Abstract
Background
Plaque psoriasis is a chronic inflammatory disorder affecting the skin and impacting quality of life. Tildrakizumab (TIL) is an IL‐23 inhibitor licensed for moderate‐to‐severe plaque psoriasis. Regulatory approval of medicinal products is based on safety and efficacy data from randomized controlled trials (RCTs) which impose stringent selection criteria. Long‐term non‐interventional studies (NIS) are needed to establish effectiveness and safety in daily practice bridging the gap between RCTs and the real‐world setting.
Objectives
This analysis of the NIS TILOT seeks to evaluate effectiveness and safety of TIL in patients with moderate‐to‐severe plaque psoriasis in a real‐world setting. Secondary objectives include the assessment of the Dermatology Life Quality Index (DLQI), treatment satisfaction and course of scalp and nail disease using Physician Global Assessment (PGA).
Methods
Interim analysis at 52 weeks (W) of the ongoing non‐interventional, prospective, long‐term multicentre study TILOT.
Results
The effectiveness analysis included 412 patients. The mean [standard deviation, SD] Psoriasis Area and Severity Index (PASI) score was 16.0 [9.1] at baseline improving by 82.4% (95% confidence interval [CI], 78.9–86.0) to 2.1 [2.9] at W52. The proportion of patients achieving PASI scores of <3 and <5 increased over time peaking at 74.6% (95% CI, 69.3–79.4) and 88.4% (95% CI, 84.3–91.8) at W52. Scalp‐PGA and nail‐PGA improved by 79.8% (95% CI, 75.6–84.0) and 72.7% (95% CI, 63.9–81.6), respectively. DLQI of 0/1 was achieved by 48.2% (95% CI, 42.3–54.2). Nine out of 10 physicians and patients expressed a high level of treatment satisfaction. No new safety signals were observed.
Conclusions
This prospective cohort study demonstrates a high degree of effectiveness and a reassuring safety profile of TIL in a real‐world setting over 52 weeks. Patients with scalp and nail involvement or pruritus showed marked improvements.
INTRODUCTION
Psoriasis is a chronic systemic inflammatory skin disorder estimated to affect more than 125 million people worldwide, 1 with a prevalence up to 8%–11% in some Northern European countries. 2 The most common form, plaque psoriasis, occurs in about 80%–90% of psoriatic patients 3 and is characterized by erythematous lesions that commonly affect the elbows, knees, and exposed sensitive areas such as the scalp and nails. 4 , 5 Plaque psoriasis is also associated with pain and pruritus that profoundly affect the quality of life and overall well‐being of most patients. 6 , 7 , 8 , 9 The understanding of the pathogenesis of plaque psoriasis, especially on the role of the interleukin (IL)‐23/IL‐17 axis of which IL‐23 is considered the master cytokine has resulted in the development of highly efficacious classes of biologic drugs. Among these, the humanized monoclonal antibody tildrakizumab (TIL), targeting the IL‐23p19 subunit, has demonstrated safety and efficacy in treating psoriasis in large scale randomized controlled phase III trials (reSURFACE 1 and reSURFACE 2). 10 Recently, results from the five‐year long‐term extension of the reSURFACE 1 and 2 trials were published, confirming sustained disease control and a reassuring safety profile of TIL in long‐term treatment of plaque psoriasis. 11 High efficacy has also been reported for other IL‐23 inhibitors. 12 , 13 Although efficacy and safety profiles of biological therapies are commonly established in phase III studies, concerns have been raised regarding the external validity of these trials due to the strict selection criteria for inclusion and exclusions of psoriasis patients. 14 , 15 Recent large registry studies have demonstrated that a significant proportion of patients (up to 78.4%) receiving systemic drugs for psoriasis in clinical practice is not adequately represented in randomized controlled trials (RCTs) because of non‐eligibility. 16 , 17 Reasons for exclusion include low disease burden and presence of comorbidities such as diabetes, hypertension, cardiac and liver dysfunctions. 17 Non‐eligible psoriasis patients seem to be more likely to develop adverse effects, 15 suggesting that real‐world safety might not be appropriately reflected by a clinical trial population. Moreover, patients not eligible for participation in RCTs showed significantly smaller changes in Psoriasis Area and Severity Index (PASI) score as compared to patients meeting common inclusion and exclusion criteria of RCTs. 16 Thus, prospective observational data of novel biologics including TIL are needed to provide valuable insight on real‐world safety and on the efficacy‐effectiveness gap that might exist between RCTs and clinical practice. Here, we present interim data at Week 52 of the large non‐interventional study (NIS) TILOT that seeks to elucidate long‐term effectiveness and safety of TIL in routine practice. An area of special interest is the impact of TIL on nail and scalp involvement, both known to pose a significant burden of disease. Moreover, this study aimed to assess patient‐reported outcomes such as health‐related quality of life (HRQoL), itch and skin pain as well as overall treatment satisfaction.
MATERIALS AND METHODS
Study design and endpoints
This is an interim analysis of the ongoing prospective, multicentre NIS TILOT for the treatment of patients with moderate‐to‐severe plaque psoriasis with TIL for 144 weeks in clinical practice. This analysis includes patients who had been enrolled in the study at least 52 weeks prior to data cut‐off. Analysis of effectiveness is based on the full analysis set that includes 412 patients. The safety analysis also includes patients who entered the study after initiation of TIL treatment, in total 441. After approval by the ethics committee, the study started in December 2018 and the end of the recruitment phase was in October 2021. The observation period will be approximately 3 years per patient (~36 months). The overall goal of the study is the assessment of the effectiveness and safety profile of TIL in a large study population (~900 subjects) when treated under routine clinical conditions. Psoriasis Area and Severity Index was assessed for all subjects as part of routine clinical practice. Other measures of clinical effectiveness included: Body Surface Area (BSA), Physician Global Assessment (PGA), scalp‐ and nail‐PGA, Dermatology Life Quality Index (DLQI), patient and physician's treatment satisfaction assessment. Itch and skin pain assessments were performed using a visual analogue scale (VAS). For itch and skin pain response analyses, these readings were converted to numerical rating scale (NRS). The cut‐off point for the NRS was <3 to identify patients with mild or moderate itch or pain. 18
Subject selection
Male or female subjects aged >18 years old with moderate‐to‐severe plaque psoriasis by investigator's clinical assessment, able to provide written informed consent for the collection, evaluation, storage and transmission of clinical data who were candidates for systemic therapy and receive TIL in routine care, were included in the study. Subjects were excluded from the study if they had a contraindication according to the Summary of Product Characteristics. 19
Data collection and monitoring
Data were collected using paper‐based case report forms. All concomitant medications were coded using the drug dictionary of the World Health Organization. All adverse drug reactions (ADRs), deaths, other reportable events and medical histories were coded according to the Medical Dictionary for Regulatory Activities. The monitoring was conducted at 10% of the participation sites as a risk‐based approach.
Statistical analysis
According to the non‐interventional character of the study, the statistical analysis was descriptive and explorative, and no statistical hypotheses were formulated. All parameters were either nominally or ordinally scaled, tabulated by absolute and relative frequencies and percentages were calculated as observed. For continuous variables mean and standard deviation (SD) or median are applied, post hoc statistical test is conducted using t‐tests. For discrete variables and responder analyses, 95% confidence intervals (CI) are provided. Presented analysis was based on observed cases (OC) and last observation carried forward (LOCF). A modified non‐responder imputation (mNRI) assessment, where missing values because of treatment discontinuation until Week 52 due to “Lack of efficacy of therapy” or “Intolerance” were imputed as non‐response, and a worst case imputation (WCI) analysis of the data, where missing values because of treatment discontinuation until Week 52 due to “Lack of efficacy of therapy” or “Intolerance” were replaced by the worst available value per patient, are also included as supplementary material. Analysis was generated using the SAS‐software, version 9.2.
RESULTS
Subject disposition and baseline characteristics
412 patients who were newly prescribed TIL at study enrolment were available for the effectiveness analysis. Most patients (82.3%) continued TIL treatment at 52 weeks, 17.7% of the patients had withdrawn from the study before Week 52, with the most common reasons for discontinuation being lack of efficacy and intolerance. Baseline characteristics of the study population are shown in Table 1. Thirty‐nine patients received TIL before the start of the study and, according to study protocol, were not included in the full analysis set, but instead included in the safety analysis set (n = 441). Out of 412 subjects, 246 (59.7%) suffered from comorbidities with hypertension being the most prevalent (n = 124, 30.1%). Overall, 361 (87.6%) of subjects had received prior systemic therapy and 103 (25.0%) subjects had received prior biologics treatment.
TABLE 1.
Patient characteristics and demographic data (full analysis set)
| Parameter | n | Mean ± SD | % |
|---|---|---|---|
| Age (years) | 412 | 47.8 ± 14.9 | |
| Gender (male) | 412 | 61.7 | |
| Height (cm) | 408 | 174.0 ± 9.4 | |
| Weight (kg) | 407 | 88.2 ± 19.2 | |
| Plaque psoriasis diagnosis since (years) | 411 | 18.8 ± 14.3 | |
| BMI (kg/m2) | 407 | 29.1 ± 6.0 | |
| PASI | 412 | 16.0 ± 9.1 | |
| PGA | 411 | 3.1 ± 0.6 | |
| BSA (%) | 401 | 26.2 ± 17.8 | |
| DLQI | 409 | 13.7 ± 7.3 | |
| Patients with nail psoriasis (nail‐PGA >0) | 411 | 44.3 | |
| Patients with scalp psoriasis (scalp‐PGA >0) | 411 | 81.0 | |
| Itching (VAS) | 397 | 56.8 ± 26.3 | |
| Pre‐treatment with conventional systemic therapy | 412 | 87.6 | |
| Pre‐treatment with biologics | 412 | 25.0 | |
| Concomitant disease | 412 | 59.7 |
Abbreviations: BMI, body mass index; BSA, body surface area; DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area and Severity Index; PGA, Physician Global Assessment; SD, standard deviation; VAS, visual analogue scale.
Effectiveness
Clinical response
The mean (SD) total PASI score decreased from 16.0 (9.1) at baseline to 4.5 (5.6) at week 16 and to 2.1 (2.9) at Week 52 corresponding to an improvement of 82.4%. A similar trend was seen using LOCF with mean PASI scores of 5.5 (7.1) at week 16 and 3.6 (5.8) at Week 52 as depicted in Figure 1 (p < 0.0001, for absolute and relative difference versus baseline for both OC and LOCF). The proportion of patients with PASI <1, PASI <3 (Figure 2a) and PASI <5 (Figure 2b) increased from 1.0 (95% CI, 0.3–2.5), 1.9 (95% CI, 0.8–3.8) and 6.8 (95% CI, 4.6–9.7) at baseline to 47.9% (95% CI, 42.1–53.6), 74.6% (95% CI, 69.3–79.4) and 88.4% (95% CI, 84.3–91.8) at Week 52, respectively. Using LOCF 43.9% (95% CI, 39.1–48.9), 67.7% (95% CI, 63.0–72.2) and 79.1% (95% CI, 74.9–83.0) achieved a PASI <1, <3, <5 response at Week 52. The percentage of patients with PASI 75 and PASI 90 response increased over time to 78.7% (95% CI, 73.6–83.2) and 57.7 (95% CI, 51.9–63.3) at Week 52, respectively. mNRI/WCI data are presented in Table S1. The mean (SD) affected BSA with TIL was reduced from 26.2 (17.8) at baseline to 9.3 (12.5) [7.2 (11.4) using LOCF] at week 16 and 4.0 (7.1) [6.4 (11.3) using LOCF] at Week 52 (p < 0.0001 for both OC and LOCF). The percentage of patients with clear or almost clear skin (PGA 0/1) increased from 1.7% (95% CI, 0.7–3.5) at baseline to 51.8% (95% CI, 46.7–56.9) at week 16 [48.9.1% (95% CI, 44.0–53.9), LOCF] and to 72.6% (95% CI, 67.3–77.5) at Week 52 [64.2% (95% CI, 59.4–68.9), LOCF]. mNRI data are presented in Table S1. In patients with scalp involvement at baseline, the scalp‐PGA dropped from 2.7 (95% CI, 2.6–2.8) at baseline to 0.6 (95% CI, 0.5–0.7) at Week 52 with an overall improvement of 79.8% (95% CI, 75.6–84.0; Figure S1a). Of these, 63.8% (95% CI, 57.2–70.0) had no scalp involvement at Week 52 (Figure 3a). Very similar results were obtained using LOCF with scalp‐PGA dropping by 70.6% (95% CI, 66.2–74.9) to a mean scalp‐PGA of 0.8 (95% CI, 0.7–0.9) at Week 52. In patients with nail disease at baseline (nail‐PGA >0), mean nail‐PGA improved from 1.8 (95% CI, 1.7–2.0) at baseline to 0.4 (95% CI, 0.3–0.6) at Week 52 (Figure S1b). This corresponds to an improvement of 72.7% (95% CI, 63.9–81.6) with 66.7 (95% CI, 57.4–75.1) of patients being clear and 24.8% (95% CI, 17.3–33.6) showing only mild disease (Figure 3b). Using LOCF, the nail‐PGA improved to a similar extent by 64.8% (95% CI, 57.4–72.3) resulting in a mean nail‐PGA of 0.6 (95% CI, 0.5–0.7) at Week 52. Median itch as assessed on the VAS decreased from 63.0 at baseline to 4.0 [6.0 LOCF] at Week 52 (p < 0.0001, for both OC and LOCF; Figure 4a). For responder analysis, VAS was transformed to NRS. No or only mild pruritus as defined by Reich et al. 18 was reported by 81.9% (95% CI, 76.9–86.3) of patients at Week 52 with a similar degree of response seen when using LOCF [74.1% (95% CI, 69.4–78.3)] (Figure 4b). Skin pain also improved in patients receiving TIL with 92.8% patients showing no or only mild skin pain (skin pain‐NRS <3) at Week 52 (data not shown).
FIGURE 1.
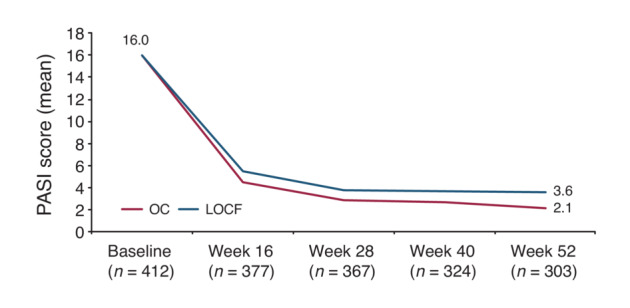
Evolution of absolute PASI over time (mean). LOCF, Last Observation Carried Forward; OC, Observed Cases; PASI, Psoriasis Area and Severity Index. LOCF: n = 412.
FIGURE 2.
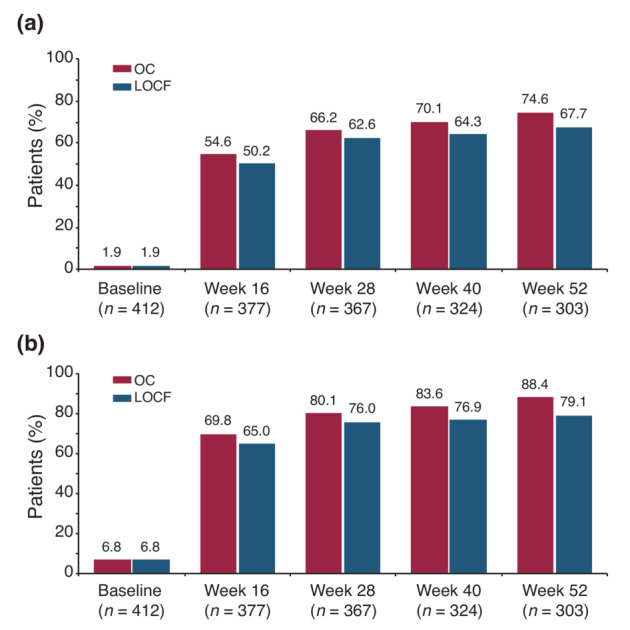
(a) Proportion of patients with PASI <3 from baseline through Week 52. (b) Proportion of patients with PASI <5 from baseline through Week 52. LOCF, Last Observation Carried Forward; OC, Observed Cases; PASI, Psoriasis Area and Severity Index. LOCF: n = 412.
FIGURE 3.
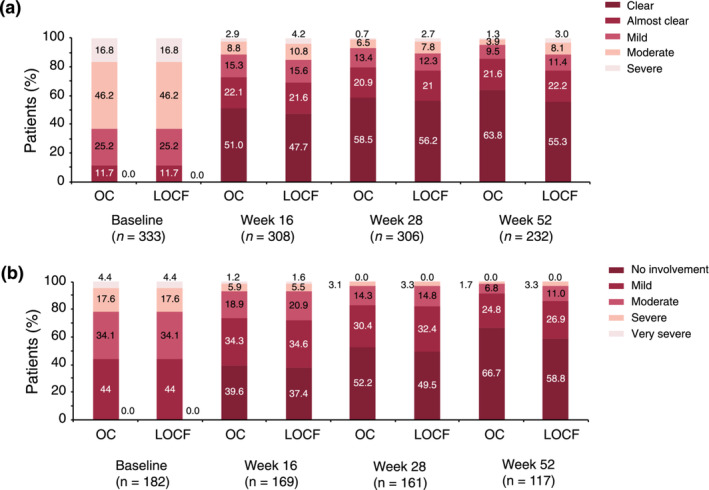
(a) Course of the scalp‐PGA in patients with scalp involvement at baseline. (b) Course of the nail‐PGA in patients with nail involvement at baseline. LOCF, Last Observation Carried Forward; OC, Observed Cases; PGA, Physician Global Assessment. Scalp‐PGA, LOCF: n = 333; nail‐PGA, LOCF: n = 182.
FIGURE 4.
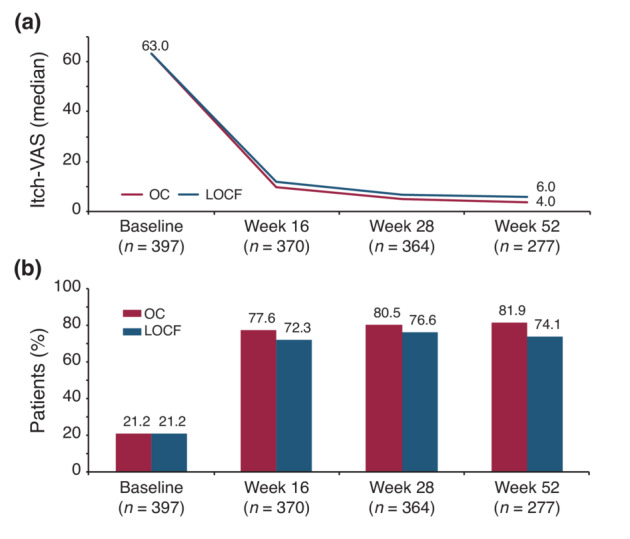
(a) Itch‐VAS up to Week 52 (median). (b) Proportion of patients with itch‐NRS <3 from baseline through Week 52. LOCF, Last Observation Carried Forward; NRS, Numerical Rating Scale; OC, Observed Cases; VAS, Visual Analogue Scale. LOCF: n = 397.
DLQI response
The median DLQI score (OC and LOCF) improved from 13.0 at baseline to 2.0 at Week 52 (p < 0.0001, for both OC and LOCF; Figure 5). The percentage of patients with no impairment of their HRQoL (DLQI 0/1) increased to 48.2% (95% CI, 42.3–54.2) and 42.5% (95% CI, 37.7–47.5) using LOCF at Week 52. mNRI data are presented in Table S1. The proportion of patients with DLQI 0/1 is presented in Figure S2. Overall, DLQI changes correlated with PASI improvement. With a Pearson correlation coefficient of r = 0.2549 at baseline and r = 0.53098 at Week 52, however, a certain degree of heterogeneity of DLQI‐scores became apparent (Figure S3).
FIGURE 5.
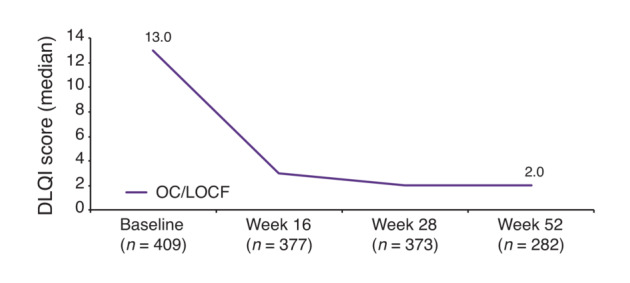
DLQI score up to Week 52 (median). OC and LOCF values are the same. DLQI, Dermatology Life Quality Index; LOCF, Last Observation Carried Forward; OC, Observed Cases. LOCF: n = 409.
Treatment satisfaction of physician and patients
At Week 52, 92.2% of physicians were satisfied or very satisfied with the effectiveness and 99.3% were satisfied or very satisfied with the tolerability of TIL (Figure 6a). Similarly, among participants, 91.4% were satisfied or very satisfied with effectiveness and 97.1% were satisfied or very satisfied with tolerability (Figure 6b).
FIGURE 6.
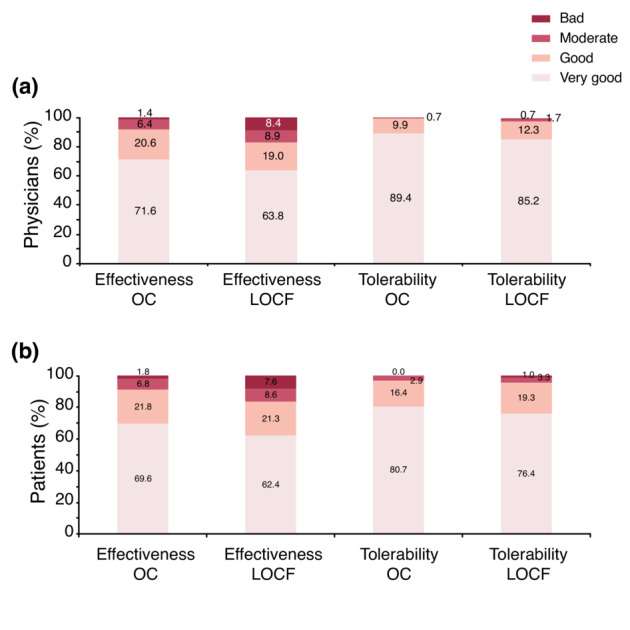
(a) Physician satisfaction—effectiveness and tolerability at Week 52. (b) Patient satisfaction—effectiveness and tolerability at Week 52. locf, Last Observation Carried Forward; OC, Observed Cases. LOCF: n = 406, OC: n = 282.
Safety
Safety data are summarized in Table 2. At the point of this interim analysis, 68 patients (15.4%) experienced an ADR. Exposure‐adjusted incidence rate (EAIR) of patients with ADR per 100 patient‐years (PY) was 16.5. Serious ADR occurred in 14 subjects (3.2%) with an EAIR of 3.4. There were three deaths during the period covered by this interim analysis and EAIR per 100 PY was 0.8. The cause of death was colon cancer in one patient and could not be discerned for the other. All patients had multiple preexisting comorbidities. The three deaths were assessed to not be related to the study drug and caused by worsening of comorbidities. Moreover, deaths in this study were in line with average annual death rate in Germany 20 (11 per 1000 people). The most common adverse drug reactions were nausea (n = 3 subjects, 0.7%) and skin infection [folliculitis (n = 3 subjects, 0.7%)].
TABLE 2.
Summary of safety
| Tildrakizumab (n = 441, 380 PY) | |||
|---|---|---|---|
| n | % | EAIR/100 PY | |
| Patients with any ADR | 68 | 15.4 | 16.5 |
| Patients with any serious ADR | 14 | 3.2 | 3.4 |
| Deaths a | 3 | 0.7 | 0.8 |
Abbreviations: ADR, adverse drug reaction; EAIR, exposure‐adjusted incidence rate; PY, patient‐years.
The three deaths were assessed as not related to the study drug.
DISCUSSION
Here, we show interim data at Week 52 of the ongoing prospective, NIS TILOT conducted in Germany to assess the effectiveness and safety of long‐term therapy with TIL in patients with moderate‐to‐severe plaque psoriasis in routine practice. We found a significant improvement of overall disease severity with a trend of continuous reduction in disease severity throughout the study, as assessed by PASI, PGA and BSA. Importantly, patients with involvement of impactful areas like scalp and nails also benefitted from TIL treatment with 6 out of 10 patients being cleared at Week 52. These changes were accompanied by improvement of HRQoL and other patient reported outcomes such as pruritus and skin pain. TIL was well tolerated with no new safety signals.
In TILOT, patient baseline characteristics were similar to those reported by the German psoriasis registry (PsoBest), which suggests that TILOT is representative of the psoriasis population receiving systemic therapy in Germany. 21 However, in comparison with the reSURFACE program some differences become evident. Patients in TILOT frequently suffered from comorbidities. In fact, cardiovascular and metabolic comorbidities such as hypertension and diabetes were more common in TILOT (30.1% and 9%) than in the reSURFACE 2 trial (25.5% and 3.6%). 10 While the presence of certain comorbid conditions is known to distinguish patients eligible for RCTs from those who are not, 17 this insight underpins the relevance of evidence from real‐world studies such as TILOT. Moreover, in this study, the mean baseline PASI and BSA (%) of 16.0 and 26.2, respectively, were lower than that of clinical trial populations, which were 20.2 and 32.0, respectively. 11 These differences might be related to the study types including the wash‐out periods commonly used in RCTs that might lead to more exacerbated baseline severity. In addition, more patients in TILOT had previously received conventional systemic (87.6% of patients vs 31.8%) or biologic therapies (25% vs 17.9%) compared with patients from reSURFACE trials. Consequently, many patients in TILOT received TIL as a later treatment line for plaque psoriasis. Despite such differences, the effectiveness results among patients in TILOT were comparable or slightly higher to those observed in clinical trials. 10 At week 28, the percentage of patients with PASI <3 (66.2%) and PASI <5 (80.1%) were higher than what was observed in RCTs 22 (62.8% and 75.3%, respectively). Interestingly, a trend towards further improvement beyond week 28 was observed for several parameters including, for example, PASI response rate (PASI <3 66.2% at week 28 vs 74.6% at Week 52). This observation is in line with data from the reSURFACE trials showing further improvement in at least a subset of patients beyond week 28. 23 Involvement of visible areas such as scalp and nails is frequently associated with a high burden of disease due to frequent stigmatization, pruritus or functional impairment and pain, respectively. Therefore, effective treatment of these manifestations is key. 4 , 24 , 25 Importantly, in this NIS, TIL demonstrated effectiveness in improving nail and scalp disease as well as mitigating plaque psoriasis related symptoms such as itch and skin pain. This study also provides insights into the impact of TIL on impairment of HRQoL experienced by plaque psoriasis patients. Although the impact of TIL on HRQoL in TILOT was significant with 48.2% of patients achieving a DLQI 0/1 at Week 52, other large real‐world studies found even higher response rates. A recently published cohort of TIL‐treated patients reported DLQI 0/1 in 61% of patients after 28 weeks of treatment. 26
Overall, TIL was well tolerated and had a favourable safety profile among patients with plaque psoriasis treated during routine clinical practice in a real‐world setting. ADRs were infrequent, mild or moderate in severity and consistent with those observed in the reSURFACE studies. 27 Treatment satisfaction in terms of effectiveness and tolerability was very high as 9 out 10 physicians and patients were satisfied or very satisfied with TIL treatment. As discussed above, effectiveness seen in TILOT compares well with data gained in RCTs. Consequently, we found no efficacy‐effectiveness gap for TIL, which stands in contrast to other biological compounds that achieved a smaller absolute change in PASI and showed higher rates of SAEs in patients ineligible for enrolment in clinical trials, compared with those eligible for participation in RCTs, as revealed by analyses of PSOBIOTEQ and BADBIR registries. 16 , 17 This also holds true for more recently anti‐IL‐23 drugs showing PASI response rates falling short of that seen in RCTs, 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 thereby highlighting the importance of performing studies in the real‐world population. Although RCTs may show differences in the efficacy of IL‐23 inhibitors, a recent retrospective observational study enrolling psoriasis patients treated with guselkumab, risankizumab and tildrakizumab showed that there were not any significant differences in terms of real‐life effectiveness between them. 36 Overall, our results are fairly in line with prior reports on effectiveness and safety of TIL in routine practice, all showing similar or even better response rates than reported in RCTs. 26 , 37 , 38
Our study has several limitations. These include the observational character, lack of a control group and mono‐national setup. All reported outcomes were documented according to routine practice. As a consequence, more data gaps may result than in strictly controlled RCTs. Further, measured variables may have been influenced by responder bias, that is, patients experiencing a favourable treatment response may have been more likely to continue treatment, while those who were not experiencing a favourable treatment response may have tended to discontinue treatment. However, to address this shortcoming, in addition to OC, more conservative imputation methods have been used to carry forward or impute less favourable results in patients with missing data/who terminated prematurely.
In conclusion, this is the largest prospective cohort study published to date within the class of IL‐23 inhibitors, confirming the effectiveness and safety of TIL. This study also supports the real‐world performance of TIL in reducing subjective symptoms of disease such as itch and improving psoriasis in visible areas such as the scalp and nails. 39 , 40 Longer‐term data from TILOT and other studies will deepen the understanding of the real‐world effectiveness and impact on the quality of life of TIL for the treatment of plaque psoriasis.
FUNDING INFORMATION
Almirall Hermal GmbH.
CONFLICT OF INTEREST
AT is the National coordinator of the performed study; US received funds from AbbVie, Deutschland GmbH, Almirall Hermal GmbH, Astellas Pharma GmbH, Beiersdorf Dermo Medical GmbH, Celgene GmbH, Janssen Cilag GmbH, Johnson & Johnson GmbH, LEO Pharma GmbH, L'Oréal GmbH, MEDA Pharma GmbH, Medical Project Design GmbH, Merz, Pharmaceuticals GmbH, MSD SHARP & DOHME GmbH, Novartis Pharma GmbH, and Pfizer GmbH, Berufsverband der deutschen Dermatologen Bundesvorstand und Landesvorsitz Bremen Vertreterversammlung der kassenärztlichen Vereinigung Bremen, President HVM‐Ausschuss der kassenärztlichen Vereinigung Bremen, Member Finanzausschuss der kassenärztlichen Vereinigung Bremen, and holds stocks for Medical project design GmbH CEO and stockholder; SD is an employee of Almirall Hermal GmbH; BK received funds from Almirall Hermal GmbH, Abbvie, Amgen, Almirall Hermal, Beiersdorf Dermo Medical, Biogen, Celgene, Dr. Pfleger, Galderma, Janssen‐Cilag, Leo Pharma, Lilly, Novartis. PP and TH have no conflicts of interest to declare.
Supporting information
Appendix S1
ACKNOWLEDGEMENTS
Medical writing support was provided by Stefania Ippati, PhD, TFS HealthScience and Eva Mateu, PhD, TFS HealthScience in accordance with Good Publication Practice (GPP3) guidelines.
Tsianakas A, Schwichtenberg U, Pierchalla P, Hinz T, Diemert S, Korge B. Real‐world effectiveness and safety of tildrakizumab in long‐term treatment of plaque psoriasis: Results from the non‐interventional, prospective, multicentre study TILOT . J Eur Acad Dermatol Venereol. 2023;37:85–92. 10.1111/jdv.18572
DATA AVAILABILITY STATEMENT
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Griffiths CEM, van der Walt JM, Ashcroft DM, Flohr C, Naldi L, Nijsten T, et al. The global state of psoriasis disease epidemiology: a workshop report. Br J Dermatol. 2017;177:e4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Damiani G, Bragazzi NL, Karimkhani Aksut C, Wu D, Alicandro G, McGonagle D, et al. The global, regional, and national burden of psoriasis: results and insights from the global burden of disease 2019 study. Front Med. 2021;8:743180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feldman SR, Goffe B, Rice G, Mitchell M, Kaur M, Robertson D, et al. The challenge of managing psoriasis: unmet medical needs and stakeholder perspectives. Am Health Drug Benefits. 2016;9:504–13. [PMC free article] [PubMed] [Google Scholar]
- 4. Wozel G. Psoriasis treatment in difficult locations: scalp, nails, and intertriginous areas. Clin Dermatol. 2008;26:448–59. [DOI] [PubMed] [Google Scholar]
- 5. Segaert S, Calzavara‐Pinton P, de la Cueva P, Jalili A, Danic DL, Pink AE, et al. Long‐term topical management of psoriasis: the road ahead. J Dermatolog Treat. 2020;33:111–20. [DOI] [PubMed] [Google Scholar]
- 6. Bhosle MJ, Kulkarni A, Feldman SR, Balkrishnan R. Quality of life in patients with psoriasis. Health Qual Life Outcomes. 2006;4:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lewis‐Beck C, Abouzaid S, Xie L, Baser O, Kim E. Analysis of the relationship between psoriasis symptom severity and quality of life, work productivity, and activity impairment among patients with moderate‐to‐severe psoriasis using structural equation modeling. Patient Prefer Adherence. 2013;7:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ljosaa TM, Mork C, Stubhaug A, Moum T, Wahl AK. Skin pain and skin discomfort is associated with quality of life in patients with psoriasis. J Eur Acad Dermatol Venereol. 2012;26:29–35. [DOI] [PubMed] [Google Scholar]
- 9. Martin ML, Gordon K, Pinto L, Bushnell DM, Chau D, Viswanathan HN. The experience of pain and redness in patients with moderate to severe plaque psoriasis. J Dermatolog Treat. 2015;26:401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reich K, Papp KA, Blauvelt A, Tyring SK, Sinclair R, Thaçi D, et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet. 2017;390:276–88. [DOI] [PubMed] [Google Scholar]
- 11. Thaci D, Piaserico S, Warren RB, Gupta AK, Cantrell W, Draelos Z, et al. Five‐year efficacy and safety of tildrakizumab in patients with moderate‐to‐severe psoriasis who respond at week 28: pooled analyses of two randomized phase III clinical trials (reSURFACE 1 and reSURFACE 2). Br J Dermatol. 2021;185:323–34. [DOI] [PubMed] [Google Scholar]
- 12. Blauvelt A, Leonardi CL, Gooderham M, Papp KA, Philipp S, Wu JJ, et al. Efficacy and safety of continuous risankizumab therapy vs treatment withdrawal in patients with moderate to severe plaque psoriasis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reich K, Griffiths CEM, Gordon KB, Papp KA, Song M, Randazzo B, et al. Maintenance of clinical response and consistent safety profile with up to 3 years of continuous treatment with guselkumab: results from the VOYAGE 1 and VOYAGE 2 trials. J Am Acad Dermatol. 2020;82:936–45. [DOI] [PubMed] [Google Scholar]
- 14. Schmitt J, Zhang Z, Wozel G, Meurer M, Kirch W. Efficacy and tolerability of biologic and nonbiologic systemic treatments for moderate‐to‐severe psoriasis: meta‐analysis of randomized controlled trials. Br J Dermatol. 2008;159:513–26. [DOI] [PubMed] [Google Scholar]
- 15. Garcia‐Doval I, Carretero G, Vanaclocha F, Ferrandiz C, Daudén E, Sánchez‐Carazo JL, et al. Risk of serious adverse events associated with biologic and nonbiologic psoriasis systemic therapy: patients ineligible vs eligible for randomized controlled trials. Arch Dermatol. 2012;148:463–70. [DOI] [PubMed] [Google Scholar]
- 16. Mason KJ, Barker JNWN, Smith CH, Hampton PJ, Lunt M, McElhone K, et al. Comparison of drug discontinuation, effectiveness, and safety between clinical trial eligible and ineligible patients in BADBIR. JAMA Dermatol. 2018;154:581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Masson Regnault M, Castañeda‐Sanabria J, Diep Tran MHT, Beylot‐Barry M, Bachelez H, Beneton N, et al. Users of biologics in clinical practice: would they be eligible for phase III clinical studies? Cohort Study in the French Psoriasis Registry PSOBIOTEQ. J Eur Acad Dermatol Venereol. 2020;34:293–300. [DOI] [PubMed] [Google Scholar]
- 18. Reich A, Chatzigeorkidis E, Zeidler C, Osada N, Furue M, Takamori K, et al. Tailoring the cut‐off values of the visual analogue scale and numeric rating scale in itch assessment. Acta Derm Venereol. 2017;97:759–60. [DOI] [PubMed] [Google Scholar]
- 19. European Medicines Agency . Ilumetri. 2018. https://www.ema.europa.eu/en/medicines/human/EPAR/ilumetri. Accessed 16 Dec 2021.
- 20. World Bank Group – International Development, Poverty, & Sustainability . World Bank. https://www.worldbank.org/en/home. Accessed 23 Feb 2022.
- 21. Augustin M, Spehr C, Radtke MA, Boehncke WH, Luger T, Mrowietz U, et al. German psoriasis registry PsoBest: objectives, methodology and baseline data. J Dtsch Dermatol Ges. 2014;12:48–57. [DOI] [PubMed] [Google Scholar]
- 22. Gordon KB, Reich K, Crowley JJ, Korman NJ, Murphy FT, Poulin Y, et al. Disease activity and treatment efficacy using patient‐level Psoriasis Area and Severity Index scores from tildrakizumab phase 3 clinical trials. J Dermatolog Treat. 2022;33:219–28. [DOI] [PubMed] [Google Scholar]
- 23. Elewski B, Menter A, Crowley J, Tyring S, Zhao Y, Lowry S, et al. Sustained and continuously improved efficacy of tildrakizumab in patients with moderate‐to‐severe plaque psoriasis. J Dermatolog Treat. 2020;31:763–8. [DOI] [PubMed] [Google Scholar]
- 24. Reich K. Approach to managing patients with nail psoriasis. J Eur Acad Dermatol Venereol. 2009;23(Suppl 1):15–21. [DOI] [PubMed] [Google Scholar]
- 25. de Jong EM, Seegers BA, Gulinck MK, Boezeman JB, van de Kerkhof PC. Psoriasis of the nails associated with disability in a large number of patients: results of a recent interview with 1,728 patients. Dermatology. 1996;193:300–3. [DOI] [PubMed] [Google Scholar]
- 26. Drerup KA, Seemann C, Gerdes S, Mrowietz U. Effective and safe treatment of psoriatic disease with the Anti‐IL‐23p19 Biologic tildrakizumab: results of a real‐world prospective cohort study in nonselected patients. Dermatology. 2021;238:615–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Blauvelt A, Reich K, Papp K, Kimball AB, Gooderham M, Tyring SK, et al. Safety of tildrakizumab for moderate‐to‐severe plaque psoriasis: pooled analysis of three randomized controlled trials. Br J Dermatol. 2018;179:615–22. [DOI] [PubMed] [Google Scholar]
- 28. Gerdes S, Bräu B, Hoffmann M, Korge B, Mortazawi D, Wiemers F, et al. Real‐world effectiveness of guselkumab in patients with psoriasis: Health‐related quality of life and efficacy data from the noninterventional, prospective, German multicenter PERSIST trial. J Dermatol. 2021;48:1854–62. [DOI] [PubMed] [Google Scholar]
- 29. Gkalpakiotis S, Cetkovska P, Arenberger P, Dolezal T, Arenbergerova M, Velackova B, et al. Risankizumab for the treatment of moderate‐to‐severe psoriasis: real‐life multicenter experience from The Czech Republic. Dermatol Ther (Heidelb). 2021;11:1345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Borroni RG, Malagoli P, Gargiulo L, Valenti M, Pavia G, Facheris P, et al. Real‐life effectiveness and safety of risankizumab in moderate‐to‐severe plaque psoriasis: a 40‐week multicentric retrospective study. Acta Derm Venereol. 2021;101:adv00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Benhadou F, Ghislain PD, Guiot F, Willaert F, del Marmol V, Lambert J, et al. Real‐life effectiveness and short‐term (16‐week) tolerance of guselkumab for psoriasis: a Belgian retrospective multicentre study. J Eur Acad Dermatol Venereol. 2020;34:e837–9. [DOI] [PubMed] [Google Scholar]
- 32. Fougerousse A‐C, Ghislain P‐D, Reguiai Z, Maccari F, Parier J, Bouilly Auvray D, et al. Effectiveness and short‐term (16‐week) tolerance of guselkumab for psoriasis under real‐life conditions: a retrospective multicenter study. J Eur Acad Dermatol Venereol. 2020;34:e644–6. [DOI] [PubMed] [Google Scholar]
- 33. Hung Y‐T, Lin Y‐J, Chiu H‐Y, Huang Y‐H. Impact of previous biologic use and body weight on the effectiveness of guselkumab in moderate‐to‐severe plaque psoriasis: a real‐world practice. Ther Adv Chronic Dis. 2021;12:20406223211046684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Malara G, Trifirò C, Bartolotta A, Giofré C, D'Arrigo G, Testa A, et al. Real‐world effectiveness and safety of Guselkumab for the treatment of psoriasis: a 6‐month prospective study in a series of psoriatic patients. Eur Rev Med Pharmacol Sci. 2021;25:406–12. [DOI] [PubMed] [Google Scholar]
- 35. Megna M, Fabbrocini G, Cinelli E, Camela E, Ruggiero A. Guselkumab in moderate to severe psoriasis in routine clinical care: an Italian 44‐week real‐life experience. J Dermatolog Treat. 2022;33:1074–8. [DOI] [PubMed] [Google Scholar]
- 36. Megna M, Tommasino N, Potestio L, Battista T, Ruggiero A, Noto M, et al. Real‐world practice indirect comparison between guselkumab, risankizumab, and tildrakizumab: results from an Italian 28‐week retrospective study. J Dermatolog Treat. 2022;1–8. 10.1080/09546634.2022.2081655 [DOI] [PubMed] [Google Scholar]
- 37. Galán‐Gutierrez M, Rodriguez‐Fernandez Freire L, Ruiz‐Villaverde R. Tildrakizumab: short‐term efficacy and safety in real clinical practice. Int J Dermatol. 2021;61;e355–57. [DOI] [PubMed] [Google Scholar]
- 38. Burlando M, Castelli R, Cozzani E, Parodi A. Treatment of moderate‐to‐severe plaque psoriasis with tildrakizumab in the real‐life setting. Drugs Context. 2021;10:2021‐2‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blome C, Augustin M, Klein TM. Nail psoriasis and quality‐of‐life measurement in clinical trials: call for the use of nail‐specific instruments. Am J Clin Dermatol. 2021;22:747–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zampieron A, Buja A, Fusco M, Linder D, Bortune M, Piaserico S, et al. Quality of life in patients with scalp psoriasis. G Ital Dermatol Venereol. 2015;150:309–16. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.


