Abstract
Background
The genotypic and histological evolution of non-small cell lung cancer (NSCLC) to small cell lung cancer (SCLC) has been described as a mechanism of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors (TKIs). However, it was extremely rare in anaplastic lymphoma kinase (ALK) positive NSCLC, and the follow-up care and outcomes of patients with this rare condition were unclear. This case was the first described the effectiveness of combined chemo-immunotherapy in a patient, with a transformed ALK positive NSCLC into SCLC after the administration of an ALK-TKIs.
Case Description
We described a unique case in which a patient with ALK-positive NSCLC underwent SCLC transformation at a metastatic site and remained ALK positive after TKI treatment. In July 2019, a 77-year-old man was diagnosed with ALK-positive stage IVB NSCLC, received alectinib and responded to alectinib. It was not until more than 7 months later that a cranial MRI showed brain metastases. And whole-brain radiotherapy was administered, and secondary epilepsy and metastatic progression occurred. One year later, computed tomography showed a left submandibular mass with multiple lymph node metastases, a left lower lung mass, and right pleura thickening. A left submandibular biopsy revealed SCLC. Echinoderm microtubule-associated protein-like 4-ALK (EML4-ALK) fusion and low tumor mutation burden (2.23 muts/Mb) were identified by next generation sequencing. The patient was administered atezolizumab (1,200 mg, d1) in combination with etoposide (0.13 g, d1–d3) and carboplatin (350 mg, d1). The left neck mass was reduced significantly, showing a partial response. Serum NSE (from 106 to 15 ng/mL), CA19-9 (from 49.4 to 34.6 U/mL) and CEA (from 4.18 to 3.09 ng/mL) returned to normal. Only mild myelosuppression (Grade 1), fatigue (Grade 1), and anorexia (Grade 1) were present. The patient had an overall survival time of 21 months.
Conclusions
This case highlighted the importance of re-biopsies to reveal pathological SCLC transformation after ALK-TKI resistance, and suggested the treatment of atezolizumab in combination with etoposide and carboplatin were potentially helpful for this phenotype.
Keywords: Non-small cell lung cancer (NSCLC), small cell lung cancer transformation, ALK fusion, atezolizumab, case report
Highlight box.
Key findings
• This appears to be the first described case of the effectiveness of combined chemo-immunotherapy in a patient, with a transformed ALK positive NSCLC into small cell lung cancer (SCLC) after the administration of an ALK-tyrosine kinase inhibitor (TKI).
What is known and what is new?
• SCLC transformation was a rare mechanism of resistance to ALK-TKIs. To date, only 5 such cases have been described. However, all these patients failed to respond to targeted therapy or chemotherapy after histological transformation, and the disease progressed rapidly.
• An ALK-positive SCLC patient who experienced histological transformation after ALK-TKI treatment responded to chemo-immunotherapy.
What is the implication, and what should change now?
• Our case suggested that combined chemo-immunotherapy might be useful in ALK-positive transformed-SCLC patients, even if these tumors show a low tumor mutational burden.
Introduction
Anaplastic lymphoma kinase (ALK), a receptor tyrosine kinase, plays an important role in the development and function of the nervous system, but is not expressed in normal lymphoid and lung tissues. ALK rearrangement can cause the enhancement of tyrosine kinase activity, resulting in the uncontrolled proliferation of tumor cells and tumor formation. The “diamond mutation” occurs in about 5% of non-small cell lung cancer (NSCLC) patients, and echinoderm microtubule-associated protein-like 4-ALK (EML4-ALK) is the most common of fusion (1). ALK-tyrosine kinase inhibitors (ALK-TKIs) have been shown to have significant and long-lasting clinical efficacy in treating ALK-positive NSCLC. This made ALK-positive patients expected to be the first subtype of NSCLC to achieve “chronic disease” (2,3). However, the vast majority of initial responders still developed drug resistance. The resistance to ALK-TKIs occurs due to secondary ALK gene mutations (e.g., ALK G1202R and ALK amplification) and off-target mechanisms (4,5).
The histological transformation from NSCLC to small cell lung cancer (SCLC) was a common mechanism of resistance to TKIs in epidermal growth factor receptor (EGFR)-mutant NSCLC and occurs in approximately 4–14% of patients (6). However, SCLC transformation was a rare resistance response to ALK-TKIs. To date, there appeared to be only 5 relevant case reports on SCLC transformation. However, after the histological transformation, targeted therapy or chemotherapy failed in all these patients, and the disease progressed rapidly (Table 1) (7-11).
Table 1. Summary of cases of SCLC transformed from ALK-positive NSCLC.
| Patient No. | Sex | Age, year | Origin | CNS metastasis* | Prior treatment | ALK rearrangement remained in transformed SCLC | Subsequent SCLC therapy |
|---|---|---|---|---|---|---|---|
| Patient 1# (7) | Male | 35 | US | Yes | First-line: ceritinib; second-line: alectinib; third-line: lorlatinib | Yes | Fourth-line: carboplatin/etoposide; fifth-line: alectinib |
| Patient 2# (8) | Female | 73 | US | No | Wedge resection; first-line: carboplatin/pemetrexed | Yes | Second-line: carboplatin + etoposide; third-line: alectinib |
| Patient 3# (9) | Male | 41 | Japan | No | First-line: cisplatin plus pemetrexed; second-line: S-1; third-line: amrubicin; fourth-line: docetaxel; fifth-line: alectinib | Yes | Sixth-line: cisplatin plus irinotecan; seventh-line: amrubicin |
| Patient 4# (10) | Male | 62 | Japan | No | First-line: carboplatin, pemetrexed, and bevacizumab, alectinib; second-line: radiotherapy + alectinib | Yes | Third-line: alectinib, ceritinib; Fourth-line: cisplatin + etoposide Fifth-line and posterior-line: amrubicin, nivolumab, and irinotecan |
| Patient 5# (11) | Female | 67 | Japan | Yes | Cisplatin + vinorelbine + radiation, Radiotherapy + paclitaxel + bevacizumab, crizotinib; multiple lines: pemetrexed, gemcitabine, docetaxel, alectinib |
Yes | Irinotecan and alectinib |
| Our case | Male | 77 | China | Yes | First-line: alectinib; second-line: radiotherapy | Yes | Third-line: atezolizumab+ etoposide + carboplatin |
*, at the time of diagnosis. ALK, anaplastic lymphoma kinase; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; CNS, central nervous system.
We reported a case of ALK-TKI-induced transformation of ALK-positive SCLC, which showed abnormal levels of neuron specific enolase (NSE), carbohydrate antigen 19-9 (CA19-9), and carcinoembryonic antigen (CEA), suggesting that these may be predictors of SCLC transformation during the development of TKI resistance. In addition, this report suggested that combined chemo-immunotherapy might be useful in treating ALK-positive transformed-SCLC patients. We present the following article in accordance with the CARE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-154/rc).
Case presentation
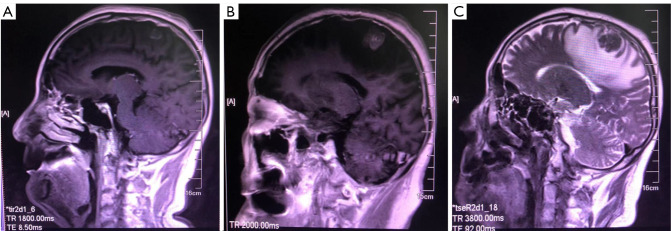
Due to left submandibular and cervical lymphadenopathy, a 77-year-old man was admitted to our hospital in July 2020. One year ago, the patient had left lower limb weakness, and was then diagnosed with stage IVB (cT4N3M1c) NSCLC with lymph node and multiple brain metastases (Figure 1A,1B) at his local hospital. Meanwhile, lung puncture specimens were confirmed positive for ALK (D5F3®, Cell Signaling) by immunohistochemistry (Figure 1C). The serum levels of NSE, CA19-9 and CEA were higher than the reference values, with 49.64 ng/mL, 615.5 U/mL and 164.6 ng/mL, respectively. Based on the diagnosis, alectinib (600 mg twice daily) was administered, and the best response was a partial remission (Figure 2A). After 11 months of treatment, brain magnetic resonance imaging revealed the progression of the brain metastases (Figure 2B). Whole-brain radiotherapy (30 Gy/10 fractions) was administered but stopped due to seizures and metastatic progression 1 month later (Figure 2C). Alectinib was continued.
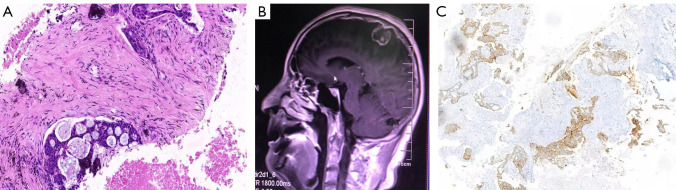
Figure 1.
The patient’s initial diagnosis. (A) Hematoxylin and eosin staining of the left lung (×100). (B) Magnetic resonance imaging (MRI) from initial diagnosis on July 20, 2019 showed brain metastases. (C) Immunohistochemistry showed that the lung puncture specimen was positive for anaplastic lymphoma kinase (ALK, ×20).
Figure 2.
Lesion changes during alectinib treatment. (A) Optimal outcome assessment of alectinib treatment. (B) Progression of brain lesions after alectinib treatment. (C) Magnetic resonance imaging (MRI) showed perimetastatic edema and disease progression.
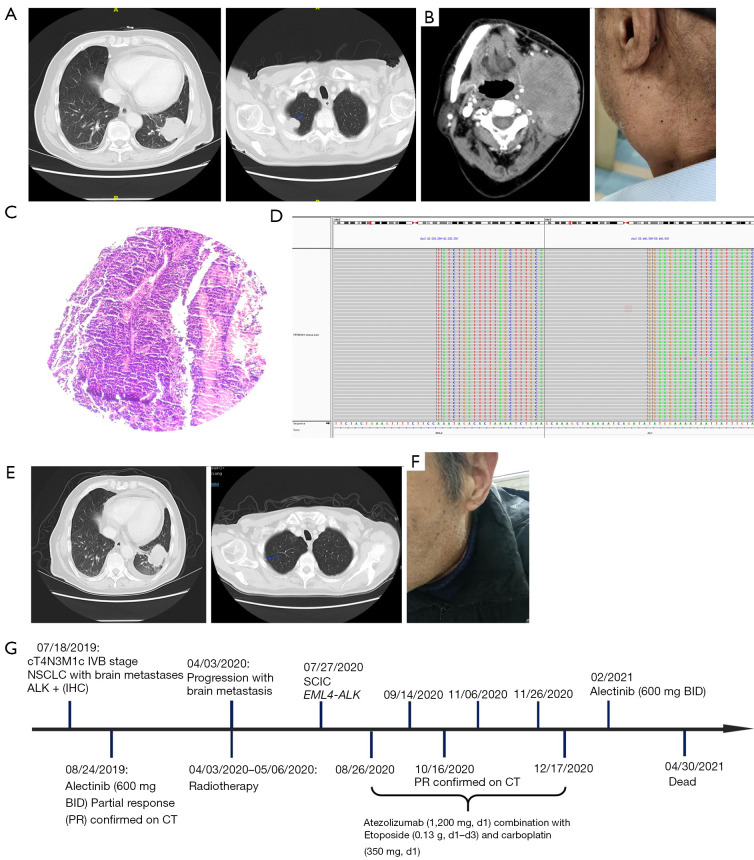
At physical examination on July 27, 2020, the patient showed an enlarged left submaxillary lymph node that was hard, fixed, tender, and had a poor range of motion. Chest computed tomography (CT) showed a mass lesion (3.87 cm × 3.42 cm) in the left lower lung, with enlargement of the left hilar and supraclavicular lymph nodes, thickened right thoracoapical pleura with a patchy shadow (Figure 3A). Jaw CT showed a mass in the left mandible, 8.52 cm × 5.95 cm in size, with multiple lymph node metastases (Figure 3B). In addition, a left submandibular biopsy revealed SCLC (Figure 3C) with immunoreactivity to CD56, synaptophysin (Syp), chromograninA (CgA), TTF-1, and Ki-67 (about 80% positive), and negative results for cytokeratin 7 (CK7), p40 and PD-L1 staining. The laboratory examinations revealed elevated serum NSE (106 ng/mL), CA19-9 (49.4 U/mL), and CEA (4.18 ng/mL) levels. The biopsy was subjected to next generation sequencing (NGS) using panels designed with 733 genes, which showed an EML4-ALK fusion variant 1 (v1, i.e., exon 1–13 of EML4 fused to exon 20–29 of ALK) fusion, V-Crk avian sarcoma virus CT10 oncogene homolog-like (CRKL) amplification, vascular endothelial growth factor receptor 1 (VEGFR1) amplification, and loss of RB1 were found (Figure 3D). Meanwhile low tumor mutation burden (TMB-L, 2.23 muts/Mb) and microsatellite stability (MSS) were identified, while TP53 was wild-type. The patient was administered atezolizumab (1,200 mg, d1) plus etoposide (130 mg, d1–d3), and carboplatin (350 mg, d1) based on the IMpower133 study.
Figure 3.
Brain lesion changes during atezolizumab combined therapy. (A) CT results before atezolizumab combined therapy. Blue arrow indicates the tumor. (B) Jaw CT results and physical examination. (C) Hematoxylin and eosin staining of the left submandibular (×100). (D) Next-generation sequencing results showing break point of echinoderm microtubule-associated protein-like 4-ALK (EML4-ALK) fusion. (E) CT results after atezolizumab combined therapy. Blue arrow indicates the tumor. (F) Physical examination after atezolizumab combined therapy. (G) Timeline of the clinical course in this patient. CT, computed tomography; NSCLC, non-small cell lung cancer; IHC, immunohistochemistry; SCLC, small cell lung cancer.
After 2 treatment cycles, the thickened pleural plaque near the right thorax had receded markedly, and the size of the left lower lung lesion was similar to that before treatment. More importantly, the left neck mass was also significantly reduced (Figure 3E), and the neck compression symptoms had significantly improved (Figure 3F). The levels of NSE (15 ng/mL), CA19-9 (34.6 U/mL), and CEA (3.09 ng/mL) all fell to normal levels. No treatment-related adverse events (such as vomiting and diarrhea) occurred during treatment. Only mild myelosuppression (Grade 1), fatigue (Grade 1), and anorexia (Grade 1) were present. There were no abnormalities in the routine blood and biochemical tests.
After completing the last treatment on December 17, 2020, the patient refused to continue treatment and was followed-up at a local hospital. In February 2021, the patient reappeared with lower left weakness, but his symptoms resolved with the oral administration of the remaining alectinib (600 mg, twice a day). Finally, due to personal reasons, the patient stopped taking the drug and passed away on April 30, 2021. The patient had an overall survival time of 21 months (Figure 3G).
All the procedures performed in this study were conducted in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
This appeared to be the first report of NSCLC transforming into SCLC after ALK-TKI and responding to combined chemotherapy and immunotherapy. Our case described both the profile of transformation from NSCLC to SCLC and the importance of repeat biopsies to manage disease progression. In particular, we demonstrate that the secondary SCLC retained the original ALK fusion, while also showing typical histologic characteristics of neuroendocrine differentiation, like expression of CD56, Syp, CgA, as well increased serum levels of NSE. On the other hand, reliable NGS testing with a 733-gene panel showed no TP53 mutations as well as a low TMB of 2.23 mut/Mb (12), which, however, did not preclude benefit from chemoimmunotherapy.
SCLC transformation has been a controversial topic. It has been theorized that initially diagnosed biopsies did not provide sufficient specimens to diagnose the mixed histology of tumors, and since the NSCLC component was inhibited by ALK-TKI, the subsequent SCLC component dominates. However, this patient was initially responsive to alectinib. In addition, the ALK fusion was still present when the NSCLC had transformed to SCLC as revealed by the second biopsy, while the biopsy at initial diagnosis showed the presence of NSCLC without any sign of SCLC. These findings did not support the theory that the 2 tumor subtypes coexist. Another theory was that the tumor pathological type changes from NSCLC to SCLC after ALK-TKI treatment. It was generally believed that NSCLC and SCLC cells were derived from the same tumor stem cells and thus have a “common origin”. Tatematsu et al. also reported that the origin cells of tumor cells carrying EGFR sensitive mutations had the potential to differentiate into neuroendocrine tumor cells (13). NSCLC was more likely to transform into SCLC under the exposure pressure of targeted therapy (14).
It has been reported that the transformation of NSCLC to SCLC appeared to occur more easily after treatment with 2nd-generation ALK-TKIs. This might be because the tumor cells are under more severe exposure pressure during the treatment of 2nd-generation TKIs. To overcome this exposure pressure, the cells may undergo biological changes or histological transformations (15). In our case, the patient, who received 2nd-generation alectinib treatment, developed SCLC transformation. Nevertheless, the more potent second-generation ALK inhibitors or lorlatinib are preferred first-line options today based on a longer patient survival in phase 3 trials compared to upfront crizotinib (16).
Questions to be further discussed and considered
Question 1: How to distinguish NSCLC complicated with SCLC from NSCLC transformed to SCLC after drug resistance?
Anna Grenda: To differentiate NSCLC complicated with SCLC from NSCLC transformed to SCLC after drug resistance, it is necessary to have samples taken at the appropriate time in terms of the clinical course of the disease (at the time of diagnosis and the point of drug resistance occurred). Further, important will be the histopathological staining and comparison of the input results of the reactions in the originally diagnosed cancer and score at the drug resistance point. In addition, the performance of a next-generation sequencing study, in particular, broad in terms of the analyzed genes, can provide valuable information on the molecular landscape of NSCLC complicated with SCLC and NSCLC transformed to SCLC. Just as the immunohistochemical reactions will differ between the two cancer subtypes, the profiles may also be different. What is more, immunohistochemical reactions do not always give a clear answer as to histological type, and NGS testing can complement such diagnostics. It would be ideal to have the result of the NGS test available on the material in which the cancer was originally diagnosed, but it is not always performed a priori, primarily because of the single-gene testing regimens implemented and used. However, it seems that tumor tissue collected at the right time in the clinical course of the disease, histopathological staining and genetic testing are all very important factors in determining whether we are dealing with NSCLC complicated with SCLC or NSCLC transformed to SCLC after drug resistance.
Paul Hofman: Specific ALK mutations (associated or not with persistence of ALK rearrangement).
Petros Christopoulos: According to WHO, combined SCLC (c-SCLC) is SCLC with additional presence of a morphologically defined NSCLC component, which should comprise ≥10% of the tumor in case of the large-cell neuroendocrine (LCNEC) or not-otherwise-specified lung carcinoma (NSCLC-NOS), but can be of any size for other NSCLC histotypes (17). In contrast, the previous diagnosis of pure NSCLC is necessary in order to document “SCLC after transformation of NSCLC”. This distinction can be difficult for metastatic lung cancer patients, because the SCLC component might be missed by the initial small biopsy due to the spatial tumor heterogeneity. Of note, the presence of molecular features, like simultaneous TP53/Rb1 mutations, in lung adenocarcinoma cannot be used as a surrogate for unrecognized concomitant SCLC, as only 18% of EGFR/TP53/Rb1 triple mutant tumors will develop SCLC transformation under treatment (18); also, the absence TP53/Rb1 mutations in lung adenocarcinoma does not exclude the simultaneous presence of occult SCLC either, as comparative studies have demonstrated different mutations in the adenocarcinoma and SCLC components of c-SCLC (19). Clinically, the presumable initial presence of c-SCLC can be inferred retrospectively by the early (within a few weeks) outgrowth of SCLC in a patient initially diagnosed with NSCLC, as the median time for the development of “SCLC after transformation” is much longer, i.e., >1 year in median (18). The mere immunohistochemical detection of neuroendocrine markers in NSCLC without SCLC morphology is not sufficient for c-SCLC, as these “NSCLC with neuroendocrine differentiation” have similar clinical courses as other NSCLC (17). In contrast, c-SCLC are very aggressive neoplasms with survival shorter than NSCLC and comparable to that of SCLC (20,21).
Author response: For newly diagnosed patients, it is relatively easy to distinguish c-SCLC or SCLC alone in clinical practice. The emphasis is on pathological diagnosis of tissue specimens and observation of cell morphology and composition. Because according to the WHO definition, c-SCLC has a clear definition (Thoracic Tumours, WHO Classification of Tumours, 5th Edition, Volume 5). Of course, it is also possible to retrospectively speculate whether the initial diagnosis was c-SCLC. Because “SCLC after transformation” takes time to develop, and the prognosis varies widely. For patients with metastasis, this distinction can be difficult because the SCLC component may be missed on the initial small biopsy due to the spatial heterogeneity of the tumor. In order to distinguish NSCLC complicated with SCLC and NSCLC transformed into SCLC after drug resistance, it is necessary to take samples at appropriate time according to the clinical course of the disease (diagnosis time and point of occurrence of drug resistance), and distinguish the two through comprehensive evaluation through histopathological staining and genetic testing, clinical characteristics and treatment process of results.
Question 2: Is gene variation one of the important reasons for the difference in biological function between NSCLC complicated with SCLC and NSCLC transformed into SCLC after drug resistance?
Anna Grenda: It is interesting at which molecular moment the transformation of NSCLC to SCLC occurs and what causes it. Could this be due to the common cellular origin of NSCLC and SCLC described in the literature (type II alveolar cells, or multipotent stem cells)? Every cell in the body has the same set of genes, and cancer cells use them to make themselves malignant by activating or deactivating certain key genes for cellular transformation. Transformation of NSCLC EGFR(+) to SCLC has been reported as one of the mechanisms of resistance to treatment with TKI inhibitors. The genetic profile of the tumor tissue may vary depending on when the material was collected for testing (at the time of initial diagnosis, whether while monitoring the course of the disease and the effects of treatment, or at the time of progression). Inactivation of TP53 and RB1 is indicated to be characteristic of primary SCLC, but inactivation of these genes is also observed in NSCLC transformed to SCLC, both in the resistance to TKI treatment and EGFR(−) NSCLC transformed to SCLC. These two tumor suppressors (TP35 and RB1) are indicated as markers associated with small cell lung cancer, where the degree of malignancy is very high, among others, by the lack of their protein products and thus lack of the blockade of cell division with damaged DNA.
Moreover, SCLC is diagnosed at an advanced stage and the question arises, what percentage of patients with small cell carcinoma are advanced-transformed-malignant NSCLC? This is difficult to verify because SCLC is usually detected at a very late stage, with a characteristic genetic profile: loss of TP53 and RB1 function, MYC amplifications or PIK3CA mutations, or the molecularly disrupted PI3K/AKT/mTOR pathway in general. Genetic analysis may help in the selection of a therapeutic strategy, focused on molecular targets, or in the future, the use of routine immunotherapy, with an increased supply of neoantigens, appearing as a result of the accumulation of a large number of genetic changes observed in highly malignant cells. NSCLC complicated with SCLC and NSCLC transformed into SCLC may differ in genetic profile depending on the molecular moment of NSCLC transformation into SCLC in which the tumor is. NSCLC transformed to SCLC may have a maintained genetic pattern of NSCLC (e.g., retain the EGFR activating mutation or as in the case presented here maintain ALK rearrangement), but in addition, overexpression of oncogenes or inactivation of tumor suppressors may appear. Thus, information about genetic variants can, on the one hand, differentiate NSCLC complicated with SCLC and NSCLC transformed into SCLC (if the patient has previously been diagnosed with NDRP), and on the other hand, allow implementing the best available therapy.
Paul Hofman: Quite not sure and this need higher number of cases for a validation.
Petros Christopoulos: “SCLC after transformation” of previous NSCLC has generally a worse prognosis and response to treatment than combined SCLC (c-SCLC), with a median OS approximately 10 months for EGFR-positive disease (22). There are certain molecular features that can in part account for these differences. For example, although the SCLC component of c-SCLC and “SCLC after transformation” share many common characteristics, like a high frequency of concomitant TP53/RB1 mutations, the “SCLC after transformation” generally loses important NSCLC-related therapeutic susceptibilities, like the sensitivity to EGFR or ALK inhibitors, even in cases where the original EGFR or ALK mutation is preserved; this is partly attributable to a downregulation of the respective oncoproteins in transformed tumors (23). Besides, the sensitivity of the same driver mutation to therapy can vary according to the histological subtype, for example ALK-mutated lung neuroendocrine tumors are less sensitive to ALK inhibitors than their adenocarcinoma counterparts (24).
Author response: Genetic analysis may help in the selection of therapeutic strategies, especially molecular targets or immunotherapy. This is why, in recent years, attention has been paid not only to genetic analysis of NSCLC, but also to patients with SCLC or c-SCLC. c-SCLC and “post-transformed SCLC” share many molecular characteristics, such as a high frequency of concomitant TP53/RB1 mutations. While “post-transformed SCLC” may have a sustained NSCLC genetic pattern (e.g., EGFR-activated mutation or ALK fusion in this case), including overexpression of oncogenes or inactivation of tumor suppressor genes may also occur, but post-transformed SCLC often loses important NSCLC-related treatment sensitivity. Such as sensitivity to EGFR or ALK inhibitors, even when the original EGFR or ALK mutation is retained.
Question 3: Is the treatment of NSCLC with SCLC the same as that of NSCLC with drug-resistant SCLC?
Anna Grenda: The given question is closely related to therapeutic regimens and physical treatment. As a molecular geneticist not directly involved in the treatment of patients, I leave the discussion of therapeutic strategies to clinicians qualified in this area.
Paul Hofman: Probably no.
Petros Christopoulos: One important difference is that for c-SCLC with actionable driver alterations, like EGFR mutations, the respective TKIs can be administered and may elicit transient responses of both the SCLC and NSCLC components in some patients (13). On the other hand, “SCLC after transformation” is invariably TKI-resistant, as can also be the SCLC component of oncogene-mutated c-SCLC, or the rare oncogene-mutated pure SCLCs (25). For cases with TKI resistance, including “SCLC after transformation” and most c-SCLC, a reasonable empiric choice is chemoimmunotherapy, which is the preferred modality for both SCLC and non-TKI eligible NSCLC currently. Besides etoposide, paclitaxel is also a reasonable platinum partner, since it is active against both SCLC and NSCLC. Of note, as the current case report shows, “SCLC after transformation” can respond to chemoimmunotherapy even in case of a low tumor mutational burden.
Author response: A reasonable option for “post-transformed SCLC” and most c-SCLC is chemotherapy-immunotherapy, which is currently the preferred modality for SCLC and NSCLC without driver genes. However, TKIs may benefit c-SCLC with driver genes, such as EGFR/ALK mutations. However, post-transformed SCLC is always resistant to TKI, so these patients may not benefit from TKI.
Conclusions
This appears to be the first described case of the effectiveness of combined immunotherapy and chemotherapy in a patient, with a transformed ALK positive NSCLC into SCLC after the administration of an ALK-TKI. A treatment strategy of repeat biopsies at the advanced stage may be useful for ALK-positive NSCLC patients with SCLC transformation.
Supplementary
The article’s supplementary files as
Acknowledgments
The abstract has been presented at IASLC 2022 World Conference on Lung Cancer.
The authors appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work, including ensuring that any questions related to the accuracy or integrity of any part of the work have been appropriately investigated and resolved. All the procedures performed in this study were conducted in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-154/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-154/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-23-154/coif). PC has received research funding from AstraZeneca, Amgen, Boehringer Ingelheim, Novartis, Roche, and Takeda; speaker’s honoraria from AstraZeneca, Novartis, Roche, Pfizer, Takeda; support for attending meetings from AstraZeneca, Eli Lilly, Daiichi Sankyo, Gilead, Novartis, Pfizer, Takeda; and personal fees for participating to advisory boards from Boehringer Ingelheim, Chugai, Pfizer, Novartis, MSD, Takeda and Roche, all outside the submitted work. JZ and MH are from 3D Medicines Inc., Shanghai. The other authors have no conflicts of interest to declare.
(English Language Editor: L. Huleatt)
References
- 1.Wu J, Savooji J, Liu D. Second- and third-generation ALK inhibitors for non-small cell lung cancer. J Hematol Oncol 2016;9:19. 10.1186/s13045-016-0251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon BJ, Kim DW, Wu YL, et al. Final Overall Survival Analysis From a Study Comparing First-Line Crizotinib Versus Chemotherapy in ALK-Mutation-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:2251-8. 10.1200/JCO.2017.77.4794 [DOI] [PubMed] [Google Scholar]
- 3.Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. 10.1056/NEJMoa1704795 [DOI] [PubMed] [Google Scholar]
- 4.Shaw AT, Solomon BJ, Besse B, et al. ALK Resistance Mutations and Efficacy of Lorlatinib in Advanced Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. J Clin Oncol 2019;37:1370-9. 10.1200/JCO.18.02236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinto JA, Raez LE, Domingo G. Clinical consequences of resistance to ALK inhibitors in non-small cell lung cancer. Expert Rev Respir Med 2020;14:385-90. 10.1080/17476348.2020.1721285 [DOI] [PubMed] [Google Scholar]
- 6.Calabrese F, Pezzuto F, Lunardi F, et al. Morphologic-Molecular Transformation of Oncogene Addicted Non-Small Cell Lung Cancer. Int J Mol Sci 2022;23:4164. 10.3390/ijms23084164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ou SI, Lee TK, Young L, et al. Dual occurrence of ALK G1202R solvent front mutation and small cell lung cancer transformation as resistance mechanisms to second generation ALK inhibitors without prior exposure to crizotinib. Pitfall of solely relying on liquid re-biopsy? Lung Cancer 2017;106:110-4. 10.1016/j.lungcan.2017.02.005 [DOI] [PubMed] [Google Scholar]
- 8.Balla A, Khan F, Hampel KJ, et al. Small-cell transformation of ALK-rearranged non-small-cell adenocarcinoma of the lung. Cold Spring Harb Mol Case Stud 2018;4:a002394. 10.1101/mcs.a002394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamagata A, Yokoyama T, Fukuda Y, et al. Alectinib re-challenge in small cell lung cancer transformation after chemotherapy failure in a patient with ALK-positive lung cancer: A case report. Respir Med Case Rep 2021;33:101440. 10.1016/j.rmcr.2021.101440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oya Y, Yoshida T, Uemura T, et al. Serum ProGRP and NSE levels predicting small cell lung cancer transformation in a patient with ALK rearrangement-positive non-small cell lung cancer: A case report. Oncol Lett 2018;16:4219-22. 10.3892/ol.2018.9158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujita S, Masago K, Katakami N, Yatabe Y. Transformation to SCLC after Treatment with the ALK Inhibitor Alectinib. J Thorac Oncol. 2016;11(6):e67-72. 10.1016/j.jtho.2015.12.105 [DOI] [PubMed] [Google Scholar]
- 12.Budczies J, Kazdal D, Allgäuer M, et al. Quantifying potential confounders of panel-based tumor mutational burden (TMB) measurement. Lung Cancer 2020;142:114-9. 10.1016/j.lungcan.2020.01.019 [DOI] [PubMed] [Google Scholar]
- 13.Tatematsu A, Shimizu J, Murakami Y, et al. Epidermal growth factor receptor mutations in small cell lung cancer. Clin Cancer Res 2008;14:6092-6. 10.1158/1078-0432.CCR-08-0332 [DOI] [PubMed] [Google Scholar]
- 14.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science 2009;324:1670-3. 10.1126/science.1171837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobeika C, Rached G, Eid R, et al. ALK-rearranged adenocarcinoma transformed to small-cell lung cancer: a new entity with specific prognosis and treatment? Per Med 2018;15:111-5. 10.2217/pme-2017-0069 [DOI] [PubMed] [Google Scholar]
- 16.Elsayed M, Christopoulos P. Therapeutic Sequencing in ALK(+) NSCLC. Pharmaceuticals (Basel) 2021;14:80. 10.3390/ph14020080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO Classification of Tumours Editorial Board. WHO classification of tumours. Thoracic Tumours. 5th ed. Lyon: IARC Press, 2019. [Google Scholar]
- 18.Offin M, Chan JM, Tenet M, et al. Concurrent RB1 and TP53 Alterations Define a Subset of EGFR-Mutant Lung Cancers at risk for Histologic Transformation and Inferior Clinical Outcomes. J Thorac Oncol 2019;14:1784-93. 10.1016/j.jtho.2019.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murase T, Takino H, Shimizu S, et al. Clonality analysis of different histological components in combined small cell and non-small cell carcinoma of the lung. Hum Pathol 2003;34:1178-84. 10.1053/j.humpath.2003.05.001 [DOI] [PubMed] [Google Scholar]
- 20.Qin J, Lu H. Combined small-cell lung carcinoma. Onco Targets Ther 2018;11:3505-11. 10.2147/OTT.S159057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisch D, Christopoulos P. Double trouble: combined large-cell neuroendocrine and small-cell lung carcinoma. Transl Cancer Res 2022;11:3006-11. 10.21037/tcr-22-1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W, Xu C, Chen H, et al. Genomic alterations and clinical outcomes in patients with lung adenocarcinoma with transformation to small cell lung cancer after treatment with EGFR tyrosine kinase inhibitors: A multicenter retrospective study. Lung Cancer 2021;155:20-7. 10.1016/j.lungcan.2021.03.006 [DOI] [PubMed] [Google Scholar]
- 23.Yin X, Li Y, Wang H, et al. Small cell lung cancer transformation: From pathogenesis to treatment. Semin Cancer Biol 2022;86:595-606. 10.1016/j.semcancer.2022.03.006 [DOI] [PubMed] [Google Scholar]
- 24.Wiedemann C, Kazdal D, Cvetkovic J, et al. Lorlatinib and compound mutations in ALK+ large-cell neuroendocrine lung carcinoma: a case report. Cold Spring Harb Mol Case Stud 2022;8:a006234. 10.1101/mcs.a006234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Dong F, Su J, et al. Resistance to Both Chemotherapy and EGFR-TKI in Small Cell Lung Cancer With EGFR 19-Del Mutation: A Case Report. Front Oncol 2020;10:1048. 10.3389/fonc.2020.01048 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as