Ovarian cancer (OC), breast cancer (BC), endometrial cancer (EC) and colorectal cancer (CRC) account for approximately 50% of cancers in women. 1 A total of 2.9 million women worldwide and approximately 88 000 women in the UK are diagnosed with these cancers annually, and 1.05 million women worldwide and 25 000 women in the UK die from them per year. 1 , 2 GLOBOCAN predicts that the number of these cancer cases will rise by 27%–53% worldwide (and by 20%–36% in women in the UK) and deaths by 49%–69% worldwide (and by 36%‐47% in women in the UK) over the next 20 years. 2 ‘Pathogenic and likely pathogenic variants’, herein termed ‘pathogenic variants’ or ‘PVs’, in a number of high–moderate penetrance cancer susceptibility genes (CSGs) can cause high‐risk breast and/or ovarian cancer syndrome or Lynch syndrome (caused by mismatched repair genes). High‐risk breast and ovarian cancer syndrome is associated with an increased risk of developing BC and/or OC. Lynch syndrome is associated mainly with an increased risk of CRC, EC and OC (see Table 1). Overall, CSGs account for around 15%–20% of OC, 3 4% of BC, 4 3% of EC, 5 and 3%–4% of CRC, 6 , 7 and a majority of these cancers are potentially preventable. High‐risk breast and ovarian cancer syndrome and Lynch syndrome fall under tier‐1 genomic applications, defined by the Centers for Disease Control and Prevention and the Office of Public Health Genomics as those having significant potential for positive impact on public health based on existing evidence‐based guidelines and recommendations. Effective preventive therapy options, including risk‐reducing surgery (mastectomy, risk‐reducing salpingo‐oophorectomy (RRSO) or hysterectomy), chemoprevention (e.g. aspirin or selective estrogen receptor modulators) and screening (for women at high risk of BC or CRC), to reduce these CSG carrier‐associated cancer risks are available in the UK National Health Service (NHS) and other health systems (Table 1). Women can also make lifestyle, contraceptive and reproductive choices, including prenatal/pre‐implantation genetic diagnosis, all of which can impact cancer risk.
TABLE 1.
Tier‐1 syndromes, cancer susceptibility genes, cancer risks and management options
| Genes | Cancer risks % | Risk‐management options | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BC | OC | CRC | EC | BC | OC | CRC | EC | Other | ||
| HBOC | BRCA1 | 72 | 44 | RRM, chemoprevention (SERM, aromatase inhibitors), screening (MRI, mammogram) a | RRSO, RRESDO | Lifestyle, reproduction, contraception, PND, PGD | ||||
| BRCA2 | 69 | 17 | ||||||||
| PALB2 | 53 | 5 | ||||||||
| RAD51C | 21 | 11 | Screening (mammogram) a | |||||||
| RAD51D | 20 | 13 | ||||||||
| BRIP1 | 6 | |||||||||
| LS | MLH1 | 11 | 48 | 37 | Hysterectomy & BSO | Screening (colonoscopy), chemoprevention (aspirin), surgical prevention | Hysterectomy, annual USS, hysteroscopy & endometrial biopsy | |||
| MSH2 | 17 | 47 | 49 | |||||||
| MSH6 | 11 | 20 | 41 | |||||||
| PMS2 b | 3 | 10 | 13 | |||||||
Abbreviations: BSO, bilateral salpingo‐oophorectomy; CP, chemoprevention; HBOC, high‐risk breast and/or ovarian cancer syndrome; LS, Lynch syndrome; PGD, pre‐implantation genetic diagnosis; PND, prenatal diagnosis; RRESDO, risk‐reducing early salpingectomy and delayed oophorectomy; RRM, risk‐reducing mastectomy; RRSO, risk‐reducing salpingo‐oophrectomy; SERM, selective estrogen receptor modulators.
NHS High Risk Breast Cancer Screening Programme.
BSO is not recommended for PMS2 as ovarian cancer risk is similar to population‐level risk.
The traditional model of genetic testing to identify CSG carriers involves accessing genetic testing through high‐risk cancer genetics clinics/services and is based on fulfilling a strong three‐generational family history or standardized clinical criteria. This process is complex, can vary regionally and internationally, and has been shown to be hampered by limited public and health professional awareness, restricted access, inadequate uptake and a huge underutilisation of genetic testing. Besides family history, clinical criteria are only moderately effective at identifying PV carriers and have an extremely poor ability to rule out a PV carrier. 8 Additionally, the traditional genetic testing thresholds have been set too high (e.g. 10% combined probability for ‘BRCA1 and BRCA2’ testing). We and others have shown that around 50% of breast and ovarian CSG carriers do not fulfil current clinical/family history‐based genetic testing criteria, and are missed. 3 , 9 , 10 Far greater numbers of carriers are missed through population‐based ascertainment. 11 For Lynch syndrome, the Bethesda molecular criteria and Amsterdam‐II clinical criteria miss 12%–30% and 55%–70% of carriers, respectively. 5 Recent data show that traditional family history guidelines may further magnify health inequalities for minority communities like non‐Hispanic Black populations, by identifying proportionally fewer high‐risk women in these populations. 12 We showed that despite 25 years of a well‐structured national service for clinical genetics, free at the point of care, over 97% of BRCA carriers remain undetected in a population of 16 million in London. 13 Forecasting models suggest that current detection rates are inadequate, and even doubling the rates would take 165 years to identify the ‘clinically detectable’ proportion of BRCA carriers, with 50% remaining unidentifiable as they don't fulfil the testing criteria. Given the effective risk management including screening (for BC/CRC) and the preventive therapy options available for CSG carriers, this highlights the inadequacy of our current approach and the massive scale of missed opportunities for cancer prevention. Next‐generation sequencing technologies, falling costs, advancements in bioinformatics, our increasing understanding and applicability of genetics, coupled with rising public awareness, now permits large‐scale, high‐throughput, population‐based genetic testing (‘population testing’). Why should we wait for someone to develop cancer in order to identify people in whom we can prevent cancer? Identifying a woman as a CSG carrier after she develops cancer is a failure of cancer prevention!
Changing the paradigm to population testing can address the limitations in the current clinical genetic testing model for CSGs across health systems and provides a forward‐looking strategy to maximise precision prevention. Precision prevention encompasses a prevention strategy that incorporates individual variation in genetic, epigenetic and non‐genetic (environmental, hormonal, reproductive and lifestyle) factors. Half a century ago Wilson and Jungner provided the initial guiding principles for population testing for disease. 14 These have been modified over the years and the UK National Screening Committee has established criteria for UK screening programmes. Over the years, additional adaptations to these principles have been developed for screening for genetic susceptibility, including important principles such as ‘analytic validity, clinical validity, clinical utility and associated ethical, legal and social implications’ (ACCE framework), 15 and other modifications.
The development of any population‐testing framework needs to consider both benefits and harms and only include testing for CSGs with well‐established clinical utility. There should be effective interventions to reduce cancer risk and the risk conferred by the CSGs should lie above the risk thresholds for undertaking these interventions. For example, RRSO is now recommended for women at greater than 4%–5% lifetime OC risk in the UK, 16 or at greater than 3%–4% lifetime OC risk in the USA, 17 thus providing clinical utility for testing newer, moderate‐penetrance CSGs.
1. THE JEWISH MODEL FOR POPULATION‐BASED GENETIC TESTING (POPULATION TESTING)
The greatest wealth of data supporting population testing comes from BRCA‐testing in the Jewish population. Around 1 in 40 Ashkenazi Jewish (AJ) individuals carry one of three Jewish BRCA founder mutations. 9 , 18 Our UK randomised trial (GCaPPS) showed that population‐based BRCA‐testing (compared with family history‐based testing) in the AJ community is feasible, acceptable, safe, has high satisfaction, does not harm quality of life or psychological well‐being, reduces long‐term anxiety, reduces uncertainty, more than doubles the BRCA carriers identified, and can be delivered in a community setting. 9 , 19 These findings are corroborated and complemented by data from large‐cohort studies from Australia, Canada, Israel and the USA. 18 , 20 Jewish population BRCA‐testing has been demonstrated to be extremely cost‐effective and in fact is cost saving in most scenarios. 21 In all, 10% of BC and 40% of OC in the Jewish population are caused by BRCA founder mutations and are potentially preventable. 22 , 23 We and others have long advocated changing policy to offer population‐based BRCA‐testing in the Jewish community. Consequently, Israel has recently changed policy and now offers population BRCA founder mutation testing to all Jewish individuals. Pilot sites offering BRCA‐testing for the Jewish population are expected to be implemented in the UK health service in 2023. The Jewish population is the first population worldwide to undergo population testing in a clinical/healthcare setting.
2. BIOBANKS/GENOMIC POPULATION COHORTS
Additional secondary findings, including PVs in CSGs, have been returned to patients/populations recruited to large biobanks and/or population cohorts, for example the UK Biobank, the 100,000 Genomes Project, the Geisenger MyCode Initiative, the LifePool Study and the Healthy Nevada Project. Although these data are complementary, add to the increasing evidence base and address the population PV prevalence for established CSGs, this bolt‐on return of undertaking additional ‘secondary findings’ is not equivalent to the prospective uptake of testing CSGs in an unselected unaffected population. A selective subgroup opting for the return of incidental/secondarily looked for findings is not generalisable to an unselected unaffected general population. Post‐hoc sequencing and/or analysis does not address, in a prospective unbiased fashion, the key issues and problems related to: (i) the logistics of population testing; (ii) information giving, consent and the uptake of testing; (iii) the uptake of screening and preventive options; (iv) the management of variants of unknown significance; and (v) the long‐term outcomes.
3. POPULATION TESTING IN THE GENERAL POPULATION
Findings from the AJ population cannot be directly extrapolated to the general non‐Jewish population. The Canadian ‘Screen Project’ provided a direct‐to‐consumer BRCA‐testing option in the general population and has been the first of its kind. However, participants (rather than the health system) were expected to pay for their test through out‐of‐pocket costs. A total of 1269 individuals were tested over 2 years. Although this approach may be helpful for improving access for some, a health system‐funded population screening programme is what is needed to maximise uptake, to ensure equity of access and downstream management, and to maximise the population impact. We demonstrated the potential cost‐effectiveness and beneficial population impact of population BRCA‐testing across multiple health systems in high‐income and upper/middle‐income countries. 24 This approach is potentially cost‐saving for the Netherlands and the USA, and is cost‐effective for the UK, Brazil and China. 24 The cost of testing needs to fall further for it to be cost‐effective in low‐income countries like India. 24 This strategy can prevent tens of thousands more BC and OC cases compared with current clinical strategies. We estimate the total general population prevalence of tier‐1 CSGs associated with BRCA1, BRCA2, PALB2, RAD51C, RAD51D, BRIP1, MLH1, MSH2, MSH6 and PMS2 CSGs, listed in Table 1, to be around 1.3%. 25 , 26 Data from large biobank/cohort studies show that approximately 75% of CSG carriers do not fulfil traditional family history‐based clinical criteria and would be missed. 11 Relatives of PV carriers identified can undergo cascade testing. Unaffected relatives of PV carriers identified through cascade testing can also access risk management and preventive interventions (Table 1). Not all CSG carriers identified will develop cancer as these genes have variable penetrance. All at‐risk individuals should have informed counselling of the pros and cons of risk‐management options, including surgical prevention. Undergoing preventive surgery can be a complex and difficult decision‐making process, which changes with time. Different individuals may opt for it at different time points, and some may make an informed choice not to undergo it. Expanding on our earlier modelling with current clinical uptake rates for surgical prevention, we estimate that testing 10 000 women could potentially lead to preventing, in total, approximately 210 cases of BC, OC, EC and CRC combined. 24 , 25 We previously demonstrated the cost‐effectiveness of population‐based testing for a panel of tier‐1 high‐risk breast/ovarian CSGs genes (BRCA1, BRCA2, RAD51C, RAD51D, BRIP1 and PALB2) in the UK and USA healthcare settings, with an incremental cost‐effectiveness ratio (ICER) of £21,599.96 per quality‐adjusted life‐year (QALY), or $54,769.78/QALY, with 83.7% and 92.7% of simulations being cost‐effective on probabilistic sensitivity analysis. 25 The potential cost‐effectiveness of testing for BRCA1, BRCA2, MLH1, MSH2, FXS and CF has also been highlighted for the Australian population. 27
Complex risk models incorporating genetic, family history, epidemiological and clinical variables are now being used to predict personalised absolute cancer risk. These have been developed and validated for a number of cancers, including BC, EC and OC. Although good validation data are available for BC and are beginning to emerge for OC, more robust validation data are needed for other cancers. This approach enables population stratification for risk‐adapted screening and/or risk‐adapted prevention. BC risk models incorporating a single‐nucleotide polymorphism (SNP)‐based polygenic risk score (PRS), mammographic density and epidemiological variables are currently being used to implement risk‐adapted BC screening in large‐scale population cohorts (UK PROCAS study) and in clinical trials such as WISDOM (USA) and MyPeBS (European). Our pilot population‐testing study to predict personalised OC risk using a validated OC‐risk model incorporating CSGs, PRS and epidemiological/reproductive risk factors recruited women through primary care using a web‐based decision tool, and demonstrated feasibility, acceptability, high satisfaction and a reduction in cancer worry with this approach. 28
More real‐world multidisciplinary implementation studies are needed to evaluate the impact of population testing for CSGs. Research needs to evaluate the psychological and socio‐ethical outcomes of population testing. Although initial modelling has highlighted the potential cost‐effectiveness of this approach, real‐world studies with long‐term outcomes of screening and prevention are needed to confirm that the model assumptions are valid and will translate to patient benefit and a reduction in cancer incidence, reconfirming the cost‐effectiveness. It is likely that population‐testing implementation models will vary by country and health system, as they will need to be context specific while following the common core principles of population testing (for an example, see Figure 1). The simplification and mainstreaming of such large‐scale testing will require the digitisation of the process of information giving, consent and a direct‐to‐patient (saliva‐based) testing approach, with more intensive counselling and support reserved for those testing positive.
FIGURE 1.
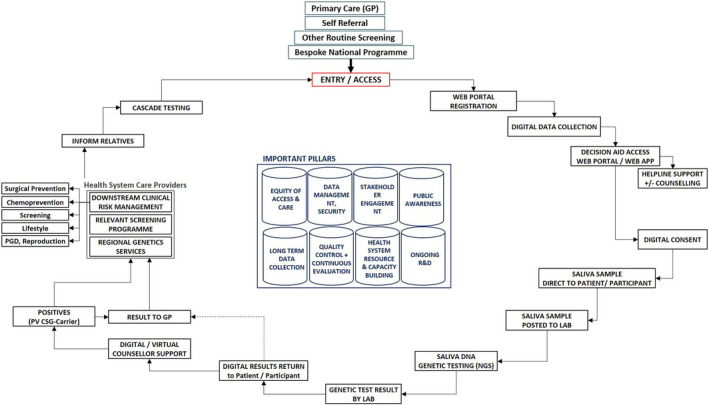
Population‐based testing pathway
Other challenges that need to be tackled include a method for the management of variants of unknown significance and developing a structure or framework for safe data management, data protection, consenting and the delivery of results. Subsequent scaling up for implementation across the health system will have additional challenges, including stakeholder engagement, awareness campaigns, an expansion in health workforce infrastructure, laboratory/testing services, and downstream screening and prevention infrastructure. The future potential for population testing to maximise precision prevention globally across high‐income, middle‐income and low‐income health systems is exciting and bright. The costs of genetic testing have fallen tenfold over the last decade. Although currently cost‐effective for high/middle‐income countries, a price point of approximately $100 a test can make this approach potentially affordable in low‐income countries too. We believe that this will be achievable in the future.
Two prospective general population‐testing studies are being implemented over the next year that will provide an initial evidence base for the assessment of population testing. The Australian ‘DNA screen pilot study’ will recruit 10 000 healthy individuals between 18 and 40 years of age through social media and offer testing for high‐risk BC/OC, Lynch syndrome and familial hypercholesterolaemia CSGs. 29 Our UK PROTECT (population‐based germline testing for early detection and cancer prevention) trial will evaluate the impact of implementing a population‐based panel genetic testing strategy for high‐ and moderate‐penetrance high‐risk BC/OC and Lynch syndrome CSGs in more than 5000 women aged >18 years recruited through primary care using a web‐based digitally enabled direct‐to‐patient saliva‐based DNA testing approach. PROTECT will address current knowledge gaps for population testing by evaluating the incremental PVs detected, uptake of testing, acceptability, satisfaction, psychosocial well‐being, overall impact, socio‐ethics, management strategy for variants of unknown significance, long‐term uptake of screening and prevention interventions, and health‐economic outcomes of population‐based genetic testing.
ACKNOWLEDGEMENT
None.
AUTHOR CONTRIBUTIONs
RM and MS drafted and wrote the commentary.
FUNDING INFORMATION
This work is supported by a grant from Yorkshire Cancer Research.
CONFLICT OF INTERESTS
RM is currently funded by Yorkshire Cancer Research for research into population testing. RM has previously received research funding from The Eve Appeal and Cancer Research UK into population testing. RM is supported by an NHS Innovation Accelerator (NIA) Fellowship for population testing. RM has research funding from Barts & the London Charity, Rose Trees Trust and BGCS outside this work, an honorarium for grant review from Israel National Institute for Health Policy Research and an honorarium for advisory board membership or lectures from Astrazeneca/MSD/GSK/EGL. Completed disclosure of interests form available to view online as supporting information.
Supporting information
Appendix S1
Appendix S2
Manchanda R, Sideris M. Population‐based genetic testing for cancer susceptibility genes: quo vadis? BJOG. 2023;130(2):125–130. 10.1111/1471-0528.17283
DATA AVAILABILITY STATEMENT
Data sharing not applicable ‐ no new data generated.
REFERENCES
- 1. CRUK . Cancer Incidence Statistics. cancer cases and rates by country in the UK. London, UK: Cancer Research UK; 2018. [Google Scholar]
- 2. International Agency for Research on Cancer . Cancer Tomorrow. A tool that predicts the future cancer incidence and mortality burden worldwide from the current estimates in 2018 up until 2040. Lyon, France: International Agency for Research on Cancer (IARC); 2018. [Google Scholar]
- 3. Chandrasekaran D, Sobocan M, Blyuss O, Miller RE, Evans O, Crusz SM, et al. Implementation of multigene germline and parallel somatic genetic testing in epithelial ovarian cancer: SIGNPOST Study. Cancers (Basel). 2021;13(17):4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Breast Cancer Association C , Dorling L, Carvalho S, Allen J, Gonzalez‐Neira A, Luccarini C, et al. Breast cancer risk genes – association analysis in more than 113,000 women. N Engl J Med. 2021;384(5):428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ryan NAJ, Glaire MA, Blake D, Cabrera‐Dandy M, Evans DG, Crosbie EJ. The proportion of endometrial cancers associated with Lynch syndrome: a systematic review of the literature and meta‐analysis. Genet Med. 2019;21(10):2167–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hampel H, Frankel WL, Martin E, Arnold M, Khanduja K, Kuebler P, et al. Feasibility of screening for Lynch syndrome among patients with colorectal cancer. J Clin Oncol. 2008;26(35):5783–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pearlman R, Frankel WL, Swanson BJ, Jones D, Zhao W, Yilmaz A, et al. Prospective statewide study of universal screening for hereditary colorectal cancer: the Ohio colorectal cancer prevention initiative. JCO Precis Oncol. 2021;5:PO.20.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kang HH, Williams R, Leary J, Ringland C, Kirk J, Ward R. Evaluation of models to predict BRCA germline mutations. Br J Cancer. 2006;95(7):914–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Manchanda R, Burnell M, Gaba F, Desai R, Wardle J, Gessler S, et al. Randomised trial of population‐based BRCA testing in Ashkenazi Jews: long‐term outcomes. BJOG. 2020;127(3):364–75. [DOI] [PubMed] [Google Scholar]
- 10. Sun L, Brentnall A, Patel S, DSM B, EJA B, DGR E, et al. A cost‐effectiveness analysis of multigene testing for all patients with breast cancer. JAMA Oncol. 2019;5:1718–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grzymski JJ, Elhanan G, Morales Rosado JA, Smith E, Schlauch KA, Read R, et al. Population genetic screening efficiently identifies carriers of autosomal dominant diseases. Nat Med. 2020;26(8):1235–9. [DOI] [PubMed] [Google Scholar]
- 12. Jakuboski SH, McDonald JA, Terry MB. Do current family history‐based genetic testing guidelines contribute to breast cancer health inequities? NPJ Breast Cancer. 2022;8(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manchanda R, Blyuss O, Gaba F, Gordeev VS, Jacobs C, Burnell M, et al. Current detection rates and time‐to‐detection of all identifiable BRCA carriers in the Greater London population. J Med Genet. 2018;55(8):538–545. [DOI] [PubMed] [Google Scholar]
- 14. Wilson J, Jungner G. Principles and practice of screening for disease. Geneva: World Health Organisation; 1968. [Google Scholar]
- 15. CDC . ACCE Model Process for Evaluating Genetic Tests. Genomic Testing. Atlanta, GA: The Office of Public Health Genomics (OPHG), Centers for Disease Control and Prevention (CDC); 2010. [Google Scholar]
- 16. Manchanda R, Gaba F, Talaulikar V, Pundir J, Gessler S, Davies M, et al. Risk‐reducing salpingo‐oophorectomy and the use of hormone replacement therapy below the age of natural menopause: scientific impact paper No. 66. BJOG. 2022;129(1):e16–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu YL, Breen K, Catchings A, Ranganathan M, Latham A, Goldfrank DJ, et al. Risk‐reducing bilateral salpingo‐oophorectomy for ovarian cancer: a review and clinical guide for hereditary predisposition genes. JCO Oncol Pract. 2022;18(3):201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gabai‐Kapara E, Lahad A, Kaufman B, Friedman E, Segev S, Renbaum P, et al. Population‐based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci USA. 2014;111(39):14205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Manchanda R, Loggenberg K, Sanderson S, Burnell M, Wardle J, Gessler S, et al. Population testing for cancer predisposing BRCA1/BRCA2 mutations in the Ashkenazi‐Jewish community: a randomized controlled trial. J Natl Cancer Inst. 2015;107(1):379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Manchanda R, Gaba F. Population based testing for primary prevention: a systematic review. Cancers (Basel). 2018;10(11):424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Manchanda R, Patel S, Antoniou AC, Levy‐Lahad E, Turnbull C, Evans DG, et al. Cost‐effectiveness of population based BRCA testing with varying Ashkenazi Jewish ancestry. Am J Obstet Gynecol. 2017;217(5):578 e1–e12. [DOI] [PubMed] [Google Scholar]
- 22. King MC, Marks JH, Mandell JB. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302(5645):643–6. [DOI] [PubMed] [Google Scholar]
- 23. Modugno F, Moslehi R, Ness RB, Nelson DB, Belle S, Kant JA, et al. Reproductive factors and ovarian cancer risk in Jewish BRCA1 and BRCA2 mutation carriers (United States). Cancer Causes Control. 2003;14(5):439–46. [DOI] [PubMed] [Google Scholar]
- 24. Manchanda R, Sun L, Patel S, Evans O, Wilschut J, De Freitas Lopes AC, et al. Economic evaluation of population‐based BRCA1/BRCA2 mutation testing across multiple countries and health systems. Cancers (Basel). 2020;12(7):1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manchanda R, Patel S, Gordeev VS, Antoniou AC, Smith S, Lee A, et al. Cost‐effectiveness of Population‐Based BRCA1, BRCA2, RAD51C, RAD51D, BRIP1, PALB2 mutation testing in unselected general population women. J Natl Cancer Inst. 2018;110(7):714–25. [DOI] [PubMed] [Google Scholar]
- 26. Win AK, Jenkins MA, Dowty JG, Antoniou AC, Lee A, Giles GG, et al. Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2017;26(3):404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang L, Bao Y, Riaz M, Tiller J, Liew D, Zhuang X, et al. Population genomic screening of all young adults in a health‐care system: a cost‐effectiveness analysis. Genet Med. 2019;21(9):1958–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gaba F, Blyuss O, Liu X, Goyal S, Lahoti N, Chandrasekaran D, et al. Population study of ovarian cancer risk prediction for targeted screening and prevention. Cancers (Basel). 2020;12(5):1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lacaze PA, Tiller J, Winship I, Group DNASI . Population DNA screening for medically actionable disease risk in adults. Med J Aust. 2022;216(6):278–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Data Availability Statement
Data sharing not applicable ‐ no new data generated.


