Abstract
Objective
To assess whether post‐stroke epilepsy (PSE) is associated with neuroimaging findings of hemosiderin in a case–control study, and whether the addition of hemosiderin markers improves the risk stratification models of PSE.
Methods
We performed a post‐hoc analysis of the PROgnosis of POST‐Stroke Epilepsy study enrolling PSE patients at National Cerebral and Cardiovascular Center, Osaka, Japan, from November 2014 to September 2019. PSE was diagnosed when one unprovoked seizure was experienced >7 days after the index stroke, as proposed by the International League Against Epilepsy. As controls, consecutive acute stroke patients with no history or absence of any late seizure or continuing antiseizure medications at least 3 months after stroke were retrospectively enrolled during the same study period. We examined cortical microbleeds and cortical superficial siderosis (cSS) using gradient‐echo T2*‐weighted images. A logistic regression model with ridge penalties was tuned using 10‐fold cross‐validation. We added the item of cSS to the existing models (SeLECT and CAVE) for predicting PSE and evaluated performance of new models.
Results
The study included 180 patients with PSE (67 women; median age 74 years) and 1,183 controls (440 women; median age 74 years). The cSS frequency was higher in PSE than control groups (48.9% vs 5.7%, p < 0.0001). Compared with the existing models, the new models with cSS (SeLECT‐S and CAVE‐S) demonstrated significantly better predictive performance of PSE (net reclassification improvement 0.63 [p = 0.004] for SeLECT‐S and 0.88 [p = 0.001] for CAVE‐S at the testing data).
Interpretation
Cortical superficial siderosis was associated with PSE, stratifying stroke survivors at high risk of PSE. ANN NEUROL 2023;93:357–370
Post‐stroke epilepsy, a major complication after stroke, affects approximately 5 to 10% of stroke survivors, 1 , 2 and approximately half of the new‐onset epilepsy cases are attributable to stroke in the elderly. 3 , 4 Post‐stroke epilepsy is linked to worse clinical outcomes, 1 , 5 , 6 , 7 and patients with post‐stroke epilepsy have approximately twofold higher mortality 1 year after stroke than those without post‐stroke epilepsy. 5 Moreover, post‐stroke epilepsy adversely affects the quality of life due to unpredictable seizure recurrence, driving restriction, and unemployment. Therefore, with the increased survival rate of stroke patients, identifying patients at high risk of post‐stroke epilepsy is crucial for managing stroke survivors.
Although the underlying mechanisms of post‐stroke epilepsy remain to be fully elucidated, different risk factors for post‐stroke epilepsy, such as stroke type, stroke severity, cortical involvement, vascular risk factors, and early seizure, have been reported, 8 and risk models, such as CAVE score 9 or SeLECT score, 10 have been established to identify patients at high risk of post‐stroke epilepsy. However, because of the relatively low frequency of post‐stroke epilepsy during the study period (the prevalence of late seizures was 8% for ischemic stroke and 11.8% for intracerebral hemorrhage 5 years after stroke), these previous studies recruited <100 cases of post‐stroke epilepsy in a derivation or validation cohort, which might not have been sufficient to assess brain imaging risk prediction biomarkers of post‐stroke epilepsy beyond stroke lesions per se.
Possible pathomechanisms of post‐stroke epilepsy include the disruption of neuronal networks for ischemic post‐stroke epilepsy, hemosiderin depositions for hemorrhagic post‐stroke epilepsy, and gliotic scarring within the cortex for both. 11 Intriguingly, post‐stroke epilepsy is more prevalent in hemorrhagic stroke than in ischemic stroke, 1 , 11 , 12 and hemorrhagic transformation after endovascular therapy substantially increases the rate of post‐stroke seizures by fivefold within 2 years. 13 Consistently, studies showed that intracranial injection of hemoglobin provoked focal spike activity in 89% of rats, 14 and seizure outcomes improved in cases of cerebral cavernous malformation with intractable focal epilepsy after complete removal of the hemosiderin fringe brain tissue. 15 These findings strongly suggest that hemosiderin may cause epileptogenesis in post‐stroke epilepsy.
Accordingly, we hypothesized that hemosiderin deposition is essential for the development of post‐stroke epilepsy after stroke. To address this hypothesis, we explored the association between post‐stroke epilepsy and neuroimaging findings of hemosiderin, and investigated whether the addition of hemosiderin markers improves the risk models of post‐stroke epilepsy in a case–control study using our post‐stroke epilepsy registry.
Methods
Study Design and Population
A retrospective case–control study was carried out at the National Cerebral and Cardiovascular Center in Osaka, Japan. Patients with post‐stroke epilepsy were retrieved from the PROgnosis of Post‐Stroke Epilepsy (PROPOSE) study, which was conducted from November 2014 to September 2019. 16 In accordance with the clinical definition from the International League Against Epilepsy, one unprovoked seizure >7 days after an index stroke was diagnosed as post‐stroke epilepsy. 17 We excluded patients with only early seizure within 7 days following a stroke onset, another etiology of epilepsy, history of only asymptomatic stroke, or potentially epileptogenic comorbidities (alcohol or drug abuse, traumatic brain injury, brain tumors, or other probable sources). All diagnoses of post‐stroke epilepsy were verified at consensus conferences attended by two board‐certified epileptologists (K.K. and A.S.) and neurologists based on electroencephalogram (EEG), seizure semiology, and clinical course during admission, and therapeutic response. To assess hemorrhagic imaging brain biomarkers, we excluded patients without gradient‐echo T2*‐weighted images (GRET2*WI) on magnetic resonance imaging (MRI). For the control group, we retrospectively enrolled consecutive patients who were admitted to our hospital due to acute stroke between November 2014 to September 2019. The eligibility criteria for the controls included patients with no history or the absence of any seizure, or continuing antiseizure medications at least 3 months after stroke. We excluded patients with <3 months of monitoring at our hospital, those who died during admission, or those who did not receive GRET2*WI on MRI. Patient clinical data were retrospectively collected from our Stroke Registry database (https://www.clinicaltrials.gov; unique identifier: NCT02251665) and medical records.
This study was approved by our institutional ethical committee according to the institutional guidelines (M26‐093‐7). Informed consent was waived by the ethical committees under the “opt‐out” principle.
Patients' Clinical Characteristics
We acquired data on demographics, including age, sex, comorbidities (hypertension, atrial fibrillation, dyslipidemia, diabetes, dementia, chronic kidney diseases, and liver cirrhosis), family history of epilepsy, and history of craniotomy. Index strokes were categorized as ischemic stroke, intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), and transient ischemic attack. For ischemic stroke, we also evaluated subtypes, such as cardiac embolism and large‐artery atherosclerosis. The severity of stroke was measured using the National Institutes of Health Stroke Scale (NIHSS) at admission for index stroke and stratified into mild (≤3), moderate (4–10), and severe (≥11). 10 The modified Rankin Scale was used to assess functional disability at discharge. 18 Moreover, the history of early seizure (onset within 7 days after stroke) was examined. Standard scalp EEG was carried out after admission, and analyzed for epileptiform activities as ictal and postictal epileptiform discharges (spike and sharp waves) based on Salzburg EEG criteria. 19 Postictal single‐photon emission computed tomography (SPECT) was performed and visually evaluated to identify the hyperperfusion area in endorsing the post‐stroke epilepsy diagnosis. If it was difficult to confirm hyperperfusion by postictal SPECT alone, interictal SPECT was performed and compared with the postictal SPECT by the subtraction method, as reported previously. 20
MRI Evaluations
We assessed GRET2*WI and fluid‐attenuated inversion recovery obtained after admission following stroke or post‐stroke epilepsy. With regard to the timing of MRI, we used MRI scans of post‐stroke epilepsy during patient hospitalization due to seizures, and MRI scans of controls as close as possible to the final follow up. Brain MRI was performed either with 1.5‐T (Magnetom Sonata or Vision; Siemens Medical Solutions, Erlangen, Germany) or 3.0‐T (Magnetom Verio or Spectra; Siemens Medical Solutions, Erlangen, Germany) scanners within a few days after admission. The sequence parameters of GRET2*WI were as follows: slice thickness, 4.0mm; interslice gap, 2.0mm; echo time, 12ms; repetition time, 550ms; and flip angle, 20°. The sequence parameters of fluid‐attenuated inversion recovery images were as follows: slice thickness, 5.0mm; interslice gap, 1.0mm; echo time, 94 to 114ms; and repetition time, 12,000ms. MR images were assessed by two experienced certified neurologists (T.T. and K.F.) who were blinded to the clinical information of participants. We evaluated the location of stroke lesions (anterior cerebral artery, middle cerebral artery, and posterior cerebral artery territories) and size in the major axis (categorized as <15mm, 15–30mm, and >30mm). The presence of cortical lesions was also assessed. Using GRET2*WI, cerebral microbleeds (CMBs) were identified in the supratentorial brain area and classified into three groups: strictly lobar CMBs (presence of CMBs exclusively in the lobar region: frontal, parietal, temporal, occipital, and insula), strictly deep CMBs (presence of CMBs exclusively in deep regions: basal ganglia and internal capsule), and mixed‐location CMBs (both in the lobar and deep locations). 21 , 22 Subtentorial CMBs were not counted in this study, because they might not affect the etiology of post‐stroke epilepsy. Cortical superficial siderosis (cSS) was defined as homogeneous curvilinear hypointensities (black) along with the cortical superficial layers, within the subarachnoid space, or both. 23 According to the established criteria, 23 we closely identified cSS to rule out cortical veins, thrombosed vessels, and superficial clusters of CMBs, as well as cortical laminar necrosis, based on their characteristics (hyperintense on fluid‐attenuated inversion recovery and hypointense on GRET2*WI), as described in Supplementary Figure 1 in Appendix S1. Infratentorial cSS was excluded from the assessment. The results of the inter‐rater reliability (T.T. and F.K.) of the status of cSS was demonstrated to be excellent (kappa = 0.83, 95% confidence interval [95% CI] 0.78–0.88), and the intra‐rater variability (T.T.) was also excellent (kappa = 0.92, 95% CI 0.89–0.96).
Siderosis Features Associated with EEG and SPECT
Focal cSS was defined as being limited to no more than three sulci, and disseminated cSS was defined as affecting four or more sulci with the modified Boston criteria. 24 Locations of cSS were defined as the frontal, temporal, parietal, and occipital lobes. We also categorized the etiologies of cSS into four groups: (1) hemorrhagic transformation after ischemic stroke, (2) subarachnoid extension of ICH, (3) SAH, and (4) cerebral amyloid angiopathy (CAA). Probable or possible CAA was identified using the modified Boston criteria. 25 The laterality of the locations of cSS was compared with that of the symptomatogenic zone (lateralization of convulsion), epileptiform activity on EEG, and hyperperfusion area on SPECT.
New Prediction‐Scoring Models of Post‐Stroke Epilepsy
Using a recently developed scoring system to predict late seizures after stroke (SeLECT score 10 and CAVE score 9 ), we determined whether the addition of cSS into the existing system improves risk stratification of post‐stroke epilepsy. The SeLECT and CAVE scores have been externally validated and commonly used. The SeLECT score, comprising the severity of stroke (NIHSS ≤3, 4–10, and ≥11), large‐artery atherosclerosis, early seizure, cortical involvement, and territory of the middle cerebral artery, 10 is suitable for ischemic stroke. The CAVE score is a predictor of late seizures after ICH based on cortical involvement, younger age (<65 years), amount of bleeding (>10ml), and history of early seizure. 9 Comparisons were made between cases with and without the addition of cSS to the SeLECT in ischemic stroke (not including transient ischemic attack) and CAVE scores in ICH (not including SAH).
Statistical Analysis
Based on a previous report about predictive factors in post‐stroke seizures, 26 we assumed that the prevalence of hemosiderin deposition in patients with post‐stroke epilepsy and controls was 38% and 18%, respectively. Based on these event rates, we calculated the sample size for a two‐sided Fisher's exact test of the proportion using PASS 11 (NCSS, LLC, Kaysville, UT, USA) with alpha = 0.05 and power = 0.90. 27 A total of 114 patients were required in each group. Considering the possible dropout rate (approximately 20% of participants), we aimed to include at least 142 patients in each group. Data are presented as median (interquartile range [IQR]) or counts (%). We compared the characteristics between the post‐stroke epilepsy and control groups using Pearson's χ2‐test or Fisher's exact test for categorical variables, and Wilcoxon's rank‐sum test for continuous variables. We examined the percentage of lateralization concordance between locations of cSS, semiology‐based symptomatogenic zone, epileptiform discharges on EEG, and hyperperfusion area on SPECT. Univariable and multivariable logistic regression analyses were performed to assess the importance of cSS in the prediction of post‐stroke epilepsy. To assess the heterogeneous factors that underpin cSS, we conducted a forest plot analysis. Each group was distinguished by age, sex, and potential confounders, and odds ratios (ORs) were calculated from the logistic regression analyses. The variance inflation factor was calculated to measure the impact of multicollinearity among the variables.
The dataset was split randomly into a training set (70%) and a testing set (30%). We developed the prediction models of post‐stroke epilepsy according to the following two steps. In the first step, we built the prediction model by using logistic regression with ridge penalties. The variables used in the analyses included the presence of cSS and the SeLECT or CAVE score. To select the optimal hyperparameter, lambda, that produces models with the best area under the curve (AUC) on the training dataset, we used 10‐fold cross‐validation. Based on the best model, we calculated β coefficients of cSS alongside the SeLECT or CAVE score. In the second step, we determined the optimal cSS scores by dividing the coefficients of cSS by those of SeLECT and CAVE scores, and rounding these resulting values to the nearest integer. Finally, we named the new models SeLECT‐S and CAVE‐S, obtained after cSS was added to the existing models (SeLECT and CAVE).
The prediction performances of the models were assessed in the training set and testing set by the AUC using the pROC package. 28 To compare new models with the existing models, we measured the category‐free net reclassification improvement, 29 a valid approach for evaluating model improvement by the inclusion of cSS as a new biomarker. Two‐sided values of p < 0.05 were regarded as significant. All statistical analyses were performed using Stata 15.1 (Stata Corp, College Station, TX, USA), the JMP 12.2.0 software package (SAS Institute, Cary, NCm USA), and the R statistical software version 4.2.0 (The R Foundation for Statistical Computing, Vienna, Austria). 30
Results
Demographics of Participants
Of the 197 patients with post‐stroke epilepsy from the PROPOSE study, 17 (8.6%) patients were excluded, because GRET2*WI was not performed during admission (Fig 1). All patients in the control group underwent GRET2*WI and were followed up for at least 3 months at our hospital. Finally, this study included 180 patients with post‐stroke epilepsy (67 women; median age 74 years) and 1,183 controls (440 women; median age 74 years). Of these 180 patients with post‐stroke epilepsy, 117 (65.0%) were those with their first‐ever seizure, and 63 (35.0%) were with a prior diagnosis of PSE and recurrent seizure at the time of registration. The median (IQR) follow‐up time of controls was 865 (378–1,279) days.
FIGURE 1.
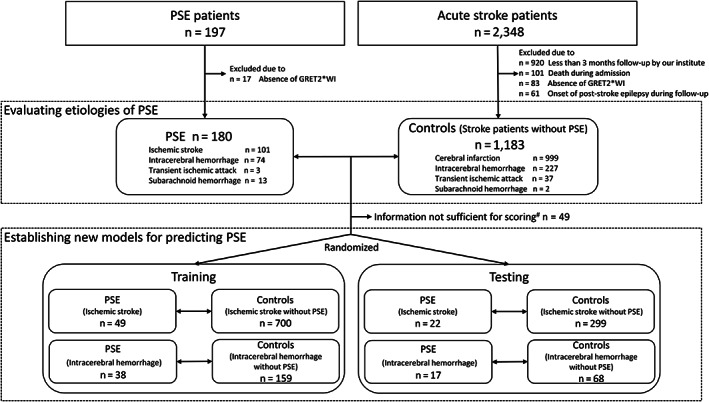
Study flow chart. To evaluate etiologies of post‐stroke epilepsy (PSE), we recruited 180 PSE patients and 1,183 controls. To establish new models for predicting PSE, we focused on ischemic stroke (not including transient ischemic attack) and intracerebral hemorrhage (not including subarachnoid hemorrhage). The dataset was split randomly into a training set (70%) and a testing set (30%). #Lack of information on history of early seizure and National Institutes of Health Stroke Scale in 49 post‐stroke epilepsy participants after ischemic stroke and intracerebral hemorrhage because of earlier stroke admission in a different hospital.
Factors Related to Post‐Stroke Epilepsy
Comparisons of baseline characteristics between stroke patients with and without post‐stroke epilepsy are shown in Table 1. Compared with the control group, the post‐stroke epilepsy group was more likely to have atrial fibrillation, dyslipidemia, dementia, chronic kidney disease, family history of epilepsy, and history of craniotomy. With regard to stroke types, hemorrhagic stroke (ICH and SAH) was more commonly observed in the post‐stroke epilepsy group. In addition, high stroke severity (NIHSS), functional disability (modified Rankin Scale), and history of early seizures were also more frequently observed in the post‐stroke epilepsy group. The median (IQR) time interval between seizure admission and the MRI scan in the post‐stroke epilepsy group, and that between stroke onset and MRI scan in controls was 0 (0–0) and 439 (91–1,080) days, respectively. Regarding MRI findings, cortical involvement, especially in the middle cerebral artery, was more frequent, and the stroke size was larger in the post‐stroke epilepsy group (Table 2). Among ICH patients, the median volume of hemorrhage was greater in the post‐stroke epilepsy group than in the control group (median 34.8 vs 6.5ml). The presence of CMBs was not different, but the presence of cSS differed significantly between the two groups (48.9% vs 5.7%, p < 0.0001). In the forest plot analysis, cSS showed a significant OR for post‐stroke epilepsy in individual (within‐group) factors, except for SAH (Supplemental Fig 2 in Appendix S1).
TABLE 1.
Demographics of the Participants
| Variables | Post‐stroke epilepsy | Controls (without post‐stroke epilepsy) | p |
|---|---|---|---|
| n = 180 | n = 1,183 | ||
| Age, years, median (IQR) | 74 (64.3–82) | 74 (65–81) | 0.76 |
| Female sex, n (%) | 67 (37.2) | 440 (37.2) | 0.99 |
| Comorbidities, n (%) | |||
| Hypertension | 150 (83.3) | 953 (80.6) | 0.38 |
| Dyslipidemia | 103 (57.2) | 756 (63.9) | 0.08 |
| Diabetes | 38 (21.1) | 281 (23.8) | 0.44 |
| Chronic kidney disease | 90 (50.0) | 426 (36.0) | 0.0003 |
| Liver cirrhosis | 6 (3.3) | 33 (2.8) | 0.68 |
| Atrial fibrillation | 61 (33.9) | 249 (21.1) | 0.0058 |
| Dementia | 48 (26.7) | 101 (8.5) | <0.0001 |
| Craniotomy | 26 (14.4) | 15 (1.3) | <0.0001 |
| Family history of epilepsy | 3 (1.7) | 2 (0.2) | 0.020 |
| Etiology of stroke subtype, n (%) | |||
| Cerebral infarction | 101 (56.1) | 999 (84.5) | <0.0001 |
| Cardiac embolism | 38 (21.1) | 205 (17.3) | 0.22 |
| Large‐artery atherosclerosis | 17 (9.4) | 132 (11.6) | 0.36 |
| Intracerebral hemorrhage | 74 (41.1) | 227 (19.2) | <0.0001 |
| Subcortical hemorrhage | 30 (15.2) | 39 (3.3) | <0.0001 |
| Transient ischemic attack | 3 (1.7) | 37 (3.1) | 0.28 |
| Subarachnoid hemorrhage | 13 (7.2) | 2 (0.2) | <0.0001 |
| NIHSS on admission, median (IQR) | 13 (3–20) | 3 (1–9) | <0.0001 |
| NIHSS grade, n (%) | |||
| ≤3 | 34 (27.4) | 604 (51.1) | <0.0001 |
| 4–10 | 21 (16.9) | 326 (27.6) | |
| ≥11 | 69 (55.7) | 253 (21.4) | |
| History of early seizure, n (%) | 22 (20.4) a | 25 (2.1) | <0.0001 |
| mRS at discharge, median (IQR) | 3 (1–4) | 2 (1–3) | <0.0001 |
Data are expressed as median (interquartile range) or absolute (percentage) values.
Lack of information on history of early seizure and NIHSS in 68 patients in the post‐stroke epilepsy group because of earlier stroke admission in a different hospital.
IQR = interquartile range; mRS = modified Rankin Scale; NIHSS = National Institutes of Health Stroke Scale.
TABLE 2.
Magnetic Resonance Imaging Findings
| Imaging features | Post‐stroke epilepsy | Controls (without post‐stroke epilepsy) | p |
|---|---|---|---|
| n = 180 | n = 1,183 | ||
| Stroke lesion, n (%) | |||
| ACA territory involvement | 24 (13.3) | 56 (4.7) | <0.0001 |
| MCA territory involvement | 138 (76.7) | 459 (38.8) | <0.0001 |
| PCA territory involvement | 19 (10.6) | 70 (5.9) | 0.019 |
| Cortical involvement, n (%) | 149 (82.8) | 493 (41.7) | <0.0001 |
| Stroke size, n (%) | <0.0001 | ||
| <15mm | 19 (10.6) | 613 (51.8) | |
| 15–30mm | 37 (20.6) | 288 (24.3) | |
| >30mm | 124 (68.9) | 282 (23.8) | |
| Volume of ICH (ml), median (IQR) | 34.8 (18.0–73) | 6.5 (2.0–14.4) | <0.0001 |
| Presence of CMBs, n (%) | 76 (42.2) | 481 (40.7) | 0.69 |
| CMBs types, n (%) | 0.09 | ||
| Strictly deep | 22/76 (29.0) | 194/481 (40.3) | |
| Strictly lobar | 15/76 (19.7) | 101/481 (21.0) | |
| Mixed | 39/76 (51.3) | 186/481 (38.7) | |
| Presence of cSS, n (%) | 88 (48.9) | 67 (5.7) | <0.0001 |
| Etiology of cSS, n (%) | 0.027 | ||
| HT of ischemic stroke | 40/88 (45.5) | 37/67 (55.2) | |
| ICH | 18/88 (20.5) | 10/67 (14.9) | |
| SAH | 9/88 (10.2) | 0/67 (0) | |
| Probable/possible CAA with cSS | 21/88 (23.9) | 18/67 (26.9) | |
| Unknown | 0/88 (0) | 2/67 (3.0) | |
| Grade of cSS, n (%) | 0.49 | ||
| Focal | 41/88 (46.6) | 35/67 (52.2) | |
| Disseminated | 47/88 (53.4) | 32/67 (47.8) | |
| Location of cSS, n (%) | |||
| Frontal | 61/88 (69.3) | 38/67 (56.7) | 0.11 |
| Temporal | 37/88 (42.1) | 25/67 (37.3) | 0.55 |
| Parietal | 26/88 (29.5) | 30/67 (44.8) | 0.051 |
| Occipital | 3/88 (3.4) | 3/67 (4.5) | 0.73 |
ACA = anterior cerebral artery; CAA = cerebral amyloid angiopathy; CMB = cerebral microbleed; cSS = cortical superficial siderosis; HT = hemorrhagic transformation; ICH = intracerebral hemorrhage; IQR = interquartile range; MCA = middle cerebral artery; MRI = magnetic resonance imaging; PCA = posterior cerebral artery; SAH = subarachnoid hemorrhage.
cSS in Patients with Post‐Stroke Epilepsy
In the post‐stroke epilepsy group, the most common etiology of cSS was hemorrhagic transformation of ischemic stroke (40 of 88 cases, 45.5%), followed by probable CAA (21 cases, 23.9%) and ICH (18 cases, 20.5%; Table 2). All patients with CAA in the post‐stroke epilepsy group fulfilled the modified Boston criteria for probable CAA. A total of 13 cases of subarachnoid extension of CAA‐associated lobar ICH were categorized as CAAs. Typical examples of post‐stroke epilepsy with cSS in each stroke etiology are shown in Figure 2. The grade or location of cSS was not different between the post‐stroke epilepsy and control groups. However, cSS lateralization was concordant with the symptomatogenic zone in 80.0% (40/50) of patients, epileptiform activity on EEG in 81.5% (22/27) of patients, and hyperperfusion area on SPECT in 87.2% (34/39) of patients (Table 3).
FIGURE 2.
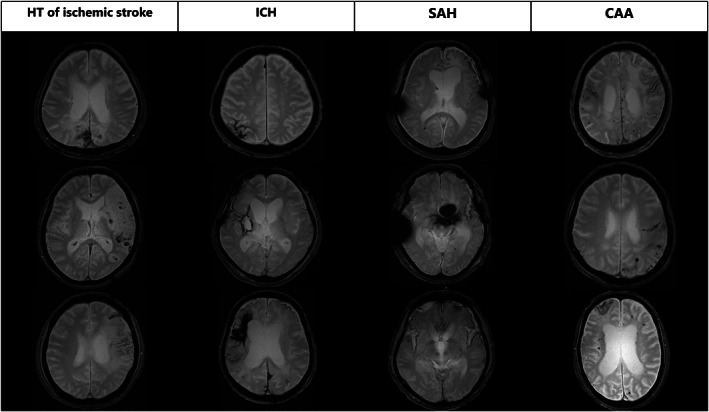
Typical examples of post‐stroke epilepsy with cortical superficial siderosis in each stroke etiology. Four pathologies (hemorrhagic transformation of ischemic stroke, intracerebral hemorrhage, subarachnoid hemorrhage, and cerebral amyloid angiopathy) in stroke patients contribute to the development of cortical superficial siderosis, leading to hemosiderin deposition in the pial and subpial regions of the brain. CAA = cerebral amyloid angiopathy; HT = hemorrhagic transformation; ICH = intracerebral hemorrhage; SAH = subarachnoid hemorrhage.
TABLE 3.
Lateralization Concordance of cSS, Symptomatogenic Zone, Epileptiform Activity on Electroencephalography, and Hyperperfusion on Single‐Photon Emission Computed Tomography
| Clinical and imaging features | cSS location a | ||
|---|---|---|---|
| Right (n = 55) | Left (n = 48) | Lateralization concordance | |
| Symptomatogenic zone (convulsion dominant) | 23/28 (82.1%) | 17/22 (77.3%) | 40/50 (80.0%) |
| Epileptiform activity on EEG | 11/14 (78.6%) | 11/13 (84.6%) | 22/27 (81.5%) |
| Hyperperfusion on SPECT | 20/24 (83.3%) | 14/15 (93.3%) | 34/39 (87.2%) |
Of 88 post‐stroke epilepsy cases of cSS, 15 had bilateral cSS.
cSS = cortical superficial siderosis; EEG = electroencephalography; SPECT = single‐photon emission computed tomography.
cSS as an Imaging Biomarker in Post‐Stroke Epilepsy
Within the post‐stroke epilepsy group (n = 180), 71 participants with post‐stroke epilepsy following ischemic stroke and 55 participants with post‐stroke epilepsy after ICH were detailed clinical and imaging information, and were further assessed to establish a scoring system (Fig 1). Univariable analyses found significant associations between ischemic post‐stroke epilepsy and some predictor items of the SeLECT score (NIHSS, history of early seizure, cortical involvement, and location in the middle cerebral artery territory), and between post‐stroke epilepsy after ICH and some predictor items of the CAVE score (history of early seizure, ICH volume >10ml, and cortical involvement; Table 4). The presence of cSS was significantly associated with post‐stroke epilepsy in both the ischemic stroke and ICH groups. All items of the SeLECT/CAVE score and cSS were included in the variance inflation factor analysis. After adjusting for all items of the SeLECT/CAVE score, the presence of cSS remained a substantial predictor for post‐stroke epilepsy after ischemic stroke (OR 11.05, 95% CI 5.96–20.49) and after ICH (OR 6.73, 95% CI 2.86–15.84), respectively. We assigned 6 points to cSS, dividing β coefficients of cSS (2.10) by that of SeLECT (0.33). We assigned 1 point to cSS, dividing β coefficients of cSS (0.89) by that of CAVE (0.63). Figure 3 shows the distribution of the actual probability by each prediction score. The AUC of the SeLECT‐S model was higher than those of the SeLECT model in the training data (AUC 0.84 [95% CI 0.78–0.90] vs AUC 0.78 [95% CI 0.72–0.84]; Fig 3A), and the testing data (AUC 0.83 [95% CI 0.73–0.92] vs AUC 0.78 [95% CI 0.68–0.88] Fig 3B), showing the better predictive ability of the SeLECT‐S scoring system (Table 5). The net reclassification improvement for the SeLECT‐S score compared with the SeLECT was 0.81 (p < 0.0001) at the training data, and 0.63 (p = 0.004) at the testing data. Additionally, the AUC of the CAVE‐S score was higher than those of the CAVE model in the training data (AUC 0.88 [95% CI 0.82–0.95] vs AUC 0.84 [95% CI 0.77–0.91]), and at the testing data (AUC 0.87 [95% CI 0.79–0.95] vs AUC 0.83 [95% CI 0.72–0.93]; Fig 3C, D). The net reclassification improvement for the CAVE‐S score compared with the CAVE was 0.89 (p < 0.0001) in the training data, and 0.88 (p = 0.001) in the testing data.
TABLE 4.
Univariable and Multivariable Logistic Regression Analyses for Post‐Stroke Epilepsy
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| β | Odds ratio (95% CI) | p | β | Odds ratio (95% CI) | p | |
| Ischemic stroke (n = 1,070) | ||||||
|
NIHSS High versus low |
1.22 | 3.40 (1.97–5.87) | <0.001 | 0.51 | 1.05 (0.55–2.01) | 0.88 |
|
NIHSS Middle versus low |
0.64 | 1.07 (0.55–2.07) | 0.85 | −0.38 | 0.68 (0.33–1.43) | 0.31 |
| Early seizure | 2.14 | 8.46 (3.77–18.97) | <0.001 | 1.80 | 6.02 (2.40–15.15) | <0.001 |
| Cortical involvement | 2.40 | 10.98 (4.98–24.19) | <0.001 | 1.59 | 4.90 (1.73–13.83) | 0.003 |
| Large‐artery atherosclerosis | 0.57 | 1.76 (0.97–3.20) | 0.06 | 0.58 | 1.79 (0.90–3.55) | 0.10 |
| MCA | 1.97 | 7.19 (3.73–13.84) | <0.001 | 0.49 | 1.64 (0.68–3.93) | 0.27 |
| cSS | 2.74 | 15.51 (8.88–27.10) | <0.001 | 2.40 | 11.05 (5.96–20.49) | <0.001 |
| Intracerebral hemorrhage (n = 282) | ||||||
| Age <65 years | 0.21 | 1.23 (0.67–2.26) | 0.51 | 0.67 | 1.95 (0.85–4.51) | 0.12 |
| Early seizure | 2.22 | 9.21 (3.24–26.21) | <0.001 | 1.66 | 5.25 (1.33–20.69) | 0.018 |
| Cortical involvement | 2.50 | 12.21 (5.91–25.25) | <0.001 | 1.32 | 3.72 (1.53–9.09) | 0.004 |
| Over 10cc hemorrhage | 2.47 | 11.76 (5.78–23.93) | <0.001 | 1.69 | 5.44 (2.25–13.18) | <0.001 |
| cSS | 2.26 | 9.57 (4.91–18.65) | <0.001 | 1.91 | 6.73 (2.86–15.84) | <0.001 |
CI = confidence interval; cSS = cortical superficial siderosis; MCA = middle cerebral artery; NIHSS = National Institutes of Health Stroke Scale.
FIGURE 3.
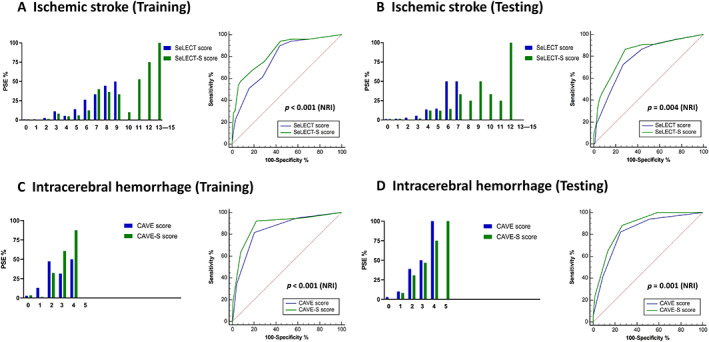
New prediction scores and receiver operating characteristic curves for post‐stroke epilepsy. (A, B) Incidence of PSE (%) stratified by the SeLECT score and SeLECT plus cSS (SeLECT‐S) score in patients with ischemic stroke at the training data (A, left) and the testing data (B, left). Receiver operating characteristic curve analyses showing comparisons of area under the curve between the SeLECT (blue) and SeLECT‐S (green) scores for detecting PSE after ischemic stroke at the training data (A, right) and the testing data (B, right). The SeLECT‐S model significantly improved the prediction performance in net reclassification improvement (p < 0.0001 at the training data, p = 0.004 at the testing data). (C, D) Incidence of PSE (%) stratified by the CAVE score and CAVE plus cSS (CAVE‐S) score in patients with intracerebral hemorrhage at the training data (C, left) and the testing data (D, left). Receiver operating characteristic curve analyses showing the area under the curve of the comparison between the CAVE score (blue) and CAVE‐S score (green) for detecting PSE after intracerebral hemorrhage at the training data (C, right) and the testing data (D, right). The CAVE‐S model significantly improved the prediction performance in net reclassification improvement (p < 0.0001 at the training data, p = 0.001 at the testing data). NRI = net reclassification improvement; PSE = post‐stroke epilepsy.
TABLE 5.
Comparisons of the Predicted Risks in Models Based on SeLECT and CAVE Scores with or without Cortical Superficial Siderosis
| Ischemic stroke | Training data (70%, n = 749) | Testing data (30%, n = 321) | ||
|---|---|---|---|---|
| AUC (95% CI) | NRI (p value) | AUC (95% CI) | NRI (p value) | |
| SeLECT score | 0.78 (0.72–0.84) | – | 0.78 (0.68–0.88) | – |
| SeLECT‐S score | 0.84 (0.78–0.90) | 0.81 (<0.001) | 0.83 (0.73–0.92) | 0.63 (0.004) |
| Intracerebral hemorrhage | Training data (70%, n = 197) | Testing data (30%, n = 85) | ||
|---|---|---|---|---|
| AUC (95% CI) | NRI (p value) | AUC (95% CI) | NRI (p value) | |
| CAVE score | 0.84 (0.77–0.91) | – | 0.83 (0.72–0.93) | – |
| CAVE‐S score | 0.88 (0.82–0.95) | 0.89 (<0.001) | 0.87 (0.79–0.95) | 0.88 (0.001) |
AUC = area under the curve; CI = confidence interval; cSS = cortical superficial siderosis; NRI = net reclassification improvement.
Discussion
The present study suggests that cSS is strongly associated with post‐stroke epilepsy, even when compared with the conventional predictors for post‐stroke epilepsy. This finding indicates that cSS is implicated in post‐stroke epilepsy development, and may be a novel imaging biomarker to identify stroke survivors at high risk of post‐stroke epilepsy. Using the CAVE‐S and SeLECT‐S scores may help stratify the risk of post‐stroke epilepsy in stroke survivors.
Cortical superficial siderosis is characterized by hemosiderin deposition in the pial or subpial layers of the central nervous system. 23 Compared with CMBs, cSS is rare in the normal population. For example, in the population‐based Rotterdam Scan Study, only 0.7% of the elderly populations had cSS; 31 in a Japanese cohort study, which evaluated the prevalence of cSS in acute stroke, 32 cSS was found in 1.0% of patients with ischemic stroke or transient ischemic attack and 4.7% of patients with ICH. As shown in the present study, cSS in post‐stroke epilepsy commonly has four types of etiologies: hemorrhagic transformation after ischemic stroke, a subarachnoid extension of ICH, SAH, and CAA. Indeed, cSS is typically identified as a key feature of CAA in the elderly population, 23 and SAH and subarachnoid extension of ICH underlie cSS.
Notably, we found that hemorrhagic transformation after ischemic stroke accounted for approximately half of the cSS cases. Consistently, a post‐mortem MRI and neuropathological study in patients with diverse cerebrovascular and neurodegenerative diseases showed that cSS was associated with both hemorrhagic stroke and hemorrhagic transformation of cortical infarcts. 33
The present study showed that the presence of cSS was independently related to post‐stroke epilepsy. Previous studies have demonstrated that the patients with lobar ICH and SAH have a high frequency of cSS (58.3–68% for lobar ICH 34 , 35 and 50.0–54.2% for SAH 36 , 37 ). A history of cranial surgery also represents one of the important risk factors for the development of cSS. 38 Convexity SAH, which could lead to cSS, was frequently found among stroke patients who underwent thrombectomy or thrombolysis (9.8–13.9%). 39 , 40 Indeed, our post‐stroke epilepsy group tended to demonstrate heterogeneous etiologies of cSS, such as SAH, subcortical hemorrhage, history of craniotomy, or a larger stroke (Table 1). Nevertheless, the relationship between cSS and post‐stroke epilepsy was still significant in each within‐group factor, except for SAH (Supplementary Fig 2 in Appendix S1).
cSS may represent a common feature of post‐stroke epilepsy, irrespective of the underlying etiologies of cSS. Moreover, the laterality of cSS had a high concordance rate with the symptomatogenic zone (convulsion‐dominant), epileptiform activity on EEG, and hyperperfusion area on SPECT, suggesting that cSS might play a significant role in epileptogenesis in stroke survivors. Epileptogenesis in post‐stroke epilepsy is postulated to result from gliotic scarring, chronic inflammation, deafferentation, selective neuronal loss, disruption of the blood–brain barrier, collateral synaptic sprouting, altered synaptic plasticity, and siderosis, although the exact mechanisms of post‐stroke epilepsy remain unclear. 41 A recent study showed that hemosiderosis was significantly correlated with post‐SAH epilepsy, regardless of the location of aneurysms, severity of SAH, and disability (modified Rankin Scale) after SAH. 36 In addition, a histopathological study of cSS in CAA found that iron deposits were mainly found across superficial cortical layers both extracellularly and intracellularly (engulfed by microglia), and increased severity of cSS was associated with upregulation of reactive astrocytes in the same cortical areas. 42 Moreover, experimental studies have demonstrated that microglia and astrocytes induce an inflammatory response and contribute to the development of epilepsy after SAH, 43 and reactive astrocytes reduce glutamate and potassium uptake within the synaptic clefts, which contribute to hyperexcitability of neurons. 44 Hence, cSS in the perilesional parenchyma may lead to epileptogenesis.
Notably, electrographic seizures were frequently observed in lobar intraparenchymal hemorrhage cases (46.6%) compared with deep hemorrhage cases without any effects on the cerebral cortex or subarachnoid/subdural spaces (20%), possibly because lobar hemorrhage is strongly predisposed to cSS compared with deep hemorrhage. 45 In addition, subarachnoid extension of hemorrhage was significantly associated with early seizure after ICH, regardless of the location of the hematoma. 46 These results further support the association between the cSS and post‐stroke epilepsy.
No study has confirmed the relationship between post‐stroke epilepsy in ischemic stroke and cSS, although hemorrhagic transformation is independently associated with post‐stroke epilepsy after stroke thrombolysis. 47 Cardiac embolism or thrombolysis is commonly associated with an elevated risk of hemorrhagic transformation, which may be implicated in cSS after ischemic stroke. 48 In fact, the most common underlying mechanism of cSS in the post‐stroke epilepsy group was hemorrhagic transformation of ischemic stroke in the present study. Moreover, a previous study showed that over half of patients with malignant middle cerebral artery infarction treated by decompressive craniectomy developed post‐stroke epilepsy, regardless of stroke severity, age, sex, and recanalization. 49 These findings suggest that surgical procedures cause vascular damage or blood leakage, thereby leading to cSS.
Cortical superficial siderosis restricted to the convexities of cerebral hemispheres and the supratentorial region may manifest as episodes of transient focal neurological episodes (TFNEs). 50 TFNEs, as one of the clinical features, were found in 14% of CAA cases; 51 34% of patients with cSS developed TFNEs, which often occurred repeatedly (53%), and 4% of patients with cSS had a generalized seizure. 52 Additionally, a recent report suggested that cortical spreading depolarization or depression (CSD) underlies TFNEs, 50 CSD can be caused by seizures, 53 and the relationship between cSS and TFNEs in CAA may be mediated by cSS‐induced seizure activity and resultant CSD. 50 Indeed, CSD and seizure activity co‐occur in the acutely injured human cerebral cortex. 54 Moreover, there is a case report of CAA accompanied by TFNEs and epileptiform EEG activity. 55 In the present study, 39.1% of post‐stroke epilepsy patients with cSS caused by CAA had epileptiform activity on EEG, and 75.0% of them had hyperperfusion area on SPECT, which supports the presence of epileptic seizures.
Notably, TFNEs are mostly recurrent, stereotyped, and short‐lasting (usually <30 minutes), and have a wide spectrum of clinical features, which are suspected to be focal seizure activities. 51 Furthermore, several reports suggest that patients with cSS develop seizures spontaneously or without other triggers. 56 , 57 , 58 Therefore, although it is difficult to distinguish post‐stroke epilepsy seizures from TFNEs secondary to cSS, cSS might underlie the epileptogenesis of post‐stroke epilepsy.
For the optical management of stroke survivors, an effective predictor of post‐stroke epilepsy is extremely important for avoiding risks of seizure, ensuring effective prevention, and considering drug intervention. Moreover, post‐stroke epilepsy is associated with increased short‐term and long‐term mortality. 59 Currently, there are many predictive models of post‐stroke epilepsy, among which the SeLECT score is one of the best for ischemic post‐stroke epilepsy, and the CAVE score is one of the best for post‐stroke epilepsy after ICH, in terms of high specificity and high negative predictive value. 9 , 10
Our univariable and multivariable analyses showed that cSS conferred the highest risk factor for post‐stroke epilepsy following ischemic stroke among various risk factors in the SeLECT score. For ICH, multivariable analysis revealed that cSS was a significant risk factor for post‐stroke epilepsy. The incorporation of cSS into the SeLECT and CAVE scores yielded significantly improved novel prediction models named the SeLECT‐S score for post‐stroke epilepsy after ischemic stroke, and the CAVE‐S score for post‐stroke epilepsy after ICH, both of which may enable risk stratification of post‐stroke epilepsy in stroke survivors and contribute to strategic post‐stroke epilepsy prevention.
There were several limitations to the present study. First, it was a retrospective study, and the participants were a selected group of patients who underwent GRET2*WI, which might have limited the generalizability of the present findings. However, due to the relatively low frequency of post‐stroke epilepsy (<10% in 10 years) among stroke survivors, the use of the post‐stroke epilepsy registry consisting of 200 participants might offset this limitation. Second, differences in background between post‐stroke epilepsy and controls may have influenced the impact of cSS on post‐stroke epilepsy. Given the results of the forest plot analysis in our study, cSS still shows a strong association with post‐stroke epilepsy, irrespective of the underlying conditions. However, further studies are warranted to clarify the causality of cSS with post‐stroke epilepsy. This study, which was based on the Japanese population, may not have highly generalizable results, given that different ethnicities can affect the development of small vessel diseases differently. 60 Third, we defined patients who were seizure‐free for at least 3 months as controls, which might have included those with a high probability of future post‐stroke epilepsy; however, the median follow‐up period in the control group was 865 days (IQR 378–1,279 days), which is acceptable for post‐stroke epilepsy investigation. Fourth, in the control group, there may also have been a selection bias due to lost to follow‐up. Fifth, overdiagnosis and underdiagnosis of post‐stroke epilepsy might have been made due to the diverse semiology of post‐stroke epilepsy. To provide greater reliability, we performed EEG and brain SPECT during admission, and certified epileptologists or neurologists carefully confirmed these clinical findings. Sixth, it remained unknown whether cSS was present before the occurrence of stroke. In fact, the post‐stroke patients with cSS showed a high prevalence of probable/possible CAA (23.9%) and dementia (26.1%). In some cases, cSS may have existed before the stroke, which should be clarified in a further study. Finally, differences in magnetic field strength, echo time, and spatial resolution may have led to different detection rates of signal changes. Although susceptibility‐weighted imaging sequences are known to have superior sensitivity to GRET2*WI sequences, we used only GRET2*WI sequences in all cases to minimize variability in detection rates of cSS.
The present results may yield new insights into the prediction of post‐stroke epilepsy. Accurately predicting a high risk of post‐stroke epilepsy in advance may reduce the risk of injury or death due to unpredictable seizures in daily life. Further prospective studies are warranted to investigate the causality of cSS with post‐stroke epilepsy. In addition, deferiprone, an iron‐chelating drug, has recently been found to be promising for cSS. 61 It should be explored whether iron‐chelators can prevent post‐stroke epilepsy in stroke survivors.
In conclusion, cSS may be a contributing factor for the development of post‐stroke epilepsy. Identifying cSS may enable risk stratification of post‐stroke epilepsy in stroke survivors, and contribute to strategic prevention of post‐stroke epilepsy.
Author Contributions
T.T., K.F., K.K., A.S., K.N., R.M., A.I., and M.I. contributed to the conception and design of the study; T.T., K.F., S.A., S.M., S.I., N.K., H.I., S.H., K.K., A.S., Y.N., K.N., A.I., and M.I. contributed to the acquisition and analysis of data; and T.T., K.F., H.I., S.H., K.K., A.S., Y.N., S.O., K. N., M.K., K.T., R.M., R.T., A.I., and M.I. contributed to drafting a significant portion of the manuscript or figures.
Potential Conflicts of Interest
A.I. belongs to the Department of Epilepsy, Movement Disorders and Physiology, Kyoto University Graduate School of Medicine, an Industry‐Academia Collaboration Course, and was supported by a grant from Eisai Corporation, Nihon Kohden Corporation, Otsuka Pharmaceutical Co., and UCB Japan Co., Ltd. The other authors report no conflicts of interest for this work.
Supporting information
Appendix S1 Supporting Information.
Acknowledgments
This research was funded by the Practical Research Project for Lifestyle‐related Diseases including Cardiovascular Diseases and Diabetes Mellitus from the Japan Agency for Medical Research and Development (JP18ek0210057), and partially supported by the Japan Society for the Promotion of Science (19K16888). Role of the Funder/Sponsor: the Japan Agency for Medical Research and Development had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Mariko Yasui worked as a data manager. Dr Maya Tojima helped with the EEG readings. Yoko Ohashi and Sayaka Wada contributed to the secretarial work.
Contributor Information
Tomotaka Tanaka, Email: tanakat@ncvc.go.jp.
Masafumi Ihara, Email: ihara@ncvc.go.jp.
References
- 1. Bladin CF, Alexandrov AV, Bellavance A, et al. Seizures after stroke: a prospective multicenter study. Arch Neurol 2000;57:1617–1622. 10.1001/archneur.57.11.1617. [DOI] [PubMed] [Google Scholar]
- 2. Zou S, Wu X, Zhu B, et al. The pooled incidence of post‐stroke seizure in 102 008 patients. Top Stroke Rehabil 2015;22:460–467. 10.1179/1074935715Z.00000000062. [DOI] [PubMed] [Google Scholar]
- 3. Sen A, Jette N, Husain M, Sander JW. Epilepsy in older people. Lancet 2020;395:735–748. 10.1016/S0140-6736(19)33064-8. [DOI] [PubMed] [Google Scholar]
- 4. Liu S, Yu W, Lu Y. The causes of new‐onset epilepsy and seizures in the elderly. Neuropsychiatr Dis Treat 2016;12:1425–1434. 10.2147/NDT.S107905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Burneo JG, Fang J, Saposnik G, Investigators of the Registry of the Canadian Stroke Network . Impact of seizures on morbidity and mortality after stroke: a Canadian multi‐centre cohort study. Eur J Neurol 2010;17:52–58. 10.1111/j.1468-1331.2009.02739.x. [DOI] [PubMed] [Google Scholar]
- 6. Labovitz DL, Hauser WA, Sacco RL. Prevalence and predictors of early seizure and status epilepticus after first stroke. Neurology 2001;57:200–206. 10.1212/wnl.57.2.200. [DOI] [PubMed] [Google Scholar]
- 7. Yoshimura H, Tanaka T, Fukuma K, et al. Impact of seizure recurrence on 1‐year functional outcome and mortality in patients with poststroke epilepsy. Neurology 2022;99:e376–e384. 10.1212/WNL.0000000000200609. [DOI] [PubMed] [Google Scholar]
- 8. Tanaka T, Ihara M. Post‐stroke epilepsy. Neurochem Int 2017;107:219–228. 10.1016/j.neuint.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 9. Haapaniemi E, Strbian D, Rossi C, et al. The CAVE score for predicting late seizures after intracerebral hemorrhage. Stroke 2014;45:1971–1976. 10.1161/STROKEAHA.114.004686. [DOI] [PubMed] [Google Scholar]
- 10. Galovic M, Dohler N, Erdelyi‐Canavese B, et al. Prediction of late seizures after ischaemic stroke with a novel prognostic model (the SeLECT score): a multivariable prediction model development and validation study. Lancet Neurol 2018;17:143–152. 10.1016/S1474-4422(17)30404-0. [DOI] [PubMed] [Google Scholar]
- 11. Doria JW, Forgacs PB. Incidence, implications, and management of seizures following ischemic and hemorrhagic stroke. Curr Neurol Neurosci Rep 2019;19:37. 10.1007/s11910-019-0957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li X, Liu R, Luo ZY, et al. Comparison of the predictive abilities of pharmacogenetics‐based warfarin dosing algorithms using seven mathematical models in Chinese patients. Pharmacogenomics 2015;16:583–590. 10.2217/pgs.15.26. [DOI] [PubMed] [Google Scholar]
- 13. Thevathasan A, Naylor J, Churilov L, et al. Association between hemorrhagic transformation after endovascular therapy and poststroke seizures. Epilepsia 2018;59:403–409. 10.1111/epi.13982. [DOI] [PubMed] [Google Scholar]
- 14. Rosen AD, Frumin NV. Focal epileptogenesis after intracortical hemoglobin injection. Exp Neurol 1979;66:277–284. 10.1016/0014-4886(79)90080-3. [DOI] [PubMed] [Google Scholar]
- 15. Wang X, Tao Z, You C, et al. Extended resection of hemosiderin fringe is better for seizure outcome: a study in patients with cavernous malformation associated with refractory epilepsy. Neurol India 2013;61:288–292. 10.4103/0028-3886.115070. [DOI] [PubMed] [Google Scholar]
- 16. Tanaka T, Fukuma K, Abe S, et al. Antiseizure medications for post‐stroke epilepsy: a real‐world prospective cohort study. Brain Behav 2021;11:e2330. 10.1002/brb3.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014;55:475–482. 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 18. van Swieten JC, Koudstaal PJ, Visser MC, et al. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–607. 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 19. Hirsch LJ, LaRoche SM, Gaspard N, et al. American clinical neurophysiology Society's standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol 2013;30:1–27. 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- 20. Fukuma K, Kajimoto K, Tanaka T, et al. Visualizing prolonged hyperperfusion in post‐stroke epilepsy using postictal subtraction SPECT. J Cereb Blood Flow Metab 2021;41:146–156. 10.1177/0271678X20902742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gyanwali B, Shaik MA, Tan CS, et al. Mixed‐location cerebral microbleeds as a biomarker of neurodegeneration in a memory clinic population. Aging (Albany NY) 2019;11:10581–10596. 10.18632/aging.102478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gyanwali B, Shaik MA, Venketasubramanian N, et al. Mixed‐location cerebral microbleeds: an imaging biomarker for cerebrovascular pathology in cognitive impairment and dementia in a memory clinic population. J Alzheimers Dis 2019;71:1309–1320. 10.3233/JAD-190540. [DOI] [PubMed] [Google Scholar]
- 23. Charidimou A, Linn J, Vernooij MW, et al. Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain 2015;138:2126–2139. 10.1093/brain/awv162. [DOI] [PubMed] [Google Scholar]
- 24. Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010;74:1346–1350. 10.1212/WNL.0b013e3181dad605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Greenberg SM, Charidimou A. Diagnosis of cerebral amyloid angiopathy: evolution of the Boston criteria. Stroke 2018;49:491–497. 10.1161/STROKEAHA.117.016990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Castro‐Apolo R, Huang JF, Vinan‐Vega M, Tatum WO. Outcome and predictive factors in post‐stroke seizures: a retrospective case‐control study. Seizure 2018;62:11–16. 10.1016/j.seizure.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 27. D'Agostino RB, Chase W, Belanger A . The appropriateness of some common procedures for testing the equality of two independent binomial populations, The American Statistician 1988;42:198–202. 10.2307/2685002. [DOI] [Google Scholar]
- 28. Robin X, Turck N, Hainard A, et al. pROC: an open‐source package for R and S + to analyze and compare ROC curves. BMC Bioinformatics 2011;12:77. 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–172; discussion 207–212. 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 30. RC Team. A language and environment for statistical computing. 2020. Available at: http://www.r-project.org/index.html. Accessed June 2, 2022.
- 31. Vernooij MW, Ikram MA, Hofman A, et al. Superficial siderosis in the general population. Neurology 2009;73:202–205. 10.1212/WNL.0b013e3181ae7c5e. [DOI] [PubMed] [Google Scholar]
- 32. Suda S, Shimoyama T, Suzuki S, et al. Prevalence and clinical characteristics of cortical superficial siderosis in patients with acute stroke. J Neurol 2017;264:2413–2419. 10.1007/s00415-017-8646-1. [DOI] [PubMed] [Google Scholar]
- 33. De Reuck J, Deramecourt V, Cordonnier C, et al. Superficial siderosis of the central nervous system: a post‐mortem 7.0‐tesla magnetic resonance imaging study with neuropathological correlates. Cerebrovasc Dis 2013;36:412–417. 10.1159/000355042. [DOI] [PubMed] [Google Scholar]
- 34. Pinho J, Araujo JM, Costa AS, et al. Intracerebral hemorrhage recurrence in patients with and without cerebral amyloid angiopathy. Cerebrovasc Dis Extra 2021;11:15–21. 10.1159/000513503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Renard D, Parvu T, Tatu L, Thouvenot E. Subarachnoid extension of lobar hemorrhage on acute/subacute MRI is associated with cerebral amyloid angiopathy criteria. Acta Neurol Belg 2020;120:863–866. 10.1007/s13760-018-01060-9. [DOI] [PubMed] [Google Scholar]
- 36. Hirano T, Enatsu R, Iihoshi S, et al. Effects of hemosiderosis on epilepsy following subarachnoid hemorrhage. Neurol Med Chir (Tokyo) 2019;59:27–32. 10.2176/nmc.oa.2018-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lummel N, Bernau C, Thon N, et al. Prevalence of superficial siderosis following singular, acute aneurysmal subarachnoid hemorrhage. Neuroradiology 2015;57:349–356. 10.1007/s00234-014-1480-6. [DOI] [PubMed] [Google Scholar]
- 38. Kumar N. Superficial siderosis: associations and therapeutic implications. Arch Neurol 2007;64:491–496. 10.1001/archneur.64.4.491. [DOI] [PubMed] [Google Scholar]
- 39. Enomoto M, Shigeta K, Ota T, et al. Predictors of intracranial hemorrhage in acute ischemic stroke after endovascular thrombectomy. Interv Neuroradiol 2020;26:368–375. 10.1177/1591019920926335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tian B, Tian X, Shi Z, et al. Clinical and imaging indicators of hemorrhagic transformation in acute ischemic stroke after endovascular Thrombectomy. Stroke 2022;53:1674–1681. 10.1161/STROKEAHA.121.035425. [DOI] [PubMed] [Google Scholar]
- 41. Feyissa AM, Hasan TF, Meschia JF. Stroke‐related epilepsy. Eur J Neurol 2019;26:18–e3. 10.1111/ene.13813. [DOI] [PubMed] [Google Scholar]
- 42. Charidimou A, Perosa V, Frosch MP, et al. Neuropathological correlates of cortical superficial siderosis in cerebral amyloid angiopathy. Brain 2020;143:3343–3351. 10.1093/brain/awaa266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang J, Liang J, Deng J, et al. Emerging role of microglia‐mediated neuroinflammation in epilepsy after subarachnoid hemorrhage. Mol Neurobiol 2021;58:2780–2791. 10.1007/s12035-021-02288-y. [DOI] [PubMed] [Google Scholar]
- 44. David Y, Cacheaux LP, Ivens S, et al. Astrocytic dysfunction in epileptogenesis: consequence of altered potassium and glutamate homeostasis? J Neurosci 2009;29:10588–10599. 10.1523/JNEUROSCI.2323-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sheikh ZB, Stretz C, Maciel CB, et al. Deep versus lobar intraparenchymal hemorrhage: seizures, hyperexcitable patterns, and clinical outcomes. Crit Care Med 2020;48:e505–e513. 10.1097/CCM.0000000000004317. [DOI] [PubMed] [Google Scholar]
- 46. Guth JC, Gerard EE, Nemeth AJ, et al. Subarachnoid extension of hemorrhage is associated with early seizures in primary intracerebral hemorrhage. J Stroke Cerebrovasc Dis 2014;23:2809–2813. 10.1016/j.jstrokecerebrovasdis.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 47. Brondani R, de Almeida AG, Cherubini PA, et al. Risk factors for epilepsy after thrombolysis for ischemic stroke: a cohort study. Front Neurol 2019;10:1256. 10.3389/fneur.2019.01256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pande SD, Win MM, Khine AA, et al. Haemorrhagic transformation following ischaemic stroke: a retrospective study. Sci Rep 2020;10:5319. 10.1038/s41598-020-62230-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Santamarina E, Sueiras M, Toledo M, et al. Epilepsy in patients with malignant middle cerebral artery infarcts and decompressive craniectomies. Epilepsy Res 2015;112:130–136. 10.1016/j.eplepsyres.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 50. Smith EE, Charidimou A, Ayata C, et al. Cerebral amyloid angiopathy‐related transient focal neurologic episodes. Neurology 2021;97:231–238. 10.1212/WNL.0000000000012234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Charidimou A, Peeters A, Fox Z, et al. Spectrum of transient focal neurological episodes in cerebral amyloid angiopathy: multicentre magnetic resonance imaging cohort study and meta‐analysis. Stroke 2012;43:2324–2330. 10.1161/STROKEAHA.112.657759. [DOI] [PubMed] [Google Scholar]
- 52. Lummel N, Wollenweber FA, Demaerel P, et al. Clinical spectrum, underlying etiologies and radiological characteristics of cortical superficial siderosis. J Neurol 2015;262:1455–1462. 10.1007/s00415-015-7736-1. [DOI] [PubMed] [Google Scholar]
- 53. Dreier JP, Major S, Pannek HW, et al. Spreading convulsions, spreading depolarization and epileptogenesis in human cerebral cortex. Brain 2012;135:259–275. 10.1093/brain/awr303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fabricius M, Fuhr S, Willumsen L, et al. Association of seizures with cortical spreading depression and peri‐infarct depolarisations in the acutely injured human brain. Clin Neurophysiol 2008;119:1973–1984. 10.1016/j.clinph.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mattavelli D, Mele F, Bertora P, et al. Epileptiform activity during transient focal neurologic episodes in cerebral amyloid angiopathy. Neurol Clin Pract 2021;11:e43–e45. 10.1212/CPJ.0000000000000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang K, Xu Z, Xiong G, Benyan L. Superficial siderosis of the central nervous system manifested with seizures. J Clin Neurosci 2010;17:277–278. 10.1016/j.jocn.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 57. Iannaccone S, Golzi V, Sferrazza B, et al. Central nervous system superficial siderosis, headache, and epilepsy. Headache 1999;39:666–669. 10.1046/j.1526-4610.1999.3909666.x. [DOI] [PubMed] [Google Scholar]
- 58. Chen CY, Xiao F, Liu JL. Superficial siderosis of the central nervous system with seizures onset. Singapore Med J 2015;56:590–591. 10.11622/smedj.2015158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Arntz RM, Rutten‐Jacobs LC, Maaijwee NA, et al. Poststroke epilepsy is associated with a high mortality after a stroke at young age: follow‐up of transient ischemic attack and stroke patients and unelucidated risk factor evaluation study. Stroke 2015;46:2309–2311. 10.1161/STROKEAHA.115.010115. [DOI] [PubMed] [Google Scholar]
- 60. Wolma J, Nederkoorn PJ, Goossens A, et al. Ethnicity a risk factor? The relation between ethnicity and large‐ and small‐vessel disease in white people, black people, and Asians within a hospital‐based population. Eur J Neurol 2009;16:522–527. 10.1111/j.1468-1331.2009.02530.x. [DOI] [PubMed] [Google Scholar]
- 61. Flores Martin A, Shanmugarajah P, Hoggard N, Hadjivassiliou M. Treatment response of deferiprone in infratentorial superficial siderosis: a systematic review. Cerebellum 2021;20:454–461. 10.1007/s12311-020-01222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.


