Abstract
Prior research on early‐life exposures to famine has established in utero development as a critical period of vulnerability to malnutrition. Yet, previous research tends to focus narrowly on this stage, at the expense of a more comprehensive examination of childhood. As a result, the literature has yet to compare the severity of the consequences of exposure to malnutrition across developmentally salient periods. Such comparison is crucial not only in the magnitude of effects but also in the nature of outcomes. Using a restricted population registry‐linked health survey, this study examines the Dutch Hunger Winter to provide a comprehensive examination of the long‐term consequences of in utero, infant, childhood, and adolescent exposure to famine. The results show malnutrition leads to heterogeneous effects depending on when the exposure occurs. In utero exposure to malnutrition leads to deleterious conditions in physical health and lower socioeconomic attainment. For older cohorts, results suggest a resilience to the effects of malnutrition on physical health in late life, but a higher vulnerability to socioeconomic stunting. Furthermore, the results suggest important gender differences in the long‐term impact of malnutrition. Males consistently show stronger negative consequences across a wider array of conditions.
Introduction
The Developmental Origins of Health and Disease (DOHaD) highlights the role that material conditions in early life play in the genesis of chronic disease and mortality risk. It emphasizes gestation as a life stage especially vulnerable to insults such as malnutrition (Barker 2004, 1990; Kuh and Shlomo 2004; Palloni et al. 2009). Accordingly, DOHaD demonstrates in utero malnutrition increases the propensity to develop an array of conditions, including cardiovascular disease, cerebrovascular disease, and diabetes, as well as reducing cognitive ability and stunting socioeconomic attainment (Almond and Currie 2011; Basso 2008; Painter, Roseboom, and Bleker 2005; Roseboom et al. 2001, 2011).
Though implicitly based on a developmental perspective, DOHaD has tended to focus narrowly on in utero exposures and cardiometabolic outcomes, at the expense of a more comprehensive developmental approach. Like gestation, infancy, childhood, and adolescence are periods of key developmental change (Nelson 2013, 2017). Focusing exclusively on the gestational period leaves crucial questions underexplored. To what extent does vulnerability due to malnutrition extend beyond gestation? How does the impact of early‐life malnutrition vary across developmental domains, either in magnitude or in heterogeneous timing effects?
The Dutch Hunger Winter has played an important role in DOHaD research, linking in utero famine exposure to adult glucose intolerance, coronary heart disease, atherogenic lipid profiles, disrupted blood coagulation, elevated stress response, obesity, and lower cognitive abilities (Lumey and Stein 1997; Lumey, Stein, and Susser 2011; de Rooij et al. 2007; Roseboom et al. 2011; Stein et al. 2006). Adopting a natural experiment framework and using unique population‐health survey data linked to the Dutch population registry, we reexamine the famine to investigate the heterogeneous effects of malnutrition beyond gestation. We also explore a wider array of late‐life health and socioeconomic outcomes than have previously been studied. Furthermore, we test for gender differences in the impact of famine exposure. While famine is a rare event in high‐income contexts, globally hundreds of millions of people struggle with hunger, severe food insecurity, and malnutrition with nearly a quarter of children under the age of 5 experiencing stunting or wasting (FAO 2021). How such exposures will affect their life trajectories remains an open question with substantial policy implications.
Background
The Dutch famine context
The Dutch Hunger Winter has been instrumental in characterizing the long‐term physiological consequences of early‐life famine exposure. At the outbreak of World War II, the Dutch government began rationing food (Banning 1946; Dols and Arcken 1946). In September 1944, Germany imposed a food embargo on the Western provinces (i.e., Noord‐Holland, Zuid‐Holland, and Utrecht; see Figure 1). An early extreme winter further precluded supplying the Western provinces from other areas, exposing approximately 4.5 million people to a severe famine. Average caloric intake in these provinces declined from approximately 1500 calories/day in September 1944 to 700 calories for children and just over 500 calories for adolescents and adults during January–March 1945 (Dols and Arcken 1946). Pregnant/lactating women were entitled to extra rations; however, at the peak of the famine these extra rations were unsustainable. The nutrient composition also suffered, as rations shifted heavily away from meat and dairy and towards cereals and potatoes. This increased the proportionate intake of carbohydrates and decreased the consumption of proteins, fats, and key vitamins and minerals (particularly calcium) (Dols and Arcken 1946). The caloric intake would not recover until June 1945. As a result, between January and July 1945 the Netherlands experienced 45,000 excess deaths, with 68 percent of occurring in famine‐affected regions (Ekamper et al. 2017). Among war‐related mortality, hunger/thirst accounted for less than 1 percent of deaths in 1944 but rose to 23 percent in 1945 (Ekamper et al. 2017).
FIGURE 1.
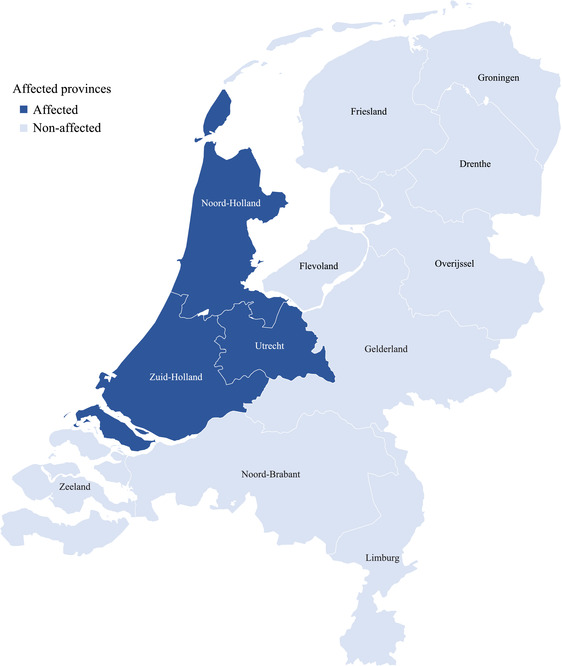
Famine‐affected provinces of the Netherlands during the 1944–1945 Winter
SOURCE: Authors' own elaboration sourcing data from Dols and Arken 1946.
Although food scarcity was the greatest danger, the famine period was also characterized by violence, infrastructure/institutional failure, severe cold, military executions, and sanitary system breakdown (Lumey, Stein, and Susser 2011). External causes of death related to injury and poisoning were one of the main sources of mortality associated with the famine period, accounting for 14.8 percent and 18 percent of all deaths in 1944 and 1945 (Ekamper et al. 2017). Furthermore, causes of death related to war and its consequences highlight substantial violence (Ekamper et al. 2017). In 1944, 60 percent of war‐related deaths were due to deaths of civilians related to operations of war. Therefore, the estimated impacts of Dutch famine exposure are likely capturing both the direct effects of severe malnutrition, as well as the additional deleterious impacts of the broader toxic milieu of war, stress, and trauma.
Windows of vulnerability in early life
A core life course principal asserts that the impact that events, exposures, or transitions have on life trajectories depends on the timing at which they occur (Elder 1998). As development unfolds from conception to adulthood, individuals traverse multiple critical/sensitive periods that shape their social and biological development. During these critical/sensitive periods, windows of vulnerability open to adversity. While individuals may be vulnerable to adverse experiences during a given developmental interval, they may be resilient to the same experiences outside of it (Kuh et al. 2003; Kuh and Shlomo 2004).
In utero vulnerability and cardiometabolic processes
David Barker and colleagues have argued since the 1980s that the principal causes of adult cardiometabolic disorders originate in utero (Barker 2004; Hales and Barker 1992). The “fetal origins” or “thrifty phenotype” hypothesis posits that fetal growth restrictions trigger fetal predictive adaptive responses. Predictive adaptive responses consist of in utero phenotypic adjustments to experienced or predicted environmental challenges (Bateson and Gluckman 2012a; Gluckman et al. 2008; Gluckman, Hanson, and Spencer 2005). This includes reordering developmental organogenetic priorities (e.g., disruptions of organ formation, growth, and functional specialization) and alterations of basal metabolic function, including blood pressure, heart rate, as well glucose and lipid metabolism (Bateson and Gluckman 2012a). While predictive adaptive responses increase fetal survival in the short term (Kahn, Narayan, and Valdez 1998), they are maladaptive in the long term, increasing the risk of cardiovascular and cerebrovascular disease, diabetes, and other chronic diseases (Almond and Currie 2011; Basso 2008; Palloni et al. 2020; Roseboom et al. 2001, 2011; Rasmussen 2001). Results from human observational studies and natural experiments have demonstrated adverse effects of in utero nutritional deprivation on later life health across a variety of famine contexts (e.g., the Leningrad siege, the Rwandan civil war, and the Chinese revolution) (Akresh, Verwimp, and Bundervoet 2011; Lumey et al. 2011; Ramirez and Haas 2021; de Rooij et al. 2007; Schulz 2010; Stanner and Yudkin 2001; Stein et al. 2006; Zhang, Gu, and Hayward 2010).
In addition to nutritional deprivation, maternal exposure to acute stress during pregnancy can also affect birth outcomes and subsequent offspring biology via alterations of the hypothalamic‐pituitary‐adrenal (HPA) axis. During periods of acute stress, mothers can release excess cortisol, which can reach the fetus via the placenta, and have strong effects on offspring biology and health. A large number of animal studies show that young adults exposed to maternal psychosocial stress during and after pregnancy experience dysregulation in key physiological systems, raising the risk of elevated body‐mass index (BMI) and body fat composition, insulin resistance, and lipid profiles consistent with the metabolic syndrome (Entringer, Buss, and Wadhwa 2015; Kuzawa and Quinn 2009a; Thayer and Kuzawa 2015). Similarly, human research demonstrates the impact of maternal stress on birth outcomes (Hobel et al. 2003; Lockwood and Kuczynski 1999). For example, Lauderdale (2006) demonstrated that stress experienced by pregnant Arabic‐named women post‐9/11 significantly increased their risk of poor birth outcomes. During the Dutch famine, both the famine and the wider context of war may have induced elevated maternal stress, permanently altering the functioning of the HPA axis in offspring, predisposing children to glucose intolerance, insulin resistance, and type‐2 diabetes.
Famine, neurological development, and socioeconomic stunting
Looking beyond cardiometabolic processes, the period from conception to approximately age 2 is also a critical period for neurological/cognitive development. Brain cell growth is most rapid during the first two years of a child's life and then rapidly slows down afterwards (Ampaabeng and Tan 2013; Beshir and Maystadt 2020). Caloric restrictions or nutrient imbalances may hinder brain development and may have an effect on overall cognitive performance, which in turn adversely affects socioeconomic attainment (Belli 1971, 1975). In utero malnutrition also has particularly strong effects on primary sensory information processing capabilities, including auditory and visual abilities (Gladstone et al. 2014; Kamel et al. 2016; Ntim‐Amponsah and Amoaku 2008). In addition to nutritional deprivation, elevated maternal cortisol can traverse the placenta and blood–brain barriers and accumulate in areas of the brain critical to cognitive development (Beshir and Maystadt 2020; Valdez Jiménez et al. 2017). Adverse effects of famine on cognitive development and subsequent educational attainment have been observed across various contexts (Ampaabeng and Tan 2013; Arage et al. 2020; Beshir and Maystadt 2020; Roseboom et al. 2011; Neelsen 2011; Rong et al. 2019; Xu et al. 2018; Zhang et al. 2010). Beyond extreme cases of famine, low‐birthweight children have lower IQs, perform substantially worse on academic achievement tests, are less likely to graduate high school, and have reduced earnings (Currie and Hyson 1999; Almond and Currie 2011; Black et al. 2005).
Beyond the womb
Beyond the interuterine environment, malnutrition and stress during subsequent stages of development may also adversely impact adult health and socioeconomic outcomes. The gut microbiome plays a critical role in physiological functions throughout the life course and influences a wide array of chronic diseases in late life, including obesity, diabetes, and cardiovascular disease (Devaraj et al. 2013; Alur 2019). Nutrient acquisition and energy harvesting that are intimately related to physical growth and weight regulation also rely on processes embedded within the microbiome (Herd et al. 2018). As humans are born microbially sterile, infant and childhood nutrition is central to building the microbiome, implicating the postnatal developmental environment (Burmeister et al. 2020; Devaraj, Hemarajata, and Versalovic 2013; Herd et al. 2018). Similarly, infancy and childhood are crucial to muscle and bone development, as those exposed to poor nutrition in early childhood experience greater loss of muscle strength and bone mass in late life among (Bartz et al. 2014; Huang, Soldo, and Elo 2011). Low calcium and protein intake during infancy, childhood, and adolescence are key determinants of bone density and muscle development (McFie and Welbourn 1962; Sayer and Cooper 2002). Bone stability and height have also been directly linked to the quantity and composition of childhood nutrition (Akachi and Canning 2007; Deaton 2007). Stress exposure throughout childhood and adolescence has further been shown to contribute to obesity. Chronically increased cortisol levels enhance hunger, promote insulin hypersecretion, and ultimately result in insulin resistance and (visceral) fat formation (McEwen and McEwen 2017; Ridout, Khan, and Ridout 2018).
Accordingly, research in the past decade has extended critical periods up through adolescence as well, as brain plasticity and rapid pubertal maturation of all organ systems occur during this period (Barouki et al. 2012; Bateson and Gluckman 2012b; Belsky and Pluess 2009). Biological embedding, a more general developmental perspective than DOHaD, asserts that childhood environments unconducive to healthy development may lead to adverse physiological and neurocognitive outcomes, as well as poor emotional and psychosocial coping mechanisms, and thus higher lifetime levels of stress and subsequently poor health (Cynader 1994; Hertzman 1999). For example, executive function, which regulates how individuals respond to social and emotional stimuli, develops between ages 3 and 9 (Vineis et al. 2016). Cognitive capacities related to attention, memory, sensory function, coordination, and broad motor skills—key variables for school and occupational success—develop during midchildhood (Demetriou et al. 2015; Hertzman 1999; Hertzman and Keating 1999).
Outside of famine contexts, there is also a well‐documented relationship between child hunger, nutritional status, and school outcomes. Children experiencing hunger and nutritional deficits fare worse along numerous dimensions of academic achievement and performance (Kleinman et al. 2002; Taras 2005). Hunger can also induce psychosocial behavioral problems in children, further hindering school performance (Kleinman et al. 1998). Furthermore, given the famine occurred within the broader context of the war school‐aged children also had to potentially cope with institutional breakdowns and disruptions to schooling trajectories if schools ceased to function if parents were reluctant to send their children to school under conditions of violence and uncertainty. Finally, older children may have left school early to help augment family resources in difficult times. Thus, it is important to extend our examination of long‐term famine effects beyond the womb.
Gender differences
A key limitation of prior research on the long‐term effects of early‐life famine exposure has been the inability to examine gender differences. This is largely due to the lack of adequate data on exposure to early‐life nutritional deprivation among cohorts of older adults. However, there is evidence in the demographic and developmental biology literature of a “frail male” phenomenon. According to the developmental biology literature, males tend to show a higher resilience during the first trimester of gestation compared to their female counterparts. However, females exhibit a stronger resilience during the fetal‐to‐neonatal transition, in infancy, and in the first years of life (Alves et al. 2019; Rosenfeld 2015). Such differences are suspected to result from sex differences in speed of maturation. Slower maturation translates to a wider time window during which insults may occur. Functional and structural development of both the cardiovascular and respiratory systems mature faster in female fetuses, shortening the window of vulnerability to insults during critical developmental periods related to cardiometabolic maturation (Aiken and Ozanne 2013; Franzek 2019; Heijmans et al. 2008; Lorente‐Pozo et al. 2018). Animal models suggest the placenta of the female fetus may be more efficient at extracting and transporting nutrients from maternal circulation under conditions of malnutrition (O'Connell et al. 2011).
In demographic research, the “frail male” hypothesis, is empirically grounded in imbalanced sex ratios at birth and disproportionate male infant mortality during times of hardship (Bisioli 2004; James 2009; Schacht, Tharp, and Smith 2019). That literature has focused almost exclusively on gender differences in survival in utero, and in the neonatal period, as such, it is unclear how gender may shape the effects of early‐life famine exposure over the long term among those who survive into adulthood. Do gender differences in the impact of famine exposure follow the same pattern in later life as they do in early childhood? The present study's use of much larger population‐based data sources overcomes this limitation.
The present study
We propose that the exposure to malnutrition and stress brought forth by the Dutch Famine, yielded differential consequences in terms of health and socioeconomic attainment depending on its timing. Because in utero stages imply organogenesis developmental saliency, we hypothesize that in utero exposure to famine‐induced malnutrition and stress results in an increased risk of cardiometabolic, muscular‐skeletal physical problems, sensory impairment, and stunted socioeconomic attainment in later life. Following previous literature, we expect increased risks to be highest for those exposed during the first trimester. Specifically, we hypothesize:
-
H1:
The largest adverse impacts of famine exposure on cardiometabolic conditions, functional limitations, sensory impairment, and self‐rated health will be for those exposed in utero. Furthermore, we hypothesize first trimester exposures will yield the largest effects.
However, we also hypothesize that the window of vulnerability associated with famine exposure extends beyond gestation and as the timing of exposure shifts to childhood and adolescence, different developmental processes come to the fore. Because organogenesis is mostly carried out during in utero developmental periods, we expect diseases related to fetal constraints to be less likely to develop in older cohorts. Accordingly, we hypothesize:
-
H2a:
Exposures to famine during childhood and adolescence will increase the risk of developing cardiometabolic diseases, functional limitations, sensory impairment, and lower self‐rated health. However, the impact of exposure for these cohorts will be smaller than for those exposed in utero.
Because older cohorts would have entered formal schooling at the time of the war/famine and thus been subject to the impact of the famine on schooling performance, schooling interruptions, institutional breakdown, and familial disruptions, creating additional barriers to socioeconomic attainment, we hypothesize:
-
H2b:
Cohorts exposed to the famine during childhood and adolescence will display larger reductions in educational attainment and income than those exposed in utero.
Finally, following previous research emphasizing male vulnerability to in utero insults we hypothesize:
-
H3:
Males exposed to the Dutch famine in utero will show greater risks of developing cardiometabolic conditions, functional limitations, sensory impairment, lower self‐rated health, and lower socioeconomic attainment than their female peers. The male disadvantage will extend to exposure beyond gestation.
Data
This study relies on the unique linkage of two sources of data: The Adult and Elderly Health Monitor (GEMON) and the Dutch Census. GEMON is a nationally representative survey of the Dutch population aged 18 years and older. We utilize the latest available waves from 2012 and 2016, which provide information on approximately 400,000 individuals. The second data source is the restricted Dutch Census data provided by Microdata CBS (Statistics Netherlands). 1 When linked to GEMON, this provides date and place of birth of respondents, allowing the identification of the location and age of the respondent—including those in utero—during the Dutch Hunger Winter. Because we are interested in studying those cohorts who had potential exposure to the Dutch Hunger Winter or those cohorts born immediately after, we exclude any respondents born after 1949. Overall missing data accounts for approximately 15 percent. 2 . After excluding individuals born after 1949 and missing data, we obtain a total sample of 123,789 individuals. 3
Exposure to famine: Cohort and gestation groups
The study focuses on individuals at risk of exposure to the Dutch Hunger Winter; hence with the exception of a control group comprised of cohorts born 1945–1949, we exclude individuals born after 1949. Following previous research, this study distinguishes between three groups (1) cohorts in utero during famine, (2) cohorts born before the famine period, and (3) cohorts born between 1945 and 1949. We further partition in utero cohorts by trimester of first exposure. Figure 2 presents a diagram pertaining to differential groups based on the timing of exposure. The shaded area refers to the famine period for which average daily rations fell below 1,000 calories (i.e., November 1944 to June 1945) (Dols et al. 1946). Assuming a nine‐month gestation interval, the study identifies differential timing of exposure based on the trimester in which famine began. For instance, respondents are considered to be at risk of exposure during the third trimester if they were born one to three months after October 1944. All others are grouped into five‐year age cohorts corresponding to different stages of childhood including infancy and early childhood (ages 1–4), the early school years (ages 5–9), and early adolescence (ages 10–14). In terms of current age, this study examines individuals aged 72–87 in 2016.
FIGURE 2.
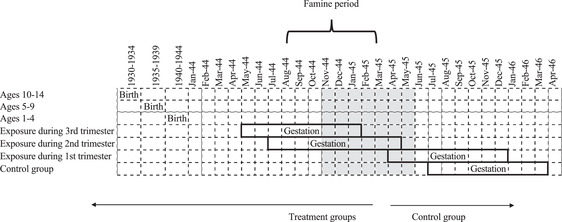
Cohort delimitation chart based on timing of exposure to famine
Dependent variables 4
Self‐rated health (SRH). A self‐assessed summary statement of the respondent's overall health status was dichotomized to express positive health (excellent/very good = 1; otherwise = 0). SRH is a widely utilized indicator of health that has been deemed a valid approximation of overall health that successfully predicts health conditions as well as mortality (Idler and Benyamini 1997; Jylhä 2009). Self‐rated health captures a wide range of both health conditions and underlying mechanisms. Utilizing an ordinal form of SRH yielded similar results.
Cardiometabolic and muscular‐skeletal physical health conditions
GEMON respondents were asked if they had ever been diagnosed by a doctor with a series of health conditions and diseases. Indicator variables identify those affirming diagnosis for cardiovascular disease or diabetes (yes = 1; no = 0). A third indicator identifies those with a BMI equal to or above 30.0 as obese. As a measure of muscular‐skeletal health, we use a number of functional limitations (0–3). The measure is based on three items that assess whether the individual has difficulty walking 400 meters without stopping, carrying an object of 5 kg a distance of 10 meters, and bending to pick up something from the ground.
Sensory impairment
Visual impairment is a dichotomous measure of whether individuals have issues reading small letters or whether they have trouble recognizing people at a distance of 4 meters. Auditory impairment is a dichotomous indicator designating whether the respondent has trouble following a conversation with three or more people.
Socioeconomic outcomes
Educational attainment is an ordinal measure capturing respondents’ highest educational degree achieved, where 1 = secondary or less, 2 = professional degree (equivalent to an associate degree), 3 = bachelor's degree, and 4 = postgraduate degree (MA/PhD) 5 . Income quintile indicates the quintile the respondent occupies in the Dutch income distribution.
Analytic approach
We utilize a difference‐in‐difference (DID) approach to estimate the treatment effect of being exposed to a context of famine, using the following equation:
where represents the outcome of interest j for individual i. represents a vector of dummy variables that stem from the categorical variable that delimits the timing of exposure to famine for individual i, and represents a vector of dummy variables that stem from the categorical variable for a region of birth for individual i. These two sources of variation—time and place of birth—are random in nature, allowing a DID estimator. The parameters of interest are the set of interaction terms (i.e., ) representing the treatment effect of being a particular age in the Western provinces of the Netherlands during the winter of 1944–1945. They represent the effect of famine exposure over and above the effect of birth in a region in the West, and the period effect of being born at a particular moment in time. Finally, represents survey fixed effects variables which capture the variation between the two waves of GEMON.
Results
Table 1 provides descriptive statistics for all outcomes by age during the exposure period and by treatment group. For simplicity, we summarize descriptive statistics for childhood as one group. In the analysis, we disaggregate by timing of exposure. Overall, children living in famine regions show a higher prevalence of negative health outcomes, including poor SRH, cardiovascular disease, diabetes, and auditory and visual problems. However, there are no differences in socioeconomic standing. Those exposed in utero show stark differences in self‐rated health, cardiovascular disease, diabetes, obesity, and auditory problems. Conversely, the differences between famine areas and nonfamine areas for those born postfamine show the reverse tendency, with the areas previously effected by famine demonstrating better health across nearly every outcome. Sixty‐two percent of those living in the famine region reported positive SRH, compared to 72 percent among those living in nonfamine provinces. Similarly, those who were in utero in the famine region also had lower SRH than those in nonfamine regions, though the gap was smaller. Conversely, for the postfamine control group those residing in the region previously affected by famine show better SRH than in the nonfamine region. Similar patterns between famine and nonfamine regions in distinct age groups can be found across multiple outcomes. For instance, cardiovascular disease prevalence is 11.4 percent for those who lived in a famine region during childhood, whereas their unexposed counterparts had a 7.8 percent prevalence.
TABLE 1.
Descriptive statistics
| Childhood (birth‐Age 14) | In Utero | Postfamine | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Famine region | Non‐famine | Famine region | Non‐famine | Famine region | Non‐famine | |||||||||||||
| Variable | (67,568) | region (32,953) | (3,041) | region (7,017) | (4,672) | region (8,903) | ||||||||||||
| Percentage | Mean | SD | Percentage | Mean | SD | Percentage | Mean | SD | Percentage | Mean | SD | Percentage | Mean | SD | Percentage | Mean | SD | |
| Physical health | ||||||||||||||||||
| Positive self‐rated health | 62.10 | 71.80 | 70.60 | 72.50 | 65.30 | 61.90 | ||||||||||||
| Cardiovascular disease | 11.40 | 7.80 | 7.60 | 6.80 | 9.90 | 10.40 | ||||||||||||
| Diabetes | 16.70 | 14.60 | 13.30 | 12.80 | 15.70 | 16.40 | ||||||||||||
| Obesity | 13.50 | 16.20 | 17.50 | 15.70 | 14.70 | 15.20 | ||||||||||||
| Functional limitations | 1.4 | 0.02 | 1.2 | 0.01 | 1.3 | 0.01 | 0.6 | 0.02 | 0.7 | 0.02 | 0.7 | 0.03 | ||||||
| Mental and sensory health | ||||||||||||||||||
| Depression and Anxiety | 40.60 | 32.00 | 31.50 | 31.70 | 37.70 | 40.00 | ||||||||||||
| Auditory impairment | 7.40 | 4.20 | 5.90 | 4.40 | 6.60 | 8.20 | ||||||||||||
| Visual impairment | 6.30 | 4.00 | 4.10 | 4.10 | 5.90 | 6.80 | ||||||||||||
| Socioeconomic status | ||||||||||||||||||
| Education | 2.5 | 0.01 | 2.7 | 0.01 | 2.6 | 0.01 | 2.7 | 0.02 | 2.6 | 0.01 | 2.4 | 0 | ||||||
| Income quintile | 3.3 | 0.01 | 3.5 | 0.01 | 3.5 | 0.02 | 3.7 | 0.03 | 3.4 | 0.01 | 3.2 | 0.01 | ||||||
Note: Total N = 123,794.
Table 2 presents DID estimates for positive self‐rated health. The treatment effect of being exposed to famine for each age group is reflected in the interaction terms. Each of these is estimated separately by gender. As can be seen in the table, there are no significant effects of famine exposure at any stage for females. However, for males there are effects for those exposed in the first and the second trimesters. Those exposed in the first trimester had a 27 percent (e – 0.32 = (1 − 0.73 * 100)) lower odds of reporting a positive self‐rated health above and beyond regional and cohort differences. Similarly, those exposed during the second trimester had 24 percent (e –0.27 = (1 − 0.76 *100)) lower odds of a positive self‐rated health above and beyond regional and cohort differences.
TABLE 2.
Difference‐in‐difference logistic regression estimates for positive self‐rated health
| Females | Males | |||
|---|---|---|---|---|
| Interactions | β | SE | β | SE |
| Famine region × first trimester | −0.02 | (0.14) | −0.32** | (0.14) |
| Famine region × second trimester | 0.07 | (0.13) | −0.27* | (0.13) |
| Famine region × third trimester | −0.09 | (0.13) | −0.07 | (0.13) |
| Famine region × Ages 1–4 | 0.1 | (0.06) | −0.04 | (0.06) |
| Famine region × Ages 5–9 | 0.11 | (0.07) | 0.01 | (0.07) |
| Famine region × Ages 10 – 14 | 0.07 | (0.07) | 0.01 | (0.07) |
| Famine region | 0.11+ | (0.06) | 0.04 | (0.06) |
| Birth groups | ||||
| First trimester | −0.25** | (0.09) | −0.139 | (0.10) |
| Second trimester | −0.25** | (0.76) | −0.023 | (0.08) |
| Third trimester | −0.02 | (0.07) | −0.01 | (0.07) |
| Ages 1–4 | −0.24*** | (0.04) | −0.247*** | (0.04) |
| Ages 5–9 | −0.51*** | (0.04) | −0.642*** | (0.05) |
| Ages 10–14 | −0.88*** | (0.05) | −1.045*** | (0.05) |
Table 3 presents DID estimates of effects of famine exposure on physical health for each cohort by gender. The results show a pattern where the earlier the timing of exposure, the higher the risk of developing diseases due to famine exposure. This is true not only because most effects are found among respondents who lived through the famine while in utero, but additionally because the results show the strongest effects concentrate in the first and second trimesters of gestation. Males exposed in the first trimester had a 33.6 percent (e0 .29 = (1 − 1.336 * 100)) higher odds of reporting a cardiovascular disease above and beyond regional and cohort differences. There is a similar effect for females as well, as they show 35 percent higher odds of reporting cardiovascular disease if they were exposed in their first trimester. Additionally, males exposed in their second trimester had a 55.3 percent (e0 .44 = (1 − 1.553 * 100)) higher odds of reporting diabetes above and beyond regional and cohort differences.
TABLE 3.
Difference in difference regression estimates for physical health
|
Cardiovascular disease (logistic regression) |
Diabetes (logistic regression) |
Obesity (logistic regression) |
Functional lim. (Neg. Binomial) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | Females | Males | Females | Males | |||||||||
| Interactions | β | SE | β | SE | β | SE | β | SE | β | SE | β | SE | β | SE | β | SE |
| Famine region × first trimester | 0.30 * | (0.14) | 0.29 * | (0.14) | 0.17 | (0.21) | 0.44 ** | (0.14) | 0.29 † | (0.17) | −0.06 | −0.15 | 0.27 * | −0.12 | −0.05 | −0.1 |
| Famine region × second trimester | −0.14 | (0.21) | −0.1 | (0.17) | −0.21 | (0.21) | 0.29 | (0.20) | 0.08 | (0.18) | 0.42 ** | (0.15) | −0.17 | −0.16 | 0.12 | −0.1 |
| Famine region × third trimester | −0.09 | (0.25) | −0.06 | (0.22) | −0.13 | (0.23) | 0.15 | (0.20) | 0.1 | (0.20) | 0.25 | (0.17) | 0.22 | −0.17 | 0.30 ** | −0.11 |
| Famine region × Ages 1–4 | −0.03 | (0.13) | 0.18 | (0.14) | 0.03 | (0.15) | 0.16 | (0.12) | 0.28 ** | (0.11) | 0.07 | (0.08) | 0.06 | −0.1 | 0.09 | −0.06 |
| Famine region × Ages 5–9 | −0.05 | (0.13) | 0.13 | (0.14) | 0.03 | (0.15) | 0.06 | (0.12) | 0.06 | (0.12) | 0 | (0.08) | 0.05 | −0.1 | 0.02 | −0.06 |
| Famine region × Ages 10 – 14 | −0.08 | (0.13) | 0.12 | (0.14) | −0.07 | (0.15) | −0.05 | (0.12) | 0.16 | (0.12) | −0.09 | (0.09) | 0.06 | −0.1 | 0.02 | −0.06 |
| Famine region | −0.04 | (0.12) | −0.14 | (0.12) | 0.02 | (0.14) | −0.15 | (0.11) | −0.18 | (0.10) | −0.05 | (0.07) | −0.1 | −0.09 | −0.04 | −0.05 |
| Birth groups | ||||||||||||||||
| First trimester | 0.01*** | (0.09) | 0.47*** | (0.08) | −0.18 | (0.12) | −0.56*** | (0.10) | 0.21*** | (0.07) | 0.05 | (0.08) | 0.33*** | −0.07 | 0.61*** | −0.03 |
| Second trimester | 0.09*** | (0.08) | 0.81*** | (0.08) | 0.08 | (0.12) | −0.51*** | (0.12) | 0.32*** | (0.07) | 0.02 | (0.09) | 0.74*** | −0.06 | 1.07*** | −0.03 |
| Third trimester | 0.33*** | (0.08) | 1.08*** | (0.29) | 0.01 | (0.14) | −0.63*** | (0.14) | 0.46*** | (0.06) | 0.11 | (0.11) | 1.22*** | −0.06 | 1.54*** | −0.03 |
| Ages 1–4 | 0.65*** | (0.08) | 1.37*** | (0.08) | 0.07 | (0.09) | −0.33*** | (0.07) | 0.61*** | (0.12) | 0.11 * | (0.05) | 1.65*** | −0.06 | 1.77*** | −0.07 |
| Ages 5–9 | 0.65*** | (0.12) | 1.52*** | (0.19) | 0.15 | (0.09) | −0.09 | (0.07) | 0.65*** | (0.10) | 0.11 * | (0.05) | 1.82*** | −0.1 | 1.68*** | −0.06 |
| Ages 10–14 | 0.99*** | (0.14) | 1.76*** | (0.18) | 0.31 | (0.09) | 0.14 | (0.09) | 0.68*** | (0.09) | 0.19*** | (0.05) | 1.63*** | −0.09 | 1.74*** | −0.05 |
Notes: Standard errors in parentheses;
† p < 0.1, * p < 0.05, ** p < 0.01, *** p < 0.001.
Ref. group for cohort variables: born 1–4 years after the famine. Survey fixed effects to capture potential differences through GEMON waves are included (not shown).
Beyond an enhanced vulnerability as a function of how early on the exposure to famine occurs, there are stark gender differences. Males are considerably more vulnerable to famine effects while in utero overall. Results suggest males experience increased probabilities of developing diabetes, cardiovascular disease, obesity, as well as a higher number of functional limitations as a cause of famine exposure. For females, there seems to be greater resilience in terms of developing most physical conditions. Females who were in utero during the famine show increased probabilities of developing cardiovascular disease and having a higher number of functional limitations.
Figure 3 presents average marginal effects for the DiD regression estimates of famine exposure on various later life physical health outcomes for each exposure and gender group. Overall, effects of famine above and beyond cohort and regional differences are concentrated within in utero periods. The only condition that registers statistically significant treatment effects outside of the in utero stage is obesity, where women exposed at ages 1–4 experience higher risk. The results show exposure to famine in the first trimester increases the risk of developing cardiovascular disease by approximately 7 percent for males and females. For diabetes, the first trimester exposure increases risk by approximately 6 percent for males. Similar and magnitude of effects are found for obesity and functional limitations.
FIGURE 3.
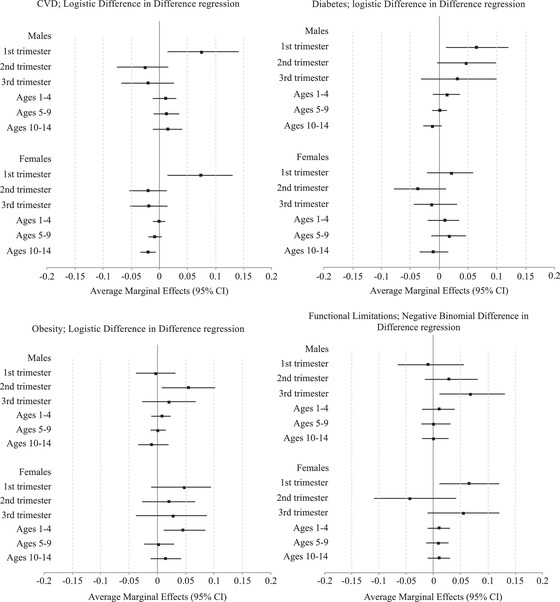
Average marginal effects of famine exposure on physical health by timing of exposure and gender
Table 4 presents DID estimates of auditory and visual impairments. For visual impairment, there are no treatment effects that are statistically significant either. Yet, famine exposure does increase the risk of developing auditory impairment for males. Males exposed during the second trimester show 71 percent (e 0.535 = (1.71 − 1 * 100)) higher odds of developing an auditory impairment.
TABLE 4.
Difference in difference logistic regression estimates for auditive impairment and visual impairment
| Auditive impairment | Visual impairment | |||
|---|---|---|---|---|
| Females | Males | Females | Males | |
| Interactions | ||||
| Famine region × first trimester | 0.22 | 0.32 | 0.31 | −0.04 |
| (0.29) | (0.31) | (0.31) | (0.28) | |
| Famine region × second trimester | −0.03 | 0.535 ** | −0.3 | 0.02 |
| (0.24) | (0.23) | (0.30) | (0.22) | |
| Famine region × third trimester | −0.04 | −0.29 | −0.16 | 0.1 |
| (0.26) | (0.32) | (0.31) | (0.23) | |
| Famine region × Ages 1–4 | −0.01 | 0.13 | −0.06 | 0.06 |
| (0.10) | (0.11) | (0.12) | (0.09) | |
| Famine region × Ages 5–9 | 0 | −0.04 | 0.02 | −0.12 |
| (0.10) | (0.10) | (0.12) | (0.09) | |
| Famine region × Ages 10–14 | 0.01 | 0.14 | −0.06 | −0.08 |
| (0.10) | (0.09) | (0.12) | (0.09) | |
| Famine region | −0.249 ** | −0.21 ** | −0.14 | −0.09 |
| (0.08) | (0.07) | (0.09) | (0.06) | |
| Birth groups | ||||
| First trimester | −1.191 *** | −1.702 *** | 1.114 *** | −1.227 *** |
| (0.05) | (0.19) | (0.07) | (0.05) | |
| Second trimester | −0.871 *** | −1.203 *** | 0.918 *** | −0.819 *** |
| (0.05) | (0.17) | (0.07) | (0.05) | |
| Third trimester | −0.39449 | −0.680 *** | 0.421 *** | −0.371 *** |
| (0.05) | (0.14) | (0.07) | (0.05) | |
| Ages 1–4 | −1.508 *** | −1.6 *** | 1.293 *** | −1.25 *** |
| (0.17) | (0.06) | (0.20) | (0.16) | |
| Ages 5–9 | −1.224 *** | −1.755 *** | 1.049 *** | −1.09 *** |
| (0.12) | (0.05) | (0.15) | (0.12) | |
| Ages 10–14 | −1.446 *** | −1.633 *** | 1.261 *** | −1.423 *** |
| (0.12) | (0.05) | (0.14) | (0.12) | |
NOTES: Standard errors in parentheses;
† p < 0.1, * p < 0.05, ** p < 0.01, *** p < 0.001.
Ref. group for cohort variables: born 1–4 years after famine. Survey fixed effects to capture potential differences through GEMON waves are included (not shown).
Table 5 presents estimated famine effects on socioeconomic outcomes. As seen in the first set of columns, with the exception of females exposed in the first trimester of gestation or at ages 10–14 exposure to famine decreases the odds of attaining a higher educational degree for all individuals. The severity of the impact is larger for males than for females. Importantly, as predicted the postnatal famine exposure is more consequential for educational attainment than in utero. For instance, the largest treatment effect for males is observed among those exposed at ages 1–4. Those exposed to famine at ages 1–4 show increased odds by 47 percent (e –0.64 = 1 − 0.53 *100)) to be in a lower educational category compared to those unexposed. Those exposed in the third trimester as well as in ages 5–14 show slightly smaller effects.
TABLE 5.
Difference in difference ordered logistic regression estimates for education and income quintile
| Education | Income quintile | |||||||
|---|---|---|---|---|---|---|---|---|
| Females | Males | Females | Males | |||||
| Interactions | β | SE | β | SE | β | SE | β | SE |
| Famine region × first trimester | −0.39 ** | (0.13) | −0.27 *** | (0.08) | 0.02 | (0.10) | −0.08 | (0.12) |
| Famine region × second trimester | −0.29 * | (0.13) | −0.46 *** | (0.08) | −0.12 | (0.10) | −0.12 | (0.11) |
| Famine region × Third trimester | −0.4 ** | (0.14) | −0.59 *** | (0.08) | −0.12 | (0.12) | −0.27 ** | (0.10) |
| Famine region × Ages 1–4 | −0.26 ** | (0.09) | −0.64 *** | (0.14) | −0.08 | (0.05) | −0.17 ** | (0.06) |
| Famine region × Ages 5–9 | −0.19 * | (0.10) | −0.51 *** | (0.12) | 0.02 | (0.05) | −0.09 † | (0.06) |
| Famine region × Ages 10–14 | −0.11 | (0.10) | −0.57 *** | (0.12) | 0.09 † | (0.05) | −0.04 | (0.06) |
| Famine region | 0.55 *** | (0.09) | 0.86 *** | (0.08) | 0.25 | (0.04) | 0.43 | (0.05) |
| Birth groups | ||||||||
| First trimester | 0.73 *** | (0.07) | 1.63 *** | (0.07) | 1.0 *** | (0.05) | 1.01 *** | (0.05) |
| Second trimester | 0.78 *** | (0.08) | 1.63 *** | (0.07) | 1.08 *** | (0.06) | 1.03 *** | (0.06) |
| Third trimester | 0.83 *** | (0.09) | 1.65 *** | (0.08) | 1.12 *** | (0.07) | 0.98 *** | (0.07) |
| Ages 1–4 | 0.66 *** | (0.06) | 1.49 *** | (0.05) | 0.81 *** | (0.03) | 0.77 *** | (0.03) |
| Ages 5–9 | 0.36 *** | (0.06) | 1.01 *** | (0.05) | 0.34 *** | (0.03) | 0.29 *** | (0.03) |
| Ages 10–14 | 0.19 ** | (0.06) | 0.62 *** | (0.05) | 0.1 *** | (0.03) | 0.04 | (0.04) |
NOTES: Standard errors in parentheses;
† p < 0.1, * p < 0.05, ** p < 0.01, *** p < 0.001.
Ref. group for cohort variables: born 1–4 years after famine. Survey fixed effects to capture potential differences through GEMON waves are included (not shown).
The results for income there share notable similarities to those for education. Effects are concentrated among males, with no statistically significant treatment effects for females (though coefficients were in the expected direction). The largest treatment effect is found for those who were exposed in the third trimester of gestation. Those exposed at this stage of gestation show a 24 percent (e –0.27 = (1 − 0.76 *100)) increase in the odds of being in a lower income. To a lesser extent, there are famine effects concentrated among individuals who lived through the famine at ages 1–4 and 5–9 as well. For an easier appreciation of these differences, we plot the treatment effects in Figure 4 for each cohort and gender. The results show there is a decrease in educational attainment for most cohort groups due to exposure to famine. 6 The largest effect is located in males exposed at ages 1–4, where famine exposure decreased the likelihood of having a higher educational attainment category by approximately 22 percent
FIGURE 4.
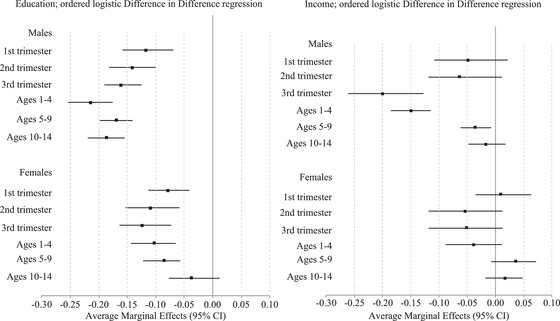
Average marginal effects of famine exposure on socioeconomic indicators by timing of exposure and gender
To formally test gender differences, we estimated pooled‐gender models and we include gender X age X famine region three‐way interaction terms. For simplicity, we display these results in the Appendix. We find males presented a higher risk of developing diabetes, functional limitations, auditory impairment, lower self‐rated health, and lower income if exposed in utero. For education, we find males display lower levels if exposed from ages 1 to 9. We find no statistically significant differences for the rest of outcomes in the study.
Sensitivity analysis
The DID approach used here rests on a set of assumptions. Most crucial is the parallel trend assumption (Branas et al. 2011; Gangl 2010; King et al. 2013). That is, in the absence of the treatment, the trends in the outcome between the control group and treatment group are the same. In the current case, suggestive evidence that this assumption is met can be seen in overall differences in health in between famine regions and nonfamine regions. They are either nonexistent or are positively higher in famine regions for those cohorts who were born after the famine period.
In addition, we perform two placebo tests based on variation in time and in place to provide further evidence. The first test restricts the sample to those individuals outside of the affected areas. Subsequently, we randomly dichotomize regions into “affected” and “nonaffected” regions within the nonaffected areas, this is all regions besides the Western provinces (i.e., Noord‐Holland, Zuid‐Holland, and Utrecht). These estimates provide a “placebo” test for the source of variation that stems from the region of birth. The second robustness test consists of restricting the analysis to the post‐WWII cohorts and randomly dichotomizing individuals into treated and control groups. These estimates provide a “placebo” test for the source of variation that comes from the timing of birth for different cohorts. The results of these tests show the “placebos” had no effect on either of the samples, indicating differences are attributable to famine exposure. These results are available in Appendix.
We conducted additional analyses on ancillary measures of health, including a number of chronic conditions, stroke, and risky behaviors (i.e., excessive drinking and daily smoking). In regard to the number of chronic conditions, we find a similar pattern to the results obtained in studying physical health. Males exposed to famine during the second and third trimesters of gestation show a higher number of chronic conditions. We did not find any effects on stroke for any cohort group or gender. In relation to risky behaviors, despite coefficients in the expected direction (i.e., famine exposure increases risky behaviors), there are none that are statistically significant.
Selection effects
A potential threat to the validity of the results is selection effects due to differential mortality, migration, and fertility. Selection effects related to mortality can pertain to immediate impacts on mortality or to delayed impacts on mortality (i.e., longevity). Previous research has found immediate excess mortality across all age groups in response to the Dutch Famine, yet; there was a heightened excess mortality for those who were exposed during their first year of life and for those who were 70 years of age and above (Ekamper et al. 2017; Lumey and Van Poppel 1994).
In terms of longevity, those exposed during adolescence and early childhood show small effects of famine exposure on mortality, and such effects become more pronounced for cohorts exposed during infancy and in utero. Shortened longevity essentially disappears for cohorts born immediately after the famine. Considering the results show heightened vulnerabilities for cohorts exposed in utero and infancy, and a resilience for cohorts exposed at older ages, it is likely the effects shown in this study's estimations are understated. Appendix B presents survival analysis estimates of differential hazard rates by gender and by timing of exposure. We find males experience a reduction in longevity as a consequence of the exposure to famine, with heightened effects if exposure occurred in utero. This implies our findings in this study are like to be underestimates, as those males that were most affected by the famine were less likely to survive to participate in 2016.
In terms of fertility, previous research has shown famine exposures can have strong effects on both fertility and fecundity. Stein and Suzzer (1975) found steep drops in births during the Dutch Hunger Winter, followed by rapid catch‐up in births once the famine ended. They also observed distinct social class differences, where parents in manual labor occupations experienced the most pronounced drops in fertility during the famine and the largest catch‐up fertility afterwards. Razzaque (2008) found similar patterns in rural fertility during the 1974 Bangladesh famine. Hence, it is possible there are strong selection effects regarding Socioeconomic Status (SES). Potentially, a larger number of babies selected into the treatment groups come from a higher SES background as fertility in high SES was more resilient to famine effects. Similarly, the control group is potentially comprised of a greater proportion of babies born to lower SES backgrounds. If this were the case, it is likely the famine effect presented above is understated.
Our estimates are also potentially subject to selection effects due to migration. Specifically, there are two patterns of migration that would threaten the validity of our estimates. The first is if exposed pregnant women living in the famine region migrated to the nonfamine region during the famine period, then gave birth in the nonfamine region. As a result, the later life outcomes from those births would be incorrectly counted among the nonexposed group, biasing our estimates downwards. The second problematic pattern would be if there was differential permanent migration out of the Netherlands by famine exposure. Those that permanently left the Netherlands prior to GENMON enrollment would not show up in the data. If that group was more heavily concentrated among those exposed or not exposed to the famine then our results would be biased downwards or upwards, respectively. Otherwise, assuming the Dutch census accurately records the place of residence during the famine period, subsequent internal migration after the famine would have no adverse impact on our estimates, as these are identified based exclusively on residence during the famine. In order to assess potential migration effects, we examined patterns of internal migration of the Dutch population for each cohort using Survey of Health, Ageing, and Retirement in Europe life history data. Approximately, 4–6 percent of the Dutch population experienced migration prior to the beginning of WWII. During WWII, this percentage rises to approximately 10 percent. External migration was unlikely at that time, as only 2 percent of the Dutch population left the country. Furthermore, we analyzed migration patterns for those exposed to famine from postfamine periods to 2016. The average proportion of migration from the western Netherlands to other regions is approximately 20 percent. However, migration patterns are stable across cohorts, indicating the likelihood of our estimates being biased is unlikely. We further test potential effects by formally modeling the impact of famine exposure on postfamine migration. We find no statistically significant effects. Furthermore, we also modeled the effect of postfamine migration on health outcomes in later life and find no statistically significant associations. In light of these tests, we believe migration is not substantially affecting our estimates. These results are available in the Appendix.
Famine treatment effects may also be confounded by outside war‐related stressors. For example, if the famine regions were more likely to experience other stressors, such as violence and proximity to combat, then what is characterized as a treatment effect of famine may be the result of the war itself. To examined this, we looked at historical data on the location and timing of conflict events (bombing, frontline movements, battles, etc.) in the Netherlands between 1941 and 1945. Provinces with the highest concentration of combat events were the Southern and Northern provinces especially in around the Arnhem area during the Allies failed Operation Market Garden. The Western (famine‐affected) and Eastern provinces experienced lower concentrations of combat events throughout the war period (Ellis 1994; Cheng 2004). 7 Data on excess mortality during the war further bears this out. Famine‐affected Western regions experienced less war‐related excess mortality from external causes (e.g., injuries) and from infectious/parasitic diseases (Ekamper 2017). These imply that our estimates are most likely biased downward, as the implicit comparison groups in nonfamine regions were simultaneously experience conditions likely to adversely impact the outcomes examined.
Discussion
While prior research has established the in utero period as highly sensitive to malnutrition, this work tends to focus narrowly on the gestational window, eschewing a more comprehensive developmental approach. Furthermore, most extant research has only examined one or two specific health conditions at a time in isolation, with few comparisons across a wider array of exposure timings and life course outcomes. As a result, questions about how various early life sensitive periods across developmental domains differ in terms of vulnerability to the effects of malnutrition remain largely underexplored. In addition, scant research has examined the extent to which there are gender differentials in the long‐term effects of early life malnutrition.
Using a unique dataset combining a large, nationally representative health survey and population registry data, the current study attempted to fill these gaps by re‐examining the Dutch Hunger Winter. This study highlights the notion that windows of vulnerability to malnutrition open and close contingent on the stage of development not only in terms of severity but also in regard to the nature of consequences. We hypothesized that in utero exposure to malnutrition results in biological adaptations, increasing risks of cardiometabolic, muscular‐skeletal physical problems, and sensorial impairment in later life. However, as the timing of exposure shifts to the postnatal and later childhood periods, different developmental processes come into play. As such, we hypothesized vulnerabilities to famine exposure shift from being primarily biological in nature to increasingly impacting processes implicated in social development and outcomes (education and income).
Our results show the in utero window is most vulnerable to malnutrition effects that lead to physiological consequences consistent with metabolic syndrome in later life (e.g., diabetes, cardiovascular disease, obesity) as well as muscular‐skeletal deficiencies and auditory impairment. Conversely, for cohorts exposed at later developmental stages (childhood and adolescence), results suggest a resilience to the effects of malnutrition on physical health in late life, but a higher vulnerability with regard to socioeconomic indicators.
Finally, research from developmental biology has shown that some structures and systems develop faster in female fetuses, shortening the window of vulnerability to insults during critical developmental periods for males. Therefore, we hypothesized important gender differentials in which famine exposure would result in more severe and frequent deleterious physical health conditions in later life for males relative to females. The results support this hypothesis. Male babies exposed in utero show stronger negative consequences across a wider array of conditions (self‐rated health, cardiovascular disease, obesity, functional limitations, and auditory impairment) than their female peers.
We also found important gender differences in the impact of on socioeconomic outcomes. Compared to their similarly exposed female peers, males show substantially larger reductions in educational attainment resulting from famine exposure. Interestingly, the differences are especially pronounced if the exposure occurred during childhood and adolescence. Furthermore, in terms of reductions in income, we only find effects among males. We believe part of the gap is likely due to differential gender and biological processes contingent on when the exposure occurred in the life course. Famine exposure occurring in utero or early childhood is likely to impact brain development and cognitive abilities, which would in turn affect educational attainment. However, for exposures occurring during later childhood and adolescence, processes related to social and gender role expectations are likely to be more important. The cohorts examined here were brought up prior to the gender revolution of the 1960s and 1970s (Goldin 2006). Hence, it is likely that highly gendered social and educational expectations played an important role in the extent to which early life exposure to famine impacted socioeconomic attainment differentially among boys and girls. If educational expectations and investments were substantially curtailed for girls relative to boys, then early life exposures which stunted the top of cognitive and achievement distributions would appear to be less consequential for the socioeconomic outcomes of girls.
The Dutch Hunger Winter literature often overlooks the fact that war and famine are contextual phenomena that exceed individuals’ exposure to physical violence or food shortages. Although the primary hazard was a severe food shortage, the period of the Dutch Hunger Winter was further characterized by high levels of violence, infrastructure and institutional breakdown, extreme cold, overcrowding, military executions, and breakdown of sanitation systems (Ekamper et al. 2017; Lumey et al. 2011). Such contextual exposures are likely to have important implications for cognitive development as well as educational and occupational outcomes.
As such war‐related exposures can induce high levels of stress, which may have long‐lasting physiological impacts apart from malnutrition or which may compound famine effects. A wide array of animal studies show that young adults exposed in utero to maternal psychosocial stress—and postnatal stress—lead to a dysregulation in key physiological systems, increasing the risk for developing higher BMI and percent body fat, primary insulin resistance, and a lipid profile consistent with the metabolic syndrome (Entringer and Wadhwa 2013; Kuzawa and Quinn 2009b; Paternain et al. 2013; Thayer and Kuzawa 2015). While we are not able to test differential effects via malnutrition or via psychological stress, it is important to recognize that stress processes induced by the famine and the broader context of war it accompanied likely contributed to adverse health in later life, independently of those related to nutritional deprivation presented here. In other words, the estimated long‐term effects of the Dutch Hunger Winter are not likely to be purely the result of famine/nutrition‐related processes. Rather, malnutrition is likely to be but one axis within a broader deleterious social, material, and psychological milieu cascading across the life course of effected cohorts.
It is worth noting the Dutch Hunger Winter lasted approximately —six to seven months. While the effects found in this study might seem small at a first glance, if one takes into consideration the duration of the famine, it is clear the effect sizes are quite substantial. One can only imagine the long‐term effects of famines of longer durations (e.g., the Spanish post‐Civil War famine lasted from 1939 to 1950) or persistent and prolonged malnutrition experienced in many contemporary low‐income contexts. As such the findings have important policy implications. While substantial progress has occurred over the past two decades in reducing malnutrition, particularly in Asia, Latin America, and the Caribbean, globally, stunting and wasting continue to be a major concern. Around the world, approximately 150 million children under the age of 5 suffer from stunting 8 another 45 million suffer from wasting 9 (FAO 2021). UNICEF, WHO, and other organizations specializing in famines, wars, and natural disasters have placed their focus on what is known as the essential nutrition actions (ENA). These policy actions provide nutrition interventions targeting the first 1,000 days of life with the aim of reducing infant and child mortality, improving physical and mental growth and development, and improving productivity. While the first 1,000 days of life are crucial, the present study shows interventions should also focus on expectant mothers and women in childbearing ages. Additionally, children outside the 1,000 first days of life window are also vulnerable, and at least for some outcomes, maybe even more so than infants and those in utero. Our results present compelling evidence supporting the notion that exposure during childhood and adolescence has the greatest effects on reductions in educational attainment. These education effects are likely to have lasting impacts on a wide variety of health and social outcomes across the life course. Overall, the results highlight that policies to improve early life nutrition and eliminate malnutrition are likely to yield large long‐term population health benefits beyond specific improvements to childhood health and survival.
Ultimately, the present study highlights the importance of considering multiple windows of vulnerability, spanning various developmental domains. As individuals move through different developmental stages, a multiplicity of critical periods can lead to heterogeneous outcomes contingent on when the exposure to malnutrition occurs. It further emphasizes the important role that gender plays in modulating malnutrition‐induced alterations to developmental trajectories.
Supporting information
Supporting Information
Acknowledgments
This project has received funding from the European Research Council under the European Union's Horizon 2020 research and innovation programme (grant agreement No 788582). This publication reflects only the author(s)'s view and the Research Executive Agency and the Commission are not responsible for any use that may be made of the information it contains.
Daniel Ramirez, Institute of Economy, Geography and Demography (IEGD), CSIC‐CCHS, Madrid, 28037, Spain. E‐mail: danielramirezsmith@gmail.com. Steven A Haas, Department of Sociology and Criminology, Pennsylvania State University, University Park, PA, 16802, USA.
Notes
Results based on calculations by *authors* , using nonpublic microdata from Statistics Netherlands. Under certain conditions, these microdata are accessible for statistical and scientific research. For further information: microdata@cbs.nl.
Previous research has asserted percentages of missing data between 10% and 15% are inconsequential for the results of analyses (Dong and Peng 2013; Enders 2010; Johnson and Young 2011). For this reason, we opted to utilize listwise deletion approaches in our analysis.
Sample size by cohort is available in Table 1. descriptive statistics.
With the exception of education and income, all measures included in the study are of a dichotomous nature. While the dataset used for the study only offers such measures in a dichotomous format, in particular for diagnoses, it is worth noting that continuous or ordinal measurements could yield richer differences in severity of conditions.
This educational scale is taken as provided by the GEMON dataset and is particular to the Dutch society.
It is important to note we do not explore indirect effects acting via educational attainment. It is possible reductions in income (and other outcomes related health) may operate via indirect effects. Because this expands beyond the scope of this particular study, we opted to exclude such analysis.
A deeper discussion on the matter as well as descriptive statistics sourced from Ekamper (2017), Ellis (1994), and Cheng (2004) is available in the Appendix.
Stunting is defined as an impaired growth and development that children experience as a consequence of poor nutrition and infections and can have irreversibly effects on cognitive and physical development, as well as an increased risk of chronic and degenerative diseases.
Low weight‐for‐height is known as wasting. It usually indicates recent and severe weight loss because a person has not had enough food to eat and/or they have had an infectious disease (e.g., diarrhea).
References
- Aiken, Catherine E. , and Ozanne Susan E.. 2013. “Sex Differences in Developmental Programming Models.” Reproduction 145(1): R1–13. 10.1530/REP-11-0489. [DOI] [PubMed] [Google Scholar]
- Akachi, Yoko , and Canning David. 2007. “The Height of Women in Sub‐Saharan Africa: The Role of Health, Nutrition, and Income in Childhood.” Annals of Human Biology 34(4): 397–410. 10.1080/03014460701452868. [DOI] [PubMed] [Google Scholar]
- Akresh, Richard , Verwimp Philip, and Bundervoet Tom. 2011. “Civil War, Crop Failure, and Child Stunting in Rwanda.” Economic Development and Cultural Change 59(4): 777–810. 10.1086/660003. [DOI] [Google Scholar]
- Almond, Douglas , and Currie Janet. 2011. “Killing Me Softly: The Fetal Origins Hypothesis.” The Journal of Economic Perspectives 25(3): 153–172. 10.1257/jep.25.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alur, Pradeep . 2019. “Sex Differences in Nutrition, Growth, and Metabolism in Preterm Infants.” Frontiers in Pediatrics 7: 22. 10.3389/fped.2019.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves, Jasmin M. , Luo Shan, Chow Ting, Herting Megan, Xiang Anny H., and Page Kathleen A.. 2019. “Sex Differences in the Association between Prenatal Exposure to Maternal Obesity and Hippocampal Volume in Children.” Brain and Behavior 10(2): e01522. 10.1002/brb3.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampaabeng, Samuel K. , and Tan Chih Ming. 2013. “The Long‐Term Cognitive Consequences of Early Childhood Malnutrition: The Case of Famine in Ghana.” Journal of Health Economics 32(6): 1013–1027. 10.1016/j.jhealeco.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Arage, Getachew , Belachew Tefera, Abera Mubarek, Abdulhay Fedilu, Abdulahi Misra, and Hassen Abate Kalkidan. 2020. “Consequences of Early Life Exposure to the 1983–1985 Ethiopian Great Famine on Cognitive Function in Adults: A Historical Cohort Study.” BMJ Open 10(9) :e038977. 10.1136/bmjopen-2020-038977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banning, C. 1946. “Food Shortage and Public Health, First Half of 1945.” The Annals of the American Academy of Political and Social Science 245(1): 93–110. 10.1177/000271624624500114. [DOI] [Google Scholar]
- Barker, D. 2004. “Developmental Origins of Adult Health and Disease.” Journal of Epidemiology and Community Health 58(2): 114–115. 10.1136/jech.58.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker, D. J. 1990. “The Fetal and Infant Origins of Adult Disease.” BMJ (Clinical Research Ed.) 301(6761): 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouki, Robert , Gluckman Peter D., Grandjean Philippe, Hanson Mark, and Heindel Jerrold J.. 2012. “Developmental Origins of Non‐Communicable Disease: Implications for Research and Public Health.” Environmental Health: A Global Access Science Source 11: 42. 10.1186/1476-069X-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz, Sarah , Mody Aaloke, Hornik Christoph, Bain James, Muehlbauer Michael, Kiyimba Tonny, Kiboneka Elizabeth, Stevens Robert, Bartlett John, Peter John V. St, Newgard Christopher B., and Freemark Michael. 2014. “Severe Acute Malnutrition in Childhood: Hormonal and Metabolic Status at Presentation, Response to Treatment, and Predictors of Mortality.” The Journal of Clinical Endocrinology & Metabolism 99(6): 2128–2137. 10.1210/jc.2013-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso, Olga . 2008. “Birth Weight Is Forever.” Epidemiology (Cambridge, Mass.) 19(2): 204–205. 10.1097/EDE.0b013e31816379d9. [DOI] [PubMed] [Google Scholar]
- Bateson, Patrick , and Gluckman Peter. 2012a. “Plasticity and Robustness in Development and Evolution.” International Journal of Epidemiology 41(1):219–223. 10.1093/ije/dyr240. [DOI] [PubMed] [Google Scholar]
- Bateson, Patrick , and Gluckman Peter. 2012b. “Plasticity and Robustness in Development and Evolution.” International Journal of Epidemiology 41(1):219–223. 10.1093/ije/dyr240. [DOI] [PubMed] [Google Scholar]
- Belli, Pedro . 1971. “The Economic Implications of Malnutrition: The Dismal Science Revisited.” Economic Development and Cultural Change 20(1):1–23. 10.1086/450527. [DOI] [Google Scholar]
- Belli, Pedro . 1975. “The Economic Implications of Malnutrition: Reply.” Economic Development and Cultural Change 23(2):353–357. 10.1086/450795. [DOI] [Google Scholar]
- Belsky, Jay , and Pluess Michael. 2009. “The Nature (and Nurture?) of Plasticity in Early Human Development.” Perspectives on Psychological Science 4(4):345–351. 10.1111/j.1745-6924.2009.01136.x. [DOI] [PubMed] [Google Scholar]
- Beshir, Habtamu Ali , and Maystadt Jean‐François. 2020. “In Utero Seasonal Food Insecurity and Cognitive Development: Evidence on Gender Imbalances From Ethiopia.” Journal of African Economies 29(4):412–431. 10.1093/jafeco/ejz028. [DOI] [Google Scholar]
- Bisioli, Claudio . 2004. “Sex Ratio of Births Conceived during Wartime.” Human Reproduction 19(1):218–219. 10.1093/humrep/deh027. [DOI] [PubMed] [Google Scholar]
- Black, Sandra E. , Devereux Paul J., and Salvanes Kjell. 2005. From the Cradle to the Labor Market? The Effect of Birth Weight on Adult Outcomes. Working Paper 11796. National Bureau of Economic Research. 10.3386/w11796. [DOI] [Google Scholar]
- Branas, Charles C. , Cheney Rose A., MacDonald John M., Tam Vicky W., Jackson Tara D., and Have Thomas R. Ten. 2011. “A Difference‐in‐Differences Analysis of Health, Safety, and Greening Vacant Urban Space.” American Journal of Epidemiology 174(11): 1296–1306. 10.1093/aje/kwr273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister, David M. , Johnson Taylor R., Lai Zhao, Scroggins Shannon R., DeRosa Mark, Jonas Rachelle B., Zhu Caroline, Scherer Elizabeth, Stewart Ronald M., Schwacha Martin G., Jenkins Donald H., Eastridge Brian J., and Nicholson Susannah E.. 2020. “The Gut Microbiome Distinguishes Mortality in Trauma Patients upon Admission to the Emergency Department.” Journal of Trauma and Acute Care Surgery 88(5): 579–587. 10.1097/TA.0000000000002612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, C. P. (2004). World War II database (Library of Congress License Control No. 2011214255). Retrieved from https://ww2db.com, April 8th 2022.
- Currie, Janet , and Hyson Rosemary. 1999. “Is the Impact of Health Shocks Cushioned by Socioeconomic Status? The Case of Low Birthweight.” Child Welfare 89(2): 6. [Google Scholar]
- Cynader, Max S. 1999. “Mechanisms of Brain Development and Their Role in Health and Well‐Being.” Daedalus 123(4): 155–165. [Google Scholar]
- Deaton, Angus . 2007. “Height, Health, and Development.” Proceedings of the National Academy of Sciences 104(33): 13232–13237. 10.1073/pnas.0611500104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriou, Christiana A. , van Veldhoven Karin, Relton Caroline, Stringhini Silvia, Kyriacou Kyriacos, and Vineis Paolo. 2015. “Biological Embedding of Early‐Life Exposures and Disease Risk in Humans: A Role for DNA Methylation.” European Journal of Clinical Investigation 45(3): 303–332. 10.1111/eci.12406. [DOI] [PubMed] [Google Scholar]
- Devaraj, Sridevi , Hemarajata Peera, and Versalovic James. 2013. “The Human Gut Microbiome and Body Metabolism: Implications for Obesity and Diabetes.” Clinical Chemistry 59(4): 617–628. 10.1373/clinchem.2012.187617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dols, M. J. L. , and van Arcken D. J. A. M.. 1946. “Food Supply and Nutrition in the Netherlands during and Immediately after World War II.” The Milbank Memorial Fund Quarterly 24(4): 319–358. 10.2307/3348196. [DOI] [PubMed] [Google Scholar]
- Dominguez, Tyan Parker , Dunkel‐Schetter Christine, Glynn Laura M., Hobel Calvin, and Sandman Curt A.. 2008. “Racial Differences in Birth Outcomes: The Role of General, Pregnancy, and Racism Stress.” Health Psychology 27(2): 194–203. 10.1037/0278-6133.27.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Yiran , and Peng Chao‐Ying Joanne. 2013. “Principled Missing Data Methods for Researchers.” SpringerPlus 2: 222. 10.1186/2193-1801-2-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij, Susanne R. , Painter Rebecca C., Holleman Frits, Bossuyt Patrick MM, and Roseboom Tessa J.. 2007. “The Metabolic Syndrome in Adults Prenatally Exposed to the Dutch Famine.” The American Journal of Clinical Nutrition 86(4): 1219–1924. 10.1093/ajcn/86.4.1219. [DOI] [PubMed] [Google Scholar]
- Ekamper, Peter , Bijwaard Govert, van Poppel Frans, and Lumey L. H.. 2017. “War‐Related Excess Mortality in The Netherlands, 1944–45: New Estimates of Famine‐ and Non‐Famine‐Related Deaths from National Death Records.” Historical Methods 50(2): 113–128. 10.1080/01615440.2017.1285260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekamper, Peter , Bijwaard Govert, van Poppel Frans, and Lumey L. H.. 2017. “War‐Related Excess Mortality in The Netherlands, 1944‐45: New Estimates of Famine‐ and Non‐Famine‐Related Deaths from National Death Records.” Historical Methods 50(2): 113–128. 10.1080/01615440.2017.1285260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder, Glen H. 1998. “The Life Course as Developmental Theory.” Child Development 69(1): 1–12. 10.2307/1132065. [DOI] [PubMed] [Google Scholar]
- Ellis, John . 1994. World War II: A Statistical Survey: The Essential Facts and Figures for All the Combatants. First edition. New York, NY: Facts on File. [Google Scholar]
- Enders, Craig K. 2010. Applied Missing Data Analysis. 1 edition. New York: The Guilford Press. [Google Scholar]
- Entringer, Sonja , Buss Claudia, and Wadhwa Pathik D.. 2015. “Prenatal Stress, Development, Health and Disease Risk: A Psychobiological Perspective – 2015 Curt Richter Award Winner.” Psychoneuroendocrinology 62: 366–375. 10.1016/j.psyneuen.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer, Sonja , and Wadhwa Pathik D.. 2013. “Developmental Programming of Obesity and Metabolic Dysfunction: Role of Prenatal Stress and Stress Biology.” In Nestlé Nutrition Institute Workshop Series, Vol. 74, edited by Bhatia J., Bhutta Z. A., and Kalhan S. C., 107–120. Basel: S. Karger AG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO, IFAD . 2021. The State of Food Security and Nutrition in the World 2021: Transforming food systems for food security, improved nutrition and affordable healthy diets for all. The State of Food Security and Nutrition in the World (SOFI) 2021. Rome, Italy: FAO. 10.4060/cb4474en. [DOI]
- Franzek, Ernst J. 2019. “Prenatal Malnutrition and Its Devastating Consequences on Mental Health Later in Life.” Open Journal of Nutrition and Food Sciences 1: 6. [Google Scholar]
- Gangl, Markus . 2010. “Causal Inference in Sociological Research.” Annual Review of Sociology 36: 21–47. [Google Scholar]
- Gladstone, Melissa , Mallewa Mac, Jalloh Alhaji Alusine, Voskuijl Wieger, Postels Douglas, Groce Nora, Kerac Marko, and Molyneux Elizabeth. 2014. “Assessment of Neurodisability and Malnutrition in Children in Africa.” Seminars in Pediatric Neurology 21(1): 50–57. 10.1016/j.spen.2014.01.002. [DOI] [PubMed] [Google Scholar]
- Gluckman, Peter D. , Hanson Mark A., Cooper Cyrus, and Thornburg Kent L.. 2008. “Effect of in Utero and Early‐Life Conditions on Adult Health and Disease.” The New England Journal of Medicine 359(1): 61–73. 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman, Peter D. , Hanson Mark A., and Spencer Hamish G.. 2005. “Predictive Adaptive Responses and Human Evolution.” Trends in Ecology & Evolution 20(10): 527–533. 10.1016/j.tree.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Goldin, Claudia . 2006. The Quiet Revolution That Transformed Women's Employment, Education, and Family. Working Paper 11953. National Bureau of Economic Research. [Google Scholar]
- Hales, C. N. , and Barker D. J.. 1992. “Type 2 (Non‐Insulin‐Dependent) Diabetes Mellitus: The Thrifty Phenotype Hypothesis.” Diabetologia 35(7): 595–601. 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- Heijmans, B. T. , Tobi E. W., Stein A. D., Putter H., Blauw G. J., Susser E. S., Slagboom P. E., and Lumey L. H.. 2008. “Persistent Epigenetic Differences Associated with Prenatal Exposure to Famine in Humans.” Proceedings of the National Academy of Sciences 105(44): 17046–17049. 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd, Pamela , Palloni Alberto, Rey Federico, and Dowd Jennifer B.. 2018. “Social and Population Health Science Approaches to Understand the Human Microbiome.” Nature Human Behaviour 2(11): 808–815. 10.1038/s41562-018-0452-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzman, Clyde . 1999. “The Biological Embedding of Early Experience and Its Effects on Health in Adulthood.” Annals of the New York Academy of Sciences 896(1): 85–95. 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- Hertzman, Clyde . 1999. Population health and human development. In Developmental Health and the Wealth of Nations: Social, Biological, and Educational Dynamics, edited by Keating D. P. and Hertzman C., pp. 21–40. New York: The Guilford Press, [Google Scholar]
- Hertzman, Clyde , and Keating Daniel P.., eds. 1999. Developmental Health and the Wealth of Nations: Social, Biological, and Educational Dynamics. New York: The Guilford Press. [Google Scholar]
- Hobel, Calvin , and Culhane Jennifer. 2003. “Role of Psychosocial and Nutritional Stress on Poor Pregnancy Outcome.” The Journal of Nutrition 133(5): 1709S‐1717S. 10.1093/jn/133.5.1709S. [DOI] [PubMed] [Google Scholar]
- Huang, Cheng , Beth J. Soldo, and Elo Irma T.. 2011. “Do Early‐Life Conditions Predict Functional Health Status in Adulthood? The Case of Mexico.” Social Science & Medicine (1982) 72(1): 100–107. 10.1016/j.socscimed.2010.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idler, Ellen L. , and Benyamini Yael. 1997. “Self‐Rated Health and Mortality: A Review of Twenty‐Seven Community Studies.” Journal of Health and Social Behavior; Albany, N.Y. 38(1): 21. [PubMed] [Google Scholar]
- James, William H. 2009. “The Variations of Human Sex Ratio at Birth during and after Wars, and Their Potential Explanations.” Journal of Theoretical Biology 257(1):116–123. 10.1016/j.jtbi.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Johnson, David R. , and Young Rebekah. 2011. “Toward Best Practices in Analyzing Datasets with Missing Data: Comparisons and Recommendations.” Journal of Marriage and Family 73(5): 926—945. 10.1111/j.1741-3737.2011.00861.x [DOI] [Google Scholar]
- Jylhä, Marja . 2009. “What Is Self‐Rated Health and Why Does It Predict Mortality? Towards a Unified Conceptual Model.” Social Science & Medicine 69(3): 307–316. 10.1016/j.socscimed.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Kahn, H. S. , Narayan K. M. V., and Valdez R.. 1998. “Prenatal Exposure to Famine and Health in Later Life.” Lancet 351(9112): 1360–1361. 10.1016/S0140-6736(05)79093-0. [DOI] [PubMed] [Google Scholar]
- Kamel, Terez Boshra , Deraz Tharwat Ezzat, Elkabarity Rasha H., and Ahmed Rasha K.. 2016. “Protein Energy Malnutrition Associates with Different Types of Hearing Impairments in Toddlers: Anemia Increases Cochlear Dysfunction.” International Journal of Pediatric Otorhinolaryngology 85: 27–31. 10.1016/j.ijporl.2016.03.018. [DOI] [PubMed] [Google Scholar]
- King, Marissa , Essick Connor, Bearman Peter, and Ross Joseph S.. 2013. “Medical School Gift Restriction Policies and Physician Prescribing of Newly Marketed Psychotropic Medications: Difference‐in‐Differences Analysis.” BMJ 346: f264. 10.1136/bmj.f264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman, Ronald E. , Murphy J. Michael, Little Michelle, Pagano Maria, Wehler Cheryl A., Regal Kenneth, and Jellinek Michael S.. 1998. “Hunger in Children in the United States: Potential Behavioral and Emotional Correlates.” Pediatrics 101(1): e3. 10.1542/peds.101.1.e3. [DOI] [PubMed] [Google Scholar]
- Kleinman, R.E. , Hall S., Green H., Korzec‐Ramirez D., Patton K., Pagano M.E., and Murphy J.M.. 2002. “Diet, Breakfast, and Academic Performance in Children.” Annals of Nutrition & Metabolism 46(1): 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh, D. , Ben‐Shlomo Y., Lynch J., Hallqvist J., and Power C.. 2003. “Life Course Epidemiology.” Journal of Epidemiology and Community Health 57(10): 778–783. 10.1136/jech.57.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh, Diana , and Ben Shlomo Yoav. 2004. A Life Course Approach to Chronic Disease Epidemiology. Oxford, UK: Oxford University Press. [Google Scholar]
- Kuzawa, Christopher W. , and Quinn Elizabeth A.. 2009a. “Developmental Origins of Adult Function and Health: Evolutionary Hypotheses.” Annual Review of Anthropology 38(1): 131–147. 10.1146/annurev-anthro-091908-164350. [DOI] [Google Scholar]
- Kuzawa, Christopher W. , and Quinn Elizabeth A.. 2009b. “Developmental Origins of Adult Function and Health: Evolutionary Hypotheses.” Annual Review of Anthropology 38(1): 131–147. 10.1146/annurev-anthro-091908-164350. [DOI] [Google Scholar]
- Lauderdale, Diane S. 2006. “Birth Outcomes for Arabic‐Named Women in California before and after September 11.” Demography 43(1): 185–201. 10.1353/dem.2006.0008. [DOI] [PubMed] [Google Scholar]
- Lockwood, C. J. , and Kuczynski E.. 1999. “Markers of Risk for Preterm Delivery” 27(1): 5–20. 10.1515/JPM.1999.001. [DOI] [PubMed] [Google Scholar]
- Lorente‐Pozo, Sheila , Parra‐Llorca Anna, Torres Begoña, Torres‐Cuevas Isabel, Nuñez‐Ramiro Antonio, Cernada María, García‐Robles Ana, and Vento Maximo. 2018. “Influence of Sex on Gestational Complications, Fetal‐to‐Neonatal Transition, and Postnatal Adaptation.” Frontiers in Pediatrics 6: 63. 10.3389/fped.2018.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumey, L. H. , and Stein Aryeh D.. 1997. “Offspring Birth Weights after Maternal Intrauterine Undernutrition: A Comparison within Sibships.” American Journal of Epidemiology 146(10): 810–819. 10.1093/oxfordjournals.aje.a009198. [DOI] [PubMed] [Google Scholar]
- Lumey, L. H. , Stein Aryeh D., and Susser Ezra. 2011. “Prenatal Famine and Adult Health.” Annual Review of Public Health 32: 237—262. 10.1146/annurev-publhealth-031210-101230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumey, L. H. , and Van Poppel F. W. A.. 1994. “The Dutch Famine of 1944–45: Mortality and Morbidity in Past and Present Generations.” Social History of Medicine 7(2): 229–246. 10.1093/shm/7.2.229. [DOI] [PubMed] [Google Scholar]
- McEwen, Craig A. , and McEwen Bruce S.. 2017. “Social Structure, Adversity, Toxic Stress, and Intergenerational Poverty: An Early Childhood Model.” Annual Review of Sociology 43(1): 445–472. 10.1146/annurev-soc-060116-053252. [DOI] [Google Scholar]
- McFie, John , and Welbourn Hebe F.. 1962. “Effect of Malnutrition in Infancy on the Development of Bone, Muscle and Fat.” The Journal of Nutrition 76(2): 97–105. 10.1093/jn/76.2.97. [DOI] [Google Scholar]
- Neelsen, Sven . 2011. “Effects of Prenatal and Early Life Malnutrition: Evidence from the Greek Famine.” Journal of Health Economics, 30(3): 479–488. [DOI] [PubMed] [Google Scholar]
- Nelson, Charles A. 2013. “Biological Embedding of Early Life Adversity.” JAMA Pediatrics 167(12): 1098–1100. 10.1001/jamapediatrics.2013.3768. [DOI] [PubMed] [Google Scholar]
- Nelson, Charles A. 2017. “Hazards to Early Development: The Biological Embedding of Early Life Adversity.” Neuron 96(2): 262–266. 10.1016/j.neuron.2017.09.027. [DOI] [PubMed] [Google Scholar]
- Ntim‐Amponsah, C. T. , and Amoaku W. M. K.. 2008. “Causes of Childhood Visual Impairment and Unmet Low‐Vision Care in Blind School Students in Ghana.” International Ophthalmology 28(5): 317–323. 10.1007/s10792-007-9134-x. [DOI] [PubMed] [Google Scholar]
- O'Connell, Bree A. , Moritz Karen M., Roberts Claire T., Walker David W., and Dickinson Hayley. 2011. “The Placental Response to Excess Maternal Glucocorticoid Exposure Differs Between the Male and Female Conceptus in Spiny Mice.” Biology of Reproduction 85(5): 1040–1047. 10.1095/biolreprod.111.093369. [DOI] [PubMed] [Google Scholar]
- Painter, Rebecca C. , Roseboom Tessa J., and Bleker Otto P.. 2005. “Prenatal Exposure to the Dutch Famine and Disease in Later Life: An Overview.” Reproductive Toxicology (Elmsford, N.Y.) 20(3): 345–352. 10.1016/j.reprotox.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Palloni, Alberto , McEniry Mary, Huangfu Yiyue, and Beltran‐Sanchez Hiram. 2020. “Impacts of the 1918 Flu on Survivors’ Nutritional Status: A Double Quasi‐Natural Experiment.” PLoS ONE 15(10): e0232805. 10.1371/journal.pone.0232805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palloni, Alberto , Milesi Carolina, White Robert G., and Turner Alyn. 2009. “Early Childhood Health, Reproduction of Economic Inequalities and the Persistence of Health and Mortality Differentials.” Social Science & Medicine 68(9): 1574–1582. 10.1016/j.socscimed.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternain, L. , de la Garza A. L., Batlle M. A., Milagro F. I., Martínez J. A., and Campión J.. 2013. “Prenatal Stress Increases the Obesogenic Effects of a High‐Fat‐Sucrose Diet in Adult Rats in a Sex‐Specific Manner.” Stress 16(2): 220–232. 10.3109/10253890.2012.707708. [DOI] [PubMed] [Google Scholar]
- Ramirez, Daniel , and Haas Steven A.. 2021. “The Long Arm of Conflict: How Timing Shapes the Impact of Childhood Exposure to War.” Demography 58(3): 951–974. 10.1215/00703370-9114715. [DOI] [PubMed] [Google Scholar]
- Razzaque, A. 2008. “Effect of Famine on Fertility in a Rural Area of Bangladesh.” Journal of Biosocial Science 20(3): 287–294. 10.1017/s0021932000006623. [DOI] [PubMed] [Google Scholar]
- Ridout, Kathryn K. , Khan Mariam, and Ridout Samuel J.. 2018. “Adverse Childhood Experiences Run Deep: Toxic Early Life Stress, Telomeres, and Mitochondrial DNA Copy Number, the Biological Markers of Cumulative Stress.” BioEssays 40(9): 1800077. 10.1002/bies.201800077. [DOI] [PubMed] [Google Scholar]
- Rong, Hongguo , Lai Xiaozhen, Mahmoudi Elham, and Fang Hai. 2019. “Early‐Life Exposure to the Chinese Famine and Risk of Cognitive Decline.” Journal of Clinical Medicine 8(4): 484. 10.3390/jcm8040484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseboom, T. J. , van der Meulen J. H., Ravelli A. C., Osmond C., Barker D. J., and Bleker O. P.. 2001. “Effects of Prenatal Exposure to the Dutch Famine on Adult Disease in Later Life: An Overview.” Molecular and Cellular Endocrinology 185(1–2): 93–98. [DOI] [PubMed] [Google Scholar]
- Roseboom, Tessa J. , Painter Rebecca C., van Abeelen Annet F. M., Veenendaal Marjolein V. E., and de Rooij Susanne R.. 2011. “Hungry in the Womb: What Are the Consequences? Lessons from the Dutch Famine.” Maturitas 70(2): 141–145. 10.1016/j.maturitas.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Roseboom, Tessa J. , van der Meulen Jan H. P., Ravelli Anita C. J., Osmond Clive, Barker David J. P., and Bleker Otto P.. 2001. “Effects of Prenatal Exposure to the Dutch Famine on Adult Disease in Later Life: An Overview.” Molecular and Cellular Endocrinology 185(1): 93–98. 10.1016/S0303-7207(01)00721-3. [DOI] [PubMed] [Google Scholar]
- Rosenfeld, Cheryl S. 2015. “Sex‐Specific Placental Responses in Fetal Development.” Endocrinology 156(10): 3422—3434. 10.1210/en.2015-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen, K. M. 2001. “The ‘Fetal Origins’ Hypothesis: Challenges and Opportunities for Maternal and Child Nutrition.” Annual Review of Nutrition 21): 73–95. 10.1146/annurev.nutr.21.1.73. [DOI] [PubMed] [Google Scholar]
- Sayer*, A. Aihie , and Cooper C.. 2002. “Early Diet and Growth: Impact on Ageing.” Proceedings of the Nutrition Society 61(1): 79–85. 10.1079/PNS2001138. [DOI] [PubMed] [Google Scholar]
- Schacht, Ryan , Tharp Douglas, and Smith Ken R.. 2019. “Sex Ratios at Birth Vary with Environmental Harshness but Not Maternal Condition.” Scientific Reports 9(1): 9066. 10.1038/s41598-019-45316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz, Laura C. 2010. “The Dutch Hunger Winter and the Developmental Origins of Health and Disease.” Proceedings of the National Academy of Sciences of the United States of America 107(39): 16757–16758. 10.1073/pnas.1012911107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanner, Sara A. , and Yudkin John S.. 2001. “Fetal Programming and the Leningrad Siege Study.” Twin Research and Human Genetics 4(5): 287–292. 10.1375/twin.4.5.287. [DOI] [PubMed] [Google Scholar]
- Stein, Aryeh D. , Zybert Patricia A., Bruin Karin van der Pal‐de, and Lumey L. H.. 2006. “Exposure to Famine during Gestation, Size at Birth, and Blood Pressure at Age 59 y: Evidence from the Dutch Famine.” European Journal of Epidemiology 21(10): 759–765. 10.1007/s10654-006-9065-2. [DOI] [PubMed] [Google Scholar]
- Stein, Zena , and Susser Mervyn. 1975. “Fertility, Fecundity, Famine: Food Rations in the Dutch Famine 1944/5 Have a Causal Relation to Fertility, and Probably to Fecundity.” Human Biology 47(1): 131–154. [PubMed] [Google Scholar]
- Taras, Howard. 2005. “Nutrition and Student Performance at School.” Journal of School Health 75(6): 199–213. 10.1111/j.1746-1561.2005.tb06674.x. [DOI] [PubMed] [Google Scholar]
- Thayer, Zaneta M. , and Kuzawa Christopher W.. 2015. “Ethnic Discrimination Predicts Poor Self‐Rated Health and Cortisol in Pregnancy: Insights from New Zealand.” Social Science & Medicine 128: 36–42. 10.1016/j.socscimed.2015.01.003. [DOI] [PubMed] [Google Scholar]
- Jiménez Valdez, L., Guzmán O. D. López, Cervantes Flores M., Costilla‐Salazar R., Calderón Hernández J., Alcaraz Contreras Y., and Rocha‐Amador D. O.. 2017. “In Utero Exposure to Fluoride and Cognitive Development Delay in Infants.” NeuroToxicology 59: 65—70. 10.1016/j.neuro.2016.12.011 [DOI] [PubMed] [Google Scholar]
- Vineis, Paolo , Kelly‐Irving Michelle, Rappaport Stephen, and Stringhini Silvia. 2016. “The Biological Embedding of Social Differences in Ageing Trajectories.” Journal of Epidemiology and Community Health 70(2): 111–113. 10.1136/jech-2015-206089. [DOI] [PubMed] [Google Scholar]
- Xu, Hongwei , Zhang Zhenmei, Li Lydia, and Liu Jinyu. 2018. “Early Life Exposure to China's 1959–61 Famine and Midlife Cognition.” International Journal of Epidemiology 47(1): 109–120. 10.1093/ije/dyx222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Zhenmei , Gu Danan, and Hayward Mark D.. 2010. “Childhood Nutritional Deprivation and Cognitive Impairment among Older Chinese People.” Social Science & Medicine 71(5): 941–949. 10.1016/j.socscimed.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


