Abstract
There is no prospective, randomised head‐to‐head trial comparing first‐line FOLFIRINOX and gemcitabine/nab‐paclitaxel in advanced pancreatic cancer. We assess real‐world effectiveness and quality of life (QoL) of both regimens using a new prognostic score. This analysis includes 1540 patients with advanced pancreatic cancer from the prospective, clinical cohort study Tumour Registry Pancreatic Cancer separated into learning (n = 1027) and validation sample (n = 513). The Pancreatic Cancer Score (PCS) was developed using multivariate Cox regression. We compared overall survival (OS) and time to deterioration (TTD) for longitudinal QoL between first‐line FOLFIRINOX (n = 407) and gemcitabine/nab‐paclitaxel (n = 655) according to patients' prognostic risk, after inverse probability of treatment weighting (IPTW) by propensity score analysis. The PCS includes nine independent prognostic factors for survival: female sex, BMI ≥24/unknown, ECOG performance status ≥1, Charlson comorbidity index ≥1, tumour staging IV/unknown at primary diagnosis, liver metastases, bilirubin >1.5× upper limit of normal (ULN), leukocytes >ULN and neutrophil‐to‐lymphocyte ratio ≥4. Median OS of the validation sample was 11.4 (95% confidence interval [CI]: 10.4‐14.4), 8.5 (95% CI: 6.8‐9.6) and 5.9 months (95% CI: 4.0‐7.4) for favourable‐ (0‐3 risk factors), intermediate‐ (4‐5 factors) and poor‐risk group (6‐9 factors), respectively. After IPTW, only poor‐risk patients had significantly longer median OS and TTD of overall QoL with FOLFIRINOX (OS: 6.9 months, 95% CI: 3.9‐13.3; TTD: 10.6 months, 95% CI: 2.0‐14.1) vs gemcitabine/nab‐paclitaxel (OS: 4.0 months, 95% CI: 2.8‐4.8; TTD: 4.1 months, 95% CI: 2.4‐4.5). Our novel PCS may facilitate treatment decisions in clinical routine of advanced pancreatic cancer, since only poor‐risk, but not favourable‐risk patients, seem to benefit from intensified treatment with FOLFIRINOX.
Keywords: cohort studies, FOLFIRINOX, gemcitabine/nab‐paclitaxel, pancreatic carcinoma, prognostic score
What's new?
Prospective, randomised head‐to‐head trials comparing first‐line FOLFIRINOX and gemcitabine/nab‐paclitaxel in advanced pancreatic cancer are lacking. Here, real‐world data on more than 1000 patients from the prospective German TPK clinical cohort study suggest that only patients stratified as having a poor survival risk by a newly developed prognostic score may benefit from intensified treatment with FOLFIRINOX. Poor‐risk patients had significantly longer median overall survival and time‐to‐deterioration of overall quality of life with FOLFIRINOX vs gemcitabine/nab‐paclitaxel, after standardising treatment groups using a propensity score. The novel prognostic score may facilitate treatment decisions in the routine clinical management of advanced pancreatic cancer.
Abbreviations
- BMI
body mass index
- CCI
Charlson comorbidity index
- CEA
carcinoembryonic antigen
- CI
confidence interval
- ECOG
Eastern Cooperative Oncology Group
- EORTC
European Organisation for Research and Treatment of Cancer
- FOLFIRINOX
leucovorin, fluorouracil, irinotecan and oxaliplatin
- GEMNAB
gemcitabine combined with nab‐paclitaxel
- HR
hazard ratio
- HRQoL
health‐related quality of life
- IPTW
inverse probability of treatment weighting
- LAPC
locally advanced, unresectable pancreatic cancer
- LLN
lower limit of normal
- MPC
metastatic pancreatic cancer
- NLR
neutrophil‐to‐lymphocyte ratio
- OS
overall survival
- PCS
Pancreatic Cancer Score
- PS
propensity score
- QoL
quality of life
- SMD
standardised mean difference
- TPK
Tumour Registry Pancreatic Cancer
- TTD
time to deterioration
- ULN
upper limit of normal
1. INTRODUCTION
Pancreatic cancer is a highly fatal disease with poor outcomes, increasing incidence and mortality rates. 1 Since more than 80% of tumours are locally advanced, unresectable (LAPC) or metastatic (MPC) at diagnosis, the relative 5‐year survival rate is only 10% for all disease stages, both in Germany 2 and the United States. 3 , 4 Pancreatic cancer is predicted to soon become the second leading cause of cancer‐related death in the European Union. 1 , 5 In Germany, approximately 19 000 new cases and almost as many deaths due to pancreatic cancer were registered in 2018. 2
To alleviate cancer‐related symptoms and prolong life, systemic chemotherapy remains the primary treatment option for LAPC and MPC. 1 Two treatment strategies, FOLFIRINOX (leucovorin, fluorouracil, irinotecan and oxaliplatin) and the combination of nab‐paclitaxel and gemcitabine (GEMNAB), have been shown to improve outcomes of patients with MPC. 6 , 7 , 8 Until today, however, there has been no head‐to‐head phase III randomised trial comparing first‐line FOLFIRINOX and GEMNAB and the choice of one regimen over the other is based on clinical parameters. 1 , 9 , 10 Real‐world data from retrospective studies indicate similar effectiveness of both treatments or a trend towards better survival with FOLFIRINOX. 11 , 12 , 13 , 14 , 15 , 16 However, most studies only focus on patients with MPC, and, even more important, imbalances in patient characteristics were often not considered. Proper patient selection is essential to identify those who will benefit most from a particular treatment. 17 Although several prognostic and predictive factors have been evaluated, 18 to our knowledge, there are no prospectively validated models for risk stratification that help in decision‐making for FOLFIRINOX or GEMNAB in routine practice of advanced pancreatic cancer.
Here, we present real‐world data on 1540 nonselected patients with LAPC or MPC treated in office‐based oncology practices and hospitals in Germany from the prospective clinical cohort study TPK (Tumour Registry Pancreatic Cancer). We developed a prognostic score for patients with LAPC/MPC, regardless of the type of systemic palliative first‐line treatment, and compared OS and time to deterioration (TTD) as a measure of longitudinal health‐related quality of life (HRQoL) with FOLFIRINOX or GEMNAB according to patient's prognostic risk. To adjust for confounding factors and to standardise the sample by balanced treatment groups, we used inverse probability of treatment weighting (IPTW) by a propensity score (PS). Our results provide important insights into which patients might benefit most from either treatment and may help to fill the evidence gap, as randomised trials are missing.
2. PATIENTS AND METHODS
2.1. Study design
The Tumour Registry Pancreatic Cancer (TPK, AMETHYST) is an open, longitudinal, multicentre, observational, prospective cohort study (NCT02089269). Patients were recruited from February 2014 until July 2018, from October 2019 until August 2020 and from June 2021 until April 2022; follow‐up is ongoing. For the present analysis, all those patients who had been recruited until the data cut on 30 June 2020 were included. Patients recruited afterwards will be subject of future interim analyses. Eligible patients were ≥18 years of age with previously untreated LAPC or MPC at start of palliative first‐line treatment. Further details on the methodology of the TPK have previously been published. 19
2.2. Cohort definition
Until data cut for this interim analysis (30 June 2020), a total of 1978 patients with LAPC/MPC were prospectively recruited into the TPK by 120 office‐based oncology practices, hospitals and medical care centres all over Germany. For the present analysis, the study cohort consists of 1540 patients with documented palliative first‐line treatment but without any prior (neo)adjuvant systemic treatment (prognosis score population; Figure 1). Of these patients, 1062 received FOLFIRINOX or GEMNAB as first‐line treatment (IPTW population), with 38% of patients (n = 407) treated with FOLFIRINOX and 62% (n = 655) with GEMNAB (Figure 1).
FIGURE 1.
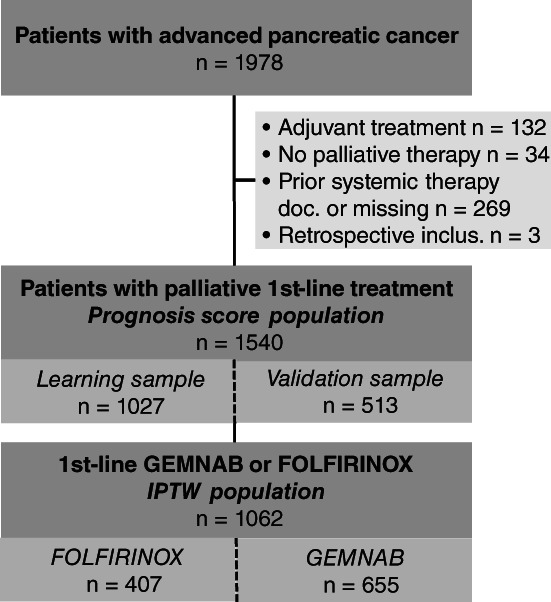
Flow chart. Patient flow chart of all patients with advanced pancreatic cancer included in this analysis, starting from the total number of patients recruited into the TPK registry from February 2014 until June 2020. The study cohort consists of all patients with advanced pancreatic cancer and palliative first‐line treatment who had not received any prior (neo)adjuvant systemic treatment (n = 1540, prognosis score population) including 1062 patients treated with FOLFIRINOX or GEMNAB in first line (IPTW population). doc., documented; GEMNAB, gemcitabine plus nab‐paclitaxel; inclus., inclusion
2.3. Statistical analysis
2.3.1. Time to event endpoints
All time to event endpoints were estimated using the Kaplan‐Meier method. 20 OS was defined as the interval between start of first‐line treatment and the date of death from any cause. Patients alive or lost to follow‐up at data cut were censored at last contact. Start of first‐line treatment was defined as first application of any systemic palliative treatment.
2.3.2. Development of the prognostic Pancreatic Cancer Score (PCS)/real‐world
The following 18 variables documented in the TPK were identified as potentially prognostic based on experts' opinion and/or previous publication as a prognostic factor: age, sex, body mass index (BMI), ECOG performance status, any comorbidity, Charlson comorbidity index (CCI), type of health insurance, tumour localisation at primary diagnosis, tumour staging (based on TNM) and grading at primary diagnosis, resection status of primary tumour, presence of liver metastases, number of metastatic sites, bilirubin, haemoglobin below lower limit of normal, thrombocytes higher upper limit of normal (ULN), leukocytes above ULN and neutrophil‐to‐lymphocyte ratio (NLR; unless otherwise stated, all variables had been documented at start of first‐line treatment). The patient sample was randomly split up 2:1 into a learning sample (2/3) and a validation sample (1/3). Prognostic factors were identified in the learning sample by Cox regression with backward elimination, 21 employing a cut‐off of P < .05. Six factors (sex, localisation of primary tumour, ECOG, BMI, presence of liver metastases and number of metastatic sites) were excluded from variable selection and directly included in the prognostic model. To simplify the score for clinical use, variables were dichotomised before entering the score. The construction of a simple classification rule has been published before. 22
Based on the prognostic factors identified, a sum score (number of risk factors) was built. For practical reasons, prognostic factors were not weighted and each factor contributed with one unit. This approach was further justified by the fact that hazard ratios (HR) were in a similar range for all identified prognostic factors. Based on the number of prognostic factors, patients were stratified into three risk groups by quartile splitting (lower quartile: favourable, second + third quartile: intermediate, upper quartile: poor risk). The predictive performance of the identified risk groups was tested in the validation sample using log‐rank test and Kaplan‐Meier method to compare both favourable‐ vs intermediate‐, favourable‐ vs poor‐ and intermediate‐ vs poor‐risk groups. Adjustment for multiplicity was done for all three comparisons according to Bonferroni. A similar prognostic score has previously been developed by our group for metastatic colorectal cancer. 22
2.3.3. Inverse probability of treatment weighting
To adjust for confounding in the two treatment groups and to standardise the sample by balanced groups, IPTW based on a PS approach was used. For each patient, a PS was estimated using logistic regression containing all 18 variables included for the development of the prognostic score plus T, N and M categories at primary diagnosis, presence of lymphogenic metastases as well as the interaction terms age with ECOG and M category with number of metastatic sites. The logistic regression model was fitted iteratively until sufficient balance for covariates between the two groups was reached. Standardised differences d < 0.1 were considered as sufficient balance. 23 , 24 The IPTW applied for each patient was calculated by taking the inverse of the PS. For the IPTW cohort, the sum of weights refers to the number of patients.
2.3.4. Quality of life
All patients enrolled in the TPK were asked about their HRQoL at start of treatment and subsequently every 2 months using the validated questionnaires European Organisation for Research and Treatment of Cancer (EORTC) QLQ‐C15‐PAL 25 and the pancreatic cancer‐specific module EORTC QLQ‐PAN26. In the present analysis, we focussed on the EORTC QLQ‐C15‐PAL questionnaire, consisting of a single item on overall QoL (global health status), two functional scales (physical and emotional function), two symptom scales (fatigue and pain) and five single items (nausea and vomiting, dyspnoea, sleeping difficulties, constipation and loss of appetite). TTD was implemented as a measure of longitudinal HRQoL and defined as the time from start of first‐line treatment to the time of first clinically relevant deterioration. Deterioration was considered to be clinically relevant for a given dimension, if a decrease of ≥10 points from baseline (≥10% of the score range) was observed at any time point after baseline. 26 , 27 According to the Kaplan‐Meier method, an event was defined as deterioration of ≥10 points or death; patients without such an event were censored at the time of completion of the last questionnaire. All patients who had completed the baseline and ≥1 further questionnaire were included in the TTD analysis (FOLFIRINOX: n = 272; GEMNAB: n = 288).
All analyses were calculated using SAS software, Version 9.4 of the SAS System for Windows. Copyright © 2002‐2012 SAS Institute Inc. SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, North Carolina.
3. RESULTS
3.1. The Pancreatic Cancer Score
As described in the methods section, the patient sample was randomly split up into learning sample (n = 1027) and validation sample (n = 513). Patients in the validation and learning sample did not substantially differ (Table S1). Eleven variables were identified as promising prognostic factors and thus dichotomised; a multivariate Cox regression model with the resulting binary variables was calculated (Table 1).
TABLE 1.
Prognostic factors
| Hazard ratio | 95% CI | P‐value | |
|---|---|---|---|
| Sex | |||
| Female vs male | 1.26 | [1.08‐1.47] | .003* |
| Body mass index (BMI) | |||
| ≥24/unknown vs <24 | 1.22 | [1.06‐1.42] | .007* |
| ECOG performance status | |||
| ≥1 vs 0 | 1.43 | [1.23‐1.67] | <.001* |
| Charlson comorbidity index (CCI) | |||
| ≥1 vs 0/unknown | 1.32 | [1.13‐1.55] | <.001* |
| Localisation of tumour at primary diagnosis | |||
| Pancreas body/tail/unknown vs pancreas head | 1.05 | [0.90‐1.22] | .528 |
| Tumour staging at primary diagnosis | |||
| IV/unknown vs I‐III | 1.50 | [1.18, 1.92] | .001* |
| Metastases | |||
| Yes vs no/unknown | 1.13 | [0.89, 1.44] | .322 |
| Liver metastases | |||
| Yes vs no/unknown | 1.23 | [1.04‐1.46] | .018* |
| Bilirubin | |||
| >1.5× ULN vs ≤1.5× ULN/unknown | 1.74 | [1.38‐2.19] | <.001* |
| Leukocytes above ULN | |||
| Yes/unknown vs no | 1.39 | [1.18‐1.64] | <.001* |
| Neutrophil‐to‐lymphocyte ratio | |||
| ≥4 vs <4/unknown | 1.57 | [1.34‐1.83] | <.001* |
Note: n = 1027, thereof 286 (27.8%) censored cases. Efron method was used to control for ties. Variables shown had been documented at patients' start of first‐line treatment, unless otherwise indicated. The following covariates were not subjected to variable selection: sex, localisation of tumour, liver metastases, metastases, BMI and ECOG Performance Status. The following effects have been removed in backward selection: age at therapy start, any comorbidities, grading at primary diagnosis, haemoglobin below LLN, insurance type, resection status at primary diagnosis, thrombocytes above LLN. *Significant results (P < .05).
Abbreviations: CI, confidence interval; LLN, lower limit of the norm; ULN, upper limit of the norm.
Employing a cut‐off of P < .05, nine variables were identified as independent prognostic factors for survival (marked with an asterisk, Table 1): female sex (HR 1.26, 95% confidence interval [CI]: 1.08‐1.47, P = .003), BMI ≥24/unknown (HR 1.22, 95% CI: 1.06‐1.42, P = .007), ECOG ≥1 (HR 1.43, 95% CI: 1.23‐1.67, P < .001), CCI ≥1 (HR 1.32, 95% CI: 1.13‐1.55, P < .001), tumour staging IV/unknown at primary diagnosis (HR 1.50, 95% CI: 1.18‐1.92, P = .001), presence of liver metastases (HR 1.23, 95% CI: 1.04‐1.46, P = .018), bilirubin >1.5x ULN (HR 1.74, 95% CI: 1.38‐2.19, P < .001), leukocytes >ULN (HR 1.39, 95% CI: 1. 18‐1.64, P < .001) and NLR ≥4 (HR 1.57, 95% CI: 1.34‐1.83, P < .001). Based on these prognostic factors, the PCS was developed, counting each risk factor as one unit. A score of 0‐9 was calculated per patient and three risk groups for survival were identified employing a quartile split of the observed risk scores: 0‐3 risk factors (favourable risk), 4‐5 risk factors (intermediate risk) and 6‐9 risk factors (poor risk) [Table S2, questionnaire for the clinical routine use]. About one third of patients were of favourable risk, approximately 50% of intermediate and nearly 20% of poor risk in both learning and validation sample (Table S3).
For each risk group, OS was calculated for the learning sample (Figure 2A). The validity of the PCS was subsequently tested in the validation sample (Figure 2B). Median OS in the validation sample was 11.4 months (95% CI: 10.4‐14.4), 8.5 months (95% CI: 6.8‐9.6) and 5.9 months (95% CI: 4.0‐7.4) for patients with favourable, intermediate or poor risk, respectively. OS differences between favourable‐ vs intermediate‐ and favourable‐ vs poor‐risk groups were statistically significant (P = .009 and P < .001, respectively, adjusted for multiplicity).
FIGURE 2.
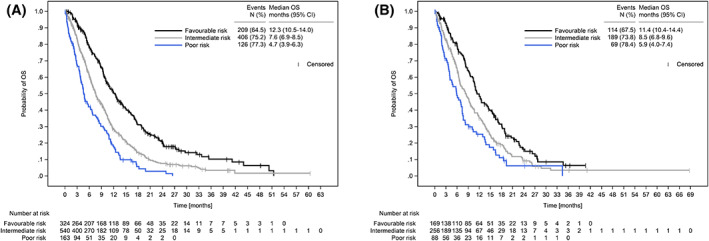
Overall survival of learning and validation sample. (A) OS of learning (n = 1027) and (B) validation sample (n = 513) by risk groups according to the PCS. CI, confidence interval; OS, overall survival; PCS, Pancreatic Cancer Score
3.2. Patient and tumour characteristics before and after IPTW
Looking at the original population before IPTW, patients with FOLFIRINOX (n = 407) differed from those receiving GEMNAB (n = 655) by several patient characteristics (Table 2). Patients receiving FOLFIRINOX were younger (median age of 61 vs 71 years; median age of the total cohort: 70 years), more often presented with ECOG 0 (46% vs 34%) and had less often comorbidities (78% vs 85%). After IPTW, the two treatment groups (n = 514.8 for FOLFIRINOX, n = 547.2 for GEMNAB; numbers refer to the sum of weights) were comparable for all characteristics (Table 2).
TABLE 2.
Patient and tumour characteristics before and after IPTW
| Characteristic at start of first‐line treatment | Original cohort | PS‐IPTW cohort | ||||
|---|---|---|---|---|---|---|
| FOLFIRINOX | GEMNAB | SMD | FOLFIRINOX | GEMNAB | SMD | |
| n = 407 | n = 655 | n a = 514.8 | n a = 547.2 | |||
| Age (y), mean (±SD) | 61.3 (±8.13) | 69.2 (±7.87) | −1.072 | 66.0 (±9.65) | 66.2 (±8.63) | −0.028 |
| Sex | ||||||
| Female | 40.5% | 45.3% | −0.097 | 43.2% | 44.5% | −0.026 |
| Male | 59.5% | 54.7% | 0.097 | 56.8% | 55.5% | 0.026 |
| BMI | ||||||
| Low (<24) | 46.9% | 44.7% | 0.044 | 45.6% | 46.0% | −0.008 |
| Normal (24‐29) | 38.6% | 36.5% | 0.043 | 36.2% | 37.6% | −0.030 |
| High (>29) | 14.3% | 18.6% | −0.118 | 18.1% | 16.3% | 0.050 |
| Missing | 0.2% | 0.2% | 0.021 | 0.1% | 0.1% | 0.002 |
| ECOG performance status | ||||||
| ECOG 0 | 45.7% | 33.7% | 0.246 | 38.5% | 38.8% | −0.006 |
| ECOG 1 | 49.4% | 54.8% | −0.109 | 49.2% | 52.3% | −0.062 |
| ECOG 2 | 4.7% | 11.3% | −0.246 | 12.0% | 8.6% | 0.125 |
| Any comorbidity | ||||||
| Yes | 77.6% | 85.2% | −0.195 | 81.7% | 80.8% | 0.025 |
| No | 22.1% | 14.8% | 0.189 | 18.2% | 19.2% | −0.027 |
| Missing | 0.2% | 0.0% | 0.070 | 0.1% | 0.0% | 0.028 |
| CCI b | ||||||
| 0 | 76.9% | 70.2% | 0.152 | 74.3% | 73.3% | 0.022 |
| 1‐2 | 15.5% | 22.6% | −0.182 | 19.4% | 20.3% | −0.024 |
| ≥3 | 7.4% | 7.2% | 0.008 | 6.3% | 6.4% | −0.004 |
| Missing | 0.2% | 0.0% | 0.070 | 0.1% | 0.0% | 0.028 |
| Health insurance | ||||||
| Not private | 88.0% | 89.2% | −0.038 | 88.5% | 88.9% | −0.012 |
| Private | 10.1% | 8.9% | 0.042 | 10.0% | 9.4% | 0.020 |
| Unknown to site | 2.0% | 2.0% | −0.001 | 1.5% | 1.7% | −0.014 |
| Localisation of tumour | ||||||
| Pancreas body | 18.7% | 23.4% | −0.115 | 22.2% | 21.3% | 0.020 |
| Pancreas head | 50.4% | 49.8% | 0.012 | 48.9% | 49.9% | −0.020 |
| Pancreas tail | 25.6% | 20.5% | 0.121 | 23.2% | 21.8% | 0.033 |
| Unknown to site | 5.4% | 6.4% | −0.043 | 5.7% | 6.9% | −0.050 |
| Tumour staging at diagnosis | ||||||
| I/II | 3.4% | 4.6% | −0.058 | 3.6% | 4.1% | −0.022 |
| III | 5.4% | 4.9% | 0.024 | 4.2% | 5.2% | −0.046 |
| IV | 76.2% | 74.4% | 0.042 | 79.5% | 74.7% | 0.112 |
| Unknown | 15.0% | 16.2% | −0.033 | 12.7% | 16.1% | −0.093 |
| Tumour grading at diagnosis | ||||||
| G1 | 1.2% | 1.7% | −0.038 | 0.9% | 1.4% | −0.043 |
| G2 | 18.2% | 21.8% | −0.091 | 22.0% | 20.2% | 0.044 |
| G3 | 20.6% | 18.3% | 0.059 | 19.4% | 18.5% | 0.022 |
| G4 | 1.2% | 1.8% | −0.049 | 1.7% | 1.9% | −0.019 |
| GX | 55.5% | 55.4% | 0.002 | 54.4% | 57.0% | −0.052 |
| Missing | 3.2% | 0.9% | 0.161 | 1.6% | 0.9% | 0.052 |
| Resection status of primary tumour | ||||||
| R0 | 2.7% | 2.6% | 0.007 | 3.1% | 3.1% | 0.003 |
| R1 | 2.0% | 1.2% | 0.059 | 1.3% | 1.4% | −0.006 |
| R2 | 0.0% | 0.3% | −0.078 | 0.0% | 0.2% | −0.048 |
| RX | 0.7% | 2.9% | −0.162 | 1.5% | 2.1% | −0.041 |
| No resection | 94.3% | 93.0% | 0.056 | 94.0% | 93.3% | 0.027 |
| Missing | 0.2% | 0.0% | 0.070 | 0.1% | 0.0% | 0.028 |
| Liver metastases | ||||||
| Yes | 59.5% | 58.3% | 0.023 | 57.6% | 57.2% | 0.008 |
| No | 24.6% | 28.7% | −0.094 | 28.0% | 28.9% | −0.020 |
| Missing | 16.0% | 13.0% | 0.085 | 14.4% | 13.9% | 0.015 |
| Lymphogenous metastases | ||||||
| Yes | 21.1% | 19.4% | 0.043 | 19.1% | 19.8% | −0.016 |
| No | 62.9% | 67.6% | −0.100 | 66.5% | 66.4% | 0.003 |
| Missing | 16.0% | 13.0% | 0.085 | 14.4% | 13.9% | 0.015 |
| Metastatic sites | ||||||
| 0 | 1.7% | 2.3% | −0.041 | 1.8% | 1.9% | −0.009 |
| 1 | 17.7% | 16.5% | 0.032 | 19.1% | 17.8% | 0.035 |
| 2 | 37.3% | 43.1% | −0.117 | 36.8% | 40.8% | −0.080 |
| ≥3 | 27.3% | 25.2% | 0.047 | 27.9% | 25.7% | 0.050 |
| Missing | 16.0% | 13.0% | 0.085 | 14.4% | 13.9% | 0.015 |
| Bilirubin | ||||||
| ≤1.5× ULN | 80.8% | 75.6% | 0.128 | 76.6% | 77.5% | −0.023 |
| 1.5‐3× ULN | 6.9% | 7.3% | −0.017 | 9.2% | 7.2% | 0.079 |
| >3× ULN | 2.7% | 4.7% | −0.107 | 4.5% | 3.9% | 0.033 |
| Unknown/missing | 9.6% | 12.4% | −0.089 | 9.7% | 11.4% | −0.055 |
| Haemoglobin <LLN | ||||||
| Yes | 9.8% | 9.0% | 0.028 | 8.1% | 8.7% | −0.022 |
| No | 86.7% | 88.5% | −0.055 | 89.2% | 87.9% | 0.038 |
| Missing/unknown | 3.4% | 2.4% | 0.059 | 2.8% | 3.4% | −0.036 |
| Thrombocytes >LLN | ||||||
| Yes | 52.6% | 54.8% | −0.045 | 51.5% | 52.2% | −0.015 |
| No | 43.7% | 42.6% | 0.023 | 45.6% | 44.3% | 0.027 |
| Missing/unknown | 3.7% | 2.6% | 0.063 | 2.9% | 3.5% | −0.033 |
| Leukocytes >ULN | ||||||
| Yes | 22.6% | 22.4% | 0.004 | 21.1% | 21.1% | −0.001 |
| No | 73.5% | 75.4% | −0.045 | 76.0% | 75.7% | 0.006 |
| Missing/unknown | 3.9% | 2.1% | 0.105 | 3.0% | 3.2% | −0.013 |
| Neutrophil‐to‐lymphocyte ratio | ||||||
| <4 | 47.4% | 47.3% | 0.002 | 46.7% | 46.0% | 0.013 |
| ≥4 | 39.3% | 38.2% | 0.023 | 40.3% | 38.4% | 0.039 |
| Missing | 13.3% | 14.5% | −0.036 | 13.0% | 15.6% | −0.074 |
Abbreviations: BMI, body mass index; CCI, Charlson comorbidity index; ECOG, Eastern Cooperative Oncology Group; GEMNAB, gemcitabine plus nab‐paclitaxel; LLN, lower limit of the norm; PS, propensity score; SMD, standardised mean difference; ULN, upper limit of the norm.
For the IPTW cohort, n refers to sum of weights; in order to increase readability, we have refrained from presenting the patient numbers (n) for the given characteristics.
Charlson comorbidity index (CCI) according to Quan et al. 46
3.3. Overall survival according to prognostic risk after IPTW
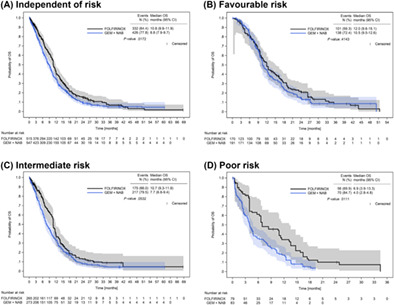
The Kaplan‐Meier probability curves for OS after IPTW are shown in Figure 3A to D. IPTW‐weighted median OS for favourable‐risk patients was quite similar in both treatment groups (12.0 months [95% CI: 9.6‐15.1] with FOLFIRINOX vs 10.5 months [95% CI: 9.5‐12.6] with GEMNAB, Figure 3B), while in the poor‐risk group, median OS with FOLFIRINOX was higher than that with GEMNAB (6.9 months [95% CI: 3.9‐13.3] vs 4.0 months [95% CI: 2.8‐4.8], Figure 3D). A trend for a better median OS in patients receiving FOLFIRINOX was already seen in the intermediate‐risk group (Figure 3C).
FIGURE 3.
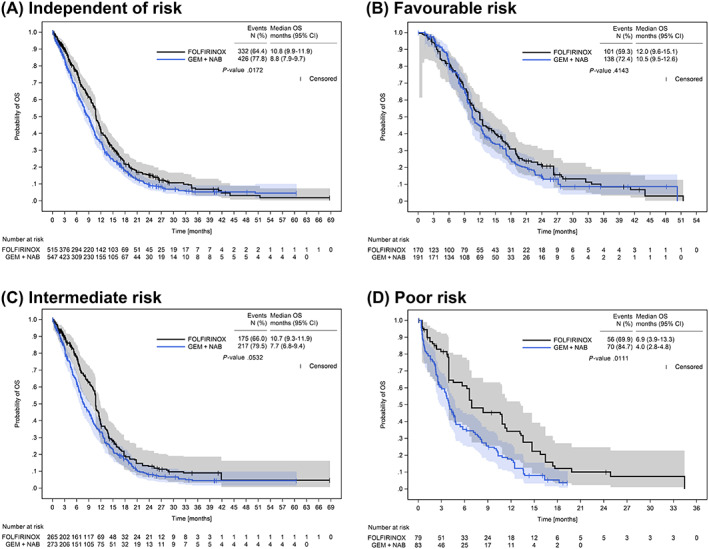
Overall survival after IPTW. OS after IPTW in patients receiving first‐line treatment with either FOLFIRINOX or GEMNAB independent of prognostic risk (A), by favourable risk (B), intermediate risk (C) and poor risk (D) according to the PCS. Numbers at risk refer to the sum of weights of the respective patients at risk for a given time point. Due to rounding, the weights of the three risk groups do not exactly add up to the sum of weights calculated for the FOLFIRINOX‐ and the GEMNAB‐IPTW cohorts, as shown in (A). P‐values were calculated with the log‐rank test. CI, confidence interval; GEMNAB, gemcitabine plus nab‐paclitaxel; OS, overall survival; PCS, Pancreatic Cancer Score
3.4. Quality of life
For HRQoL, the Kaplan‐Meier probability curves for TTD after IPTW are shown in Figure 4A‐D. While for patients with favourable risk, median TTD hardly differed by the type of treatment (7.1 months [95% CI: 4.0‐8.7] with FOLFIRINOX vs 6.1 months [95% CI: 4.7‐8.3] with GEMNAB, Figure 4B), for poor‐risk patients, median TTD of overall QoL was more than twice as long with FOLFRINOX as with GEMNAB (10.6 months [95% CI: 2.0‐14.1] vs 4.1 months [95% CI: 2.4‐4.5], Figure 4D). A trend for a longer median TTD of overall QoL in patients receiving FOLFIRINOX was already seen in the intermediate‐risk group (Figure 4C). For the functional scales of the EORTC QLQ‐C15‐PAL, there was a similar trend of longer median TTD in poor‐risk patients receiving FOLFIRINOX (data not shown). Due to low numbers of events, no conclusions can be drawn for the remaining scales.
FIGURE 4.
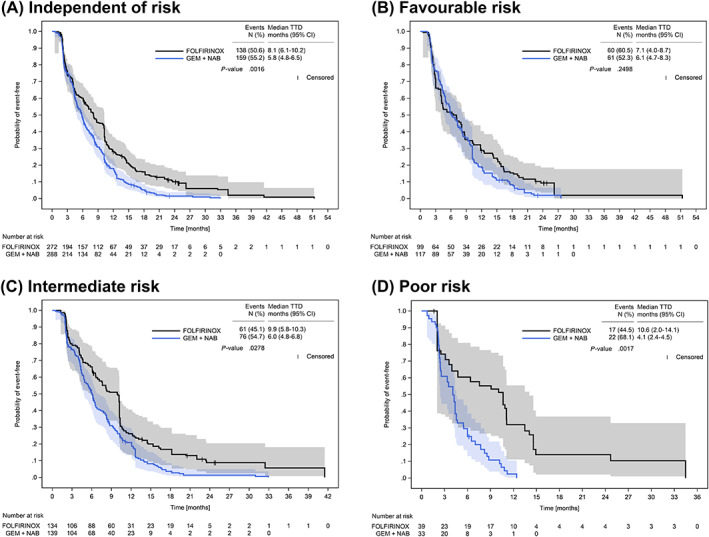
Time to deterioration of overall quality of life after IPTW. TTD of overall QoL (global health status) after IPTW in patients receiving first‐line treatment with either FOLFIRINOX or GEMNAB independent of prognostic risk (A), by favourable risk (B), intermediate risk (C) and poor risk (D) according to the PCS. Numbers at risk refer to the sum of weights of the respective patients at risk for a given time point. Due to rounding, the weights of the three risk groups do not exactly add up to the sum of weights calculated for the FOLFIRINOX‐ and the GEMNAB‐IPTW cohorts, as shown in (A). P‐values were calculated with the log‐rank test. CI, confidence interval; GEMNAB, gemcitabine plus nab‐paclitaxel; PCS, Pancreatic Cancer Score; QoL, quality of life; TTD, time to deterioration
4. DISCUSSION
In patients with advanced pancreatic cancer, FOLFIRINOX and GEMNAB are first‐line standard of care, however, there are no phase III randomised trials comparing the efficacy of both regimens. To the best of our knowledge, this is the first comparative effectiveness analysis of FOLFIRINOX and GEMNAB in real‐world patients with LAPC/MPC according to patients' prognostic risk, after adjustment for baseline imbalances between the two groups.
While several prognostic and predictive factors have been studied over the last decade, in clinical practice, there are neither clear factors, nor prospectively validated prognostic risk scores from large cohort studies that could help choosing the best treatment for the individual pancreatic cancer patient. 17 , 18 In a smaller retrospective study on 123 relatively nonselected patients with LAPC/MPC, a prognostic score combining BMI >25 and carcinoembryonic antigen (CEA) elevation was developed to identify patients with the highest benefit from aggressive chemotherapy. 17 Inclusion, however, was limited to patients receiving FOLFIRINOX. By combining nine independent prognostic features, which are routinely available prior to start of treatment and as long as whole genome sequencing of pancreatic cancer is not yet standard of care, our new PCS provides an easy‐applicable and comprehensive tool that efficiently separates patients into three survival risk groups, regardless of the type of first‐line treatment. All factors, that is, patient, tumour and clinical characteristics, have previously been published to be prognostically relevant in (advanced) pancreatic cancer: female sex, 28 BMI, 17 ECOG, 29 , 30 CCI, 31 tumour stage, 32 , 33 presence of liver metastases, 30 , 34 bilirubin level, 30 leukocyte/white blood cell count 35 and NLR. 29 , 36
With 70 years in median, the age of our original patient population was representative of that of the average pancreatic cancer cohort, as most patients are a median of 71 years old at diagnosis. 1 In addition, the median age of patients receiving FOLFIRINOX (61 years) and GEMNAB (71 years), respectively, is in line with the age ranges reported for FOLFIRINOX (59‐64 years) 11 , 12 , 13 , 17 , 37 , 38 , 39 and GEMNAB (68‐71 years). 11 , 12 , 13 , 37 , 38 , 39 Patients receiving FOLFIRINOX more often presented with ECOG 0, whereas patients with GEMNAB were more likely to be ECOG 2, which agrees with other real‐word data. 11 , 13 , 37 Hence, our results confirm the observation that patients receiving FOLFIRINOX are rather younger and fitter. 1 , 14 This is crucial to withstand the toxicities associated with FOLFIRINOX 37 and corresponds to guideline recommendations, 6 , 9 , 10 , 40 but also fits to physicians' preferences in clinical routine. 13 , 19 , 41 To overcome these imbalances between treatment groups that could result in an overestimation of the effect for FOLFIRINOX, we used IPTW based on a PS approach. The PS method is a robust alternative for the analysis of nonrandomised intervention trials, with advantages over conventional regression modelling. 42
Overall effectiveness of FOLFIRINOX and GEMNAB shown by our real‐world data was largely comparable, with a slightly longer IPTW‐weighted median OS observed for FOLFIRINOX (10.8 vs 8.8 months). Outcome results closely reflect those of the pivotal phase III PRODIGE 4 and MPACT trials reporting a median OS of 11.1 months for FOLFIRINOX 6 and 8.7 months for GEMNAB, 7 , 8 respectively, even though inclusion was limited to highly selected patients with MPC. Our findings also broadly agree with previous retrospective data: a systemic review of retrospective studies comparing first‐line GEMNAB and FOLFIRINOX in MPC or LAPC revealed a slightly longer median OS for FOLFIRINOX (15.9 vs 14.4 months), but the differences, when available, were not statistically significant. 14 In most of these studies, however, differences in patient characteristics may also have confounded results. There are two comparative studies including patients with both LAPC and MPC which used a PS approach by IPTW as well 11 , 13 : with a IPTW‐weighted median OS of 10.1 months for both treatment groups, effectiveness of FOLFIRINOX and GEMNAB was equal in a study on 455 patients (158 FOLFIRINOX, 297 GEMNAB) treated at three academic centres in Austria. 13 In a Canadian study including 1130 patients (632 FOLFIRINOX, 498 GEMNAB) from six cancer centres in British Columbia, crude median OS was 9.6 and 6.1 months for FOLFIRINOX and GEMNAB, respectively (HR 0.60; 95% CI: 0.53‐0.69); after IPTW, OS was significantly improved for patients treated with FOLFIRINOX vs GEMNAB (HR 0.77; 95% CI: 0.70‐0.85). 11
As far as we know, this is the first comparative analysis evaluating effectiveness according to patients' prognostic risk. Poor‐risk patients as defined by the PCS appear to benefit from aggressive first‐line chemotherapy with FOLFIRINOX not only by significantly prolonged survival (median OS of 6.9 vs 4.0 months), but also by better overall HRQoL (median TTD of 10.6 vs 4.1 months). Of note, some phase II/III randomised trials are ongoing to compare the efficacy of modified FOLFIRINOX vs GEMNAB in advanced pancreatic cancer. 43 , 44 Although not considering patients' prognostic risk, the findings of these trials will be crucial to determine whether our real‐world results can be confirmed. Although the safety of FOLFIRINOX was less favourable than gemcitabine in the phase III PRODIGE 4/ACCORD 11 trial, FOLFIRINOX reduced QoL impairment compared to gemcitabine. 45 Since survival can only be prolonged to a limited extent in advanced pancreatic cancer, main goals should focus on alleviation of tumour symptoms and stabilisation of QoL. The identification of patients who will benefit most from either treatment, together with patient's preferences should be the basis for treatment decisions, which may be supported by the PCS.
4.1. Limitations
A limitation of this analysis is that only those variables were considered as potential prognostic factors which had been documented in the TPK. Furthermore, using weighted prognostic factors could have improved the precision of the PCS, but would have made the score less easy‐to‐use. Given that HRs did not greatly differ between prognostic factors, the approach of an unweighted score seems appropriate. A further limitation is that validation was performed on a second data set from the same data source and not on a truly independent, external data source. Thus, an additional external validation of our results would further strengthen the generalisability of the PCS. Since the number of poor‐risk patients is relatively low, the interpretation of results might be hampered. However, these limitations should be weighed against the strengths of this work which include the prospective, longitudinal design and the analysis of data from a large real‐world sample. Combining risk stratification and IPTW to account for imbalances between treatment groups is a major advantage of this work. The inclusion of patients with LAPC is another strength and adds further insights into the effectiveness of FOLFIRINOX and GEMNAB beyond MPC.
5. CONCLUSIONS
We propose the new PCS combining nine independent prognostic factors, which are usually available prior to start of first‐line treatment in patients with LAPC or MPC. The PCS can be of great help in predicting the prognosis of patients with advanced pancreatic cancer and in treatment decision‐making. It represents an easy applicable, comprehensive tool, not only for routine clinical use, but possibly also for risk stratification in clinical trials. Assuming balanced treatment groups, our real‐world data suggest no general advantage from first‐line FOLFIRINOX over GEMNAB. Only poor‐risk patients seem to benefit from FOLFIRINOX, whereas for patients with favourable risk, OS and overall QoL appear to be comparable in both treatment groups. Our data warrant further assessment of this newly defined risk scores in future randomised trials.
AUTHOR CONTRIBUTIONS
Norbert Marschner: Conceptualisation, funding acquisition, investigation, resources, supervision, writing—original draft. Susanna Hegewisch‐Becker: Conceptualisation, investigation, resources, writing—review & editing. Marcel Reiser: Investigation, resources, writing—review & editing. Eyck von der Heyde: Investigation, resources, writing—review & editing. Mathias Bertram: Investigation, resources, writing—review & editing. Stephan H. Hollerbach: Investigation, resources, writing—review & editing. Stephan Kreher: Investigation, resources, writing—review & editing. Thomas Wolf: Investigation, resources, writing—review & editing. Adrian Binninger: Conceptualisation, data curation, methodology, project administration, visualisation, writing—original draft. Marco Chiabudini: Conceptualisation, data curation, formal analysis, methodology, validation, writing—original draft. Anja Kaiser‐Osterhues: Conceptualisation, methodology, visualisation, writing—original draft. Martina Jänicke: Conceptualisation, methodology, supervision, visualisation, writing—original draft. The work reported in the article has been performed by the authors, unless clearly specified in the text.
FUNDING INFORMATION
The TPK and its satellite projects are designed, managed and analysed by iOMEDICO and have received financial support from Baxter Deutschland GmbH, Celgene GmbH and Servier Deutschland GmbH. None of the funding companies had any role in study design, data collection and analysis, interpretation of results or decision to publish.
CONFLICT OF INTEREST
Marcel Reiser, Eyck von der Heyde, Mathias Bertram, Stephan H. Hollerbach, Stephan Kreher, Thomas Wolf, Adrian Binninger, Marco Chiabudini, Anja Kaiser‐Osterhues and Martina Jänicke declare no conflict of interest concerning the topic of this publication. Outside of the published work, the institutions of Susanna Hegewisch‐Becker, Marcel Reiser, Eyck von der Heyde, Mathias Bertram, Stephan H. Hollerbach, Stephan Kreher and Thomas Wolf received remuneration for the documentation of patient data. Norbert Marschner reported receiving honoraria for talks and attendance of conferences from Celgene and Servier; he is Chief executive officer and shareholder of iOMEDICO AG. Susanna Hegewisch‐Becker reported a consulting or advisory role for Servier.
ETHICS STATEMENT
The TPK/AMETHYST was approved by the responsible ethics committee and is registered at ClinicalTrials.gov (NCT02089269). Written informed consent was obtained from all patients.
Supporting information
Appendix S1 Supporting Information.
ACKNOWLEDGEMENTS
The authors thank all patients, physicians and study teams participating in the TPK. We thank Dr David Hamm (iOMEDICO) for support and comments during design and set‐up of the project and Dr Karin Potthoff (iOMEDICO) for critical comments on the article. The authors thank Dr Stephanie Dille (iOMEDICO) for support in the preparation of this article.
Marschner N, Hegewisch‐Becker S, Reiser M, et al. FOLFIRINOX or gemcitabine/nab‐paclitaxel in advanced pancreatic adenocarcinoma: A novel validated prognostic score to facilitate treatment decision‐making in real‐world. Int J Cancer. 2023;152(3):458‐469. doi: 10.1002/ijc.34271
Funding information Servier Deutschland GmbH; Celgene GmbH; Baxter Deutschland GmbH
DATA AVAILABILITY STATEMENT
The data that support the findings of our study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008‐2020. [DOI] [PubMed] [Google Scholar]
- 2. Robert Koch‐Institut . Gesellschaft der Epidemiologischen Krebsregister in Deutschland e.V. Krebs in Deutschland 2017/2018. Häufigkeiten und Trends [Internet]. 13. Ausgabe. Berlin: Robert Koch‐Institut. https://www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/krebs_in_deutschland_node.html. Accessed December 2, 2021.
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7‐30. [DOI] [PubMed] [Google Scholar]
- 4. National Cancer Institute . SEER Cancer Statistics Review, 1975‐2018 [Internet]; 2021. https://seer.cancer.gov/csr/1975_2018/sections.html. Accessed December 2, 2021.
- 5. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 6. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817‐1825. [DOI] [PubMed] [Google Scholar]
- 7. Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab‐paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691‐1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goldstein D, El‐Maraghi RH, Hammel P, et al. nab‐paclitaxel plus gemcitabine for metastatic pancreatic cancer: long‐term survival from a phase III trial. J Natl Cancer Inst. 2015;107:dju413. [DOI] [PubMed] [Google Scholar]
- 9. Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2015;26(Suppl 5):v56‐v68. [DOI] [PubMed] [Google Scholar]
- 10. Martín AM, Hidalgo M, Alvarez R, et al. From first line to sequential treatment in the management of metastatic pancreatic cancer. J Cancer. 2018;9:1978‐1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chan KKW, Guo H, Cheng S, et al. Real‐world outcomes of FOLFIRINOX vs gemcitabine and nab‐paclitaxel in advanced pancreatic cancer: a population‐based propensity score‐weighted analysis. Cancer Med. 2020;9:160‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williet N, Saint A, Pointet A‐L, et al. Folfirinox versus gemcitabine/nab‐paclitaxel as first‐line therapy in patients with metastatic pancreatic cancer: a comparative propensity score study. Therap Adv Gastroenterol. 2019;12:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Riedl JM, Posch F, Horvath L, et al. Gemcitabine/nab‐paclitaxel versus FOLFIRINOX for palliative first‐line treatment of advanced pancreatic cancer: a propensity score analysis. Eur J Cancer. 2021;151:3‐13. [DOI] [PubMed] [Google Scholar]
- 14. Chiorean EG, Cheung WY, Giordano G, Kim G, Al‐Batran S‐E. Real‐world comparative effectiveness of nab‐paclitaxel plus gemcitabine versus FOLFIRINOX in advanced pancreatic cancer: a systematic review. Ther Adv Med Oncol. 2019;11:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chun JW, Lee SH, Kim JS, et al. Comparison between FOLFIRINOX and gemcitabine plus nab‐paclitaxel including sequential treatment for metastatic pancreatic cancer: a propensity score matching approach. BMC Cancer. 2021;21:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pusceddu S, Ghidini M, Torchio M, et al. Comparative effectiveness of gemcitabine plus nab‐paclitaxel and FOLFIRINOX in the first‐line setting of metastatic pancreatic cancer: a systematic review and meta‐analysis. Cancer. 2019;11:E484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schlick K, Magnes T, Ratzinger L, et al. Novel models for prediction of benefit and toxicity with FOLFIRINOX treatment of pancreatic cancer using clinically available parameters. PLoS One. 2018;13:e0206688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dell'Aquila E, Fulgenzi CAM, Minelli A, et al. Prognostic and predictive factors in pancreatic cancer. Oncotarget. 2020;11:924‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hegewisch‐Becker S, Aldaoud A, Wolf T, et al. Results from the prospective German TPK clinical cohort study: treatment algorithms and survival of 1,174 patients with locally advanced, inoperable, or metastatic pancreatic ductal adenocarcinoma. Int J Cancer. 2019;144:981‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457‐481. [Google Scholar]
- 21. Heinze G, Wallisch C, Dunkler D. Variable selection: a review and recommendations for the practicing statistician. Biom J. 2018;60:431‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marschner N, Frank M, Vach W, et al. Development and validation of a novel prognostic score to predict survival in patients with metastatic colorectal cancer: the metastatic colorectal cancer score (mCCS). Colorectal Dis. 2019;21:816‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083‐3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Austin PC. The use of propensity score methods with survival or time‐to‐event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Groenvold M, Petersen MA, Aaronson NK, et al. The development of the EORTC QLQ‐C15‐PAL: a shortened questionnaire for cancer patients in palliative care. Eur J Cancer. 2006;42:55‐64. [DOI] [PubMed] [Google Scholar]
- 26. Cocks K, King MT, Velikova G, Martyn St‐James M, Fayers PM, Brown JM. Evidence‐based guidelines for determination of sample size and interpretation of the European organisation for the research and treatment of cancer quality of life questionnaire Core 30. J Clin Oncol. 2011;29:89‐96. [DOI] [PubMed] [Google Scholar]
- 27. Maringwa JT, Quinten C, King M, et al. Minimal important differences for interpreting health‐related quality of life scores from the EORTC QLQ‐C30 in lung cancer patients participating in randomized controlled trials. Support Care Cancer. 2011;19:1753‐1760. [DOI] [PubMed] [Google Scholar]
- 28. Matsumoto I, Kamei K, Omae K, et al. FOLFIRINOX for locally advanced pancreatic cancer: results and prognostic factors of subset analysis from a nation‐wide multicenter observational study in Japan. Pancreatology. 2019;19:296‐301. [DOI] [PubMed] [Google Scholar]
- 29. Dogan M, Algin E, Guven ZT, et al. Neutrophil‐lymphocyte ratio, platelet‐lymphocyte ratio, neutrophil‐platelet score and prognostic nutritional index: do they have prognostic significance in metastatic pancreas cancer? Curr Med Res Opin. 2018;34:857‐863. [DOI] [PubMed] [Google Scholar]
- 30. Ter Veer E, van Rijssen LB, Besselink MG, et al. Consensus statement on mandatory measurements in pancreatic cancer trials (COMM‐PACT) for systemic treatment of unresectable disease. Lancet Oncol. 2018;19:e151‐e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakai Y, Isayama H, Sasaki T, et al. Comorbidity, not age, is prognostic in patients with advanced pancreatic cancer receiving gemcitabine‐based chemotherapy. Crit Rev Oncol Hematol. 2011;78:252‐259. [DOI] [PubMed] [Google Scholar]
- 32. Qi Q, Geng Y, Sun M, Wang P, Chen Z. Clinical implications of systemic inflammatory response markers as independent prognostic factors for advanced pancreatic cancer. Pancreatology. 2015;15:145‐150. [DOI] [PubMed] [Google Scholar]
- 33. Yu S‐L, Xu L‐T, Qi Q, et al. Serum lactate dehydrogenase predicts prognosis and correlates with systemic inflammatory response in patients with advanced pancreatic cancer after gemcitabine‐based chemotherapy. Sci Rep. 2017;7:45194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ploquin A, Truant S, Piessen G, et al. Locally advanced or metastatic pancreatic adenocarcinoma: easily available factors of predictive prolonged survival under gemcitabine. In Vivo. 2017;31:731‐735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Feng L, Gu S, Wang P, et al. White blood cell and granulocyte counts are independent predictive factors for prognosis of advanced pancreatic caner. Gastroenterol Res Pract. 2018;2018:8096234‐8096236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gargiulo P, Dietrich D, Herrmann R, et al. Predicting mortality and adverse events in patients with advanced pancreatic cancer treated with palliative gemcitabine‐based chemotherapy in a multicentre phase III randomized clinical trial: the APC‐SAKK risk scores. Ther Adv Med Oncol. 2019;11:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang Y, Camateros P, Cheung WY. A real‐world comparison of FOLFIRINOX, gemcitabine plus nab‐paclitaxel, and gemcitabine in advanced pancreatic cancers. J Gastrointest Cancer. 2019;50:62‐68. [DOI] [PubMed] [Google Scholar]
- 38. Cartwright TH, Parisi M, Espirito JL, et al. Clinical outcomes with first‐line chemotherapy in a large retrospective study of patients with metastatic pancreatic cancer treated in a US Community oncology setting. Drugs Real World Outcomes. 2018;5:149‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maeda O, Yokoyama Y, Yamaguchi J, et al. Real‐world experience with FOLFIRINOX and gemcitabine plus nab‐paclitaxel in the treatment of pancreatic cancer in Japan. Ann Oncol. 2017;28:x69. [Google Scholar]
- 40. Sohal DPS, Kennedy EB, Khorana A, et al. Metastatic pancreatic cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36:2545‐2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kim S, Signorovitch JE, Yang H, et al. Comparative effectiveness of nab‐paclitaxel plus gemcitabine vs FOLFIRINOX in metastatic pancreatic cancer: a retrospective nationwide chart review in the United States. Adv Ther. 2018;35:1564‐1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kuss O, Blettner M, Börgermann J. Propensity score: an alternative method of analyzing treatment effects. Dtsch Arzteblatt. 2016;113:597‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ohba A, Ozaka M, Mizusawa J, et al. Randomized multicenter phase II/III study of gemcitabine plus nab‐paclitaxel or modified FOLFIRINOX or S‐IROX in patients with metastatic or recurrent pancreatic cancer (JCOG1611, GENERATE). J Clin Oncol. 2022;40:TPS627. [Google Scholar]
- 44. Ozaka M, Ueno M, Ishii H, et al. Randomized phase II study of modified FOLFIRINOX versus gemcitabine plus nab‐paclitaxel combination therapy for locally advanced pancreatic cancer (JCOG1407). J Clin Oncol. 2021;39:4017. [Google Scholar]
- 45. Gourgou‐Bourgade S, Bascoul‐Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol. 2013;31:23‐29. [DOI] [PubMed] [Google Scholar]
- 46. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676‐682. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information.
Data Availability Statement
The data that support the findings of our study are available from the corresponding author upon reasonable request.


