Summary
We conducted a systematic review to evaluate the effect of high‐flow nasal oxygen and conventional oxygen therapy during procedural sedation amongst adults and children. We searched MEDLINE, EMBASE and CINAHL for randomised controlled trials that reported the effects of high‐flow nasal oxygen during procedural sedation. The primary outcome measure was hypoxaemia and the secondary outcomes were minimum oxygen saturation; hypercarbia; requirement for airway manoeuvres; and procedure interruptions. The quality of evidence was assessed using the revised Cochrane risk‐of bias tool and grading of recommendations, assessment, development and evaluation (GRADE). Nineteen randomised controlled trials (4121 patients) including three in children were included. Administration of high‐flow nasal oxygen reduced hypoxaemia, risk ratio (95%CI) 0.37 (0.24–0.56), p < 0.001; minor airway manoeuvre requirements, risk ratio (95%CI) 0.26 (0.11–0.59), p < 0.001; procedural interruptions, risk ratio (95%CI) 0.17 (0.05–0.53), p = 0.002; and increased minimum oxygen saturation, mean difference (95%CI) 4.1 (2.70–5.50), p < 0.001; as compared with the control group. High‐flow nasal oxygen had no impact on hypercarbia, risk ratio (95%CI) 1.24 (0.97–1.58), p = 0.09, I2 = 0%. High‐flow nasal oxygen reduced the incidence of hypoxaemia regardless of the procedure involved, degree of fractional inspired oxygen, risk‐profile of patients and mode of propofol administration. The evidence was ascertained as moderate for all outcomes except for procedure interruptions. In summary, high‐flow nasal oxygen compared with conventional oxygenation techniques reduced the risk of hypoxaemia, increased minimum oxygen saturation and reduced the requirement for airway manoeuvres. High‐flow nasal oxygen should be considered in patients at risk of hypoxaemia during procedural sedation.
Keywords: bronchoscopy, endoscopy, high‐flow nasal oxygen, hypoxaemia, procedural sedation
Introduction
Procedural sedation facilitates simple as well as complex interventions through the application of sedative‐hypnotic and/or analgesic medications [1]. Medications used to achieve this sedative state can induce respiratory depression and upper airway obstruction, both of which may lead to hypoxaemia. Analysis of case records of 8000 procedural sedations across 39 countries revealed oxygen desaturation as the most prevalent adverse event during procedural sedation, followed by airway obstruction and apnoea [2]. Varying definitions of hypoxaemia have been used in this context, with the incidence being reported as high as 60% [3]. Risk factors for hypoxaemia include: higher ASA physical status; reduced cardiopulmonary reserve; known or suspected sleep apnoea; obesity; and prolonged procedural duration [4, 5]. Extended periods of hypoxaemia can result in myocardial ischaemia and cardiac arrhythmias [6, 7].
Conventional methods of oxygen supplementation during sedation include low‐flow oxygen delivery devices such as standard nasal prongs [8] and simple facemasks [9]. With these devices, the maximum flow rate is within 10–15 l.min‐1, whereby FiO2 is dependent on the inspiratory flow rate and usually lies within 0.3–0.6 [10]. High‐flow nasal oxygen (HFNO) has been recently adopted as an alternative to conventional oxygen delivery methods due to several advantageous features [8, 11, 12, 13]. These include delivery of FiO2 up to 1.0 with flows up to 70 l.min‐1 to overcome a patient's peak inspiratory flow rate, flushing out anatomical dead space and generating flow‐dependent positive airway pressure [14].
Six systematic reviews have been published exploring the effects of HFNO during gastrointestinal, bronchoscopy and dental interventions [15, 16, 17, 18, 19, 20]. Although these reviews were in favour of HFNO in mitigating the risk of hypoxaemia, there was a paucity of subgroup analyses exploring the influence of covariates and how it may influence the overall interpretation of data. Since then, eight new trials, including three in children, both in favour of [21, 22, 23, 24, 25, 26, Scheuermann‐Jahn et al, preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1] and against [27] the application of HFNO have been published, which adds to the body of evidence. A comprehensive review reporting the effect of HFNO across all procedures and age groups using grading of recommendations, assessment, development, and evaluation (GRADE) recommendations is lacking. This systematic review and meta‐analysis had the primary objective of evaluating the efficacy of HFNO in reducing hypoxaemia in both adults and children undergoing sedation for various types of procedures when compared with conventional oxygen delivery.
Methods
An a priori protocol was established based on the PRISMA statement [28]. We searched MEDLINE, EMBASE and CINAHL databases for randomised controlled trials (RCTs) from database inception to December 2021 (online Supporting Information, Appendix S1). The titles and abstracts of all relevant articles were screened, and the reference lists of studies selected for critical appraisal were screened for additional studies. Grey literature and unpublished articles were searched for through Google Scholar, the PROSPERO registry and the International Trials Registry Platform of the World Health Organisation (https://trialssearch.who.int).
The included studies consisted of RCTs that investigated the effects of HFNO use in any age group which underwent elective procedures under sedation. Trials comparing HFNO against any single or combined conventional oxygen delivery therapy (simple facemask, standard nasal cannula or oxygenating mouth guard) were included. Studies were deemed to clearly define the patient characteristics, intervention types and technical aspects of sedation. We did not anaylse studies that used airway topicalisation with local anaesthesia without pharmacological sedation, those reporting emergency procedures, procedures requiring neuromuscular blockade drugs (e.g. electroconvulsive therapy) and those comparing tracheal intubation or supraglottic airway insertion. Studies involving patients who were pregnant or critically ill were also not analysed. Titles and abstracts were screened for and assessed against inclusion criteria by two authors (VT and VS). The methodological quality of the included studies were assessed using the Cochrane Collaboration's new version risk of bias 2 tool for assessing risk of bias in randomised trials [29]. The domains assessed were: randomisation process; deviations from intended interventions; missing outcome data; measurement of the outcome; and selection of the reported result. A series of questions with the answers (‘yes’; ‘probably yes’; ‘no information’; ‘probably no’; and ‘no’) determined the risk of bias as low risk, some concerns, and high risk. If we estimated one domain as ‘some concerns’ or ‘high risk’, this judgement was taken for the entire outcome.
The following data were extracted for each trial: study design; procedure type; patient characteristics; oxygen supplementation; FiO2; sedation technique; description of desaturation; minimum SpO2; hypercarbia; airway manoeuvres; procedural interruptions and other reported adverse events. For the complete analysis, see online Supporting Information (Table S1). Disagreements were resolved by consensus within the group.
Our primary outcome measure was hypoxaemia (as a desaturation event) defined as a dichotomous outcome as outlined by the primary studies. Secondary outcomes were: minimum SpO2 during the procedure; hypercarbia; requirement of minor airway manoeuvres, defined as chin lift/jaw thrust or insertion of oral/nasopharyngeal airway; and procedural interruptions. Trials reporting one or more of these outcomes were included. Our definition of hypoxaemia and hypercarbia was in congruence with that of the original included trials and no specific SpO2 or end‐tidal carbon dioxide (ETCO2) thresholds were adopted. For trials that reported hypoxaemia with more than one threshold SpO2, such as set values of SpO2 above 90%, described as mild, moderate and severe, we extracted the threshold value closest to 90% for our analyses.
We performed a meta‐analysis based on the Cochrane Handbook for Systematic Reviews of Interventions when the outcomes were reported by two or more trials [30]. Statistical analysis was performed using ReviewManager (version 5.3, The Cochrane Collaboration, Oxford, UK). For binary outcomes, both numerator and denominator were extracted for each of the randomised groups. For continuous outcomes, mean, standard deviation (SD) and sample size were extracted from each of the randomised groups. Where studies reported median and ranges or interquartile range, we derived mean and standard deviation as described by Wan et al. [31]. We calculated risk ratios (RR) with 95%CI for binary outcomes and mean differences with 95%CI for continuous outcomes. A random effect Mantel–Haenszel model was used to calculate RR and the inverse variance method was applied for mean difference. Heterogeneity was assessed using I2 statistics and values between 50% and 90% were considered to represent substantial heterogeneity. We expected clinical heterogeneity between the trials, as the effect estimates were likely to be derived from diverse patient characteristics, procedure types, and sedation techniques. This was our rationale for choosing a random effects meta‐analysis model and exploring heterogeneity with sensitivity and subgroup analysis. Publication bias was examined visually using Begg's funnel plot, which is recommended when outcomes are reported by at least 10 trials. Plots fulfilling these criteria were considered symmetrical: similar number of trials being present on either side of the effect estimate; effect estimates of predominantly larger studies being close to the RR; and effect estimates scattering widely at the bottom from a few smaller studies being minimal. A priori sensitivity analysis was planned to analyse the outcome of hypoxaemia by excluding studies with a high risk of bias. A sensitivity analysis was also planned by repeating the analysis by deleting one study at a time (leave‐one‐method) for all outcomes reported by at least two trials. We planned a priori subgroup analysis for the outcome of hypoxaemia regardless of the heterogeneity to assess potential confounding factors and to distinguish the subpopulations that were most likely to benefit from HFNO therapy. The groups were stratified as: procedure sub‐types; participants deemed at risk of hypoxaemia vs. not at risk (as judged by the included trials); FiO2 administration in the HFNO group – 1.0 vs. < 1.0; mode of propofol administration – intermittent bolus vs. continuous infusion including target‐controlled infusions; and adults vs. children. We considered a p value of <0.05 to be statistically significant.
We used the GRADE recommendations [32] to categorise the certainty of evidence for each outcome as high, moderate, low and very low. Certainty of the evidence indicates how certain one can be that an effect estimate represents the true effect and was assessed for individual outcomes from pooled estimates. Two review authors independently assessed certainty to downgrade the quality of evidence. This involved examination of limitations in five domains: study design (risk of bias or methodological issues); directness of the evidence; consistency across studies; precision of estimates; and probability of publication bias. A pre‐specified subgroup analysis was also used to assess the study weights of trials with high risk of bias for the outcome of hypoxaemia, as guided by a recent editorial [33]. If there was no subgroup effect identified in terms of risk of bias, we decided to infer our GRADE judgements based on all trials with increased precision without rating down this domain for this outcome. Conversely, if a subgroup difference for this domain was encountered, we decided to exclude trials with high risk of bias as per the recommendations from the GRADE working group [33].
Results
Of the 129 studies identified, 19 RCTs published between 2015 and 2021 were included with a total of 4121 patients, of which 2059 received HFNO and 2062 received conventional oxygenation, deemed to be the control group (Fig. 1). The sample sizes ranged from 30 to 1994 patients. For the complete analysis see online Supporting Information (Table S1). The included studies comprised eight gastrointestinal [8, 9, 12, 22, 34, 35, 36, 37], five bronchoscopy [11, 24, 26, 38, 39], three cardiology [21, 27, Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1], two dental trials [23, 40] and one endovascular trial [25]. Three of the trials studied children [21, 23, 26], one each in cardiology [21], bronchoscopy [26] and dental procedures [23] with a total sample size of 329 patients. Four trials assessed patients at high risk of developing hypoxaemia [9, 22, 35, 37] and one trial studied such patients when they presented for transcatheter aortic valve implantation [Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1]. Within the HFNO group, nine trials used FiO2 at 1.0 [11, 12, 26, 27, 34, 36, 37, 38, 39] and nine used below 1.0 [9, 21, 22, 23, 24, 25, 35, 40, Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1], with this information being unavailable in one trial [8]. The flow rates in the HFNO groups varied between 30–70 l.min‐1 in studies on adults while weight‐based rates were used in children. The control groups received oxygen through a low‐flow nasal cannula at 1–10 l.min‐1 in 14 trials [8, 11, 12, 22, 23, 24, 25, 26, 34, 35, 36, 39, 40, Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1], simple facemask at ≥ 5 l.min‐1 in two [21, 27], bite block oxygen insufflation at 10–15 l.min‐1 in one [38] and a combination of low‐flow nasal oxygen and bite block oxygen insufflation in one [37]. In one multicentre trial, oxygen administration in the control group was left to the discretion of the participating centres and consisted of standard nasal cannulae, facemask and nasopharyngeal catheters [9].
Figure 1.
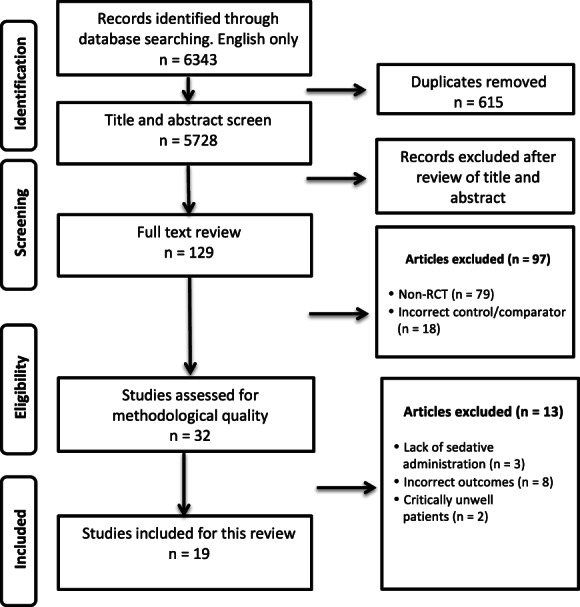
Flow diagram of study selection. RCT, randomised controlled trial.
Sedation regimens included varying combinations of propofol, fentanyl, alfentanil, remifentanil, midazolam, ketamine and phencyclidine, with standardised protocols in 12 trials. All except three trials [24, 39, Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1] used propofol‐based sedation regimens. Of the 16 trials that reported propofol‐based sedation approaches, either a target‐controlled infusion or a weight‐based manual infusion regimen was employed in nine [9, 21, 23, 25, 34, 35, 36, 37, 40] while an intermittent bolus approach was utilised in seven trials [8, 11, 12, 22, 26, 27, 38]. The depth of sedation was titrated to pre‐defined clinical endpoints (Ramsay sedation scale, modified observer's alertness/sedation scale, sedation state scale, Richmond Agitation Score); EEG monitoring (bispectral index); or society‐based recommendations (e.g. ASA guidelines) in all the included trials. The average durations of the procedures were available in 16 trials. They were <10 min in two trials [12, 36], 10–20 min in four trials [22, 24, 26, 34], 20–30 min in two trials [9, 38], 30–40 min in three trials [11, 23, 37], 40–50 min in one trial [40] and 50–60 min in three trials [8, 21, 35]. The cardiology trial had a mean procedural duration of 113 min [Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1]. The primary outcome measure of hypoxaemia was available in 17 trials, but not in two [23, 40] and 16 trials reported more than one outcome. Mean and SD of the continuous outcome had to be imputed from reported median and range or interquartile range in four trials [11, 21, 23, 38]. In one trial, the HFNO group was divided into two, based on two different flow rates of 30 l.min‐1 and 50 l.min‐1 and the minimum SpO2 was represented in a graphical format [40]. For this trial, we adopted the combined data that were extracted and used by Liu et al. in their recent meta‐analysis [16].
Across trials, overall risk of bias was predominantly of some concern in 10 trials [11, 12, 21, 22, 23, 24, 26, 34, 38, 39]. Five trials had low risk of bias [8, 9, 27, 37, Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1] and four had high risk of bias [25, 35, 36, 40] (Fig. 2). The risk of randomisation bias was adjudged as low in all but one trial that did not describe the randomisation sequence generation and allocation concealment [40]. Random sequence generation and allocation concealment were adequately described in 10 trials [8, 11, 25, 26, 27, 35, 37, 38, 39, Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1]. Details about allocation concealment were not available in eight trials [9, 12, 21, 22, 23, 24, 34, 36]; however, there were no baseline imbalances to suggest a problem with randomisation. The risk bias of deviation from intended intervention/oxygenation method was deemed low in 17 trials. Seven trials applied an intention to treat analysis [9, 23, 24, 25, 34, 39, 40] and 10 [8, 11, 12, 21, 22, 26, 27, 36, 37, Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1] applied a modified intention to treat principle where participants with missing outcome data were excluded after randomisation. The reasons for data exclusion or missing data in the studies included: failure of electronic data capture; cancellation or change of procedure; withdrawal of consent; improper fit of the device; discontinued assigned oxygenation method due to use of argon plasma coagulation; discomfort from device; and abandonment of the intended oxygen delivery method within 1 min. The data exclusion rates were < 5% in all trials and the risk of bias for missing outcome data and measurement of the outcome were deemed low for all the included trials. The assessors were blinded to the intervention in only two trials [9, 26] and presumably blinding of the oxygen delivery apparatus was impractical. The objective outcome measurements were unlikely to be influenced by lack of blinding. The risk of bias of selection of the reported results were judged as being ‘low’ for seven, ‘some concerns’ for nine, and ‘high’ for three. Although an a priori defined protocol was available for most trials, many lacked a pre‐specified statistical plan. Deviations from the outcome measures between pre‐specified protocols and reported results were noted in nine trials [11, 21, 22, 25, 34, 35, 36, 39, Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1]. Publication bias was low for the outcomes hypoxaemia and minimum SpO2 but was evident for minor airway manoeuvres as trials were unevenly distributed on either side of the effect estimate. For funnel plot analysis, see online Supporting Information (Fig. S1). This asymmetry was attributed to methodological and statistical heterogeneity as well as differences in the underlying risks between trials of different sizes. Hence, a statistical evaluation of the funnel plot asymmetry was not attempted [41, 42].
Figure 2.
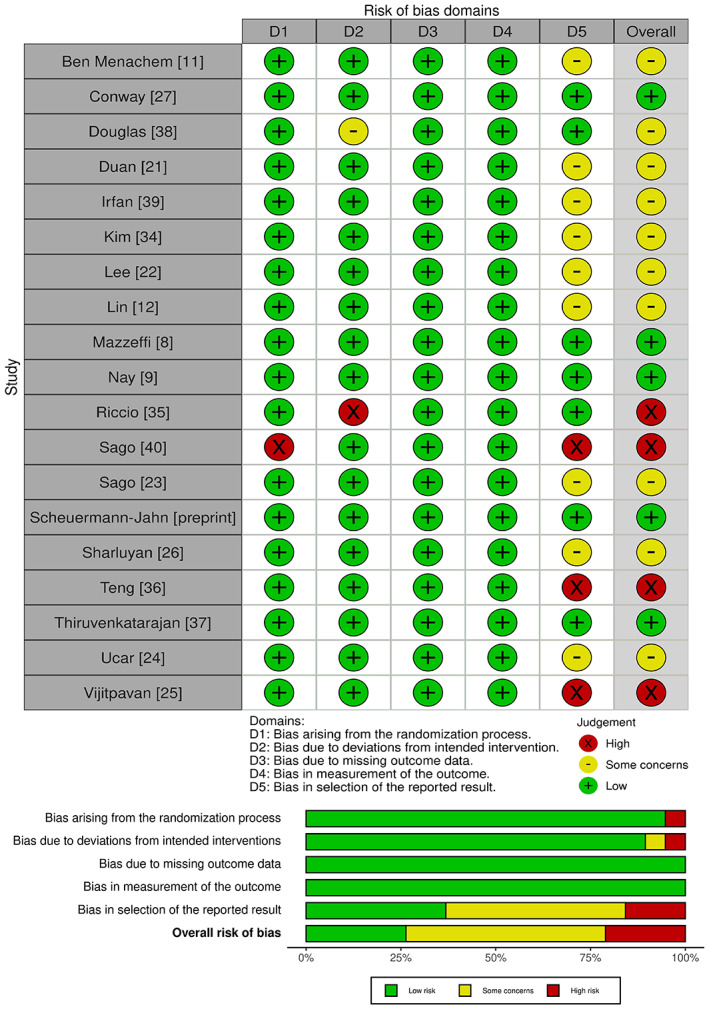
Risk of bias summary.
Seventeen trials, including three in children, reported data on oxygen desaturation in 2024 patients who received HFNO and 2037 in the control group [8, 9, 11, 12, 21, 22, 24, 25, 26, 27, 34, 35, 36, 37, 38, 39, Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1]. Any SpO2 drop below 90% regardless of duration was defined as hypoxaemia in 10 trials [21, 22, 24, 27, 34, 35, 36, 37, 38, 39]; a decline below 92% irrespective of duration of the event in three [8, 9, 25]; below 93% regardless of duration in one [Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1]; and over 15 s in another [34]. Hypoxaemia was reported with more than one cut‐off in three trials, was stratified as below 94% and 90% in two trials [11, 26] and the third trial had categories described as 75–90% and below 75% [12]. Pooled analysis showed that HFNO significantly reduced hypoxaemia as compared with the control group, RR (95%CI) 0.37 (0.24–0.56), p < 0.001, risk reduction 63%, GRADE = moderate. However, there was substantial heterogeneity, I2 = 72% (Fig. 3, online Supporting Information, Table S2). There were no subgroup differences noted when studies were stratified based on the risk of bias (p = 0.71) (online Supporting Information, Fig. S2).
Figure 3.
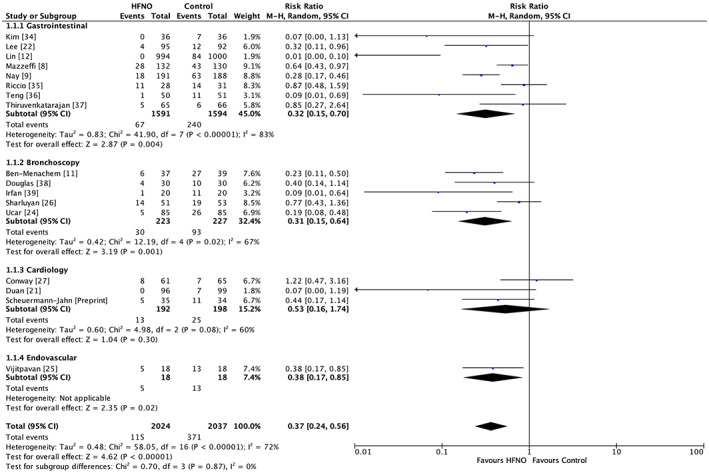
Forest plot comparing risk of hypoxaemia between the HFNO and control groups. HFNO, high‐flow nasal oxygen; M‐H, Mantel–Haenszel.
The subgroup of gastrointestinal procedures consisted of eight trials (3185 patients) and the RR (95%CI) was 0.32 (0.15–0.70), I2 = 83% vs. a RR (95%CI) of 0.31 (0.15–0.64), I2 = 67% and 0.53 (0.16–1.74), I2 = 60% in bronchoscopy (five trials, 450 patients) and cardiology procedures (three trials, 390 patients), respectively. High‐flow nasal oxygen was beneficial in reducing hypoxaemia in all trials except the cardiology procedures. A subgroup difference was not evident (Chi2 = 0.70, degrees of freedom (df) = 3, p = 0.87, I2 = 0%) (Fig. 3, online Supporting Information, Table S2). A sensitivity analysis by the leave‐one‐out method did not alter the results. The FiO2 values in the HFNO group were reported by 16 trials, of which FiO2 of 1.0 was used in nine [11, 12, 26, 27, 34, 36, 37, 38, 39] while seven used FiO2 < 1.0 in the range of 0.28–0.50 [9, 21, 22, 24, 25, 35, Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1]. Both subgroups had results in favour of the HFNO intervention, and no subgroup effect was noted (Chi2 = 0.33, df = 1, p = 0.56, I2 = 0%) (Table 1, online Supporting Information, Table S2 and Fig. S3).
Table 1.
Subgroup analysis of the primary outcome of hypoxaemia based on prespecified group stratifications.
| Group stratification | No. of trials | HFNO; n | Control; n |
Effect estimates of individual subgroups RR (95%CI), p value, I2 |
Overall effect estimate RR (95%CI), p value, I2 |
Subgroup difference; p value |
|---|---|---|---|---|---|---|
| Procedure types | 0.37 (0.24–0.56), < 0.001, 72% | 0.87 | ||||
| Gastrointestinal | 8 | 1591 | 1594 | 0.32 (0.15–0.70), = 0.004, 83% | ||
| Bronchoscopy | 5 | 223 | 227 | 0.31 (0.15–0.64), = 0.001, 67% | ||
| Cardiology | 3 | 192 | 198 | 0.53 (0.16–1.74), = 0.30, 60% | ||
| Endovascular | 1 | 18 | 18 | 0.38 (0.17–0.85), = 0.02, n/a | ||
| FiO2 in HFNO group | 0.34 (0.21–0.54), < 0.001, 72% | 0.56 | ||||
| FiO2 = 1.0 | 9 | 1344 | 1360 | 0.27 (0.10–0.69), = 0.006, 82% | ||
| FiO2 < 1.0 | 7 | 548 | 547 | 0.37 (0.23–0.59), < 0.001, 55% | ||
| Hypoxaemia risk | 0.37 (0.24–0.56), < 0.001, 72% | 0.22 | ||||
| Deemed at risk | 5 | 414 | 411 | 0.48 (0.28–0.85), = 0.01, 61% | ||
| Deemed not at risk | 12 | 1610 | 1626 | 0.28 (0.15–0.53), < 0.001, 78% | ||
| Mode of propofol administration | 0.40 (0.25–0.64), = 0.0002, 75% | 0.85 | ||||
| Manual or target‐controlled infusion | 7 | 484 | 489 | 0.37 (0.19–0.72), = 0.003, 64% | ||
| Bolus | 7 | 1400 | 1409 | 0.41 (0.19–0.87), = 0.02, 83% | ||
| Adult vs. children | 0.37 (0.24–0.56), < 0.001, 72% | 0.95 | ||||
| Trials in adults | 15 | 1877 | 1885 | 0.35 (0.22–0.55), < 0.001, 73% | ||
| Trials in children | 2 | 147 | 152 | 0.33 (0.03–3.85), = 0.37, 68% |
HFNO, high‐flow nasal oxygen; FiO2, fraction of inspired oxygen.
There were five trials (825 patients) enrolling those at risk of hypoxaemia, with four recruiting patients deemed high risk [9, 22, 35, 37], while the fifth trial was designed for patients with cardiac risk factors prone to hypoxaemia who presented for transcatheter aortic valve replacement [Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1]. Older patients aged >65 y were recruited in one study [22] and those with a BMI > 40 kg m‐2 in another [35]. While the trial by our group enrolled participants with one of the three characteristics (ASA physical status ≥ 3; known or suspected obstructive sleep apnoea; and BMI > 30 kg m‐2 [37]) the trial by Nay et al. [9] recruited patients who were aged >60 y; those with cardiorespiratory comorbidities; higher ASA classification; BMI > 30 kg m‐2; and known or suspected obstructive sleep apnoea. Twelve trials enrolled 3236 patients deemed as at low‐risk for hypoxaemia. High‐flow nasal oxygen was beneficial in both groups in reducing hypoxaemia and a subgroup difference was not evident (Chi2 = 1.54, df = 1, p = 0.22, I2 = 34.9%) (Table 1, online Supporting Information, Table S2 and Fig. S3).
Seven trials administered propofol as an infusion either in the form of a target‐controlled infusion [25, 36, 37] or as a manually controlled infusion [9, 21, 34, 35]. An intermittent bolus technique was used in seven trials [8, 11, 12, 22, 26, 27, 38]. Results were similar within both the subgroups in favour of the HFNO group, and there was no subgroup difference (Chi2 = 0.04, df = 1, p = 0.85, I2 = 0%) (Table 1, online Supporting Information, Table S2 and Fig. S3). There were 15 trials in adults of 3762 patients and two trials in children within the age range of 38 months [26] to 13 y [21] of 299 patients. The paediatric subgroup showed no statistically significant difference between the interventions, RR (95%CI) 0.33 (0.03–3.85), p = 0.37, I2 = 68%. Nonetheless, there were no significant subgroup differences in terms of age group (Chi2 = 0.00, df = 1, p = 0.95, I2 = 0%) (Table 1, online Supporting Information, Table S2 and Fig. S3). As heterogeneity could not be explained by sensitivity/subgroup analysis, we judged the overall quality of evidence for the outcome hypoxaemia across all procedures as moderate certainty due to inconsistency.
Ten trials including two in children totalling 880 patients reported minimum SpO2 [11, 21, 22, 23, 34, 35, 37, 38, 39, 40]. The minimum SpO2 was significantly higher in the HFNO group in comparison to the control group, mean difference (95%CI) 4.10 (2.70–5.50), p < 0.001, I2 = 82%, GRADE = moderate. Although a significant subgroup difference (Chi2 = 16.50, df = 3, p = 0.009, I2 = 81.8%) was noted, it must be interpreted with caution in view of the heterogeneity and imprecision from the wide 95%CI of the effect estimate for bronchoscopy specific interventions. Sensitivity analysis by the leave‐one‐out method did not alter the results (Fig. 4, online Supporting Information, Table S2).
Figure 4.
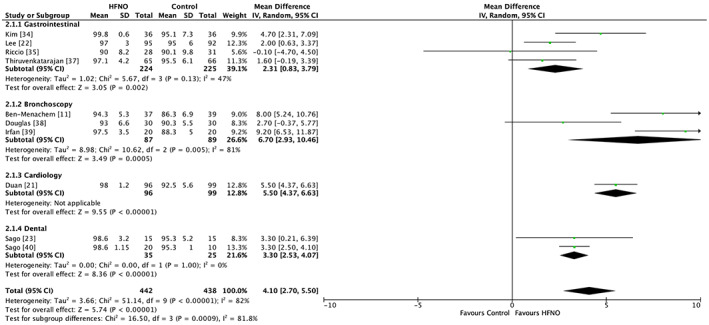
Forest plot comparing minimum SpO2 between the HFNO and control groups. HFNO, high‐flow nasal oxygen; IV, inverse variance.
Ten trials including two involving children (3027 patients), reported the requirement of minor airway manoeuvres such as jaw thrust/chin lift or insertion of oral or nasopharyngeal airways to maintain airway patency and improve ventilation [9, 12, 21, 23, 25, 34, 35, 36, 37, 40]. Six trials reported an objective trigger for these manoeuvres based on a decline in SpO2. Of these trials, two had cut‐offs below 95% [23, 40], one had threshold levels below 92% [25] and three below 90% [12, 21, 35]. High‐flow nasal oxygen significantly reduced the need for minor airway manoeuvres, RR (95%CI) 0.26 (0.11–0.59), p < 0.001, I2 = 88%, GRADE = moderate. A sensitivity analysis by the leave‐one‐out approach showed similar results (Fig. 5, online Supporting Information, Table S2). Six trials comprising 878 patients (including one in children) reported data on procedure interruptions [9, 11, 22, 26, 34, 38] as either withdrawal of equipment (e.g. bronchoscope) [11] or interruptions to improve airway management. Meta‐analysis revealed a significant reduction in procedure interruptions in the HFNO group, RR (95%CI) 0.17 (0.05–0.53), p = 0.002. Although the heterogeneity was 0%, we downgraded the GRADE evidence to low in view of imprecision related to very few events and wide 95%CI. Sensitivity analysis by the leave‐one out strategy showed similar results (Fig. 5, online Supporting Information, Table S2).
Figure 5.
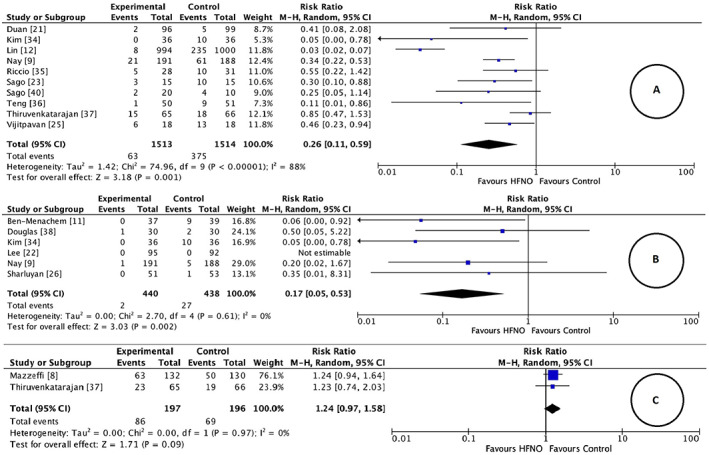
Forest plot comparing (a) requirement of minor airway manoeuvre; (b) procedure interruption; (c) risk of hypercarbia between the HFNO and control groups. HFNO, high‐flow nasal oxygen; M‐H, Mantel–Haenszel.
Two trials involving 393 adult patients reported data on hypercarbia [8, 37]. The trial by Mazzeffi et al. assessed patients during oesophagogastroduodenoscopy with an unspecified propofol‐based sedation regimen [8]. In comparison, the trial by our group examined patients at risk of desaturation during endoscopic retrograde cholangiopancreatography using target‐controlled administration of propofol [37]. Both trials employed transcutaneous carbon dioxide monitoring and an increase of > 2.66 kPa from baseline was deemed as hypercarbia. There was no significant difference in hypercarbia between the intervention groups, RR (95%CI) 1.24 (0.97–1.58), p = 0.09, I2 = 0%, GRADE = moderate (Fig. 5, online Supporting Information, Table S2). Although there was no statistical heterogeneity, methodological heterogeneity should be considered. The impact of carbon dioxide insufflation as a confounding factor should also be taken into consideration. Two more trials reported ETCO2 measured at the end of the procedure and were excluded as the sampling methods were not consistent between the trials. This was in addition to concerns that ETCO2 sampled from nasal cannulae may not reliably reflect blood carbon dioxide levels [34, 38].
Adverse events were not consistently reported across trials. The requirement of upgrade to facemask, tracheal intubation, bag‐mask ventilation, positive pressure ventilation and non‐invasive ventilation were described in six trials [9, 12, 25, 26, 37, Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1] with reported cumulative rates of 5/1354 (0.4%) and 14/1359 (1.0%) in the HFNO and the control groups respectively. Airway dryness reported as xeromycteria (i.e. dry nose), dry mouth/throat was described in the HFNO group in four trials and the event rate was 68/1112 (6.1%) [12, 25, 37, Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1]. A 1.7% rate of xeromycteria resolving within 30 min was reported in the largest included gastroscopy trial on 1994 patients where the mean procedure duration was 5 min [12]. Two trials reported abdominal bloating with the event rates being 9/100 and 11/100 in the HFNO and control groups respectively [37, Scheuermann‐Jahn et al, preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1].
Discussion
Our review and meta‐analysis across adults and children showed that the use of HFNO when compared with conventional oxygen therapy led to a reduction in hypoxaemia. High‐flow nasal oxygen application also resulted in higher values of minimum SpO2, a reduction in the requirements for minor airway manoeuvres and fewer procedural interruptions. High‐flow nasal oxygen had no impact on hypercarbia. The certainty of evidence was adjudged moderate for all the outcomes except for procedural interruptions, which was deemed to be low. With moderate quality of evidence, our subgroup analysis revealed that HFNO was beneficial in reducing hypoxaemia regardless of the baseline risk of developing hypoxaemia, irrespective of the FiO2 used or the mode of propofol administration. This indicates that these potentially confounding factors had no influence on the effect of HFNO therapy.
Two paediatric trials that reported hypoxaemia failed to show a difference between the HFNO and control groups [21, 26]. Even though these trials showed less heterogeneity, they were underpowered for this outcome, and this limitation was acknowledged by the study authors in one of the trials [26].
Six reviews accumulating evidence from 14 studies have been published on this topic [15, 16, 17, 18, 19, 20], of which 11 eligible RCTs [8, 9, 11, 12, 34, 35, 36, 37, 38, 39, 40] have been included in the present review. The first review involving 2123 patients from three studies showed that HFNO compared with conventional oxygenation reduced the risk of hypoxaemia [15]. A subsequent review [16] involving three gastrointestinal procedures, two bronchoscopy [11, 38] and one dental procedure [40] showed similar results. The review, however, excluded three studies in children. Concurrently, three gastrointestinal [18, 19, 20] and one bronchoscopy‐related review [17] also revealed results in favour of HFNO in mitigating the risk of hypoxaemia. In the bronchoscopy‐specific review, one of the included studies used only topical lignocaine in the absence of sedative‐hypnotics or analgesic drugs, thereby attributing to methodological heterogeneity [43].
Our results on the effect of HFNO on hypoxaemia are in keeping with that of the previous reviews. A comparable magnitude of heterogeneity for the effect estimate hypoxaemia was observed between the current review and five previous meta‐analyses [15, 16, 18, 19, 20]. Our results on minimum SpO2 are also consistent with all except one previous review [18]. The meta‐analysis by Hung et al. on gastrointestinal endoscopy procedures across three trials involving 262 participants, revealed no difference between HFNO and conventional oxygen therapy for the outcome of minimum SpO2. While our results were in favour of the HFNO intervention group in reducing minor airway manoeuvres (11 trials, 3145 patients), the meta‐analysis by Liu et al. [16] (4 trials, 2184 patients) found no difference. It appears that the additional trials included in our review enhanced the information size to provide more conclusive evidence in favour of HFNO for a range of outcome estimates.
The present review findings on procedure interruptions across six trials of 878 patients were similar to that reported by the meta‐analysis of Hung et al. [18], consisting of two trials of 451 patients. The event rates were one and two respectively in the meta‐analysis by Hung et al. [18] and this review. The CIs were noted to be wider in both. Based on these limitations, we downgraded the certainty of this evidence to low. The results of hypercarbia were pooled from just two trials, and thus had very little power for this outcome.
Our systematic review has several strengths. It incorporates the largest possible number of trials for procedural sedation and patient subpopulations including those at higher risk of desaturation. Eight new RCTs [21, 22, 23, 24, 25, 26, 27, Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1] were evaluated in addition to the 14 RCTs analysed by previous reviews. The trials had participants from adult and paediatric populations, with ages ranging from 38 months [26] to 83 y [Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1]. Our search strategy using various databases and a pre‐registration site allowed us to retrieve a trial [Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1] listed in a pre‐publication repository prior to peer review, possibly reducing publication bias. We preferred a larger inclusive review as there is little evidence that many procedural sedation interventions exert different effects in different procedure types [44]. Additionally, we ascertained that any difference in effect estimate is unlikely to represent an interaction between participants' age and the effect of HFNO intervention [44]. This was our reasoning to include participants across all age groups. We used the recently introduced Cochrane risk of bias 2 tool which is likely to overcome some of the challenges encountered by the old tool in the domains of incomplete outcome data and selective outcome reporting [29]. This was supplemented by applying the GRADE criteria to assess the certainty of evidence.
Our review also has shortcomings. Firstly, varying definitions of hypoxaemia were reported suggesting that it is context‐ as well as investigator‐ specific. Secondly, oxygen flow rates and FiO2 were inconsistent across trials. Thirdly, substantial heterogeneity was still present for most outcome measures even after sensitivity and subgroup analysis. However, the direction or size of the summary estimate did not change. As previously outlined, the trials included were also clinically and methodologically heterogeneous with a range of procedures and sedation protocols across diverse population groups with the proceduralists ranging from anaesthetists to physicians. Fourthly, the effects of duration of sedation as well the impact of various positions such as supine, lateral, or prone of the eight gastrointestinal trials on the outcomes of interest were not explored. Lastly, the trials originated from various geographical locations, nine from Asia [12, 21, 22, 23, 24, 25, 34, 36, 40], four from Europe [9, 26, 39, Scheuermann‐Jahn et al., preprint, https://doi.org/10.21203/rs.3.rs‐989822/v1] and three each from North America [8, 27, 35] and Australia [11, 37, 38]. Nevertheless, these factors enhance the external validity and applicability of our findings.
Our review has implications for clinical practice. There is moderate certainty of evidence that HFNO reduces the risk of hypoxaemia and increases minimum SpO2 across a range of procedural sedation types. Hence, it should be considered in high‐risk patients such as those with higher ASA physical status, critical illness, obstructive sleep apnoea, obesity or during high risk procedures such as advanced gastrointestinal and bronchoscopy procedures. It is worth stating that the increased FiO2 and physiologic benefits of HFNO may not overcome hypoxaemia due to pulmonary shunt [45, 46] or drug‐induced hypoventilation [38]. Further, HFNO will not be able to maintain oxygenation in sustained upper airway obstruction. While the cost implications of using HFNO were not formally evaluated in the current review, it is imperative that its usage should be tailored to case‐ specific requirements in both resource‐rich as well as resource‐deplete settings. Adequately powered trials in the paediatric population as well those deemed at risk of developing hypoxaemia are required to establish its role.
In conclusion, this systematic review presents moderate quality of evidence that the application of HFNO is an effective intervention to reduce the incidence of hypoxaemia during procedural sedation. There is moderate evidence that it can increase the minimum oxygen saturation observed and reduce the requirement of minor airway manoeuvres. A low quality of evidence also suggests that it reduces procedural interruptions.
Supporting information
Appendix S1 Search strategy.
Figure S1 Funnel plots for hypoxaemia, minimum SpO2 and airway manoeuvres.
Figure S2 Forest plot for risk of bias.
Figure S3 Subgroup analysis for hypoxaemia forest plots.
Table S1 Data extraction table.
Table S2 GRADE summary table.
Acknowledgements
A protocol was prospectively registered with PROSPERO (CRD42022302623). The authors acknowledge and thank Ms K. Rough, Research Librarian Knowledge Resources, Australian and New Zealand College of Anaesthetists and Ms A. Holasek, Reference and Training Librarian, Queen Elizabeth Hospital, Adelaide, Australia for their contributions, guidance and feedback in developing a search strategy. No competing interests declared. Open access publishing facilitated by The University of Adelaide, as part of the Wiley ‐ The University of Adelaide agreement via the Council of Australian University Librarians.
References
- 1. van Haperen M, Preckel B, Eberl S. Indications, contraindications, and safety aspects of procedural sedation. Current Opinion in Anaesthesiology 2019; 32: 769–75. [DOI] [PubMed] [Google Scholar]
- 2. Mason KP, Roback MG, Chrisp D, et al. Results from the adverse event sedation reporting tool: a global anthology of 7952 records derived from> 160,000 procedural sedation encounters. Journal of Clinical Medicine 2019; 8: 2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Daskaya H, Uysal H, Çiftçi T, Baysal B, İdin K, Karaaslan K. Use of the gastro‐laryngeal tube in endoscopic retrograde cholangiopancreatography cases under sedation/analgesia. Turkish Journal of Gastroenterology 2016; 27: 246–51. [DOI] [PubMed] [Google Scholar]
- 4. Sidhu R, Turnbull D, Newton M, et al. Deep sedation and anaesthesia in complex gastrointestinal endoscopy: a joint position statement endorsed by the British Society of Gastroenterology (BSG), joint advisory group (JAG) and Royal College of Anaesthetists (RCoA). Frontline Gastroenterology 2019; 10: 141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hinkelbein J, Schmitz J, Lamperti M, Fuchs‐Buder T. Procedural sedation outside the operating room. Current Opinion in Anesthesiology 2020; 33: 533–8. [DOI] [PubMed] [Google Scholar]
- 6. Holm C, Christensen M, Rasmussen V, Schulze S, Rosenberg J. Hypoxaemia and myocardial ischaemia during colonoscopy. Scandinavian Journal of Gastroenterology 1998; 33: 769–72. [DOI] [PubMed] [Google Scholar]
- 7. Johnston S, McKenna A, Tham T. Silent myocardial ischaemia during endoscopic retrograde cholangiopancreatography. Endoscopy 2003; 35: 1039–42. [DOI] [PubMed] [Google Scholar]
- 8. Mazzeffi MA, Petrick KM, Magder L, et al. High‐flow nasal cannula oxygen in patients having anesthesia for advanced esophagogastroduodenoscopy: HIFLOW‐ENDO, a randomized clinical trial. Anesthesia and Analgesia 2021; 132: 743–51. [DOI] [PubMed] [Google Scholar]
- 9. Nay MA, Fromont L, Eugene A, et al. High‐flow nasal oxygenation or standard oxygenation for gastrointestinal endoscopy with sedation in patients at risk of hypoxaemia: a multicentre randomised controlled trial (ODEPHI trial). British Journal of Anaesthesia 2021; 127: 133–42. [DOI] [PubMed] [Google Scholar]
- 10. Bateman NT, Leach RM. ABC of oxygen. Acute oxygen therapy. British Medical Journal 1998; 317: 798–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ben‐Menachem E, McKenzie J, O'Sullivan C, Havryk AP. High‐flow nasal oxygen versus standard oxygen during flexible bronchoscopy in lung transplant patients: a randomized controlled trial. Journal of Bronchology and Interventional Pulmonology 2020; 27: 259–65. [DOI] [PubMed] [Google Scholar]
- 12. Lin Y, Zhang X, Li L, et al. High‐flow nasal cannula oxygen therapy and hypoxia during gastroscopy with propofol sedation: a randomized multicenter clinical trial. Gastrointestinal Endoscopy 2019; 90: 591–601. [DOI] [PubMed] [Google Scholar]
- 13. Gotera C, Lobato SD, Pinto T, Winck J. Clinical evidence on high flow oxygen therapy and active humidification in adults. Revista Portuguesa de Pneumologia 2013; 19: 217–27. [DOI] [PubMed] [Google Scholar]
- 14. Lodeserto FJ, Lettich TM, Rezaie SR. High‐flow nasal cannula: mechanisms of action and adult and pediatric indications. Cureus 2018; 10: e3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spence EA, Rajaleelan W, Wong J, Chung F, Wong DT. The effectiveness of high‐flow nasal oxygen during the intraoperative period: a systematic review and meta‐analysis. Anesthesia and Analgesia 2020; 131: 1102–10. [DOI] [PubMed] [Google Scholar]
- 16. Liu H‐Y, Tam K‐W, Loh E‐W, et al. High‐flow nasal oxygenation reduces the risk of desaturation in adults receiving procedural sedation: a meta‐analysis of randomized controlled trials. Perioperative Medicine 2021; 10: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Su CL, Chiang LL, Tam KW, Chen TT, Hu MC. High‐flow nasal cannula for reducing hypoxemic events in patients undergoing bronchoscopy: a systematic review and meta‐analysis of randomized trials. PLoS One 2021; 16: e0260716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hung KC, Chang YJ, Chen IW, et al. Efficacy of high flow nasal oxygenation against hypoxemia in sedated patients receiving gastrointestinal endoscopic procedures: a systematic review and meta‐analysis. Journal of Clinical Anesthesia 2022; 77: 110651. [DOI] [PubMed] [Google Scholar]
- 19. Doulberis M, Sampsonas F, Papaefthymiou A, et al. High‐flow versus conventional nasal cannula oxygen supplementation therapy and risk of hypoxia in gastrointestinal endoscopies: α systematic review and meta‐analysis. Expert Review of Respiratory Medicine 2022; 16: 323–32. [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y‐X, He X‐X, Chen Y‐P, Yang S. The effectiveness of high‐flow nasal cannula during sedated digestive endoscopy: a systematic review and meta‐analysis. European Journal of Medical Research 2022; 27: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Duan X, Wei N, Wei J, et al. Effect of high‐flow nasal cannula oxygen therapy on pediatric patients with congenital heart disease in procedural sedation: a prospective, randomized trial. Journal of Cardiothoracic and Vascular Anesthesia 2021; 35: 2913–9. [DOI] [PubMed] [Google Scholar]
- 22. Lee MJ, Cha B, Park JS, et al. Impact of high‐flow nasal cannula oxygenation on the prevention of hypoxia during endoscopic retrograde cholangiopancreatography in elderly patients: a randomized clinical trial. Digestive Diseases and Sciences 2022; 67: 4154–60. [DOI] [PubMed] [Google Scholar]
- 23. Sago T, Watanabe K, Kawabata K, Shiiba S, Maki K, Watanabe S. A nasal high‐flow system prevents upper airway obstruction and hypoxia in pediatric dental patients under intravenous sedation. Journal of Oral and Maxillofacial Surgery 2021; 79: 539–45. [DOI] [PubMed] [Google Scholar]
- 24. Yilmazel Ucar E, Araz Ö, Kerget B, Akgun M, Saglam L. Comparison of high‐flow and conventional nasal cannula oxygen in patients undergoing endobronchial ultrasonography. Internal Medicine Journal 2021; 51: 1935–9. [DOI] [PubMed] [Google Scholar]
- 25. Vijitpavan A, Kooncharoensuk Y. High flow versus conventional nasal cannula for oxygenation and ventilation maintenance during surgery with intravenous deep sedation by propofol: a randomized controlled study. BMC Anesthesiology 2021; 21: 214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sharluyan A, Osona B, Frontera G, et al. High flow nasal cannula versus standard low flow nasal oxygen during flexible bronchoscopy in children: a randomized controlled trial. Pediatric Pulmonology 2021; 56: 4001–10. [DOI] [PubMed] [Google Scholar]
- 27. Conway A, Collins P, Chang K, Kamboj N, Filici AL, Lam P, Parotto M. High flow nasal oxygen during procedural sedation for cardiac implantable electronic device procedures: a randomised controlled trial. European Journal of Anaesthesiology 2021; 38: 839–49. [DOI] [PubMed] [Google Scholar]
- 28. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. British Medical Journal 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Flemyng E, Dwan K, Moore TH, Page MJ, Higgins JP. Risk of bias 2 in Cochrane reviews: a phased approach for the introduction of new methodology. Cochrane Database of Systematic Reviews 2020; 10: ED000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Chicester, UK: John Wiley & Sons, 2019. [Google Scholar]
- 31. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014; 14: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Siemieniuk R, Guyatt G. What is GRADE. British Medical Journal Best Practice 2019. https://bestpractice.bmj.com/info/toolkit/learn-ebm/what-is-grade/ [Google Scholar]
- 33. Granholm A, Alhazzani W, Møller MH. Use of the GRADE approach in systematic reviews and guidelines. British Journal of Anaesthesia 2019; 123: 554–9. [DOI] [PubMed] [Google Scholar]
- 34. Kim SH, Bang S, Lee KY, et al. Comparison of high flow nasal oxygen and conventional nasal cannula during gastrointestinal endoscopic sedation in the prone position: a randomized trial. Canadian Journal of Anesthesia 2021; 68: 460–6. [DOI] [PubMed] [Google Scholar]
- 35. Riccio CA, Sarmiento S, Minhajuddin A, Nasir D, Fox AA. High‐flow versus standard nasal cannula in morbidly obese patients during colonoscopy: a prospective, randomized clinical trial. Journal of Clinical Anesthesia 2019; 54: 19–24. [DOI] [PubMed] [Google Scholar]
- 36. Teng W‐N, Ting CK, Wang Y‐T, et al. High‐flow nasal cannula and mandibular advancement bite block decrease hypoxic events during sedative esophagogastroduodenoscopy: a randomized clinical trial. BioMed Research International 2019; 2019: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thiruvenkatarajan V, Dharmalingam A, Arenas G, et al. Effect of high‐flow vs. low‐flow nasal plus mouthguard oxygen therapy on hypoxaemia during sedation: a multicentre randomised controlled trial. Anaesthesia 2022; 77: 46–53. [DOI] [PubMed] [Google Scholar]
- 38. Douglas N, Ng I, Nazeem F, et al. A randomised controlled trial comparing high‐flow nasal oxygen with standard management for conscious sedation during bronchoscopy. Anaesthesia 2018; 73: 169–76. [DOI] [PubMed] [Google Scholar]
- 39. Irfan M, Ahmed M, Breen D. Assessment of high flow nasal cannula oxygenation in endobronchial ultrasound bronchoscopy: a randomized controlled trial. Journal of Bronchology and Interventional Pulmonology 2021; 28: 130–7. [DOI] [PubMed] [Google Scholar]
- 40. Sago T, Harano N, Chogyoji Y, Nunomaki M, Shiiba S, Watanabe S. A nasal high‐flow system prevents hypoxia in dental patients under intravenous sedation. Journal of Oral and Maxillofacial Surgery 2015; 73: 1058–64. [DOI] [PubMed] [Google Scholar]
- 41. Song F, Hooper L, Loke Y. Publication bias: what is it? How do we measure it? How do we avoid it? Open Access Journal of Clinical Trials 2013; 2013: 71–81. [Google Scholar]
- 42. Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. British Medical Journal 2006; 333: 597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Longhini F, Pelaia C, Garofalo E, et al. High‐flow nasal cannula oxygen therapy for outpatients undergoing flexible bronchoscopy: a randomised controlled trial. Thorax 2022; 77: 58–64. [DOI] [PubMed] [Google Scholar]
- 44. Smith A, Carlisle J. Reviews, systematic reviews and Anaesthesia . Anaesthesia 2015; 70: 644–50. [DOI] [PubMed] [Google Scholar]
- 45. Sarkar M, Niranjan N, Banyal PK. Mechaniss of hypoxemia. Lung India 2017; 34: 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goligher EC, Slutsky AS. Not just oxygen? Mechanisms of benefit from high‐flow nasal cannula in hypoxemic respiratory failure. American Journal of Respiratory and Critical Care Medicine 2017; 195: 1128–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Search strategy.
Figure S1 Funnel plots for hypoxaemia, minimum SpO2 and airway manoeuvres.
Figure S2 Forest plot for risk of bias.
Figure S3 Subgroup analysis for hypoxaemia forest plots.
Table S1 Data extraction table.
Table S2 GRADE summary table.


