ABSTRACT
Understanding how species respond to climate change is key to informing vulnerability assessments and designing effective conservation strategies, yet research efforts on wildlife responses to climate change fail to deliver a representative overview due to inherent biases. Bats are a species‐rich, globally distributed group of organisms that are thought to be particularly sensitive to the effects of climate change because of their high surface‐to‐volume ratios and low reproductive rates. We systematically reviewed the literature on bat responses to climate change to provide an overview of the current state of knowledge, identify research gaps and biases and highlight future research needs. We found that studies are geographically biased towards Europe, North America and Australia, and temperate and Mediterranean biomes, thus missing a substantial proportion of bat diversity and thermal responses. Less than half of the published studies provide concrete evidence for bat responses to climate change. For over a third of studied bat species, response evidence is only based on predictive species distribution models. Consequently, the most frequently reported responses involve range shifts (57% of species) and changes in patterns of species diversity (26%). Bats showed a variety of responses, including both positive (e.g. range expansion and population increase) and negative responses (range contraction and population decrease), although responses to extreme events were always negative or neutral. Spatial responses varied in their outcome and across families, with almost all taxonomic groups featuring both range expansions and contractions, while demographic responses were strongly biased towards negative outcomes, particularly among Pteropodidae and Molossidae. The commonly used correlative modelling approaches can be applied to many species, but do not provide mechanistic insight into behavioural, physiological, phenological or genetic responses. There was a paucity of experimental studies (26%), and only a small proportion of the 396 bat species covered in the examined studies were studied using long‐term and/or experimental approaches (11%), even though they are more informative about the effects of climate change. We emphasise the need for more empirical studies to unravel the multifaceted nature of bats' responses to climate change and the need for standardised study designs that will enable synthesis and meta‐analysis of the literature. Finally, we stress the importance of overcoming geographic and taxonomic disparities through strengthening research capacity in the Global South to provide a more comprehensive view of terrestrial biodiversity responses to climate change.
Keywords: bats, climate change, conservation, life traits, physiology, species range
I. INTRODUCTION
Climate change is currently recognised as one of the main threats to global biodiversity (Schmittner & Galbraith, 2008; Wolkovich et al., 2014) with impacts expected to become more severe before the end of the century (Urban, 2015). Yet research efforts on wildlife responses to climate change fail to deliver a representative overview because studies are taxonomically and geographically biased (e.g. Pacifici et al., 2015; Scheffers et al., 2016; Winter et al., 2016; Feeley, Stroud & Perez, 2017), often focus on particular species (e.g. Lane et al., 2012; Tafani et al., 2013; Plard et al., 2014), or are primarily based on correlative modelling approaches (e.g. Warren et al., 2018). The threats that stem from climate change are diverse and multifaceted, resulting in complicated direct and indirect effects on wildlife. Anthropogenically driven climate change has already resulted in increased temperature, changes in precipitation patterns and increased intensity and frequency of extreme events, such as heatwaves, droughts, or wildfires (Allan et al., 2021); all are factors that strongly influence wildlife by increasing mortality, reducing reproductive success, or altering species–environment relationships and biotic interactions, among other effects (Montoya & Raffaelli, 2010; Moreno & Møller, 2011).
Bats (Chiroptera) are the second most species‐rich taxonomic order of mammals, accounting for one‐fifth of global mammalian diversity (>1430 species; Simmons & Cirranello, 2021). Bats are globally distributed and inhabit a wide diversity of habitats, spanning from tropical rainforests to arid regions, boreal forests, and oceanic islands (Altringham, 2011). As bats occur in a diverse range of environmental conditions, they represent an excellent study system for assessing the effects of climate change on vertebrates. Bats display a variety of dietary adaptations including insectivory, carnivory, nectarivory and haematophagy (Altringham, 2011), thus playing key roles in ecosystems and providing ecosystem services such as suppression of pest populations, seed dispersal, pollination, enhancement of soil fertility and nutrient distribution (Kunz et al., 2011; Ramírez‐Fráncel et al., 2021).
Globally, over 16% of bat species are classified as threatened by the IUCN Red List (2022). However, the conservation status of many bats is unknown, with 18% classified as data deficient and 57% with unknown population trends, a higher proportion than other mammals and birds (Frick, Kingston & Flanders, 2020). Bats are especially threatened by habitat loss (43% of species) and bushmeat hunting (33%; IUCN, 2022). New threats have emerged in recent years, including wind energy installations (Frick et al., 2017), emerging infectious diseases (O'Shea et al., 2016), climate change (Sherwin, Montgomery & Lundy, 2013) and increased frequency of heatwaves and fires, resulting in mass mortality events (Welbergen et al., 2008; Ward et al., 2020).
Bats are expected to be particularly sensitive to climate change because they are prone to dehydration due to high surface‐to‐volume ratios caused by their generally small body mass and their large wing and tail membranes (Korine et al., 2016). Moreover, their slow, K‐selected reproductive strategy (e.g. Famoso, Hopkins & Davis, 2018) makes them more susceptible to extinction under rapid environmental changes (Frick et al., 2020; O'Grady et al., 2004). Understanding how bats are affected by and cope with climate change is thus key to informing more accurate conservation assessments and management strategies from the global to the local level.
Here, we present a systematic review of bat responses to climate change to provide an overview of the current state of knowledge, identify research gaps and biases, and highlight future research needs. First, we identified studies focusing on the impacts of climate change on bats and classified them based on the methods used, studied taxa and evidence of response to climate change. Second, we summarised the evidence of bat responses to climate change from the individual to the assemblage levels and their response type, from behavioural to evolutionary, and quantified taxonomic and geographic biases. Finally, we tested for associations between the studied climate change events and the observed bat responses.
Overall, our review was guided by the following hypotheses and associated predictions.
Bats are long‐lived mammals that may show a time lag in their functional responses to climate change, and therefore may require long‐term (e.g. >20 years) studies to identify responses to climate change. Given the lack of long‐term monitoring programmes of bats across the world, we predicted that there will be limited concrete evidence in the literature for bat responses to climate change.
Given the expected sensitivity of bats to climate change due to their high surface‐to‐volume ratios we hypothesised that bats will predominately show negative responses to climate change, in the form of population declines and range contractions.
Given the well‐documented mass mortality events of bats, in particular pteropodids, under heat waves (Welbergen et al., 2008), we hypothesised that bat responses to extreme events will be more negative than their responses to temperature and rainfall changes. We predicted that the family Pteropodidae will show particularly negative responses in the form of population declines and range losses in response to extreme events.
II. METHODS
(1). Literature search
We followed the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) protocols (Page et al., 2021). We searched the Web of Science and Scopus databases using the following search string: ‘Chiroptera’ OR ‘bat*’ AND ‘climate change*’ OR ‘global warming’ OR ‘drought*’ OR ‘extreme event*’ OR ‘windthrow’ OR ‘fire*’ OR ‘mortality’ OR ‘extreme temperature*’. We also searched for relevant grey literature, using the same search terms and the advanced search features in Google, to identify additional papers that had been missed, however, no new documents were identified through our Google search. Searches were performed in English, Spanish, Portuguese and Polish. The final search was carried out in August 2020.
(2). Articles screening and classification
The review team consisted of 17 reviewers who held regular meetings to set the inclusion criteria, establish a common review protocol, and discuss any possible divergences in the relevance of some studies. To ensure consistency among reviewers, 46% of the documents were independently reviewed by at least two reviewers. If the reviewers disagreed about whether the paper should be included, they discussed the paper, and if opinions were still divergent, the paper was reviewed by a third reviewer. All reviewers were given clear protocols to follow with detailed descriptions of the inclusion criteria and practice papers to assess consistency among reviewers.
We first screened the title and abstract of the studies retrieved in our search and excluded documents that did not study the impacts of climate change on bats. Specifically, we interpreted as an impact of climate change any measured change, whether positive (e.g. range expansion, population increase), negative (e.g. range contraction, population decrease) or neutral (e.g. no change or unclear trend), in response to climatically driven pressures. We included impacts that were either explicitly observed and measured or predicted based on modelling approaches. We then read the full text to assess its relevance according to the following four criteria, at least one of which had to be fulfilled for the study to be included in the review: (C1) the paper focused on assessing bat responses to the effects of climate change, including predictive modelling studies (speculative studies without fact‐based evidence were excluded); (C2) the paper reported quantitative data on morphology, behaviour, colony size and location or hibernation in relation to climate change; (C3) the paper clearly mentioned before–after, control–impact experiments or a long‐term monitoring design to tease apart the effects of climate change from the other confounding factors; (C4) the paper reported the impacts of a single extreme event putatively linked to climate change.
Because our aim was to establish a baseline on the overall current state of knowledge on bat responses to climate change, our retained studies reported myriad exposures (increase in temperature, changes in precipitation, etc.) and/or multiple outcomes (spatial, demographic, physiological, etc.) based on different methodological approaches (empirical, model‐based, etc.). Hence, our studies were not homogenous and sample sizes for each response were too low to allow systematic extraction of quantitative data that could be summarised as standardised effect sizes for a meta‐analysis (Gurevitch et al., 2018). This would require a narrower, more focused question that could direct the literature search and data extraction, and prior identification of common metrics reported across several studies to address that question. We instead report our findings as a qualitative synthesis. From each retained paper, we extracted information on the bat species investigated, the geographic scope of the study, study type, evidence level, methods, temporal period, climate change events, bat responses and interaction with other drivers (see online Supporting Information, Table S1). Chi‐squared tests were used to test our predictions and identify associations between the studied climate change event, study type, recorded responses and the studied biome.
Where possible, we also assessed whether the observed responses were positive or negative. Changes in diversity or community structure, as well as individual behaviour, are difficult to evaluate in terms of their conservation implications. Thus, this classification was applied only to studies reporting demographic and spatial responses. We considered as positive those responses that potentially, but clearly, favour the long‐term persistence of populations or species (e.g. range expansions, increased population size, increased reproductive success, decreased mortality), and as negative those responses that can lead to local or global extinction (e.g. range contractions, decreased population size, decreased reproductive success, increased mortality). Mixed or ambiguous responses were classified as no change or unclear.
III. RESULTS
We retrieved 1081 papers, 926 of which were rejected based on their title and abstract or because they could not be accessed (two books and one unidentified reference). Of the remaining 155 papers, 77 were excluded after reading the full text because they did not satisfy at least one of our inclusion criteria. The final data set included 78 papers (Fig. 1; Table S2). See Table S3 for a list of excluded studies, with reasons for exclusion.
Fig. 1.
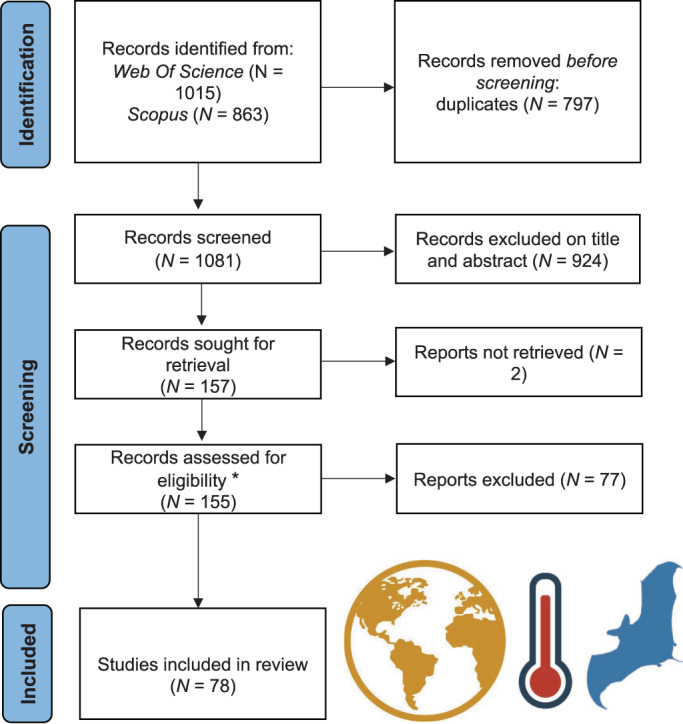
PRISMA flow diagram illustrating the systematic review process for studies investigating bats and climate change. *Inclusion criteria: (1) paper focuses on assessing bat responses to climate change; (2) paper reports quantitative data on bats in relation to climate change; (3) paper reports evidence from long‐term monitoring or field experiment; (4) paper reports on the impacts on bats due to single extreme events putatively linked to climate change.
The retained papers were published between 1932 and 2020, but the majority (>80%) were published during the last 10 years (Fig. 2B). Most studies relied on species distribution modelling (26%), field experiments (24%) and field observations (23%), with other types of approaches representing a minor portion of the published evidence (Fig. 2G). The type of evidence retrieved was mainly observational (72% of papers), followed by experimental approaches (26%). In terms of the level of evidence (Fig. 2H), 44% of papers reported a concrete response (i.e. recorded a phenotypic or behavioural response to climate change through either field or laboratory experiments or field observations), while 40% reported a response based only on model predictions [predictive species distribution models (SDMs) or demographic simulations]. Around 15% of studies were classified as speculative, i.e. they interpreted observed changes as responses to climate change without supporting evidence (Fig. 2H).
Fig. 2.

Summary of published evidence for the impacts of climate change on bats, according to (A) inclusion criterion (see Section II.2); (B) year of publication; (C) biome investigated, Temp., temperate; Trop., tropical; (D) climate change event studied; (E) observed specific response by bats; (F) general category of type of response (note that mortality here refers to mass mortality events, usually in response to extreme events); (G) type of study; (H) evidence level; and (I) level of response.
The reviewed studies reported evidence of the impact of climate change on 396 bat species (see full list in Table S4), 93% (370 species) of which were studied using SDM approaches, 52% using field observations (206 species), 11% using experimental approaches (43 species), and less than 1% (three species) using genetic methods. More than a third of the species studied (151 species, 38%) were only studied using SDM approaches, and only 38 species (<10%) were featured in more than three papers (Table S4). The most frequently studied species was the North American little brown bat (Myotis lucifugus; 10 studies), followed by four European species (Rhinolophus ferrumequinum, Hypsugo savii, Myotis daubentonii and Pipistrellus kuhlii) and by Myotis thysanodes (all featuring in eight studies each). Of the retrieved papers, 18% covered a time span of less than 1 year (Fig. S1). After removing these short‐term studies and two studies dealing with palaeoclimatic data, the median time span of studies was 22 years (range: 1–139), which dropped to 10 years (range: 1–132) after excluding modelling papers projecting climatic conditions under future scenarios (e.g. to 2070). Only nine field studies covered periods >20 years (Fig. S1). The most frequently reported responses by bats were at both the population (28%) and species (45%) levels and mostly consisted of range shifts (57%), changes in patterns of diversity (26%) and demographic changes (20%) (Fig. 2E, F). Only four papers provided evidence of no response (from 12 species), all dealing with a lack of demographic responses or community changes in relation to climate change. The climatic event reported most frequently as affecting bats was increased mean temperature (50%), followed by change in rainfall regime (23%) (Fig. 2D). The most studied continents were Europe (40%, 27 studies), North America (27%, 18 studies) and Oceania (19%, 13 studies), while the least studied were South America and Africa (two and three studies, respectively) and Asia (6%, four studies; Fig. 3). Correspondingly, the most studied biomes were temperate coniferous and broadleaf forest (47%) and Mediterranean (20%) (Fig. 2C).
Fig. 3.
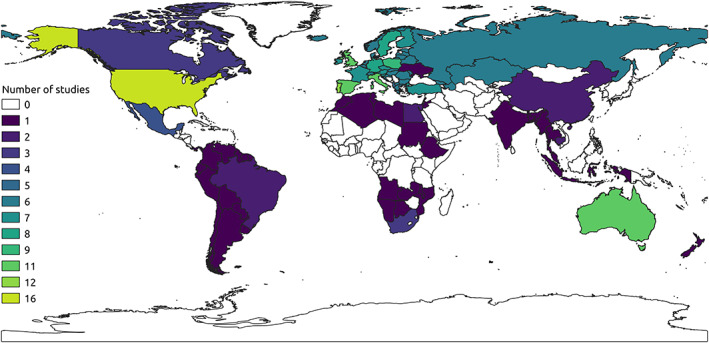
Global distribution of studies on bats and climate change according to country/region of study. Countries with no studies included in this review are depicted with no colour.
We found significant associations between the studied climate change events and the identified responses (χ 2 = 76.35, df = 42, P < 0.005). Studies of the effects of heatwaves primarily reported mass mortality events and physiological changes, but not range changes. By contrast, studies of the effects of temperature increase were associated with range changes but not mortality. We also found significant associations between study type and the climate change event examined (χ 2 = 52.65, df = 36, P < 0.05), whereby the effects of heatwaves and wildfires were primarily examined using experimental studies. Despite the lack of significant associations between biome and study type (P > 0.05), only field observations and SDM studies were carried out in the tropics, while experiments were mainly carried out in temperate environments (14 papers, 70% of experimental studies).
The direction of the reported consequences of climate change on bats varied with the type of response (Fig. 4). A prevalence of negative effects was consistent for reported demographic and spatial responses. Of the demographic responses to all climate change events, 61% were negative, including increased mortality and decreased reproductive success leading to population decline, the remaining 39% included both mixed, neutral and positive effects. Of 160 retrieved spatial responses, 30% reported actual or potentially positive effects (i.e. range expansion), 40% negative (i.e. range contraction), and the remaining 30% provided mixed or unclear evidence. In line with our hypothesis, all responses to extreme events were negative (range contraction or population decrease) or unclear/no change (Fig. 4). In addition, while spatial responses showed high variability in their direction and across bat families, demographic responses were mostly negative, particularly among Pteropodidae and Molossidae. Positive demographic responses (population increases) were identified for only some vespertilionid and rhinolophid bats, mostly associated with temperature increases. Twenty‐five papers highlighted that climate change may interact with other drivers potentially affecting bats, the most frequently reported being land‐use change and associated habitat fragmentation (40% of papers).
Fig. 4.
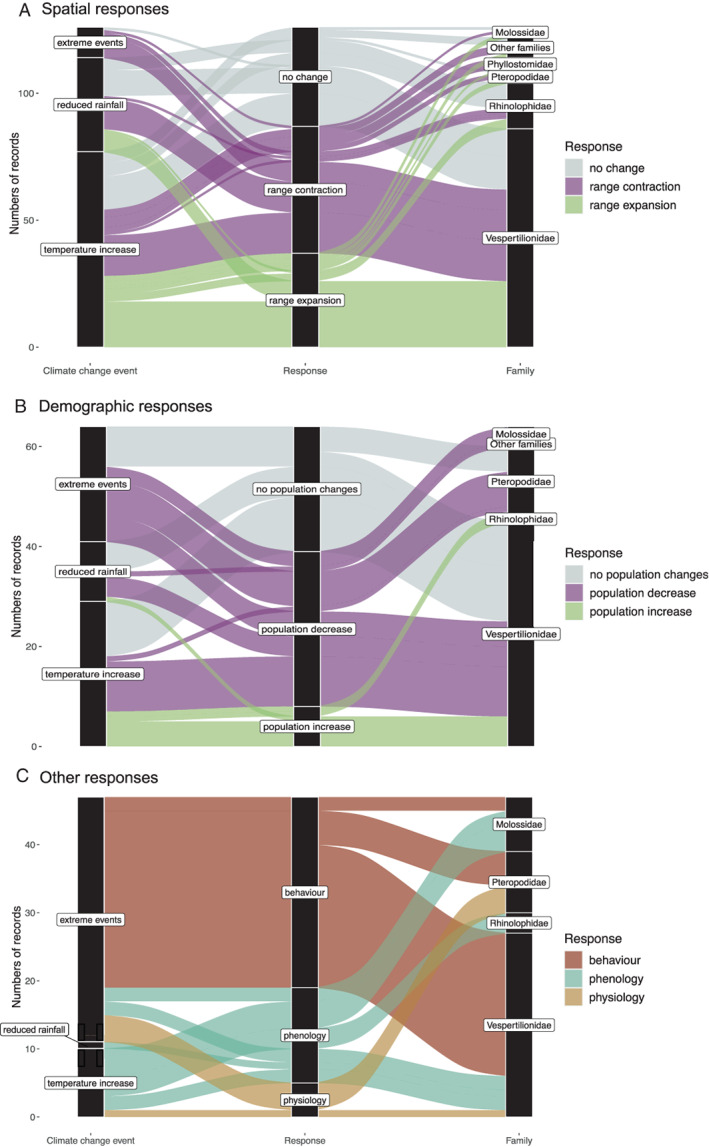
Effects of climate change on bats, weighted according to the number of records, for (A) spatial responses, (B) demographic responses, and (C) other responses (behaviour, physiology and phenology). Plots show bat family, reported climate change events, and bat response. In (A) and (B), responses are classified as either positive (range expansion or population increase, in green), negative (range contraction or population decrease, in pink) or neutral (no change, in grey). In (C), climate change events are related to three specific types of response (behaviour, physiology, phenology) due to the more variable nature of these responses and the low number of studies for each category. Note that only responses with N > 5 are included in this figure.
IV. DISCUSSION
Understanding how bats respond to climate change is key to informing vulnerability assessments and to designing effective conservation strategies, not only for bats but also for other terrestrial vertebrate groups experiencing similar pressures. Our analysis represents the first comprehensive systematic review of the evidence for how bats are responding to climate change, moving our understanding forward from previous work based on predictions and scattered evidence (Jones & Rebelo, 2013; Sherwin et al., 2013). Since those earlier reviews, the number of studies addressing this topic has greatly increased, but only a small proportion provides concrete evidence for bat responses to climate change. Only around 7% of papers and reports published in the last 90 years that mentioned bats and climate change added evidence on the topic, a rather limited body of literature when compared to other topics relating to bats (e.g. López‐Baucells, Rocha & Fernández‐Llamazares, 2018). Indeed, the overall research effort on bats and climate change is rather low in comparison to other vertebrate groups. For example, and notwithstanding a total number of species that is nearly an order of magnitude greater than the total number of bats, Møller, Fiedler & Berthold (2010) retrieved >2800 papers dealing with the effects of climate change on birds, compared with our final data set of 78 papers identified as actually addressing this topic for bats. As a consequence of this lower research effort, the available evidence only includes ca. 27% of bat species (396 species out of >1430), more than a third of which were only studied using modelling approaches.
Nearly half of the evidence for the effects of climate change on bats comes from modelling studies, primarily based on projections of species distributions under future climate change scenarios. While modelling studies can provide useful estimates for conservation assessments and planning, they inevitably rely on critical assumptions and can have a limited predictive capacity (Sofaer et al., 2018; Venne & Curie, 2021). Thus they do not offer concrete evidence for the effects of climate change, but rather predictions of what could happen provided that the equilibrium assumption holds, the data upon which they were based are representative and sufficient, and all relevant environmental variables are included (Santini et al., 2021). The high proportion of modelling and speculative studies (Fig. 2H) underlines the limited scientific evidence available on the effects of climate change on bats.
Ideally, bat responses to climate change (spatial, phenological, demographic, etc.) should be quantitatively synthesised using a meta‐analysis. However, we found that the empirical evidence to date is sparse and not sufficiently homogeneous to derive common effect sizes across studies suitable for a meta‐analysis. For example, a meta‐analysis addressing the effects of climate change on range and elevational shifts across taxa included 764 and 1367 individual species responses (measured as km/decade), respectively (Chen et al., 2011). Similarly, evidence for elevational shifts in birds (m/year) was synthesised from data for hundreds of species and dozens of studies worldwide (Scridel et al., 2018; Neate‐Clegg et al., 2021), whereas phenological shifts (reported as days of advancement/delay per decade) were analysed from a similarly large database (127 studies; Cohen, Lajeunesse & Rohr, 2018). A similar approach is desirable for bat species, yet our review reveals that much of the evidence for the effects of climate change on bats is based on modelling rather than long‐term observational studies. We call for increased efforts to design studies that are aimed at understanding bat responses to climate change and for the collection and reporting of results in a standardised manner. Publications should provide sufficient raw data to facilitate their use in future meta‐analyses (Radchuk et al., 2019).
We identified geographic biases in the study of bats and climate change. A bias towards the Global North (high‐income countries, covering primarily temperate, Mediterranean and boreal biomes) is common in biodiversity research (e.g. Blettler et al., 2018; Titley, Snaddon & Turner, 2017; Winter et al., 2016), likely related to socioeconomic disparities. A large portion of bat diversity is concentrated in the tropics (West Africa, Central and South America, Southeast Asia), with diversity hotspots in tropical forest biomes (Cooper‐Bohannon et al., 2016; Myers et al., 2000). Hence, the current evidence base for the effects of climate change on bats is unlikely to represent their full range of responses. Species at higher latitudes may experience different pressures from climate change compared to those in the tropics, for example in terms of the types and intensity of extreme events or changes to weather patterns (Wigley, 2009), and may require different adaptations to compensate accordingly (Pacifici et al., 2015).
(1). Bat responses to climate change
Like other animals, bats may respond to climate change either by moving to different areas, adapting to new conditions, or going extinct. We identified a wide range of responses by bats to different climate change events, including positive, negative and neutral responses. Our review identified a strong prevalence of spatial (range) changes (Fig. 2E, F), including both range shifts and expansions. This may reflect a genuine tendency of bats to move in response to environmental change due to their relatively high mobility, but this may not apply to all species and regions. Evidence for extensive range expansion comes from ecologically plastic species, such as Pipistrellus nathusii, P. kuhlii, H. savii and Tadarida brasiliensis, which have expanded their ranges rapidly towards higher latitudes during the past few decades (Ancillotto et al., 2016; Lundy, Montgomery & Russ, 2010; McCracken et al., 2018; Uhrin et al., 2016). By contrast, evidence of range losses was primarily derived from correlative model predictions rather than observational studies (Rebelo, Tarroso & Jones, 2010; Arumoogum, Schoeman & Ramdhani, 2019; Razgour et al., 2021).
The evolutionary adaptation of wildlife to climate change is hard to quantify because the data necessary to disentangle phenotypic plasticity and evolution are scarce or difficult to collect (Radchuk et al., 2019). Razgour et al. (2019) highlighted the role of climate‐adapted genotypes in species' responses to climate change. Bats can react rapidly to changing weather patterns by modifying their phenology, as has been documented by several studies. For example, during the last 22 years, T. brasiliensis has advanced summer migration and parturition timing by around 2 weeks and begun to overwinter in areas previously occupied exclusively during the summer months, presumably in response to climate change‐related temperature increases (Stepanian & Wainwright, 2018). Such changes are common in vertebrates [e.g. birds (Crick, 2004); amphibians and reptiles (Winter et al., 2016)], particularly in temperate biomes where seasonal environmental conditions are less predictable and species inhabiting these areas may have evolved the flexibility to change their life cycles (Reed et al., 2010). Phenological changes that seem adaptive to climate change in the short term may prove to be ecological traps if mismatches occur between the phenology of the bats and the key resources they rely on (e.g. water, arthropods or fruiting plants), with unpredictable effects on species persistence in a given area (Renner & Zohner, 2018; Stenseth & Mysterud, 2002).
Extinction is the most dramatic response of wildlife to climate change. Several modelling studies predict substantial range contractions, and a consequent increase in extinction risk, for bat species such as the African fruit bats Epomophorus wahlbergii and E. angolensis (Arumoogum et al., 2019) due to the disappearance of suitable habitat soon. Increased and unusual mortality rates have been reported for at least eight species [e.g. Myotis nattereri (Reusch et al., 2019) and Pteropus giganteus (Dey, Roy & Chattopadhyay, 2015)] in response to extreme events directly associated with climate change, including heatwaves and wildfires. Extreme events can result in the loss of individuals due to direct mortality (e.g. heat stress, roost burning) or resource depletion (e.g. water availability). Such extreme events are not a new stressor for wildlife, but with climate change their frequency and intensity are predicted to increase (Ummenhofer & Meehl, 2017), placing wildlife under stronger selection and with the potential to lead to extinctions of endemic species with narrow or insular ranges (Leclerc, Courchamp & Bellard, 2018). There is increasing evidence that extreme events related to climate change have played a key role in the local extinction of several species, including bumblebees (Rasmont & Iserbyt, 2012) and corals (Sheppard, Sheppard & Fenner, 2020), and even to total extinction, as in the case of the Bramble Cay mosaic‐tailed rat, Melomys rubicola (Waller et al., 2017). Although the extinction of bat species or extirpation of bat populations due to climate change has yet to be documented, a recent study predicted dramatic population crashes and high extinction risk during the next few decades for the Mediterranean island‐endemic Sardinian long‐eared bat, Plecotus sardus (Ancillotto et al., 2021).
(2). Gaps in the literature and future research
Obtaining more physiological and molecular data on species responses to climate change will be essential for improving the ability of models to predict future changes in distributions and abundance (Bozinovic & Pörtner, 2015; Urban et al., 2016), but both fields are underrepresented in the bat literature. Only three studies included in this review addressed genetic responses to climate change. This reflects the general paucity of studies applying genomic and genetic approaches in climate change research (Pauls et al., 2013). Threats to hotspots of bat genetic diversity under climate change could limit the evolutionary potential of these species and their ability to respond to environmental changes (Razgour et al., 2013). This highlights the importance of integrating genetic/genomic tools to assess species' long‐term viability under climate change. Genomic tools not only provide information on losses of genetic diversity and changes in genetic composition but also on changes in effective population size and genomic vulnerability resulting from maladaptation to changing conditions (Hohenlohe, Funk & Rajora, 2021). Adaptive genetic variation associated with current local climatic conditions can be used to infer the sensitivity of populations to future climatic conditions, and therefore to guide conservation efforts (Razgour et al., 2018). In addition, genomic tools can provide evidence of adaptive responses to climate change (Hoffmann & Sgrò, 2011), which is currently absent from the bat climate change literature. Initiatives to generate genomic databases for bats across the globe such as the Bat1K project (Teeling et al., 2018) will facilitate future research into the genomic responses of bats to climate change.
Similarly, the physiological responses of bats to climate change have been neglected. The available information suggests that bats respond to increasing environmental temperatures by reducing their torpor bout duration (Stawski & Geiser, 2012) and increasing their metabolic rate (Humphries, Thomas & Speakman, 2002); heatwaves leading to heat stress often result in mass mortality (Pruvot et al., 2019). Species are likely to respond differently to climate change based on their mobility and thermal tolerance, and therefore more research is needed on a wider range of bat species. The physiological impacts of climate change have been studied mostly through examining the effects of increased temperature and aridity, while other important factors, such as phenology, are often neglected (Feder, 2010); yet the latter is particularly relevant for bats that enter torpor/hibernation or migrate seasonally in response to changing food availability. Physiological traits can limit species distribution ranges because they influence the survival and reproduction of organisms under specific environmental conditions. Therefore, to predict responses to climate change accurately we need to have a full understanding of their physiological capacities (Chown et al., 2010). The general paucity of data on bat physiological traits, even for well‐studied species, is likely to impede our ability to improve model predictions and incorporate mechanistic modelling approaches.
Species‐specific life histories associated with environmental constraints, like hibernation, can influence the way climate influences population dynamics. However, the influence of hibernation on responses to climate change is poorly understood. We know little about the plasticity of hibernation behaviours and the capacity of hibernators to respond to climate change. Bats face extreme energetic challenges during the winter period, when the highest mortality rates are recorded (Lentini et al., 2015; Fritze & Puechmaille, 2018). Hibernators may be particularly susceptible to changes in seasonal climate as they have a relatively short activity season in which to reproduce and gain sufficient mass to survive the following winter. Hibernating bats periodically arouse from hibernation, but arousals are energetically expensive and can account for around 75% of winter energy expenditure (Kayser, 1953). More frequent extreme temperature changes during winter could cause more premature arousals and an increased risk of water loss (Thomas & Geiser, 1997), which can result in dehydration or depletion of critical energy reserves to the extent that the bat is unable to survive the winter. Limited food availability when bats arouse from hibernation can also cause significant mortalities (Jones et al., 2009). An understanding of natural activity patterns and whether and how seasonal climate variability can affect the fitness of hibernators will be essential to understanding bat responses to climate change.
Climate change is causing phenological mismatches between interacting species whose activity is triggered by different environmental stimuli. While this phenomenon has received substantial attention in migrating birds (e.g. Saino et al., 2011; Visser, Holleman & Gienapp, 2006), fleshy‐fruited plants (González‐Varo et al., 2021) and insect pollinators (e.g. Forrest, 2016; Memmott et al., 2007), we found no studies investigating phenological mismatches in bats. Many tropical bats are nectarivorous and changes in flowering patterns (e.g. due to precipitation regime shifts) may affect their resource use throughout the year. Similarly, for temperate bat species that enter torpor or migrate to avoid thermal stress during the coldest season (Krauel & McCracken, 2013; Meyer, Senulis & Reinartz, 2016), changes in seasonal temperatures may create mismatches between bat emergence from torpor or return from migration and seasonal resource availability.
Changes in interspecific interactions under climate change may alter the ecosystem services provided by animals (e.g. Prather et al., 2013). Whether the ecosystem services delivered by bats (e.g. pest suppression, pollination, seed dispersal) will be affected by climate change is unknown, yet very likely. Models predict that climate change will lead to a more than 75% reduction in the overlap between the ranges of the bat Leptonycteris nivalis and the Agave plants they pollinate during their annual migration, which may drive the bat to extinction and adversely affect the sexual reproduction and genetic variation of the pollinated plant (Gómez‐Ruiz & Lacher, 2019). Similarly, insectivorous bat species are predicted to shift their ranges to higher latitudes to track suitable conditions, such as common pipistrelles in Europe (Smeraldo et al., 2021). Their pest suppression ecosystem service would cease in the abandoned regions, with potentially negative consequences for agriculture, forests and human health. Yet, all these effects are currently hypothetical, and our review reveals the need for empirical studies.
Indirect effects of climate change on bats from interspecific interactions other than resource availability also have been understudied. Climate change may impact other species that naturally interact with bats, like their natural predators, or foster novel interactions with species that did not previously interact with bats. Climate change may modify interspecific competition between bats and other organisms (including other bat species), or generate completely novel competitive scenarios, the outcomes of which are hard to predict (Salinas‐Ramos et al., 2020). Impacts on bats from expanding populations of introduced species (Welch & Leppanen, 2017) may also be exacerbated because many invasive species thrive in altered environments and may exploit conditions related to climate change (Gallardo & Aldridge, 2013).
A critical obstacle in assessing the response of bats to climate change is that reported evidence may represent a response to a short‐term process, such as weather fluctuations, rather than to long‐term climatic changes. Longitudinal studies reporting actual (field) evidence of responses by bats represent only a minor fraction of the total studies (12%), yet they are extremely valuable to provide clearer insights into the differential effects of short‐ and long‐term fluctuations in environmental conditions. For example, changes in species distributions or phenology, which are critical to understanding and monitoring the effectiveness of adaptation, can be detected only via long‐term surveys. However, most of the papers in our review that included time‐series information consisted of comparisons between the recent or current distribution of bats and predicted changes in climatic suitability in future decades; only a few studies analysed the results of longitudinal studies lasting 10–30 years to investigate how species have already been impacted by climate change. Expanding or establishing systematic monitoring of the abundance and distribution of species and populations is a key priority to validate assessments of species' vulnerability to climate change. Monitoring efforts should be targeted at areas where the effects of climate change are likely to be most prominent to expand empirical knowledge about climate change impacts on wildlife and provide a more solid basis for improving future projections.
There is mounting evidence that the intensity, frequency and duration of extreme weather events are increasing around the globe (Allan et al., 2021). Identifying the populations most at risk is important because such events can have a devastating impact on small populations, in particular for species with low reproductive and population growth rates, like bats. Populations in tropical, subtropical and arid regions are likely to be most at risk because tropical storms and droughts are predicted to become more frequent and severe (Sherwin et al., 2013). O'Shea et al. (2016) reported 114 mass bat mortality events from abiotic factors, including heatwaves, droughts, extremely cold weather, flooding and storms, but no data were available from Europe, Africa or South America. We still do not know how heatwaves impact the reproductive success of bats. Collecting data on the effects of extreme weather events will be crucial for mitigating impacts and implementing conservation actions in high‐risk areas. For example, creating artificial water sources to provide drinking water and foraging opportunities for bats in arid environments could support populations during drought events (Korine et al., 2016).
V. CONCLUSIONS
(1) Several biological and ecological traits of bats may make them sensitive to climate change, yet there is surprisingly little evidence on how these mammals respond to this anthropogenic environmental pressure. Our systematic review of published evidence for bat responses to climate change highlights important gaps in the literature and the way we study the impacts of climate change.
(2) The focus on correlative predictive modelling approaches (SDMs), whilst allowing the study of impacts on species at local‐to‐global scales, does not facilitate an in‐depth understanding of behavioural, physiological, phenological and genetic responses.
(3) Longitudinal studies are particularly challenging in long‐lived organisms like bats, especially given the short timeframe of most research grants. However, they are essential for tracking species' responses to climate change. Similarly, experimental approaches can provide concrete evidence for species responses but are challenging in long‐lived species and species of conservation concern.
(4) Genomic approaches could help bridge the gap by providing a snapshot of both evolutionary adaptations and phenotypic plasticity (e.g. through differential gene expression analysis or epigenetic approaches), although such studies should be performed in addition to investigations of phenotypic responses in the field.
(5) More attention should be given to the interactions between climate change and other drivers that may impact bats, such as land‐use change, biological invasions, or perturbations in interspecific and ecosystem‐wide interactions between bats and their environment.
(6) Geographic and taxonomic disparities must be overcome to enable a more comprehensive view of biodiversity responses to climate change through strengthening research capacity in the Global South, where a large proportion of biodiversity is found.
Supporting information
Fig. S1. Distribution of study duration (in years) extracted from studies (N = 46) about bats and climate change, after excluding modelling and palaeoclimatic works.
Table S1. List of the variables extracted from each paper analysed, with name and description.
Table S2. List of studies that met our inclusion criteria.
Table S3. List of studies excluded from analysis, with reasons for exclusion.
Table S4. List of bat species for which evidence regarding the impact of climate change was reported in the literature.
ACKNOWLEDGEMENTS
This article is based on work from COST Action CA18107 ‘Climate change and bats: from science to conservation – ClimBats’ (https://climbats.eu/), supported by COST (European Cooperation in Science and Technology). L. S. was supported by the MUR Rita Levi Montalcini program, O. R. by a Natural Environment Research Council Independent Research Fellowship (NE/M018660/20, R. R. by a fellowship from the Portuguese Foundation for Science and Technology (2020.01129.CEECIND/CP1601/CT0004), A. B. ‐L. by a Juan de la Cierva‐Incorporación grant (IJCI‐2017‐31419) from the Spanish Ministry of Science, Innovation and Universities and an EMERGIA grant (EMERGIA20_00252) from the Junta de Andalucía) and N. T. by the Bulgarian National Science Fund (CP‐06‐COST/15 from 16.12.2020) and а PhD Fellowship from Karoll Knowledge Foundation. We are indebted to Dr. John Welch, Dr. Brock Fenton and an anonymous reviewer for valuable comments. Open Access Funding provided by Universita degli Studi di Napoli Federico II within the CRUI‐CARE Agreement.
REFERENCES
References identified with an asterisk (*) were included in our systematic review.
- * Adams, R. A. (2010). Bat reproduction declines when conditions mimic climate change projections for western North America. Ecology 91(8), 2437–2445. [DOI] [PubMed] [Google Scholar]
- * Adams, R. A. & Hayes, M. A. (2008). Water availability and successful lactation by bats as related to climate change in arid regions of western North America. Journal of Animal Ecology 77(6), 1115–1121. [DOI] [PubMed] [Google Scholar]
- * Adams, R. A. & Hayes, M. A. (2018). Assemblage‐level analysis of sex‐ratios in Coloradan bats in relation to climate variables: a model for future expectations. Global Ecology and Conservation 14, e00379. [Google Scholar]
- * Aguado‐Bautista, Ó. & Escalante, T. (2015). Changes on patterns of endemism of the Mexican land mammals by global warming. Revista Mexicana de Biodiversidad 86(1), 99–110. [Google Scholar]
- Allan, R. P. , Hawkins, E. , Bellouin, N. & Collins, B. (2021). IPCC, 2021: Summary for Policymakers. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre‐Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Res; IPCC: Geneva, Switzerland, 2018.
- Altringham, J. D. (2011). Bats: From Evolution to Conservation. New York: Oxford University Press. [Google Scholar]
- * Amorim, F. , Carvalho, S. B. , Honrado, J. & Rebelo, H. (2014). Designing optimized multi‐species monitoring networks to detect range shifts driven by climate change: a case study with bats in the North of Portugal. PLoS One 9(1), e87291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Amorim, F. , Jorge, I. , Beja, P. & Rebelo, H. (2018). Following the water? Landscape‐scale temporal changes in bat spatial distribution in relation to Mediterranean summer drought. Ecology and Evolution 8(11), 5801–5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Amorim, F. , Mata, V. A. , Beja, P. & Rebelo, H. (2015). Effects of a drought episode on the reproductive success of European free‐tailed bats (Tadarida teniotis). Mammalian Biology 80(3), 228–236. [Google Scholar]
- * Ancillotto, L. , Budinski, I. , Nardone, V. , Di Salvo, I. , Della Corte, M. , Bosso, L. & Russo, D. (2018). What is driving range expansion in a common bat? Hints from thermoregulation and habitat selection. Behavioural Processes 157, 540–546. [DOI] [PubMed] [Google Scholar]
- Ancillotto, L. , Fichera, G. , Pidinchedda, E. , Veith, M. , Kiefer, A. , Mucedda, M. & Russo, D. (2021). Wildfires, heatwaves and human disturbance threaten insular endemic bats. Biodiversity and Conservation 30(14), 4401–4416. [Google Scholar]
- * Ancillotto, L. , Santini, L. , Ranc, N. , Maiorano, L. & Russo, D. (2016). Extraordinary range expansion in a common bat: the potential roles of climate change and urbanisation. The Science of Nature 103(3‐4), 15. [DOI] [PubMed] [Google Scholar]
- * Arumoogum, N. , Schoeman, M. C. & Ramdhani, S. (2019). The relative influence of abiotic and biotic factors on suitable habitat of Old World fruit bats under current and future climate scenarios. Mammalian Biology 98(1), 188–200. [Google Scholar]
- * Austin, L. V. , Silvis, A. , Ford, W. M. & Powers, K. E. (2020). Effects of historic wildfire and prescribed fire on site occupancy of bats in Shenandoah National Park, Virginia, USA. Journal of Forestry Research 31(4), 1255–1270. [Google Scholar]
- * Bilgin, R. , Keşişoğlu, A. & Rebelo, H. (2012). Distribution patterns of bats in the Eastern Mediterranean Region through a climate change perspective. Acta Chiropterologica 14(2), 425–437. [Google Scholar]
- * Blakey, R. V. , Webb, E. B. , Kesler, D. C. , Siegel, R. B. , Corcoran, D. & Johnson, M. (2019). Bats in a changing landscape: linking occupancy and traits of a diverse montane bat community to fire regime. Ecology and Evolution 9(9), 5324–5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blettler, M. C. , Abrial, E. , Khan, F. R. , Sivri, N. & Espinola, L. A. (2018). Freshwater plastic pollution: recognizing research biases and identifying knowledge gaps. Water Research 143, 416–424. [DOI] [PubMed] [Google Scholar]
- * Blomberg, A. S. , Vasko, V. , Salonen, S. , Pētersons, G. & Lilley, T. M. (2021). First record of a Nathusius' pipistrelle (Pipistrellus nathusii) overwintering at a latitude above 60° N. Mammalia 85(1), 74–78. [Google Scholar]
- * Bondareco, A. , Körtner, G. & Geiser, F. (2014). Hot bats: extreme thermal tolerance in a desert heat wave. Naturwissenschaften 101(8), 679–685. [DOI] [PubMed] [Google Scholar]
- * Bosso, L. , Ancillotto, L. , Smeraldo, S. , D'arco, S. , Migliozzi, A. , Conti, P. & Russo, D. (2018). Loss of potential bat habitat following a severe wildfire: a model‐based rapid assessment. International Journal of Wildland Fire 27(11), 756–769. [Google Scholar]
- * Bourne, S. & Hamilton‐Smith, E. (2007). Miniopterus schreibersii bassanii and climate change. Australasian Bat Society Newsletter 28, 67–69. [Google Scholar]
- Bozinovic, F. & Pörtner, H. O. (2015). Physiological ecology meets climate change. Ecology and Evolution 5(5), 1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Burles, D. W. , Brigham, R. M. , Ring, R. A. & Reimchen, T. E. (2009). Influence of weather on two insectivorous bats in a temperate Pacific Northwest rainforest. Canadian Journal of Zoology 87(2), 132–138. [Google Scholar]
- * Burns, C. E. , Johnston, K. M. & Schmitz, O. J. (2003). Global climate change and mammalian species diversity in US national parks. Proceedings of the National Academy of Sciences 100, 11474–11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Chattopadhyay, B. , Garg, K. M. , Ray, R. & Rheindt, F. E. (2019). Fluctuating fortunes: genomes and habitat reconstructions reveal global climate‐mediated changes in bats' genetic diversity. Proceedings of the Royal Society B: Biological Sciences 286(1911), 20190304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, I. C. , Hill, J. K. , Ohlemüller, R. , Roy, D. B. & Thomas, C. D. (2011). Rapid range shifts of species associated with high levels of climate warming. Science 333(6045), 1024–1026. [DOI] [PubMed] [Google Scholar]
- Chown, S. L. , Hoffmann, A. A. , Kristensen, T. N. , Angilletta, M. J. Jr. , Stenseth, N. C. & Pertoldi, C. (2010). Adapting to climate change: a perspective from evolutionary physiology. Climate Research 43(1‐2), 3–15. [Google Scholar]
- Cohen, J. M. , Lajeunesse, M. J. & Rohr, J. R. (2018). A global synthesis of animal phenological responses to climate change. Nature Climate Change 8(3), 224–228. [Google Scholar]
- Cooper‐Bohannon, R. , Rebelo, H. , Jones, G. , Cotterill, F. , Monadjem, A. , Schoeman, M. C. & Park, K. (2016). Predicting bat distributions and diversity hotspots in southern Africa. Hystrix, the Italian Journal of Mammalogy 27(1), 1–11722. [Google Scholar]
- * Costa, W. F. , Ribeiro, M. , Saraiva, A. M. , Imperatriz‐Fonseca, V. L. & Giannini, T. C. (2018). Bat diversity in Carajás National Forest (Eastern Amazon) and potential impacts on ecosystem services under climate change. Biological Conservation 218, 200–210. [Google Scholar]
- Crick, H. Q. (2004). The impact of climate change on birds. Ibis 146, 48–56. [Google Scholar]
- Dey, S. , Roy, U. S. & Chattopadhyay, S. (2015). Effect of heat wave on the Indian flying fox Pteropus giganteus (Mammalia: Chiroptera: Pteropodidae) population from Purulia district of West Bengal, India. Journal of Threatened Taxa 7, 7029–7033. [Google Scholar]
- * Doty, A. C. , Stawsky, C. , Law, B. S. & Geiser, F. (2016). Post‐wildfire physiological ecology of an Australian microbat. Journal of Comparative Physiology 186(7), 937–946. [DOI] [PubMed] [Google Scholar]
- * Downs, C. T. , Awuah, A. , Jordaan, M. , Magagula, L. , Mkhize, T. , Paine, C. & Hart, L. A. (2015). Too hot to sleep? Sleep behaviour and surface body temperature of Wahlberg's epauletted fruit bat. PLoS One 10(3), e0119419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famoso, N. A. , Hopkins, S. S. & Davis, E. B. (2018). How do diet and body mass drive reproductive strategies in mammals? Biological Journal of the Linnean Society 124(2), 151–156. [Google Scholar]
- Feder, M. E. (2010). Physiology and global climate change. Annual Review of Physiology 72, 123–125. [DOI] [PubMed] [Google Scholar]
- Feeley, K. J. , Stroud, J. T. & Perez, T. M. (2017). Most ‘global’ reviews of species' responses to climate change are not truly global. Diversity and Distributions 23(3), 231–234. [Google Scholar]
- Forrest, J. R. (2016). Complex responses of insect phenology to climate change. Current Opinion in Insect Science 17, 49–54. [DOI] [PubMed] [Google Scholar]
- Frick, W. F. , Baerwald, E. F. , Pollock, J. F. , Barklay, R. M. R. , Szymanski, J. A. , Weller, T. J. , Russel, A. L. , Loeb, S. C. , Medellin, R. A. & McGuire, L. P. (2017). Fatalities at wind turbines may threaten population viability of a migratory bat. Biological Conservation 209, 172–177. [Google Scholar]
- Frick, W. F. , Kingston, T. & Flanders, J. (2020). A review of the major threats and challenges to global bat conservation. Annals of the New York Academy of Sciences 1469(1), 5–25. [DOI] [PubMed] [Google Scholar]
- * Frick, W. F. , Reynolds, D. S. & Kunz, T. H. (2010). Influence of climate and reproductive timing on demography of little brown myotis Myotis lucifugus . Journal of Animal Ecology 79(1), 128–136. [DOI] [PubMed] [Google Scholar]
- * Frick, W. F. , Stepanian, P. M. , Kelly, J. F. , Howard, K. W. , Kuster, C. M. , Kunz, T. H. & Chilson, P. B. (2012). Climate and weather impact timing of emergence of bats. PLoS One 7(8), e42737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritze, M. & Puechmaille, S. J. (2018). Identifying unusual mortality events in bats: a baseline for bat hibernation monitoring and white‐nose syndrome research. Mammal Review 48(3), 224–228. [Google Scholar]
- * Froidevaux, J. S. , Boughey, K. L. , Barlow, K. E. & Jones, G. (2017). Factors driving population recovery of the greater horseshoe bat (Rhinolophus ferrumequinum) in the UK: implications for conservation. Biodiversity and Conservation 26(7), 1601–1621. [Google Scholar]
- Gallardo, B. & Aldridge, D. C. (2013). The ‘dirty dozen’: socio‐economic factors amplify the invasion potential of 12 high‐risk aquatic invasive species in Great Britain and Ireland. Journal of Applied Ecology 50(3), 757–766. [Google Scholar]
- * Gómez‐Ruiz, E. P. & Lacher, T. E. Jr. (2019). Climate change, range shifts, and the disruption of a pollinator‐plant complex. Scientific Reports 9(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González‐Varo, J. P. , Rumeu, B. , Albrecht, J. , Arroyo, J. M. , Bueno, R. S. , Burgos, T. & Traveset, A. (2021). Limited potential for bird migration to disperse plants to cooler latitudes. Nature 595(7865), 75–79. [DOI] [PubMed] [Google Scholar]
- * Gottfried, I. , Gottfried, T. , Lesinski, G. , Hebda, G. , Ignaczak, M. , Wojtaszyn, G. & Kowalski, M. (2020). Long‐term changes in winter abundance of the barbastelle Barbastella barbastellus in Poland and the climate change–are current monitoring schemes still reliable for cryophilic bat species? PLoS One 15(2), e0227912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevitch, J. , Koricheva, J. , Nakagawa, S. & Stewart, G. (2018). Meta‐analysis and the science of research synthesis. Nature 555(7695), 175–182. [DOI] [PubMed] [Google Scholar]
- * Haest, B. , Stepanian, P. M. , Wainwright, C. E. , Liechti, F. & Bauer, S. (2021). Climatic drivers of (changes in) bat migration phenology at Bracken Cave (USA). Global Change Biology 27(4), 768–780. [DOI] [PubMed] [Google Scholar]
- * Hall, L. K. , Lambert, C. T. , Larsen, R. T. , Knight, R. N. & Mcmillan, B. R. (2016). Will climate change leave some desert bat species thirstier than others? Biological Conservation 201, 284–292. [Google Scholar]
- * Hayes, M. A. & Adams, R. A. (2017). Simulated bat populations erode when exposed to climate change projections for western North America. PLoS One 12(7), e0180693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, A. A. & Sgrò, C. M. (2011). Climate change and evolutionary adaptation. Nature 470(7335), 479–485. [DOI] [PubMed] [Google Scholar]
- Hohenlohe, P. A. , Funk, W. C. & Rajora, O. P. (2021). Population genomics for wildlife conservation and management. Molecular Ecology 30(1), 62–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Hughes, A. C. , Satasook, C. , Bates, P. J. , Bumrungsri, S. & Jones, G. (2012). The projected effects of climatic and vegetation changes on the distribution and diversity of Southeast Asian bats. Global Change Biology 18(6), 1854–1865. [Google Scholar]
- * Humphries, M. M. , Thomas, D. W. & Speakman, J. R. (2002). Climate‐mediated energetic constraints on the distribution of hibernating mammals. Nature 418(6895), 313–316. [DOI] [PubMed] [Google Scholar]
- IUCN (2022). The IUCN Red List of Threatened Species. Version 2022‐1. Electronic file available at https://www.iucnredlist.org. Accessed on 31.7.2022.
- * Jones, G. , Barlow, K. , Ransome, R. D. & Gilmour, L. (2015). Greater horseshoe bats and their insect prey: the impact and importance of climate change and agri‐environment schemes. Final Report to PTES. Electronic file available at http://ptes.org. Accessed on 31.8.2020.
- Jones, G. , Jacobs, D. S. , Kunz, T. H. , Willig, M. R. & Racey, P. A. (2009). Carpe noctem: the importance of bats as bioindicators. Endangered Species Research 8(1‐2), 93–115. [Google Scholar]
- * Jones, G. & Rebelo, H. (2013). Responses of bats to climate change: learning from the past and predicting the future. In Bat Evolution, Ecology, and Conservation, pp. 457–478. Springer, New York. [Google Scholar]
- Kayser, C. (1953). L'hibernation des mammiferes. Annales de Biologie 29, 109–150. [Google Scholar]
- Korine, C. , Adams, R. , Russo, D. , Fisher‐Phelps, M. & Jacobs, D. (2016). Bats and water: anthropogenic alterations threaten global bat populations. In Bats in the Anthropocene: Conservation of Bats in a Changing World, pp. 215–241. Springer, Cham. [Google Scholar]
- Krauel, J. J. & McCracken, G. F. (2013). Recent advances in bat migration research. In Bat Evolution, Ecology, and Conservation, pp. 293–313. Springer, New York. [Google Scholar]
- * Kravchenko, K. A. , Vlaschenko, A. S. , Lehenert, L. S. , Courtiol, A. & Voight, C. C. (2020). Generational shift in the migratory common noctule bat: first‐year males lead the way to hibernacula at higher latitudes. Biology Letters 16(9), 20200351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz, T. H. , Braun de Torrez, E. , Bauer, D. , Lobova, T. & Fleming, T. H. (2011). Ecosystem services provided by bats. Annals of the New York Academy of Sciences 1223(1), 1–38. [DOI] [PubMed] [Google Scholar]
- * Lambert, C. T. , Hall, L. K. , Larsen, R. T. , Knight, R. N. & Mcmillan, B. R. (2018). Temporal partitioning and the effects of climate change on two ecologically similar desert bats. Journal of Mammalogy 99(6), 1486–1494. [Google Scholar]
- Lane, J. E. , Kruuk, L. E. B. , Charmantier, A. , Murie, J. O. & Dobson, F. S. (2012). Delayed phenology and reduced fitness associated with climate change in a wild hibernator. Nature 489, 554–557. [DOI] [PubMed] [Google Scholar]
- * Law, B. , Doty, A. , Chidel, M. & Brassil, T. (2018). Bat activity before and after a severe wildfire in Pilliga forests: resilience influenced by fire extent and landscape mobility? Austral Ecology 43(6), 706–718. [Google Scholar]
- Leclerc, C. , Courchamp, F. & Bellard, C. (2018). Insular threat associations within taxa worldwide. Scientific Reports 8(1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentini, P. E. , Bird, T. J. , Griffiths, S. R. , Godinho, L. N. & Wintle, B. A. (2015). A global synthesis of survival estimates for microbats. Biology Letters 11(8), 20150371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Linton, D. M. & Macdonald, D. W. (2018). Spring weather conditions influence breeding phenology and reproductive success in sympatric bat populations. Journal of Animal Ecology 87(4), 1080–1090. [DOI] [PubMed] [Google Scholar]
- * Loeb, S. C. & Winters, E. A. (2013). Indiana bat summer maternity distribution: effects of current and future climates. Ecology and Evolution 3(1), 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * López‐Baucells, A. , Flaquer, C. , Mas, M. , Pons, P. & Puig‐Montserrat, X. (2021). Recurring fires in Mediterranean habitats and their impact on bats. Biodiversity and Conservation 30(2), 385–402. [Google Scholar]
- López‐Baucells, A. , Rocha, R. & Fernández‐Llamazares, Á. (2018). When bats go viral: negative framings in virological research imperil bat conservation. Mammal Review 48(1), 62–66. [Google Scholar]
- * Lučan, R. K. , Weiser, M. & Hanák, V. (2013). Contrasting effects of climate change on the timing of reproduction and reproductive success of a temperate insectivorous bat. Journal of Zoology 290(2), 151–159. [Google Scholar]
- * Lundy, M. , Montgomery, I. & Russ, J. (2010). Climate change‐linked range expansion of Nathusius' pipistrelle bat, Pipistrellus nathusii (Keyserling & Blasius, 1839). Journal of Biogeography 37(12), 2232–2242. [Google Scholar]
- * Luo, J. , Koselj, K. , Zsebok, S. , Siemers, B. M. & Goerlitz, H. R. (2014). Global warming alters sound transmission: differential impact on the prey detection ability of echolocating bats. Journal of the Royal Society Interface 11(91), 20130961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Martin, G. , Yanez‐Arenas, C. , Chen, C. , Plowright, R. K. , Webb, R. J. & Skerratt, L. F. (2018). Climate change could increase the geographic extent of Hendra virus spillover risk. EcoHealth 15(3), 509–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken, G. F. , Bernard, R. F. , Gamba‐Rios, M. , Wolfe, R. , Krauel, J. J. , Jones, D. N. & Brown, V. A. (2018). Rapid range expansion of the Brazilian free‐tailed bat in the southeastern United States, 2008–2016. Journal of Mammalogy 99(2), 312–320. [Google Scholar]
- Memmott, J. , Craze, P. G. , Waser, N. M. & Price, M. V. (2007). Global warming and the disruption of plant–pollinator interactions. Ecology Letters 10(8), 710–717. [DOI] [PubMed] [Google Scholar]
- Meyer, G. A. , Senulis, J. A. & Reinartz, J. A. (2016). Effects of temperature and availability of insect prey on bat emergence from hibernation in spring. Journal of Mammalogy 97(6), 1623–1633. [Google Scholar]
- Møller, A. P. , Fiedler, W. & Berthold, P. (eds) (2010). Effects of Climate Change on Birds. Oxford University Press, Oxford. [Google Scholar]
- Montoya, J. M. & Raffaelli, D. (2010). Climate change, biotic interactions and ecosystem services. Philosophical Transactions of the Royal Society B: Biological Sciences 365, 2013–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, J. & Møller, A. P. (2011). Extreme climatic events in relation to global change and their impact on life histories. Current Zoology 57, 375–389. [Google Scholar]
- Myers, N. , Mittermeier, R. A. , Mittermeier, C. G. , Da Fonseca, G. A. B. & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature 403, 853–858. [DOI] [PubMed] [Google Scholar]
- * Nagy, J. A. , Bartholy, J. , Pongracz, R. , Pieczka, I. , Breuer, H. & Hufnagel, L. (2017). Analysis of the impacts of global warming on European bat species's range area in the 21st century using regional climate model simulation. Quarterly Journal of the Hungarian Meteorological Service 121(3), 285–301. [Google Scholar]
- Neate‐Clegg, M. H. , Jones, S. E. , Tobias, J. A. , Newmark, W. D. & Şekercioğlu, Ç. H. (2021). Ecological correlates of elevational range shifts in tropical birds. Frontiers in Ecology and Evolution 9, 215. [Google Scholar]
- O'Grady, J. J. , Reed, D. H. , Brook, B. W. & Frankham, R. (2004). What are the best correlates of predicted extinction risk? Biological Conservation 118(4), 513–520. [Google Scholar]
- * Ortega‐Garcia, S. , Guevara, L. , Arroyo‐Cabrales, J. , Lindig‐Cisneros, R. , Martinez‐Meyer, E. , Vega, E. & Schondube, J. E. (2017). The thermal niche of Neotropical nectar‐feeding bats: its evolution and application to predict responses to global warming. Ecology and Evolution 7(17), 6691–6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea, T. J. , Cryan, P. M. , Hayman, D. T. S. , Plowright, R. K. & Streicker, D. G. (2016). Multiple mortality events in bats: a global review. Mammal Review 46, 175–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici, M. , Foden, W. B. , Visconti, P. , Watson, J. E. , Butchart, S. H. , Kovacs, K. M. & Rondinini, C. (2015). Assessing species vulnerability to climate change. Nature Climate Change 5(3), 215–224. [Google Scholar]
- Page, M. J. , Mckenzie, J. E. , Bossuyt, P. M. , Boutron, I. , Hoffmann, T. C. , Mulrow, C. D. & Moher, D. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Systematic Reviews 10(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Parris, K. M. & Hazell, D. L. (2005). Biotic effects of climate change in urban environments: the case of the grey‐headed flying‐fox (Pteropus poliocephalus) in Melbourne, Australia. Biological Conservation 124, 267–276. [Google Scholar]
- Pauls, S. U. , Nowak, C. , Bálint, M. & Pfenninger, M. (2013). The impact of global climate change on genetic diversity within populations and species. Molecular Ecology 22(4), 925–946. [DOI] [PubMed] [Google Scholar]
- * Pierson, E. D. , Elmgyist, T. , Rainey, W. E. & Cox, P. A. (1996). Effects of tropical cyclonic storms on flying fox populations on the South Pacific islands of Samoa. Conservation Biology 10(2), 438–451. [Google Scholar]
- * Piksa, K. & Gubała, W. J. (2021). First record of Miniopterus schreibersii (Chiroptera: Miniopteridae) in Poland—a possible range expansion? Mammal Research 66(1), 211–215. [Google Scholar]
- * Pio, D. V. , Engler, R. , Linder, H. P. , Monadiem, A. , Cotteril, F. P. , Taylor, P. J. & Guisan, A. (2014). Climate change effects on animal and plant phylogenetic diversity in southern Africa. Global Change Biology 20(5), 1538–1549. [Google Scholar]
- Plard, F. , Gaillard, J.‐M. , Coulson, T. , Hewison, J. M. , Delorme, D. , Warnant, C. & Bonenfant, C. (2014). Mismatch between birth date and vegetation phenology slows the demography of roe deer. PLoS Biology 12, e1001828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prather, C. M. , Pelini, S. L. , Laws, A. , Rivest, E. , Woltz, M. , Bloch, C. P. & Joern, A. (2013). Invertebrates, ecosystem services and climate change. Biological Reviews 88(2), 327–348. [DOI] [PubMed] [Google Scholar]
- * Pruvot, M. , Cappelle, J. , Furey, N. , Hul, V. , Heng, H. S. , Duong, V. , Dussart, P. & Horwood, P. (2019). Extreme temperature event and mass mortality of insectivorous bats. European Journal of Wildlife Research 65(3), 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Pryde, M. A. , O'Donnell, C. F. J. & Barker, R. J. (2005). Factors influencing survival and long‐term population viability of New Zealand long‐tailed bats (Chalinolobus tuberculatus): implications for conservation. Biological Conservation 126, 175–185. [Google Scholar]
- Radchuk, V. , Reed, T. , Teplitsky, C. , Van De Pol, M. , Charmantier, A. , Hassall, C. , Adamík, P. , Adriaensen, F. , Ahola, M. P. , Arcese, P. , Miguel Avilés, J. , Balboltin, J. , Berg, K. S. , Borras, A. , Burthe, S. , et al. (2019). Adaptive responses of animals to climate change are most likely insufficient. Nature Communications 10, 3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez‐Fráncel, L. A. , García‐Herrera, L. V. , Losada‐Prado, S. , Reinoso‐Flórez, G. , Sánchez‐Hernández, A. , Estrada‐Villegas, S. & Guevara, G. (2021). Bats and their vital ecosystem services: a global review. Integrative Zoology 17, 2–23. [DOI] [PubMed] [Google Scholar]
- Rasmont, P. & Iserbyt, S. (2012). The bumblebees scarcity syndrome: are heat waves leading to local extinctions of bumblebees (Hymenoptera: Apidae: Bombus)? Annales de la Société Entomologique de France 48, 275–280. [Google Scholar]
- * Ratcliffe, F. (1932). Notes on the fruit bats (Pteropus spp.) of Australia. The Journal of Animal Ecology 1, 32–57. [Google Scholar]
- * Razgour, O. (2015). Beyond species distribution modeling: a landscape genetics approach to investigating range shifts under future climate change. Ecological Informatics 30, 250–256. [Google Scholar]
- * Razgour, O. , Forester, B. , Taggart, J. B. , Bekaert, M. , Juste, J. , Ibáñez, C. , Puechmaille, S. J. , Novella‐Fernandez, R. , Alberdi, A. & Manel, S. (2019). Considering adaptive genetic variation in climate change vulnerability assessment reduces species range loss projections. Proceedings of the National Academy of Sciences 116(21), 10418–10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Razgour, O. , Juste, J. , Ibáñez, C. , Kiefer, A. , Rebelo, H. , Puechmaille, S. J. , Arlettaz, R. , Burke, T. , Dawson, D. A. , Beaumont, M. & Jones, G. (2013). The shaping of genetic variation in edge‐of‐range populations under past and future climate change. Ecology Letters 16(10), 1258–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razgour, O. , Kasso, M. , Santos, H. & Juste, J. (2021). Up in the air: threats to Afromontane biodiversity from climate change and habitat loss revealed by genetic monitoring of the Ethiopian Highlands bat. Evolutionary Applications 14(3), 794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Razgour, O. , Taggart, J. B. , Manel, S. , Juste, J. , Ibanez, C. , Rebelo, H. , Alberdi, A. , Jones, G. & Park, K. (2018). An integrated framework to identify wildlife populations under threat from climate change. Molecular Ecology Resources 18(1), 18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Rebelo, H. , Tarroso, P. & Jones, G. (2010). Predicted impact of climate change on European bats in relation to their biogeographic patterns. Global Change Biology 16(2), 561–576. [Google Scholar]
- Reed, T. E. , Waples, R. S. , Schindler, D. E. , Hard, J. J. & Kinnison, M. T. (2010). Phenotypic plasticity and population viability: the importance of environmental predictability. Proceedings of the Royal Society B: Biological Sciences 277(1699), 3391–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner, S. S. & Zohner, C. M. (2018). Climate change and phenological mismatch in trophic interactions among plants, insects, and vertebrates. Annual Review of Ecology, Evolution, and Systematics 49, 165–182. [Google Scholar]
- * Reusch, C. , Gampe, J. , Scheuerlein, A. , Meier, F. , Grosche, L. & Kerth, G. (2019). Differences in seasonal survival suggest species‐specific reactions to climate change in two sympatric bat species. Ecology and Evolution 9, 7957–7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Roche, N. , Langton, S. , Aughney, T. , Lynn, D. & Marnell, F. (2019). Elucidating the consequences of a warming climate for common bat species in north‐western Europe. Acta Chiropterologica 21(2), 359–373. [Google Scholar]
- * Rydell, J. , Eklof, J. , Fransson, H. & Lind, S. (2018). Long‐term increase in hibernating bats in Swedish mines—effect of global warming? Acta Chiropterologica 20(2), 421–426. [Google Scholar]
- * Sachanowicz, K. & Ciechanowski, M. (2006). First winter record of the migratory bat Pipistrellus nathusii (Keyserling and Blasius 1839) (Chiroptera: Vespertilionidae) in Poland: yet more evidence of global warming? Mammalia 70, 168–169. [Google Scholar]
- * Sachanowicz, K. , Ciechanowski, M. , Tryjanowski, P. & Kosicki, J. Z. (2019). Wintering range of Pipistrellus nathusii (Chiroptera) in Central Europe: has the species extended to the north‐east using urban heat islands? Mammalia 83(3), 260–271. [Google Scholar]
- Saino, N. , Ambrosini, R. , Rubolini, D. , von Hardenberg, J. , Provenzale, A. , Hüppop, K. & Sokolov, L. (2011). Climate warming, ecological mismatch at arrival and population decline in migratory birds. Proceedings of the Royal Society B: Biological Sciences 278(1707), 835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Salinas‐Ramos, V. B. , Agnelli, P. , Bosso, L. , Ancillotto, L. & Russo, D. (2021). Body size of Italian greater horseshoe bats (Rhinolophus ferrumequinum) increased over one century and a half: a response to climate change? Mammalian Biology 101(6), 1127–1131. [Google Scholar]
- Salinas‐Ramos, V. B. , Ancillotto, L. , Bosso, L. , Sánchez‐Cordero, V. & Russo, D. (2020). Interspecific competition in bats: state of knowledge and research challenges. Mammal Review 50(1), 68–81. [Google Scholar]
- Santini, L. , Benítez‐López, A. , Maiorano, L. , Čengić, M. & Huijbregts, M. A. (2021). Assessing the reliability of species distribution projections in climate change research. Diversity and Distributions 27(6), 1035–1050. [Google Scholar]
- * Scheel, D. , Vincent, T. L. S. & Cameron, G. N. (1996). Global warming and the species richness of bats in Texas. Conservation Biology 10(2), 452–464. [Google Scholar]
- Scheffers, B. R. , De Meester, L. , Bridge, T. C. , Hoffmann, A. A. , Pandolfi, J. M. , Corlett, R. T. & Watson, J. E. (2016). The broad footprint of climate change from genes to biomes to people. Science 354(6313), aaf7671. [DOI] [PubMed] [Google Scholar]
- Schmittner, A. & Galbraith, E. D. (2008). Glacial greenhouse‐gas fluctuations controlled by ocean circulation changes. Nature 456(7220), 373–376. [DOI] [PubMed] [Google Scholar]
- Scridel, D. , Brambilla, M. , Martin, K. , Lehikoinen, A. , Iemma, A. , Matteo, A. , Jähnig, S. , Caprio, E. , Bogliani, G. , Pedrini, P. , Rolando, A. , Arlettaz, R. & Chamberlain, D. (2018). A review and meta‐analysis of the effects of climate change on Holarctic mountain and upland bird populations. Ibis 160(3), 489–515. [Google Scholar]
- Sheppard, C. , Sheppard, A. & Fenner, D. (2020). Coral mass mortalities in the Chagos Archipelago over 40 years: regional species and assemblage extinctions and indications of positive feedbacks. Marine Pollution Bulletin 154, 111075. [DOI] [PubMed] [Google Scholar]
- Sherwin, H. A. , Montgomery, W. I. & Lundy, M. G. (2013). The impact and implications of climate change for bats. Mammal Review 43(3), 171–182. [Google Scholar]
- Simmons, N.B. & Cirranello, A.L. (2021). Bat Species of the World: a taxonomic and geographic database. Electronic file available at https://batnames.org/ Accessed on 6.12.2021.
- * Smeraldo, S. , Bosso, L. , Salinas‐Ramos, V. B. , Ancillotto, L. , Sánchez‐Cordero, V. , Gazaryan, S. & Russo, D. (2021). Generalists yet different: distributional responses to climate change may vary in opportunistic bat species sharing similar ecological traits. Mammal Review 51(4), 571–584. [Google Scholar]
- * Snoyman, S. , Muhic, J. & Brown, C. (2012). Nursing females are more prone to heat stress: demography matters when managing flying‐foxes for climate change. Applied Animal Behaviour Science 142(1‐2), 90–97. [Google Scholar]
- Sofaer, H. R. , Jarnevich, C. S. , Pearse, I. S. , Smyth, R. L. , Auer, S. , Cook, G. L. & Hamilton, H. (2018). Development and delivery of species distribution models to inform decision‐making. BioScience 69(7), 544–557. [Google Scholar]
- * Stawski, C. & Geiser, F. (2012). Will temperature effects or phenotypic plasticity determine the thermal response of a heterothermic tropical bat to climate change? PLoS One 7(7), e40278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenseth, N. C. & Mysterud, A. (2002). Climate, changing phenology, and other life history traits: nonlinearity and match–mismatch to the environment. Proceedings of the National Academy of Sciences 99(21), 13379–13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Stepanian, P. M. & Wainwright, C. E. (2018). Ongoing changes in migration phenology and winter residency at Bracken Bat Cave. Global Change Biology 24(7), 3266–3275. [DOI] [PubMed] [Google Scholar]
- Tafani, M. , Cohas, A. , Bonenfant, C. , Gaillard, J.‐M. & Allainé, D. (2013). Decreasing litter size of marmots over time: a life history response to climate change? Ecology 94, 580–586. [DOI] [PubMed] [Google Scholar]
- * Tapia‐Palacios, M. A. , Garcia‐Suarez, O. , Sotomayor‐Bonilla, J. , Silva‐Magaña, M. A. , Perez‐Ortiz, G. , Espinosa‐Garcia, A. C. & Mazari‐Hiriart, M. (2018). Abiotic and biotic changes at the basin scale in a tropical dry forest landscape after Hurricanes Jova and Patricia in Jalisco, Mexico. Forest Ecology and Management 426, 18–26. [Google Scholar]
- Teeling, E. C. , Vernes, S. C. , Dávalos, L. M. , Ray, D. A. , Gilbert, M. T. P. , Myers, E. & Bat1K Consortium (2018). Bat biology, genomes, and the Bat1K project: to generate chromosome‐level genomes for all living bat species. Annual Review of Animal Biosciences 6, 23–46. [DOI] [PubMed] [Google Scholar]
- * Thomas, C. D. , Hill, J. K. , Anderson, B. J. , Bailey, S. , Beale, C. M. , Bradbury, R. B. & Yardley, T. (2011). A framework for assessing threats and benefits to species responding to climate change. Methods in Ecology and Evolution 2(2), 125–142. [Google Scholar]
- Thomas, D. W. & Geiser, F. (1997). Periodic arousals in hibernating mammals: is evaporative water loss involved? Functional Ecology 11(5), 585–591. [Google Scholar]
- Titley, M. A. , Snaddon, J. L. & Turner, E. C. (2017). Scientific research on animal biodiversity is systematically biased towards vertebrates and temperate regions. PLoS One 12(12), e0189577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Tomassini, A. , Colangelo, P. , Agnelli, P. , Jones, G. & Russo, D. (2014). Cranial size has increased over 133 years in a common bat, Pipistrellus kuhlii: a response to changing climate or urbanization? Journal of Biogeography 41(5), 944–953. [Google Scholar]
- Uhrin, M. , Hüttmeir, U. , Kipson, M. , Estók, P. , Sachanowicz, K. , Bücs, S. & Benda, P. (2016). Status of Savi's pipistrelle Hypsugo savii (Chiroptera) and range expansion in Central and south‐eastern Europe: a review. Mammal Review 46(1), 1–16. [Google Scholar]
- Ummenhofer, C. C. & Meehl, G. A. (2017). Extreme weather and climate events with ecological relevance: a review. Philosophical Transactions of the Royal Society B: Biological Sciences 372, 20160135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban, M. C. (2015). Accelerating extinction risk from climate change. Science 348(6234), 571–573. [DOI] [PubMed] [Google Scholar]
- Urban, M. C. , Bocedi, G. , Hendry, A. P. , Mihoub, J. B. , Pe'er, G. , Singer, A. & Travis, J. M. J. (2016). Improving the forecast for biodiversity under climate change. Science 353(6304), 1113–1122. [DOI] [PubMed] [Google Scholar]
- Venne, S. & Currie, D. J. (2021). Can habitat suitability estimated from MaxEnt predict colonizations and extinctions? Diversity and Distributions 27(5), 873–886. [Google Scholar]
- Visser, M. E. , Holleman, L. J. & Gienapp, P. (2006). Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia 147(1), 164–172. [DOI] [PubMed] [Google Scholar]
- Waller, N. L. , Gynther, I. C. , Freeman, A. B. , Lavery, T. H. & Leung, L. K. P. (2017). The Bramble Cay melomys Melomys rubicola (Rodentia: Muridae): a first mammalian extinction caused by human‐induced climate change? Wildlife Research 44(1), 9–21. [Google Scholar]
- Ward, M. , Tulloch, A. I. , Radford, J. Q. , Williams, B. A. , Reside, A. E. , Macdonald, S. L. & Watson, J. E. (2020). Impact of 2019–2020 mega‐fires on Australian fauna habitat. Nature Ecology & Evolution 4(10), 1321–1326. [DOI] [PubMed] [Google Scholar]
- Warren, R. , Price, J. , Graham, E. , Forstenhaeusler, N. & VanDerWal, J. (2018). The projected effect on insects, vertebrates, and plants of limiting global warming to 1.5 C rather than 2 C. Science 360, 791–795. [DOI] [PubMed] [Google Scholar]
- * Welbergen, J. A. (2012). The impacts of extreme events on biodiversity–lessons from die‐offs in flying‐foxes. In Proceedings of the International Symposium on the Importance of Bats as Bioindicators. Granollers, Barcelona. [Google Scholar]
- * Welbergen, J. A. , Klose, S. M. , Markus, N. & Ebby, P. (2008). Climate change and the effects of temperature extremes on Australian flying‐foxes. Proceedings of the Royal Society B: Biological Sciences 275, 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch, J. N. & Leppanen, C. (2017). The threat of invasive species to bats: a review. Mammal Review 47(4), 277–290. [Google Scholar]
- * Wheeler, B. H. (2012). Modeling range shifts for North American bats under climate change. North American bat ranges and climate change. Dissertation Project: University of Berkley.
- Wigley, T. M. (2009). The effect of changing climate on the frequency of absolute extreme events. Climatic Change 97(1), 67–76. [Google Scholar]
- Winter, M. , Fiedler, W. , Hochachka, W. M. , Koehncke, A. , Meiri, S. & De la Riva, I. (2016). Patterns and biases in climate change research on amphibians and reptiles: a systematic review. Royal Society Open Science 3(9), 160158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Wolff, J. M. , Battaglia, L. , Carter, T. C. , Rodman, L. B. , Britzke, E. R. & Feldhamer, G. A. (2009). Effects of tornado disturbance on bat communities in southern Illinois. Northeastern Naturalist 16(4), 553–562. [Google Scholar]
- Wolkovich, E. M. , Cook, B. I. , McLauchlan, K. K. & Davies, T. J. (2014). Temporal ecology in the Anthropocene. Ecology Letters 17(11), 1365–1379. [DOI] [PubMed] [Google Scholar]
- * Wu, J. (2016). Detection and attribution of the effects of climate change on bat distributions over the last 50 years. Climatic Change 134(4), 681–696. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Distribution of study duration (in years) extracted from studies (N = 46) about bats and climate change, after excluding modelling and palaeoclimatic works.
Table S1. List of the variables extracted from each paper analysed, with name and description.
Table S2. List of studies that met our inclusion criteria.
Table S3. List of studies excluded from analysis, with reasons for exclusion.
Table S4. List of bat species for which evidence regarding the impact of climate change was reported in the literature.


