Abstract
Neuralgic amyotrophy (NA), also referred to as idiopathic brachial plexitis and Parsonage‐Turner syndrome, is a peripheral nerve disorder characterized by acute severe shoulder pain followed by progressive upper limb weakness and muscle atrophy. While NA is incompletely understood and often difficult to diagnose, early recognition may prevent unnecessary tests and interventions and, in some situations, allow for prompt treatment, which can potentially minimize adverse long‐term sequalae. High‐resolution ultrasound (HRUS) has become a valuable tool in the diagnosis and evaluation of NA. Pathologic HRUS findings can be grouped into four categories: nerve swelling, swelling with incomplete constriction, swelling with complete constriction, and fascicular entwinement, which may represent a continuum of pathologic processes. Certain ultrasound findings may help predict the likelihood of spontaneous recovery with conservative management versus the need for surgical intervention. We recommend relying heavily on history and physical examination to determine which nerves are clinically affected and should therefore be assessed by HRUS. The nerves most frequently affected by NA are the suprascapular, long thoracic, median and anterior interosseous nerve (AIN) branch, radial and posterior interosseous nerve (PIN) branch, axillary, spinal accessory, and musculocutaneous. When distal upper limb nerves are affected (AIN, PIN, superficial radial nerve), the lesion is almost always located in their respective fascicles within the parent nerve, proximal to its branching point. The purpose of this review is to describe a reproducible, standardized, ultrasonographic approach for evaluating suspected NA, and to share reliable techniques and clinical considerations when imaging commonly affected nerves.
Keywords: brachial plexus, hourglass constriction, neuromuscular, parsonage‐turner syndrome, ultrasound imaging
1. INTRODUCTION
Neuralgic amyotrophy (NA), also referred to as idiopathic brachial plexitis and Parsonage‐Turner syndrome, is an inflammatory peripheral nerve disorder that typically affects the upper limb. The classic presentation consists of acute severe shoulder pain followed by patchy upper limb weakness and muscle atrophy, often involving winging of the scapula. 1 , 2 The incidence could be as high as 1:1000. 2 NA is usually idiopathic. More than half of patients describe some type of antecedent event such as recent infection, strenuous exercise, surgery, trauma, vaccination, rheumatologic disease, hepatitis E infection, pregnancy, or childbirth. 3 , 4 Hereditary NA, seen in less than 10% of cases, is linked to an autosomal dominant mutation in the SEPT9 gene. 5 , 6 , 7
The pathophysiology of neuropathy in NA is incompletely understood, but likely involves a complex interplay of genetic, mechanical, immunologic, and inflammatory factors. 2 , 5 , 6 , 7 , 8 The nerves most frequently affected are the suprascapular (SSN), long thoracic (LTN), median and anterior interosseous nerve (AIN) branch, radial and posterior interosseous nerve (PIN) branch, axillary (AN), spinal accessory (SAN), and musculocutaneous. 2 , 3 , 9 , 10 Nerve involvement outside of the distribution of the upper limb can occur and has been reported in the lumbosacral plexus, phrenic (PN), and laryngeal nerves. 11 , 12 , 13 The diagnostic work up for NA begins with a thorough history and physical exam, and often includes some combination of electrodiagnostic testing (EDX), magnetic resonance imaging (MRI), and high‐resolution ultrasound (HRUS). The rapid improvement in high‐resolution transducers and image processing has led to ultrasound becoming the modality of choice for imaging most superficial peripheral nerves not obscured by bone, and there is an increasing volume of literature illustrating the role of ultrasound in the assessment of NA. 9 , 14 , 15 While NA is often difficult to diagnose, early recognition may prevent unnecessary tests and interventions, and allow for prompt treatment, which can potentially minimize adverse long‐term sequalae. 16 , 17 The purpose of this review is to describe a reproducible, standardized ultrasonographic approach for evaluating suspected NA, and to share reliable techniques and clinical considerations when imaging nerves commonly affected by NA.
2. PLANNING THE ULTRASONOGRAPHIC APPROACH
A thorough history and physical examination are critical in recognizing NA, as it is primarily a clinical diagnosis. 1 , 8 , 18 Typical NA, which encompasses approximately 70% of cases, follows a three phase disease progression. 1 Phase one is characterized by acute‐onset, severe, neuropathic pain in the shoulder, lasting an average of 28 days. Pain is typically unilateral with an average numerical rating scale score of 8/10 at onset, continuous, and often accompanied by sensory abnormalities. Phase two is defined by muscle paresis, typically within 2 wk but sometimes as early as 24 h, followed by progressive muscle atrophy. In one large study evaluating 246 patients with NA, the infraspinatus (71.8%), serratus anterior muscle (SAM) (70.0%), supraspinatus muscle (SSM) (65.7%), biceps brachii (61.0%), rhomboids (54.2%), and pronator teres (52.3%) were among the most frequently involved muscles. 12 More recent literature suggests AIN and PIN syndromes, although previously thought to be “isolated” nerve palsies or entrapment syndromes, may actually represent one of the more common manifestations of NA. 19 , 20 , 21 Phase three is characterized by motor recovery, which typically occurs over several months. Approximately one third of patients will have persistent symptoms beyond 6 mo, and a small percentage will have residual weakness years later. Some patients, particularly those with hereditary NA, may follow an atypical course presenting with painless onset, pure sensory involvement, diaphragm dysfunction, or lumbosacral trunk involvement. 2
EDX testing is frequently used in the evaluation of NA. However, there are inherent challenges of EDX testing that make it difficult to routinely rely on for diagnosis. 1 , 18 , 22 Electromyography (EMG) can lack initial sensitivity when performed in acute‐onset neuropathy, and the widely practiced method of analyzing “routine” muscles may result in sampling error and misdiagnosis. 23 Sensory nerve conduction studies may show abnormalities in as few as 20% of clinically affected nerves. 24 Abnormalities, when present, may be technically challenging to detect due to the proximal anatomic location of the affected nerves. 6 The utility of EDX is likely to be highest in atypical cases in which the diagnosis cannot be confidently made from history and physical examination alone, or as a tool to rule out alternate diagnoses. 1 , 18 , 22
Historically, the diagnostic relevance of imaging in NA has been unclear, with its main role being to exclude alternate diagnoses. As our imaging capabilities evolve, researchers have noted important diagnostic and prognostic clues that may revolutionize the way we evaluate and treat NA. 25 MRI can now more reliably identify nerve changes in acute, subacute, and chronic phases of NA. 26 , 27 However, MRI is time consuming, costly, and lacks sensitivity for identifying detailed nerve structure and pathology. 22 , 25 HRUS is an inexpensive, real‐time, point of care modality that identifies findings specific to NA and may help predict prognosis and need for ultrasound‐guided procedures or surgical intervention. 9 , 14 , 15 , 28
Pathologic HRUS findings in NA can be grouped into four categories: nerve swelling, swelling with incomplete constriction, swelling with complete constriction, and fascicular entwinement. Nerve swelling alone is the most common sonographic finding in NA (Figure 1). Swelling is defined as hypo‐echogenicity and loss of fascicular structure with nerve enlargement. Nerve constriction, or “hourglass constriction,” is best seen longitudinally on HRUS (Figure 2). Incomplete constriction is defined as a focal decrease in nerve diameter but with internal continuity of the nerve preserved, whereas complete constriction demonstrates a hyperechogenic division of the nerve with internal continuity disrupted. Fascicular entwinement is defined as rotation of individual nerve fascicles within a nerve, seen best on dynamic cross‐sectional imaging (Supporting Information Video S1, which is available online). 9 , 14 It is postulated that these HRUS findings represent a continuum of pathological processes that begins with nerve inflammation, edema, and microcompartment syndrome, causing adhesions and local fixation of nerve fascicles, ultimately resulting in thinning and constriction of the nerve. 29 Notably, complete hourglass constriction and fascicular entwinement have been linked to nerve torsion, a poorly understood gross finding that has been described in several case reports and is thought to carry a poor prognosis. 30 , 31 Nerve thinning and constriction is thought to be a precursor to nerve torsion, which may represent the most severe manifestation of this process. 9 , 29 While hourglass constrictions and fascicular entwinement are less common, these features are critical to recognize as they may provide insight regarding prognosis and need for intervention beyond conservative measures. 6 , 14 , 32
FIGURE 1.
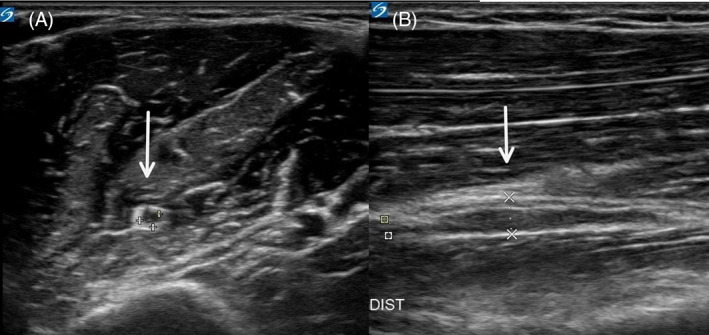
Pathologic nerve swelling with increased hypo echogenicity and loss of fascicular structure of the musculocutaneous nerve (white arrows). A. short axis view. B longitudinal axis view
FIGURE 2.
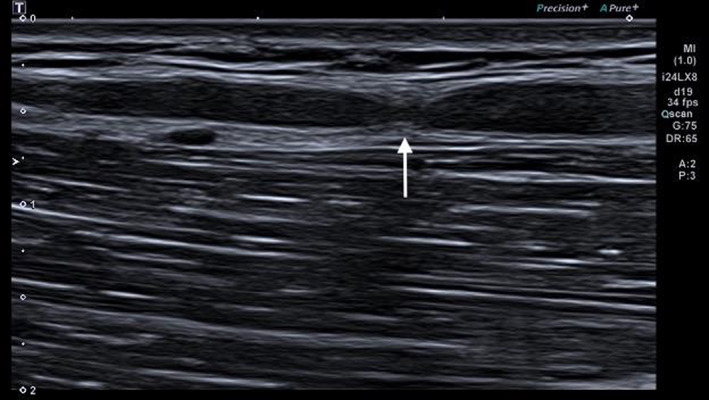
Hourglass constriction of the median nerve (white arrow) seen in the longitudinal axis view
We recommend relying heavily on history and physical examination to determine which nerves are affected and should therefore be assessed with ultrasound. EDX testing may be used as an adjuvant if diagnosis is unclear, whether because the patient is in the ~30% minority that follow an atypical disease course or there is a genuine differential diagnosis to explore, or if the affected nerves cannot be determined from history and physical examination alone. A rigid scanning protocol and bilateral studies for every patient with suspected NA would be insensitive and inefficient as a wide variety of nerves can be affected, and without clinical context, small abnormalities may be difficult to detect. 3
3. ULTRASONOGRAPHIC APPROACH
Peripheral nerves are best visualized with a high‐frequency (≥12 MHz) linear‐array transducer. 33 The images in this manuscript were obtained using a logic E ultrasound device and 12 MHz linear array transducer (GE Healthcare, Chicago, IL). Detailed information on patient positioning is discussed in each individual section below. A simple way to initiate the examination is to first locate the nerve at a well‐defined anatomic landmark using the short axis view (transverse plane). From this site, the nerve can be followed proximally and distally. We recommend scanning the nerve along its entire course or to the extent of which a clear image can be obtained. The sonographer should look for areas of nerve enlargement and constriction. To determine if nerve swelling is present, measure the cross‐sectional area of the nerve and compare to normal reference values at that location, and consider comparing to the contralateral side. 34 At sites of constriction, slowly rotate the probe 90 degrees with the indicator toward the patient's head to obtain the long axis view to determine if hourglass constriction is present. At a pathologic site, slow dynamic short‐axis scanning should be performed to look for fascicular entwinement. Importantly, when NA involves distal upper limb nerves (AIN, PIN, superficial radial nerve), the lesion is almost always located in their respective fascicles within the parent nerve itself, proximal to its branching point. 6 , 14 , 19 , 20 Therefore, we recommend prioritizing scanning the parent nerve in these situations. For smaller nerves that may be more difficult to visualize and obtain a reliable cross‐sectional area (SSN, LTN, accessory, and PN), we recommend comparing to the contralateral side for differences in size, contour, echogenicity, and structure. 14 In the following sections, a systematic approach for imaging nerves commonly affected by NA and other important clinical considerations will be described.
3.1. Common upper limb nerves
Ultrasound techniques for common upper limb nerves such as the musculocutaneous, median, and radial nerve are well described and therefore mentioned in brief in this manuscript. Refer to supplemental images and referenced manuscripts for more detailed information.
To scan the musculocutaneous nerve (MCN), the patient should be supine with the shoulder slightly abducted and externally rotated (Supporting Information Figure S1). The MCN nerve can be identified in the medial proximal arm where it pieces the coracobrachialis. The nerve can be followed proximally to its origin from the lateral cord where it joins the lateral root of the median nerve at the level of the axilla, or distally where it travels through the flexor compartment of the arm between the brachialis and biceps brachii. 35
To scan the median nerve, the patient should be supine with the shoulder slightly abducted and externally rotated (Supporting Information Figure S2). The median nerve can be located at the wrist crease where it travels through the carpal tunnel between the flexor retinaculum and flexor muscle tendons. The nerve can be followed proximally through the forearm between the superficial and deep flexor digitorum muscles. Near the antecubital fossa, the median nerve is medial to the brachial artery between the two heads of the pronator teres muscles, and at the mid‐portion of the arm, the median nerve traverses lateral to the brachial artery. 35 , 36 The AIN branches from the median nerve in the proximal forearm just distal to the pronator teres. In one NA study, the AIN was the most frequently affected nerve and had the most sonographic abnormalities compared to all others. Clinically, this can present as profoundly as the inability to flex the thumb and index finger, or as minimally as a flexion deficit in one distal phalanx, mimicking a tendon rupture. Swelling without constriction was seen in >60% of the patients with AIN deficits. 14 Importantly, when the AIN is affected, the lesion is almost always found in the AIN fascicles within the median nerve, proximal to its branching point. 6 , 14 , 37 Therefore, we do not recommend routinely scanning the AIN proper.
To scan the radial nerve, the patient should be supine with the shoulder slightly abducted and externally rotated, and the forearm pronated (Supporting Information Figure S3). The radial nerve can be located near the elbow anterior to the lateral epicondyle. Near the antecubital fossa, the radial nerve bifurcates into the superficial radial nerve and deep radial nerve, which is referred to as the PIN once passed the supinator tunnel. The radial nerve should be reidentified near the elbow and followed proximally through the spiral groove of the humerus, where it travels alongside the deep brachial artery between the triceps brachii. The patient's shoulder may be placed in a flexed and abducted position to locate the radial nerve and brachial artery in the axilla. 35 Similar to the AIN, when the PIN and superficial radial nerves are affected, the lesions are almost always found in their respective fascicles within the radial nerve, proximal to its branching point. 14 , 20 In the study discussed above, the radial nerve/PIN were the second most frequently affected nerves and had the second highest number of sonographic abnormalities, behind the AIN. 14 Of note, the presence of hourglass constrictions and fascicular entwinement in the radial nerve is significantly more frequent than in other nerves and most often involves the PIN fascicle within the radial nerve. 6 , 14
3.2. Suprascapular nerve
To scan the SSN at its origin, the patient should be supine with the head turned to the opposite side (Figure 3). The origin of the SSN can be located by identifying and following the C5 nerve root laterally until the nerve is visualized branching from the superior trunk. Techniques to locate the cervical nerve roots in the neck using ultrasound have been well described. 38 After branching from the superior trunk, the SSN can be followed in its course through the posterior triangle of the neck along the omohyoid muscle belly. To locate the SSN at the scapula, the patient should be seated upright facing away from the sonographer. The probe can be placed just superior to the spine of the scapula (which can be identified via palpation) over the suprascapular fossa. At this level, medial and lateral scanning can locate suprascapular notch at the superior border of the scapula. Here, the SSN runs under the transverse scapular ligament before entering the supraspinous fossa. Doppler ultrasound can be used to identify the suprascapular artery directly above the SSN and ligament. The SSN travels underneath the SSM and continues through the spinoglenoid foramen adjacent to the suprascapular vessels into the infraspinatus fossa. 35 , 39 , 40 In one large NA study, the infraspinatus and SSM were the first and third most commonly affected muscles, respectively. 2
FIGURE 3.
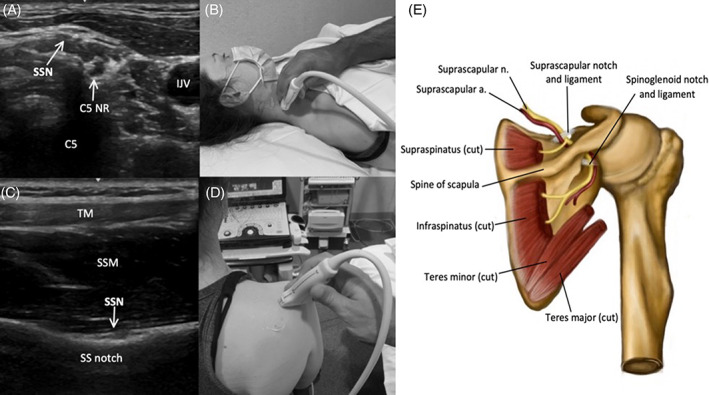
Image 3A demonstrates the SSN in the posterior triangle of the neck, shortly after branching from the C5 nerve root (C5 NR) (C5 = shadow from posterior tubercle of C5 foramen, and Image 3B shows the corresponding probe placement. Image 3C demonstrates the SSN passing through the suprascapular notch (SS notch), deep to the TM and SSM, and Image 3D shows the corresponding probe placement. Image 3E depicts the SSN and relevant anatomy in the posterior scapular region
3.3. Axillary nerve
To scan the AN, the patient should sit upright with the shoulder in neutral position (Figure 4). The AN can be identified in the quadrangular space, which is formed by teres minor superiorly, teres major inferiorly, the long head of triceps brachii medially, and the humerus laterally. The transducer should be positioned on the posterior arm near the axilla to obtain a sagittal view. The nerve can be identified adjacent to the posterior circumflex humeral artery (PCHA) using Doppler ultrasound. The AN and PCHA traverse laterally and wrap around the surgical neck of the humerus where the AN divides into the anterior and posterior terminal divisions to innervate the deltoid and teres minor. 35
FIGURE 4.
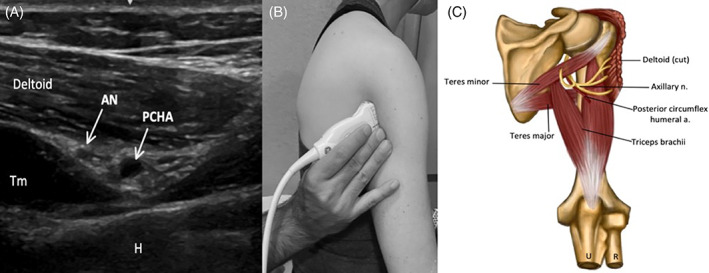
Image 4A demonstrates the AN and PCHA in the quadrangular space of the posterior axilla, inferior to the deltoid (Tm = teres minor muscle, H = humerus) (here the probe is placed superior to the level of teres major, the inferior border of the quadrangular space), and Image 4B shows the corresponding probe placement. Image 4C depicts the AN and relevant anatomy in quadrangular space of the posterior upper limb
3.4. Long thoracic nerve
To scan the LTN, the patient should be supine with the head turned to the opposite side (Figure 5). The LTN can be identified in the neck piercing through the middle scalene muscle (MS). Identify the MS posterolateral to the brachial plexus (BP) in the neck and slide the transducer in the short‐axis view along the C5‐C7 levels. Due to the proximity of the LTN and dorsal scapular nerve in this region, careful attention should be paid to the cervical level and depth. The dorsal scapular nerve is usually located more superficially in the MS at the C5 and C6 level, whereas the LTN is deeper at the C6 and C7 level. The LTN can be followed as it traverses parallel to the MS until reaching the clavicle. With the patient in the lateral decubitus position, the LTN can be identified as it travels inferiorly along the lateral ribcage. The nerve joins the path of the long thoracic artery (LTA) at the level of the fourth or fifth rib, and this neurovascular bundle can be identified between the more superficial latissimus dorsi and deep SAM distal to the level of the eighth rib. 41 , 42 While the LTN is one of the most frequently affected nerves in NA, abnormalities on HRUS are less common compared to other highly affected nerves. This may be partially explained by the technical difficulty of visualizing the LTN with ultrasound due to its small size and limited window for reliable visualization. 14
FIGURE 5.
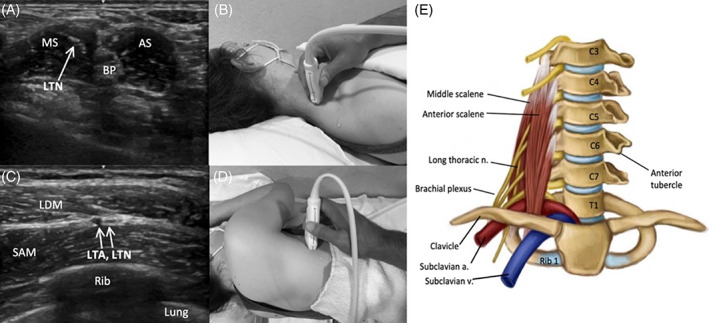
Image 5A demonstrates the LTN piercing the MS in the distal lateral neck, and Image 5B shows the corresponding probe placement. Image 5C demonstrates the LTN traveling adjacent LTA as it passes between the latissimus dorsi muscle (LDM) and the SAM along the lateral rib cage, and Image 5D shows the corresponding probe placement. Image 5E depicts the LTN and relevant anatomy in anterior neck
3.5. Spinal accessory nerve
To scan the SAN, the patient should be supine with the head turned to the opposite side (Figure 6). The SAN initially descends alongside the internal jugular vein (IJV) before branching to innervate the sternocleidomastoid muscle (SCM). The SAN can be identified in the posterior triangle of the neck at the posterolateral border of the SCM midway between the ear and clavicle. The sonographer can scan cranially toward the skull base and caudally as the nerve runs through or deep to the SCM, superficial to the levator scapulae muscle (LSM). It then pierces and innervates the trapezius muscle (TM). 36 Notably, one study identified NA as the most common medical cause of unilateral TM palsy. 43
FIGURE 6.
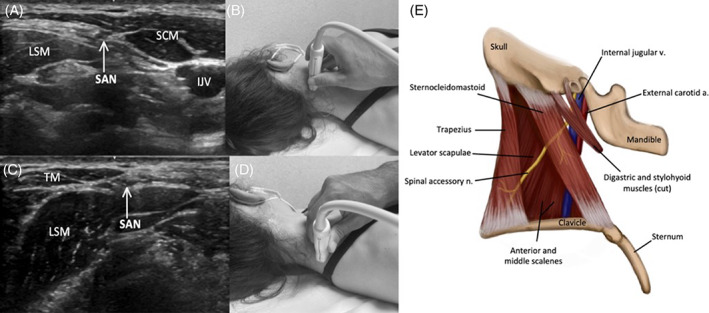
Image 6A demonstrates the SAN along the lateral border of the SCM in the proximal lateral neck, and Image 6B shows the corresponding probe placement. Image 6C demonstrates the SAN superficial to the LSM and deep to the TM in the distal lateral neck, and Image 6D shows the corresponding probe placement. Image 6E depicts the SA and relevant anatomy in lateral neck
3.6. Phrenic nerve
To scan the PN, the patient should be supine with the head turned to the opposite side (Figure 7). The PN originates from the C3, C4, and C5 nerve roots and can be tracked from the C4 extraforaminal root or identified in the lateral neck superficial to the anterior scalene muscle (AS) and medial to the supraclavicular nerve branches. The AS can be identified by locating the BP, which is seen between the anterior and MS. 38 The PN descends along the AS, and then between the subclavian vein (anterior) and artery (posterior) before entering the thorax. 38 , 44 Of note, ultrasound can also be used to effectively assess the bilateral hemi‐diaphragm muscles. The presence of severe, early onset diaphragm muscle atrophy may be an important clue in diagnosing NA, and regular monitoring of diaphragm function with ultrasound can help determine prognosis and inform decision making in NA. 13 The technique for scanning the diaphragm is described at length in other publications. 45
FIGURE 7.
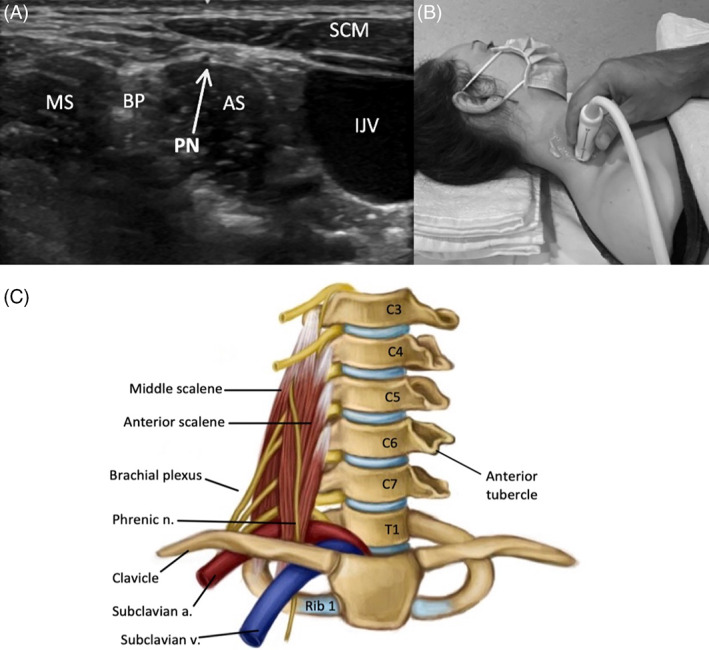
Image 7A demonstrates the PN superficial to the AS in the lateral neck, and Image 7B shows the corresponding probe placement. Image 7C depicts the PN and relevant anatomy in the anterior neck
4. DISCUSSION
Ultrasound can provide important diagnostic and prognostic clues in NA. Aranyi et al. reported noteworthy associations among HRUS findings and the degree of clinical recovery. In their study, nerve swelling alone and swelling with incomplete constriction showed the greatest degree of spontaneous recovery at 6 mo. In contrast, patients with complete hourglass constrictions showed negligible improvement with conservative management and often progressed to surgical intervention. 14 Although recently associated with NA, hourglass constrictions are not a novel concept; isolated cases of hourglass constrictions were described as early as 1966. 46 Several case reports and small case series have described the frequent need for surgical intervention in this cohort, thus reshaping our understanding of how to manage NA. 29 , 32 , 47 , 48 Recently, Gstoettner et al. established a surgical treatment algorithm for patients with NA and hourglass constrictions. The authors suggest that if patients fail conservative treatment for 3 mo, high‐resolution imaging should be performed to evaluate for hourglass constrictions and, depending on the degree of constriction, patients should be treated with neurolysis or neurorrhaphy/grafting. 6 HRUS has proven to be a dependable option to detect hourglass constrictions and should therefore become the standard of care in the diagnosis and follow‐up of NA, 14 , 47 , 48 , 49 , 50 Some clinicians suggest skipping conservative management altogether and progressing to immediate surgical exploration in the small minority of patients with complete hourglass constrictions or rotational phenomena on HRUS. However, this approach will require further investigation. 14
While hourglass constrictions and fascicular entwinement may be pathognomonic for NA, these findings are likely not sensitive, as many individuals with NA do not have these changes, even with detailed high‐resolution imaging. What is typically seen is focal nerve enlargement, often with only select fascicular enlargement. 14 Further research is needed to understand the optimal timing to perform HRUS imaging, the diagnostic accuracy and prognostic meaning of all these findings, and a clear timeline for follow up studies. Large, prospective trials are needed to better understand this condition and, most importantly, optimize treatments and interventions.
Abbreviations
- AIN
anterior interosseous nerve
- AN
axillary nerve
- AS
anterior scalene muscle
- BP
brachial plexus
- EDX
electrodiagnostic testing
- EMG
electromyography
- HRUS
high‐resolution ultrasound
- IJV
internal jugular vein
- LSM
levator scapulae muscle
- LTA
long thoracic artery
- LTN
long thoracic nerve
- MCN
musculocutaneous
- MRI
magnetic resonance imaging
- MS
middle scalene muscle
- NA
neuralgic amyotrophy
- PCHA
posterior circumflex humeral artery
- PIN
posterior interosseous nerve
- PN
phrenic nerve
- SAM
serratus anterior muscle
- SAN
spinal accessory nerve
- SCM
sternocleidomastoid muscle
- SSM
supraspinatus muscle
- SSN
suprascapular nerve
- TM
trapezius muscle
CONFLICT OF INTEREST
Dr. Cignetti, Ms. Cox, and Drs. Baute, McGhee, Strakowski, Boon, Norbury, and Cartwright have no conflicts of interest. Dr. van Alfen works as an ultrasound consultant for Dynacure and performs editorial services for Wiley Publishing; all payments go to their employer.
ETHICS STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
FINANCIAL DISCLOSURE
Dr. Cignetti, Ms. Cox, and Drs. Baute, McGhee, van Alfen, Strakowski, Boon, Norbury, and Cartwright have no disclosures.
Supporting information
Figure S1 Image 1A demonstrates the musculocutaneous nerve (MCN) piercing the coracobrachialis muscle (CBM) deep to the biceps brachii muscle in the medial proximal arm (BA = brachial artery, H = humerus), and Image 1B shows the corresponding transducer placement. Image 1C demonstrates the longitudinal view of the MCN traveling superficial to the CBM in the medial proximal arm, and Image 1D shows the corresponding transducer placement. Image 1E depicts the musculocutaneous nerve and relevant anatomy in the anterior upper limb.
Figure S2 Image 2A demonstrates the median nerve (MN) superficial to the flexor tendons and carpal bones in the carpal tunnel at the wrist, and Image 2B shows the corresponding transducer placement. Image 2C demonstrates the MN with its branch, the anterior interosseous nerve (AIN), alongside the anterior interosseous artery (AIA) (R = radius) in the anterior forearm, and Image 2D shows the corresponding transducer placement. Image 2E demonstrates the MN situated medial to the brachial artery (BA) in the distal medial arm (H = humerus), and Image 2F shows the corresponding transducer placement. Image 2G demonstrates the MN situated lateral to the BA in the medial proximal arm, and Image 2H shows the corresponding transducer placement. Image 2I depicts the median nerve and relevant anatomy in the anterior upper limb (left) and the anterior interosseous nerve and relevant anatomy in the anterior forearm (right).
Figure S3 Image 3A demonstrates the radial nerve (RN) superficial to the lateral distal humerus (H) proximal to the antecubital fossa (BRM = brachioradialis muscle), and Image 3B shows the corresponding transducer placement. Image 3C demonstrates the posterior interosseus nerve (PIN) traveling between the superficial and deep heads of the supinator muscle (SM) in the proximal anterior forearm (ECRL = extensor carpi radialis longus, R = radius), and image 3D shows the corresponding transducer placement. Image 3E demonstrates the RN within the spiral groove of the humerus (H) in the lateral arm, and Image 3F shows the corresponding transducer placement. Figure 5G demonstrates the longitudinal view of the RN superficial to the triceps muscle in the lateral arm, and Image 3H shows the corresponding transducer placement. Image 3I demonstrates the MN and brachial artery (BA) in the axilla, and Image 3 J shows the corresponding transducer placement. Image 3 K depicts the radial nerve and relevant anatomy in the anterior upper limb with pronated forearm (left), and in the triangular interval of the posterior upper limb (right).
Video S1 Fascicular entwinement of the median nerve seen in the short axis view.
ACKNOWLEDGMENT
None.
Cignetti NE, Cox RS, Baute V, et al. A standardized ultrasound approach in neuralgic amyotrophy. Muscle & Nerve. 2023;67(1):3‐11. doi: 10.1002/mus.27705
Answer questions and earn CME https://education.aanem.org/URL/JR100.
The objectives of this activity are to: 1)Be able to plan an appropriate ultrasonographic approach to studying the patient with suspected neuralgic amyotrophy; 2) Be able to perform technically satisfactory scans of the musculocutaneous, median, and radial nerves in patients with suspected neuralgic amyotrophy; 3) Be able to perform technically satisfactory scans of less commonly studied upper extremity nerves in these patients: suprascapular, axillary, long thoracic, spinal accessory, and phrenic.
The AANEM is accredited by the American Council for Continuing Medical Education (ACCME) to providing continuing education for physicians. AANEM designates this Journal‐based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study
REFERENCES
- 1. IJspeert J, RMJ J, van Alfen N. Neuralgic amyotrophy. Curr Opin Neurol. 2021;34:605‐612. [DOI] [PubMed] [Google Scholar]
- 2. van Alfen N, van Engelen BGM. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain. 2006;129(Pt 2):438‐450. [DOI] [PubMed] [Google Scholar]
- 3. Ferrante MA, Wilbourn AJ. Lesion distribution among 281 patients with sporadic neuralgic amyotrophy. Muscle Nerve. 2017;55(6):858‐861. [DOI] [PubMed] [Google Scholar]
- 4. Zanette G, Rasera A, Tamburin S. Selective atrophy of the brachialis muscle in neuralgic amyotrophy: ultrasound imaging of fascicular nerve damage. J Neurol Neurosurg Psychiatry. 2020;91(10):1118‐1119. [DOI] [PubMed] [Google Scholar]
- 5. van Alfen N. Clinical and pathophysiological concepts of neuralgic amyotrophy. Nat Rev Neurol. 2011;7(6):315‐322. [DOI] [PubMed] [Google Scholar]
- 6. Gstoettner C, Mayer JA, Rassam S, et al. Neuralgic amyotrophy: a paradigm shift in diagnosis and treatment. J Neurol Neurosurg Psychiatry. 2020;91(8):879‐888. [DOI] [PubMed] [Google Scholar]
- 7. Kuhlenbäumer G, Hannibal MC, Nelis E, et al. Mutations in SEPT9 cause hereditary neuralgic amyotrophy. Nat Genet. 2005;37(10):1044‐1046. [DOI] [PubMed] [Google Scholar]
- 8. Van Eijk JJJ, Groothuis JT, Van Alfen N. Neuralgic amyotrophy: an update on diagnosis, pathophysiology, and treatment. Muscle Nerve. 2016;53(3):337‐350. [DOI] [PubMed] [Google Scholar]
- 9. Arányi Z, Csillik A, Dévay K, et al. Ultrasonographic identification of nerve pathology in neuralgic amyotrophy: enlargement, constriction, fascicular entwinement, and torsion. Muscle Nerve. 2015;52(4):503‐511. [DOI] [PubMed] [Google Scholar]
- 10. Ferrante MA. The distribution of neuralgic amyotrophy lesions is overwhelmingly extraplexal. Muscle Nerve. 2018;58(3):325‐326. [DOI] [PubMed] [Google Scholar]
- 11. van Alfen N, Doorduin J, van Rosmalen MHJ, et al. Phrenic neuropathy and diaphragm dysfunction in neuralgic amyotrophy. Neurology. 2018;91(9):e843‐e849. [DOI] [PubMed] [Google Scholar]
- 12. PJB D, Thaisetthawatkul P. Lumbosacral plexopathy. Contin Minneap Minn. 2014;20(5 Peripheral Nervous System Disorders):1343‐1358. [DOI] [PubMed] [Google Scholar]
- 13. Farr E, D'Andrea D, Franz CK. Phrenic nerve involvement in neuralgic Amyotrophy (parsonage‐turner syndrome). Sleep Med Clin. 2020;15(4):539‐543. [DOI] [PubMed] [Google Scholar]
- 14. ArÁnyi Z, Csillik A, DéVay K, et al. Ultrasonography in neuralgic amyotrophy: sensitivity, spectrum of findings, and clinical correlations. Muscle Nerve. 2017;56(6):1054‐1062. [DOI] [PubMed] [Google Scholar]
- 15. van Rosmalen M, Lieba‐Samal D, Pillen S, van Alfen N. Ultrasound of peripheral nerves in neuralgic amyotrophy. Muscle Nerve. 2019;59(1):55‐59. [DOI] [PubMed] [Google Scholar]
- 16. Cup EH, Ijspeert J, Janssen RJ, et al. Residual complaints after neuralgic amyotrophy. Arch Phys Med Rehabil. 2013;94(1):67‐73. [DOI] [PubMed] [Google Scholar]
- 17. van Alfen N, van Engelen BGM, Hughes RAC. Treatment for idiopathic and hereditary neuralgic amyotrophy (brachial neuritis). Cochrane Database Syst Rev. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al Khalili Y, Jain S, Lam JC, DeCastro A. Brachial Neuritis [Internet]. StatPearls. StatPearls Publishing; 2021. [cited 2021 Sep 29]. http://www.ncbi.nlm.nih.gov/books/NBK499842/.Available from: [PubMed] [Google Scholar]
- 19. Pham M, Bäumer P, Meinck H‐M, et al. Anterior interosseous nerve syndrome: fascicular motor lesions of median nerve trunk. Neurology. 2014;82(7):598‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bäumer P, Kele H, Xia A, et al. Posterior interosseous neuropathy: supinator syndrome vs fascicular radial neuropathy. Neurology. 2016;87(18):1884‐1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sneag DB, Arányi Z, Zusstone EM, et al. Fascicular constrictions above elbow typify anterior interosseous nerve syndrome. Muscle Nerve. 2020;61(3):301‐310. [DOI] [PubMed] [Google Scholar]
- 22. Ortiz Torres M, Gudlavalleti A, Mesfin FB. Brachial Plexitis [Internet]. StatPearls. StatPearls Publishing; 2021. [cited 2021 Sep 29]. http://www.ncbi.nlm.nih.gov/books/NBK448114/.Available from: [PubMed] [Google Scholar]
- 23. van Eijk JJJ, Groothuis JT, van Alfen N. Reply. Muscle Nerve. 2016;54(2):342‐343. [DOI] [PubMed] [Google Scholar]
- 24. van Alfen N, Huisman WJ, Overeem S, van Engelen BGM, Zwarts MJ. Sensory nerve conduction studies in neuralgic amyotrophy. Am J Phys Med Rehabil. 2009;88(11):941‐946. [DOI] [PubMed] [Google Scholar]
- 25. Lieba‐Samal D, Jengojan S, Kasprian G, Wöber C, Bodner G. Neuroimaging of classic neuralgic amyotrophy. Muscle Nerve. 2016;54(6):1079‐1085. [DOI] [PubMed] [Google Scholar]
- 26. Park MS, Kim DH, Sung DH. Magnetic resonance neurographic findings in classic idiopathic neuralgic amyotrophy in subacute stage: a report of four cases. Ann Rehabil Med. 2014;38(2):286‐291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim DH, Kim J, Sung DH. Hourglass‐like constriction neuropathy of the suprascapular nerve detected by high‐resolution magnetic resonance neurography: report of three patients. Skeletal Radiol. 2019;48(9):1451‐1456. [DOI] [PubMed] [Google Scholar]
- 28. Abraham A, Izenberg A, Dodig D, Bril V, Breiner A. Peripheral nerve ultrasound imaging shows enlargement of peripheral nerves outside the brachial plexus in neuralgic Amyotrophy. J Clin Neurophysiol. 2016;33(5):e31‐e33. [DOI] [PubMed] [Google Scholar]
- 29. Lundborg G. Commentary: hourglass‐like fascicular nerve compressions. J Hand Surg. 2003;28(2):212‐214. [DOI] [PubMed] [Google Scholar]
- 30. Krishnan KR, Sneag DB, Feinberg JH, et al. Outcomes of microneurolysis of hourglass constrictions in chronic neuralgic Amyotrophy. J Hand Surg Am. 2021;46(1):43‐53. [DOI] [PubMed] [Google Scholar]
- 31. Pan Y‐W, Wang S, Tian G, Li C, Tian W, Tian M. Typical brachial neuritis (Parsonage‐Turner syndrome) with hourglass‐like constrictions in the affected nerves. J Hand Surg Am. 2011;36(7):1197‐1203. [DOI] [PubMed] [Google Scholar]
- 32. Wu P, Yang JY, Chen L, Yu C. Surgical and conservative treatments of complete spontaneous posterior interosseous nerve palsy with hourglass‐like fascicular constrictions: a retrospective study of 41 cases. Neurosurgery. 2014;75(3):250‐257. discussion 257. [DOI] [PubMed] [Google Scholar]
- 33. Brown JM, Yablon CM, Morag Y, Brandon CJ, Jacobson JA. US of the peripheral nerves of the upper extremity: a landmark approach. Radiographics. 2016;36(2):452‐463. [DOI] [PubMed] [Google Scholar]
- 34. Won SJ, Kim B‐J, Park KS, Yoon JS, Choi H. Reference values for nerve ultrasonography in the upper extremity. Muscle Nerve. 2013;47(6):864‐871. [DOI] [PubMed] [Google Scholar]
- 35. Wu C‐H, Chang K‐V, Özçakar L, et al. Sonographic tracking of the upper limb peripheral nerves: a pictorial essay and video demonstration. Am J Phys Med Rehabil. 2015;94(9):740‐747. [DOI] [PubMed] [Google Scholar]
- 36. Youngner JM, Matsuo K, Grant T, Garg A, Samet J, Omar IM. Sonographic evaluation of uncommonly assessed upper extremity peripheral nerves: anatomy, technique, and clinical syndromes. Skeletal Radiol. 2019;48(1):57‐74. [DOI] [PubMed] [Google Scholar]
- 37. Krishnan KR, Sneag DB, Feinberg JH, Wolfe SW. Anterior interosseous nerve syndrome reconsidered: a critical analysis review. JBJS Rev. 2020;8(9):e2000011. [DOI] [PubMed] [Google Scholar]
- 38. Baute V, Strakowski JA, Reynolds JW, et al. Neuromuscular ultrasound of the brachial plexus: a standardized approach. Muscle Nerve. 2018;58(5):618‐624. [DOI] [PubMed] [Google Scholar]
- 39. Basta M, Sanganeria T, Anatomy VM. Shoulder and upper limb, Suprascapular nerve [Internet]. StatPearls. StatPearls Publishing; 2021. [cited 2021 Sep 30]. http://www.ncbi.nlm.nih.gov/books/NBK557880/.Available from: [PubMed] [Google Scholar]
- 40. Gruber L, Loizides A, Löscher W, Glodny B, Gruber H. Focused high‐resolution sonography of the suprascapular nerve: a simple surrogate marker for neuralgic amyotrophy? Clin Neurophysiol. 2017;128(8):1438‐1444. [DOI] [PubMed] [Google Scholar]
- 41. Chang K‐V, Wu W‐T, Mezian K, Naňka O, Özçakar L. Sonoanatomy revisited: the long thoracic nerve. Med Ultrason. 2019;21(3):349‐352. [DOI] [PubMed] [Google Scholar]
- 42. Kim H. Dorsal scapular and long thoracic nerves during ultrasound‐guided interscalene brachial plexus block. Asian J Anesthesiol. 2017;55(1):26‐27. [DOI] [PubMed] [Google Scholar]
- 43. Seror P, Stojkovic T, Lefevre‐Colau MM, Lenglet T. Diagnosis of unilateral trapezius muscle palsy: 54 cases. Muscle Nerve. 2017;56(2):215‐223. [DOI] [PubMed] [Google Scholar]
- 44. Zhang Y, Duan F, Ma W. Ultrasound‐guided phrenic nerve block for intraoperative persistent hiccups: a case report. BMC Anesthesiol. 2018;18:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sarwal A, Walker FO, Cartwright MS. Neuromuscular ultrasound for evaluation of the diaphragm. Muscle Nerve. 2013;47(3):319‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Abe T, Hoshiko M, Shinohara N, Takamatsu T. Isolated posterior interosseous nerve palsy caused by entrapment neuropathies Isolated paralysis of the deep branch of the radial nerve thought to be the entrapment neuropathy. Rinsho Seikei Geka. 1966;1:617‐21. [Google Scholar]
- 47. Wang Y, Liu T, Song L, et al. Spontaneous peripheral nerve palsy with hourglass‐like fascicular constriction in the upper extremity. J Neurosurg. 2019;131(6):1876‐1886. [DOI] [PubMed] [Google Scholar]
- 48. Pan Y, Wang S, Zheng D, et al. Hourglass‐like constrictions of peripheral nerve in the upper extremity: a clinical review and pathological study. Neurosurgery. 2014;75(1):10‐22. [DOI] [PubMed] [Google Scholar]
- 49. Deng H, Lu B, Yin C, et al. The effectiveness of ultrasonography in the diagnosis of spontaneous Hourglasslike constriction of peripheral nerve in the upper extremity. World Neurosurg. 2020;134:e103‐e111. [DOI] [PubMed] [Google Scholar]
- 50. Qi HT, Wang XM, Li SY, et al. The role of ultrasonography and MRI in patients with non‐traumatic nerve fascicle torsion of the upper extremity. Clin Radiol. 2013;68(9):e479‐e483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Image 1A demonstrates the musculocutaneous nerve (MCN) piercing the coracobrachialis muscle (CBM) deep to the biceps brachii muscle in the medial proximal arm (BA = brachial artery, H = humerus), and Image 1B shows the corresponding transducer placement. Image 1C demonstrates the longitudinal view of the MCN traveling superficial to the CBM in the medial proximal arm, and Image 1D shows the corresponding transducer placement. Image 1E depicts the musculocutaneous nerve and relevant anatomy in the anterior upper limb.
Figure S2 Image 2A demonstrates the median nerve (MN) superficial to the flexor tendons and carpal bones in the carpal tunnel at the wrist, and Image 2B shows the corresponding transducer placement. Image 2C demonstrates the MN with its branch, the anterior interosseous nerve (AIN), alongside the anterior interosseous artery (AIA) (R = radius) in the anterior forearm, and Image 2D shows the corresponding transducer placement. Image 2E demonstrates the MN situated medial to the brachial artery (BA) in the distal medial arm (H = humerus), and Image 2F shows the corresponding transducer placement. Image 2G demonstrates the MN situated lateral to the BA in the medial proximal arm, and Image 2H shows the corresponding transducer placement. Image 2I depicts the median nerve and relevant anatomy in the anterior upper limb (left) and the anterior interosseous nerve and relevant anatomy in the anterior forearm (right).
Figure S3 Image 3A demonstrates the radial nerve (RN) superficial to the lateral distal humerus (H) proximal to the antecubital fossa (BRM = brachioradialis muscle), and Image 3B shows the corresponding transducer placement. Image 3C demonstrates the posterior interosseus nerve (PIN) traveling between the superficial and deep heads of the supinator muscle (SM) in the proximal anterior forearm (ECRL = extensor carpi radialis longus, R = radius), and image 3D shows the corresponding transducer placement. Image 3E demonstrates the RN within the spiral groove of the humerus (H) in the lateral arm, and Image 3F shows the corresponding transducer placement. Figure 5G demonstrates the longitudinal view of the RN superficial to the triceps muscle in the lateral arm, and Image 3H shows the corresponding transducer placement. Image 3I demonstrates the MN and brachial artery (BA) in the axilla, and Image 3 J shows the corresponding transducer placement. Image 3 K depicts the radial nerve and relevant anatomy in the anterior upper limb with pronated forearm (left), and in the triangular interval of the posterior upper limb (right).
Video S1 Fascicular entwinement of the median nerve seen in the short axis view.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study


