Summary
Classical Hodgkin lymphoma (cHL) is characterised by malignant Hodgkin Reed–Sternberg cells located in an inflammatory microenvironment. Blood biomarkers result from active cross‐talk between malignant and non‐malignant cells. One promising biomarker in adult patients with cHL is thymus and activation‐regulated chemokine (TARC). We investigated TARC as marker for interim and end‐of‐treatment response in paediatric cHL. In this multicentre prospective study, TARC levels were measured among 99 paediatric patients with cHL before each cycle of chemotherapy and were linked with interim and end‐of‐treatment remission status. TARC levels were measured by enzyme‐linked immunosorbent assay. At diagnosis, TARC levels were elevated in 96% of patients. Plasma TARC levels declined significantly after one cycle of chemotherapy (p < 0.01 vs. baseline) but did not differ at interim assessment by positron emission tomography (p = 0.31). In contrast, median plasma TARC at end of treatment was significantly higher in three patients with progressive disease compared to those in complete remission (1.226 vs. 90 pg/ml; p < 0.001). We demonstrate that, in paediatric patients, plasma TARC is a valuable response marker at end‐of‐treatment, but not at interim analysis after the first two chemotherapy cycles. Further research is necessary to investigate TARC as marker for long‐term progression free survival.
Keywords: lymphoma biomarker, Paediatric Hodgkin lymphoma, response marker, TARC, thymus and activation‐regulated chemokine
INTRODUCTION
Paediatric classical Hodgkin lymphoma (cHL) is currently a highly curable disease with an overall survival of >90%. 1 , 2 Still, 10%–15% of patients experience relapse or progressive disease. 3 , 4 , 5 Moreover, in paediatric patients, long‐term toxicities such as cardiac toxicity, are of great concern. 6 , 7 , 8 The challenge remains to tailor treatment to eradicate malignancy with minimal side‐effects and to identify those patients who will need treatment intensification as early as possible.
In paediatric cHL, chemotherapy intensity is based on initial staging, the presence of B‐symptoms, E‐lesions, bulky disease, and erythrocyte sedimentation rate (ESR) level at diagnosis. Radiotherapy (RT) can be omitted depending on the response to chemotherapy, which is assessed by combined fluorodeoxyglucose‐positron emission tomography with computed tomography (FDG‐PET/CT) after the first two cycles of chemotherapy. 2 PET/CT has a high negative predictive value; however, its positive predictive value (PPV) is modest. 9 , 10 Other disadvantages of using PET/CT are exposure to radiation, time consumption, and high costs. 11 Due to the lack of fully accurate predictive factors, there is a medical need to identify prognostic (bio)markers.
Blood biomarkers hold promise to be practical, patient‐friendly and cost‐effective, with a high reproducibility and comparability between laboratories. They result from the communication of Hodgkin Reed–Sternberg (HRS) cells and the tumour microenvironment (TME) and therefore potentially reflect lymphoma viability. 12 , 13 They could be used as serial markers before, during and after treatment to determine early treatment response and to detect disease recurrence after end of treatment (EOT). However, such markers must be at least as specific and sensitive as FDG‐PET imaging.
One biomarker reported in adult patients with cHL is thymus and activation‐regulated chemokine (TARC, also termed CCL17). 14 , 15 , 16 , 17 TARC is produced by HRS cells and antigen‐presenting cells and attracts T‐helper type 2 cells. 18 We recently demonstrated that TARC is a sensitive and specific marker for the diagnosis of paediatric cHL. 19 Additionally, we established normal values for TARC in paediatric patients and set a cut‐off level for elevation of TARC in paediatric patients. 19 In adult patients with cHL, TARC is also correlated with treatment response. 15 , 16 , 17 , 20 , 21 High post‐therapy TARC levels were associated with poorer survival, 22 whereas early reduction of TARC after one cycle of chemotherapy was associated with treatment success. 20 Finally, Plattel et al. 23 concluded in 2020 that the PPV of interim TARC levels was higher than the PPV of the interim PET scan (iPET) after two cycles of chemotherapy.
One biomarker reported in adult cHL patients is thymus and activation‐regulated chemokine (TARC, also termed CCL17). 14 , 15 , 16 , 17 TARC is produced by HRS cells and antigen‐presenting cells and attracts T‐helper type 2 cells. 18 We recently demonstrated that TARC is a sensitive and specific marker for the diagnosis of paediatric cHL. 19 Additionally, we established normal values for TARC in paediatric patients and set a cut‐off level for elevation of TARC in paediatric patients. 19 In adult cHL patients, TARC is also correlated with treatment response. 15 , 16 , 17 , 20 , 21 High post‐therapy TARC levels were associated with poorer survival, 22 whereas early reduction of TARC after one cycle of chemotherapy was associated with treatment success. 20 Finally, Plattel et al. concluded in 2020 that the positive predictive value (PPV) of interim TARC levels was higher than the PPV of the interim PET‐scan after two cycles of chemotherapy (iPET). 23
To our knowledge, TARC levels during treatment in paediatric patients with cHL have not yet been reported. We therefore analysed serial TARC levels before, during and after treatment, and compared them with treatment response determined by radiological and pathological assessment in a large cohort of paediatric patients with cHL.
METHODS
Study population
We conducted a prospective, multicentre study in the Erasmus Medical Centre—Sophia Children's Hospital (EMC—Sophia) in Rotterdam, the Princess Máxima Centre for Paediatric Oncology in Utrecht, and the Amsterdam University Medical Centre, location VUmc, in Amsterdam (all in the Netherlands). Patients were enrolled from 1 November 2016 until 1 January 2022. As there are no studies of TARC as response marker in children reported, this is a pilot study to investigate if there is an association between TARC and treatment response. A total of 99 paediatric patients with cHL who finished treatment were included; baseline TARC data for 47 of these patients have been reported previously. 19 For the description of inclusion and exclusion criteria please refer to that report. 19
Baseline characteristics including age, sex, presence of B‐symptoms, ESR, C‐reactive protein, stage, bulky disease and E‐lesions were collected. All patients and/or parents or guardians provided written informed consent according to national laws. This study is Institutional Review Board‐approved and registered under Dutch Trial registry number 6876.
Initial staging and treatment and response assessment
The staging procedures were described in our previously published report. 19 All patients received two cycles of OEPA chemotherapy (containing prednisolone, vincristine, doxorubicin and etoposide). After these cycles an iPET was performed. For all sites with visibly enhanced FDG‐uptake the Deauville score was determined. Deauville scores were defined as (i) no uptake above background, (ii) uptake equal to or lower than mediastinum, (iii) uptake between mediastinum and liver uptake, (iv) uptake moderately increased compared to the liver and (v) uptake markedly increased compared to the liver. 24 , 25 The cut‐off for PET positivity was set at Deauville score of ≥4. Patients were classified as partial metabolic remission (PMR) when they had any sites of Deauville uptake of ≥4. Patients with lower Deauville scores were defined as complete metabolic remission (CMR).
After two cycles of OEPA chemotherapy, patients continued with one, two or four cycles of consolidation polychemotherapy. In most patients RT could be omitted, based on their response to treatment, i.e., patients with a CMR by iPET. When chemotherapy was finished, radiological assessment was repeated: patients with partial remission at interim analysis were assessed with a PET and magnetic resonance imaging (MRI) scan and patients with complete remission (CR) at interim analysis were assessed with MRI or CT. Patients were divided in two subgroups at EOT; CR or no CR. No CR was defined as refractory disease or progressive disease/relapse. Progression or relapse was suspected if at least one initially involved mass increased by >25% compared to the best previous response or when new lymphatic or extra‐lymphatic lesions occurred. If there were signs of progression or relapse found by radiological assessment, additional investigations and biopsy were necessary to confirm this.
Sample collection and enzyme‐linked immunosorbent assay
Plasma and serum TARC samples were collected at diagnosis, prior to the commencement of every subsequent cycle of chemotherapy, prior to the commencement of RT and at the end of all treatment. As patients received one, two or four cycles of consolidation chemotherapy depending on their extent of disease, and as not all patients received RT, we did not obtain the same amount of TARC samples from all patients. Patients with one cycle of consolidation chemotherapy and no RT had four time‐points (TPs) of TARC available (TP1, TP2, TP3, TP8), patients with one cycle of consolidation chemotherapy and additional RT had five TPs available (TP1, TP2, TP3, TP4, TP8), patients with two cycles of consolidation chemotherapy and no RT had five TPs available (TP1, TP2, TP3, TP4, TP8), patients with two cycles of consolidation chemotherapy and additional RT had six TPs available (TP1, TP2, TP3, TP4, TP5, TP8), patients with four cycles of consolidation chemotherapy and no RT had seven TPs available (TP1, TP2, TP3, TP4, TP5, TP6, TP8), and finally patients with four cycles of consolidation chemotherapy and additional RT had eight TPs available (TP1, TP2, TP3, TP4, TP5, TP6, TP7, TP8). For all patients TP1 was taken before start of treatment, TP3 before start of the third chemotherapy cycle (at time of iPET) and TP8 was taken after finishing treatment (including RT). The procedure of sampling and analysis is described in our previous report. 19 TARC values of >875 pg/ml at diagnosis were considered elevated. 19
Outcome
The outcome measures of this study were as follows: (i) association of pre‐treatment TARC levels with established prognostic risk factors, (ii) evaluate TARC levels to predict treatment response, and (iii) TARC levels as an indicator for remission status at the EOT.
Statistical analyses
Statistical inter‐group comparison of categorical variables was done using Pearson's chi‐squared test or Fisher's exact test (in case of sparse data). A rank‐sum test was used to compare continuous non‐normally distributed variables. TARC levels were compared to iPET results and to the results of the radiological evaluation and pathological outcome at end of therapy. We analysed the relative change of TARC response between TPs by calculating the difference between TARC values between TP1 (baseline) and TP3 (iPET) and for the period between TP3 and TP8 (EOT). For binary outcome, logistic regression was used to assess a potential association. The area under the curve (AUC) of TARC values from initiation until termination of treatment and from interim assessment until termination was used as a single overall response feature during the clinical course. A p < 0.05 was considered statistically significant. Data analysis was performed with Stata 17.1 SE for Windows (StataCorp.).
RESULTS
Patient characteristics
In total, 99 patients with newly diagnosed cHL who finished treatment were included. Baseline characteristics of these patients are summarised in Table 1. The median (range) age was 14 (6–17) years and there were slightly more females (n = 52 [52.5%]), than males.
TABLE 1.
Patients' baseline characteristics
| Characteristic | Value |
|---|---|
| Number of patients | 99 |
| Age, years, median (range) | 14 (6–17) |
| Male, n (%) | 47 (47.5) |
| B‐symptoms, n (%) | |
| Fever | 17 (17.2) |
| Drenching night sweats | 31 (31.3) |
| Weight loss | 12 (12.1) |
| ESR >30 mm/h | 66 (66.7) |
| Stage, n (%) | |
| 1 | 0 (0.0) |
| 2 | 37 (37.4) |
| 3 | 33 (33.3) |
| 4 | 29 (29.3) |
| E‐lesions, n (%) | 19 (19.4) |
| Bulky disease, n (%) | 38 (38.4) |
| Radiotherapy, n (%) | 27 (27) |
Plasma TARC at diagnosis in patients with cHL
Plasma TARC in newly diagnosed patients was measured at diagnosis (TP1) in 96 patients. In three patients, TARC samples were taken after the start of chemotherapy, and therefore these values were excluded. The median (range) value was 14 073 (139–142 276) pg/ml. Plasma TARC was elevated (≥875 pg/ml) in 96/99 (97%) of patients. The plasma TARC levels at baseline were significantly higher in patients with an ESR of >30 mm/h (p = 0.01), with E‐lesions (p = 0.001), with bulky disease (p < 0.001) and with B‐symptoms (p = 0.02). The plasma TARC levels were not correlated with staging (p = 0.50). TARC data in serum were comparable with plasma.
TARC levels and treatment response at iPET
The TARC levels were measured before each cycle of chemotherapy (Figure 1). As patients received one, three or four cycles of consolidation chemotherapy and some patients received also additional RT, the number of TARC samples per patients differed. Table 2 shows the number of TARC samples available per TP and the missing cases.
FIGURE 1.
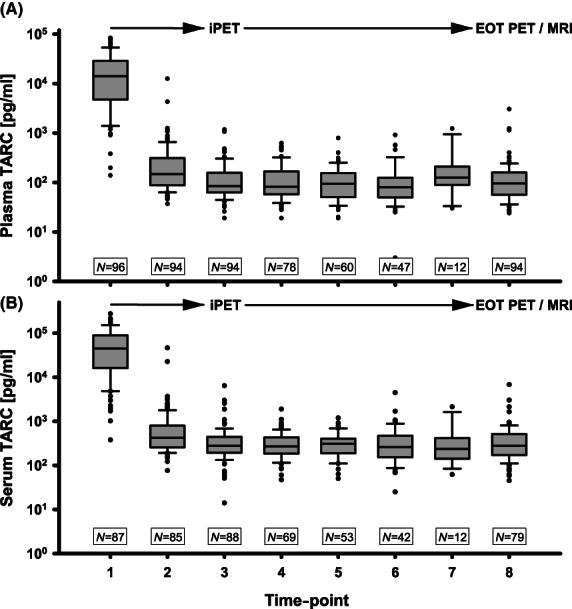
Thymus and activation‐regulated chemokine (TARC) levels at diagnosis and during treatment. (A) Serial plasma TARC levels at diagnosis and during treatment; time‐point 1 (TP1) to TP8 (end of treatment). (B) Serial serum TARC levels. EOT, end of treatment; MRI, magnetic resonance imaging; (i)PET, (interim) positron emission tomography.
TABLE 2.
Number of thymus and activation‐regulated chemokine samples available per time‐point
| TP1 | TP2 | TP3 | TP4 | TP5 | TP6 | TP7 | TP8 | |
|---|---|---|---|---|---|---|---|---|
| No. of samples | 96 | 94 | 94 | 78 | 60 | 47 | 12 | 94 |
| No. of missing | 3 | 3 | 3 | 9 | 7 | 7 | 1 | 3 |
| No. of NA | 0 | 2 | 2 | 12 | 32 | 43 | 88 | 2 |
Notes: A TP is not applicable when a patient does not receive therapy at that TP as described in the methods section. One patient died at the beginning of treatment, the samples of this patient from TP2–T8 are registered as NA in this table.
Samples were categorised as missing when they were not taken or taken after the start of the chemotherapy cycle. All samples were randomly missing; most patients had one TP missing, three patients had three TPs missing and one patient had six TPs missing.
Abbreviations: NA, not applicable; No., number; TP, time‐point.
The iPET after two cycles of chemotherapy (at TP3) was performed in 98 out of 99 patients. One patient passed away during treatment due to complications of the chemotherapy, and therefore TARC values or radiological results were not available from TP2 to TP8.
In all, 56 (57%) patients achieved CMR at iPET and 42 (43%) achieved PMR.
Plasma TARC samples were available at baseline in 96 patients, and at TP2 and TP3 in 94 patients. In all the patients, TARC levels had already decreased after one cycle of chemotherapy (TP2) (p < 0.01; Figure 1). At TP2, the median (range) value of TARC was 148 (37–12 535) pg/ml (n = 99). After two cycles of chemotherapy (TP3), the median (range) TARC value was 85 (19–1174) pg/ml (n = 99). In 94 patients, data of both TARC at TP3 and the results of the iPET were available; patients with CMR (n = 55) had a median (range) value of 77 (32–469) pg/ml compared to a median (range) TARC value of 101 (19–1174) pg/ml in patients with PMR (n = 39) (p = 0.31; Figure 2). Logistic regression for iPET and TARC at TP3 showed an odds ratio (OR) of 1.00 (95% confidence interval [CI] 0.99–1.00, p = 0.1). There was also no statistical difference in the relative change of TARC values between TP1 and TP3 between patients with CMR and PMR status on PET scan (98.9% vs. 99.3%, p = 0.44). Data in serum were comparable.
FIGURE 2.
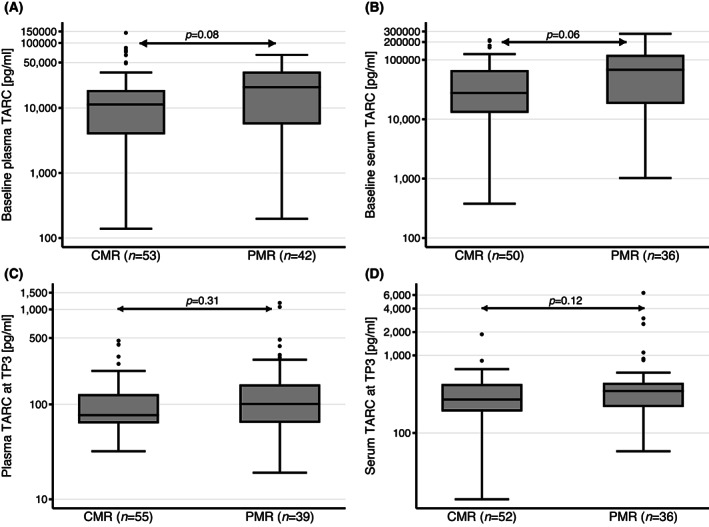
Thymus and activation‐regulated chemokine (TARC) levels at baseline (time‐point 1 [TP1]) and TP3 associated with the remission status on interim PET (iPET) scan. (A) Plasma TARC levels at baseline (TP1) were not associated with remission status on iPET scan (p = 0.08). Logistic regression for iPET and plasma TARC at TP1 showed also no meaningful association with an odds ratio (OR) of 1.00 (95% confidence interval [CI] 0.99–1.00, p = 0.49). (B) Serum TARC levels at TP1 were not associated with remission status on iPET scan (p = 0.06; OR 1.00, 95% CI 1.00–1.00, p = 0.03). (C) Plasma TARC levels at TP3 were not associated with remission status on iPET scan (p = 0.31). Logistic regression for iPET and plasma TARC at TP3 showed also no meaningful association with an OR of 1.00 (95% CI 0.99–1.00, p = 0.1). (D) Serum TARC levels at TP3 were not associated with remission status on iPET scan (p = 0.12; OR 1.00, 95% CI 0.99–1.00, p = 0.1). CMR, complete metabolic remission; PMR, as partial metabolic remission.
We investigated a potential association of TARC levels at diagnosis (baseline TARC at TP1) with the result of the iPET. TARC levels at TP1 did not statistically significantly differ by iPET status: patients with CMR showed a median (range) baseline TARC of 11 336 (139–142 276) pg/ml compared to 20 841 (197–65 545) pg/ml for patients with PMR (p = 0.09) (Figure 2). An additional logistic regression failed to demonstrate a significant association with an OR of 1.00 (95% CI 0.99–1.00, p = 0.49). Also, the receiver operating characteristic analysis showed insufficient discrimination with an AUC of 0.60 (95% CI 0.48–0.72). Data in serum showed no significant association as well.
TARC levels associated with treatment response at EOT
A total of 98 patients finished treatment at time of analysis and had radiological assessment and TARC analysis, data of one patient were missing as this patient died during treatment. In all, 91 patients were in CR at the EOT‐radiological assessment. In seven patients radiological imaging was suspicious for progressive disease. This was confirmed by biopsy in three patients. The other four patients did not receive a biopsy and were instead reassessed radiologically after 3 months and did not show any signs of progressive disease anymore. Thus, three of 95 patients had biopsy‐confirmed progressive disease. Two of these patients showed PMR at their iPET and one patient CMR. TARC values were analysed at EOT (TP8) and were associated with the pathology results. The median (range) TARC values at TP8 (n = 94) were 90 (24–400) pg/ml in patients with CR and 1226 (1135–3041) pg/ml in the three patients with progressive disease (p < 0.001; Figure 3); all three patients with progressive disease had a TARC value at EOT of >1000 pg/ml. In these three patients, TARC levels first normalised after the start of chemotherapy and then rose again between TP3 and TP8 (Figure 3). The ‘distance’ between the lowest TARC for a patient with progressive disease and the highest TARC for a patient with CR was several 100 units of TARC. The relative change of plasma TARC between TP3 and EOT was also significantly different between the two groups; the median TARC changed only 3.1% for patients in CR compared with 187%–330% for patients with progressive disease (p = 0.002).
FIGURE 3.
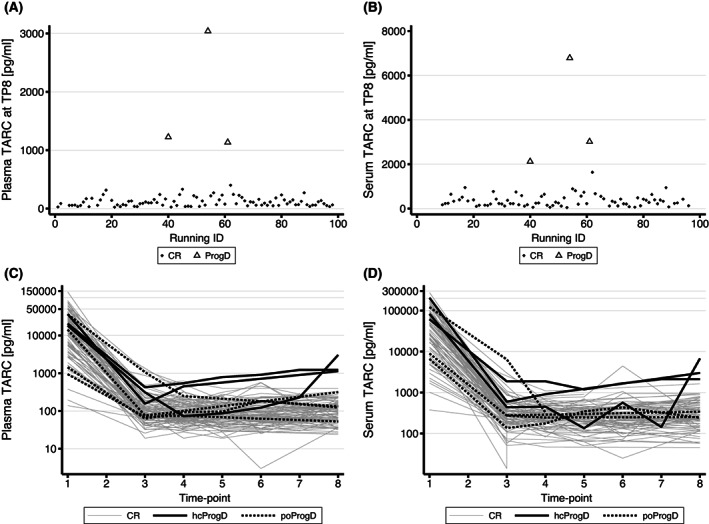
Thymus and activation‐regulated chemokine (TARC) levels at end of treatment (EOT) for patients with complete remission (CR) versus progressive disease (ProgD) based on pathological confirmation. (A) Plasma TARC levels at EOT for patients with CR versus ProgD. (B) Serum TARC levels at EOT for patients with CR versus ProgD. (C) All available serial plasma TARC values for patients. (D) All available serial serum TARC values for all patients. Grey lines represent patients in CR, the black lines represent the patients with histologically confirmed ProgD (hcProgD) and the dashed lines represent the patients with suspected ProgD at radiological assessment, but not histologically confirmed (poProgD). All patients with ProgD have a clear increase of TARC, compared to the patients in CR. Patients with a false positive radiological outcome, show no increase of TARC.
Serum TARC values (TP8) also differed by pathological outcome; the median (range) serum TARC values were 255 (45–1640) pg/ml in patients with CR and 3018 (2122–6789) pg/ml in patients with progressive disease (p < 0.001). However, the ‘distance’ between the lowest serum TARC value for a patient with progressive disease and the highest serum TARC for a patient in CR was rather small compared to the plasma TARC values (Figure 3).
Based on the currently low number of patients with progressive disease, the identification of early treatment failure (before TP8) was not feasible yet.
Both plasma TARC and serum TARC were found to be a more specific marker with a much higher PPV for progressive disease than the radiology assessment. There were seven patients with signs of progressive disease on radiology assessment at EOT. However, only three of them had confirmed progressive disease, confirmed with additional investigations and biopsy, and therefore four patients were false positive at radiological assessment. The sensitivity of radiological assessment was therefore 100%, the specificity 95.8% and the PPV 42.9%. Plasma TARC had a 100% sensitivity, specificity and PPV in our sample using a threshold of >1000 pg/ml.
There were 27 patients who received RT, after completing chemotherapy cycles. All these patients already had normalised TARC levels before starting RT. One of the patients with progressive disease at EOT received RT, this patient showed a TARC level of 229 pg/ml before starting RT. The other two patients with progressive disease did not receive RT.
Early TARC reduction associated with treatment response at EOT
We also investigated the association between early plasma TARC reduction (after one cycle of chemotherapy) and treatment outcome at iPET and EOT. Logistic regression of iPET on TARC levels at TP2 showed an OR of 1.00 (95% CI 0.99–1.00, p = 0.21). Logistic regression of iPET on the relative change of TARC from TP1 to TP2 resulted in an OR of 1.8 (95% CI 0.06–51.1, p = 0.73). The logistic regression of the EOT‐radiological outcome on the absolute and relative change of TARC from TP1 to TP2 also showed no significant association (OR 0.99, 95% CI 0.99–1.00, p = 0.69; and OR 0.84, 95% CI 0.001–572, p = 0.95 respectively). Data in serum were comparable.
Area under the curve of TARC values as a single response feature and clinical outcome
Finally, we investigated if the AUC of TARC levels as a single summary response feature was correlated with clinical outcome. There was no statistically significant difference in the AUC of TARC from TP1 to EOT, when stratified by outcome. We hypothesised that the period between iPET (TP3) and EOT might play a more important role, as iPET showed no signs of progressive disease. We differentiated between patients who received two cycles of consolidation chemotherapy and patients who received four cycles of consolidation chemotherapy, as the patients with four cycles had got more TPs of TARC available. For patients who received two consolidation chemotherapy cycles, we then included patients with complete TARC profiles for TP3, TP4 and TP8. The one patient with pathologically confirmed progressive disease had a much higher AUC for TARC of 3476 compared to an AUC of 647 (range 170–2601, n = 26) for patients with CR at EOT. For patients who received four cycles of consolidation chemotherapy, we then included patients with complete TARC profiles for TP3, TP5 and TP8. The two patients with pathologically confirmed progressive disease showed a significantly higher AUC of 4665 (range 4240–5091) pg/ml for plasma TARC compared to an AUC of 580 (range 121–1726) pg/ml for patients (n = 49) with CR at EOT.
DISCUSSION
This TARC analysis is unique, as to the best of our knowledge it is the first study performed in paediatric patients with cHL. There is increasing evidence for TARC as a response marker in adult patients with cHL. 14 , 15 , 23 Because adult and paediatric cHL differ partly with respect to histology, clinical presentation, staging and prognosis, 26 , 27 , 28 it is important that TARC as a response marker is evaluated in a paediatric cohort. Additionally, for a pilot study the size of this study is adequate, considering the lower incidence of cHL in the paediatric population compared to the adult population. In this study, we showed that TARC is a reliable marker for early progression at EOT in paediatric patients with cHL. We also found that TARC is a more specific marker for such early progression at EOT than the radiology assessment. At the same time, TARC appeared not to be helpful in evaluating induction response.
In our study, TARC levels were elevated in 96% of paediatric patients with primary diagnosis of cHL, which makes TARC a very suitable biomarker for cHL. We demonstrated that TARC levels already drop significantly after one cycle of chemotherapy. This is consistent with the studies of Plattel et al. 16 , 21 in adult patients with cHL.
Guidetti et al. 20 did find an association with TARC levels and interim remission status in adult patients with cHL. Viviani et al. 29 found an association between early reduction of TARC and treatment response. Plattel et al. 23 found an association between interim TARC levels and treatment outcome. We could not confirm these findings in our study. It is unclear if these differences between paediatric and adult patients are caused by pathophysiological differences between these groups or due to extrinsic factors. Possible explanations for this could be differences in therapy; paediatric patients receive two cycles of OEPA chemotherapy, whereas adult patients mostly receive ABVD cycles (adriamycin, bleomycin, vinblastine, and dacarbazine). Secondly, it could be possible that FDG‐PET avidity at iPET is partly caused by inflammation due to a more active immune system in children and not by Hodgkin activity, therefore resulting in low TARC levels. 30 , 31 Thirdly, paediatric and adult cHL differ also biologically. 27 , 28 , 32 Taking this together, more studies with a larger sample size of paediatric patients are necessary to draw definite conclusion about this.
The TARC levels at EOT were associated with loss of remission status. As there were only three patients with progressive disease at EOT in this study, a larger series is needed to confirm that TARC can be used as a marker for progressive disease. However, our findings are in line with the data of adult patients and therefore it is plausible that TARC is a marker for treatment response in the paediatric population as well. 14 , 16 , 23 In our study, TARC was found to be more predictive for loss of response than radiology assessment. This is in line with studies in adult patients. Plattel et al. 23 found a concordance of response on iPET and TARC levels in 87% of adult patients with cHL. They also found that TARC‐based response evaluation provided an improved PPV for 5‐year progression‐free survival and similarly, an improved negative predictive value compared to PET‐based response evaluation. Hsi et al. 23 found that TARC is the best prognostic factor for progression‐free survival. 33 , 34 These results could be explained by the fact that PET scans have a high false positive rate.
Consistent with adult studies, TARC levels in paediatric patients with cHL were associated with bulky disease 16 , 20 , 35 and with B‐symptoms and ESR. 16 , 17 An association with disease stage was not found unlike results from most studies in adults. 16 , 20 , 22 However, these results were in line with our previous study in paediatric patients. 19 In the present study and in our previous study, there were no patients with Stage 1 disease included. Moreover, 25 of the 37 patients with Stage II disease in this study had either B‐symptoms, bulky disease, E‐lesion or elevated ESR. This might contribute to the fact that we did not find an association of TARC levels with disease stage.
This study demonstrates that both plasma and serum can be used to evaluate TARC in paediatric patients with cHL. Outcomes of plasma TARC and serum TARC in this study were mostly comparable. However, for the patients with progressive disease versus those with CR at EOT, plasma TARC was more discriminative than serum TARC. Therefore, we recommend using plasma TARC for disease monitoring.
A limitation of this study is the small number of cases with progressive disease. This is due to the low incidence of progressive disease in the paediatric population. Larger powered studies are necessary to draw conclusions about the value of TARC as a marker for long‐term progression‐free survival. Studies in adult patients also show that there is a correlation with TARC and the 5‐year progression‐free survival. 14 , 23
With the outcome of this study and the studies in adult patients in mind, we suggest that TARC measurement should be added to the routine assessment in paediatric patients with cHL at diagnosis and during treatment. The costs of TARC measurements are much lower than radiological assessment; one TARC measurement costs ~€28 versus ~€1200 for a PET‐scan and ~€700 for a full‐body MRI. Many hospitals can perform TARC analysis, as it is also used as biomarker for other diseases, among which atopic dermatitis. 18 However, if TARC analysis is not possible at a hospital, plasma can be sent easily to a neighbouring hospital for analysis. The plasma samples should be centrifuged by the local laboratory and transported to the hospital that can perform TARC analysis. When TARC levels increase above 1000 pg/ml after a previous decrease, further research would be recommended. On the other hand, a low TARC with a suspicious radiological outcome, without clinical signs of a relapse, could justify a wait‐and‐see policy. Further research is necessary to decide if TARC can replace radiological assessment, which would drastically decrease costs. There are no data in paediatric patients on TARC levels during follow‐up, so therefore we cannot draw precise conclusions on the interpretation and recommendations of TARC assessment during follow‐up. However, we suggest that TARC measurements should also be added to the routine surveillance during follow‐up, with the same recommendation as during treatment.
We found that TARC is a marker for outcome at EOT in children with cHL. Because almost all children have elevated TARC levels at diagnosis, this makes TARC a very suitable biomarker. Future studies in paediatric cHL should further explore the reliability of TARC levels and FDG‐PET imaging in relation to long‐term treatment outcomes. Moreover, it could be worthwhile to study TARC as a follow‐up marker for early detection of relapse instead of radiological assessments.
AUTHOR CONTRIBUTIONS
Auke Beishuizen, Friederike A. G. Meyer‐Wentrup and C. Michel Zwaan conceived the project and provided leadership. Eline A. M. Zijtregtop, M.V., Friederike A. G. Meyer‐Wentrup. and Auke Beishuizen organised the study. Claudius Diez and Eline A. M. Zijtregtop analysed the data and contributed to the manuscript; Claudius Diez made the figures. Eline A. M. Zijtregtop wrote the manuscript, Claudius Diez, Friederike A. G. Meyer‐Wentrup and Auke Beishuizen supervised. All authors reviewed the manuscript and accepted the contents of the article.
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose.
ACKNOWLEDGEMENT
This work was financially supported by the Ferenc Foundation and the Erasmus MC Foundation, enabled by a legacy of the family Etienne‐van Dijk. We would like to thank them both. The funding source was not involved on the study design, data collection, data analysis, interpretation of data, writing the report or the decision to submit the paper for publication. The visual abstract was created with BioRender.com
Zijtregtop EAM, Diez C, Zwaan CM, Veening MA, Beishuizen A, Meyer‐Wentrup FAG. Thymus and activation‐regulated chemokine (TARC) as treatment response marker for paediatric Hodgkin lymphoma: A pilot study. Br J Haematol. 2023;200(1):70–78. 10.1111/bjh.18473
Auke Beishuizen and Friederike A. G. Meyer‐Wentrup contributed equally.
DATA AVAILABILITY STATEMENT
Fully de‐identified data can be made available from the corresponding author on reasonable request. For these data, please contact a.beishuizen-2@prinsesmaximacentrum.nl.
REFERENCES
- 1. Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, et al. Cancer Statistics Review, 1975‐2014. Bethesda, MD: National Cancer Institute; 2017. https://seer.cancer.gov/archive/csr/1975_2014/ [Google Scholar]
- 2. Mauz‐Korholz C, Landman‐Parker J, Balwierz W, Ammann RA, Anderson RA, Attarbaschi A, et al. Response‐adapted omission of radiotherapy and comparison of consolidation chemotherapy in children and adolescents with intermediate‐stage and advanced‐stage classical Hodgkin lymphoma (EuroNet‐PHL‐C1): a titration study with an open‐label, embedded, multinational, non‐inferiority, randomised controlled trial. Lancet Oncol. 2022;23(1):125–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brenner H, Gondos A, Pulte D. Ongoing improvement in long‐term survival of patients with Hodgkin disease at all ages and recent catch‐up of older patients. Blood. 2008;111(6):2977–83. [DOI] [PubMed] [Google Scholar]
- 4. Reedijk AMJ, Zijtregtop EAM, Coebergh JWW, Meyer‐Wentrup FAG, Hebeda KM, Zwaan CM, et al. Improved survival for adolescents and young adults with Hodgkin lymphoma and continued high survival for children in The Netherlands: a population‐based study during 1990‐2015. Br J Haematol. 2020;189(6):1093–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. von Tresckow B, Moskowitz CH. Treatment of relapsed and refractory Hodgkin lymphoma. Semin Hematol. 2016;53(3):180–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aleman BM, van den Belt‐Dusebout AW, De Bruin ML, van't Veer MB, Baaijens MH, de Boer JP, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109(5):1878–86. [DOI] [PubMed] [Google Scholar]
- 7. Castellino SM, Geiger AM, Mertens AC, Leisenring WM, Tooze JA, Goodman P, et al. Morbidity and mortality in long‐term survivors of Hodgkin lymphoma: a report from the childhood cancer survivor study. Blood. 2011;117(6):1806–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Brien MM, Donaldson SS, Balise RR, Whittemore AS, Link MP. Second malignant neoplasms in survivors of pediatric Hodgkin's lymphoma treated with low‐dose radiation and chemotherapy. J Clin Oncol. 2010;28(7):1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barnes JA, LaCasce AS, Zukotynski K, Israel D, Feng Y, Neuberg D, et al. End‐of‐treatment but not interim PET scan predicts outcome in nonbulky limited‐stage Hodgkin's lymphoma. Ann Oncol. 2011;22(4):910–5. [DOI] [PubMed] [Google Scholar]
- 10. Hutchings M, Mikhaeel NG, Fields PA, Nunan T, Timothy AR. Prognostic value of interim FDG‐PET after two or three cycles of chemotherapy in Hodgkin lymphoma. Ann Oncol. 2005;16(7):1160–8. [DOI] [PubMed] [Google Scholar]
- 11. Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, et al. Early interim 2‐[18F]fluoro‐2‐deoxy‐D‐glucose positron emission tomography is prognostically superior to international prognostic score in advanced‐stage Hodgkin's lymphoma: a report from a joint Italian‐Danish study. J Clin Oncol. 2007;25(24):3746–52. [DOI] [PubMed] [Google Scholar]
- 12. Kuppers R, Engert A, Hansmann ML. Hodgkin lymphoma. J Clin Invest. 2012;122(10):3439–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skinnider BF, Mak TW. The role of cytokines in classical Hodgkin lymphoma. Blood. 2002;99(12):4283–97. [DOI] [PubMed] [Google Scholar]
- 14. Hsi ED, Li H, Nixon AB, Schoder H, Bartlett NL, LeBlanc M, et al. Serum levels of TARC, MDC, IL‐10, and soluble CD163 in Hodgkin lymphoma: a SWOG S0816 correlative study. Blood. 2019;133(16):1762–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jones K, Vari F, Keane C, Crooks P, Nourse JP, Seymour LA, et al. Serum CD163 and TARC as disease response biomarkers in classical Hodgkin lymphoma. Clin Cancer Res. 2013;19(3):731–42. [DOI] [PubMed] [Google Scholar]
- 16. Plattel WJ, Alsada ZN, van Imhoff GW, Diepstra A, van den Berg A, Visser L. Biomarkers for evaluation of treatment response in classical Hodgkin lymphoma: comparison of sGalectin‐1, sCD163 and sCD30 with TARC. Br J Haematol. 2016;175(5):868–75. [DOI] [PubMed] [Google Scholar]
- 17. Sauer M, Plutschow A, Jachimowicz RD, Kleefisch D, Reiners KS, Ponader S, et al. Baseline serum TARC levels predict therapy outcome in patients with Hodgkin lymphoma. Am J Hematol. 2013;88(2):113–5. [DOI] [PubMed] [Google Scholar]
- 18. Zijtregtop EAM, van der Strate I, Beishuizen A, Zwaan CM, Scheijde‐Vermeulen MA, Brandsma AM, et al. Biology and clinical applicability of plasma thymus and activation‐regulated chemokine (TARC) in classical Hodgkin lymphoma. Cancers (Basel). 2021;13(4):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zijtregtop EAM, Meyer‐Wentrup F, Wong W‐C, Hoogendijk R, Lopez‐Yurda M, Zwaan CM, et al. Plasma thymus and activation‐regulated chemokine (TARC) as diagnostic marker in pediatric Hodgkin lymphoma. eJHaem. 2020;1(1):152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guidetti A, Mazzocchi A, Miceli R, Paterno E, Taverna F, Spina F, et al. Early reduction of serum TARC levels may predict for success of ABVD as frontline treatment in patients with Hodgkin lymphoma. Leuk Res. 2017;62:91–7. [DOI] [PubMed] [Google Scholar]
- 21. Plattel WJ, van den Berg A, Visser L, van der Graaf AM, Pruim J, Vos H, et al. Plasma thymus and activation‐regulated chemokine as an early response marker in classical Hodgkin's lymphoma. Haematologica. 2012;97(3):410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weihrauch MR, Manzke O, Beyer M, Haverkamp H, Diehl V, Bohlen H, et al. Elevated serum levels of CC thymus and activation‐related chemokine (TARC) in primary Hodgkin's disease: potential for a prognostic factor. Cancer Res. 2005;65(13):5516–9. [DOI] [PubMed] [Google Scholar]
- 23. Plattel WJ, Visser L, Diepstra A, Glaudemans A, Nijland M, van Meerten T, et al. Interim thymus and activation regulated chemokine versus interim (18) F‐fluorodeoxyglucose positron‐emission tomography in classical Hodgkin lymphoma response evaluation. Br J Haematol. 2020;190(1):40–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gallamini A, Fiore F, Sorasio R, Meignan M. Interim positron emission tomography scan in Hodgkin lymphoma: definitions, interpretation rules, and clinical validation. Leuk Lymphoma. 2009;50(11):1761–4. [DOI] [PubMed] [Google Scholar]
- 25. Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C. Report on the first international workshop on interim‐PET‐scan in lymphoma. Leuk Lymphoma. 2009;50(8):1257–60. [DOI] [PubMed] [Google Scholar]
- 26. Alexander FE, Jarrett RF, Lawrence D, Armstrong AA, Freeland J, Gokhale DA, et al. Risk factors for Hodgkin's disease by Epstein‐Barr virus (EBV) status: prior infection by EBV and other agents. Br J Cancer. 2000;82(5):1117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bazzeh F, Rihani R, Howard S, Sultan I. Comparing adult and pediatric Hodgkin lymphoma in the surveillance, epidemiology and end results program, 1988‐2005: an analysis of 21734 cases. Leuk Lymphoma. 2010;51(12):2198–207. [DOI] [PubMed] [Google Scholar]
- 28. Parkes SE, Coad NA, Muir KR, Jones TJ, Cameron AH, Mann JR. Hodgkin's disease in children in the west midlands, 1957‐1986: a large population‐based study. Pediatr Hematol Oncol. 1994;11(5):471–86. [DOI] [PubMed] [Google Scholar]
- 29. Viviani S, Mazzocchi A, Pavoni C, Taverna F, Rossi A, Patti C, et al. Early serum TARC reduction predicts prognosis in advanced‐stage Hodgkin lymphoma patients treated with a PET‐adapted strategy. Hematol Oncol. 2020;38(4):501–8. [DOI] [PubMed] [Google Scholar]
- 30. Linton PJ, Dorshkind K. Age‐related changes in lymphocyte development and function. Nat Immunol. 2004;5(2):133–9. [DOI] [PubMed] [Google Scholar]
- 31. Valiathan R, Ashman M, Asthana D. Effects of ageing on the immune system: infants to elderly. Scand J Immunol. 2016;83(4):255–66. [DOI] [PubMed] [Google Scholar]
- 32. Jarrett RF. Viruses and Hodgkin's lymphoma. Ann Oncol. 2002;13(Suppl 1):23–9. [DOI] [PubMed] [Google Scholar]
- 33. Andre MPE, Girinsky T, Federico M, Reman O, Fortpied C, Gotti M, et al. Early positron emission tomography response‐adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol. 2017;35(16):1786–94. [DOI] [PubMed] [Google Scholar]
- 34. Johnson P, Federico M, Kirkwood A, Fossa A, Berkahn L, Carella A, et al. Adapted treatment guided by interim PET‐CT scan in advanced Hodgkin's lymphoma. N Engl J Med. 2016;374(25):2419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cuccaro A, Annunziata S, Cupelli E, Martini M, Calcagni ML, Rufini V, et al. CD68+ cell count, early evaluation with PET and plasma TARC levels predict response in Hodgkin lymphoma. Cancer Med. 2016;5(3):398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Fully de‐identified data can be made available from the corresponding author on reasonable request. For these data, please contact a.beishuizen-2@prinsesmaximacentrum.nl.


