Abstract
Osteoporosis is a systemic bone‐resorbing disease that easily causes subsequent risk of fracture. Hence, the substantial physical burden of osteoporosis makes it an important public health issue. Seborrheic dermatitis (SD) is a chronic, recurrent, inflammatory skin disease. Despite the advances in medication for treating osteoporosis, identifying undiagnosed osteoporosis patients is still challenging. Since osteoporosis and SD share a similar pathobiology, e.g. inflammation and hormonal imbalance, we aimed to investigate whether the existence of SD increases osteoporosis risk by using the Taiwan National Health Insurance Research Database. A total of 7831 patients aged 18–50 years with SD and a control group of 31 324 patients without SD matched by age, gender, Charlson Comorbidity Index, and index date at a ratio of 1:4 during 1996–2010 were recruited in the study. To measure the cumulative incidence and compare the hazard ratios of osteoporosis between each group, the Kaplan–Meier method and Cox proportional hazard regression models were utilized. It was found that 0.98% of SD patients had osteoporosis. Compared to the non‐SD group, the SD group had a 5.95‐fold higher osteoporosis risk after adjustment for variables. The impact of SD on osteoporosis risk was largest in the female and young age groups. In addition, the presence of hyperlipidemia, hyperthyroidism, and epilepsy synergistically increased osteoporosis incidence in the SD group. This first large cohort study demonstrated an association between SD and osteoporosis. Since the effect on bone health in SD patients with concomitant diseases is largest in early life, diet or lifestyle recommendations as well as regular bone examinations are advised during follow‐up of SD.
Keywords: bone health, inflammation, osteoporosis, population, seborrheic dermatitis
1. INTRODUCTION
The substantial physical burden of osteoporosis makes its growing incidence in the general population an important public health issue. 1 Osteoporosis is a systemic bone‐resorbing disease that causes bone fragility and subsequent risk of fracture. 2 Globally, osteoporosis is the most prevalent bone disorder. 3 The disease is more common in females than in males, 4 and osteoporotic fracture occurs in more than half of all females older than 50 years. 5 The disease results from an osteoclast–osteoblast imbalance caused by hormonal deficiency and abnormal increase in systemic pro‐inflammatory cytokines. 6 Despite recent remarkable advances in medications for treating osteoporosis, 7 identifying undiagnosed osteoporosis patients is still challenging.
Seborrheic dermatitis (SD) is an inflammatory skin disease. Clinical manifestations include ill‐defined erythematous exfoliative scaling patches accompanied by variable pruritus. The disease is often chronic, and relapse is common. 8 The most common sites of SD, known as “seborrheic areas,” are the anterior chest, axilla, back, groin, central area of the face, and scalp. 9 , 10 Its incidence is highest in infants, adolescents, and adults aged 30–60 years. 11 The prevalence of SD is approximately 5% in adults but is even higher in immunocompromised individuals and in those with neurologic diseases. 12 Although its pathobiology has not been precisely determined, the most common etiology of SD is Malassezia yeast infection. Other potential causative factors include compromise of epidermal barrier integrity or skin microbiota composition, androgen or sebaceous activity, host immune response, and environmental changes. 13 Currently, SD is mainly treated with anti‐inflammatory medication and topical corticosteroid agents.
Since osteoporosis and SD share a similar pathobiology, e.g. inflammation and hormonal imbalance, the aim of this study was to use the Taiwan National Health Insurance Research Database (NHIRD) to investigate whether the existence of SD increases osteoporosis risk.
2. METHODS
2.1. Data sources
The Taiwan National Health Insurance (NHI) program implemented in March 1995 covers >99% of the 23.74 million residents of Taiwan. The NHIRD published by the National Health Research Institute is an encrypted secondary database containing all records for the NHI program. The NHIRD is a large‐sample sized database of real‐world evidence made available for use in medical research. This study was performed using the Longitudinal Health Insurance Databases (LHID) 2010, which contains data for 1 000 000 beneficiaries randomly sampled from the original NHIRD. All diseases were coded based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM).
2.2. Study population
This study recruited 7831 patients aged 18–50 years with SD defined as a record of ICD‐9‐CM codes 706.3 and 690.1 entered by a dermatologist during 1996–2010 in two or more consecutive ambulatory visits or in one or more inpatient visits. The index date was the date of the first SD diagnosis. Propensity score matching was used to match the SD group with a control group of 31 324 patients without SD by age, gender, Charlson Comorbidity Index (CCI), and index date at a ratio of 1:4.
2.3. Main outcome
Both the SD group and the non‐SD group (controls) were followed up until the date of the first osteoporosis diagnosis or until December 31, 2010. An osteoporosis diagnosis was defined as an ICD‐9‐CM code 733.0 entered by an orthopedic surgeon in two or more consecutive ambulatory visits or in one or more inpatient visits and at least one record of a bone mineral density examination. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21
2.4. Comorbidities
Baseline comorbidities identified as potential confounders in this study were diabetes mellitus (ICD‐9‐CM code 250), hyperlipidemia (ICD‐9‐CM code 272), hypertension (ICD‐9‐CM codes 401–405), chronic liver disease (ICD‐9‐CM codes 456, 571, and 572), hyperthyroidism (ICD‐9‐CM code 242), chronic kidney disease (ICD‐9‐CM codes 582, 583, 585, 586, and 588), chronic pulmonary disease (ICD‐9‐CM codes 490–496), depression (ICD‐9‐CM codes 296.2, 296.3, 300.4, and 311), stroke (ICD‐9‐CM codes 430–438), epilepsy (ICD‐9‐CM code 345), dementia (ICD‐9‐CM codes 290, 294.1, 331.0, and 331.2), and psoriasis (ICD‐9‐CM code 696.0, 696.1, and 696.8). The severity of comorbidities was classified into four levels according to the CCI: 0, 1–2, 3–4, and >5.
2.5. Statistical analysis
Chi‐square test and Student's t‐test were used as appropriate in comparisons of categorical and continuous variables of demographic characteristics between the SD and non‐SD groups. In each group, overall incidence rates specific to gender and age were estimated per 1000 person‐years. The cumulative incidence of osteoporosis was determined in each group using the Kaplan–Meier method and compared between groups using the log‐rank test. Cox proportional hazard regression models were used to compare hazard ratio (HRs) and 95% confidence intervals (CIs) for osteoporosis between the two groups with adjustments for age, gender, CCI, and relevant comorbidities (diabetes mellitus, hyperlipidemia, hypertension, chronic liver disease, hyperthyroidism, chronic kidney disease, chronic pulmonary disease, depression, stroke, epilepsy, dementia, and psoriasis) in the multivariable model. A P value <0.05 was considered statistically significant. Statistical Analysis Software 9.4 (SAS Institute, Cary, NC, USA) was used to process all data analyses.
3. RESULTS
The 39 155 patients enrolled in this study included 7831 patients with SD (SD group) and a control group of 31 324 without SD (non‐SD group). In each group, the majority (53.74%) of patients was female (Table 1). Compared to the non‐SD group, the SD group had a higher prevalence of diabetes mellitus, hyperlipidemia, hypertension, chronic liver disease, hyperthyroidism, depression, stroke, dementia, and psoriasis.
TABLE 1.
Demographic data between patients with seborrheic dermatitis and controls
| Characteristics | With seborrheic dermatitis (n = 7831) | Controls (n = 31 324) | P |
|---|---|---|---|
| Mean age at enrolled (SD), years | 33.1 (9.0) | 33.3 (9.1) | 0.1339 |
| Age subgroup, n (%) | |||
| 18–29 | 3334 (42.57) | 13 336 (42.57) | 1.000 |
| 30–39 | 2327 (29.72) | 9308 (29.72) | |
| 40–49 | 2170 (27.71) | 8680 (27.71) | |
| Gender, n (%) | |||
| Males | 3623 (46.26) | 14 492 (46.26) | 1.000 |
| Females | 4208 (53.74) | 16 832 (53.74) | |
| Charlson Comorbidity Index, n (%) | |||
| 0 | 2307 (29.46) | 9228 (29.46) | 1.000 |
| 1–2 | 3725 (47.57) | 14 900 (47.57) | |
| 3–4 | 1292 (16.50) | 5168 (16.50) | |
| ≥5 | 507 (6.47) | 2028 (6.47) | |
| Comorbidity, n (%) | |||
| Diabetes mellitus | 759 (9.69) | 2402 (7.67) | <0.001 |
| Hyperlipidemia | 1628 (20.79) | 4525 (14.45) | <0.001 |
| Hypertension | 1110 (14.17) | 2991 (9.55) | <0.001 |
| Hyperthyroidism | 556 (7.10) | 1529 (4.88) | <0.001 |
| Chronic liver disease | 2367 (30.23) | 8175 (26.10) | <0.001 |
| Chronic kidney disease | 445 (5.68) | 1738 (5.55) | 0.644 |
| Chronic pulmonary disease | 2559 (32.68) | 10 496 (33.51) | 0.163 |
| Depression | 960 (12.26) | 2543 (8.12) | <0.001 |
| Stroke | 165 (2.11) | 472 (1.51) | <0.001 |
| Epilepsy | 133 (1.70) | 523 (1.67) | 0.859 |
| Dementia | 41 (0.52) | 78 (0.25) | <0.001 |
| Psoriasis | 670 (8.56) | 299 (0.95) | <0.001 |
Abbreviation: SD, standard deviation.
The incidence of osteoporosis was significantly higher in the SD group (0.98%, n = 77) compared to the non‐SD group (0.66%, n = 206) (Table 2). Additionally, the SD group tended to develop osteoporosis more rapidly (2.2 years after enrolment) compared to the control group (8.9 years after enrolment).
TABLE 2.
Characteristic of osteoporosis events between patients with seborrheic dermatitis and controls
| Characteristics | With seborrheic dermatitis (n = 7831) | Controls (n = 31 324) | P |
|---|---|---|---|
| Osteoporosis event, n (%) | 77 (0.98) | 206 (0.66) | 0.002 |
| Period of developing osteoporosis median (IQR), years | 2.2 (1.1–5.1) | 8.9 (5.5–12.2) | <0.001 |
Abbreviation: IQR, interquartile range.
Table 3 shows that, during the follow‐up period, the SD group had a 5.95‐fold higher osteoporosis risk compared to the non‐SD group (2.42 vs 0.44 per 1000 person‐years, respectively) after adjustment for age, gender, CCI, and comorbidities. Stratified analysis also revealed a higher osteoporosis risk in the SD group compared to the non‐SD group. In gender‐specific analyses of SD patients, the incidence of osteoporosis was higher in females compared to males (3.69 vs 1.04 per 1000 person‐years, respectively); in addition, osteoporosis risk in SD patients was higher in females compared to males (adjusted HR = 7.36, 95% CI = 5.15–10.52 for females; adjusted HR = 3.80, 95% CI = 1.95–7.38 for males). In age‐specific analysis, the incidence of osteoporosis substantially and consistently increased with age. However, the impact of SD on osteoporosis risk was higher in younger‐aged patients (adjusted HR = 8.05, 95% CI = 4.28–15.12) than in older‐aged patients (adjusted HR = 6.16, 95% CI = 4.31–8.81).
TABLE 3.
Comparison of osteoporosis events between patients with seborrheic dermatitis and controls
| Variables | With seborrheic dermatitis | Controls | Crude HR a (95% CI) | Adjusted HR a (95% CI) | P | ||||
|---|---|---|---|---|---|---|---|---|---|
| Osteoporosis events | PYs | Rate | Osteoporosis events | PYs | Rate | ||||
| Overall | 77 | 31 874.07 | 2.42 | 206 | 468 494.47 | 0.44 | 8.05 (5.93–10.92) | 5.95 (4.35–8.13) | <0.001 |
| Gender | |||||||||
| Males | 16 | 15 325.62 | 1.04 | 55 | 216 992.88 | 0.25 | 6.12 (3.24–11.54) | 3.80 (1.95–7.38) | <0.001 |
| Females | 61 | 16 548.45 | 3.69 | 151 | 251 501.59 | 0.60 | 9.15 (6.43–13.01) | 7.36 (5.15–10.52) | <0.001 |
| Age subgroup | |||||||||
| 18–29 | 8 | 13 286.19 | 0.60 | 26 | 19 9854.95 | 0.13 | 6.78 (3.02–15.23) | 6.03 (2.68–13.58) | <0.001 |
| 30–39 | 15 | 9258.64 | 1.62 | 35 | 139 398.47 | 0.25 | 9.39 (5.02–17.54) | 8.05 (4.28–15.12) | <0.001 |
| 40–49 | 54 | 9329.24 | 5.79 | 145 | 129 241.04 | 1.12 | 7.31 (5.17–10.34) | 6.16 (4.31–8.81) | <0.001 |
Abbreviations: 95% CI, 95% confidence interval; HR, hazard ratio; PY, person‐year; Rate, incidence rate in per 1000 person‐years.
Model adjusted for age, gender, CCI and relevant comorbidities.
Figure 1 shows that the Kaplan–Meier method with log‐rank test further revealed a significantly higher cumulative incidence rate of osteoporosis in the SD group compared to the non‐SD patients (P < 0.001).
FIGURE 1.
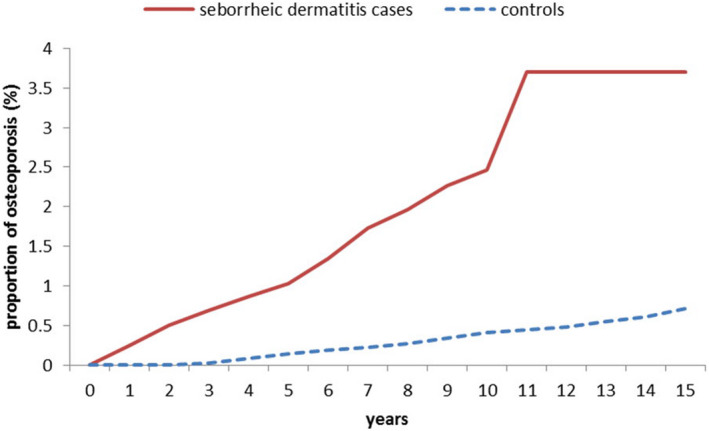
Comparison of Kaplan–Meier curves for osteoporosis risk between patients with seborrheic dermatitis and controls during the 15‐year follow‐up period
Table 4 shows that, in patients diagnosed with SD, the risk factors for osteoporosis included advanced age, female, high CCI, hyperlipidemia, hyperthyroidism, and epilepsy.
TABLE 4.
Significant predictors of osteoporosis after seborrheic dermatitis diagnosis
| Variables | Adjusted HR a | 95% CI | P |
|---|---|---|---|
| Age | 2.56 | (1.78–3.70) | <0.001 |
| Sex | 4.44 | (2.53–7.79) | <0.001 |
| Charlson Comorbidity Index | 1.37 | (1.05–1.79) | 0.019 |
| Hyperlipidemia | 2.13 | (1.28–3.54) | 0.004 |
| Hyperthyroidism | 2.02 | (1.16–3.50) | 0.012 |
| Epilepsy | 4.09 | (1.76–9.50) | 0.001 |
Abbreviations: 95% CI, 95% confidence interval; HR, hazard ratio.
The adjusted HR and 95% CI were estimated by a stepwise Cox proportional hazards regression method; model adjusted for age, gender, CCI and relevant comorbidities.
4. DISCUSSION
This study was the first large retrospective cohort study to demonstrate an association between SD and osteoporosis: 0.98% of SD patients aged 18–50 years had osteoporosis. This SD group has a 5.95‐fold higher risk of osteoporosis than the control cohort after adjustment for comorbidity. Among the SD group, osteoporosis was more common in females than in males. Additionally, the effect of SD on osteoporosis was more prominent in younger patients than older patients. Hyperlipidemia, hyperthyroidism, and epilepsy synergistically increased the incidence of osteoporosis in the SD group.
The mechanism of the increased osteoporosis risk in SD patients is likely multifactorial. First, Malassezia yeasts are already known to be a major causative factor in SD. In Malassezia species, lipase secretion hydrolyses free fatty acids, which induces inflammation by generating large quantities of oleic acids and arachidonic acids. 22 By inducing assembly of inflammasomes and activation of pattern recognition receptors, immune system dysregulation causes keratinocyte differentiation and proliferation. 23 , 24 Studies of SD patients also show that high androgen activity causes sebaceous gland activity and lipid composition, which then induces Malassezia proliferation and a continual cycle of inflammation. 13 , 25 Hence, the receptor activator of nuclear factor κB ligand (RANKL), tumor necrosis‐α, and interleukin‐6 are not only inflammatory cytokines, but also pro‐osteoclastogenic factors that promote osteoclast activation and differentiation. 26 In a mouse model of skin inflammation, KCASP1Tg mice exhibited osteoporosis and increased expression of inflammatory cytokines in the femur. 27 Hence, SD increases osteoporosis risk by generating an inflammatory response.
Second, since both SD and psoriasis are diseases of chronic inflammation, SD patients and psoriasis patients share common clinical characteristics, and co‐presence of SD and psoriasis is occasionally reported. 28 , 29 By examining employees in a voluntary company database in Germany, Zander et al. reported that approximately 2.7% of SD patients had psoriasis, suggesting that SD patients had a higher prevalence of psoriasis (odds ratio [OR] = 1.2, 95% CI = 1.0–1.5). 29 In a cross‐sectional study through analyzing the Clalit Health Services database in Israel, SD patients were more likely to have psoriasis (1.6% vs 0.8%). 28 In our study, SD patients were also prone to develop psoriasis. Psoriasis is a is not only a chronic multisystem inflammatory disease but is also related to vitamin D deficiency. Keller et al. have shown that osteoporotic patients had a higher prevalence of psoriasis (OR = 1.65, 95% CI = 1.42–1.94). 18 Additionally, psoriasis was reported to be related to male osteoporotic patients (OR = 1.86, 95% CI = 1.44–2.39) in a case–control study analyzing a database in Israel. 30 Attia et al. reported that psoriasis patients with or without arthritis were prone to have lower bone mineral density than healthy people. 31 Antonio et al. demonstrated that patients with long‐term psoriasis tended to develop decreased bone mineral density relative to healthy individuals. 32 In the meta‐analysis by Su et al., psoriatic patients were likely to have fractures relative to nonpsoriatic people (adjusted OR = 1.09, 95% CI = 1.06–1.12). 33 Also, in a cross‐sectional study of Arias‐Santiago et al., patients with psoriatic disease had lower bone mineral density than healthy people. 34 By analyzing the Korean National Health Insurance Service – Health Screening Cohort, Choi et al. reported that psoriasis increased the risk of osteoporosis (OR = 1.21, 95% CI = 1.16–1.27). 35 Patients with extensive and chronic psoriasis are known to have increased risks of osteopenia and osteoporosis. 36 Studies indicate that SD is also a predictor of metabolic syndrome, 37 which is characterized by a cluster of disorders, including abdominal obesity, hypertension, hyperlipidemia, and insulin resistance. Since insulin resistance and bone metabolism share a similar pathophysiology, abnormal signaling of insulin could cause dysregulation of osteoblast activity and osteoclast differentiation, leading to bone damage and osteoporosis. 9 , 10 Third, SD is readily aggravated by stress. A cross‐sectional study of a Chinese population of SD patients reported that nearly half of patients had severe emotional disorders, 38 including anxiety, depression, and obsessive–compulsive disorder. 39 In osteoporosis patients, depression can contribute to bone loss and osteoporotic fractures by activating the hypothalamo–pituitary–adrenal (HPA) axis and sympathetic system. 40 , 41 The SD patients in this study had a high prevalence of depression, hypertension, hyperlipidemia, and diabetes mellitus. Seborrheic dermatitis may contribute to osteoporosis risk by increasing the risks of metabolic syndrome, psoriasis, and depression. 42 However, these complex associations need further clarification.
In our study, the influence of SD on osteoporosis risk was higher in patients aged 30–39 years compared to other age groups. In all SD patients, the osteoporosis incidence rate increased with age. Compared to younger patients, however, older patients are more likely to have multiple comorbidities that contribute to osteoporosis. Thus, the role of SD in accelerating bone loss may not significantly increase with age. These data indicate that the pathobiological role of SD in osteoporosis may differ by age. Patients in puberty or in younger age groups are in a vulnerable phase of skeletal bone mineralization. In younger age groups, a high rate of vitamin D turnover results in a high prevalence of hypovitaminosis D. 43 Abnormally high vitamin D turnover also decreases osteoblast function, which causes deficient bone acquisition. 44
Moreover, the osteoporosis incidence in SD patients is reportedly increased in the presence of hyperlipidemia, hyperthyroidism, and epilepsy. The biologically active thyroid hormone 3,5,3′‐l‐triiodothyronine regulates the rates of bone maturation and mineralization. 45 The accelerated bone turnover rate in hyperthyroidism patients increases bone resorption and mineralized bone lost. 46 , 47 , 48 Cholesterol and its metabolites also affect bone homeostasis by modulating the activation and differentiation of osteoblasts and osteoclasts. 49 Oxidized lipid accumulation in bone tissues attenuates osteogenic differentiation and parathyroid hormone resistance, both of which promote osteoporosis. 50 , 51 Serum lipid levels negatively correlate with bone mineral density. 51 Notably, people with epilepsy are often deficient in vitamin D, which decreases bone mineral density and increases fracture risk. In epilepsy patients, the osteoporosis rate tends to be highest in young age groups and in patients treated with multiple antiepileptic drugs, 52 , 53 therefore concomitant diseases in patients with SD may be predictive factors for osteoporosis, which leads to increased fracture. 46 , 48 , 54 , 55 , 56
The biggest strength of our study is that it is the first population‐based study with a large sample size to investigate the interplay between SD and osteoporosis risk. However, a limitation is that diagnoses of SD and osteoporosis were both defined by ICD‐9‐CM codes from claims records. Hence, the numbers of patients with SD and patients with osteoporosis may have been underestimated because patients with untreated SD are also at risk of undiagnosed osteoporosis. If the SD patients have higher consciousness of medical problems than non‐SD patients, they are keen to receive osteoporosis examination and have more chance to be diagnosed as osteoporosis. Yet, Taiwanese national health insurance offers far lower payments to boast universal health coverage and easy access to any medical institutions. Although we could not completely exclude the interference of the increased exposure of SD patients to the medical community, most Taiwanese are indeed quick to seek medical attention when suffering from any discomfort based on the convenience and low cost of the health system. To minimize the bias, we also utilized Cox proportional hazard regression models to compare the hazard ratio and 95% confidence intervals for osteoporosis between the two groups with adjustment for age, gender, CCI, and relevant comorbidities in the multivariable model. These limitations are common in studies that have used electronic health insurance databases in other countries. Since the dataset did not include SD severity, the association between SD severity and outcome could not be determined. However, the LHID has already been used for various scientific studies of osteoporosis risk factors 57 , 58 , 59 ; our study provides additional scientific information. A final limitation is that the database lacked detailed data for osteoporosis risk factors that may have influenced the results, including relevant genetic factors, family history, sun exposure, diet/exercise habits, tobacco and alcohol consumption, and body mass index. 58
In conclusion, this study is the first cohort study to demonstrate that SD patients have an increased risk of osteoporosis, especially in the presence of hyperlipidemia, hyperthyroidism, and epilepsy. Notably, the influence of SD on osteoporosis risk was largest in young age groups. Since the influence of bone mass and overall bone health in SD patients with concomitant diseases is assumedly largest at an early age, lifestyle and diet recommendations as well as regular bone metabolism/mineral density examinations are warranted in routine treatment of SD.
CONFLICT OF INTEREST
None declared.
ETHICS STATEMENT
All insurance reimbursement claims data used in this study were collected from the Taiwan NHIRD. The study was assessed and approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB‐EXEMPT‐[I] 20150040) in accordance with Declaration of Helsinki principles. Based on the regulations of the Institutional Review Board, the informed consent requirement was waived.
ACKNOWLEDGMENTS
This work was supported by grants MOST‐111‐2314‐B‐075B‐016‐MY3, MOST‐110‐2314‐B‐037‐095, and MOST‐111‐2314‐B‐037‐097 from the Taiwan National Science and Technology Council, KMUH110‐0R31 from Kaohsiung Medical University Hospital, KSVGH111‐094 from Kaohsiung Veterans General Hospital, and KMU‐TC111B05 from Kaohsiung Medical University Research Grant.
Lu Y‐Y, Lu C‐C, Tsai C‐Y, Liu Y‐J, Huang C‐L, Wang W‐T, et al. Impact of seborrheic dermatitis on osteoporosis risk: A population‐based cohort study. J Dermatol. 2022;49:1291–1298. 10.1111/1346-8138.16578
REFERENCES
- 1. Nitta K, Yajima A, Tsuchiya K. Management of osteoporosis in chronic kidney disease. Intern Med. 2017;56:3271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang CJ, McCauley LK. Osteoporosis and periodontitis. Curr Osteoporos Rep. 2016;14:284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nih consensus development panel on osteoporosis prevention D, therapy: osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–95. [DOI] [PubMed] [Google Scholar]
- 4. Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. 2014;29:2520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coughlan T, Dockery F. Osteoporosis and fracture risk in older people. Clin Med. 2014;14:187–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pacifici R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J Bone Miner Res. 1996;11:1043–51. [DOI] [PubMed] [Google Scholar]
- 7. Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5:898–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bukvic Mokos Z, Kralj M, Basta‐Juzbasic A, Lakos Jukic I. Seborrheic dermatitis: an update. Acta Dermatovenerol Croat. 2012;20:98–104. [PubMed] [Google Scholar]
- 9. Borda LJ, Perper M, Keri JE. Treatment of seborrheic dermatitis: a comprehensive review. J Dermatolog Treat. 2019;30:158–69. [DOI] [PubMed] [Google Scholar]
- 10. Janniger CK, Schwartz RA. Seborrheic dermatitis. Am Fam Physician. 1995;52:159–60. [PubMed] [Google Scholar]
- 11. Kang S. Fitzpatrick's dermatology. 9th ed. New York, NY: McGraw‐Hill Education; 2019. p. 1. [Google Scholar]
- 12. Mameri ACA, Carneiro S, Mameri LMA, Telles da Cunha JM, ESM R. History of seborrheic dermatitis: conceptual and clinico‐pathologic evolution. Skinmed. 2017;15:187–94. [PubMed] [Google Scholar]
- 13. Wikramanayake TC, Borda LJ, Miteva M, Paus R. Seborrheic dermatitis‐looking beyond Malassezia. Exp Dermatol. 2019;28:991–1001. [DOI] [PubMed] [Google Scholar]
- 14. Hu LY, Lu T, Chen PM, Shen CC, Hung YM, Hsu CL. Should clinicians pay more attention to the potential underdiagnosis of osteoporosis in patients with ankylosing spondylitis? a national population‐based study in Taiwan. PLoS One. 2019;14:e0211835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klahan S, Kuo CN, Chien SC, Lin YW, Lin CY, Lin CH, et al. Osteoporosis increases subsequent risk of gallstone: a nationwide population‐based cohort study in Taiwan. BMC Gastroenterol. 2014;14:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Syu DK, Hsu SH, Yeh PC, Kuo YF, Huang YC, Jiang CC, et al. The association between coronary artery disease and osteoporosis: a population‐based longitudinal study in Taiwan. Arch Osteoporos. 2022;17:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen SJ, Lin CS, Lin CL, Kao CH. Osteoporosis is associated with high risk for coronary heart disease: a population‐based cohort study. Medicine. 2015;94:e1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keller JJ, Kang JH, Lin HC. Association between osteoporosis and psoriasis: results from the longitudinal health insurance database in Taiwan. Osteoporos Int. 2013;24:1835–41. [DOI] [PubMed] [Google Scholar]
- 19. Chen HJ, Yang HY, Hsueh KC, Shen CC, Chen RY, Yu HC, et al. Increased risk of osteoporosis in patients with nonalcoholic fatty liver disease: a population‐based retrospective cohort study. Medicine. 2018;97:e12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hong‐Jhe C, Chin‐Yuan K, Ming‐Shium T, Fu‐Wei W, Ru‐Yih C, Kuang‐Chieh H, et al. The incidence and risk of osteoporosis in patients with anxiety disorder: a population‐based retrospective cohort study. Medicine. 2016;95:e4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu CY, Lu YY, Lu CC, Su YF, Tsai TH, Wu CH. Osteoporosis in adult patients with atopic dermatitis: a nationwide population‐based study. PLoS One. 2017;12:e0171667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Riciputo RM, Oliveri S, Micali G, Sapuppo A. Phospholipase activity in Malassezia furfur pathogenic strains. Mycoses. 1996;39:233–5. [DOI] [PubMed] [Google Scholar]
- 23. Adalsteinsson JA, Kaushik S, Muzumdar S, Guttman‐Yassky E, Ungar J. An update on the microbiology, immunology and genetics of seborrheic dermatitis. Exp Dermatol. 2020;29:481–9. [DOI] [PubMed] [Google Scholar]
- 24. Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140:805–20. [DOI] [PubMed] [Google Scholar]
- 25. Arsic Arsenijevic VS, Milobratovic D, Barac AM, Vekic B, Marinkovic J, Kostic VS. A laboratory‐based study on patients with Parkinson's disease and seborrheic dermatitis: the presence and density of Malassezia yeasts, their different species and enzymes production. BMC Dermatol. 2014;14:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Montalcini T, Romeo S, Ferro Y, Migliaccio V, Gazzaruso C, Pujia A. Osteoporosis in chronic inflammatory disease: the role of malnutrition. Endocrine. 2013;43:59–64. [DOI] [PubMed] [Google Scholar]
- 27. Mizutani K, Isono K, Matsushima Y, Okada K, Umaoka A, Iida S, et al. Inflammatory skin‐derived cytokines accelerate osteoporosis in mice with persistent skin inflammation. Int J Mol Sci. 2020;21:3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Linder D, Dreiher J, Zampetti A, Sampogna F, Cohen AD. Seborrheic dermatitis and hypertension in adults: a cross‐sectional study. J Eur Acad Dermatol Venereol. 2014;28:1450–5. [DOI] [PubMed] [Google Scholar]
- 29. Zander N, Sommer R, Schafer I, Reinert R, Kirsten N, Zyriax BC, et al. Epidemiology and dermatological comorbidity of seborrhoeic dermatitis: population‐based study in 161 269 employees. Br J Dermatol. 2019;181:743–8. [DOI] [PubMed] [Google Scholar]
- 30. Dreiher J, Weitzman D, Cohen AD. Psoriasis and osteoporosis: a sex‐specific association? J Invest Dermatol. 2009;129:1643–9. [DOI] [PubMed] [Google Scholar]
- 31. Attia EA, Khafagy A, Abdel‐Raheem S, Fathi S, Saad AA. Assessment of osteoporosis in psoriasis with and without arthritis: correlation with disease severity. Int J Dermatol. 2011;50:30–5. [DOI] [PubMed] [Google Scholar]
- 32. D'Epiro S, Marocco C, Salvi M, Mattozzi C, Luci C, Macaluso L, et al. Psoriasis and bone mineral density: implications for long‐term patients. J Dermatol. 2014;41:783–7. [DOI] [PubMed] [Google Scholar]
- 33. Chen TL, Lu JW, Huang YW, Wang JH, Su KY. Bone mineral density, osteoporosis, and fracture risk in adult patients with psoriasis or psoriatic arthritis: a systematic review and meta‐analysis of observational studies. J Clin Med. 2020;9:3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martinez‐Lopez A, Blasco‐Morente G, Giron‐Prieto MS, Arrabal‐Polo MA, Luque‐Valenzuela M, Luna‐Del Castillo JD, et al. Linking of psoriasis with osteopenia and osteoporosis: a cross‐sectional study. Indian J Dermatol Venereol Leprol. 2019;85:153–9. [DOI] [PubMed] [Google Scholar]
- 35. Lee JW, Min C, Bang CH, Kwon BC, Choi HG. Psoriasis is associated with an increased risk of osteoporosis: follow‐up and nested case‐control studies using a national sample cohort. Osteoporos Int. 2021;32:529–38. [DOI] [PubMed] [Google Scholar]
- 36. Wi D, Wilson A, Satge F, Murrell DF. Osteoporosis and psoriasis: a literature review. Clin Exp Dermatol. 2022;47:1438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Imamoglu B, Hayta SB, Guner R, Akyol M, Ozcelik S. Metabolic syndrome may be an important comorbidity in patients with seborrheic dermatitis. Arch Med Sci Atheroscler Dis. 2016;1:e158–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Xuan M, Lu C, He Z. Clinical characteristics and quality of life in seborrheic dermatitis patients: a cross‐sectional study in China. Health Qual Life Outcomes. 2020;18:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Comert A, Akbas B, Kilic EZ, Akin O, Gokce E, Goktuna Z, et al. Psychiatric comorbidities and alexithymia in patients with seborrheic dermatitis: a questionnaire study in Turkey. Am J Clin Dermatol. 2013;14:335–42. [DOI] [PubMed] [Google Scholar]
- 40. Cizza G, Primma S, Csako G. Depression as a risk factor for osteoporosis. Trends Endocrinol Metab. 2009;20:367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cizza G, Primma S, Coyle M, Gourgiotis L, Csako G. Depression and osteoporosis: a research synthesis with meta‐analysis. Horm Metab Res. 2010;42:467–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. He B, Lyu Q, Yin L, Zhang M, Quan Z, Ou Y. Depression and osteoporosis: a mendelian randomization study. Calcif Tissue Int. 2021;109:675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arora E, Singh H, Gupta YK. Impact of antiepileptic drugs on bone health: need for monitoring, treatment, and prevention strategies. J Family Med Prim Care. 2016;5:248–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rozenberg S, Bruyere O, Bergmann P, Cavalier E, Gielen E, Goemaere S, et al. How to manage osteoporosis before the age of 50. Maturitas. 2020;138:14–25. [DOI] [PubMed] [Google Scholar]
- 45. Bassett JH, Williams GR. Role of thyroid hormones in skeletal development and bone maintenance. Endocr Rev. 2016;37:135–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bassett JH, Williams GR. Critical role of the hypothalamic‐pituitary‐thyroid axis in bone. Bone. 2008;43:418–26. [DOI] [PubMed] [Google Scholar]
- 47. Bauer DC, Ettinger B, Nevitt MC, Stone KL. Study of osteoporotic fractures research G: risk for fracture in women with low serum levels of thyroid‐stimulating hormone. Ann Intern Med. 2001;134:561–8. [DOI] [PubMed] [Google Scholar]
- 48. Apostu D, Lucaciu O, Oltean‐Dan D, Muresan AD, Moisescu‐Pop C, Maxim A, et al. The influence of thyroid pathology on osteoporosis and fracture risk: a review. Diagnostics. 2020;10:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yin W, Li Z, Zhang W. Modulation of bone and marrow niche by cholesterol. Nutrients. 2019;11:1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sage AP, Lu J, Atti E, Tetradis S, Ascenzi MG, Adams DJ, et al. Hyperlipidemia induces resistance to PTH bone anabolism in mice via oxidized lipids. J Bone Miner Res. 2011;26:1197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tanko LB, Bagger YZ, Christiansen C. Low bone mineral density in the hip as a marker of advanced atherosclerosis in elderly women. Calcif Tissue Int. 2003;73:15–20. [DOI] [PubMed] [Google Scholar]
- 52. Ucan Tokuc FE, Fatma G, Abidin E, Yasemin GB. Management of bone metabolism in epilepsy. Ideggyogy Sz. 2021;74:257–65. [DOI] [PubMed] [Google Scholar]
- 53. Likasitthananon N, Nabangchang C, Simasathien T, Vichutavate S, Phatarakijnirund V, Suwanpakdee P. Hypovitaminosis D and risk factors in pediatric epilepsy children. BMC Pediatr. 2021;21:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Petty SJ, Wilding H, Wark JD. Osteoporosis associated with epilepsy and the use of anti‐epileptics – a review. Curr Osteoporos Rep. 2016;14:54–65. [DOI] [PubMed] [Google Scholar]
- 55. Mirza F, Canalis E. Management of endocrine disease: secondary osteoporosis: pathophysiology and management. Eur J Endocrinol. 2015;173:R131–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhou Y, Deng T, Zhang H, Guan Q, Zhao H, Yu C, et al. Hypercholesterolaemia increases the risk of highturnover osteoporosis in men. Mol Med Rep. 2019;19:4603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lin TL, Wu CY, Chen YJ. Fracture risks in patients with atopic dermatitis: a nationwide matched cohort study‐reply. Ann Allergy Asthma Immunol. 2022;128:231. [DOI] [PubMed] [Google Scholar]
- 58. Lu CC, Qin H, Zhang ZH, Zhang CL, Lu YY, Wu CH. The association between keloid and osteoporosis: real‐world evidence. BMC Musculoskelet Disord. 2021;22:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hu SC, Lin CL, Tu HP. Association between psoriasis, psoriatic arthritis and gout: a nationwide population‐based study. J Eur Acad Dermatol Venereol. 2019;33:560–7. [DOI] [PubMed] [Google Scholar]


