Summary
While there is a burgeoning body of research linking smoking during pregnancy to problem behaviour in offspring, a major criticism of this work has been the crude measurement of exposure in these studies (e.g. retrospective, self-reported only) that could lead to biased estimates. To address this issue, we used a pregnancy cohort with repeated prospective measures of exposure as well as biological assays to generate estimates of exposure patterns using a range of modelling techniques. In this paper we report on the analytical approaches we have developed, including patterns of exposure over time and best-estimate approaches that combine self-report and cotinine measures, and compare their predictive value in relation to different dimensions of fetal growth as a first step towards examining the utility of greater precision of exposure measurement.
Surprisingly, in this sample the more complex assessments of exposure, including biological measures, generally did not perform better than simple indicators of exposure based on repeated self-report measures, with one exception: a combined self-report cotinine ‘best estimate’ of third trimester exposure was uniquely associated with lower brain : body ratio. Further study is needed using more sophisticated cotinine assays and testing prediction of a range of outcomes to ascertain whether these findings represent true differences or are specific to the sample, methods and outcomes used. Such research will inform the development of guidelines for adequate exposure characterisation in developmental studies.
Keywords: maternal smoking, urinary cotinine, birthweight, birth length, brain, body ratio
Introduction
The increased risks of low birthweight, spontaneous abortion, perinatal and neonatal mortality among infants exposed to maternal smoking during pregnancy are well known,1 but whether or not exposure plays a causal role in affecting intelligence and behaviour in offspring is more controversial.2,3 One of the barriers to advancing aetiological research in these areas has been the relatively crude measurement of exposure (e.g. retrospective, self-reported typical number of cigarettes per day). Evidence of non-disclosure, recall bias and fluctuations in patterns of smoking across pregnancy suggests that this approach may introduce substantial bias in defining exposure status. However, to date there has been a lack of existing cohorts in which smoking during pregnancy has been measured prospectively and in sufficient detail to allow the examination of variations in timing, intensity and duration of exposure in relation to long-term outcomes.
Adult smokers tend to maintain fairly stable smoking patterns and nicotine levels over time. However, pregnant women exhibit complex patterns of smoking in pregnancy with repeated attempts to quit and fluctuations in the numbers of cigarettes smoked.4,5 These complicated patterns of smoking may be more common in contemporary than in historical cohorts as the pressure not to smoke during pregnancy has increased in most social groups.6
Epidemiological studies of maternal smoking during pregnancy have measured smoking in a variety of ways. At the simplest level, researchers may know only if a woman reported ever smoking during the pregnancy and this information may be retrospective, or from a secondary source such as the medical record or birth certificate. Other studies may have information on the average number of cigarettes smoked each day and, again, such a measure may be as simple as a single, retrospective data item in a secondary source; at best, a study may have repeated, prospective self-report measures. Increasingly, epidemiological studies acquire both self-report and biological assays, generally of cotinine.
Assays of metabolised cotinine can be thought of as a ‘gold standard’ measure of current exposure to cigarette smoke, but as cotinine has a half-life of only 9 h in pregnant women no single measure can be informative about historical exposure.7 How cotinine measures relate to fetal exposure is also complicated by womens’ individual differences in smoking topography (i.e. number and duration of puffs, amount of each cigarette smoked) and metabolism, and the role of pregnancy in accelerating the metabolism of nicotine. Repeated and frequent cotinine assays might seem helpful but in many studies cost and feasibility issues limit the number of assays per subject. In addition, it has been shown that the correlation between self-reported number of cigarettes and cotinine levels across pregnancy, for a given woman, is weaker than the correlation between self-reported number of cigarettes and cotinine between different women, at any given time.4 This is not to suggest that cotinine measures are not useful. They may provide valuable additional sources of information about nicotine metabolism inside the body (and therefore fetal exposure) if collections are made on precise timing schedules tied to daily routines of women, as well as their cigarette and water consumption.8 Thus, it has previously been suggested that integrating self-reported and biological measures of exposure will yield ‘best estimates’ of exposure, as each provides unique information and has unique sources of error.4,8
In this paper we use data from a pregnancy cohort to compare predictions from simple measures of smoking (based on self-report or cotinine) with more sophisticated analytical methods that incorporate patterns of smoking across time and integrate self-report and urinary cotinine. Here we choose to examine predictive utility in relation to fetal growth as a first step towards examining whether precision of exposure measurement enhances prediction because the adverse impact of exposure to cigarettes on these growth parameters is well characterised and because different dimensions of fetal growth ought to be sensitive to differences in the timing, duration and intensity of exposure.
Methods
Data and study sample
For these analyses we used a prospective cohort study of fetal and postnatal exposure to cigarette smoke. The study was originally established as the Maternal and Infant Smoking Study of East Boston to compare the relative effects of in utero and early-life exposure to cigarette smoke on infant lung function;9,10 it is now being followed as the offspring enter adolescence as the East Boston Family Study. Pregnant women were enrolled in the study at a neighbourhood health clinic between March 1986 and October 1992. They were eligible for the study if they were less than 20 weeks pregnant, spoke English or Spanish, would be 19 years of age by time of delivery and planned to return to the clinic for paediatric care. During the enrolment period, 1365 eligible women presented at the clinic and 1000 enrolled in the study. In order to provide maximal power for estimating smoking patterns, all women who provided pregnancy smoking data were included in these analyses (one woman did not provide any smoking information). Thus, the potential sample for analysis of pregnancy smoking patterns was 999; however, sample sizes for particular analyses differ, because of exclusions and missing outcome data as outlined below.
Measurement of smoking and cotinine levels
Women were asked at baseline about their past and current smoking status. At each subsequent prenatal visit [modal number of visits = 7, range 1–12 (Fig. 1)], women reported current smoking habits, including the number of cigarettes being smoked per day. The study aimed to capture all scheduled prenatal visits, the number of which would vary with timing of entry into prenatal care and medical considerations. Variation in the number of visits is also due to women’s non-attendance at scheduled visits, women’s unwillingness at a given visit to complete the survey questions and provide a urine sample, and the inability of research assistants to capture all subjects at all visits. A spot urine sample was collected and analysed for measurement of cotinine by radioimmunoassay at each of these visits. Urine cotinine values were corrected for urine concentration, and expressed as ng/mg of urinary creatinine.
Figure 1.
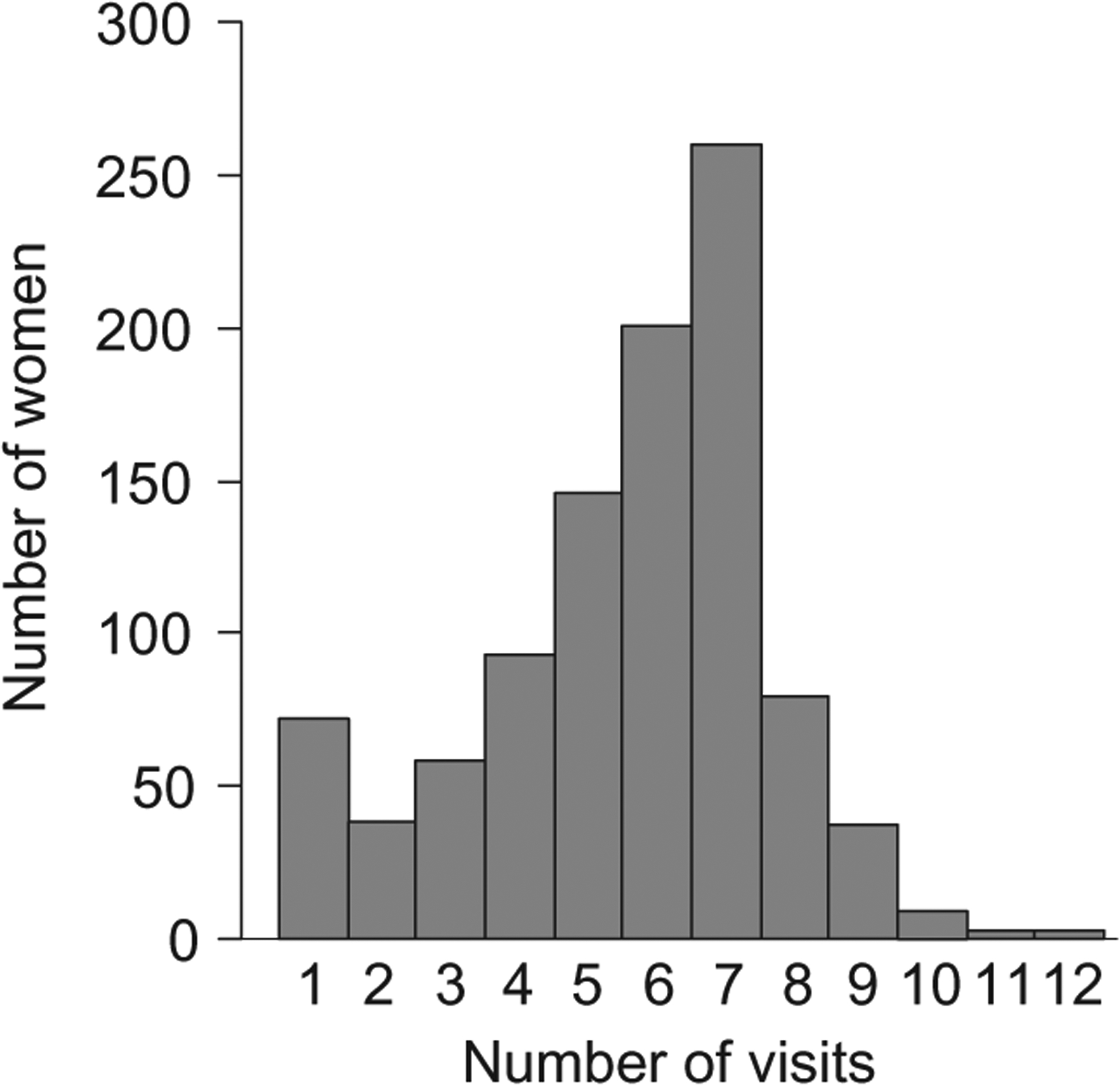
Frequency distribution of number of prenatal visits.
Indicators of maternal smoking during pregnancy
Ten measures of maternal smoking during pregnancy are compared in this study (Table 1). We first assessed exposure based on maternal self-report. To calculate two simple measures of exposure, we used information from the repeated maternal reports of smoking at their prenatal visits to generate (i) an indicator of whether or not a woman ever smoked during the pregnancy (Model 1A) and (ii) a measure of the average number of cigarettes smoked per day (Model 2A). We used a square-root transformation of the average number of cigarettes in analyses to normalise the distribution of values.
Table 1.
Measurements of maternal smoking during pregnancy
| Maternal smoking parameter | No. of women with data available for estimate | No. of women who smoked by measure | Value among smokers |
|---|---|---|---|
| Mean (SD) | |||
| Ever smoked during pregnancy (Model 1A) | 981 | 296 (30%) | |
| Number of cigarettes per day | |||
| Average | 981 | 296a | 11.5 (7.8) |
| Square root of average (Model 2A) | 3.0 (1.3) | ||
| Trimester 1–2 self-report (Model 3A1) – square root | 909 | 266b | 3.0 (1.1) |
| Trimester 3 self-report (Model 3A2) – square root | 909 | 266b | 2.9 (1.1) |
| Ever positive cotinine during pregnancy (Model 1B) | 959 | 315 (33%) | |
| Cotinine value | |||
| Average | 959 | 315c | 34.9 (32.5) |
| Log of average (Model 2B) | 3.1 (1.2) | ||
| Trimester 1–2 cotinine (Model 3B1) – log | 909 | 292d | 2.7 (1.0) |
| Trimester 3 cotinine (Model 3B2) – log | 909 | 292d | 3.0 (0.9) |
| Combined self-report/cotinine | |||
| Trimester 1/2 (Model 4A) | 909 | 307e | 21.8 (8.5) |
| Trimester 3 (Model 4B) | 909 | 264e | 20.9 (9.2) |
Sub-sample of women who ever self-reported smoking in pregnancy.
Sub-sample of women with more than one visit and with evidence of smoking by self-report.
Sub-sample of women with at least one cotinine value indicating smoking.
Sub-sample of women with more than one visit and with evidence of smoking by cotinine.
Sub-sample of women with self-report and cotinine data and evidence of smoking in relevant trimester.
We then generated more complex estimates that would allow us to examine the effects of the intensity of early and late fetal exposure (timing) to cigarette smoke on growth by maternal self-reported smoking. For women with more than one visit, who had some evidence of smoking, we calculated further measures of smoking using the repeated self-reports of average number of cigarettes smoked per day.
A multivariable model for both self-reported smoking (square-root transformed) and cotinine (log transformed) was developed using the SAS PROC MIXED procedure. The model consisted of separate population mean trajectories as functions of gestational age for self-reported smoking and for cotinine. In addition, for each subject, a random effect was added for combined trimesters 1 and 2, and for trimester 3, separately for smoking and cotinine. This allowed each woman’s smoking pattern to deviate from the population mean. These random effects were allowed to be correlated with one another in the model. Finally, the individual observations for each subject were allowed to be correlated with each other following an AR(1) correlation structure, allowing a stronger correlation between observations close together in time compared with observations farther apart in time. The model also included measurement error components for both smoking and cotinine.
From this model, a woman’s self-report and cotinine trajectories as a function of gestational age could be predicted along with standard errors of these predictions. This was accomplished for trimesters 1–2 (Model 3A1) by computing the average of the fitted values (for either smoking or cotinine) from the mean model for gestational ages ≤26 weeks, and adding to this the subject-specific random effects for trimesters 1–2. Because the random effects in the model are allowed to be correlated with one another (i.e. cotinine and smoking effects are correlated, as are trimester 1–2 and 3 effects), this procedure borrows information across the subject’s entire range of observations in order to generate these predictions, thereby increasing their accuracy relative to simple averages of self-reports or cotinine measures for each trimester. For example, for a woman who only has one visit during trimesters 1–2, but several during trimester 3, her predictions for trimesters 1–2 will be more accurate than just using the single time point because the predictions will draw on information available in trimester 3, which is correlated with smoking behaviour in trimesters 1–2. The resulting estimates of the number of cigarettes smoked and the cotinine level were used as predictors in models 3A1 and 3A2.
We used a parallel process with the cotinine measures. A urinary creatinine-adjusted cotinine cut-off value of 200 ng/mg has been validated for these data.4 To generate simple indicators of exposure based on cotinine values, we created a measure of whether or not women ever had a cotinine value that was at or above this cut-point during the pregnancy (Model 1B) and a continuous measure of the average cotinine value during pregnancy (Model 2B). We used a natural log transformation of cotinine values in analyses to normalise the distribution of values.
As with self-reported smoking, we calculated two further more complex measures of exposure based on repeated cotinine values to assess intensity and timing effects. For the first and second trimesters of pregnancy, we found the average of the fitted values from a mean model of the cotinine values for these trimesters, adding a subject-specific random component (Trimester 1–2 Cotinine, Model 3B1). For the third trimester, we repeated the process, using the average of fitted values for the last trimester, adding a subject-specific random component (Trimester 3 Cotinine, Model 3B2).
Finally, we used a timing-specific ‘best estimate’ measure of exposure that combines self-report and biological measures, as we hypothesised that a measure that incorporated both would be a better predictor of fetal growth than measures based on either alone. Recently, Dukic et al. proposed a best estimate method to mathematically combine self-reported and biological exposure measures.8 This deterministic method estimates the average relationship between urine cotinine (taking into account its exponential decay7,11) and the number of cigarettes reported smoked in a sample of pregnant women. In the absence of observed smoking pattern, Dukic et al. suggest assuming constant pattern over the past 3 days, and uniform over the day.8 The adjustments are obtained by first classifying women into categories of ‘under-reporters’, ‘accurate reporters’ and ‘over-reporters’ by comparing their cotinine level with the ‘average’ cotinine concentration per cigarette in a pregnant population,8 and then adjusting the actual woman’s self-report based on the average deviation compared with all women in the same category. Thus, this adjustment combines information about the ‘average woman’s behaviour’ and the information obtained from the sample of women in the dataset. The classification used a deterministic scheme utilising the average cotinine per cigarette (Model 4A for trimesters 1–2 and Model 4B for Trimester 3).8
Indicators of fetal growth
Information on infant anthropometry was abstracted from hospital delivery records and includes birthweight (g), crown-heel length (cm) and head circumference (cm). Two measures of body proportionality are also examined: ponderal index and brain : body ratio.
Infant birthweight reflects the growth and development of all body dimensions, including those that peak earlier in pregnancy, such as growth in length, and those that peak later, such as lean body mass, fat tissue and brain tissue.12–16 As growth in length peaks earlier in pregnancy and growth in head circumference peaks later, we expected measures of early and late exposure, respectively, to perform better than measures that are averaged across pregnancy. We hypothesised that any measure of exposure to cigarette smoke will be a significant predictor of birthweight.
Ponderal index is defined as 100 × [birthweight (g)/length (cm)3] and is sensitive to timing of insults to fetal growth.17 Infants with a low ponderal index are longer and thinner, indicating compromised growth during later pregnancy, whereas infants with a high ponderal index are shorter and fatter, suggesting compromised growth in early pregnancy. We would expect measures that reflect late exposure to perform better in predicting relatively long, thin infants and measures that reflect early exposure to perform better in predicting relatively short, fatter infants.
Brain weight can be estimated as 0.037 × head circumference (cm), according to the model of fetal brain growth developed by the National Institute of Neurological and Communicative Disorders and Stroke’s Collaborative Perinatal Project.18 Brain : body ratio is defined as 100 × [0.037 × head circumference (cm)2.57]/birthweight (g). A higher brain : body ratio indicates a greater proportion of body weight residing in the brain; values for full term, healthy infants average 9–10%.19 We hypothesised that late exposure would be most predictive of infants with relatively lower brain : body ratio.
The deleterious direct effects of smoking on fetal growth are thought to be due to hypoxia, and the toxic effects of nicotine and carbon monoxide,20 but smoking also has effects on the duration of pregnancy and gestational age is therefore a potential mediator of the effects of smoking on fetal growth.21 To account for this, we used gestational age-adjusted outcomes in all analyses. We estimated models regressing each fetal growth parameter on gestational age in weeks and used the residuals from these models as dependent variables in all models examining the effects of smoking. For length and head circumference, the effect of gestational age on growth was modelled with a linear term. For birthweight, we also included a quadratic term for gestational age in the model. Previous studies have reported that the effect of smoking during pregnancy on measures of fetal body proportionality is additionally affected by fetal growth retardation; therefore our models of ponderal index and brain : body ratio are adjusted for birthweight z-score, as well as for gestational age.19,22
Statistical analysis
Descriptive summaries, including means and proportions, were used to describe the characteristics of the study sample. We examined the distribution of the fetal growth parameters and excluded outliers with unlikely values [length < 40 cm (n = 11), head circumference < 30 cm (n = 6), ponderal index > 4 (n = 9), brain : body ratio > 15 (n = 3)]. We used linear regression models to assess the impact of different smoking parameters (self-report, cotinine and combined measures) on each of the five measures of fetal growth (birthweight, length, head circumference, ponderal index and brain : body ratio). We report robust estimates of standard errors for all models to account for unmeasured variation due to measurement error in self-reported smoking and cotinine, and measurement error due to estimation of our first, second and third trimester, and combined models.
Aside from adjustment of each outcome for gestational age (and birthweight z-score in some cases), we include no covariates in the models reported here. Potential confounding factors are those which are associated with smoking during pregnancy and independently associated with fetal growth. We found that maternal age, race/ethnicity and education were unrelated to fetal growth in this sample and therefore could not confound associations between smoking and fetal growth. Similarly, we found that maternal complications of pregnancy known to be associated with fetal growth (hypertension, eclampsia, gestational diabetes and pre-existing diabetes) were not associated with smoking in this cohort; neither was parity nor infant sex, so none of these potential confounding factors could act as confounders in our models.
Results
The sample for analysis comprised 54% White and 40% Hispanic, with 6% women of other race or ethnic status. The cohort was primarily of low socio-economic status; 37% of the women had less than a high school education. At time of enrolment, the mean age was 26.5 years, with a standard deviation of 5 years.
At enrolment 28% reported current smoking. Of these, 75% continued to report smoking at every prenatal visit, whereas 14% reported smoking at some visits but not others, and 11% did not return for another visit. Half of the sample reported ever smoking in their lifetime. The median number of cigarettes per day among smokers at enrolment was 10 per day. Maternal smoking variables used in the analyses of timing, intensity and duration of smoking on fetal growth are reported in Table 1. It should be noted that the mean number of cigarettes smoked for Model 2A is based on a larger sample than the means for models 3A1 and 3A2, which are restricted to women with more than one measure; the same restriction applies to the estimates of mean cotinine for models 3B1 and 3B2, vis-à-vis the mean cotinine level for all women with at least one measure (Model 2B).
Table 2 shows infant growth outcomes among the 835 mothers with infants for whom at least one outcome was recorded. Compared with infants whose mothers never smoked in pregnancy, for infants whose mothers smoked (by either positive self-report or positive cotinine value), birthweight was 126 g [95% CI 51, 202 g] lower (P = 0.001), length was 0.6 cm [95% CI 0.2, 0.9 cm] shorter (P = 0.005) and head circumference was 0.3 cm [95% CI 0.1, 0.6 cm] smaller (P = 0.002). There were no statistically significant differences in ponderal index or brain : body ratio. These simple comparisons (exposed or not based on self-report and cotinine) form a basis against which to judge the performance of our other measures of maternal smoking status.
Table 2.
Unadjusted fetal growth outcomes among women who smoked at all during pregnancy and non-smokers as assessed by self-report or cotinine
| Fetal growth | Smoked during pregnancy (n = 270)a | Never smoked during pregnancy (n = 565)a |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Birthweight (g) | 3303 (498) | 3429 (531) |
| Crown-heel length (cm) | 50.0 (2.5) | 50.6 (2.5) |
| Head circumference (cm) | 34.1 (1.6) | 34.4 (1.5) |
| Ponderal indexb | 2.6 (0.3) | 2.7 (0.3) |
| Brain: body ratioc | 9.8 (1.2) | 9.7 (1.2) |
The sample sizes given are for birthweight; sample sizes are slightly different for other fetal growth outcomes as not all measures were recorded for all infants.
100 × [birthweight (g)/length (cm)3].
100 × [0.037 × head circumference (cm)2.57]/birthweight (g).
Tables 3–5 show the effects on fetal growth of various measures of maternal smoking by self-report (Table 3: models 1A, 2A, 3A), cotinine values (Table 4: models 1B, 2B, 3B) and best-estimate measures combining self-report and cotinine measures (Table 5: models 4A and 4B). In models using average number of cigarettes per day (models 2A, 3A) and average cotinine values across pregnancy (models 2B, 3B), the coefficients given in the tables are for the square root- and log-transformed variables, respectively.
Table 3.
Effects of maternal smoking measures, based on self-report, on fetal growth outcomes
| Self-reported smoking in pregnancy | Model 1A ever smoked | Model 2A square root of average cigs per day | Model 3A1 1st, 2nd trimester estimate | Model 3A2 3rd trimester estimate | |
|---|---|---|---|---|---|
| Birthweight (g) | b (SE) | −181 (35) | −54 (10) | −58 (10) | −62 (11) |
| t | −5.16 | −5.45 | −5.59 | −5.9 | |
| P | <0.001 | <0.001 | <0.001 | <0.001 | |
| R 2 | 0.03 | 0.03 | 0.03 | 0.04 | |
| Crown-heel length (cm) | b (SE) | −0.8 (0.2) | −0.2 (0.05) | −0.3 (0.1) | −0.3 (0.1) |
| t | −4.44 | −4.72 | −4.63 | −4.91 | |
| P | <0.001 | <0.001 | <0.001 | <0.001 | |
| R 2 | 0.02 | 0.03 | 0.03 | 0.03 | |
| Head circumference (cm) | b (SE) | −0.4 (0.1) | −0.1 (0.03) | −0.1 (0.04) | −0.1 (0.04) |
| t | −3.44 | −3.51 | −3.3 | −3.51 | |
| P | 0.001 | <0.001 | 0.001 | <0.001 | |
| R 2 | 0.02 | 0.02 | 0.01 | 0.02 | |
| Ponderal index (g/cm3) | b (SE) | 0.02 (0.02) | 0.01 (0.01) | 0.01 (0.01) | 0.01 (0.01) |
| t | 1.02 | 1.5 | 1.32 | 1.42 | |
| P | 0.307 | 0.133 | 0.186 | 0.155 | |
| R 2 | 0.001 | 0.002 | 0.002 | 0.002 | |
| Brain : body ratio (cm2.57/g) | b (SE) | −0.05 (0.1) | −0.02 (0.02) | −0.02 (0.02) | −0.02 (0.02) |
| t | −0.72 | −1.08 | −0.86 | −1.03 | |
| P | 0.469 | 0.282 | 0.393 | 0.304 | |
| R 2 | 0.001 | 0.001 | 0.001 | 0.001 |
All outcomes are adjusted for gestational age at delivery. Models estimating effects on ponderal index and brain : body ratio are also adjusted for birthweight z-score. See Table 1 for sample sizes.
Table 5.
Effects of maternal smoking measures, based on ‘best estimate’ combined self-report and urine cotinine measures, on fetal growth outcomes
| ‘Best estimate’ measures of smoking in pregnancy (cotinine adjusted cigarettes per day) | Model 4A Deterministic method −1st, 2nd trimesters | Model 4B Deterministic method −3rd trimester | |
|---|---|---|---|
| Birthweight (g) | b (SE) | −7.4 (1.5) | −8.3 (1.5) |
| t | −5.08 | −5.62 | |
| P | <0.001 | <0.001 | |
| R 2 | 0.03 | 0.04 | |
| Crown-heel length (cm) | b (SE) | −0.03 (0.01) | −0.04 (0.01) |
| t | −3.93 | −4.9 | |
| P | <0.001 | <0.001 | |
| R 2 | 0.02 | 0.03 | |
| Head circumference (cm) | b (SE) | −0.02 (0.01) | −0.02 (0.01) |
| t | −3.83 | −4.6 | |
| P | <0.001 | <0.001 | |
| R 2 | 0.02 | 0.03 | |
| Ponderal index (g/cm3) | b (SE) | 0.001 (0.001) | 0.001 (0.001) |
| t | 0.72 | 1.34 | |
| P | 0.469 | 0.181 | |
| R 2 | 0.001 | 0.002 | |
| Brain : body ratio(cm2.57/g) | b (SE) | −0.004 (0.003) | −0.006 (0.003) |
| t | −1.36 | −2.05 | |
| P | 0.174 | 0.04 | |
| R 2 | 0.002 | 0.006 |
All outcomes are adjusted for gestational age at delivery. Models estimating effects on ponderal index and brain : body ratio are also adjusted for birthweight z-score. See Table 1 for sample sizes.
Table 4.
Effects of maternal smoking measures, based on cotinine values, on fetal growth outcomes
| Cotinine levels in pregnancy | Model 1B ever smoked by cotinine | Model 2B log of average cotinine | Model 3B1 1st, 2nd trimester estimate | Model 3B2 3rd trimester estimate | |
|---|---|---|---|---|---|
| Birthweight (g) | b (SE) | −154 (34) | −38 (7) | −65 (11) | −64 (11) |
| t | −4.54 | −5.35 | −5.85 | −5.86 | |
| P | <0.001 | <0.001 | <0.001 | <0.001 | |
| R 2 | 0.02 | 0.03 | 0.04 | 0.04 | |
| Crown-heel length (cm) | b (SE) | −0.6 (0.2) | −0.14 (0.03) | −0.3 (0.1) | −0.3 (0.06) |
| t | −3.42 | −4.25 | −4.7 | −4.73 | |
| P | 0.001 | <0.001 | <0.001 | <0.001 | |
| R 2 | 0.01 | 0.02 | 0.03 | 0.03 | |
| Head circumference (cm) | b (SE) | −0.4 (0.1) | −0.1 (0.02) | −0.15 (0.04) | −0.15 (0.04) |
| t | −3.47 | −4.81 | −3.82 | −3.91 | |
| P | 0.001 | <0.001 | <0.001 | <0.001 | |
| R 2 | 0.02 | 0.03 | 0.02 | 0.02 | |
| Ponderal index (g/cm3) | b (SE) | 0.01 (0.02) | 0.001 (0.004) | 0.01 (0.01) | 0.01 (0.01) |
| t | 0.56 | 0.97 | 1.14 | 1.17 | |
| P | 0.572 | 0.796 | 0.256 | 0.243 | |
| R 2 | 0.001 | 0.001 | 0.001 | 0.001 | |
| Brain : body ratio(cm2.57/g) | b (SE) | −0.09 (0.07) | −0.02 (0.01) | −0.03 (0.02) | −0.03 (0.02) |
| t | −1.26 | −2.19 | −1.29 | −1.4 | |
| P | 0.207 | 0.081 | 0.197 | 0.163 | |
| R 2 | 0.002 | 0.005 | 0.002 | 0.002 |
All outcomes are adjusted for gestational age at delivery. Models estimating effects on ponderal index and brain : body ratio are also adjusted for birthweight z-score. See Table 1 for sample sizes.
Birthweight
Having reported ever smoking at all in pregnancy is associated with a reduction in birthweight of 181 g (Model 1A). The effects of average number of cigarettes smoked per day (Model 2A) and the estimates of self-reported average early (Model 3A1) and late (Model 3A2) pregnancy smoking are all statistically significant and of similar magnitude. Having a positive cotinine value at some point in pregnancy is associated with a reduction in birthweight of 154 g (Model 1B). The effects of the average cotinine value across visits (Model 2B) and the estimates of average early (Model 3B1) and late (Model 3B2) pregnancy cotinine values are all statistically significant. Models 3B1 and 3B2 have very similar magnitudes of effect, which are somewhat larger than that estimated from Model 2B. The best-estimate measure shows similar effect patterns: Model 4A shows a 7-g decrease in birthweight for every additional cotinine-corrected cigarette smoked per day during the first 2 trimesters, while there is an 8-g decrease associated with each additional cigarette smoked in the last trimester (Model 4B). Smoking measures explain between 2% and 4% of variance in birthweight.
Crown-heel length
Having smoked at all in pregnancy is associated with a reduction in length of almost 1 cm. The effects of average number of cigarettes smoked per day (Model 2A) and the estimates of self-reported average early (Model 3A1) and late (Model 3A2) pregnancy smoking are all statistically significant and of similar magnitude. Results are almost identical using cotinine values. Having a positive cotinine value at least once during pregnancy is associated with a reduction in length of almost 0.6 cm. The effects of the average cotinine value across visits (Model 2B) and the estimates of average early (Model 3B1) and late (Model 3B2) pregnancy cotinine values are all statistically significant. Models 3B1 and 3B2 have identical magnitude of effect, somewhat larger than that estimated from Model 2B. The best-estimate measures show similar effect patterns: Model 4A shows a 0.03 cm decrease in average length for every additional cotinine-corrected cigarette smoked per day during the first 2 trimesters, while there is a 0.04 cm decrease associated with each additional cigarette smoked in the last trimester (Model 4B). Smoking measures account for between 1% and 3% of variance in length.
Head circumference
Having smoked at all in pregnancy is associated with a reduction in head circumference of 0.4 cm. The effects of average number of cigarettes smoked per day (Model 2A) and the estimates of self-reported average early (Model 3A1) and late (Model 3A2) pregnancy smoking are all statistically significant and of identical magnitude. Having a positive cotinine value at some point in pregnancy is associated with a reduction in head circumference of almost half a centimetre. The effects of the average cotinine value across visits (Model 2B) and the estimates of average early (Model 3B1) and late (Model 3B2) pregnancy cotinine values are all statistically significant. Models 3B1 and 3B2 have identical magnitude of effect, again somewhat larger than that estimated from Model 2B. The best-estimate measures again show similar effect patterns: there is a 0.02 cm decrease in average circumference for every additional cotinine-corrected cigarette smoked per day during the first 2 trimesters, as well as with every additional cigarette smoked in the last trimester. Smoking measures account for between 1.5% and 3% of variance in head circumference.
Ponderal index
None of the smoking variables has a significant effect on ponderal index. In our sample, estimates of smoking for both early and late pregnancy are associated with increased ponderal index, although these associations are not significant. None of the cotinine measures has a significant effect on ponderal index, although again all the coefficients have a positive sign, indicating a shorter, fatter infant. Smoking variables account for less than 1% of variance in ponderal index in all models.
Brain : body ratio
Only the best-estimate measure for the third trimester is significantly related to brain: body ratio, although all models have a negative sign, indicating relatively less weight residing in the brain and compromise of later growth. Smoking variables account for less than 1% of the variance in brain : body ratio.
Discussion
In summary, models of the impact of smoking in pregnancy on fetal growth were generally consistent whether or not smoking was measured via self-report, cotinine assay or measures combining the two. We were able to detect significant effects of smoking on birthweight, length and head circumference, but no significant impact on ponderal index and only one measure was significantly related to brain : body ratio. For the fetal growth parameters where smoking was a significant predictor, measures of exposure in the first and second trimester combined vs. third trimester were not related to substantial differences in growth, except for brain : body ratio, for which only third trimester exposure was significant. We had hypothesised that our more complex measures, that either focused on particular timing of exposure during pregnancy (Model 3) or our best-estimate measures that incorporated both self-report and urine cotinine measures (Model 4) might add precision for all estimates, including sufficient power to detect effects on ponderal index and brain : body ratio. These hypotheses were not well supported. In addition, as cotinine measures can reflect fetal exposure to passive smoking as well as exposure to active maternal smoking, and both can influence fetal growth, we might have expected models based on cotinine to perform better than those based on self-report. However, England et al. did not find that cotinine measures explained more variation in birthweight than self-reported smoking in a study of over 3000 US women.23
These generally null findings suggest the possibility that in epidemiological studies of outcomes where exposure is expected or known to have a substantial impact, simple measures of average cigarette consumption may be sufficient for the estimation of effects or for inclusion as potential confounding variables or effect modifiers in models of other aetiological associations. However, we note that our single indicator model (ever exposed or not) was more precise than such measures in many epidemiological studies that have used a single question at the end of pregnancy to determine ‘average exposure’; here, these simple indicators of exposure and average exposure were based on prospective, repeated measures of smoking across the pregnancy. The fact that we were able to detect a subtle effect of exposure on brain : body ratio only with the best-estimate indicator of third trimester exposure suggests that such measures may have the potential for identifying further subtle effects of smoking in pregnancy.
This pattern of findings may also, to some extent, reflect measurement artefacts including lower precision of cotinine assays using radioimmunassay vs. the current gas chromatography mass-spectrometry (GCMS) methods,24 and sample size limitations (e.g. only approximately one-third of the sample were pregnancy smokers) that constrained detection of more subtle effects and/or the nature of the outcomes (e.g. restricted range in outcomes such as ponderal index or brain : body ratio). Despite previous studies demonstrating fluctuations in smoking across pregnancy in this cohort,4 most women who smoked in the first and second trimesters also smoked in the third and much larger samples may be needed to establish the effect of variations in the timing, intensity and duration of fetal exposure.
Methods of measurement error correction vary in their impact on precision. In a review of methods, Thurigen et al. reflect on the choice of methods in practice and state that ‘two conflicting aspects have to be balanced: ease of use on one hand, causing a certain loss in precision of estimation, versus theoretical precision of modelling on the other hand, resulting in more precise and valid estimates, but causing difficulties in implementation’.25 Our two different approaches to adjustment (models 3A and 3B, and models 4A and 4B) may result in less precise estimates, although we certainly hope they result in less biased estimates. If they are less precise, then we are at least conservative in our inference. However, arguments in favour of a non-artefactual interpretation of our results are that we were able to rule out confounding by a number of maternal and infant factors and both the parameter estimates and explanatory power of our models (R2) were generally consistent across models, as well as significance levels.
If these findings reflect true effects, more parsimonious assessments of exposure may be adequate. Replication of these findings in studies with larger samples of smokers and with more sophisticated cotinine assays will be important before reaching a definitive conclusion.
Previous studies of the effects on fetal growth of smoking during different time periods of pregnancy have mostly looked at the timing of quitting smoking, generally at one or two defined cut-points, rather than variations in continuous exposure. MacArthur and Knox reported that stopping smoking prior to 16 weeks gestation resulted in birthweights similar to non-smokers among British women.26 Quitting by 30 weeks was as predictive of birthweight as any dose measure of exposure in a study by Hebel et al.27 and Cliver et al. found that, for women who stopped smoking after the first trimester, quitting was more predictive of fetal growth than the amount smoked previous to quitting.28 Lindley et al. studied more than 15 000 Swedish births and found quitting smoking before 32 weeks prevented deficits in birthweight, head circumference and brain : body weight ratio, although these women had babies with shorter lengths and higher ponderal index than non-smokers.19 A study of over 7000 pregnant Dutch women found that smoking in late pregnancy had stronger associations with low birthweight than smoking in mid-pregnancy and that smoking in early pregnancy did not increase the risk.29
Although the present study focuses on prediction of fetal growth outcomes, the broader goal in developing our more complex measures was to contribute to epidemiological studies of the teratogenic effect of smoking in pregnancy on long-term developmental outcomes such as intelligence, temperament and antisocial behaviour. These are in many ways more subtle relationships than that between smoking and fetal growth – partly because the causal pathways are likely to be complex, partly because measurement of the outcomes can also be complex, and also because some outcomes are not measurable until many years after exposure. Estimating exposure with precision will be important in such studies, which are likely to be affected by complicated confounding phenomena, and methods such as our ‘best estimate’ methods that incorporate information from both self-report and biological assays may prove useful.
Our more complex measures of exposure patterns perform at least as well as, and in one case better than, simpler measures for straightforward outcomes but in the present study do not demonstrate clear advantage. It remains to be seen whether or not they allow better prediction of more subtle, long-term developmental outcomes of smoking in pregnancy. There is often a tendency to assume that ‘more’ is ‘better’ when it comes to scientific measurement. Multiple measurements and biological assays add substantial costs to research investigations. As we continue to examine the ‘added value’ of more complex estimations of exposure for prediction, we hope to provide empirical evidence that can inform determinations of the level of information necessary for adequately characterising fetal exposure to smoking in pregnancy.
Acknowledgements
The writing of this paper was supported by grants from the National Institute of Drug Abuse to Dr Pickett (R03 DA14334–01) and Dr Wakschlag (R01 DA15223). Dr Pickett is supported by a UK NIHR (National Institute for Health Research) Career Scientist Award. The authors acknowledge the contributions of: Dr Ira Tager for consultation on the Maternal and Infant Smoking Study of East Boston, and East Boston Family Study collaborators, Gretchen Biesecker, PhD and Bennett Leventhal, MD. The ongoing support of Vince Smeriglio, PhD of NIDA for this programme of research is gratefully acknowledged.
References
- 1.Walsh RA. Effects of maternal smoking on adverse pregnancy outcomes: examination of the criteria of causation. Human Biology 1994; 66:1059–1092. [PubMed] [Google Scholar]
- 2.Olds D Tobacco exposure and impaired development: a review of the evidence. Mental Retardation and Developmental Disabilities Research Reviews 1997; 3:257–269. [Google Scholar]
- 3.Wakschlag LS, Pickett KE, Cook E Jr, Benowitz BL, Leventhal NL. Maternal smoking during pregnancy and severe antisocial behavior in offspring: a review. American Journal of Public Health 2002; 92:966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickett KE, Rathouz PJ, Kasza K, Wakschlag LS, Wright R. Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy. Paediatric and Perinatal Epidemiology 2005; 19:368–376. [DOI] [PubMed] [Google Scholar]
- 5.Pickett KE, Wakschlag LS, Dai L, Leventhal BL. Fluctuations of maternal smoking during pregnancy. Obstetrics and Gynecology 2003; 101:140–147. [DOI] [PubMed] [Google Scholar]
- 6.Orleans CT, Barker DC, Kaufman NJ, Marks JF. Helping pregnant smokers quit: meeting the challenge in the next decade. Tobacco Control 2000; 9:iii6–iii11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dempsey D, Jacob P 3rd, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. Journal of Pharmacology and Experimental Therapeutics 2002; 301:594–598. [DOI] [PubMed] [Google Scholar]
- 8.Dukic VM, Niessner M, Benowitz N, Hans S, Wakschlag L. Modeling the relationship of cotinine and self-reported measures of maternal smoking during pregnancy: a deterministic approach. Nicotine and Tobacco Research 2007; 9:453–465. [DOI] [PubMed] [Google Scholar]
- 9.Tager IB, Ngo L, Hanrahan JP. Maternal smoking during pregnancy. Effects on lung function during the first 18 months of life. American Journal of Respiratory and Critical Care Medicine 1995; 152:977–983. [DOI] [PubMed] [Google Scholar]
- 10.Hanrahan JP, Tager IB, Segal MR, Tosteson TD, Castile RG, Van Vunakis H, et al. The effect of maternal smoking during pregnancy on early infant lung function. American Review of Respiratory Disease 1992; 145:1129–1135. [DOI] [PubMed] [Google Scholar]
- 11.Benowitz NL, Jacob P 3rd. Nicotine metabolism in non-smokers. Clinical Pharmacology and Therapeutics 1990; 48:473–474. [DOI] [PubMed] [Google Scholar]
- 12.Poissonnet CM, Burdi AR, Garn SM. The chronology of adipose tissue appearance and distribution in the human fetus. Early Human Development 1984; 10:1–11. [DOI] [PubMed] [Google Scholar]
- 13.Toft PB, Leth H, Ring PB, Peitersen B, Lou HC, Henriksen O. Volumetric analysis of the normal infant brain and in intrauterine growth retardation. Early Human Development 1995; 43:15–29. [DOI] [PubMed] [Google Scholar]
- 14.Falkner F Ultrasonography and fetal growth: key perinatal factors. Journal of Perinatology 1995; 15:114–118. [PubMed] [Google Scholar]
- 15.Bernstein IM, Goran MI, Amini SB, Catalano PM. Differential growth of fetal tissues during the second half of pregnancy. American Journal of Obstetrics and Gynecology 1997; 176:28–32. [DOI] [PubMed] [Google Scholar]
- 16.Bernstein IM, Blake K, Wall B, Badger GJ. Evidence that normal fetal growth can be noncontinuous. Obstetrical and Gynecological Survey 1996; 51:213–214. [DOI] [PubMed] [Google Scholar]
- 17.Caulfield LE, Haas JD, Belizan JM, Rasmussen KM, Edmonston B. Differences in early postnatal morbidity risk by pattern of fetal growth in Argentina. Paediatric and Perinatal Epidemiology 1991; 5:263–275. [DOI] [PubMed] [Google Scholar]
- 18.McLennan JE, Gilles FH, Neff RK. A model of growth of the human fetal brain. In: The Developing Human Brain: Growth and Epidemiologic Neuropathy. Editors: Gilles FH, Leviton A, Dooling EC. Boston, MA: Wright PG. 1983; pp. 43–58. [Google Scholar]
- 19.Lindley AA, Becker S, Gray RH, Herman AA. Effect of continuing or stopping smoking during pregnancy on infant birth weight, crown-heel length, head circumference, ponderal index, and brain : body weight ratio. American Journal of Epidemiology 2000; 152:219–225. [DOI] [PubMed] [Google Scholar]
- 20.Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? Journal of Pharmacology and Experimental Therapeutics 1998; 285:931–945. [PubMed] [Google Scholar]
- 21.Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. American Journal of Obstetrics and Gynecology 2000; 182:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kramer MS, Olivier M, McLean FH, Dougherty GE, Willis DM, Usher RH. Determinants of fetal growth and body proportionality. Pediatrics 1990; 86:18–26. [PubMed] [Google Scholar]
- 23.England LJ, Kendrick JS, Gargiullo PM, Zahniser SC, Hannon WH. Measures of maternal tobacco exposure and infant birth weight at term. American Journal of Epidemiology 2001; 153:954–960. [DOI] [PubMed] [Google Scholar]
- 24.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine and Tobacco Research 2002; 4:149–159. [DOI] [PubMed] [Google Scholar]
- 25.Thurigen D, Spiegelman D, Blettner M, Heuer C, Brenner H. Measurement error correction using validation data: a review of methods and their applicability in case-control studies. Statistical Methods in Medical Research 2000; 9:447–474. [DOI] [PubMed] [Google Scholar]
- 26.MacArthur C, Knox EG. Smoking in pregnancy: effects of stopping at different stages. British Journal of Obstetrics and Gynaecology 1988; 95:551–555. [DOI] [PubMed] [Google Scholar]
- 27.Hebel JR, Fox NL, Sexton M. Dose-response of birth weight to various measures of maternal smoking during pregnancy. Journal of Clinical Epidemiology 1988; 41:483–489. [DOI] [PubMed] [Google Scholar]
- 28.Cliver SP, Goldenberg RL, Cutter GR, Hoffman HJ, Davis RO, Nelson KG. The effect of cigarette smoking on neonatal anthropometric measurements. Obstetrics and Gynecology 1995; 85:625–630. [DOI] [PubMed] [Google Scholar]
- 29.Jaddoe VW, Troe EJ, Hofman A, Mackenbach JP, Moll HA, Steegers EA, et al. Active and passive maternal smoking during pregnancy and the risks of low birthweight and preterm birth: the Generation R Study. Paediatric and Perinatal Epidemiology 2008; 22:162–171. [DOI] [PubMed] [Google Scholar]


