Abstract
Lentigo maligna (LM) is a subtype of in situ melanoma that classically presents in elderly patients as a slowly growing lesion on sun‐exposed areas that may evolve to invasive melanoma. Line‐field confocal optical coherence tomography (LC‐OCT) is a new non‐invasive technique for a real‐time, vertical, and horizontal skin imaging with high resolution close to conventional histopathology. We present the LC‐OCT features of an LM of the nose in a 49‐year‐old white man along with their horizontal and vertical histopathological correlations. LC‐OCT was able to detect in vivo, in both horizontal and vertical imaging, the main microscopic features typical of LM by showing, in the epidermis and around the hair follicles, the presence of large, bright roundish, or dendritic atypical cells, with evident nuclei, corresponding to atypical melanocytes with a tendency toward folliculotropism. A strong correspondence between LC‐OCT images and vertical and horizontal histopathological sections was observed. Our study, although limited to a single case, is indicative of the great potential of LC‐OCT to improve the non‐invasive diagnosis of LM.
Keywords: dermoscopy, diagnosis, histopathology, lentigo maligna, line‐field confocal optical coherence tomography
1. INTRODUCTION
Lentigo maligna (LM) is a subtype of in situ melanoma that classically presents in elderly patients as a slowly growing lesion on sun‐exposed areas and that may evolve to invasive melanoma (LMM). It usually occurs on the head and neck, and clinically appears as an asymmetric pigmented macule or patch of increasing size with irregular borders. LM can be present for years before being diagnosed. Histopathologically, increased proliferation of atypical melanocytes along the basal layer of the epidermis with extension along adnexal structures is the main feature, and associated solar elastosis is typically seen.
The diagnosis of pigmented facial lesions, mainly including LM, pigmented actinic keratosis, and solar lentigo, is challenging and may require a timely recognition in order to define the most correct approach. In the past years, the introduction of innovative imaging techniques, especially dermoscopy and reflectance confocal microscopy (RCM), has made a significant contribution in the differential diagnosis of these lesions. 1 , 2 , 3 , 4 , 5 , 6 , 7 Such techniques are able to improve the diagnostic accuracy and allow a prompt diagnosis by reducing invasive procedures at critical sites on the face. Dermoscopy is easy to use and extensively and routinely used in clinical practice; on the other hand, RCM, being more costly and time consuming, has a higher specificity and sensitivity and is mainly used in dermatology centers with advanced technologies.
Line‐field confocal optical coherence tomography (LC‐OCT) is a new non‐invasive system for a real‐time, vertical, and horizontal skin imaging with high resolution close to conventional histopathology. LC‐OCT systems have recently been marketed, and preliminary studies have reported its use in selected cutaneous disorders, including malignant and benign neoplasms 8 , 9 , 10 , 11 , 12 , 13 , 14 and some inflammatory and infectious diseases. 15 , 16 , 17 , 18
We herein present the LC‐OCT features of a case of LM involving the nose along with their horizontal and vertical histopathological correlations.
2. CASE REPORT
A 49‐year‐old white man presented with a 3‐year history of a slowly enlarging pigmented lesion on his left nasal region. Past medical history was non‐contributory. At physical examination, an irregular, dark, brownish/black patch with ill‐defined borders was noted (Figure 1A). Polarized light dermoscopy (Illuco IDS‐1100, Tre T Medical) revealed the presence of a diffuse dark brown area interrupted by hypopigmented holes of varying width (creating a “pseudonetwork”), asymmetric pigmented follicular openings, dark rhomboidal structures, and gray globules (Figure 1B). LC‐OCT examination was performed with the commercially available DeepLive (DAMAE Medical), which provides images with an axial resolution of 1.1 μm, a lateral resolution of 1.3 μm, and a field of view of 1.2 × 0.5 × 0.5 mm; it is equipped with an integrated dermoscopic camera allowing a precise positioning over the area to examine. DeepLive is a medical device with CE (Conformité Européenne) mark in Europe, where it is commercially available throughout and can be used in daily clinical practice. It is not FDA (Food and Drug Administration) approved and can only be used for clinical research purposes in the USA.
FIGURE 1.
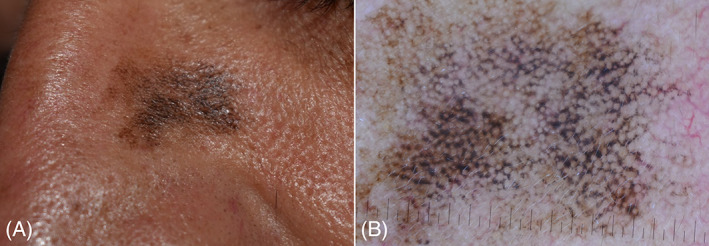
(A) Lentigo maligna of the nose: irregular dark brownish/black patch with ill‐defined borders. (B) Dermoscopy showing a dark brown irregular pseudonetwork, asymmetric pigmented follicular openings, dark rhomboidal structures, and gray globules
LC‐OCT showed, in the vertical images, the presence of bright, atypical, roundish cells linearly distributed along the dermo‐epidermal junction; similar scattered cells were also visible within the epidermis (Figure 2A). In the horizontal view, LC‐OCT showed, across the viable epidermis, at the dermo‐epidermal junction and around the hair follicles, the presence of bright, atypical, large, roundish, or dendritic cells with evident nuclei, causing architectural disarray (Figures 3A, 4A, and 5A). No atypical cells were observed in the superficial dermis in either vertical or horizontal sections. After obtaining an informed consent, an LC‐OCT‐guided incisional biopsy was performed in a target area stained with surgical ink, and vertical and horizontal histopathological sections showed in the epidermis, lentiginous proliferation of rounded to elongated, poorly pigmented melanocytes with hyperchromatic nuclei and prominent nucleoli, singly arranged or in small nests along the basal layer; above the basal layer, moderate pagetoid spread, along with adnexal involvement; in the superficial dermis, some melanophages along with signs of solar elastosis. Based on histopathology, the diagnosis of LM was rendered. Both vertical and horizontal histopathological sections showed a strong correspondence with LC‐OCT images (Figures 2B, 3B, 4B, and 5B).
FIGURE 2.
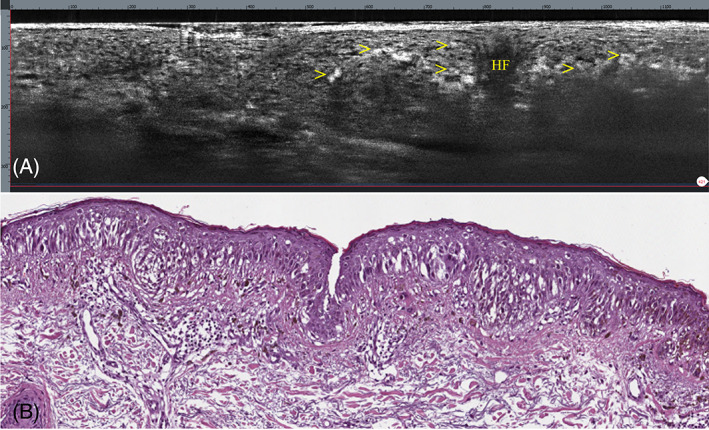
Lentigo maligna. (A) Vertical LC‐OCT showing, along the dermo‐epidermal junction and within the epidermis, bright atypical, roundish cells (arrows); HF, hair follicle. (B) Vertical histopathology showing a lentiginous proliferation of atypical melanocytes crowding the basal layer of the epidermis; moderate pagetoid spread above the basal layer is seen. Underlying superficial dermis exhibits solar elastosis. H&E: original magnification, ×150
FIGURE 3.
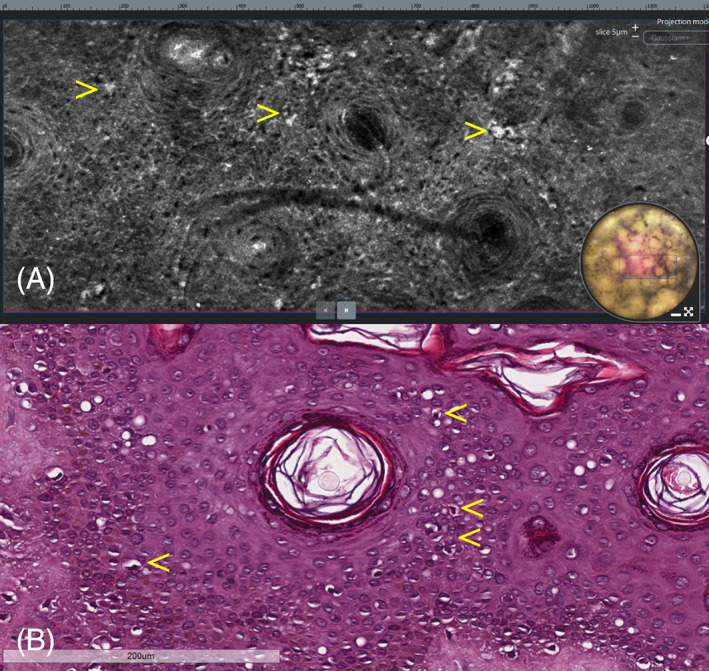
Lentigo maligna. (A) Horizontal LC‐OCT at the level of the stratum spinosum showing bright atypical, roundish cells with evident nuclei (arrows), causing architectural disarray. (B) Horizontal histopathologic section at the same level showing intraepidermal pagetoid spread of scattered neoplastic melanocytes (arrows). H&E: original magnification, ×150
FIGURE 4.
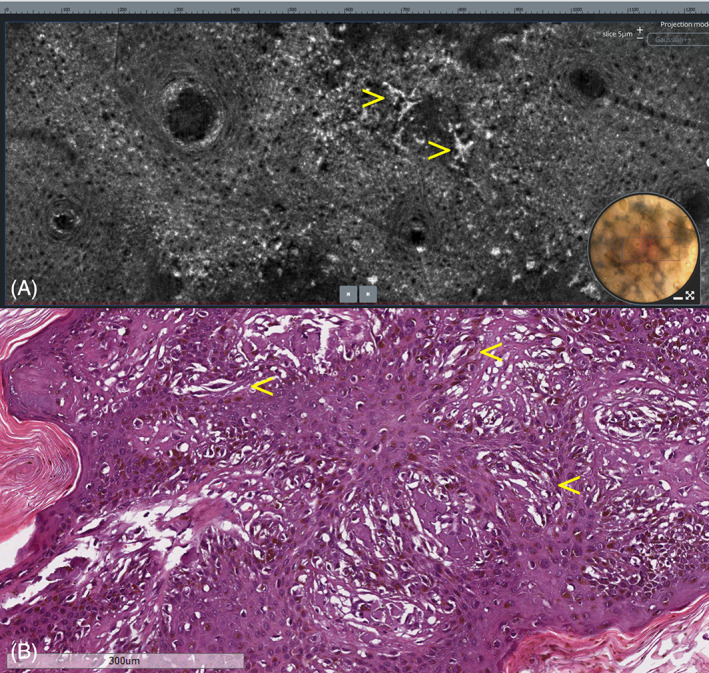
Lentigo maligna. (A) Horizontal LC‐OCT at the base of the epidermis showing large pleomorphic cells of dendritic morphology (arrows). (B) Horizontal histopathologic section at the same level showing proliferation of rounded to elongated, poorly pigmented melanocytes with hyperchromatic nuclei and prominent nucleoli (arrows), singly arranged or reunited in small nests. H&E: original magnification, ×150
FIGURE 5.
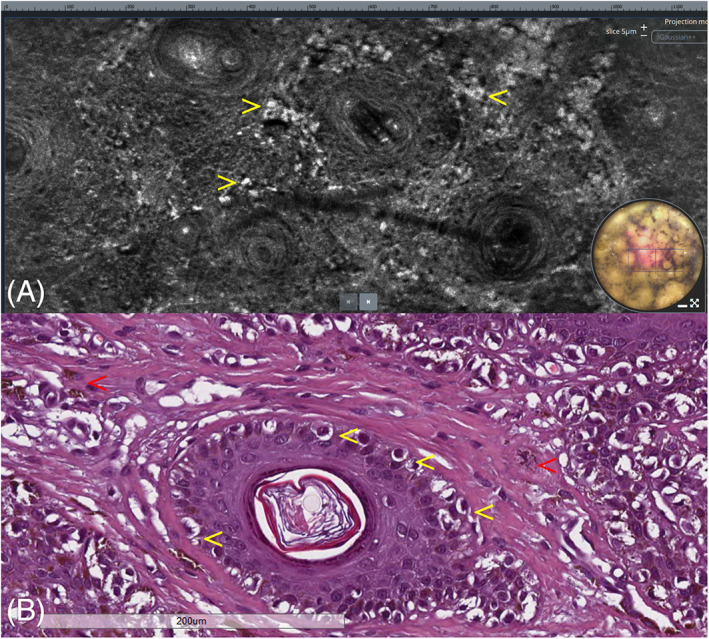
Lentigo maligna. (A) Horizontal LC‐OCT at the level of the dermo‐epidermal junction showing bright atypical, roundish, and dendritic cells with evident nuclei located around the hair follicles (arrows). (B) Horizontal histopathologic section at the same level showing adnexal involvement by neoplastic cells (yellow arrows) along with dermal melanophages (red arrows). H&E: original magnification, ×200
3. DISCUSSION
We describe the LC‐OCT features of LM. In our case, LC‐OCT was able to detect in vivo, in both horizontal and vertical imaging, the main microscopic features typical of LM by showing, in the epidermis and around the hair follicles, the presence of large, bright, roundish, or dendritic atypical cells, with evident nuclei, corresponding to atypical melanocytes with a tendency toward folliculotropism. From our results, some considerations follow: the features observed by LC‐OCT in the horizontal sections are similar to those normally obtained by RCM, 3 , 4 , 5 , 6 , 7 which has been demonstrated to be useful in the non‐invasive diagnosis of LM by showing the characteristic atypical melanocytes mainly distributed around the hair follicles. Compared with RCM, LC‐OCT has a slightly lower resolution (1.3 vs. 0.8 μm, respectively) and a higher penetration depth (~500 vs. ~200 μm). In addition, LC‐OCT has the great advantage of providing vertical images that allow an immediate view of the different layers of the skin corresponding to the same perspective of conventional histopathology. In the case of LM, vertical sections provided by LC‐OCT allow a more prompt and accurate evaluation of the dermo‐epidermal junction, crucial for the assessment of dermal invasion.
Similarly to the available handheld version of the RCM system, the LC‐OCT device is equipped with a handheld probe that does not require to be fixed on the skin. The probe can be easily moved for the rapid examination of different fields, including difficult‐to‐access, small areas with convexity/concavity such as the face.
Although further studies on larger case series are necessary, our preliminary results indicate that LC‐OCT might improve the non‐invasive diagnosis of LM. We are aware that our study is related to a single case; however, it is indicative of the great potential of this new imaging technique to provide an almost perfect correlation with horizontal and vertical histopathological sections. The gold standard for the final diagnosis of LM remains surgical excision and histopathology, which also provide reliable information on early dermal invasion and progression to LMM. However, this invasive procedure may be problematic, especially for large lesions of the face in elderly patients with comorbidities, therefore LC‐OCT may be crucial for narrowing down the number of unnecessary biopsies by ruling out other disorders such as pigmented actinic keratosis and solar lentigo/seborrheic keratosis. 9 , 10 In actinic keratosis, LC‐OCT shows, similarly to RCM, a variable degree of hyperkeratosis, acanthosis, and cellular and nuclear pleomorphism of keratinocytes, as reported by some studies. 10 , 19 , 20 , 21 As regards solar lentigo/seborrheic keratosis, although a specific LC‐OCT study has not been conducted so far, the expected findings, based on our experience, would be similar to those obtained with RCM, that is, hyperpigmentation of the basal layer, polycyclic papillary contour, bright branching tubular structures, and bulbous projections. 21 Finally, thanks to the integrated dermoscopic camera, LC‐OCT may be useful to detect the most appropriate areas for biopsy as well as for surgical margins delineation, and for topical treatment monitoring.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGEMENT
Open Access Funding provided by Universita degli Studi di Catania within the CRUI‐CARE Agreement.
Verzì AE, Broggi G, Caltabiano R, Micali G, Lacarrubba F. Line‐field confocal optical coherence tomography of lentigo maligna with horizontal and vertical histopathologic correlations. J Cutan Pathol. 2023;50(2):118‐122. doi: 10.1111/cup.14321
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Schiffner R, Schiffner‐Rohe J, Vogt T, et al. Improvement of early recognition of lentigo maligna using dermatoscopy. J Am Acad Dermatol. 2000;42(1 Pt 1):25‐32. [DOI] [PubMed] [Google Scholar]
- 2. Stolz W, Schiffner R, Burgdorf WH. Dermatoscopy for facial pigmented skin lesions. Clin Dermatol. 2002;20(3):276‐278. [DOI] [PubMed] [Google Scholar]
- 3. Cinotti E, Labeille B, Debarbieux S, et al. Dermoscopy vs. reflectance confocal microscopy for the diagnosis of lentigo maligna. J Eur Acad Dermatol Venereol. 2018;32(8):1284‐1291. [DOI] [PubMed] [Google Scholar]
- 4. Zubair R, Haroon A, Rao B. Bedside diagnosis of lentigo maligna with reflectance confocal microscopy. J Cutan Pathol. 2018;45(7):553‐555. [DOI] [PubMed] [Google Scholar]
- 5. Carapeba MOL, Alves Pineze M, Nai GA. Is dermoscopy a good tool for the diagnosis of lentigo maligna and lentigo maligna melanoma? A meta‐analysis. Clin Cosmet Investig Dermatol. 2019;12:403‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cinotti E, Fiorani D, Labeille B, et al. The integration of dermoscopy and reflectance confocal microscopy improves the diagnosis of lentigo maligna. J Eur Acad Dermatol Venereol. 2019;33(10):e372‐e374. [DOI] [PubMed] [Google Scholar]
- 7. Nie T, Jiang X, Zheng B, Zhang Y. Effect of reflectance confocal microscopy compared to dermoscopy in the diagnostic accuracy of lentigo maligna: a meta‐analysis. Int J Clin Pract. 2021;75(8):e14346. [DOI] [PubMed] [Google Scholar]
- 8. Suppa M, Fontaine M, Dejonckheere G, et al. Line‐field confocal optical coherence tomography of basal cell carcinoma: a descriptive study. J Eur Acad Dermatol Venereol. 2021;35(5):1099‐1110. [DOI] [PubMed] [Google Scholar]
- 9. Ruini C, Schuh S, Gust C, et al. Line‐field confocal optical coherence tomography for the in vivo real‐time diagnosis of different stages of keratinocyte skin cancer: a preliminary study. J Eur Acad Dermatol Venereol. 2021;35(12):2388‐2397. [DOI] [PubMed] [Google Scholar]
- 10. Cinotti E, Tognetti L, Cartocci A, et al. Line‐field confocal optical coherence tomography for actinic keratosis and squamous cell carcinoma: a descriptive study. Clin Exp Dermatol. 2021;46(8):1530‐1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verzì AE, Micali G, Lacarrubba F. Line‐field confocal optical coherence tomography may enhance monitoring of superficial basal cell carcinoma treated with imiquimod 5% cream: a pilot study. Cancers. 2021;13(19):4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schuh S, Ruini C, Perwein MKE, et al. Line‐field confocal optical coherence tomography: a new tool for the differentiation between nevi and melanomas? Cancers. 2022;14(5):1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lenoir C, Diet G, Cinotti E, et al. Line‐field confocal optical coherence tomography of sebaceous hyperplasia: a case series. J Eur Acad Dermatol Venereol. 2021;35(8):e509‐e511. [DOI] [PubMed] [Google Scholar]
- 14. Lacarrubba F, Verzì AE, Puglisi DF, Broggi G, Caltabiano R, Micali G. Line‐field confocal optical coherence tomography of xanthogranuloma: correlation with vertical and horizontal histopathology. J Cutan Pathol. 2021;48(9):1208‐1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tognetti L, Cinotti E, Suppa M, et al. Line field confocal optical coherence tomography: an adjunctive tool in the diagnosis of autoimmune bullous diseases. J Biophotonics. 2021;14(5):e202000449. [DOI] [PubMed] [Google Scholar]
- 16. Lacarrubba F, Verzì AE, Puglisi DF, Micali G. Line‐field confocal optical coherence tomography: a novel, non‐invasive imaging technique for a rapid, in‐vivo diagnosis of herpes infection of the skin. J Eur Acad Dermatol Venereol. 2021;35(6):e404‐e406. [DOI] [PubMed] [Google Scholar]
- 17. Ruini C, Schuh S, Hartmann D, et al. Noninvasive real‐time imaging of mite skin infestations with line‐field confocal optical coherence tomography. Br J Dermatol. 2021;184(1):e3. [DOI] [PubMed] [Google Scholar]
- 18. Verzì AE, Micali G, Lacarrubba F. Line‐field confocal optical coherence tomography in molluscum contagiosum: a case series. J Eur Acad Dermatol Venereol. 2021;35(12):e934‐e936. [DOI] [PubMed] [Google Scholar]
- 19. Lenoir C, Cinotti E, Tognetti L, et al. Line‐field confocal optical coherence tomography of actinic keratosis: a case series. J Eur Acad Dermatol Venereol. 2021;35(12):e900‐e902. [DOI] [PubMed] [Google Scholar]
- 20. Tang Z, Kang L, Zhang Y, et al. The diagnostic value of in vivo reflectance confocal microscopy in actinic keratosis. Skin Res Technol. 2021;27(1):80‐85. [DOI] [PubMed] [Google Scholar]
- 21. Farnetani F, Manfredini M, Chester J, Ciardo S, Gonzalez S, Pellacani G. Reflectance confocal microscopy in the diagnosis of pigmented macules of the face: differential diagnosis and margin definition. Photochem Photobiol Sci. 2019;18(5):963‐969. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


