Abstract
Posttransplant diabetes mellitus (PTDM) and prediabetes (impaired glucose tolerance [IGT] and impaired fasting glucose [IFG]) are associated with cardiovascular events. We assessed the diagnostic performance of fasting plasma glucose (FPG) and HbA1c as alternatives to oral glucose tolerance test (OGTT)‐derived 2‐hour plasma glucose (2hPG) using sensitivity and specificity in 263 kidney transplant recipients (KTRs) from a clinical trial. Between visits at 6, 12, and 24 months after transplantation, 28%–31% of patients switched glycemic category (normal glucose tolerance [NGT], IGT/IFG, PTDM). Correlations of FPG and HbA1c against 2hPG were lower at 6 months (r = 0.59 [FPG against 2hPG]; r = 0.45 [HbA1c against 2hPG]) vs. 24 months (r = 0.73 [FPG against 2hPG]; r = 0.74 [HbA1c against 2hPG]). Up to 69% of 2hPG‐defined PTDM cases were missed by conventional HbA1c and FPG thresholds. For prediabetes, concordance of FPG and HbA1c with 2hPG ranged from 6%–9%. In conclusion, in our well‐defined randomized trial cohort, one‐third of KTRs switched glycemic category over 2 years and although the correlations of FPG and HbA1c with 2hPG improved with time, their diagnostic concordance was poor for PTDM and, especially, prediabetes. Considering posttransplant metabolic instability, FPG's and HbA1c's diagnostic performance, the OGTT remains indispensable to diagnose PTDM and prediabetes after kidney transplantation.
Short abstract
Analyses of data from kidney transplant recipients who participated in a randomized controlled trial show that the oral glucose tolerance test is indispensable to diagnose diabetes and pre‐diabetes because concordance between hemoglobin A1c and fasting plasma glucose tests is poor.
Abbreviations
- 2hPG
2‐hour plasma glucose
- ADA
American Diabetes Association
- AUC
area under the curve
- HbA1c
glycated hemoglobin
- IFG
impaired fasting glucose
- IGT
impaired glucose tolerance
- ITP‐NODAT
insulin therapy for the prevention of new onset diabetes after transplantation
- KTRs
kidney transplant recipients
- OGTT
oral glucose tolerance test
- PPV
positive predictive value
- PTDM
posttransplant diabetes mellitus
- ROC
receiver operating characteristic
- STARD
standards for the reporting of diagnostic accuracy studies
- WHO
World Health Organization
1. INTRODUCTION
Persistent hyperglycemia after solid‐organ transplantation has been recognized as an own diabetes entity 1 and is referred to as posttransplant diabetes mellitus (PTDM). Pathophysiologically, PTDM is linked to glucocorticoids and calcineurin inhibitors, which are required immunosuppressants after transplantation but have diabetogenic properties. 2 Predisposing factors that associate with diabetes in the general population and additional transplant‐specific factors add to individual PTDM risk. 3 PTDM has long been underdiagnosed due to a lack of standardized definition or diagnosis, 4 despite its clinical relevance, especially regarding increased risk for cardiovascular events and mortality. 5 , 6 , 7
At an international PTDM consensus guidelines meeting held in 2003, 8 it was recommended that PTDM be diagnosed with the diabetes criteria established by the American Diabetes Association (ADA) 9 and World Health Organization (WHO). 10 The diagnostic glycemia thresholds for type 2 diabetes in the general population were justified by their association with increased risk for microvascular complication (retinopathy). 11 Glycated hemoglobin (HbA1c) for diabetes diagnosis was recommended in 2008 by an international expert committee, 12 again considering retinopathy. A large study published in 2010 confirmed that HbA1c is also associated with cardiovascular risk. 13
In contrast to type 2 diabetes, the evidence linking PTDM to microvascular complications is scarce, 14 and retinopathy after transplantation is understudied, 15 as was acknowledged at a subsequent PTDM expert consensus meeting, held in 2014. 16 The pitfalls of using HbA1c in diagnosing PTDM during the early posttransplant phase, 17 and studies assessing HbA1c's diagnostic accuracy in kidney transplant recipients (KTRs) 18 , 19 , 20 led to the recommendation that the OGTT should be considered the most important diagnostic test. 16 Indeed, not only studies of undiagnosed type 2 diabetes 21 , 22 and impaired glucose tolerance (IGT) 23 in the general population, but also two studies from KTRs in particular 18 , 19 observed relatively low sensitivity of fasting plasma glucose (FPG) compared with the OGTT in identifying PTDM. Subsequent to the 2014 PTDM consensus meeting, two additional studies used single measurements of HbA1c in KTRs and found sensitivities of only 38% at 10 weeks 24 and 43% at 1 year 25 after transplant, compared with the OGTT.
However, a meta‐analysis investigating type 2 diabetes 26 and the study of KTRs at 10 weeks 18 indicated that lowered thresholds for HbA1c and FPG could be used in the early detection of previously undiagnosed individuals and, ultimately, could reduce the number of OGTTs needed. Indeed, the authors of all previous receiver operating characteristic (ROC) curve analyses 18 , 19 , 20 , 24 , 25 have eventually asked the same question: Under which circumstances could FPG and HbA1c be used safely to diagnose PTDM without missing patients at risk? Alternatively, might the diagnostic thresholds have to be altered for this purpose? To arrive at a definite conclusion, we here employed well‐characterized participants of a randomized controlled trial 27 to evaluate FPG and HbA1c against the OGTT at 6, 12, and 24 months after transplantation. Our specific aim was to determine the ability of FPG and HbA1c to identify PTDM and posttransplant prediabetes, in view of the fact that IGT and/or impaired fasting glucose (IFG) 6 are also strong predictors of cardiovascular events.
2. MATERIALS AND METHODS
2.1. Study participants
KTRs of this post‐hoc analysis of a multicenter randomized clinical trial previously participated in the Insulin Therapy for the Prevention of New Onset Diabetes After Transplantation study (ITP‐NODAT, ClinicalTrials.gov NCT03507829). 27 In ITP‐NODAT, individuals were randomized into two groups before transplantation. Participants allocated to the intervention group had received capillary blood glucose measurements and intermediate‐acting insulin isophane therapy for afternoon glucose surpassing 140 mg/dl (7.8 mmol/L). Standard‐of‐care participants had been allowed short‐acting insulin and sulfonylureas upon discharge. The ITP‐NODAT trial was performed from November 21, 2012, through May 22, 2018, at four European transplant centers (Medical University of Vienna, Austria; Medical University of Graz, Austria; Hospital del Mar Barcelona; and Charité Universitätsmedizin Berlin), with 2 years' follow‐up for each patient. Of all 263 adult KTRs in ITP‐NODAT (all without previously known impairment of glucose metabolism before transplantation, receiving glucocorticoids, mycophenolate acid, and tacrolimus), diagnostic test accuracy was evaluated in 217 participants. These were participants without continuous glucose‐lowering treatment and who had OGTT and HbA1c data available at 6, 12, and 24 months. All ITP‐NODAT trial participants provided written informed consent following approval from the institutional review board at each participating center. Approval for the present retrospective analysis of ITP‐NODAT trial data was additionally obtained from the ethics committee of the Medical University of Vienna (EK 1611/2020 [protocol and ethics vote available from the authors upon request]).
2.2. Laboratory measurements and definitions
For the evaluation of PTDM during the ITP‐NODAT trial, three OGTTs and HbA1c measurements had been scheduled at months 6, 12, and 24 after transplantation for all participants. As part of the previous clinical trial, the results of the OGTT at month 6 were blinded to study participants and study investigators and were revealed only in the final analysis. OGTTs were performed using a glucose load containing the equivalent of 75 g anhydrous glucose dissolved in water, as described by the WHO. 28 Plasma samples before glucose intake and after 2 h were separated promptly from whole blood and stored at −80°C for the subsequent study‐related measurements at the central laboratory site (Medical University of Vienna).
Glucose was assessed by the hexokinase method, and HbA1c was assessed by high‐performance liquid chromatography separation of hemoglobin fractions. 29 OGTTs and HbA1c measurements were obtained at the day of the study visit and were not available to the performers of the tests. Missing OGTT or HbA1c data led to exclusion of the patient for the respective time point. An independent investigator assessed the conclusiveness of the test results (e.g., whether unphysiological glucose values existed), during the data extraction process of the original study.
We used ADA criteria 1 and defined OGTT‐derived 2hPG ≥200 mg/dl (11.1 mmol/L) as PTDM and 140–199 mg/dl (7.8–11.0 mmol/L) as IGT in the absence of glucose‐lowering treatment (reference standard). PTDM can also be defined by FPG during an OGTT, and the combined FPG and 2hPG criteria are in this paper referred to as plasma glucose criteria to diagnose PTDM. FPG and HbA1c were evaluated as index tests, and besides HbA1c ≥ 6.5% (48 mmol/mol) and FPG ≥ 126 mg/dl (7.0 mmol/L), a previously proposed, lowered screening threshold for HbA1c of 6.2% (44 mmol/mol) and a combined screening criterion (FPG ≥ 126 mg/dl [7.0 mmol/L] and/or HbA1c ≥ 6.5% [48 mmol/mol]) 24 were assessed. Apart from PTDM, we evaluated thresholds for increased diabetes risk using ADA criteria for prediabetes, on the basis that IGT 5 as well as IGT and/or IFG pose specific risk factors after transplantation. 6 In the general population, IGT and IFG have also been shown to be characterized by distinct metabolic characteristics. 30 HbA1c was not added as a reference criterion for prediabetes because, to our knowledge, HbA1c 5.7%–6.4% (39–47 mmol/mol) has not been linked to cardiovascular events after transplantation 6 and was only evaluated as an index test, in contrast to IGT and IFG. The diagnostic accuracy of FPG 100–125 mg/dl (5.6–6.9 mmol/L) and HbA1c 5.7%–6.4% (39–47 mmol/mol) 1 were therefore assessed in the absence of PTDM and compared with the OGTT‐derived diagnosis of IGT and IGT and/or IFG.
Using these cut‐offs, we longitudinally evaluated HbA1c and FPG as index tests intended for use in diagnosis and screening and as a potential replacement for OGTT.
2.3. Statistical analyses
Categorical outcomes were described using frequencies and proportions, while continuous variables were described using means ± standard deviations (SDs) or medians and interquartile ranges (IQR) when appropriate. Group differences were evaluated using 95% confidence intervals, and p‐values were reported according to two‐tailed analysis and considered statistically significant when <.05. Linear correlations among FPG, HbA1c, and 2hPG were reported using Pearson's r. Classifier performance was assessed by the area under the curve (AUC) obtained by ROC curve analysis, and exploratory cut‐off points were identified for clinical application. For the diagnosis of IGT and IGT and/or IFG, the ordinal variables were dichotomized by excluding patients with diabetes. Area‐proportional Venn diagrams were drawn using eulerAPE_3.0.0. 31 Calculations were performed using Microsoft Excel 2020 for macOS (Microsoft Corporation), IBM SPSS Statistics for macOS Version 26.0 (IBM), and R 4.0.3 (R Core Team, 2020).
We used the Standards for the Reporting of Diagnostic Accuracy Studies (STARD) statement to ensure completeness of reporting 32 (Appendix S1).
3. RESULTS
3.1. Characteristics of the trial participants
Detailed information on the patient flow of 263 KTRs leading to the study population is provided in Figure S1. After omitting participants with continuous glucose‐lowering medication or missing OGTT and HbA1c data, diagnostic accuracy was analyzed in n = 217 participants (n = 188 participants at month 6, n = 183 at month 12, and n = 163 at month 24). The clinical and transplant‐specific characteristics of the trial participants at baseline are reported in Table 1. Steroid doses decreased from month 6 until month 24 (mean 5.2 mg vs. 4.0 mg prednisone per day in paired observations, p < .001).
TABLE 1.
Participant characteristics at baseline
| Characteristics | All (n = 263) | Study population (n = 217) |
|---|---|---|
| Demographic and metabolic characteristics | ||
| Female, n (%) | 100 (39.8) | 85 (39.5) |
| Age, years | 53.0 (40.0–61.0) | 51.0 (39.3–60.0) |
| ≥60 years, n (%) | 76 (30.0) | 55 (25.5) |
| BMI, kg/m2 | 25.2 (22.1–29.1) | 25.2 (22.1–28.4) |
| ≥30 kg/m2, n (%) | 48 (20.6) | 37 (18.7) |
| Plasma glucose, mg/dl | 94 (84–110) | 93 (84–106) |
| Plasma glucose, mmol/L | 5.2 (4.7–6.1) | 5.2 (4.7–5.9) |
| ≥100 mg/dl (5.6 mmol/mol), n (%) | 90 (37.0) | 70 (33.5) |
| NGSP HbA1c, % | 5.2 (4.9–5.5) | 5.1 (4.8–5.4) |
| IFCC HbA1c, mmol/mol | 33.3 (30–36.6) | 32.2 (29–35.5) |
| ≥5.7% (39 mmol/mol), n (%) | 36 (15.7) | 27 (13.8) |
| Pre‐transplant history | ||
| Primary kidney disease | ||
| Glomerular disease, n (%) | 91 (39.4) | 79 (40.5) |
| Vascular disease, n (%) | 29 (12.6) | 25 (12.8) |
| Tubulointerstitial disease, n (%) | 16 (6.9) | 16 (8.2) |
| Polycystic disease, n (%) | 39 (16.9) | 28 (14.4) |
| Unknown, n (%) | 53 (22.9) | 44 (22.6) |
| Other primary disease, n (%) | 3 (1.3) | 3 (1.5) |
| Family history of diabetes, n (%) | 38 (18.7) | 32 (18.5) |
| Chronic hepatitis C, n (%) | 3 (1.2) | 3 (1.4) |
| CMV antibody positive, n (%) | 171 (69.0) | 147 (69.3) |
| Transplantation information | ||
| Living donor, n (%) | 53 (21.2) | 49 (22.8) |
| Graft number | ||
| First, n (%) | 218 (82.9) | 187 (86.2) |
| Second, n (%) | 32 (12.2) | 26 (12.0) |
| More than two, n (%) | 3 (1.2) | 3 (1.4) |
| CMV high risk, n (%) | 42 (17.0) | 33 (15.6) |
| PRA highest ≥ 10%, n (%) | 26 (11.8) | 22 (11.7) |
| Immunosuppression early after transplantation | ||
| Tacrolimus, n (%) | 254 (100.0) | 217 (100.0) |
| Mycophenolate mofetil, n (%) | 120 (50.2) | 102 (50.2) |
| Mycophenolic sodium, n (%) | 119 (49.8) | 101 (49.8) |
| Glucocorticosteroid, n (%) | 253 (100.0) | 216 (100.0) |
Note: Values are presented as median (interquartile range) or number (percent). Missing entries early after transplantation were excluded, therefore percentages of immunosuppression are slightly deviating from the original publication, where percentages refer to 263 participants.
Abbreviations: BMI, body mass index; CMV, cytomegalovirus; IFCC, International Federation of Clinical Chemistry and Laboratory Medicine; KTR, kidney transplant recipients; NGSP, National Glycohemoglobin Standardization Program; PRA, panel reactive antibody.
3.2. Evolution of posttransplant diabetes mellitus
The evolution of PTDM defined by the glucose‐lowering medication or OGTT (plasma glucose criteria) of the clinical trial population is provided in Figure 1A, and detailed classification is provided in Figure S2. PTDM was present in n = 43 (19.5%), n = 34 (16.1%), and n = 33 (18.1%) participants, and IGT and/or IFG was present in n = 71 (32.3%), n = 65 (30.8%), and n = 58 (31.9%) participants at months 6, 12, and 24, respectively. Between visits at 6, 12, and 24 months after transplantation, 28%–31% of patients switched glycemic category (normal glucose tolerance [NGT], IGT/IFG, PTDM). Specifically, n = 11 (28.2%) and n = 5 (17.2%) participants with PTDM improved their glucose metabolism while, conversely, n = 4 (2.5%) and n = 9 (6.0%) participants without diabetes developed PTDM after months 6 and 12. Among trial participants without PTDM at month 6 who were classified as having PTDM at months 12 and 24, the majority had both IGT and IFG at the previous month‐6 visit (n = 3 [75.0%] and n = 7 [77.8%] individuals at months 12 and 24, respectively). Among trial participants without PTDM at month 12 who developed PTDM at month 24, n = 5 (55.6%) had both IGT and IFG at month 12. Two participants (2.2%) developed PTDM after having normal glucose tolerance at month 12. When we compared male and female KTRs at 6–24 months, women more often than men had PTDM defined by 2hPG and PTDM in the absence of glucose‐lowering medication. Specifically, at month 12, 4 (57.1%) men versus 9 (100.0%) women had diabetic 2hPG (p = .06) and 18 (51.4%) men versus 21 (70%) women had IGT in the group of individuals with prediabetes (p = .13). There were 7 (35.0%) men versus 9 (64.3%) women who had PTDM in the absence of glucose‐lowering medication at month 12 (p = .09) (Figure S3).
FIGURE 1.
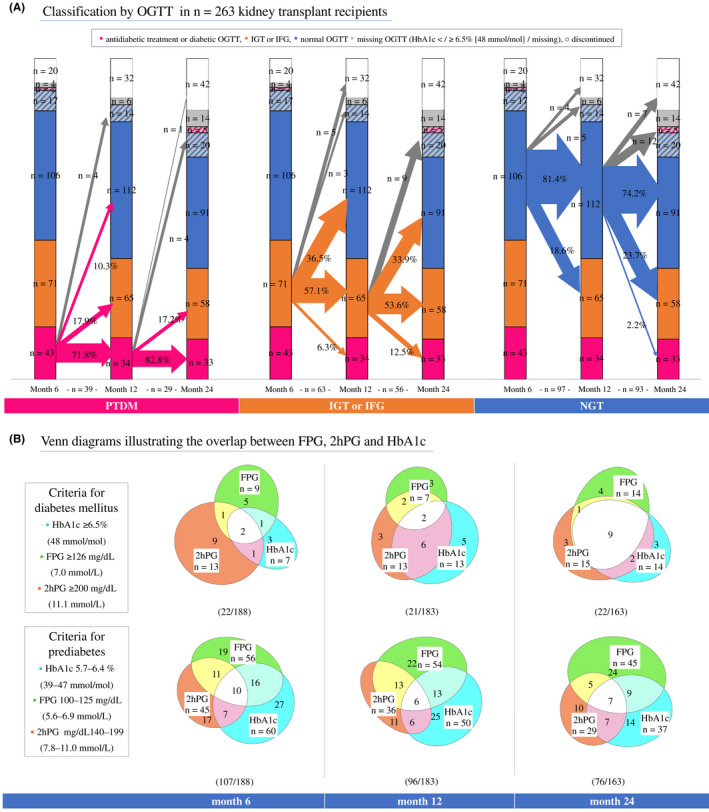
Posttransplant diabetes mellitus evolution and concordance of diabetes criteria. (A) Diabetic status and the metabolic instability after transplantation using oral glucose tolerance testing (OGTT). The size of the crossing arrows is proportional to the number of patients changing category. Proportions of patients within the deriving category changing to another category are displayed on the arrows (excluding missing OGTT or discontinuations, gray arrows). Number of patients from which proportions are derived are below the baseline. Categories arranged from bottom up and divided into subgroups: posttransplant diabetes mellitus (pink, defined by glucose‐lowering medication or diabetic OGTT—diabetic 2hPG and/or FPG), prediabetes (IGT and/or IFG, orange), NGT = normal glucose tolerance (blue, normal 2hPG and FPG values). HbA1c measurements in participants with missing OGTT (gray) and with diabetic HbA1c (pink stripes) or normal HbA1c (blue stripes). Discontinuations (white) refer to the clinical trial dropouts (Schwaiger et al.) and not to the study flow in Figure S1. (B) Overlap of HbA1c, fasting plasma glucose (FPG), and 2‐h plasma glucose (2hPG) diagnostic criteria in participants who had both OGTT and HbA1c data available. Numbers within the categories refer to patient numbers. Diabetes and prediabetes criteria were overlapping in most patients. Therefore, the sum of patients in the upper (diabetes) and lower (prediabetes) diagrams within a group are not equivalent to the total number of patients with diabetes or prediabetes. [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Diagnostic criteria for posttransplant diabetes mellitus and prediabetes
Diagnostic criteria were not perfectly concordant (Figure 1 panels B), showing that over the course of 24 months up to 69% of transplanted patients with PTDM (defined by 2hPG) remained below the diagnostic thresholds of FPG or HbA1c. The sensitivity of HbA1c and FPG criteria identifying PTDM defined by 2hPG (Table 2) or by plasma glucose criteria (Table S4) increased through 24 months. Still, at month 24, 20% of ITP‐NODAT trial participants with PTDM (defined by 2hPG) would have been missed without an OGTT.
TABLE 2.
Diagnostic accuracy of HbA1c and FPG criteria for PTDM and IGT
| TPR (95% CI) | TNR (95% CI) | LR+ | PPV | TP | FN | TN | FP | |
|---|---|---|---|---|---|---|---|---|
| PTDM (2hPG reference) | ||||||||
| FPG ≥ 126 mg/dl (7.0 mmol/L) | ||||||||
| Month 6 | 0.23 (0.05, 0.54) | 0.97 (0.93, 0.99) | 6.8 | 0.34 | 3 | 10 | 169 | 6 |
| Month 12 | 0.31 (0.09, 0.61) | 0.98 (0.95, 1.00) | 17.1 | 0.57 | 4 | 9 | 167 | 3 |
| Month 24 | 0.67 (0.38, 0.88) | 0.97 (0.93, 0.99) | 24.7 | 0.71 | 10 | 5 | 144 | 4 |
| HbA1c ≥ 6.5% (48 mmol/mol) | ||||||||
| Month 6 | 0.23 (0.05, 0.54) | 0.98 (0.94, 0.99) | 10.0 | 0.43 | 3 | 10 | 171 | 4 |
| Month 12 | 0.62 (0.32, 0.86) | 0.97 (0.93, 0.99) | 21.2 | 0.62 | 8 | 5 | 165 | 5 |
| Month 24 | 0.73 (0.45, 0.92) | 0.98 (0.94, 1.00) | 36.7 | 0.79 | 11 | 4 | 145 | 3 |
| FPG ≥ 126 mg/dl (7.0 mmol/L) or HbA1c ≥ 6.5% (48 mmol/mol) | ||||||||
| Month 6 | 0.31 (0.09, 0.61) | 0.95 (0.90, 0.98) | 6.0 | 0.31 | 4 | 9 | 166 | 9 |
| Month 12 | 0.77 (0.46, 0.95) | 0.95 (0.91, 0.98) | 16.4 | 0.56 | 10 | 3 | 162 | 8 |
| Month 24 | 0.80 (0.52, 0.96) | 0.95 (0.90, 0.98) | 17.0 | 0.63 | 12 | 3 | 141 | 7 |
| Impaired glucose tolerance | ||||||||
| ≥100 mg/dl (5.6 mmol/L) | ||||||||
| Month 6 | 0.58 (0.42, 0.72) | 0.79 (0.71, 0.86) | 2.8 | 0.49 | 26 | 19 | 103 | 27 |
| Month 12 | 0.58 (0.41, 0.74) | 0.80 (0.72, 0.86) | 2.9 | 0.44 | 21 | 15 | 107 | 27 |
| Month 24 | 0.52 (0.33, 0.71) | 0.75 (0.66, 0.82) | 2.1 | 0.33 | 15 | 14 | 89 | 30 |
| HbA1c ≥ 5.7% (39 mmol/mol) | ||||||||
| Month 6 | 0.44 (0.30, 0.60) | 0.72 (0.64, 0.80) | 1.6 | 0.36 | 20 | 25 | 94 | 36 |
| Month 12 | 0.44 (0.28, 0.62) | 0.75 (0.66, 0.82) | 1.8 | 0.32 | 16 | 20 | 100 | 34 |
| Month 24 | 0.55 (0.36, 0.74) | 0.82 (0.73, 0.88) | 3.0 | 0.42 | 16 | 13 | 97 | 22 |
| FPG ≥ 100 mg/dl (5.6 mmol/L) or HbA1c ≥ 5.7% (39 mmol/mol) | ||||||||
| Month 6 | 0.67 (0.51, 0.80) | 0.60 (0.51, 0.68) | 1.7 | 0.37 | 30 | 15 | 78 | 52 |
| Month 12 | 0.78 (0.61, 0.90) | 0.63 (0.55, 0.72) | 2.1 | 0.36 | 28 | 8 | 85 | 49 |
| Month 24 | 0.69 (0.49, 0.85) | 0.63 (0.54, 0.72) | 1.9 | 0.31 | 20 | 9 | 75 | 44 |
Note: Reference standard: 2hPG (2‐h plasma glucose) derived by an oral glucose tolerance test (OGTT). Exact confidence intervals (95% CI) were based on binomial probabilities. Impaired glucose tolerance (IGT): ordinal variables were dichotomized by excluding individuals with diabetes mellitus defined by 2hPG.
Abbreviations: FN, false negative; FP, false positive; FPG, fasting plasma glucose; HbA1c, hemoglobin A1c; LR+, positive likelihood ratio (shift from pretest to post‐test probability); PPV, positive predictive value (precision); TN, true negative; TNR, true negative rate (without exclusions = specificity); TP, true positive; TPR, true positive rate (without exclusions = sensitivity).
IGT, IFG, and HbA1c 5.7%–6.4% (39 mmol/mol) were more inhomogeneous than diabetes criteria. Over the course of 24 months, concordance between FPG, HbA1c, and 2hPG ranged from 6% to 9% (Figure 1) and up to 33% of IGT would have been missed in the absence of an OGTT (Table 2). At month 24, HbA1c ≥5.7% (39 mmol/mol) identified 55% of individuals with IGT, with a positive predictive value (PPV) of 0.42.
3.4. Relationship of fasting plasma glucose and HbA1c versus 2hPG
Scatter plots showing individual values for 2hPG, FPG, and HbA1c and their corresponding Pearson correlation coefficients are provided in Figure 2. The correlation between HbA1c and 2hPG improved considerably from month 6 to month 24 after transplant (r = 0.45 [95% confidence interval 0.31, 0.56] vs. r = 0.74 [0.60, 0.82]). We also observed that in patients with PTDM, median 2hPG, HbA1c, and FPG results increased over time (Table S5). Reflecting the correlation coefficients, the discriminatory power (area under the curve [AUC, 95% CI]) of FPG and HbA1c were also lowest in month 6 for both HbA1c (0.85 [0.77, 0.93]) and FPG (0.89 [0.82, 0.96]), and both tests improved until month 24, but their 95% CIs indicated no statistically significant difference (Table S6).
FIGURE 2.
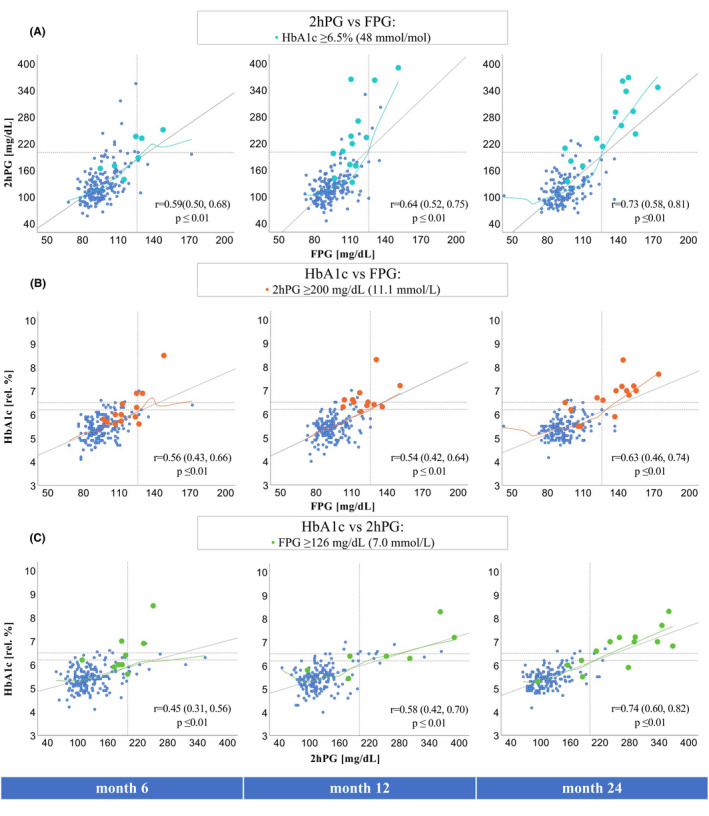
Linear correlations of FPG, 2hPG, and HbA1c. r = Pearson correlation coefficient and 95% confidence interval (95% CI) obtained by bootstrapping. (A) Correlation of 2hPG versus FPG, blue dots (= HbA1c ≥ 6.5% [48 mmol/mol]). (B) Correlation of HbA1c versus FPG, orange dots (= 2hPG ≥ 200 mg/dl [11.1 mmol/L]). (C) Correlation of HbA1c versus 2hPG, green dots (= FPG ≥ 126 mg/dl [7.0 mmol/L]). Non‐linearity was described by loess curves (colored lines, locally estimated scatterplot smoothing). Gray line = linear regression line, dotted lines = diabetes mellitus (DM) thresholds (FPG ≥ 126 mg/dl [7.0 mmol/L], 2hPG ≥ 200 mg/dl [11.1 mmol/L], HbA1c ≥ 6.2% [44 mmol/mol], ≥6.5% [48 mmol/mol]). 2hPG, 2‐h plasma glucose; FPG, fasting plasma glucose. [Color figure can be viewed at wileyonlinelibrary.com]
The predictive strength for IGT and IGT/IFG were similarly low at each time point and FPG was generally better than HbA1c. However, the AUC of HbA1c identifying IGT increased at month 24.
Lowered HbA1c and FPG diabetes thresholds for screening purposes derived by ROC curve analysis are provided in Table S7. HbA1c ≤5.5% (37 mmol/mol) and FPG ≤95 mg/dl (5.3 mmol/L) always ruled out PTDM (defined by 2hPG). The sensitivity and specificity of HbA1c ≥6.2% (44 mmol/mol) are provided in Table S8.
4. DISCUSSION
We observed metabolic instability in the extended posttransplant period and persistently decreased diagnostic performance of single FPG and HbA1c measurements. These findings imply that the OGTT remains the most important diagnostic test after kidney transplantation. Our results may be applied to populations of White individuals without a history of diabetes before transplantation.
The proportion of individuals with 2hPG‐defined PTDM not reaching the diagnostic thresholds of FPG or HbA1c ranged from 20% to 69%. In line, Ussif et al. identified 26% of patients with PTDM by 2hPG only, and not by FPG, at 1 year after transplant in a large study cohort. 25 The proportion of PTDM (10% vs. 8%) and the sensitivity of HbA1c ≥6.5% in identifying PTDM (43% vs. 50%) were similar to our findings. 25 Other studies investigating month 12 had considerably lower sample sizes and HbA1c showed sensitivities ≤50%. 20 , 33 These findings indicate that FPG and HbA1c are not optimal replacements for the OGTT to diagnose PTDM. However, it should be noted that the proportion of patients with PTDM, identified by FPG alone, is also not small, and that those individuals with isolated 2hPG ≥200 mg/dl (≥11.1 mmol/L) are more likely to revert to prediabetes. 34
Recent studies showed that IGT and prediabetes are not only intermediate stages in the transition to PTDM, but specific risk factor for adverse outcomes such as cardiovascular disease 6 and death. 5 These studies go against previous efforts that sought a replacement for the OGTT. In fact, Valderhaug et al. 5 found evidence that 2hPG is superior for the prediction of death, compared to FPG. For the general population the DECODE 35 and DECODA 36 trials, as well as a previous meta‐analysis of 20 studies 37 also showed that IGT is a stronger predictor for death and cardiovascular disease than IFG. Individuals with HbA1c measurements just below the diabetes threshold might even represent a population with exceptionally high cardiovascular risk compared to those above this threshold. 38 No similar finding correlating HbA1c with adverse outcomes has been shown in transplanted patients. Finally, a recent epidemiological study of type 2 diabetes showed that 2hPG will improve prediction of adverse outcomes in models that include FPG and HbA1c. 39
Another principal finding of the present analysis is that prediabetes and especially IGT may only be identified by an OGTT. Specifically, the concordance of FPG, HbA1c, and 2hPG criteria for prediabetes ranged from only 6% to 9% with all three criteria and as many as 45% to 56% of individuals with IGT had an HbA1c value <5.7% (39 mmol/mol). Poor correlation might be why Porrini et al. 6 found no association of HbA1c at 12 months with cardiovascular events in individuals without PTDM, while prediabetes (IGT and/or IFG) was significantly associated. The second, important message regarding prediabetes is, that we observed high metabolic instability and that one in three KTRs switched glycemic category between 6, 12, and 24 months. Similarly, high fluctuations between 3 and 36 months after transplant have been shown by Porrini et al., 34 which indicates that the OGTT is even more important in the identification of prediabetes in a metabolically unstable period. The practical implication of these findings is, that the OGTT should be repeated, for example, annually, in patients with high risk for PTDM.
Analyzing even more closely those individuals who progressed to PTDM, we could observe that patients with PTDM often had combined IGT + IFG at the previous visit and, although our number of observations was small, these results reflect studies showing that subjects with IGT + IFG have a higher risk of proceeding to type 2 diabetes. 40 Moreover, women were more often identified by isolated 2hPG ≥200 mg/dl (11.1 mmol/L) and isolated IGT than men, but again this finding was limited by the number of observations. In the general population, IGT is also more prevalent in women and at older ages, while IFG is considerably more common in men. 40 It has also been shown that diabetes, especially, in elderly women is rather identified by isolated 2hPG than by FPG 41 in comparison with younger individuals and elderly men. These findings might be partially explained by the standardized glucose load and studies that showed slower intestinal glucose absorption in females, explained by height and fat‐free mass. 42
Apart from statistical associations, it is important that clinicians are aware of influences that may affect the individual patient. Except for possible pathophysiological influences on FPG, there may be analytical difficulties. These include intraindividual variability of 5.7%–8.3% 43 and reproducibility of diabetic results of only 70% that have been reported in the general population. 44 Also, interindividual variation of glucose measurements of up to 12.5% 43 and decay of glucose in test tubes in the range of 5% to 7% per hour have been reported in previous studies. 43 Factors influencing the pre‐analytical variation include medications, food ingestion, prolonged fasting or exercise, and stress hyperglycemia due to illness. 45
The diagnostic accuracy of HbA1c was also considerably lower at month 6 compared with month 24. HbA1c potentially under‐ or over‐diagnoses diabetes due to ethnicity, sex, age, or BMI, 46 of which the relevance of ethnicity is controversial as these differences have not been linked to cardiovascular outcomes. 47 , 48 , 49 Other reported influential factors are erythropoiesis, altered hemoglobin, glycation, erythrocyte destruction, and assay interference. 50 Assays are now greatly unaffected by carbamylation, labile intermediates, and common hemoglobin variants. 51 However, alteration in erythrocyte lifespan, ethnicity, age, and method‐specific alterations due to homozygous variants 43 could influence results. HbA1c shows less intra‐individual variability and interference during infections or illness than glucose measurements, and samples are more stable at room temperature. 43 , 44 , 52 However, the within‐variation of HbA1c has been shown to be increased in KTRs compared with the general population 53 and might be additionally influenced by late posttransplant anemia, which is reported in 20% to 57% of patients at 1 year after transplant. 17 After transplantation, loss of kidney function and endogenous erythropoietin production, blood loss and iron deficiency, medications, bone marrow suppression, chronic inflammation, and viral infections could be involved. 54
The clinical implications of our findings thus are the following: that PTDM and especially prediabetes are best identified by an OGTT and that the OGTT should be repeated due to metabolic instability. This is particularly true for patients with prediabetes or high cardiovascular risk. Although the OGTT has been recommended for some time, 16 not all transplant centers implemented regular screening, which may partially be due to structural difficulties, depending on the center or country. In the general population, the OGTT is actually an inexpensive screening method, 55 but there are no cost‐effectiveness studies available after transplantation. Stratifying for OGTT referral using FPG and HbA1c could be helpful, 18 whereas this method would ignore the presence of IGT. Patients with NGT are unlikely to develop PTDM later on. Those without NGT may be in need for closer follow‐up. Using an early OGTT (e.g., at 3 months) has the benefit of being able to intervene early, which may be important because PTDM is more reversible when diagnosed early. 34 Patient perspectives in prenatal screening indicate that the OGTT is not well tolerated by some patients and may be experienced as burdensome. 56 , 57 Therefore, successful screening may also involve active patient education and uncomplicated access.
4.1. Study strengths and limitations
Strengths of the present study include its cohort of well‐defined kidney‐transplanted individuals receiving three diagnostic OGTTs over a period of 2 years. No other study has longitudinally compared the diagnostic test accuracy of FPG and HbA1c versus OGTT at 6, 12, and 24 months after transplantation. Only a few studies have explicitly evaluated prediabetes in the posttransplant setting and this focus was chosen in context of a recent publication that showed increased risk for cardiovascular events. 6
This study is primarily limited by its retrospective design and relatively low sample size. Any proportion based on PTDM cases alone, such as sensitivity, could only be estimated with low precision. Further, the OGTT is strongly influenced by intraindividual fluctuation, 44 , 52 thereby limiting the use of a single glucose measurement as a reference test. Of note, the trial intervention led to lower odds for PTDM and might have led to less severe stages of disease and fewer undetected PTDM cases due to the extensive screening. Additionally, most treatment group participants (about 50% of total) received insulin, but it should be recognized that most of these participants were weaned off insulin before month 6 and therefore the influence on the test results should be limited. In comparison, the number of patients with PTDM identified at the 1‐year OGTT was only slightly lower, as in the study by Ussif et al. (8.3% vs. 10.3%, respectively). 25
The fact that ethnicity was not collected, was a drawback of our study, but it is likely that most patients were European White individuals and our results might not be reproducible in cohorts of different ethnicity. Studies in the general population showed a relevant effect of ethnicity on HbA1c results. 47 Posttransplant, ethnic disparities are discussed in graft adverse outcomes 58 , 59 and future studies are needed to investigate diagnosis and its relation to PTDM adverse outcomes in other ethnic groups. As long as these data are lacking for transplanted patients and because of influences on HbA1c after transplant, accuracy of diagnostic criteria in non‐White populations is uncertain.
We acknowledge the fact that use of the term prediabetes has been controversial and might be misleading, as most individuals with prediabetes will in fact never develop diabetes mellitus. A previous study showed that the risk for cardiovascular events in individuals with prediabetes after transplantation is increased to a degree similar to that in individuals with PTDM, 6 and for consistency we followed its use of the term prediabetes.
5. CONCLUSION
In our well‐defined randomized trial cohort, one in three KTRs switched glycemic category over 2 years. Analysis of three consecutive OGTTs revealed that up to 69% of patients with PTDM and 33% of IGT would have been missed had no OGTT been performed. Although the correlations between FPG, HbA1c, and 2hPG improved with time, their diagnostic concordance was poor for PTDM and, especially, prediabetes. Metabolic instability in the extended posttransplant period and the persistently decreased diagnostic performance of single FPG and HbA1c measurements imply that the OGTT remains the most important diagnostic test after kidney transplantation.
AUTHOR CONTRIBUTIONS
Amelie Kurnikowski, Espen Nordheim, Trond Geir Jenssen, and Manfred Hecking designed the study and wrote and submitted the study protocol. Kathrin Eller, Klemens Budde, Julio Pascual, Manfred Hecking, and Johannes Werzowa enrolled participants into the trial and actively treated the previous trial participants. Elisabeth Schwaiger, Simon Krenn, Jürgen Harreiter, Alexandra Kautzky‐Willer, Michael Leutner, Johannes Werzowa, Andrea Tura, Klemens Budde, Kathrin Eller, and Julio Pascual retrieved the data. Amelie Kurnikowski, Espen Nordheim, Trond Geir Jenssen, and Manfred Hecking analyzed the data. Amelie Kurnikowski, Espen Nordheim, Trond Geir Jenssen, and Manfred Hecking wrote the manuscript. E. Schwaiger, Simon Krenn, Jürgen Harreiter, Alexandra Kautzky‐Willer, Michael Leutner, Johannes Werzowa, Andrea Tura, Klemens Budde, Kathrin Eller, Julio Pascual, and Michael Krebs corrected the manuscript. All authors approved the final version of the manuscript prior to submission.
FUNDING INFORMATION
This was an Investigator‐Initiated Trial with financial support provided by University of Michigan subcontract No. 3002300292 to NIH grant R01DK092475, Astellas Pharma GmbH, and Eli Lilly GmbH. We are grateful to all trial sponsors, especially to the National Institute Of Diabetes And Digestive And Kidney Diseases. The project described was supported by Grant Number R01DK092475 from the National Institute Of Diabetes And Digestive And Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute Of Diabetes And Digestive And Kidney Diseases or the National Institutes of Health.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation. None of the authors received financial support from the involved companies (Astellas Pharma GmbH and Eli Lilly GmbH), related to the present work.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
We thank Prof. Florian Frommlet for helpful discussions and statistical advice.
Kurnikowski A, Nordheim E, Schwaiger E, et al. Criteria for prediabetes and posttransplant diabetes mellitus after kidney transplantation: A 2‐year diagnostic accuracy study of participants from a randomized controlled trial. Am J Transplant. 2022;22:2880‐2891. doi: 10.1111/ajt.17187
Amelie Kurnikowski and Espen Nordheim share equal authorship.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.
REFERENCES
- 1. Classification and Diagnosis of Diabetes . Standards of medical care in diabetes‐2021. Diabetes Care. 2021;44(Suppl 1):S15‐s33.33298413 [Google Scholar]
- 2. Hecking M, Sharif A, Eller K, Jenssen T. Management of post‐transplant diabetes: immunosuppression, early prevention, and novel antidiabetics. Transpl Int. 2021;34(1):27‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hecking M, Werzowa J, Haidinger M, et al. Novel views on new‐onset diabetes after transplantation: development, prevention and treatment. Nephrol Dial Transplant. 2013;28(3):550‐566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Werzowa J, Hecking M, Haidinger M, et al. The diagnosis of posttransplantation diabetes mellitus: meeting the challenges. Curr Diab Rep. 2015;15(5):601. [DOI] [PubMed] [Google Scholar]
- 5. Valderhaug TG, Hjelmesæth J, Hartmann A, et al. The association of early post‐transplant glucose levels with long‐term mortality. Diabetologia. 2011;54(6):1341‐1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Porrini E, Diaz JM, Moreso F, et al. Prediabetes is a risk factor for cardiovascular disease following renal transplantation. Kidney Int. 2019;96(6):1374‐1380. [DOI] [PubMed] [Google Scholar]
- 7. Eide IA, Halden TA, Hartmann A, et al. Mortality risk in post‐transplantation diabetes mellitus based on glucose and HbA1c diagnostic criteria. Transpl Int. 2016;29(5):568‐578. [DOI] [PubMed] [Google Scholar]
- 8. Davidson J, Wilkinson A, Dantal J, et al. New‐onset diabetes after transplantation: 2003 International consensus guidelines. Proceedings of an international expert panel meeting. Barcelona, Spain, 19 February 2003. Transplantation. 2003;75(10 Suppl):SS3‐SS24. [DOI] [PubMed] [Google Scholar]
- 9. Diagnosis TECot, Mellitus CoD . Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(suppl_1):s5‐s20. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization . Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1, Diagnosis and classification of diabetes mellitus. World Health Organization; 1999. [Google Scholar]
- 11. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20(7):1183‐1197. [DOI] [PubMed] [Google Scholar]
- 12. International expert committee report on the role of the A1c assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327‐1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Selvin E, Steffes MW, Zhu H, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362(9):800‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Londero TM, Giaretta LS, Farenzena LP, et al. Microvascular complications of posttransplant diabetes mellitus in kidney transplant recipients: a longitudinal study. J Clin Endocrinol Metab. 2019;104(2):557‐567. [DOI] [PubMed] [Google Scholar]
- 15. Jenssen T, Hartmann A. Post‐transplant diabetes mellitus in patients with solid organ transplants. Nat Rev Endocrinol. 2019;15(3):172‐188. [DOI] [PubMed] [Google Scholar]
- 16. Sharif A, Hecking M, de Vries AP, et al. Proceedings from an international consensus meeting on posttransplantation diabetes mellitus: recommendations and future directions. Am J Transplant. 2014;14(9):1992‐2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sharif A, Baboolal K. Diagnostic application of the A(1c) assay in renal disease. J Am Soc Nephrol. 2010;21(3):383‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Valderhaug TG, Jenssen T, Hartmann A, et al. Fasting plasma glucose and glycosylated hemoglobin in the screening for diabetes mellitus after renal transplantation. Transplantation. 2009;88(3):429‐434. [DOI] [PubMed] [Google Scholar]
- 19. Armstrong KA, Prins JB, Beller EM, et al. Should an oral glucose tolerance test be performed routinely in all renal transplant recipients? Clin J Am Soc Nephrol. 2006;1(1):100‐108. [DOI] [PubMed] [Google Scholar]
- 20. Shabir S, Jham S, Harper L, Ball S, Borrows R, Sharif A. Validity of glycated haemoglobin to diagnose new onset diabetes after transplantation. Transpl Int. 2013;26(3):315‐321. [DOI] [PubMed] [Google Scholar]
- 21. Cowie CC, Rust KF, Byrd‐Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1c criteria in the U.S. population in 1988‐2006. Diabetes Care. 2010;33(3):562‐568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Will new diagnostic criteria for diabetes mellitus change phenotype of patients with diabetes? Reanalysis of European epidemiological data. DECODE Study Group on behalf of the European Diabetes Epidemiology Study Group. BMJ. 1998;317(7155):371‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Carnevale Schianca GP, Rossi A, Sainaghi PP, Maduli E, Bartoli E. The significance of impaired fasting glucose versus impaired glucose tolerance: importance of insulin secretion and resistance. Diabetes Care. 2003;26(5):1333‐1337. [DOI] [PubMed] [Google Scholar]
- 24. Eide IA, Halden TA, Hartmann A, et al. Limitations of hemoglobin A1c for the diagnosis of posttransplant diabetes mellitus. Transplantation. 2015;99(3):629‐635. [DOI] [PubMed] [Google Scholar]
- 25. Ussif AM, Åsberg A, Halden TAS, Nordheim E, Hartmann A, Jenssen T. Validation of diagnostic utility of fasting plasma glucose and HbA1c in stable renal transplant recipients one year after transplantation. BMC Nephrol. 2019;20(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kaur G, Lakshmi PVM, Rastogi A, et al. Diagnostic accuracy of tests for type 2 diabetes and prediabetes: a systematic review and meta‐analysis. PLoS One. 2020;15(11):e0242415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schwaiger E, Krenn S, Kurnikowski A, et al. Early postoperative basal insulin therapy versus standard of care for the prevention of diabetes mellitus after kidney transplantation: a multicenter randomized trial. J Am Soc Nephrol. 2021;32(8):2083‐2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mellitus WHOECoD, World Health Organisation . WHO Expert Committee on Diabetes Mellitus [meeting held in Geneva from 25 September to 1 October 1979]: second report. World Health Organization; 1980. [Google Scholar]
- 29. Goldstein DE, Little RR, Wiedmeyer HM, England JD, McKenzie EM. Glycated hemoglobin: methodologies and clinical applications. Clin Chem. 1986;32(10 Suppl):B64‐B70. [PubMed] [Google Scholar]
- 30. Tura A, Grespan E, Göbl CS, et al. Profiles of glucose metabolism in different prediabetes phenotypes, classified by fasting glycemia, 2‐hour OGTT, Glycated hemoglobin, and 1‐hour OGTT: an IMI DIRECT study. Diabetes. 2021;70(9):2092‐2106. [DOI] [PubMed] [Google Scholar]
- 31. Micallef L, Rodgers P. eulerAPE: drawing area‐proportional 3‐Venn diagrams using ellipses. PLoS One. 2014;9(7):e101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yates CJ, Fourlanos S, Colman PG, Cohney SJ. Screening for new‐onset diabetes after kidney transplantation: limitations of fasting glucose and advantages of afternoon glucose and glycated hemoglobin. Transplantation. 2013;96(8):726‐731. [DOI] [PubMed] [Google Scholar]
- 34. Porrini EL, Diaz JM, Moreso F, et al. Clinical evolution of post‐transplant diabetes mellitus. Nephrol Dial Transplant. 2016;31(3):495‐505. [DOI] [PubMed] [Google Scholar]
- 35. Group DS, Group obotEDE . Glucose tolerance and cardiovascular mortality: comparison of fasting and 2‐hour diagnostic criteria. Arch Intern Med. 2001;161(3):397‐405. [DOI] [PubMed] [Google Scholar]
- 36. DECODA Study Group, International Diabetes Epidemiology Group . Cardiovascular risk profile assessment in glucose‐intolerant Asian individuals—an evaluation of the World Health Organization two‐step strategy: the DECODA Study (Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Asia). Diabet Med. 2002;19(7):549‐557. [DOI] [PubMed] [Google Scholar]
- 37. Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 1999;22(2):233‐240. [DOI] [PubMed] [Google Scholar]
- 38. Yahyavi SK, Snorgaard O, Knop FK, et al. Prediabetes defined by first measured HbA(1c) predicts higher cardiovascular risk compared with HbA(1c) in the diabetes range: a cohort study of nationwide registries. Diabetes Care. 2021;44(12):2767‐2774. [DOI] [PubMed] [Google Scholar]
- 39. Lu J, He J, Li M, et al. Predictive value of fasting glucose, postload glucose, and hemoglobin A(1c) on risk of diabetes and complications in Chinese adults. Diabetes Care. 2019;42(8):1539‐1548. [DOI] [PubMed] [Google Scholar]
- 40. Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19(9):708‐723. [DOI] [PubMed] [Google Scholar]
- 41. Hutchinson MS, Joakimsen RM, Njølstad I, et al. Effects of age and sex on estimated diabetes prevalence using different diagnostic criteria: the Tromsø OGTT study. Int J Endocrinol. 2013;2013:613475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kautzky‐Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37(3):278‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sacks DB. A1c versus glucose testing: a comparison. Diabetes Care. 2011;34(2):518‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Selvin E, Crainiceanu CM, Brancati FL, Coresh J. Short‐term variability in measures of glycemia and implications for the classification of diabetes. Arch Intern Med. 2007;167(14):1545‐1551. [DOI] [PubMed] [Google Scholar]
- 45. Dungan KM, Braithwaite SS, Preiser J‐C. Stress hyperglycaemia. Lancet (London, England). 2009;373(9677):1798‐1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gonzalez A, Deng Y, Lane AN, et al. Impact of mismatches in HbA(1c) vs glucose values on the diagnostic classification of diabetes and prediabetes. Diabet Med. 2020;37(4):689‐696. [DOI] [PubMed] [Google Scholar]
- 47. Selvin E. Are there clinical implications of racial differences in HbA1c? A difference, to be a difference, must make a difference. Diabetes Care. 2016;39(8):1462‐1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Herman WH. Are there clinical implications of racial differences in HbA1c? Yes, to not consider can do great harm! Diabetes Care. 2016;39(8):1458‐1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Selvin E, Rawlings AM, Bergenstal RM, Coresh J, Brancati FL. No racial differences in the association of glycated hemoglobin with kidney disease and cardiovascular outcomes. Diabetes Care. 2013;36(10):2995‐3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes. 2009;1(1):9‐17. [DOI] [PubMed] [Google Scholar]
- 51. Sacks DB. Hemoglobin A1c in diabetes: panacea or pointless? Diabetes. 2013;62(1):41‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gambino R. Glucose: a simple molecule that is not simple to quantify. Clin Chem. 2007;53(12):2040‐2041. [DOI] [PubMed] [Google Scholar]
- 53. Pimentel AL, Camargo JL. Variability of glycated hemoglobin levels in the first year post renal transplantation in patients without diabetes. Clin Biochem. 2017;50(18):997‐1001. [DOI] [PubMed] [Google Scholar]
- 54. Winkelmayer WC, Chandraker A. Pottransplantation anemia: management and rationale. Clin J Am Soc Nephrol. 2008;3(Suppl 2):S49‐S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Icks A, Haastert B, Gandjour A, et al. Cost‐effectiveness analysis of different screening procedures for type 2 diabetes: the KORA survey 2000. Diabetes Care. 2004;27(9):2120‐2128. [DOI] [PubMed] [Google Scholar]
- 56. Alecrim MJ, Mattar R, Torloni MR. Pregnant women's experience of undergoing an oral glucose tolerance test: a cross‐sectional study. Diabetes Res Clin Pract. 2022;189:109941. [DOI] [PubMed] [Google Scholar]
- 57. Lachmann EH, Fox RA, Dennison RA, Usher‐Smith JA, Meek CL, Aiken CE. Barriers to completing oral glucose tolerance testing in women at risk of gestational diabetes. Diabet Med. 2020;37(9):1482‐1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Taber DJ, Gebregziabher M, Payne EH, Srinivas T, Baliga PK, Egede LE. Overall graft loss versus death‐censored graft loss: unmasking the magnitude of racial disparities in outcomes among US kidney transplant recipients. Transplantation. 2017;101(2):402‐410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Williams A, Richardson C, McCready J, et al. Black ethnicity is not a risk factor for mortality or graft loss after kidney transplant in the United Kingdom. Exp Clin Transplant. 2018;16(6):682‐689. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


