Abstract
Background
Age‐related changes of facial soft tissue cause clinical signs of facial aging such as lip atrophy, marionette lines, and an accentuated nasolabial fold. These changes can be modified using dermal fillers.
Aims
To evaluate efficacy, longevity, and safety of a cross‐linked hyaluronic acid‐based filler with Tri‐Hyal technology in the treatment of lips, nasolabial folds, and marionette lines.
Materials and Methods
This prospective, multi‐center trial evaluated injections of three different areas (lips, nasolabial fold alone, or with marionette wrinkles) with a soft tissue filler containing 25 mg/ml cross‐linked hyaluronic acid and 0.3% lidocaine. Primary endpoint was the aesthetic correction 3 weeks after one injection session without touch‐up. Follow‐up was 18 months. Assessments were performed using the Global Aesthetic Score (GAS), clinical scoring based on photographic scales, high‐frequency ultrasound imaging, and the Global Aesthetic Improvement Scale (GAIS).
Results
In total, 100 subjects were injected. GAS improved significantly for all treatment indications at 3 weeks (p < 0.0001). Success rates were highest for nasolabial folds (98.4%), followed by marionette lines (94.4%) and lips (73.5%). After 18 months post‐injection, success was observed in 91%, 88%, and 33% of subjects injected into nasolabial folds, marionette lines, and lips, respectively. GAIS scored highest for nasolabial folds (SGAIS: 71%; IGAIS: 40%), followed by marionette lines (SGAIS: 56%; IGAIS: 33%) and lips (SGAIS: 30%; IGAIS: 22%) at 18 months follow‐up.
Conclusions
The filler demonstrated high efficacy and safety in all indications. Regional differences in longevity were evident. Thus, the necessity of regional retreatments should be discussed with patients before injection.
Keywords: dermal filler, facial aging, hyaluronic acid, lips volume, perioral rejuvenation
1. INTRODUCTION
Soft‐tissue filler injections are among the most popular procedures in esthetic medicine. 1 There has been a continuous increase in demand over recent years, fueled by an ever‐growing desire for eternal youth. According to market research, this desire is facilitated by an increase of availability, provided by a growing number of aesthetic practitioners, an increase in number, variety, and quality of injectables, as well as an increase in social acceptance of rejuvenating procedures. 2
Facial aging is a multifactorial process involving all facial tissues. Bone remodeling, muscle atrophy, deflation and displacement of fat compartments, loss of skin elasticity, hydration, and texture contribute to this natural process. 3 These age‐related changes cause clinical signs of facial aging such as lip atrophy, marionette lines, and an accentuated nasolabial fold, which are associated with a decline in attractiveness, psychological well‐being, and quality of life. 4 , 5 , 6 , 7
Consequently, frequently demanded treatments in clinical practice include volumizing and contouring of lips, as well as amelioration of nasolabial fold and marionette line severeness. If performed by trained practitioners, hyaluronic‐acid‐based soft‐tissue filler injections provide a cost‐effective, safe, reliable, and reproducible means to meet patients’ expectations and provide facial rejuvenation in a minimally invasive manner. 8
Given the plethora of injectable medical devices available on the market, clinical outcome studies exploring the short‐ and long‐term effects of these devices are required, to allow for informed decision making and product choice of both patients and practitioners.
This prospective, multicenter clinical trial evaluated the same cross‐linked hyaluronic acid‐based filler in the treatment of lips, nasolabial folds, and marionette lines over a time period of 18‐months. The aim was to determine the efficacy, longevity, and safety after injection and to gather reference data on the effect obtained for different treatment indications.
2. MATERIALS AND METHODS
2.1. Study design
This type 2 interventional study was designed as a prospective, nonrandomized, multicenter clinical trial evaluating the efficacy and safety of a hyaluronic acid‐based filler in the treatment of lips, nasolabial folds, and marionette lines. A total of 15 study centers based in France enrolled subjects between April 2019 and June 2019. The study duration was 20 months till December 2020. Subjects, in whom a correction of at least one of the studied zones (lips or nasolabial fold) was sought, were included. The treatment of marionette lines was optional for the subjects recruited for nasolabial fold treatment. Healthy male and female subjects ≥19 years of age, with a Fitzpatrick phototype of I–IV, a score of ≥1 in the GAS on at least one area of interest and at least one of the scales by clinical scoring (a grade ≥0 and ≤2 on the lip volume fullness scale from Medicis; for women, a grade of 5 on the nasolabial folds scale from Bazin; For men, a grade ≥5 on the nasolabial folds scale from Bazin) were included in this study. Subjects had not received any facial cosmetic procedure (surgery, laser, botulinum toxin, or filler injections) for at least 6 months prior to inclusion into the study and had never received a nonresorbable filler. Patients did not have any contraindications for HA injections. Detailed inclusion and exclusion criteria are provided in Tables S1 and S2. Subjects provided written and informed consent prior to inclusion into the study. Ethical approval was granted by a national independent review board (CPP Ile de France V, Saint‐Antoine hospital, 284 rue du Faubourg Saint Antoine, 75 012 Paris; ID‐RCB: 2018‐A02466‐49). The trial was registered within the the ClinicalTrials.gov public registry under the identifier NCT04647513.
2.2. Intervention
Each subject received injection of the same soft tissue filler (Art Filler® Lips, Laboratoires FILLMED) containing 25 mg/ml cross‐linked hyaluronic acid with Tri‐Hyal technology and 0.3% lidocaine. Injections were performed at baseline, and no corrective injections with the study product or any other product during the period of the study were allowed. Each subject could be injected either each zone separately (Lips or Nasolabial Fold with or without Marionette line) or both zones together. Intradermal injection with a 27G × 13 mm needle (TSK Laboratory Europe B.V.) or a 25G × 55 mm cannula (Softfil Medical Aesthetics) was performed at all injection sites. Volume and technique of injection were performed upon the injectors’ discretion. The maximum amount of product injected was limited to 1.0 ml for lips, 2.0 ml for nasolabial folds alone (or 1.0 ml per side), or 4.0 ml for nasolabial folds combined with marionette lines (2.0 ml per side; 1.0 ml per nasolabial fold/ marionette line).
2.3. Assessments and outcomes
Subjects were followed up over a duration of 18 months. The esthetic correction was evaluated at 3 weeks (D21) after injection. The persistence of the correction was evaluated at 3 (D90), 6 (D180), 9 (D270), 12 (D360), 15 (D450), and 18 (D540) months. The following assessments were performed:
Global Aesthetic Score (GAS): The esthetic result of the injection was assessed using the 7‐point (0–3) Global Aesthetic Score (Table S3) by the injector.
Digital photographs: Standardized digital photography was performed at each visit.
Clinical scoring based on photographic scales: Bazin nasolabial fold scale, Bazin marionette wrinkles scales, 9 , 10 as well as Medicis lips fullness scale (MLFS) 11 were assessed on each visit by the injector.
Investigator and Subject Global Aesthetic Improvement Scale (GAIS): The 7‐point GAIS score (+3: very much improved to −3: for very much worse) was assessed by the injector and subject at each follow‐up visit for each treated area concerned to provide the overall satisfaction rate of both investigators and subjects.
High‐frequency ultrasound imaging: High‐frequency ultrasound imaging (20 MHz DermaScan C USB; Monaderm) produced 2D visualizations to measure the dermal density (%). 12 The dermal density was assessed at each visit for 16 subjects randomly selected from those who received injection for the nasolabial folds. The intensity of the reflected echoes (corresponding to the tissue density) was assessed via an integrated microprocessor and visualized using the integrated software as color‐graded 2D‐images. The color scale of echogenicity (from hyperechogenic to hypoechogenic) ranged from white‐yellow–red–green–blue–black. 12 , 13
2.4. Primary objective
The primary objective was to measure the aesthetic improvement according to the GAS from D0 (Baseline) to D21, of lips volume and nasolabial folds alone or with marionette wrinkles, after injection of the soft tissue filler. A decrease (for nasolabial folds and marionette lines) or increase (for lips) of at least one grade (±0.5 point among a 0–3 points scale) defined a successful correction.
2.5. Secondary objectives
Secondary objectives were defined as follows:
To measure the remanence (at D90, D180, D270, D360, D450, and D540) of restoration of studied areas after soft‐tissue filler injections from baseline according to GAS.
- To evaluate the efficacy, longevity, and duration of volume correction:
- For the lips volume according to the MLFS,
- For the nasolabial folds and the marionette wrinkles according to the Bazin photographic Scales
To assess the SGAIS/ IGAIS between D21 and the follow up visits (D90, D180, D270, D360, D450, and D540) per zone
To evaluate skin density using high‐frequency ultrasound imaging.
To assess the safety per zone and per treatment.
2.6. Safety
Safety was evaluated by the investigator through assessing the frequency, intensity and causal relationship of adverse events (AEs) including erythema, ecchymosis, hematoma, oedema, dyschromia, irregularity at palpation, necrosis, tyndall effect, and over‐correction during the entire study period. The aspect and sensitivity of treated areas were assessed according to the criteria presented in Tables S4 and S5. They were scored by the investigator at each visit from the first injection till the end of the study. Subjects were also asked to report any local or systemic reaction / sensation they encounter (i.e., bruise / redness / swelling / pain / sensitivity / itching / others on 4 levels: 0 = no problem / 3 = intense) in a daily log during the study.
2.7. Sample size calculation
Sample size calculation for the primary objective was based on previously published data. 14 With an alpha of 0.05 and a power of 0.90, a sample size of at least 20 subjects (per area and treatment) was needed to detect a mean difference of 1 grade (SD 1.5, effect size 0.66) on the 7‐point (0 to 3) GAS score. In order to obtain a sufficient number of subjects for a long‐term performance and tolerance assessment (18‐month), it was planned to include at least 30 subjects per injection site.
2.8. Statistical analyses
Continuous variables were summarized using descriptive statistics, including number of subjects (n), mean, standard deviation (SD), median, minimum, maximum, and 95% confidence interval. For categorical variables, summaries included counts of subjects and percentages in corresponding categories. The efficacy and longevity of the effect were analyzed on the Per Protocol (PP) population. For each follow‐up time, D21, D90, D180, D270, D360, D450, and D540, the per‐protocol population (PP) was defined by all the subjects seen at this visit and having an evaluation of the GAS. The respective success percentages were calculated by the ratio of satisfactory responses on each treated area. The statistical significance of the primary objective was calculated by a Wilcoxon test. The significance level was set at 0.05. GAS was also assessed using the intention‐to‐treat population (all subjects enrolled and for whom at least one injection of one of the studied products has been performed and at least one evaluation of the main criteria at D0 has been performed) to be able to conclude unambiguously on the response rate. In cases of missing data, the subject was considered as having not reached the efficiency criterion for the ITT analysis. Safety assessments were evaluated using the safety analysis set. All statistical procedures were performed using Statistical Analysis Software (MATLAB. MathWorks® version 2020b).
3. RESULTS
3.1. Subject disposition and demographics
A total of 100 subjects (male: n = 7; female: n = 93) with a mean age of 55.5 ± 9.8 [range: 27–74] and a mean BMI of 23.9 ± 3.2 [range: 18.8–36.3] were injected with the investigational device. Further demographic data are summarized in Table 1. Nasolabial folds were treated most frequently (n = 67 subjects) followed by marionette lines (n = 52 subjects) and lips (n = 34 subjects). Subjects received a mean of 2.0 ± 0.9 ml [range: 0.6–4] of soft‐tissue filler injection. The mean quantity of injected product per area is depicted in Table 2. Of the subjects included, 86 subjects completed the study at D540 (18 months).
TABLE 1.
Patient characteristics and demographic data (ITT population)
| Variable | |
|---|---|
| Mean age (years) | 55.5 (9.8) |
| Gender (n) | |
| Male | 7 |
| Female | 93 |
| Mean weight (kg) | 63.7 (12.1) |
| Mean height (cm) | 162.5 (6.7) |
| Mean BMI (kg/m2) | 23.9 (3.2) |
| Skin Phototype | |
| I | 1 |
| II | 36 |
| III | 50 |
| IV | 12 |
| Missing data | 1 |
Abbreviations: n, number; y, years.
TABLE 2.
Volume of injected product per area (ITT population)
| Lips | Nasolabial folds | Marionette lines | All (sum of all the injected volumes for the same patient) | |
|---|---|---|---|---|
| N | 34 | 67 | 52 | 100 |
| Mean Volume (ml) | 0.9 | 1.6 | 1.1 | 2.0 |
| Standard deviation | 0.1 | 0.5 | 0.5 | 0.9 |
Abbreviations: n, number; ml, milliliter.
3.2. Primary endpoint – Global aesthetic clinical scoring
A significant improvement of the GAS compared to baseline was achieved for all investigated treatment indications at 21 days post intervention (all p < 0.0001) with a mean difference of ~1 grade (0.5 point among 0–3 scale) on means and medians for nasolabial folds, marionette lines and lips. Nasolabial folds demonstrated the highest success rates (98.4%), followed by marionette lines (94.4%) and lips (73.5%) (Table 3). Success rates were consistent with results from the ITT population (Nasolabial folds: 95.5%; Marionette lines: 88.6%; Lips: 73.5%).
TABLE 3.
Significant improvement of the GAS between baseline and 21 days post‐injection (PP population)
| Global aesthetic clinical score | Lips | Nasolabial folds | Marionette lines | ||||
|---|---|---|---|---|---|---|---|
| D0 | D21 | D0 | D21 | D0 | D21 | ||
| 0 | N | 4 | ‐ | ‐ | 3 | ‐ | 2 |
| % | 12% | ‐ | ‐ | 5% | ‐ | 6% | |
| 0.5 | N | 3 | 2 | ‐ | 3 | ‐ | 5 |
| % | 9% | 6% | ‐ | 5% | ‐ | 14% | |
| 1 | N | 16 | 5 | ‐ | 24 | ‐ | 10 |
| % | 47% | 15% | ‐ | 39% | ‐ | 28% | |
| 1.5 | N | 11 | 12 | 1 | 15 | 1 | 8 |
| % | 32% | 35% | 2% | 24% | 3% | 22% | |
| 2 | N | ‐ | 11 | 7 | 12 | 7 | 6 |
| % | ‐ | 32% | 11% | 19% | 19% | 17% | |
| 2.5 | N | ‐ | 4 | 22 | 5 | 10 | 5 |
| % | ‐ | 12% | 35% | 8% | 28% | 14% | |
| 3 | N | ‐ | ‐ | 32 | ‐ | 18 | ‐ |
| % | ‐ | ‐ | 52% | ‐ | 50% | ‐ | |
| Success (at least −1 grade vs. D0) a | N | 25 | 61 | 34 | |||
| % | 73.5% | 98.4% | 94.4% | ||||
| N | 34 | 34 | 62 | 62 | 36 | 36 | |
| Mean | 1.0 | 1.6 | 2.7 | 1.4 | 2.6 | 1.4 | |
| SD | 0.5 | 0.5 | 0.4 | 0.6 | 0.4 | 0.7 | |
| Min | 0.0 | 0.5 | 1.5 | 0.0 | 1.5 | 0.0 | |
| Median | 1.0 | 1.5 | 3.0 | 1.5 | 2.8 | 1.5 | |
| Max | 1.5 | 2.5 | 3.0 | 2.5 | 3.0 | 2.5 | |
| p‐Value | <0.0001 | <0.0001 | <0.0001 | ||||
+1 grade for the lips.
3.3. Secondary endpoints
3.3.1. Remanence of restoration (based on Global Aesthetic Scale)
Long‐term results were best for subjects injected into nasolabial folds. Totally, 18 months after injection, a decrease (increase for lips) of at least one grade of the GAS was still observed in 91%, 88%, and 33% of subjects injected into nasolabial folds, marionette lines, and lips, respectively. Improvements were highly significant compared to baseline for nasolabial folds (mean D0: 2.7 (0.4) vs. mean D540: 1.7 (0.6), p < 0.0001) and marionette lines (mean D0: 2.6 (0.4) vs. mean D540: 1.7 (0.7), p < 0.0001) at 540 days post‐injection and at 450 days post‐injection for lips (mean D0: 1.0 (0.5) vs. mean D450: 1.2 (0.5), p = 0.0326). (Figure 1, Table S6).
FIGURE 1.
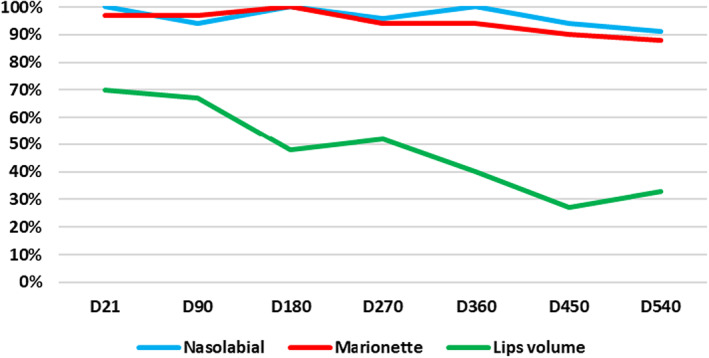
Success rate (%; at least ‐ (nasolabial fold and marionette lines) or + (lips) 1 grade on GAS vs. D0) over the entire study period according to GAS with respect to each facial region injected.
3.4. Photographic scales
The Bazin nasolabial fold and marionette lines scores both showed significant improvement compared to baseline over all follow‐up visits (all p ≤ 0.0002). Mean values for nasolabial folds improved from a mean of 4.9 ± 0.4 to 2.2 ± 1.0 at 3 weeks (Figure 2) and to 2.9 ± 0.9 at 18‐months post‐injection. Mean values for marionette lines improved from a mean of 4.7 ± 0.8 to 2.3 ± 1.0 at 3 weeks and 3.1 ± 0.9 at 18‐months post‐injection. The MLFS showed significant improvement compared to baseline at all follow‐up visits (p < 0.0001), except for D360 (not sufficient subjects visited at this time point due to COVID‐19 lockdown in France). Mean values for lips improved from a mean of 1.2 ± 0.8 to 2.2 ± 0.7 at 3 weeks (Figure 2) and 1.5 ± 0.6 at 18‐months post‐injection. Further results are summarized in Tables S7–S9.
FIGURE 2.
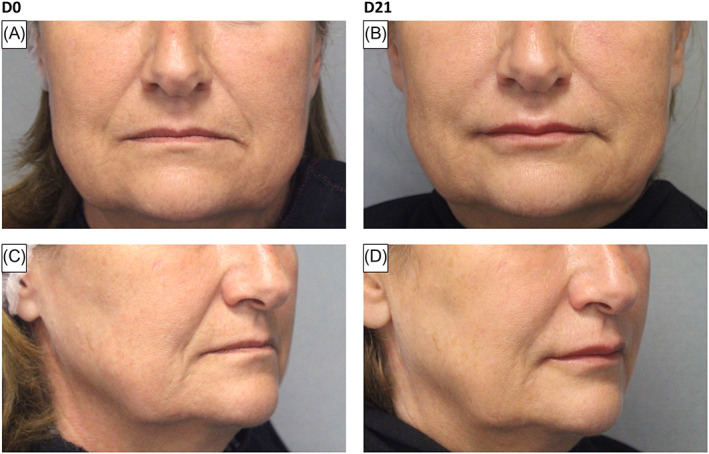
Exemplary photographs illustrating treatment in a 63‐year‐old female with baseline MLFS of 1 and Bazin nasolabial folds scale of 3 (Panels A and C) and at D21, MLFS of 2 and Bazin nasolabial folds scale of 1.5 (Panels B and D).
3.5. GAIS
Aesthetic Improvement as measured using the GAIS was highest at 3 weeks post‐intervention for all indications, irrespective of injector or subject assessments. (Table S10) A continuous decrease of GAIS rating (% total of subjects improved compared to D0) was observed up until 18‐months post‐intervention, with improvement rates remaining highest for nasolabial folds (SGAIS: 71%; IGAIS: 40%), followed by marionette lines (SGAIS: 56%; IGAIS: 33%) and lips (SGAIS: 30%; IGAIS: 22%) at the end of the study period (Figure 3).
FIGURE 3.
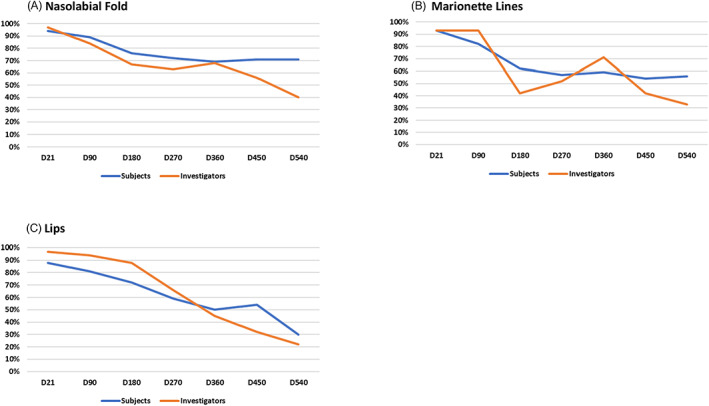
Aesthetic Improvement as measured using the GAIS (subject and investigator) over the entire study period. Values are shown as the % total of subjects that improved compared to D0. ((A) Nasolabial Fold; (B) Marionette Lines; (C) Lips).
3.6. Dermal density
High‐frequency dermal ultrasound showed significant increases in collagen and new collagen density following soft‐tissue filler treatment for nasolabial folds. A significant increase of dermal density (tissue echogenicity) compared to baseline (D0) was found in nasolabial folds at all follow‐up visits (n = 16 all p ≤ 0.0025) (Figures 4 and 5). Density was highest at D90, with 50.9% ± 13.6% and decreased continuously up until D540 with 44.6 %± 11.9% compared to D0 with 32.9% ± 9.8%.
FIGURE 4.

Dermal density (%) measured in nasolabial folds using high‐frequency ultrasound imaging over the entire study period.
FIGURE 5.
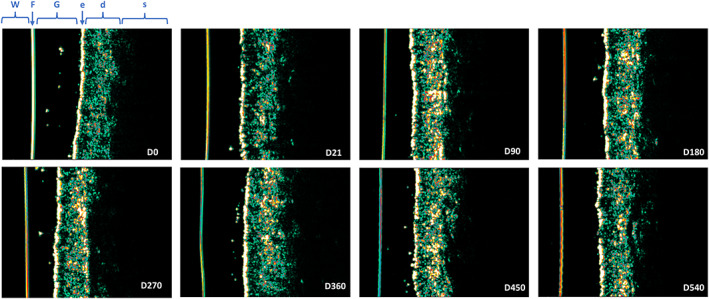
High‐frequency ultrasound images of the left nasolabial fold in one representative subject over the whole study period (D0 to D540) after one single injection of AF Lips at D0. The color scale of echogenicity (from hyperechogenic to hypoechogenic) ranges from white‐yellow–red–green–blue–black. The echogenic density of ultrasound is based on the quantity of collagen. d, dermis; e, epidermis; F, Film; G, gel; s, subcutaneous adipose tissue; W, water.
3.7. Safety assessments
A total of 54 patients reported 133 AEs during the study period. Two SAEs were reported, including pregnancy and COVID‐19 infection. Of the AEs reported, 87 were study product or injection procedure related treatment‐emergent adverse events reported by 28 patients injected. These most frequently included swelling (n = 23) hematoma (n = 18), pain (n = 12), irregularities at palpation (n = 11), erythema (n = 5), nodules (n = 5), tingling sensation (n = 5), and induration (n = 2). All of these AEs were resolved in several days/weeks.
3.8. Local aspect and sensitivity
The majority of local reactions resolved within 3 weeks. After the D21 visit, irregularity of palpation and/ or over‐correction was reported in 4 subjects injected into lips, all other local reactions had resolved. Overcorrection and irregularity at palpation were reported in one subject treated for nasolabial folds and marionette lines after the D21 visit, respectively. All reactions were resolved at the end of the study.
4. DISCUSSION
This prospective, multicenter clinical trial evaluated the efficacy and safety of a hyaluronic acid‐ based soft tissue filler with Tri‐Hyal technology in the treatment of lips, nasolabial folds, and marionette lines. While all injections showed satisfactory results, there were intriguing differences in the efficacy depending on the facial region injected. Injections into the nasolabial fold displayed highest success rates and significant improvements in the respective outcome parameters including subjective and objective assessments, both in the short‐ and long term. A recent systematic review on soft‐tissue fillers for the nasolabial fold measured outcomes on aesthetic improvement using the Wrinkle Severity Rating Scale (WSRS) and GAIS scales. The data show pooled mean GAIS scores of 1.27 at the 12‐month post‐injection visit, 15 which compares to data reported in this study demonstrating mean values of 0.96 and 1.16 for the investigator and subject GAIS rating, respectively. The follow‐up time of 18 months in the presented trial can be considered a major strength. Similar follow‐up times were investigated by Rzany et al. who studied two hyaluronic acid‐based soft tissue fillers for the treatment of nasolabial folds; however, a retreatment was performed after 9 months. 16 They found response rates of approximately 80% (as assessed using the WSRS) 18 months after the initial treatment. While the assessments were based on the GAS in this study, success rates were as high as 93% in this study population, without any touch up/ re‐injection. Injections into marionette lines were correspondingly promising in the presented subject population; however, treatment of the lips revealed inferior longevity. This inferiority could be explained by the low authorized volume of injection (max 1.0 ml) for lips and also the high mobility of the treated zone (lips) compared to the other zones (nasolabial fold and marionette lines). Czumbel et al. performed a meta‐analysis on HA‐based dermal filler for lip augmentation. Similar to the results presented here, they report that treatments remained effective in only about half of the treated subjects after 1 year. 17
Filler longevity is one of the key factors considered by patients and practitioners prior to injection. As hyaluronic acid is a component of the skin extracellular matrix, it is naturally subjected to enzymatic and nonenzymatic degradation. 18 This physiologic process limits the longevity of hyaluronic acid‐based soft tissue fillers and can be considered a central drawback of its application. Several reports have investigated degradation resistance of HA‐based soft‐tissue fillers. Thereby, they found that the physico‐chemical properties, i.e., the degree of cross‐linking, gel concentration, cohesiveness, hardness and capacity for swelling significantly determined the degradation rate. 19 , 20 , 21 , 22 In this study, however, the same product was used for all perioral regions, which poses the question as to why differences in longevity were found. Possibly, the underlying facial anatomy, the mobility of the treated zone, the volume of injection and the injection technique with respect to the injection site play a role in the extent of product degradation. It is believed that mobility of the facial area treated plays a major role in product degradation, 22 , 23 and studies have suggested that shear forces can change soft‐tissue fillers rheologic properties. 24 The mouth is an area of extremely high mobility, required for verbal and nonverbal communication, food intake and digestion. Muscle action of the orbicularis oris muscle complex causes continuous stretching and compression of the lips, and the physical shear forces can consequently contribute to HA‐filler break down. 24 Therefore, the lips require more frequent correction and more volume of injection compared to facial folds which are considered more stable. 12 Future studies will need to elaborate on this finding and investigate the longevity of the same HA‐based soft‐tissue filler injected into different anatomic sites using different injection techniques to shed further light on this.
Previously, it has been suggested that high‐frequency ultrasound imaging is a suitable application to objectively determine the tissue structure of the skin. 13 Dermal density, defined by the components of the extracellular matrix including collagen, elastin, and hyaluronic acid, can therefore be regarded as an important parameter to monitor regeneration following rejuvenating procedures. 25 , 26 The unprecedented results of this study demonstrate significant increase of dermal density at all follow‐up visits, as compared to baseline. This provides evidence of the structural tissue remodeling induced by the injection of the HA filler, particularly by Tri‐Hyal technology which contains 3 different types of HA. 27 The soft‐tissue filler utilized in this study is manufactured using three different sizes of nonanimal origin hyaluronic acid chains. These include BDDE cross‐linked very‐long chain and long‐chain HA, as well as free HA. Several advantages have been discussed regarding this manufacturing technology. Exemplary, studies have suggested the minimization of cross‐linking agent that could reduce potential toxicity, the slow release of free HA which can promote extra‐cellular matrix production by fibroblasts and longer‐lasting stimulation of dermal growth. 27 , 28 , 29 However, more basic science research needs to be conducted to further define the molecular mechanisms by which soft‐tissue fillers manufactured using Tri‐Hyal technology improve skin quality.
This study is not free of limitations. Overall, the study lacks merit of a blinded, randomized, controlled trial. Evaluations were not performed by blinded investigators, which could have added further strength to the data. In addition, objective volume analysis using state‐of the art three‐dimensional surface analysis could have added further objective support to the findings of this study.
The safety profile of the investigated hyaluronic acid‐based soft‐tissue filler corresponds to reports of other devices which are frequently utilized for facial rejuvenation. The treatment emergent adverse events were as expected after hyaluronic‐acid based soft‐tissue filler injections and resolved in most cases 21 days after injection.
5. CONCLUSIONS
The investigated hyaluronic acid‐based soft tissue filler with Tri‐Hyal technology demonstrated high efficacy and safety in the restoration of lips volume and treatment of nasolabial folds and marionette lines. Regional differences in longevity were evident, with long‐term results being best for nasolabial folds and marionette lines. Matching previously published data, degradation of hyaluronic acid‐based soft‐tissue filler can be an issue in lip augmentation and the necessity of retreatments should be discussed with the subject prior to injection.
AUTHOR CONTRIBUTIONS
A.E.‐D., M.G., F.B., F.L., A.G.‐V., P.G., M.T., L.B., I.C., E.R.M., J.D., P.B., T.V., M.D., and H.C. performed the research; K.N. and F.F. supervised the study and made substantial contributions to study conception and design; and K.N., N.M., and F.F. were involved in drafting the manuscript or revising it critically for important intellectual content. Each author has participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
CONFLICT OF INTEREST
F.F. and K.N. are employees of Fillmed Laboratories. A.E.‐D., M.G., F.B., F.L., A.G.‐V., P.G., M.T., L.B., I.C., E.R.M., J.D., P.B., T.V., M.D., and H.C. received a fee for conducting the study. None of the other authors declared any potential conflicts of interest with respect to the research, authorship, and publication of this article.
ETHICAL APPROVAL
Ethical approval was granted by a national independent review board (CPP Ile de France V, Saint‐Antoine hospital, 284 rue du Faubourg Saint Antoine, 75012 Paris, ID‐RCB: 2018‐A02466‐49). The trial was registered within the the ClinicalTrials.gov public registry under the identifier NCT04647513.
Supporting information
Tables S1–S10
ACKNOWLEDGEMENT
A special thanks to Sabrina Lekcir, Annabelle Vicente, and Justine Page for their remarkable help for monitoring and data recovery. The authors deeply thank Sophie Mac, Jean‐Marie Sainthillier, Marie‐José Moschetti, Ana De Lemos, Luc Sentier, Elizabetta Izzo, and Bérengère Boucly for their dynamic support from concept to execution of the study.
Ehlinger‐David A, Gorj M, Braccini F, et al. A prospective multicenter clinical trial evaluating the efficacy and safety of a hyaluronic acid‐based filler with Tri‐Hyal technology in the treatment of lips and the perioral area. J Cosmet Dermatol. 2023;22:464‐472. doi: 10.1111/jocd.15169
Funding information
This study was funded by Laboratoires FILLMED Paris, France
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. https://www.isaps.org/wp‐content/uploads/2022/01/ISAPS‐Global‐Survey_2020.pdf. Accessed Jan 09, 2022.
- 2. https://www.biospace.com/article/fact‐mr‐says‐growth‐of‐lip‐filler‐market‐propelled‐by‐increasing‐number‐of‐aesthetic‐procedures/. Accessed Jan 09, 2022.
- 3. Cotofana S, Fratila AA, Schenck TL, Redka‐Swoboda W, Zilinsky I, Pavicic T. The anatomy of the aging face: a review. Facial Plast Surg. 2016;32(3):253‐260. [DOI] [PubMed] [Google Scholar]
- 4. Cotofana S, Lachman N. Anatomy of the facial fat compartments and their relevance in aesthetic surgery. J Dtsch Dermatol Ges. 2019;17(4):399‐413. [DOI] [PubMed] [Google Scholar]
- 5. Schenck TL, Koban KC, Schlattau A, et al. The functional anatomy of the superficial fat compartments of the face: a detailed imaging study. Plast Reconstr Surg. 2018;141(6):1351‐1359. [DOI] [PubMed] [Google Scholar]
- 6. Alghoul MS, Vaca EE, Mioton LM, Zins JE. The functional anatomy of the deep facial fat compartments: a detailed imaging‐based investigation. Plast Reconstr Surg. 2020;145(4):870e‐871e. [DOI] [PubMed] [Google Scholar]
- 7. Cohen JL, Rivkin A, Dayan S, et al. Multimodal facial aesthetic treatment on the appearance of aging, social confidence, and psychological wellbeing: HARMONY study. Aesthet Surg J. 2021;42:NP115‐NP124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Snozzi P, van Loghem JAJ. Complication management following rejuvenation procedures with hyaluronic acid fillers‐an algorithm‐based approach. Plast Reconstr Surg Glob Open. 2018;6(12):e2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bazin R, Doublet E. Atlas du Vieillissement Cutané: Population Européenne. Éditions Med'Com; 2007. [Google Scholar]
- 10. Flament F, Bazin R, Oiu H. Skin Aging Atlas. Édition Med'com; 2017. [Google Scholar]
- 11. Carruthers A, Carruthers J, Hardas B, et al. A validated lip fullness grading scale. Dermatol Surg. 2008;34(Suppl 2):S161‐S166. [DOI] [PubMed] [Google Scholar]
- 12. Kleinerman R, Whang TB, Bard RL, Marmur ES. Ultrasound in dermatology: principles and applications. J Am Acad Dermatol. 2012;67(3):478‐487. [DOI] [PubMed] [Google Scholar]
- 13. Crisan D, Crisan M, Moldovan M, Lupsor M, Badea R. Ultrasonographic assessment of the cutaneous changes induced by topical flavonoid therapy. Clin Cosmet Investig Dermatol. 2012;5:7‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kopera D, Palatin M, Bartsch R, et al. An open‐label uncontrolled, multicenter study for the evaluation of the efficacy and safety of the dermal filler princess VOLUME in the treatment of nasolabial folds. Biomed Res Int. 2015;2015:195328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stefura T, Kacprzyk A, Dros J, et al. Tissue fillers for the nasolabial fold area: a systematic review and meta‐analysis of randomized clinical trials. Aesthetic Plast Surg. 2021;45(5):2300‐2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rzany B, Bayerl C, Bodokh I, et al. An 18‐month follow‐up, randomized comparison of effectiveness and safety of two hyaluronic acid fillers for treatment of moderate nasolabial folds. Dermatol Surg. 2017;43(1):58‐65. [DOI] [PubMed] [Google Scholar]
- 17. Czumbel LM, Farkasdi S, Gede N, et al. Hyaluronic acid is an effective dermal filler for lip augmentation: a meta‐analysis. Front Surg. 2021;8:681028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol. 2012;4(3):253‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Park S, Park KY, Yeo IK, et al. Investigation of the degradation‐retarding effect caused by the low swelling capacity of a novel hyaluronic acid filler developed by solid‐phase crosslinking technology. Ann Dermatol. 2014;26(3):357‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ibrahim S, Kang QK, Ramamurthi A. The impact of hyaluronic acid oligomer content on physical, mechanical, and biologic properties of divinyl sulfone‐crosslinked hyaluronic acid hydrogels. J Biomed Mater Res A. 2010;94(2):355‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kablik J, Monheit GD, Yu L, Chang G, Gershkovich J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol Surg. 2009;35(Suppl 1):302‐312. [DOI] [PubMed] [Google Scholar]
- 22. da Costa A, Biccigo DGZ, de Souza Weimann ET, et al. Durability of three different types of hyaluronic acid fillers in skin: are there differences among biphasic, monophasic Monodensified, and monophasic Polydensified products? Aesthet Surg J. 2017;37(5):573‐581. [DOI] [PubMed] [Google Scholar]
- 23. Matarasso SL, Carruthers JD, Jewell ML, Restylane CG. Consensus recommendations for soft‐tissue augmentation with nonanimal stabilized hyaluronic acid (Restylane). Plast Reconstr Surg. 2006;117(3 Suppl):3S‐34S. [DOI] [PubMed] [Google Scholar]
- 24. Cotofana S, Hamade H, Bertucci V, et al. Change in rheologic properties of facial soft‐tissue fillers across the physiologic angular frequency Spectrum. Plast Reconstr Surg. 2021;148(2):320‐331. [DOI] [PubMed] [Google Scholar]
- 25. Ha JM, Lim CA, Han K, et al. The effect of micro‐spicule containing epidermal growth factor on periocular wrinkles. Ann Dermatol. 2017;29(2):187‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crisan D, Lupsor M, Boca A, Crisan M, Badea R. Ultrasonographic assessment of skin structure according to age. Indian J Dermatol Venereol Leprol. 2012;78(4):519. [DOI] [PubMed] [Google Scholar]
- 27. Trevidic P, Andre P, Benadiba L, et al. Objective 18‐month comparison of the tolerability of 2 dermal fillers formulated with tri‐Hyal technology. Plast Reconstr Surg Glob Open. 2020;8(12):e3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen R, Yang W, Sun J, et al. Triple cross‐linked hyaluronic acid based on tri‐Hyal technique has more durable effect on dermal renewal. Clin Cosmet Investig Dermatol. 2022;15:691‐701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Trevidic P, Andre P, Benadiba L, et al. Prospective, Split‐face, randomized, long‐term blinded objective comparison of the performance and tolerability of two new hyaluronic acid fillers. Dermatol Surg. 2017;43(12):1448‐1457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S10
Data Availability Statement
Research data are not shared.


