Abstract
Background
One in seven couples are impacted by infertility in the UK, and female infertility is often associated with several health conditions impacted by nutrition. Despite many studies aimed at identifying the critical role of nutrition in infertility, there is currently no screening tool that identifies nutritional risk factors for infertility.
Aim
To propose a self‐administered screening tool to identify women who would benefit from nutritional intervention to promote fertility.
Methods
A narrative review was carried out to identify and summarise modifiable nutritional risk factors that can influence female fertility, including comorbidities that can influence nutrition intake, absorption, and metabolism.
Key Findings
A nutrition screening tool outlining modifiable nutrition risk factors potentially improving female fertility has been proposed, comprising of BMI, medical history and quality of diet and lifestyle which would aid in designing evidence based dietetic services for female infertility.
Keywords: artificial fertility treatments, diet, dietitian, female infertility, nutrition, screening tool
Fertility is influenced by many modifiable nutritional risk factors and medical health conditions that can influence nutritional intake, absorption, or metabolism as well as other lifestyle factors. We propose a screening tool to easily identify women going through infertility who would benefit from dietetic referral and intervention.

The key points from the paper
Nutrition plays a critical role in managing infertility; however, there is currently no screening tool that identifies nutritional risk factors for infertility. We identify and summarise modifiable nutritional risk factors influencing female fertility and propose a self‐administered screening tool to identify women who would benefit from nutritional intervention to promote fertility.
INTRODUCTION
The prevalence of infertility in the UK is approximately 1 in 7 couples, 1 with an increased prevalence associated with later cohabitation with a partner, higher socio‐economic status, higher educational attainment, higher occupational status, and for those with children, becoming parents at an older age. 2 UK live birth rates are declining with 681,560 live births in 2020, a reduction of 4.4% from 2019 and the lowest number of live births since 2002. 3 In addition to increasing age of conception, fertility health care professionals (HCPs) face further issues in the UK, including Clinical Commissioning Groups (CCGs) cutting funding for fertility treatments available through the National Health Service (NHS). Curbs in funding will promote further health inequalities between those able to afford private treatment and those who cannot self‐fund their fertility treatment. Few NHS services offer dietetic support within fertility services. When support exists, the focus is mainly on weight management.
Despite many studies identifying the critical role of nutrition in infertility, there is currently no screening tool that identifies nutritional risk factors for infertility. Given the increasing evidence of the impact of nutrition on infertility, it is timely and essential to develop evidence‐based pathways and education materials promoting optimal nutritional status for those seeking support with both natural and artificial reproductive therapies.
AIMS AND OBJECTIVES:
Within this article we will be outlining the health conditions, nutritional risk factors, and lifestyle factors linked to infertility which can be positively impacted by nutritional intervention.
This narrative review aims to:
identify and summarise modifiable nutritional risk factors which can influence female fertility (including comorbidities that can influence nutrition intake, absorption, and metabolism),
discuss the outcomes achieved by nutritional intervention, and
propose a screening tool to identify women who would benefit from nutritional interventions to promote fertility.
Health Conditions Which Can Be Nutritionally Optimised to Improve Fertility
Female infertility may be associated with several health conditions impacted by nutrition (summarised in Figure 1); their appropriate medical and nutritional management can help improve fertility and other associated health outcomes. This clinical consideration would help HCPs manage beyond the immediate reproductive needs and consider overall health and long‐term implications. 4 Examples include the following.
Figure 1.
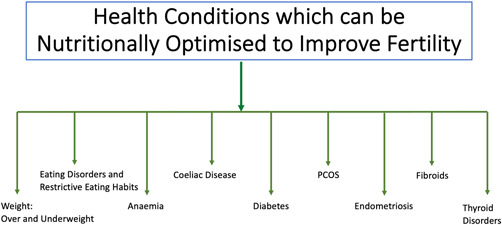
Summary of health conditions which can be nutritionally optimised to improve fertility
Weight and Infertility
Obesity is a well‐documented risk factor for infertility and birth complications; and, being underweight is also linked to poorer fertility and birth outcomes. Chavarro et al. (2007) found a J‐shaped relationship between weight and infertility due to ovulatory disorders. Women with a body mass index (BMI) > 25 kg/m2 and <20 kg/m2 had a higher risk of infertility, which increased further when BMI was >30 kg/m2. 5 Van der Steeg et al. (2017) demonstrated that women with a BMI > 29 kg/m2 experienced lower fecundity, with the probability of conception linearly declining with each increased BMI point. 6 National Institute for Health & Care excellence (NICE) guidelines suggest that women with a BMI of 30 kg/m2 or more should be informed that (i) they are likely to take longer to conceive; (ii) if they are also not ovulating, then losing weight (5–7% body weight) is likely to increase their chance of conception; and (iii) they are likely to have reduced chances of success with assisted reproductive procedures. 7
A recent systematic review and meta‐analysis evaluated the effectiveness of weight management interventions in infertility and found that a reduced‐calorie diet and increased aerobic exercise improved pregnancy rates and ovulation status. The authors highlight that the dietary intervention should not be overly restrictive and would be more effective with regular, long‐term support (e.g. weekly coaching for 6 months) to improve compliance, with better adherence seen with the dual enrolment of patient and partner. 8
The highest risk of infertility is seen in women with BMI < 20 kg/m2. 5 Amenorrhoea (the absence of menstruation) and menometrorrhagia (excessive uterine bleeding) are seen more frequently in women with a low BMI, 9 with a low BMI also linked to poor oocyte quality. 10 Functional hypothalamic amenorrhoea (FHA) is a leading cause of secondary amenorrhoea, the three main types being related to weight loss, stress, or exercise. 11 The US Endocrine Society Clinical Practice Guidelines recommend that the minimum threshold for a woman's BMI be 18.5 kg/m2 to optimise her chances of fertility. 12 They suggest that increasing body weight can improve regular ovulation, conception, and uncomplicated pregnancy; this should be done by detailed assessment and counselling by a registered dietitian. 12 NICE guidelines suggest that increasing body weight is likely to improve the chances of conception of women with a BMI < 19 kg/m2 with irregular menstruation. 13
Eating Disorders
The importance of dietetic support during weight loss is evidenced by the four‐fold higher prevalence of eating disorders (ED) among women with infertility. 14 Given the benefits of a healthy BMI and with fertility centres focusing on weight loss as part of their eligibility criteria, some women will ‘diet’ – with the intention of weight loss but without adequate advice and support. This can increase their risk of disordered eating or an eating disorder (ED), which are often undetected or untreated. EDs are associated with high‐risk pregnancies and complications such as preterm delivery, low birth weight, intrauterine growth restriction, caesarean birth, low Apgar scores, and negative impacts on IVF outcomes. 14 Prevalence of EDs within infertile women is between 5.5% and 20.7%, 14 , 15 , 16 , 17 particularly in those with ovulatory disorders. In addition, a tendency towards non‐disclosure of their ED to their healthcare provider emphasises the need for ED screening. 16
NICE guidelines suggest that in women with a history of EDs planning a pregnancy, the GP/midwife should advise on healthy eating and avoiding any unhealthy weight loss measures. 18 It is also essential to consider psychological input when indicated 18 ; therefore HCPs managing infertility should be proficient in identifying signs of EDs and refer to specialist services when required.
Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) is one of the most common endocrine conditions among women of childbearing age, with a prevalence of 2.2%–26%. 19 It is characterised by two or more of the following: hyperandrogenism, anovulation, and polycystic ovaries. A crucial metabolic complication of PCOS is insulin resistance (IR) which is thought to be a pivotal contributor to the pathogenesis, with 40%–70% of women with PCOS having IR independent of BMI. 20 Around 40%–60% of women with PCOS live with obesity, which itself leads to insulin insensitivity, metabolic syndrome, and increased cardiovascular risk. 21 The prevalence of infertility in women with PCOS is 70%–80%, 22 with PCOS causing 75% of cases of anovulatory infertility. 23 IR is thought to play a role in PCOS‐related infertility, perhaps through its impact on hyperandrogenism, contributing to anovulation. 24 Furthermore, both obesity and PCOS independently influence smaller oocyte size, thereby affecting maturation promotion activity. 25
In a secondary analysis of two randomised controlled trials (RCT) studying women living with overweight/obesity (age 18–40 years) with PCOS and infertility, a benefit of improved ovulation and live birth rate was seen when lifestyle modification with weight loss preceded infertility treatment with clomiphene citrate compared with medical management alone. Weight loss interventions included caloric restriction, anti‐obesity medication, behavioural modification, and exercise during a 16‐week preconception intervention, after which clomiphene citrate was administered. The cumulative ovulation rate increased from 45% to 62%, and the live birth rate improved from 8.5% to 25% with lifestyle intervention. 26 Traditional weight‐loss strategies at preconception were based on a low‐fat, calorie‐deficit diet 27 ; however, a recent meta‐analysis conducted on data from eight randomised controlled trials (n = 327) suggested that calorie deficit from a low‐fat and low‐CHO diet (fat <35% and CHO <45%) had a more significant effect on the levels of follicle‐stimulating hormone (FSH) (MD = 0.40, 95% CI (0.09, 0.71)) and sex hormone‐binding globulin (SHBG) (MD = 6.20, 95% CI (3.68, 8.72)) than a high‐fat and low‐CHO diet (fat >35% and CHO <45%). 28 Further research is required to evaluate the optimal amount of carbohydrate and duration of low carbohydrate intake. 27
Diabetes
Increasing maternal age combined with a higher prevalence of obesity means an increased chance that women trying to conceive will be affected by metabolic disorders, such as type 2 diabetes. IR and obesity are risk factors for both PCOS and type 2 diabetes. Achieving a 5%–7% weight loss before planning conception aids in improving metabolic control. 29 HbA1C concentration correlates positively with the presence of menstrual irregularities and hypothalamic anovulation, and better glycaemic control is associated with a more regular menstrual cycle 30 and improved reproductive function. 31 A systematic review conducted by Franz et al. (2017), including 60 studies, evaluated the effectiveness of dietetic input in the management of diabetes. The findings suggested a reduction in HbA1C, ranging from 0.3% to 1.8%, with ongoing dietetic care at 12 months. 32 Undernutrition in type 1 diabetes is also associated with female infertility. 29
Coeliac Disease
Coeliac disease (CD) in women can lead to delayed puberty, infertility, amenorrhea, and precocious menopause. 33 CD‐related malabsorption can lead to a deficiency of critical childbearing nutrients such as folate, iron, and vitamin K. 34 Though the prevalence of infertility is similar among those with CD and the general population, there is a higher maternal age among those with CD, as shown by the relative fertility rate. 35 The risk of miscarriage in infertile women with CD can be reduced nine‐fold by strict adherence to a gluten‐free diet. 36 It is advisable to screen women presenting with unexplained infertility or recurrent miscarriages for subclinical CD using serological tests. 37
Anaemia
Epidemiological and animal studies suggest that iron deficiency anaemia may be linked to infertility, and case study reports have suggested a link between pernicious anaemia and infertility. 38 , 39 , 40 The mechanisms through which iron deficiency anaemia impacts fertility are not clear; however, it has been shown that iron‐containing proteins are essential for ovum development and follicle maturation 41 and that in pigs, iron is involved in hormone secretion and cell proliferation. 42 Supplementation with nonheme iron may decrease the risk of ovulatory infertility, 43 and iron‐rich water has been successful as prophylaxis for pregnant women to prevent iron deficiency anaemia; it is gentler on the gut compared to oral iron supplementation. 44
Although pernicious anaemia is rare, once identified and treated with vitamin B12, women experiencing unexplained infertility can become pregnant within months. 45 Those at risk of vitamin B12 deficiency include individuals with malabsorption or restrictive dietary patterns, such as vegans. Ensuring nutritional adequacy of iron, folic acid, vitamin B12, protein, and vitamin C is essential for preventing and treating all types of anaemia.
Endometriosis
Endometriosis (a condition whereby endometrial tissue grows outside the uterus, in the surrounding organs and structures) affects about 10% of women of childbearing age 46 and is a chronic inflammatory, oxidative stress, oestrogen‐dependent condition associated with infertility. 47 It accounts for up to 50% of infertility in women 46 ; it is estimated that 30%–50% of women with endometriosis are infertile. 48
Endometriosis presents with symptoms including chronic pelvic pain, painful menstruation and ovulation, painful defecation, and persistent or recurrent genital pain occurring before, during, or after intercourse. Many of these features are similar to irritable bowel syndrome (IBS) and pelvic inflammatory disease (PID), and the probability of comorbidity between endometriosis and these two conditions is high. 49 Interventions with a low FODMAP diet conducted in a subgroup with IBS showed improvement in symptoms related to visceral hypersensitivity in 72% of women who presented with both endometriosis and IBS, whereas only 49% women had improvement who presented with IBS alone. 50
Observational studies have shown that the following are associated with a reduced risk of endometriosis: limiting red meat, 51 endocrine disruptors, and increased vitamin D intake. 52 , 53 Qualitative interviews suggest potential benefits of dietary changes reducing endometriosis symptoms such as pain and menstrual cyclicity and increasing well‐being. These changes include excluding or decreasing intake of certain foods, particularly gluten, dairy, and carbohydrates, and increasing intake of fruit, vegetables, and fish 54 ; however, further research is needed in this area.
Fibroids
Uterine leiomyomata, commonly known as fibroids, are estimated to be present in 4.5%–68.6% of women under 50 years, 30% of which are asymptomatic. 55 The symptoms of fibroids include heavy bleeding, anaemia, extreme tiredness, painful periods, and infertility. Fibroids are present in 5%–10% of infertile women and may be the sole cause of infertility in 1%–2.4%. 56 Their impact on fertility depends on their location and if they are large enough to distort the endometrial cavity. 57 Vitamin D deficiency is believed to be related to uterine fibroids 58 ; and optimal vitamin D status may restrict their growth. 59 Baird et al. found that for every 25 nmol/L increase in serum 25(OH)D, there was a 20% reduction in the risk of having fibroids. They also found that if a woman's serum 25(OH)D level was >50 nmol/L, there was a 36% less risk of having fibroids, and the effect was independent of ethnicity. 60 An RCT supplementing 1250 ug/week vitamin D3 over 12 weeks in women with vitamin D deficiency resulted in halting fibroids' progression compared to the control group, where a significant increase in progression was seen. 61
Thyroid Disorders
Medical management of thyroid disorders is essential for those with infertility as studies suggest that pregnancy outcomes are improved when TSH levels are kept <2.5 mIU/L, which is stricter than the clinical guideline recommendation of <4.12 mIU/L for those with hypothyroidism alone. 62 , 63 , 64 , 65
Despite a growing interest in the impact of nutrition on thyroid disorders, there is a lack of studies specifically exploring the impact of dietetic intervention for thyroid conditions among women with infertility, outside of weight management. However, it should be noted that lower vitamin D levels have been associated with hypothyroidism, autoimmune thyroid diseases and Hashimoto's thyroiditis, and iodine deficiency and iodine excess can both have negative implications for thyroid function. 66 , 67
Nutritional Factors
Energy Availability
Intentionally or unintentionally undereating (not consuming enough calories, food, or nutrients to meet the body's requirements) is linked to functional hypothalamic amenorrhea (FHA). 11 It is well established that starvation is associated with amenorrhea. Hypocaloric intakes and reduced energy availability (EA) can be linked to ‘dieting’, which itself may be associated with restriction of specific foods, food groups, or nutrients leading to negative impacts on fertility. 68 Dieting may also be associated with EDs, which are associated with high‐risk pregnancies, several adverse perinatal outcomes, 69 , 70 and an elevated prevalence of iron deficiency anaemia and coffee/caffeine consumption (both nutritional risk factors in infertility). 71 Even short‐term undereating can negatively affect menstrual cycles prior to weight loss occurring, and modest reductions in energy availability over prolonged periods have been associated with menstrual disturbances and decreased LH pulse frequency. 72 , 73 Avoidance of foods or food groups can lead to nutritional inadequacies with negative effects on fertility. The importance of nutritional adequacy for fertility is discussed below.
Macronutrient Intakes and Fertility
Carbohydrate
There is evidence suggesting that consuming a diet with a low glycaemic load (GL), high in fibre and with plenty of whole grains, may have beneficial effects on fecundity and oestrogen levels. 74 , 75 , 76 , 77 In those with PCOS, a reduction in carbohydrate intake led to improved insulin sensitivity and reduced testosterone; however, these effects were not seen in healthy menstruating women. 78 , 79 , 80 Some studies suggest that sugar‐sweetened beverages (SSB) are detrimental to fertility, with intakes of SSBs being associated with reduced fecundability and reduced reproductive success in those undergoing IVF. 81 , 82
Protein
In a study by Chavarro and colleagues, 18,555 women with a history of infertility were followed as they attempted a pregnancy during an 8‐year period. It was found that consuming vegetable protein instead of carbohydrates or animal protein was associated with a substantially lower risk of ovulatory infertility. 83 In a study considering 2696 embryos from 269 patients undergoing intracytoplasmic sperm injection cycles, red meat had a negative effect on blastocyst formation, implantation rates, and the probability of a live birth, 84 whereas a study of 351 women showed that fish intake was associated with a higher probability of live birth following assisted reproductive technology (ART), especially when fish replaced processed meat. 85 A differential effect of varying proteins on insulin sensitivity may explain these findings, in addition to the replacement of carbohydrate sources with vegetable protein, likely reducing the glycemic index (GI) of a meal. 86
Fat
Fatty acids (FA) are known to play an important role in reproductive function, with evidence suggesting that increased omega‐3 polyunsaturated fatty acid (PUFA) intakes, reduced trans FAs and saturated FA intakes may enhance fertility. 5 , 87 , 88 , 89 Increased PUFA intakes have been associated with higher fecundity, shorter time‐to‐pregnancy, and better ART outcomes; however, no dose‐response relationship has been established. 89 , 90 , 91 Trans‐FAs are known to increase insulin resistance and, in the NHS‐II study, were associated with higher risks of ovulatory infertility. 5 Minimal work has been done looking specifically at the effects of saturated fat on fertility, though a recent study showed that higher intakes of saturated fat were associated with an adjusted relative risk of 0.67 for clinical pregnancy in women undergoing ART. 92 The effects of other FAs on fertility, including omega‐6 polyunsaturated FAs and monounsaturated FAs, are not yet clear. 90 , 93
Foods and Food Groups
Seafood Consumption
There is growing evidence showing an association between fish intake and improved fertility. Nassan et al. showed that replacing meat with fish improves the probability of live births following ART. 85 , 88 , 89 Hsi and colleagues assessed the MeHg concentration in the hair of infertile women versus pregnant women (n = 224), with the infertile cohort showing significantly greater levels. 94 The potential negative effects of environmental toxins from seafood consumption are not clear, but overall, there are clear benefits to oily fish consumption with low mercury levels. Although the link between mercury and fertility is inconclusive, fish containing lower levels of mercury are recommended for women who wish to conceive. 95 , 96
Soy
Although controversy exists around soy intake and reproduction, with more research needed, 97 , 98 observational evidence suggests that higher intakes of soy isoflavones have been associated with 77% higher fertilisation rate in those undergoing ART compared to not consuming soy. 99 Interventional studies show positive effects of soy supplementation in those undergoing ART with Unfer et al. showing lower rates of miscarriage and higher rates of pregnancy with 1500 mg/day phytoestrogen supplementation in those undergoing IUI, compared to placebo 100 , 101 and a study by Shahin and colleagues finding that 120 mg/day oral phytoestrogen in those with unexplained infertility undergoing ovulation induction was associated with shorter induction cycles, and higher endometrial thickness and pregnancy rates. 100 , 101 Although the mechanisms for these positive effects are not clear, it is suggested that phytoestrogens may have oestrogenic effects or act as oestrogenic agonists in addition to being potent antioxidants.
Dairy
The fertility risk factor study by Greenlee and colleagues examined agricultural and residential exposures associated with female infertility; the study of 322 women found that consuming three or more glasses of milk per day is protective of female fertility, with consumers having a 70% lower risk of infertility than non‐consumers. 102 Chavarro et al. (2007) followed 18,555 married, premenopausal women without a history of infertility who attempted pregnancy or got pregnant within an 8‐year period and found that high‐fat dairy intake compared to low‐fat dairy intake has been associated with a lower risk of ovulatory infertility. 103 , 104 Conversely, results from a study that considered food‐frequency questionnaire data from two cohort studies in Denmark and North America did not support the hypothesis that full‐fat dairy was superior to low‐fat dairy in promoting fertility, 105 so no strong conclusions can currently be made regarding the type of dairy intake.
Alcohol
Fan et al. (2017) showed that alcohol intake was associated with reduced fecundability, with risk increasing in a dose‐response manner. 106 Heavy alcohol use is thought to diminish the ovarian reserve and is associated with multiple reproductive risks, including decreased chance of having a live birth and increased risk of foetal loss and having a child with foetal alcohol syndrome. 107 There is substantial evidence that alcohol intake, even moderate consumption, can negatively affect ART outcomes. 108 , 109 The expert opinion from the Maternal and Fertility Nutrition Specialist Group from British Dietetic Association (BDA) and Royal College of Obstetricians and Gynaecologists (RCOG) suggests it is safest to avoid alcohol intake prior to treatment.
Caffeine
Bolumar et al. 110 suggested that a high intake of caffeine (>500 mg per day) increased time‐to‐pregnancy, but there is inconsistent evidence regarding the effect of moderate caffeine intake on fertility outcomes. 110 , 111 Beyond fertility, the link between caffeine and pregnancy outcomes is clearer, with increased caffeine consumption linked to spontaneous abortion. 112 Evidence regarding caffeine intake and IVF outcomes remains inconsistent. 113 , 114 As cited by the European Food Safety Authority, the Belgian Superior Health Authority (2012) recommends that women of childbearing age consume <200 mg caffeine per day. 115 , 116 This precautionary advice is supported by others, 117 with some authors suggesting this intake may still be too high. 112
Micronutrients
Vitamin D
Recommended intake (RNI) of vitamin D for UK adults is 10ug/day unless deficiency is present. 118 There is no specific recommendation for those trying to conceive or experiencing infertility; however, vitamin D deficiency and insufficiency (serum level <75 nmol/L) is linked to lower success rates for women undergoing fertility treatments. 119 Research suggests that vitamin D may be beneficial for women with PCOS, insulin resistance, or low levels of anti‐Mullerian hormone (AMH), and a deficiency of vitamin D is associated with the pathogenesis of endometriosis. 120 , 121 The authors propose that the improved fertility rates may be due to the immunomodulatory effect of vitamin D via the reduction of inflammatory cytokines 122 and a direct impact on the endometrium. 123
Vitamin D may be beneficial only for women with disorders like PCOS, insulin resistance, or low levels of AMH.
Folate and Folic Acid
Folate is the natural form of vitamin B9 found in foods, and folic acid is the synthetic version found in supplements and fortified foods. Both forms can prevent folate deficiency, which currently occurs in 20%–40% of women of reproductive age. 124 Current UK recommendations advise women planning to conceive to take 400ug/day folic acid from 3 months prior to conception and during pregnancy and consume a folate‐rich diet to prevent neural tube defects (NTD). For those at higher risk of NTDs (those with previous pregnancy of NTD, male partner with a history of NTD, periconceptional antiepileptic drug exposure, pre‐existing diabetes, and pre‐pregnancy obesity 125 ), a higher dose of 5 mg/day is recommended. 125 , 126 However, these women should also be assessed for vitamin B12 deficiency as high dose of folic acid supplementation may mask vitamin B12 deficiency. 127 Where deficiency is found, a vitamin B12 supplement of 2.6ug/day should be recommended. 125
Supplemental intake of folic acid has been shown not only to prevent NTDs 125 but also to reduce the risk of infertility and improve outcomes of infertility treatment. Fertility outcomes appear to favour supplemental folate over dietary folate, 128 with higher synthetic folate intake associated with higher luteal progesterone and decreased odds of anovulation and so may improve chances of conception further. 88
Vitamin B12
Although rare, vitamin B12 deficiency is associated with infertility, abnormal egg development, and miscarriage. 38 In those undergoing ART, a higher serum B12 concentration is associated with higher live birth rates. 129 Current UK consensus on recommended intake of vitamin B12 is 1.5 μg/day for those trying to conceive (2.6 µg if taking a high dose of folic acid); however, given the high prevalence of deficiency in women of childbearing age, it has been suggested that the RNI be increased to around 5–7 μg/day, or at least to the European recommendations of 4.5 μg/day. 130 As previously mentioned, this is of particular importance in those trying to conceive who are at higher risk of B12 deficiency.
Iron
Iron deficiency is associated with ovulatory infertility and reduced conception rates, 40 , 43 and in pregnancy, with low birth weight and developmental delay. 131 Ideally, iron status should be optimised prior to conception as supplemental iron can cause GI distress and interfere with nutrient absorption. RNI of iron in women of childbearing age is 14.8 μg/day, higher in those with measured deficiency.
Iodine
Moderate to severe iodine deficiency is associated with a 46% decrease in fecundability, 132 and in pregnancy, it is associated with adverse effects on foetal growth and cognitive development, and an increase in preeclampsia and preterm delivery. 133 , 134 , 135 Studies suggest that initiating iodine supplement use in pregnancy may be too late and that supplementation with 150 μg/day should begin preconception.
Zinc
Zinc plays a key role in many processes involved in female fertility and pregnancy, including ovulation and oocyte maturation. 136 , 137 However, there is a lack of supplementation studies in women, preventing any specific recommendations. 138
Vitamin A
Fertility nutrition is not limited to successful conception but aims towards a live pregnancy and healthy offspring. Vitamin A is associated with a teratogenic impact when supplemented in high doses such as 15,000 IU (4500 ug retinol equivalents [RE]) from diet or more than 10,000 IU (3000 RE) from supplements. 139 , 140 These levels of intake are not rare in high‐income countries, especially with habitual multivitamin supplementation and/or intake of organ meats (e.g., liver). Increased circulating retinoic acid in the first trimester can lead to miscarriage and congenital malformations. 141 The UK NICE guidelines advise avoiding supplementation of more than 5000 IU vitamin A (1500 ug). 142 Prenatal supplementations containing beta carotene are not associated with negative outcomes. 141 Women still need to meet their recommended daily allowance from food sources. Animal studies suggest the importance of vitamin A during the implantation stages 143 ; human trials are needed to evaluate the relevance.
Dietary Patterns
Despite growing evidence to suggest that plant‐based diets have beneficial health effects and the suggestion that vegetable protein is superior to animal protein for fertility, 85 there are also data to suggest that vegetarians are more likely to have menstrual irregularities than non‐vegetarians, 26.5% vs 4.9%, respectively. 144 High fibre, low fat, and no meat consumption seen in vegetarians have been associated with lower oestrogen levels. 144 However, more recent research suggests that in long‐term, weight‐stable vegetarians with a healthy BMI, vegetarianism per se is not associated with increased menstrual cycle disturbances, 145 suggesting that a well‐planned nutritionally adequate vegetarian diet may not be detrimental to fertility. Particular importance should be paid to vitamin B12, selenium, iodine, iron, choline, and omega‐3 PUFA which may be deficient in a plant‐based diet.
Regarding dietary patterns and protective effects on fertility and conception, a Mediterranean diet of whole grains, unsaturated fats, vegetables, and fish is shown to be effective. 88 , 146 Furthermore, the Mediterranean diet has been shown to improve outcomes in women undergoing IVF treatment. 147 , 148 , 149 A lower risk of difficulty conceiving was seen in subjects with high adherence to the Mediterranean diet compared to those with the least adherence. 150
A 2007 study by Chavarro et al. showed a ‘fertility diet’ (consisting of high consumption of MUFA with reduced trans‐fat consumption, high vegetable protein and less animal protein intake, high‐fat dairy, low glycaemic carbohydrates and high whole grain and fibre intake, multivitamin intake as well as more vegetarian sources of iron) was associated with 66% lower risk of ovulatory fertility and 27% reduced risk of infertility from other causes. 5
Summary of Nutritional Factors
As summarised in Table 1, female fertility is negatively affected by reduced energy availability, high glycaemic load, and high carbohydrate intake, high trans‐fat intake, and high animal protein intake. Positive effects are seen with intakes of omega‐3 PUFAs, especially oily fish intake, low GI load, high intake of whole grains, reduced carbohydrate intake, vegetable protein intake, and high antioxidant intakes. Soy intake may be beneficial to those struggling with infertility. Many micronutrient deficiencies have been associated with infertility, and optimising intakes and status of micronutrients such as vitamin D, folate, vitamin B12, iron, iodine, and zinc may offer improvements in fertility, though further research is required to make specific recommendations regarding multivitamin/mineral supplementation. An overall dietary pattern in accordance with the Mediterranean diet style of eating has the most evidence in support of improved fertility. Further research is needed to decipher the effects of full‐fat dairy intake on fertility, though there are some promising data.
Table 1.
Summary of nutritional factors affecting fertility
| Nutritional factor | Suggested intake for fertility | Reported impact on fertility | References |
|---|---|---|---|
| Energy availability | Ensure adequate energy intake | Ensure energy provision for reproductive function | 11, 68, 72, 72, |
| Carbohydrate | Low glycemic index preferable, reduce high GI, include whole grains, high fibre | Beneficial effects on fecundity and oestrogen levels | 74‐77 |
| Protein | Plant based > animal based. Include fish | Replacing animal sources of protein with plant‐based sources of protein may reduce ovulatory infertility risk. | 83, 84, 85, 86 |
| Fat | MUFA/PUFA > SFA and trans fat | Leads to higher fecundity, shorter time‐to‐pregnancy, and better ART outcomes | 5, 87‐92 |
| Foods and food groups | |||
| Seafood | 1–2 portions fish per week 1 oily fish (max 2 due to heavy metals) | Fish consumption linked to shorter time to pregnancy and increased probability of live births following ART | 85, 88, 89 |
| Soy | Soy intake from foods may be beneficial to those with infertility, though evidence is not conclusive. | Linked to higher ART success | 99, 100, 101, |
| Dairy | 2–3 servings of dairy/day | Dairy is protective of fertility. | 102, 103, 104, 105 |
| Whole grains | Include whole grains as per healthy eating guidelines. | Linked to beneficial effects on fecundity and oestrogen levels | 74‐77 |
| Red meat | As per healthy eating guidelines, replace some animal protein with plant protein. | Linked to infertility and associated with reduced ART success | 84, 85 |
| Alcohol | Avoid | Linked to reduced fecundability with risk increasing in a dose‐response manner, and to reduced ART success | 106, 107, 108, 109 |
| Caffeine | Limit to <200 µg/day | Increased time‐to‐pregnancy | 110, 111 |
| Micronutrients | |||
| Vitamin D | 10 µg/day. Test to identify any deficiency and supplement further as necessary. | Improves reproductive health; deficiency and insufficiency linked to lower ART success rates | 118, 119 |
| Folate/folic acid | 400 µg per day, 5 mg if special conditions | Reduces risk of neural tube defects and promotes egg quality | 125, 126 |
| Vitamin B12 | 1.5 µg (2.6 µg if taking high dose of folic acid) | Important for implantation and maintaining a healthy pregnancy | 38, 125, 127 |
| Iron | 14.8 mg or address deficiency | Essential for healthy and regular ovulation. Deficiency linked to ovulatory infertility and reduced conception rates. | 40, 43 |
| Iodine | 150 µg | Ensures healthy menstruation and improves chances of pregnancy; important for healthy development of baby | 132, 133‐135 |
| Zinc | Insufficient evidence to make a recommendation | Important for healthy ovulation and menstruation, as well as early development of embryo | 136, 137, 138 |
| Vitamin A | Avoid supplementation of >1500 µg vitamin A. Beta carotene is preferred source for supplementation. | Teratogenic effects in high doses | 139, 140, 141, 142 |
Nutritional Inadequacy
In addition to the medical conditions already mentioned, other health conditions are often linked to nutritional inadequacies, including swallowing difficulties, food allergies/intolerances, restrictive diets, malabsorption conditions, post‐bariatric surgery, and post active oncology treatments, all of which could benefit from dietetic assessment and intervention, ensuring nutritional adequacy for the first 1000 days of life. Therefore the proposed self‐screening tool needs to accompany clinical judgement.
Lifestyle Factors
Physical Activity
Evidence suggests that although moderate, regular physical activity (PA) can positively influence fertility and ART outcomes, high‐intensity PA has been associated with poorer fertility outcomes. 151 Inactivity and sedentary behaviours are also associated with higher rates of infertility. 152 Decreasing PA can be particularly effective in women with functional hypothalamic amenorrhea to improve energy balance and reverse amenorrhea. 12
Stress
There is a well‐known association between infertility and stress, with infertile individuals reporting higher levels of depression and anxiety than fertile individuals 153 ; however, it has been less clear if stress causes infertility. Recent reviews suggest that psychological interventions can be effective in reducing anxiety and depression, which could improve pregnancy rates in infertile women, 154 supporting a holistic approach to fertility management.
Smoking
Strong evidence exists to suggest that smoking has an adverse effect on reproductive capabilities and promotes infertility in men and women. 155 , 156 Smoking can also have adverse effects on ARTs, affecting ovarian reserves and decreasing fertilisation rates. 157
PROPOSED FERTILITY NUTRITION SCREENING TOOL
To identify those in need of nutritional support in their fertility journey, a simple, self‐administered nutrition screening tool is proposed in Table 2.
Table 2.
Proposed fertility nutrition screening tool
| Section and question | Score | ||
|---|---|---|---|
| |||
| BMI can be calculated using weight (kg)/height (m) 2 . You could ask your nurse to help you out with it. | |||
| Yes | No | Yes = 1, No = 0 | |
|
|||
| Yes | No | Yes = 2, No = 0 | |
|
|||
| |||
| Do you have any of the following conditions which could influence your nutrition status? | Yes | No | Yes = 2, No = 0 |
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
| |||
| Have you been following each of the following preconception practices in the past 3–6 months? | |||
| Nutrition adequacy | Yes | No | Yes = 0, No = 1 |
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
| Lifestyle factors | Yes | No | Yes = 0, No = 1 |
|
|||
|
|||
| Total score (maximum score = 28) | |||
A score of 1 or above demonstrates nutrition risk that the dietitian could organise self‐help resources such as a group education, webinar, or factsheet. A score of 2 and above requires a dietetic referral to be made. Further research is required to validate the tool.
CONCLUSION
Given the lack of gold standard for comparison, validating this tool is currently difficult. As a self‐administered tool, it requires further review with clinical judgement on the type and extent of dietetic involvement. The scope of this tool is limited to identify gaps in nutrition status and a potential link to subclinical and clinical infertility. It could also help identify women who would benefit from a higher folic acid supplementation, identify the need for monitoring serum levels of specific nutrients, and prioritise where a fertility dietitian can provide nutrition intervention with educational material, group or one‐on‐one consultations. Further research is mandated to adapt the screening tool in different settings and to assess the impact of the tool in identifying nutritional needs in those with infertility, perhaps through comparison to dietetic nutrition assessments as previously done with tools such as SGA.
AUTHOR CONTRIBUTIONS
The manuscript was written and reviewed by all authors.
DECLARATION OF INTEREST
None.
FUNDING AND SPONSORSHIP
None.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/jhn.13055
ACKNOWLEDGEMENTS
Special thanks to Sarah Colebrook and Robert Hickey from Lister Hospital for the their contributions in building the concept of the screening tool.
Biographies
Komal Deepak Kumar is a UK registered dietitian with clinical experience in male and female infertility, pregnancy with diabetes, and research interests in endometriosis. Joint Chair BDA Maternal and Fertility Specialist group (2020–2024).
Ro Huntriss is a registered dietitian and chief nutrition officer at the global healthtech firm, Palta. She has a special interest in fertility nutrition, sharing tips on her Instagram page @fertility.dietitian.uk
Eulalee Green is a consultant dietitian and health coach with extensive experience in maternal, paediatric, and adult clinical nutrition; nutritional education training; public health nutrition; and managing public health programs.
Dr Shabana Bora is a consultant gynaecologist and fertility specialist at the Lister Fertility Clinic, London, with a special interest in early pregnancy, recurrent miscarriage, endometriosis, and reproductive medicine, and is a BFS and RCOG member.
Dr Claire Pettitt is a UK registered dietitian and nutritionist and an associate professor at the Singapore Institute of Technology. She has a special interest in women's health nutrition, including PCOS and fertility.
Deepak Kumar K, Huntriss R, Green E, Bora S, Pettitt C. Development of a nutrition screening tool to identify need for dietetic intervention in female infertility. J Hum Nutr Diet. 2023;36:154–168. 10.1111/jhn.13055
REFERENCES
- 1. Overview | Fertility problems : assessment and treatment | Guidance | NICE. (2017).
- 2. Datta J, Palmer MJ, Tanton C, Gibson LJ, Jones KG, Macdowall W, et al. Prevalence of infertility and help seeking among 15 000 women and men. Hum Reprod. 2016;31:2108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown P. Vital statistics in the UK: births, deaths and marriages. (2021).
- 4. Hanson B, Johnstone E, Dorais J, Silver B, Peterson CM, Hotaling J. Female infertility, infertility‐associated diagnoses, and comorbidities: a review. J Assist Reprod Genet. 2017;34:167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chavarro JE, Rich‐Edwards JW, Rosner BA, Willett WC. Diet and lifestyle in the prevention of ovulatory disorder infertility. Obstet Gynecol. 2007;110:1050–58. [DOI] [PubMed] [Google Scholar]
- 6. van der Steeg JW, Steures P, Eijkemans MJC, Habbema JDF, Hompes PGA, Burggraaff JM, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2007;23:324–328. [DOI] [PubMed] [Google Scholar]
- 7. Overview | Fertility problems : assessment and treatment | Guidance | NICE.
- 8. Best D, Avenell A, Bhattacharya S. How effective are weight‐loss interventions for improving fertility in women and men who are overweight or obese? A systematic review and meta‐analysis of the evidence. Hum Reprod Update. 2017;23:681–705. [DOI] [PubMed] [Google Scholar]
- 9. Aladashvili‐Chikvaidze N, Kristesashvili J, Gegechkori M. Types of reproductive disorders in underweight and overweight young females and correlations of respective hormonal changes with BMI. Iran . J Reprod Med. 2015;13:135–40. [PMC free article] [PubMed] [Google Scholar]
- 10. Brower M, Wang E, Alexander C, Hill D, Danzer H, Surrey M, et al. The effect of low body mass index on pregnancy outcomes and ooctye quality in IVF cycles. Fertil Steril. 2014;102:338. [Google Scholar]
- 11. Meczekalski B, Katulski K, Czyzyk A, Podfigurna‐Stopa A, Maciejewska‐Jeske M. Functional hypothalamic amenorrhea and its influence on women's health. J Endocrinol Invest. 2014;37:1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gordon CM, Ackerman KE, Berga SL, Kaplan JR, Mastorakos G, Misra M, et al. Functional Hypothalamic Amenorrhea: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2017;102:1413–39. [DOI] [PubMed] [Google Scholar]
- 13. NICE . Fertility problems: assessment and treatment. https://www.nice.org.uk/guidance/cg156?unlid=86583397720167208641
- 14. Langley S. A Nutrition Screening Form for Female Infertility Patients. Can J Diet Pract Res. 2014;75:195–201. [DOI] [PubMed] [Google Scholar]
- 15. Rodino IS, Byrne S, Sanders KA. Obesity and psychological wellbeing in patients undergoing fertility treatment. Reprod Biomed Online. 2016;32:104–12. [DOI] [PubMed] [Google Scholar]
- 16. Freizinger M, Franko DL, Dacey M, Okun B, Domar AD. The prevalence of eating disorders in infertile women. Fertil Steril. 2010;93:72–8. [DOI] [PubMed] [Google Scholar]
- 17. Bruneau M, Colombel A, Mirallié S, Fréour T, Hardouin JB, Barrière P, et al. Desire for a child and eating disorders in women seeking infertility treatment. PLoS One. 2017;12:e0178848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. NICE . Eating disorders: recognition and treatment. (National Institute for Health and Care Excellence (UK), 2017). [PubMed]
- 19. Conway G, Dewailly D, Diamanti‐Kandarakis E, Escobar‐Morreale HF, Franks S, Gambineri A, et al. The polycystic ovary syndrome: a position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014;171:P1–29. [DOI] [PubMed] [Google Scholar]
- 20. Evanthia Diamanti‐Kandarakis AD. Insulin Resistance and the Polycystic Ovary Syndrome Revisited: An Update on Mechanisms and Implications. Endocr Rev. 2012;33:981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sawant S, Bhide P. Fertility Treatment Options for Women With Polycystic Ovary Syndrome. Clin Med Insights Reprod Health. 2019;13:1179558119890867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joham AE, Teede HJ, Ranasinha S, Zoungas S, Boyle J. Prevalence of infertility and use of fertility treatment in women with polycystic ovary syndrome: data from a large community‐based cohort study. J Womens Health. 2015;24:299–307. [DOI] [PubMed] [Google Scholar]
- 23. Balen A. Polycystic Ovary Syndrome and Secondary Amenorrhoea. Dewhurst's Textbook of Obstetrics & Gynaecology 9th edition . 2018. 513–33. 10.1002/9781119979449.ch41 [DOI]
- 24. Farid NR, Diamanti‐Kandarakis E. Diagnosis and Management of Polycystic Ovary Syndrome. Springer; 2011. [Google Scholar]
- 25. Polycystic ovary syndrome and maternal obesity affect oocyte size in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril. 2011;95:2146–49.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Legro RS, Dodson WC, Kunselman AR, Stetter CM, Kris‐Etherton PM, Williams NI, et al. Benefit of Delayed Fertility Therapy With Preconception Weight Loss Over Immediate Therapy in Obese Women With PCOS. J Clin Endocrinol Metab. 2016;101:2658–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McGrice M, Porter J. The Effect of Low Carbohydrate Diets on Fertility Hormones and Outcomes in Overweight and Obese Women: A Systematic Review. Nutrients. 2017;9:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang X, Zheng Y, Guo Y, Lai Z. The Effect of Low Carbohydrate Diet on Polycystic Ovary Syndrome: A Meta‐Analysis of Randomized Controlled Trials. Int J Endocrinol. 2019;2019:4386401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Livshits A, Seidman DS. Fertility Issues in Women with Diabetes. Women's Health. 2009;5:701–707. [DOI] [PubMed] [Google Scholar]
- 30. Strotmeyer ES, Steenkiste AR, Foley TP, Berga SL, Dorman JS. Menstrual Cycle Differences Between Women With Type 1 Diabetes and Women Without Diabetes. Diabetes Care. 2003;26:1016–21. [DOI] [PubMed] [Google Scholar]
- 31. Marca A, la, la Marca A, Morgante G. & De Leo, V. Evaluation of hypothalamic pituitary adrenal axis in amenorrhoeic women with insulin‐dependent diabetes. Hum Reprod. 1999;14:298–302. [DOI] [PubMed] [Google Scholar]
- 32. Franz MJ, MacLeod J, Evert A, Brown C, Gradwell E, Handu D, et al. Academy of Nutrition and Dietetics Nutrition Practice Guideline for Type 1 and Type 2 Diabetes in Adults: Systematic Review of Evidence for Medical Nutrition Therapy Effectiveness and Recommendations for Integration into the Nutrition Care Process. J Acad Nutr Diet. 2017;117:1659–79. [DOI] [PubMed] [Google Scholar]
- 33. Stazi AV, Mantovani A. A risk factor for female fertility and pregnancy: celiac disease. Gynecol Endocrinol. 2000;14:454–63. [DOI] [PubMed] [Google Scholar]
- 34. Internal Clinical Guidelines Team (UK) . Coeliac Disease: Recognition, Assessment and Management. (National Institute for Health and Care Excellence (UK), 2015). [PubMed]
- 35. Tata LJ, Card TR, Logan RFA, Hubbard RB, Smith CJP, West J. Fertility and pregnancy‐related events in women with celiac disease: a population‐based cohort study. Gastroenterology. 2005;128:849–55. [DOI] [PubMed] [Google Scholar]
- 36. Ciacci C, Cirillo M, Auriemma G, Di Dato G, Sabbatini F, Mazzacca G. Celiac disease and pregnancy outcome. Am J Gastroenterol. 1996;91:718–22. [PubMed] [Google Scholar]
- 37. Collin P, Vilska S, Heinonen PK, Hällström O, Pikkarainen P. Infertility and coeliac disease. Gut. 1996;39:382–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bennett M. Vitamin B12 deficiency, infertility and recurrent fetal loss. J Reprod Med. 2001;46:209–12. [PubMed] [Google Scholar]
- 39. El‐Nemr A, Al‐Shawaf T, Sabatini L, Wilson C, Lower AM, Grudzinskas JG. Effect of smoking on ovarian reserve and ovarian stimulation in in‐ vitro fertilization and embryo transfer. Hum Reprod. 1998;13:2192–98. [DOI] [PubMed] [Google Scholar]
- 40. Li YQ, Cao XX, Bai B, Zhang JN, Wang MQ, Zhang YH. Severe Iron Deficiency Is Associated with a Reduced Conception Rate in Female Rats. GOI. 2014;77:19–23. [DOI] [PubMed] [Google Scholar]
- 41. Briggs DA, Sharp DJ, Miller D, Gosden RG. Transferrin in the developing ovarian follicle: evidence for de‐novo expression by granulosa cells. Mol Hum Reprod. 1999;5:1107–14. [DOI] [PubMed] [Google Scholar]
- 42. Kolesarova A, Capcarova M, Medvedova M, Sirotkin AV, Kovacik J. In Vitro Assessment of Iron Effect on Porcine Ovarian Granulosa Cells: Secretory Activity, Markers of Proliferation and Apoptosis. Physiol Res. 2011;60:503–10. 10.33549/physiolres.931969 [DOI] [PubMed] [Google Scholar]
- 43. Chavarro JE, Rich‐Edwards JW, Rosner BA, Willett WC. Iron intake and risk of ovulatory infertility. Obstet Gynecol. 2006;108:1145–52. [DOI] [PubMed] [Google Scholar]
- 44. McKenna D, Spence D, Haggan SE, McCrum E, Dornan JC, Lappin TR. A randomized trial investigating an iron‐rich natural mineral water as a prophylaxis against iron deficiency in pregnancy. Clin Lab Haematol. 2003;25:99–103. [DOI] [PubMed] [Google Scholar]
- 45. Jackson ID, Doig W, Mcdonald G. PERNICIOUS ANÆEMIA AS A CAUSE OF INFERTILITY. The Lancet. 1967;290:1159–60. [DOI] [PubMed] [Google Scholar]
- 46. Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K, et al. Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract Res Clin Obstet Gynaecol. 2018;51:1–15. [DOI] [PubMed] [Google Scholar]
- 47. Nnoaham KE, Sivananthan S, Hummelshoj L, Jenkinson C, Webster P, Kennedy SH, et al. MULTI‐CENTRE STUDIES OF THE GLOBAL IMPACT OF ENDOMETRIOSIS AND THE PREDICTIVE VALUE OF ASSOCIATED SYMPTOMS. J Endometr. 2009;1:36–45. [PMC free article] [PubMed] [Google Scholar]
- 48. Bulletti C, Coccia ME, Battistoni S, Borini A. Endometriosis and infertility. J Assist Reprod Genet. 2010;27:441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Seaman HE, Ballard KD, Wright JT, de Vries CS. Endometriosis and its coexistence with irritable bowel syndrome and pelvic inflammatory disease: findings from a national case‐control study‐‐Part 2. BJOG. 2008;115:1392–96. [DOI] [PubMed] [Google Scholar]
- 50. Moore JS, Gibson PR, Perry RE, Burgell RE. Endometriosis in patients with irritable bowel syndrome: Specific symptomatic and demographic profile, and response to the low FODMAP diet. Aust N Z J Obstet Gynaecol. 2017;57:201–205. [DOI] [PubMed] [Google Scholar]
- 51. Yamamoto A, Harris HR, Vitonis AF, Chavarro JE, Missmer SA. A prospective cohort study of meat and fish consumption and endometriosis risk. Am J Obstet Gynecol. 2018;219:178.e1–178.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kalaitzopoulos DR, Lempesis IG, Athanasaki F, Schizas D, Samartzis EP, Kolibianakis EM, et al. Association between vitamin D and endometriosis: a systematic review. Hormones. 2020;19:109–21. [DOI] [PubMed] [Google Scholar]
- 53. Harris HR, Chavarro JE, Malspeis S, Willett WC, Missmer SA. Dairy‐food, calcium, magnesium, and vitamin D intake and endometriosis: a prospective cohort study. Am J Epidemiol. 2013;177:420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Karlsson JV, Patel H, Premberg A. Experiences of health after dietary changes in endometriosis: a qualitative interview study. BMJ Open. 2020;10:e032321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Giuliani E, As‐Sanie S, Marsh EE. Epidemiology and management of uterine fibroids. Int J Gynaecol Obstet. 2020;149:3–9. [DOI] [PubMed] [Google Scholar]
- 56. Guo XC, Segars JH. The impact and management of fibroids for fertility: an evidence‐based approach. Obstet Gynecol Clin North Am. 2012;39:521–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cook H, Ezzati M, Segars JH, McCarthy K. The impact of uterine leiomyomas on reproductive outcomes. Minerva Ginecol. 2010;62:225–36. [PMC free article] [PubMed] [Google Scholar]
- 58. Skowrońska P, Pastuszek E, Kuczyński W, Jaszczoł M, Kuć P, Jakiel G, et al. The role of vitamin D in reproductive dysfunction in women‐a systematic review. Ann Agric Environ Med. 2016;23:671–676. [DOI] [PubMed] [Google Scholar]
- 59. Sharan C, Halder SK, Thota C, Jaleel T, Nair S, Al‐Hendy A. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol‐O‐methyltransferase. Fertil Steril. 2011;95:247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Baird DD, Hill MC, Schectman JM, Hollis BW. Vitamin d and the risk of uterine fibroids. Epidemiology. 2013;24:447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Arjeh S, Darsareh F, Asl ZA, Azizi Kutenaei M. Effect of oral consumption of vitamin D on uterine fibroids: A randomized clinical trial. Complement Ther Clin Pract. 2020;39:101159. [DOI] [PubMed] [Google Scholar]
- 62. Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro‐Green A. Universal screening versus case finding for detection and treatment of thyroid hormonal dysfunction during pregnancy. J Clin Endocrinol Metab. 2010;95:1699–1707. [DOI] [PubMed] [Google Scholar]
- 63. Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, Mechanick JI, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American. Thyroid Association. Thyroid. 2012;22:1200–35. [DOI] [PubMed] [Google Scholar]
- 64. Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105:239–45. [DOI] [PubMed] [Google Scholar]
- 65. De Groot L, Abalovich M, Alexander EK, Amino N, Barbour L, Cobin RH, et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:2543–65. [DOI] [PubMed] [Google Scholar]
- 66. Taheriniya S, Arab A, Hadi A, Fadel A, Askari G. Vitamin D and thyroid disorders: a systematic review and Meta‐analysis of observational studies. BMC Endocr Disord. 2021;21:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chung HR. Iodine and thyroid function. Ann Pediatr Endocrinol Metab. 2014;19:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Laughlin GA, Dominguez CE, Yen SS. Nutritional and endocrine‐metabolic aberrations in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 1998;83:25–32. [DOI] [PubMed] [Google Scholar]
- 69. Mantel Ä, Hirschberg AL, Stephansson O. Association of Maternal Eating Disorders With Pregnancy and Neonatal Outcomes. JAMA Psychiatry. 2020;77:285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Linna MS, Raevuori A, Haukka J, Suvisaari JM, Suokas JT, Gissler M. Pregnancy, obstetric, and perinatal health outcomes in eating disorders. Am J Obstet Gynecol. 2014;211(392):e1–8. [DOI] [PubMed] [Google Scholar]
- 71. Dörsam AF, Preißl H, Micali N, Lörcher SB, Zipfel S, Giel KE. The Impact of Maternal Eating Disorders on Dietary Intake and Eating Patterns during Pregnancy: A Systematic Review. Nutrients. 2019;11:840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Loucks AB, Heath EM. Induction of low‐T3 syndrome in exercising women occurs at a threshold of energy availability. Am J Physiol. 1994;266:R817–23. [DOI] [PubMed] [Google Scholar]
- 73. Koltun KJ, Souza De, Scheid MJ, JL, Williams . N. I. Energy Availability Is Associated With Luteinizing Hormone Pulse Frequency and Induction of Luteal Phase Defects. J Clin Endocrinol Metab. 2020;105:185‐ 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Chavarro JE, Rich‐Edwards JW, Rosner BA, Willett WC. A prospective study of dietary carbohydrate quantity and quality in relation to risk of ovulatory infertility. Eur J Clin Nutr. 2009;63:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Agarwal A, Aponte‐Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gaskins AJ, Chiu YH, Williams PL, Keller MG, Toth TL, Hauser R, et al. Maternal whole grain intake and outcomes of in vitro fertilization. Fertil Steril. 2016;105:1503 – 1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gaskins AJ, Mumford SL, Zhang C, Wactawski‐Wende J, Hovey KM, Whitcomb BW, et al. Effect of daily fiber intake on reproductive function: the BioCycle Study. Am . J Clin Nutr. 2009;90:1061–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gower BA, Chandler‐Laney PC, Ovalle F, Goree LL, Azziz R, Desmond RA, et al. Favourable metabolic effects of a eucaloric lower‐carbohydrate diet in women with PCOS. Clin Endocrinol. 2013;79:550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mehrabani HH, Salehpour S, Amiri Z, Farahani SJ, Meyer BJ, Tahbaz F. Beneficial effects of a high‐protein, low‐glycemic‐load hypocaloric diet in overweight and obese women with polycystic ovary syndrome: a randomized controlled intervention study. J Am Coll Nutr. 2012;31:117–25. [DOI] [PubMed] [Google Scholar]
- 80. Gorczyca AM, Sjaarda LA, Mitchell EM, Perkins NJ, Schliep KC, Wactawski‐Wende J, et al. Dietary Carbohydrate Intake Does Not Impact Insulin Resistance or Androgens in Healthy, Eumenorrheic Women. J Clin Endocrinol Metab. 2015;100:2979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hatch EE, Wesselink AK, Hahn KA, Michiel JJ, Mikkelsen EM, Sorensen HT, et al. Intake of Sugar‐sweetened Beverages and Fecundability in a North American Preconception Cohort. Epidemiology. 2018;29:369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Machtinger R, Gaskins AJ, Mansur A, Adir M, Racowsky C, Baccarelli AA, et al. Association between preconception maternal beverage intake and in vitro fertilization outcomes. Fertil Steril. 2017;108:1026–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chavarro JE, Rich‐Edwards JW, Rosner BA, Willett WC. Protein intake and ovulatory infertility. Am J Obstet Gynecol. 2008;198(210):e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Braga DP, Halpern G, Setti AS, Figueira RC, Iaconelli A Jr, Borges E Jr. The impact of food intake and social habits on embryo quality and the likelihood of blastocyst formation. Reprod Biomed Online. 2015;31:30–8. [DOI] [PubMed] [Google Scholar]
- 85. Nassan FL, Chiu YH, Vanegas JC, Gaskins AJ, Williams PL, Ford JB, et al. Intake of protein‐rich foods in relation to outcomes of infertility treatment with assisted reproductive technologies. Am J Clin Nutr. 2018;108:1104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gannon MC, Nuttall FQ, Saeed A, Jordan K, Hoover H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am J Clin Nutr. 2003;78:734–41. [DOI] [PubMed] [Google Scholar]
- 87. Nehra D, Le HD, Fallon EM, Carlson SJ, Woods D, White YA, et al. Prolonging the female reproductive lifespan and improving egg quality with dietary omega‐3 fatty acids. Aging cell. 2012;11:1046–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gaskins AJ, Chavarro JE. Diet and fertility: a review. Am J Obstet Gynecol. 2018;218:379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wise LA, Wesselink AK, Tucker KL, Saklani S, Mikkelsen EM, Cueto H, et al. Dietary Fat Intake and Fecundability in 2 Preconception Cohort Studies. Am J Epidemiol. 2018;187:60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chiu Y‐H, Chavarro JE, Souter I. Diet and female fertility: doctor, what should I eat? Fertil Steril. 2018;110:560–569. [DOI] [PubMed] [Google Scholar]
- 91. Lass A, Belluzzi A. Omega‐3 polyunsaturated fatty acids and IVF treatment. Reprod Biomed Online. 2019;38:95–9. [DOI] [PubMed] [Google Scholar]
- 92. De Cosmi V, Cipriani S, Parazzini F, Ricci E, Esposito G, Noli S, et al. Fatty acids intake and outcomes of assisted reproduction in women referring to an Italian Fertility Service: cross‐sectional analysis of a prospective cohort study. J Hum Nutr Diet . Forthcoming 2021. 10.1111/jhn.12982 [DOI] [PubMed]
- 93. Moran LJ, Tsagareli V, Noakes M, Norman R. Altered Preconception Fatty Acid Intake Is Associated with Improved Pregnancy Rates in Overweight and Obese Women Undertaking in Vitro Fertilisation. Nutrients. 2016;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hsi H‐C, Hsu Y‐W, Chang T‐C, Chien L‐C. Methylmercury Concentration in Fish and Risk‐Benefit Assessment of Fish Intake among Pregnant versus Infertile Women in Taiwan. PLoS One. 2016;11:e0155704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. FDA . Eating Fish Advice. (2019).
- 96. Fish and Shellfish Nutrition . NHS UK https://www.nhs.uk/live-well/eat-well/fish-and-shellfish-nutrition/ (2018).
- 97. Wesselink AK, Hatch EE, Mikkelsen EM, Trolle E, Willis SK, McCann SE, et al. Dietary phytoestrogen intakes of adult women are not strongly related to fecundability in 2 preconception cohort studies. J Nutr. 2020;150:1240–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Rizzo G, Feraco A, Storz MA, Lombardo M. The role of soy and soy isoflavones on women's fertility and related outcomes: an update. J Nutr Sci. 2022;11:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Vanegas JC, Afeiche MC, Gaskins AJ, Mínguez‐Alarcón L, Williams PL, Wright DL, et al. Soy food intake and treatment outcomes of women undergoing assisted reproductive technology. Fertil Steril. 2015;103):749‐55.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Shahin AY, Ismail AM, Zahran KM, Makhlouf AM. Adding phytoestrogens to clomiphene induction in unexplained infertility patients‐‐a randomized trial. Reprod Biomed Online. 2008;16:580–588. [DOI] [PubMed] [Google Scholar]
- 101. Unfer Vittorio, Casini Maria. Luisa, Costabile, Loredana, Mignosa, Sandro Gerli, Di Renzo, Gian Carlo. High dose of phytoestrogens can reverse the antiestrogenic effects of clomiphene citrate on the endometrium in patients undergoing intrauterine insemination: a randomized trial. The Journal of the Society for Gynecologic Investigation: JSGI. 2004;11:323–328. [DOI] [PubMed] [Google Scholar]
- 102. Greenlee AR, Arbuckle TE, Chyou P‐H. Risk Factors for Female Infertility in an Agricultural Region. Epidemiology. 2003;14:429–36. [DOI] [PubMed] [Google Scholar]
- 103. Chavarro JE, Rich‐Edwards JW, Rosner B, Willett WC. A prospective study of dairy foods intake and anovulatory infertility. Hum Reprod. 2007;22:1340–47. [DOI] [PubMed] [Google Scholar]
- 104. Kim K, Wactawski‐Wende J, Michels KA, Plowden TC, Chaljub EN, Sjaarda LA, et al. Dairy Food Intake Is Associated with Reproductive Hormones and Sporadic Anovulation among Healthy Premenopausal Women. J Nutr. 2017;147:218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wise LA, Wesselink AK, Mikkelsen EM, Cueto H, Hahn KA, Rothman KJ, et al. Dairy intake and fecundability in 2 preconception cohort studies. Am J Clin Nutr. 2017;105:100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fan D, Liu L, Xia Q, Wang W, Wu S, Tian G, et al. Female alcohol consumption and fecundability: a systematic review and dose‐response meta‐analysis. Sci Rep. 2017;7:13815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Van Heertum K, Rossi B. Alcohol and fertility: how much is too much? Fertil Res Pract. 2017;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Klonoff‐Cohen H, Lam‐Kruglick P, Gonzalez C. Effects of maternal and paternal alcohol consumption on the success rates of in vitro fertilization and gamete intrafallopian transfer. Fertil Steril. 2003;79:330–339. [DOI] [PubMed] [Google Scholar]
- 109. Rooney KL, Domar AD. The impact of lifestyle behaviors on infertility treatment outcome. Curr Opin Obstet Gynecol. 2014;26:181–185. [DOI] [PubMed] [Google Scholar]
- 110. Bolúmar F, Olsen J, Rebagliato M, Bisanti L. & European Study Group on Infertility and Subfecundity. Caffeine Intake and Delayed Conception: A European Multicenter Study on Infertility and Subfecundity. Am J Epidemiol. 1997;145:324–34. [DOI] [PubMed] [Google Scholar]
- 111. Gaskins AJ, Rich‐Edwards JW, Williams PL, Toth TL, Missmer SA, Chavarro JE. Pre‐pregnancy caffeine and caffeinated beverage intake and risk of spontaneous abortion. Eur J Nutr. 2018;57:107–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lyngsø J, Ramlau‐Hansen CH, Bay B, Ingerslev HJ, Hulman A, Kesmodel US. Association between coffee or caffeine consumption and fecundity and fertility: a systematic review and dose‐response meta‐analysis. Clin Epidemiol. 2017;9:699–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Klonoff‐Cohen H, Bleha J, Lam‐Kruglick P. A prospective study of the effects of female and male caffeine consumption on the reproductive endpoints of IVF and gamete intra‐Fallopian transfer. Hum Reprod. 2002;17:1746–54. [DOI] [PubMed] [Google Scholar]
- 114. Choi JH, Ryan LM, Cramer DW, Hornstein MD, Missmer SA. Effects of Caffeine Consumption by Women and Men on the Outcome of In Vitro Fertilization. 10.1089/caf.2011.0001 [DOI] [PMC free article] [PubMed]
- 115.European Food Safety Authority. Scientific Opinion on the safety of caffeine. (2015). 10.2903/j.efsa.20YY.NNNN [DOI]
- 116.Superior Health Council, Brussels. The use of caffeine in foodstuffs. (2012).
- 117. Macit MS, Akdevelioglu Y. An Overview of The Relationship Between Fertility and Caffeine Intake. Clinical and Experimental Health Sciences. 2017;50:138. [Google Scholar]
- 118.SACN. Vitamin D and Health. (2016).
- 119. Chu J, Gallos I, Tobias A, Tan B, Eapen A, Coomarasamy A. Vitamin D and assisted reproductive treatment outcome: a systematic review and meta‐analysis. Hum Reprod. 2018;33:65–80. [DOI] [PubMed] [Google Scholar]
- 120. Aoun A, Khoury VE, Malakieh R. Can Nutrition Help in the Treatment of Infertility? Prev Nutr Food Sci. 2021;26:109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Muscogiuri G, Altieri B, de Angelis C, Palomba S, Pivonello R, Colao A, et al. Shedding new light on female fertility: The role of vitamin D. Rev Endocr Metab Disord. 2017;18:273–83. [DOI] [PubMed] [Google Scholar]
- 122. Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- 123. Evans KN, Bulmer JN, Kilby MD, Hewison M. Vitamin D and placental‐decidual function. J Soc Gynecol Investig. 2004;11:263–71. [DOI] [PubMed] [Google Scholar]
- 124. Rogers LM, Cordero AM, Pfeiffer CM, Hausman DB, Tsang BL, De‐Regil LM, et al Global folate status in women of reproductive age: a systematic review with emphasis on methodological issues. Ann N Y Acad Sci. 2018;1431:35–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wilson RD, Genetics C, Wilson RD, Audibert F, Brock JA, Carroll J, et al. Pre‐conception Folic Acid and Multivitamin Supplementation for the Primary and Secondary Prevention of Neural Tube Defects and Other Folic Acid‐Sensitive Congenital Anomalies. J Obstet Gynaecol Can. 2015;37:534–52. [DOI] [PubMed] [Google Scholar]
- 126. Kennedy D, Koren G. Identifying women who might benefit from higher doses of folic acid in pregnancy. Can Fam Physician. 2012;58:394–7. [PMC free article] [PubMed] [Google Scholar]
- 127.Joint Formulary Committee (Great Britain). BNF 76 (British National Formulary) September 2018. (British National Formulary, 2018).
- 128. Gaskins AJ, Mumford SL, Chavarro JE, Zhang C, Pollack AZ, Wactawski‐Wende J, et al. The impact of dietary folate intake on reproductive function in premenopausal women: a prospective cohort study. PLoS One. 2012;7:e46276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Gaskins AJ, Chiu YH, Williams PL, Ford JB, Toth TL, Hauser R, et al Association between serum folate and vitamin B‐12 and outcomes of assisted reproductive technologies. Am J Clin Nutr. 2015;102:943–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Sukumar Nithya, Adaikalakoteswari Antonysunil, Venkataraman Hema, Maheswaran Hendramoorthy. Saravanan, Ponnusamy. Vitamin B12 status in women of childbearing age in the UK and its relationship with national nutrient intake guidelines: results from two National Diet and Nutrition Surveys. BMJ Open. 2016;8(12):768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Berglund SK, Torres‐Espínola FJ, García‐Valdés L, Segura MT, Martínez‐Zaldívar C, Padilla C, et al The impacts of maternal iron deficiency and being overweight during pregnancy on neurodevelopment of the offspring. Br J Nutr. 2017;118:533–40. [DOI] [PubMed] [Google Scholar]
- 132. Mills JL, Buck Louis GM, Kannan K, Weck J, Wan Y, Maisog J, et al. Delayed conception in women with low‐urinary iodine concentrations: a population‐based prospective cohort study. Hum Reprod. 2018;33:426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bath SC, Rayman MP. A review of the iodine status of UK pregnant women and its implications for the offspring. Environ Geochem Health. 2015;37:619–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Bath SC, Steer CD, Golding J, Emmett P, Rayman MP. Effect of inadequate iodine status in UK pregnant women on cognitive outcomes in their children: results from the Avon Longitudinal Study of Parents and Children (ALSPAC). Lancet. 2013;382:331–337. [DOI] [PubMed] [Google Scholar]
- 135. Abel MH, Caspersen IH, Sengpiel V, Jacobsson B, Meltzer HM, Magnus P, et al Insufficient maternal iodine intake is associated with subfecundity, reduced foetal growth, and adverse pregnancy outcomes in the Norwegian Mother, Father and Child Cohort Study. BMC Med. 2020;18:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Chatzicharalampous C, Jeelani R, Mikhael S, Aldhaheri S, Najeemudin S, Morris RT, et al. Zinc: an essential metal for maintenance of female fertility. Fertil Steril. 2018;109:e19. [Google Scholar]
- 137. Ebisch IMW, Thomas CMG, Peters WHM, Braat DDM, Steegers‐Theunissen RPM. The importance of folate, zinc and antioxidants in the pathogenesis and prevention of subfertility. Hum Reprod Update. 2007;13:163–74. [DOI] [PubMed] [Google Scholar]
- 138.The Fertility Society of Australia‐Pre‐Conception Health Special Interest Group. Micronutrient (Zinc and Selenium) supplements and subfertility. (2015).
- 139. Hunt JR. Teratogenicity of high vitamin A intake. N Engl J Med. 1996;334:1197. [DOI] [PubMed] [Google Scholar]
- 140. Rothman KJ, Moore LL, Singer MR, Nguyen US, Mannino S, Milunsky A. Teratogenicity of high vitamin A intake. N Engl J Med. 1995;333:1369–73. [DOI] [PubMed] [Google Scholar]
- 141. Maia SB, et al. Vitamin A and Pregnancy: A Narrative Review. Nutrients. 2019;11:681. 10.3390/nu11030681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. National Institute for Health and Clinical Excellence (Great Britain) . Antenatal Care: Routine Care for the Healthy Pregnant Woman. (2008).
- 143. Gómez E, Caamaño JN, Rodríguez A, De Frutos C, Facal N, Díez C. Bovine early embryonic development and vitamin A. Reprod Domest Anim. 2006;41:63–71. [DOI] [PubMed] [Google Scholar]
- 144. Pedersen AB, Bartholomew MJ, Dolence LA, Aljadir LP, Netteburg KL, Lloyd T. Menstrual differences due to vegetarian and non vegetarian diets. Am J Clin Nutr. 1991;53:879–85. [DOI] [PubMed] [Google Scholar]
- 145. Barr SI. Vegetarianism and menstrual cycle disturbances: is there an association? Am. J Clin Nutr. 1999;70:549S–554S. [DOI] [PubMed] [Google Scholar]
- 146. Panth N, Gavarkovs A, Tamez M, Mattei J. The Influence of Diet on Fertility and the Implications for Public Health Nutrition in the United States. Front Public Health. 2018;6:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Sun H, Lin Y, Lin D, Zou C, Zou X, Fu L, et al Mediterranean diet improves embryo yield in IVF: a prospective cohort study. Reprod Biol Endocrinol. 2019;17:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Karayiannis D, Kontogianni MD, Mendorou C, Mastrominas M, Yiannakouris N. Adherence to the Mediterranean diet and IVF success rate among non‐obese women attempting fertility. Hum Reprod. 2018;33:494–502. [DOI] [PubMed] [Google Scholar]
- 149. Vujkovic M, de Vries JH, Lindemans J, Macklon NS, van der Spek PJ, Steegers EA, et al The preconception Mediterranean dietary pattern in couples undergoing in vitro fertilization/intracytoplasmic sperm injection treatment increases the chance of pregnancy. Fertil Steril. 2010;94:2096–2101. [DOI] [PubMed] [Google Scholar]
- 150. Toledo E, Lopez‐del Burgo C, Ruiz‐Zambrana A, Donazar M, Navarro‐Blasco I, Martínez‐González MA, et al Dietary patterns and difficulty conceiving: a nested case‐control study. Fertil Steril. 2011;96:1149–53. [DOI] [PubMed] [Google Scholar]
- 151.The Fertility Society of Australia. The role of exercise in improving fertility, quality of life and emotional well‐being. (2016).
- 152. Foucaut A‐M, Faure C, Julia C, Czernichow S, Levy R, Dupont C, et al Sedentary behavior, physical inactivity and body composition in relation to idiopathic infertility among men and women. PLoS One. 2019;14:e0210770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. De Berardis D, Mazza M, Marini S, Del Nibletto L, Serroni N, Pino MC, et al Psychopathology, emotional aspects and psychological counselling in infertility: a review. Clin Ter. 2014;165:163–169. [DOI] [PubMed] [Google Scholar]
- 154. Rooney KL, Domar AD. The relationship between stress and infertility. Dialogues Clin Neurosci. 2018;20:41–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Homan GF, Davies M, Norman R. The impact of lifestyle factors on reproductive performance in the general population and those undergoing infertility treatment: a review. Hum Reprod Update. 2007;13:209–23. [DOI] [PubMed] [Google Scholar]
- 156. Augood C, Duckitt K, Templeton AA. Smoking and female infertility: a systematic review and meta‐analysis. Hum Reprod. 1998;13:1532–39. [DOI] [PubMed] [Google Scholar]
- 157. Firns S, Cruzat VF, Keane KN, Joesbury KA, Lee AH, Newsholme P, et al. The effect of cigarette smoking, alcohol consumption and fruit and vegetable consumption on IVF outcomes: a review and presentation of original data. Reprod Biol Endocrinol. 2015;13:134. [DOI] [PMC free article] [PubMed] [Google Scholar]


