Abstract
Background:
Smoking during pregnancy has been consistently associated with risk of problem behaviour in offspring. There is debate about whether this association reflects a teratological effect or is a marker for problematic maternal characteristics. We test these “competing” hypotheses by examining whether (1) exposure is associated with an early risk pathway by testing its association with infant temperamental difficultness, and (2) whether pregnancy quitting is associated with an early protective pathway, testing its association with easy infant temperament.
Methods:
We used the 9-month-old sweep of the Millennium Cohort Study, a cohort of over 18 000 infants born in 2000–2. Mothers were classified as pregnancy non-smokers, quitters and light or heavy smokers. Temperamental positive mood, receptivity to novelty and regularity were assessed with the Carey Infant Temperament Scale.
Results:
Pregnancy quitters had infants with the highest scores of easy temperament and heavy smokers had infants with the lowest scores (F = 28.51, p<0.001). Pregnancy smoking also predicted difficult temperament: heavy smoking was associated with increased risk of low positive mood (OR = 1.17, p = 0.09). In contrast, pregnancy quitting exerted a protective effect with decreased risk of distress to novelty (OR = 0.79, p<0.01) and irregularity (OR = 0.89, p = 0.02) in these infants.
Conclusions:
Pathways from pregnancy smoking to offspring behaviour are complex and multi-determined. These findings suggest that both exposure and maternal characteristics associated with pregnancy smoking status contribute to offspring behavioural patterns. Research that characterises differences between quitters and persistent smokers and examines the role of these differences in prediction of early vulnerabilities and problems in adaptation over time will be important for elucidating these pathways.
Maternal smoking during pregnancy has been consistently associated with increased risk of problem behaviour in offspring, including antisocial behaviour, smoking and substance abuse.1–4 These associations have generally been robust to confounding and demonstrated in multiple, independent, cross-national samples.4 5
A central question that remains is whether prenatal smoking has an aetiological role in pathways to problem behaviours via teratological effects or whether it is a marker for intergenerational transmission processes associated with both the tendency to smoke persistently during pregnancy and to have offspring with problem behaviour. Although these have often been proposed as “competing” hypotheses, we suggest that an “either/or” conceptualisation is probably too simplistic—that is, it is likely that the association of prenatal smoking and offspring problem behaviour reflects the influence of both teratological processes and maternal characteristics.6
In order to advance understanding of this question, we juxtapose these alternative hypotheses by examining hypothesised pathways by which exposure and maternal characteristics would exert their effects. Firstly, we examine whether exposure is associated with an early risk pathway, by comparing the risk of difficult temperament in the infants of women who smoke during pregnancy (pregnancy smokers), women who give up smoking during pregnancy (pregnancy quitters) and non-smokers. Secondly, we examine whether pregnancy quitting is associated with an early protective pathway, by comparing the association of quitting (compared to not smoking or persistent smoking) with infant easy temperament.
Exposure and behavioural disruption
From a behavioural teratological perspective, a coherent pattern of behavioural effects would be expected across development—that is, teratological effects would be hypothesised to occur via a disruption of fetal brain development, which in turn would disrupt the regulation of postnatal behavioural processes. In fact, there is substantial evidence from animal studies that nicotine is a behavioural teratogen.7 The majority of studies on exposure and behavioural risk have not had a developmental focus.8 Little is known about the early emergence of exposure-related behavioural risk because problem behaviours have been studied primarily in older youths when these behaviours are already well entrenched and multiple intervening risk processes have exerted their effects. Thus, examination of exposure-related patterns as they unfold in early life provides a more direct examination of these processes.
Infant temperament reflects biologically determined predispositions towards patterns of behavioural regulation and reactivity that are evident at birth and are relatively stable across the lifespan.9 Since temperament has been extensively studied, is relatively stable and has well developed measurement methods it provides a useful measure for examining exposure-related differences in early behaviour.10 Although a wide variety of terms have been used to describe various dimensions of infant temperament, three central dimensions are emotionality/mood, receptivity/distress to novelty and regularity.11 12 Emotionality reflects the infant’s characteristic manner of emotional response, including typical mood and the intensity of emotional expressions in terms of both negative and positive poles.13 Receptivity/distress to novelty reflects the infant’s characteristic response to novel stimuli ranging from receptivity to environmental changes to distress to novelty. Regularity reflects the extent to which infants’ biological rhythms are consistent or unpredictable.14 The pattern of these temperamental dimensions has also been described in terms of an overall “easy temperament” (for example, positive mood, receptivity to novelty, regularity) and “difficult temperament” (for example, low positive/high negative mood, distress to novelty, irregularity).14 Difficult infant temperament is a well established risk factor for later maladaptation (for example, antisocial behaviour) whereas easy temperament is associated with positive adaptation over time.10 14 15
Although several studies have demonstrated that exposure is associated with infant irritability,16 17 to our knowledge only one study has examined the relation of exposure to specific dimensions of difficult temperament.18 In this study of a Finnish birth cohort, exposure was associated with particular dimensions of temperament and these associations were robust to confounding; although the nature of particular associations varied across developmental periods. To our knowledge, no studies have tested the effects of exposure and pregnancy quitting simultaneously in relation to early temperament and the relation to easy temperament has not been studied. Since sex differences have been demonstrated in patterns of exposure and problem behaviour in older youths, examination of sex differences in early exposure-related patterns is also important for elucidating whether there are divergent pathways for boys and girls from early in life.4
Maternal characteristics that distinguish pregnancy quitters from persistent pregnancy smokers
Smoking cessation during pregnancy appears to reflect a deliberate effort to protect the baby rather than a long-term intention to quit; postpartum relapse rates are extremely high.19 This capacity to “suspend smoking”19 is likely to be one manifestation of maternal personality characteristics such as the capacity to adapt flexibly to environmental circumstances and the ability to plan and delay gratification.6 Consistent with this, women who quit smoking in pregnancy have better general functioning, including more sustained relationships, more skilfulness in use of community resources and less disrupted and stressful life circumstances and are less likely to have a history of social problems and antisocial behaviour compared to pregnancy smokers.20 21 These differences in functioning may reflect stable differences in personality that are linked to a protective behavioural style in the infant both via heritable processes and via association with quality of parenting and family environment. However, as women who quit have generally been grouped either with non-smokers or with continuing smokers, in studies of prenatal smoking, the independent contribution of quitting to behavioural pathways has not been examined.
In this paper, we examine the relation of maternal smoking patterns during pregnancy to infant temperament in a large birth cohort. In particular, we test our hypothesis that heavy exposure and quitting smoking in pregnancy will make independent contributions to prediction of temperament. In so doing, we are suggesting that pregnancy quitting is not merely a reflection of the absence of exposure—rather we hypothesise that it is a marker for maternal personality characteristics such as constraint and flexibility.22 If this hypothesis is correct, the behavioural patterns of the infants of quitters would differ not only from those of the exposed infants but also from those of the never-exposed infants. In particular, we hypothesise that (1) heavy exposure will be associated with difficult infant temperament and; (2) that pregnancy quitting will independently predict infant easy temperament. We also explore sex differences in these patterns.
METHODS
Data and study sample
Data for these analyses came from the Millennium Cohort Study (MCS), a large prospective study of infants born in 2000–2 in the United Kingdom. The first wave of data collection took place when the infants were around 9 months old and includes data on 18 819 infants in 18 533 families. The sampling design allowed for over-representation of areas (electoral wards) with high levels of childhood deprivation and high proportions of ethnic minorities. Infants born on eligible dates in eligible areas were selected from the Child Benefit Register (child benefit is a universal benefit payable from birth). Detailed information collected from parents during this first wave included information on pregnancy, birth, parental and infant health, infant development and social and economic circumstances. The response rate was 72%; non-respondents were more likely to be without a fixed residence, living in ethnic minority areas in England or living in advantaged areas in Northern Ireland.23 Full details of the Millennium Cohort Study have been published previously24 and are also supplied in documentation deposited with the data at the UK Data Archive. This study excludes families with multiple births (n = 256), families where someone other than the natural mother was the respondent (n = 30) and families with missing information on maternal smoking during pregnancy (n = 4), giving an analytical sample of 18 263 mother-infant pairs.
Measurement of maternal smoking during pregnancy
Retrospective information was collected on smoking during pregnancy by women’s self-report. Mothers were asked whether or not they had smoked before pregnancy, whether they had quit smoking or continued to smoke during pregnancy and the average number of cigarettes they had smoked per day when pregnant. Following previous studies examining smoking within the MCS,25 we classified mothers as (1) never smoked during pregnancy, (2) quit smoking during pregnancy, (3) continuous light smoker during pregnancy (less than 10 cigarettes per day) and (4) continuous heavy smoker during pregnancy (10+ cigarettes per day) (based on consistent findings of a half pack per day being the level of exposure at which negative behavioural effects have been reported).4
Measurement of infant temperament
Infant temperament was assessed using 14 questions from the Carey Infant Temperament Scale.26 27 The Carey scale is a widely used temperament measure that has demonstrated good reliability and validity.12 27 Difficult temperament on the Carey scale has been associated with maladaptation over time, including antisocial behaviour.15 Three dimensions of temperament were assessed. Positive mood assessed the extent to which the infant is characteristically cheerful across a range of daily care contexts (five items). Receptivity to novelty assessed the infant’s characteristic manner of responding to environmental changes ranging from comfortable with novelty to likely to become distressed (five items). High scores indicate distress and withdrawal from new situations. Regularity indicated the extent to which the infant’s physiological functions are regular and rhythmic (four items) or irregular. High scores indicate regularity. For each temperament question/statement, for example
he is pleasant (smiles, laughs) when first arriving in unfamiliar places (friend’s house, shop)”
mothers rated the infant on a frequency ranging from 1–5: “almost never,” “rarely,” “usually does not,” “often,” “almost always” or “can’t say.” Each answer was assigned a score (answers for the receptivity to novelty dimension were reverse-coded (high scores reflected lack of distress to novelty) and for all dimensions “can’t say” was coded as missing) and scores summed to give three-dimensional subscores and an overall score of temperamental robustness. Higher overall scores were considered indicators of “easy temperament.”
In order to test our hypothesis about exposure and temperamental difficultness and because, in a large sample meaningless small differences in continuous scores can be statistically significant, we also created indicators of “difficult” temperament. Carey has described the “difficult child” as one whose temperament scores fall below the population norm. We label infants as having “difficult” temperament on any of the three dimensions if their score is below the sample mean for that dimension (that is, we categorise infants as exhibiting low positive mood if they are below the sample mean on mood score, distress to novelty if they are below the mean for receptivity to novelty and irregularity if they are below the mean for regularity) and as having “difficult” overall temperament if their scores are classified as difficult on all three dimensions.
Measurement of potential confounding variables
Variables were examined as potential confounding factors on the basis of previously established relations with either smoking during pregnancy or problems of temperament or behaviour in infancy or childhood. These included: birth weight (in grams), length of gestation (days), mother’s age (years) and maternal self-reports of alcohol consumption during pregnancy (yes/no), history of depression (yes/no) and partner-perpetrated domestic violence (yes/no/don’t want to answer). Marital status is defined as married, cohabiting or single. Three measures of socioeconomic status were examined: income-related poverty is defined as household income below 60% of the median; mother’s educational attainment is categorised into six groups on the National Vocational Qualification scale, ranging from no qualifications to degree level28; and mother’s social class is categorised according to the National Statistics Socio-Economic Classification (NS-SEC),29 collapsed for these analyses into routine and semi-routine occupations (characterised by moderate-low job security, career prospects and autonomy) versus all others. Mother’s ethnic group is classified into six categories: white, mixed, Indian, Pakistani/Bangladeshi, black/black British and other.
Statistical analysis
All analyses were carried out using survey weights to correct for the complex sampling design of the study. Sample characteristics are described using means and proportions, with differences tested by Wald tests and χ2 tests, respectively. Unadjusted odds ratios are estimated for difficult temperament in relation to categories of maternal smoking during pregnancy. Logistic regression models were used to examine the effects of maternal smoking on difficult infant temperament, adjusting for potential confounding factors. To investigate any moderating effect all analyses were repeated, stratified by infant sex.
RESULTS
Table 1 shows the characteristics of mothers and infants by categories of smoking during pregnancy. Over a third (35.7%) of mothers reported smoking at some time during pregnancy, although almost half of these quit smoking, and only a small proportion of the sample (9.95%) smoked heavily throughout pregnancy. Women who smoked throughout pregnancy had significantly smaller infants and shorter gestations, although women who quit smoking had infants with birth weights and gestations comparable to non-smokers. Women who smoked at any time in pregnancy were also significantly younger, more likely to have a history of depression and more likely to have reported being a victim of domestic violence. They were less likely to be married or to have educational qualifications, and more likely to be poor or in a routine or semi-routine occupation. Mothers of white or mixed ethnicity were much more likely to smoke than women in any other ethnic group. There was no significant association between alcohol consumption and smoking in pregnancy—in fact, the highest prevalence of alcohol consumption was reported by women who quit smoking.
Table 1.
Characteristics of mothers and infants by maternal smoking status during pregnancy
| Never smoked (n = 11 747) 64.3% | Quit smoking (n = 2320) 12.7% | Light smoker (n = 2378) 13.0% | Heavy smoker (n = 1818) 9.95% | |
|---|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | |
| Infant birth weight (kg)** | 3.37 (0.05) | 3.41 (0.02) | 3.20 (0.02) | 3.17 (0.02) |
| Infant length of gestation (days)* | 277 (0.18) | 278 (0.33) | 276 (0.31) | 276 (0.30) |
| Mother’s age (years)*** | 30.2 (0.62) | 27.2 (0.53) | 26.8 (0.40) | 27.4 (0.32) |
| Mother consumed alcohol during pregnancy | 26.3% | 34.9% | 31.2% | 33.8% |
| History of maternal depression*** | 18.9% | 29.3% | 35.4% | 41.4% |
| Partner-perpetrated domestic violence*** | ||||
| Yes | 2.6% | 4.3% | 4.6% | 6.7% |
| Prefer not to answer | 2.3% | 2.6% | 3.8% | 4.8% |
| Marital status*** | ||||
| Married | 70.7% | 39.3% | 31.3% | 27.9% |
| Cohabiting | 17.4% | 35.0% | 35.5% | 34.7% |
| Single | 11.9% | 25.7% | 33.1 % | 37.4% |
| Household income-related poverty*** | 27.3% | 34.9% | 51.6% | 62.9% |
| Mother’s educational qualifications** | ||||
| None | 13.7% | 12.0% | 21.9% | 36.3% |
| NVQ1 | 6.4% | 10.2% | 14.1 % | 14.6% |
| NVQ2 | 26.8% | 37.0% | 37.2% | 31.0% |
| NVQ3 | 14.9% | 16.7% | 14.1 % | 10.4% |
| NVQ4 | 33.4% | 21.8% | 11.9% | 7.2% |
| NVQ5 | 4.7% | 2.3% | 0.9% | 0.4% |
| Mother’s social class*** | ||||
| Routine and semi-routine occupation | 28.6% | 42.5% | 53.2% | 58.5% |
| Mother’s ethnic group*** | ||||
| White | 59.6% | 14.2% | 14.6% | 11.6% |
| Mixed | 52.7% | 19.1% | 19.7% | 8.5% |
| Indian | 93.9% | 3.8% | 1.7% | 0.8% |
| Pakistani/Bangladeshi | 96.0% | 1.0% | 2.2% | 0.7% |
| Black/black British | 81.8% | 8.9% | 7.5% | 1.8% |
| Other | 87.0% | 7.5% | 4.3% | 1.2% |
NVQ, national vocational qualification.
Differences were statistically significant at *p<0.01, **p<0.001, ***p<0.0001.
The overall temperament score for the sample was 56.6 (SD 6.5), on a scale ranging from 14–70. The top part of table 2 shows the unadjusted associations between categories of maternal smoking during pregnancy and continuous scores of easy temperament. Consistent with our hypotheses, the highest temperament scores are reported by mothers who quit smoking (57.3) and the lowest among heavy smokers (55.7) an adjusted Wald test showed that this pairwise contrast is significantly different (F = 28.51, p<0.001 but not the pairwise contrast between non-smokers and heavy smokers (F = 3.4, p = 0.10). Pregnancy smoking status was also associated with particular subdimensions of continuous temperament scores. Again, there are significant differences between the infants of mothers who quit and mothers who smoked heavily (F = 9.07, p = 0.02), but not between non-smokers and heavy smokers (F = 2.20, pe = 0.018), with infants of women who quit smoking having the most positive mood. A similar pattern is seen for both the receptivity to novelty and regularity subdimensions; the tests for overall differences are significant, the differences between non-smokers and heavy smokers are not significant, and the biggest differences are between women who quit and heavy smokers and these are statistically significant (F = 7.8, p = 0.02 for receptivity to novelty; F = 33.06, p<0.001 for regularity).
Table 2.
Infant temperament by categories of maternal smoking in pregnancy
| Never smoked | Quit smoking | Light smoker | Heavy smoker | p Value for test of overall differences | |
|---|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) | ||
| Easy temperament | 56.7 (55.2 to 58.2) | 57.3 (56.4 to 58.1) | 56.5 (55.8 to 57.1) | 55.7 (55.2 to 56.2) | <0.01 |
| Dimensions of easy temperament | |||||
| Positive mood | 19.2 (18.9 to 19.5) | 19.3 (19.0 to 19.5) | 19.3 (19.0 to 19.5) | 19.0 (18.8 to 19.2) | 0.01 |
| Receptivity to novelty | 20.2 (19.6 to 20.9) | 20.7 (20.3 to 21.1) | 20.1 (19.9 to 21.1) | 20.03 (19.8 to 20.3) | 0.05 |
| Regularity | 17.0 (16.4 to 17.7) | 17.2 (16.9 to 17.5) | 16.9 (16.7 to 17.2) | 16.6 (16.4 to 16.8) | 0.01 |
| Reference | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Difficult temperament | – | 0.82 (0.52 to 1.28) | 0.99 (0.68 to 1.44) | 0.97 (0.60 to 1.58) | |
| Categories of difficult temperament | |||||
| Low positive mood | – | 0.95 (0.81 to 1.10) | 0.96 (0.87 to 1.07) | 1.05 (0.93 to 1.20) | |
| Distress to novelty | – | 0.91 (0.77 to 1.08) | 1.04 (0.97 to 1.11) | 0.97 (0.83 to 1.30) | |
| Irregularity | – | 0.91 (0.68 to 1.23) | 1.10 (0.83 to 1.47) | 1.28 (0.95 to 1.72) |
Differences were statistically significant at *p<0.01, **p<0.001.
The lower half of table 2 presents the odds ratios for various measures of “difficult” temperament by smoking categories. Compared to infants whose mothers never smoked during pregnancy (reference category), there is a trend towards a lower likelihood of difficult temperament among infants whose mothers quit smoking and a higher risk among infants of pregnancy smokers. In terms of particular dimensions of difficultness, infants whose mothers smoked heavily during pregnancy were more likely to have irregularity. Conversely, infants of women who quit were less likely to show distress to novelty and irregularity.
We examined whether or not associations between the different dimensions of difficult temperament and smoking status were robust to confounding in multiple logistic regression models (table 3). An increased trend of low positive mood among infants whose mothers smoked heavily was amplified when other factors were included in the model (fig 1). Higher birth weight and alcohol consumption during pregnancy were also associated with low positive mood; compared to white mothers, Pakistani/Bangladeshi mothers, black/black British mothers and mothers with other ethnicity were significantly more likely to report having an infant with low positive mood. Interestingly, for distress to novelty and irregularity, controlling for confounders amplified the protective association for these dimensions of difficult temperament among women who quit smoking in pregnancy (fig 1). Mothers’ age, low violence and non-white ethnicity were also associated with distress to novelty. Unmarried mothers, poor mothers, mothers without qualifications and ethnic minority mothers were more likely to have infants with irregular temperament. We found similar results (not presented) in multiple linear regression models predicting continuous temperament scores.
Table 3.
Multiple logistic regression models for difficult temperament dimensions
| Low positive mood | Distress to novelty | Irregularity | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Smoking status during pregnancy | ||||||
| Never | Ref | Ref | Ref | |||
| Quit | 0.98 (0.79 to 1.21) | 0.84 | 0.79 (0.67 to 0.93) | 0.01 | 0.89 (0.80 to 0.97) | 0.02 |
| Light smoker | 1.04 (0.86 to1.24) | 0.65 | 0.97 (0.92 to 1.03) | 0.29 | 1.02 (0.95 to 1.10) | 0.49 |
| Heavy smoker | 1.17 (0.97 to 1.42) | 0.09 | 0.92 (0.78 to 1.08) | 0.27 | 1.05 (0.96 to 1.16) | 0.22 |
| Birth weight (kg) | 1.07 (1.00 to 1.14) | 0.05 | 1.00 (0.92 to 1.08) | 0.89 | 0.97 (0.90 to 1.03) | 0.29 |
| Gestation (days) | 1.00 (0.99 to 1.00) | 0.44 | 1.00 (0.99 to 1.00) | 0.26 | 1.00 (0.99 to 1.00) | 0.52 |
| Mother’s age (years) | 1.00 (0.99 to 1.01) | 0.20 | 0.98 (0.97 to 0.99) | 0.01 | 1.00 (1.00 to 1.01) | 0.07 |
| Alcohol consumption during pregnancy | 1.21 (1.12 to 1.31) | <0.001 | 0.99 (0.90 to 1.09) | 0.93 | 1.03 (0.96 to 1.10) | 0.30 |
| History of maternal depression | 1.11 (0.96 to 1.30) | 0.13 | 1.04 (0.98 to 1.10) | 0.14 | 1.04 (0.93 to 1.10) | 0.44 |
| Partner-perpetrated domestic violence | ||||||
| No | Ref | Ref | Ref | |||
| Yes | 1.14 (0.96 to 1.37) | 0.12 | 1.11 (1.03 to 1.20) | 0.01 | 0.95 (0.77 to 1.17) | 0.58 |
| Prefer not to answer | 1.23 (0.98 to 1.55) | 0.07 | 0.99 (0.78 to 1.27) | 0.98 | 1.14 (0.86 to 1.51) | 0.31 |
| Marital status | ||||||
| Married | Ref | Ref | Ref | |||
| Cohabiting | 0.96 (0.85 to 1.09) | 0.52 | 1.04 (0.90 to 1.19) | 0.57 | 1.22 (1.07 to 1.40) | 0.01 |
| Single | 1.23 (0.92 to 1.66) | 0.13 | 1.17 (0.91 to 1.49) | 0.18 | 2.03 (1.76 to 2.33) | <0.001 |
| Household income-related poverty | 1.04 (0.95 to 1.14) | 0.38 | 1.06 (0.98 to 1.14) | 0.11 | 1.37 (1.18 to 1.60) | 0.001 |
| Mother’s education (NVQ grade) | 1.05 (0.99 to 1.11) | 0.09 | 0.98 (0.93 to 1.02) | 0.32 | 0.90 (0.87 to 0.94) | 0.001 |
| Mother’s social class | ||||||
| Routine and semi-routine occupation | 1.01 (0.86 to 1.18) | 0.93 | 1.02 (0.93 to 1.12) | 0.09 | 1.04 (096 to 1.12) | 0.29 |
| Mother’s ethnic group | ||||||
| White | Ref | Ref | Ref | |||
| Mixed | 1.46 (0.93 to 2.28) | 0.09 | 1.81 (1.40 to 2.33) | 0.001 | 1.65 (1.28 to 2.14) | <0.01 |
| Indian | 1.29 (0.90 to 1.82) | 0.14 | 2.17 (1.82 to 2.58) | <0.001 | 2.85 (2.24 to 3.62) | <0.001 |
| Pakistani/Bangladeshi | 1.70 (1.53 to 1.88) | <0.001 | 1.69 (1.50 to 1.91) | <0.001 | 2.73 (1.88 to 3.97) | <0.001 |
| Black/black British | 2.03 (1.78 to 2.32) | <0.001 | 1.29 (1.07 to 1.55) | 0.01 | 3.23 (2.72 to 3.82) | <0.001 |
| Other | 1.43 (1.29 to 1.58) | <0.001 | 1.35 (1.03 to 1.78) | 0.03 | 2.85 (1.89 to 4.30) | <0.001 |
Figure 1.
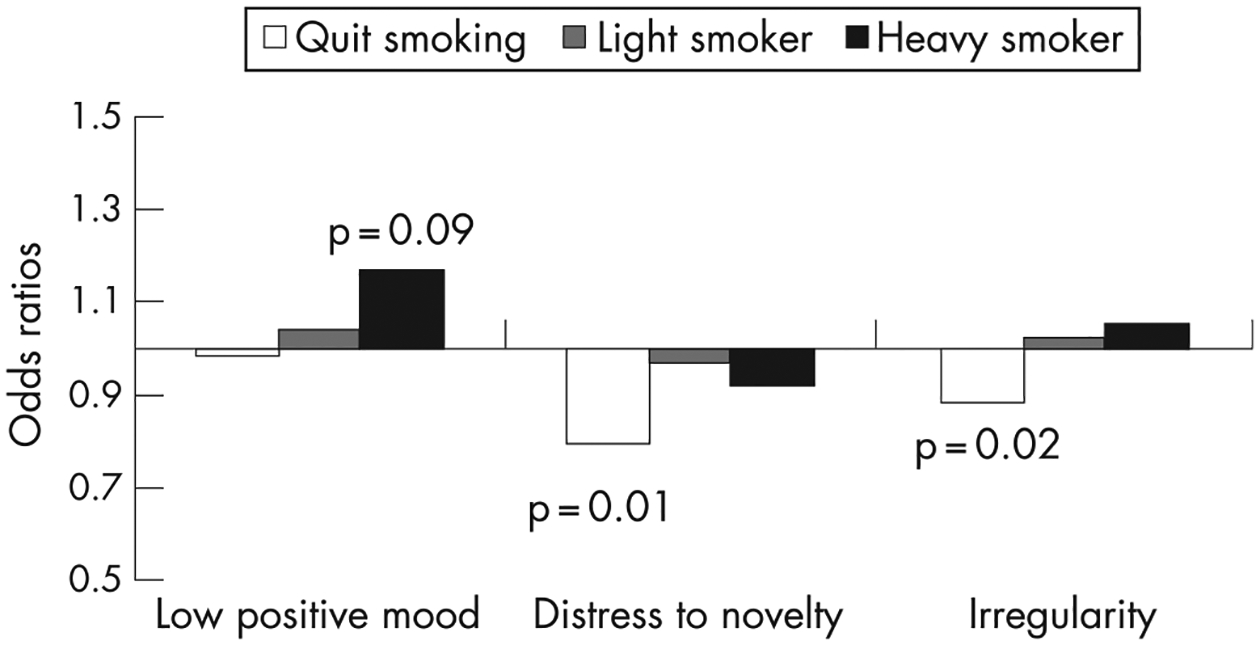
Odds ratios for categories of difficult temperament by smoking categories, reference category is women who never smoked during pregnancy.
We also examined sex differences in temperament and in the relation of smoking in pregnancy to temperament. Although the overall temperament scores were significantly different (p = 0.003) these differences were extremely small (boys: mean 56.8, SD 6.5; girls: mean 56.5, SD 6.5). There were no significant sex differences in scores on the positive mood and regularity scores, but girls scored as significantly less receptive to novelty than boys, p<0.001, although again these differences were small (boys mean 20.5, SD 3.6; girls: mean 20.0, SD 3.7). In sex-stratified multivariable analyses of the effect of smoking in pregnancy on infant temperament, we found that effects were stronger in infant boys than in infant girls. For low positive mood, the odds ratio associated with heavy smoking was 1.3 for boys (p = 0.09) and 1.06 for girls (p = 0.50). For distress to novelty, the protective association of quitting smoking was stronger in boys (OR = 0.74, p = 0.001) than in girls (OR = 0.83, p = 0.21), and the same pattern was seen for irregularity (boys: OR = 0.84, p = 0.002; girls: OR = 0.94, p = 0.30).
DISCUSSION
To our knowledge, this is the first study to go beyond testing for “exposure” effects to a more nuanced exploration of pregnancy smoking status and behavioural outcomes. In particular, we have demonstrated that both exposure and quitting smoking during pregnancy have independent effects on temperament. Strikingly, quitting smoking during pregnancy appears to denote a protective effect via increased likelihood of having an infant with easy temperament in comparison to women who never smoke, and with persistent exposure and confounders controlled. Conversely, persistent heavy smoking in pregnancy is associated with a more difficult temperamental style, a well established developmental antecedent to the types of problem behaviours that previous research has consistently shown to be sequelae of heavy smoking.30 31 The stronger pattern of difficultness evident for boys relative to girls is also consistent with research on older youths. This adds to an emerging body of evidence that the disrupted behavioural patterns associated with exposure are evident and coherent across developmental periods.6 8 18
In the juxtaposition of two competing hypotheses about “mechanism” of effect, we have provided evidence which suggests that both maternal characteristics that distinguish persistent smoking from quitting and teratological effects are potentially at work. In particular, the association of quitting smoking during pregnancy with easy temperament suggests that both quitting and easy temperament may reflect heritable, stable aspects of personality encompassing flexibility, regularity and positive mood.32 This is perhaps best highlighted by the fact that pregnancy quitting predicted lower likelihood of distress to novelty relative to heavy smokers. As women who quit smoking in pregnancy are exposing their unborn infants to some degree, and as many will subsequently relapse and expose their infants to environmental tobacco smoke,19 our findings suggest that maternal characteristics can potentially override teratological effects of low-grade or intermittent exposure. Thus, the capacity to “suspend smoking” on behalf of fetal wellbeing appears to represent not only the absence of problem behaviour characteristics traditionally associated with persistent pregnancy smoking but also adaptive maternal characteristics such as self-restraint and the ability to shift behaviour based on changing environmental contingencies. These heritable characteristics may be linked to easy temperament in the infants via multiple processes. Firstly, the “positive” characteristics of quitters are very similar to the “easy temperament” in their infants, suggesting genetic transmission of these traits. Secondly, this more “agreeable” personality style may be associated with a positive rating bias. Findings from behaviour genetic studies provide support for these dual processes that maternal personality characteristics are associated with systematic rating biases but also with “objective” ratings of child behaviour.33 34
The trend of an association of heavy smoking with infant difficult mood adds weight to the burgeoning evidence that pregnancy smoking may have an aetiological role in the development of problem behaviours. Most notably, the present findings converge with other recent studies using a variety of methods and designs, which demonstrate that atypical behavioural patterns in the offspring of persistent pregnancy smokers are evident as early as the first years of life.8 35 Characterising maternal personality differences across smoking groups will be an important next step in future studies designed to identify pathways by which prenatal smoking and behavioural risk in offspring are linked, as will be the use of genetically informative designs.
Strengths of the present study include the large, representative sample and the ability to detect relatively subtle yet robust associations with early infant temperament. Several limitations of the present study must also be noted. As noted above, sole reliance on maternal report of infant temperament may introduce informant biases (for example, response sets and social desirability) that are correlated with maternal smoking behaviour. Follow-up studies using multi-method, multi-informant assessments of infant temperament (see Rothbart and Bates12) will be optimal for characterising unique variance contributed by maternal smoking status. Maternal smoking assessment was also brief, self-reported and retrospective and did not capture the fluctuations in smoking that are common across pregnancy.36 However, non-disclosure rates are lower in non-clinical epidemiological studies than in clinic-based smoking cessation studies, and have been reported to be as low as 5% in some studies.37 The use of biomarkers such as cotinine to validate longitudinal patterns of smoking in pregnancy is of questionable validity; studies have shown that women self-report changes in pregnancy smoking that are not captured with even substantial numbers of repeated cotinine measures.38 Replication in a sample with prospective, repeated measures of smoking and objective measures of temperament will be important.
Findings reported here represent yet another “piece of the puzzle” in an ongoing programme of research examining complex pathways from maternal health behaviours during pregnancy to developmental disruptions in offspring.4 6 39 The challenges of “unpacking” the multiple convergent influences at work demands integrated multidisciplinary efforts combining epidemiological, teratological, psychiatric and psychosocial perspectives. Fundamentally, these findings underscore that pregnancy smoking (or quitting) behaviour is not only an individual but a maternal behaviour with complex determinants and sequelae. Enhancing understanding of these complex, multi-determined phenomena will importantly inform research on prevention and effects on offspring.
What this paper adds.
Heavy smoking in pregnancy (more than 10 cigarettes/day) may be associated with infant temperamental difficultness, a precursor to antisocial behaviour.
Women who quit smoking during pregnancy have temperamentally easier infants compared to persistent smokers and non-smokers.
These patterns cannot be explained by sociodemographic correlates of maternal pregnancy smoking status.
Policy implications.
Psychological characteristics that differentiate women who persist in smoking from women who quit because of pregnancy may have important implications for pregnancy cessation programmes and for the long-term outcomes of their offspring.
Acknowledgements:
This research was supported by a postgraduate Advanced Studentship in Health Services Research from the Medical Research Council, UK to CW. KEP and LSW were supported by grant 1R01DA15223 from the US National Institute for Drug Abuse. The authors also thank the ESRC Data Archive at Essex University for providing the Millennium Cohort Study data.
Footnotes
Competing interests: None.
REFERENCES
- 1.Brennan PA, Grekin ER, Mortensen EL, et al. Relationship of maternal smoking during pregnancy with criminal arrest and hospitalization for substance abuse in male and female adult offspring. Am J Psychiatry 2002;159:48–54. [DOI] [PubMed] [Google Scholar]
- 2.Buka SL, Shenassa ED, Niaura R. Elevated risk of tobacco dependence among offspring of mothers who smoked during pregnancy: a 30-year prospective study. Am J Psychiatry 2003;160:1978–84. [DOI] [PubMed] [Google Scholar]
- 3.Monuteaux MC, Blacker D, Biederman J, et al. Maternal smoking during pregnancy and offspring overt and covert conduct problems: a longitudinal study. J Child Psychol Psychiatry 2006;47:883–90. [DOI] [PubMed] [Google Scholar]
- 4.Wakschlag LS, Pickett KE, Cook E Jr, et al. Maternal smoking during pregnancy and severe antisocial behavior in offspring: a review. Am J Public Health 2002;92:966–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huizink AC, Mulder EJ. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev 2006;30:24–41. [DOI] [PubMed] [Google Scholar]
- 6.Wakschlag LS, Pickett KE, Kasza KE, et al. Is prenatal smoking associated with a developmental pattern of conduct problems in young boys? J Am Acad Child Adolesc Psychiatry 2006;45:461–7. [DOI] [PubMed] [Google Scholar]
- 7.Benowitz N Nicotine safety and toxicity. New York: Oxford University Press, 1998. [Google Scholar]
- 8.Wakschlag LS, Leventhal BL, Pine DS, et al. Elucidating early mechanisms of developmental psychopathology: the case of prenatal smoking and disruptive behavior. Child Dev 2006;77:893–906. [DOI] [PubMed] [Google Scholar]
- 9.Rothbart MK, Ahadi SA. Temperament and the development of personality. J Abnorm Psychol 1994;103:55–66. [DOI] [PubMed] [Google Scholar]
- 10.Rothbart MK, Posner M, Hershey K. Temperament, attention, and developmental psychopathology. In: Cicchetti D, Cohen D, eds. Developmental psychopathology. New York: John Wiley, 1995:315–40. [Google Scholar]
- 11.Buss A The EAS theory of temperament. In: Strelau J, Angleitner A, eds. Explorations in temperament. London: Plenum, 1991. [Google Scholar]
- 12.Rothbart MK, Bates J. Temperament. In: Damon W, Eisenberg N, eds. Handbook of child psychology: social, emotional and personality development. 5th ed. New York: Wiley, 1998:105–76. [Google Scholar]
- 13.Hane AA, Fox NA, Polak-Toste C, et al. Contextual basis of maternal perceptions of infant temperament. Dev Psychol 2006;42:1077–88. [DOI] [PubMed] [Google Scholar]
- 14.Chess S, Thomas A. Continuities and discontinuities in temperament. In: Robins L, Ruttter M, eds. Straight and devious pathways from childhood to adulthood. Cambridge: Cambridge University Press, 1990. [Google Scholar]
- 15.Coon H, Carey G, Corley R, et al. Identifying children in the Colorado Adoption Project at risk for conduct disorder. J Am Acad Child Adolesc Psychiatry 1992;31:503–11. [DOI] [PubMed] [Google Scholar]
- 16.Fried PA, Makin JE. Neonatal behavioural correlates of prenatal exposure to marihuana, cigarettes and alcohol in a low risk population. Neurotoxicol Teratol 1987;9:1–7. [DOI] [PubMed] [Google Scholar]
- 17.Wakschlag LS, Hans SL. Maternal smoking during pregnancy and conduct problems in high-risk youth: a developmental framework. Dev Psychopathol 2002;14:351–69. [DOI] [PubMed] [Google Scholar]
- 18.Martin RP, Dombrowski SC, Mullis C, et al. Smoking during pregnancy: association with childhood temperament, behavior, and academic performance. J Pediatr Psychol 2006;31:490–500. [DOI] [PubMed] [Google Scholar]
- 19.DiClemente CC, Dolan-Mullen P, Windsor RA. The process of pregnancy smoking cessation: implications for interventions. Tob Control 2000;9(Suppl 3):iii16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kodl MM, Wakschlag LS. Does a childhood history of externalizing problems predict smoking during pregnancy? Addict Behav 2004;29:273–9. [DOI] [PubMed] [Google Scholar]
- 21.Wakschlag LS, Pickett KE, Middlecamp MK, et al. Pregnant smokers who quit, pregnant smokers who don’t: does history of problem behavior make a difference? Soc Sci Med 2003;56:2449–60. [DOI] [PubMed] [Google Scholar]
- 22.Krueger RF, Caspi A, Moffitt TE. Epidemiological personology: the unifying role of personality in population-based research on problem behaviors. J Pers 2000;68:967–98. [DOI] [PubMed] [Google Scholar]
- 23.Dex S, Joshi H, eds. Children of the 21st century: from birth to nine months. Bristol: Policy Press, 2005. [Google Scholar]
- 24.Smith K, Joshi H. The Millennium Cohort Study. Popul Trends 2002;(107):30–4. [PubMed] [Google Scholar]
- 25.Kiernan K, Pickett KE. Marital status disparities in maternal smoking during pregnancy, breastfeeding and maternal depression. Soc Sci Med 2006;63:335–46. [DOI] [PubMed] [Google Scholar]
- 26.Carey WB. Measuring infant temperament. J Pediatr 1972;81:414. [DOI] [PubMed] [Google Scholar]
- 27.Carey WB, McDevitt SC. Revision of the Infant Temperament Questionnaire. Pediatr 1978;61:735–9. [PubMed] [Google Scholar]
- 28.Qualifications and Curriculum Authority. National vocational qualifications. [6 February 2007]; available from: http://www.qca.org.uk/610.html
- 29.Office for National Statistics. The national statistics socio-economic classification: user manual. London: Palgrave McMillan, 2005. [Google Scholar]
- 30.Bates J, Bayles K, Bennett D, et al. Origins of externalizing behavior at eight years of age. In: Pepler D, Rubin K, eds. The development and treatment of childhood aggression. Hillsdale, NJ: Erlbaum, 1991. [Google Scholar]
- 31.Shaw DS, Keenan K, Vondra JI. Developmental precursors of externalizing behavior: ages 1 to 3. Dev Psychol 1994;30:355–64. [Google Scholar]
- 32.Krueger RF, Caspi A, Moffitt TE. Epidemiological personology: the unifying role of personality in population-based research on problem behaviors. J Pers 2000;68:967–98. [DOI] [PubMed] [Google Scholar]
- 33.Burt S, McGue M, Krueger R, et al. Sources of covariation among the child externalizing disorders: informant effects and shared environment. Psychol Med 2005;35:1133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matheny A, Wilson R, Thoben A. Home and mother: relations with infant temperament. Dev Psych 1987;23:323–31. [Google Scholar]
- 35.Tremblay RE, Nagin DS, Seguin JR, et al. Physical aggression during early childhood: trajectories and predictors. Pediatrics 2004;114:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pickett KE, Wakschlag LS, Dai L, et al. Fluctuations of maternal smoking during pregnancy. Obstet Gynecol 2003;101:140–7. [DOI] [PubMed] [Google Scholar]
- 37.Klebanoff MA, Levine RJ, Morris CD, et al. Accuracy of self-reported cigarette smoking among pregnant women in the 1990s. Paediatr Perinat Epidemiol 2001;15:140–3. [DOI] [PubMed] [Google Scholar]
- 38.Pickett KE, Rathouz PJ, Kasza K, et al. Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy. Paediatr Perinat Epidemiol 2005;19:368–76. [DOI] [PubMed] [Google Scholar]
- 39.Pickett KE, Wakschlag LS, Rathouz PJ, et al. The working-class context of pregnancy smoking. Health Place 2002;8:167–75. [DOI] [PubMed] [Google Scholar]


