Abstract
Objective
Management of hypothyroidism is controversial because of medication cost pressures and scientific uncertainty on how to address treatment dissatisfaction experienced by some patients. The objective was to investigate the experience and preferences of UK endocrinologists in use of thyroid hormones.
Design
Web‐based survey.
Patients
UK endocrinologists were invited to participate.
Measurements
Responses to questionnaire.
Results
The response rate was 21% (272/1295). While levothyroxine monotherapy is regarded as the treatment of choice for hypothyroidism, 51% of respondents stated that combined treatment with levothyroxine and liothyronine could be considered for levothyroxine‐treated patients whose symptoms persist despite normalisation of serum thyroid stimulating hormone (TSH) concentration. However, only 40% are currently prescribing such treatment, and just 23% would consider taking it themselves. A small minority prescribe desiccated thyroid extract, and those most likely to do so are aged over 60 years. Most respondents stated that they have no influence over brand or formulation of levothyroxine dispensed to their patients and expect no major differences in efficacy between different formulations. A total of 9% would prescribe levothyroxine for euthyroid enlarging goitre, and 29% for euthyroid female infertility with high titre thyroid peroxidase antibodies, despite recent trials finding no benefit.
Conclusions
UK endocrine practice in management of hypothyroidism is broadly in line with international guidance. However, a minority of respondents would consider thyroid hormone supplementation in euthyroid individuals for female infertility, enlarging goitre, and other indications in which evidence of efficacy is lacking. Willingness to consider prescribing combined levothyroxine and liothyronine, for hypothyroid symptoms which persist despite normalised TSH, has increased in comparison to previous international surveys, despite inconsistent evidence of benefit.
Keywords: desiccated thyroid extract, goitre, hypothyroidism, infertility, survey, thyroxine, triiodothyronine
1. INTRODUCTION
The UK prevalence of hypothyroidism was estimated recently at 3.6% overall, increasing from around 1 in 1000 children aged up to 10 years, to approximately 1 in 10 adults aged over 70 years. 1 This disease burden occurs within a population that had in recent decades been regarded as iodine replete, however, concern about gradually falling iodine intake, particularly in women of child‐bearing age, 2 is borne out by the latest results of the UK National Diet and Nutrition Survey, confirming that this cohort (females aged 16–49 years) now meets World Health Organisation criteria for iodine deficiency. 3
Primary hypothyroidism in the United Kingdom is usually diagnosed and managed by general practitioners, within the National Health Service (NHS), which provides tax‐funded healthcare. General practitioners tend to refer to NHS endocrinologists only if difficulties arise in diagnosis or management. The latest UK National Institute for Health and Care Excellence (NICE) guidance recommends levothyroxine (LT4) as first‐line treatment. Prescribers are directed to achieve thyroid stimulating hormone (TSH) concentrations within the reference interval, measuring TSH every 3 months until stable, and annually thereafter. 4
NICE guidance does not address switching between LT4 tablet brands or formulations, despite several other European countries experiencing widespread difficulties after enforced switches. 5 However, the UK Medicines and Healthcare products Regulatory Agency (MHRA) advises that, while generic prescribing is routinely appropriate, a specific, tolerated product can be prescribed for patients reporting persistent symptoms when switching between different LT4 tablets, and liquid LT4 can be considered if symptoms or poor control of thyroid function persist despite adherence to a specific product. 6 LT3 is not recommended for routine use but NICE guidelines nevertheless direct readers toward NHS England Specialist Pharmacy Services (SPS) guidance, which, in limited circumstances, provides for long‐term NHS‐funded LT4 + LT3 combination therapy, after a trial of at least 3 months' duration under supervision of an endocrinology consultant.
LT4 tablets are among the most prescribed medications in the United Kingdom. Liquid and soft‐gel capsule preparations are available, though their current cost in the United Kingdom is several times greater than tablets. The bioavailability of LT4 in liquid and soft‐gel capsules is reported to be greater than for tablets, but their cost‐effectiveness in adult hypothyroidism remains uncertain, even when absorption is impaired by comorbidities, concomitant medications, or behavioural factors. 7 Nevertheless, from 2016 to 2021, nontablet LT4 preparations have taken an increasing share of the market and, together with liothyronine (LT3) and desiccated thyroid extract (DTE), have consumed 35% of UK National Health Service (NHS) expenditure in England on thyroid hormones, despite representing just 0.36% of items dispensed. 8
The current study is part of the Treatment of Hypothyroidism in Europe by Specialists: an International Survey (THESIS) project, which is designed to investigate attitudes and practice in controversial aspects of thyroidology, most notably in the use of thyroid hormones and nutritional supplements for hypothyroidism, and the management of euthyroid disorders, including goitre and female infertility.
2. METHOD
We used Qualtrics to host a web‐based survey consisting of 12 demographics questions, followed by 23 questions on thyroid hormone therapy. The survey text is provided in a Supportiing Information: online appendix. Individual electronic links were sent by email to clinical members of the Society for Endocrinology and/or British Thyroid Association, followed by two reminders, between 5th March 2021 and 27th April 2021. Repeat submissions from the same IP address were blocked automatically. Anonymous responses were collected and electronically stored by the survey service. Approval for distribution of invitations was granted by Society and Association officers and, as the study constituted a membership survey, research ethics committee approval was not required.
2.1. Statistical analysis
Responses were considered valid for analysis only if demographic data were complete. Data were analysed using GraphPad Prism version 8.3.4 software. Descriptive statistics were prepared for responses to each question. The χ 2 test and Fisher's exact test were used to compare frequencies, and the χ 2 test for trend was used to test for linear relationships with ordinal variables. A two‐sided p < .05 was considered statistically significant.
3. RESULTS
From 1295 invitations, 283 (22%) responses were received, of which 272 (21%) included complete demographic details and were thus eligible for analysis (Figure 1). Of these valid responses, 230/272 (85%) provided a response to every question. Participants' professional and demographic characteristics are summarised in Table 1. A total of 264/272 (97%) participants reported treating thyroid patients on a daily or weekly basis.
Figure 1.
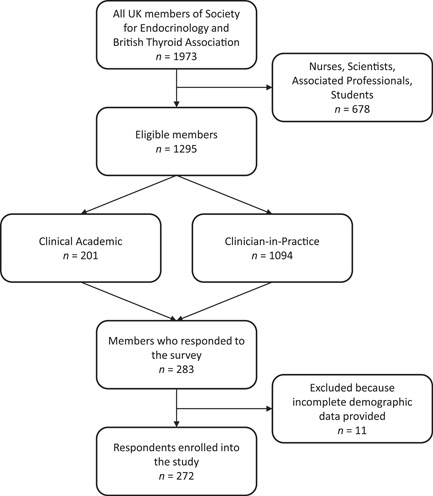
Flowchart of respondents
Table 1.
Demographic characteristics of respondents
| Characteristic | Category | Female | Male | Total | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age in years | 21–30 | 3 | 1.1 | 3 | 1.1 | 6 | 2.2 |
| 31–40 | 32 | 11.8 | 36 | 13.2 | 68 | 25 | |
| 41–50 | 44 | 16.2 | 52 | 19.1 | 96 | 35 | |
| 51–60 | 26 | 9.6 | 47 | 17.3 | 73 | 27 | |
| 61–70 | 2 | 0.7 | 23 | 8.5 | 25 | 9.2 | |
| >70 | 0 | 0 | 4 | 1.5 | 4 | 1.5 | |
| Total | 107 | 39.3 | 165 | 60.7 | 272 | 100 | |
| Years in medical practice | 0–10 | 14 | 5.1 | 18 | 6.6 | 32 | 11.8 |
| 11–20 | 48 | 17.6 | 49 | 18.0 | 97 | 35.7 | |
| 21–30 | 36 | 13.2 | 52 | 19.1 | 88 | 32.4 | |
| 31–40 | 9 | 3.3 | 35 | 12.9 | 44 | 16.2 | |
| >40 | 0 | 0 | 11 | 4.0 | 11 | 4.0 | |
| Specialisationa | Endocrinology | 104 | 38.2 | 160 | 58.8 | 264 | 97.1 |
| Internal medicine | 54 | 19.9 | 91 | 33.5 | 145 | 53.3 | |
| Others | 9 | 3.3 | 9 | 3.3 | 18 | 6.6 | |
| Place of practicea | University centre | 38 | 14.0 | 74 | 27.2 | 112 | 41.2 |
| Regional hospital | 66 | 24.3 | 96 | 35.3 | 162 | 59.6 | |
| Private clinic | 6 | 2.2 | 27 | 9.9 | 33 | 12.1 | |
| General Practice | 1 | 0.4 | 0 | 0 | 1 | 0.4 | |
| Basic researcher | 0 | 0 | 1 | 0.4 | 1 | 0.4 | |
| Specialist Practice | 7 | 2.6 | 22 | 8.1 | 29 | 10.7 | |
| Professional society membershipa | National endocrine societies | 82 | 30.1 | 139 | 51.1 | 221 | 81.3 |
| European Thyroid Association | 4 | 1.5 | 17 | 6.3 | 21 | 7.7 | |
| American Thyroid Association | 1 | 0.4 | 3 | 1.1 | 4 | 1.5 | |
| Asian and Oceanian Thyroid Association | 1 | 0.4 | 0 | 0 | 1 | 0.4 | |
| Frequency of treating thyroid patients | Daily | 50 | 18.4 | 93 | 34.2 | 143 | 52.6 |
| Weekly | 55 | 20.2 | 66 | 24.3 | 121 | 44.5 | |
| Rarely | 2 | 0.7 | 6 | 2.2 | 8 | 2.9 | |
| Number of patients treated for hypothyroidism | >100 per year | 28 | 10.3 | 67 | 24.6 | 95 | 34.9 |
| 51–100 per year | 25 | 9.2 | 53 | 19.5 | 78 | 28.7 | |
| 10–50 per year | 51 | 18.8 | 39 | 14.3 | 90 | 33.1 | |
| Rarely treat hypothyroid patients | 3 | 1.1 | 6 | 2.2 | 9 | 3.3 | |
Respondents were permitted to select more than one option.
3.1. Treatment of hypothyroidism
Almost all respondents (256/258; 99%) consider LT4 to be the treatment of choice for hypothyroidism. One participant indicated a preference for LT4 + LT3 combination, and one for LT3 monotherapy. No one suggested DTE.
Regarding decisions on the type of LT4 dispensed, most respondents stated either that they have no control (118/257; 46%) or that the choice is usually made by general practitioners (93/257; 36%). When asked about the relative merits of LT4 tablets, liquid, and soft‐gel capsules, most favoured LT4 tablets, and expected no major differences in absorption between formulations in any clinical context (Table 2). There was no association between demographic characteristics and views on LT4 preparations.
Table 2.
Preferred LT4 formulations by UK endocrinologists in different clinical conditions
| Tablets/tablets from another manufacturer | Soft‐gel capsule | Liquid solution | I expect no major changes with different formulations | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| Interfering drugs may influence the stability of therapy. Which LT4 preparation is in your experience least likely to be subject to variable absorption? | 53 (20.7) | 5 (2.0) | 38 (14.8) | 160 (62.5) |
| Which of the following preparations of LT4 would you prescribe in case of first diagnosis of hypothyroidism when the patient self‐reports intolerance to various foods raising the possibility of coeliac disease, malabsorption, lactose intolerance, or intolerance to common excipients? | 154 (60.6) | 10 (3.9) | 29 (11.4) | 61 (24.0) |
| Which of the following preparations of LT4 would you prescribe for a patient established on LT4 who has unexplained poor biochemical control of hypothyroidism? | 110 (43.7) | 11 (4.4) | 26 (10.3) | 105 (41.7) |
| Which of the following preparations of LT4 would you prescribe for a patient with poor biochemical control who is unable (due to busy lifestyle) to take LT4 fasted and separate from food/drink? | 83 (33.3) | 22 (8.8) | 35 (14.1) | 109 (43.8) |
| Which of the following preparations of LT4 would you prescribe for a patient established on LT4 tablets who has good biochemical control of hypothyroidism but continues to have symptoms? | 52 (21.1) | 0 (0) | 2 (0.8) | 193 (78.1) |
Many respondents (127/246; 52%) regard dietary supplements, such as selenium and iodine, as having no adjunctive role to thyroid hormones. A significant minority (93/246; 38%) would accept their use at patient request or as a complementary treatment. Only small numbers regard them as useful in subclinical hypothyroidism (7/246; 2.8%) or with coexisting autoimmune thyroiditis (19/246; 7.7%). No association was found with any demographic characteristic.
After starting LT4 replacement therapy, 136/247 (55%) would recheck TSH after 4–6 weeks, and 109/247 (44%) after 8 weeks. After switching to a different LT4 formulation, or from one manufacturer's tablets to another, 114/246 (46%) would recheck TSH after 4–6 weeks, and 102/246 (41%) after 8 weeks, while 16/246 (6.5%) would recommend not rechecking at all, if the nominal dosage is unchanged, and 14/246 (5.7%) would test TSH based on clinical evaluation.
3.2. Persistent symptoms of hypothyroidism
Participants were asked to outline their experience of managing patients whose hypothyroid symptoms persist despite LT4 treatment returning serum TSH concentration to the reference interval (Figure 2A). Most (175/239; 73%) respondents stated that such patients represent 10% or less of their caseload, but 50% (119/238) reported that the prevalence has increased over the past 5 years. No associations between demographic variables and responses were identified.
Figure 2.
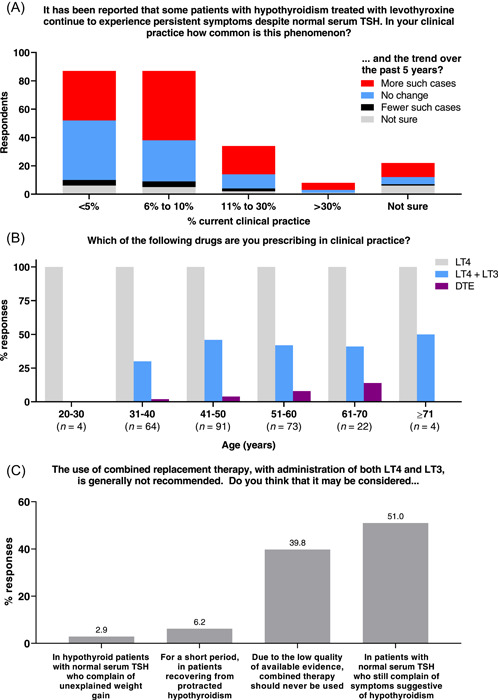
(A) Respondents' reports of prevalence (n = 239) and trend (n = 238) in patients experiencing persistent hypothyroid symptoms despite LT4 treatment returning serum TSH concentration to the reference interval. (B) Current prescribing practice, by age of respondent (n stated separately for each age group). (C) Willingness to consider prescribing combined LT4 + LT3 therapy in specified circumstances (n = 241).
Opinions were elicited on eight hypothetical explanations for the persistence of hypothyroid symptoms despite normalised serum TSH. Participants were asked to indicate their level of agreement with each statement (choosing “Strongly agree,” “Agree,” “Neutral,” “Disagree,” or “Strongly disagree”) and to rank them in terms of perceived aetiological importance. A comparison of the sum of counts of “Strongly agree” and “Agree” with the sum of counts of the top three ranks revealed similar results. “Psychological factors,” “Comorbidities” and “Unrealistic patient expectations” were regarded as the most likely explanations for persistent symptoms, while “The inability of LT4 to restore normal physiology,” “The presence of underlying inflammation due to autoimmunity,” and “The burden of having to take medication” were regarded as least likely (Table 3).
Table 3.
Perceptions of the relative importance of eight potential causes of symptoms of hypothyroidism persisting despite LT4 treatment returning serum TSH to the reference interval
| Possible cause | Sum of “Strongly agree” + “Agree” (n) | Sum of top 3 ranks (n) |
|---|---|---|
| Psychosocial factors | 134 | 166 |
| Comorbidities | 123 | 130 |
| Patient unrealistic expectation | 113 | 129 |
| Burden of chronic disease | 93 | 62 |
| Chronic fatigue syndrome | 69 | 61 |
| Inability of levothyroxine to restore normal physiology | 39 | 58 |
| Presence of underlying inflammation due to autoimmunity | 38 | 44 |
| Burden of having to take medication | 27 | 40 |
3.3. Combination therapy with LT4 and LT3
When asked about their current practice, all 258 respondents stated that they prescribe LT4, whereas only 103/258 (40%) prescribe LT4 + LT3 combination therapy, and just 14/258 (5.4%) also prescribe DTE (Figure 2B). The likelihood of prescribing DTE was greater with increasing age (p = .0318) and with private practice (p = .0190). Significant associations were not identified with other demographic characteristics, nor for any characteristic with the likelihood of prescribing LT4 + LT3 combination therapy.
Notwithstanding that 60% of respondents do not currently prescribe LT4 + LT3 combination therapy, only 96/241 (40%) agreed with the statement that “Due to the low quality of available evidence, combined therapy should never be used.” A greater proportion (123/241; 51%) stated that it may be considered “In patients with normal serum TSH who still complain of symptoms suggestive of hypothyroidism” (Figure 2C). This opinion was more common in those treating >100 patients annually for hypothyroidism, compared to those treating fewer (62% vs. 45%, respectively; p = .0111). No other associations with demographic characteristics were identified.
Lastly, participants were asked about their attitude to taking LT4 + LT3 combination or DTE therapy themselves. Statistical disclosure control is applied to avoid any risk of respondents with a diagnosis of hypothyroidism (8/230; 3.5%) being identified. However, amongst respondents without hypothyroidism, 170/222 (77%) would not consider either treatment if they were themselves to develop hypothyroidism, citing safety concerns, dosing inconvenience, and lack of evidence of efficacy. The remainder (52/222; 23%) stated that they would consider such treatment; many indicated that their opinions were based on direct experience of patient benefit.
3.4. Prescribing thyroid hormones for euthyroid patients
When asked for their views on thyroid hormone therapy in biochemically euthyroid patients, most respondents (163/259; 63%) answered that treatment is never indicated, but a substantial minority (74/259; 29%) would consider thyroid hormone therapy for female infertility with high levels of thyroid antibodies, and smaller proportions for other scenarios (Figure 3). There was no association between any demographic characteristic and the likelihood of considering thyroid hormone therapy for any indication in euthyroid individuals.
Figure 3.
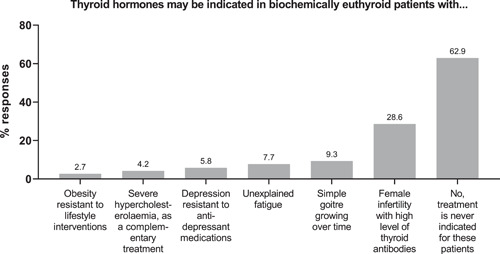
Views on prescribing for euthyroid individuals
4. DISCUSSION
This survey of UK‐based clinical members of the Society for Endocrinology and the British Thyroid Association received valid responses from 21% of those eligible to respond. This crude response rate is a limitation of the study but is comparable to other reports 9 , 10 , 11 emerging from the pan‐European THESIS collaboration 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 and to similar North American 24 and European Thyroid Association surveys. 25 , 26 Furthermore, it might underestimate the proportion of thyroid specialists responding if, as seems likely, the clinical membership includes individuals with limited or no thyroid practice who chose not to participate. This is illustrated by the differing approach to the THESIS survey taken in Germany, surveying only members of the thyroid subsection (n = 206) of the Deutsche Gesellschaft für Endokrinologie (total membership approximately 1660) and obtaining a 79.1% response rate with 163 responses. 23
A further limitation of the study is that the views of general practitioners, who successfully manage most hypothyroidism in the United Kingdom, were not canvassed because it was not within the scope of the pan‐European THESIS project to extend the survey to nonspecialists. The proportion of thyroid disease managed by specialists, compared to generalists, differs between countries across the continent; international comparisons should be viewed in this light. The results of the current study are biased by design to reflect the views of UK endocrinologists managing people with complex thyroid disease.
Stringent measures ensured that responses were received only from eligible members and excluded duplicates. Almost all respondents reported managing thyroid patients weekly or daily. Over half the respondents practised in internal medicine and endocrinology, most commonly in a regional hospital, followed by a university centre, with private practice being uncommon. The age–sex distribution was broadly even below 40 years but skewed increasingly towards men in older bands. We, therefore, consider that the results capture the attitudes and current practice of a representative sample of UK endocrinologists, provided by them in a spirit of collective scientific curiosity, and in the prevailing context of COVID‐19 pandemic restrictions on clinical practice and normal daily life. 27
4.1. Treatment of hypothyroidism
Most respondents indicated a preference for prescribing LT4 tablets for hypothyroidism, whether or not absorption is impaired. This aligns with the British Thyroid Association (BTA) Executive Committee statement on management of primary hypothyroidism 28 but differs from THESIS survey results in Greece 19 and Hungary, 21 where a majority would prescribe soft‐gel capsules if impaired absorption is suspected, and with equivalent results from Italy 12 and Romania, 13 where the preference is for liquid LT4. Given that the number of NHS prescriptions of nontablet LT4 formulations has risen in England in the past 5 years by over 10%, 8 and that such formulations are approximately two orders of magnitude more expensive than tablets, there seems to be an urgent need for informed debate among UK endocrinologists and primary care prescribers about the utility and cost‐effectiveness of nontablet LT4.
Compared to thyroid hormones, prescriptions in England for selenium and iodine supplements are rare, and costs to the NHS negligible. 8 Nevertheless, 38% of respondents would agree to their use “at the patient's request, or as a complementary treatment.” This disparity suggests a lack of opposition to dietary supplements, in the context of widespread use of consumer dietary supplements among the UK population, rather than any intention actively to prescribe them. Such a position would reflect the absence of evidence for selenium supplementation in hypothyroidism 29 and is in keeping with BTA Executive Committee guidance. 28 This differs from practice in Greece, 19 Poland, 15 and Romania, 13 where supplements are prescribed more commonly in autoimmune hypothyroidism. It has been speculated that this may be because nutritional deficiencies are more common in those populations. 15
Current practice with respect to monitoring of TSH after starting LT4 treatment, and after a change of brand or formulation, is in line with current NICE and BTA guidance, 4 , 28 and in keeping with other THESIS collaboration publications to date.
4.2. Persistent symptoms of hypothyroidism
Notwithstanding compelling evidence for the efficacy of thyroid hormone replacement in profound (but not subclinical) hypothyroidism, there is a complex and sometimes inconsistent relationship between treatment satisfaction, quality of life, and specific and nonspecific hypothyroid symptoms, serum TSH concentration, and nonthyroid factors such as age and comorbidities. 30 Recent online surveys, recruiting through patient‐facing websites, demonstrate dissatisfaction with treatment and with treating healthcare professionals among self‐selecting respondents. 31 , 32 Notable among the findings of Mitchell et al. 31 was that 89.2% of respondents rated information provided by their general practitioners less highly than that obtained from other sources, among which, internet/media, followed by patient organisations, and then other patients, were judged most frequently to be superior.
In the current survey, most endocrinologists reported that 10% or fewer of their LT4‐treated hypothyroid patients experience persistent symptoms despite normal TSH. Nevertheless, 50% indicated that the frequency of this phenomenon has increased over the past 5 years. Similar patterns have been observed in Denmark and Sweden, 14 , 17 whereas THESIS survey reports from Greece, 19 Romania, 13 and Belarus 20 indicate lower prevalence.
While the combined caseload of all UK endocrinologists represents only a small minority of patients with hypothyroidism in the United Kingdom, the findings are consistent with the presence of significant morbidity within the specific patient group. A potential limitation is that some responses might have been based on conjecture, rather than on systematic, detailed assessment of caseload, introducing the risk of bias if patients experiencing persistent symptoms are reviewed more frequently and thus come more swiftly than others to mind.
Accepting that the survey underscores the need to ameliorate persistent hypothyroid symptoms, it is noteworthy that few respondents agreed that “in most patients treated with levothyroxine who achieve normal serum TSH, persistent symptoms are due to inability of levothyroxine to restore normal physiology.” It can be inferred that a majority do not believe that more complex thyroid hormone preparations than LT4 monotherapy are likely to be helpful. Similarly, the relative lack of priority given to the “presence of underlying inflammation due to autoimmunity,” suggests that few, if any, would consider pursuing an immunotherapeutic approach to autoimmune thyroiditis. Instead, respondents tended to regard nonthyroid factors as most important, a finding consistent with almost every THESIS collaboration national publication to date 9 , 10 , 11 , 13 , 14 , 15 , 17 , 18 , 19 , 20 , 21 , 22 , 23 and with the conclusions of a recent narrative review. 33
4.3. Therapy with combined LT4 and LT3 or with DTE
Access to combination LT4 + LT3 therapy in the United Kingdom was reduced, and debate about its contested benefits sharpened, by a 6021% price rise for generic LT3 tablets between 2007 and 2017. At the end of this period, LT3 20 mcg tablets were over 100 times more expensive in the United Kingdom than in France or the Czech Republic. 34 This cost pressure substantially distorted clinical decision‐making, with the greatest impact falling on the least affluent regions. 35 An inquiry by the UK Competition and Markets Authority found that an excessive and unfair pricing strategy had been pursued; fines totalling £101,442,899 (equivalent to approximately €121 m and US$132 m at time of writing) were imposed on the manufacturer and associated companies. 34 This ruling is gradually affecting prices but the differential between LT3 and LT4 remains extraordinary: having fallen from £258.19 (approximately €310 and US$340) in 2016/17, 34 the ballpark NHS cost of a single 28‐tablet pack of LT3 20 mcg tablets at time of writing is £71.90 (approximately €86 and US$95), compared to £0.89 (approximately €1.06 or US$1.17) for the same number of LT4 100 mcg tablets. 36
The current study demonstrates that, while LT4 tablet monotherapy is the treatment of choice for hypothyroidism, 51% of respondents will consider using combined LT4 + LT3 for people whose hypothyroid symptoms persist despite serum TSH concentration being returned to the reference interval. While no previous British data are available, comparison with a survey in 2013 of members of North American specialist societies, which found that just 3.6% of respondents would consider prescribing combined LT4 + LT3 in this situation, 24 suggests that a shift in attitude towards combination therapy may be occurring. A striking range of responses has emerged on this topic from other national surveys in the THESIS collaboration, with over 70% of respondents in Denmark 14 and Sweden 17 being willing to use combined LT4 + LT3, whereas equivalent results from France, 11 Belgium, 18 Greece, 19 and Bulgaria 9 are around 25% or fewer. These proportions do not correlate closely with the corresponding national perceptions of prevalence of persistent symptoms, and thus may reflect societal pressures as well as inconsistent evidence of benefit of combined LT4 + LT3 in randomised, controlled trials to date. 30
As the proportion of respondents in the current study indicating willingness to prescribe is greater than the proportion currently prescribing combined LT4 + LT3 (40%), a possible inference is that specialist assessment by UK endocrinologists identifies relatively few patients who derive unequivocal benefit from such treatment. Consistent with findings from other THESIS collaboration national surveys 9 , 11 , 14 , 15 , 17 , 20 , 21 , 23 the proportion of UK endocrinologists who would themselves consider taking combined LT4 + LT3 treatment, in the event of developing hypothyroidism, is smaller still than the proportion currently prescribing. While it is apparent that, for many, concerns about safety, dosing inconvenience, and lack of evidence of efficacy inform this opinion, the questionnaire was not designed to determine whether a perception of low personal risk of suffering persistent symptoms might also be relevant. The hypothetical nature of responses to this question must therefore be acknowledged as a limitation of the study.
4.4. Prescribing thyroid hormones for euthyroid patients
A definitive, UK multicentre, randomised, double‐blind, placebo‐controlled trial of LT4 treatment in 952 euthyroid women with thyroid peroxidase antibodies and a history of miscarriage or infertility was published in 2019, 37 demonstrating no improvement in live birth rate with LT4 treatment. This confirmed the findings of an earlier, open‐label trial in 600 women in China with similar baseline characteristics, 38 and both publications were reflected in the European Thyroid Association's 2021 guideline, which recommends against systematic LT4 supplementation of euthyroid women undergoing fertility treatment. 39 Despite this, 29% of respondents in the current study indicated that they considered thyroid hormone supplementation to be indicated for female infertility in euthyroid women with high levels of thyroid antibodies. In THESIS collaboration results to date, just 18% of Bulgarian endocrinologists considered this to be an indication, 9 whereas the proportions are greater elsewhere than in the United Kingdom, particularly Denmark (42%), Sweden (47%), Spain (49%), Hungary (59%), Poland and Germany (63%), and the Czech Republic (78%). 14 , 15 , 16 , 17 , 21 , 22 , 23
With respect to thyroid hormone treatment for euthyroid goitre, the absence of long‐term efficacy after treatment withdrawal, 40 coupled with evidence that protracted TSH reduction below the reference interval causes loss of bone mineral density in postmenopausal women, adverse cardiac effects in the elderly, and increased mortality, 41 has led to such treatment being discouraged in recent guidelines. 42 Reports from the THESIS collaboration have again revealed substantial variation between countries, but without close correlation to willingness to prescribe for euthyroid female infertility with positive antibodies. A majority of Czech, German and Bulgarian endocrinologists reported willingness to prescribe for euthyroid goitre, 9 , 22 , 23 whereas corresponding figures for Hungary, 21 France, 11 Finland, 10 Poland, 15 and Belgium 18 were between one‐third and one‐half of participants, and, for Belarus, 20 Greece, 19 Spain, 16 Italy, 12 Sweden, 17 and Denmark, 14 between 12% and 25%. Longstanding differences between European countries in prevalence and, hence, specialists' clinical experience, of endemic goitre might influence this area of practice. At 9.3%, the proportion of UK respondents advocating thyroid hormone treatment for euthyroid goitre is low, and the lack of association with demographic characteristics suggests that the rationale against this practice is widely accepted among UK endocrinologists.
Evidence is weak or nonexistent that any hypothetical benefits of thyroid hormone treatment might outweigh adverse effects in euthyroid patients with the other surveyed conditions. It is therefore reassuring that respondents in the current study overwhelmingly regard such treatment as inappropriate.
5. CONCLUSIONS
Our major findings are, first, that UK endocrinologists have confidence in the bioequivalence of LT4 tablets and do not regard LT4 liquid or soft‐gel capsule formulations as standard therapy; second, that the phenomenon of persistent hypothyroid symptoms on sufficient LT4 treatment to “normalise” serum TSH concentration is widely recognised; third, that nonthyroid factors are regarded as most likely to explain such symptoms; fourth, that while many will consider prescribing combined LT4 + LT3, but not DTE, for this indication, fewer UK endocrinologists actually prescribe it and fewer still would take it themselves, and, finally, that a substantial minority have yet to adjust their practice in prescribing thyroid hormones for euthyroid patients, particularly with respect to euthyroid women with thyroid peroxidase antibodies and a history of miscarriage or infertility.
For UK endocrinologists, the role of combined LT4 + LT3 treatment for hypothyroidism clearly remains a live issue for debate, brought uniquely into national focus by excessive, unfair pricing. Further analysis within the THESIS collaboration may reveal if the UK is an outlier with respect to prescribing. Meanwhile, the current study suggests that there will be enthusiasm among UK endocrinologists for testing the efficacy of psychological therapies for persistent hypothyroid symptoms. Whether this is true across Europe will become apparent as further publications emerge from the THESIS collaboration.
AUTHOR CONTRIBUTIONS
Petros Perros, Laszlo Hegedüs, Enrico Papini, Endre V. Nagy, Roberto Attanasio and Roberto Negro conceived the study. Younes R. Younes and Benjamin C. T. Field collected and analysed the data. Younes R. Younes wrote the first draft of the manuscript. All authors commented on drafts and approved the final version.
CONFLICT OF INTEREST
LH, EVN, EP and PP are members of the scientific board for, and have received consultancy fees from, IBSA Institut Biochimique SA (Lugano, Switzerland), a manufacturer of liquid and soft‐gel capsule formulations of LT4. IBSA had no role in the design of the study, in data collection, analysis, interpretation or presentation, in writing of the manuscript, or in the decision to submit for publication. The remaining authors declare no conflict of interest.
Supporting information
Supporting information.
ACKNOWLEDGEMENTS
We would like to thank all the physicians who responded to our survey. This study did not receive any specific grant from any funding agency in the public, commercial, or not‐for‐profit sector.
Younes YR, Perros P, Hegedüs L, et al. Use of thyroid hormones in hypothyroid and euthyroid patients: A THESIS questionnaire survey of UK endocrinologists. Clin Endocrinol (Oxf). 2023;98:238‐248. 10.1111/cen.14812
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ingoe L, Phipps N, Armstrong G, Rajagopal A, Kamali F, Razvi S. Prevalence of treated hypothyroidism in the community: analysis from general practices in North‐East england with implications for the United Kingdom. Clin Endocrinol. 2017;87:860‐864. [DOI] [PubMed] [Google Scholar]
- 2. Vanderpump M. Thyroid and iodine nutritional status: a UK perspective. Clin Med. 2014;14:s7‐s11. [DOI] [PubMed] [Google Scholar]
- 3. Public Health England . NDNS: results from years 9 to 11 (combined) ‐ statistical summary (published 11 December 2020). Accessed May 1, 2022. https://www.gov.uk/government/statistics/ndns-results-from-years-9-to-11-2016-to-2017-and-2018-to-2019/ndns-results-from-years-9-to-11-combined-statistical-summary
- 4. National Institute for Health and Care Excellence . NICE guideline 145. Thyroid disease: assessment and management. 2019. Accessed January 23, 2022. www.nice.org.uk/guidance/ng145 [PubMed]
- 5. Fliers E, Demeneix B, Bhaseen A, Brix TH. European thyroid association (ETA) and thyroid federation international (TFI) joint position statement on the interchangeability of levothyroxine products in EU countries. Eur Thyr J. 2018;7:238‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Medicines and Healthcare products Regulatory Agency . Levothyroxine: new prescribing advice for patients who experience symptoms on switching between different levothyroxine products. 2021. Accessed March 18, 2022. www.gov.uk/drug-safety-update/levothyroxine-new-prescribing-advice-for-patients-who-experience-symptoms-on-switching-between-different-levothyroxine-products
- 7. Nagy EV, Perros P, Papini E, Katko M, Hegedüs L. New formulations of levothyroxine in the treatment of hypothyroidism: trick or treat? Thyroid. 2021;31:193‐201. [DOI] [PubMed] [Google Scholar]
- 8. The DataLab, University of Oxford . 2022. Accessed March 18, 2022. www.OpenPrescribing.net
- 9. Borissova A‐M, Boyanov MA, Attanasio R, et al. Use of thyroid hormones in hypothyroid and euthyroid patients: a THESIS questionnaire survey of Bulgarian physicians. Endocrinology. 2020;25:229‐309. [Google Scholar]
- 10. Metso S, Hakala T, Attanasio R, et al. Use of thyroid hormones in the treatment of hypothyroidism: THESIS questionnaire survey for Finnish specialists. Finnish Med J. 2021;76:2885. Accessed 19 March 19, 2022. www.laakarilehti.fi/tieteessa/alkuperaistutkimukset/thesis-kyselytutkimuskilpirauhashormonien-kaytto-kilpirauhasen-vajaatoiminnan-hoidossa/en [Google Scholar]
- 11. Buffet C, Belin L, Attanasio R, et al. Real‐life practice of thyroid hormone use in hypothyroid and euthyroid patients: a detailed view from the THESIS* questionnaire survey in France. Ann Endocrinol. 2022;83:27‐34. [DOI] [PubMed] [Google Scholar]
- 12. Negro R, Attanasio R, Nagy EV, Papini E, Perros P, Hegedüs L. Use of thyroid hormones in hypothyroid and euthyroid patients; the 2019 Italian survey. Eur Thyr J. 2020;9:25‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Niculescu DA, Attanasio R, Hegedüs L, et al. Use of thyroid hormones in hypothyroid and euthyroid patients: a THESIS questionnaire survey of Romanian physicians. Acta Endocrinol. 2020;16:462‐469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Riis KR, Frølich JS, Hegedüs L, et al. Use of thyroid hormones in hypothyroid and euthyroid patients: a 2020 THESIS questionnaire survey of members of the danish endocrine society. J Endocrinol Invest. 2021;44:2435‐2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bednarczuk T, Attanasio R, Hegedüs L, et al. Use of thyroid hormones in hypothyroid and euthyroid patients: a THESIS* questionnaire survey of polish physicians *THESIS: treatment of hypothyroidism in Europe by specialists: an international survey. Endokrynol Polska. 2021;72:357‐365. [DOI] [PubMed] [Google Scholar]
- 16. Galofré JC, Attanasio R, Hegedüs L, et al. Use of thyroid hormone in hypothyroid and euthyroid patients in Spain. A THESIS questionnaire survey. Endocrinol Diabetes Nutr. 2021;69(21):520‐529. 10.1016/j.endinu.2021.07.003 [DOI] [PubMed] [Google Scholar]
- 17. Planck T, Lantz M, Perros P, et al. Use of thyroid hormones in hypothyroid and euthyroid patients: a 2020 THESIS questionnaire survey of members of the Swedish endocrine society. Front Endocrinol. 2021;12:795111. 10.3389/fendo.2021.795111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burlacu M‐C, Attanasio R, Hegedüs L, et al. Use of thyroid hormones in hypothyroid and euthyroid patients: a THESIS* survey of Belgian specialists *THESIS: treatment of hypothyroidism in Europe by specialists: an international survey. Thyr Res. 2022;15:3. 10.1186/s13044-022-00121-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paschou SA, Alevizaki M, Attanasio R, et al. Use of thyroid hormones in hypothyroid and euthyroid patients: a 2020 THESIS questionnaire survey of members of the hellenic endocrine society. Hormones. 2022;21:103‐111. [DOI] [PubMed] [Google Scholar]
- 20. Shepelkevich AP, Dydyshka YV, Yurenya EV, et al. Features of the use of synthetic analogues of thyroid hormones: a 2020 THESIS* questionnaire survey of members of the Belarusian public medical association of endocrinology and metabolism. Probl Endocrinol. 2022;68:18‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Berta E, Lengyel IM, Hegedűs L, et al. Pajzsmirigyhormon‐kezelési szokások magyarországon. A THESIS kérdöíves felmérés eredményei. Orv Hetil. 2022;163:463‐472. [DOI] [PubMed] [Google Scholar]
- 22. Jiskra J, Paleček J, Attanasio R, et al. Use of thyroid hormones in hypothyroid and euthyroid patients: a 2020 THESIS questionnaire survey of members of the Czech Society of endocrinology. BMC Endocr Disord. 2022;22:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vardarli I, Brandenburg T, Hegedüs L, et al. A questionnaire survey of German thyroidologists on the use of thyroid hormones in hypothyroid and euthyroid patients: the THESIS (treatment of hypothyroidism in Europe by specialists: an international survey) collaborative. Exp Clin Endocrinol Diabetes . 2022. (Online ahead of print) 10.1055/a-1832-0644 [DOI] [PubMed]
- 24. Burch HB, Burman KD, Cooper DS, Hennessey JV. A 2013 survey of clinical practice patterns in the management of primary hypothyroidism. J Clin Endocrinol Metab. 2014;99:2077‐2085. [DOI] [PubMed] [Google Scholar]
- 25. Negro R, Hegedüs L, Attanasio R, Papini E, Winther KH. A 2018 european thyroid association survey on the use of selenium supplementation in graves’ hyperthyroidism and graves’ orbitopathy. Eur Thyroid J. 2019;8:7‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hegedüs L, Frasoldati A, Negro R, Papini E. European thyroid association survey on use of minimally invasive techniques for thyroid nodules. Eur Thyroid J. 2020;9:194‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pavlatou MG, Žarković M, Hegedüs L, Priestley J, McMullan C, Perros P. A survey on the psychological impact and access to health care of thyroid patients during the first SARS‐COV‐2 lockdown. Clin Endocrinol. 2021;96:869‐877. [DOI] [PubMed] [Google Scholar]
- 28. Okosieme O, Gilbert J, Abraham P, et al. Management of primary hypothyroidism: statement by the British thyroid association executive committee. Clin Endocrinol. 2016;84:799‐808. [DOI] [PubMed] [Google Scholar]
- 29. Winther KH, Rayman MP, Bonnema SJ, Hegedüs L. Selenium in thyroid disorders—essential knowledge for clinicians. Nat Rev Endocrinol. 2020;16:165‐176. [DOI] [PubMed] [Google Scholar]
- 30. Hegedüs L, Bianco AC, Jonklaas J, Pearce SH, Weetman AP, Perros P. Primary hypothyroidism and quality of life. Nat Rev Endocrinol. 2022;18:230‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mitchell AL, Hegedüs L, Žarković M, Hickey JL, Perros P. Patient satisfaction and quality of life in hypothyroidism: an online survey by the British thyroid foundation. Clin Endocrinol. 2021;94:513‐520. [DOI] [PubMed] [Google Scholar]
- 32. Peterson SJ, Cappola AR, Castro MR, et al. An online survey of hypothyroid patients demonstrates prominent dissatisfaction. Thyroid. 2018;28:707‐721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perros P, Van Der Feltz‐Cornelis C, Papini E, Nagy EV, Weetman AP, Hegedüs L. The enigma of persistent symptoms in hypothyroid patients treated with levothyroxine: a narrative review. Clin Endocrinol . 2021. (Online ahead of print). 10.1111/cen.14473 [DOI] [PubMed]
- 34. Competition and Markets Authority . Decision of the Competition and Markets Authority. Excessive and unfair pricing with respect to the supply of liothyronine tablets in the UK. Case 50395. Non‐confidential decision. Accessed January 23, 2022. www.gov.uk/cma-cases/pharmaceutical-sector-anti-competitive-conduct
- 35. Taylor PN, Razvi S, Muller I, et al. Liothyronine cost and prescriptions in England. Lancet Diab Endocrinol. 2019;7:11‐12. [DOI] [PubMed] [Google Scholar]
- 36. National Institute for Health and Care Excellence . British National Formulary. 2022. Accessed March 18, 2022. bnf.nice.org.uk
- 37. Dhillon‐Smith RK, Middleton LJ, Sunner KK, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Eng J Med. 2019;380:1316‐1325. [DOI] [PubMed] [Google Scholar]
- 38. Wang H, Gao H, Chi H, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer. JAMA. 2017;318:2190‐2198. [DOI] [PubMed] [Google Scholar]
- 39. Poppe K, Bisschop P, Fugazzola L, Minziori G, Unuane D, Weghofer A. 2021 European Thyroid Association guideline on thyroid disorders prior to and during assisted reproduction. Eur Thyr J. 2021;2020(9):281‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hegedüs L, Bonnema SJ, Bennedbæk FN. Management of simple nodular goiter: current status and future perspectives. Endocr Rev. 2003;24:102‐132. [DOI] [PubMed] [Google Scholar]
- 41. Lillevang‐Johansen M, Abrahamsen B, Jørgensen HL, Brix TH, Hegedüs L. Over‐ and under‐replacement of hypothyroidism is associated with excess mortality: a register‐based cohort study. Thyroid. 2018;28:566‐574. [DOI] [PubMed] [Google Scholar]
- 42. Gharib H, Papini E, Garber JR, et al. American association of clinical endocrinologists, American college of endocrinology, and associazione medici endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules—2016 update. Endocr Pract. 2016;22(suppl 1):1‐60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


