Abstract
Mass spectrometry is widely used for quantitative proteomics studies, relative protein quantification, and differential expression analysis of proteins. There is a large variety of quantification software and analysis tools. Nevertheless, there is a need for a modular, easy-to-use application programming interface in R that transparently supports a variety of well principled statistical procedures to make applying them to proteomics data, comparing and understanding their differences easy. The prolfqua package integrates essential steps of the mass spectrometry-based differential expression analysis workflow: quality control, data normalization, protein aggregation, statistical modeling, hypothesis testing, and sample size estimation. The package makes integrating new data formats easy. It can be used to model simple experimental designs with a single explanatory variable and complex experiments with multiple factors and hypothesis testing. The implemented methods allow sensitive and specific differential expression analysis. Furthermore, the package implements benchmark functionality that can help to compare data acquisition, data preprocessing, or data modeling methods using a gold standard data set. The application programmer interface of prolfqua strives to be clear, predictable, discoverable, and consistent to make proteomics data analysis application development easy and exciting. Finally, the prolfqua R-package is available on GitHub https://github.com/fgcz/prolfqua, distributed under the MIT license. It runs on all platforms supported by the R free software environment for statistical computing and graphics.
Keywords: proteomics, statistical software, differential expression analysis
Introduction
Proteins carry out the most crucial functions and give structure to living cells. Hence, measuring changes in protein abundance is the subject of active research.1 Bottom-up mass spectrometric methods, where proteins are specifically and reproducibly digested into protein fragments—peptides, are employed to identify and quantify proteins in complex samples containing hundreds to thousands of proteins.2,3 The peptides are first separated by their chemical and physical properties using liquid chromatography (LC). Afterward, they are ionized, weighed, identified, and quantified using mass spectrometric techniques, e.g., electro-spray-ionization mass spectrometry (ESI-MS). Finally, peptide identification is achieved by fragmenting and matching the measured fragment masses to theoretical masses computed from known peptide sequences.4−6 For quantification, intact peptide ions7,8 or products of peptide ion fragmentation9,10 are counted and aggregated to obtain peptide abundances. Finally, the identified and quantified peptides are assigned to proteins based on protein sequence information.11
Proteomics quantification experiments enable monitoring of the relative abundances of thousands of proteins in biological samples. Most studies use parallel-group designs, where one or many treatment groups are compared to the control group.12,13 More recently, more complex experimental designs with an increasing number of samples have been studied, e.g., factorial designs and longitudinal studies (time series), sometimes with repeated measurements on the same subject.14,15 The data can be modeled using linear fixed-, mixed-, or random-effects models.16 Based on these models, tests can be applied to examine whether specific factors and factor interactions are significant; e.g., it can be tested if differences in protein abundance between groups are statistically significant.
An important aspect of mass spectrometric data are missing peptide and protein quantifications. Rubin17 classified missing data problems into three categories: missing completely at random (MCAR), missing at random (MAR), and missing not at random (MNAR). For instance, in data-dependent acquisition (DDA) MS, only a limited number of MS1 signals are selected for fragmentation and identified. Peptide quantification algorithms transfer identification information between MS1 features in different samples by aligning the data using retention time and mass information, thus reducing the amount of missing data. Nevertheless, highly abundant proteins can suppress the detection of other proteins in a sample, a MAR mechanism. Furthermore, a weak correlation between the number of missing measurements in a group and the abundance of the quantified protein can be observed, caused by the limit of detection (LOD), an MNAR mechanism.18
Several data analysis packages exist to model MS protein quantification experiments, e.g., limma,19MSstats,20PECA,21msqrob2,22 or proDA,23 to mention some, all implemented in R.24 Each of them has some unique features; for example, MSstats determines the statistical model from the structure of the sample annotation, which allows users with limited statistical knowledge to perform differential expression analysis (DEA). At the same time, limma enables the specification of a design matrix using a linear model formula and implements the empirical Bayes variance shrinkage method. The package PECA performs a roll-up of peptide level differences and peptide level p-value estimates obtained from limma or PECA, to protein level estimates. Furthermore, msqrob2 combines robust linear models fitted to protein abundances and a quasibinomial generalized linear model fitted to peptide counts into Hurdle model. Finally, the proDA package implements a linear probabilistic dropout model to account for missing data without imputation.
Of note are the various approaches to handling missing observations, which are inherent in mass spectrometric bottom-up experiments. For instance, MSstats handles missing data by feature filtering and imputation. Other means of modeling missing observations are the Hurdle models discussed by Goeminne et al.,25 while the R-package proDA models missingness using probabilistic dropout models.23
We can use all the R-packages discussed when analyzing parallel-group designs using a single explanatory variable and contrasting groups. However, we can use only some of them to model factorial designs or repeated measurements. Table 1 gives an overview of the models and features these packages support. We see that, for instance, limma and proDA allow us to fit a comprehensive variety of models and test various hypotheses; however, good knowledge of the design matrix specification using the R formula interface is required.26
Table 1. Models supporteda by R-Packages used for differential protein expression analysis.
| pd | rm | eb | fd | int | mem | md | |
|---|---|---|---|---|---|---|---|
| PECA | Y | Y | Y | NA | NA | NA | NA |
| limma | Y | Y | Y | Y | Y | NA | NA |
| MSstats | Y | Y | NA | Y | Y | Y | NA |
| proDA | Y | Y | Y | Y | Y | NA | Y |
| msqrob2 | Y | Y | Y | Y | Y | Y | Y |
| prolfqua | Y | Y | Y | Y | Y | Y | Y |
pd, parallel design; rm, repeated measurements; fd, factorial design; int, interactions among factors; mem, mixed effect models; eb, empirical Bayes; md, missing data modelling (no imputation needed); Y, yes.
When developing the R-package prolfqua, we were inspired by the R-package caret(27) which enables us to call various machine learning (ML) methods and makes selecting the best ML algorithm for your problem easy. We aimed for a similar R-package for the DEA of quantitative proteomics data. However, the existing packages differ widely regarding supported designs, model specifications, and output formats. At the same time, they share the following features: fitting linear models to either peptide or protein abundances, determining differences among groups, and afterward applying empirical Bayes variance shrinkage. Therefore, the revised goal was to provide a modular object-oriented design, with R linear models as a core, and add features such as p-value aggregation, variance shrinkage, or modeling of missing observations.
Furthermore, prolfqua also includes methods specific to proteomics data. For example, we implemented strategies to estimate protein intensities from peptide intensities: top N,28 Tukey’s median polish,29 and robust linear models.25 Peptide or protein abundances can then be scaled and transformed using robust scaling, quantile normalization, or vsn to remove systematic differences among samples and heteroscedasticity. In this respect, prolfqua can be compared with R-packages such as DEP(30) or POMA(31), which support the entire DEA pipeline.
Since group sizes are relatively small, typically with four or five subjects per group, the high power of the tests is a relevant criterion to assess the modeling method. The quantified proteins can be ranked using the estimated fold-change, t-statistics, or scaled p-value and subjected to gene set enrichment (GSEA) or over-representation analysis32 to determine up or downregulated groups of proteins. Furthermore, the statistical model must provide an unbiased estimate of the false discovery rate (FDR) to manage expectations when selecting protein lists for follow-up experiments. We will use the partial area under the receiver operator curve (ROC) to assess the power of the tests and compare the FDR with the false discovery proportion (FDP). We use the IonStar(33) and CPTAC(34) data sets, processed with MaxQuant and FragPipe, to benchmark the modeling methods implemented in prolfqua and to compare our results with those of MSstats, msqrob2, and proDA. Although other benchmark data sets exist,35,36 the IonStar data set has the advantage that the expected differences, for the spike in proteins, among groups are small compared to other benchmark data sets, making DEA more difficult and enabling us to see performance differences among the modeling methods.
Methods
Implementation
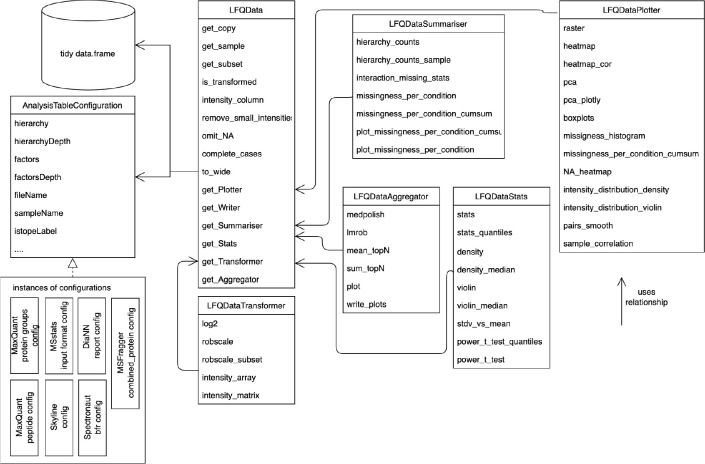
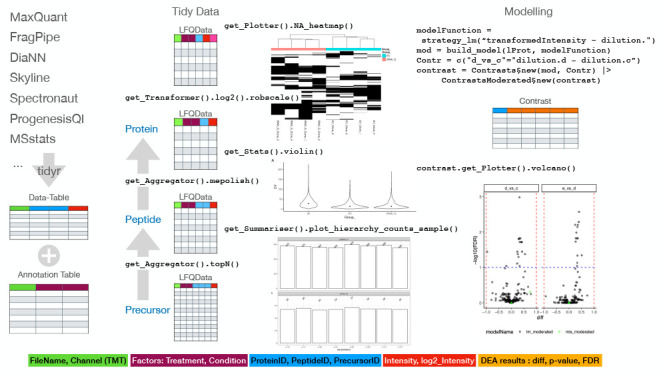
We store all the data needed for analysis in a data frame as tidy data; i.e., every column is a variable, every row an observation, and every cell a single value.37 Using an R638 configuration object (Figure 1), we specify which variable is in which column making it easy to integrate new inputs in prolfqua if provided as tidy data. For example, to visualize tidy Spectronaut,39 DiaNN,10 Skyline40 outputs, or data in MSstats(20) format, only a few lines of code are needed to update the prolfquaAnalysisTableConfiguration configuration. The configuration encapsulates the differences among the various input formats in column names and enables the using methods without additional arguments. An example code for creating a FragPipe(7) configuration can be found in Section S3, “Creating a Prolfqua Configuration”. We implemented methods that transform the data into tidy data for popular software like MaxQuant,8 or FragPipe, which stores the same variable, e.g., intensity, in multiple columns, one for each sample. Relying on the tidy data table enables us to interface with many data manipulation, visualization, and modeling methods, implemented in base R(24) and the tidyverse41 easily. We use R6 classes to structure the functionality of the package (see Figures 1 and 2). R6 classes are well supported by command-line completion features (see Figure S8 in the SI) in RStudio,42 and help to implement argument-free functions.
Figure 1.
Class diagram of classes representing the proteomics data. The LFQData class encapsulates the quantitative proteomics data stored in a table of tidy data. An instance of the AnalysisTableConfiguration class specifies a mapping of table columns to sample names, peptide or protein identifiers, explanatory variables, and response variables. The LFQDataPlotter class and other classes decorate the LFQData class with additional functionality. For instance, the LFQDataStats and LFQDataSummary reference the LFQData class and group methods for variance and sample size estimation or summarizing peptide and protein counts. Furthermore, the LFQDataTransformer and LFQDataAggregator classes group functions for data normalization and estimating protein from peptide intensities.
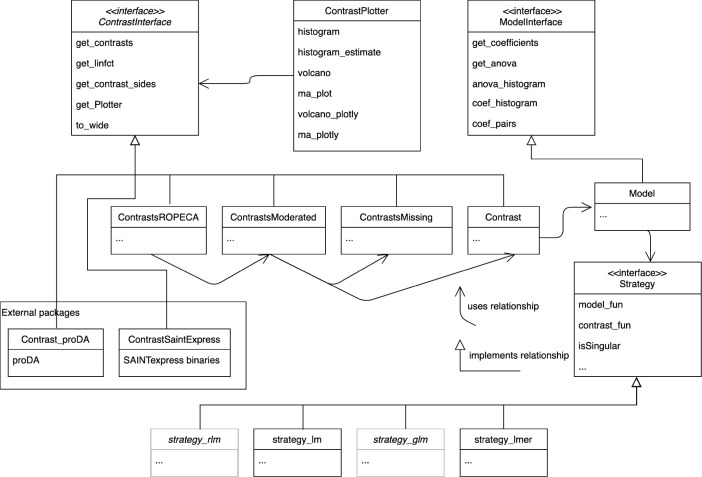
Figure 2.
Unified modeling language (UML) diagram of modeling- and contrast-related classes. Different strategies, e.g., lm, lmer, and glm (Table 2), reference methods to fit models, and compute contrasts. The model builder method fits the statistical model given the data and a strategy. The models are used to analyze variance (ANOVA) or to estimate contrasts. All classes estimating contrasts implement the ContrastsInterface. Results of external tools, e.g., SAINTexpress, or proDA are adapted to implement the Contrasts interface.
R’s formula interface for linear models is flexible, widely used, and well documented.26,43 We use the formula interface to specify the models, making it easy to reproduce an analysis performed with prolfqua in other statistical programming languages. In addition, we implement features specific to high-throughput experiments, such as the empirical Bayes variance and p-value moderation, which utilizes the parallel structure of the protein measurements and the analysis.19 We also compute probabilities of differential protein regulation based on peptide-level models.21 We used R6 classes to encapsulate the statistical modeling functionality in prolfqua (see Figure 2). We specify a contrast interface (ContrastsInterface). Several implementations allow the performance of DEA given linear or mixed effect models (Contrasts), variance shrinkage (ContrastsModerated), or to estimate contrasts in cases when observations are missing for an entire group of samples (ContrastsMissing). Further implementations of the interface encapsulate and integrate DEA results of external tools such as proDA or SAINTexpress(44) used to analyze data from protein interaction studies.
Data Sets for Benchmarking
IonStar
To evaluate the performance of DEA, we use the IonStar benchmark data set,33 available from the Proteomics Identifications Database (PRIDE) identifier PXD003881. DH5α Escherichia coli lysate was spiked in human pancreatic cancer cells (Panc-1) lysate at five levels: 3%, 4.5%, 6%, 7.5%, and 9% E. coli. We annotated these dilutions from smallest to largest with the letters a–e. By comparing the various dilutions, we obtain different effect sizes; e.g., when comparing dilution e (9%) against dilution d (7.5%), the expected difference is 1.2 for E. coli proteins and 1 for human proteins. There are four technical replicates for each dilution, hence 20 raw files in total. To compare the performance of the various methods implemented in prolfqua, we use only the contrasts resulting in minor differences Δ = (1.20, 1.25, 1.30, 1.50), because for bigger differences, all models perform similarly.
IonStar/MaxQuant
We processed the raw data of the IonStar data set using MaxQuant(8) Version 1.6.10.43, with MaxQuant default settings for Orbitrap data. The text files generated by MaxQuant are available in the prolfquadata R-package.45MaxQuant produces various output files which can be used for DEA. We are using the quantification results reported in the “peptide.txt” file for DEA. However, MSstats is using the “evidence.txt” file for the DEA.
IonStar/FragPipe
We processed the raw data of the IonStar data set using FragPipe(7) Version 14, with the default workflow for label-free quantification with match between runs enabled. The text files generated by FragPipe are available in the prolfquadata R-package.45 Similarly to MaxQuant, the FragPipe software produces various outputs which can be used for DEA. We used the total protein intensities reported in the “combined_protein.tsv” file as input for the DEA and called this data set IonStar/FragPipe/combined_protein.tsv. Alternatively, we benchmarked the DEA using the “MSstats.tsv” file as input and called this data set IonStar/FragPipe/MSstats.tsv.
CPTAC/MaxQuant
We used the CPTAC data set, available in the R-package msdata, and described in reference (22). In brief, the Sigma Universal Protein Standard mixture 1 (UPS1) containing 48 different human proteins was spiked in a protein background of 60 ng/μL Saccharomyces cerevisiae strain BY4741. Two different spike-in concentrations were used, 6A (0.25 fmol UPS1 proteins/μL) and 6B (0.74 fmol UPS1 proteins/μL). Three replicates are available for each concentration. The data were searched with MaxQuant version 1.5.2.8.
Data Preprocessing for Model Comparison
The peptide abundances (from the MaxQuant peptide.txt file) were log2 transformed and subsequently scaled, where median and the mean absolute deviation was obtained from the human proteins only. We removed one-hit wonders, i.e., proteins with a single peptide assignment. Protein abundances are inferred from the peptide intensities using Tukey’s median polish. Finally, we fitted the fixed effect linear models, the dropout model proDA to protein abundances, the mixed effect linear model, the ROPECA model, and the hurdle model implemented in msqrob2 to peptide intensities.
Benchmark Metrics
The IonStar data set contains Homo sapiens proteins with constant concentrations and E. coli proteins with varying concentrations. We know that for H. sapiens proteins, the difference β between two dilutions should be β = 0, while for E. coli proteins, we know that the difference between dilutions should be β ≠ 0.
We can use various
statistics to examine the alternative hypothesis β ≠
0: the contrast estimate, i.e., the log2 fold-change β,
the t-statistic 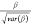 , or the p-value and moderated p-value. For each statistic and each value of the statistics
we then compute a confusion matrix (see Table 3). From the confusion matrix we obtain measures
such as true positive rate (TPR), false positive
rate (FPR), or false discovery proportion (FDP) which are given in Table 3 with
, or the p-value and moderated p-value. For each statistic and each value of the statistics
we then compute a confusion matrix (see Table 3). From the confusion matrix we obtain measures
such as true positive rate (TPR), false positive
rate (FPR), or false discovery proportion (FDP) which are given in Table 3 with
| 1 |
| 2 |
| 3 |
Table 3. Confusion Matrixa.
| prediction/truth | E. coli | H. sapiens | total |
|---|---|---|---|
| beta != 0 | TP | FP | R |
| beta == 0 | FN | TN | |
| total | P | N | m |
TP, true positive; FP, false positive; FN, false negative; TN, true negatives; P, all positive cases (all E. coli proteins); N, all negative cases (all H. sapiens proteins); m, all proteins.
In order to compute the confusion matrices based on the p-value we first need to rescale it (see eq 11).
By plotting the TPR versus the FPR we obtain the receiver operator characteristic curve (ROC curve).46 The area under the curve (AUC) or partial areas under the curve (pAUC), at various values of the FPR, are measures of performance derived from the ROC curve. Using these measures, we can compare the performances of the statistics for a single model or the various models and test if the differences are statistically significant, using a test to compare ROC curves.
Modeling
Robust Scaling of the Data
Välikangas et al.47 discuss and benchmark various methods of peptide or protein intensity normalization, such as variance stabilizing normalization48 or quantile normalization.49 In this work, we use a robust version of the z-score, where instead of the mean we use the median x̃, and instead of the standard deviation we use the median absolute deviation S̃:
| 4 |
Because we need to estimate the differences among groups on the original scale, we must multiply the z-score by the average standard deviation of all the n samples in the experiment.
| 5 |
To apply this transformation, we need to estimate two parameters per sample; therefore, it works for experiments with thousands of proteins and experiments where only a few hundred proteins per sample are measured. For the Ionstar data set, we used the intensities of H. sapiens proteins, whose concentrations do not change, to determine x̃ and S̃ and then applied it to all the intensities (including E. coli) in the sample.
Estimating Differences between Groups
Given a linear model y = βX, we can compute the difference βc between two groups by the dot product of weights c and model parameters β, where c is a column vector with as many elements as there are coefficients β in the linear model. If c has 0 for one or more of its rows, then the corresponding coefficient in β is not involved in determining the contrast.50
The difference estimate βc is given by the dot product
| 6 |
and the variance of βc by
| 7 |
with X being the design matrix. The degrees of freedom for the contrast are equal to the residual degrees of freedom of the linear model. For estimating contrasts from mixed effects models we used the function contest implemented in the R-package lmerTest and used the Satterthwaite51 method to estimate the denominator degrees of freedom. These methods are available in the class Contrast (see Figure 2).
The package prolfqua provides functions to determine the vector of parameter weights c, from a linear model and a contrast specification string. In section Material S10 in the SI, we provided an example of how to specify contrasts for a data set with two explanatory variables and an interaction term.
Contrast Estimation in the Presence of Missing Data Using LOD
Missing observations lead to different group sizes, which results in unbalanced designs. Linear and mixed effect models can handle unbalanced designs. As long as at least one observation in a group is available, and sufficient observations to estimate the variance are available, they will produce unbiased estimates. Therefore, no imputation is needed.
However, if there is no observation in a group the model fit fails. For example, suppose a protein is unobserved in all the samples of a group. In that case, a plausible explanation is that the protein abundance is below the limit of detection (LOD) of the MS instrument. In such a case, we will substitute the group mean using the expected protein abundance A at the LOD ALOD. To estimate ALOD we are using the protein abundances of those groups where the protein was observed in only a single sample (see section Material S8 “Estimating ALOD”, in the SI). Typically there are many such cases, and hence we take the median.
When
computing differences Δ among two groups a and b, we will use either the group mean a̅ or b̅ estimated from the
data. However, if for instance no observations are present in group b, we will use 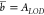 . Furthermore, if
. Furthermore, if 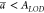 , we also set
, we also set 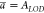 , or more formally
, or more formally
We use the pooled variance in all groups to estimate the protein variance, assuming they are the same. The pooled variance sp2 is given by
| 8 |
with ni the number of observations, and si the standard deviation in each group. The standard deviation for the t-statistics is then given by
| 9 |
Where ng is the number of groups, and n is the number of observations. If variance cannot be estimated for a protein, because there are too few observations in other groups, we use the median pooled variance of all other proteins in the data set. This method is implemented in the class ContrastsMissing (see Figure 2).
p-Value Moderation
From the linear and the mixed effect models, we can obtain the residual standard deviation σ, and degrees of freedom df. Smyth52 discuss how to use the σ and df of all models to estimate the corresponding priors and posterior σ̃. These can be used to moderate the t-statistics by
| 10 |
We implemented this method in the class ContrastModerated (Figure 2).
Summarizing Peptide Level Differences and p-Values on Protein Level
To summarize peptide level models to protein models, we apply the method suggested by Suomi and Elo21 that uses the median scaled p-value of the peptide models and the cumulative distribution function of the Beta distribution (CDF) to determine a regulation probability of the protein.
To obtain the p̃ of a protein we first rescaled the peptide p-values by taking the sign of the fold-change β̂ into account, i.e.:
| 11 |
Afterward, the median scaled p-value 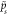 is determined and, using the transformation
below, transformed back onto the original scale:
is determined and, using the transformation
below, transformed back onto the original scale:
| 12 |
Because we use the median with the ith order statistic 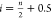 , we parametrize the CDF of the Beta distribution
with
, we parametrize the CDF of the Beta distribution
with 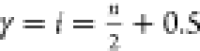 and
and 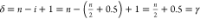 . We implemented this method in the class ContrastROPECA (Figure 2).
. We implemented this method in the class ContrastROPECA (Figure 2).
Results and Discussion
Example Analysis Workflow
The code snippets in this
section demonstrate how a DEA workflow can be implemented using the prolfqua R-package (see Material S1 "How to Install prolfqua and prolfquabenchmark" in
the
SI). To speed up the computation of these examples, we use a subset
of the Ionstar data set generated by randomly selecting 400 proteins.
First, we remove all proteins with a single peptide and all observations
for which MaxQuant reports zero intensities, leaving
332 proteins. Next, peptide abundances are log2 transformed
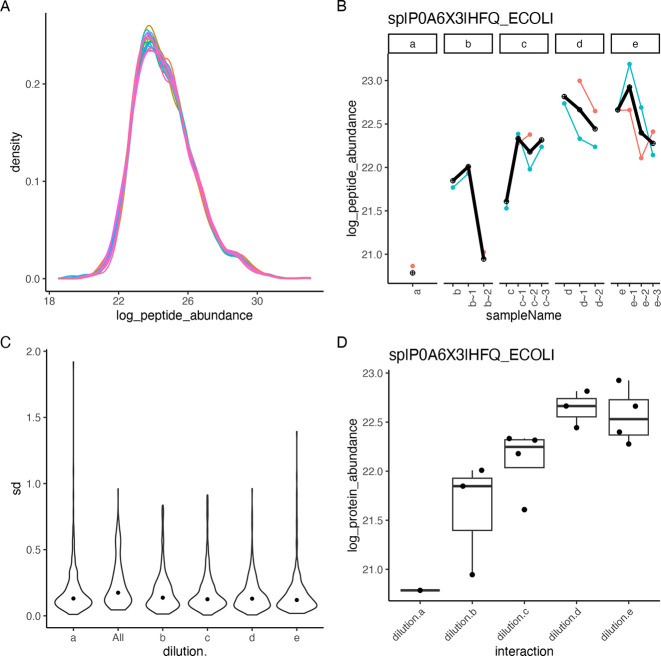
and robust z-score scaled using the method robscale. Then, using the LFQDataPlotter class, we show the distribution of the normalized peptide abundances
in Figure 3A. Afterward,
protein intensities are estimated from peptide intensities using Tukey’s
median polish. Figure 3B shows the peptide intensities and the estimated protein intensities.
Next, we compute the standard deviation of all the proteins in each
group and display their distribution using violin plots (Figure 3C). Finally, we create
a box plot (Figure 3D) showing the abundance of one protein.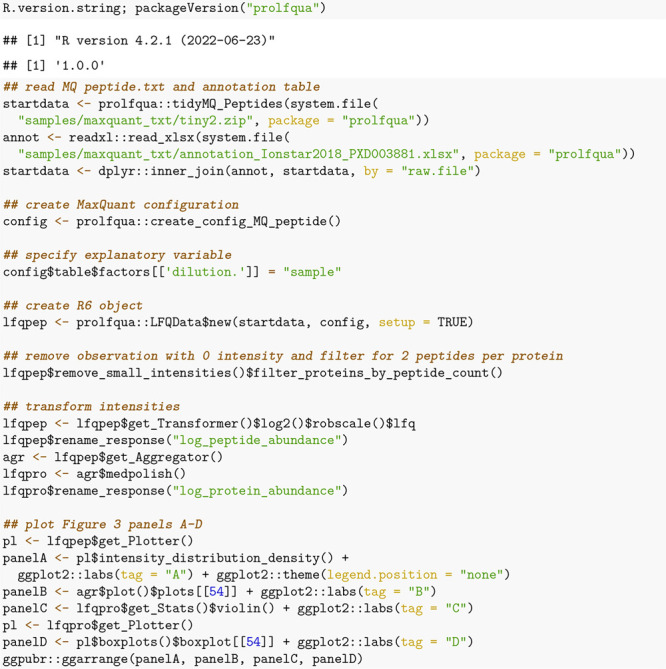
Figure 3.
(A) Density plot of peptide intensity distributions for 20 samples. For each sample a line with a different color is shown. (B) Peptide intensities for protein HFQ_ECOLI are shown using lines of different colors, and the protein intensity estimate is shown using a fat black line. (C) Distribution of standard deviations of all proteins in each dilution group (a–e) and overall (all). (D) Distribution of protein intensities of Protein HFQ_ECOLI in each dilution group.
The following code example illustrates how we compute
differences
among groups. First, the linear model and the differences are specified.
Afterward, the model is fitted to the data using the build_model function. Next, we estimate the contrasts from the linear model
using the Contrasts class or directly from
the data using the ContrastsMissing class.
Afterward, we apply t-statistic moderation using
the ContrastModerated class. Finally, the merge_contrasts_results function merges both sets of
contrast estimates, preferring the one obtained from the linear model
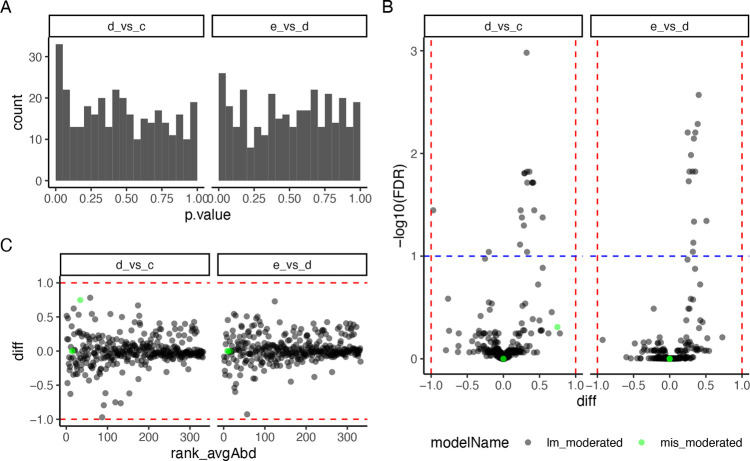
if both are available. Then we create the plots shown in Figure 4. Figure 4A shows the distribution of
the p-values, Figure 4B is the volcano plot for each comparison, and Figure 4C is a Bland–Altman
plot reporting the difference between groups as a function of the
rank of the protein abundance.
Figure 4.
(A) Histogram showing the distribution of p-values for 332 proteins for contrasts “e_vs_d” and “d_vs_c”. (B) Volcano plot showing −log10 transformed FDR as a function of the difference between groups for 332 proteins. With black dots, we show effect size and FDR estimates obtained from the linear model, while in green, we plot those obtained using imputation. (C) Difference between groups, as a function of the rank of the abundance of the proteins.
The R linear and mixed effect models allow modeling parallel designs, repeated measurements, factorial designs, and many more features. Models in prolfqua are specified using R’s linear and mixed model formula interface. Therefore, knowledge of the R regression model infrastructure43,53 is advantageous when using our package. Furthermore, this glass box approach should make it easy to reimplement an analysis performed with prolfqua using base R or other programming languages by reading the analysis script. However, in the package documentation, we showcase how a user, without this knowledge, can analyze experiments with a parallel-group design and a factorial design.
Using the data frame of tidy data ensures interoperability with other proteomics-related packages that manage their data with tidy-tables, e.g., protti.54 To simplify the integration of prolfqua with Bioconductor-based workflows, we provide a method that converts the LFQData class into a SummarizedExperiment. The use of R6 classes, which encapsulate the configuration and the data, allow for writing very concise code where functions can have few arguments. Autocompletion support for R6 classes in the RStudio editor makes it easy for novices to explore prolfqua’s functionality (see Figure S8 in the SI).
To ease the usage barriers of the R-package to users not familiar with statistics and R programming, we developed an application based on the prolfqua package into our data management platform B-Fabric.55,56 The B-Fabric system runs a computing infrastructure controlled by a local resource management system that supports cloud-bursting.57 This integration enables users to select the input data and basic settings in a graphical user interface (GUI). This way, prolfqua, and B-Fabric help scientists meet requirements from funding agencies, journals, and academic institutions while publishing their data according to the FAIR58 data principles. We are working on creating a shiny standalone application with the described functionality and making it available soon.
Benchmarking Modeling Approaches
Using a benchmark data set with known ground truth (see the Methods section), we assessed the performance of different modeling approaches implemented in prolfqua, MSstats, proDA, and msqrob2. Table 4 summarizes which methods we have evaluated, which MaxQuant files we used as input, and if the models are fitted to peptide or protein intensities. We make the R-markdown files to replicate the benchmarking available at prolfquabenchmark (see Material S2 “Benchmark Vignettes (IonStar/MaxQuant)” in the SI).
Table 4. All Benchmarked Modelsa.
| label | description | abundance | input file |
|---|---|---|---|
| MSstats | preprocess with default parameters | precusor | evidence.txt |
| msqrob2 | merge of msqrobHurdleIntensity and msqrobHurdleCount (msqrobHurdle) | protein and peptide | peptide.txt |
| proDA | probabilistic dropout model | protein | peptide.txt |
| prolfqua_missing | ContrastsMissing, ContrastsModerated | protein | peptide.txt |
| prolfqua_lm_mod | strategy_lm, Contrasts, ContrastsModerated | protein | peptide.txt |
| prolfqua_merged | addContrastResults(prefer = proflqua_lm_mod, add = prolfqua_missing)b | protein | peptide.txt |
| prolfqua_mix_eff_mod | strategy_lmer, Contrasts, ContrastsModerated | peptide | peptide.txt |
| prolfqua_ropeca | strategy_lm, Contrasts, ContrastsModerated, ContrastsROPECA | peptide | peptide.txt |
Label, name of the method; description, functions used in the respective packages; abudances, indicates if model is fitted to peptide or protein abundances; input file, name of MaxQuant file used as input.
“prolfqua_merged”, augments estimates which are missing in “prolfqua_lm_mod” with those from “prolfqua_missing”.
The IonStar/MaxQuant data set (see the Methods section) captures only the variance from the chromatography, electrospray, and mass spectrometric measurements since only technical replicates are available for each dilution. Therefore, essential sources of variation typically present in other experiments, such as biochemical and biological ones, are not measured. Furthermore, this data set with a parallel-group design does not allow for benchmarking models with interactions. Thus, while we can extrapolate some of the results to more realistic data sets, we must be careful not to overinterpret our findings. Specifically, the observed variances will be higher in data sets with biological replicates, and the power will be lower for the same number of samples. Furthermore, the proportion of missing observations in real-life data sets might be higher or distributed differently in groups.
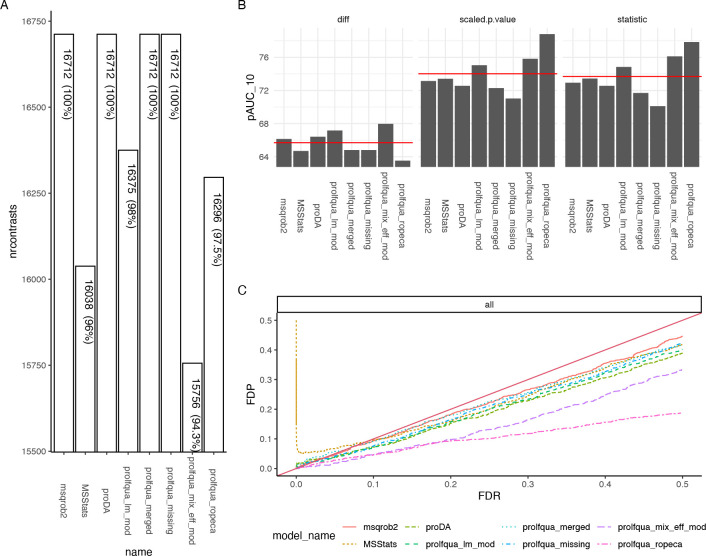
When comparing DEA performance, a relevant parameter is the number of differences among conditions a method can estimate (see Figure 5A). For each protein, we tried to determine four differences [Δ = (1.20, 1.25, 1.30, 1.50)], and therefore, given 4178 proteins with at least two peptides, there are, in total, 16712 possible differences. Since msqrob2, proDA, proflqua_missing, and prolfqua_merged directly model missing observations, they estimate all possible contrasts. However, some models fail to estimate differences when abundances are unobserved or rely on imputation. For instance, when using the mixed effect models, sensitive to missing data, we estimate the fewest number of contrasts with 15756.
Figure 5.
(A) Number of estimated contrasts for each modeling method (higher is better). (B) Partial area under the ROC curve at 10% FPR (pAUC10) for all contrasts and three different statistics: the difference among groups (diff, panel B left), the scaled p-value (sign(diff)·p.value) (scaled.p.value, panel B center), and the t-statistics (statistic, panel B right), where a higher pAUC10 is better. The red line indicates the average area under the curve of all methods. (C) Plot of the false discovery proportion (FDP) as a function of the FDR. Ideally, the FDR should be equal to the FDP. Therefore, larger distances from the diagonal are worse.
The benchmark functionality of prolfqua includes receiver operator curves (ROC) and computes partial areas under those curves (pAUC) and other scores, e.g., the false discovery proportion FDP. Since the set of effect size estimates will differ for some methods, e.g., 16712 vs. 15756 (see Figure 5A), this introduces a bias when computing receiver operator curves and the pAUC. Hence, to conclude that one method performs better, it does not suffice if the pAUC is greater, but the number of proteins with differential expression results needs to be equal or larger. However, for proDA, msqrob2, and prolfqua_merged, we can compare the pAUC to asses which method performs best.
Figure 5B shows how various estimates obtained from the models, i.e., the difference between groups, t-statistics, and scaled p-values, allow identifying true positives (TP) given a false positive rate (FPR) of 10% by displaying the partial area under the ROC (pAUC10). Ordering proteins by the t-statistic or p-value leads to a higher pAUC10 than when ordering by the estimated difference among groups.
We can conclude that if we want to sort the proteins according to the likelihood of being differentially regulated to perform gene set enrichment analysis,32 the t-statistic is better suited than the fold-change estimate. When computing the p-values from the t-statistics, we incorporate the degrees of freedom, improving the inferences (see Figure 5B, center versus left). There is no such improvement for the mixed effect model. The reason is an erroneous denominator degree of freedom estimation for many proteins, a known problem in the case of mixed effect models. Furthermore, for the fixed effect linear model, the empirical Bayes variance shrinkage, as suggested by Smyth,52 consistently improves the ranking of proteins compared with the unmoderated estimates (not shown). However, since also for this method, a correct degree of freedom estimate is required, it does not work for mixed effect models.
Suppose an accurate estimate of the difference among groups is essential. In that case, among the models fitted to protein intensities, calculated using Tukey’s median polish, the proDA model performed best (see Figure 5B left). The dropout model more accurately models the posterior protein intensities, compared with prolfqua_missing, which uses a point estimate of the LOD. Furthermore, the prolfqua_ropeca model that first fits peptide level models and then summarizes differences performed worst. We speculate that the peptide-level outliers do not affect the protein estimates when using Tukey’s median polish method.
We also benchmark if the FDR obtained from a model is an unbiased estimate of the false discovery proportion FDP. Figure 5C shows the FDP, obtained from the confusion matrix, as a function of the FDR determined from the model. Most lines are below the diagonal, which indicates that the FDR estimates are modestly conservative for this particular benchmark data set. In the case of MSstats, we observe a high proportion of false discoveries for small FDR values. In the case of the prolfqua_ropeca method, the FDR estimates, obtained by applying the Benjamini–Hochberg correction to the Beta distribution-based regulation probabilities, strongly overestimate the FDP.
However, computing the t-statistics at the peptide level and then summarizing it for each protein using their median produces the highest pAUC10 scores among all the tested models (see Figure 5B prolfqua_ropeca). Furthermore, by using the Beta distribution to model the number of peptides observed, we can further improve the pAUC scores (see Figure 5B center). However, the properties of Beta-based probabilities need to be better understood; their distribution is not uniform under the null hypothesis (see section Material S9 “The probabilities produced by ROPECA are not p-values” in the SI). Therefore, the resulting FDR estimates are biased (see Figure 5C). Consequently, we cannot recommend this method if an unbiased estimate of FDR is essential, which is frequently the case. In addition, peptides are more strongly affected by missing values, reducing the number of contrasts we could estimate for the data set using this method (see Figure 5C).
The R-packages proDA, msqrob2, and prolfqua do not impute missing data but integrate them into the statistical model, while MSstats filters and imputes the data using an accelerated failure model. Despite imputation, MSstats estimates fewer group differences (16038) and does not achieve a higher pAUC10 (see Figure 5). Furthermore, Figure 5C shows that when using MSstats, the proportion of false discoveries might be very high for a low FDR because of false positives. Hence, augmenting the linear model for handle missing observations using the quasi-binomial generalized linear model, the dropout model, or estimating missing differences using the LOD simplifies the analysis pipeline since no imputation is needed and improves the quality of the estimates.
Of note, MSStats uses the evidence.txt file, while all the other methods use peptide.txt files as input (see Table 4). Furthermore, MSstats uses equalized medians normalization, while all the other methods use robust scaling (see the Methods section). These are possible confounding factors to consider. Finally, while prolfqua, as well as proDA, is highly modular, and to a lesser extent msqrob2, enabling us to use the same data preprocessing and normalization, MSstats is monolithic, making it unfeasible to use a preprocessing or normalization method not available in MSstats.
We obtained difference and FDR estimates for all proteins and comparisons, as shown in Figure 5A when using (a) the probabilistic dropout model (proDA), (b) the hurdle model (msqrobHurdle), and (c) prolfqua_merged. We observe that the performance of the scaled p-values or the t-statistics are comparable among these three methods (Figure 5B). We tested if there was a significant difference between the pAUC10 for all three methods, but did not reject the null hypothesis that there is no such difference (section Material S3 “DEA benchmark: IonStar/MaxQuant/peptide.txt - Significance test” in the SI). Also, the FDR estimates (Figure 5C) are comparable for all three methods.
Furthermore, all three models perform similarly when examined using a different benchmark data set CPTAC/MaxQuant (see the Methods section). For this data set, proDA performed slightly but not significantly better than prolfqua and msqrobHurdle (and section Material S4 “DEA benchmark: CPTAC/MaxQuant/peptide.txt” in the SI).
In addition, we examined the DEA performance when using protein intensities reported by quantification software FragPipe for the IonStar data set as input. Using protein abundances as input significantly simplifies the analysis and interpretation and might benefit from optimization implemented in the quantification software. However, we can only fit the proDA and prolfqua_merged (see Table 4) models to protein abundances, while MSstats and msqrobHurdle require peptide spectrum match or peptide level abundances. In this DEA benchmark, prolfqua performed slightly but not significantly better than proDA (section Material S5 “DEA benchmark: IonStar/FragPipeV14/combined_protein.tsv” in the SI).
Finally, we also compared the DEA performances when starting the analysis from the precursor abundances reported in the “MSstats.tsv” file, generated by FragPipe v14, from the IonStar data set. Since MSstats, msqrob2, proDA, and prolfqua all read MStstats.tsv files, we could eliminate a confounding factor, i.e., different input abundances (section Material S6 “DEA benchmark: IonStar/FragPipeV14/MSstats.tsv” in the SI). In this DEA benchmark, msqrob2 and prolfqua_merged perform best but not significantly better than proDA or MSstats. Furthermore, by processing the IonStar data set with both MaxQuant and FragPipe, we can compare their performances (section Material S7 “Comparing DEA results for MaxQuant and FragPipe” in the SI).
We focused our benchmark on comparing the statistical modeling methods while we fixed the preprocessing steps. However, some of these steps are of utmost significance when performing differential expression analysis.59 One of them is the normalization of the abundances within the samples to remove systematic differences.60 The method used to infer proteins from peptide identifications11 and protein abundances from peptide abundances is an additional important factor.28 For instance, the original proDA publication uses MaxLFQ61 protein estimates. However, when using MaxLFQ abundances reported by MaxQuant, the pAUC10 is lower [pAUC10(t-statistics) = 66%] compared with results obtained when protein abundances are estimated from peptide abundances using Tukey’s median polish [pAUC10(t-statistics) = 72%]. Last but not least, the software7,8 used to identify and quantify proteins significantly contributes to the entire pipeline’s performance altering the number of identified proteins and the sensitivity and specificity of the differential expression analysis. In section Material S7 “Comparing DEA results for MaxQuant and FragPipe” in the SI, we compare DEA benchmarking results for the quantification software. While the number of proteins identified with two peptides is practically the same, the DEA benchmark performance differs significantly by ∼10% for the pAUC10 score. This difference is more significant than the differences due to the choice of the modeling method.
Conclusion
prolfqua is a feature-rich, object-oriented, and modular R-package to analyze quantitative mass spectrometric data with simple or complex experimental designs. While other R-packages for differential expression analysis of proteins typically only implement one modeling approach, prolfqua supports various models (see Figure 2 and Table 2). Furthermore, the contrast specification is explicit and consistent for all models and allows for testing interactions. The modular design of prolfqua enables adding new features, e.g., generalized linear models to model the presence or absence of a protein quantification, or robust linear models, in the future. Furthermore, the developed framework can integrate other modeling methods, e.g., the probabilistic dropout model23 or accurate variance estimation.62 Hence, prolfqua enables the implementation of applications where the user can select an alternative normalization method, protein abundance estimation method, or DEA algorithms. Furthermore, this R-package can analyze other types of quantitative proteomics data, e.g., label-free DIA or labeling-based TMT data.
Table 2. prolfqua Functions That Can Be Used to Fit Various Models.
| prolfqua functions | model |
|---|---|
| strategy_lm, Contrasts | linear modeling of peptide or protein abundances and group difference estimation |
| strategy_lmer, Contrasts | mixed effect modeling of peptide or protein abundances and group differences estimation |
| ContrastsMissing | group difference estimation when no observations in one of the groups |
| ContrastsROPECA | estimating group differences for proteins by summarizing peptide differences |
| ContrastsModerated | empirical Bayes variance shrinkage for group difference estimates (limma) |
| runSaint, ContrastsSAINTexpress | protein interaction scoring (SAINTexpress) |
| strategy_proDA,a ContrastsProDA | adapter to the probabilistic dropout model implemented in proDA |
In development.
When comparing statistical modeling methods for the DEA, we assessed performance measures such as the number of estimated contrasts, the pAUC, and if the FDR is an unbiased estimate of the FDP. It is relevant that an analysis pipeline shows good performance in all these categories. The examined models prolfqua_merged, proDa, and msqrob2 performed well in all these categories. Leveraging these computational experiments, we can provide the following advice: (i) Estimate protein abundances from peptide abundances using a robust or nonparametric regression method. (ii) Fit linear models to protein abundances. (iii) Do not impute missing observation but statistically model missingness to estimate parameters, i.e., group differences. (iv) Explicitly report the model used. (v) If the measurements are correlated, as for technical replicates, mixed effect models might work if the sample sizes are large; if not, aggregate the replicates and fit a linear model instead. (vi) If you use fixed effect linear models, apply variance moderation to improve the t-statistics and p-value estimates. (vii) If you want to sort your protein lists to perform gene set enrichment analysis, use the t-statistic instead of the difference.
Acknowledgments
The authors thank the Technology Platform Fund (TPF) of the University of Zurich and all FGCZ proteomics colleagues for fruitful discussions.
Glossary
Abbreviations
- API
application programming interface
- AUC
area under the curve
- CDF
cumulative distribution function
- DEA
differential expression analysis
- DIA
data independent acquisition
- ESI-MS
electro-spray-ionization mass spectrometry
- FAIR
findable, accessible, interoperable, and reusable
- FDP
false discovery proportion
- FDR
false discovery rate
- FP
false positive
- FPR
false positive rate
- LC
liquid chromatography
- LC-MS
liquid chromatography followed by mass spectrometry
- LOD
limit of detection
- MAR
missing at random
- MCAR
missing completely at random
- ML
machine learning
- MS
mass spectrometry
- pAUC
partial area under the curve
- pAUC10
partial area under the curve for an FPR of 0–10%
- ROC
receiver operator curve
- TP
true positive
- TMT
tandem mass tag
- UML
unified modeling language
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.jproteome.2c00441.
Material S1: How to Install prolfqua and prolfquabenchmark; Material S2: Benchmark Vignettes; Material S3: DEA benchmark IonStar/MaxQuant/peptide.txt -S ignificance test; Material S4: DEA benchmark : CPTAC/MaxQuant/peptide.txt; Material S5: DEA benchmark : IonStar/FragPipeV14/combined_protein.tsv; Material S6: DEA benchmark : IonStar/FragPipeV14/MSstats.tsv; Material S7: Comparing DEA results for MaxQuant and FragPipe; Material S8: Estimating ALOD; Material S9: Probabilities produced by ROPECA, which are not p-values; Material S10: Specifying Contrasts for Models with two Factors and Interaction Term; and Material S11: Creating a prolfqua configuration (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Vidova V.; Spacil Z. A review on mass spectrometry-based quantitative proteomics: Targeted and data independent acquisition. Analytica chimica acta 2017, 964, 7–23. 10.1016/j.aca.2017.01.059. [DOI] [PubMed] [Google Scholar]
- Bubis J. A.; Levitsky L. I.; Ivanov M. V.; Tarasova I. A.; Gorshkov M. V. Comparative evaluation of label-free quantification methods for shotgun proteomics. Rapid Commun. Mass Spectrom. 2017, 31, 606–612. 10.1002/rcm.7829. [DOI] [PubMed] [Google Scholar]
- da Veiga Leprevost F.; Haynes S. E.; Avtonomov D. M.; Chang H.-Y.; Shanmugam A. K.; Mellacheruvu D.; Kong A. T.; Nesvizhskii A. I. Philosopher: a versatile toolkit for shotgun proteomics data analysis. Nat. Methods 2020, 17, 869–870. 10.1038/s41592-020-0912-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng J. K.; Hoopmann M. R.; Jahan T. A.; Egertson J. D.; Noble W. S.; MacCoss M. J. A deeper look into Comet—implementation and features. J. Am. Soc. Mass Spectrom. 2015, 26, 1865–1874. 10.1007/s13361-015-1179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F.; Li N.; Yu W. PIPI: PTM-invariant peptide identification using coding method. J. Proteome Res. 2016, 15, 4423–4435. 10.1021/acs.jproteome.6b00485. [DOI] [PubMed] [Google Scholar]
- Kong A. T.; Leprevost F. V.; Avtonomov D. M.; Mellacheruvu D.; Nesvizhskii A. I. MSFragger: ultrafast and comprehensive peptide identification in mass spectrometry–based proteomics. Nat. Methods 2017, 14, 513–520. 10.1038/nmeth.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F.; Haynes S. E.; Teo G. C.; Avtonomov D. M.; Polasky D. A.; Nesvizhskii A. I. Fast Quantitative Analysis of timsTOF PASEF Data with MSFragger and IonQuant. Molecular Cellular Proteomics 2020, 19, 1575–1585. 10.1074/mcp.TIR120.002048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J.; Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nature biotechnology 2008, 26, 1367–1372. 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- Röst H. L.; Rosenberger G.; Navarro P.; Gillet L.; Miladinovic S. M.; Schubert O. T.; Wolski W.; Collins B. C.; Malmström J.; Malmström L.; et al. OpenSWATH enables automated, targeted analysis of data-independent acquisition MS data. Nature biotechnology 2014, 32, 219–223. 10.1038/nbt.2841. [DOI] [PubMed] [Google Scholar]
- Demichev V.; Messner C. B.; Vernardis S. I.; Lilley K. S.; Ralser M. DIA-NN: neural networks and interference correction enable deep proteome coverage in high throughput. Nat. Methods 2020, 17, 41–44. 10.1038/s41592-019-0638-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesvizhskii A. I.; Aebersold R. Interpretation of shotgun proteomic data, the protein inference problem. Molecular & cellular proteomics 2005, 4, 1419–1440. 10.1074/mcp.R500012-MCP200. [DOI] [PubMed] [Google Scholar]
- de Leeuw S. M.; Kirschner A. W.; Lindner K.; Rust R.; Budny V.; Wolski W. E.; Gavin A.-C.; Nitsch R. M.; Tackenberg C. APOE2, E3, and E4 differentially modulate cellular homeostasis, cholesterol metabolism, and inflammatory response in isogenic iPSC-derived astrocytes. Stem cell reports 2022, 17, 110–126. 10.1016/j.stemcr.2021.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubscher D.; Gryder B. E.; Sunkel B. D.; Andresson T.; Wachtel M.; Das S.; Roschitzki B.; Wolski W.; Wu X. S.; Chou H.-C.; et al. BAF complexes drive proliferation and block myogenic differentiation in fusion-positive rhabdomyosarcoma. Nat. Commun. 2021, 12, 1–16. 10.1038/s41467-021-27176-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G.; Wolski W. E.; Kummer S.; Hofstetter M.; Theocharides A. P. A.; Manz M. G.; Aebersold R.; Meier-Abt F. Proteomic identification of proliferation and progression markers in human polycythemia vera stem and progenitor cells. Blood Advances 2022, 6, 3480–3493. 10.1182/bloodadvances.2021005344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Abt F.; Wolski W. E.; Tan G.; Kummer S.; Amon S.; Manz M. G.; Aebersold R.; Theocharides A. Reduced CXCL4/PF4 expression as a driver of increased human hematopoietic stem and progenitor cell proliferation in polycythemia vera. Blood cancer journal 2021, 11, 1–6. 10.1038/s41408-021-00423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D.; Mächler M.; Bolker B.; Walker S. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software, Articles 2015, 67, 1–48. 10.18637/jss.v067.i01. [DOI] [Google Scholar]
- Rubin D. B. Inference and missing data. Biometrika 1976, 63, 581–592. 10.1093/biomet/63.3.581. [DOI] [Google Scholar]
- McGurk K. A.; Dagliati A.; Chiasserini D.; Lee D.; Plant D.; Baricevic-Jones I.; Kelsall J.; Eineman R.; Reed R.; Geary B.; et al. The use of missing values in proteomic data-independent acquisition mass spectrometry to enable disease activity discrimination. Bioinformatics 2020, 36, 2217–2223. 10.1093/bioinformatics/btz898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie M. E.; Phipson B.; Wu D.; Hu Y.; Law C. W.; Shi W.; Smyth G. K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic acids research 2015, 43, e47–e47. 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M.; Chang C.-Y.; Clough T.; Broudy D.; Killeen T.; MacLean B.; Vitek O. MSstats: an R package for statistical analysis of quantitative mass spectrometry-based proteomic experiments. Bioinformatics 2014, 30, 2524–2526. 10.1093/bioinformatics/btu305. [DOI] [PubMed] [Google Scholar]
- Suomi T.; Elo L. L. Enhanced differential expression statistics for data-independent acquisition proteomics. Sci. Rep. 2017, 7, 5869. 10.1038/s41598-017-05949-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goeminne L. J.; Gevaert K.; Clement L. Peptide-level robust ridge regression improves estimation, sensitivity, and specificity in data-dependent quantitative label-free shotgun proteomics. Molecular & Cellular Proteomics 2016, 15, 657–668. 10.1074/mcp.M115.055897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlmann-Eltze C.; Anders S.. proDA: Probabilistic Dropout Analysis for Identifying Differentially Abundant Proteins in Label-Free Mass Spectrometry. bioRxiv 2020. https://doi.org/10.1101/661496.
- A Language and Environment for Statistical Computing, 2021. https://www.R-project.org/.
- Goeminne L. J.; Sticker A.; Martens L.; Gevaert K.; Clement L. MSqRob takes the missing hurdle: uniting intensity-and count-based proteomics. Anal. Chem. 2020, 92, 6278–6287. 10.1021/acs.analchem.9b04375. [DOI] [PubMed] [Google Scholar]
- Law C. W.; Zeglinski K.; Dong X.; Alhamdoosh M.; Smyth G. K.; Ritchie M. E. A guide to creating design matrices for gene expression experiments. F1000Research 2020, 9, 1444. 10.12688/f1000research.27893.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M. Building Predictive Models in R Using the caret Package. Journal of Statistical Software, Articles 2008, 28, 1–26. 10.18637/jss.v028.i05. [DOI] [Google Scholar]
- Grossmann J.; Roschitzki B.; Panse C.; Fortes C.; Barkow-Oesterreicher S.; Rutishauser D.; Schlapbach R. Implementation and evaluation of relative and absolute quantification in shotgun proteomics with label-free methods. Journal of Proteomics 2010, 73, 1740–1746. 10.1016/j.jprot.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Tukey J. W.Exploratory Data Analysis; Addison-Wesley: London, 1977. [Google Scholar]
- Zhang X.; Smits A. H.; van Tilburg G. B.; Ovaa H.; Huber W.; Vermeulen M. Proteome-wide identification of ubiquitin interactions using UbIA-MS. Nat. Protoc. 2018, 13, 530–550. 10.1038/nprot.2017.147. [DOI] [PubMed] [Google Scholar]
- Castellano-Escuder P.; Andrés-Lacueva C.; Sánchez-Pla A.. POMA: User-friendly Workflow for Metabolomics and Proteomics Data Analysis; 2021; R package version 1.2.0. [DOI] [PMC free article] [PubMed]
- Subramanian A.; Tamayo P.; Mootha V. K.; Mukherjee S.; Ebert B. L.; Gillette M. A.; Paulovich A.; Pomeroy S. L.; Golub T. R.; Lander E. S.; et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 15545–15550. 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X.; Shen S.; Li J.; Hu Q.; Nie L.; Tu C.; Wang X.; Poulsen D. J.; Orsburn B. C.; Wang J.; et al. IonStar enables high-precision, low-missing-data proteomics quantification in large biological cohorts. Proc. Natl. Acad. Sci. U. S. A. 2018, 115, E4767–E4776. 10.1073/pnas.1800541115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards N. J.; Oberti M.; Thangudu R. R.; Cai S.; McGarvey P. B.; Jacob S.; Madhavan S.; Ketchum K. A. The CPTAC data portal: a resource for cancer proteomics research. J. Proteome Res. 2015, 14, 2707–2713. 10.1021/pr501254j. [DOI] [PubMed] [Google Scholar]
- Wessels H. J.; Bloemberg T. G.; Van Dael M.; Wehrens R.; Buydens L. M.; van den Heuvel L. P.; Gloerich J. A comprehensive full factorial LC-MS/MS proteomics benchmark data set. Proteomics 2012, 12, 2276–2281. 10.1002/pmic.201100284. [DOI] [PubMed] [Google Scholar]
- O’Connell J. D.; Paulo J. A.; O’Brien J. J.; Gygi S. P. Proteome-wide evaluation of two common protein quantification methods. J. Proteome Res. 2018, 17, 1934–1942. 10.1021/acs.jproteome.8b00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H. Tidy Data. Journal of Statistical Software 2014, 59, 1–23. 10.18637/jss.v059.i10.26917999 [DOI] [Google Scholar]
- Chang W.R6: Encapsulated Classes with Reference Semantics, 2020; R package version 2.5.0.
- Bruderer R.; Bernhardt O. M.; Gandhi T.; Miladinovic S. M.; Cheng L.-Y.; Messner S.; Ehrenberger T.; Zanotelli V.; Butscheid Y.; Escher C.; et al. Extending the limits of quantitative proteome profiling with data-independent acquisition and application to acetaminophen-treated three-dimensional liver microtissues. Molecular & Cellular Proteomics 2015, 14, 1400–1410. 10.1074/mcp.M114.044305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean B.; Tomazela D. M.; Shulman N.; Chambers M.; Finney G. L.; Frewen B.; Kern R.; Tabb D. L.; Liebler D. C.; MacCoss M. J. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010, 26, 966–968. 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H.; et al. Welcome to the tidyverse. Journal of Open Source Software 2019, 4, 1686. 10.21105/joss.01686. [DOI] [Google Scholar]
- RStudio: Integrated Development Environment for R. RStudio; PBC.: Boston, MA, 2022. [Google Scholar]
- Faraway J. J.Extending the Linear Model with R; Chapman and Hall/CRC, 2016. [Google Scholar]
- Teo G.; Liu G.; Zhang J.; Nesvizhskii A. I.; Gingras A.-C.; Choi H. SAINTexpress: improvements and additional features in Significance Analysis of INTeractome software. Journal of proteomics 2014, 100, 37–43. 10.1016/j.jprot.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolski W. prolfquadata, 2021; R package version 0.1.0. https://gitlab.bfabric.org/wolski/prolfquadata.
- Robin X.; Turck N.; Hainard A.; Tiberti N.; Lisacek F.; Sanchez J.-C.; Müller M. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics 2011, 12, 77. 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Välikangas T.; Suomi T.; Elo L. L. A systematic evaluation of normalization methods in quantitative label-free proteomics. Briefings in bioinformatics 2016, 19, 1–11. 10.1093/bib/bbw095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber W.; Von Heydebreck A.; Sültmann H.; Poustka A.; Vingron M. Variance stabilization applied to microarray data calibration and to the quantification of differential expression. Bioinformatics 2002, 18, S96–S104. 10.1093/bioinformatics/18.suppl_1.S96. [DOI] [PubMed] [Google Scholar]
- Bolstad B. M.; Irizarry R. A.; Åstrand M.; Speed T. P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003, 19, 185–193. 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Irizarry R.; Love M. PH525x series—Biomedical Data Science, 2018; Interactions and contrasts. http://genomicsclass.github.io/book/pages/interactions_and_contrasts.html.
- Kuznetsova A.; Brockhoff P.; Christensen R. lmerTest Package: Tests in Linear Mixed Effects Models. Journal of Statistical Software, Articles 2017, 82, 1–26. 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- Smyth G. K. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Statistical applications in genetics and molecular biology 2004, 3, 1–25. 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- Venables W. N.; Ripley B. D.. Modern Applied Statistics with S, 4th ed.; Springer: New York, 2002; ISBN 0-387-95457-0. [Google Scholar]
- Quast J.-P.; Schuster D.; Picotti P. protti: an R package for comprehensive data analysis of peptide-and protein-centric bottom-up proteomics data. Bioinformatics Advances 2022, 2, vbab041. 10.1093/bioadv/vbab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Türker C.; Akal F.; Joho D.; Panse C.; Barkow-Oesterreicher S.; Rehrauer H.; Schlapbach R. In B-Fabric: the Swiss Army Knife for life sciences, Proceedings of the 13th International Conference on Extending Database Technology—EDBT, 2010.
- Panse C.; Trachsel C.; Türker C. Bridging data management platforms and visualization tools to enable ad-hoc and smart analytics in life sciences. Journal of Integrative Bioinformatics 2022, 19, 20220031. 10.1515/jib-2022-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleksiev T.; Barkow-Oesterreicher S.; Kunszt P.; Maffioletti S.; Murri R.; Panse C.. Lecture Notes in Computer Science; Springer Berlin Heidelberg, 2013; pp 447–461. [Google Scholar]
- Wilkinson M. D. The FAIR Guiding Principles for scientific data management and stewardship. Scientific Data 2016, 3, 160018. 10.1038/sdata.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich K.; Brombacher E.; Fahrner M.; Vogele D.; Kook L.; Pinter N.; Bronsert P.; Timme-Bronsert S.; Schmidt A.; Bärenfaller K.; et al. Benchmarking of analysis strategies for data-independent acquisition proteomics using a large-scale dataset comprising inter-patient heterogeneity. Nat. Commun. 2022, 13, 1–13. 10.1038/s41467-022-30094-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursiheimo A.; Vehmas A. P.; Afzal S.; Suomi T.; Chand T.; Strauss L.; Poutanen M.; Rokka A.; Corthals G. L.; Elo L. L. Optimization of statistical methods impact on quantitative proteomics data. J. Proteome Res. 2015, 14, 4118–4126. 10.1021/acs.jproteome.5b00183. [DOI] [PubMed] [Google Scholar]
- Cox J.; Hein M. Y.; Luber C. A.; Paron I.; Nagaraj N.; Mann M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Molecular & cellular proteomics 2014, 13, 2513–2526. 10.1074/mcp.M113.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y.; Orre L. M.; Tran Y. Z.; Mermelekas G.; Johansson H. J.; Malyutina A.; Anders S.; Lehtiö J. DEqMS: a method for accurate variance estimation in differential protein expression analysis. Molecular & Cellular Proteomics 2020, 19, 1047–1057. 10.1074/mcp.TIR119.001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.