Abstract
Implementation of the kidney allocation system in 2014 greatly reduced access disparity due to human leukocyte antigen (HLA) sensitization. To address persistent disparity related to candidate ABO blood groups, herein we propose a novel metric termed “ABO‐adjusted cPRA,” which simultaneously considers the impact of candidate HLA and ABO sensitization on the same scale. An ethnic‐weighted ABO‐adjusted cPRA value was computed for 190 467 candidates on the kidney waitlist by combining candidate's conventional HLA cPRA with the remaining fraction of HLA‐compatible donors that are ABO‐incompatible. Consideration of ABO sensitization resulted in higher ABO‐adjusted cPRA relative to conventional cPRA by HLA alone, except for AB candidates since they are not ABO‐sensitized. Within cPRA Point Group = 99%, 43% of the candidates moved up to ABO‐adjusted cPRA Point Group = 100%, though this proportion varied substantially by candidate blood group. Nearly all O and most B candidates would have elevated ABO‐adjusted cPRA values above this policy threshold for allocation priority, but relatively few A candidates displayed this shift. Overall, ABO‐adjusted cPRA more accurately measures the proportion of immune‐compatible donors compared with conventional HLA cPRA, especially for highly sensitized candidates. Implementation of this novel metric could enable the development of allocation policies permitting more ABO‐compatible transplants without compromising equity.
Keywords: ABO incompatibility, ethics and public policy, genetics, histocompatibility, immunogenetics, organ allocation, organ procurement and allocation, panel reactive antibody (PRA), registry/registry analysis, translational research/science
Short abstract
A metric for both HLA and ABO immune sensitization can be applied in organ allocation systems to address disparities in transplant access among candidates in different ABO blood groups.
Abbreviations
- cPRA
calculated panel reactive antibody
- HLA
human leukocyte antigen
- KAS
kidney allocation system
- KPSAM
kidney–pancreas simulated allocation model
- NMDP
National Marrow Donor Program
- OPTN
Organ Procurement and Transplantation Network
- UNOS
United Network for Organ Sharing
- SRTR
Scientific Registry for Transplant Recipients
1. INTRODUCTION
Kidney transplantation is a life‐saving therapy for patients with end‐stage kidney disease. 1 , 2 , 3 , 4 , 5 Unfortunately, there are numerous biological and socioeconomic factors which impact a patient's access to transplantation. The primary immunologic determinants of access to kidney transplantation are candidates' level of sensitization to human leukocyte antigens (HLAs) and ABO blood group antigens. 1 Historically, kidney transplant candidates broadly sensitized to HLAs generally faced prolonged wait‐times, 6 reduced access to transplantation, 7 and increased mortality on the waitlist. 8 The implementation of the 2014 kidney allocation system (KAS) led to a substantial decrease in access disparity based on HLA compatibility. 9 , 10 , 11 Specifically, candidates received allocation points on a sliding scale based on the percentage of the donor pool with whom they were HLA‐incompatible, a metric referred to as calculated panel reactive antibody (cPRA). 12 In addition to allocation points, highly sensitized candidates (cPRA = 100%) are prioritized for national sharing of organs, as the local donor pool is unlikely to yield the HLA‐compatible donors needed for these candidates. 13
In contrast to the expansive accommodations addressing HLA disparities with cPRA, disparities according to candidate ABO blood group were not addressed by the KAS and, as such, have persisted. 14 , 15 In the United States, blood group B and O candidates experience lower access to transplant compared with those with blood groups A and AB. 16 Because the size of the deceased donor pool differs by ABO category, current policy limits organ allocation to ABO‐identical transplants (at least in most circumstances) to ensure that A and AB candidates do not divert organs from other blood group categories. Blood group O candidates are biologically compatible only with the O donor pool, but there is an asymmetry where O donors are compatible for candidates of all blood groups, meaning allocation to other blood groups decreases access for O candidates. ABO policy restrictions are only loosened to improve transplant opportunities for certain categories of candidates who have the greatest needs or benefits, such as zero‐ABDR mismatched transplants. 17
ABO‐compatible transplants that are not ABO‐identical are currently restricted by policy rather than biology, effectively constraining the pool of potential donors. A more ideal allocation system would maximize opportunities for transplant by removing ABO restrictions but without increasing disparity. We propose that a population genetic adjustment for ABO compatibility is the missing piece that would help achieve equity while improving access.
Herein we present a novel unified metric for immunologic compatibility, simultaneously considering the contribution of both candidate HLA and ABO sensitization on the same scale. Furthermore, we measured the impact of this unified cPRA on candidates within different ABO blood groups, focusing especially on highly sensitized candidates where small differences in cPRA could have a large effect on the priority points assigned under the current KAS policy.
2. METHODS
This study received ethics approval from the Tulane University Institutional Review Board. The study was based on OPTN data as of December 2021. The most recent waitlist entries for US kidney candidates within the years of 2019 and 2020 were selected. 190 467 candidates had at least one listing in this time period, and 78 186 candidates had unacceptable HLAs listed.
2.1. Measuring HLA antibody sensitization using stem cell donor typing
The current OPTN cPRA calculation relies on using the HLA haplotype frequencies of deceased kidney donors recovered from January 1, 2007, to December 31, 2008. 18 Due to the lack of donor HLA typing entered for HLA‐DQA1, ‐DPB1, ‐DPA1, and/or allele‐specific antigens during this timeframe, the OPTN cPRA calculator does not account for candidates with anti‐HLA antibodies formed against these antigens. 19 Based on our previous work, 20 which is currently disseminated for OPTN public comment, 21 utilization of the National Marrow Donor Program (NMDP) registry as a data source for HLA frequencies might represent a more precise method to quantify sensitization to all HLA loci/alleles relevant for solid organ transplantation. Thus, in the present study, candidate cPRA values by HLA were computed using population‐specific, allele‐level HLA genotype frequencies covering all classical HLA loci, namely, HLA‐A, B, C, DRB1, DRB3/4/5, DQA1, DQB1, DPA1, DPB1, based on over 2 million US stem cell donors from the NMDP registry, 20 and accounting for the proportion of the donor's race/ethnicity in the deceased donor population. 18
2.2. Measuring ABO blood group sensitization using phenotype frequencies
We computed population‐specific ABO phenotype frequencies using deceased OPTN kidney donors recovered from 2000 to 2021. ABO frequencies were similar to a previous US blood banking dataset. 22 In this analysis, candidates of blood group O are considered to be sensitized to donors with A, B, and AB phenotypes. Candidates of blood group A are sensitized to donors with B and AB phenotypes. Candidates of blood group B are sensitized to donors with A or AB phenotypes. Candidates of blood group AB are not sensitized to antigens in the ABO system.
2.3. The ABO‐adjusted cPRA metric and web calculator
Figure 1 is a schematic depicting the approach used to compute the ABO‐adjusted cPRA for each waitlisted candidate: ABO‐adjusted cPRA value is computed by upward adjusting the candidate's conventional HLA cPRA by the remaining fraction of HLA‐compatible donors that are ABO‐incompatible (Figure 1). In the illustrated example, a blood group O candidate with unacceptable antigen HLA‐DR53 has an HLA cPRA of 50%. Among the remaining fraction of the HLA‐compatible donor pool however, the candidate is incompatible with blood group A, B, and AB donors. This is termed ABOadj to represent the percentage of donor pool that is HLA‐compatible but ABO‐incompatible with the candidate. ABOadj is calculated by multiplying the percentage of donor pool that is HLA‐compatible (indicated by 100% ‐ cPRAHLA) with the phenotype frequency of donor pool that is ABO‐incompatible with the candidate (PhenoFreqiABO). The ABO‐adjusted cPRA is the sum of cPRAHLA and ABOadj = 50% + 26% = 76%.
FIGURE 1.
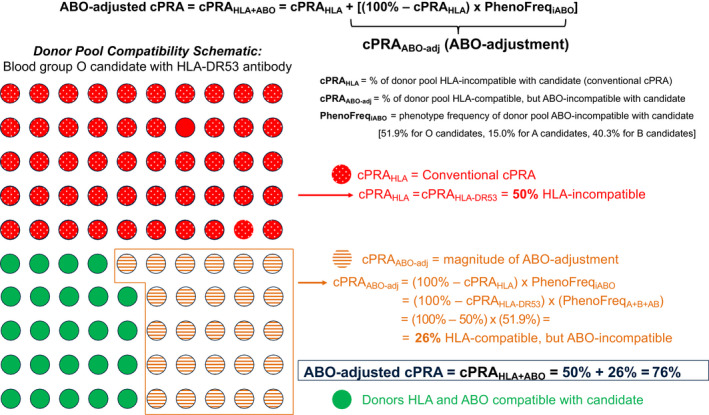
Schematic depicting the approach of computing ABO‐adjusted cPRA in an illustrated example (blood group O candidate with HLA‐DR53 antibody). There are 100 circles in a 10 × 10 grid, each representing 1% of the deceased donor pool. The dotted red circles denote donors that carry HLA‐DR53 and thus are HLA incompatible with the candidate. Orange circles with horizontal lines denote donors that are HLA compatible but ABO incompatible with the candidate. The candidate's ABO‐adjusted cPRA is the sum of all immunologically incompatible donors (red + orange circles). The proportion of immune compatible donors for a candidate of blood group O with HLA unacceptable antigen of HLA‐DR53 is shown in green. [Color figure can be viewed at wileyonlinelibrary.com]
The example calculation in Figure 1 is slightly simplified because ethnic‐specific cPRA values are calculated first, then an overall cPRA value is computed based on a weighted average using the ethnic composition of the deceased donor pool (Table 1). Because there are differences among ethnic categories in both ABO and HLA frequencies, these two genetic systems are not completely independent in the ABO‐adjusted cPRA metric.
TABLE 1.
ABO blood group phenotype frequencies by US kidney donor race/ethnic group
| OPTN ethnic category | Deceased donor ethnic proportions (%) | ABO blood group phenotype proportions (%) | |||
|---|---|---|---|---|---|
| O | A | B | AB | ||
| White | 66.71 | 45.3 | 41.4 | 9.9 | 3.4 |
| Black | 14.61 | 50.7 | 25.8 | 19.7 | 3.9 |
| Hispanic | 14.43 | 59.2 | 29.4 | 9.4 | 2.0 |
| Asian | 2.49 | 39.7 | 26.9 | 26.9 | 6.6 |
| Multi‐ethnic | 0.85 | 51.4 | 33.4 | 12.5 | 2.7 |
| Native American | 0.62 | 58.9 | 32.0 | 6.3 | 2.9 |
| Hawaiian and Pacific Islander | 0.29 | 46.1 | 33.3 | 16.8 | 3.8 |
Note: Blood group phenotype frequencies based on 190 954 OPTN deceased kidney/pancreas donors recovered from 2000 to 2021.
A web calculator for ABO‐adjusted cPRA calculations was developed using the Python Django web application framework and is publicly available at http://transplanttoolbox.org/abo_hla_cpra. The input to the calculator is a combination of candidate's unacceptable HLA antigens and their ABO phenotype. The output is the fraction of the donor pool that will be incompatible based on candidate's HLA and/or ABO sensitization.
2.4. Waitlist analysis of kidney candidate unacceptable antigens with ABO adjustment
ABO‐adjusted cPRA values were analyzed for a data set of 190 467 candidates with listings in 2019 and 2020, based on the candidate ABO phenotype and their most recent listed unacceptable HLAs. 20 cPRA values were computed for both ABO‐adjusted cPRA and HLA cPRA alone. Scatterplots and point‐group reclassification matrices were generated to illustrate the impact of merging ABO into an overall cPRA metric for antibody sensitization.
3. RESULTS
3.1. Both ABO blood group and HLA phenotype frequencies vary by population
We computed population‐specific ABO blood group frequencies based on 190 954 OPTN deceased kidney donors recovered from 2000 to 2021. In Table 1, we provide ethnic group proportions for the seven categories used for the proposed HLA cPRA as well as ABO phenotype frequencies within each category.
3.2. A unified metric for immunologic compatibility would result in relative increases in cPRA based on candidate ABO sensitization
Candidate ABO sensitization based on natural antibodies was computed from population‐specific ABO phenotype frequencies using a weighted average given the ethnic composition of the donor pool, provided in Table 2. For O candidates, we find that 51.9% of HLA‐compatible donors are ABO‐incompatible; for B candidates 40.3% of donors are ABO‐incompatible; and for A candidates 15.0%. For AB candidates, their ABO‐adjusted cPRA is the same as their HLA cPRA.
TABLE 2.
Frequency of incompatible donor ABO phenotypes based on candidate's ABO blood group
| Candidate ABO blood group | Incompatible donor ABO phenotypes | Frequency of incompatible donor ABO phenotypes |
|---|---|---|
| O | A + B + AB | 51.9% |
| A | B + AB | 15.0% |
| B | A + AB | 40.3% |
| AB | Not sensitized | 0.0% |
Note: Based on each candidate ABO blood group phenotype category, the incompatible ABO donor phenotypes and their frequencies in the US deceased donor pool population are shown.
3.3. ABO adjustment leads to an increase in cPRA values relative to HLA cPRA alone and most candidates would move up to higher point groups
For the waitlist data set of 190 467 candidates' unacceptable HLAs, we plotted the ABO‐adjusted cPRA values versus the conventional HLA cPRA values (Figure 2). The figure illustrates the proportion of immune‐incompatible donors given both ABO and HLA, compared with the proportion incompatible based on HLA antibodies alone. The relative increase in ABO‐adjusted cPRA, versus conventional HLA cPRA on the diagonal, is the most drastic for O candidates, where on average 51.9% of HLA‐compatible donors are ABO‐incompatible.
FIGURE 2.
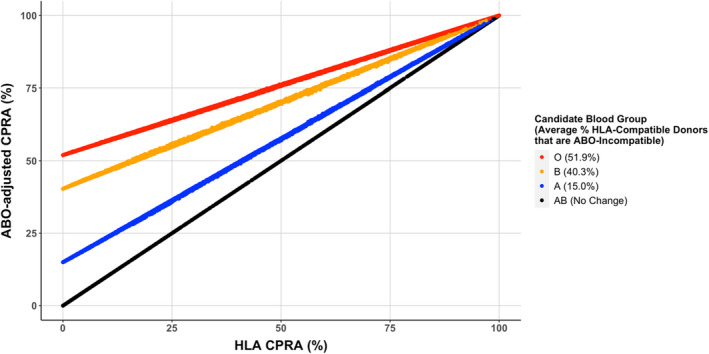
Scatterplot of kidney waitlist candidate unified ABO‐adjusted cPRA versus conventional HLA cPRA values. Candidate ABO blood groups are highlighted in different colors, and the ABO‐adjustment results in a differential average lift off the diagonal depending on blood group. [Color figure can be viewed at wileyonlinelibrary.com]
The majority of candidates would move to a higher point discrete group when ABO is considered with HLA sensitization (Figure 3 and Figure S1). The absolute change in cPRA is non‐uniform: candidates who are not HLA‐sensitized receive the maximum absolute increase in ABO‐adjusted cPRA value relative to candidates who have a high HLA cPRA at baseline. Overall, this ABO‐adjusted cPRA is expected to result in a significant shift toward more candidates being recognized as highly sensitized, an effect that is driven principally by blood group O and B candidates on the waitlist.
FIGURE 3.
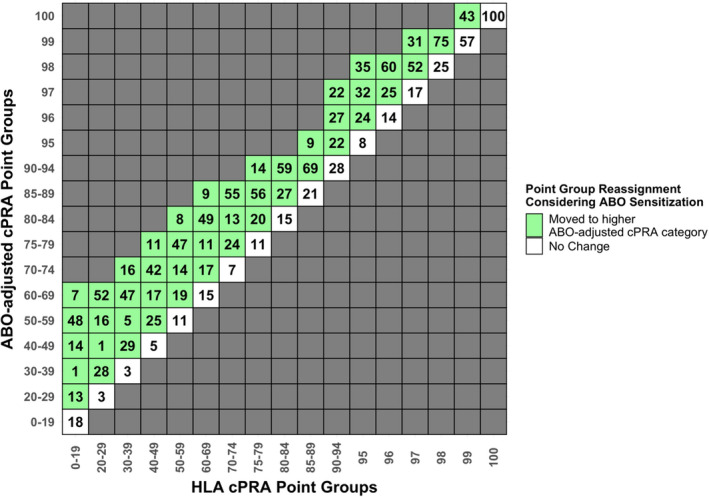
Percentage of waitlist candidates moving to higher point groups under the current system of discrete cPRA Point Group categories. Within each column representing the current HLA cPRA metric, the percentage of candidates who would remain in the same point group after ABO adjustment is shown in the white boxes on the diagonal. The percentage of candidates who would move up to higher point groups after ABO‐adjustment is shown in the green boxes. [Color figure can be viewed at wileyonlinelibrary.com]
Capturing the variable impact of candidate ABO could more equitably determine allocation points, especially for highly sensitized candidates near point group boundaries. For instance, within the conventional HLA cPRA Point Group = 99%, 43% of candidates are elevated to ABO‐adjusted cPRA Point Group = 100% after consideration of ABO sensitization, though this proportion varied substantially by candidate ABO blood group (Figure 4 dotted vertical line). After adjusting for ABO, nearly all O and most B candidates would move across the boundary between cPRA 99 and 100, compared with relatively few blood group A candidates and none that are AB.
FIGURE 4.
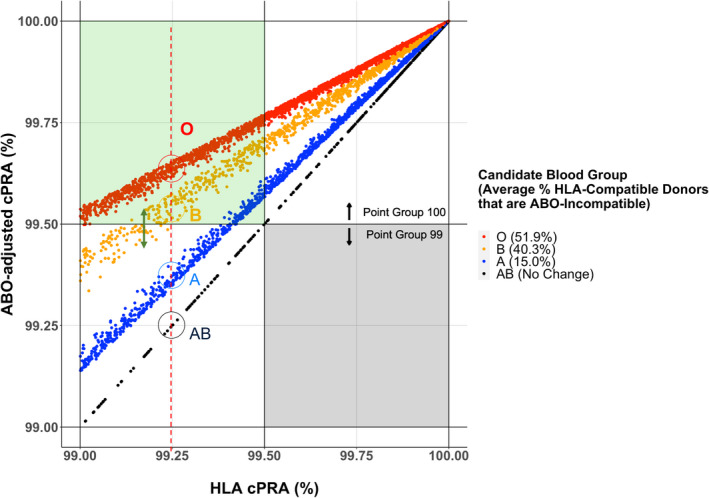
Scatterplot of ABO‐adjusted cPRA versus HLA cPRA values among highly sensitized waitlist candidates with HLA cPRA ≥ 99%. The dotted red line denotes the differential impact of the unified cPRA metric on candidates within different blood groups with the same HLA cPRA value. The green arrow indicates ethnic‐specific cPRA scatter due to the interaction of ABO and HLA frequencies. The green box indicates waitlist candidates currently in HLA cPRA Point Group 99 that move up to Point Group 100 after ABO adjustment. [Color figure can be viewed at wileyonlinelibrary.com]
While genes encoding ABO and HLAs reside on different chromosomes, there is some ethnic‐specific variation in HLA and ABO frequencies. We observed that candidates with the same HLA cPRA values and ABO group may have slightly different ABO‐adjusted cPRA depending on the specific combination of HLA alleles and blood groups, which manifests as a scatter from the diagonal line in Figure 2. Ethnic‐specific frequency variation may have a larger impact for highly HLA‐sensitized candidates, particularly in those where the cPRA values fall within the boundary of cPRA groups (i.e., 99%–100%) that qualify for increased allocation points and/or priority for broader geographical sharing (Figure 4, green arrow).
4. DISCUSSION
The Organ Procurement and Transplantation Network (OPTN) has proposed a continuous distribution framework for the organ allocation system where every candidate would receive a composite allocation score based on multiple factors that influence patient survival, medical urgency, biological compatibility, patient access, and placement efficiency. 23 OPTN committees are using an analytic hierarchy process to incorporate community input on weighting the importance of each factor relative to one another. The current KAS involves a multitude of allocation sequence classifications, resulting in hard categorical boundaries. For example, in OPTN Policies there are currently 43 allocation sequence classifications for kidney donor profile index (KDPI) < 20% determined in the match results based on donor and candidate factors. 17 The ABO and HLA sensitization metrics discussed here represent but two of the candidate factors that contribute to the biological compatibility component of continuous distribution, but these compatibility factors deserve special attention because of their outsized contributions to access disparity under the current system.
In this study, we present a framework to apply a novel unified metric of immune compatibility that accounts for both the candidate's HLA and ABO sensitization status. The standard HLA‐based cPRA metric provides only a limited quantification of access to immune‐compatible donors in the KAS, as kidney candidates do not currently receive allocation points based on blood group sensitization. Currently, AB candidates experience significantly higher transplant rates relative to candidates in other blood groups. 1 Under a unified cPRA metric for immunologic sensitization, AB candidates are not sensitized to blood group, thus only HLA antibodies can increase their ABO‐adjusted cPRA. Similarly, blood group A candidates who also have higher than average transplant rates, have a smaller ABO sensitization component that eliminates only 15.0% of HLA‐compatible donors on average, compared with 40.3% ABO sensitization for B candidates and 51.9% for O candidates.
In the United States, deceased donor kidneys are primarily allocated to ABO‐identical waitlist candidates with the exception of (1) blood type O donors into non‐O candidates who have zero‐ABDR mismatches with the donor; (2) blood type B donors to non‐B candidates who have zero‐ABDR mismatches with the donor; (3) blood type A donors into AB candidates; and (4) blood type A, non‐A1 and AB, non‐A1B donors into B candidates. 17 Equitable sharing and allocation of these blood group permissible but non‐identical kidneys requires consideration of the combined HLA + ABO sensitization status of the candidate. This is currently not possible with standard cPRA measurements, which fail to account for ABO disparity. Applying the ABO‐adjusted cPRA metric to waitlist candidates would result in more O and B candidates receiving points for their ABO disadvantage, with some receiving higher priority for broader geographic allocation, proportionate with their limited donor pool.
The relevance and utility of the ABO‐adjusted cPRA metric is even greater as OPTN considers a more flexible continuous distribution model which aims to remove hard boundaries between allocation sequence categories. This is particularly important for the most disadvantaged groups—such as extremely highly sensitized and medically urgent patients—who are currently precluded from being offered otherwise HLA and ABO compatible donors that are blood group non‐identical. For instance, a blood group A candidate with 99.99% cPRA is currently unable to receive an HLA‐ and ABO‐compatible blood group O donor offer, outside of being 0‐ABDR mismatched with the donor. With these ABO policy restrictions removed, some of the more highly sensitized or medically urgent non‐O candidates could access O donors ahead of less sensitized O candidates. Critically, removal of this restriction can only be implemented equitably if the totality of immune compatibility with ABO and HLA are considered in the same metric.
As the transplant community considers allowing ABO‐compatible transplants to maximize transplant opportunities especially for currently disadvantaged populations, it is important to safeguard against inappropriate diversion of blood group O donors to non‐O candidates. Equity is a critically important consideration, as 53.7% of the candidates on the US kidney waitlist are blood group O, of which 67.4% are identified as ethnic minorities based on OPTN data as of July 7, 2022. While a previous analysis found that ethnicity had little influence on disparity in access after adjusting for blood group and other candidate factors, 1 42.1% of candidates in the most disadvantaged B blood group are of Black ethnicity, even though Black patients represent 31% of kidney candidates. A major function of the ABO‐adjusted cPRA metric is that it would ensure vulnerable patient groups that are blood group O and B and also highly HLA‐sensitized would be appropriately recognized for their overall immune sensitization. Thus, in terms of the contribution of candidate biology to the composite allocation score under continuous distribution, non‐O candidates would only outrank O candidates in scenarios where the donor pool for the non‐O candidates is smaller based on combined HLA and ABO factors.
Our analysis reveals that the ABO‐adjusted cPRA metric is a better representation of a candidate's overall immune compatibility than conventional HLA cPRA. Nonetheless, prospective modeling to determine the impact of any policy changes on equity is essential prior to implementation. Given the complexity of the current US KAS and the proposed major changes that will soon be incorporated into continuous distribution, effective modeling will best be accomplished through application of the SRTR kidney‐pancreas simulated allocation model (KPSAM) package. 24 If prioritized by OPTN, we anticipate that modeling will find that ABO‐adjusted cPRA yields more equitable transplant rates than HLA cPRA alone for highly sensitized candidates. Such modeling would necessitate removal of policy rules on the current stringent definition of ABO permissibility. Alternatively, informative modeling could also be achieved using datasets from other systems where kidneys are already allocated to ABO‐compatible candidates 19 (rather than ABO‐identical)—this schema is more representative of the proposed KAS under continuous distribution and may serve to complement US KPSAM modeling results.
In summary, we describe an approach to removing policy restrictions against ABO compatible transplants that might decrease disparity in access among ABO blood groups while at the same time improve access for candidates most broadly sensitized against HLAs. Removing such restrictions could also conceivably increase organ utilization by providing organ procurement organizations more potential “homes” for hard‐to‐place blood type O and B kidneys. Our proposed unified immunologic sensitization metric, the “ABO‐adjusted” cPRA, has the potential to be an integral component of OPTN's continuous distribution framework as the transplant community works toward a more equitable allocation system.
DISCLOSURE
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. Dr. Gragert was supported by an OPTN subcontract for “Update CPRA Calculator.”
Supporting information
Figure S1
ACKNOWLEDGMENTS
NIAID 1U01AI152960‐01 “MHC and KIR Sequencing and Association Analyses in the iGeneTRAiN Studies.” This work was made possible by a Scholarly Retreat at Tulane's A Studio in the Woods. JHL is supported by a Michael Smith Foundation for Health Scholar award. MK is supported by a Michael Smith Health Professional Investigator award. This analysis was based on OPTN data as of March 13, 2022. This work was supported in part by Health Resources and Services Administration contract HHSH250‐2019‐00001C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Gragert L, Kadatz M, Alcorn J, et al. ABO‐adjusted calculated panel reactive antibody (cPRA): A unified metric for immunologic compatibility in kidney transplantation. Am J Transplant. 2022;22:3093‐3100. doi: 10.1111/ajt.17175
DATA AVAILABILITY STATEMENT
Python and R scripts to perform secondary data analysis on the OPTN / UNOS STAR dataset will be made available upon request.
REFERENCES
- 1. Stewart DE, Wilk AR, Toll AE, et al. Measuring and monitoring equity in access to deceased donor kidney transplantation. Am J Transplant. 2018;18(8):1924‐1935. [DOI] [PubMed] [Google Scholar]
- 2. Kulkarni S, Ladin K, Haakinson D, Greene E, Li L, Deng Y. Association of racial disparities with access to kidney transplant after the implementation of the new kidney allocation system. JAMA Surg. 2019;154(7):618‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hamoda RE, McPherson LJ, Lipford K, et al. Association of sociocultural factors with initiation of the kidney transplant evaluation process. Am J Transplant. 2020;20(1):190‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ku E, McCulloch CE, Adey DB, Li L, Johansen KL. Racial disparities in eligibility for preemptive Waitlisting for kidney transplantation and modification of eGFR thresholds to equalize waitlist time. J Am Soc Nephrol. 2021;32(3):677‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhou S, Massie AB, Luo X, et al. Geographic disparity in kidney transplantation under KAS. Am J Transplant. 2018;18(6):1415‐1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gebel HM, Kasiske BL, Gustafson SK, et al. Allocating deceased donor kidneys to candidates with high panel‐reactive antibodies. Clin J Am Soc Nephrol. 2016;11(3):505‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gebel HM, Bray RA. HLA antibody detection with solid phase assays: great expectations or expectations too great? Am J Transplant. 2014;14(9):1964‐1975. [DOI] [PubMed] [Google Scholar]
- 8. Sapir‐Pichhadze R, Tinckam KJ, Laupacis A, Logan AG, Beyene J, Kim SJ. Immune sensitization and mortality in wait‐listed kidney transplant candidates. J Am Soc Nephrol. 2016;27(2):570‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jackson KR, Covarrubias K, Holscher CM, et al. The national landscape of deceased donor kidney transplantation for the highly sensitized: transplant rates, waitlist mortality, and posttransplant survival under KAS. Am J Transplant. 2019;19(4):1129‐1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stewart DE, Kucheryavaya AY, Klassen DK, Turgeon NA, Formica RN, Aeder MI. Changes in deceased donor kidney transplantation one year after KAS implementation. Am J Transplant. 2016;16(6):1834‐1847. [DOI] [PubMed] [Google Scholar]
- 11. Cecka JM, Kucheryavaya AY, Reinsmoen NL, Leffell MS. Calculated PRA: initial results show benefits for sensitized patients and a reduction in positive crossmatches. Am J Transplant. 2011;11(4):719‐724. [DOI] [PubMed] [Google Scholar]
- 12. Friedewald JJ, Samana CJ, Kasiske BL, et al. The kidney allocation system. Surg Clin North Am. 2013;93(6):1395‐1406. [DOI] [PubMed] [Google Scholar]
- 13. Israni AK, Salkowski N, Gustafson S, et al. New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. J Am Soc Nephrol. 2014;25(8):1842‐1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Organ Transplantation Report on Equity in Access 2016. Accessed January 31, 2022. https://optn.transplant.hrsa.gov/media/2842/equity_in_access_report_201611.pdf.
- 15. Maldonado AQ, Sjöholm K, Lee J, Olsson H, Kjellman C, Stewart DE. Beyond CPRA: identifying sensitized kidney candidates with markedly low access to deceased donor transplantation by granular CPRA and blood type. OBM Transplantation. 2021;5(2):1. [Google Scholar]
- 16. Bryan CF, Cherikh WS, Sesok‐Pizzini DA. A2 /A2 B to B renal transplantation: past, present, and future directions. Am J Transplant. 2016;16(1):11‐20. [DOI] [PubMed] [Google Scholar]
- 17. Organ Procurement and Transplantation Network (OPTN) Policies. Effective Date: 09/01/2021. Accessed January 31, 2022. https://optn.transplant.hrsa.gov/media/eavh5bf3/optn_policies.pdf.
- 18. Proposal to Update the Calculated PRA (CPRA). OPTN Histocompatibility Committee Report to the Board of Directors. 14 November 2011.
- 19. Tinckam KJ, Liwski R, Pochinco D, et al. cPRA increases with DQA, DPA, and DPB unacceptable antigens in the Canadian cPRA calculator. Am J Transplant. 2015;15(12):3194‐3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kransdorf EP, Pando MJ, Stewart D, et al. Stem cell donor HLA typing improves CPRA in kidney allocation. Am J Transplant. 2021;21(1):138‐147. [DOI] [PubMed] [Google Scholar]
- 21. Notice of OPTN Policy Changes: Change Calculated Panel Reactive Antibody (CPRA) Calculation. Accessed March 25, 2022. https://optn.transplant.hrsa.gov/media/nlqdyd1o/policy‐notice_change‐cpra‐calculation_histo.pdf.
- 22. Garratty G, Glynn SA, McEntire R, Study RED . ABO and rh(D) phenotype frequencies of different racial/ethnic groups in the United States. Transfusion. 2004;44(5):703‐706. [DOI] [PubMed] [Google Scholar]
- 23. Organ Procurement and Transplantation Network Continuous Distribution. Accessed January 31, 2022. https://optn.transplant.hrsa.gov/governance/key‐initiatives/continuous‐distribution/.
- 24. Scientific Registry of Transplant Recipients Simulated Allocation Models. Accessed February 23, 2022. https://www.srtr.org/requesting‐srtr‐data/simulated‐allocation‐models/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Data Availability Statement
Python and R scripts to perform secondary data analysis on the OPTN / UNOS STAR dataset will be made available upon request.


