Summary
Background
Primary cutaneous peripheral T‐cell lymphomas with a T‐follicular helper phenotype (pcTFH‐PTCL) are poorly characterized, and often compared to, but not corresponding with, mycosis fungoides (MF), Sézary syndrome, primary cutaneous CD4+ lymphoproliferative disorder, and skin manifestations of angioimmunoblastic T‐cell lymphomas (AITL).
Objectives
We describe the clinicopathological features of pcTFH‐PTCL in this original series of 23 patients, and also characterize these cases molecularly.
Methods
Clinical and histopathological data of the selected patients were reviewed. Patient biopsy samples were also analysed by targeted next‐generation sequencing.
Results
All patients (15 men, eight women; median age 66 years) presented with skin lesions, without systemic disease. Most were stage T3b, with nodular (n = 16), papular (n = 6) or plaque (atypical for MF, n = 1) lesions. Three (13%) developed systemic disease and died of lymphoma. Nine (39%) patients received more than one line of chemotherapy. Histologically, the lymphomas were CD4+ T‐cell proliferations, usually dense and located in the deep dermis (n = 14, 61%), with the expression of at least two TFH markers (CD10, CXCL13, PD1, ICOS, BCL6), including three markers in 16 cases (70%). They were associated with a variable proportion of B cells. Eight patients were diagnosed with an associated B‐cell lymphoproliferative disorder (LPD) on biopsy, including Epstein–Barr virus (EBV)‐positive diffuse large B‐cell lymphoma (n = 3), EBV+ LPD (n = 1) and monotypic plasma cell LPD (n = 4). Targeted sequencing showed four patients to have a mutated TET2–RHOAG17V association (as frequently seen in AITL) and another a TET2/DNMT3A/PLCG1/SETD2 mutational profile. The latter patient, one with a TET2–RHOA association, and one with no detected mutations, developed systemic disease and died. Five other patients showed isolated mutations in TET2 (n = 1), PLCG1 (n = 2), SETD2 (n = 1) or STAT5B (n = 1).
Conclusions
Patients with pcTFH‐PTCL have pathological and genetic features that overlap with those of systemic lymphoma of TFH derivation. Clinically, most remained confined to the skin, with only three patients showing systemic spread and death. Whether pcTFH‐PTCL should be integrated as a new subgroup of TFH lymphomas in future classifications is still a matter of debate.
What is already known about this topic?
There is a group of cutaneous lymphomas that express T‐follicular helper (TFH) markers that do not appear to correspond to existing World Health Organization diagnostic entities.
These include mycosis fungoides, Sézary syndrome, or primary cutaneous CD4+ small/medium‐sized T‐cell lymphoproliferative disorder or cutaneous extensions of systemic peripheral T‐cell lymphomas (PTCL) with TFH phenotype.
What does this study add?
This is the first large original series of patients with a diagnosis of primary cutaneous PTCL with a TFH phenotype (pcTFH‐PTCL) to be molecularly characterized.
pcTFH‐PTCL may be a standalone group of cutaneous lymphomas with clinicopathological and molecular characteristics that overlap with those of systemic TFH lymphomas, such as angioimmunoblastic T‐cell lymphoma, and does not belong to known diagnostic groups of cutaneous lymphoma.
This has an impact on the treatment and follow‐up of patients; the clinical behaviour needs to be better clarified in further studies to tailor patient management.
There is a group of cutaneous lymphomas that express TFH markers that do not appear to correspond to existing WHO diagnostic entities. This is the first large original series of patients with a diagnosis of primary cutaneous peripheral T‐cell lymphomas with a T‐follicular helper phenotype (pcTFH‐PTCL) to be molecularly characterised. pcTFH‐PTCL may be a standalone group of cutaneous lymphomas with clinicopathological and molecular characteristics that overlap with those of systemic TFH lymphomas, and does not belong to known diagnostic groups of cutaneous lymphoma.
Linked Comment: W. Kempf. Br J Dermatol 2022; 187:841–842.
Plain language summary available online
Among lymphomas with nodal presentation commonly associated with systemic symptoms, three groups of lymphomas of T‐follicular helper (TFH) cell origin are recognized in the current World Health Organization (WHO) classification: angioimmunoblastic T‐cell lymphoma (AITL), follicular T‐cell lymphoma and other nodal peripheral T‐cell lymphoma (PTCL) with a TFH phenotype. 1 The TFH phenotype is defined as the detection of at least two, ideally three, TFH markers among CD10, CXCL13, PD1, ICOS and BCL6. 1 The three lymphomas show similar genetic profiles, characterized by recurrent mutations in the TET2, DNMT3A and RHOA genes. 2 Skin involvement may occur in all three subtypes, and is especially common in AITL. 3 Among cutaneous T‐cell lymphomas (CTCLs), the classification does not yet include a specific category for those with a TFH phenotype. Sézary syndrome (SS) and transformed mycosis fungoides (MF) are known to express PD1. 4 , 5 Other studies show the rate for full TFH marker expression (three of five) to be 19–56%. 6 , 7 Primary cutaneous CD4+ small‐/medium‐sized cell lymphoproliferative disorder (pcSMLPD), a disease generally presenting as solitary, self‐contained lesions with distinctly indolent behaviour, is also considered to belong to the TFH lineage, 8 , 9 , 10 , 11 although it does not share the genetic mutations reported in systemic TFH‐PTCL. 12
A subset of lymphoma with predominant or primary cutaneous presentation bearing a TFH phenotype (pcTFH‐PTCL) cannot be reliably classified into any of the recognized entities of the current WHO classification. They are likely to differ from MF, SS and pcSMLPD by clinical presentation of lesions (multiple, with varied appearances, not restricted to patches/plaques), their histology and their outcomes (protracted disease with sometimes aggressive evolution), and, in contrast with other PTCLs, are defined by the absence of systemic disease and nodal involvement. Until now, molecular alterations associated with these lymphomas are largely unknown from published cases. 13 , 14 , 15 , 16 Buder et al. have described a case that has failed to respond to multiple lines of treatment, highlighting the need for further characterization to help in patient care. 5
This prompted us to undertake a comprehensive clinical, pathological and molecular study of 23 cases of pcTFH‐PTCL collected within the framework of the French Study Group of Cutaneous Lymphomas network (Groupe Francais d’Etude des Lymphomes Cutanés, GFELC), with the aim to (i) better define their clinical and pathological presentation, (ii) characterize their molecular alterations, and (iii) discuss how they compare with other PTCLs with a TFH derivation.
Materials and methods
Patient selection and data recording
Initial case screening from the databases of the GFELC from 2010 to 2021 targeted histological diagnoses of PTCL with a TFH phenotype with a primarily cutaneous presentation. Multiple cutaneous lesions must have been present at diagnosis or during follow‐up (to exclude cases of pcSMLPD). Tissue biopsies must have shown evidence of neoplastic T‐cell infiltration, T‐cell antigen loss (among CD3, CD5, CD2 and CD7) and/or a clonal T‐cell population, with expression of at least two TFH markers in neoplastic T cells among CD10, CXCL13, BCL6, ICOS and PD1. No patient had been included in previous studies. The study was registered and performed according to the guidelines of the French Bioethics Law for retrospective noninterventional research studies after the absence of objection. The GFELC database was ruled by the French data protection authority (CNIL, Commission Nationale de l’Informatique et des Libertés) in 2017 to be compliant with legislation on the confidentiality and proper use of individual patient data.
A second selection was performed after pathological review using inclusion criteria based on clinical and follow‐up information (Figure 1). The criteria were designed with particular care to eliminate cases that may have raised differential diagnostic issues with early MF (typical patch/plaques) or SS (erythroderma); it was also ensured that they did not meet criteria for systemic TFH lymphoma by the absence of concomitant nodal/systemic disease at diagnosis.
Figure 1.
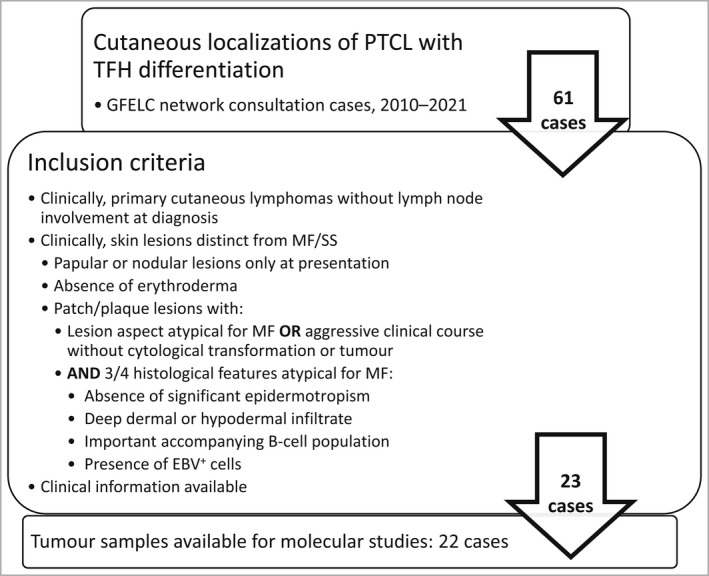
Case selection and inclusion criteria. EBV, Epstein–Barr virus; GFELC, Groupe Francais d’Etude des Lymphomes Cutanés [French Study Group of Cutaneous Lymphomas network]; MF, mycosis fungoides; PTCL, peripheral T‐cell lymphoma; TFH, T‐follicular helper; SS; Sézary syndrome.
A standardized form, sent to the physicians in charge of the patients, was used to collect clinical, demographic and laboratory data. Tumour–node–metastasis (TNM) staging for non‐MF/Sézary CTCL 17 was recorded based on clinical examination and computed or positron emission tomography imaging. The date of the initial diagnosis for follow‐up, treatments and disease progression data was defined as the date of the first histopathological report of lymphoma.
Morphological and immunophenotypic studies
All patient cases were signed out by expert dermatopathologists from the GFELC network, including co‐authors in this paper, in accordance with routine diagnostic procedures. Slides were stained with haematoxylin–eosin–saffron. Immunohistochemistry panels were performed according to the usual diagnostic standards for lymphoma. Immunohistochemical systems and antibodies were all validated for routine clinical diagnostic use. In situ hybridization (ISH) for Epstein–Barr virus (EBV) using Epstein–Barr encoding region (EBER) probes and kappa and lambda light chains was performed as previously described. 3
Slides and pathology reports of the initial diagnostic biopsy were reviewed and the data collected using a standardized datasheet. Expression of the phenotypic markers was scored semi‐quantitatively based on the proportion of positive cells within the T‐cell infiltrate (0, no staining; 1, < 10%; 2, 10–50%; 3, > 50%). Each TFH marker was scored positive when present in > 10% of the cells (scores 2 or 3). The CD4/CD8 ratio was estimated visually.
Clonality studies and molecular characterization
DNA was extracted from formalin‐fixed paraffin‐embedded (FFPE) skin biopsies (initial diagnosis) using Maxwell 16 IVD and Maxwell 16 FFPE Plus LEV DNA Purification Kits (Promega; Madison, WI, USA) according to the manufacturer’s instructions. T‐ and B‐cell clonality studies were performed as previously reported. 18 Targeted gene sequencing was performed, as previously reported, 19 on an Ion S5 platform (ThermoFisher Scientific; Waltham, MA, USA) at an expected depth of 1000x with an amplicon‐based library constitution based on a custom panel of the main recurrently mutated genes in PTCL, especially AITL and other nodal PTCLs of TFH phenotype (TET2, DNMT3A, IDH2, RHOA, CD28, SETD2, PLCG1, STAT3 and STAT5B).
Statistical analysis
All characteristics are described by patient. Categorical variables are reported as numbers and percentages, and continuous variables as means and medians. Two groups were compared in terms of aggressiveness of the disease. The aggressive group was defined as death due to disease, development of systemic disease or progression after one line of chemotherapy. Statistical tests consisted of Fisher exact or χ2 tests for categorical variables and Wilcoxon tests for continuous variables, with P‐value cut‐off of 0·05. Analyses were performed using the Stataid package in the R environment. 20
Results
Clinical features and disease course
The clinical features of the patients are summarized in Table 1. The patients were eight women and 15 men, with a mean age of 67 years and median of 66 (range 25–95) years. Skin lesions were predominantly nodules (n = 16, 70%) or papules (n = 6, 26%), whereas plaques were observed in one patient. In this last patient (# 9), the plaques were raised, thick and purple, which did not favour the diagnosis of MF. All but one patient (# 15) had multiple lesions at presentation, with a predominance of stage T3 (T3b: n = 13, 57%; T3a: n = 3, 13%; T1–2: n = 7, 30%). The initial histological diagnosis of patient 15 was not compatible with pcSMLPD, and he eventually developed other skin lesions. Sixteen patients did not have systemic symptoms [Eastern Cooperative Oncology Group (ECOG) 0, 70%], whereas B symptoms were present for seven (30%). In accordance with our inclusion criteria, no patients had nodal or extracutaneous tissue involvement at initial staging. Two patients (# 15 and 21) had detectable peripheral blood populations of abnormal T cells by flow cytometry, without lymphocytosis, phenotypically consistent with the neoplastic cells found in the skin, and remained without any other systemic manifestations during follow‐up. Serum lactate dehydrogenase levels, when available, were normal for most patients (n = 9 of 13, 69%). Other notable findings at diagnosis included antineutrophil cytoplasmic antibodies detected in one patient (# 17).
Table 1.
Features of patients with pcTFH‐PTCL at first histopathological diagnosis
| Pt | Age (y)/Sex | Lesion type | Distribution of lesions | T stage (TNM) | ECOG PS scale | B symptoms | Serum LDH | Other notable features | Composite/synchronous B‐cell LPD | Duration of FU | Status at latest FU | Systemic spread during FU | Treatments (in succession) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 84/F | Nodule | Thigh | T2a | 0 | – | NA | EBV+ DLBCL | 19 mo | CR | R‐CHOP | ||
| 2 | 84/F | Plaque/ papules | Abdomen | T2b | 0 | 0 | EBV+ B‐cell LPD | 48 mo | DOD | Nodes, lungs | MTX, BEX, IFN, RTx, GEM, romidepsin | ||
| 3 | 95/F | Nodule | Trunk, upper + lower limbs | T3b | 0 | – | NA | EBV+ DLBCL | 12 mo | DOC (myeloma) | No treatment | ||
| 4 | 55/M | Nodule | Trunk | T3b | 0 | – | Normal | Clonal PC LPD | 16 mo | Pro | Gemzar | ||
| 5 | 79/M | Nodule | Disseminated lesions | T3b | 3 | + | 2.2N | – | 12 mo | DOD | Nodes, liver, bloodb | TS, BEX, CHOP | |
| 6 | 74/F | Nodule | Trunk, upper limbs, head | T3b | 0 | – | Normal | Clonal PC LPD | 38 mo | CR | TS | ||
| 7 | 78/M | Nodule | Face, upper + lower limbs | T3b | 1 | + | Normal | – | 37 mo | PR | BEX, RTx | ||
| 8 | 59/F | Papules | Thorax | T2a | 0 | + | NA | – | 3 mo | CR | TS | ||
| 9 | 57/F | Plaque (atypical for MF) | Neck, scalp, trunk | T3b | 1 | + | Normal | EBV+ DLBCL | 43 mo | PR | MTX, BEX, doxorubicin, GEM, IFN | ||
| 10 | 54/F | Nodule | Trunk, head | T2a | 2 | – | 1.6N | Clonal PC LPD | 12 mo | S | TS, MTX | ||
| 11 | 58/M | Nodule | NA | T3b | NA | NA | NA | Clonal PC LPD | 307 mo | Pro | No treatment | ||
| 12 | 54/M | Nodule | Trunk, lower limbs | T3b | 0 | – | 1.4N | – | 98 mo | DOD | Nodes, BM, meningealb | R‐CVP, CHOP, bortezomib, lenalinomide, GEM, RTx, IFN, bendamustine | |
| 13 | 52/M | Papules | Lower limbs | T3a | 0 | – | Normal | – | 74 mo | S | No treatment | ||
| 14 | 64/M | Nodule | Upper + lower limbs | T3a | 0 | – | NA | Clonal PC LPD | 78 mo | CR | CHOP, VRD, AllHSCT | ||
| 15 | 79/M | Nodule | Chest a | T1a | 0 | – | NA | PB population | – | 72 mo | PR | TS, MTX, GEM, PTx, thalidomide, mini‐CHP | |
| 16 | 78/M | Nodule | Legs | T2a | 0 | – | NA | – | 20 mo | CR | CHL, TS | ||
| 17 | 75/F | Papules | Lower limbs, face | T3b | 0 | + | 1.5N | ANCA | – | 42 mo | S | PTx | |
| 18 | 26/M | Nodule | Back, upper limbs | T3b | 0 | – | Normal | – | 18 mo | S | TS | ||
| 19 | 57/M | Nodule | Trunk, lower limbs | T3b | 0 | – | Normal | – | 32 mo | CR | TS | ||
| 20 | 80/M | Papules | Trunk, thigh, abdomen | T3b | 2 | + | NA | – | 16 mo | Pro | TS | ||
| 21 | 61/M | Nodule | Upper + lower limbs, abdomen, face | T3b | 0 | – | NA | PB population | 17 mo | CR | CHOP, stem‐cell autograft | ||
| 22 | 64/M | Papules | Lower limbs | T2a | 0 | 0 | Normal | – | 72 mo | PR | TS, MTX, PTx, CHL, BEX | ||
| 23 | 68/M | Nodule | Trunk | T2c | 2 | + | Normal | – | 30 mo | CR | MTX |
Developed other skin lesions during follow‐up; bBiopsy‐confirmed systemic involvement.
AllHSCT, allogeneic haematopoietic stem‐cell transplantation; ANCA, antineutrophil cytoplasmic antibodies; BEX, bexarotene; BM, bone marrow; CHL, chlormethine; CHP, cyclophosphamide, doxorubicin, prednisone; CR, complete remission; DLBCL, diffuse large B‐cell lymphoma; DOC, died from other cause; DOD, died from disease; ECOG, Eastern Cooperative Oncology Group; FU, follow‐up; GEM, gemcitabine; IFN, interferon‐alpha; LDH, lactate dehydrogenase; LPD, lymphoproliferative disorder; mo, months; MF, mycosis fungoides; MTX, methotrexate; N, serum LDH ratio to normal laboratory value; NA, not available; PB, peripheral blood; PC, plasma cell; pcTFH‐PTCL, primary cutaneous peripheral T‐cell lymphomas with a T‐follicular helper phenotype; PR, partial remission; Pro, progression; PS, performance status; Pt, patient; PTx, phototherapy; (R‐)CHOP, (rituximab), cyclophosphamide, doxorubicin, vincristine, prednisone; R‐CVP, rutiximab, cyclophosphamide, vincristine, prednisone; RTx, radiotherapy; S, stable disease; TNM, tumour–node–metastasis; TS, topical steroids; VRD, bortezomib, lenalidomide, dexamethasone; y, years
With a median follow‐up of 32 (6–307) months, three patients (# 2, 5 and 12) developed extracutaneous lymphoma and died of the disease. Another patient (# 3), with an indolent skin disease evolution (spontaneous regression of lesions), died from multiple myeloma. Eight patients were in complete remission at the end of the follow‐up period and four in partial remission. Treatment allowed stabilization of the lesions for four patients and three had progressive disease. In total, nine patients required more than one line of chemotherapy.
Histopathological and phenotypic features
Twenty‐nine skin biopsies at diagnosis were analysed for the 23 patients (Figure 2, Table S1; see Supporting Information). The architectural patterns included dense and diffuse in the deep dermis (14 of 23, 61%), subepidermal band‐like (five of 23, 22%), exclusively perivascular (three of 23, 13%) or nodular (one of 23, 4%); in addition, one patient showed a necrotic subcutaneous panniculitis‐like pattern. The infiltrate expanded into the hypodermis for 17 patients (74%). Significant epidermotropism was present for four patients (17%) (# 1, 8, 10 and 19) and most did not show epidermal changes (16 of 23, 70%). Follicular and/or adnexal involvement was seen for 10 patients (43%). Vascular changes included vascular hyperplasia for 12 patients (52%). Only two cases had a significant accompanying eosinophilic infiltrate.
Figure 2.
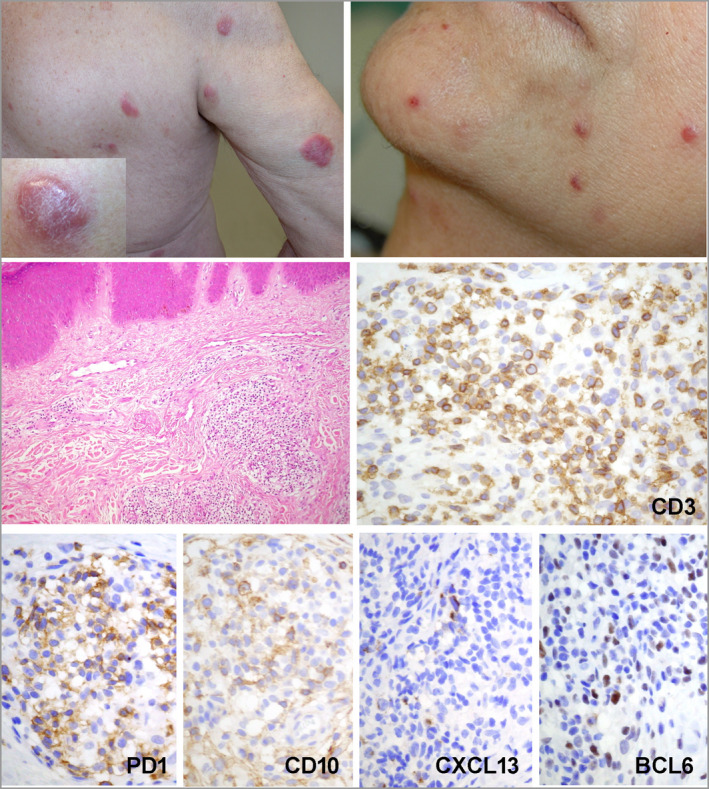
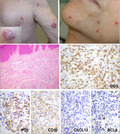
Representative clinical and histopathological aspects of pcTFH‐PTCL. Clinical photographs show multiple erythematous nodules on the trunk and the right arm in patient 5, with a ‘dome‐shaped’ erythematous and slightly squamous nodule, strongly resembling a pcSMLPD (inset). Erythematous papules on the cheek and chin were the manifestation of disease in patient 17. In patient 11’s biopsy, there is a dermal infiltrate, made of small/medium‐sized atypical lymphocytes. The neoplastic T cells show a full TFH phenotype, with positivity for CD3, PD1, CD10, CXCL13 and BCL6. pcTFH‐PTCL, primary cutaneous peripheral T‐cell lymphomas with a TFH phenotype; pcSMLPD, primary cutaneous CD4+ small‐/medium‐sized cell lymphoproliferative disorder; TFH, T‐follicular helper. [Colour figure can be viewed at wileyonlinelibrary.com]
The neoplastic T‐cell infiltrate was CD4+ in all cases. T‐cell antigen loss was seen in eight cases (35%), all with CD7 and one case with the additional loss of CD2. PD1 and ICOS were the most frequently expressed TFH markers, observed in 22 (96%) and 21 (91%) cases, respectively, vs. BCL6 (n = 11 of 18, 61%), CXCL13 (n = 15, 65%) and CD10 (n = 5 of 22, 23%). Sixteen cases (70%) significantly expressed three TFH markers, whereas the remaining cases had two out of five TFH markers. In three cases (# 4, 21 and 23), there was a significant subpopulation of CD30+ tumour cells, with one case showing sheets of large atypical CD30+ lymphocytes, together with strong diffuse expression of CXCL13, PD1 and ICOS. Seven cases showed a significant reactive CD8 population, and two cases showed a reversed CD4/CD8 ratio.
B cells were present in significant quantities for 16 patients (CD20 immunohistochemical score > 2). EBV+ large cells were observed by EBER ISH in six cases, of which three had overt EBV+ diffuse large B‐cell lymphoma (Figure 3) and one had an EBV+ polymorphic lymphoproliferative disorder. A monotypic plasma cell component was identified in another four cases, without correlating with the number of plasma cells present in the biopsy (Figure 4).
Figure 3.
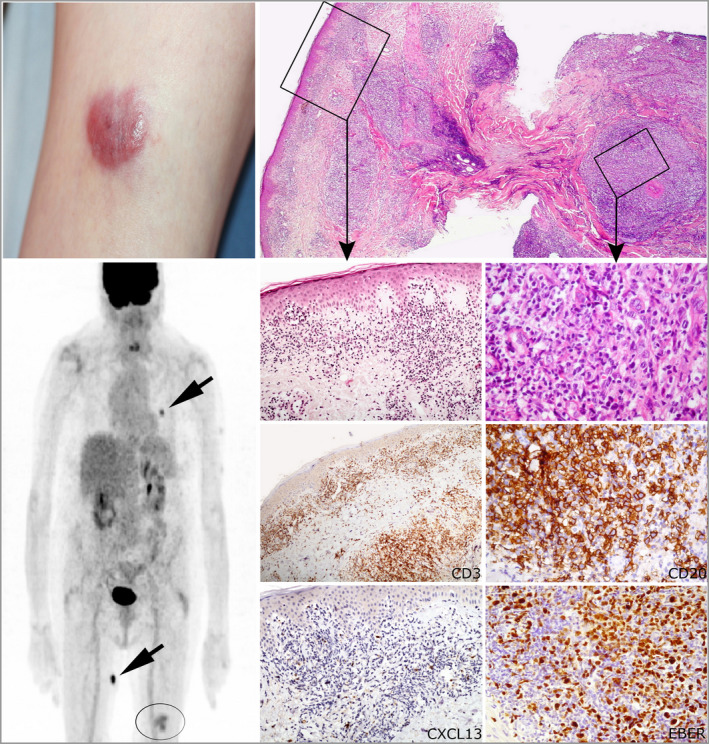
pcTFH‐PTCL associated with an EBV+ lymphoproliferation in the skin (patient 1). Erythematous nodule on the left thigh. The fluorodeoxyglucose positron emission/computed tomography imaging shows two subcutaneous hypermetabolic nodules (arrows) on the trunk and the right thigh, in addition to a skin lesion of the left thigh (circle). Histology shows a dense lymphocytic infiltrate involving all layers of skin. The superficial part of the lesion shows a T‐cell component made of small CD3+ and CXCL13+ lymphocytes with hyperchromatic nuclei. In the deep dermis and hypodermis, there are sheets of large atypical immunoblast‐like CD20+ EBV+ lymphocytes (EBER+). pcTFH‐PTCL, primary cutaneous peripheral T‐cell lymphomas with a T‐follicular helper phenotype; EBV, Epstein–Barr virus. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 4.
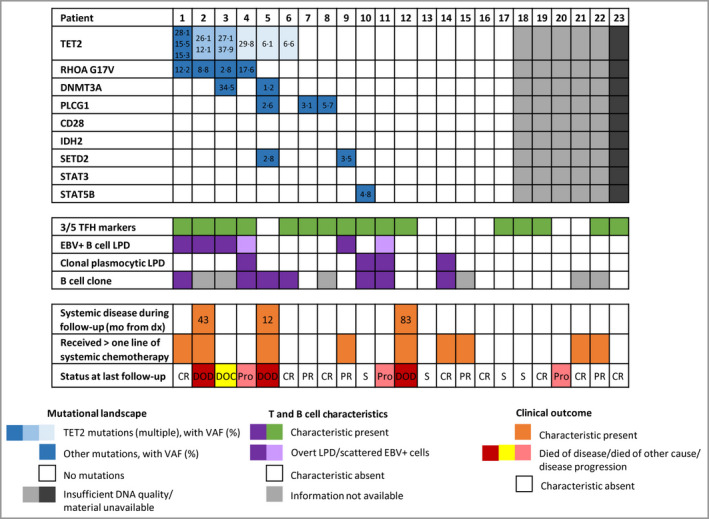
Representation of molecular data with correlation to relevant clinicopathological features. EBV, Epstein–Barr virus; LPD, lymphoproliferative disorder. [Colour figure can be viewed at wileyonlinelibrary.com]
Clonality analysis and genetic aberrations detected by targeted deep sequencing
A dominant T‐cell clone was identified in skin biopsies of 17 of 19 (89%) patients. A dominant B‐cell clone was identified in seven of 17 (41%), including those with an EBV+ or monoclonal plasma cell LPD (Figure 4). In total, 10 of 23 (43%) patients had accompanying clonal or abnormal B‐cell proliferation.
Twenty‐two patients had material available for targeted next‐generation sequencing. DNA quality for the procedure was insufficient for interpretation in five cases. Among the 17 cases successfully sequenced, 10 showed identifiable variants, observed with a mean sequencing depth per detected mutation of 2004x. Seven had no detectable mutations (Figure 4). Patients 1–4 showed a double TET2–RHOA G17V‐mutated profile. Patient 5 carried multiple mutations (TET2, DNMT3A, PLCG1, SETD2). Other anomalies included isolated TET2, PLCG1, SETD2 or STAT5B mutations. In the patients with double TET2–RHOA mutations, variant allele frequency ratios of TET2 with RHOA were generally higher than 2 : 1, supporting a role for possible clonal haematopoiesis in the development of the neoplasm, as described in AITL. 21 These pati also showed concomitant B‐cell anomalies (EBV+ B‐cell lymphoproliferative disorder, n = 3; clonal plasmocytic LPD, n = 1) and one developed systemic involvement and died of disease at 43 months.
Clinicopathological and molecular correlations
Overall survival of the cohort was 78% at 98 months. There were no statistically significant differences between the aggressive and indolent groups in terms of demographic traits. In terms of histopathological characteristics, patients with aggressive disease had less epidermotropism (P = 0·0364) and higher numbers of accompanying CD8+ cells (P = 0·0094), but no other associations were identified.
Although the statistical analyses were limited by the small number of cases, there was no significant difference in the survival of patients with or without double TET2–RHOA mutations. This mutational profile was also not an indicator of disease aggressiveness. It was strongly associated with the presence of a clonal B‐cell population (P = 0·039), but there were no other differences within the demographic or histopathological features of this subgroup and the rest of the study cohort.
Discussion
Primary cutaneous peripheral T‐cell lymphomas with a T‐follicular helper phenotype (pcTFH‐PTCL) are poorly characterized and difficult to fit into the existing categories of the current WHO classification scheme. According to the current series, they may share certain biological similarities with recognized entities of nodal T‐cell lymphomas of TFH derivation, as 10 of 23 patients had EBV+ B cells, a clonal expansion of B or plasma cells, and/or accompanying neoplastic B‐cell proliferation, and four of these cases showed an AITL‐like molecular mutational profile. These characteristics are not reported in other CTCLs known to express TFH lineage markers, including MF, SS and pcSMLPD (for comparison, see Table S2 in the Supporting Information).
AITL is a disease, usually arising in the lymph nodes, that is associated with auto‐immune manifestations and B‐cell expansion, which can result in clonal B‐cell LPD, often with EBV positivity or plasmacytic differentiation. 22 , 23 Overall, a significant proportion of the cases of pcTFH‐PTCL appears to derive, as for nodal TFH‐PTCL, from neoplastic TFH cells that retained their functional properties to recruit, support and stimulate B cells, sometimes to the point of overt lymphoma. 24 Despite these similarities, the absence of extracutaneous disease and the clinical behaviour of most of the patients with pcTFH‐PTCL is not consistent with a diagnosis of skin manifestations of AITL or other nodal PTCL‐TFH. Indeed, most patients from our series showed lesions that remained limited to the skin during follow‐up, and many also did not require aggressive treatment for disease control, including five of the 10 patients with clonal/neoplastic B‐cell expansion, EBV+ cells, or AITL‐like molecular features. A recent publication described a case with a skin biopsy (nondiagnostic for lymphoma) sampled 12 months before a diagnosis of AITL in the lymph node, in which a RHOA G17V mutation was detected. 25 This finding raises the issue of whether some cases in this series may eventually correspond to this scenario, although follow‐up times from our series generally exceeded 12 months and the diagnosis of overt lymphoma was first established in the skin. As is often the case with cutaneous lymphoma, the first manifestations of the disease and the initiation of medical treatment may have long preceded histopathological confirmation, potentially leading to an underestimation of the protracted nature of the disease.
Importantly, we applied stringent selection criteria to exclude potential confounding cases of MF and SS, especially patients who presented with patches, plaque lesions or erythroderma, as both MF and SS have been reported to express certain TFH markers. 3 , 16 , 17 Therefore, 14 patients with patches/plaques were not included, although their biopsies (data not shown) showed deep dermal infiltration by neoplastic cells expressing three TFH markers, sometimes admixed with a B‐cell component, which are not classical features of MF. 18 Four patients with erythroderma were also not included, despite the absence of circulating neoplastic cells, a classical feature of SS. Overall, despite the fact that some of these cases could correspond to true pcTFH‐PTCL, they were not included in the current series, as formally excluding a diagnosis of MF is difficult, because (i) MF is by far the most frequently occurring CTCL, (ii) there are currently no gold standard molecular/phenotypic features for diagnosing MF, and (iii) MF is clinically and histologically highly polymorphic.
pcSMLPD was introduced into the last update of the WHO classification, emphasizing their benign behaviour. A retrospective series published by our group showed a presentation with isolated self‐limited lesions, with a tendency for spontaneous regression after skin biopsy. 12 The lesions of pcTFH‐PTCL, taken individually, sometimes have the appearance of solitary dome‐shaped erythematous nodules, similar to the nodular variants of pcSMLPD. Nevertheless, these patients received chronic and even aggressive courses of treatment and were resistant to therapy, and few had nodal or systemic involvement during the evolution of the disease, making a diagnosis of pcSMLPD for such patients inappropriate and misleading in terms of clinical management.
High‐throughput sequencing studies have deciphered the mutational alterations associated with AITL, showing a unique pattern. 21 , 26 By contrast, the mutational landscape of MF and SS appears to be very heterogeneous, with rare recurrent events. 27 , 28 , 29 , 30 Using targeted deep sequencing, we identified an association of TET2 and RHOAG17V mutations in four samples from this series, which is highly characteristic of AITL or other nodal PTCLs of TFH derivation. Importantly, three had a purely cutaneous disease with no nodal involvement, with a follow‐up of 12–19 months. In addition, variant allele frequency ratios between TET2 and other mutations suggest that clonal haematopoiesis may be involved in pcTFH‐PTCL, similar to nodal PTCL of TFH derivation. 21 We found two nonsynonymous missense mutations of the PLCG1 gene and an isolated SETD2 mutation. Alterations of the PLCG1 gene were first identified in CTCL, especially in MF and SS, but were more recently shown to be present in a subset of AITL, adult T‐cell leukaemia lymphoma and PTCL‐NOS (not otherwise specified), and thus do not appear to be entirely specific to any PTCL subtype. 31 , 32 , 33 The loss of function of SETD2, a tumour‐suppressor gene, resulting in a loss of H3K36 methylation, is highly recurrent in monomorphic epitheliotropic intestinal T‐cell lymphoma, 34 and has also been reported in hepatosplenic T‐cell lymphoma, 35 and more recently in PTCL‐NOS. 36 Limitations of this study include the restricted scope of mutational studies; whole‐genome and/or transcriptome studies would be useful to identify further patterns.
In conclusion, in the current series, we describe a group of primary cutaneous lymphomas with a TFH phenotype that are not clearly categorized into any of the current entities of the WHO classification, including those often sharing a TFH phenotype, such as MF, SS and pcSMLPD. Although most patients with pcTFH‐PTCL showed a disease confined to the skin over time, the disease showed a potential for systemic dissemination in a minority of patients. Whether pcTFH‐PTCL should be considered as a separate group of cutaneous lymphomas remains an open question, but at least a portion of these patients share pathological and genetic features with nodal PTCL‐TFH, despite the absence of systemic manifestations. In addition, a number of patients in the current series showed chronic or even aggressive disease requiring long‐term treatment, including chemotherapy. Therapeutic perspectives in these patients can be explored, at least for those with AITL‐like molecular alterations of epigenetic regulators, as targeted treatments have been introduced for systemic TFH lymphomas, including histone deacetylase inhibitors 37 , 38 and hypomethylating agents (e.g. 5‐azacytidine). 39 , 40 Further characterization of this heterogeneous group is needed to improve the treatment and follow‐up of these patients.
Author contributions
Luojun Wang: Data curation (equal); formal analysis (equal); writing – original draft (equal); writing – review and editing (equal). Delphine Rocas: Data curation (equal); formal analysis (equal); writing – original draft (equal). Stéphane Dalle: Resources (equal); writing – review and editing (equal). Nouhoum Sako: Investigation (equal). Laura Pelletier: Investigation (equal). Nadine Martin: Investigation (equal). Aurélie Dupuy: Investigation (supporting). Nadia Tazi: Investigation (supporting). Brigitte Balme: Investigation (equal); resources (equal). Béatrice Vergier: Resources (equal). Marie Beylot‐Barry: Resources (equal); writing – review and editing (equal). Agnès Carlotti: Investigation (equal); resources (equal). Martine Bagot: Resources (equal); writing – review and editing (supporting). Maxime Battistella: Resources (equal); writing – review and editing (supporting). Guillaume Chaby: Resources (equal). Saskia Ingen‐Housz‐Oro: Resources (equal). Philippe Gaulard: Conceptualization (equal); funding acquisition (equal); methodology (equal); supervision (equal); writing – review and editing (lead). Nicolas Ortonne: Conceptualization (lead); funding acquisition (equal); methodology (lead); supervision (equal); writing – review and editing (lead).
Funding
This work was supported by a grant from the Société Française de Dermatologie (SFD, AO 2015), the Fondation pour la Recherche Médicale (FRM) and the Fondation ARC pour la Recherche sur le Cancer.
Conflicts of interest
The authors have no conflicts of interest to declare.
Ethics statement
The study was registered and performed according to the guidelines of the French Bioethics Law for retrospective noninterventional research studies after the absence of objection.
Supporting information
Table S1 Details of histopathological and immunophenotypic features (29 biopsies from 23 patients).
Table S2 Comparative table of the common clinical, pathological and molecular aspects of cutaneous (or cutaneous locations of) T‐cell lymphomas with TFH marker expression.
Acknowledgments
We thank Dr François Lemonnier for help with formatting of the manuscript and conception of the figures.
L.W., D.R., P.G. and N.O. contributed equally.
Plain language summary available online
Data availability
The data that support the findings of this study are available in the supplementary material of this article. More detailed data for this study are available from the corresponding author on reasonable request.
References
- 1. Swerdlow S, Campo E, Jaffe ES et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th edition. Lyon: International Agency for Research on Cancer, 2017; 585. [Google Scholar]
- 2. Dobay MP, Lemonnier F, Missiaglia E et al. Integrative clinicopathological and molecular analyses of angioimmunoblastic T‐cell lymphoma and other nodal lymphomas of follicular helper T‐cell origin. Haematologica 2017; 102:e148–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leclaire Alirkilicarslan A, Dupuy A, Pujals A et al. Expression of TFH markers and detection of RHOA p.G17V and IDH2 p.R172K/S mutations in cutaneous localizations of angioimmunoblastic T‐cell lymphomas. Am J Surg Pathol 2017; 41:1581–92. [DOI] [PubMed] [Google Scholar]
- 4. Kantekure K, Yang Y, Raghunath P et al. Expression patterns of the immunosuppressive proteins PD‐1/CD279 and PD‐L1/CD274 at different stages of cutaneous T‐cell lymphoma/mycosis fungoides. Am J Dermatopathol 2012; 34:126–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buder K, Poppe LM, Bröcker EB et al. Primary cutaneous follicular helper T‐cell lymphoma: diagnostic pitfalls of this new lymphoma subtype. J Cutan Pathol 2013; 40:903–8. [DOI] [PubMed] [Google Scholar]
- 6. Meyerson HJ, Awadallah A, Pavlidakey P et al. Follicular center helper T‐cell (TFH) marker positive mycosis fungoides/Sezary syndrome. Mod Pathol 2013; 26:32–43. https://doi.org/ 10.1038/modpathol.2012.124. [DOI] [PubMed] [Google Scholar]
- 7. Bosisio FM, Cerroni L. Expression of T‐follicular helper markers in sequential biopsies of progressive mycosis fungoides and other primary cutaneous T‐cell lymphomas. Am J Dermatopathol 2015; 37:115–21. [DOI] [PubMed] [Google Scholar]
- 8. Garcia‐Herrera A, Colomo L, Camós M et al. Primary cutaneous small/medium CD4+ T‐cell lymphomas: a heterogeneous group of tumors with different clinicopathologic features and outcome. J Clin Oncol 2008; 26:3364–71. [DOI] [PubMed] [Google Scholar]
- 9. Rodríguez Pinilla SM, Roncador G, Rodríguez‐Peralto JL et al. Primary cutaneous CD4+ small/medium‐sized pleomorphic T‐cell lymphoma expresses follicular T‐cell markers. Am J Surg Pathol 2009; 33:81–90. [DOI] [PubMed] [Google Scholar]
- 10. Cetinözman F, Jansen PM, Willemze R. Expression of programmed death‐1 in primary cutaneous CD4‐positive small/medium‐sized pleomorphic T‐cell lymphoma, cutaneous pseudo‐T‐cell lymphoma, and other types of cutaneous T‐cell lymphoma. Am J Surg Pathol 2012; 36:109–16. [DOI] [PubMed] [Google Scholar]
- 11. Ally MS, Prasad Hunasehally RY, Rodriguez‐Justo M et al. Evaluation of follicular T‐helper cells in primary cutaneous CD4+ small/medium pleomorphic T‐cell lymphoma and dermatitis. J Cutan Pathol 2013; 40:1006–13. [DOI] [PubMed] [Google Scholar]
- 12. Beltzung F, Ortonne N, Pelletier L et al. Primary cutaneous CD4+ small/medium T‐cell lymphoproliferative disorders: a clinical, pathologic, and molecular study of 60 cases presenting with a single lesion: a multicenter study of the French Cutaneous Lymphoma Study Group. Am J Surg Pathol 2020; 44:862–72. [DOI] [PubMed] [Google Scholar]
- 13. Le Tourneau A, Audouin J, Molina T et al. Primary cutaneous follicular variant of peripheral T‐cell lymphoma NOS. A report of two cases. Histopathology 2010; 56:548–51. [DOI] [PubMed] [Google Scholar]
- 14. Battistella M, Beylot‐Barry M, Bachelez H et al. Primary cutaneous follicular helper T‐cell lymphoma: a new subtype of cutaneous T‐cell lymphoma reported in a series of 5 cases. Arch Dermatol 2012; 148:832–9. [DOI] [PubMed] [Google Scholar]
- 15. Ohmatsu H, Sugaya M, Fujita H et al. Primary cutaneous follicular helper T‐cell lymphoma treated with allogeneic bone marrow transplantation: immunohistochemical comparison with angioimmunoblastic T‐cell lymphoma. Acta Derm Venereol 2014; 94:54–7. [DOI] [PubMed] [Google Scholar]
- 16. Wang JY, Nguyen GH, Ruan J, Magro CM. Primary cutaneous follicular helper T‐cell lymphoma: a case series and review of the literature. Am J Dermatopathol 2017; 39:374–83. [DOI] [PubMed] [Google Scholar]
- 17. Kim YH, Willemze R, Pimpinelli N et al. TNM classification system for primary cutaneous lymphomas other than mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007; 110:479–84. [DOI] [PubMed] [Google Scholar]
- 18. Delfau‐Larue MH, Petrella T, Lahet C et al. Value of clonality studies of cutaneous T lymphocytes in the diagnosis and follow‐up of patients with mycosis fungoides. J Pathol 1998; 184:185–90. [DOI] [PubMed] [Google Scholar]
- 19. Dupuy A, Lemonnier F, Fataccioli V et al. Multiple ways to detect IDH2 mutations in angioimmunoblastic T‐cell lymphoma from immunohistochemistry to next‐generation sequencing. J Mol Diagn 2018; 20:677–85. [DOI] [PubMed] [Google Scholar]
- 20. Alcazer V. StatAid: an R package with a graphical user interface for data analysis. J Open Source Softw 2020; 5:2630. [Google Scholar]
- 21. Sakata‐Yanagimoto M, Enami T, Yoshida K et al. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nat Genet 2014; 46:171–5. [DOI] [PubMed] [Google Scholar]
- 22. Zettl A, Lee SS, Rüdiger T et al. Epstein–Barr virus‐associated B‐cell lymphoproliferative disorders in angioimmunoblastic T‐cell lymphoma and peripheral T‐cell lymphoma, unspecified. Am J Clin Pathol 2002; 117:368–79. [DOI] [PubMed] [Google Scholar]
- 23. Balagué O, Martínez A, Colomo L et al. Epstein–Barr virus negative clonal plasma cell proliferations and lymphomas in peripheral T‐cell lymphomas: a phenomenon with distinctive clinicopathologic features. Am J Surg Pathol 2007; 31:1310–22. [DOI] [PubMed] [Google Scholar]
- 24. Gaulard P, de Leval L. Follicular helper T cells: implications in neoplastic hematopathology. Semin Diagn Pathol 2011; 28:202–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dobson R, Du PY, Rásó‐Barnett L et al. Early detection of T‐cell lymphoma with T follicular helper phenotype by RHOA mutation analysis. Haematologica 2021; 107:489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palomero T, Couronné L, Khiabanian H et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas. Nat Genet 2014; 46:166–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. da Silva Almeida AC, Abate F, Khiabanian H et al. The mutational landscape of cutaneous T cell lymphoma and Sézary syndrome. Nat Genet 2015; 47:1465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ungewickell A, Bhaduri A, Rios E et al. Genomic analysis of mycosis fungoides and Sézary syndrome identifies recurrent alterations in TNFR2. Nat Genet 2015; 47:1056–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Choi J, Goh G, Walradt T et al. Genomic landscape of cutaneous T cell lymphoma. Nat Genet 2015; 47:1011–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Woollard WJ, Pullabhatla V, Lorenc A et al. Candidate driver genes involved in genome maintenance and DNA repair in Sézary syndrome. Blood 2016; 127:3387–97. [DOI] [PubMed] [Google Scholar]
- 31. Manso R, Rodríguez‐Pinilla SM, González‐Rincón J et al. Recurrent presence of the PLCG1 S345F mutation in nodal peripheral T‐cell lymphomas. Haematologica 2015; 100:e25–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kataoka K, Nagata Y, Kitanaka A et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet 2015; 47:1304–15. [DOI] [PubMed] [Google Scholar]
- 33. Wang M, Zhang S, Chuang SS et al. Angioimmunoblastic T cell lymphoma: novel molecular insights by mutation profiling. Oncotarget 2017; 8:17763–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roberti A, Dobay MP, Bisig B et al. Type II enteropathy‐associated T‐cell lymphoma features a unique genomic profile with highly recurrent SETD2 alterations. Nat Commun 2016; 7:12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McKinney M, Moffitt AB, Gaulard P et al. The genetic basis of hepatosplenic T‐cell lymphoma. Cancer Discov 2017; 7:369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ji MM, Huang YH, Huang JY et al. Histone modifier gene mutations in peripheral T‐cell lymphoma not otherwise specified. Haematologica 2018; 103:679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ghione P, Faruque P, Mehta‐Shah N et al. T follicular helper phenotype predicts response to histone deacetylase inhibitors in relapsed/refractory peripheral T‐cell lymphoma. Blood Adv 2020; 4:4640–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bachy E, Camus V, Thieblemont C et al. Romidepsin plus CHOP versus CHOP in patients with previously untreated peripheral T‐cell lymphoma: results of the Ro‐CHOP phase III study (conducted by LYSA). J Clin Oncol 2022; 40:242–51. [DOI] [PubMed] [Google Scholar]
- 39. Lemonnier F, Dupuis J, Sujobert P et al. Treatment with 5‐azacytidine induces a sustained response in patients with angioimmunoblastic T‐cell lymphoma. Blood 2018; 132:2305–9. [DOI] [PubMed] [Google Scholar]
- 40. Falchi L, Ma H, Klein S et al. Combined oral 5‐azacytidine and romidepsin are highly effective in patients with PTCL: a multicenter phase 2 study. Blood 2021; 137:2161–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Details of histopathological and immunophenotypic features (29 biopsies from 23 patients).
Table S2 Comparative table of the common clinical, pathological and molecular aspects of cutaneous (or cutaneous locations of) T‐cell lymphomas with TFH marker expression.
Data Availability Statement
The data that support the findings of this study are available in the supplementary material of this article. More detailed data for this study are available from the corresponding author on reasonable request.


