Abstract
Background
Cigarette smoking is one of the leading causes of preventable death world wide. There is good evidence that brief interventions from health professionals can increase smoking cessation attempts. A number of trials have examined whether skills training for health professionals can lead them to have greater success in helping their patients who smoke.
Objectives
To determine the effectiveness of training health care professionals in the delivery of smoking cessation interventions to their patients, and to assess the additional effects of training characteristics such as intervention content, delivery method and intensity.
Search methods
The Cochrane Tobacco Addiction Group’s Specialised Register, electronic databases and the bibliographies of identified studies were searched and raw data was requested from study authors where needed. Searches were updated in March 2012.
Selection criteria
Randomized trials in which the intervention was training of health care professionals in smoking cessation. Trials were considered if they reported outcomes for patient smoking at least six months after the intervention. Process outcomes needed to be reported, however trials that reported effects only on process outcomes and not smoking behaviour were excluded.
Data collection and analysis
Information relating to the characteristics of each included study for interventions, participants, outcomes and methods were extracted by two independent reviewers. Studies were combined in a meta‐analysis where possible and reported in narrative synthesis in text and table.
Main results
Of seventeen included studies, thirteen found no evidence of an effect for continuous smoking abstinence following the intervention. Meta‐analysis of 14 studies for point prevalence of smoking produced a statistically and clinically significant effect in favour of the intervention (OR 1.36, 95% CI 1.20 to 1.55, p= 0.004). Meta‐analysis of eight studies that reported continuous abstinence was also statistically significant (OR 1.60, 95% CI 1.26 to 2.03, p= 0.03).
Healthcare professionals who had received training were more likely to perform tasks of smoking cessation than untrained controls, including: asking patients to set a quit date (p< 0.0001), make follow‐up appointments (p< 0.00001), counselling of smokers (p< 0.00001), provision of self‐help material (p< 0.0001) and prescription of a quit date (p< 0.00001). No evidence of an effect was observed for the provision of nicotine gum/replacement therapy.
Authors' conclusions
Training health professionals to provide smoking cessation interventions had a measurable effect on the point prevalence of smoking, continuous abstinence and professional performance. The one exception was the provision of nicotine gum or replacement therapy, which did not differ between groups.
Plain language summary
Can training health professionals to ask people if they smoke increase offers of advice and help patients quit?
Training programs are used to encourage health professionals to ask their patients if they smoke, and then offer advice to help them quit. The review of 17 trials found that these training programs help health professionals to identify smokers and increase the number of people who quit smoking. The programs also increase the number of people offered advice and support for quitting by health professionals.
Summary of findings
Summary of findings for the main comparison. Training health professionals for smoking cessation.
| Training health professionals for smoking cessation | ||||||
| Patient or population: Smokers treated by health professionals Intervention: Training | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Training health professionals | |||||
| Point prevalence of smoking cessation self‐report and some biologically validated Follow‐up: 6 to 14 months | 78 per 1000 | 107 per 1000 (88 to 131) | OR 1.41 (1.13 to 1.77) | 13459 (14 studies) | ⊕⊕⊕⊝ moderate1,2 | |
| Continuous smoking abstinence self‐report and some biologically validated Follow‐up: 6 to 14 months | 27 per 1000 | 42 per 1000 (28 to 62) | OR 1.60 (1.26 to 2.03) | 9443 (8 studies) | ⊕⊕⊕⊝ moderate1,2 | |
| Number of smokers counselled self‐report Follow‐up: 6 to 48 months | 465 per 1000 | 664 per 1000 (578 to 739) | OR 2.28 (1.58 to 3.27) | 8531 (14 studies) | ⊕⊕⊝⊝ low1,3 | |
| Patients asked to make a follow‐up appointment self‐report Follow‐up: 6 to 12 months | 166 per 1000 | 400 per 1000 (233 to 593) | OR 3.34 (1.52 to 7.30) | 3114 (7 studies) | ⊕⊝⊝⊝ very low1,2,3 | |
| Number of smokers receiving self‐help material self‐report Follow‐up: 6 to 48 months | 134 per 1000 | 351 per 1000 (227 to 500) | OR 3.51 (1.90 to 6.47) | 4925 (9 studies) | ⊕⊝⊝⊝ very low1,2,3 | |
| Number of smokers receiving nicotine gum/replacement therapy self‐report Follow‐up: 12 to 48 months | 312 per 1000 | 416 per 1000 (283 to 563) | OR 1.57 (0.87 to 2.84) | 5073 (9 studies) | ⊕⊕⊝⊝ low1,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Unclear methods of sequence generation and allocation concealment in the majority of studies and all studies had inadequate blinding of participants 2 Wide confidence intervals around the estimate of effect 3 Significantly large amounts of heterogeneity were observed (I² >90%)
Background
Description of the condition
Every year approximately 5.4 million people die from tobacco‐related diseases, translating to 1 in every 10 deaths among adults world wide (Mathers 2006; WHO 2008). Approximately 80% of those deaths are from people living in less developed countries and by 2030 this figure will increase to more than 8 million per year if no action is taken (Mathers 2006). If current trends continue on this trajectory, an estimated 500 million people alive today will be killed by tobacco. In the 27 countries that form the European Union, over 25% of cancer deaths and 15% of all deaths can be attributed to smoking (European Commission 2004). Smoked tobacco is known to cause up to 90% of all lung cancers and is a significant risk factor for strokes and fatal heart attacks. In addition, tobacco use is linked to the development and treatment of many oral diseases (Bergstrom 2000; Balaji 2008; Petersen 2009) including oral cancer, delayed wound healing and peridentitis contributing to loss of teeth and edentulism (Tomar 2000; Mohammad 2006; Gordon 2009).
Description of the intervention
Health professionals are at the forefront of tobacco epidemics as they consult millions of people and can encourage them to quit smoking (WHO 2005; Zwar 2009). In developed countries, more than 80% of the population will see a primary care physician at least once a year, with doctors perceived to be influential sources of information on smoking cessation (Mullins 1999; Richmond 1999; Zwar 2009). It has been reported that most dentists and dental hygienists believe the lack of skills and training is a significant barrier to effectively providing tobacco cessation interventions into routine care (Gelskey 2002; Warnakulasuriya 2002; Gordon 2009; Rosseel 2009).
Providing training in smoking cessation care is one possible method for increasing the number and quality of delivered interventions by primary care health professionals, and a variety of training methods are available (Anderson 2004; Twardella 2004; Stead 2009). To date, individual studies have shown an effect of training on physician's activities, but there have been doubts about the extent to which this translates into changes in patient behaviour and actual smoking abstinence (Kottke 1989; Cummings 1989a; Cummings 1989b). Training health professionals to deliver smoking cessation messages has been known to increase the frequency with which interventions are offered to patients in the clinical context (Thorogood 2006).
How the intervention might work
Provision of advice and support to smokers by healthcare professionals in primary care settings has been shown to be the most cost‐effective preventive service and has a small but significant effect on cessation rates (Maciosek 2006; Solberg 2006; Stead 2008). Even though these rates appear low from the perspective of many clinicians, they could translate into a substantial public health benefit if consistently provided, as approximately 70‐80% of adults have contact with a health care practitioner, usually in primary care, at least once each year (Mullins 1999; Richmond 1999; Hung 2009; Zwar 2009). It is therefore disappointing that despite ongoing developments in this field worldwide, the number of patients who report receiving advice on smoking cessation from health professionals is still low (CDC 2007).
Why it is important to do this review
On a worldwide scale, tobacco use currently costs hundreds of billions of dollars each year (WHO 2008). Data on the global impact of tobacco is incomplete, however it is known to be high, with annual tobacco related health care costs being US$81 billion for the USA, US$7 billion for Germany and US$1 billion for Australia (Guindon 2008).
The first systematic review on this topic was published over a decade ago and showed that training health professionals to provide smoking cessation interventions had a positive effect on professional performance. However, there was no strong evidence that it changed smoking behavior of patients (Lancaster 2008). Since then, a number of new trials have examined whether specific skills training for health professionals leads them to overcome frequently mentioned barriers and to have greater success in helping their patients to quit smoking.
We therefore systematically identified and reviewed the evidence from new published randomized controlled trials that have studied the effects of training and supporting health care professionals in providing smoking cessation advice. Furthermore, we assessed the effects of training characteristics, such as the content, setting, and intensity.
Objectives
The aim of this review was to assess the effectiveness of training health care professionals to deliver smoking cessation interventions to their patients, and to assess the effects of training characteristics (such as contents, setting, delivery and intensity).
Methods
Criteria for considering studies for this review
Types of studies
We considered only randomized controlled trials.
Types of participants
We considered trials in which the unit of randomization was a healthcare practitioner or practice, and that reported the effects on patients who were smokers.
Types of interventions
We considered interventions in which health care professionals were trained in methods to promote smoking cessation among their patients. To be included in the review studies had to have allocated healthcare professionals to at least two groups (including one which received some form of training) by a formal randomization process. Studies that used historical controls were excluded. We included studies that compared a trained group to an untrained control group, and studies that examined the effectiveness of adding prompts and reminders to training.
Types of outcome measures
Primary outcomes
The primary outcome measure was abstinence from smoking six months or more after the start of the intervention, assessed as:
point prevalence (defined as not smoking at a set period (e.g., seven days) prior to the follow‐up), and
continuous abstinence (defined as not smoking for an extended/prolonged period at follow‐up)
The definition of point prevalence and continuous abstinence for each study can be found in the 'Outcomes' section of the Characteristics of included studies table.
The strictest available criteria to define abstinence were used. In studies where biochemical validation of cessation was available, only those participants who met the criteria for biochemically confirmed abstinence were regarded as being abstinent. Those lost to follow‐up were regarded as being continuing smokers.
Secondary outcomes
Secondary ‘patient level’ outcome measures included process variables such as the number of smokers who were:
asked to set a date for stopping (quit date)
given a follow‐up appointment
counselled
given self‐help materials
offered nicotine gum/replacement therapy
prescribed a quit date, and
cost effectiveness for interventions.
Secondary ‘physician level’ outcome measures include the number of referrals made (to local smoking cessation services).
To be included in the review, studies had to assess changes in the long term smoking behaviour of patients. Studies which only assessed the effect of training on the consultation process were excluded.
Search methods for identification of studies
We identified potentially relevant study reports from the Cochrane Tobacco Addiction Group Specialised Register. This Register includes reports of trials and other evaluations of interventions for smoking cessation and prevention, based on regular highly sensitive searches of multiple electronic databases including MEDLINE, EMBASE, PsycINFO and CENTRAL, and handsearches of conference abstracts. For details of search strategies and dates see the Cochrane Tobacco Addiction Group Module in the Cochrane Library. The most recent search of the Register was in March 2012. Records were identified from the Register as potentially relevant if they included the free text terms ‘training’ or ‘trained’ or the MeSH keywords ‘Education, Premedical’ or ‘Education, Professional’ or ‘Inservice Training’ or ‘Physician's Practice Patterns’ or ‘Dentist's Practice Patterns’ or ‘Delivery of Health Care’ or ‘Comprehensive Health Care’ or ‘Critical Pathways’ or ‘Disease Management’ or the EMBASE indexing terms ‘clinical education’ or ‘continuing education provider’ or ‘continuing education’ or ‘medical education’ as indexing terms. We conducted an additional search of MEDLINE (via OVID, to 2012 Feb week 5) exploding the same MeSH keywords in combination with the terms for smoking cessation and controlled trials used in the regular search of MEDLINE for the Specialised Register. See Appendix 1 for this strategy. Records included definite and probable reports of randomized trials, and reviews.
Data collection and analysis
Selection of studies
Two reviewers (KC, MV) prescreened all study reports identified from the Specialised Register (limited to papers published after 1999 for this update). Articles were rejected if the title and/or abstract did not meet the inclusion/exclusion criteria. In instances where the study could not be categorically rejected, the full text was obtained and screened. Reference lists of screened articles were scanned for other potentially relevant articles.
Two reviewers then independently assessed the relevant studies for inclusion (KC and MV), with discrepancies resolved by consensus. Studies which were excluded though relevant to the review topic are listed in the Characteristics of excluded studies table, with the reason for their exclusion described.
Data extraction and management
A combination of two reviewers independently extracted data from published reports (KC, MV, and MB). Disagreements were resolved by referral to a third party. No attempt was made to blind any of these reviewers to either the results of the primary studies or the intervention the subjects received.
The data extraction process identified information on the following design characteristics:
Country and setting of study
Description of training delivery method, duration, content
Number of therapists (intervention, control, post randomization dropouts)
Number of patient participants (intervention, control, losses to follow‐up in each condition), method of identification/enrolment
Number of patients per therapist (range and/or average)
Description of intervention and control conditions
Definition of abstinence for smoking cessation outcome(s), duration of follow‐up, method of biochemical validation if used
Secondary outcomes reported
Data was extracted and entered into Review Manager for the following outcome variables, where reported:
Point prevalence abstinence at longest follow‐up (preferred outcome for meta‐analysis is continuous or sustained abstinence)
Continuous or sustained smoking abstinence at longest follow‐up
Cost effectiveness analysis for intervention
We also extracted data on process outcomes where reported. These included patient reported or documented delivery of interventions, such as: setting a quit date, making a follow‐up appointment, number of smokers counselled, provision of self‐help materials, prescription of nicotine replacement therapy and/or prescription of a quit date.
Assessment of risk of bias in included studies
Two reviewers independently assessed the full text versions for of all included papers for risk of bias using the Cochrane Handbook guidelines, using a domain‐based evaluation (Higgins 2009). In addition, extra criteria developed by the Cochrane EPOC Group (EPOC 2009) were used to address potential sources of bias related to clustering effects. These domains included sequence generation, allocation concealment, blinding for participants, blinding for outcome assessors, incomplete outcome data, selective reporting, imbalance of outcome measures at baseline, comparability of intervention and control group characteristics at baseline, protection against contamination, selective recruitment of participants and any other sources of potential biases. The risk of bias was assessed for each domain as 'high risk', 'low risk', and 'unclear risk' (using the guidelines from Table 8.5.c of the Cochrane Handbook, Higgins 2009). Two of three reviewers (KC, MV or MB) independently assessed the included studies for risk of bias. Conflicts were resolved by consensus or by referring to a third party if disagreement persisted.
Unit of analysis issues
The trials included in the review used cluster randomization. Outcomes relate to individual patients whilst allocation to the intervention is by provider or practice, and ignoring this may introduce unit of analysis errors. Using statistical methods which assume for example that all patients’ chances of quitting are independent ignores the possible similarity between outcomes for patients seen by the same provider. This may underestimate standard errors and give misleadingly narrow confidence intervals, leading to the possibility of a type 1 error (Altman 1997). All trials were expected to be cluster randomized studies, with analysis performed at the level of individuals whilst accounting for the clustering in the data. This was performed by using a random effects model for pooled meta‐analysis as recommended in the Cochrane Handbook (Chapter 16.3.3, Higgins 2009) and checked by a statistician (AE). For those studies which did not adjust for clustering the actual sample size was replaced with the effective sample size (ESS), calculated using a rho= 0.02 as per Campbell 2000. Trials may use a variety of statistical methods to investigate or compensate for clustering; we have recorded whether studies used these and whether the significance of any effect was altered. In instances where the studies appeared homogenous via a combination of the statistical I² test in addition to homogeneity expressed in the visual inspection of a Funnel plot we meta‐analysed using a fixed effect model. However in the presence of significant heterogeneity (as defined below under ‘Data Synthesis’) the random effects model was used.
In the case of multi‐arm trials each pair‐wise comparison was included separately, but with shared intervention groups divided out approximately evenly among the comparators. However, if the intervention groups were deemed similar enough to be pooled, the groups were combined using appropriate formulas in the Cochrane Handbook (Table 7.7.a for continuous data and Chapter 16.5.4 for dichotomous data, Higgins 2009).
Dealing with missing data
Missing participant data were evaluated on an available case analysis basis as described in Chapter 16.2.2 of the Cochrane Handbook (Higgins 2009). Missing standard deviations were addressed by imputing data from the studies within the same meta‐analysis or from a different meta‐analysis as long as these use the same measurement scale, have the same degree of measurement error and the same time periods (between baseline and final value measurement, as per Chapter 16.1.3.2 of the Cochrane Handbook, Higgins 2009). Where statistics essential for analysis were missing (e.g. group means and standard deviations for both groups are not reported) and could not be calculated from other data, we attempted to contact the authors to obtain data. Loss of participants that occurred prior to performance of baseline measurements was assumed to have no effect on the eventual outcome data of the study. Losses after the baseline measurement were taken were assessed and discussed. Studies that had more than 30% attrition (i.e., deaths and withdrawals) were reported in text only and excluded from the meta‐analysis.
We made an attempt to contact all authors for verification of methodological quality, classification of the intervention(s) and outcomes data. We attempted to contact the second author if we were unsuccessful in contacting the first author.
Assessment of heterogeneity
The review was expected to have some heterogeneity due to factors such as differing characteristics of clinics, practices and medical surgeries, differences in intervention characteristics and varying measurement tools used to assess outcomes. The Chi² and I² statistic (Higgins 2009) were used to quantify inconsistency across studies. The presence of significant heterogeneity was further explored through subgroup analyses. These were conducted for:
‘treatment type’ (e.g., counselling alone, counselling plus nicotine replacement therapy, counselling plus request for additional appointments, etc.)
‘treatment intensity’ (number of sessions)
‘treatment intensity’ (total exposure)
‘mode of delivery’ (e.g., face‐to‐face, group sessions or both)
‘behavioural change techniques’ (e.g., prompting, providing feedback, use of behavioural change theories)
‘type of professional being trained’ (e.g., dentist, doctor, health care worker etc.)
‘length of follow‐up’ (i.e., >6 to <9 months, >9 to <12 months, >12 to <24 months), and
‘risk of bias’ (i.e., high risk of bias for: < 2 domains, 3 – 5 domains, 6 – 8 domains or > 9 domains).
The likelihood of false positive results among subgroup analyses increase with the number of potential effect modifiers being investigated (Higgins 2009). As such we have adjusted these analyses using a Holm‐Bonferroni method (Holm 1979) using α= 0.05.
Assessment of reporting biases
With the inclusion of more than ten included studies, potential reporting biases were assessed using a funnel plot. Asymmetry in the plot could be attributed to publication bias, but may well be due to true heterogeneity, poor methodological design or artefact. Contour lines corresponding to perceived milestones of statistical significance (p= 0.01, 0.05, 0.1 etc.) were applied to funnel plots, which may help to differentiate between asymmetry due to publication bias from that due to other factors (Higgins 2009).
Data synthesis
1. For dichotomous outcomes the fixed effect model with an odds ratio (OR) was calculated with 95% confidence interval (CI), which was synthesised using inverse variance. However for outcomes with greater than 10 included studies a test for heterogeneity was conducted using a combination of two methods. If heterogeneity was found (defined as the I² test >60% and visual inspection of the funnel plot indicating no clustering of large or small studies) the random effects model was used in place of the fixed effect model, as suggested by the Cochrane Handbook (Section 9.5.2 and 9.5.3, Higgins 2009). Reasons for heterogeneity are further explored in the discussion. When studies appeared homogenous, the meta‐analysis was redone using the fixed effect model.
2. For continuous outcomes, a fixed effect model with a weighted mean difference (WMD) or standardised mean difference (SMD) with 95% confidence intervals were calculated as appropriate. However, in the presence of significant heterogeneity (as defined above) the random effects model was used in place of the fixed effect model.
Sensitivity analysis
Sensitivity analysis was conducted on studies with an unclear or high risk of bias for sequence generation and/or allocation concealment.
We include the Tobacco Addiction Group glossary of tobacco‐specific terms (Appendix 2).
Results
Description of studies
See the Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
Of 381 articles screened, 17 studies met all of the inclusion criteria (see Figure 1 for PRISMA diagram). Detailed information relating to each included study is reported in the Characteristics of included studies table (for information relating to the 65 excluded studies see Characteristics of excluded studies).
1.

Study flow diagram
Included studies
Design
All 17 included studies used a randomized controlled trial design with clustering and eleven studies also adopted nesting of participants within practices/hospitals (Wilson 1988; Cohen (Dent) 1989; Cohen (Doc) 1989; Cummings (Priv) 1989; Kottke 1989; Lennox 1998; Strecher 1991; Hymowitz 2007; Twardella 2007; Unrod 2007; Gordon 2010). One study (Twardella 2007) incorporated a 2x2 factorial design with randomization to: training plus incentive, training plus medication, training plus incentive and medication or usual care.
Sample sizes
In total 28,531 patients were assessed at baseline (following randomization) with 21,031 remaining in the studies at final follow‐up. Authors report a total of 1,434 individual health professionals recruited at baseline (across a known 260 practices) with follow‐up available for 1,204. Sample sizes for individual studies were medium to large, with the smallest number of patients (randomized at baseline) found in the Wang 1994 study (n= 93) and the largest in the Kottke 1989 study. The smallest sample at follow‐up remained with the Wang 1994 study (n= 82), and the largest remained with the Kottke 1989 study (n= 5266) . At the health professional level, the Hymowitz 2007 study had the largest number of residents randomized at baseline (n= 275) and follow‐up (n= 235) and likewise, Wang 1994 had the smallest number of residents at baseline and follow‐up (n= 27 for both). Seven studies also reported baseline cluster sizes at the practice level: Lennox 1998 (n= 16); Sinclair 1998 (n= 62); Swartz 2002 (n= 50); Joseph 2004 (n= 20); Hymowitz 2007 (n= 16); Twardella 2007 (n= 82); and Gordon 2010 (n= 14).
Setting
Eleven of the 17 studies were conducted in the USA, one in Canada (Wilson 1988), one in Taiwan (Wang 1994), one in Scotland (Sinclair 1998), one in the United Kingdom (Lennox 1998), one in Switzerland (Cornuz 2002) and one in Germany (Twardella 2007). Two studies were performed in a dentistry setting (Cohen (Dent) 1989; Gordon 2010), whilst the remaining 15 were conducted within primary care clinics, HMO (Health Maintenance Organisation) medical centres (Cummings 1989; Swartz 2002), VAMC's (Veterans Affairs Medicial Centres) (Joseph 2004) and one in a pharmacy setting (Sinclair 1998).
Participants
At the health professional level, two studies were performed with dentists (Cohen (Dent) 1989; Gordon 2010), six studies included only primary care physicians (Wilson 1988; Cohen (Doc) 1989; Cummings (Priv) 1989; Kottke 1989Twardella 2007; Unrod 2007), two studies were conducted with residents (Cornuz 2002 and paediatric residents in Hymowitz 2007), three studies incorporated a combination of primary care physicians and internists (Cummings 1989; Strecher 1991; Wang 1994), one study used pharmacists (Sinclair 1998), whilst the remaining three studies used a combination of health professionals including physicians, nurse practitioners, physician assistants, psychologists, pharmacists and other health visitors (Lennox 1998; Swartz 2002; Joseph 2004).
The individual patients in 16 of the 17 included studies were those visiting their health professional during the recruitment phase of each study. They were recruited during standard GP, dentist or outpatient visits, emergency department visits or from waiting rooms. The Hymowitz 2007 study was the only one to perform the training in a paediatric setting, targeting the parents/guardians of children visiting 16 primary care clinics.
Interventions
Treatment type
Six studies provided patients with a counselling plus nicotine replacement therapy intervention arm (Wilson 1988; Cohen (Dent) 1989; Cohen (Doc) 1989; Sinclair 1998; Joseph 2004; Twardella 2007). The two Cohen et al studies had a second intervention arm of counselling plus a reminder for physicians to ask about smoking (chart prompt), and a third intervention arm combining the counselling, nicotine replacement therapy and chart prompt (Cohen (Dent) 1989; Cohen (Doc) 1989). Another study (Twardella 2007) also had three intervention arms: counselling plus nicotine replacement therapy; counselling plus a monetary incentive to the physician following study completion per successful smoke‐free participant (€130); and a counselling plus nicotine replacement therapy plus incentive arm. The Wilson 1988 study had two intervention arms in addition to usual care: counselling and nicotine gum (as mentioned above) and a second arm of nicotine gum plus usual care (i.e., physicians were not trained in counselling). Three studies included multiple intervention methods to curtail smoking including counselling, nicotine replacement therapy, request for additional follow‐up appointments and provision of self‐help materials (Cummings (Priv) 1989; Cummings 1989; Gordon 2010), whilst one study combined three of those four (counselling, nicotine replacement therapy, and self‐help materials, Cornuz 2002). Five studies used counselling alone (Strecher 1991; Wang 1994; Lennox 1998; Swartz 2002; Unrod 2007) and two studies used counselling with the addition of self‐help materials (Kottke 1989; Hymowitz 2007).
Treatment intensity
The level of training intensity for health professionals ranged from one 40‐minute session in the Unrod 2007 study, to 'four or five' day long sessions in the Joseph 2004 study. Nine studies had a training session for one day or less: Wilson 1988 (four hours), Cohen (Dent) 1989 (one hour), Cohen (Doc) 1989 (one hour), Kottke 1989 (6 hours), Lennox 1998 (one day), Sinclair 1998 (two hours), Twardella 2007 (two hours), Unrod 2007 (40 minutes) and Gordon 2010 (three hours). Four studies had two separate sessions: Strecher 1991 (two, one hour sessions scheduled two weeks apart), Wang 1994 (two sessions of unknown duration), Cornuz 2002 (two, four hour training sessions scheduled two weeks apart) and Swartz 2002 (two, 20 minute training sessions and another session of unknown duration, where residents were able to practice counselling techniques with standardised patients). Four studies had three or more sessions: Cummings (Priv) 1989 and Cummings 1989 both had three, one hour sessions over a four to five week period, Hymowitz 2007 had four, one hour sessions, four times a year and Joseph 2004 had four to five, day long sessions within six months.
Mode of intervention delivery
Three different modes of intervention delivery were used being groups sessions, one‐on‐one or a combination of the two. Two studies only used one‐on‐one sessions (Joseph 2004; Unrod 2007), eleven studies delivered the intervention in a group setting only (Wilson 1988; Cummings 1989; Kottke 1989; Strecher 1991; Wang 1994; Lennox 1998; Sinclair 1998; Swartz 2002; Hymowitz 2007; Twardella 2007; Gordon 2010) with an eighth study using group delivery as the primary mode, however doctors who were unable to attend received a private session in their office (Cummings (Priv) 1989). Finally three studies used both modes of intervention delivery (Cohen (Dent) 1989; Cohen (Doc) 1989; Cornuz 2002), with health professionals in the two Cohen et al studies provided the option of a group or individual session.
Theoretical model ‐ behavioural change technique
Nine studies used behavioural change theories to underpin the intervention techniques. These included the 'stages of change' (also known as the trans‐theoretical) model (Kottke 1989; Strecher 1991; Wang 1994; Lennox 1998; Sinclair 1998; Cornuz 2002; Twardella 2007) and the '5A' (Ask, Assess, Advise, Assist and Arrange) approach (Unrod 2007; Gordon 2010). Three studies incorporated prompting or reminders to ask about tobacco use (Cohen (Dent) 1989; Cohen (Doc) 1989; Hymowitz 2007) and four provided feedback to the health providers, for example number of patients counselled (Cornuz 2002; Swartz 2002; Joseph 2004; Unrod 2007).
Type of professional being trained:
Two studies only focused on dentists (Cohen (Dent) 1989; Gordon 2010), one focused on pharmacists (Sinclair 1998), and the remaining fourteen studies all involved doctors. Five of these fourteen studies included doctors still undergoing training, either residents (Strecher 1991; Wang 1994; Cornuz 2002; Hymowitz 2007) or a combination of physicians and internists (Cummings 1989). Three other studies included training to other health care workers as well as doctors: Lennox 1998 also involved nurses and other health visitors; Swartz 2002 also trained nurse practitioners, physicians assistants and other health professionals; and, in addition to doctors, Joseph 2004 included nurses, psychologists and pharmacists.
Length of follow‐up
Eight studies reported follow‐up periods between six and nine‐months post intervention (Cohen (Dent) 1989; Cohen (Doc) 1989; Strecher 1991; Wang 1994; Lennox 1998; Sinclair 1998; Unrod 2007; Gordon 2010), eleven studies presented 12 month follow‐up data (Wilson 1988; Cohen (Dent) 1989; Cohen (Doc) 1989; Cummings 1989; Kottke 1989; Wang 1994; Cornuz 2002; Swartz 2002; Joseph 2004; Twardella 2007; Gordon 2010) and two studies assessed extended follow‐up periods of 14 months (Lennox 1998) and four years (Hymowitz 2007). However, only two‐year post intervention data was available for Hymowitz 2007 at the time of writing.
Outcomes
Smoking abstinence was assessed in all included studies through self‐report of either continuous abstinence (no smoking for an extended period of time) or point prevalence (for example, no smoking for seven days prior to the time of outcome collection). Of the eight studies that reported continuous abstinence, six (Cummings (Priv) 1989; Cummings 1989; Gordon 2010; Lennox 1998; Sinclair 1998; Wilson 1988) also reported a point prevalence measure of abstinence. Ten of the included studies used biochemical validation through either exhaled carbon monoxide (Cohen (Dent) 1989; Cohen (Doc) 1989; Strecher 1991; Cornuz 2002), serum cotinine (Kottke 1989; Twardella 2007), saliva cotinine (Wilson 1988; Unrod 2007) or a combination of exhaled carbon monoxide and serum cotinine (Cummings (Priv) 1989; Cummings 1989). A number of secondary outcomes measures were reported by some studies including: patients asked to set a quit date; patients asked to make a follow‐up appointment; number of smokers counselled; number of smokers receiving self‐help material; number of smokers receiving nicotine gum/replacement therapy; and number of smokers prescribed a quit date.
Two studies reported n‐values as a total across both intervention and control arms (Cohen (Dent) 1989; Cohen (Doc) 1989) and six studies reported n‐values as percentages, which had to be transformed into whole numbers (Wilson 1988; Cornuz 2002; Swartz 2002; Joseph 2004; Hymowitz 2007; Unrod 2007). As such there is likely to be some small variance between actual n‐values and those reported in these analyses, but this is not significant. Seven studies had multiple intervention arms, which were considered similar enough to be pooled together, two in the Wilson 1988, Kottke 1989 and Wang 1994 studies and three intervention arms in the Cohen (Dent) 1989, Cohen (Doc) 1989, Strecher 1991 and Twardella 2007 studies. One study did not report the n‐value for subjects at randomization, and hence this was calculated based on the number eligible for study and the number at follow‐up (Strecher 1991). The Kottke 1989 study reported all outcome data as continuous variables, as such it was unable to be pooled in the meta‐analyses. Smoking related outcomes in the Hymowitz 2007 study were unable to be pooled as only change scores from baseline were presented.
Excluded studies
Sixty‐five studies (71 articles) were excluded for the following reasons: 21 included consultation process only, 18 did not include a control group, 13 failed to measure smoking related outcome data, 12 were considered to be inadequately randomized and one only reported on smokeless tobacco use. See the Characteristics of excluded studies table for more detailed information relating to each excluded study.
Risk of bias in included studies
Methodological details for the 17 included studies are provided in the 'risk of bias table' at the end of the Characteristics of included studies tables. Key methodological features are also summarised in Figure 2.
2.

Risk of bias graph: review authors' judgements about each risk of bias judgement presented as percentages across all included studies
Random sequence generation (selection bias)
Five studies reported adequate methods of sequence generation (Cummings 1989; Cornuz 2002; Hymowitz 2007; Twardella 2007; Unrod 2007), two had inadequate methods (Kottke 1989; Strecher 1991) whilst the remaining ten did not provide enough information to assess risk of bias for sequence generation and were hence judged to be at unclear risk in this category. Adequate methods included the use of a random number generator or coin toss, whilst unclear methods were described as being 'random' in design, however methods were not described. The Kottke 1989 study required some physicians to be re‐assigned due to inappropriate allocation methods during assignment. For the Strecher 1991 study appropriate randomization did not occur as residents were randomly assigned by clinic half‐day session to one of four groups, which risks introducing bias. All 17 trials used cluster randomization, with five studies inadequately accounting for potential clustering effects in the data, requiring manual clustering adjustments (Wilson 1988; Cummings (Priv) 1989; Cummings 1989; Kottke 1989; Wang 1994). Only two studies (Kottke 1989; Hymowitz 2007) reported outcome data at the level of randomization. No authors reported that differences in the method of analysis affected the results.
Allocation concealment (selection bias)
Allocation concealment was unclear in all 17 included studies as authors did not describe methods of allocation concealment. Authors of the Lennox 1998 study report that physicians were randomly and blindly allocated to control or intervention groups, however the methods were not described. Another study mentioned that an independent research assistant concealed the result of randomization until two weeks before the intervention, when residents were provided with details about training sessions, however, methods of concealment were again not reported (Cornuz 2002).
Blinding (performance bias and detection bias) of participants
Only one study reported adequately blinding participants to the intervention (Cornuz 2002), as residents were not informed about the aim of the trial and were advised only that a survey on cardiovascular risk factors and prevention would be conducted. Authors announced that a training program in clinical prevention that included sessions on smoking cessation and management of dyslipidaemia was being conducted. Authors also report that patients were blinded to the aim of the study and group allocation of their physician. Due to the nature of the intervention, blinding of participants was not possible for the remaining 16 studies. An attempt was made to blind physicians in the Unrod 2007 study, with physicians learning their group assignment only after signing the informed consent, however they were not blinded during the study intervention period and follow‐up.
Blinding (performance bias and detection bias) of outcome assessors
Three studies reported methods blinding of outcome assessors that we judged at low risk of bias. Authors of Cummings (Priv) 1989 stated that 'outcome assessors were blinded', authors of the Joseph 2004 study report interviewers collecting patient outcomes were blinded to subject treatment status and authors in the Strecher 1991 study report that telephone interviewers, who were blinded to residents’ and patients’ group assignments, obtained the patient reports. The remaining 14 studies did not report any attempts to blind outcome assessors and as such are unclear for this category.
Incomplete outcome data (attrition bias)
Incomplete outcome data was adequately addressed in three studies (Cummings (Priv) 1989; Cummings 1989; Gordon 2010) and unclear in the remaining 14 studies. The Cummings (Priv) 1989 and Cummings 1989 studies reported that missing data was accounted for in analyses, whilst the Gordon 2010 study reported the use of multiple imputation procedures to account for missing data with participants lost to attrition discussed in the text. All unclear studies failed to mention if there was any missing outcome data and if so, how this was addressed when reporting results.
Selective reporting (reporting bias)
Selective reporting was evident in three studies (Hymowitz 2007; Unrod 2007; Gordon 2010), unclear in three studies (Kottke 1989; Strecher 1991; Wang 1994) and not detected in the remaining 11. Although all pre‐specified outcomes were addressed in the four year follow‐up for the Hymowitz 2007 study, the authors mention that outcome data for year one was omitted in order to provide a 'cleaner look' at the progress of the data. In the Unrod 2007 study, smoking abstinence from baseline to follow‐up (an outcome that would be expected to have been assessed in this study) was not reported. The Gordon 2010 authors report that secondary participant outcomes were examined with no significant differences on any variables, and that therefore they were not presented in the publication. Also, receipt of intervention was reported in text as percentages, however no information regarding this outcome was reported for the control.
Imbalance of outcome measures at baseline
One study did not report data for baseline smoking and made no mention of statistical analyses to potentially adjust for any imbalances (Wang 1994), as such the risk of bias category was assessed as unclear. All remaining studies adequately addressed imbalances of outcome measures at baseline. Thirteen studies accounted for baseline imbalances through analysis of covariance, regression analyses or other analysis techniques, whilst three studies reported outcomes at baseline to be similar across groups and as such did not require adjustment (Cummings (Priv) 1989; Lennox 1998; Sinclair 1998).
Comparability of intervention and control group characteristics at baseline
Five studies had unclear comparability between intervention and control groups at baseline (Wilson 1988; Cohen (Dent) 1989; Cohen (Doc) 1989; Cummings 1989; Twardella 2007) and the remaining twelve studies adequately addressed any differences found between groups via appropriate analysis methods.
Protection against contamination
Two studies reported contamination. In Gordon 2010, authors reported contamination due to a tax increase on cigarettes in New York, which resulted in a drop in smoking prevalence from 18.4% in 2006 to 15.8% in 2008. Authors believed that this tax increase contributed to the unusually high rate of smoking cessation in the usual care patients, thereby affecting the relative impact of the intervention. Authors of the second study, Strecher 1991, mention that "all four groups worked closely with one another at each site", leading to the possibility of contamination, however they also state that “...the effects appeared to be slight.” Nine studies had unclear risk of bias for contamination with insufficient information to permit a judgement of yes or no, whilst the remaining six studies (Wilson 1988; Cummings (Priv) 1989; Cummings 1989; Kottke 1989; Lennox 1998; Cornuz 2002) reported no potential contamination during the study period.
Selective recruitment of participants
Although no studies were identified as having selectively recruited participants, this could not be completely ruled out for eleven studies, which were determined to have an unclear risk of bias for this outcome (Wilson 1988; Cohen (Dent) 1989; Cohen (Doc) 1989; Cummings (Priv) 1989; Kottke 1989; Strecher 1991; Wang 1994; Sinclair 1998; Swartz 2002; Twardella 2007; Gordon 2010). The sampling frames in these studies were unclear and as such, generalisability is of a potential concern. The remaining six studies adequately reported recruitment methods and were determined as having a low risk of bias.
Other bias
No other biases were identified for the 17 included studies.
Effects of interventions
See: Table 1
Intervention effectiveness was assessed in all seventeen included studies through smoking prevalence, as well as through multiple secondary outcomes (see Table 1). All data were analysed as per the pre‐defined methodology outlined in the Methods section. For a summary of intervention effectiveness for each of these outcomes see Table 2.
1. Summary of individual study outcomes.
| Study ID/sub‐headings: | Detailed synthesis of intervention effectiveness: |
|
Cohen (Dent) 1989 Point prevalence/ continuous abstinence |
One year follow‐up: At 12 month follow‐up there was a significant interaction between subjects receiving the gum compared to control (7.7% and 3.1% for gum and control groups respectively, p< 0.05). When the three intervention groups were combined together as per the methods outlined in this review, point prevalence of smoking at 12 month follow‐up was 5.1%, compared to the control of 3.1%, which failed to reach statistical significance. Six months follow‐up: At 6 month follow‐up the coefficient for the reminder effect was negative, which authors state is likely to be caused by high cessation in the gum group coupled with the lower percentages in the gum and reminders group (9% for gum only, 3.2% for reminder only, 3% for gum and reminder and 3.1% for control). |
| Cohen (Dent) 1989 Secondary outcomes |
Patient asked to set a quit date: Prompted dentists were more likely to ask patients to set a quit date (6% for gum only, 14% for reminder only, 31% for both reminder and gum and 3% for control) Number of smokers counselled: Prompted conditions increased the likelihood of dentists advising their patients to quit (72% for gum only, 59% for reminder only, 85% for both reminder and gum and 37% for controls) |
|
Cohen (Doc) 1989 Point prevalence/ continuous abstinence |
One year follow‐up: The combination of gum and reminders did not increase the percent of patients who quit smoking compared to either condition alone. At 1 year follow‐up significant negative interaction between gum and reminders were found (p< 0.05). Pair‐wise comparisons among the groups showed that the three intervention groups were not significantly different from each other (reminder 15%, gum 8.8%, both 9.6%), however, each of them were significantly different from the control for analyses based on returnees and on all patients (control 2.7%, p< 0.05). Twelve month quit percentages for point prevalence were significantly higher for the reminders group (7.9%), compared to those using gum (4.7%), those using a combination of the two (5.2%) and control (1.5%), p< 0.05. When the three intervention groups were combined together as per the methods outlined in this review, point prevalence of smoking at 12 months follow‐up was 5.9%, compared to the control of 1.5%, which statistically favoured the intervention, p= 0.002. |
| Cohen (Doc) 1989 Secondary outcomes |
Patient asked to set a quit date: Prompted doctors were more likely to ask patients to set a quit date (10% for gum only, 33% for reminder only, 58% for both reminder and gum and 2% for control) Number of smokers counselled: Both the gum and prompted conditions increased the likelihood of doctors advising patients to quit (84% for gum only, 75% for reminder only, 95% for both reminder and gum and 41% for control) |
|
Cornuz 2002 Point prevalence/ continuous abstinence |
One year follow‐up: At 12 month follow‐up, 7 day point prevalence was significantly higher in the intervention group (15 of 115 patients [13%, 95% CI 7% to 12%]) compared to the control group (7 of 136 patients [5%, 95% CI 1% to 9%]). The eight‐percentage point difference between groups translates to a resident needing to counsel 13 patients to gain 1 additional former smoker. |
| Cornuz 2002 Secondary outcomes |
Patient asked to set a quit date: The short‐term effect of the training program performed by the resident was statistically significant in favour of the intervention with 8% compared to 2% for the intervention and control groups respectively Patient asked to make follow‐up appointment: Short‐term effect was not significantly different between groups with 7% of the intervention and 3% of the control population asked by their physician to make a follow‐up appointment Number of smokers counselled: Not statistically significant with 39% of intervention patients and 29% of control patients counselled not to smoke Number of smokers receiving self‐help materials: Short‐term effects were statistically significant between groups with 14% of intervention subjects provided with a brochure compared to 1% of control |
|
Cummings (Priv) 1989 Point prevalence/ continuous abstinence |
One year follow‐up: There was no statistical significance on 7 day point prevalence for validated smoking cessation at one year follow‐up, with 6.7% for trained group patients quit compared to 8.2% for control. Biochemically validated continuous abstinence (defined as > 9 months abstinence) results were similar with 3.2% for intervention subjects and 2.5% for control (95% CI for the 0.7% difference= ‐1.7 to +3.1%) |
| Cummings (Priv) 1989 Secondary outcomes |
Patient asked to set a quit date: Physicians in the experimental group asked more smokers to set quit dates with 100 out of 261 for intervention and 22 out of 177 for control Patient asked to make follow‐up appointment: Trained physicians were significantly more likely to arrange a follow‐up appointment to discuss smoking with 50 out of 261 for the intervention and 19 out of 177 for control Number of smokers counselled: Trained physicians were significantly more likely to discuss smoking (64%) compared to the control (44%) Number of smokers receiving self‐help materials: Physicians in the experimental group gave self‐help booklets to more smokers with 151 out of 411 for intervention compared to 38 out of 407 for control Number of smokers receiving nicotine gum/replacement therapy: There was no significant difference in the prescription of nicotine gum; Control group patients with whom smoking was discussed were more likely to be prescribed it (19%) than the trained group (13%) |
|
Cummings 1989 Point prevalence/ continuous abstinence |
One year follow‐up: There was no significant effect on validated abstinence at one year follow‐up, with 8.0% of trained group patients quitting versus 7.1% of control. |
| Cummings 1989 Secondary outcomes |
Patient asked to set a quit date: Trained physicians were significantly more likely to ask patients to set a quit date with 37.6% of intervention subjects and 11.1% of control subjects asked Patient asked to make follow‐up appointment: Significantly more subjects in the intervention group had a follow‐up appointment arranged with 15.2% compared to 5% in the control population Number of smokers counselled: Trained and control physicians were similar in terms of asking patients to discuss smoking (50.1% vs 44.9% respectively) Number of smokers receiving self‐help materials: Physicians in the intervention arm were more likely to provide patients with self‐help materials with 24.9% compared to control physicians with 8.4% Number of smokers receiving nicotine gum/replacement therapy: There was no significant difference in the prescription of nicotine gum; Approximately 10% of patients with whom smoking was discussed were prescribed gum Number of smokers prescribed a quit date: Trained physicians were significantly more likely to prescribe patients with a quit date (16.1%) compared to control physicians (1.2%) |
|
Gordon 2010 Point prevalence/ continuous abstinence |
Six months follow‐up: Significantly higher abstinence levels were reported for both continuous abstinence and point prevalence at 7.5 month (six months post‐enrolment plus six week grace period) follow‐up (continuous abstinence: 74 out of 1394 for intervention and 22 out of 1155 control, p< 0.01; Point prevalence: 158 out of 1394 for intervention and 79 out of 1155 for control, p< 0.05) |
| Gordon 2010 Secondary outcomes |
No secondary outcomes reported across both groups, however two outcomes reported for intervention group only: Number of smokers receiving self‐help materials: Among intervention patients, 66.5% reported receiving the self‐help reading materials and 96.7% reported reading them Number of smokers receiving nicotine gum/replacement therapy: Of the intervention subjects 16.9% reported using nicotine replacement therapy |
|
Hymowitz 2007 Point prevalence/ continuous abstinence |
One year follow‐up: There was an increase in the special training condition of reported quitting during the past year of 3.8% (an 8.5% increase over baseline levels), however the change from baseline failed to achieve statistical significance. Among parents associated with standard training, the change was only 0.8%. |
| Hymowitz 2007 Secondary outcomes |
Number of smokers counselled: There was a significant increase in the percentage of parents counselled at both intervention and control training sites from baseline, however absolute levels of this activity for residents in each conditions was low (intervention 21.4% (OR 2.08, 95% CI 1.12 to 3.87), control 16.7% (OR 1.84, 95% CI 0.84 to 4.02)). There was no significant difference between groups. Number of smokers receiving self‐help materials: Provision of cessation materials increased significantly across both groups over the four year period when compared to baseline values (intervention 28.8% (OR 1.95, 95% CI 1.10 to 3.46), control 17.6% (OR 1.76, 95% CI 0.76 to 4.08)). There was no significant difference between groups. Number of smokers receiving nicotine gum/replacement therapy: Few parents in either condition reported that residents prescribed nicotine replacement therapy (intervention n= 7.6%, control n= 5.9%) |
|
Joseph 2004 Point prevalence/ continuous abstinence |
One year follow‐up: At follow‐up the point prevalence of smoking cessation did not significantly improve for the intervention subjects, over that of control (intervention 11.4%, control 13.2% (p= 0.51 for Pearson Chi² test)). |
| Joseph 2004 Secondary outcomes |
Number of smokers counselled: During the intervention period, 59% of subjects in the intervention arm received behavioural support to stop smoking in comparison to 55% in the control (p= NS) Number of smokers receiving nicotine gum/replacement therapy: Twenty‐one percent of subjects reported receiving medications for smoking cessation in the intervention arm whilst 19% received medication in the control group (p= NS) |
|
Kottke 1989 Point prevalence/ continuous abstinence |
One year follow‐up: Almost half of the participants in each group who were smoking at baseline reported quit attempts for at least 24 hours during the previous year, with a mean duration of cessation of 2‐months. No differences between the three groups were identified. |
| Kottke 1989 Secondary outcomes |
Patient asked to set a quit date: Almost 20% of patients seen in the workshop group reported being asked to set a quit date, compared to 10% in the materials group and 5% in the no‐assistance group (p< 0.005) Patient asked to make follow‐up appointment: Greater proportions of patients in the workshop group were asked to make a follow‐up appointment compared to the other two groups but this was not significant Number of smokers counselled: Slightly over half of the patients interviewed reported that they had been ‘asked if they smoked’ when visiting their physicians during the campaign (p< 0.025); This did not differ significantly between intervention groups Number of smokers receiving self‐help materials: One third of patients in the workshop group reported receiving self‐help material compared to 11% in the no‐assistance group (p< 0.001) |
|
Lennox 1998 Point prevalence/ continuous abstinence |
Fourteen months follow‐up: There was no significant difference in sustained abstinence at 14 months between intervention (3.6%) and control (4.7%). Eight months follow‐up: No significant difference was observed between intervention and control groups as to whether an attempt was made to give up smoking at any time during the study period. |
| Lennox 1998 Secondary outcomes |
Number of smokers counselled: No significant difference in discussion of smoking with doctors, nurses or health visitors, however results in both groups were above 70%; Intervention subjects who smoked were more likely than control subjects who smoked to recall smoking having been mentioned in a consultation during the 14‐month follow‐up period (significant for GP consultations at the 10 percent level, but not for consultations with practice nurses or health visitors |
|
Sinclair 1998 Point prevalence/ continuous abstinence |
Nine month follow‐up: There was no significant difference in nine month continuous abstinence with Intervention group 12%, control 7.4%, and no difference in one month point prevalence. |
| Sinclair 1998 Secondary outcomes |
Number of smokers counselled: Patients consulting training pharmacists were significantly more likely to report discussion of smoking (85% vs 62.3%) Number of smokers receiving nicotine gum/replacement therapy: Anti‐smoking products were bought by most subjects following enrolment, however, intervention subjects were significantly more likely to make a purchase (p = 0.0085); There was a significantly greater use of nicotine patches relative to nicotine gum in the intervention group compared with the control group (p = 0.029). Overall, approximately three‐quarters of the customers used patches compared with a quarter using gum |
|
Stretcher 1991 Point prevalence/ continuous abstinence |
Six months follow‐up: There were no significant differences between 6 month validated abstinence rates, which ranged from 1.7% to 5.7% |
| Stretcher 1991 Secondary outcomes |
Patient asked to set a quit date: Trained physicians were significantly more likely to advise smokers to quit (73% vs 58%) based on physician reported outcomes, however patient reports of this outcome are not significant Patient asked to make follow‐up appointment: Overall there were no significant differences in scheduling follow‐up appointments; According to patient outcomes however, more tutorial physicians asked to schedule follow‐up appointments compared to non‐tutorial physicians (p< 0.05) Number of smokers counselled: A prompt alone achieved similar counselling levels compared to control (75% vs 70% respectively) and there was no significant interaction between tutorial and prompt; After adjusting for pre‐test scores and speciality, physicians receiving the tutorial reported a significantly greater number of patients advised to quit (76%) compared to non‐tutorial physicians (69%) (p< 0.05) Number of smokers receiving self‐help materials: All physicians were equally likely to give self help materials Number of smokers receiving nicotine gum/replacement therapy: There were no differences in the proportion of physicians who prescribed nicotine gum Number of smokers prescribed a quit date: There were no differences in advice to set a quit day, but the trained group was significantly more likely to write a quit day prescription according to physicians; Patients reported that significantly more tutorial physicians prescribed a quit date than non‐tutorial physicians, however when groups were combined (tutorial and prompt, prompt only and tutorial only) this was not significant |
|
Swartz 2002 Point prevalence/ continuous abstinence |
One year follow‐up: Intervention subjects were more likely to quit at follow‐up (14.8% quit percentage) compared to control subjects (10.7%). Although this result was not statistically significant (p= 0.08), authors of the study report long‐term clinically important reductions. |
| Swartz 2002 Secondary outcomes |
Patient asked to set a quit date: There was no significant difference between intervention and control groups for patients being advised to quit smoking (OR 1.22, 95% CI 0.81 to 1.83) Patient asked to make follow‐up appointment: No significant difference was observed between intervention and control groups for patients asked to make a follow‐up appointment (OR 1.08, 95% CI 0.77 to 1.51) Number of smokers counselled: Providers discussed counselling more in the intervention group compared to control (27.7% vs. 20.8%; OR 1.39, 95% CI 0.96 to 2.02) Number of smokers receiving self‐help materials: There was no statistically significant difference between groups for the prevision of self‐help materials (OR 1.04, 95% CI 0.76 to 1.43) Number of smokers receiving nicotine gum/replacement therapy: Subjects in both intervention and control groups had similar offers for the provision of nicotine replacement therapy (intervention 46.2%, control 18.6%, OR 0.89, 95% CI 0.63 to 1.25) |
|
Twardella 2007 Point prevalence/ continuous abstinence |
One year follow‐up: Point prevalence of smoking abstinence was 3%, 3%, 12% and 15% for the control, treatment plus incentive (TI), treatment plus medication (TM) and treatment plus incentive and medication (TI+TM) arms respectively. There were statistically significant differences between the TM, TI+TM and control arms (p= 0.046 and p= 0.02, respectively). Continuous abstinence (for at least 6‐months) was higher in the TM arm (13/140, 9%) and TI+TM arm (17/219, 8%) compared to the control arm (1/74, 1%) and TI arm (2/144, 1%), however this difference was not statistically significant. |
| Twardella 2007 Secondary outcomes |
Number of smokers counselled: No significant differences were observed for number of smokers counselled between the four groups (control 59%, TI 73%, TM 67%, TI+TM 65%) Number of smokers receiving self‐help materials: A significant difference was observed when comparing TM group to control group (p=0.03), however no other between group difference were observed (control 15%, TI 32%, TM 31%, TI+TM 24%) Number of smokers receiving nicotine gum/replacement therapy: There was a significant difference between groups for prescription of nicotine replacement therapy, particularly for those provided with reimbursement for costs of the medication (TM and TI+TM) (control 7%, TI 13%, TM 30%, TI+TM 22%) |
|
Unrod 2007 Point prevalence/ continuous abstinence |
Six months follow‐up: Seven day point prevalence of abstinence results were higher in the intervention group (12%) than the control group (8%), however this difference approached but did not reach significance (OR 1.77, 95% CI 0.94 to 3.34, p= 0.078). |
| Unrod 2007 Secondary outcomes |
Patient asked to make follow‐up appointment:Intervention physicians were five times more likely to arrange a follow‐up appointment (47.5%) compared to control (9.7%) (OR 8.14, 95% CI 3.98 to 16.68, p< 0.0001) Number of smokers counselled: Significantly more intervention physicians provided quit smoking assistance to their patients (55.1%) compared to control physicians (20.2%) (OR 4.31, 95% CI 2.59 to 7.16, p< 0.0001) Number of smokers receiving self‐help materials: Physicians in the intervention group were more than three times as likely to provide self‐help materials to patients (32.3%) compared to control physicians (6.9%) (OR 5.14, 95% CI 2.60 to 10.14, p< 0.0001) |
|
Wang 1994 Point prevalence/ continuous abstinence |
Six months follow‐up: Statistically significant difference favouring the lesson intervention over the control (p=0.02) and significant difference (p=0.054) between lessons (G1) and poster (G2), however there was no significant difference between group 2 and control. When group 1 and group 2 were combined in meta‐analyses and adjusted for potential clustering effects, no significant differences were observed. |
| Wang 1994 Secondary outcomes |
No secondary outcomes were reported |
|
Wilson 1988 Point prevalence/ continuous abstinence |
One year follow‐up: Differences between the training arm and the other two arms were significant for sustained abstinence at one year and for 2 point prevalence, but not for one year point prevalence. Results were similar when mean cessation percentages were adjusted for baseline values. Twelve month sustained abstinence results were 8.8% for the intervention group, compared to 6.1% and 4.4% in the two comparison arms. However, when the two intervention groups were combined and adjustments for potential clustering effects taken into account, these results were no longer significant for point prevalence or continuous abstinence. |
| Wilson 1988 Secondary outcomes |
Patient asked to make follow‐up appointment: Training groups more likely to ask for a quit date (54%) and arrange follow‐up (12%) than gum only (12%/22%) or usual care (2%/4%) Number of smokers counselled: Training (85%) and gum (70%) groups more likely to mention smoking than usual care (31%) Number of smokers receiving nicotine gum/replacement therapy: Training (63%) and gum (59%) groups more likely to suggest use of gum than usual care (9%) |
Overall summary of smoking behaviour
Four out of 13 studies detected significant intervention effectiveness in training health professionals to influence point prevalence of smoking in their patients at primary follow‐up (Cohen (Doc) 1989; Cornuz 2002; Twardella 2007; Gordon 2010). Out of the eight studies reporting continuous abstinence at primary follow‐up, only one reported a statistically significant effect in favour of the intervention (Gordon 2010). Fifteen of the 17 included studies (the exceptions being Kottke 1989 and Hymowitz 2007) could be included in a meta‐analysis for the primary outcome of smoking (Analysis 1.1). Using a fixed effect model there was a statistically and clinically significant effect in favour of the intervention for point prevalence abstinence (OR 1.36, 95% CI 1.20 to 1.55, 14 trials, I² = 57%) and continuous abstinence (OR 1.60, 95% CI 1.26 to 2.03, 8 trials, I² = 59%) (Figure 3). Using only the stricter outcome of continuous abstinence for studies reporting both types of cessation, a pooled estimate for all 15 trials gave a similar estimate (OR 1.60, 95% CI 1.35 to 1.89, I² = 55%, data not displayed). Since the heterogeneity in this analysis approached the level at which we proposed a random‐effects model we did a sensitivity analysis; the point estimates were similar and the wider confidence intervals continued to exclude no effect. The trial contributing most evidently to the heterogeneity, particularly for the continuous outcome, was Lennox 1998 in which the point estimates for both abstinence outcomes favoured the control group.
1.1. Analysis.
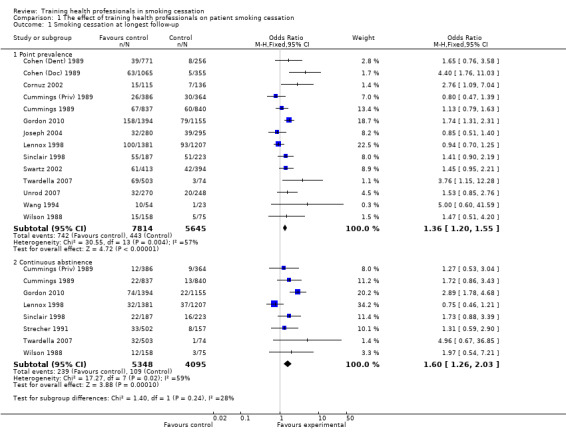
Comparison 1 The effect of training health professionals on patient smoking cessation, Outcome 1 Smoking cessation at longest follow‐up.
3.
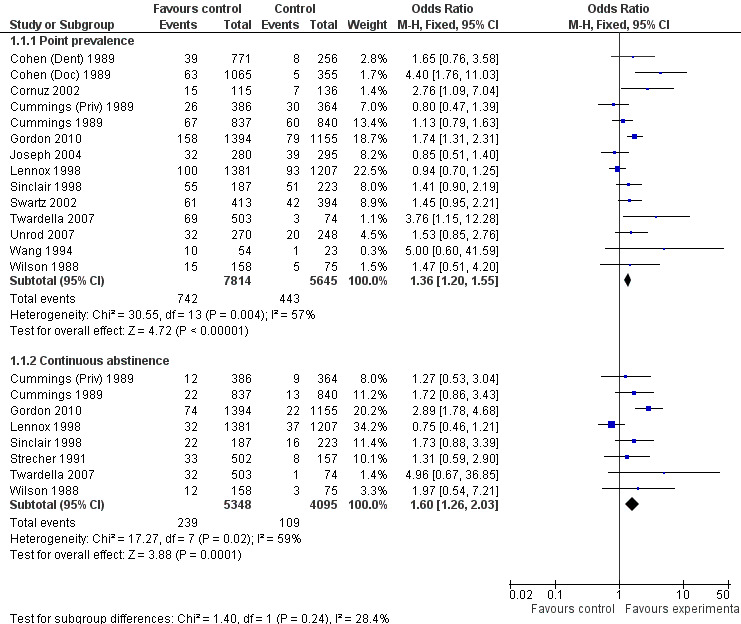
Forest plot of comparison: 1 The effect of training health professionals on patient smoking cessation
Two studies could not be included in the meta‐analyses. In the Kottke 1989 study at one year follow‐up almost half of the participants in each group who were smoking at baseline reported quit attempts for at least 24 hours during the previous year, with a mean duration of cessation of two months. No differences between the three groups were identified. For the Hymowitz 2007 study there was an increase in the special training condition of reported quitting during the past year of 3.8% (an 8.5% increase over baseline levels), however the change from baseline failed to achieve statistical significance. Among parents associated with standard training, the change was only 0.8%.
As per pre‐specified methodology, a funnel plot examined the primary outcome of smoking cessation using contour lines to assess the presence of reporting biases. No publication biases were identified (Figure 4).
4.

Funnel plot of comparison: 1 The effect of training health professionals on patient smoking cessation
Overall summary of secondary outcomes
Asked to set a quit date for stopping (quit date)
Nine studies reported the effect of training health professionals on the number of patients being asked to set a quit date, eight of which could be included in the meta‐analysis producing a significant result (random effects OR 4.98, 95% CI 2.29 to 10.86, Analysis 1.2). Only three of the seven studies crossed the line of no effect (Strecher 1991; Cornuz 2002; Swartz 2002) but there was a very high level of heterogeneity (I² = 90%) suggesting that not all interventions had the same impact on this outcome. Subgroup analyses suggest that some of the heterogeneity might be due to whether or not the patient intervention included an offer of NRT. The two studies (Strecher 1991; Swartz 2002) that reported this outcome and did not include NRT showed no difference between groups. The other studies showed more consistent evidence that intervention increased numbers although the size of effect remained variable (Analysis 2.1). Contrary to what might have been expected, the studies where training took only a single session had higher effect sizes (Cohen (Dent) 1989; Cohen (Doc) 1989; Wilson 1988, Analysis 3.1) compared to the five studies using multiple sessions. Duration of training was similar for the three sub‐groups being examined (Analysis 4.1) as was intervention delivery via one‐on‐one compared to group sessions (Analysis 5.1). There was a large amount of variability between the use of prompting and provision of feedback, however this difference was not significant (Analysis 6.1). Intervention delivery by a doctor (six studies) or dentist (one study) produced a larger effect size compared to delivery by a healthcare worker (Swartz 2002), which may also explain some of the heterogeneity (Analysis 7.1). When comparing follow‐up periods, studies reporting between six and nine months (Cohen (Dent) 1989; Cohen (Doc) 1989; Strecher 1991) and between nine and 12 months (seven studies) produced similar effect sizes and large amounts of variability (Analysis 8.1). Studies judged to be at lower risk of bias were more likely to show evidence of an effect (seven studies) compared to studies with between three and five categories rated at high risk of bias (Strecher 1991), however the between group analysis did not suggest that this was a source of heterogeneity (Analysis 9.1).
1.2. Analysis.

Comparison 1 The effect of training health professionals on patient smoking cessation, Outcome 2 Patient asked to set a quit date.
2.1. Analysis.

Comparison 2 Sub‐group: treatment type, Outcome 1 Patient asked to set a quit date.
3.1. Analysis.

Comparison 3 Sub‐group: treatment intensity ‐ Number of sessions, Outcome 1 Patient asked to set a quit date.
4.1. Analysis.

Comparison 4 Sub‐group: treatment intensity ‐ Total exposure, Outcome 1 Patient asked to set a quit date.
5.1. Analysis.

Comparison 5 Sub‐group: mode of intervention delivery, Outcome 1 Patient asked to set a quit date.
6.1. Analysis.

Comparison 6 Sub‐group: behavioural change technique used, Outcome 1 Patient asked to set a quit date.
7.1. Analysis.

Comparison 7 Sub‐group: type of professional being trained, Outcome 1 Patient asked to set a quit date.
8.1. Analysis.
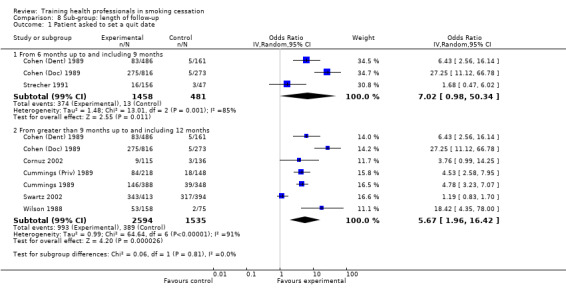
Comparison 8 Sub‐group: length of follow‐up, Outcome 1 Patient asked to set a quit date.
9.1. Analysis.

Comparison 9 Sub‐group: risk of bias in the studies, Outcome 1 Patient asked to set a quit date.
Given a follow‐up appointment
There was a significant increase in the intervention arm for patients being asked to make a follow‐up appointment, as reported in seven studies available for meta‐analysis (random effects OR 3.34, 95% CI 1.51 to 7.37, Analysis 1.3), although significant heterogeneity was observed (I² =92%). When comparing interventions using NRT with those that used counselling alone, an I² of 96% was observed, meaning any results from a pooled analysis would be too unreliable. As such only a visual analysis of odds ratios and confidence intervals are presented, showing similar variability between sub‐groups (Analysis 2.2). Subgroup analyses for treatment intensity suggest that some of the heterogeneity might be due to whether or not the training sessions were single or multiple. Two studies that employed single sessions (Wilson 1988; Unrod 2007) were more likely to show an effect (although variability was observed), compared to five studies using multiple sessions, which produced a smaller effect estimate with less variability (Analysis 3.2). When comparing the duration of the training, significant heterogeneity was once again observed between groups, with studies presenting large amounts of variability, resulting in a pooled estimate being unreliable for comparison (Analysis 4.2). There was little difference between delivery by one‐on‐one compared to group sessions (Analysis 5.2), and due to significant heterogeneity (I² =96%) the pooled comparison of prompting and provision of feedback was not possible, although a visual display shows variability is mostly due to the Unrod 2007 study (Analysis 6.2). Similar to other outcomes, delivery of the intervention by a doctor (assessed in seven studies) meant that more patients were likely to have a follow‐up appointment compared to intervention delivery by a healthcare worker (one study), however the Swartz 2002 study was present in both sub‐groups as the intervention included delivery by both a doctor and healthcare worker, as such a statistical between group comparison was not performed (Analysis 7.2). Reporting of results at different follow‐up periods were similar between sub‐groups, although the five studies with follow‐up between nine and 12 months had similar distributions with the exception of the Wilson 1988 study, which significantly favoured the intervention and had wide confidence intervals (Analysis 8.2). No between group differences were observed for quality of the studies (Analysis 9.2).
1.3. Analysis.

Comparison 1 The effect of training health professionals on patient smoking cessation, Outcome 3 Patient asked to make a follow‐up appointment.
2.2. Analysis.
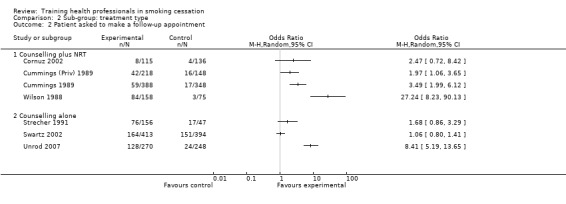
Comparison 2 Sub‐group: treatment type, Outcome 2 Patient asked to make a follow‐up appointment.
3.2. Analysis.

Comparison 3 Sub‐group: treatment intensity ‐ Number of sessions, Outcome 2 Patient asked to make a follow‐up appointment.
4.2. Analysis.
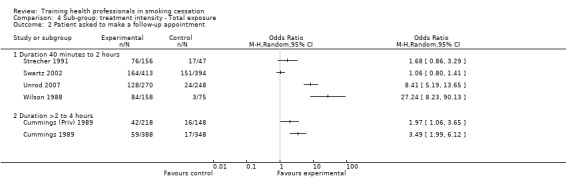
Comparison 4 Sub‐group: treatment intensity ‐ Total exposure, Outcome 2 Patient asked to make a follow‐up appointment.
5.2. Analysis.
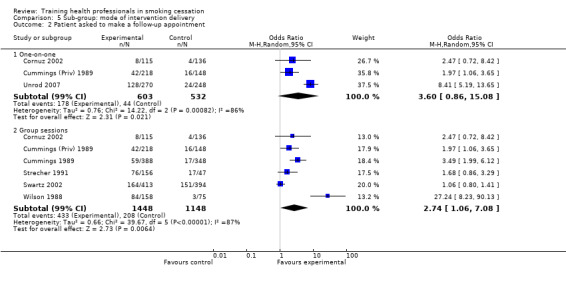
Comparison 5 Sub‐group: mode of intervention delivery, Outcome 2 Patient asked to make a follow‐up appointment.
6.2. Analysis.

Comparison 6 Sub‐group: behavioural change technique used, Outcome 2 Patient asked to make a follow‐up appointment.
7.2. Analysis.

Comparison 7 Sub‐group: type of professional being trained, Outcome 2 Patient asked to make a follow‐up appointment.
8.2. Analysis.

Comparison 8 Sub‐group: length of follow‐up, Outcome 2 Patient asked to make a follow‐up appointment.
9.2. Analysis.

Comparison 9 Sub‐group: risk of bias in the studies, Outcome 2 Patient asked to make a follow‐up appointment.
Counselled
Fourteen of the fifteen studies reporting on the number of smokers counselled were meta‐analysed (Analysis 1.4). Overall, a statistically and clinically significant effect in favour of the intervention was observed (OR 2.28, 95% CI 1.58 to 3.27, p< 0.00001), assessed using the random effects model due to significant heterogeneity (I²= 93%). An investigation into the causes of heterogeneity found no differences between counselling with and without nicotine replacement therapy (Analysis 2.3), however implementation via multiple sessions or single sessions did produce between group differences, with a larger effect size for single session delivery (Analysis 3.3). Duration of intervention delivery also produced significant differences with total exposure of between 40 minutes and two hours producing a larger effect size compared to durations of between two and four hours and greater than four hours (Analysis 4.3). Mode of intervention delivery (one‐on‐one compared to group sessions) produced very similar effect sizes (Analysis 5.3), as did the provision of feedback and prompting to aid intervention delivery by the health professional (Analysis 6.3). The type of health professional being trained may contribute to the heterogeneity with the one study evaluating dentists (Cohen (Dent) 1989) producing a larger effect size compared to those with doctors and other health professionals which showed a more conservative effect with narrow confidence intervals (Analysis 7.3). When examining follow‐up periods, there was a slightly larger effect and more variability in the studies reporting results between six and nine months compared to results between nine and twelve months and 12 and 24 months (Analysis 8.3). No sub‐group differences were observed when analysing studies based on risks of bias (Analysis 9.3).
1.4. Analysis.

Comparison 1 The effect of training health professionals on patient smoking cessation, Outcome 4 Number of smokers counselled.
2.3. Analysis.
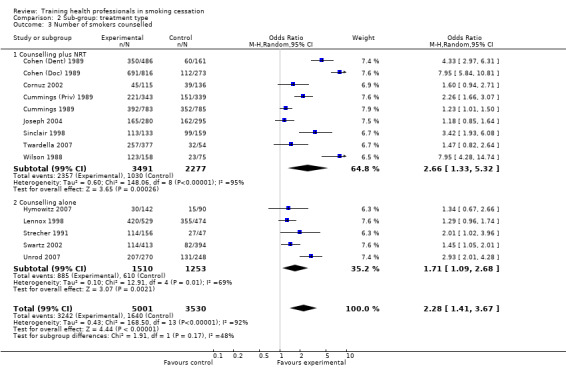
Comparison 2 Sub‐group: treatment type, Outcome 3 Number of smokers counselled.
3.3. Analysis.

Comparison 3 Sub‐group: treatment intensity ‐ Number of sessions, Outcome 3 Number of smokers counselled.
4.3. Analysis.

Comparison 4 Sub‐group: treatment intensity ‐ Total exposure, Outcome 3 Number of smokers counselled.
5.3. Analysis.

Comparison 5 Sub‐group: mode of intervention delivery, Outcome 3 Number of smokers counselled.
6.3. Analysis.
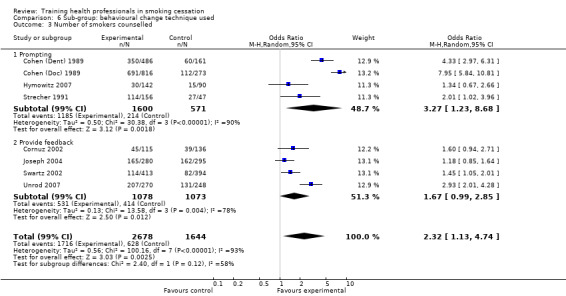
Comparison 6 Sub‐group: behavioural change technique used, Outcome 3 Number of smokers counselled.
7.3. Analysis.
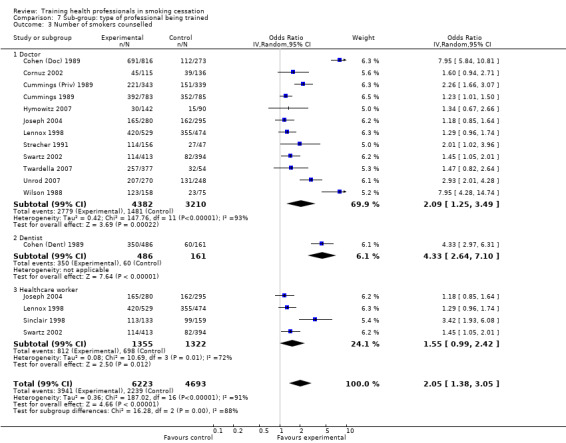
Comparison 7 Sub‐group: type of professional being trained, Outcome 3 Number of smokers counselled.
8.3. Analysis.

Comparison 8 Sub‐group: length of follow‐up, Outcome 3 Number of smokers counselled.
9.3. Analysis.

Comparison 9 Sub‐group: risk of bias in the studies, Outcome 3 Number of smokers counselled.
Given self‐help materials
The number of smokers receiving self‐help material increased significantly in favour of the intervention for the nine studies able to be included in the meta‐analysis (OR 3.52, 95% CI 1.90 to 6.52, p< 0.0001, Analysis 1.5). Provision of cessation materials in the Hymowitz 2007 study, which could not be included in the meta‐analysis, did increase significantly across both groups over the four year study period when compared to baseline values (intervention 28.8%, control 17.6%) however, this interaction was not statistically different between groups. The other study unable to be meta‐analysed (Kottke 1989) also produced a statistically significant effect (p< 0.001). Signficant heterogeneity was observed in the meta‐analysis (I²= 91%) which was explored through sub‐group analyses. The type of treatment did not show a significant difference between groups, although the counselling plus nicotine replacement therapy group did have a larger effect size compared to counselling alone (Analysis 2.4). Likewise, no differences were observed for single compared to multiple session delivery (Analysis 3.4) or duration of delivery (Analysis 4.4), although the Cornuz 2002 study with a total exposure over four hours did produce a very large effect with wide confidence intervals. No differences were observed for the mode of intervention delivery (Analysis 5.4) or provision of prompting or feedback to aid health professionals in the provision of self‐help materials (Analysis 6.4). The one study (Swartz 2002) which included healthcare workers for intervention delivery produced less of an effect compared to the pooled result of studies using doctors (Analysis 7.4). No difference between sub‐groups was observed for length of follow‐up (Analysis 8.3) although studies identified as having less risk of bias did have a larger effect size compared to those with larger amounts of bias (Analysis 9.4).
1.5. Analysis.
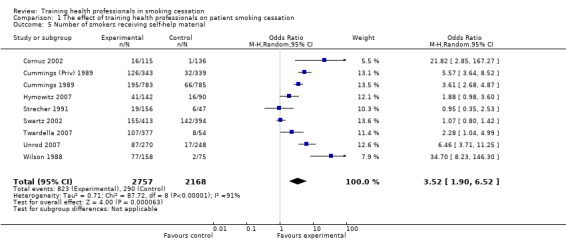
Comparison 1 The effect of training health professionals on patient smoking cessation, Outcome 5 Number of smokers receiving self‐help material.
2.4. Analysis.

Comparison 2 Sub‐group: treatment type, Outcome 4 Number of smokers receiving self‐help material.
3.4. Analysis.
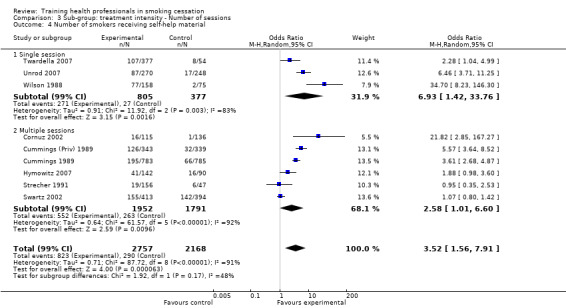
Comparison 3 Sub‐group: treatment intensity ‐ Number of sessions, Outcome 4 Number of smokers receiving self‐help material.
4.4. Analysis.
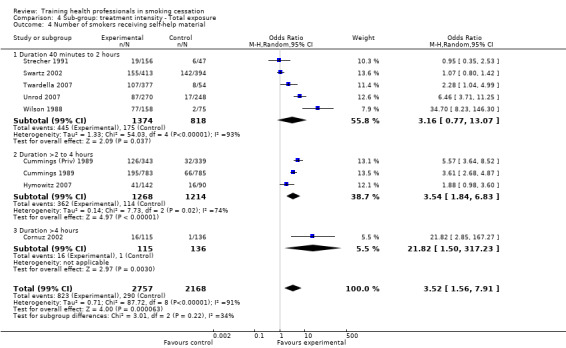
Comparison 4 Sub‐group: treatment intensity ‐ Total exposure, Outcome 4 Number of smokers receiving self‐help material.
5.4. Analysis.

Comparison 5 Sub‐group: mode of intervention delivery, Outcome 4 Number of smokers receiving self‐help material.
6.4. Analysis.
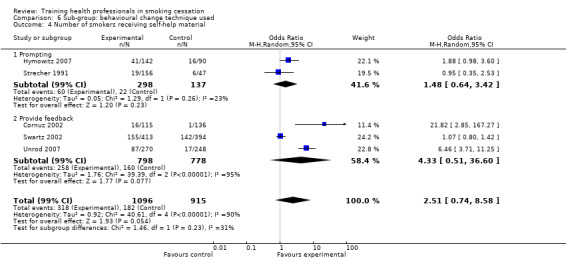
Comparison 6 Sub‐group: behavioural change technique used, Outcome 4 Number of smokers receiving self‐help material.
7.4. Analysis.

Comparison 7 Sub‐group: type of professional being trained, Outcome 4 Number of smokers receiving self‐help material.
9.4. Analysis.

Comparison 9 Sub‐group: risk of bias in the studies, Outcome 4 Number of smokers receiving self‐help material.
Offered nicotine gum/replacement therapy
Nine studies were pooled to assess the number of smokers receiving nicotine gum/replacement therapy (Analysis 1.6). The meta‐analysis did not produce evidence of an effect (OR 1.57, 95% CI 0.87 to 2.84, p= NS), but significant heterogeneity was detected (I²= 91%). The Hymowitz 2007 study also assessed this outcome with few parents in either condition reporting that residents prescribed nicotine replacement therapy (intervention 7.6%, control 5.9%). An exploration into the possible sources of heterogeneity found no difference between interventions containing counselling with or without nicotine replacement therapy (Analysis 2.5), however surprising results were observed with much larger effect sizes for single session intervention delivery compared to multiple session (Analysis 3.5), which could account for some of the heterogeneity. No differences were observed between sub‐groups for treatment intensity (Analysis 4.5), mode of intervention delivery (Analysis 5.5), use of feedback or prompting (Analysis 6.5), type of professional being trained (Analysis 7.5) or length of follow‐up (Analysis 8.5). However studies with less risk of bias did produce larger effect sizes compared to studies with three to five sources of bias identified, which could also contribute to some of the observed heterogeneity (Analysis 9.5).
1.6. Analysis.
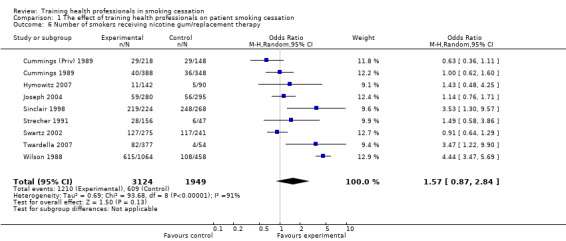
Comparison 1 The effect of training health professionals on patient smoking cessation, Outcome 6 Number of smokers receiving nicotine gum/replacement therapy.
2.5. Analysis.

Comparison 2 Sub‐group: treatment type, Outcome 5 Number of smokers receiving nicotine gum/replacement therapy.
3.5. Analysis.
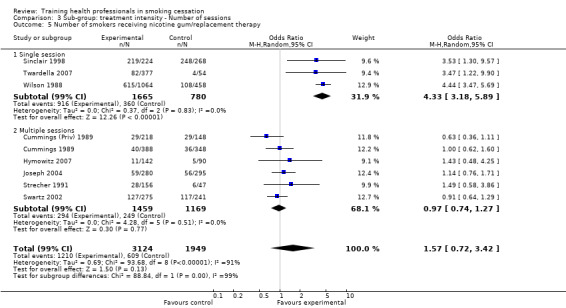
Comparison 3 Sub‐group: treatment intensity ‐ Number of sessions, Outcome 5 Number of smokers receiving nicotine gum/replacement therapy.
4.5. Analysis.
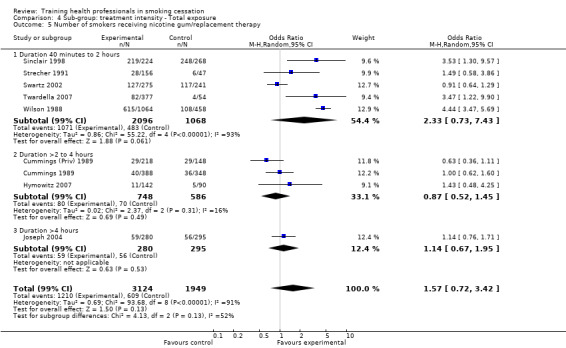
Comparison 4 Sub‐group: treatment intensity ‐ Total exposure, Outcome 5 Number of smokers receiving nicotine gum/replacement therapy.
5.5. Analysis.
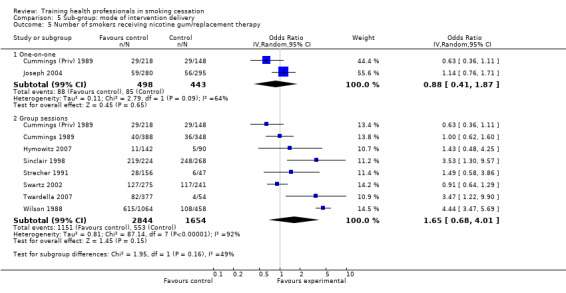
Comparison 5 Sub‐group: mode of intervention delivery, Outcome 5 Number of smokers receiving nicotine gum/replacement therapy.
6.5. Analysis.

Comparison 6 Sub‐group: behavioural change technique used, Outcome 5 Number of smokers receiving nicotine gum/replacement therapy.
7.5. Analysis.

Comparison 7 Sub‐group: type of professional being trained, Outcome 5 Number of smokers receiving nicotine gum/replacement therapy.
8.5. Analysis.

Comparison 8 Sub‐group: length of follow‐up, Outcome 5 Number of smokers receiving nicotine gum/replacement therapy.
9.5. Analysis.

Comparison 9 Sub‐group: risk of bias in the studies, Outcome 5 Number of smokers receiving nicotine gum/replacement therapy.
Prescribed a quit date
Only three studies reported on smokers being prescribed a quit date (Wilson 1988; Cummings 1989; Strecher 1991). Pooling these together produced a statistically and clinically significant effect in favour of the intervention (OR 14.18, 95% CI 6.57 to 30.61, p< 0.00001, Analysis 1.7), with minimal observed heterogeneity. As such, sub‐group analyses were not necessary for this outcome.
1.7. Analysis.

Comparison 1 The effect of training health professionals on patient smoking cessation, Outcome 7 Number of smokers prescribed a quit date.
Cost effectiveness of interventions
Cost effectiveness data was presented in one study (Cornuz 2002), with the incremental cost of the intervention reported to amount to (U.S.) $2.58 per consultation by a smoker. When considering 'cost per life‐year saved', this translated to (U.S.) $25.40 for men and $35.20 for women, with one‐way sensitivity analyses yielding a range of $4.00 to $107.10 in men and $9.70 to $148.60 in women. The Joseph 2004 study reported that the dollar spent per 1000 primary care patients did increase in the intervention sites and decrease in control sites, however this was not significant.
Number of referrals made
No studies reported on the number of referrals made to local smoking cessation services.
Statistical analyses and cluster adjustments
All 17 studies used a cluster randomized design for practical reasons, with the unit of randomization being the health care practitioner or practice. However, in 15 of the 17 studies patients were the unit of analysis. Hymowitz 2007 and Kottke 1989 were the exceptions, reporting outcomes at the level of randomization (the doctor/resident). The majority of studies that reported outcomes at the level of patient accounted for potential clustering effects within their reported results, with four studies (three in the late 1980's Wilson 1988; Cummings (Priv) 1989; Cummings 1989 and one in the mid‐1990's Wang 1994) being the exceptions. The two Cummings et al studies did perform clustering analyses, however they were not included in the published results as they were seen to have had no effect on the final outcome. As such, the data for these studies were manually adjusted for potential clustering effects as per the pre‐specified methodology outlined in the Unit of analysis issues section of this review.
Sub‐group analyses
Multiple sub‐group analyses have been considered as per the pre‐defined methodology to further explore heterogeneity. When considering these outcomes the level of statistical significance should be considered at p< 0.01, to account for potential false positive results (as per the Bonferroni adjustment described Assessment of heterogeneity), which increase with the number of potential effect modifiers being investigated. Total study confidence intervals were assessed at the 99% level for all sub‐group analyses. Significant heterogeneity was determined through a combination of the I² statistic (I² >60%), Chi² statistic and visual inspection of the Forest plots, and was present for all outcomes with the exception of 'Smoking cessation at longest follow‐up' and 'Number of smokers prescribed a quit date' where significant heterogeneity was not identified. In the presence of heterogeneity based on the I² statistic of > 96%, the pooled estimate has been removed, as the outcomes are considered too different to be combined in meta‐analysis. Likewise, when a comparison contained the same study in different sub‐groups, the pooled estimate was not used.
Discussion
Summary of main results
Seventeen completed studies (total 28,531 subjects) assessed the benefits of interventions to train health professionals to provide smoking cessation initiatives to their patients. Whilst some methodological variations occurred between studies in relation to intervention, delivery mode, type of health professional and duration, they were all aimed at training health professionals to help their patients stop smoking. The primary outcome of smoking cessation was presented in pooled meta‐analyses as point prevalence (14 studies) and continuous abstinence (eight studies). A statistically and clinically significant effect in favour of the intervention was observed for both of these outcomes at final follow‐up (see Table 1). All secondary outcomes (with one exception) produced a statistically and clinically significant effect in favour of the intervention at final follow‐up. These outcomes include asking patients to set a quit date, asking patients to make follow‐up appointments, counselling of smokers, provision of self‐help material and prescription of a quit date. No evidence of an effect was observed for the secondary outcome of providing patients with nicotine gum/replacement therapy. No studies were able to be meta‐analysed to assess the cost effectiveness of interventions.
Overall completeness and applicability of evidence
In the context of current practice, this review should be used to provide readers with an outline of what interventions have a proven effect, and where resources need to be directed for future investigations. Studies which incorporated multiple intervention components such as provision of nicotine replacement therapy, requests for follow‐up appointments and provision of self‐help material were more likely to be successful than those with interventions of counselling alone. Surprisingly, health professionals who were trained using only a single session and in a group setting were just as likely if not more likely to have patients quit smoking as those being trained with multiple delivery sessions and one‐on‐one training (i.e., face to face with the trainer). Similarly, the duration of training for the health professional of between 40 minutes to two hours was just as effective, and in some cases more so, than a duration of greater than two hours. Studies with multiple follow‐up periods and closer monitoring of outcomes by investigators (including the provision of feedback) were more successful than those of lesser intensity. Smoking cessation interventions delivered by a doctor or dentist were more likely to produce successful quit attempts than those delivered by other health care workers.
To ensure methodological rigour, future studies should aim to incorporate the following into the study design:
Report patient level outcomes (e.g., smoking cessation) as well as health professional outcomes (e.g., physician report of number of smokers counselled) rather than providing details only relating to the consultation process
Adequate methods of randomization and allocation concealment
Report smoking related outcome data both pre and post intervention
Incorporate a control group which adequately matches the demographic characteristics of the intervention population.
Quality of the evidence
Study quality was a potential issue in this review with many of the studies being of unclear methodological design. It is extremely difficult to blind participants in relation to what intervention they will be receiving, as there are two levels to consider: the health professional and the patient. All 17 included studies had unclear allocation concealment whilst only five studies adequately reported methods of random sequence generation, two had a high risk of bias with the remaining ten studies being unclear. Overall, the body of evidence identified permits a moderately robust conclusion regarding the objectives of this review, with 17 included studies (28,531 participants).
Evidence presented in the summary of findings table was downgraded to take into account:
limitations in design: methods of randomization, allocation concealment and/or blinding were not described or inadequate for the majority of studies assessing the particular outcome (‐1)
Inconsistencies: significant heterogeneity (‐1)
Imprecision: only few participants in few studies available to assess the outcome (‐1)
Potential biases in the review process
A potential bias in the review process is exclusion of studies examining interventions that train health professionals in smoking cessation that are of questionable methodological design. This review does sacrifice inclusion of some relevant information, however the trade off is a meta‐analysis of higher quality evidence on which future investigations can be based. Some of the pertinent information from these studies is discussed below under Agreements and disagreements with other studies or reviews though results should be interpreted with caution. Another limitation to the review is the under‐reporting of the intervention for included studies. This means that some studies may have indeed included additional intervention components that, had we known they existed, would have led us to classify the study differently within the sub‐groups. One key strength of the review process to address potential biases is the use of two experienced and independent review authors who assessed the studies for risk of bias, although this can do little to account for biases which occur in the methodological designs of the included studies.
Agreements and disagreements with other studies or reviews
A compilation of systematic reviews and surveys of key informants were published as a special edition in the journal 'Drug and Alcohol Review' in 2009, relating to the education and training of health professionals and students in tobacco, alcohol and other drugs (Richmond 2009a). The first published survey of 21 key informants from eight countries found a high level of consistency in the content of the smoking cessation interventions, with 72% of programs using the 5A (Ask, Assess, Advise, Assist, Arange) model, 64% using the stages of change (trans‐theoretical) model, 84% including pharmacotherapies, with 84% having some reference to clinical practice guidelines (Zwar 2009). Only five of the seventeen included studies in our review had reference to any particular behavioural change technique, however it is quite likely that the majority of studies are based around some kind of theoretical behavioural change context, which is not reported in the publication. These results are similar to the those reported in Richmond 2009b. The authors identified a lack of interest (with other continuing education topics considered to be a higher priority) and lack of funding for interventions to be the major barriers for the uptake and sustainability of training programs (Zwar 2009). Some possible solutions were provided to address these barriers including raising awareness of the importance of smoking cessation for the health of patients and incorporating education on smoking cessation into vocational courses for specialties. Another systematic review of postgraduate smoking cessation training for physicians in 28 European countries found nine studies which met all of the inclusion criteria containing a total of 170 postgraduate training programs (Kralikova 2009). The key implications reported by the authors were that postgraduate training in smoking cessation may not be reaching physicians and was not rigorously evaluated. To combat this problem multiple authors suggest that future research needs to incorporate methods of disseminating effective educational activities with the intention of increasing participation (Kralikova 2009; Muramoto 2009). It is also imperative that health professional organisations advocate for the systematic implementation of comprehensive tobacco cessation training programs to increase the number of patients receiving tobacco cessation interventions (Botelho 2009). Another study using direct observation of physician‐patient encounters found similar results and concluded that strategies are needed to assist physicians to incorporate systematic approaches that will standardise smoking cessation care (Ellerbeck 2001). In this investigation, discussions around tobacco were more common in practices that utilised standard forms for recording smoking status and during new patient visits. Interestingly, the authors also found that discussions around tobacco use occurred less often among physicians in practice for more than 10 years and with older patients (Ellerbeck 2001), which is similar to an observational study by Bertakis 2007 investigating the factors associated with physician discussion of tobacco use with patients. Considerable resistance was also observed in a cohort of physicians receiving academic detailing to promote tobacco‐use cessation counselling in dental offices. Dental staff members (including receptionists, office managers, dental assistants and dental hygienists) were reluctant to participate in the interventions due to increased paperwork, having to deal with uncooperative patients, and the perception that only a few patients use tobacco anyway and that counselling does not work (Albert 2004). However, the resistance observed did decrease as follow‐up visits progressed and staff became more comfortable with the intervention and the procedures involved. This evidence suggests that through the provision of first‐hand experience prior to guiding patients through the same process, physicians may feel more comfortable in implementing smoking cessation interventions into standard practice, which has the potential to be highly cost‐effective. One of the included studies by Cornuz 2002 reported that training residents in smoking cessation counselling is very cost‐effective and may be more efficient than the majority of currently accepted tobacco control interventions. This has also been supported by more recent systematic reviews and investigations (Maciosek 2006; Solberg 2006; Stead 2008). As such, the provision of counselling, advice and/or offers of assistance to the patient has the potential to significantly increase the number of quit attempts, which subsequently has the potential to reduce health related costs as well as morbidity and mortality associated with ongoing chronic tobacco use.
The previous version of this Cochrane review (New Reference) included eight studies with six finding no effect of intervention. The authors also stated that effects of training on process outcomes increased if prompts and reminders were used, however they concluded that there was no strong evidence that training health professionals to provide smoking cessation interventions changed smoking behaviour. With the addition of nine studies (more than half the initial number of inclusions), the findings of this review have now changed to support the training health professionals in smoking cessation interventions.
Authors' conclusions
Implications for practice.
Overall, a moderately large amount of methodologically rigorous evidence has been presented to support the effectiveness of training health professionals in smoking cessation. The following program characteristics could be considered for individuals involved in future clinical practice initiatives:
Combination of multiple intervention components including the provision of counselling, offer of follow‐up appointments, setting or being prescribed a quit date and provision of self‐help material
A one‐off group training session for health professionals of between one to two hours duration, providing there is adequate follow‐up and monitoring of progress. This will need to include provision of follow‐up feedback to health professionals and resources such as patient self‐help materials, with consideration given to other intervention components as mentioned above.
Consider organisational factors to ensure that smoking cessation messages are reliably delivered. Training can be expensive, and simply providing programs for health care professionals, without addressing the constraints imposed by the conditions in which they practise, is unlikely to be a wise use of health care resources.
Implications for research.
Multi‐component investigations incorporating new pharmacological interventions for smoking cessation (such as varenicline tartrate and bupropion) or other cessation aids (such as electronic cigarettes) alongside physician training should be considered to determine if any additional benefit in long‐term abstinence can be obtained. Future research needs to ensure that adequate methodological rigour is met with considerations relating to:
Sequence generation and allocation concealment
Demographics and comparability of the control comparison
Reporting of smoking related outcome data
Collection of data both pre and post intervention implementation.
So as to enable interventions to be replicated in clinical practice, it is also important that authors of future trial reports describe the content of the training in sufficient detail, for example detailing the educational methods, strategies and theories used to train the professionals.
What's new
| Date | Event | Description |
|---|---|---|
| 5 December 2013 | Amended | Correction to Summary of Findings Table (confidence interval for continuous abstinence) |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 2, 1996
| Date | Event | Description |
|---|---|---|
| 30 March 2012 | New search has been performed | Seven new studies added; SOF table, meta‐analyses and summary of individual study effectiveness table added. |
| 30 March 2012 | New citation required and conclusions have changed | Structure of review changed, body of text updated and re‐written; Conclusions changed. |
| 4 August 2008 | Amended | Converted to new review format |
| 31 May 2000 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Firstly we would like to thank Monaz Mehta, Lindsay Stead, Elizabeth Arnold, Jamie Hartmann‐Boyce and the Tobacco Addiction Group editors and personnel for their ongoing assistance and guidance throughout the review process and peer reviewers for their useful comments.
We would also like to acknowledge Dr Tim Cowan and Judith Gordon for supplying unpublished data from their research.
The late Chris Silagy was the second author on the first version of this review. Data analysis in the previous version of this review changed substantially in response to comments received from Jeremy Grimshaw, and discussion at a workshop on analysis of clustered data organised by the Cochrane Statistical Methods Working Group.
Appendices
Appendix 1. MEDLINE search strategy
1 RANDOMIZED‐CONTROLLED‐TRIAL.pt. (223948) 2 CONTROLLED‐CLINICAL‐TRIAL.pt. (38083) 3 CLINICAL‐TRIAL.pt. (265615) 4 Meta analysis.pt. (29188) 5 exp Clinical Trial/ (457811) 6 Random‐Allocation/ (38507) 7 randomized‐controlled trials/ (69081) 8 double‐blind‐method/ (68631) 9 single‐blind‐method/ (13151) 10 placebos/ (12338) 11 Research‐Design/ (43437) 12 ((clin$ adj$ trial$) or placebo$ or random$).ti,ab. (530665) 13 ((singl$ or doubl$ or trebl$ or tripl$) adj5 (blind$ or mask$)).ti,ab. (67270) 14 (volunteer$ or prospectiv$).ti,ab. (340629) 15 exp Follow‐Up‐Studies/ (269958) 16 exp Retrospective‐Studies/ (314812) 17 exp Prospective‐Studies/ (233927) 18 exp Evaluation‐Studies/ or Program‐Evaluation.mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] (187044) 19 exp Cross‐Sectional‐Studies/ (115024) 20 exp Behavior‐therapy/ (25130) 21 exp Health‐Promotion/ (34021) 22 exp Community‐Health‐Services/ (246874) 23 exp Health‐Education/ (69098) 24 exp Health‐Behavior/ (59981) 25 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 (1995011) 26 smoking cessation.mp. or exp Smoking Cessation/ (17529) 27 "Tobacco‐Use‐Cessation"/ (545) 28 "Tobacco‐Use‐Disorder"/ (5569) 29 Tobacco‐Smokeless/ (1457) 30 exp Tobacco‐Smoke‐Pollution/ (6538) 31 exp Tobacco‐/ (13929) 32 exp Nicotine‐/ (10241) 33 ((quit$ or stop$ or ceas$ or giv$) adj5 smoking).ti,ab. (6469) 34 exp Smoking/pc, th [Prevention & Control, Therapy] (8740) 35 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 [A category smoking terms] (50315) 36 1 or 2 or 3 [Likely CT design terms; RCTs, CCTs, Clinical trials] (384162) 37 (animals not humans).sh. [used with 'not' to exclude animal studies for each subset] (1521160) 38 (35 and 36) not 37 [Set 1: A smoking terms, likely CT design terms, human only] (3290) 39 Education, Premedical/ (192) 40 exp Education, Professional/ (102079) 41 exp Inservice Training/ (13162) 42 Physician's Practice Patterns/ (30147) 43 Dentist's Practice Patterns/ (1382) 44 exp Delivery of Health Care/ (479118) 45 exp Comprehensive Health Care/ (120957) 46 Critical Pathways/ (3744) 47 Disease Management/ (8035) 48 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 (622105) 49 (training or trained).ti,ab. (152010) 50 48 not 49 [MeSH without training text terms] (575357) 51 38 and 49 [training text terms with smoking trials] (224) 52 38 and 50 [sensitive MeSH terms, no mention of training in text] (600)
Records retrieved by this strategy that matched records in the Tobacco Addiction Group Specialised Register were screened for potential relevance. Records not already in the Register were not checked because they would previously have been retrieved during regular searches, and excluded for not being reports of controlled trials or other potentially eligible evaluations of tobacco control interventions.
Appendix 2. Glossary of tobacco‐specific terms
| Term | Definition |
| Abstinence | A period of being quit, i.e. stopping the use of cigarettes or other tobacco products, May be defined in various ways; see also: point prevalence abstinence; prolonged abstinence; continuous/sustained abstinence |
| Biochemical verification | Also called 'biochemical validation' or 'biochemical confirmation': A procedure for checking a tobacco user's report that he or she has not smoked or used tobacco. It can be measured by testing levels of nicotine or cotinine or other chemicals in blood, urine, or saliva, or by measuring levels of carbon monoxide in exhaled breath or in blood. |
| Bupropion | A pharmaceutical drug originally developed as an antidepressant, but now also licensed for smoking cessation; trade names Zyban, Wellbutrin (when prescribed as an antidepressant) |
| Carbon monoxide (CO) | A colourless, odourless highly poisonous gas found in tobacco smoke and in the lungs of people who have recently smoked, or (in smaller amounts) in people who have been exposed to tobacco smoke. May be used for biochemical verification of abstinence. |
| Cessation | Also called 'quitting' The goal of treatment to help people achieve abstinence from smoking or other tobacco use, also used to describe the process of changing the behaviour |
| Continuous abstinence | Also called 'sustained abstinence' A measure of cessation often used in clinical trials involving avoidance of all tobacco use since the quit day until the time the assessment is made. The definition occasionally allows for lapses. This is the most rigorous measure of abstinence |
| 'Cold Turkey' | Quitting abruptly, and/or quitting without behavioural or pharmaceutical support. |
| Craving | A very intense urge or desire [to smoke]. See: Shiffman et al 'Recommendations for the assessment of tobacco craving and withdrawal in smoking cessation trials' Nicotine & Tobacco Research 2004: 6(4): 599‐614 |
| Dopamine | A neurotransmitter in the brain which regulates mood, attention, pleasure, reward, motivation and movement |
| Efficacy | Also called 'treatment effect' or 'effect size': The difference in outcome between the experimental and control groups |
| Harm reduction | Strategies to reduce harm caused by continued tobacco/nicotine use, such as reducing the number of cigarettes smoked, or switching to different brands or products, e.g. potentially reduced exposure products (PREPs), smokeless tobacco. |
| Lapse/slip | Terms sometimes used for a return to tobacco use after a period of abstinence. A lapse or slip might be defined as a puff or two on a cigarette. This may proceed to relapse, or abstinence may be regained. Some definitions of continuous, sustained or prolonged abstinence require complete abstinence, but some allow for a limited number or duration of slips. People who lapse are very likely to relapse, but some treatments may have their effect by helping people recover from a lapse. |
| nAChR | [neural nicotinic acetylcholine receptors]: Areas in the brain which are thought to respond to nicotine, forming the basis of nicotine addiction by stimulating the overflow of dopamine |
| Nicotine | An alkaloid derived from tobacco, responsible for the psychoactive and addictive effects of smoking. |
| Nicotine Replacement Therapy (NRT) | A smoking cessation treatment in which nicotine from tobacco is replaced for a limited period by pharmaceutical nicotine. This reduces the craving and withdrawal experienced during the initial period of abstinence while users are learning to be tobacco‐free The nicotine dose can be taken through the skin, using patches, by inhaling a spray, or by mouth using gum or lozenges. |
| Outcome | Often used to describe the result being measured in trials that is of relevance to the review. For example smoking cessation is the outcome used in reviews of ways to help smokers quit. The exact outcome in terms of the definition of abstinence and the length of time that has elapsed since the quit attempt was made may vary from trial to trial. |
| Pharmacotherapy | A treatment using pharmaceutical drugs, e.g. NRT, bupropion |
| Point prevalence abstinence (PPA) | A measure of cessation based on behaviour at a particular point in time, or during a relatively brief specified period, e.g. 24 hours, 7 days. It may include a mixture of recent and long‐term quitters. cf. prolonged abstinence, continuous abstinence |
| Prolonged abstinence | A measure of cessation which typically allows a 'grace period' following the quit date (usually of about two weeks), to allow for slips/lapses during the first few days when the effect of treatment may still be emerging. See: Hughes et al 'Measures of abstinence in clinical trials: issues and recommendations'; Nicotine & Tobacco Research, 2003: 5 (1); 13‐25 |
| Relapse | A return to regular smoking after a period of abstinence |
| Secondhand smoke | Also called passive smoking or environmental tobacco smoke [ETS] A mixture of smoke exhaled by smokers and smoke released from smouldering cigarettes, cigars, pipes, bidis, etc. The smoke mixture contains gases and particulates, including nicotine, carcinogens and toxins. |
| Self‐efficacy | The belief that one will be able to change one's behaviour, e.g. to quit smoking |
| SPC [Summary of Product Characteristics] | Advice from the manufacturers of a drug, agreed with the relevant licensing authority, to enable health professionals to prescribe and use the treatment safely and effectively. |
| Tapering | A gradual decrease in dose at the end of treatment, as an alternative to abruptly stopping treatment |
| Titration | A technique of dosing at low levels at the beginning of treatment, and gradually increasing to full dose over a few days, to allow the body to get used to the drug. It is designed to limit side effects. |
| Withdrawal | A variety of behavioural, affective, cognitive and physiological symptoms, usually transient, which occur after use of an addictive drug is reduced or stopped. See: Shiffman et al 'Recommendations for the assessment of tobacco craving and withdrawal in smoking cessation trials' Nicotine & Tobacco Research 2004: 6(4): 599‐614 |
Data and analyses
Comparison 1. The effect of training health professionals on patient smoking cessation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Smoking cessation at longest follow‐up | 15 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Point prevalence | 14 | 13459 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [1.20, 1.55] |
| 1.2 Continuous abstinence | 8 | 9443 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [1.26, 2.03] |
| 2 Patient asked to set a quit date | 8 | 4332 | Odds Ratio (M‐H, Random, 95% CI) | 4.98 [2.29, 10.86] |
| 3 Patient asked to make a follow‐up appointment | 7 | 3114 | Odds Ratio (M‐H, Random, 95% CI) | 3.34 [1.51, 7.37] |
| 4 Number of smokers counselled | 14 | 8531 | Odds Ratio (M‐H, Random, 95% CI) | 2.28 [1.58, 3.27] |
| 5 Number of smokers receiving self‐help material | 9 | 4925 | Odds Ratio (M‐H, Random, 95% CI) | 3.52 [1.90, 6.52] |
| 6 Number of smokers receiving nicotine gum/replacement therapy | 9 | 5073 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.87, 2.84] |
| 7 Number of smokers prescribed a quit date | 3 | 1172 | Odds Ratio (M‐H, Fixed, 95% CI) | 14.18 [6.57, 30.61] |
Comparison 2. Sub‐group: treatment type.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient asked to set a quit date | 8 | 4332 | Odds Ratio (M‐H, Random, 95% CI) | 4.98 [1.79, 13.88] |
| 1.1 Counselling plus NRT | 6 | 3322 | Odds Ratio (M‐H, Random, 95% CI) | 7.45 [3.30, 16.85] |
| 1.2 Counselling alone | 2 | 1010 | Odds Ratio (M‐H, Random, 95% CI) | 1.22 [0.78, 1.92] |
| 2 Patient asked to make a follow‐up appointment | 7 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Counselling plus NRT | 4 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Counselling alone | 3 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of smokers counselled | 14 | 8531 | Odds Ratio (M‐H, Random, 95% CI) | 2.28 [1.41, 3.67] |
| 3.1 Counselling plus NRT | 9 | 5768 | Odds Ratio (M‐H, Random, 95% CI) | 2.66 [1.33, 5.32] |
| 3.2 Counselling alone | 5 | 2763 | Odds Ratio (M‐H, Random, 95% CI) | 1.71 [1.09, 2.68] |
| 4 Number of smokers receiving self‐help material | 9 | 4925 | Odds Ratio (M‐H, Random, 95% CI) | 3.52 [1.56, 7.91] |
| 4.1 Counselling plus NRT | 5 | 3165 | Odds Ratio (M‐H, Random, 95% CI) | 5.50 [2.45, 12.36] |
| 4.2 Counselling alone | 4 | 1760 | Odds Ratio (M‐H, Random, 95% CI) | 1.91 [0.56, 6.48] |
| 5 Number of smokers receiving nicotine gum/replacement therapy | 9 | 5073 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.72, 3.42] |
| 5.1 Counselling plus NRT | 6 | 4122 | Odds Ratio (M‐H, Random, 95% CI) | 1.78 [0.65, 4.91] |
| 5.2 Counselling alone | 3 | 951 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.66, 1.50] |
Comparison 3. Sub‐group: treatment intensity ‐ Number of sessions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient asked to set a quit date | 8 | 4332 | Odds Ratio (M‐H, Random, 95% CI) | 4.98 [1.79, 13.88] |
| 1.1 Single session | 3 | 1969 | Odds Ratio (M‐H, Random, 95% CI) | 14.45 [3.98, 52.48] |
| 1.2 Multiple sessions | 5 | 2363 | Odds Ratio (M‐H, Random, 95% CI) | 2.79 [1.03, 7.55] |
| 2 Patient asked to make a follow‐up appointment | 7 | 3114 | Odds Ratio (M‐H, Random, 95% CI) | 3.34 [1.18, 9.46] |
| 2.1 Single session | 2 | 751 | Odds Ratio (M‐H, Random, 95% CI) | 13.33 [2.95, 60.24] |
| 2.2 Multiple sessions | 5 | 2363 | Odds Ratio (M‐H, Random, 95% CI) | 1.88 [0.94, 3.74] |
| 3 Number of smokers counselled | 14 | 8531 | Odds Ratio (M‐H, Random, 95% CI) | 2.28 [1.41, 3.67] |
| 3.1 Single session | 7 | 4213 | Odds Ratio (M‐H, Random, 95% CI) | 3.39 [1.56, 7.37] |
| 3.2 Multiple sessions | 7 | 4318 | Odds Ratio (M‐H, Random, 95% CI) | 1.50 [1.14, 1.98] |
| 4 Number of smokers receiving self‐help material | 9 | 4925 | Odds Ratio (M‐H, Random, 95% CI) | 3.52 [1.56, 7.91] |
| 4.1 Single session | 3 | 1182 | Odds Ratio (M‐H, Random, 95% CI) | 6.93 [1.42, 33.76] |
| 4.2 Multiple sessions | 6 | 3743 | Odds Ratio (M‐H, Random, 95% CI) | 2.58 [1.01, 6.60] |
| 5 Number of smokers receiving nicotine gum/replacement therapy | 9 | 5073 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.72, 3.42] |
| 5.1 Single session | 3 | 2445 | Odds Ratio (M‐H, Random, 95% CI) | 4.33 [3.18, 5.89] |
| 5.2 Multiple sessions | 6 | 2628 | Odds Ratio (M‐H, Random, 95% CI) | 0.97 [0.74, 1.27] |
Comparison 4. Sub‐group: treatment intensity ‐ Total exposure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient asked to set a quit date | 8 | 4332 | Odds Ratio (M‐H, Random, 95% CI) | 4.98 [1.79, 13.88] |
| 1.1 Duration 40 minutes to 2 hours | 5 | 2979 | Odds Ratio (M‐H, Random, 95% CI) | 5.63 [0.71, 44.43] |
| 1.2 Duration >2 to 4 hours | 2 | 1102 | Odds Ratio (M‐H, Random, 95% CI) | 4.70 [3.08, 7.16] |
| 1.3 Duration >4 hours | 1 | 251 | Odds Ratio (M‐H, Random, 95% CI) | 3.76 [0.65, 21.65] |
| 2 Patient asked to make a follow‐up appointment | 6 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Duration 40 minutes to 2 hours | 4 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Duration >2 to 4 hours | 2 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of smokers counselled | 14 | 8531 | Odds Ratio (M‐H, Random, 95% CI) | 2.28 [1.41, 3.67] |
| 3.1 Duration 40 minutes to 2 hours | 8 | 4220 | Odds Ratio (M‐H, Random, 95% CI) | 3.25 [1.67, 6.33] |
| 3.2 Duration >2 to 4 hours | 3 | 2482 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.86, 2.86] |
| 3.3 Duration >4 hours | 3 | 1829 | Odds Ratio (M‐H, Random, 95% CI) | 1.29 [0.99, 1.68] |
| 4 Number of smokers receiving self‐help material | 9 | 4925 | Odds Ratio (M‐H, Random, 95% CI) | 3.52 [1.56, 7.91] |
| 4.1 Duration 40 minutes to 2 hours | 5 | 2192 | Odds Ratio (M‐H, Random, 95% CI) | 3.16 [0.77, 13.07] |
| 4.2 Duration >2 to 4 hours | 3 | 2482 | Odds Ratio (M‐H, Random, 95% CI) | 3.54 [1.84, 6.83] |
| 4.3 Duration >4 hours | 1 | 251 | Odds Ratio (M‐H, Random, 95% CI) | 21.82 [1.50, 317.23] |
| 5 Number of smokers receiving nicotine gum/replacement therapy | 9 | 5073 | Odds Ratio (M‐H, Random, 95% CI) | 1.57 [0.72, 3.42] |
| 5.1 Duration 40 minutes to 2 hours | 5 | 3164 | Odds Ratio (M‐H, Random, 95% CI) | 2.33 [0.73, 7.43] |
| 5.2 Duration >2 to 4 hours | 3 | 1334 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.52, 1.45] |
| 5.3 Duration >4 hours | 1 | 575 | Odds Ratio (M‐H, Random, 95% CI) | 1.14 [0.67, 1.95] |
Comparison 5. Sub‐group: mode of intervention delivery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient asked to set a quit date | 8 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 One‐on‐one | 4 | 2353 | Odds Ratio (M‐H, Random, 95% CI) | 7.52 [2.17, 26.12] |
| 1.2 Group sessions | 8 | 4332 | Odds Ratio (M‐H, Random, 95% CI) | 4.98 [1.79, 13.88] |
| 2 Patient asked to make a follow‐up appointment | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 One‐on‐one | 3 | 1135 | Odds Ratio (M‐H, Random, 95% CI) | 3.60 [0.86, 15.08] |
| 2.2 Group sessions | 6 | 2596 | Odds Ratio (M‐H, Random, 95% CI) | 2.74 [1.06, 7.08] |
| 3 Number of smokers counselled | 14 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 One‐on‐one | 6 | 3762 | Odds Ratio (M‐H, Random, 95% CI) | 2.76 [1.27, 6.01] |
| 3.2 Group sessions | 12 | 7438 | Odds Ratio (M‐H, Random, 95% CI) | 2.47 [1.41, 4.30] |
| 4 Number of smokers receiving self‐help material | 9 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 One‐on‐one | 3 | 1451 | Odds Ratio (M‐H, Random, 95% CI) | 6.09 [3.93, 9.44] |
| 4.2 Group sessions | 8 | 4407 | Odds Ratio (M‐H, Random, 95% CI) | 3.22 [1.36, 7.65] |
| 5 Number of smokers receiving nicotine gum/replacement therapy | 9 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5.1 One‐on‐one | 2 | 941 | Odds Ratio (IV, Random, 95% CI) | 0.88 [0.41, 1.87] |
| 5.2 Group sessions | 8 | 4498 | Odds Ratio (IV, Random, 95% CI) | 1.65 [0.68, 4.01] |
Comparison 6. Sub‐group: behavioural change technique used.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient asked to set a quit date | 5 | 2997 | Odds Ratio (M‐H, Random, 95% CI) | 4.19 [0.63, 28.09] |
| 1.1 Prompting | 3 | 1939 | Odds Ratio (M‐H, Random, 95% CI) | 6.99 [0.90, 54.02] |
| 1.2 Provide feedback | 2 | 1058 | Odds Ratio (M‐H, Random, 95% CI) | 1.76 [0.43, 7.17] |
| 2 Patient asked to make a follow‐up appointment | 4 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Prompting | 1 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Provide feedback | 3 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Number of smokers counselled | 8 | 4322 | Odds Ratio (M‐H, Random, 95% CI) | 2.32 [1.13, 4.74] |
| 3.1 Prompting | 4 | 2171 | Odds Ratio (M‐H, Random, 95% CI) | 3.27 [1.23, 8.68] |
| 3.2 Provide feedback | 4 | 2151 | Odds Ratio (M‐H, Random, 95% CI) | 1.67 [0.99, 2.85] |
| 4 Number of smokers receiving self‐help material | 5 | 2011 | Odds Ratio (M‐H, Random, 95% CI) | 2.51 [0.74, 8.58] |
| 4.1 Prompting | 2 | 435 | Odds Ratio (M‐H, Random, 95% CI) | 1.48 [0.64, 3.42] |
| 4.2 Provide feedback | 3 | 1576 | Odds Ratio (M‐H, Random, 95% CI) | 4.33 [0.51, 36.60] |
| 5 Number of smokers receiving nicotine gum/replacement therapy | 4 | 1526 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.76, 1.45] |
| 5.1 Provide feedback | 2 | 1091 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.71, 1.41] |
| 5.2 Prompting | 2 | 435 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.57, 3.76] |
Comparison 7. Sub‐group: type of professional being trained.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient asked to set a quit date | 8 | 4332 | Odds Ratio (M‐H, Random, 95% CI) | 4.98 [1.79, 13.88] |
| 1.1 Doctor | 6 | 2878 | Odds Ratio (M‐H, Random, 95% CI) | 6.35 [2.49, 16.19] |
| 1.2 Dentist | 1 | 647 | Odds Ratio (M‐H, Random, 95% CI) | 6.43 [1.91, 21.56] |
| 1.3 Healthcare worker | 1 | 807 | Odds Ratio (M‐H, Random, 95% CI) | 1.19 [0.74, 1.91] |
| 2 Patient asked to make a follow‐up appointment | 7 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Doctor | 7 | 3114 | Odds Ratio (M‐H, Random, 95% CI) | 3.34 [1.18, 9.46] |
| 2.2 Healthcare worker | 1 | 807 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.73, 1.54] |
| 3 Number of smokers counselled | 14 | 10916 | Odds Ratio (IV, Random, 95% CI) | 2.05 [1.38, 3.05] |
| 3.1 Doctor | 12 | 7592 | Odds Ratio (IV, Random, 95% CI) | 2.09 [1.25, 3.49] |
| 3.2 Dentist | 1 | 647 | Odds Ratio (IV, Random, 95% CI) | 4.33 [2.64, 7.10] |
| 3.3 Healthcare worker | 4 | 2677 | Odds Ratio (IV, Random, 95% CI) | 1.55 [0.99, 2.42] |
| 4 Number of smokers receiving self‐help material | 9 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Doctor | 9 | 4925 | Odds Ratio (IV, Random, 95% CI) | 3.51 [1.57, 7.85] |
| 4.2 Healthcare worker | 1 | 807 | Odds Ratio (IV, Random, 95% CI) | 1.07 [0.73, 1.55] |
| 5 Number of smokers receiving nicotine gum/replacement therapy | 9 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Doctor | 8 | 4581 | Odds Ratio (IV, Random, 95% CI) | 1.44 [0.63, 3.30] |
| 5.2 Healthcare worker | 3 | 1583 | Odds Ratio (IV, Random, 95% CI) | 1.27 [0.64, 2.53] |
Comparison 8. Sub‐group: length of follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient asked to set a quit date | 8 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 From 6 months up to and including 9 months | 3 | 1939 | Odds Ratio (IV, Random, 95% CI) | 7.02 [0.98, 50.34] |
| 1.2 From greater than 9 months up to and including 12 months | 7 | 4129 | Odds Ratio (IV, Random, 95% CI) | 5.67 [1.96, 16.42] |
| 2 Patient asked to make a follow‐up appointment | 7 | 3114 | Odds Ratio (IV, Random, 95% CI) | 3.34 [1.19, 9.34] |
| 2.1 From 6 months up to and including 9 months | 2 | 721 | Odds Ratio (IV, Random, 95% CI) | 3.82 [0.48, 30.51] |
| 2.2 From greater than 9 months up to and including 12 months | 5 | 2393 | Odds Ratio (IV, Random, 95% CI) | 3.10 [0.98, 9.75] |
| 3 Number of smokers counselled | 14 | Odds Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.1 From 6 months up to and including 9 months | 6 | 3752 | Odds Ratio (IV, Random, 95% CI) | 3.13 [1.38, 7.09] |
| 3.2 From greater than 9 months up to and including 12 months | 10 | 6575 | Odds Ratio (IV, Random, 95% CI) | 2.50 [1.34, 4.64] |
| 3.3 From greater than 12 months up to 24 months | 2 | 1235 | Odds Ratio (IV, Random, 95% CI) | 1.30 [0.91, 1.86] |
| 4 Number of smokers receiving self‐help material | 9 | 4925 | Odds Ratio (IV, Random, 95% CI) | 3.51 [1.57, 7.85] |
| 4.1 From 6 months up to and including 9 months | 2 | 721 | Odds Ratio (IV, Random, 95% CI) | 2.59 [0.22, 30.56] |
| 4.2 From greater than 9 months up to and including 12 months | 6 | 3972 | Odds Ratio (IV, Random, 95% CI) | 4.42 [1.53, 12.70] |
| 4.3 From greater than 12 months up to 24 months | 1 | 232 | Odds Ratio (IV, Random, 95% CI) | 1.88 [0.80, 4.42] |
| 5 Number of smokers receiving nicotine gum/replacement therapy | 9 | 5073 | Odds Ratio (IV, Random, 95% CI) | 1.57 [0.72, 3.41] |
| 5.1 From 6 months up to and including 9 months | 2 | 695 | Odds Ratio (IV, Random, 95% CI) | 2.27 [0.75, 6.85] |
| 5.2 From greater than 9 months up to and including 12 months | 6 | 4146 | Odds Ratio (IV, Random, 95% CI) | 1.44 [0.54, 3.81] |
| 5.3 From greater than 12 months up to 24 months | 1 | 232 | Odds Ratio (IV, Random, 95% CI) | 1.43 [0.34, 5.99] |
8.4. Analysis.

Comparison 8 Sub‐group: length of follow‐up, Outcome 4 Number of smokers receiving self‐help material.
Comparison 9. Sub‐group: risk of bias in the studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient asked to set a quit date | 8 | 4332 | Odds Ratio (IV, Random, 95% CI) | 4.97 [1.85, 13.30] |
| 1.1 Up to and including 2 domains | 7 | 4129 | Odds Ratio (IV, Random, 95% CI) | 5.67 [1.96, 16.42] |
| 1.2 From 3 to 5 domains | 1 | 203 | Odds Ratio (IV, Random, 95% CI) | 1.68 [0.31, 9.00] |
| 2 Patient asked to make a follow‐up appointment | 7 | 3114 | Odds Ratio (IV, Random, 95% CI) | 3.34 [1.19, 9.34] |
| 2.1 Up to and including 2 domains | 6 | 2911 | Odds Ratio (IV, Random, 95% CI) | 3.79 [1.14, 12.55] |
| 2.2 From 3 to 5 domains | 1 | 203 | Odds Ratio (IV, Random, 95% CI) | 1.68 [0.69, 4.06] |
| 3 Number of smokers counselled | 14 | 8531 | Odds Ratio (IV, Random, 95% CI) | 2.28 [1.41, 3.67] |
| 3.1 Up to and including 2 domains | 11 | 7804 | Odds Ratio (IV, Random, 95% CI) | 2.32 [1.34, 4.02] |
| 3.2 From 3 to 5 domains | 2 | 435 | Odds Ratio (IV, Random, 95% CI) | 1.64 [0.87, 3.10] |
| 3.3 From 6 to 8 domains | 1 | 292 | Odds Ratio (IV, Random, 95% CI) | 3.42 [1.61, 7.28] |
| 4 Number of smokers receiving self‐help material | 9 | 5157 | Odds Ratio (IV, Random, 95% CI) | 3.26 [1.57, 6.77] |
| 4.1 Up to and including 2 domains | 8 | 4722 | Odds Ratio (IV, Random, 95% CI) | 4.08 [1.75, 9.55] |
| 4.2 From 3 to 5 domains | 2 | 435 | Odds Ratio (IV, Random, 95% CI) | 1.48 [0.64, 3.42] |
| 5 Number of smokers receiving nicotine gum/replacement therapy | 9 | 5073 | Odds Ratio (IV, Random, 95% CI) | 1.57 [0.72, 3.41] |
| 5.1 Up to and including 2 domains | 6 | 4146 | Odds Ratio (IV, Random, 95% CI) | 1.44 [0.54, 3.81] |
| 5.2 From 3 to 5 domains | 2 | 435 | Odds Ratio (IV, Random, 95% CI) | 1.47 [0.57, 3.76] |
| 5.3 From 6 to 8 domains | 1 | 492 | Odds Ratio (IV, Random, 95% CI) | 3.53 [0.95, 13.09] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cohen (Dent) 1989.
| Methods |
Country: USA, Indianapolis area Design: Randomized controlled trial; Nested; Clustered Objective: To improve the effectiveness of dentists helping their patients quit smoking Methods of analysis: A generalized linear model was used to analyse the results of the quit‐smoking rates and a scale‐factor was used to reflect the expected extra variance in quit rates caused by between‐dentist variability; Chi² statistic based on changes in the deviance function for a series of nested models was used to test for main effect and interactions; Two‐way analyses of variance were calculated on the weighted data for the amount of time spent in counselling patients about their smoking Clustering adjustment made: Yes ‐ Generalised linear model allowed a scale‐factor to reflect the extra variance expected to be inflated due to variability between dentists Significance of cluster adjustment: Not reported |
|
| Participants |
Therapist description: Dentists Eligible for study: n= 54 Randomized: n= 50 Completed: Gum n= 9, reminder n= 10, gum & reminder n= 12, control n= 13 (total n= 44) Age: Not reported Gender: Not reported Patient description: n= 1027 patients from American private dental practices Eligible for study: n= 1027 Randomized: n= 1027 Completed: n= 647 Age: Mean = 37.1 (SD + 10.4) (total population only) Gender: Males= 43.2% males (total population only) |
|
| Interventions |
Setting: American private dental practices Training of those delivering the intervention to the health professional: Not reported Intervention description: Three intervention groups: Training & nicotine gum, training & reminder (chart prompt), combined training with prompt & nicotine gum Control description: Training alone (advice, quit date, follow‐up check); Dentists provided a booklet containing the four‐step care protocol and were encouraged to counsel their patients who were smokers Duration of intervention: One hour Intervention delivered by: General dentist Intensity: One lecture |
|
| Outcomes |
Pre‐specified outcome data: Point prevalence of cessation at 12 months; Number advised to quit; Number asked about setting a quit date Follow‐up period: Twelve months total: 6 months (defined as the smoking status determined at any visit that occurred at least 3 months after the initial appointment but not more than 9 months); 12 months (defined as the smoking status determined at any visit that occurred at least 9 months and 1 day and up to 15 months after the initial visit) |
|
| Notes |
Process measures: Outcomes reported in Cohen 1987; Patients not having a visit during the 6 or 12 month periods were assumed to be smokers Validation: Expired carbon monoxide The three intervention groups were combined for meta‐analyses to produce the single 'Intervention' sample |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization mentioned though methods not described: "...dentists and their entire panel of patients who smoked cigarettes were randomly assigned to one of four conditions." |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding (performance bias and detection bias) of participants | High risk | Due to the nature of the intervention blinding of participants was not possible for this study |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | No mention of attempted blinding for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Patients not having a visit during the 6 or 12 month periods were assumed to be smokers; No further information provided regarding missing or incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes, including those that were pre‐specified were reported |
| Other bias | Low risk | No other biases identified |
| Imbalance of outcome measures at baseline | Low risk | Analysis of covariance occurred |
| Comparability of intervention and control group characteristics at baseline | Unclear risk | Insufficient information reported to permit judgement of yes or no; "… participating dentists varied widely in age, types of practices, previous use of tobacco effects …" |
| Protection against contamination | Unclear risk | Insufficient information to permit judgement of yes or no |
| Selective recruitment of participants | Unclear risk | n‐values across different intervention groups not reported |
Cohen (Doc) 1989.
| Methods |
Country: USA Design: Randomized controlled trial; Nested; Clustered Objective: Evaluation of a RCT of interventions designed to improve effectiveness of physicians and dentists in helping their patients quit smoking Methods of analysis: Analysis of variance performed on percentages; Stepwise multiple regression analyses performed using the weighted number of minutes as the criterion to determine the extent to which the amount of counselling time was a function of the health professionals' initial attitudes and habits; Chi² analysis used to test main effects and interactions; Generalised linear interactive modelling (GLIM) software used Clustering adjustment made: Yes ‐ Generalised linear model allowed a scale‐factor to reflect the extra variance expected to be inflated due to variability between physicians Significance of cluster adjustment: Not reported |
|
| Participants |
Therapist description: n= 112 primary care physicians (including n= 97 physicians in training) Eligible for study: Not reported Randomized: Total= 97 internal medicine residents and 15 faculty general internists Completed: Total= 97 internal medicine residents and 15 faculty general internists Age: Not reported Gender: Not reported Patient description: n= 1420 patients receiving primary care, not selected by motivation to quit Eligible for study: Participation refusal rate was 9.7% of all eligible patients contacted Randomized: n= 1420 Completed: n= 1091 medical patients Age: 18 to 64 years; Mean = 46.2 + 11.6 years Gender: Male= 37% |
|
| Interventions |
Setting: General medicine (primary care) clinic of a city‐county teaching hospital in the USA Training of those delivering the intervention to the health professional: Registered internist Intervention description: Three intervention groups: Training & nicotine gum, training & reminder (chart prompt), combined training with prompt & nicotine gum Control description: Training alone (advice, quit date, follow‐up check); Physicians provided a booklet containing the four‐step care protocol and were encouraged to counsel their patients who were smokers Duration of intervention: One‐hour lecture or personalised instruction Intervention delivered by: David M Smith, registered internist Intensity: One, one hour lecture maximum |
|
| Outcomes |
Pre‐specified outcome data: Point prevalence of abstinence at 12 months; Patients who did not have an appointment in the period regarded as smokers; Rates also reported giving returnees as denominator; Number advised to quit; Number asked about setting a quit date; Had their doctor talked to them about smoking Follow‐up period: Six and 12 months (12 months defined as patients visited 9 and 15 months after the initial visit) |
|
| Notes |
Process measures: Outcomes reported in Cohen 1987; Patients not having a visit during the 6 or 12 month periods were assumed to be smokers Validation: Expired carbon monoxide The three intervention groups were combined for meta‐analyses to produce the single 'Intervention' sample |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization mentioned however methods not described |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding (performance bias and detection bias) of participants | High risk | Due to the nature of the intervention blinding of participants was not possible for this study |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | No mention of attempted blinding for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Patients not visiting the physicians during the 6 and 12 month visits were assumed smokers; No further information provided regarding missing or incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes, including those that were pre‐specified were reported |
| Other bias | Low risk | No other potential risks of bias identified |
| Imbalance of outcome measures at baseline | Low risk | Analysis of covariance occurred |
| Comparability of intervention and control group characteristics at baseline | Unclear risk | Insufficient information reported to permit judgement of yes or no |
| Protection against contamination | Unclear risk | Insufficient information reported to permit judgement of yes or no |
| Selective recruitment of participants | Unclear risk | Insufficient information reported to permit judgement of yes or no |
Cornuz 2002.
| Methods |
Country: Geneva and Lausanne, Switzerland, Europe Design: Randomized controlled trial; Clustered Objective: To assess the efficacy of an educational program based on behavioural theory, active learning methods, and practice with standardized patients in helping patients abstain from smoking and changing physicians’ counselling practices Methods of analysis: To compare baseline characteristics of patients and physicians’ practices between groups, the authors used the Chi² or Fisher exact tests for categorical data and the t‐test or Wilcoxon rank‐sum test for continuous data; To test the effectiveness of the training on the outcomes, the authors performed a logistic regression with generalized estimating equation to stratify by clinic and adjust for clustering on residents; Intention‐to‐treat analysis was performed for abstinence from smoking, in which smokers lost at follow‐up were considered to be continuing smokers; Because smoking abstinence was validated in a sub sample of the study participants, the authors used simulation to perform sensitivity analysis of the likelihood of smoking cessation Clustering adjustment made: Yes ‐ to test the effectiveness of the training on the outcomes, the authors performed a logistic regression with generalized estimating equation to stratify by clinic and adjust for clustering on residents Significance of cluster adjustment: Not reported |
|
| Participants |
Therapist description: Resident physicians; All residents were at the end of postgraduate training in general internal medicine or family medicine Eligible for study: n= 35 Randomized: Intervention n= 17; Control n= 18 Completed: Intervention n= 17; Control n= 18 Age: Median 31 years Gender: 18 females and 17 males Patient description: Patients aged 16 to 75 years who consulted one of the outpatient clinics for a follow‐up or an emergency visit Eligible for study: n= 1456 Randomized: Intervention n= 115; Control n= 136 Completed: Intervention n= 77; Control n= 100 Age: Range 16 to 75 years; Mean + SD: Intervention 35.1 + 14 years; Control 36.9 + 15 years Gender: Intervention = 63% male; Control= 57% male |
|
| Interventions |
Setting: Two general internal medicine clinics of the university hospitals of Lausanne and Geneva, Switzerland; Both sites are public service clinics that provide adult ambulatory care to approximately 25,000 outpatient visits per year Training of those delivering the intervention to the health professional: Teachers are two authors, who are experienced physicians active in both clinical practice and teaching; Both were previously trained in smoking cessation counselling through a Master of Public Health course and are considered national experts in smoking cessation Intervention description: The training program is based on 5 principles: 1) recent evidence‐based content on tobacco use and cessation, 2) behavioural theory (stage‐of‐change model), 3) pharmacological therapy, 4) educational methods focusing on active skills training, and 5) tobacco control context; Session 1: Video‐clips observations, interactive workshops and role plays; Session 2: practice with standardized patients; At the end of the first session, participants received a set of documents (reference manual, two algorithms of counselling strategies and pharmacological therapy, record sheet for consultations with smokers, brochures for patients and patient instructions for NRT) Control description: Training in management of dyslipidaemia with equal contact time to the intervention; This course taught residents about the Swiss guidelines on screening for and diagnosis/management of high blood levels of cholesterol; Residents that were trained in smoking cessation attended the lesson on dyslipidaemia 4 months later, and vice versa Duration of intervention: Two, 4 hour sessions scheduled 2 weeks apart Intervention delivered by: Not specified though face‐to‐face workshops took place Intensity: Two, half‐day sessions; Total contact time 8 hours |
|
| Outcomes |
Pre‐specified outcome data: Self‐reported abstinence from smoking, 1 week point prevalence of abstinence, score of overall quality of counselling based on use of 14 counselling strategies, patient willingness to quit, daily cigarette consumption, socio‐demographic data, cardiovascular risk factors, smoking history, nicotine dependence, smoking intervention Follow‐up period: Twelve months |
|
| Notes |
Process measures: None reported Validation: Exhaled carbon monoxide testing at one clinic |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent research assistant performed computer randomization stratified by clinic |
| Allocation concealment (selection bias) | Unclear risk | An independent research assistant concealed the result of randomization until 2 weeks before the intervention, when residents were provided with details about training sessions ‐ however methods not described |
| Blinding (performance bias and detection bias) of participants | Low risk | "Residents were blinded to the aim of the trial and were informed only that a survey on cardiovascular risk factors and prevention would be conducted"; "We announced only that a training program in clinical prevention that included sessions on smoking cessation and management of dyslipidaemia was being conducted"; "Patients were also blinded to the aim of the study and group allocation of their physician" |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | A research assistant was blinded to group allocation for measurement of exhaled carbon monoxide; Authors also mention that allocation of residents and patient assignment was blinded to research staff that collected data; No mention of attempts to blind outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention‐to‐treat analysis used; No further information provided regarding missing or incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes, including those that were pre‐specified were reported |
| Other bias | Low risk | No other biases identified |
| Imbalance of outcome measures at baseline | Low risk | Baseline outcome data are reported and similar |
| Comparability of intervention and control group characteristics at baseline | Low risk | The authors mention no differences at baseline between intervention and control residents or patients |
| Protection against contamination | Low risk | "Residents who first trained in smoking cessation attended the session on dyslipidaemia 4 months later, and vice versa. The second session took place after the 3 month patient recruitment period had ended" ‐ Contamination unlikely |
| Selective recruitment of participants | Low risk | "...to identify smokers and avoid revealing group assignments, we interviewed all patients, regardless their smoking status" |
Cummings (Priv) 1989.
| Methods |
Country: USA Design: Randomized controlled trial; Nested; Clustered Objective: To test if physicians who are trained to use the 'Quit for Life' (QFL) program are more effective in helping patients to quit smoking Methods of analysis: Chi² test for proportions and t‐tests for means; Multiple logistic regression (for proportions) and ordinary least‐squares (for means) and calculated adjustment rates from the partial slopes associated with a dummy variable; Individual patients were the unit of analysis Clustering adjustment made: No adjustment to presented data but separate analyses tested clustering effects Significance of cluster adjustment: Clustering effects were tested in separate analyses; These adjustments had no discernible effect on significance levels and did not alter the conclusion |
|
| Participants |
Therapist description: Primary care physicians in private practice Eligible for study: n= 844 Randomized: Intervention n= 31; Control n= 28 Completed: Intervention n= 20; Control n= 18 Age: Not reported Gender: Intervention females n= 4; Control females n= 2 Patient description: n= 916 smoking patients not selected by motivation to quit Eligible for study: Not reported Randomized: Intervention n= 470; Control n= 446 Completed: Intervention n= 360; Control n= 364 Age: Intervention mean = 43 years; Control mean = 45 years Gender: Intervention mean = 53%; Control mean = 61% |
|
| Interventions |
Setting: Private primary care internal medicine and family practice (primary care) in San Francisco, USA; Local hospitals at times that fit with the schedules of the participating physicians; Four who were unable to attend the second sessions received the training privately in their office Training of those delivering the intervention to the health professional: Not described Intervention description: Training (personalised advice, quit date, one follow‐up visit, self help materials and nicotine gum) Control description: Normal care (no training) Duration of intervention: Three, one hour seminars Intervention delivered by: Internist or psychologist Intensity: Three, one hour seminars, second seminar one or two weeks after the first, third seminar four to twelve weeks later |
|
| Outcomes |
Pre‐specified outcome data: Demographic characteristics; Smoking history; How much do you want to quit smoking; How confident are you that you will not be smoking one year from now; Pressure to quit from family and friends; Was smoking discussed; Did you receive a self‐help booklet; Did you receive a follow‐up appointment about smoking Follow‐up period: Twelve months |
|
| Notes |
Process measures: None reported Validation: Expired carbon monoxide and serum cotinine Manual adjustment for potential clustering effects performed in the meta‐analyses for primary outcome data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Authors state patients were randomly assigned however methods not described |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding (performance bias and detection bias) of participants | High risk | Due to the nature of the intervention blinding of participants was not possible for this study |
| Blinding (performance bias and detection bias) of outcome assessors | Low risk | Authors state outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants assumed smokers if lost to follow‐up or abstinence unable to be biochemically verified; Missing outcome data accounted for in analyses |
| Selective reporting (reporting bias) | Low risk | All expected outcomes, including those that were pre‐specified were reported |
| Other bias | Low risk | No other biases identified |
| Imbalance of outcome measures at baseline | Low risk | Imbalances adjusted for using logistic regression |
| Comparability of intervention and control group characteristics at baseline | Low risk | Imbalances adjusted for using logistic regression |
| Protection against contamination | Low risk | Members of the same group practice were assigned to the same condition to minimise cross‐over |
| Selective recruitment of participants | Unclear risk | More control participants were recruited by practice staff than intervention subjects; Methods of recruitment not clearly described |
Cummings 1989.
| Methods |
Country: San Francisco, California, USA Design: Randomized controlled trial; Clustered Objective: To test whether physicians who receive a continuing education program about how to counsel smokers to quit would counsel smokers more effectively and have higher rates of long‐term smoking cessation among their patients that smoke Methods of analysis: Chi² for proportions and t‐tests for means were used for significance measures; Binomial test for difference between paired proportions used to calculate confidence intervals for changes in attitudes and self‐reported counselling practices of physicians in the experimental group before and after training; To analyse differences between the groups in patient reports about physicians counselling and rates of abstinence, large‐sample difference‐of‐proportions and difference‐of‐means tests were used; To determine significance of intervention among those patients who had the greatest desire to quit, an interaction was tested between assignment to the experimental or control group and the smoker’s rating of his or her desire to quit; Multiple logistic regression analysis used to determine significance for specific counselling strategies by experimental group physicians for abstinence levels Clustering adjustment made: No ‐ The individual patient was the unit of analysis for these results; However, patients were clustered by physician and physicians were clustered by work station; "...Therefore for simplicity, we present the results with the patient as the unit of analysis" Significance of cluster adjustment: Not reported |
|
| Participants |
Therapist description: Physicians Eligible for study: n= 189 internists Randomized: n= 81; Control n= 41; Intervention n= 40 Completed: n= 81; Control n= 41; Intervention n= 40 Age: Not reported Gender: Control: 27% female; Intervention 30% female Patient description: Eligible for study: n= 2056; Control n= 1032; Intervention n= 1024 Randomized: n= 2056; Control n= 1032; Intervention n= 1024 Completed: n= 2012; Control n= 1008; Intervention n= 1004 Age: Control 45 years; Intervention 46 years Gender: Control 53% female; Intervention 58% female |
|
| Interventions |
Setting: Four Health Maintenance Organisation (HMO) medical centres in northern California
Training: Three, one hour group tutorials Training of those delivering the intervention to the health professional: Not stated but delivered by internist or psychologist Intervention description: Training (personalised advice, quit date, one follow‐up visit, self help materials and nicotine gum) Control description: Normal care (no training) Duration of intervention: Three sessions over a five to fourteen week period Intervention delivered by: Internist or psychologist Intensity: Three, one hour sessions |
|
| Outcomes |
Pre‐specified outcome data: long‐term abstinence from smoking (≥ 9 months); Number of smokers counselled; Asked to set a quit date; Asked to make a follow‐up appointment; Number receiving self help materials; Number receiving nicotine gum; Number of smokers prescribed a quit date Follow‐up period: Point prevalence abstinence at 12 months |
|
| Notes |
Process measures: None reported Validation: Expired carbon monoxide and serum cotinine Manual adjustment for potential clustering effects performed in the meta‐analyses for primary outcome data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation of physicians (by computer) to intervention or control groups |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding (performance bias and detection bias) of participants | High risk | Due to the nature of the intervention blinding of participants was not possible for this study |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | Authors report a blinded assessment of principal outcomes; Methods for blinding participants or outcome assessors were not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data obtained for 78% of surviving patients of experimental physicians and 76% of surviving controls |
| Selective reporting (reporting bias) | Low risk | All expected outcomes, including those that were pre‐specified were reported |
| Other bias | Low risk | No other biases identified |
| Imbalance of outcome measures at baseline | Low risk | Participants assumed smokers if lost‐to‐follow‐up or abstinence unable to be biochemically verified; Missing outcome data accounted for in analyses |
| Comparability of intervention and control group characteristics at baseline | Unclear risk | Except for sex, the characteristics of smokers in the experimental and control groups were similar |
| Protection against contamination | Low risk | "...To minimize exchange of information, materials, and cross‐over of patients between two groups of physicians, we grouped physicians into 22 units corresponding to existing medical stations, each with distinct space and separate office staff " |
| Selective recruitment of participants | Low risk | n‐values are similar across groups; Also, all smokers who made a visit to any doctor participating in the study were eligible for participating in the study |
Gordon 2010.
| Methods |
Country: USA Design: Randomized controlled trial; Nested; Clustered Objective: With consideration to the oral health effects associated with chronic tobacco use, the dental visit provides a "teachable moment" during which the dental team can relate oral health and systemic problems to tobacco use and provide evidence‐based brief interventions to patients who use tobacco in lower socio‐economic areas Methods of analysis: Analysis of variance with clinics as a random, nested factor within condition and patients nested within clinic for both outcomes, for all participants, and within each racial/ethnic group; Logistic regression used for baseline measures of tobacco use with condition included as a covariate Clustering adjustment made: Yes: intra cluster correlation and analysis of variance with nesting Significance of cluster adjustment: Not reported |
|
| Participants |
Therapist description: Federally funded public health dental clinics in lower socio‐economic areas Eligible for study: Not reported Randomized: Intervention n= 7 practices; Control n= 7 practices Completed: Intervention n= 7 practices; Control n= 7 practices Age: Not reported Gender: Not reported Patient description: Dental patients aged 18 years and older who were seen for a non‐emergency visit to the clinic and were self‐identified current tobacco users (within the past 7 days) Eligible for study: n= 2751 completed informed consent and baseline survey Randomized: Intervention n= 1434; Control n= 1203 Completed:Six weeks Intervention n= 1214; Control n= 1026; 7.5 months Intervention n= 990; Control n= 885 Age: Total sample only: Mean = 40.5 + 12.6 years Gender: Total sample only: Female= 45.8% n= 1508 |
|
| Interventions |
Setting: Baseline survey completed in the clinic and were mailed follow‐up surveys at 6 weeks and 7.5 months (lower socio‐economic areas) Training of those delivering the intervention to the health professional: Not reported Intervention description: '5A approach' (Ask, Advise, Assess, Assist and Arrange): Ask ‐ ask all patients about their tobacco use at every visit; Advise ‐ relating the oral effects of tobacco use to the patients’ oral health status and advising patients to quit tobacco; Assess ‐ setting a quit date, discussing pharmacotherapy, providing free self‐help materials and free nicotine replacement therapy; Arrange ‐ arranging for follow‐up by mail or phone for patients setting a quit date; Each intervention practice was provided with a supply of nicotine patches and lozenges, as well as printed patient self‐help materials and information on the local tobacco quit line, which providers were asked to give to all tobacco‐using patients Control description: Usual care ‐ delayed intervention control; Following the study period control clinics received the in‐service workshop and received all the intervention materials Duration of intervention: One workshop Intervention delivered by: Dentists, dental hygienists and dental assistants Intensity: One, 3 hour workshop |
|
| Outcomes |
Pre‐specified outcome data: Tobacco cessation, reduction in tobacco use, number of quit attempts, change in readiness to quit, number of cigarettes smoked per day, level of nicotine dependence Follow‐up period: 7.5 months (6 months post‐enrolment plus a 6 week grace period) |
|
| Notes |
Process measures: Intervention subjects only ‐ 66.5% reported receiving the reading materials and the majority of patients reported reading them (96.7%); 16.9% reported using nicotine replacement therapy and 10.9% reported receiving quit line counselling Validation: No biochemical validation n‐values re‐calculated for meta‐analysis to permit intention‐to‐treat analysis for primary outcome data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization mentioned however methods not described |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding (performance bias and detection bias) of participants | High risk | Due to the nature of the intervention blinding of participants was not possible for this study |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | No mention of attempted blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data accounted for via multiple imputation procedures; Attrition in participants discussed in text |
| Selective reporting (reporting bias) | High risk | Secondary participant outcomes were examined however authors found no significant differences on any of these variables, consequently no data was presented in the publication; Receipt of intervention secondary outcome measures were reported as percentages in text, however no information was presented for the control population |
| Other bias | Low risk | No other biases identified |
| Imbalance of outcome measures at baseline | Low risk | Imbalances at baseline were identified, however they were controlled using analysis of variance |
| Comparability of intervention and control group characteristics at baseline | Low risk | Logistic regression used to examine the effect of baseline measures on tobacco use with condition as a covariate in the model |
| Protection against contamination | High risk | Authors mention a tax increase on cigarettes in New York (2008), such that the tax on a pack of cigarettes was $5.00; The smoking prevalence in New York City dropped from 18.4% (2006) to 15.8% (2008); Authors state this likely contributed to the unusually high rate of quitting among usual care patients observed in this study, thereby affecting the relative impact of the intervention |
| Selective recruitment of participants | Unclear risk | Insufficient information to permit judgment of yes or no |
Hymowitz 2007.
| Methods |
Country: USA Design: Randomized controlled trial; Nested; Clustered Objective: The primary aim of the study was to compare the effects of the two training conditions on resident tobacco intervention as measured by annual resident tobacco survey and OSCEs, baseline, and end‐of‐study patient and parent/guardian tobacco surveys, and a survey of program graduates who enter paediatric practice Methods of analysis: Due to training site being the unit of randomization, analyses were based on aggregated data rather than on individuals; Likert scales were calculated as means; Two‐stage mixed model relationship was used for waves of residents at baseline and 2 year follow‐up Clustering adjustment made: No – However data were analysed based on aggregated data to account for unit of analysis issues; Authors state that this will provide “…an unbiased estimate of the intervention effect and standard error” (also know as a ‘mean analysis’). Significance of cluster adjustment: Not reported |
|
| Participants |
Therapist description: Paediatric residents undergoing training in the New York/New Jersey metropolitan area Eligible for study: n= 16 residency training programs; n= 2069 Residents Randomized: n= 16 residency training programs; 3rd year residents n= 140 in intervention arm; n= 135 in control arm Completed: n= 14 residency training programs; 3rd year residents n= 136 in intervention arm; n= 99 in control arm Age: Approximately 33 years of age for overall population; Intervention mean = 32.3 + 5.1 years; Control mean = 33.7 + 5.7 years Gender: Intervention female= 69.1%; Control female= 59.3% Patient description: Parent/Guardian: Parents of the patients visiting the primary care clinics Eligible for study: n= 1770 Randomized: Intervention n= 849; Control n= 776 Completed: Intervention n= 724; Control n= 617 Age: Overall= 29.88 + 8.65 years Gender: Female= 85.8% Patient description: Children: Patients (children) visiting the primary care clinics Eligible for study: n= 550 Randomized: Intervention n= 255; Control n= 300 Completed: Intervention n= 255; Control n= 300 Age: Intervention 14.89 + 1.84 years; Control 15 + 2.16 years Gender: Intervention female= 55.3%; Control female= 60% |
|
| Interventions |
Setting: New York/New Jersey metropolitan area; Continuity clinic (primary care clinic) served as the venue for resident tobacco‐intervention activities Training of those delivering the intervention to the health professional: Not specified Intervention description: Special training – ‘Solutions for Smoking’ was the main teaching tool; Also provided with assistance with clinics (e.g., take‐home educational and behavioural‐change materials available in the waiting areas, anti‐tobacco posters, marking charts of smokers etc); Packets of educational and behavioural materials designed for mothers of newborns, adolescent smokers, parents who smoke etc.; Seminar series provided opportunities to distribute program materials, highlight key concepts and aspects of the background material, and utilise role‐playing to help residents acquire interviewing, counselling and tobacco‐intervention skills; PowerPoint presentations were used during these seminars on environmental tobacco smoke, smoking cessation and prevention of smoking onset and solutions for smoking audio/visual vignettes to demonstrate and model state‐of‐the‐art counselling and intervention skills Control description: Standard training – Background reading material that included the clinical practice guideline 'Treating Tobacco Use and Dependence' and 'American Academy of Pediatrics Statement on Tobacco'; A manual entitled 'Clinical Interventions to Prevent Tobacco Use by Children and Adolescents'; A journal article on approaches to tobacco prevention and control in clinic and office settings; Standard training sites did not receive assistance with clinic mobilisation or have access to companion intervention material; They did receive pamphlets and related material to facilitate intervention on tobacco; Seminar also conducted the same as the intervention group with the exception of vignettes to demonstrate counselling and intervention skills Duration of intervention: One hour seminars, four times per year Intervention delivered by: Unclear, though the manuscript mentions ‘training directors’; Seminars delivered by senior investigators from the New Jersey Medical School Intensity: One hour seminars, four times per year |
|
| Outcomes |
Pre‐specified outcome data: Primary outcome measures included changes in resident tobacco intervention activities and skills in the area of environmental tobacco smoke, tobacco‐use prevention and tobacco‐use cessation; Demographic information, knowledge and attitudes about tobacco prevention and control, tobacco‐intervention activities during the past year, use of specific tobacco‐intervention skills and strategies, and beliefs about the efficacy of tobacco intervention in patients and parents Follow‐up period: Four years in total; Outcome data for participants only published for 2 year follow‐up |
|
| Notes |
Process measures: Sixty percent of residents in the special training condition reported review of ‘Solutions for Smoking’, although a higher proportion attended the seminar series (80%) and had access to companion intervention material in the clinic Validation: No biochemical validation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed according to coin toss |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding (performance bias and detection bias) of participants | High risk | Due to the nature of the intervention blinding of participants was not possible for this study |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | No mention of attempts to blind outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One site withdrew from the study following the events of 9/11/2001 and another withdrew later; No further information provided regarding missing or incomplete outcome data |
| Selective reporting (reporting bias) | High risk | All pre specified outcomes were addressed in the 4 year outcome findings; However, the authors mention that outcome data for year 1 were omitted in order to provide a ‘cleaner look’ at the progress of the study |
| Other bias | Low risk | No other biases identified |
| Imbalance of outcome measures at baseline | Low risk | All within‐condition analyses controlled for residents’ gender, smoking status and ethnic status |
| Comparability of intervention and control group characteristics at baseline | Low risk | The two conditions differed with respect to racial composition, however analyses adjusted to account for residents' gender, smoking status and ethnic status |
| Protection against contamination | Unclear risk | The control and intervention residents all arrived at the medical school and attended the one hour seminar together at the same time |
| Selective recruitment of participants | Low risk | n‐values reported and similar across groups |
Joseph 2004.
| Methods |
Country: USA Design: Randomized controlled trial; Clustered Objective: To test the effect of modest intensity, practical systems changes that might increase the delivery of smoking cessation treatment within VAMCs (Veterans' Medical Centres); Authors hypothesized that an intervention addressing common barriers to delivery of smoking cessation treatment at the organisation level (as opposed to provider or patient level) might be an effective strategy to improve compliance with guideline recommendations; The trial was designed to test the effectiveness of this intervention Methods of analysis: McNemar odds on change to assess differences in the change between intervention groups; Pearson Chi² statistic to compute the significant of the resulting odds ratio between the intervention and control group; Differences in smoking cessation rates were determined via the Pearson Goodness‐of‐Fit Chi² statistic; Change scores were used for continuous variables and the relative difference in change was measured using the Wilcoxon rank sum test; Logistic regression was used for binary outcomes; SAS glimmix macro was used to incorporate the design effect and allow for the binary outcome Clustering adjustment made: Yes ‐ SAS glimmix macros used to incorporate the design effects Significance of cluster adjustment: Not reported |
|
| Participants |
Therapist description: Physicians, nurses, psychologists and pharmacists were present at the training meeting Eligible for study: n= 164 VAMCs (Veteran Medical Centres) nationwide Randomized: Intervention n= 10; Control n= 10 Completed: Intervention n= 10; Control n= 10 Age: Not reported Gender: Not reported Patient description: A random selection of patients who had seen their primary care provider (at VAMCs) within 6 weeks were phoned for baseline surveys; Current smokers were identified and underwent 1 year follow‐up also via phone Eligible for study: Cohort n= 5793; Eligible n= 5367 Randomized: Intervention n= 2112; Control n= 2142 Completed: Intervention n= 641; Control n= 783 Age:Baseline ‐ Intervention 64.6 years; Control 63.1 years; Follow‐up ‐ Intervention 64.9 years; Control 63.8 years Gender:Baseline (male) ‐ Intervention 96.1%; Control 95.3%; Follow‐up ‐ Intervention 95.8%; Control 98.0% |
|
| Interventions |
Setting: Veterans Affairs Medical Centers (VAMCs) Training of those delivering the intervention to the health professional: Registered nurse who was trained in smoking cessation methods and had considerable administrative experience within Veteran Affairs Intervention description: Intervention sites received 5 copies of the AHCPR Smoking Cessation Guideline for distribution; Plus a multi‐component intervention designed to increase implementation of 3 specific Guideline recommendations: 1) documentation of tobacco use status in the medical record 2) delivery of intervention to all smokers and 3) liberal use of smoking cessation medications; The organisational support included a training meeting, site visits and a study interventionist at the coordinating site in Minneapolis; Removal of formulary restrictions were encouraged for smoking cessation aids as were the requirements for attendance at a cessation class to access pharmacotherapies; Bupropion SR was suggested as an addition to formulary; However approaches were individualised for each site Control description: Control sites also received 5 copies of the AHCPR Smoking Cessation Guideline for distribution Duration of intervention: Authors state intervention lasted through a 6 month period, however level of exposure for participants not specified Intervention delivered by: Registered nurse face‐to‐face through 2 to 3 site visits within the first 6 months to communicate with directors of primary care, pharmacy service chiefs, smoking cessation coordinators and primary care nurses, as well as the 2 day training meeting Intensity: One, 2 day training meeting held in Minneapolis for the site‐based principal investigator; 2 to 3 day visit to each site by the interventionist within the first 6 months |
|
| Outcomes |
Pre‐specified outcome data: General health, smoking history/status, nicotine dependence, services provided at the last primary care visit, mood, alcohol use and demographics, provision of counselling, referred to a smoking cessation clinic, provided advice or medications and cessation discussed (documented in medical records) Follow‐up period: Twelve months |
|
| Notes |
Process measures: None reported Validation: No biochemical validation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization mentioned, however methods not described |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding (performance bias and detection bias) of participants | High risk | Due to the nature of the intervention blinding of participants was not possible for this study |
| Blinding (performance bias and detection bias) of outcome assessors | Low risk | Interviewers were blinded to subjects’ site treatment status |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition appears to be higher in the intervention arm than the control arm based on n‐values; No further information provided regarding missing or incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes, including those that were pre‐specified were reported |
| Other bias | Low risk | No other biases identified |
| Imbalance of outcome measures at baseline | Low risk | A logistics regression analysis was used to account for imbalances in outcome measures at baseline |
| Comparability of intervention and control group characteristics at baseline | Low risk | No significant differences between subject characteristics were identified |
| Protection against contamination | Unclear risk | Insufficient information to permit judgement of yes or no |
| Selective recruitment of participants | Low risk | Baseline n‐values appear similar, methods for recruitment of participants are the same across groups |
Kottke 1989.
| Methods |
Country: USA Design: Randomized controlled trial; Clustered Objective: "...the task of Doctors Helping Smokers was to be the development and testing of a program to help physicians incorporate currently identified smoking cessation intervention into their practice routine." Hypothesis: that physicians trained in a workshop would be more effective in helping their smoking patients quit than would similar volunteer physicians who received only patient education materials or a group of physicians that received no assistance Methods of analysis: Data presented as proportions were analysed with the Chi² analysis; Data reported as means and SDs were analysed with analysis of variance; Life‐table analysis used to examine relapse patterns of the patients who attempted to quit smoking Clustering adjustment made:Physicians unit of analysis; Multivariate regression used to adjust for confounding effects of differences among the groups of doctors and their patients Significance of cluster adjustment: Not reported |
|
| Participants |
Therapist description: n= 109 family practitioners Eligible for study; n‐value: 1110; n= 109 physicians returned postcards Randomized; n‐value: Workshop group n= 27; No‐assistance group n= 17; Materials group n= 22 Completed; n‐value: Workshop group n= 27; No‐assistance group n= 17; Materials group n= 22 Age: Workshop group 37.9 + 9.7; No‐assistance group 39.5 + 7.7; Materials group 44.3 +11.7 Gender: Workshop group female=22.2%; No‐assistance group female=9.1%; Materials group female=11.8% Patient description:n= 1653 primary care smoking patients not selected by motivation to quit Eligible for study; n‐value: Not reported Randomized; n‐value: 6053 total (89.4% of patients whose names were submitted by the physicians) Completed; n‐value: 87% of the n= 6053 were available for follow‐up; 86.8%, 87.5% and 86.8% for the workshop, materials and no‐assistance groups respectively Age: 18 to 70 years; Mean= slightly over 40 Gender: 2/3 female |
|
| Interventions |
Setting: Private family practice (primary care) in Minnesota, USA; Workshop site not described though likely centralised Training of those delivering the intervention to the health professional: Not described Intervention description: Two intervention groups: Materials group ‐ physicians given self‐help manuals to distribute; Workshop group ‐ self‐help manuals plus 6 hour group workshop Control description: Normal care Duration of intervention:Workshop group: 6‐hour workshop given on two occasions. Workshop started in the morning with two presentations of 30‐minutes about the effects of smoking, chronic disease and organisation for smoking cessation interventions; 1‐hour presentation on doctor‐patient intervention skills; 1‐hour introduction to smoking cessation techniques; Two 1‐hour small‐group workshop sessions on counselling sessions and planning for smoking cessation interventions and 30‐minutes for summary and discussion; Materials group: 100 copies of Quit‐and‐Win, a smoking cessation manual Intervention delivered by: Not described Intensity: Workshop: 6‐hr workshop given on 2 occasions; Materials group: None; No assistance: None |
|
| Outcomes |
Pre‐specified outcome data: Physicians: Characteristics, knowledge, skills, confidence and beliefs about smoking cessation in relation to their performance during the trial Patients: demographics, smoking habits, health status, details about visit with physician, prevalence of smoking in their social environment and support received from spouse or others who were emotionally important to them; Four questions about extent to which they felt in control of their life, the confidence they felt about handling personal problems, extent that “things were going [their] way,” and the extent to which difficulties were piling up; Serum cotinine levels Follow‐up period: 12months |
|
| Notes |
Process measures: None Validation: Serum cotinine Not able to be meta‐analysed due to unit of analysis being the practitioners instead of the individuals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Some physicians were re‐assigned to groups due to inappropriate allocation methods during assignment |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding (performance bias and detection bias) of participants | High risk | Due to the nature of the intervention it is not possible to blind participants |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | No mention of attempted blinding for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participants lost‐to‐follow‐up were assumed smokers; No information on how missing data from questionnaires were handled |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of yes or no |
| Other bias | Low risk | No other potential threats to validity identified |
| Imbalance of outcome measures at baseline | Low risk | Analysis of covariance conducted |
| Comparability of intervention and control group characteristics at baseline | Low risk | Characteristics of physicians reported and comparable; Multivariate regression analysis conducted to adjust for confounding factors |
| Protection against contamination | Low risk | Physicians within the same practice were all within the same arm of the study |
| Selective recruitment of participants | Unclear risk | Insufficient reporting to permit judgement of yes or no |
Lennox 1998.
| Methods |
Country: United Kingdom Design: Randomized controlled trial; Nested; Clustered Objective: To assess the impact of the training intervention on both health professionals and smoking subjects Methods of analysis: Comparison of binary outcomes were analysed using the Chi² test; Logistic and multiple regression analyses were carried out where appropriate for these outcome measures; Comparisons of continuous outcomes were analysed using t‐tests and multiple linear regression; Confounders were adjusted including age, sex and deprivation score for the regression analysis as well as for indicators for the intervention group Clustering adjustment made: Yes ‐ GLMM (Generalised linear mixed model) approach used for regression techniques which added the general practice as a random factor nested within the treatment groups to the other fixed‐effect factors Significance of cluster adjustment: Regression techniques used to explore clustering effects for variables significant in individual level analyses; No significant difference in point prevalence of abstinence after adjustment |
|
| Participants |
Therapist description: n= 16 general practices with training for doctors, nurses and health visitors Eligible for study: n= 26 practices Randomized: n= 16 practices Completed: n= 16 practices Age: Not reported Gender: Not reported Patient description: Smoking patients of the practices identified from questionnaires to random sample Eligible for study: Not reported Randomized: Number of patients surveyed: Intervention n= 6631; Control n= 6631; Number of patients responding: Intervention n= 5022; Control n= 5217; Number of smokers identified: Intervention n= 1381; Control n= 1207 Completed: Eight months ‐ Intervention n= 941; Control n= 864; 14 months ‐ Intervention n= 898; Control n= 795 Age: Not reported Gender: Not reported |
|
| Interventions |
Setting: Primary care medical practices in Aberdeen, UK Training of those delivering the intervention to the health professional: Two authors conducted the training, one a senior health promotion officer experienced in group work with primary health care teams and the other a GP Intervention description: One day training workshop based on stages of change model Control description: Usual care control group Duration of intervention: Six identical one day training workshops were held within a three week period based on stages of change model Intervention delivered by: Two authors, one a senior health promotion officer experienced in group work with primary health care teams and the other a GP Intensity: One day training workshop |
|
| Outcomes |
Pre‐specified outcome data: Changes in attitudes, self‐reported behaviour, change in readiness to change, cessation attempt made, point prevalence, continuous abstinence Follow‐up period: 8 and 14 months post workshop for patient questionnaires |
|
| Notes |
Process measures: Some subjects did not attend their practice during the study and therefore were not exposed to the effects of the training Validation: No biochemical validation n‐values re‐calculated for meta‐analysis to permit intention‐to‐treat analysis for primary outcome data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Physicians were randomly allocated to control or intervention however method not described; Patients were randomly selected via a computer‐generated randomization program for every 1 in 6 drawn from the patient lists |
| Allocation concealment (selection bias) | Unclear risk | Physicians were randomly and blindly allocated to control or intervention however methods not described |
| Blinding (performance bias and detection bias) of participants | High risk | Due to the nature of the intervention blinding of participants was not possible for this study |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | No mention of attempted blinding for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No further information provided regarding missing or incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes, including those that were pre‐specified were reported |
| Other bias | Low risk | No other biases identified |
| Imbalance of outcome measures at baseline | Low risk | No significant differences between groups |
| Comparability of intervention and control group characteristics at baseline | Low risk | No significant differences between groups however intervention subjects less affluent than controls which was adjusted for in regression analyses |
| Protection against contamination | Low risk | Practices containing staff who attended pilot workshops or staff whom worked for more than one participating practice were excluded |
| Selective recruitment of participants | Low risk | Patients were randomly selected from practices using a computer‐generated randomization program; n‐values are similar across groups |
Sinclair 1998.
| Methods |
Country: Scotland Design: Randomized controlled trial Objective: To evaluate a training workshop for community pharmacy personnel to improve their counselling in smoking cessation based on the stage‐of‐change model Methods of analysis: To demonstrate the differences between intervention and control groups, parametric tests (t‐tests for quantitative variables) and non‐parametric tests (Mann‐Whitney tests for quantitative variables) were used. Multiple logistic regression was carried out for the binary outcomes of point prevalence at one month, and continuous abstinence at four and nine months, and to assess the effect of potential confounders Clustering adjustment made: Yes; authors mention that the effect of cluster randomization was assessed by firstly calculating the degree of intra‐cluster correlation for each of the binary outcomes of abstinence. Secondly, regression techniques, adding the pharmacy as a random factor nested within the treatment groups to the other fixed effect factors, were considered leading to a generalised linear mixed model. The authors mention that intra‐cluster correlations for the outcomes at each time point were calculated. The estimated values were less than 0.0001 and therefore negligible Significance of cluster adjustment: No; authors mention that trends in outcome were not affected by potential confounders or adjustment for clustering Setting: Residents and physicians in family medicine, Taiwan Training: Two lessons |
|
| Participants |
Therapist description: Eligible for study; n‐value: n= 76 pharmacies Randomized; n‐value: Intervention n= 32 pharmacies; Control n= 30 pharmacies Completed; n‐value: Intervention n= 32 pharmacies (specify: n= 94 (54 assistants, 40 pharmacists); Control n= 29 pharmacies Age: Not described Gender: Intervention: 54 female assistants; 25 female pharmacists; Control: not described Patient description: Eligible for study; n‐value: n= 775 smokers Randomized; n‐value: Intervention n= 224; Control n= 268 Completed; n‐value: Intervention n= 159; Control n= 188 Age: Intervention 41.7 (17‐74); Control 41.5 (17‐77) Gender: Intervention 61.2% men; Control 62.7% men |
|
| Interventions |
Setting: Eight workshops were scheduled with a choice of dates, times and location (Aberdeen or Elgin ‐ the major population centres which are located 70 miles apart at apposite ends of the study area) Training of those delivering the intervention to the health professional: Not described Intervention description: Training in stages of change approach to smoking cessation Control description: Usual care Duration of intervention: Two‐hour workshop Intervention delivered by: Not described Intensity: One workshop |
|
| Outcomes |
Pre‐specified outcome data: Self‐reported point prevalence smoking cessation rates at one month; Self‐reported continuous abstinence from zero to four months and from zero to nine months; The pharmacy support process (registration, counselling and client record) Follow‐up period: 1, 4, 9 months; Point prevalence of abstinence at 12 months No process outcomes |
|
| Notes |
Validation: none n‐values re‐calculated for meta‐analysis to permit intention‐to‐treat analysis for primary outcome data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Pharmacy recruits were stratified by type … and ranked according to the pharmacists' level of motivation … They were then randomized to either the intervention or control group by sequential allocation" |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding (performance bias and detection bias) of participants | High risk | Authors state "Pharmacists and pharmacy assistants were aware of group by virtue of intervention design" |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | Methods for blinding participants for outcome assessors were not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of yes or no |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported |
| Other bias | Low risk | No other sources of bias were identified |
| Imbalance of outcome measures at baseline | Low risk | The potential confounders of age, sex, socio‐economic status, and nicotine dependence showed no difference between intervention and controls |
| Comparability of intervention and control group characteristics at baseline | Low risk | There was no significant difference between the characteristics of the intervention and control customers |
| Protection against contamination | Unclear risk | To minimise inter‐group contamination, both leaflets requested customers to return to that same pharmacy for any further advise and for subsequent purchase(s) of anti‐smoking products |
| Selective recruitment of participants | Unclear risk | During the 12‐month customer recruitment period, all smokers who sought advice on stopping smoking or those who bought an OTC anti‐smoking product in preparation for a new attempt to stop smoking were eligible for inclusion |
Strecher 1991.
| Methods |
Country: USA Design: Randomized Controlled Trial; Factorial design; Nested; Cluster Objective: The study evaluated the effectiveness of training and prompting under realistic conditions, including: the use of simple and generalisable interventions; training conducted by existing faculty; and evaluation at several sites with residents from three primary care specialties Methods of analysis: Contingency tables with Chi² tests, t‐tests, and analysis of variance (ANCOVA) were used to investigate the pre‐test equivalencies of the four groups and all outcomes for selected other variables; ANCOVA compared the effects of the two interventions, alone and in combination, whilst controlling for pre‐test scores and physician speciality Clustering adjustment made: No Significance of cluster adjustment: N/A (Physician speciality adjusted for but not individual physician clustering effects) |
|
| Participants |
Therapist description: 250 residents in internal medicine, family practice and paediatrics Eligible for study; n= 261 Randomized; n= 250; Tut (Tutilage) and Pro (Prompt) n= 66; Tut only n= 66; Pro only n= 60; Control n= 58 Completed; n= 234; Tut and Pro n= 62; Tut only n= 63; Pro only n= 55; Control n= 54 Age: Not reported Gender: Not reported Patient description: 937 patients from American primary care medical practice Eligible for study; n= 937; Tut and Pro n= 250; Tut only n= 243; Pro only n= 228; Control n= 225 Randomized; n= 843 Completed; n= 659; Tut and Pro n= 184; Tut only n= 156; Pro only n= 162; Control n= 157 Age: 17 to 75 years; Mean age = 45 years Gender: 63% female |
|
| Interventions |
Setting: American primary care residency programs (physicians in training) Training of those delivering the intervention to the health professional: Not specified though one of the authors in each instance conducted the tutorial Intervention description: Three intervention groups: Tutilage only (minimal contact counselling); Prompt only (chart‐reminder and advice sheet); Tutilage and Prompt Control description: Normal care Duration of intervention: Only held once, two sessions in total ‐ the first included slide presentations the second group discussions Intervention delivered by: One of the authors, usually a clinic director or a faculty member conducted the tutorial Intensity: Tutorial: two sessions ‐ initial one‐hour long, second session two weeks later |
|
| Outcomes |
Pre‐specified outcome data: Self‐administered questionnaires requesting self‐reports on smoking‐cessation counselling frequency, content, attitude and training; Patients were asked about smoking habits and physicians advice to stop smoking Follow‐up period: 6‐months |
|
| Notes |
Process measures: None Validation: Expired CO; Biochemical verification was obtained where possible The three intervention groups were combined for meta‐analyses to produce the single 'Intervention' sample; n‐values re‐calculated for meta‐analysis to permit intention‐to‐treat analysis for primary outcome data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Authors state "After the pre‐test, residents were randomly assigned by clinic half‐day session to one of four groups” |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding (performance bias and detection bias) of participants | High risk | Due to the nature of the intervention it was not possible to blind participants |
| Blinding (performance bias and detection bias) of outcome assessors | Low risk | Authors state “...telephone interviewers, who were blinded to residents’ and patients’ group assignments, obtained patient reports...” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of data containing any missing variables; Missing outcome data not described |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of yes or no |
| Other bias | Low risk | No other sources of bias were identified |
| Imbalance of outcome measures at baseline | Low risk | All groups were reported as similar for baseline outcomes; Analysis of variance also conducted |
| Comparability of intervention and control group characteristics at baseline | Low risk | All groups were reported as similar for baseline characteristics; Analyses to test pre‐test equivalence were conducted |
| Protection against contamination | High risk | Authors state contamination occurred as all four groups worked closely with one another at each site though they also state that “...the effects appeared to be slight.” |
| Selective recruitment of participants | Unclear risk | Insufficient information to permit judgement of yes or no |
Swartz 2002.
| Methods |
Country: USA Design: Randomized controlled trial; Clustered Objective: Primary goal of this study was to determine if in‐person feedback intervention, compared to mailed feedback, would lead to a higher use of tobacco treatments by patients who smoke Method of Analysis: Odds ratios, 95% confidence intervals and p‐values were calculated to evaluate intervention effects on patient and provider behaviour; Unadjusted models and models adjusted for age, insurance at baseline, practice speciality and region of the state were calculated using logistic regression; All analyses were completed with SAS statistical software Clustering adjustments made: Yes – survey logistic procedures Significance of clustering: Not reported |
|
| Participants |
Therapist description: Primary care providers with practices of at least 75% internal medicine or family medicine clinicians providers combined with Medicaid and HMO panel size of at least 200 adults; n= 176 were physicians, n= 26 nurse practitioners, n= 20 physician assistants, n= 3 unknown classification Eligible for study: n= 150 practices; n= 230 providers within the 50 practices recruited were eligible Randomized: n= 50 practices; n= 225 providers Completed: n= 50 practices; n= 179 providers Age: Not reported Gender: Not reported Patient description: Patients were adults receiving primary care by a study practice aged 18 years and older who were seen within the prior year Eligible for study: n= 17318 identified as receiving primary care by a study practice; n= 11547 eligible Randomized: n= 7461 completed baseline survey; n= 1238 patients identified as smokers at baseline Completed: n= 807 reporting provider visit in the year proceeding follow‐up; n= 516 smokers with baseline and follow‐up surveys reporting one serious quit attempt Age: Intervention mean age= 41.9 years; Control mean age= 42.9 years Gender: Intervention male= 26.4%; Control male= 23.2% |
|
| Interventions |
Setting: Maine Medicaid and Maine HMO, USA Training of those delivering the intervention to the health professional: Not reported Intervention description: Experimental study practices received two educational office sessions, with data feedback presented during the first visit; Second visit reinforced the guidelines and discussed office systems to improve tobacco treatment Control description: Control practices received information and feedback data by mail Duration of intervention: For the intervention: Two educational office sessions, the second occurred five months after the first Intervention delivered by: One nurse practitioner well‐versed in motivational interviewing and tobacco guidelines Intensity: Twenty minute slide presentation followed by feedback and discussions for the first visit; Second visit discussions time not stated |
|
| Outcomes |
Pre‐specified outcome data: Reports of provider asking about tobacco, advice to quit, spending time talking about smoking or quitting, discussing tobacco treatment medications, and discussing counselling services or programs; Smokers were asked about serious attempts at quitting for 24 hours or longer, use of medication or counselling to aid quitting, and use of any tobacco in the previous week (7 day point prevalence) Follow‐up Period: Fifteen to 18 months later which corresponded to 12 months following the practice intervention |
|
| Notes |
Process measures: None reported Validation: No biochemical validation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization mentioned however methods not described |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding (performance bias and detection bias) of participants | High risk | Due to the nature of the intervention blinding of participants was not possible for this study |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | No mention of attempted blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No further information provided regarding missing or incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes, including those that were pre‐specified were reported |
| Other bias | Low risk | No other biases identified |
| Imbalance of outcome measures at baseline | Low risk | Differences in intervention effect were adjusted for baseline outcomes |
| Comparability of intervention and control group characteristics at baseline | Low risk | Data were adjusted for age, gender and insurance to account for patient differences |
| Protection against contamination | Unclear risk | Insufficient information to permit judgement of yes or no |
| Selective recruitment of participants | Unclear risk | Methods of recruitment not described |
Twardella 2007.
| Methods |
Country: Germany Design: Randomized controlled trial; Nested; Clustered; Factorial design 2x2 Objective: The aim of this study was to examine whether and to what extent structural changes could enhance promotion of smoking cessation in general practice. In particular, we aimed to investigate the effect of the following strategies on smoking cessation rates: (1) specific training of general practitioners in methods of promoting smoking cessation and a financial incentive to general practitioners for each recruited patient who successfully quits and (2) specific training of general practitioners in promotion of smoking cessation and the cost‐free prescription of drugs proved effective in supporting smoking cessation Methods of analysis: Primary end‐point data were assessed on an intention‐to‐treat basis; Smoking abstinence at 12 months was assessed using a mixed logistic regression model accounting for cluster randomization including a random effect for medical practice in the model; Baseline imbalances between intervention arms were adjusted using multivariate analyses; The effect of drug use during follow‐up, as recorded by general practitioners, was evaluated in a bivariate mixed logistic regression model Clustering adjustment made: Yes ‐ mixed logistic regression model, using PROC NLMIXED in "SAS V8.1" (including a random effect for medical practice) Significance of cluster adjustment: Not reported |
|
| Participants |
Therapist description: General practitioners in the Rhine‐Neckar region located in southwest Germany Eligible for study: n= 174 met the inclusion criteria Randomized: Total= 94 general practitioners from n= 82 practices; Usual care: n= 21 therapists (20 practices); Training + incentive: n= 24 therapists (21 practices); Training + medication: n= 23 therapists (21 practices); Training, incentive + medication: n= 26 therapists (20 practices) Completed: n= 59 practices; Usual care: n= 14 practices; Training + incentive: n= 16 practices; Training + medication: n= 11 practices; Training, incentive + medication: n= 18 practices Age: Not reported Gender: Not Reported Patient description: Patients visiting the practices and who smoked at least 10 cigarettes per day and aged between 36 to 75 years, were recruited by participating general practitioners, irrespective of intention to quit smoking and conditional on written informed consent Eligible for study: n= 587 Randomized: n= 587; Usual care: n= 76; Training + incentive: n= 146; Training + medication: n= 144; Training, incentive + medication: n= 221 Completed: n= 488; Usual care: n= 61; Training + incentive: n= 123; Training + medication: n= 121; Training, incentive + medication: n= 183 Age: Range 36 to 75 years;<45 years: Usual care n= 30; Training + incentive n= 55; Training + medication n= 59; Training, incentive + medication n= 95; 45 to 54 years: Usual care n= 24; Training + incentive n= 63; Training + medication n= 44; Training, incentive + medication n= 86; > 55 years: Usual care n= 22; Training + incentive n= 28; Training + medication n= 41; Training, incentive + medication n= 40 Gender: Female: Usual care n= 38; Training + incentive n= 74; Training + medication n= 71; Training, incentive + medication n= 121 |
|
| Interventions |
Setting: Not reported Training of those delivering the intervention to the health professional: Not reported Intervention description: Three intervention groups: Training + incentive – Two hour cost‐free group tutorial for general practitioners in methods of promoting smoking cessation including stages of change model, approaches for counselling in general practice and potential of pharmacological support; Financial remuneration of €130 after study completion per smoke‐free participant; Training + medication – Same group tutorial as above plus general practitioners could offer cost‐free prescription of drugs proved effective in supporting smoking cessation; Training, incentive + medication – All of the above Control description: Usual care Duration of intervention: A single 2 hour tutorial available at two session times Intervention delivered by: Not reported Intensity: Two‐hour workshop |
|
| Outcomes |
Pre‐specified outcome data: Primary outcome measure ‐ Self‐reported point prevalence of smoking abstinence obtained at 12 months follow‐up Second outcome measure ‐ Continuous smoking abstinence for at least 6 months (183 days) at 12 months follow‐up; Frequency of the use of methods to support smoking cessation among patients during the follow‐up period as reported by general practitioners Follow‐up period: Twelve months |
|
| Notes |
Process measures: None reported Validation: Serum cotinine Other: Definition of abstinence ‐ Participants were categorised as ‘at least 6 months abstinent’ if they were smoke free at 12 months follow‐up, validated by serum cotinine, and, according to self‐report, had stopped smoking at least 6 months before the date of follow‐up The three intervention groups were combined for meta‐analyses to produce the single 'Intervention' sample |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization performed centrally at the German Centre for Research on Aging using PROC PLAN in SAS |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding (performance bias and detection bias) of participants | High risk | Due to the nature of the intervention blinding of participants was not possible for this study |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | Authors report serum cotinine levels determined in a blinded fashion, though methods not described; No mention of blinding for assessors of the other outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two participants died during follow‐up and five participants could not be located; Three participants in whom smoking abstinence could not be validated as a result of current use of nicotine replacement therapy were excluded; No further information provided regarding missing or incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes, including those that were pre‐specified were reported |
| Other bias | Low risk | No other biases identified |
| Imbalance of outcome measures at baseline | Low risk | Patients did substantially differ at baseline regarding the stage of change for smoking cessation; A multivariate analysis was conducted in which the authors adjusted for all baseline factors that were unequally distributed between intervention arms, as assessed by Mantel‐Haenszel Chi² statistic |
| Comparability of intervention and control group characteristics at baseline | Unclear risk | "We found no significant differences between the four groups of GPs with respect to the number of GPs per practice (p= 0.13), location (p= 0.62), sex (p= 0.38), age (p= 0.19) or smoking status (p= 0.21)"; 13 GPs withdrew and 13 GPs had no referrals of eligible patients, leaving a total of 68 GPs, unequally divided across the different arms |
| Protection against contamination | Unclear risk | Insufficient information to permit judgement of yes or no |
| Selective recruitment of participants | Unclear risk | Authors state that a possibility exists for selective recruitment, however statistical adjustments for this at follow‐up still produce a significant result; n‐values are different between the three intervention groups in comparison to the usual care arm |
Unrod 2007.
| Methods |
Country: USA Design: Randomized controlled trial; Nested; Clustered Objective: To bolster the rate at which physicians delivered smoking cessation services and to increase patients' quit rates Methods of analysis: Descriptive statistics for characterisation of sample at baseline; Pearson's Chi² test and independent sample t‐test to measure differences between groups; Hierarchic generalised linear model analysis of variance controlling for baseline variables used to measure physician performance; Abstinence analysed via generalised linear model Clustering adjustment made: Yes ‐ Mixed linear modelling with physician as clustering variable used for smoking related outcomes Significance of cluster adjustment: Not reported |
|
| Participants |
Therapist description: Primary care physicians recruited from the four largest metropolitan boroughs, Bronx, Brooklyn, Manhattan and Queens Eligible for study: n= 579 Randomized: Intervention n= 35; Control n= 35 Completed: Intervention n= 35; Control n= 35 Age: Mean = 51.1 + 8.1 years (total population only) Gender: Males= 74% (total population only) Patient description: Patients in primary care physician waiting rooms who were identified as smokers Eligible for study: n= 5826 Randomized: Intervention n= 270; Control n= 248 Completed: Intervention n= 237; Control n= 228 Age: Intervention mean= 43.5 + 14.7 years; Control mean= 42.8 + 14.2 years Gender: Intervention 58% male; Control 64% male |
|
| Interventions |
Setting: Training conducted during a 40 minute visit to the physicians office Training of those delivering the intervention to the health professional: Not reported Intervention description: Physician training in brief smoking cessation counselling based on the 5As Clinical Practice Guideline algorithm; Patients and physicians provided with a one page report containing smoking‐related information and recommendations based on the information provided during the patient assessment Control description: Physicians in the control condition were not given any training and were instructed to continue their usual smoking cessation practices; Patients completed the same assessments but did not receive the report (being the one page report characterising patients smoking habits) Duration of intervention: One session only Intervention delivered by: Health educator Intensity: One, 40 minute session |
|
| Outcomes |
Pre‐specified outcome data: Patients asked ‐ Did your doctor... ask whether you smoke, ask whether you are ready to quit, advise you to quit smoking, help you to quit smoking, help you set goals about quitting, give you written materials about quitting, refer you to a quit smoking program, talk to you about quit‐smoking medications, make a follow‐up appointment to discuss smoking Primary outcome measure ‐ 7 day point prevalence abstinence; Longest quit attempt (in days); Total number of 25 hour quit attempts, stage‐of‐change progression Follow‐up period: Six months |
|
| Notes |
Process measures: None reported Validation: For sub‐group of participants ‐ Saliva‐cotinine test; 14 of 16 samples confirmed abstinence (88%) n‐values re‐calculated for meta‐analysis to permit intention‐to‐treat analysis for primary outcome data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding (performance bias and detection bias) of participants | High risk | Physicians learned their group assignment after signing the informed consent; Due to the nature of the intervention blinding of participants was not possible for this study |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | No mention of attempts to blind outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 62 subjects were withdrawn due to computer malfunction, scheduling and time constraints; No further information provided regarding missing or incomplete outcome data |
| Selective reporting (reporting bias) | High risk | Smoking abstinence from baseline to follow‐up has not been reported, which is an outcome that would be expected to have been assessed for such a study |
| Other bias | Low risk | No other biases identified |
| Imbalance of outcome measures at baseline | Low risk | Patient intervention and control groups differed on the amount of daily smoking with the intervention group having more smokers with >25 year smoking history which was subsequently controlled in all smoking outcome analyses |
| Comparability of intervention and control group characteristics at baseline | Low risk | Both physician and patient characteristics were reported and no differences were found |
| Protection against contamination | Unclear risk | Geographic location of intervention and control physicians not described |
| Selective recruitment of participants | Low risk | Project staff offered participation to all identified smokers |
Wang 1994.
| Methods |
Country: Taiwan Design: Randomized Controlled Trial Objective: To assess the stages‐of‐change model in cigarette smoking and practice guidelines for practicing cigarette smoking cessation counselling in a short training program, designed to make physicians more willing to help their patients to quit smoking and increase success rates Methods of analysis: All data were analysed using either the Chi² or Fisher’s exact tests Clustering adjustment made: No Significance of cluster adjustment: Not applicable |
|
| Participants |
Therapist description: Residents and physicians in family medicine Eligible for study; n‐value not reported Randomized; n‐value: Group one: lessons n= 9, Group two: posters n= 9, Group three: usual care n= 9 Completed; n‐value: Group one: lessons n= 9, Group two: posters n= 9, Group three: usual care n= 9 Age: Not reported Gender: Not reported Patient description: Eligible for study; n‐value not reported Randomized; n‐value: n= 93, Group one: n= 39, Group two: n= 26, Group three: n= 28 Completed; n‐value: n= 82, Group one: n= 35, Group two: n= 24, Group three: n= 23 Age: Group one: <40 n= 14, 40‐59 n= 17, > 60 n= 8; Group two: <40 n= 14, 40‐59 n= 8, > 60 n= 4; Group three: <40 n= 7, 40‐59 n= 12, > 60 n= 9 Gender: Group one: male n= 38 female n= 1; Group two: male n= 24 female n= 2; Group three: male n= 27 female n= 1 Therapists: 27 physicians Patients: 93 patients |
|
| Interventions |
Setting: Not reported Training of those delivering the intervention to the health professional: Not reported Intervention description: Two intervention groups: Training ‐ stages of change model and practice guidelines; Poster ‐ used as a reminder to give advice Control description: Usual care Duration of intervention: Group one: two lessons; Group two: provided with poster only; Group three: no intervention Intervention delivered by: Not reported Intensity: Group one: two lessons; Group two: provided with poster only; Group three: no intervention |
|
| Outcomes |
Pre‐specified outcome data: Demographic data, cigarette‐smoking habits and health beliefs Follow‐up period: 6‐months; Point prevalence of abstinence at 12 months No process outcomes |
|
| Notes |
Validation: None Process measures: None reported Manual adjustment for potential clustering effects performed in the meta‐analyses for primary outcome data; The two intervention groups were combined for meta‐analyses to produce the single 'Intervention' sample; n‐values re‐calculated for meta‐analysis to permit intention‐to‐treat analysis for primary outcome data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Physicians were randomized “…to one of three groups by number of years in practice.” No other information provided |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding (performance bias and detection bias) of participants | High risk | Due to the nature of the intervention it was not possible to blind participants |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | No mention of blinding for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of missing outcome data or how any missing variables were handled |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of yes or no |
| Other bias | Low risk | No other biases identified |
| Imbalance of outcome measures at baseline | Unclear risk | Data not reported for baseline smoking; No mention of analyses of covariance |
| Comparability of intervention and control group characteristics at baseline | Low risk | Authors reported no significant differences between patient demographic characteristics |
| Protection against contamination | Unclear risk | Insufficient information to permit judgement of yes or no |
| Selective recruitment of participants | Unclear risk | Methods of recruitment not described; n‐values are different between groups |
Wilson 1988.
| Methods |
Country: Canada Design: Randomized controlled trial; Nested; Clustered Objective: To investigate the effects of a smoking cessation workshop on physician practices and on patients’ smoking behaviour Methods of analysis: Analysis of covariance – Obtained by averaging patient values within the practice; Analysis of differences between groups – If there was no difference between the usual care and gum only groups (untrained cohorts) these would be combined and compared with the gum plus (trained cohort); Regression analysis performed on practice unit, adjusting for the effects of predictor variables and treatment Clustering adjustment made: No ‐ None reported Significance of cluster adjustment: Not reported |
|
| Participants |
Therapist description: Physicians Eligible for study: n= 460 Family physicians Randomized: n= 90 Physicians Completed: n= 83 Physicians; Usual care n= 27; Gum only n= 29; Gum plus n= 27 Age: Usual care: Mean = 41.64 years; Gum only: Mean = 41.77 years; Gum plus: Mean = 40.57 years Gender: Usual care: Male 92.6%; Gum only: Male 93.1%; Gum plus: Male 81.5% Patient description: Eligible for study: Not stated as n‐value; Participation consent rates were: Usual care 91%; Gum only 83%; Gum plus 76% Randomized: Not reported Completed: Usual care n= 601; Gum only n= 726; Gum plus n= 606 (total n= 1933) Age:<25 years: Usual care 22%; Gum only 19%; Gum plus 17%; 25 to 44 years: Usual care 50%; Gum only 54%; Gum plus 56%; ≥ 45 years: Usual care 27%; Gum only 27%; Gum plus 27% Gender: Male: Usual care 39%; Gum only 42%; Gum plus 33% |
|
| Interventions |
Setting: Clinical practice setting – Participation during routine physician consultation; Based in Ontario, Hamilton Training of those delivering the intervention to the health professional: Not described; CME Protocol Intervention description: Two intervention groups: Gum only ‐ Physicians instructed to approach patients in their usual manner about quitting smoking and to offer nicotine gum as an aid to quitting; Gum Plus Training ‐ Gum in addition to training Control description: Usual care Duration of intervention: One, 4 hour training workshop to Gum plus physician cohort Intervention delivered by: Not described Intensity: Control ‐ Not explicitly reported; Gum only ‐ Not explicitly reported; Gum plus ‐ One, 4 hour workshop for physicians; For patients ‐ Use of gum, 1 to 6 follow‐up visits and quit dates |
|
| Outcomes |
Pre‐specified outcome data: Three‐month self reported sustained abstinence prior to biochemically validated cessation at 12 months; Smoking behaviour, cessation attempts and nicotine gum use measured by telephone interviews; Physicians performance measured by patient flow sheets and patient telephone exit interviews Follow‐up period: Point prevalence of abstinence at 12 months |
|
| Notes |
Process measures: None reported Validation: Salivary cotinine The two intervention groups were combined for meta‐analyses to produce the single 'Intervention' sample; Manual adjustment for potential clustering effects performed in the meta‐analyses for primary outcome data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization mentioned as 'allocated by practice to one of the three treatment groups' however methods not described |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding (performance bias and detection bias) of participants | High risk | Due to the nature of the intervention blinding of participants was not possible for this study |
| Blinding (performance bias and detection bias) of outcome assessors | Unclear risk | No mention of attempted blinding for outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | A total of 87 patients (4.5%) who may have been non‐smokers were classified as cigarette smokers for the purpose of the analysis; No further information provided regarding missing or incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes, including those that were pre‐specified were reported |
| Other bias | Low risk | No other biases identified |
| Imbalance of outcome measures at baseline | Low risk | Adequately described in text and adjusted for using analysis of variance |
| Comparability of intervention and control group characteristics at baseline | Unclear risk | Carried out a comparison of demographic characteristics of the cohorts; Baseline characteristics not fully reported |
| Protection against contamination | Low risk | Clinical practice level randomization suitable for this type of study; No indication of contamination from external sources during study period; All participants were family physicians within a 40 mile radius of the McMaster University in Hamilton, Ontario |
| Selective recruitment of participants | Unclear risk | Physician n‐values across different groups not reported; Participation consent rates were 91%, 83% and 76%, respectively, in the usual care, gum only, and gum plus groups |
HMO: Health Maintenance Organization; OTC: over the counter
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albert 2006 | No patient smoking related outcomes reported separately for intervention and control groups |
| Allen 1998 | Unit of randomization was patients not health care providers; No patient level outcome data reported |
| Andrews 1999 | No smoking related outcomes reported as interventions for smokeless tobacco only |
| Andrews 2001 | Consultation process only ‐ No smoking related outcomes reported |
| Ballbe 2008 | Sample not randomly allocated ‐ consultation process only |
| Bernstein 2009 | Sample not randomly allocated ‐ consultation process only |
| Bobo 1997 | Consultation process only |
| Campbell 1997 | Consultation process only ‐ No smoking related outcomes reported |
| Caplan 2011 | No control group |
| Carney 1995 | Consultation process only ‐ No smoking related outcomes reported |
| Cockburn 1992 | Study compared academic detailing, courier delivery and direct mailing of a new smoking cessation program for use in primary care; Did not include any measure of the extent to which physicians changed their counselling, or the number of smokers who stopped smoking in the 3 groups |
| Depue 2002 | No control group |
| Dietrich 1992 | Consultation process only ‐ No smoking related outcomes reported |
| Dunkley 1997 | Sample not randomly allocated ‐ midwives selected into intervention and control groups |
| Etter 2000 | No smoking related outcomes reported |
| Etter 2006 | No smoking related outcomes reported |
| Giuntini 2001 | No smoking related outcomes reported |
| Goldberg 1994 | Training not randomized |
| Gordon 2005a | Sample not randomly allocated ‐ historical control only |
| Gordon 2005b | Investigation of smokeless tobacco cessation only |
| Graham 2011 | Consultation process only ‐ No smoking related outcomes reported |
| Guo 2010 | No control group |
| Haresaku 2010 | No control group and no patient related smoking outcomes reported |
| Keller 2000 | Consultation process only ‐ No smoking related outcomes reported |
| Kerr 2011 | Consultation process only ‐ No smoking related outcomes reported |
| Leong 2008 | No smoking related outcomes reported ‐ only patient movement across stages of change model |
| Lindsay 1997 | Consultation process only ‐ No smoking related outcomes reported |
| Little 2009 | Consultation process only ‐ No smoking related outcomes reported |
| Manfredi 2011 | No control group |
| Martin 2010 | Consultation process only ‐ No smoking related outcomes reported |
| Matten 2011 | No control group |
| McEwen 2002 | Consultation process only ‐ No smoking related outcomes reported |
| McEwen 2006 | Consultation process only ‐ No long‐term smoking related outcomes reported |
| McIntosh 2004 | Consultation process only ‐ No smoking related outcomes reported |
| McRobbie 2008 | Consultation process only ‐ No long‐term smoking related outcomes reported |
| Meyer 2008 | Sample not randomly allocated ‐ unit of randomization weeks 1, 2, and 3 within the 'randomly selected' practices |
| Moore 2005 | Consultation process only ‐ No smoking related outcomes reported |
| Morgan 1996 | Both groups of physicians received training; Delayed intervention group asked to give usual care |
| Moss 2009 | No control group |
| Ockene 1991 | Physicians not randomly allocated to training; Patients were randomly allocated to different types of physician counselling with or without nicotine gum |
| Patwardhan 2010 | Consultation process only ‐ No smoking related outcomes reported |
| Pereira 2006 | No patient related smoking outcome data available |
| Prokhorov 2010 | No outcome data available on matched cohort ‐ follow‐up data only presented for cross‐sectional sample of patients |
| Pronk 2006 | No control group and no patient outcome data presented |
| Rankin 2010 | Sample not randomly allocated and no patient outcome data presented |
| Richmond 1998 | No control group: All physicians trained to provide Smokescreen intervention; Intervention consisted of telephone calls to ask about use of program; Patient smoking outcomes not given separately for intervention groups |
| Roche 1996 | No control group: Comparison of different methods of training, with no patient quit rate outcomes |
| Royce 1995 | No control group |
| Russos 1999 | Consultation process only ‐ No smoking related outcomes reported |
| Schmelz 2010 | No control group and no patient related smoking outcomes reported |
| Schnoll 2003 | Level of randomization not healthcare practitioner or practice; Level of randomization is patient |
| Secker Walker 1992 | The study involved training residents in obstetrics and family practice to give advice about stopping smoking during pre‐natal care; However, training was not the variable that was randomized |
| Sheffer 2009 | No control group and no patient related smoking outcomes reported |
| Sheffer 2011 | No control group |
| Sohn 2010 | No control group and no patient related smoking outcomes reported |
| Steinemann 2005 | Consultation process only ‐ No smoking related outcomes reported |
| Stolz 2012 | No true control group and no patient related smoking outcomes reported |
| Targhetta 2011 | Sample not randomly allocated |
| Von Garnier 2010 | Historical control group only |
| Walsh 2010 | No patient smoking related outcomes reported |
| Ward 1996 | No smoking related outcome data |
| Wisborg 1998 | Sample not randomly allocated ‐ Midwives working on Thursdays were considered to be the intervention group |
| Young 2002 | Consultation process only ‐ No smoking related outcomes reported |
Differences between protocol and review
RevMan version 5.1 was upgraded to version 5.1.2 during the review update, as such risk of bias domain categories were altered from 'yes', 'no' and 'unclear' to 'high risk', 'low risk' and 'unclear risk'.
Contributions of authors
Kristin Carson updated the protocol, reviewed the literature, identified studies for inclusion, extracted data, entered and analysed data and updated the text of the manuscript.
Marjolein Verbiest updated the protocol, reviewed the literature, identified studies for inclusion, extracted data and updated the text of the manuscript.
Mathilde Crone updated the protocol, identified studies for inclusion and updated the text of the manuscript.
Malcolm Brinn extracted data, entered and analysed data and updated the text of the manuscript.
Adrian Esterman updated the protocol, analysed data and updated the text of the manuscript.
Willem Assendelft assisted in updating the protocol and updating the text of the manuscript.
Brian Smith assisted in updating the protocol and updating the text of the manuscript.
Sources of support
Internal sources
The Respiratory Medicine Unit, The Queen Elizabeth Hospital, Australia.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Cohen (Dent) 1989 {published data only}
- Cohen SJ, Stooky GK, Katz BP, Drook CA, Christen AG. Helping smokers quit: a randomized controlled trial with private practice dentists. J Am Dent Assoc 1989;118:41‐5. [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Stooky GK, Katz BP, Drook CA, Smith DM. Encouraging primary care physicians to help smokers quit. A randomised, controlled trial. Ann Intern Med 1989;110:648‐52. [DOI] [PubMed] [Google Scholar]
Cohen (Doc) 1989 {published data only}
- Cohen SJ, Christen AG, Katz BP, Drook CA, Davis BJ, Smith DM, et al. Counseling medical and dental patients about cigarette smoking: the impact of nicotine gum and chart reminders. Am J Public Health 1987;77:313‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen SJ, Stooky GK, Katz BP, Drook CA, Smith DM. Encouraging primary care physicians to help smokers quit. A randomised, controlled trial. Ann Intern Med 1989;110:648‐52. [DOI] [PubMed] [Google Scholar]
Cornuz 2002 {published data only}
- Cornuz J, Humair JP, Seematter L, Stalder H, Pecoud A. A randomized controlled trial of resident training in smoking cessation using standardized patients. 11th World Conference on Tobacco or Health 6‐11 August Chicago Abstract book. Chicago, Illinois, USA, 2000.
- Cornuz J, Humair JP, Seematter L, Stoianov R, Melle GV, Stalder H, Pecoud A. Efficacy of resident training in smoking cessation: a randomized, controlled trial of a program based on application of behavioral theory and practice with standardized patients. Annals of Internal Medicine 2002;136:429‐37. [DOI] [PubMed] [Google Scholar]
- Cornuz J, Humair JP, Seematter L, Stoianov R, Melle G, Stalder H, Pecoud A. Summaries for Patients: Effect of a training program for resident physicians in improving success rate in helping patients quit smoking. Annals of Internal Medicine 2002;136(6):I‐31. [Google Scholar]
- Cornuz J, Zellweger JP, Mounoud C, Decrey H, Pecoud A, Burnand B. Smoking cessation counseling by residents in an outpatient clinic. Preventive Medicine 1997;26:292‐6. [DOI] [PubMed] [Google Scholar]
- Humair J, Sadowski J, Zellweger J, Kuenzi B, Burkhalter A, Cornuz J. Dissemination of an effective smoking cessation training program for Swiss primary care physicians. Journal of General Internal Medicine 2005;20(Supplement 1):31. [Google Scholar]
- Humair JP, Cornuz J. A new curriculum using active learning methods and standardized patients to train residents in smoking cessation. Journal of General Internal Medicine 2003;18:1023‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinget C, Martin E, Wasserfallen JB, Humair JP, Cornuz J. Cost‐effectiveness analysis of a European primary‐care physician training in smoking cessation counseling. European Journal of Cardiovascular Prevention and Rehabilitation 2007;14:451‐5. [DOI] [PubMed] [Google Scholar]
Cummings (Priv) 1989 {published data only}
- Cummings SR, Richard RJ, Duncan CL, Hansen B, Vander Martin R, Gerber B, et al. Training physicians about smoking cessation: a controlled trial in private practice. Journal of General Internal Medicine 1989;4:482‐9. [DOI] [PubMed] [Google Scholar]
Cummings 1989 {published data only}
- Cummings SR, Coates TJ, Richard RJ, Hansen B, Zahnd EG, Vander Martin R, et al. Training physicians in counseling about smoking cessation. A randomized trial of the "Quit for Life" program. Annals of Internal Medicine 1989;110:640‐7. [DOI] [PubMed] [Google Scholar]
Gordon 2010 {published and unpublished data}
- Gordon JS, Albert DA, Andrews JA, Crews KM, Payne TJ, Severson HH. Tobacco cessation in public dental clinics: short‐term outcomes. Society for Research on Nicotine and Tobacco 14th annual Meeting. Portland, Oregon, 2008 Feb 26‐ Mar 1.
- Gordon JS, Andrews JA, Albert DA, Crews KM, Payne TJ, Severson HH. Tobacco Cessation via Public Dental Clinics: Results of a Randomized Trial. American Journal of Public Health 2010;100(7):1307‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hymowitz 2007 {published data only}
- Hymowitz N, Schwab J, Haddock CK, Burd KM, Pyle S. The Pediatric Residency Training on Tobacco Project: baseline findings from the resident tobacco survey and observed structured clinical examinations. Preventive Medicine 2004;39:507‐16. [DOI] [PubMed] [Google Scholar]
- Hymowitz N, Schwab J, Haddock CK, Pyle S, Meshberg S. The Pediatric Resident Training on Tobacco Project: Interim Findings. Journal of the National Medical Association Feb 2006;92(8):190‐203. [PMC free article] [PubMed] [Google Scholar]
- Hymowitz N, Schwab J, Haddock CK, Pyle S, Meshberg S. The pediatric residency training on tobacco project: baseline findings from the patient tobacco survey. Preventive Medicine 2005;41:159‐66. [DOI] [PubMed] [Google Scholar]
- Hymowitz N, Schwab J, Haddock CK, Pyle S, Moore G, Meshberg S. The pediatric resident training on tobacco project: baseline findings from the Parent/Guardian Tobacco Survey. Preventive Medicine 2005;41:334‐41. [DOI] [PubMed] [Google Scholar]
- Hymowitz N, Schwab JV, Haddock CK, Pyle SA, Schwab LM. The pediatric residency training on tobacco project: four‐year resident outcome findings. Preventive Medicine 2007;45(6):481‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Joseph 2004 {published data only}
- Joseph AM, Arikian NJ, An LC, Nugent SM, Sloan RJ, Pieper CF and the GIFT Research Group. Results of a Randomized Controlled Trial of Intervention to Implement Smoking Guidelines in Veterans Affairs Medical Centers. Medical Care 2004;42(11):1100‐10. [DOI] [PubMed] [Google Scholar]
Kottke 1989 {published data only}
- Kottke TE, Brekke ML, Solberg LI, Hughes JR. A randomised controlled trial to increase smoking intervention by physicians. Doctors helping smokers, round 1. JAMA 1989;261:2101‐6. [PubMed] [Google Scholar]
Lennox 1998 {published data only}
- Lennox AS, Bain N, Taylor RJ, McKie L, Donnan PT, Groves J. Stages of Change training for opportunistic smoking intervention by the primary health care team. Part I: randomised controlled trial of the effect of training on patient smoking outcomes and health professional behaviour as recalled by patients. Health Educ J 1998;57:140‐149. [Google Scholar]
Sinclair 1998 {published data only}
- Sinclair HK, Bond CM, Lennox AS. The long‐term learning effect of training in stage of change for smoking cessation: a three year follow up of community pharmacy staff's knowledge and attitudes. International Journal of Pharmacy Practice 1999;7:1‐11. [Google Scholar]
- Sinclair HK, Bond CM, Lennox AS, Silcock J, Winfield AJ, Donnan PT. Training pharmacists and pharmacy assistants in the stage‐of‐change model of smoking cessation: a randomised controlled trial in Scotland. Tob Control 1998;7:253‐261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strecher 1991 {published data only}
- Campbell EE, Lyles MF, Strecher VJ, Gonzalez JJ. Teaching smoking cessation counseling skills to resident physicians. Clin Res 1989;37:805A. [Google Scholar]
- Campbell EE, Villagra VG, Rgers CS and Other Members of the North Carolina Primary Care Research Consortium. Teaching and promoting smoking cessation counseling in primary care residencies: description of a method. Teaching and Learning in Medicine 1991;3(1):20‐7. [Google Scholar]
- Strecher VJ, O'Malley MS, Villagra VG. Can residents be trained to counsel patients about quitting smoking? Results from a randomized trial. J Gen Intern Med 1991;6:9‐17. [DOI] [PubMed] [Google Scholar]
Swartz 2002 {published and unpublished data}
- Swartz S, Cowan T. A Randomized Trial of Academic Profiling increases provider tobacco intervention. Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15‐18, Orlando, Florida. Orlando, Florida, 2006.
- Swartz S, Cowan T, Thompson D, Leighton A, Goldstein M, DePue J, Salem‐Schatz S. A Randomized Trial of Academic Profiling to Increase Tobacco Interventions in Primary Care. Supplied by author.
- Swartz S, Cowan T, Thompson D, Leighton A, Goldstein M, DePue J, Salem‐Schatz S. Academic Detailing and Data Feedback to Increase Tobacco Treatment. Supplied by author.
- Swartz SH, Cowan TM, DePue J, Goldstein G. Academic profiling of tobacco‐related performance measures in primary care. Nicotine & Tobacco Research 2002;4(S1):S39‐45. [DOI: 10.1080/14622200210128018] [DOI] [PubMed] [Google Scholar]
Twardella 2007 {published data only}
- Breitling LP, Twardella D, Raum E, Brenner H. Situational Temptation Scores and Smoking Cessation in General Care. Psychology of Addictive Behaviors 2009;23(2):362‐7. [DOI: 10.1037/a0015715] [DOI] [PubMed] [Google Scholar]
- Salize HJ, Merkel S, Reinhard I, Twardella D, Mann K, Brenner J. Cost‐effective primary care‐based strategies to improve smoking cessation: more value for money. Archives of Internal Medicine 2009;169(3):230‐5. [DOI] [PubMed] [Google Scholar]
- Twardella D, Brenner H. Effects of practitioner education, practitioner payment and reimbursement of patients' drug costs on smoking cessation in primary care: a cluster randomised trial. Tobacco Control 2007;16:15‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Unrod 2007 {published data only}
- Unrod M, Smith M, Spring B, DePue J, Redd W, Winkel G. Randomized Controlled Trial of a Computer‐Based, Tailored Intervention to Increase Smoking Cessation Counseling by Primary Care Physicians. Journal of General Internal Medicine 2007;22:478‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 1994 {published data only}
- Wang WD. Feasibility and effectiveness of a stages‐of‐change model in cigarette smoking cessation counseling. J Formos Med Assoc 1994;93:752‐757. [PubMed] [Google Scholar]
Wilson 1988 {published data only}
- Lindsay EA, Wilson DM, Best JA, Willms DG, Singer J, Gilbert JR, Taylor DW. A randomized trial of physician training for smoking cessation. Am J Health Promot 1989;3:11‐8. [DOI] [PubMed] [Google Scholar]
- Wilson DM, Taylor DW, Gilbert JR, Best JA, Lindsay EA, Willms DG, Singer J. A randomized trial of a family physician intervention for smoking cessation. JAMA 1988;260:1570‐4. [PubMed] [Google Scholar]
References to studies excluded from this review
Albert 2006 {published data only}
- Albert DA. Tobacco and the Dental Office: Encouraging Patients to Quit. http://ncth.confex.com/ncth/viewHandout.epl?uploadid=679 [accessed 17/03/2011].
- Albert DA, Gordon J, Andrews J, Severson H, Ward A, Sadowsky D. Use of electronic continuing education in dental offices for tobacco cessation. Society for Research on Nicotine and Tobacco 12th Annual Meeting February 15‐18, Orlando, Florida. 2006.
Allen 1998 {published data only}
- Allen B, Pederson LL, Leonard EH. Effectiveness of physicians‐in‐training counseling for smoking cessation in African Americans. Journal of the National Medical Association 1998;90:597‐604. [PMC free article] [PubMed] [Google Scholar]
Andrews 1999 {published data only}
- Andrews JA, Severson HH, Lichtenstein E, Gordon JS, Barckley MF. Evaluation of a dental office tobacco cessation program: effects of smokeless tobacco use. Annals of Behavioral Medicine 1999;21(1):48‐53. [DOI] [PubMed] [Google Scholar]
Andrews 2001 {published data only}
- Andrews JO, Tingen MS, Waller JL, Harper RJ. Provider feedback improves adherence with AHCPR Smoking Cessation Guideline. Preventive Medicine 2001;33:415‐21. [DOI] [PubMed] [Google Scholar]
Ballbe 2008 {published data only}
- Ballbe M, Mondon S, Nieva G, Walther M, Salto E, Gual A. Evaluation of a training programme for health professionals on smoking cessation in hospitalized patients [Evaluacion de un programa de formacion de profesionales sanitarios sobre abordaje del tabaquismo en pacientes hospitalizados]. Adicciones 2008;20:125‐30. [PubMed] [Google Scholar]
Bernstein 2009 {published data only}
- Bernstein SL, Boudreaux ED, Cabral L, Cydulka RK, Schwegman D, Larkin GL, et al. Efficacy of a brief intervention to improve emergency physicians' smoking cessation counseling skills, knowledge, and attitudes. Substance Abuse 2009;30:158‐81. [DOI] [PubMed] [Google Scholar]
Bobo 1997 {published data only}
- Bobo JK, Anderson JR, Bowman A. Training chemical dependency counselors to treat nicotine dependence. Addictive Behaviors 1997;22:23‐30. [DOI] [PubMed] [Google Scholar]
Campbell 1997 {published data only}
- Campbell HS, Petty TL, Meadows LM, Simpson L, Jannett PA. Improving tobacco cessation services in rural dental offices ‐ recruitment to a randomized controlled trial. Canadian Journal of Community Dentistry 1997;12(2):15‐22. [Google Scholar]
Caplan 2011 {published data only}
- Caplan L, Stout C, Blumenthal DS. Training physicians to do office‐based smoking cessation increases adherence to PHS guidelines. Journal of Community Health 2011;36(2):238‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carney 1995 {published data only}
- Carney PA, Dietrich AJ, Freeman DH Jr, Mott LA. A standardized‐patient assessment of a continuing medical education program to improve physicians' cancer‐control clinical skills. Academic Medicine 1995;70(1):52‐8. [DOI] [PubMed] [Google Scholar]
Cockburn 1992 {published data only}
- Cockburn J, Ruth D, Silagy C, Dobbin M, Reid Y, Scollo M, et al. Randomised trial of three approaches for marketing smoking cessation programmes to Australian general practitioners. BMJ 1992;304:691‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Depue 2002 {published data only}
- Depue JD, Goldstein MG, Schilling A, Reiss P, Papandonatos G, Sciamanna C, et al. Dissemination of the AHCPR clinical practice guideline in community health centres. Tobacco Control 2002;11:329‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dietrich 1992 {published data only}
- Dietrich AJ, O'Connor GT, Keller A, Carney PA, Levy D, Whaley FS. Cancer: improving early detection and prevention. A community practice randomised trial. British Medical Journal 1992;304(6828):687‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunkley 1997 {published data only}
- Dunkley J. Training midwives to help pregnant women stop smoking. Nursing Times Jan 1997;93(5):1‐3. [PubMed] [Google Scholar]
Etter 2000 {published data only}
- Etter JF, Rielle JC, Perneger TV. Labeling smokers' charts with a "smoker" sticker: results of a randomized controlled trial among private practitioners. Journal of General Internal Medicine 2000;15(6):421‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Etter 2006 {published data only}
- Etter JF. Impact of educational outreach visits on smoking cessation activities performed by specialist physicians: A randomized trial. Education for Heatlh 2006;19(2):155‐65. [DOI] [PubMed] [Google Scholar]
Giuntini 2001 {published data only}
- Giuntini C, Barrueco M, Astbury C, Hider AE, Hogue SL. Involvement of healthcare professionals in a smoking cessation programme positively benefit attitudes towards smoking. Society for Research on Nicotine and Tobacco 3rd Europe Conference, Paris, 19‐22 September. 2001.
Goldberg 1994 {published data only}
- Goldberg DN, Hoffman AM, Farinha MF, Marder DC, Tinson‐Mitchem L, Burton D, Smith EG. Physician delivery of smoking‐cessation advice based on the stages‐of‐change model. American Journal of Preventive Medicine 1994;10:267‐74. [PubMed] [Google Scholar]
Gordon 2005a {published data only}
- Gordon JS, Andrews JA, Lichtenstein E, Severson HH. The impact of a brief tobacco‐use cessation intervention in public health dental clinics. Journal of the American Dental Association 2005;136:179‐86. [DOI] [PubMed] [Google Scholar]
Gordon 2005b {published data only}
- Gordon JS, Andrews JA, Lichtenstein E, Severson HH, Akers L. Disseminating a smokeless tobacco cessation intervention model to dental hygienists: A randomized comparison of personalized instruction and self‐study methods. Health Psychology 2005;24:447‐55. [DOI] [PubMed] [Google Scholar]
Graham 2011 {published data only}
- Graham GF. Nursing student training, perception, and behavior in tobacco cessation counseling: A randomized experimental study. Dissertation Abstracts International: Section B: The Sciences and Engineering 2011;71(7‐B):4171. [Google Scholar]
Guo 2010 {published data only}
- Guo FR, Hung LY, Chang CJ, Leung KK, Chen CY. The evaluation of a Taiwanese training program in smoking cessation and the trainees' adherence to a practice guideline. BMC Public Health 2010;10:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haresaku 2010 {published data only}
- Haresaku S, Hanioka T, Yamamoto M, Ojima M. Impact of a tobacco curriculum on smoking behavior and attitudes toward smoking in dental students in Japan: a three‐year follow‐up study. International Dental Journal 2010;60(2):99‐105. [PubMed] [Google Scholar]
Keller 2000 {published data only}
- Keller S, Donnerbanzhoff N, Kaluza G, Baum E, Basler HD. Improving physician‐delivered counseling in a primary care setting: Lessons from a failed attempt. Education for Health 2000;13(3):387‐97. [DOI] [PubMed] [Google Scholar]
Kerr 2011 {published data only}
- Kerr S, Whyte R, Watson H, Tolson D, McFadyen AK. A mixed‐method evaluation of the effectiveness of tailored smoking cessation training for healthcare practitioners who work with older people. Worldviews on Evidence‐Based Nursing 2011;8(3):177. [DOI] [PubMed] [Google Scholar]
Leong 2008 {published data only}
- Leong SL, Lewis PR, Curry WJ, Gingrich DL. Tobacco world: evaluation of a tobacco cessation training program for third‐year medical students. Academic Medicine Oct 2008;83(10 Suppl):S25‐8. [DOI] [PubMed] [Google Scholar]
Lindsay 1997 {published data only}
- Lindsay EA, Hymowitz N, Giffen C, Purcell T, Pomrehan P, Pechacek T. Tobacco control activities of primary‐care physicians in the Community Intervention Trial for Smoking Cessation. COMMIT Research Group. Tobacco Control 1997;6(Suppl 2):S49‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Little 2009 {published data only}
- Little SJ, Hollis JF, Fellows JL, Snyder JJ, Dickerson JF. Implementing a tobacco assisted referral program in dental practices. Journal of Public Heatlh Dentistry 2009;69(3):149‐55. [DOI] [PubMed] [Google Scholar]
Manfredi 2011 {published data only}
- Manfredi C, Cho YI, Warnecke R, Saunders S, Sullivan M. Dissemination strategies to improve implementation of the PHS smoking cessation guideline in MCH public health clinics: experimental evaluation results and contextual factors. Health Education Research 2011;26(2):349‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 2010 {published data only}
- Martin BA, Bruskiewitz RH, Chewning BA. Effect of a tobacco cessation continuing professional education program on pharmacists' confidence, skills, and practice‐change behaviors. Journal of the American Pharmacists Association Jan 2010;50(1):9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matten 2011 {published data only}
- Matten P, Morrison V, Rutledge DN, Chen T, Chung E, Wong SF. Evaluation of tobacco cessation classes aimed at hospital staff nurses. Oncology Nursing Forum 2011;38(1):67‐73. [DOI] [PubMed] [Google Scholar]
McEwen 2002 {published data only}
- McEwen A, Preston A, West R. Effect of a GP desktop resource on smoking cessation activities of general practitioners. Addiction 2002;97(5):595‐7. [DOI] [PubMed] [Google Scholar]
McEwen 2006 {published data only}
- McEwen A, West R, Preston A. Triggering anti‐smoking advice by GPs: Mode of action of an intervention stimulating smoking cessation advice by GPs. Patient Education and Counseling 2006;62:89‐94. [DOI: 10.1016/j.pec.2005.06.008] [DOI] [PubMed] [Google Scholar]
McIntosh 2004 {published data only}
- McIntosh S, Ossip‐Klein DJ, Hazel‐Fernandez L, Spada J, McDonald PW, Klein JD. Recruitment of physician offices for an office‐based adolescent smoking cessation study. Nicotine & Tobacco Research 2005;7(3):405‐12. [DOI] [PubMed] [Google Scholar]
McRobbie 2008 {published data only}
- McRobbie H, Hajek P, Feder G, Eldridge S. A cluster‐randomised controlled trial of a brief training session to facilitate general practitioner referral to smoking cessation treatment. Tobacco Control 2008;17:173‐6. [DOI] [PubMed] [Google Scholar]
Meyer 2008 {published data only}
- Meyer C, Ulbricht S, Baumeister SE, Schumann A, Ruge J, Bischof G, Rumpf HJ, John U. Proactive interventions for smoking cessation in general medical practice: a quasi‐randomized controlled trial to examine the efficacy of computer‐tailored letters and physician‐delivered brief advice. Addiction 2008;103:294‐304. [DOI: 10.1111/j.1360-0443.2007.02031.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2005 {published data only}
- Moore E, Keerbs A. The impact of training medical assistants to provide smoking cessation counseling to an underserved patient population. Journal of Investigative Medicine 2005;53:S81. [Google Scholar]
Morgan 1996 {published data only}
- Morgan GD, Noll EL, Orleans CT, Rimer BK, Amfoh K, Bonney G. Reaching midlife and older smokers ‐ tailored interventions for routine medical care. Preventive Medicine 1996;25:346‐54. [DOI] [PubMed] [Google Scholar]
Moss 2009 {published data only}
- Moss DR, Cluss PA, Watt‐Morse M, Pike F. Targeting pregnant and parental smokers: Long‐term outcomes of a practice‐based intervention. Nicotine & Tobacco Research 2009;11(3):278‐85. [DOI] [PubMed] [Google Scholar]
Ockene 1991 {published data only}
- Ockene JK, Adams A, Pbert L, Luippold R, Hebert JR, Quirk M, Kalan K. The Physician‐Delivered Smoking Intervention Project: factors that determine how much the physician intervenes with smokers. J Gen Intern Med 1994;9:379‐84. [DOI] [PubMed] [Google Scholar]
- Ockene JK, Kristeller J, Goldberg R, Amick TL, Pekow PS, Hosmer D, et al. Increasing the efficacy of physician‐delivered smoking interventions: a randomized clinical trial. J Gen Intern Med 1991;6:1‐8. [DOI] [PubMed] [Google Scholar]
Patwardhan 2010 {published data only}
- Patwardhan P, Chewing B. Randomized controlled trial evaluating the effect of a multimodal training intervention on brief tobacco cessation counseling in community chain pharmacies. Journal of the American Pharmacists Association 2010;50(2):260. [Google Scholar]
Pereira 2006 {published data only}
- Pereira BC, Stoebner‐Dalbarre A, Gourgou S, Sancho‐Garnier H, Rijnoveanu A, Slama K, et al. A cluster randomised controlled trial of a continuing education programme ‐ Smoking cessation intervention. European Journal of Epidemiology 2006;21:99. [Google Scholar]
Prokhorov 2010 {published data only}
- Prokhorov AV, Hudmon KS, Marani S, Foxhall L, Ford KH, Luca NS, et al. Engaging physicians and pharmacists in providing smoking cessation counseling. Archives of Internal Medicine 2010;170(18):1640‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pronk 2006 {published data only}
- Pronk NP, Havlicek D, Stafne E. Tobacco cessation interventions in dental networks: a practice‐based evaluation of the impact of education on provider knowledge, referrals, and pharmacotherapy use. Preventing Chronic Disease 2006;3(3):A96. [PUBMED: PMC1637804] [PMC free article] [PubMed] [Google Scholar]
Rankin 2010 {published data only}
- Rankin KV, Jones DL, Crews KM. Tobacco cessation education for dentists: An evaluation of the lecture format. Journal of Cancer Education 2010;25:282‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Richmond 1998 {published data only}
- Richmond R, Mendelsohn C, Kehoe L. Family physicians' utilization of a brief smoking cessation program following reinforcement contact after training: A randomized trial. Prev Med 1998;27:77‐83. [DOI] [PubMed] [Google Scholar]
Roche 1996 {published data only}
- Roche AM, Eccleston P, Sanson Fisher RW. Teaching smoking cessation skills to senior medical students ‐ a block‐randomized controlled trial of 4 different approaches. Prev Med 1996;25:251‐8. [DOI] [PubMed] [Google Scholar]
Royce 1995 {published data only}
- Royce JM, Ashford A, Resnicow K, Freeman HP, Caesar AA, Orlandi MA. Physician and nurse assisted smoking cessation in Harlem. J Nat Med Assoc 1995;87:291‐300. [PMC free article] [PubMed] [Google Scholar]
Russos 1999 {published data only}
- Russos S, Keating K, Hovell MF, Jones JA, Slymen DJ, Hofstetter CR, Rubin B, Morrison T. Counseling youth in tobacco‐use prevention: Determinants of clinician compliance. Preventive Medicine 1999;29:13‐21. [DOI] [PubMed] [Google Scholar]
Schmelz 2010 {published data only}
- Schmelz AN, Nixon B, McDaniel A, Hudmon KS, Zillich AJ. Evaluation of an online tobacco cessation course for health professions students. American Journal of Pharmaceutical Education 2010;74(2):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schnoll 2003 {published data only}
- Schnoll RA, Zhang B, Rue M, Krook JE, Spears WT, Marcus AC, Engstrom PF. Brief physician‐initiated quit‐smoking strategies for clinical oncology settings: a trial coordinated by the Eastern Cooperative Oncology Group. Journal of Clinical Oncology 2003;21(2):355‐65. [DOI] [PubMed] [Google Scholar]
Secker Walker 1992 {published data only}
- Secker Walker RH, Solomon LJ, Flynn BS, et al. Training obstetric and family practice residents to give smoking cessation advice during prenatal care. Am J Obstet Gynecol 1992;166:1356‐63. [DOI] [PubMed] [Google Scholar]
- Secker‐Walker RH, Dana GS, Solomon LJ, Flynn BS, Geller BM. The role of health professionals in a community‐based program to help women quit smoking. Preventive Medicine 2000;30(2):126‐37. [DOI] [PubMed] [Google Scholar]
Sheffer 2009 {published data only}
- Sheffer CE, Barone CP, Anders ME. Training health care providers in the treatment of tobacco use and dependence: pre‐ and post‐training results. Journal of Evaluation In Clinical Practice Aug 2009;15(4):607‐13. [DOI] [PubMed] [Google Scholar]
Sheffer 2011 {published data only}
- Sheffer CE, Barone C, Anders ME. Training nurses in the treatment of tobacco us and dependence: pre‐ and post‐training results. Journal of Advanced Nursing 2011;67(1):176‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sohn 2010 {published data only}
- Sohn M, Ahn Y, Park H. A pilot study to evaluate a simulation based smoking cessation intervention training for Korean nursing students. International Journal of Stroke 2010;5:276. [Google Scholar]
Steinemann 2005 {published data only}
- Steinmann S, Roytman T, Chang J, Holzman J, Hishinuma E, Nagoshi M, et al. Impact of education on smoking cessation counseling by surgical residents. American Journal of Surgery 2005;189(1):44‐6. [DOI] [PubMed] [Google Scholar]
Stolz 2012 {published data only}
- Stolz D, Langewitz W, Meyer A, Pierer K, Tschudi P, S'ng CT, et al. Enhanced didactic methods of smoking cessation training for medical students ‐ A randomized study. Nicotine & Tobacco Research 2012;14(2):224‐8. [DOI] [PubMed] [Google Scholar]
Targhetta 2011 {published data only}
- Targhetta R, Bernhard L, Sorokaty JM, BAlmes JL, Nalpas B, Perney P. Intervention study to improve smoking cessation during hospitalization. Public Health 2011;125(7):457‐63. [DOI] [PubMed] [Google Scholar]
Von Garnier 2010 {published data only}
- Garnier C, Meyer M, Leuppi J, Battegay E, Zeller A. Smoking cessation counselling: impact of chart stickers and resident training. Swiss Medical Weekly March 2010;140(11‐12):175‐80. [DOI] [PubMed] [Google Scholar]
Walsh 2010 {published data only}
- Walsh MM, Belek MG, Prakash P, Grimes B, Heckman B, Kaufman N, et. al. Changing dentists' tobacco control attitudes and behaviors. Society for Research on Nicotine and Tobacco 16th Annual Meeting. 2010 Feb 24‐27.
Ward 1996 {published data only}
- Ward J, Sanson Fisher RW. Does a 3‐day workshop for family medicine trainees improve preventive care ‐ a randomized control trial. Prev Med 1996;25:741‐7. [DOI] [PubMed] [Google Scholar]
Wisborg 1998 {published data only}
- Wisborg K, Henriksen TB, Secher NJ. A prospective intervention study of stopping smoking in pregnancy in a routine antenatal care setting. British Journal of Obstetrics and Gynaecology Nov 1998;105:1171‐6. [DOI] [PubMed] [Google Scholar]
- Wisborg K, Henriksen TB, Secher NJ. A trial of smoking cessation in a routine antenatal care setting. Acta Obstetricia et Gynecologica Scandinavica 1997;76(167 (Part 2)):86. [Google Scholar]
Young 2002 {published data only}
- Young JM, D'Este C, Ward JE. Improving family physicians use of evidence‐based smoking cessation strategies: a cluster randomization trial. Preventive Medicine 2002;35(6):572‐83. [DOI] [PubMed] [Google Scholar]
- Young JM, Ward J. Can distance learning improve smoking cessation advice in family practice? A randomized trial. J Contin Educ Health Prof 2002;22(2):84‐93. [DOI] [PubMed] [Google Scholar]
Additional references
Albert 2004
- Albert DA, Ahluwalia KP, Ward A, Sadowsky D. The use of 'academic detailing' to promote tobacco‐use cessation counseling in dental offices. The Journal of the American Dental Association 2004;135:1700‐6. [http://jada.ada.org/cgi/content/full/135/12/1700] [DOI] [PubMed] [Google Scholar]
Altman 1997
- Altman DG, Bland JM. Statistics notes. Units of analysis. BMJ 1997;314:1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2004
- Anderson P, Jane‐Llopis E. How can we increase the involvement of primary health care in the treatment of tobacco dependence? A meta‐analysis. Addiction 2004;99(3):299‐312. [DOI] [PubMed] [Google Scholar]
Balaji 2008
- Balaji SM. Tobacco smoking and surgical healing of oral tissues: A review. Indian Journal of Dental Research http://www.ijdr.in/article.asp?issn=0970‐9290;year=2008;volume=19;issue=4;spage=344;epage=348;aulast=Balaji (accessed 10/10/2011) 2008;19(4):344‐8. [DOI] [PubMed] [Google Scholar]
Bergstrom 2000
- Bergstrom J, Eliasson S, Dock J. A 10‐year prospective study of tobacco smoking and periodontal health. Journal of Periodontol 2000;71:1338‐47. [DOI] [PubMed] [Google Scholar]
Bertakis 2007
- Bertakis KD, Azari R. Determinants of physician discussion regarding tobacco and alcohol abuse. Journal of Health Communication 2007;12(6):513‐25. [DOI: 10.1080/10810730701508187] [DOI] [PubMed] [Google Scholar]
Botelho 2009
- Botelho R, Wassum K, Benzian H, Selby P, Chan S. Address the gaps in tobacco cessation training and services: Developing professional organisational alliances to create social movements. Drug and Alcohol Review 2009;28:558‐66. [DOI: 10.1111/j.1465-3362.2009.00112.x] [DOI] [PubMed] [Google Scholar]
Campbell 2000
- Campbell M, Grimshaw J, Steen N. Sample size calculations for cluster randomised trials. Changing Professional Practice in Europe Group (EU BIOMED II Concerted Action) Health Services Research Unit, University of Aberdeen, UK. J Health Serv Res Policy Jan 2000;5(1):12‐6. [DOI] [PubMed] [Google Scholar]
CDC 2007
- Centers for Disease Control and Prevention (CDC). Smoking‐cessation advice from health‐care providers‐Canada, 2005. Morbidity & Mortality Weekly Report (CDC); http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5628a3.htm [accessed 31/07/2011] 2007; Vol. 56, issue 28:708‐12. [PubMed]
Cummings 1989a
- Cummings SR, Coates TJ, Richard RJ, Hansen B, Zahnd EG, VanderMartin R, et al. Training physicians in counseling about smoking cessation. A randomized trial of the "Quit for Life" program. Annual Internal Medicine 1989;110(8):640‐7. [DOI] [PubMed] [Google Scholar]
Cummings 1989b
- Cummings SR, Richard RJ, Duncan CL, Hansen B, Vander MR, Gerbert B, et al. Training physicians about smoking cessation: a controlled trial in private practice. Journal of General Internal Medicine 1989;4(6):482‐9. [DOI] [PubMed] [Google Scholar]
Ellerbeck 2001
- Ellerbeck EF, Ahluwalia JS, Jolicoeur DG, Gladden J, Mosier MC. Direct observation of smoking cessation activities in primary care practice. The Journal of Family Practice 2001;50(8):688‐93. [PubMed] [Google Scholar]
EPOC 2009
- Cochrane EPOC Group 2009. Cochrane Effective Practice and Organisation of Care Group. Available from: http://www.epoc.cochrane.org (accessed 1 November 2009).
European Commission 2004
- European Commission, ASPECT Consortium. Tobacco or Health in the European Union: Past, present and future. http://ec.europa.eu/health/ph_determinants/life_style/Tobacco/Documents/tobacco_fr_en.pdf (accessed 08/10/2011) 2004:1‐295. [ISBN: 92‐894‐8219‐2]
Gelskey 2002
- Gelskey SC. Impact of a dental/dental hygiene tobacco‐use cessation curriculum on practice. Journal of Dental Education 2002;66:1074‐8. [PubMed] [Google Scholar]
Gordon 2009
- Gordon JS, Albert DA, Crews KM, Fried J. Tobacco education in dentistry and dental hygiene. Drug and Alcohol Review 2009;28:517‐32. [DOI: 10.1111/j.1465-3362.2009.00105.x] [DOI] [PubMed] [Google Scholar]
Guindon 2008
- Guindon GE, et al. World Health Organization. The cost attributable to tobacco use:a critical review of the literature. Population and Development Review; Geneva, World Health Organization http://www.jstor.org/pss/25434675 (accessed 08/10/2011) 2008;34(1):188‐94. [Google Scholar]
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Available from www.cochrane‐handbook.org] [Google Scholar]
Holm 1979
- Holm S. A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics 1979;6(2):65‐70. [Google Scholar]
Hung 2009
- Hung DY, Shelley DR. Multilevel analysis of the chronic care model and 5A services for treating tobacco use in urban primary care clinics. BMC Health Services Research 2009;44(1):103‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kralikova 2009
- Kralikova E, Bonevski B, Stepankova L, Pohlova L, Mladkova N. Postgraduate medical education on tobacco and smoking cessation in Europe. Drug and Alcohol Review 2009;28:474‐83. [DOI: 10.1111/j.1465-3362.2009.00104.x] [DOI] [PubMed] [Google Scholar]
Maciosek 2006
- Maciosek MV, Coffield AB, Edwards NM, Flottemesch TJ, Goodman MJ, Solberg LI. Priorities among effective clinical preventive services: results of a systematic review and analysis. American Journal of Preventive Medicine 2006;31(1):52‐61. [DOI] [PubMed] [Google Scholar]
Mathers 2006
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine http://www.plosmedicine.org/article/info:doi/10.1371/journal.pmed.0030442 (accessed 08/10/2011) 2006;3:e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohammad 2006
- Mohammad A, Crews K, Taybos G, Vancevitch P. Academy of oral medicine clinician's guide to tobacco cessation: Integrating medicine and dentistry. Hamilton, Ontario: BC Decker Inc, 2006. [ISBN: 978‐1‐936176‐19‐9] [Google Scholar]
Mullins 1999
- Mullins R, Livingston P, Borland R. A strategy for involving general practitioners in smoking control. Australian and New Zealand Journal of Public Health 1999;23:249‐51. [DOI] [PubMed] [Google Scholar]
Muramoto 2009
- Muramoto ML, Lando H. Faculty development in tobacco cessation: Training health professionals and promoting tobacco control in developing countries. Drug and Alcohol Review 2009;28:498‐506. [DOI: 10.1111/j.1465-3362.2009.00106.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Petersen 2009
- Petersen PE. Global policy for improvement of oral health in the 21st century – implications to oral health research of World Health Assembly 2007, World Health Organization. Community Dentistry and Oral Epidemiology http://onlinelibrary.wiley.com/doi/10.1111/j.1600‐0528.2008.00448.x/full (accessed 10/10/2011) 2009;37(1):1‐8. [DOI] [PubMed] [Google Scholar]
Richmond 1999
- Richmond RL. The physician can make a difference with smokers: evidence based clinical approaches. International Journal of Tuberculosis and Lung Disease 1999;3:100‐12. [PubMed] [Google Scholar]
Richmond 2009a
- Richmond R. Education and training for health professionals and students in tobacco, alcohol and other drugs. Drug and Alcohol Review 2009;28:463‐5. [DOI: 10.1111/j.1465-3362.2009.00115.x] [DOI] [PubMed] [Google Scholar]
Richmond 2009b
- Richmond R, Zwar N, Taylor R, Hunnisett J, Hyslop F. Teaching about tobacco in medical schools: A worldwide study. Drug and Alcohol Review 2009;28:484‐97. [DOI: 10.1111/j.1465-3362.2009-00105.x] [DOI] [PubMed] [Google Scholar]
Rosseel 2009
- Rosseel JP, Jacobs JE, Hilberink SR, Maassen IM, Allard HB, Plasschaert AJM, Grol RPTM. What determines the provision of smoking cessation advice and counselling by dental care teams?. British Dental Journal http://www.nature.com/bdj/journal/v206/n7/abs/sj.bdj.2009.272.html (accessed 10/10/2011) 2009;206:E13. [DOI: 10.1038/sj.bdj.2009.272] [DOI] [PubMed] [Google Scholar]
Solberg 2006
- Solberg LI, Maciosek MV, Edwards NM, Khanchandani HS, Goodman MJ. Repeated tobacco‐use screening and intervention in clinical practice: health impact and cost effectiveness. American Journal of Preventive Medicine 2006;31(1):62‐71. [DOI] [PubMed] [Google Scholar]
Stead 2008
- Stead LF, Bergson G, Lancaster T. Physician advice for smoking cessation. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD000165.pub3] [DOI] [PubMed] [Google Scholar]
Stead 2009
- Stead M, Angus K, Holme I, Cohen D, Tait G. Factors influencing European GPs' engagement in smoking cessation: a multi‐country literature review. British Journal General Practice 2009;59(566):682‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorogood 2006
- Thorogood M, Hillsdon M, Summerbell C. Changing behaviour. Clinical Evidence BMJ http://clinicalevidence.bmj.com/ceweb/conditions/cvd/0203/0203_I8.jsp (accessed 10/10/2011) 2006;08:203. [Google Scholar]
Tomar 2000
- Tomar SL, Asma S. Smoking‐attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. Journal of Periodontology 2000;71:743‐51. [DOI] [PubMed] [Google Scholar]
Twardella 2004
- Twardella D, Brenner H. Lack of training as a central barrier to the promotion of smoking cessation: a survey among general practitioners in Germany. European Journal Public Health 2004;15(2):140‐5. [DOI] [PubMed] [Google Scholar]
Warnakulasuriya 2002
- Warnakulasuriya S. Effectiveness of tobacco counseling in the dental office. Journal of Dental Education 2002;66:1079‐87. [PubMed] [Google Scholar]
WHO 2005
- World Health Organization. The role of health professionals in tobacco control. http://www.who.int/mediacentre/news/releases/2005/pr22/en/index.html (accessed 10/10/2011) 2005.
WHO 2008
- World Health Organization. WHO report on the global tobacco epidemic. The MPOWER package. http://www.who.int/tobacco/mpower/mpower_report_full_2008.pdf (accessed 08/10/2011) 2008.
Zwar 2009
- Zwar NA, Richmond RL, Davidson D, HasanI. Postgraduate education for doctors in smoking cessation. Drug and Alcohol Review 2009;28:466‐73. [DOI: 10.1111/j.1465-33662.2009.00103.x] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lancaster 2000
- Lancaster T, Fowler G. Training health professionals in smoking cessation. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD000214] [DOI] [PubMed] [Google Scholar]
Lancaster 2008
- Lancaster T, Silagy C, Fowler G. Training health professionals in smoking cessation. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD000214] [DOI] [PubMed] [Google Scholar]
Silagy 1994
- Silagy C, Lancaster T, Gray S, Fowler G. Effectiveness of training health professionals to provide smoking cessation interventions: systematic review of randomised controlled trials. Quality in Health Care 1994;3:193‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]


