Hui T, Yingcai Z, et al. Programmed death receptor(PD)‐1 monoclonal antibody‐induced acute immune hepatitis in the treatment of recurrent hepatocellular carcinoma after liver transplantation: a case report. Organ Transplantation. 2016;7:44‐47. [Google Scholar]
- 40. Gassmann D, Weiler S, Mertens JC, et al. Liver allograft failure after nivolumab treatment‐a case report with systematic literature research. Transplant Direct. 2018;4(8):e376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rammohan A, Reddy MS, Farouk M, Vargese J, Rela M. Pembrolizumab for metastatic hepatocellular carcinoma following live donor liver transplantation: the silver bullet? Hepatology. 2018;67:1166‐1168. [DOI] [PubMed] [Google Scholar]
- 42. Kuo JC, Lilly LB, Hogg D. Immune checkpoint inhibitor therapy in a liver transplant recipient with a rare subtype of melanoma: a case report and literature review. Melanoma Res. 2018;28(1):61‐64. [DOI] [PubMed] [Google Scholar]
- 43. Biondani P, De Martin E, Samuel D. Safety of an anti‐PD‐1 immune checkpoint inhibitor in a liver transplant recipient. Ann Oncol. 2018;29(1):286‐287. [DOI] [PubMed] [Google Scholar]
- 44. Varkaris A, Lewis DW, Nugent FW. Preserved liver transplant after PD‐1 pathway inhibitor for hepatocellular carcinoma. Am J Gastroenterol. 2017;112(12):1895‐1896. [DOI] [PubMed] [Google Scholar]
- 45. Friend BD, Venick RS, McDiarmid SV, et al. Fatal orthotopic liver transplant organ rejection induced by a checkpoint inhibitor in two patients with refractory, metastatic hepatocellular carcinoma. Pediatr Blood Cancer. 2017;64(12):e26682. doi: 10.1002/pbc.26682 [DOI] [PubMed] [Google Scholar]
- 46. Dueland S, Guren TK, Boberg KM, et al. Acute liver graft rejection after ipilimumab therapy. Ann Oncol. 2017;28(10):2619‐2620. [DOI] [PubMed] [Google Scholar]
- 47. Schvartsman G, Perez K, Sood G, Katkhuda R, Tawbi H. Immune checkpoint inhibitor therapy in a liver transplant recipient with melanoma. Ann Intern Med. 2017;167(5):361‐362. [DOI] [PubMed] [Google Scholar]
- 48. Morales RE, Shoushtari AN, Walsh MM, Grewal P, Lipson EJ, Carvajal RD. Safety and efficacy of ipilimumab to treat advanced melanoma in the setting of liver transplantation. J Immunother Cancer. 2015;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ranganath HA, Panella TJ. Administration of ipilimumab to a liver transplant recipient with unresectable metastatic melanoma. J Immunother. 2015;38(5):211. [DOI] [PubMed] [Google Scholar]
- 50. Mancuso A, Mazzola A, Cabibbo G, et al. Survival of patients treated with sorafenib for hepatocellular carcinoma recurrence after liver transplantation: a systematic review and meta‐analysis. Dig Liver Dis. 2015;47(4):324‐330. [DOI] [PubMed] [Google Scholar]
- 51. Smedman TM, Guren TK, Line PD, Dueland S. Transplant oncology: assessment of response and tolerance to systemic chemotherapy for metastatic colorectal cancer after liver transplantation—a retrospective study. Transpl Int. 2019;32(11):1144‐1150. [DOI] [PubMed] [Google Scholar]
- 52. Hu B, Yang XB, Sang XT. Liver graft rejection following immune checkpoint inhibitors treatment: a review. Med Oncol. 2019;36(11):94. [DOI] [PubMed] [Google Scholar]
- 53. Ho CM, Chen HL, Hu RH, Lee PH. Harnessing immunotherapy for liver recipients with hepatocellular carcinoma: a review from a transplant oncology perspective. Ther Adv Med Oncol. 2019;11:1758835919843463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kittai AS, Oldham H, Cetnar J, Taylor M. Immune checkpoint inhibitors in organ transplant patients. J Immunother. 2017;40(7):277‐281. [DOI] [PubMed] [Google Scholar]
- 55. Yang Z, Sun J, Zhuang L, Mou H, Zheng S. Preliminary evaluation of Atezolizumab plus bevacizumab as salvage treatment for recurrent hepatocellular carcinoma after liver transplantation. Liver Transpl. 2022;28(5):895‐896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.


