Abstract
Background
Anaphylaxis is the most acute and life‐threatening manifestation of allergic disorders. Currently, there is a need to improve its medical management and increase the understanding of its molecular mechanisms. This study aimed to quantify the extravasation underlying human anaphylactic reactions and propose new theragnostic approaches.
Methods
Molecular determinations were performed in paired serum samples obtained during the acute phase and at baseline from patients presenting with hypersensitivity reactions. These were classified according to their severity as Grades 1, 2 and 3, the two latter being considered anaphylaxis. Tryptase levels were measured by ImmunoCAP, and serum protein concentration was quantified by Bradford assay. Human serum albumin (HSA) and haemoglobin beta subunit (HBB) levels were determined by Western blot and polyacrylamide gel electrophoresis, respectively.
Results
A total of 150 patients were included in the study. Of them, 112 had experienced anaphylaxis (83 and 29 with Grade 2 and 3 reactions, respectively). Tryptase diagnostic efficiency substantially improved when considering patients' baseline values (33%–54%) instead of the acute value threshold (21%). Serum protein concentration and HSA significantly decreased in anaphylaxis (p < .0001). HSA levels dropped with the severity of the reaction (6% and 15% for Grade 2 and 3 reactions, respectively). Furthermore, HBB levels increased during the acute phase of all hypersensitivity reactions (p < .0001).
Conclusions
For the first time, the extravasation underlying human anaphylaxis has been evaluated based on the severity of the reaction using HSA and protein concentration measurements. Additionally, our findings propose new diagnostic and potential therapeutic approaches for this pathological event.
Keywords: anaphylaxis, haemoglobin, human serum albumin, protein concentration, tryptase
This study aimed to assess the extravasation underlying anaphylaxis reactions and propose new theragnostic approaches. Acute and baseline serum samples were obtained from 150 patients with hypersensitivity reactions. The diagnostic capacity of tryptase improved with thresholds considering the patients baseline status. Protein and HSA levels decreased in anaphylaxis reactions according to their severity. HBB levels increased in all hypersensitivity reactions.Abbreviations: HBB, haemoglobin subunit β; HSA, human serum albumin
Abbreviations
- HBB
haemoglobin subunit β
- HSA
human serum albumin
1. INTRODUCTION
Anaphylaxis is the most severe acute manifestation of allergic disorders, being a life‐threatening systemic hypersensitivity reaction that develops rapidly. 1 Currently, 50–112 cases per 100,000 inhabitants are registered per year, but its incidence seems to be underestimated. 2 , 3
Ongoing criteria for diagnosing anaphylaxis are clinical. However, the plethora of clinical features associated with this pathological event makes it difficult to achieve a consensus on its definition, impairing our ability to adequately manage these severe reactions. 4 Different phenotypes and underlying endotypes have been described in anaphylaxis according to a few biomarkers, 5 being serum tryptase the most widely used to complement the diagnosis of this pathological event. 6 , 7
In recent years, the upper normality reference threshold concentration for serum tryptase has decreased from 15 μg/L to 11.4 μg/L. 7 However, this biomarker has several drawbacks since it is not elevated in all cases, but mainly in most severe reactions, where clinical diagnosis is undoubtful. 6 Therefore, new diagnostic criteria have been proposed. For instance, an increase in tryptase levels of 20% plus 2 μg/L from baseline to the acute phase is used to diagnose mast cell activation syndrome. 8 In addition, the study of Borer‐Reinhold et al. established an increase above 135% of acute serum tryptase baseline values to indicate cell mast activation in patients with anaphylaxis to Hymenoptera venoms. 9
Mechanistically, different cellular and molecular pathways result in the release of several mediators leading to anaphylactic manifestations that affect various organs and systems. 10 Cutaneous symptoms are the most frequent, but respiratory and circulatory events are of utmost relevance in severe reactions. 11 Cardiovascular events, such as increased vascular permeability, extravasation of fluids, vasodilatation and hypotension, are central in the pathophysiology of anaphylaxis. 12 Among them, endothelial permeability has been associated with cutaneous, respiratory or gastrointestinal symptoms when angioedema, emphysema, diarrhoea or other manifestations appear. 13
Specifically, the opening of the endothelial barrier occurs during the first seconds of the reaction, causing leakage of vascular contents into the tissues. After this time, the endothelium closes, restoring its normal state. 14 When this occurs, according to Starling's principle, the oncotic pressure continues to be higher than the tissue pressure, so the fluid can return to the vessels by diffusion. However, as the endothelium has been closed, most proteins cannot pass through it and stay in the extravascular space. 15 , 16 Consequently, there is a reduction in serum concentration. Among the extravasated proteins, human serum albumin (HSA), the major serum protein, only re‐enters into the circulatory system via the lymphatic vessels so remains longer in the tissular compartment. 16 Therefore, measurements of protein concentration and HSA levels could be used to indirectly quantify the extravasation underlying anaphylaxis. Precisely, both determinations have been proposed as molecular markers for several pathological conditions. 17 , 18 , 19 , 20
However, most findings on the leakage of vascular fluids associated with anaphylaxis have been obtained from animal models and barely in the context of patients. 10 In particular, a study based in haemoglobin measurements demonstrated that a transfer of up to 35% of plasma to the interstitial compartment can occur in the first 10 min of the reaction in patients with anaphylaxis. 21 Haemoglobin is a tetrameric protein, composed of two alpha subunits and two beta subunits (HBB), and located within red blood cells to execute the oxygen transport. 22 Therefore, its elevation in serum is a result of haemolysis, which can be induced by several factors such as the actions of complement and coagulation. 23 Both processes are highly correlated with anaphylaxis and are particularly activated in most severe cases. 24 , 25
This study aimed to assess the extravasation underlying human anaphylaxis according to the severity of the reaction and the systems affected, as well as to evaluate different serum tryptase thresholds and propose new diagnostic tools and potential therapeutic approaches for the clinical management of anaphylaxis.
2. MATERIALS AND METHODS
2.1. Study design and patients
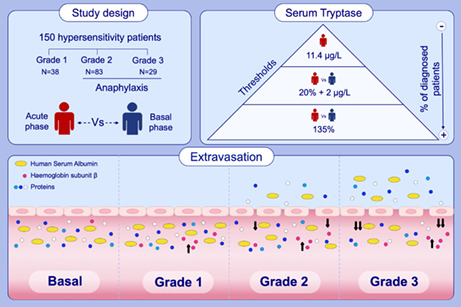
This was an observational study in patients with hypersensitivity reactions who attended seven Spanish hospitals (Fundación Jiménez Díaz University Hospital, Cruz Roja Central Hospital, Niño Jesús University Children's Hospital, Navarre University Clinic, Ramón y Cajal Hospital, Reina Sofía of Córdoba Hospital and Guadalajara's University Hospital) between June 2016 and June 2021.
All patients were followed up in the clinical setting according to medical practice. An allergy work‐up was performed as needed, and the diagnosis was confirmed by an allergist. Patients' management and treatment administration were carried out according to clinical guidelines.
Severity criteria were applied based on clinical symptoms and Brown's classification system. 26 Consequently, reactions were categorized as mild (Grade 1), moderate (Grade 2) or severe (Grade 3). However, only Grade 2 and Grade 3 events were considered anaphylaxis, according to the criteria established by the National Institute of Allergy and Infectious Disease and the Food Allergy and Anaphylaxis Network. 27
All study participants signed the corresponding informed consent before their inclusion. The study was performed in accordance with the parameters established by the Helsinki Declaration. The protocol was approved by the Ethics Committee (CEIm FJD, PIC057‐19).
2.2. Serum sample collection
For all patients, serum was collected in the acute phase of the hypersensitivity episode and at baseline (at least 14 days after the reaction). Acute samples were obtained in the emergency departments when allergen exposure was accidental and at the allergy units if the hypersensitive reaction was observed after a controlled challenge test. Considering the heterogeneity of patients, a baseline phase was used as control for each corresponding acute sample.
2.3. Serum tryptase determinations
Tryptase levels were measured from serum samples at the Clinical Analysis Service of the Fundación Jiménez Díaz Hospital using ImmunoCAP Phadia 1000 (Thermo Scientific).
2.4. Quantification of serum protein concentrations
Protein concentrations were assessed by Bradford colorimetric assay. 28 For this purpose, a standard curve and previously diluted (1:200) sera from both phases of the reaction were added in duplicate to a plate with the reagent Coomassie Plus (Thermo Scientific). The absorbance at 595 nm of all standards and samples was measured with an Infinite F200 spectrophotometer (Tecan).
2.5. Serum protein profile evaluation
Serum proteins were separated by electrophoresis in polyacrylamide gels (SDS‐PAGE) at 12.5% or 15% concentration. In all cases, acute and baseline samples from each patient were diluted and run together on the same gel under identical conditions. The protein's molecular weight was determined by the addition of a commercial pre‐stained marker (Thermo Scientific). Furthermore, the protein profile was stained and visualized using the reagent Coomassie Brilliant Blue G‐250 Dye (ICN Biomedicals, Inc.). Finally, gels were scanned using the Amersham Imager 600 system (GE Healthcare).
2.6. Human serum albumin measurements
Proteins from patients' serum were separated by SDS‐PAGE, as previously described, and transferred by the Semi‐Dry method to nitrocellulose membranes (Bio‐Rad). Then, HSA detection was performed by incubating the membrane with an anti‐HSA primary antibody, which was produced following the technique described by Albar et al. in 1981. 29 Subsequently, an appropriate goat anti‐rabbit HRP‐conjugated secondary antibody (Jackson Laboratory) was added. Finally, proteins were visualized using the chemiluminescent ECL Prime Western Blotting Detection Reagent (Thermo Scientific) and the Amersham Imager 600 system (GE Healthcare). The quantification of the bands was carried out with the ImageJ software. 30
2.7. Protein identification
Protein identification and characterization were performed after their separation by SDS‐PAGE (15%). Protein bands were stained with PageBlue Protein Staining Solution (Fermentas International), and spots of interest were cut out from the gels with a sterile scalpel and sent for analysis to the Proteomic Service of the Complutense University of Madrid (UCM, Spain). There, the samples were reduced, alkylated and digested with trypsin, according to Sechi 2002. 31 Afterwards, the supernatant was collected and placed on a matrix‐assisted laser desorption/ionization (MALDI) plate with a matrix of 3 mg/ml of α‐cyano‐4‐hydroxycinnamic acid (Sigma) in 50% acetonitrile. Analyses were performed in a 4800 Plus Proteomics Analyzer MALDI‐TOF/TOF mass spectrometer (Applied Biosystems) operated in positive reflector mode with an accelerating voltage of 20,000 V. In addition, all mass spectra were calibrated internally using peptides from the auto digestion of trypsin. For protein identification, the SwissProt database was searched with the Human taxonomy restriction (20,370 sequences; 11,359,082 residues) using MASCOT 2.6 (http://www.matrixscience.com) via the Global Protein Server v3.6 software (ABSciex).
2.8. Haemoglobin subunit beta determination
HBB levels were determined from 1 μl of patients' serum at baseline and during the acute phase, which were run together on the same gel under identical conditions. Proteins were separated by SDS‐PAGE at 15% concentration and stained with Coomassie Brilliant Blue G‐250 Dye (ICN Biomedicals, Inc.), as previously described. The resulting gels were scanned on the Amersham Imager 600 system (GE Healthcare), and the protein bands were quantified using the ImageJ software. 30
2.9. Statistical analysis
Categorical variables were described as the frequency and percentage, whereas continuous data were presented as the median and the interquartile range. All analyses and graphics were performed using Graph Pad Prism 8 software.
Non‐parametric tests were used in all cases because data did not follow a normal distribution. To determine the significant differences between the acute and baseline phases in each group, a two‐tailed Wilcoxon matched pairs signed rank test was performed. However, for comparison between groups, data were normalized for each patient by attributing the value of 1 to baseline and a proportional increase or decrease to the acute phase (acute/basal ratio), and unpaired tests were used. To evaluate the significant differences between anaphylaxis and Grade 1 conditions, a two‐tailed Mann–Whitney test was applied. Furthermore, when three or more groups were compared, the Dunn's Kruskal–Wallis multiple comparison test was performed. In addition, a two‐tailed Spearman correlation was used to assess the linear relationship of the different variables. The threshold of statistical significance was established at p < .05.
3. RESULTS
3.1. Characteristics of patients and hypersensitivity reactions
The characteristics of patients and their hypersensitivity reactions are summarized in Table 1. Our study included 150 patients aged between 5 and 81 years old; more than half were females. Regarding hypersensitivity reactions, the most frequent trigger was drugs, and approximately three out of four were classified as anaphylaxis (Grade 2 and Grade 3 events). Grade 1 reactions were only triggered by drugs and involved cutaneous and mucosal manifestations. In contrast, in anaphylactic reactions, the most common symptoms were cutaneous and respiratory, followed by digestive and/or mucosal; cardiovascular and nervous symptoms were the least frequent. However, together with cutaneous symptoms, cardiovascular manifestations were the most frequent in Grade 3 reactions. Treatment was administered prior to obtaining the acute sample in more than half of patients for the sake of their safety. In these cases, antihistamines and corticosteroids were the drugs used in the major number of patients, followed by epinephrine and β2‐adrenergic agonist.
TABLE 1.
Characteristics of patients and their hypersensitivity reactions
| Total | Grade 1 | Grade 2 | Grade 3 | Anaphylaxis a | p‐Value b | |
|---|---|---|---|---|---|---|
| Number | 150 | 38 | 83 | 29 | 112 | – |
| Gender (female) | 61% | 68% | 59% | 58% | 62% | .3394 |
| Age (years), mean (SD) | 39.5 ± 18.3 | 46.0 ± 18.8 | 34.4 ± 16.9 | 42.8 ± 18.8 | 37.3 ± 17.6 | .0382* |
| Atopy | 49% | 29% | 58% | 48% | 55% | .0083* |
| Basal Allergies | 46% | 29% | 58% | 48% | 55% | .0083* |
| Asthma | 27% | 13% | 35% | 21% | 31% | .0339* |
| Rhinitis | 37% | 21% | 47% | 31% | 43% | .0197* |
| Atopic dermatitis | 6% | 0% | 6% | 14% | 8% | .1157 |
| Trigger | ||||||
| Drug | 66% | 100% | 52% | 62% | 55% | <.0001* |
| AINES | 45% | 50% | 47% | 28% | 41% | .3504 |
| Antibiotics | 37% | 42% | 34% | 33% | 34% | .4348 |
| Contrasts | 5% | 3% | 5% | 11% | 7% | .4496 |
| Chemotherapies | 7% | 0% | 7% | 22% | 11% | .0374* |
| Others c | 6% | 5% | 7% | 6% | 7% | .7387 |
| Food | 29% | 0% | 40% | 35% | 38% | <.0001* |
| Nuts | 23% | 0% | 21% | 30% | 23% | .0008* |
| Fish/Seafood | 23% | 0% | 25% | 20% | 23% | .0008* |
| Fruits | 14% | 0% | 15% | 10% | 14% | .0122* |
| Milk | 19% | 0% | 21% | 10% | 19% | .0013* |
| Egg | 14% | 0% | 12% | 20% | 14% | .0122* |
| Others d | 7% | 0% | 6% | 10% | 7% | .1921 |
| Others e | 5% | 0% | 8% | 3% | 7% | .1921 |
| Symptoms | ||||||
| Cutaneous | 89% | 92% | 87% | 90% | 88% | .5698 |
| Mucosal | 49% | 42% | 53% | 45% | 51% | .4528 |
| Digestive | 37% | 0% | 49% | 52% | 50% | <.0001* |
| Respiratory | 64% | 0% | 88% | 79% | 86% | <.0001* |
| Neurological | 19% | 0% | 19% | 41% | 25% | .0004* |
| Cardiovascular | 23% | 0% | 11% | 86% | 30% | <.0001* |
| Treatment f | 62% | 24% | 75% | 73% | 79% | <.0001* |
| Epinephrine | 61% | 11% | 61% | 83% | 67% | <.0001* |
| H1R antagonist | 81% | 100% | 79% | 78% | 79% | .0013* |
| H2R antagonist | 18% | 11% | 11% | 39% | 19% | .3173 |
| Corticosteroids | 80% | 100% | 75% | 83% | 77% | .0008* |
| β2‐adrenergic agonist | 17% | 0% | 20% | 17% | 19% | .0051* |
Abbreviations: AINES, non‐steroidal anti‐inflammatory drugs; H1R, histamine type 1 receptor; H2R, histamine type 2 receptor; SD, standard deviation.
Patients with Grade 2 and Grade 3 reactions.
Statistical differences between patients with Grade 1 events and those with anaphylaxis (*p value < .05).
Anaphylaxis induced by analgesics, anaesthetics, proton‐pump inhibitors or angiotensin‐converting‐enzyme inhibitors.
Anaphylaxis induced by mustard and honey.
Anaphylaxis induced by vaccines, insect stings or idiopathic.
Percentage of patients treated prior to acute sample collection in each group and treatments administered. The remaining patients received treatment after obtaining the sample.
3.2. Tryptase concentrations in anaphylaxis
First, serum tryptase was measured in the study population to determine its effectiveness as the main biomarker of anaphylaxis. In Grade 1 reactions, tryptase concentrations did not increase from baseline to the acute phase (Figure 1A). Conversely, in Grade 2 and Grade 3 anaphylactic reactions, tryptase levels showed a statistically significant increase during the acute phase (Figure 1B).
FIGURE 1.
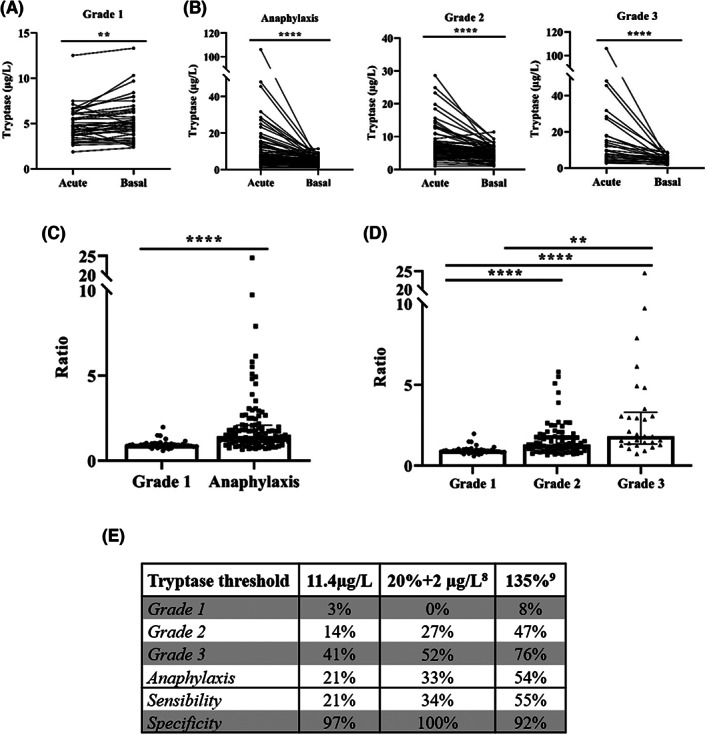
Baseline serum tryptase levels enrich the diagnosis of anaphylaxis. (A) Comparison of serum tryptase concentrations between the acute phase and at baseline in Grade 1 reactions (**p = .0029). (B) Serum tryptase concentrations in total anaphylactic samples and stratified by Grade 2 and Grade 3 reactions (****p < .0001). (C) Comparison of acute/basal ratios of serum tryptase concentrations between Grade 1 reactions and patients with anaphylaxis (****p < .0001). (D) Differences in acute/basal ratios of serum tryptase concentrations between Grade 1, Grade 2 (**p = .0091) and Grade 3 (****p < .0001) reactions. (E) Percentage of patients considered positive based on the current reference threshold of tryptase (11.4 μg/L) and on its elevation over baseline levels (20% + 2 μg/L, and/or 135%). Percentage of sensitivity and specificity for the diagnosis of anaphylaxis in each cut‐off point.
When comparing the ratio of tryptase (acute/basal) concentrations, patients with anaphylaxis showed a significant two‐fold elevation compared with Grade 1 reactions (Figure 1C). Furthermore, tryptase ratios during the acute phase increased with the severity of the reaction, rising up to 60% and 240% in Grade 2 and Grade 3 reactions, respectively (Figure 1D). Precisely, such elevation is significantly higher in drug‐induced anaphylaxis than in food‐mediated cases, as described by Cardona et al. 6
In addition, the diagnostic capability of tryptase levels was evaluated comparing the reference threshold during the acute phase (≥11.4 μg/L) against approaches that consider its elevation over baseline levels (20% + 2 μg/L, or 135%). Results showed a substantially better sensibility and correlation with the clinical diagnosis of anaphylaxis when considering the percentage of increase instead of the reference threshold. Specifically, more than twice of patients were correctly diagnosed considering the 135% increment in the acute phase (Figure 1E).
3.3. Serum protein concentrations drop in anaphylaxis
Increased vascular permeability, one of the main pathophysiological mechanisms underlying anaphylaxis, is ruled by the Starling Principle. 15 This process is regulated by differences in hydrostatic and oncotic pressures. Consequently, the comparison of serum protein concentrations between the acute and baseline phases would indirectly support a molecular value of the extravasation occurring in the studied reactions. In patients with Grade 1 reactions, serum protein concentrations did not experience any change between the baseline and acute phases (Figure 2A). However, the acute phase of anaphylactic reactions showed a significantly decrease of protein concentrations compared with baseline (Figure 2B,C). Specifically, when comparing the acute/basal ratio values, patients who experienced an anaphylactic reaction showed a 6% decrease compared with those with Grade 1 reactions (Figure 2D). Moreover, serum protein determinations decreased with severity, dropping 4% in Grade 2 and 11% in Grade 3, while there was no change in Grade 1 (Figure 2E). However, no correlation was observed between reduced protein concentration ratio and increased tryptase ratio (R: −0.1638; p = .0872).
FIGURE 2.
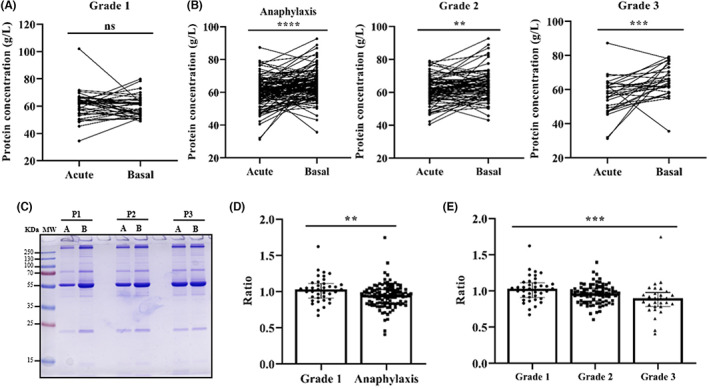
Serum protein concentration decreases according to the severity of anaphylaxis. Determination of acute and basal protein concentrations in Grade 1 reactions (A) in all patients with anaphylaxis (****p < .0001), as well as Grade 2 (**p = .0015) and Grade 3 (***p = .0005) reactions (B). (C) Coomassie blue‐stained SDS‐PAGE serum protein profile in the acute phase (A) and at baseline (B) of three representative patients with anaphylaxis (P1, P2 and P3). KDa, kilodalton; MW, molecular weight. (D) Comparison of acute/basal ratios of serum protein concentrations between anaphylactic and Grade 1 reactions (**p = .0044). (E) Differences in acute/basal ratios of serum protein concentrations between Grade 1, Grade 2 and Grade 3 reactions. Grade 1 vs. Grade 2 (ns p = .1137); Grade 1 vs. Grade 3 (***p = .0009); Grade 2 vs. Grade 3 (ns p = .0765). ns: not statistically significant.
3.4. HSA levels decrease in anaphylaxis
Due to the previous observation, it was considered relevant to determine the levels of HSA, as it alone accounts for more than 50% of the total serum protein. 32 HSA levels significantly dropped during the acute phase of anaphylaxis and correlated with the protein concentration decrease previously observed (Figure 3A,B). However, they did not correlate with the increase of tryptase ratio (R: −0.09407; p = .3283). After evaluating HSA concentrations according to the severity of the reaction, no changes from baseline to the acute phase were identified for Grade 1 events, but a statistically significant decrease was observed for Grade 2 and Grade 3 reactions (Figure 3C). Similarly, as seen with serum protein determinations, the acute/basal ratio of HSA levels of patients who suffered anaphylaxis was 8% lower compared with those who experienced a Grade 1 reaction (Figure 3D). Furthermore, this drop in HSA ratios during the acute phase was commensurate with the severity of the reaction, being unaffected in Grade 1 reactions, but reduced 6% and 15% in Grade 2 and Grade 3 cases, respectively (Figure 3E).
FIGURE 3.
Human serum albumin (HSA) levels decreased with the severity of anaphylaxis. (A) HSA levels during the acute phase and at baseline in patients with anaphylaxis (****p < .0001). AU, arbitrary units. (B) Correlation between HSA values and serum protein concentration (****p < .0001). (C) The images above show a representative Western blot of HSA in the acute phase (A) and at baseline (B) of three patients (P1, P2 and P3) with Grade 1, Grade 2 and Grade 3 reactions. Quantification of HSA levels in the acute phase and at baseline of Grade 1, Grade 2 (****p < .0001) and Grade 3 (****p < .0001) reactions. (D) Acute/basal ratio of HSA in Grade 1 and anaphylactic reactions (***p = .0005). (E) Stratification of HSA ratio values according to severity classification. Grade 1 vs. Grade 2 (*p = .0416); Grade 1 vs. Grade 3 (****p < .0001); Grade 2 vs. Grade 3 (*p = .0106). (F) Acute/basal ratio of HSA in Grade 1, Grade 2 and Grade 3 reactions based on the affected organs/systems. Grade 1C vs. Grade 3V (***p = .0003); Grade 1M vs. Grade 3V (*p = .0110). Grade 1C (n = 22), Grade 1M (n = 16), Grade 2R (n = 72), Grade 2D (n = 40), Grade 2N (n = 17), Grade 3R (n = 10), Grade 3V (n = 22). C, cutaneous; D, digestive; M, mucosal; N, neurological; ns, not statistically significant; R, respiratory; V, vascular.
Given that the classification of hypersensitivity and anaphylactic reactions includes a variety of organs involved in each grade, we evaluated HSA values according to the individual systems affected. We did not observe any changes in the Grade 1 group, which included cutaneous or mucosal manifestations, but Grade 2 reactions showed a 6%, 7% and 10% decrease of HSA in patients with respiratory, digestive and neurological manifestations, respectively. Finally, Grade 3 samples showed the highest drop of this protein, being 11% in patients with respiratory symptoms and 17% in those with cardiovascular affectation. Specifically, such gradual drop results statistically significant in Grade 3 patients with vascular involvement compared with those with Grade 1 reactions (Figure 3F).
3.5. HBB increases in hypersensitivity reactions
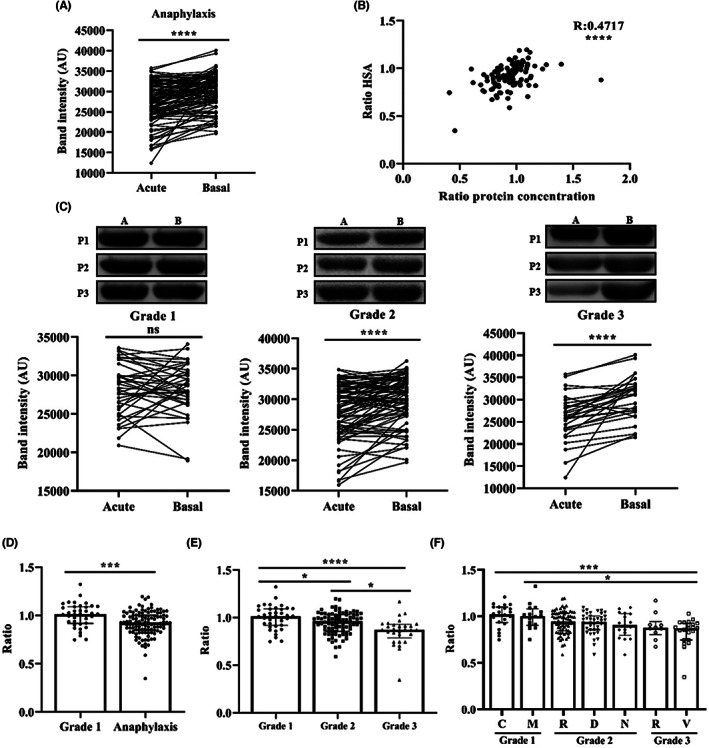
Serum protein profiling by SDS‐PAGE revealed an increase of an approximately 14‐KDa protein during the acute phase (Figure 4A). Subsequently, this band was identified and characterized as HBB (Figure 4B and supplementary material). Remarkably, the quantification of vascular fluid extravasation in anaphylaxis performed by Fisher in 1986 was determined by measurements of haemoglobin. 21 The analysis of acute and baseline serum samples from patients presenting Grade 1 reactions showed a significant increase of HBB concentrations during the acute phase (Figure 4C). This elevation was also found in all anaphylactic cases, as well as when splitting them into Grade 2 and Grade 3 reactions (Figure 4D). However, no differences were found when comparing the acute/baseline ratio of HBB between patients with anaphylaxis and those with Grade 1 reactions, or between the three degrees of severity (Figure 4E,F). In addition, no correlation was observed with the ratios of serum tryptase (R: 0.1308; p = .1308), protein concentration (R: −0.0005; p = .9948) or HSA (R: −0.1409; p = .0855).
FIGURE 4.
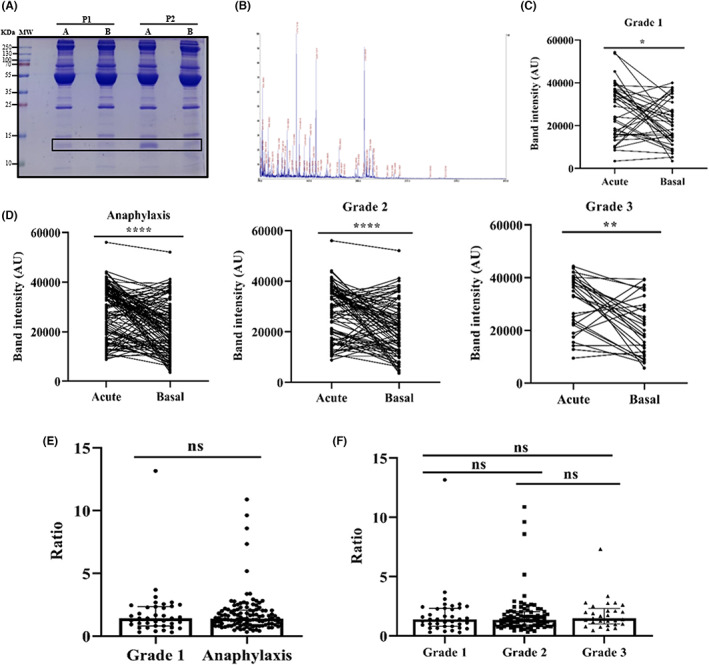
Haemoglobin beta subunit (HBB) serum levels increased in the acute phase of all hypersensitivity reactions. (A) Coomassie blue‐stained SDS‐PAGE serum protein profile in the acute (A) phase and at baseline (B) of two patients with anaphylaxis (P1 and P2). The rectangle indicates the HBB protein. KDa: kilodalton; MW: molecular weight. (B) Peptide spectrum determined by a dual time‐of‐flight (TOF/TOF) reflector. The x‐axis represents the peptide mass (m/z), while the y‐axis shows the percentage of intensity (%). (C) Determination of HBB levels in the acute phase and at baseline of Grade 1 reactions (*p = .0148). AU, arbitrary units. (D) HBB quantification of the total anaphylactic (****p < .0001), Grade 2 (****p < .0001), and Grade 3 (**p = .0012) reactions. (E) Acute/basal ratio of HBB in anaphylactic and Grade 1 reactions. (F) Stratification of HBB values according to severity classification. Grade 1 vs. Grade 2 (ns p > .9999); Grade 1 vs. Grade 3 (ns p > .9999); Grade 2 vs. Grade 3 (ns p > .9999). ns: not statistically significant.
4. DISCUSSION
Anaphylaxis is a systemic hypersensitivity reaction with pleiotropic characteristics. Therefore, the focus of its clinical approaches is evolving towards precision medicine. 33 In our study, we have demonstrated a better diagnostic efficiency of tryptase when it is based on personal values (acute/basal ratio and percentage increase compared to baseline) than if thresholds recommended in guidelines are used. Furthermore, molecular determinations support a drop in serum protein concentration and HSA, as well as an increase in HBB in the acute phase.
As previously described by Sala‐Cunill et al., our results concur with an increase in serum tryptase during anaphylaxis that is well associated with the severity of the reaction. 6 Currently, the existing clinical reference threshold for serum tryptase in this pathological event takes into account only the acute condition. 7 However, it has been shown that serum tryptase measurements obtained during the acute episode should be compared with patients' baseline values. 34 These have already been considered for the diagnosis of mast cell activation syndromes and Hymenoptera venom‐induced anaphylaxis. 8 , 9 Consequently, all three criteria were evaluated in our sample cohorts, and results obtained showed that patients clinically characterized as anaphylaxis were better diagnosed considering their baseline values. Specifically, more than twice were correctly classified considering the increment of 135%. Nevertheless, acute and basal tryptase levels may also be elevated in several non‐anaphylactic cases. 35 Therefore, it is imperative to find new and better diagnostic biomarkers.
To our knowledge, this is the first time that serum protein concentration and HSA levels have been evaluated in anaphylaxis. Results obtained showed a decrease in both factors during the acute phase compared to baseline and according to the severity of the reaction. Serum protein concentration has been proposed as a diagnostic indicator for other acute conditions such as pancreatitis and ascites. 17 , 18 Similarly occurs with HSA, where a decrease in its levels has been related to several pathological processes like operative stress and liver surgery. 19 , 20 Therefore, the use of both measurements as complementary biomarkers to serum tryptase could improve and increase the accuracy of the current diagnosis of anaphylaxis. However, additional studies with different cohorts of patients and a larger number of samples are still needed to determine the sensitivity and specificity of these molecular markers individually and in combination with serum tryptase.
On the contrary, increased HBB levels were observed during the acute phase in patients with anaphylaxis. Systemic inflammation has been shown to lead a massive release of haemoglobin from red blood cells. 23 Therefore, haemolysis could be an indicator of inflammatory processes. Specifically, it has been shown that free haemoglobin can increase vascular permeability in the pulmonary microvasculature. 36 In addition, HBB has been proposed as a diagnostic marker for sepsis. 37 However, haemolysis can also occur during obtaining, processing and storage of the samples, leading to alterations in measurements and detracting the value of haemoglobin as a diagnostic molecular marker. 38 Our results showed that HBB levels were also increased in mild hypersensitivity reactions and did not differ according to severity, so their measurement could have been altered by this factor. Interestingly, relevant observations carried out in perioperative patients with anaphylaxis determined fluid loss during anaphylaxis by using haemoglobin measurements. 21 To our knowledge, this is the only research that has indirectly quantified said process in humans. However, it did not consider these possible interferences caused by the quality of the samples. Therefore, we aimed to indirectly measure endothelial permeability and fluid extravasation by quantifying serum protein concentration and determining HSA levels.
In anaphylaxis, disruption of the endothelial barrier leads to leakage of vascular contents into the tissues. 14 This event results in a reduction of the serum concentration and an unspecific decrease of all circulating proteins, as observed in our results. Among the extravasated proteins, HSA accounts for more than 50% of total serum protein. 32 Specifically, several techniques have been based on this fact, such as the Miles assay, where albumin staining with Evans blue dye allows visualization and quantification of its extravasation. 39 This method has been widely used to evaluate the endothelial permeability in animal models of anaphylaxis. 40 Therefore, the drop of HSA in anaphylactic serum would allow to indirectly quantify the levels of extravasation occurring during the reaction. Our results also show a clear association between this fact and the severity of said pathological event, being higher in most severe cases. Furthermore, since the classification of hypersensitivity and anaphylactic reactions includes a variety of organs involved in each grade, we evaluated HSA values according to the individual systems affected in each reaction. These findings have allowed to elucidate the pathophysiology of human anaphylaxis after many years. However, although the collection of samples is arduous and complicated, studies in a larger population of patients are needed.
In addition, our results regarding HSA open a new field for research and clinical management of anaphylaxis. The treatment of this pathological event is based on the injection of adrenaline, but other complementary therapies are used to alleviate the loss of volume, such as the administration of intravenous fluids. 33 Nevertheless, no attempts have been made to correct or palliate the hypoalbuminemia underlying this process. Currently, several clinical trials disagree on the effectiveness of albumin therapies. 16 However, a relevant study in 1818 patients with severe sepsis showed that the addition of albumin did not improve the treatment of hypovolaemia compared to crystalloids, but it had several haemodynamic advantages in these patients. 41 HSA benefits are attributed to its physiological functions as an antioxidant, anti‐inflammatory and endothelial barrier stabilizer, among others. 42 In addition, it plays a key role as a scavenger of nitric oxide, the main vasodilator mediator in anaphylaxis. 42 , 43 Therefore, therapeutic supplementation with HSA is postulated as a promising complementary alternative for this pathological event, although clinical trials are needed to evaluate it.
In conclusion, we have evaluated different serum tryptase thresholds for the diagnosis of anaphylaxis, pointing out that it is critical to consider the patient's baseline status. Furthermore, we have observed a decrease in protein concentration and HSA levels during this pathological event, which allowed us to indirectly determine the extravasation during the reaction based on its severity, being higher in most severe cases. Moreover, an increase in HBB has been found in samples from patients with hypersensitivity reactions. Results obtained represent a breakthrough in the understanding of the endothelial permeability and extravasated vascular volume underlying human anaphylaxis, a topic in which no progress has been made for a long time. In addition, even considering that our findings are of an exploratory nature, their clinical potential could be critical for the development of new diagnostic and therapeutic strategies in anaphylaxis.
AUTHOR CONTRIBUTIONS
Emilio Nuñez‐Borque and Carlos Pastor‐Vargas performed the experimental work with participations of Sergio Fernández‐Bravo, Natalia Casado‐Navarro and Paloma Tramón. Vanesa Esteban coordinated the work. Diana Betancor, Alicia Gómez‐López, Pablo Rodríguez Del Río, Juan María Beitia, Carmen Moreno‐Aguilar, David González‐de‐Olano, María José Goikoetxea, María Dolores Ibáñez‐Sandín, Javier Cuesta‐Herranz and José Julio Laguna provided the clinical support and the recruitment of human serum samples. Ariadna Martin‐Blazquez and David López‐Domínguez participated in the statistical analysis. Vanesa Esteban and Emilio Nuñez‐Borque designed the experiments and interpreted the results with participations of Diana Betancor, Carlos Pastor‐Vargas and Javier Cuesta‐Herranz. Vanesa Esteban and Emilio Nuñez‐Borque wrote the manuscript. All the authors reviewed and approved the manuscript.
CONFLICT OF INTEREST
EN‐B was funded from FOOD‐AL (CM_P2018/BAAA‐4574). DB was granted by Rio Hortega Research Contract, from the Instituto Carlos III. All other authors have no conflict of interest within the scope of the submitted work.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
This research was supported by grants from the Instituto de Salud Carlos III (PI18/00348, PI21/00158) and FEDER Thematic Networks and Cooperative Research Centers RETICS ARADyAL (RD16/0006/0013, RD16/0006/0018, RD16/0006/0023, RD16/0006/0026, RD16/0006/0031, RD16/0006/0033). This work was also sustained by the SEAIC (19_A08) and Alfonso X el Sabio University Foundations. The authors wish to thank i2e3 for providing medical writing assistance on behalf of Allergy Therapeutics (BEC‐AT) and Irene Márquez for carried out the proof of concept of the study.
Nuñez‐Borque E, Betancor D, Pastor‐Vargas C, et al. Personalized diagnostic approach and indirect quantification of extravasation in human anaphylaxis. Allergy. 2023;78:202‐213. doi: 10.1111/all.15443
REFERENCES
- 1. Cardona V, Ansotegui IJ, Ebisawa M, et al. World allergy organization anaphylaxis guidance 2020. World Allergy Organ J. 2020;13(10):100472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tejedor‐Alonso MA, Moro‐Moro M, Múgica‐García MV. Epidemiology of anaphylaxis: contributions from the last 10 years. J Investig Allergol Clin Immunol. 2015;25(3):163‐175. quiz follow 174–175. [PubMed] [Google Scholar]
- 3. Tanno LK, Bierrenbach AL, Simons FER, et al. Critical view of anaphylaxis epidemiology: open questions and new perspectives. Allergy Asthma Clin Immunol. 2018;14:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Atanaskovic‐Markovic M, Gomes E, Cernadas JR, et al. Diagnosis and management of drug‐induced anaphylaxis in children: an EAACI position paper. Pediatr Allergy Immunol. 2019;30(3):269‐276. [DOI] [PubMed] [Google Scholar]
- 5. Agache I, Akdis CA. Precision medicine and phenotypes, endotypes, genotypes, regiotypes, and theratypes of allergic diseases. J Clin Invest. 2019;129(4):1493‐1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sala‐Cunill A, Cardona V, Labrador‐Horrillo M, et al. Usefulness and limitations of sequential serum tryptase for the diagnosis of anaphylaxis in 102 patients. Int Arch Allergy Immunol. 2013;160(2):192‐199. [DOI] [PubMed] [Google Scholar]
- 7. Platzgummer S, Bizzaro N, Bilò MB, et al. Recommendations for the use of tryptase in the diagnosis of anaphylaxis and clonal mastcell disorders. Eur Ann Allergy Clin Immunol. 2020;52(2):51‐61. [DOI] [PubMed] [Google Scholar]
- 8. Valent P, Akin C, Bonadonna P, et al. Proposed diagnostic algorithm for patients with suspected mast cell activation syndrome. J Allergy Clin Immunol Pract. 2019;7(4):1125‐1133.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Borer‐Reinhold M, Haeberli G, Bitzenhofer M, et al. An increase in serum tryptase even below 11.4 ng/mL may indicate a mast cell‐mediated hypersensitivity reaction: a prospective study in Hymenoptera venom allergic patients. Clin Exp Allergy J Br Soc Allergy Clin Immunol. 2011;41(12):1777‐1783. [DOI] [PubMed] [Google Scholar]
- 10. Reber LL, Hernandez JD, Galli SJ. The pathophysiology of anaphylaxis. J Allergy Clin Immunol. 2017;140(2):335‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simons FER. Anaphylaxis. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S161‐S181. [DOI] [PubMed] [Google Scholar]
- 12. Khan BQ, Kemp SF. Pathophysiology of anaphylaxis. Curr Opin Allergy Clin Immunol. 2011;11(4):319‐325. [DOI] [PubMed] [Google Scholar]
- 13. Nuñez‐Borque E, Fernandez‐Bravo S, Yuste‐Montalvo A, Esteban V. Pathophysiological, cellular, and molecular events of the vascular system in anaphylaxis. Front Immunol. 2022;13:836222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Claesson‐Welsh L, Dejana E, McDonald DM. Permeability of the endothelial barrier: identifying and reconciling controversies. Trends Mol Med. 2021;27(4):314‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Michel CC, Woodcock TE, Curry F‐RE. Understanding and extending the Starling principle. Acta Anaesthesiol Scand. 2020;64(8):1032‐1037. [DOI] [PubMed] [Google Scholar]
- 16. Erstad BL. The revised starling equation: the debate of albumin versus crystalloids continues. Ann Pharmacother. 2020;54(9):921‐927. [DOI] [PubMed] [Google Scholar]
- 17. Yoon J‐S, Kim S, Kang J‐H, Park J, Yu D. Alterations in serum protein electrophoresis profiles during the acute phase response in dogs with acute pancreatitis. Can J Vet Res. 2020;84(1):74‐78. [PMC free article] [PubMed] [Google Scholar]
- 18. Prabhu M, Gangula RS, Stanley W. Diagnostic utility of serum ascites lipid and protein gradients in differentiation of ascites. Int J Hepatol. 2019;2019:8546010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hübner M, Mantziari S, Demartines N, Pralong F, Coti‐Bertrand P, Schäfer M. Postoperative albumin drop is a marker for surgical stress and a predictor for clinical outcome: a pilot study. Gastroenterol Res Pract. 2016;2016:8743187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Labgaa I, Joliat G‐R, Demartines N, Hübner M. Serum albumin is an early predictor of complications after liver surgery. Dig Liver Dis. 2016;48(5):559‐561. [DOI] [PubMed] [Google Scholar]
- 21. Fisher MM. Clinical observations on the pathophysiology and treatment of anaphylactic cardiovascular collapse. Anaesth Intensive Care. 1986;14(1):17‐21. [DOI] [PubMed] [Google Scholar]
- 22. Ahmed MH, Ghatge MS, Safo MK. Hemoglobin: structure, function and allostery. Subcell Biochem. 2020;94:345‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Effenberger‐Neidnicht K, Hartmann M. Mechanisms of hemolysis during sepsis. Inflammation. 2018;41(5):1569‐1581. [DOI] [PubMed] [Google Scholar]
- 24. Nguyen SMT, Rupprecht CP, Haque A, Pattanaik D, Yusin J, Krishnaswamy G. Mechanisms governing anaphylaxis: inflammatory cells, mediators, endothelial gap junctions and beyond. Int J Mol Sci. 2021;22(15):7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sala‐Cunill A, Björkqvist J, Senter R, et al. Plasma contact system activation drives anaphylaxis in severe mast cell‐mediated allergic reactions. J Allergy Clin Immunol. 2015;135(4):1031‐1043.e6. [DOI] [PubMed] [Google Scholar]
- 26. Brown SGA. Clinical features and severity grading of anaphylaxis. J Allergy Clin Immunol. 2004;114(2):371‐376. [DOI] [PubMed] [Google Scholar]
- 27. Sampson HA, Muñoz‐Furlong A, Campbell RL, et al. Second symposium on the definition and management of anaphylaxis: summary report—Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391‐397. [DOI] [PubMed] [Google Scholar]
- 28. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Anal Biochem. 1976;72:248‐254. [DOI] [PubMed] [Google Scholar]
- 29. Albar JP, Juarez C, Vivanco‐Martínez F, Bragado R, Ortíz F. Structural requirements of rabbit IgG F(ab′)2 fragment for activation of the complement system through the alternative pathway‐I. Disulfide bonds. Disulfide bonds Mol Immunol. 1981;18(10):925‐934. [DOI] [PubMed] [Google Scholar]
- 30. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sechi S. A method to identify and simultaneously determine the relative quantities of proteins isolated by gel electrophoresis. Rapid Commun Mass Spectrom RCM. 2002;16(15):1416‐1424. [DOI] [PubMed] [Google Scholar]
- 32. Anderson NL, Polanski M, Pieper R, et al. The human plasma proteome: a nonredundant list developed by combination of four separate sources. Mol Cell Proteomics MCP. 2004;3(4):311‐326. [DOI] [PubMed] [Google Scholar]
- 33. Castells M. Diagnosis and management of anaphylaxis in precision medicine. J Allergy Clin Immunol. 2017;140(2):321‐333. [DOI] [PubMed] [Google Scholar]
- 34. Simons FER, Frew AJ, Ansotegui IJ, et al. Risk assessment in anaphylaxis: current and future approaches. J Allergy Clin Immunol. 2007;120(1 Suppl):S2‐S24. [DOI] [PubMed] [Google Scholar]
- 35. Lee AYS. Elevated serum tryptase in non‐anaphylaxis cases: a concise review. Int Arch Allergy Immunol. 2020;181(5):357‐364. [DOI] [PubMed] [Google Scholar]
- 36. Shaver CM, Wickersham N, McNeil JB, et al. Cell‐free hemoglobin‐mediated increases in vascular permeability. a novel mechanism of primary graft dysfunction and a new therapeutic target. Ann Am Thorac Soc. 2017;14(Supplement_3):S251‐S252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yoo H, Ku S‐K, Kim S‐W, Bae J‐S. Early diagnosis of sepsis using serum hemoglobin subunit Beta. Inflammation. 2015;38(1):394‐399. [DOI] [PubMed] [Google Scholar]
- 38. Gislefoss RE, Berge U, Lauritzen M, Langseth H, Wojewodzic MW. A simple and cost‐effective method for measuring hemolysis in biobank serum specimens. Biopreserv Biobank. 2021;19(6):525‐530. [DOI] [PubMed] [Google Scholar]
- 39. Brash JT, Ruhrberg C, Fantin A. Evaluating vascular hyperpermeability‐inducing agents in the skin with the miles assay. J Vis Exp. 2018;19(136):57524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mendez‐Barbero N, Yuste‐Montalvo A, Nuñez‐Borque E, et al. The TNF‐like weak inducer of the apoptosis/fibroblast growth factor‐inducible molecule 14 axis mediates histamine and platelet‐activating factor‐induced subcutaneous vascular leakage and anaphylactic shock. J Allergy Clin Immunol. 2020;145(2):583‐596.e6. [DOI] [PubMed] [Google Scholar]
- 41. Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370(15):1412‐1421. [DOI] [PubMed] [Google Scholar]
- 42. Rozga J, Piątek T, Małkowski P. Human albumin: old, new, and emerging applications. Ann Transplant. 2013;18:205‐217. [DOI] [PubMed] [Google Scholar]
- 43. Cauwels A, Janssen B, Buys E, Sips P, Brouckaert P. Anaphylactic shock depends on PI3K and eNOS‐derived NO. J Clin Invest. 2006;116(8):2244‐2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


