Abstract
Aim
The voltage‐gated potassium channel Kv11.1 is important for repolarizing the membrane potential in excitable cells such as myocytes, pancreatic α‐ and β‐cells. Moxifloxacin blocks the Kv11.1 channel and increases the risk of hypoglycaemia in patients with diabetes. We investigated glucose regulation and secretion of glucoregulatory hormones in young people with and without moxifloxacin, a drug known to block the Kv11.1 channel.
Materials and Methods
The effect of moxifloxacin (800 mg/day for 4 days) or placebo on glucose regulation was assessed in a randomized, double‐blind, crossover study of young men and women (age 20‐40 years and body mass index 18.5‐27.5 kg/m2) without chronic disease, using 6‐h oral glucose tolerance tests and continuous glucose monitoring.
Results
Thirty‐eight participants completed the study. Moxifloxacin prolonged the QTcF interval and increased heart rate. Hypoglycaemia was more frequently observed with moxifloxacin, both during the 8 days of continuous glucose monitoring and during the oral glucose tolerance tests. Hypoglycaemia questionnaire scores were higher after intake of moxifloxacin. Moxifloxacin reduced the early plasma‐glucose response (AUC0‐30 min) by 7% (95% CI: −9% to −4%, p < .01), and overall insulin response (AUC0‐360 min) decreased by 18% (95% CI: −24% to −11%, p < .01) and plasma glucagon increased by 17% (95% CI: 4%‐33%, p = .03). Insulin sensitivity calculated as the Matsuda index increased by 11%, and MISI, an index of muscle insulin sensitivity, increased by 34%.
Conclusions
In young men and women, moxifloxacin, a drug known to block the Kv11.1 channel, increased QT interval, decreased glucose levels and was associated with increased muscle insulin sensitivity and more frequent episodes of hypoglycaemia.
Keywords: fluoroquinolones, hERG, hypoglycaemia, insulin sensitivity, KCNH2, Kv11.1, long QT syndrome, moxifloxacin
1. INTRODUCTION
The voltage‐gated potassium channel Kv11.1 plays a crucial role in the repolarization phase in the heart 1 , 2 and is expressed in a range of electrically excitable cells important for glucose homeostasis, such as pancreatic α‐ and β‐cells, 3 , 4 , 5 intestinal L‐cells 6 and skeletal muscle cells. 7
Various pharmaceuticals have the off‐target effect of blocking Kv11.1 channels, e.g. certain antibiotics, antiarrhythmics, antidepressants and antipsychotics, 8 , 9 causing prolongation of the QT‐interval, i.e. acquired long QT syndrome (LQTS), with an increased risk of life‐threatening arrhythmias. Because of the increased risk, all new pharmaceutical drugs are tested for their adverse effect of blocking the Kv11.1 channel. 10
Fluoroquinolones, a class of antibiotics, block Kv11.1 channels and have been associated with episodes of severe hypoglycaemia and hyperglycaemia in patients with diabetes mellitus. 11 , 12 In a register‐based study of patients with diabetes (n = 78 433), the use of fluoroquinolones was associated with severe hypoglycaemia (causing a visit to an emergency department or hospitalization), most commonly in patients treated with moxifloxacin. 11 In 2018, the FDA announced that safety labels of fluoroquinolones should include a warning about the risk of hypoglycaemia. 13 Furthermore, a recent study has used the Food and Drug Administration Adverse Event Reporting System (FAERS) to evaluate the risk of reporting hypoglycaemia as an adverse event with the different antibiotics. 12 They found an increased risk of reporting hypoglycaemia (n = 60) identified from preferred terms, from patients reporting adverse events in relation to moxifloxacin (n = 3728), although this was mainly observed in patients also treated with a sulphonylurea or a meglitinide. 12
Dysfunctional Kv11.1 channels, because of loss‐of‐function (LoF) mutations in the KCNH2 (hERG) gene, cause LQTS type 2 (LQT2), which is associated with ventricular tachycardia, syncope and sudden death. 1 , 14 Furthermore, patients with LQT2 have symptomatic postprandial hypoglycaemia compared with matched controls. 15 Similar findings have been made in patients with LQTS type 1 because of LoF mutations in the KCNQ1 gene encoding the voltage‐gated potassium channel Kv7.1. 16
Higher 3H‐deoxy‐glucose uptake into skeletal muscle, liver, kidney and lung tissue has been found in Kcnq1‐deficient mice lacking the Kv7.1 channel than wild‐type littermates. 17 Furthermore, these mice had increased insulin sensitivity with lower glucose levels, despite higher plasma glucagon. 17 Kv11.1 has recently been hypothesized to have a role in excitation‐contraction coupling in skeletal muscle cells. 18
Thus, the Kv11.1 channel appears to be involved in glucose regulation; however, exactly how the blockade of the Kv11.1 channel affects glucose homeostasis in men and women has not been studied in detail before. Therefore, we studied the metabolic response to glucose during pharmacological blockade of Kv11.1 channels in young men and women without chronic disease.
2. METHODS
2.1. Study design
The study was a double‐blind, placebo‐controlled, crossover study. Block randomization by sex was used to assign participants to one of the two sequences from an allocation list provided by the pharmacy formulating the capsules and containers, but not otherwise associated with the study. Participants and research personnel were blinded to the sequence and study medication, and unblinding occurred after the final visit of the last participant. Two test days were conducted, separated by a washout period of minimum 3 weeks, sufficient to eliminate any carry‐over effect because the elimination half‐life of moxifloxacin in healthy individuals ranges from 8.2 to 15.1 h. 19 The study is reported according to the CONSORT 2019 extension for randomized crossover trials guidelines. 20
2.2. Participants
Inclusion criteria for participants were: 20‐40 years of age and body mass index 18.5‐27.5 kg/m2 to represent the general young population in Denmark. 21 , 22 Exclusion criteria were: chronic disease such as heart disease, diabetes and other metabolic diseases, or liver disease, a family history of congenital LQTS, QTc‐interval >440 ms for men and >450 ms for women, clinically important and/or symptomatic bradycardia, regular medication (contraceptive pills allowed), pregnancy or breastfeeding. Recruitment was conducted through the website “www.forsøgspersoner.dk” and bulletin boards across the University of Copenhagen's facilities. Participants were recruited from the Capital Region of Denmark between December 2018 and July 2019. The study was conducted at the Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen. Before participating in the study, the participants provided written oral informed consent after receiving written and oral information about the study. According to Danish law and The National Committee on Health Research Ethics in Denmark consent to participate in biomedical research must be based on an interest in participating in research and the possible benefit of the scientific area moving forward. Participants received remuneration for study participation with a maximum of 3000 DKK/~410 USD before tax, for the time used in the trial at the test days during working hours, which was approved by the National Committee on Health Research Ethics in Denmark. The study was conducted in accordance with the Declaration of Helsinki principles and approved by the Research Ethics Committee in Denmark (H‐17013807) and registered at ClinicalTrials.gov NCT03868657.
2.3. Interventions
The fluoroquinolone moxifloxacin (Teva Pharmaceuticals, Tel Aviv, Israel) was used to block the Kv11.1 channel. 23 , 24 , 25 A dose of 800 mg was administered per day, which previously has been given safely to participants. 26 Placebo was 800 mg of calcium carbonate per day. Moxifloxacin and placebo were prepared by the pharmacy in identical capsules (400 mg in each capsule). On the 3 days leading up to each test day, the participants were instructed to take two capsules each morning. On the study day, they were instructed to ingest the capsules 2 h before starting the test day. Adverse events were reported to the medical personnel in the trial. In case of significant adverse events, participants were withdrawn from the trial. The participants were instructed to refrain from eating or drinking 10 h before the test days and refrain from taking acetaminophen 24 h previously. In addition, participants were instructed not to exercise 24 h before the test days.
2.4. Continuous glucose monitoring
A Dexcom G6 sensor (Dexcom Inc., San Diego, CA, USA) was placed subcutaneously on the abdomen of study participants 4 days before each test day. The sensor was pre‐calibrated and connected by Bluetooth to a monitor device handed out to participants. Monitor devices did not show glucose readings but gave participants warnings if the connection was lost. After recording for 8 days, the sensor was removed, and data were extracted using Dexcom Clarity software. Data were analysed using the R‐package CGManalysis. 27 Excursions were registered when a time period of 10 min was below the thresholds (3.0, 3.3 and 3.9 mmol/L).
2.5. Outcomes measured on each test day
First, on each test day, participants completed a hypoglycaemia questionnaire, including frequency and severity of 18 symptoms, used in previous studies 15 , 16 and translated into Danish. A copy of the questionnaire is included in the Supplementary appendix.
Next, an intravenous catheter was inserted into a cubital vein, and baseline electrocardiograms and blood samples were collected 10 min, 5 min and immediately before the oral glucose tolerance test (OGTT) began. One gram of ground acetaminophen was added to 75 g of glucose dissolved in 250 ml of water and consumed evenly over 3 min. Acetaminophen was added to later measure the serum levels, as an indirect measure of gastric emptying. 28 Within the next 6 h, samples were collected as follows: 8, 15, 30, 45, 60, 90, 120, 150, 180, 210, 240, 270, 300, 330 and 360 min.
Before collecting blood samples, electrocardiograms were collected from participants resting in a supine position. The 12‐lead electrocardiograms were recorded in triplicates (MAC VU360, GE Healthcare).
Blood samples were drawn from an intravenous catheter at each time point. EDTA tubes were immediately centrifuged, and plasma was assayed for plasma glucose during test days using enzyme electrode technology (YSI model 2300 STAT plus; YSI Inc., Yellow Springs, OH, USA) and an aliquot was stored at −20°C for glucagon‐like peptide‐1 (GLP‐1) and glucagon measurements. Serum tubes were centrifuged after 30 min. Serum insulin and C‐peptide were measured using electro‐chemiluminescence‐immunoassay technology (Cobas 8000 e801; Roche Diagnostics, Rotkreuz, Switzerland). Plasma GLP‐1 and glucagon were analysed using in‐house radioimmunoassays targeting the C‐terminals of the hormones (antiserum code no. 89390 and 4305, respectively). 29 , 30 Serum acetaminophen was measured using enzymatic spectrophotometry (Acetaminophen L3K® kit; SEKISUI Diagnostic, Burlington, MA, USA).
2.6. Calculations
Fridericia formula (QTcF = QT/[RR]1/3) was used to correct QT interval for heart rate. 31 Fasting hormone and substrate levels were calculated as an average of samples taken 10 min, 5 min and immediately before the OGTT. The trapezoidal rule was used to calculate total and incremental/decremental area under the curve (AUC) of hormone and substrate levels. If values were missing for single time points, substitute values were calculated using linear interpolation. Homeostatic model assessment of insulin resistance (HOMA‐IR) was calculated using fasting glucose (mmol/L) multiplied by fasting insulin (μU/ml) divided by 22.5. 32 The Matsuda index was assessed by dividing 10 000 by the square root of the product of fasting glucose, fasting insulin, and mean glucose and mean insulin from time 0 to 120 min. 33 ISEC software was used to calculate the pre‐hepatic insulin secretion rates (ISR) expressed in pmol/kg × min for each test day based on the C‐peptide levels and the participants' sex, age, height and weight. 34 The insulinogenic index, an index of the early insulin response, was calculated as ΔISR from baseline until 30 min divided by the corresponding Δ glucose (ΔISR0‐30 min/Δglucose0‐30 min). Muscle insulin sensitivity index (MISI) was calculated using the MISI calculator. 35 The insulin conversion factor was 1 μU/ml = 6.00 pmol/L and glucose conversion factor was 1 mg/dl = 0.05551 mmol/L.
2.7. Statistical analysis
We estimated a required sample size of 34 participants provide 80% power, with an alpha level = 0.05, to detect a change of one‐third (823 pmol/L × min) in postprandial insulin (AUC0‐30 min) and postprandial glucose AUC0‐30 min (10 mmol/L × min) based on the observations seen in patients with LQT2 compared with matched control subjects. 15 Values for p were adjusted for multiple testing according to Benjamini and Hochberg 36 and denoted p‐adj. Values p‐adj < 0.05 were considered statistically significant. Changes in metabolic responses evaluated as the AUC were analysed using linear mixed models, each with an unstructured covariance pattern and with treatment, period and gender as fixed effects. Changes in normally distributed variables were reported as the estimated mean difference with 95% confidence intervals (CIs). Non‐normally distributed variables were logarithmically transformed before analysing, and estimates were back‐transformed and reported as ratios with 95% CI. Statistical analyses and figures were conducted using SAS Enterprise Guide version 7.15 (SAS Institute Inc., Cary, NC, USA) and R version 4.1.2.
3. RESULTS
3.1. Study flow and participant characteristics
Forty‐four participants were included in the trial and randomized. Four participants dropped out: two because of gastrointestinal adverse events (moxifloxacin), one because of influenza‐like symptoms (placebo) and one before treatment. In addition, two participants were excluded from analyses because of incorrect intake of study medication. Thirty‐eight participants, including women (n = 19) and men (n = 19), completed the two test days per protocol and were analysed (Figure 1). At inclusion, the mean ± SD age was 25.7 ± 4.7 years, body mass index was 23.6 ± 2.4 kg/m2 and baseline QTcF of 405 ± 19 ms (Table S1). None of the participants had impaired fasting glucose (>5.6 mmol/L). One participant had impaired glucose tolerance, with a 2‐h glucose value of 8.2 mmol/L, above the 7.8 mmol/L threshold, on the placebo test day, but not after the intake of moxifloxacin.
FIGURE 1.
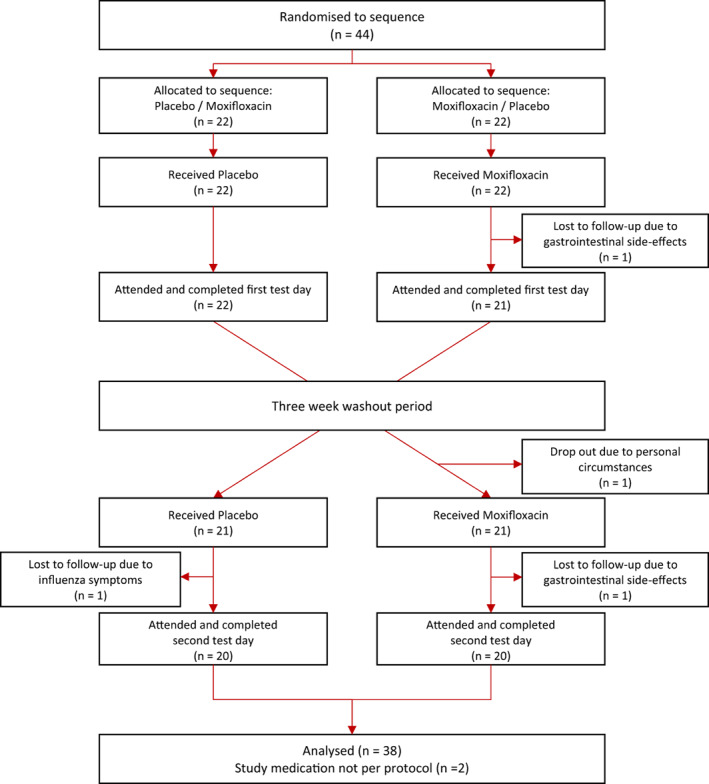
Flow chart
3.2. Continuous glucose monitoring reveals more frequent episodes of hypoglycaemia during intake of moxifloxacin
Continuous glucose monitoring was performed on all participants for 8 days during moxifloxacin and placebo treatment. During moxifloxacin treatment the mean difference in plasma glucose excursions per day below 3.3 mmol/L (0.09 n/day, 95% CI: 0.01‐0.17) and 3.0 mmol/L (0.05 n/day, 95% CI: 0.02‐0.08) were more frequent compared with placebo (Table 1). All measurements from the 8 days of recording during moxifloxacin and placebo treatment are shown in Figure S1.
TABLE 1.
CGM during moxifloxacin or placebo treatment
| Moxifloxacin (n = 37) | Placebo (n = 37) | Difference | |
|---|---|---|---|
| Average glucose mmol/L | 6.0 ± 0.5 | 6.1 ± 0.5 | −0.1 (−0.3; 0.1) |
| Maximum glucose mmol/L | 10.6 ± 1.2 | 10.9 ± 1.4 | −0.3 (−0.7; 0.2) |
| Minimum glucose mmol/L | 3.4 ± 0.7 | 3.6 ± 0.5 | −0.2 (−0.4; 0.0) |
| Total min spent | |||
| >10 mmol/L | 50 ± 62 | 46 ± 56 | 3.9 (−15.7; 23.6) |
| 7.9‐10 mmol/L | 457 ± 505 | 510 ± 388 | −54 (−253.2; 145.3) |
| 3.9‐7.8 mmol/L | 8285 ± 1458 | 8317 ± 899 | −31.4 (−623.4; 560.6) |
| 3.0‐3.8 mmol/L | 70 ± 110 | 48 ± 89 | 20.9 (−24.1; 65.8) |
| <3.0 mmol/L | 12 ± 23 | 1 ± 5 | 10.9 (3.5; 18.2) |
| Percentage of time spent | |||
| >10 mmol/L | 0.6 ± 0.7 | 0.5 ± 0.6 | 0.1 (−0.2; 0.3) |
| 7.9‐10 mmol/L | 5.2 ± 5.3 | 5.7 ± 4.3 | −0.5 (−2.6; 1.6) |
| 3.9‐7.8 mmol/L | 93.4 ± 5.4 | 93.2 ± 4.4 | 0.2 (−2; 2.3) |
| 3.0‐3.8 mmol/L | 0.8 ± 1.2 | 0.5 ± 1 | 0.2 (−0.3; 0.7) |
| <3.0 mmol/L | 0.1 ± 0.3 | 0 ± 0.1 | 0.1 (0; 0.2) |
| Number of excursions | |||
| <3.9 mmol/L | 111 | 75 | ‐ |
| <3.3 mmol/L | 33 | 7 | ‐ |
| <3.0 mmol/L | 14 | 1 | ‐ |
| Mean excursions | |||
| <3.9 mmol/L | 3 ± 4.3 | 2 ± 2.9 | 0.9 (−0.6; 2.5) |
| <3.3 mmol/L | 0.9 ± 1.8 | 0.2 ± 0.6 | 0.7 (0.1; 1.3) |
| <3.0 mmol/L | 0.4 ± 0.7 | 0 ± 0.2 | 0.3 (0.1; 0.6) |
| Number of excursions per day | |||
| <3.9 mmol/L | 14.90 | 10.31 | ‐ |
| <3.3 mmol/L | 4.39 | 0.98 | ‐ |
| <3.0 mmol/L | 1.93 | 0.14 | ‐ |
| Mean excursions per day | |||
| <3.9 mmol/L | 0.40 ± 0.57 | 0.28 ± 0.40 | 0.11 (−0.09; 0.33) |
| <3.3 mmol/L | 0.12 ± 0.24 | 0.03 ± 0.08 | 0.09 (0.01; 0.17) |
| <3.0 mmol/L | 0.05 ± 0.10 | 0.00 ± 0.02 | 0.05 (0.02; 0.08) |
Study participants wore the CGM for 4 days before each test day and glucose recording was performed for 8 days. The results listed are for the approximate 8 days, if not otherwise specified. values are shown as means ± SD. Differences are presented as estimated mean differences (95% CI). Excursions are registered when a time period of at least 10 min is below the threshold. Abbreviation: CGM, continuous glucose monitoring.
3.3. Hypoglycaemia questionnaire scores are higher during intake of moxifloxacin
In the hypoglycaemia questionnaire, the participants reported a higher frequency (14.4 ± 6.7 points vs. 11.3 ± 5.4 points, p < .01) and severity (11.3 ± 5.4 points vs. 9.6 ± 5.3 points, p = .02) of hypoglycaemia‐related symptoms during the 4 days of moxifloxacin intake compared with placebo (Table S2).
3.4. Increased QTcF interval after intake of moxifloxacin
QTcF interval increased by 28 ms (95% CI: 24‐31) with moxifloxacin compared with placebo, and the difference persisted throughout the 6‐h OGTT (Figure 2 and Table S3), confirming the Kv11.1 block.
FIGURE 2.
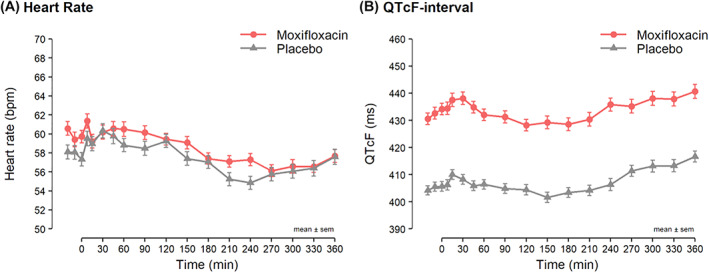
Heart rate and QTcF interval in ms during the 6 h oral glucose tolerance test divided into moxifloxacin (red circles) and placebo (grey triangles) test day. Means ± the standard error of the means are shown. QTcF is the QT corrected interval according to Fridericia's formula: QT/RR 1/3
3.5. Fasting glucose, insulin, glucagon‐like peptide‐1 and glucagon levels are unchanged after intake of moxifloxacin
Fasting glucose, insulin, GLP‐1 and glucagon levels were not different between the two test days (Table 2). The fasting C‐peptide level was 14% higher (95% CI: 8%‐21%, p < .001) on the moxifloxacin test day than on the placebo test day (Table 2).
TABLE 2.
Fasting and postprandial responses and indices during moxifloxacin or placebo treatment
| Moxifloxacin | Placebo | Difference or ratio | p‐unadj (p‐adj) | |
|---|---|---|---|---|
| Fasting | ||||
| Glucose (mmol/L) | 4.8 ± 0.4 | 4.9 ± 0.3 | −0.1 (−0.2; 0.0) | .07 (.09) |
| Insulin (pmol/L) a | 43 ± 19 | 39 ± 16 | 1.09 (0.95; 1.26) | .22 (.26) |
| C‐peptide (pmol/L) a | 630 ± 158 | 567 ± 166 | 1.14 (1.08; 1.21) | <.001 (<.01) |
| GLP‐1 (pmol/L) a | 11 ± 4 | 11 ± 5 | 0.97 (0.84; 1.13) | .72 (.72) |
| Glucagon (pmol/L) a | 11 ± 4 | 10 ± 4 | 1.04 (0.93: 1.16) | .54 (.59) |
| Total area under the curve | ||||
| 0‐30 min | ||||
| Glucose (mmol/L × min) | 172 ± 21 | 184 ± 19 | −12 (−17; −8) | <.001 (<.01) |
| Insulin (pmol/L × min) a | 4532 ± 2679 | 6200 ± 3021 | 0.71 (0.62; 0.8) | <.001 (<.01) |
| C‐peptide (pmol/L × min) a | 32 070 ± 10 881 | 36 976 ± 11 016 | 0.87 (0.8; 0.94) | <.001 (<.01) |
| GLP‐1 (pmol/L × min) a | 440 ± 130 | 541 ± 235 | 0.85 (0.76; 0.95) | <.01 (.02) |
| Glucagon (pmol/L × min) | 282 ± 95 | 260 ± 102 | 1.12 (0.99; 1.27) | .07 (.09) |
| 0‐120 min | ||||
| Glucose (mmol/L × min) | 729 ± 126 | 758 ± 126 | −28 (−60; 3) | .08 (.10) |
| Insulin (pmol/L × min) a | 25 266 ± 11 495 | 32 051 ± 12 949 | 0.77 (0.71; 0.84) | <.001 (<.01) |
| C‐peptide (pmol/L × min) a | 206 430 ± 48 092 | 240 039 ± 57 489 | 0.86 (0.81; 0.91) | <.001 (<.01) |
| GLP‐1 (pmol/L × min) a | 2371 ± 454 | 2751 ± 803 | 0.88 (0.82; 0.95) | <.01 (.02) |
| Glucagon (pmol/L × min) | 757 ± 251 | 687 ± 246 | 1.13 (1.00; 1.29) | .05 (.08) |
| 0‐360 min | ||||
| Glucose (mmol/L × min) | 1841 ± 168 | 1863 ± 185 | −22 (−64; 20) | .29 (.33) |
| Insulin (pmol/L × min) a | 37 636 ± 15 188 | 45 491 ± 18 671 | 0.82 (0.76; 0.89) | <.001 (<.01) |
| C‐peptide (pmol/L × min) a | 399 434 ± 83 430 | 439 635 ± 111 694 | 0.91 (0.87; 0.96) | <.001 (<.01) |
| GLP‐1 (pmol/L × min) a | 5624 ± 978 | 5782 ± 1317 | 0.98 (0.93; 1.04) | .58 (.61) |
| Glucagon (pmol/L × min) | 2905 ± 985 | 2522 ± 894 | 1.17 (1.04; 1.33) | .01 (.03) |
| Indices | ||||
| HOMA IR a | 1.48 ± 0.61 | 1.38 ± 0.60 | 1.11 (1.00; 1.23) | .045 (.07) |
| Matsuda index a | 7.85 ± 3.74 | 6.84 ± 2.57 | 1.11 (1.03; 1.21) | <.01 (.02) |
| MISI index | 0.32 ± 0.05 | 0.22 ± 0.02 | 1.34 (1.03; 1.75) | .03 (.05) |
| Insulinogenic index a | 2.91 ± 1.38 | 3.39 ± 1.7 | 0.85 (0.77; 0.94) | <.01 (.02) |
Values are shown as means ± SD. Differences are presented as estimated mean differences (95% CI). Abbreviation: GLP‐1, glucagon‐like peptide‐1.
Estimated geometric mean ratio. Adjusted p‐value by Benjamini‐Hochberg correction.
3.6. Decreased early phase (AUC0 ‐30 min) glucose, insulin, C‐peptide and glucagon‐like peptide‐1 responses to an oral glucose tolerance test after intake of moxifloxacin
In the early phase response (AUC0‐30 min) to an OGTT, moxifloxacin induced a decreased glucose (−12 mmol/L × min, 95% CI: −17 to −8, p < .0001), insulin secretion (−29%, 95% CI: −38% to −20%, p < .001), C‐peptide secretion (−13%, 95% CI: −20% to −6%, p < .001) and GLP‐1 secretion (−15%, 95% CI: −24%: −5%, p < .01) compared with placebo. The early phase response of glucagon was not different between moxafloxacin and placebo (Table 2 and Figure 3).
FIGURE 3.
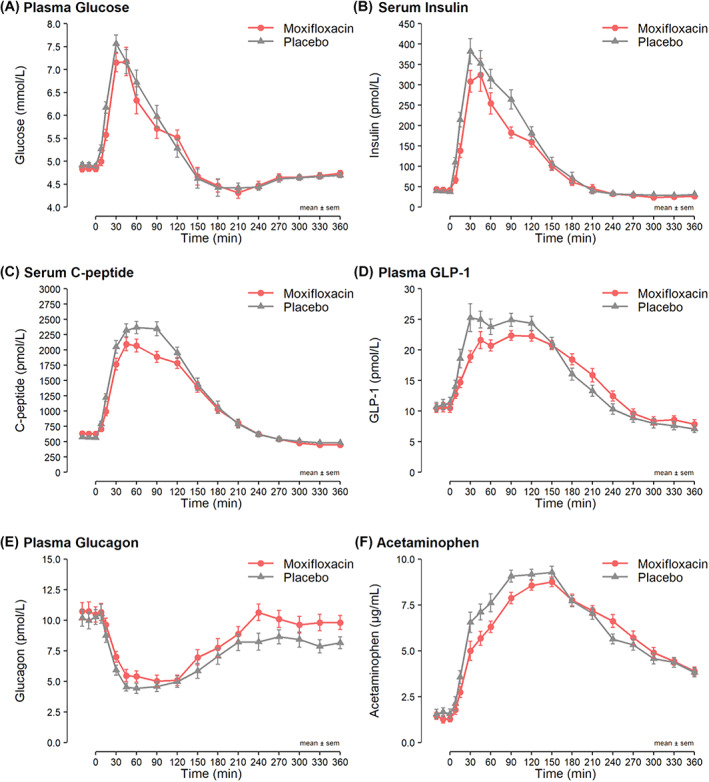
(A) Plasma glucose, (B). serum insulin, (C) serum C‐peptide, (D) plasma glucagon‐like peptide‐1 (GLP‐1), (E) plasma glucagon and (F) serum acetaminophen concentration during the 6‐h oral glucose tolerance test divided by moxifloxacin (red) and placebo (grey) test day. Means ± SEM
3.7. Decreased 2‐h (AUC0 ‐120 min) insulin, C‐peptide and glucagon‐like peptide‐1 responses to an oral glucose tolerance test after intake of moxifloxacin
Moxifloxacin decreased the 2‐h responses (AUC0‐120 min) to an OGTT of insulin secretion (−23%, 95% CI: −29% to −16%, p < .001), C‐peptide secretion (−13%, 95% CI: −20% to −6%, p < .001) and GLP‐1 secretion (−12%, 95% CI: −18%: −5%, p < .01) compared with placebo control. The 2‐h responses of glucose and glucagon were similar on moxifloxacin and placebo days (Table 2 and Figure 3).
3.8. Decreased 6‐h (AUC0 ‐360 min) insulin and C‐peptide responses, but increased glucagon responses to oral glucose tolerance test after intake of moxifloxacin
In the full 6 h after the oral glucose load (AUC0‐360 min) moxifloxacin induced a decreased insulin secretion (−18%, 95% CI: −24% to −11%, p < .001), a decreased C‐peptide secretion (−9%, 95% CI: −13% to −4%, p < .001), whereas glucagon secretion increased markedly (17%, 95% CI: 4% to 33%, p = .01) compared with placebo control. The total 6‐h responses of glucose and GLP‐1 were not different between moxifloxacin and placebo (Table 2 and Figure 3).
3.9. Increased HOMA‐IR, Matsuda index and muscle insulin sensitivity index, and lowered insulinogenic index after intake of moxifloxacin
HOMA‐IR, an estimation of hepatic insulin resistance, increased by 11% (95% CI: 0%‐23%, p = .045) compared with placebo. The Matsuda index, an index of whole‐body insulin sensitivity, increased by 11% (95% CI: 3%‐21%, p < .01) and MISI, an index of muscle insulin sensitivity, increased by 34% (95% CI: 3%‐75%, p = .03) compared with placebo. The insulinogenic index, an index of early‐phase insulin response (0‐30 min), decreased by 15% (95% CI: −23% to −6%, p < .01).
3.10. Delayed gastric emptying for the first 2 h of the oral glucose tolerance test after intake of moxifloxacin
Acetaminophen levels, an indirect measure of gastric emptying, were lower with moxifloxacin for the first 2 h of the OGTT (AUC0‐30 min: −21%, 95% CI: −35% to −4%, and AUC0‐120 min: −16%, 95% CI: −22% to −9%). Over the whole 6‐h OGTT, the uptake of acetaminophen (AUC0‐360 min) was unchanged (Figure 3 and Table S4).
4. DISCUSSION
In this crossover study, we showed that in young men and women without chronic disease, moxifloxacin, a drug known to block the Kv11.1 channel, is associated with decreased plasma glucose, increased insulin sensitivity and more frequent episodes of hypoglycaemia.
Moxifloxacin is a known blocker of the Kv11.1 channel, 37 causing prolongation of the QTcF interval, which was verified in this study where participants had marked prolongation of the QTcF interval with moxifloxacin treatment.
Episodes of hypoglycaemia in patients with type 2 diabetes have been reported more frequently after intake of fluoroquinolones. 11 , 12 The participants in the present study were young, did not have diabetes or other chronic conditions and did not take any medication. Nevertheless, continuous glucose monitoring revealed that during treatment with moxifloxacin more time was spent with a glucose level <3.0 mmol/L and with more frequent excursions <3.3 mmol/L. Moreover, glucose levels <3.9 mmol/L were more frequent during the OGTT (Table 1 and Figure S2). Hypoglycaemia questionnaires revealed a higher overall score, with higher frequency and severity.
During the first 2 h of the OGTT, plasma glucose levels and insulin, C‐peptide and GLP‐1 secretion, measured as postprandial AUCs, were lower after intake of moxifloxacin compared with placebo. As the ratio between plasma glucose levels and serum insulin changed, the Matsuda index, an index of whole‐body insulin sensitivity, increased and the MISI also increased. Kv11.1 channels have recently been found expressed in human skeletal muscle. 18 The Kv11.1 channel is more abundant in atrophied skeletal muscle of patients with cancer and in immobilization‐ and denervation‐induced atrophied skeletal muscle in mice. 7 , 38 , 39 In mice, immobilization‐induced atrophy is prevented by astemizole, an antihistamine that also blocks Kv11.1 channels. 7 Kv11.1 seems to be involved in proteolytic activity of skeletal muscles and is hypothesized to have a role in excitation‐contraction coupling. 18 Higher 3H‐deoxy‐glucose uptake into skeletal muscle, liver, kidney and lung tissue has been found in Kcnq1‐deficient mice (lacking the Kv7.1 channel) than wild‐type littermates. 17 The insulin sensitivity was increased in the Kcnq1‐deficient mice, and the shaker phenotype may also increase muscle activity. 17 Furthermore, these mice had increased insulin sensitivity with lower glucose levels, despite higher plasma glucagon. 17 Interestingly, we also observed increased insulin sensitivity and higher plasma glucagon levels during the OGTT with moxifloxacin blockade of the Kv11 channel. The observed increased insulin sensitivity may be connected to the hypothesized role in excitation‐contraction coupling. 18 HOMA‐IR, an indirect measure of hepatic insulin resistance, increased, although not significantly, after intake of moxifloxacin, possibly because of the off‐target drug effect of moxifloxacin on the liver, reflected by mild increases in alanine aminotransferase levels previously found during treatment. 40
Glucagon levels increased significantly from 120 to 360 min during the OGTT after intake of moxifloxacin, despite similar glucose levels, as also observed in the Kcnq1‐deficient mouse. 17 The increased glucagon during the OGTT may be a counter‐regulatory response to the more frequent episodes of low glucose observed both during OGTT monitoring and continuous glucose monitoring to avoid severe hypoglycaemia.
The acetaminophen AUC was reduced during the first 2 h of the OGTT after intake of moxifloxacin. Acetaminophen is absorbed in the proximal small intestine, and lower postprandial values (AUC) indicate slower gastric emptying or decreased absorption from the small intestine, 28 which also may explain the lower GLP‐1 levels during the first 2 h of the OGTT. These effects may represent general antibiotic/fluoroquinolone off‐target effects or specific Kv11.1 blockade via Kv11.1 channels expressed in the jejunum. 41 Kv11.1 blockade induced by the agents E‐4031 or MK‐499 changed human jejunal smooth muscle electrical activity. 41 At low concentrations, E‐4031 or MK‐499 increased this electrical activity, but at higher concentrations, reductions in both contractile amplitude and frequency were found until, at the highest concentration, no contraction occurred. 41 The participants in our study had a decreased glucose‐stimulated insulin secretion and increased glucagon response compared with the insulin hypersecretion and glucagon hyposecretion seen in patients with LoF mutations in KCNH2 encoding Kv11.1, particularly in those with a mutation in the PAS domain of the channel. 15
Fluoroquinolones/quinolones have previously been shown in vitro to enhance glucose‐stimulated insulin secretion in mouse and rat islets because of blockade of the KATP channels. 42 , 43 , 44 However, these studies report substantial variability in the observed effect, depending on which specific drug was investigated. 42 , 43 , 44 Thus, for moxifloxacin, in an in vitro study of mouse pancreatic islets, glucose‐stimulated insulin secretion was only enhanced at glucose levels of 5 mM with a very high concentration of moxifloxacin (500 μM) or at glucose 10 mM with a high concentration of moxifloxacin (100 μM). 44 In a clinical study in humans, a single dose of 250 mg moxifloxacin gave a peak concentration of 2.2 μg/ml = 5.5 μM, 45 thus much lower concentrations than those reported in vitro (5.5 μM in humans vs. 100‐500 μM in vitro). Thus, the lower in vivo concentration in humans of moxifloxacin may explain why our findings do not mimic the very high in vitro concentration‐induced findings. Furthermore, interestingly moxifloxacin compared with other fluoroquinolones, has been shown to influence mitochondrial function in mouse beta cells that could oppose any stimulatory effects on insulin secretion. 46 Thus moxifloxacin decreased the ATP/ADP ratio in response to 20 mM glucose in murine beta‐cells, thereby reducing the signal for closing the KATP channels and possibly lowering insulin secretion. 46 Our data support this notion and indicate that hypoglycaemia during moxifloxacin is because of increased peripheral insulin sensitivity, not increased insulin secretion.
Thus, we speculate that moxifloxacin induces a primary increase in insulin sensitivity because of KCNH2 blockade in skeletal muscle, probably leading to increased tonus because of delayed repolarization, and thereby insulin‐independent contraction‐mediated GLUT4 translocation to the membrane and enhanced insulin‐independent glucose transport.
In combination with the effect of moxifloxacin on the K‐ATP channel, perturbing the glucose stimulus‐secretion coupling that masks the effect of Kv11.1 blockade on delayed repolarization and hypersecretion in the beta‐cell, this may lead to a reduction in insulin secretion, which however is insufficient to compensate fully for the increase in peripheral insulin sensitivity, thereby leading to hypoglycaemia and a counter‐regulatory glucagon response to maintain 0‐120 min glucose normal, while the lower GLP1 response is mostly an effect of delayed gastric emptying.
Moxifloxacin‐induced impaired beta‐cell mitochondrial function could also be the reason for increased episodes of hypoglycaemia in patients with type 2 diabetes. 46 The more frequent, longer and symptomatic episodes of hypoglycaemia during moxifloxacin treatment reported here in young men and women, not on any other medication certainly warrant cautious use of fluoroquinolones in individuals with a risk of hypoglycaemia.
Strengths of the present study include the novelty of investigating the role of Kv11.1 in glucose regulation in young men and women and the discovery of moxifloxacin‐induced hypoglycaemia even in young men and women without chronic disease. Hypoglycaemia is a serious side effect, which can be life threatening in patients with diabetes. The deleterious effect is exaggerated by affecting the electrical activity of the heart in the form of a prolonged QT interval. Thus, studying effects of a commonly used antibiotic is of great clinical value. Furthermore, strengths include the crossover design, which reduces confounding covariates as each participant is his/her own control. Participants received a minor remuneration for their study participation, which was approved by the National Committee on Health Research Ethics in Denmark. Compliance to the study protocol was high, and increased QTcF (with a mean of 28 ms) in all participants, during active medication, strongly supported the intake of active medication for all participants.
Limitations of the study include the potential off‐target drug effects of the study drug. We used OGTTs to investigate glucose regulation, as it stimulates the release of incretins and therefore mimics better an everyday setting than a standard clamp experiment, therefore calculated values from OGTTs were used to assess insulin sensitivity. However, using calculated values of insulin sensitivity from an OGTT is an estimation and not as precise as euglycaemic‐hyperinsulinaemic clamp (golden standard). Continuous glucose monitors were used to evaluate glucose regulation over a longer period in everyday living. The monitors have been improved regarding accuracy, also in the lower glucose ranges; however, potential accuracy limitations, particularly within the lower ranges may still exist. 47 Acetaminophen absorption was used as a surrogate marker for gastric emptying, not as precise as scintigraphy but more durable and has proven to be reliable. 48
5. CONCLUSION
In young men and women without chronic disease, moxifloxacin, a drug known to block the Kv11.1 channel, increased the QT interval, decreased glucose levels and increase insulin sensitivity, associated with an increased risk of hypoglycaemia.
AUTHOR CONTRIBUTIONS
SST and JKK designed the study. CRJ contributed to the design of the study, the data collection, the statistical analyses, the interpretation of data and wrote the first draft of the manuscript. JB and CK contributed to the design of the study and the collection of data. JJH provided data. AFL and TMP contributed to the interpretation of the data. SV contributed to the design of the study and the interpretation of the data. JLI contributed to the statistical analyses. JJH, BH and RFS contributed to the production and interpretation of data. SST contributed to the design of the study, obtained funding, interpretation of data and contributed to the first draft of the manuscript. All authors critically reviewed the manuscript and approved the version to be published. CRJ and SST are the guarantors of the manuscript and, thereby, are guarantors of the integrity of the data and the results reported in this manuscript.
AUTHORS' RELATIONSHIPS AND ACTIVITIES
JJH was supported by the Novo Nordisk Foundation.
FUNDING
The study was funded by grants from the Independent Research Fund Denmark, the Danish Heart Association, and the Lundbeck Foundation. The funding sources for the study were not involved in the design of the study, the collection, analysis, and interpretation of data writing the report, and did not impose any restrictions regarding publication.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14851.
TRIAL REGISTRATION
The Danish National Committee on Health Research Ethics: H‐17013807. Clinicaltrial.org: NCT03868657.
Supporting information
Appendix S1. Supporting Information
ACKNOWLEDGMENTS
The authors wish to express their sincere appreciation to the study participants and biomedical laboratory scientist Desirée Hornbæk Milling for her exceptional assistance with the investigation. A poster with preliminary results of this study was presented at the 80th Scientific Session of the American Diabetes Association (ADA) and at the 56th Annual Meeting of the European Association for the Study of Diabetes (EASD).
Juhl CR, Burgdorf J, Knudsen C, et al. A randomized, double‐blind, crossover study of the effect of the fluoroquinolone moxifloxacin on glucose levels and insulin sensitivity in young men and women. Diabetes Obes Metab. 2023;25(1):98‐109. doi: 10.1111/dom.14851
Funding information Danmarks Frie Forskningsfond; Hjerteforeningen; Lundbeckfonden
DATA AVAILABILITY STATEMENT
Upon reasonable request and if following the regulations of the Danish Data Protection Agency, data may be shared.
REFERENCES
- 1. Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage‐gated potassium channel family. Science. 1995;269(5220):92‐95. doi: 10.1126/science.7604285 [DOI] [PubMed] [Google Scholar]
- 2. Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquird cardiac arrthytmia: HERG encodes the IKr potassium channel. Cell. 1995;81(2):299‐307. doi: 10.1016/0092-8674(95)90340-2 [DOI] [PubMed] [Google Scholar]
- 3. Rosati B, Marchetti P, Crociani O, et al. Glucose‐ and arginine‐induced insulin secretion by human pancreatic β‐cells: the role of HERG K + channels in firing and release. FASEB J. 2000;14(15):2601‐2610. doi: 10.1096/fj.00-0077com [DOI] [PubMed] [Google Scholar]
- 4. Hardy AB, Fox JEM, Giglou PR, et al. Characterization of Erg K + channels in α‐ and β‐cells of mouse and human islets. J Biol Chem. 2009;284(44):30441‐30452. doi: 10.1074/jbc.M109.040659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lubberding AF, Juhl CR, Skovhøj EZ, Kanters JK, Mandrup‐Poulsen T, Torekov SS. Celebrities in the heart, strangers in the pancreatic beta cell: voltage‐gated potassium channels Kv7.1 and Kv11.1 bridge long QT syndrome with hyperinsulinaemia as well as type 2 diabetes. Acta Physiol. 2022;234(3):e13781. doi: 10.1111/apha.13781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vandenberg JI, Perry MD, Perrin MJ, Mann SA, Ke Y, Hill AP. hERG K(+) channels: structure, function, and clinical significance. Physiol Rev. 2012;92(3):1393‐1478. doi: 10.1152/physrev.00036.2011 [DOI] [PubMed] [Google Scholar]
- 7. Wang X, Hockerman GH, Green HW, et al. Mergla K + channel induces skeletal muscle atrophy by activating the ubiquitin proteasome pathway. FASEB J. 2006;20(9):1531‐1533. doi: 10.1096/fj.05-5350fje [DOI] [PubMed] [Google Scholar]
- 8. Wang W, MacKinnon R. Cryo‐EM structure of the open human ether‐à‐go‐go‐related K + channel hERG. Cell. 2017;169(3):422‐430.e10. doi: 10.1016/j.cell.2017.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nielsen J, Graff C, Kanters JK, Toft E, Taylor D, Meyer JM. Assessing QT interval prolongation and its associated risks with antipsychotics. CNS Drugs. 2011;25(6):473‐490. doi: 10.2165/11587800-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 10. Valentin JP, Hoffmann P, Ortemann‐Renon C, et al. The challenges of predicting drug‐induced QTc‐prolongation in humans. Toxicol Sci. 2022;187:3‐24. doi: 10.1093/toxsci/kfac013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chou HW, Wang JL, Chang CH, Lee JJ, Shau WY, Lai MS. Risk of severe dysglycemia among diabetic patients receiving levofloxacin, ciprofloxacin, or moxifloxacin in Taiwan. Clin Infect Dis. 2013;57(7):971‐980. doi: 10.1093/cid/cit439 [DOI] [PubMed] [Google Scholar]
- 12. Kennedy KE, Teng C, Patek TM, Frei CR. Hypoglycemia associated with antibiotics alone and in combination with sulfonylureas and meglitinides: an epidemiologic surveillance study of the FDA adverse event reporting system (FAERS). Drug Saf. 2020;43(4):363‐369. doi: 10.1007/s40264-019-00901-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. FDA . FDA updates warnings for fluoroquinolone antibiotics on risks of mental health and low blood sugar adverse reactions. https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics-risks-mental-health-and-low-blood-sugar-adverse. 2018. https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics-risks-mental-health-and-low-blood-sugar-adverse. Accessed March 7, 2022.
- 14. Curran ME, Splawski I, Timothy KW, Vincen GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80(5):795‐803. doi: 10.1016/0092-8674(95)90358-5 [DOI] [PubMed] [Google Scholar]
- 15. Hyltén‐Cavallius L, Iepsen EW, Wewer Albrechtsen NJ, et al. Patients with long‐QT syndrome caused by impaired hERG ‐encoded Kv11.1 potassium channel have exaggerated endocrine pancreatic and incretin function associated with reactive hypoglycemia. Circulation. 2017;135(18):1705‐1719. doi: 10.1161/CIRCULATIONAHA.116.024279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Torekov SS, Iepsen E, Christiansen M, et al. KCNQ1 long QT syndrome patients have hyperinsulinemia and symptomatic hypoglycemia. Diabetes. 2014;63(4):1315‐1325. doi: 10.2337/db13-1454 [DOI] [PubMed] [Google Scholar]
- 17. Boini KM, Graf D, Hennige AM, et al. Enhanced insulin sensitivity of gene‐targeted mice lacking functional KCNQ1. Am J Physiol: Regul, Integr Comp Physiol. 2009;296(6):22‐26. doi: 10.1152/ajpregu.90839.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zampieri S, Sandri M, Cheatwood JL, et al. The ERG1A K + channel is more abundant in rectus abdominis muscle from cancer patients than that from healthy humans. Diagnostics. 2021;11(10):1879. doi: 10.3390/diagnostics11101879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moise PA, Birmingham MC, Schentag JJ. Pharmacokinetics and metabolism of moxifloxacin. Drugs Today. 2000;36(4):229‐244. doi: 10.1358/dot.2000.36.4.570201 [DOI] [PubMed] [Google Scholar]
- 20. Dwan K, Li T, Altman DG, Elbourne D. CONSORT 2010 statement: extension to randomised crossover trials. BMJ. 2019;366:l4378. doi: 10.1136/bmj.l4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bentham J, Di Cesare M, Bilano V, et al. Worldwide trends in body‐mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population‐based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627‐2642. doi: 10.1016/S0140-6736(17)32129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Danskernes sundhed Den Nationale Sundhedsprofil 2021 . 2022. www.sst.dk. Accessed March 28, 2022.
- 23. Bischoff U, Schmidt C, Netzer R, Pongs O. Effects of fluoroquinolones on HERG currents. Eur J Pharmacol. 2000;406(3):341‐343. doi: 10.1016/S0014-2999(00)00693-2 [DOI] [PubMed] [Google Scholar]
- 24. Kang J, Wang L, Chen XL, Triggle DJ, Rampe D. Interactions of a series of fluoroquinolone antibacterial drugs with the human cardiac K + channel HERG. Mol Pharmacol. 2001;59(1):122‐126. doi: 10.1124/mol.59.1.122 [DOI] [PubMed] [Google Scholar]
- 25. Qiu HY, Yuan SS, Yang FY, Shi TT, Yang JK. HERG protein plays a role in moxifloxacin‐induced hypoglycemia. J Diabetes Res. 2016;2016:6741745‐6741746. doi: 10.1155/2016/6741745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Démolis JL, Kubitza D, Tennezé L, Funck‐Brentano C. Effect of a single oral dose of moxifloxacin (400 mg and 800 mg) on ventricular repolarization in healthy subjects. Clin Pharmacol Ther. 2000;68(6):658‐666. doi: 10.1067/MCP.2000.111482 [DOI] [PubMed] [Google Scholar]
- 27. Vigers T, Chan CL, Snell‐Bergeon J, et al. Cgmanalysis: an R package for descriptive analysis of continuous glucose monitor data. PLoS One. 2019;14(10):e0216851. doi: 10.1371/journal.pone.0216851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clements JA, Heading RC, Nimmo WS, Prescott LF. Kinetics of acetaminophen absorption and gastric emptying in man. Clin Pharmacol Ther. 1978;24(4):420‐431. doi: 10.1002/cpt1978244420 [DOI] [PubMed] [Google Scholar]
- 29. Ørskov C, Rabenhøj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine‐extended glucagon‐like peptide I in humans. Diabetes. 1994;43(4):535‐539. doi: 10.2337/DIAB.43.4.535 [DOI] [PubMed] [Google Scholar]
- 30. Orskov C, Jeppesen J, Madsbad S, Holst JJ. Proglucagon products in plasma of noninsulin‐dependent diabetics and nondiabetic controls in the fasting state and after oral glucose and intravenous arginine. J Clin Invest. 1991;87(2):415‐423. doi: 10.1172/JCI115012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fridericia LS. The duration of systole in an electrocardiogram in normal humans and in patients with heart disease. Ann Noninvasive Electrocardiol. 2003;8(4):343‐351. doi: 10.1046/j.1542-474X.2003.08413.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412‐419. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 33. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462‐1470. doi: 10.2337/diacare.22.9.1462 [DOI] [PubMed] [Google Scholar]
- 34. Hovorka R, Soons PA, Young MA. ISEC: A program to calculate insulin secretion. Comput Methods Programs Biomed. 1996;50(3):253‐264. doi: 10.1016/0169-2607(96)01755-5 [DOI] [PubMed] [Google Scholar]
- 35. O'Donovan SD, Lenz M, Goossens GH, et al. Improved quantification of muscle insulin sensitivity using oral glucose tolerance test data: the MISI calculator. Sci Rep. 2019;9(1):1‐10. doi: 10.1038/s41598-019-45858-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc, Ser B. 1995;57(1):289‐300. doi: 10.1111/J.2517-6161.1995.TB02031.X [DOI] [Google Scholar]
- 37. Alexandrou AJ, Duncan RS, Sullivan A, et al. Mechanism of hERG K + channel blockade by the fluoroquinolone antibiotic moxifloxacin. Br J Pharmacol. 2006;147(8):905‐916. doi: 10.1038/sj.bjp.0706678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Anderson LB, Ravara B, Hameed S, et al. Merg1a protein abundance increases in the atrophied skeletal muscle of denervated mice, but does not affect nfκb activity. J Neuropathol Exp Neurol. 2021;80(8):776‐788. doi: 10.1093/jnen/nlab062 [DOI] [PubMed] [Google Scholar]
- 39. Whitmore C, Pratt EPS, Anderson L, et al. The ERG1a potassium channel increases basal intracellular calcium concentration and calpain activity in skeletal muscle cells. Skeletal Muscle. 2020;10(1):1‐15. doi: 10.1186/s13395-019-0220-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bethesda (MD) . LiverTox: Clinical and Research Information on Drug‐Induced Liver Injury [Internet]. National Institute of Diabetes and Digestive and Kidney Diseases. [PubMed]
- 41. Farrelly AM, Ro S, Callaghan BP, et al. Expression and function of KCNH2 (HERG) in the human jejunum. Am J Physiol‐Gastrointest Liver Physiol. 2003;284(6):883‐895. doi: 10.1152/ajpgi.00394.2002 [DOI] [PubMed] [Google Scholar]
- 42. Saraya A, Yokokura M, Gonoi T, Seino S. Effects of fluoroquinolones on insulin secretion and beta‐cell ATP‐sensitive K + channels. Eur J Pharmacol. 2004;497(1):111‐117. doi: 10.1016/J.EJPHAR.2004.06.032 [DOI] [PubMed] [Google Scholar]
- 43. Maeda N, Tamagawa T, Niki I, et al. Increase in insulin release from rat pancreatic islets by quinolone antibiotics. Br J Pharmacol. 1996;117(2):372‐376. doi: 10.1111/J.1476-5381.1996.TB15201.X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ghaly H, Kriete C, Sahin S, et al. The insulinotropic effect of fluoroquinolones. Biochem Pharmacol. 2009;77(6):1040‐1052. doi: 10.1016/J.BCP.2008.11.019 [DOI] [PubMed] [Google Scholar]
- 45. Lode H. Evidence of different profiles of side effects and drug‐drug interactions among the quinolones‐the pharmacokinetic standpoint. Chemotherapy. 2001;47(3):24‐31. www.karger.comwww.karger.com/journals/che. Accessed August 13, 2022 [DOI] [PubMed] [Google Scholar]
- 46. Ghaly H, Jörns A, Rustenbeck I. Effect of fluoroquinolones on mitochondrial function in pancreatic beta cells. Eur J Pharm Sci. 2014;52(1):206‐214. doi: 10.1016/j.ejps.2013.11.011 [DOI] [PubMed] [Google Scholar]
- 47. Reddy N, Verma N, Dungan K. Monitoring technologies‐continuous glucose monitoring, Mobile technology, biomarkers of glycemic control. Endotext. 2020;16:1‐70. https://www.ncbi.nlm.nih.gov/books/NBK279046/. Accessed August 9, 2022. [PubMed] [Google Scholar]
- 48. Medhus AW, Lofthus CM, Bredesen J, Husebye E. Gastric emptying: the validity of the paracetamol absorption test adjusted for individual pharmacokinetics. Neurogastroenterol Motil. 2001;13(3):179‐185. doi: 10.1046/J.1365-2982.2001.00249.X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supporting Information
Data Availability Statement
Upon reasonable request and if following the regulations of the Danish Data Protection Agency, data may be shared.


