Abstract
Purpose
The purpose of this study is to test the hypothesis that greater habitual water intake is associated with lower risk of dry eye disease (DED).
Methods
We included 51 551 participants from the population‐based Lifelines cohort (mean age = 51.2 years) in this cross‐sectional association study. DED was assessed using the Women's Health Study (WHS) dry eye questionnaire. Water intake was calculated from food frequency questionnaires. Logistic regressions were used to analyse the relationship between DED and water intake or 24‐h urine volume, corrected for age, sex, body mass index, physical activity, smoking status, education, income, 48 comorbidities, and 15 medication groups.
The main outcome measure was WHS‐defined DED. Highly symptomatic dry eye and clinical diagnosis of DED were secondary outcomes.
Results
In total, 9.1% of the population had WHS‐defined DED. Higher water intake was associated with increased prevalence of WHS‐defined DED (OR: 1.011 per 100 ml/day, 95% CI: 1.004–1.017, p = 0.003). After excluding those with a clinical diagnosis, greater water intake was still tied to increased risk of having DED symptoms (OR: 1.010 per 100 ml/day, 95% CI: 1.006–1.015, p < 0.001). Higher 24‐h urine volumes were also associated with higher risk of WHS‐defined DED (OR: 1.010 per 100 ml/day, 95% CI: 1.005–1.015, p < 0.001).
Conclusions
In this large, population‐based study, higher water intake was not tied to reduced risk of DED. Rather, it was associated with a modest increased risk of DED. Interventional studies are needed to fully understand the effect of water intake on DED, but this study found no evidence that greater water intake is beneficial for DED.
Keywords: dry eye disease, epidemiology, hydration, urine volume, water intake
1. INTRODUCTION
Dry eye disease (DED) is a complex disease of the tear film and ocular surface that causes symptoms of discomfort, visual disturbance and tear film instability with a risk of damage to the ocular surface (Craig et al., 2017). Worldwide, DED has a prevalence ranging from 5% to 50%, depending on the population studied and definition adopted (Stapleton et al., 2017). Symptoms of dry eye, including ocular pain and irritation, can cause a substantial reduction in general well‐being and quality of life (Li et al., 2012; Mertzanis et al., 2005; Miljanovic et al., 2007). Activities such as driving, reading, watching television, and work tasks are often impacted (Miljanovic et al., 2007). In addition, DED yields a sizable economic burden, with an estimated annual cost of USD 3.84 billion in the US alone (Yu et al., 2011). Symptoms, once established, are often hard to treat, and the clinical course of DED is often chronic (McDonald et al., 2016; Messmer, 2015; Stapleton et al., 2017). It is therefore important to uncover modifiable risk factors and interventions that may prevent the development of DED.
Adequate hydration is vital for optimal physiological function (Jéquier & Constant, 2010), especially in the cardiovascular (Watso & Farquhar, 2019) and renal systems (Clark et al., 2016). It is also tied to better overall physical (Murray, 2007) and cognitive (Masento et al., 2014) performance. Additionally, increased water intake may combat dry skin (Palma et al., 2015) and higher water intake was associated with less dry mouth symptoms in a small, cross‐sectional study (Lee et al., 2020). For DED, clinicians may recommend drinking more water to alleviate dry eye symptoms. However, so far there have been few studies assessing the effect of hydration status and water intake on ocular surface health. Previous studies have found a link between plasma osmolality, a marker of hydration, and tear osmolarity (Fortes et al., 2011; Walsh et al., 2012). Yet, no population‐based study looking at the relationship between water intake or hydration status and DED has been published.
This cross‐sectional study aims to test the hypothesis that greater habitual water intake is tied to a lower risk of DED using the Lifelines cohort, a large Northern European population. The relationship between water intake and DED was investigated in multiple ways, including models correcting for a great number of confounders and stratification by sex. Additionally, as another biomarker of hydration status, we also assessed 24‐h urine volume for its association with dry eye.
2. METHODS
2.1. Lifelines cohort and participants
Lifelines is a multidisciplinary, prospective, population‐based cohort study examining the health and health‐related behaviours of 167 729 persons living in the north of the Netherlands. It uses a broad range of investigative procedures in assessing the biomedical, sociodemographic, behavioural, physical, and psychological factors which contribute to the health and disease of the general population, with a special focus on multi‐morbidity and complex genetics (Stolk et al., 2008). Participants, almost exclusively of European ancestry, were included via general practitioners or self‐enrolment between 2006 and 2013 and will be followed for at least 30 years. The cohort is described in detail elsewhere (Scholtens et al., 2015; Sijtsma et al., 2021). The Lifelines protocol has been approved by the medical ethics committee of the University Medical Center Groningen under number 2007/152 and carried out in accordance with the Declaration of Helsinki, and all participants provided written informed consent.
The first (baseline) general assessment (1A) was conducted between 2007 and 2013, with two follow‐up questionnaires sent out on average 1.5 years (1B) and 2.5 years (1C) later. The second general assessment (2A) was between 2014 and 2017. Figure 1 shows the timeline of assessments of exposure and outcome measures included in the current study.
FIGURE 1.
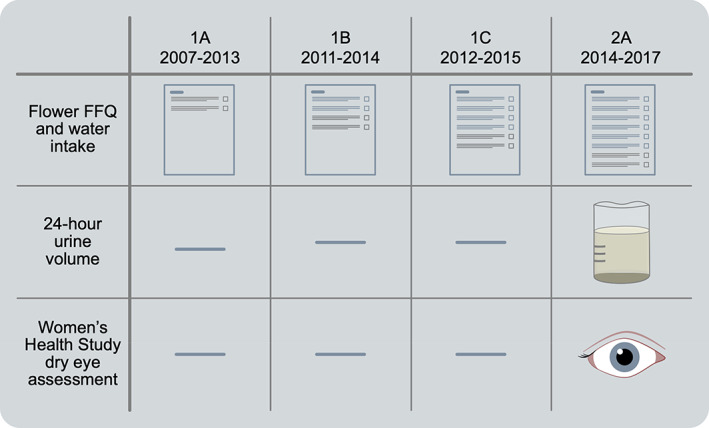
This chart shows when exposures and outcomes were assessed. The Food Frequency Questionnaire (FFQ) was administered in four parts. The first part assessed baseline dietary data at 1A. Each of the three subsequent visits at 1B, 1C, and 2A recorded additional dietary information. The complete FFQ data available at 2A was used to calculate total daily water intake for each participant. Both 24‐h urine volume and dry eye status using the Women's Health Study questionnaire were assessed at 2A.
2.2. Assessment of DED
This study used the Women's Health Study (WHS) dry eye questionnaire (Gulati et al., 2006) to assess DED at assessment 2A. This questionnaire is the most frequently used tool when assessing DED status in population‐based studies (Stapleton et al., 2017). The instrument has been validated against a standardized clinical exam and has a similar sensitivity and specificity as a 16‐item survey (Gulati et al., 2006). The WHS questionnaire consists of the following three questions: (1) “How often do your eyes feel dry (not wet enough)?” (2) “How often do your eyes feel irritated?” and (3) “Have you ever received a diagnosis of dry eye?” Question 1 and 2 have possible answers of: “Never,” “Sometimes,” “Often,” and “Constantly.” Question 3 has possible answers of: “Yes,” “No,” and “I don't know.” WHS‐defined DED was the main outcome measure of this study. WHS‐defined DED requires either a self‐reported clinical diagnosis of DED or “highly symptomatic dry eye” (both dryness and irritation at least “often”) (Bazeer et al., 2019; Gulati et al., 2006). We further defined two secondary dry eye outcomes based on questionnaire answers, described in further details elsewhere (Magno et al., 2021): (i) a reported clinical diagnosis of DED and (ii) “highly symptomatic dry eye.”
2.3. Assessment of water intake
A 110‐item semi‐quantitative food frequency questionnaire (FFQ) assessing dietary consumption over the previous month was specially developed for the Lifelines cohort study (Brouwer‐Brolsma et al., 2017). The FFQ was administered in four parts, with an assessment of major food groups at baseline (1A) and three follow‐up questionnaires investigating micronutrients at 1B, 1C, and 2A.
Total daily water intake was calculated from water stemming from: beverages consumed (“beverage water”), foods ingested (“food water”), and the metabolic breakdown of the ingested macronutrients and alcohol (“metabolic water”), similar to past work investigating hydration status in the general population (Manz et al., 2012). Food and beverage water were determined by multiplying the weight consumed of each food and beverage item with its relative water content, based on the Dutch National Institute for Public Health and the Environment's Nevo Tables (NEVO‐tabel, 2011). Metabolic water was computed based on the following equation (Manz et al., 2012):
In addition to self‐reported water intake over the last month, we also assessed 24‐h urine volume, which was collected on average half a month after the DED assessment (2A). The 24‐h urine volume strongly correlates with same‐day fluid intake and provides information about the acute hydration status (Perrier et al., 2013; Zhang et al., 2017). Despite there being no “gold standard” for assessing hydration status (Armstrong, 2007), 24‐h urine volume could be useful in validating the questionnaire‐based findings (Gandy, 2015).
Energy intake from all sources, in kcal/day, was also computed. Caloric intake is a determinant of recommended daily water intake (EFSA Panel on Dietetic Products, 2010), and a hypocaloric diet has been found to improve clinical signs of dry eye (Molina‐Leyva et al., 2020). Subjects with likely erroneous FFQ data were excluded. FFQ data were deemed unreliable if the ratio between energy intake and basal metabolic rate, calculated using the Schofield equation (Schofield, 1985), was <0.5 or >2.75, or the total energy intake was <800 kcal/day for men or <500 kcal/day for women, similar to that of past studies (Vinke et al., 2020).
2.4. Assessment of possible confounding factors
At baseline (1A), participants were asked to: “…indicate which of the following disorders you have or have had?” with a wide range of possible answers relating to cardiovascular, chronic pain, gastrointestinal, kidney and urinary, neurological, haematological, autoimmune, skin, and mental conditions. Additionally, subjects were asked to report, using free text, any other disorders that they have or have had. At repeat visits, the participants were requested to provide information about the occurrence of new conditions since their last questionnaire. A specific questionnaire assessing ocular conditions and traits was also given to the participants at the same time as the DED assessment (2A). Using this information, dichotomous variables were created for the occurrence of a broad range of diagnoses and conditions, as previously described in greater detail (Vehof et al., 2021). 48 comorbidities were found associated with WHS‐defined DED (Vehof et al., 2021). In addition, the Anatomical Therapeutic Chemical (ATC) codes for the medications used by the participants were carefully recorded at visit 1A.
2.5. Statistics
Descriptive statistics were used to assess the population characteristics. Multivariable logistic regression models were used to investigate the relationship between the dry eye phenotypes (dependent variables) and water intake (independent variable, base unit 100 ml/day). The analyses were adjusted for: age and sex only (Model 1), relevant demographic variables (age, sex, education level [low, middle, high], and net monthly household income [<2000, 2000–3000, >3000 euros/month]), body mass index (BMI), daily caloric intake, alcohol intake, and smoking status (never, current, past history of smoking; Model 2), and the factors in Model 2 and 48 medical comorbidities associated with WHS‐defined DED (Vehof et al., 2021) and 15 medications and medication groups associated with both dry eye (Wolpert et al., 2021) and dry mouth (Wolff et al., 2017) (Model 3). The medications and comorbidities corrected for are presented in Table S1a,b. The association between 24‐h urine volume and the dry eye phenotypes was assessed in a similar way. As the prevalence and risk factors of DED are highly sex‐specific (Magno et al., 2021; Sullivan et al., 2017), sex‐stratified analyses were also conducted. The interaction term [sex*water intake] was included to assess the statistical significance of any sex‐specific relationship. Age‐decade specific quintiles of water intake were made for males and females, and the effect of extreme values, 5th vs. 1st quintile was also assessed in each sex separately. The quintiles were generated in an age‐neutral way (water intake was ranked within subjects in the same decade of life before summation), as the males tended to get younger with increasing water intake, while females tended to increase in age.
Patients with a DED diagnosis or substantial symptoms of dry eye may increase their water intake which could thus affect the relationship between water intake and dry eye. Therefore, the association between “symptomatic dry eye” and water intake was additionally assessed after exclusion of diagnosed participants. “Symptomatic dry eye” was defined as having both dryness and irritation at least “Sometimes,” or having either symptom “Often” or more, similar to past work (Magno et al., 2021). Furthermore, persons with symptoms only “sometimes” (as opposed to those with more frequent symptoms) would likely not increase their water intake to alleviate symptoms. Thus, the association between water intake and experiencing symptoms of dryness “Sometimes” vs. “Never” was also additionally assessed.
A p‐value under 0.05 was regarded as statistically significant for all analyses. All analyses were conducted using SPSS software, version 25.0 (SPSS Inc.).
3. RESULTS
Of all participants, 51 551 subjects had available data and were included in this study. Table 1 provides a summary of population characteristics. The prevalence of WHS‐defined DED was 9.1%, with females more likely to have DED than men. Mean total water intake was 2491 ml/day with beverage water contributing 62% of total water consumed. The rest was made up of food water (28%) and metabolic water (10%). On average, males had a higher intake of water from all sources than females. Table S2 provides a more detailed presentation of study characteristics broken down for each age‐decade specific quintile of total water intake. Interestingly, the mean female age went up with increased water intake, while men's mean age went down. Figure 2 depicts the prevalence of WHS‐defined DED, clinical diagnosis of dry eye, and highly symptomatic dry eye in each age‐decade specific quintile of water intake for both sexes. Females had an increase in the prevalence of WHS‐defined DED with increasing water intake, with the 1st quintile (1684 ml/day) having a prevalence of 11.1% compared with 13.4% in the 5th quintile (3257 ml/day). In the male population, the prevalence of WHS‐defined DED was 4.7% in the 1st quintile (1824 ml/day) and 5.3% in the 5th quintile (3539 ml/day).
TABLE 1.
Demographics of the study population
| All (N = 51 551) | Males (N = 20 377) | Females (N = 31 174) | |
|---|---|---|---|
| Age, years, mean (standard deviation [SD]) | 51.2 (12.4) | 52.1 (12.4) | 50.5 (12.3) |
| Ethnicity—White, European, % | 98.7 | 98.9 | 98.5 |
| Income | |||
| <2000 Euro per month, % | 27.1 | 21.4 | 30.8 |
| 2000–3000 Euro per month, % | 29.8 | 33.4 | 27.5 |
| >3000 Euro per month, % | 33.7 | 38.2 | 30.7 |
| Chose not to answer, % | 9.4 | 7 | 11 |
| Smoker | |||
| Current, % | 14.1 | 15.4 | 13.3 |
| Former, % | 33.7 | 36.7 | 31.8 |
| Never, % | 52.2 | 47.9 | 54.9 |
| Dry eye | |||
| Women's Health Study‐definition, % | 9.1 | 5.0 | 11.7 |
| Highly symptomatic dry eye, % | 1.9 | 0.8 | 2.5 |
| Clinical diagnosis, % | 8.5 | 4.7 | 11.0 |
| Symptomatic dry eye, % | 30.0 | 22.3 | 34.9 |
| Comorbidities a | |||
| Number of comorbidities, mean (SD) | 2.9 (2.1) | 2.4 (1.9) | 3.3 (2.2) |
| Presence of ≥1 comorbidity, % | 89.5 | 85.7 | 92.0 |
| Food Frequency Questionnaire | |||
| Energy intake, kcal/day, mean (SD) | 2111 (619) | 2444 (658) | 1893 (479) |
| Water intake | |||
| Total water intake, ml/day, mean (SD) | 2491.3 (606.0) | 2610.7 (631.4) | 2413.3 (575.6) |
| Beverage water, ml/day, mean (SD) | 1538.9 (501.7) | 1578.2 (515.4) | 1513.3 (490.9) |
| Food water, ml/day, mean (SD) | 693.4 (195.4) | 731.4 (208.5) | 668.5 (182.0) |
| Metabolic water, ml/day, mean (SD) | 259.0 (76.7) | 301.1 (81.4) | 231.5 (59.1) |
Note: Baseline demographics. Data are mean (standard deviation), or percentage.
Contact lens wear, hypertension (measured), macular degeneration, glaucoma/ocular hypertension, eye surgery (any), allergic conjunctivitis, Bell's palsy, keratoconus, laser refractive surgery, irritable bowel syndrome, fibromyalgia, osteoarthritis, spinal disc herniation, repetitive strain injury, rheumatoid arthritis, systemic lupus erythematosus, Sjogren's disease, atherosclerosis, cardiac arrhythmia, liver cirrhosis, chronic cystitis, urinary incontinence, spasticity, migraine, chronic fatigue syndrome, depression, burnout, autism, gastric ulcer, Crohn's disease, asthma, acne, psoriasis, eczema, rosacea, hay fever, allergy (any), anaemia, diabetes mellitus, osteoporosis, thyroid disease (any), Graves' disease, carpal tunnel syndrome, obstructive sleep apnea, lichen planus, sarcoidosis, chronic back pain, and sinusitis.
FIGURE 2.
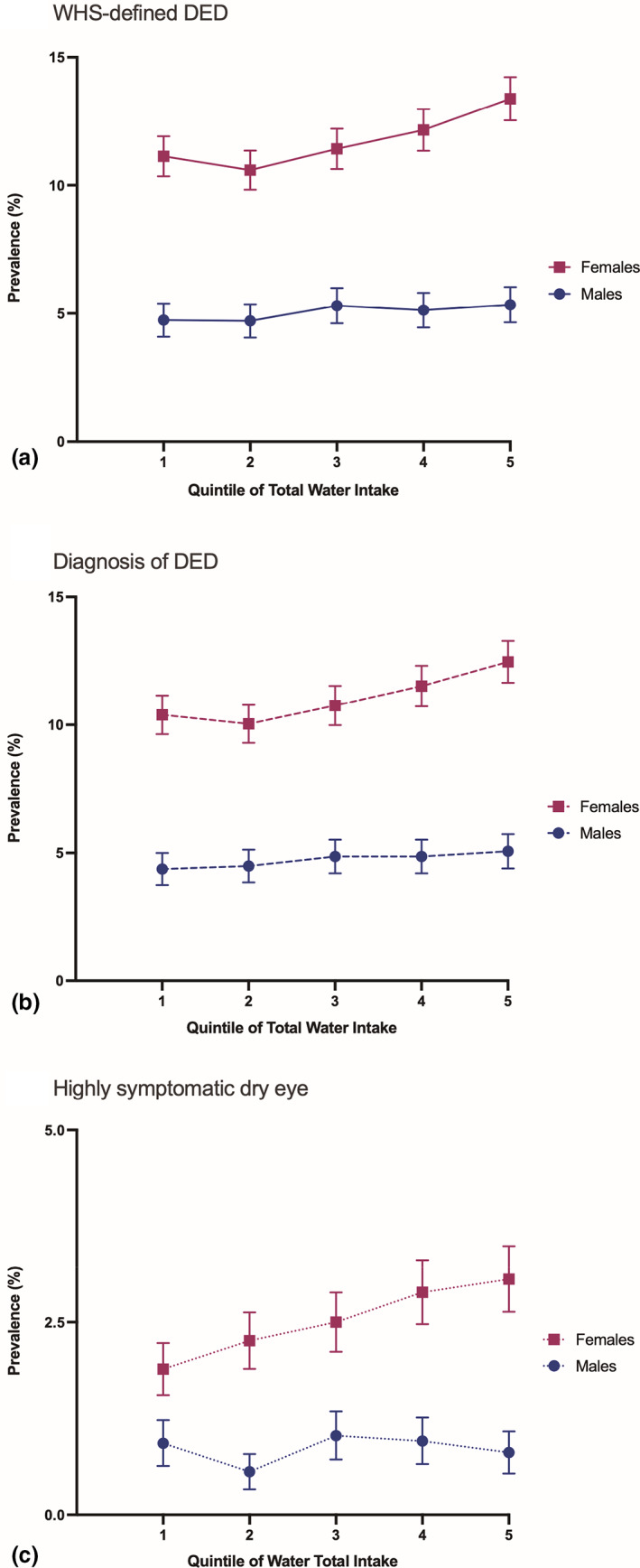
The relationship between age‐decade specific quintiles of water intake and prevalence of (a) Women's health study (WHS)‐defined dry eye (DED), (b) clinical diagnosis, and (c) highly symptomatic dry eye, in males and in females. Prevalence with 95% confidence intervals. Quintiles were generated in an age‐decade specific manner to correct for the additional effect of increasing (in females) and decreasing (in males) age with increasing water intake.
Table 2 shows the relationship between continuous total water intake and the primary and secondary outcomes. A greater total water intake was tied to a higher risk of having the main outcome variable, WHS‐defined DED, in all analyses. When correcting for comorbidities and medications, Model 3, the OR of having DED was 1.011 per 100 ml/day (95% CI: 1.004–1.017, p = 0.003). An increased total water intake was similarly associated with an increased risk of both secondary outcomes, highly symptomatic dry eye and clinical diagnosis. No significant difference in WHS‐defined DED prevalence was seen between the 5th and the 1st quintile in Model 3 (OR: 1.05, 95% CI: 0.91–1.21, p = 0.53, for females and OR: 1.25, 95% CI: 0.93–1.69, p = 0.14, for males).
TABLE 2.
Relationship between continuous total water intake (100 ml) and dry eye phenotypes
| Dry eye phenotypes | All (N = 51 551) | |||||
|---|---|---|---|---|---|---|
| OR (95% CI), Model 1 a | p‐value | OR (95% CI), Model 2 b | p‐value | OR (95% CI), Model 3 c | p‐value | |
| WHS‐defined DED | 1.013 (1.008–1.018) | <0.001 | 1.020 (1.013–1.026) | <0.001 | 1.011 (1.004–1.017) | 0.001 |
| Highly symptomatic dry eye | 1.024 (1.013–1.034) | <0.001 | 1.033 (1.020–1.046) | <0.001 | 1.020 (1.006–1.033) | 0.004 |
| Clinical diagnosis | 1.013 (1.007–1.018) | <0.001 | 1.019 (1.013–1.026) | <0.001 | 1.010 (1.004–1.017) | 0.002 |
Note: Odds ratio of having dry eye per 100 ml of total water intake per day.
Abbreviations: CI, confidence interval; OR, odds ratio; WHS, Women's Health Study.
Model 1: Corrected for age and sex only.
Model 2: Corrected for age, sex, body mass index, physical activity score, daily caloric intake, alcohol intake, smoking status, education level, and net monthly household income, full data available for 46 367 participants.
Model 3: corrected for age, sex, physical activity score, body mass index, daily caloric intake, alcohol intake, smoking status, education level, net monthly household income, 48 comorbidities associated with dry eye, and 15 medications and medication groups associated with dry eye and dry mouth, full data available for 45 730 participants.
Table 3 shows the sex‐stratified analyses. In general, results were similar in males and in females. The interaction term [sex*water intake] was not significant in any of the analyses shown in Table 2, indicating no sex‐specific effects of this relationship.
TABLE 3.
Relationship between continuous total water intake (100 ml) and dry eye phenotypes, stratified by sex
| Dry eye phenotypes | Males (N = 20 377) | Females (N = 31 174) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI), Model 1 a | p‐value | OR (95% CI), Model 2 b | p‐value | OR (95% CI), Model 3 c | p‐value | OR (95% CI), Model 1 a | p‐value | OR (95% CI), Model 2 b | p‐value | OR (95% CI), Model 3 c | p‐value | |
| WHS‐defined DED | 1.008 (0.998–1.018) | 0.11 | 1.022 (1.009–1.036) | 0.001 | 1.016 (1.002–1.029) | 0.024 | 1.013 (1.007–1.019) | <0.001 | 1.017 (1.010–1.025) | <0.001 | 1.008 (1.001–1.016) | 0.033 |
| Highly symptomatic dry eye | 1.008 (0.985–1.032) | 0.48 | 1.026 (0.996–1.058) | 0.09 | 1.024 (0.992–1.056) | 0.14 | 1.026 (1.013–1.038) | <0.001 | 1.033 (1.019–1.047) | <0.001 | 1.019 (1.004–1.034) | 0.015 |
| Clinical diagnosis | 1.009 (0.998–1.019) | 0.10 | 1.023 (1.009–1.037) | 0.001 | 1.016 (1.002–1.030) | 0.027 | 1.013 (1.007–1.019) | <0.001 | 1.017 (1.009–1.024) | <0.001 | 1.007 (1.000–1.015) | 0.060 |
Note: Odds ratio of having dry eye per 100 ml of total water intake per day.
Abbreviations: CI, confidence interval; OR, odds ratio; WHS, Women's Health Study.
Model 1: Corrected for age and sex only.
Model 2: Corrected for age, sex, body mass index, physical activity score, daily caloric intake, alcohol intake, smoking status, education level, and net monthly household income, full data available for 46 367 participants.
Model 3: corrected for age, sex, physical activity score, body mass index, daily caloric intake, alcohol intake, smoking status, education level, net monthly household income, 48 comorbidities associated with dry eye, and 15 medications and medication groups associated with dry eye and dry mouth, full data available for 45 730 participants.
Table 4 shows the relationship between sources of water and WHS‐defined DED. Greater consumption of beverage water and food water were both independently related to a higher risk of WHS‐defined DED. There was no significant association between metabolic water intake and WHS‐defined DED.
TABLE 4.
Relationship between sources of water intake (100 ml) and Women's Health Study‐defined dry eye
| Water intake | All (N = 51 551) | |||||
|---|---|---|---|---|---|---|
| OR (95% CI), Model 1 a | p‐value | OR (95% CI), Model 2 b | p‐value | OR (95% CI), Model 3 c | p‐value | |
| Beverage water (100 ml) | 1.013 (1.007–1.019) | <0.001 | 1.018 (1.011–1.025) | <0.001 | 1.009 (1.002–1.016) | 0.012 |
| Food water (100 ml) | 1.037 (1.020–1.053) | <0.001 | 1.048 (1.027–1.069) | <0.001 | 1.035 (1.014–1.057) | 0.001 |
| Metabolic water (100 ml) | 0.965 (0.920–1.012) | 0.15 | 0.967 d (0.917–1.019) | 0.21 | 0.997 d (0.944–1.052) | 0.90 |
Note: Odds ratio of having Women's Health Study‐defined dry eye per 100 ml of water intake per day.
Abbreviations: CI, confidence interval; OR, odds ratio; WHS, Women's Health Study.
Model 1: Corrected for age and sex only.
Model 2: Corrected for age, sex, body mass index, physical activity score, daily caloric intake, alcohol intake, smoking status, education level, and net monthly household income, full data available for 46 367 participants.
Model 3: corrected for age, sex, physical activity score, body mass index, daily caloric intake, alcohol intake, smoking status, education level, net monthly household income, 48 comorbidities associated with dry eye, and 15 medications and medication groups associated with dry eye and dry mouth, full data available for 45 730 participants.
Metabolic water intake was not corrected for energy intake due to high correlation between them (Pearson's r = 0.999).
Table 5 shows the relationship between 24‐h urine volume and dry eye outcomes. When adjusting for all comorbidities and medications (Model 3), each 100 ml of urine volume increased the risk of having WHS‐defined DED with around 1% (OR: 1.010 per 100 ml/day, 95% CI: 1.005–1.015, p < 0.001). Greater urine volumes were associated with a higher risk of WHS‐defined DED in both males and females, as shown in Table S3.
TABLE 5.
Relationship between 24‐h urine volume (100 ml) and dry eye phenotypes
| Dry eye phenotypes | All (N = 49 332) | |||||
|---|---|---|---|---|---|---|
| OR (95% CI), Model 1 a | p‐value | OR (95% CI), Model 2 b | p‐value | OR (95% CI), Model 3 c | p‐value | |
| WHS‐defined DED | 1.013 (1.009–1.018) | <0.001 | 1.012 (1.007–1.016) | <0.001 | 1.010 (1.005–1.015) | <0.001 |
| Highly symptomatic dry eye | 1.010 (1.001–1.020) | 0.028 | 1.013 (1.003–1.023) | 0.011 | 1.010 (1.000–1.021) | 0.06 |
| Clinical diagnosis | 1.013 (1.008–1.017) | <0.001 | 1.011 (1.006–1.016) | <0.001 | 1.009 (1.004–1.014) | <0.001 |
Note: Odds ratio of having dry eye per 100 ml of 24‐h urine volume.
Abbreviations: CI, confidence interval; OR, odds ratio; WHS, Women's Health Study.
Model 1: Corrected for age and sex only.
Model 2: corrected for age, sex, body mass index, physical activity score, daily caloric intake, alcohol intake, smoking status, education level, and net monthly household income, full data available for 44 384 participants.
Model 3: corrected for age, sex, physical activity score, body mass index, daily caloric intake, alcohol intake, smoking status, education level, net monthly household income, and 48 comorbidities associated with dry eye, and 15 medications and medication groups associated with dry eye and dry mouth, full data available for 43 780 participants.
In addition, after exclusion of participants with a reported clinical diagnosis of DED, higher total water intake was still associated with increased odds of having symptomatic dry eye in all models (OR: 1.010 per 100 ml/day, 95% CI: 1.006–1.015, p < 0.001, Model 3). Similarly, total water intake was tied to an increased likelihood of experiencing dryness “Sometimes” rather than “Never” (OR: 1.010 per 100 ml/day, 95% CI: 1.006–1.014, p < 0.001, Model 3).
4. DISCUSSION
While greater water intake has been associated with health benefits in other general populations, such as lower risk of nonalcoholic fatty liver disease (Wang et al., 2021) and chronic kidney disease (Wang & Jiang, 2021), this large epidemiological study did not find it to be associated with a reduced risk of having DED. The findings contradicted our hypothesis. In fact, both a higher self‐reported water intake and a greater measured 24‐h urine volume were tied to an increased prevalence of DED. The same relationship was found when beverage water and food water were assessed separately. However, there was no significant difference in DED prevalence in the 5th and 1st age decade‐specific quintiles of water intake in either sex after correcting for comorbidities and medications.
The European Food Safety Authority recommends daily intake of food and beverage water of 2.0 L/day for females and 2.5 L/day for males, but no upper limit was defined (EFSA Panel on Dietetic Products, 2010). This includes water from all food and beverage sources, but excludes metabolic water. In this study, when excluding metabolic water, 74.5% of males and 39.8% of females consumed less than these recommendations. Using this cutoff in a sensitivity analysis, the results were similar to that of the analyses presented in the current study, with water intake above the cut‐off being significantly associated with more WHS‐defined DED in all models (data not shown).
The biological link behind the relationship between habitual water intake and DED is unknown. One, small, hospital‐based cross‐sectional study found that patients with elevated tear osmolarity or symptoms of DED and impaired visual acuity score, had higher plasma osmolarity, a proxy of hydration status, than a non‐DED control group (Walsh et al., 2012). Plasma osmolality and tear osmolarity were also found to be strongly correlated (r = 0.93) in a different study, and the authors speculate that dehydration increases tear osmolarity through decreased lacrimal gland function (Fortes et al., 2011). However, an RCT observed no change in Schirmer's test 45 to 180 min after drinking 200 ml of water (Osei et al., 2014). Moreover, Masmali et al. found red thread test and tear ferning test scores to be unchanged before and after acute consumption of hot water (Masmali et al., 2019). Likewise, the present population‐based study did not find habitual water intake to be tied to less DED in any way. Future studies assessing the impact of water intake on dry eye should include both subjective and objective measures and include a prolonged follow‐up.
Clinicians may recommend drinking more water as supplemental therapy for dry eye. Additionally, patients often search the internet for information about symptoms and diseases (Van de Belt et al., 2013). A Google search with “dry eye treatment” yields several results suggesting drinking water as beneficial for dry eye, and it has been proposed that improving whole‐body hydration through fluid intervention could have a therapeutic effect on dry eyes (Walsh et al., 2012). It could thus be thought that drinking more water could benefit dryness‐related complaints. Indeed, an intervention of increased daily water intake improved dry skin in one small study (Palma et al., 2015). Similarly, another study found that those with dry mouth symptoms had lower water intakes than those without (Lee et al., 2020). Due to this, patients may be inclined to think that increasing water intake would alleviate dry eye symptoms (Yeo & Tong, 2018).
When analysing non‐diagnosed participants only, higher water intake was still positively correlated with symptomatic dry eye. Notwithstanding, patients experiencing dry eye without a clinical diagnosis might also try drinking more to lessen dryness symptoms. It is plausible to assume they would likely do so by drinking more water rather than eat foods with higher water content. In this study, however, higher food water intake was also associated with more DED. Additionally, participants experiencing only mild symptoms of dryness were assessed separately as they would likely not have enough symptoms to alter their lifestyle. Greater water intake was associated with an increased likelihood of experiencing ocular dryness “Sometimes” compared with “Never.” Based on these findings, reverse causality is unlikely to explain the results of this study.
There are several strengths of this study looking at the relationship between water intake and DED. First, the substantial sample size and ample information collected from the Lifelines cohort allowed us to correct for numeral confounding factors, including 48 comorbidities of dry eye and 15 different medications and medication groups associated with dry eye and dry mouth, and to stratify by sex while maintaining a large sample size. Second, we included all major sources of water when calculating total water intake, in addition to exploring the effects of each of them separately. Third, we used 24‐h urine volume as a biomarker of hydration to validate our findings. The direction of effect was the same in all analyses regardless of source of dietary water, measured urinary volume and definition of dry eye (mild and severe symptoms, diagnosis), supporting a true effect.
Our study has also some limitations. First, we assessed water intake in the Lifelines cohort through an FFQ which relies on retrospectively self‐reported dietary intake, and recall errors are possible. The assessment of water intake could have been further solidified with the inclusion of a 3‐ to 4‐day diary of water intake (Mons et al., 2007). Though we used urine volume as a criterion measurement, there is no consensus on a “gold standard” for assessing hydration status with biomarkers, and other alternatives are plasma osmolality, urine colour and salivary indices (Armstrong, 2007). Second, the collection of urine, the DED assessment visit, and the FFQ administering were at different timepoints, and changes in diet may have occurred. Third, given the cross‐sectional nature of the data, the causality of the observed relationship cannot be elucidated. Moreover, despite correcting for a large range of comorbidities and confounding factors, there could still be a residual confounding effect of other factors. Last, objective measurements of DED were not included as it was not feasible with the current large population‐wide study design. Objective measures of tear film osmolarity and stability and meibomian gland status could have given valuable information about ocular surface health.
In this large, population‐based study greater water intake was not found to be related to a decreased risk of DED. In fact, it appeared to be associated with a slightly higher prevalence of WHS‐defined DED and dry eye symptoms. Based on these findings, advising dry eye patients to increase water intake is not justifiable. To fully understand any effect of water consumption on dry eye, future interventional studies should be conducted.
Supporting information
TABLE S1
TABLE S2 Prevalence of Women’s Health Study‐defined dry eye in age‐decade specific quintiles of water intake, stratified by sex
TABLE S3 Relationship between 24‐h urine volume (100 ml) and dry eye phenotypes, stratified by sex
ACKNOWLEDGEMENTS
The authors wish to acknowledge the study participants, the services of the Lifelines Cohort Study, and the contributing research centers delivering data to Lifelines. The Lifelines Biobank initiative has been made possible by subsidy from the Dutch Ministry of Health, Welfare, and Sport, the Dutch Ministry of Economic Affairs, the University Medical Center Groningen (UMCG the Netherlands), University of Groningen, and the Northern Provinces of the Netherlands. The sponsor or funding organization had no role in the design or conduct of this research. The authors have no conflicts of interest to declare. We are very grateful to Emily Moschowits for her excellent contributions during the writing process.
Nguyen, L. , Magno, M.S. , Utheim, T.P. , Jansonius, N.M. , Hammond, C.J. & Vehof, J. (2023) The relationship between habitual water intake and dry eye disease. Acta Ophthalmologica, 101, 65–73. Available from: 10.1111/aos.15227
Long Nguyen and Morten Schjerven Magno contributed equally as cofirst authors.
REFERENCES
- Armstrong, L.E. (2007) Assessing hydration status: the elusive gold standard. Journal of the American College of Nutrition, 26(Supplement 5), 575s–584s. 10.1080/07315724.2007.10719661 [DOI] [PubMed] [Google Scholar]
- Bazeer, S. , Jansonius, N. , Snieder, H. , Hammond, C. & Vehof, J. (2019) The relationship between occupation and dry eye. The Ocular Surface, 17(3), 484–490. 10.1016/j.jtos.2019.04.004 [DOI] [PubMed] [Google Scholar]
- Brouwer‐Brolsma, E.M. , Streppel, M.T. , van Lee, L. , Geelen, A. , Sluik, D. , van de Wiel, A.M. et al. (2017) A National Dietary Assessment Reference Database (NDARD) for the Dutch population: rationale behind the design. Nutrients, 9(10), 1136. 10.3390/nu9101136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, W.F. , Sontrop, J.M. , Huang, S.H. , Moist, L. , Bouby, N. & Bankir, L. (2016) Hydration and chronic kidney disease progression: a critical review of the evidence. American Journal of Nephrology, 43(4), 281–292. 10.1159/000445959 [DOI] [PubMed] [Google Scholar]
- Craig, J.P. , Nichols, K.K. , Akpek, E.K. , Caffery, B. , Dua, H.S. , Joo, C.K. et al. (2017) TFOS DEWS II definition and classification report. The Ocular Surface, 15(3), 276–283. 10.1016/j.jtos.2017.05.008 [DOI] [PubMed] [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA) . (2010) Scientific opinion on dietary reference values for water. EFSA Journal, 8, 1459. 10.2903/j.efsa.2010.1459 [DOI] [Google Scholar]
- Fortes, M.B. , Diment, B.C. , Di Felice, U. , Gunn, A.E. , Kendall, J.L. , Esmaeelpour, M. et al. (2011) Tear fluid osmolarity as a potential marker of hydration status. Medicine and Science in Sports and Exercise, 43(8), 1590–1597. 10.1249/MSS.0b013e31820e7cb6 [DOI] [PubMed] [Google Scholar]
- Gandy, J. (2015) Water intake: validity of population assessment and recommendations. European Journal of Nutrition, 54(Supplement 2), 11–16. 10.1007/s00394-015-0944-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati, A. , Sullivan, R. , Buring, J.E. , Sullivan, D.A. , Dana, R. & Schaumberg, D.A. (2006) Validation and repeatability of a short questionnaire for dry eye syndrome. American Journal of Ophthalmology, 142(1), 125–131. 10.1016/j.ajo.2006.02.038 [DOI] [PubMed] [Google Scholar]
- Jéquier, E. & Constant, F. (2010) Water as an essential nutrient: the physiological basis of hydration. European Journal of Clinical Nutrition, 64(2), 115–123. 10.1038/ejcn.2009.111 [DOI] [PubMed] [Google Scholar]
- Lee, K.A. , Park, J.C. & Park, Y.K. (2020) Nutrient intakes and medication use in elderly individuals with and without dry mouths. Nutrition Research and Practice, 14(2), 143–151. 10.4162/nrp.2020.14.2.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M. , Gong, L. , Chapin, W.J. & Zhu, M. (2012) Assessment of vision‐related quality of life in dry eye patients. Investigative Ophthalmology & Visual Science, 53(9), 5722–5727. 10.1167/iovs.11-9094 [DOI] [PubMed] [Google Scholar]
- Magno, M.S. , Daniel, T. , Morthen, M.K. , Snieder, H. , Jansonius, N. , Utheim, T.P. et al. (2021) The relationship between alcohol consumption and dry eye. The Ocular Surface, 21, 87–95. 10.1016/j.jtos.2021.05.005 [DOI] [PubMed] [Google Scholar]
- Manz, F. , Johner, S.A. , Wentz, A. , Boeing, H. & Remer, T. (2012) Water balance throughout the adult life span in a German population. The British Journal of Nutrition, 107(11), 1673–1681. 10.1017/s0007114511004776 [DOI] [PubMed] [Google Scholar]
- Masento, N.A. , Golightly, M. , Field, D.T. , Butler, L.T. & van Reekum, C.M. (2014) Effects of hydration status on cognitive performance and mood. The British Journal of Nutrition, 111(10), 1841–1852. 10.1017/s0007114513004455 [DOI] [PubMed] [Google Scholar]
- Masmali, A.M. , Alanazi, S.A. , Alotaibi, A.G. , Fagehi, R. , Abusharaha, A. & El‐Hiti, G.A. (2019) The acute effect of a single dose of green tea on the quality and quantity of tears in normal eye subjects. Clinical Ophthalmology, 13, 605–610. 10.2147/opth.S201127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, M. , Patel, D.A. , Keith, M.S. & Snedecor, S.J. (2016) Economic and humanistic burden of dry eye disease in Europe, North America, and Asia: a systematic literature review. The Ocular Surface, 14(2), 144–167. 10.1016/j.jtos.2015.11.002 [DOI] [PubMed] [Google Scholar]
- Mertzanis, P. , Abetz, L. , Rajagopalan, K. , Espindle, D. , Chalmers, R. , Snyder, C. et al. (2005) The relative burden of dry eye in patients' lives: comparisons to a U.S. normative sample. Investigative Ophthalmology & Visual Science, 46(1), 46–50. 10.1167/iovs.03-0915 [DOI] [PubMed] [Google Scholar]
- Messmer, E.M. (2015) The pathophysiology, diagnosis, and treatment of dry eye disease. Deutsches Ärzteblatt International, 112(5), 71–82. 10.3238/arztebl.2015.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miljanovic, B. , Dana, R. , Sullivan, D.A. & Schaumberg, D.A. (2007) Impact of dry eye syndrome on vision‐related quality of life. American Journal of Ophthalmology, 143(3), 409–415. 10.1016/j.ajo.2006.11.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina‐Leyva, I. , Molina‐Leyva, A. , Riquelme‐Gallego, B. , Cano‐Ibáñez, N. , García‐Molina, L. & Bueno‐Cavanillas, A. (2020) Effectiveness of Mediterranean diet implementation in dry eye parameters: a study of PREDIMED‐PLUS trial. Nutrients, 12(5), 1289. 10.3390/nu12051289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mons, M.N. , van der Wielen, J.M. , Blokker, E.J. , Sinclair, M.I. , Hulshof, K.F. , Dangendorf, F. et al. (2007) Estimation of the consumption of cold tap water for microbiological risk assessment: an overview of studies and statistical analysis of data. Journal of Water and Health, 5(Supplement 1), 151–170. 10.2166/wh.2007.141 [DOI] [PubMed] [Google Scholar]
- Murray, B. (2007) Hydration and physical performance. Journal of the American College of Nutrition, 26(Supplement 5), 542s–548s. 10.1080/07315724.2007.10719656 [DOI] [PubMed] [Google Scholar]
- NEVO‐tabel R . (2011) Nederlands Voedingsstoffenbestand 2011. Den Haag: Voedingscentrum, p. 339. [Google Scholar]
- Osei, K.A. , Ovenseri‐Ogbomo, G. , Kyei, S. & Ntodie, M. (2014) The effect of caffeine on tear secretion. Optometry and Vision Science, 91(2), 171–177. 10.1097/opx.0000000000000129 [DOI] [PubMed] [Google Scholar]
- Palma, L. , Marques, L.T. , Bujan, J. & Rodrigues, L.M. (2015) Dietary water affects human skin hydration and biomechanics. Clinical, Cosmetic and Investigational Dermatology, 8, 413–421. 10.2147/ccid.S86822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier, E. , Rondeau, P. , Poupin, M. , le Bellego, L. , Armstrong, L.E. , Lang, F. et al. (2013) Relation between urinary hydration biomarkers and total fluid intake in healthy adults. European Journal of Clinical Nutrition, 67(9), 939–943. 10.1038/ejcn.2013.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield, W.N. (1985) Predicting basal metabolic rate, new standards and review of previous work. Human Nutrition Clinical Nutrition, 39(Supplement 1), 5–41. [PubMed] [Google Scholar]
- Scholtens, S. , Smidt, N. , Swertz, M.A. , Bakker, S.J. , Dotinga, A. , Vonk, J.M. et al. (2015) Cohort profile: lifeLines, a three‐generation cohort study and biobank. International Journal of Epidemiology, 44(4), 1172–1180. 10.1093/ije/dyu229 [DOI] [PubMed] [Google Scholar]
- Sijtsma, A. , Rienks, J. , van der Harst, P. , Navis, G. , Rosmalen, J.G.M. & Dotinga, A. (2021) Cohort profile update: lifelines, a three‐generation cohort study and biobank. International Journal of Epidemiology. 10.1093/ije/dyab257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton, F. , Alves, M. , Bunya, V.Y. , Jalbert, I. , Lekhanont, K. , Malet, F. et al. (2017) TFOS DEWS II epidemiology report. The Ocular Surface, 15(3), 334–365. 10.1016/j.jtos.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Stolk, R.P. , Rosmalen, J.G. , Postma, D.S. , de Boer, R.A. , Navis, G. , Slaets, J.P. et al. (2008) Universal risk factors for multifactorial diseases: LifeLines: a three‐generation population‐based study. European Journal of Epidemiology, 23(1), 67–74. 10.1007/s10654-007-9204-4 [DOI] [PubMed] [Google Scholar]
- Sullivan, D.A. , Rocha, E.M. , Aragona, P. , Clayton, J.A. , Ding, J. , Golebiowski, B. et al. (2017) TFOS DEWS II sex, gender, and hormones report. The Ocular Surface, 15(3), 284–333. 10.1016/j.jtos.2017.04.001 [DOI] [PubMed] [Google Scholar]
- Van de Belt, T.H. , Engelen, L.J. , Berben, S.A. , Teerenstra, S. , Samsom, M. & Schoonhoven, L. (2013) Internet and social media for health‐related information and communication in health care: preferences of the Dutch general population. Journal of Medical Internet Research, 15(10), e220. 10.2196/jmir.2607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehof, J. , Snieder, H. , Jansonius, N. & Hammond, C.J. (2021) Prevalence and risk factors of dry eye in 79,866 participants of the population‐based Lifelines cohort study in The Netherlands. The Ocular Surface, 19, 83–93. 10.1016/j.jtos.2020.04.005 [DOI] [PubMed] [Google Scholar]
- Vinke, P.C. , Navis, G. , Kromhout, D. & Corpeleijn, E. (2020) Socio‐economic disparities in the association of diet quality and type 2 diabetes incidence in the Dutch Lifelines cohort. EClinicalMedicine, 19, 100252. 10.1016/j.eclinm.2019.100252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, N.P. , Fortes, M.B. , Raymond‐Barker, P. , Bishop, C. , Owen, J. , Tye, E. et al. (2012) Is whole‐body hydration an important consideration in dry eye? Investigative Ophthalmology & Visual Science, 53(10), 6622–6627. 10.1167/iovs.12-10175 [DOI] [PubMed] [Google Scholar]
- Wang, H.W. & Jiang, M.Y. (2021) Higher volume of water intake is associated with lower risk of albuminuria and chronic kidney disease. Medicine (Baltimore), 100(20), e26009. 10.1097/md.0000000000026009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X. , Lin, S. , Gan, S. , Gu, Y. , Yang, Y. , Zhang, Q. et al. (2021) Higher plain water intake is related to lower newly diagnosed nonalcoholic fatty liver disease risk: a population‐based study. European Journal of Clinical Nutrition, 75, 1801–1808. 10.1038/s41430-021-00891-9 [DOI] [PubMed] [Google Scholar]
- Watso, J.C. & Farquhar, W.B. (2019) Hydration status and cardiovascular function. Nutrients, 11(8), 1866. 10.3390/nu11081866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff, A. , Joshi, R.K. , Ekström, J. , Aframian, D. , Pedersen, A.M. , Proctor, G. et al. (2017) A guide to medications inducing salivary gland dysfunction, xerostomia, and subjective sialorrhea: a systematic review sponsored by the world workshop on oral medicine VI. Drugs in R&D, 17(1), 1–28. 10.1007/s40268-016-0153-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert, L.E. , Snieder, H. , Jansonius, N.M. , Utheim, T.P. , Hammond, C.J. & Vehof, J. (2021) Medication use and dry eye symptoms: a large, hypothesis‐free, population‐based study in The Netherlands. The Ocular Surface, 22, 1–12. 10.1016/j.jtos.2021.06.009 [DOI] [PubMed] [Google Scholar]
- Yeo, S. & Tong, L. (2018) Coping with dry eyes: a qualitative approach. BMC Ophthalmology, 18(1), 8. 10.1186/s12886-018-0671-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J. , Asche, C.V. & Fairchild, C.J. (2011) The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea, 30(4), 379–387. 10.1097/ICO.0b013e3181f7f363 [DOI] [PubMed] [Google Scholar]
- Zhang, N. , Du, S. , Tang, Z. , Zheng, M. , Yan, R. , Zhu, Y. et al. (2017) Hydration, fluid intake, and related urine biomarkers among male college students in Cangzhou, China: a cross‐sectional study‐applications for assessing fluid intake and adequate water intake. International Journal of Environmental Research and Public Health, 14(5), 513. 10.3390/ijerph14050513 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1
TABLE S2 Prevalence of Women’s Health Study‐defined dry eye in age‐decade specific quintiles of water intake, stratified by sex
TABLE S3 Relationship between 24‐h urine volume (100 ml) and dry eye phenotypes, stratified by sex


