Abstract
Objective
To define the prevalence of subclinical synovitis on magnetic resonance imaging (MRI) in a large cohort of patients with juvenile idiopathic arthritis (JIA) in clinical remission and to evaluate its predictive value in terms of disease flare and joint deterioration.
Methods
Ninety patients with clinically inactive JIA who underwent a contrast‐enhanced (CE)–MRI of a previously affected joint were retrospectively included. Each joint was evaluated for synovitis, tenosynovitis, and bone marrow edema. Baseline and follow‐up radiographs were assessed to evaluate structural damage progression.
Results
CE‐MRI was acquired in 45 wrists, 30 hips, 13 ankles, and 2 knees. Subclinical synovitis was detected in 59 (65.5%) of 90 patients and bone marrow edema in 42 (46.7%) of 90 patients. Fifty‐seven of 90 (63.3%) patients experienced a disease flare during follow‐up. Forty‐four of 59 (74.6%) patients with subclinical synovitis experienced a disease flare versus 13 (41.9%) of 31 patients with no residual synovitis on MRI (P = 0.002). The presence of subclinical synovitis was the best predictor of disease flare on multivariable regression analysis (hazard ratio [HR] 2.45, P = 0.003). Baseline and follow‐up radiographs were available for 54 patients, and 17 (31.5%) of 54 patients experienced radiographic damage progression. The presence of bone marrow edema (HR 4.40, P = 0.045) and being >17 years old (HR 3.51, P = 0.04) were strong predictors of joint damage progression in the multivariable analysis.
Conclusion
MRI‐detected subclinical inflammation was present in a large proportion of patients with JIA despite clinical remission. Subclinical synovitis and bone marrow edema have been shown to play a role in predicting the risk of disease relapse and joint deterioration, with potential implications for patients' management of the disease.
INTRODUCTION
Juvenile idiopathic arthritis (JIA) is the most common rheumatic disease in children. Its hallmark is persistent inflammation of the synovial tissue of joints and tendon sheaths, which if left untreated, may cause damage to joint structures and physical disability (1). The primary aim of treatment is to prevent irreversible sequelae by inducing early disease remission that, owing to the significant advances in therapeutic options, has become an attainable goal for most JIA patients (2, 3, 4, 5). The accurate assessment of remission status in JIA patients is of utmost relevance to taper medications and prevent side effects from their long‐term administration. The current criteria for defining inactive disease largely rely on subjective clinical symptoms, joint examination findings, and acute‐phase reactants (6, 7). Recently the question has been raised whether current measures used to define clinical remission truly reflect the absence of synovial inflammation. In fact, MRI and musculoskeletal ultrasound (MSUS) studies have demonstrated ongoing subclinical synovitis in a sizeable proportion of adult rheumatoid arthritis (RA) patients with “clinical inactive disease” (8). An even more relevant point is that residual synovitis is responsible for disease flare and structural damage progression in RA patients, with consequent therapeutic implications (9, 10). Unlike RA, very few studies have explored the potential role of imaging in the assessment of remission status in JIA (11, 12, 13, 14, 15, 16).
SIGNIFICANCE & INNOVATIONS.
Current conventional measures used to define clinical remission are insensitive to exclude residual inflammation in juvenile idiopathic arthritis (JIA).
Subclinical synovitis on magnetic resonance imaging (MRI) is the best predictor of disease flare in JIA patients with clinically inactive disease.
Bone marrow edema on MRI plays a significant role in predicting joint damage progression.
Because of its multiplanar capabilities and excellent soft‐tissue contrast, MRI is increasingly used in the assessment of JIA patients. Contrast‐enhanced (CE) MRI is very sensitive in detecting the early signs of soft tissue inflammation and in differentiating the active hypervascular pannus from the inactive fibrotic pannus. The potential of CE‐MRI to improve the evaluation of disease activity beyond clinical examination has been suggested by the results of pilot studies reporting subclinical synovitis in up to 50% of the JIA patients who were considered to be clinically inactive (11, 12, 13, 14). In a recent prospective study on 32 JIA patients in clinical remission, patients with persistently inactive disease and those with flaring disease differed in the maximum enhancement of the synovium shown on dynamic CE‐MRIs of their knees, suggesting that the functional properties of the synovial tissue might play a role in predicting disease flare (15). However, because of the low sample size, the authors could not draw definitive conclusions on the prognostic value of subclinical synovitis in JIA. The aim of the present study is to determine the prevalence of subclinical synovitis as detected by CE‐MRI in a larger cohort of JIA patients in clinical remission and to evaluate its predictive value in terms of disease flare and joint deterioration.
PATIENTS AND METHODS
Study design
JIA patients with inactive disease who underwent a CE‐MRI of a previously affected joint between October 2012 and December 2016 were retrospectively included in the present study. MRI was requested by the treating physician to confirm the clinical remission status and/or monitor structural damage progression. Inactive disease was defined according to the Wallace criteria (6, 7), which comprise the following: no joints with active arthritis; no fever, rash, serositis, splenomegaly, or generalized lymphadenopathy secondary to JIA; no evidence of active uveitis; a normal erythrocyte sedimentation rate or C‐reactive protein level (or, if elevated, not attributable to JIA); a physician global assessment of disease activity indicating no disease activity; and/or duration of morning stiffness of ≤15 minutes. Clinical remission on medication was established when the criteria for inactive disease were met for a minimum of 6 continuous months while the patient was still taking medication, whereas clinical remission off medication was established when the criteria for inactive disease were met for a minimum of 12 continuous months while the patient had discontinued all antiarthritis and antiuveitis medications (6, 7). Flare of synovitis was defined as recurrence of clinical signs of joint inflammation, including swelling, pain on motion/tenderness, and restricted motion, that required a major therapeutic intervention (i.e., an intraarticular glucocorticoid injection, the start of a systemic therapy, or the change of the administration of methotrexate [MTX] from the oral to the subcutaneous route).
Clinical examination was performed by experienced pediatric rheumatologists (MM and SV) who were blinded with regard to imaging results. The study was performed according to good clinical practice guidelines and the Declaration of Helsinki and was approved by the institution's ethical review board.
Imaging protocol
CE‐MRI was performed using a 1.5T MRI scanner (Achieva Intera; Philips Medical Systems). The imaging protocol included the following sequences: a T1‐weighted turbo spin‐echo (TSE), a TSE T2‐weighted fat‐saturated sequence or a STIR sequence, and a T1‐weighted 3‐dimensional gradient‐echo acquired immediately after the injection of 0.1 mmoles/kg of body weight of gadolinium‐based contrast agent. Each MRI was independently scored by a pediatric musculoskeletal radiologist (FM) and a pediatric rheumatologist (CM) with more than 10 years of experience in musculoskeletal MRI. CE‐MRIs were performed on the following joints: wrist and metacarpophalangeal (MCP) joints, the ankle and midfoot, hips, and the knee. Synovitis was defined as an area in the synovial compartment that showed above normal post‐gadolinium enhancement of a thickness greater than the width of the normal synovium. A score of 0 was assessed as “normal” while scores of 1, 2, and 3, which were assessed as “mild,” “moderate,” and “severe,” respectively, increased by thirds of the presumed maximum volume of enhancing tissue in the synovial compartment.
The Outcome Measures in Rheumatology (OMERACT) Rheumatoid Arthritis Magnetic Resonance Imaging scoring system (RAMRIS), which has a 0–3 grading scale, was used to grade the severity of synovitis in 3 wrist regions, which comprised the distal radioulnar, radiocarpal, intercarpal, and carpo‐metacarpophalangeal joints, and in each MCP joint. A total synovitis score ranging from 0–24 was obtained by adding the scores of the single joint recess (16, 17, 18). The OMERACT RAMRIS 0–3 score was also used to grade synovitis at the tibio‐peroneo‐talar, talonavicular, subtalar, calcaneocuboid, and the cuneonavicular joints and at the 3 tarsometatarsal joints (the first, second to third, and fourth to fifth tarsometatarsal joints) (19, 20). The total synovitis score was calculated by adding the scores assigned to each of the 8 joint recesses, yielding a total score ranging from 0–24. The degree of synovitis in each coxofemoral joint was assessed using a 0–4 semiquantitative score, with grade 0 indicating no CE, grade 1 indicating focal synovial CE, grade 2 indicating diffuse synovial CE, grade 3 indicating diffuse enhancement with synovial thickening, and grade 4 indicating diffuse enhancement with villonodular synovial thickening (21). Synovitis was evaluated in 6 areas of the knee (patellofemoral, suprapatellar recesses, infrapatellar fat pad, the adjacent areas of the cruciate ligaments, and the adjacent areas of the medial and lateral posterior condyle), according to the Juvenile Arthritis MRI Scoring (JAMRIS) system (22), which uses a 0–2 scale.
Tenosynovitis was defined as peritendinous effusion and/or post–contrast enhancement of the tendon sheath seen on axial sequences over ≥3 consecutive slices (23, 24). Tenosynovitis was graded on a binary scale (present or absent) (23). Bone marrow edema was defined as a lesion within the trabecular bone with ill‐defined margins and signal characteristics consistent with increased water content (16). Bone marrow edema was scored proportionally by the amount of bone that had edema using the OMERACT RAMRIS 0–3 scale, with a score of 0 indicating no edema, 1 indicating 1–33% of the bone was edematous, 2 indicating 34–66% of the bone was edematous, and 3 indicating 67–100% of the bone was edematous, at 15 areas within the carpus (distal radius and ulna, carpal bones, and the bases of the metacarpal bones); bone marrow edema was also scored at 14 areas within the midfoot/ankle region (distal tibial epiphysis, distal fibula epiphysis, tarsal bones, and the bases of the metatarsal bones) (16, 19, 25, 26). A total bone marrow edema score was obtained by adding the scores for individual bones, yielding a maximum score of 45 for the wrist and of 42 for the midfoot/ankle region. The severity of bone marrow edema at the hip was assessed by assigning a 0–2 semiquantitative score (none, less than, or greater than one‐third of the epiphysis) at each femoral head (27, 28, 29). Bone marrow edema was scored semiquantitatively in 8 anatomic regions of the knee based on the JAMRIS system (22).
The presence and extent of joint changes were assessed by visual inspection of clinical remission off medication. Baseline and follow‐up radiographs of the wrist/hand and foot were scored according to the adapted versions of the modified Sharp/van der Heijde score (30), which is based on the assessment of joint space narrowing (JSN) and bone erosions/deformities on 0–4 and 0–5 severity scales, respectively. Hip damage was graded according to the Childhood Arthritis Radiographic Score of the Hip (CARSH), which assesses the following abnormalities: JSN, erosion, growth abnormalities, subchondral cysts, malalignment, sclerosis of the acetabulum, and avascular necrosis of the femoral head (31). Radiographs were assessed by a pediatric rheumatologist (AR) with >25 years of experience in reading skeletal radiographs and familiarity with radiographic scoring, who was blinded with regard to clinical and MRI findings and to the chronological sequence of the radiographs. Radiographic progression was determined by calculating the change in the modified Sharp/van der Heijde and CARSH scores between radiographs assessed at baseline and follow‐up radiographs.
Sixty‐three CE‐MRIs (7 wrists, 15 hips, 2 knees, and 39 ankles) obtained from children without musculoskeletal disease were included as a control group. CE‐MRIs were required to investigate suspected vascular malformations, cystic bone lesion, osteolytic lesions, lymphedema of the lower extremity, neurofibromas, and urinary tract abnormalities.
Statistical analysis
Descriptive statistics were reported in terms of absolute frequencies or percentages for categorical data and in terms of medians and first and third quartiles (Q1–Q3) for continuous quantitative data. Comparison of categorical data was performed by Pearson's chi‐square test (or Fisher's exact test in case of expected frequencies of <5). Comparison of quantitative data was performed by nonparametric Mann‐Whitney U test. Concordance between 2 readers for the presence or absence of synovitis and bone marrow edema was evaluated by Cohen's kappa coefficient (32) with the cut‐off values suggested by Landis and Koch (33), which were as follows: κ < 0.4 (poor), κ ≥ 0.4–0.60 (moderate), κ ≥ 0.6–0.80 (substantial), and κ ≥ 0.80 (almost perfect agreement). The synovitis and bone marrow edema total scores of different joint types were standardized on a scale with the same 0–100 range according to the following formula:
where “obs” indicates observed and “min” indicates the minimum. A similar standardization was applied to the radiographic damage score of different joint types. The standardization of the scores converts the total scores in a range that varies from 0–100 and therefore allows the comparison of different joints.
Survival analysis, considering disease flare and radiographic damage progression as the outcome variables, was performed, and curves reporting on y‐axis cumulative failure rates were drawn according to the Nelson‐Aalen method. The log‐rank test was used to compare different survival curves. Finally, Cox regression models were performed in order to identify independent predictors of disease flare and joint damage progression. Statistically significant variables in the bivariate analysis or clinically relevant variables were included in the Cox regression analysis. The following variables were considered in the Cox regression model: JIA subtype, patient's age, clinical remission duration, bone marrow edema on MRI, subclinical synovitis, and radiographic damage at study entry. Quantitative variables (i.e., MRI scores, etc.) were categorized by means of the receiver operating characteristic curve analysis that allowed to obtain the cut‐off value that discriminated best between patients who did experience disease flare or radiographic progression and those who did not (34). The likelihood ratio (LR) was used in testing the role of different variables in the model, and hazard ratios (HRs) and their 95% confidence intervals (95% CIs) were calculated and reported. All statistical tests were 2‐sided, and P values less than 0.05 were considered statistically significant. Statistica software (version 9.0; StatSoft Corporation) was used for descriptive and bivariate analyses, and Stata software (version 11; StataCorp LLC) was used in drawing the Nelson‐Aalen curves and the Cox regression model analyses.
RESULTS
A total of 90 JIA patients with clinical inactive disease were included in the study. The demographic and clinical features of the patients are shown in Table 1. Fifteen (16.7%) of 90 patients were in clinical remission off medication, while 75 (83.3%) of 90 patients were in clinical remission on medication (MTX in 21 [28%] of 75 patients, biologic agents in 40 [53.3%] of 75 patients, and combination therapies [MTX + a biologic agent] in 14 [18.7%] of 75 patients). CE‐MRIs were acquired in the following joints: 45 wrists, 30 hips, 13 ankles, and 2 knees. CE‐MRI revealed subclinical synovitis in 59 (65.5%) of 90 patients and bone marrow edema in 42 (46.7%) of 90 patients despite status of clinical remission (Figure 1). Median synovitis and bone marrow edema scores at baseline were 16.7 (95% CI 16.7–33.3) and 8.0 (95% CI 5.5–15.0), respectively. Interobserver concordance for the presence/absence of synovitis and bone marrow edema was moderate (κ = 0.74 [95% CI 0.5–0.9]) and substantial (κ = 0.82 [95% CI 0.62–1.0]), respectively. Eight (12.7%) of 63 healthy controls showed synovitis on the CE‐MRI, whereas bone marrow changes were observed in 22 (34.9%) of 63 healthy children. Median synovitis and bone marrow edema scores in healthy children were 0.0 (95% CI 0.0–0.0) and 0.0 (95% CI 0.0–4.2), respectively.
Table 1.
Demographic, clinical, and laboratory features of 90 patients with JIA*
| Variable | Value |
|---|---|
| Age, years | 13.8 (10.8, 16.5) |
| Age at JIA onset, years | 3.7 (2.1, 8.2) |
| Female sex, no (%) | 75 (83.3) |
| Male sex, no. (%) | 15 (16.7) |
| Disease duration, years | 8.5 (5.3, 12.6) |
| ILAR category, no. (%) | |
| Persisted oligoarthritis | 22 (24.4) |
| Extended oligoarthritis | 33 (36.7) |
| Polyarthritis RF− | 22 (24.4) |
| Polyarthritis RF+ | 3 (3.3) |
| Systemic | 10 (11.1) |
| Patients in clinical remission off medications, no. (%) | 15 (16.7) |
| Patients in clinical remission on medications, no. (%) | 75 (83.3) |
| MTX† | 21 (28) |
| Biologic agents (anti‐TNF or anti–IL‐6)† | 40 (53.3) |
| MTX + a biologic agent† | 14 (18.7) |
| Duration of clinical remission, months | 10.7 (7.3, 16.6) |
| CHAQ, median (minimum – maximum) score | 0 (0, 0.25) |
| No. of joints with active disease | 0 (0, 0) |
| VAS, 0–10 scale | 0 (0, 0) |
| Parent/patient's assessment of overall health, 0–10 scale | 0 (0, 0) |
| CRP, mg/dl‡ | 0 (0, 0) |
| ESR, mm/hour§ | 9 (6, 11) |
Values are the median (interquartile range [IQR]) unless indicated otherwise. Anti‐TNF = anti–tumor necrosis factor; CHAQ = Childhood Health Assessment Questionnaire; CRP = C‐reactive protein; ESR = erythrocyte sedimentation rate; ILAR = International League of Associations for Rheumatology; JIA = juvenile idiopathic arthritis; MTX = methotrexate; RF+ = positive rheumatoid factor; RF– = negative rheumatoid factor; VAS = visual analog scale.
Data were available for 75 patients.
Normal range <0.46 md/dl.
Normal range <20 mm/hour.
Figure 1.
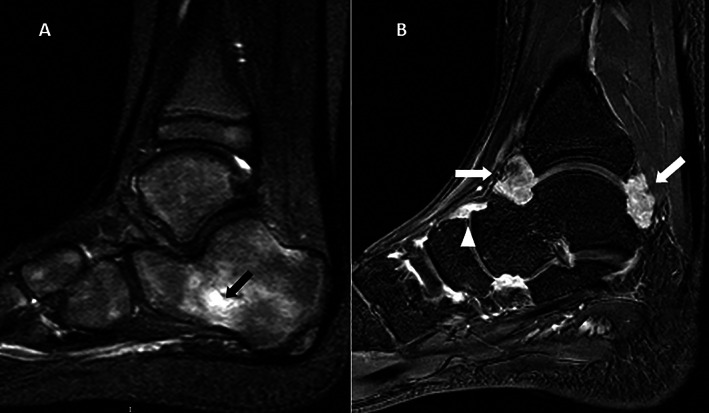
Contrast‐enhanced magnetic resonance imaging of the ankle obtained from 2 patients with juvenile idiopathic arthritis in clinical remission. A, Sagittal T2‐weighted sequence showing bone marrow edema within the calcaneus (arrow). B, Sagittal T1‐weighted gadolinium contrast‐enhanced sequence showing synovial enhancement in the tibiotalar (arrows) and talo‐navicular (arrowhead) joints.
Patients were followed clinically for a median time of 47.8 months. Fifty‐seven (63.3%) of 90 patients experienced a disease flare during follow‐up. The median time between MRI and disease flare was 1.49 years, with first and third quartiles of 0.88 and 2.08 years, respectively. Thirty‐six (63.2%) of 57 patients experienced relapse in the same joint assessed by the MRI. As anticipated, 15 (16.7%) of 90 patients were in clinical remission off medication and remained treatment‐free until disease relapse. Among the 75 patients who were in clinical remission on medication, 33 (44%) of 75 patients had discontinued treatment, 27 (36%) of 75 had reduced the dosage or frequency of drug administration, and 15 (20%) of 75 had continued therapy without changes during the follow‐up. Twenty‐four (72.7%) of 33 patients that had discontinued treatment experienced a disease flare versus 16 (59.3 %) of 27 patients and 8 (53.3 %) of 15 patients who had reduced or continued unchanged the treatment, respectively (P = 0.35).
No associations were found between disease flare and patient age (P = 0.23), JIA subtype (P = 0.45), disease duration (P = 0.16), clinical remission on or off medication (P = 0.77), type of treatment at study entry (P = 0.86), duration of clinical remission (P = 0.054), and presence of bone marrow edema (P = 0.77) and tenosynovitis (P = 0.24) on MRI. Forty‐four (74.6%) of 59 patients with synovitis as detected by MRI experienced a clinical flare versus 13 (41.9%) of 31 patients with no residual synovitis on MRI (P = 0.002). The presence of persistent synovitis on MRI was the best predictor of disease flare on multivariable analysis (HR 2.45, P = 0.003) (Table 2). As shown in Figure 2, the patient group with negative findings on MRI showed significantly lower cumulative flare rates curves than the group with subclinical synovitis (P = 0.008 by log‐rank test); we also drew separate Aalen‐Nelson curves for each type of joint (wrist, hip, and ankle) and found that this result was mostly evident for the hip joint.
Table 2.
Best‐fitted Cox regression model analysis results for clinical flare outcome*
| Disease | HR (95% CI) | P † |
|---|---|---|
| JIA subtype‡ | 0.34 | |
| Extended oligoarticular | 1.45 (0.71, 2.99) | – |
| Polyarticular RF–/RF+ | 1.95 (0.94, 4.06) | – |
| Systemic | 1.26 (0.47, 3.36) | – |
| Subclinical synovitis: yes§ | 2.45 (1.31, 4.59) | 0.003 |
95% CI = 95% confidence interval; HR = hazard ratio; JIA = juvenile idiopathic arthritis; RF–/RF+ = negative/positive rheumatoid factor.
By likelihood ratio test.
Reference category: persistent oligoarticular JIA.
Reference category: no.
Figure 2.
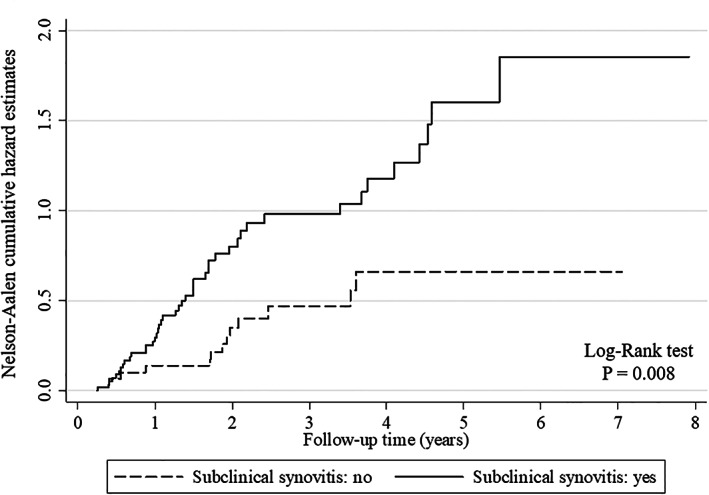
Nelson‐Aalen curves showing cumulative flare rates by magnetic resonance imaging results. The presence of subclinical synovitis was significantly related to higher rates of flares than the absence of subclinical synovitis.
Baseline and follow‐up radiographs were available for 54 patients. Seventeen (31.5%) of 54 patients experienced joint damage progression. The risk of joint deterioration was higher in patients with systemic onset JIA (P = 0.001) but was not related to disease duration (P = 0.10), duration of clinical remission (P = 0.14), or ongoing therapy (MTX only versus biologic agents only versus MTX + biologic agents versus no treatment) (P = 0.85). Moreover, there were no differences in the probability of developing joint damage deterioration in relation to changes in therapy during follow‐up, i.e., treatment stop versus treatment reduction versus unchanged treatment (P = 0.36). Subclinical synovitis was not associated with structural damage progression (P = 0.52); on the contrary, a bone marrow edema score of >4 (P = 0.0007) and the presence of radiographic joint damage (P = 0.007) at study entry were significantly related to structural deterioration. An MRI bone marrow score of >4 (HR 4.40, P = 0.043) and patient age of >17 years (HR 3.51, P = 0.04) were the best predictors of joint damage progression in the multivariable analysis (Table 3). As shown in Figure 3, patients with a bone marrow edema standardized score of >4 had significantly higher rates of bone damage progression than patients with a bone marrow edema standardized score of ≤4 (P = 0.005 by log‐rank test).
Table 3.
Best‐fitted Cox regression model analysis results of bone damage progression outcome*
| HR (95% CI) | P | |
|---|---|---|
| Age >17 years | 3.51 (1.09, 11.25) | 0.04 |
| Bone marrow edema score of >4 | 4.4 (0.87, 22.18) | 0.045 |
95% CI = 95% confidence interval; HR = hazard ratio.
Figure 3.
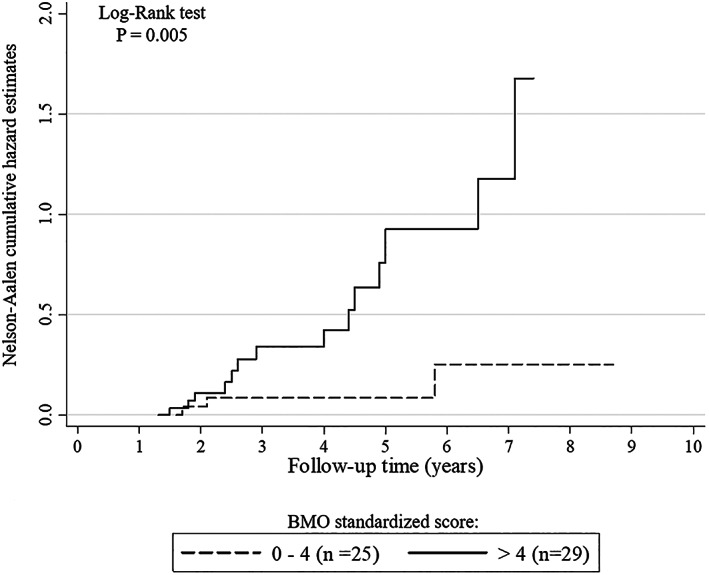
Nelson‐Aalen curves showing cumulative rates of bone damage by magnetic resonance imaging. A bone marrow edema (BMO) standardized score of >4 was significantly related to higher rates of bone damage progression than a bone marrow edema standardized score of ≤4.
DISCUSSION
In this study, we sought to explore the prognostic value of MRI in the assessment of remission status in a cohort of patients with clinically inactive JIA. Our results show that MRI plays an important role in predicting the risk of disease flare and joint deterioration, with potential implications for patients' management of disease. Complete disease quiescence is regarded as the ideal therapeutic target in JIA because its achievement helps to prevent physical disability (3). Recently, evidence has been provided that disease remission, classified according to clinical criteria, might not accurately reflect a true inflammation‐free status (11, 12, 15, 35). By visualizing inflammation at the primary site of pathologic changes, MRI and MSUS are intuitively more accurate in the evaluation of persistent synovitis than clinical and laboratory indices, which are surrogate markers of inflammation. In our study, MRI revealed persistent synovial inflammation in a substantial proportion of patients despite the fact that they were in clinically defined remission. This finding is not surprising and is consistent with MSUS studies in JIA, which showed an incidence of subclinical synovitis varying from 25–76.9% (13, 14, 35, 36, 37). Extensive data from the literature have indicated the prognostic value of subclinical inflammation in predicting disease flare and joint damage progression in RA patients with clinical inactive disease (8, 9, 10). These observations have opened a debate on the opportunity to include imaging in the criteria for defining remission (38). Unlike RA, very few studies have explored the long‐term significance of imaging findings in JIA patients who are in clinical remission, with conflicting results (13, 14, 15, 36, 37). Magni‐Manzoni and colleagues found that the presence of ultrasound‐detected synovial abnormalities did not predict disease flare in 39 patients with clinically inactive JIA (13). The results of this study have recently been challenged by De Lucia et al who demonstrated that MSUS abnormalities increased the risk of disease flare by almost 4 times in 88 JIA patients with clinically inactive disease (14); in line with findings from that study, the results from the present work show that subclinical synovitis on MRI plays a significant role in predicting disease flare in JIA patients in clinical remission, especially in those with previous incidence of hip arthritis. This finding is not surprising since hip involvement is a well‐established indicator of poor prognosis in JIA. Notably, the deep anatomical location of the hip joint makes it difficult to ascertain reliably the presence of inflammation from clinical examination, as inflamed synovium cannot be directly palpated; moreover, restricted movements with joint pain may occur in patients with joint damage, leading clinicians to underestimate active disease. Previous studies found that clinical findings are inadequate for the assessment of hip arthritis when compared to MRI (21). Our findings further emphasize the pivotal role of this imaging modality as an adjunct to clinical examination of the hip to guide treatment choices.
In contrast to RA studies (39), discontinuation of therapy was not associated with disease flare in the present work, although patients who stopped treatment revealed a greater tendency to experience a disease flare than those who had reduced the dosage or kept the therapy unchanged. Longitudinal studies aiming to evaluate the effect of treatment on subclinical synovitis are needed to define the role of imaging in guiding treatment discontinuation in JIA.
Thus far, no study has investigated the risk of joint deterioration in JIA patients in clinical remission. This issue is crucial because the definition of remission should ideally include the absence of symptoms or signs of inflammation, joint damage progression, and a stable functional status. Notably, in the present study, progression of joint damage occurred in around one‐third of JIA patients despite clinical remission. These results are consistent with previous studies in RA, in which 10–30% of patients in clinical remission continue to develop “silent” structural damage progression (9, 10, 40, 41). Subclinical synovitis was considered a plausible explanation for the apparent dissociation between clinical remission and continued structural deterioration in RA patients (10, 42). Unexpectedly, in the study cohort, subclinical synovial inflammation had no prognostic value in terms of progression of structural damage. Multivariable analysis showed that bone marrow edema was an independent predictor of radiographic damage. This result is not surprising since bone marrow edema is considered the strongest predictor of joint deterioration and disability in RA patients (43, 44, 45). Histologic studies in RA patients have shown that bone marrow edema reflects the presence of an inflammatory infiltrate that triggers an osteoclastic response, thus paving the way for the development of bone erosions (46).
A deep knowledge of the evolving patterns of skeletal maturity is of paramount importance when interpreting bone marrow edema in children. In fact, unlike adults, bone marrow changes have been described in the carpal (47, 48) and tarsal bones (49) of a relevant percentage of healthy children. Consistent with this, in the present study, bone marrow changes were found in ~35% of healthy controls, which entailed a potential risk of diluting its prognostic relevance. Although discrimination from physiologic to pathologic bone marrow changes in children is still challenging, we have identified a threshold for MRI bone marrow edema that discriminated between patients in remission state with or without the risk of radiographic progression. This study is the first to demonstrate the poor prognostic value of bone marrow edema in JIA and to suggest that MRI evaluation at the time of clinical remission could be an important tool to identify patients who are eligible for tapering and discontinuation of therapies because they have a low risk of experiencing structural deterioration.
The results of the present study should be interpreted in the context of certain limitations. First, we cannot exclude a referral bias since only patients who were candidates for MRI were enrolled. It is plausible that MRI was required by the treating physician for patients who had experienced a more severe disease course, which may explain the higher rate of disease relapses observed in the present study compared to literature data (14, 50). In addition, the majority of the joints examined by MRI were wrists or hips, and the involvement of these joints is considered a poor prognostic factor. Another point to consider is that since one of the major limitations of MRI is the ability to investigate only 1 or very few joints per session, we were not able to exclude the presence of subclinical synovitis in other joints. MSUS assessment, which allows a real‐time multiple joint assessment, could have led to a more comprehensive definition of remission status. Similarly, the integration of MRI findings with molecular biomarkers of inflammation, such as myeloid‐related protein 8 (MRP‐8) and MRP‐14, could have provided valuable information on biologic remission status. Lack of MRI follow‐up data is another limitation of the study. Evaluation of the evolution of MRI findings throughout the disease course is essential for therapeutic decision. Finally, a larger control group, matching the exact same joints evaluated in the JIA cohort, could have provided normative data useful for identifying potential joint specific cut‐off levels for imaging remission goals in JIA patients.
In summary, this study shows that MRI improves the evaluation of disease activity beyond clinical examination. MRI‐detected “subclinical inflammation” has been shown to be present in a substantial proportion of patients in clinical remission and may develop into disease flare and joint deterioration. The detection of residual inflammation on MRI may help to identify patients with sustained clinical inactive disease that could more safely undergo treatment reduction or discontinuation. Longitudinal prospective studies are needed to confirm the results of the present study as its retrospective design is not suited to explore the role of other possible confounders.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be submitted for publication. Dr. Malattia had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design
Mazzoni, Pistorio, Magnaguagno, Magnano, Ravelli, Malattia.
Acquisition of data
Mazzoni, Magnaguagno, Viola, Urru, Magnano, Ravelli, Malattia.
Analysis and interpretation of data
Mazzoni, Pistorio, Ravelli, Malattia.
Supporting information
Disclosure Form
Author disclosures are available at https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Facr.24757&file=acr24757‐sup‐0001‐Disclosureform.pdf.
REFERENCES
- 1. Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet 2007;369:767–78. [DOI] [PubMed] [Google Scholar]
- 2. Magnani A, Pistorio A, Magni‐Manzoni S, et al. Achievement of a state of inactive disease at least once in the first 5 years predicts better outcome of patients with polyarticular juvenile idiopathic arthritis. J Rheumatol 2009;36:628–34. [DOI] [PubMed] [Google Scholar]
- 3. Hinze C, Gohar F, Foell D. Management of juvenile idiopathic arthritis: hitting the target. Nat Rev Rheumatol 2015;11:290–300. [DOI] [PubMed] [Google Scholar]
- 4. Woerner A, Uettwiller F, Melki I, et al. Biological treatment in systemic juvenile idiopathic arthritis: achievement of inactive disease or clinical remission on a first, second or third biological agent. RMD Open 2015;1:e000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ravelli A, Consolaro A, Horneff G, et al. Treating juvenile idiopathic arthritis to target: recommendations of an international task force. Ann Rheum Dis 2018;77:819–28. [DOI] [PubMed] [Google Scholar]
- 6. Wallace CA, Giannini EH, Huang B, et al. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res (Hoboken) 2011;63:929–36. [DOI] [PubMed] [Google Scholar]
- 7. Wallace CA, Ruperto N, Giannini E, et al. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol 2004;31:2290–4. [PubMed] [Google Scholar]
- 8. Brown AK, Quinn MA, Karim Z, et al. Presence of significant synovitis in rheumatoid arthritis patients with disease‐modifying antirheumatic drug–induced clinical remission: evidence from an imaging study may explain structural progression. Arthritis Rheum 2006;54: 3761–73. [DOI] [PubMed] [Google Scholar]
- 9. Brown AK, Conaghan PG, Karim Z, et al. An explanation for the apparent dissociation between clinical remission and continued structural deterioration in rheumatoid arthritis. Arthritis Rheum 2008;58:2958–67. [DOI] [PubMed] [Google Scholar]
- 10. Molenaar ET, Voskuyl AE, Dinant HJ, et al. Progression of radiologic damage in patients with rheumatoid arthritis in clinical remission. Arthritis Rheum 2004;50:36–42. [DOI] [PubMed] [Google Scholar]
- 11. Van Gulik EC, Welsink‐Karssies MM, van den Berg JM, et al. Juvenile idiopathic arthritis: magnetic resonance imaging of the clinically unaffected knee. Pediatr Radiol 2018;48:333–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown A, Hirsch R, Laor T, et al. Do patients with juvenile Idiopathic arthritis in clinical remission have evidence of persistent inflammation on 3T magnetic resonance imaging? Arthritis Care Res (Hoboken) 2012;64:1846–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Magni‐Manzoni S, Scirè CA, Ravelli A, et al. Ultrasound‐detected synovial abnormalities are frequent in clinically inactive juvenile idiopathic arthritis, but do not predict a flare of synovitis. Ann Rheum Dis 2013;72:223–8. [DOI] [PubMed] [Google Scholar]
- 14. De Lucia O, Ravagnani V, Pregnolato F, et al. Baseline ultrasound examination as possible predictor of relapse in patients affected by juvenile idiopathic arthritis (JIA). Ann Rheum Dis 2018;77:1426–31. [DOI] [PubMed] [Google Scholar]
- 15. Nusman CM, Hemke R, Lavini C, et al. Dynamic contrast‐enhanced magnetic resonance imaging can play a role in predicting flare in juvenile idiopathic arthritis. Eur J Radiol 2017;88:77–81. [DOI] [PubMed] [Google Scholar]
- 16. Østergaard M, Peterfy C, Conaghan P, et al. OMERACT rheumatoid arthritis magnetic resonance imaging studies. Core set of RM acquisitions, joint pathology definitions, and the OMERACT RA‐RM scoring system. J Rheumatol 2003; 30:1385–6. [PubMed] [Google Scholar]
- 17. Malattia C, Damasio MB, Pistorio A, et al. Development and preliminary validation of a paediatric‐targeted MRI scoring system for the assessment of disease activity and damage in juvenile idiopathic arthritis. Ann Rheum Dis 2011;70:440–6. [DOI] [PubMed] [Google Scholar]
- 18. Van Dijkhuizen EH, Vanoni F, Magnano GM, et al. Effect of the inclusion of the metacarpophalangeal joints on the wrist magnetic resonance imaging scoring system in juvenile idiopathic arthritis. J Rheumatol 2018;45:1581–7. [DOI] [PubMed] [Google Scholar]
- 19. Baan H, Bezooijen R, Avenarius JK, et al. Magnetic resonance imaging of the rheumatic foot according to the RAMRIS system is reliable. J Rheumatol 2011;38:1003–8. [DOI] [PubMed] [Google Scholar]
- 20. Șerban O, Fodor D, Papp I, et al. Reasons for discordances between ultrasonography and magnetic resonance imaging in the evaluation of the ankle, hindfoot and heel of the patients with rheumatoid arthritis. Med Ultrason 2019;21:405–13. [DOI] [PubMed] [Google Scholar]
- 21. Argyropoulou MI, Fanis SL, Xenakis T, et al. The role of MRI in the evaluation of hip joint disease in clinical subtypes of juvenile idiopathic arthritis. Br J Radiol 2002;75:229–33. [DOI] [PubMed] [Google Scholar]
- 22. Hemke R, van Rossum MA, van Veenendaal M, et al. Reliability and responsiveness of the Juvenile Arthritis MRI Scoring (JAMRIS) system for the knee. Eur Radiol 2013;23:1075–83. [DOI] [PubMed] [Google Scholar]
- 23. Haavardsholm EA, Ostergaard M, Ejbjerg BJ, et al. Introduction of a novel magnetic resonance imaging tenosynovitis score for rheumatoid arthritis: reliability in a multireader longitudinal study. Ann Rheum Dis 2007;66:1216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lambot K, Boavida P, Damasio MB, et al. MRI assessment of tenosynovitis in children with juvenile idiopathic arthritis: inter‐ and intra‐observer variability. Pediatr Radiol 2013;43:796–802. [DOI] [PubMed] [Google Scholar]
- 25. Tanturri de Horatio L, Damasio MB, Barbuti D, et al. MRI assessment of bone marrow in children with juvenile idiopathic arthritis: intra‐ and inter‐observer variability. Pediatr Radiol 2012;42:714–20. [DOI] [PubMed] [Google Scholar]
- 26. Dakkak YJ, Matthijssen XM, van der Heijde D, et al. Reliability of magnetic resonance imaging (MRI) scoring of the metatarsophalangeal joints of the foot according to the rheumatoid arthritis MRI score. J Rheumatol 2020;47:1165–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kirkhus E, Flatø B, Riise O, et al. Differences in MRI findings between subgroups of recent‐onset childhood arthritis. Pediatr Radiol 2011;41:432–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. El‐Azeem MI, Taha HA, El‐Sherif AM. Role of MRI in evaluation of hip joint involvement in juvenile idiopathic arthritis. Egypt Rheumatol 2012;34:75–82. [Google Scholar]
- 29. Shelmerdine SC, Di Paolo PL, Tanturri de Horatio L, et al. Imaging of the hip in juvenile idiopathic arthritis. Pediatr Radiol 2018;48:811–7. [DOI] [PubMed] [Google Scholar]
- 30. Ravelli A, Ioseliani M, Norambuena X, et al. Adapted versions of the Sharp/van der Heijde score are reliable and valid for assessment of radiographic progression in juvenile idiopathic arthritis. Arthritis Rheum 2007;56:3087–95. [DOI] [PubMed] [Google Scholar]
- 31. Bertamino M, Rossi F, Pistorio A, et al. Development and initial validation of a radiographic scoring system for the hip in juvenile idiopathic arthritis. J Rheumatol 2010;37:432–9. [DOI] [PubMed] [Google Scholar]
- 32. Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960;20:37–46. [Google Scholar]
- 33. Landis JR, Koch GC. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–74. [PubMed] [Google Scholar]
- 34. Metz CE. Receiver operating characteristic analysis: a tool for the quantitative evaluation of observer performance and imaging systems. J Am Coll Radiol 2006;3:413–22. [DOI] [PubMed] [Google Scholar]
- 35. Rebollo‐Polo M, Koujok K, Weisser C, et al. Ultrasound findings on patients with juvenile idiopathic arthritis in clinical remission. Arthritis Care Res (Hoboken) 2011;63:1013–9. [DOI] [PubMed] [Google Scholar]
- 36. Zhao Y, Rascoff NE, Iyer RS, et al. Flares of disease in children with clinically inactive juvenile idiopathic arthritis were not correlated with ultrasound findings. J Rheumatol 2018;45:851–7. [DOI] [PubMed] [Google Scholar]
- 37. Miotto E, Silva VB, Mitraud SA, et al. Patients with juvenile idiopathic arthritis in clinical remission with positive power Doppler signal in joint ultrasonography have an increased rate of clinical flare: a prospective study. Pediatr Rheumatol Online J 2017;15:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saleem B, Brown AK, Keen H, et al. Should imaging be a component of rheumatoid arthritis remission criteria? A comparison between traditional and modified composite remission scores and imaging assessments. Ann Rheum Dis 2011;70:792–8. [DOI] [PubMed] [Google Scholar]
- 39. Haschka J, Englbrecht M, Hueber AJ, et al. Relapse rates in patients with rheumatoid arthritis in stable remission tapering or stopping antirheumatic therapy: interim results from the prospective randomised controlled RETRO study. Ann Rheum Dis 2016;75:45–51. [DOI] [PubMed] [Google Scholar]
- 40. Rezaei H, Saevarsdottir S, Forslind K, et al. In early rheumatoid arthritis, patients with a good initial response to methotrexate have excellent 2‐year clinical outcomes, but radiological progression is not fully prevented: data from the methotrexate responders population in the SWEFOT trial. Ann Rheum Dis 2012;71:186–91. [DOI] [PubMed] [Google Scholar]
- 41. Aletaha D, Smolen JS. Joint damage in rheumatoid arthritis progresses in remission according to the Disease Activity Score in 28 joints and is driven by residual swollen joints. Arthritis Rheum 2011;63:3702–11. [DOI] [PubMed] [Google Scholar]
- 42. Lillegraven S, Prince FH, Shadick NA, et al. Remission and radiographic outcome in rheumatoid arthritis: application of the 2011 ACR/EULAR remission criteria in an observational cohort. Ann Rheum Dis 2012;71:681–6. [DOI] [PubMed] [Google Scholar]
- 43. Haavardsholm EA, Bøyesen P, Østergaard M, et al. Magnetic resonance imaging findings in 84 patients with early rheumatoid arthritis: bone marrow oedema predicts erosive progression. Ann Rheum Dis 2008;67:794–800. [DOI] [PubMed] [Google Scholar]
- 44. Hetland ML, Stengaard‐Pedersen K, Junker P, et al. Radiographic progression and remission rates in early rheumatoid arthritis: MRI bone oedema and anti‐CCP predicted radiographic progression in the 5‐year extension of the double‐blind randomized CIMESTRA trial. Ann Rheum Dis 2010;69:1789–95. [DOI] [PubMed] [Google Scholar]
- 45. Bøyesen P, Haavardsholm EA, Ostergaard M, et al. MRI in early rheumatoid arthritis: synovitis and bone marrow oedema are independent predictors of subsequent radiographic progression. Ann Rheum Dis 2011;70:428–33. [DOI] [PubMed] [Google Scholar]
- 46. Dalbeth N, Smith T, Gray S, et al. Cellular characterisation of magnetic resonance imaging bone oedema in rheumatoid arthritis; implications for pathogenesis of erosive disease. Ann Rheum Dis 2009;68:279–82. [DOI] [PubMed] [Google Scholar]
- 47. Avenarius DF, Ording Müller LS, Rosendahl K. Joint fluid, bone marrow edema‐like changes and ganglion cysts in the pediatric wrist: features that may mimic pathologic abnormalities‐follow‐up of a healthy cohort. AJR Am J Roentgenol 2017;23:1–6. [DOI] [PubMed] [Google Scholar]
- 48. Müller LS, Avenarius D, Damasio B, et al. The paediatric wrist revisited: redefining MR findings in healthy children. Ann Rheum Dis 2011;70:605–10. [DOI] [PubMed] [Google Scholar]
- 49. Shabshin N, Schweitzer ME, Morrison WB, et al. High‐signal T2 changes of the bone marrow of the foot and ankle in children: red marrow or traumatic changes? Pediatr Radiol 2006;36:670–6. [DOI] [PubMed] [Google Scholar]
- 50. Guzman J, Oen K, Huber AM, et al. The risk and nature of flares in juvenile idiopathic arthritis: results from the ReACCh‐Out cohort. Ann Rheum Dis 2016;75:1092–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure Form


