Abstract
Strongyloides stercoralis is a soil-transmitted helminth that is mainly found in the tropical and subtropical regions and affects approximately 600 million people globally. The medical importance of strongyloidiasis lies in its capacity to remain asymptomatic and chronically unnoticed until the host is immunocompromised. Additionally, in severe strongyloidiasis, hyperinfection syndrome and larva dissemination to various organs can occur. Parasitological techniques such as Baermann-Moraes and agar plate culture to detect larvae in stool samples are the current gold standard. However, the sensitivity might be inadequate, especially with reduced worm burden. Complementing parasitological techniques, immunological techniques including immunoblot and immunosorbent assays are employed, with higher sensitivity. However, cross-reactivity to other parasites may occur, hampering the assay’s specificity. Recently, advances in molecular techniques such as polymerase chain reaction and next-generation sequencing technology have provided the opportunity to detect parasite DNA in stool, blood, and environmental samples. Molecular techniques, known for their high sensitivity and specificity, have the potential to circumvent some of the challenges associated with chronicity and intermittent larval output for increased detection. Here, as S. stercoralis was recently included by the World Health Organization as another soil-transmitted helminth targeted for control from 2021 to 2030, we aimed to present a review of the current molecular techniques for detecting and diagnosing S. stercoralis in a bid to consolidate the molecular studies that have been performed. Upcoming molecular trends, especially next-generation sequencing technologies, are also discussed to increase the awareness of its potential for diagnosis and detection. Improved and novel detection methods can aid in making accurate and informed choices, especially in this era where infectious and non-infectious diseases are increasingly commonplace.
Graphical Abstract
Keywords: Molecular detection, Molecular diagnosis, Soil-transmitted helminth, Strongyloides stercoralis, Strongyloidiasis
Background
Strongyloides stercoralis, a soil-transmitted helminth (STH), is responsible for human strongyloidiasis, which is estimated to affect approximately 600 million people globally [1–3]. Strongyloidiasis is endemic in tropical and subtropical regions, and foci of infections have also been found in temperate countries, including Japan, Australia, and Italy [4]. Strongyloides stercoralis infection in humans ranges from asymptomatic light infections to chronic symptomatic infections. Severe strongyloidiasis can occur as hyperinfection syndrome (increased parasite burden resulting in high parasite load) and/or disseminated strongyloidiasis (presence of larva in other ograns aside from the gastrointestinal tract). Like a silent assassin, S. stercoralis infection can remain asymptomatic and chronically unnoticed until the host is immunocompromised [5, 6]. Hyperinfection is potentially life-threatening, with mortality rates of up to 85% in immunocompromised patients [7, 8]. Moreover, the unique ability of S. stercoralis to replicate itself in the human host allows for cycles of autoinfection, where the larva attains infectivity without leaving the host [9].
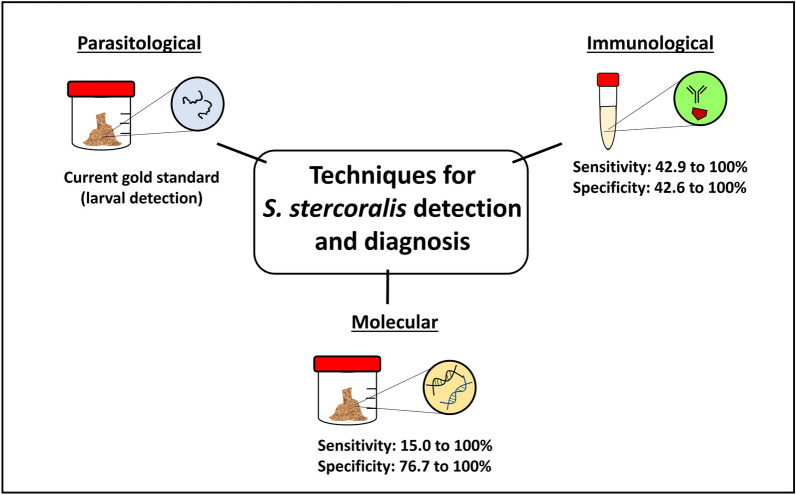
Currently, there is consensus regarding the underestimation of the actual prevalence rate of S. stercoralis, partly due to asymptomatic infections and inadequately sensitive methods for detection and diagnosis [3]. In contrast to other STHs where the gold standard of diagnosis is the presence of eggs in microscopic stool examination, S. stercoralis larvae are usually released in stool samples instead. Moreover, in asymptomatic infections where the larval output is low and intermittent, the sensitivity of stool examination may be compromised [10]. Other methods for S. stercoralis detection include immunological and molecular methods, which have been dubbed a more sensitive alternative to complement diagnosis. The current molecular methods include conventional polymerase chain reaction (PCR) and quantitative PCR (qPCR), which are widely used for the molecular detection and identification of parasitic helminths [10–12]. However, the effectiveness of PCR as a diagnostic tool for S. stercoralis diagnosis and detection remains subjective because of the differing sensitivities reported.
Recently, the World Health Organization (WHO) included S. stercoralis with the other STHs targeted for control from 2021 to 2030 [11]. Incorporating S. stercoralis into a WHO control program includes gaining knowledge of the epidemiology of S. stercoralis, conducting field evaluations and pilot projects, and finding a suitable standard diagnostic tool for detection and diagnosis [11]. Due to the inclusion of S. stercoralis as a target for control, the importance of a sensitive and accurate technique for molecular diagnosis is crucial.
In this study, to consolidate the molecular studies that have been conducted and to assist stakeholders in the WHO’s direction, we present an up-to-date review of the current molecular techniques used for detection and diagnosis of S. stercoralis. Additionally, upcoming molecular trends, especially next-generation sequencing technologies, are discussed in this context to increase awareness of their potential for diagnosis and detection.
Techniques for Strongyloides stercoralis detection
Parasitological techniques
Currently, parasitological techniques are the gold standard for detecting S. stercoralis larvae in fecal samples under microscopes [13]. Compared to other STHs, where eggs can be detected in fecal samples, S. stercoralis eggs are not usually found; thus, parasitological techniques like the simple smear or Kato-Katz are not suitable. More appropriate parasitological methods for larval detection include the Baermann-Mores and agar plate culture (APC) [14–17]. The sensitivity of the technique is crucial to make a correct diagnosis, as the failure to detect S. stercoralis does not indicate the unequivocal absence of infection [9]. Also, multiple fecal examinations have been proven to be more sensitive than a single examination [9, 18]. Knopp et al. (2008) revealed an increase in sensitivity from 6.3% (for single examination) to 10.8% (for multiple examinations) for S. stercoralis detection in a combination of Baermann-Moraes and APC [18]. Modifications in APC have also aided in improving the sensitivity and reducing bacterial contamination [19]. However, these methods are time-consuming and require trained parasitologists for detection and identification. Also, in cases where there is light infection and the larval output is intermittent and low, the sensitivity of parasitological techniques can be compromised.
Despite the low sensitivity, parasitological techniques remain the go-to method for S. stercoralis detection and diagnosis. They are commonly used as a benchmark to compare the efficacy of immunological and molecular techniques [20, 21]. Although there is a shift towards adopting combinations of various parasitological methods and immunological or molecular techniques, its specificity, low cost, and no requirement for special equipment allow for the ease of use, especially in field settings.
Immunological techniques
Immunological techniques, such as enzyme-linked immunosorbent assay (ELISA), immunofluorescence antibody test (IFAT), and western blot, have been used as alternatives for S. stercoralis diagnosis and present certain advantages over parasitological methods [22]. Various studies have shown their high sensitivity, depending on the type of test employed [9, 22, 23]. Table 1 summarizes the sensitivity and specificity of the different immunological tests for the diagnosis of human strongyloidiasis. Among the 32 studies, the sensitivity ranged from 42.9% to 100%, while the specificity ranged from 42.6% to 100%.
Table 1.
Summary of the studies on the sensitivity and specificity of immunological methods for strongyloidiasis
| Immunological methoda | Population sample | Sensitivity (%) | Specificity (%) | Reference method | References |
|---|---|---|---|---|---|
| ELISA IgG IVD commercial kit | Serum from pregnant women in the Peruvian Amazon | 63.3 | 69.6 | Parasitological | [58] |
| Serum from Center for Tropical Diseases in Italy and National Institute of Health in the USA | 91.2 | 99.1 | Parasitological | [24] | |
| Serum from Universiti Sains Malaysia in Malaysia | 84.6 | 83.6 | Parasitological | [59] | |
| ELISA IgG Bordier commercial kit | Serum from outpatients at Hospital for Tropical Diseases in London | 81 | NA | Parasitological | [60] |
| Serum from patients at Rennes University Hospital in France | 100 | 97 | Parasitological | [29] | |
| Serum from Center for Tropical Diseases in Italy and National Institute of Health in the USA | 89.5 | 98.3 | Parasitological | [24] | |
| ELISA crude antigen SciMedx commercial kit | Serum from patients in the Hospital Universitario 12 de Octubre in Spain | 89.2 to 94.7 | 72.3 to 89.3 | Composite reference and parasitological | [61] |
| ELISA IgG InBios Strongy Detect commercial kit | Serum submitted to laboratories in the USA | 80 | 90 | Parasitological | [62] |
| NIE-ELISA NovaLisa commercial kit | Serum from patients in the Hospital Universitario 12 de Octubre in Spain | 72.3 to 78.9 | 85.1 to 93.6 | Composite reference and parasitological | [61] |
| ELISA IgG Strongy Detect (both Ss-NIE and Ss-IR recombinant antigens) commercial kit | Serum from patients at the National Institute of Allergy and Infectious Diseases in the USA | 98.6 | 98.6 | Parasitological | [31] |
| ELISA IgG4 Strongy Detect (both Ss-NIE and Ss-IR recombinant antigens) commercial kit | Serum from patients at the National Institute of Allergy and Infectious Diseases in the USA | 95.9 | 100 | Parasitological | [31] |
| ELISA IgG (crude Strongyloides ratti antigen) | Urine from communities in northeast Thailand | 83 to 85 | 53 to 56 | Parasitological | [63] |
| Serum from communities in northeast Thailand | 100 | 42.6 | Parasitological | [63] | |
| Serum from communities in northeast Thailand | 84.5 | 100 | Parasitological | [64] | |
| ELISA IgG (crude Strongyloides venezuelensis antigen) | Serum from Hospital das Clinicas da Faculdade in Brazil | 95 | 97.83 | Parasitological | [65] |
| Serum from Hospital das Clinicas da Faculdade in Brazil | 92.5 | 93.48 | Parasitological | [65] | |
| Serum from Instituto de Medicina Tropical Alexander von Humboldt in Peru | 74.1 | 100 | Parasitological | [66] | |
| ELISA IgG (crude Strongyloides stercoralis antigen) | Serum from communities in northeast Thailand | 73 | 86 | Parasitological | [67] |
| Serum from communities in northeast Thailand | 83.5 | 100 | Parasitological | [64] | |
| Serum from patients with hematologic malignancy at the University Hospital in Brazil | 68.0 | 89.0 | Parasitological | [68] | |
| Serum from Universiti Sains Malaysia in Malaysia | 84.6 | 81.8 | Parasitological | [59] | |
| Serum from patients with corticosteroid therapy in primary health care centers in Egypt | 42.1 | 82.6 | Parasitological | [69] | |
| Serum from Instituto de Investigacionces de Enfermedades Tropicales in Argentina | 97 | 100 | Parasitological | [70] | |
| Serum from immunocompromised patients in Phramongkutlao Hospital in Thailand | 42.9 | 96.3 | Parasitological | [27] | |
| Centers for Disease Control and prevention EIA IgG (crude Strongyloides stercoralis antigen) | Serum from patients at Toronto General Hospital in Canada | 94.6 | NA | Parasitological | [71] |
| ELISA IgG | Serum from travelers attending the Hospital for Tropical Diseases in London | 73 | NA | Parasitological | [72] |
| ELISA IgG | Serum from immigrants attending the Hospital for Tropical Diseases in London | 98 | NA | Parasitological | [72] |
| ELISA IgG4 (crude Strongyloides stercoralis antigen) | Serum from Universiti Sains Malaysia in Malaysia | 76.9 | 92.7 | Parasitological | [59] |
| ELISA IgE (crude Strongyloides stercoralis antigen) | Serum from Universiti Sains Malaysia in Malaysia | 100 | 100 | Parasitological, molecular, immunological | [73] |
| Serum from Universiti Sains Malaysia in Malaysia | 7.7 | 100 | Parasitological | [59] | |
| ELISA IgY (crude Strongyloides venezuelensis antigen from larva) | Serum from Biological Samples Bank of Laboratório de Parasitologia in Brazil | 95.56 | 88.89 | Parasitological | [74] |
| ELISA IgY (crude Strongyloides venezuelensis antigen from adult females) | Serum from Biological Samples Bank of Laboratório de Parasitologia in Brazil | 95.56 | 91.11 | Parasitological | [74] |
| ELISA IgG (synthetic peptide C10) | Serum from patients | 95 | 89.2 | Parasitological | [75] |
| ELISA IgG (synthetic peptide D3) | Serum from patients | 95 | 92.5 | Parasitological | [75] |
| ELISA IgG4 (Strongyloides stercoralis rSs1a recombinant antigen) | Serum from Universiti Sains Malaysia in Malaysia | 96 | 93 | Parasitological, molecular, immunological | [76] |
| ELISA IgG (SsAg recombinant monoclonal antibody) | Serum bank at University Sains Malaysia in Malaysia | 100 | 100 | Parasitological and immunological | [77] |
| ELISA (Strongyloides stercoralis recombinant 14-3-3 protein) | Serum from patients | 96 | NA | Parasitology | [78] |
| NIE-ELISA | Serum from Center for Tropical Diseases in Italy and National Institute of Health in the USA | 75.4 | 94.8 | Parasitological | [24] |
| Serum from Instituto de Investigacionces de Enfermedades Tropicales in Argentina | 84 | 100 | Parasitological | [70] | |
| Serum from communities in Argentina | 76.7 | 71.6 | Bayesian latent class analysis estimates | [79] | |
| Dried blood spots from indigenous community in Australia | 85.7 | 88.9 | Parasitological | [80] | |
| NIE-LIPS | Serum submitted to laboratories in the USA | 100 | 100 | Parasitological | [62] |
| Serum from Center for Tropical Diseases in Italy and National Institute of Health in the USA | 85.1 | 100 | Parasitological | [24] | |
| Serum from Instituto de Investigacionces de Enfermedades Tropicales in Argentina | 97.8 | 100 | Parasitological | [70] | |
| NIE dot-based assay | Serum from multiple reference laboratories | 96.3 | 100 | Parasitological | [81] |
| SsIR-LIPS | Serum from Instituto de Investigacionces de Enfermedades Tropicales in Argentina | 91.2 | 100 | Parasitological | [70] |
| IFAT (Strongyloides stercoralis larva) | Serum from patients at the Centre for Tropical Diseases in Italy | 95.5 | NA | Composite reference | [16] |
| Serum from Center for Tropical Diseases in Italy and National Institute of Health in the USA | 93.9 | 92.2 | Parasitological | [24] | |
| Gelatin particle indirect agglutination assay (crude Strongyloides stercoralis antigen) | Serum from communities in northeast Thailand | 81 | 81 | Parasitological | [67] |
| Serum from patients with corticosteroid therapy in primary health care centers in Egypt | 89.4 | 81.8 | Parasitological | [69] | |
| Gelatin particle indirect agglutination (crude Strongyloides venezuelensis antigen) | Serum from Instituto de Medicina Tropical Alexander von Humboldt in Peru | 98.2 | 100 | Parasitological | [66] |
| ICT (crude Strongyloides stercoralis antigen) | Serum from Khon Kaen University in Thailand | 93.3 | 83.7 | Parasitological | [32] |
| Lateral flow rapid dipstick test IgG4 (SsRapid™) | Serum from northeast Thailand | 82 | 96 | Parasitological, immunological | [82] |
| Serum from Universiti Sains Malaysia in Malaysia | 91.3 | 100 | Parasitological, molecular, immunological | [33] |
aEIA enzyme immunosorbent assay, ELISA enzyme-linked immunosorbent assay, ICT immunochromatographic test, IFAT immunofluorescene antibody test, LIPS luciferase immunoprecipitation systems assay
The sensitivity of five immunological tests (consisting of in-house assays and commercially available ELISA tests) was compared by Bisoffi et al. (2014), and their results revealed that the sensitivity among the tests ranged from 75.4% to 93.9%, with the IFAT test being the most sensitive [24]. However, studies have also revealed cross-reactivity with other helminthic infections, such as filariasis and schistosomiasis, when crude antigens are used [5, 22, 25]. Also, immunological tests cannot distinguish between current and past infections of S. stercoralis, which can be a limiting factor in areas where strongyloidiasis is endemic [23, 26]. Moreover, the sensitivity of immunodiagnostics can be reduced in cases where the host is severely immunosuppressed. In a study performed on immunocompromised patients in Thailand, the sensitivity was reported to be 42.9% using IgG indirect ELISA [27]. Currently, newer and more convenient immunodiagnostic tests are being developed to increase the specificity and reduce the time taken for results. These include the development of a commercial ELISA and a luciferase immunoprecipitation system using recombinant antigens (LIPS-NIE) that have no cross-reactivity with other STHs [24, 28–30]. Recently, a commercial ELISA kit (Strongy Detect, Inbios) with both recombinant antigens Ss-NIE and Ss-IR showed high sensitivity and specificity for IgG and IgG4 [31]. In addition, rapid tests like point-of-care cassettes and dipstick tests have been developed to rapidly detect strongyloidiasis [32, 33]. In recent years, a combination of parasitological and immunological techniques has been used for diagnosis and has proven to be more robust than parasitological techniques alone [10]. Although immunological techniques, with their high sensitivity, present a suitable complement to parasitological techniques, their low specificity and sensitivity, especially in immunocompromised hosts, remain a current limitation.
Molecular techniques
Molecular techniques have been touted as a promising tool for S. stercoralis diagnosis and identification, with their potential for increased sensitivity and specificity [12, 20]. Table 2 summarizes the molecular-based studies conducted with their sensitivity and specificity values for S. stercoralis detection. Of the 24 studies reviewed, the sensitivity ranged from 15 to 100%, while specificity ranged from 76.7% to 100%, with different studies utilizing parasitological or immunological techniques, or both as references. The majority of studies conducted used fecal samples, while three studies used urine samples for the detection of S. stercoralis DNA. The most common genetic marker used was the nuclear 18S ribosomal RNA (rRNA) gene, with 16 out of 24 (66%) studies using the 18S primers and assay developed by Verweij et al. (2009) [34].
Table 2.
Summary of studies on the sensitivity and specificity of molecular techniques for Strongyloides stercoralis detection and diagnosis
| Genetic marker | Primer used | Type of PCRa | Sample type | Sensitivity (%) | Specificity (%) | Reference method | References |
|---|---|---|---|---|---|---|---|
| 18S | [34] | RT-PCR | Fecal | 72 to 92 | 100 | Parasitological | [34]b |
| 84.7 | 95.8 | Parasitological | [83] | ||||
| 76.8 | 89.7 | Parasitological | [84] | ||||
| 15 to 34.1 | > 99 | Parasitological | [87] | ||||
| 93.8 | 86.5 | Parasitological | [35] | ||||
| 90 | 85.7 | Parasitological | [36] | ||||
| 27.5 to 86.3 | NA | Parasitological | [45] | ||||
| 73.9 | 100 | Parasitological | [39] | ||||
| 85 | 87.3 | Parasitological | [25] | ||||
| 38 | 100 | Immunological | [85] | ||||
| 63 | NA | Immunological | [20] | ||||
| 57 | NA | Parasitological and immunological (composite reference) | [16] | ||||
| Multiplex RT-PCR | Fecal | 17.4 to 76.3 | 93.9 | Parasitological and molecular combination | [15] | ||
| 88.9 | 92.7 | Parasitological | [86] | ||||
| cPCR | Fecal | 100 | NA | Parasitological | [21] | ||
| 76.7 | 84.3 | Parasitological | [25] | ||||
| 78.8 to 84.8 | 82.5 to 95 | Parasitological | [87] | ||||
| 100 | NA | Parasitological | [88] | ||||
| [89] | cPCR | Fecal | 100 | NA | Parasitological | [89]b | |
| Nested PCR | Fecal | 75 | NA | Parasitological | [89]b | ||
| [37] | Multiplex RT-PCR | Fecal | 72 to 100 | 100 | Parasitological | [37]b | |
| [38] | RT-PCR | Fecal | 82 | 76.7 | Parasitological | [38]b | |
| ddPCR | Fecal | 98 | 90 | Parasitological | [38]b | ||
| COI | [90] | Nested PCR | Fecal | 100 | 91.6 | Parasitological | [90]b |
| ITS2 | [89] | cPCR | Fecal | 61 | NA | Parasitological | [91] |
| [92] | cPCR | Fecal | 100 | NA | Parasitological | [92]b | |
| Repetitive elements | [46] | cPCR | Urine | 93.6 | NA | Parasitological | [46]b |
| 17 | NA | Immunological | [20] | ||||
| 74.7 | 77.1 | Bayesian estimates | [79] |
aRT-PCR real-time PCR, cPCR conventional PCR, ddPCR droplet digital PCR
bThe reference indicates that the primers were originally developed in that particular study
The assay by Verweij et al. (2009) [34] targets the nuclear 18S rRNA gene using a real-time PCR (RT-PCR) assay for the detection of S. stercoralis in fecal samples [34]. Since its development, the assay and primers have been widely adopted by the scientific community, for both conventional and RT-PCR [21, 35, 36]. Also, multiplex PCR has been developed to simultaneously detect other STHs along with S. stercoralis, enhancing the utility of molecular techniques for diagnostics and detection [37]. Aside from the 18S rRNA gene primers by Verweij et al. (2009), other primers targeting the 18S rRNA gene and different PCR techniques have been employed. Of note, Iamrod et al. (2021) [38] developed and tested a droplet digital PCR (ddPCR) assay for S. stercoralis detection in fecal samples [38]. The study revealed higher sensitivity and specificity using ddPCR compared to RT-PCR and parasitological techniques. Although other genetic markers like the mitochondrial cytochrome c oxidase subunit I (COI) gene, internal transcribed spacer 2 (ITS2) region, and repetitive units have been used, the 18S rRNA gene remains a popular choice for S. stercoralis detection.
Although the sensitivity range of molecular techniques varies greatly (from 15 to 100%), molecular techniques are still highly valuable as a diagnostic tool, as only five studies reported a sensitivity of < 50%. In a systematic meta-analysis of molecular diagnostic accuracy for S. stercoralis, the accuracy was estimated to be 71.76% using parasitological techniques as the reference and 61.85% using either parasitological or immunological techniques [12]. The advantages of utilizing molecular techniques to diagnose S. stercoralis outweigh their limitations. First, molecular detection outperforms parasitological techniques such as spontaneous sedimentation in terms of sensitivity, and studies have revealed that the sensitivity and accuracy of diagnosis increase when a combination of techniques is applied in conjunction. Hailu et al. [39] tested five diagnostic methods (RT-PCR and four other parasitological methods) for S. stercoralis and revealed a higher detection rate when a combination of parasitological and molecular techniques was used as compared to a single diagnostic method [39]. The advantages and limitations of each of the three techniques for S. stercoralis detection are summarized in Table 3. Using a combination of techniques, the positivity rate increased from 10.9% (APC) or 28.8% (RT-PCR) to 36% when both APC and RT-PCR were employed. Second, DNA from dead larvae can be detected via PCR, while the larvae have to be alive for detection via APC or Baermann. Third, the simultaneous detection of other helminths and species identification can also be performed via molecular techniques, enhancing the efficiency. Finally, in terms of specificity, molecular techniques have the edge over immunological techniques. Although the sensitivity of molecular techniques is hindered by similar factors as parasitological techniques, such as low and intermittent larval output, these limitations can hopefully be overcome in the near future through the use of novel molecular methods with their increased sensitivity for detection.
Table 3.
Advantages and limitations of each technique for Strongyloides stercoralis detection
| Techniques | Advantage | Limitation |
|---|---|---|
| Parasitological |
•Lower cost compared to immunological and molecular techniques •Easily implementable in a field setting |
•Require increased sampling for higher sensitivity due to irregular larva output or asymptomatic patients •Possible misdiagnosis with hookworms due to similar morphology •Require live larva •Risk of S. stercoralis contamination when APC is used |
| Immunological |
•Higher sensitivity than parasitological and molecular techniques •Not limited by the larval output •Able to detect other pathogens through multiplex assays •Possible to detect other biological materials such as breast milk and saliva |
•Potential for cross-reactivity with other helminthiases •Persistence of antibodies renders the technique unable to distinguish between past and present infections (especially in endemic areas) •Lowered sensitivity for immunocompromised host |
| Molecular |
•Higher sensitivity than parasitological techniques (direct examination, spontaneous sedimentation, or Kato-katz) •Higher specificity than serological techniques •Lower expertise is required than parasitological techniques •Ability to detect dead larva •Increased accuracy with molecular identification •Able to detect other pathogens through multiplex assays (Multiplex PCR) •Possible to detect from other environments, not only from stool, and urine samples |
•Lack of standard for PCR and DNA extraction, causing varied sensitivity, and specificity •Require increased sampling for higher sensitivity due to irregular larva output or asymptomatic patients |
Current molecular trends and novel tools for Strongyloides stercoralis detection
Aside from diagnosis and detection, molecular techniques also allow the study of S. stercoralis molecular identification, phylogenetics, and genetic diversity. Other types of molecular-based studies performed for S. stercoralis are summarized in Table 4. These consist of cross-sectional, molecular identification, phylogenetics, genetic diversity, and molecular technique modification and improvement studies. Aside from fecal and urine samples, most studies have performed larval isolation of S. stercoralis prior to individual worm DNA extraction. Other types of sample include serum, cerebrospinal fluid (CSF), and bronchoalveolar lavage fluid to detect the presence of S. stercoralis DNA. The various types of genetic markers used include the nuclear 18S and 28S rRNA genes, ITS1 region, major sperm protein (MSP) gene, the mitochondrial COI, 12S and 16S rRNA genes, and repetitive elements. Although these genetic markers can be used for molecular identification and phylogenetic studies, the 18S rRNA and COI genes are highly popular. For the 18S rRNA gene, Hasegawa et al. (2009) [40] suggested the use of the hypervariable regions (named HVR-I, II, III, IV) to explore genetic differences between S. stercoralis populations [40]. With its high sequence variation, the mitochondrial COI gene is another genetic marker used to study the population genetics and diversity of S. stercoralis in different hosts and localities [41–43]. These genetic markers have proven helpful for the molecular identification of cryptic species and in aiding to shed light on the zoonotic potential of S. stercoralis through comparative molecular studies on dog and human isolates [42].
Table 4.
Summary of molecular studies for Strongyloides stercoralis
| Genetic marker | Type of PCRa | Sample typeb | Type of study | References |
|---|---|---|---|---|
| 18S | cPCR | Fecal | •Cross sectional | [93] |
| •Molecular technique | [21] | |||
| •Cross sectional | [94] | |||
| •Prospective | [95] | |||
| •Cross sectional | [96] | |||
| Larva | •Case report | [97] | ||
| •Cross sectional | [98] | |||
| •Case report | [99] | |||
| •Molecular technique | [40] | |||
| •Cross sectional and phylogenetics | [100] | |||
| •Cross sectional and phylogenetics | [101] | |||
| •Cross sectional, phylogenetics, and genetic diversity | [42] | |||
| •Cross sectional, phylogenetics, and genetic diversity | [102] | |||
| •Phylogenetics | [103] | |||
| •Cross sectional and genetic diversity | [104] | |||
| •Case report | [105] | |||
| •Cross sectional and phylogenetics | [106] | |||
| •Cross sectional and phylogenetics | [107] | |||
| Serum | •Cross sectional | [47] | ||
| Multiplex cPCR | Larva | •Molecular technique | [108] | |
| RT-PCR | Fecal | •Cross sectional | [109] | |
| •Cross sectional | [110] | |||
| •Cross sectional | [111] | |||
| •Cross sectional | [112] | |||
| •Cross sectional | [113] | |||
| Multiplex RT-PCR | Larva | •Molecular technique | [114] | |
| LAMP | Urine | •Molecular technique | [52] | |
| cPCR, Illumina | Fecal | •Cross sectional, phylogenetics, molecular technique | [115] | |
| cPCR, RT-PCR, Illumina | Fecal | •Cross sectional, phylogenetics, genotyping | [116] | |
| 28S | cPCR | Larva | •Cross sectional and phylogenetics | [101] |
| RT-PCR | Fecal | •Molecular technique | [117] | |
| LAMP | Larva | •Molecular technique | [118] | |
| ITS1 | Nested PCR | Fecal | •Cross sectional | [119] |
| Larva | •Cross sectional | [120] | ||
| Multiplex cPCR | Fecal | •Case report | [121] | |
| Repetitive elements | RT-PCR, Illumina | Larva | •Molecular technique | [122] |
| MSP | cPCR | Larva | •Cross sectional and phylogenetics | [101] |
| 12S | cPCR | Larva | •Phylogenetics | [123] |
| Illumina | Larva | •DNA metabarcoding | [51] | |
| 16S | cPCR | Larva | •Phylogenetics | [123] |
| Illumina | Larva | •DNA metabarcoding | [51] | |
| COI | cPCR | Larva | •Cross sectional and phylogenetics | [100] |
| •Case report | [99] | |||
| •Cross sectional and phylogenetics | [101] | |||
| •Cross sectional, phylogenetics, and genetic diversity | [42] | |||
| •Cross sectional, phylogenetics, and genetic diversity | [102] | |||
| •Phylogenetics | [124] | |||
| •Case report | [105] | |||
| •Cross sectional and phylogenetics | [106] | |||
| Serum | •Cross sectional | [47] | ||
| Nested PCR | Fecal | •Phylogenetics and genetic diversity | [41] | |
| cPCR, Illumina | Fecal | •Cross sectional, phylogenetics, molecular technique | [115] | |
| •Cross sectional, phylogenetics, genotyping | [116] | |||
| Metagenome | Illumina | Cerebrospinal fluid | •Case report | [50] |
| Bronchoalveolar lavage fluid | •Cross sectional | [49] | ||
| •Cross-sectional | [48] | |||
| Whole genome | Illumina | Larva | •Genomics | [125] |
| •Cross sectional and phylogenetics | [106] | |||
| •Phylogenetics and genomics | [126] |
aRT-PCR real-time PCR, cPCR conventional PCR
bThe fecal sample type indicates that molecular detection was performed directly from the fecal sample, while the larva sample type indicates that Strongyloides larvae were first isolated from the fecal sample and molecular identification was performed using the isolated larvae
Researchers have recently attempted to increase the diagnostic sensitivity for S. stercoralis detection. First, parasitological, immunological, and molecular techniques are increasingly employed for screening and confirmatory testing to broaden the net cast and to increase the detection accuracy rather than relying on one approach [16, 44]. Zueter et al. (2014) used fecal and serum samples collected from cancer patients to detect S. stercoralis through these three techniques [44]. Second, improvements have been made in the DNA extraction and PCR protocols for molecular detection via fecal samples. Examples include the removal of PCR inhibitors in fecal samples, enhancing DNA extraction methods, and exploring different sample types, such as urine and other bodily fluids, to determine if they can be used for diagnostics [8, 45, 46]. Cell-free DNA is also being explored, where molecular detection using the 18S rRNA and COI genes has been used to detect S. stercoralis in serum samples [47]. Third, the increasing trend in the use of next-generation sequencing (NGS) technologies for molecular-based studies is slowly gaining traction for helminth diagnostics. Illumina sequencing metagenomics were used to detect S. stercoralis in CSF and bronchoalveolar lavage fluid samples from patients, showing the high sensitivity of the technique and potential for use [48–50]. Additionally, targeted amplicon Illumina sequencing of the 12S and 16S rRNA genes through DNA metabarcoding has also demonstrated the potential of detecting S. stercoralis larvae spiked in mock helminth communities and environment matrices [51]. Although conventional molecular-based methods are still popular, the shift toward NGS is certain in the future. The use of NGS compared to conventional molecular-based methods can be highly advantageous because of their high sensitivity, decreased cost, and increased convenience.
In addition to increasing the sensitivity of S. stercoralis detection, the convenience of molecular detection in the field is another advantage. A loop-mediated isothermal amplification (LAMP) assay was successfully developed by Fernández-Soto et al. (2020) [52] using human urine and fecal samples for S. stercoralis detection [52]. Another interesting concept is the use of portable systems such as the portable Bento Lab, which is fully equipped with DNA extraction, PCR, and sequencing devices suitable for use in the field. Using the Bento Lab and the MinIon sequencer, DNA barcoding of parasitic and free-living nematode species was successfully performed directly in the field setting and was identified with 96 to 100% accuracy [53]. Lastly, as strongyloidiasis can be positively associated with hosts with underlying disease conditions, concurrent screening for strongyloidiasis and other diseases should be undertaken, especially for immunocompromised patients or patients requiring immunosuppressive drugs. Co-infection of strongyloidiasis with COVID-19 has been reported as well as Strongyloides hyperinfection syndrome resulting from treatment with corticosteroids for COVID-19 [54–56]. With infectious diseases being commonplace, there is an increasing need to screen for Strongyloides to prevent potentially fatal scenarios, especially when the use of corticosteroids is evident [57].
Conclusions
The application of molecular techniques is undoubtedly vital to determine the true prevalence and disease burden of S. stercoralis. As each technique (parasitological, immunological, and molecular) has its benefits and drawbacks, none should be used as a stand-alone test for diagnosis. Molecular techniques can play a confirmatory role in diagnosis, with their ability to circumvent both the low sensitivity of parasitological techniques and the low specificity of immunological techniques. With molecular techniques advancing at an extraordinary pace, it is certainly a keystone in strongyloidiasis detection, especially in an era where infectious diseases and zoonoses are increasing in frequency.
Acknowledgements
Not applicable.
Abbreviations
- APC
Agar plate culture
- COI
Cytochrome c oxidase subunit I
- cPCR
Conventional PCR
- CSF
Cerebrospinal fluid
- ddPCR
Droplet digital polymerase chain reaction
- ELISA
Enzyme-linked immunosorbent assay
- IFAT
Immunofluorescence antibody test
- ITS
Internal transcribed spacer
- LAMP
Loop-mediated isothermal amplification
- LIPS-NIE
Luciferase immunoprecipitation system using recombinant antigens
- MSP
Major sperm protein
- NGS
Next generation sequencing
- PCR
Polymerase chain reaction
- qPCR
Qualitative polymerase chain reaction
- rRNA
Ribosomal RNA
- RT-PCR
Real-time polymerase chain reaction
- STH
Soil-transmitted helminth
- WHO
World Health Organization
Author contributions
UT and AC conceived the study, wrote and reviewed the manuscript draft, and approved the final manuscript. Both authors have read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, and not-for-profit sectors.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abigail Hui En Chan, Email: chromatography23@gmail.com.
Urusa Thaenkham, Email: urusa.tha@mahidol.edu.
References
- 1.Bisoffi Z, Buonfrate D, Montresor A, Requena-Méndez A, Muñoz J, Krolewiecki AJ, et al. Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis. 2013;7:5. doi: 10.1371/journal.pntd.0002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, et al. Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis. 2013;7:7. doi: 10.1371/journal.pntd.0002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buonfrate D, Bisanzio D, Giorli G, Odermatt P, Fürst T, Greenaway C, et al. The global prevalence of Strongyloides stercoralis infection. Pathogens. 2020;9:6. doi: 10.3390/pathogens9060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ramanathan R, Nutman T. Strongyloides stercoralis infection in the immunocompromised host. Curr Infect Dis Rep. 2008;10:2. doi: 10.1007/s11908-008-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144:3. doi: 10.1017/S0031182016000834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schär F, Giardina F, Khieu V, Muth S, Vounatsou P, Marti H, et al. Occurrence of and risk factors for Strongyloides stercoralis infection in South-East Asia. Acta Trop. 2016 doi: 10.1016/j.actatropica.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Concha R, Harrington W, Jr, Rogers AI. Intestinal strongyloidiasis: recognition, management, and determinants of outcome. J Clin Gastroenterol. 2005;39:3. doi: 10.1097/01.mcg.0000152779.68900.33. [DOI] [PubMed] [Google Scholar]
- 8.Repetto SA, Alba Soto CD, Cazorla SI, Tayeldin ML, Cuello S, Lasala MB, et al. An improved DNA isolation technique for PCR detection of Strongyloides stercoralis in stool samples. Acta Trop. 2013;126:2. doi: 10.1016/j.actatropica.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Infect Dis. 2001;33:7. doi: 10.1086/322707. [DOI] [PubMed] [Google Scholar]
- 10.Buonfrate D, Formenti F, Perandin F, Bisoffi Z. Novel approaches to the diagnosis of Strongyloides stercoralis infection. Clin Microbiol Infect. 2015;21:6. doi: 10.1016/j.cmi.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Diagnostic methods for the control of strongyloidiasis, Virtual meeting, 29 September 2020 . 2021. https://www.who.int/publications/i/item/9789240016538. Accessed 01 Nov 2022.
- 12.Buonfrate D, Requena-Mendez A, Angheben A, Cinquini M, Cruciani M, Fittipaldo A, et al. Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection-A systematic review and meta-analysis. PLoS Negl Trop Dis. 2018;12:2. doi: 10.1371/journal.pntd.0006229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inês Ede J, Souza JN, Santos RC, Souza ES, Santos FL, Silva ML, et al. Efficacy of parasitological methods for the diagnosis of Strongyloides stercoralis and hookworm in faecal specimens. Acta Trop. 2011;120:3. doi: 10.1016/j.actatropica.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Gelaye W, Williams NA, Kepha S, Junior AM, Fleitas PE, Marti-Soler H, et al. Performance evaluation of Baermann techniques: the quest for developing a microscopy reference standard for the diagnosis of Strongyloides stercoralis. PLoS Negl Trop Dis. 2021;15:2. doi: 10.1371/journal.pntd.0009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knopp S, Salim N, Schindler T, Karagiannis Voules DA, Rothen J, Lweno O, et al. Diagnostic accuracy of Kato-Katz, FLOTAC, Baermann, and PCR methods for the detection of light-intensity hookworm and Strongyloides stercoralis infections in Tanzania. Am J Trop Med Hyg. 2014;90:3. doi: 10.4269/ajtmh.13-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buonfrate D, Perandin F, Formenti F, Bisoffi Z. A retrospective study comparing agar plate culture, indirect immunofluorescence and real-time PCR for the diagnosis of Strongyloides stercoralis infection. Parasitology. 2017;144:6. doi: 10.1017/S0031182016002559. [DOI] [PubMed] [Google Scholar]
- 17.De Carvalho GLX, Moreira LE, Pena JL, Marinho CC, Bahia MT, Machado-Coelho GLL. A comparative study of the TF-Test®, Kato-Katz, Hoffman-Pons-Janer, Willis and Baermann-Moraes coprologic methods for the detection of human parasitosis. Memórias do Instituto Oswaldo Cruz. 2012 doi: 10.1590/s0074-02762012000100011. [DOI] [PubMed] [Google Scholar]
- 18.Knopp S, Mgeni AF, Khamis IS, Steinmann P, Stothard JR, Rollinson D, et al. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis. 2008;2:11. doi: 10.1371/journal.pntd.0000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pocaterra LA, Ferrara G, Peñaranda R, Rojas E, Pérez-Chacón G, Hernán A, et al. Improved detection of Strongyloides stercoralis in modified agar plate cultures. Am J Trop Med Hyg. 2017;96:4. doi: 10.4269/ajtmh.16-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Formenti F, La Marca G, Perandin F, Pajola B, Romano M, Santucci B, et al. A diagnostic study comparing conventional and real-time PCR for Strongyloides stercoralis on urine and on faecal samples. Acta Trop. 2019 doi: 10.1016/j.actatropica.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Repetto SA, Ruybal P, Solana ME, López C, Berini CA, Alba Soto CD, et al. Comparison between PCR and larvae visualization methods for diagnosis of Strongyloides stercoralis out of endemic area: a proposed algorithm. Acta Trop. 2016 doi: 10.1016/j.actatropica.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Costa IN, Bosqui LR, Corral MA, Costa-Cruz JM, Gryschek RCB, de Paula FM. Diagnosis of human strongyloidiasis: application in clinical practice. Acta Trop. 2021 doi: 10.1016/j.actatropica.2021.106081. [DOI] [PubMed] [Google Scholar]
- 23.Montes M, Sawhney C, Barros N. Strongyloides stercoralis: there but not seen. Curr Opin Infect Dis. 2010;23:5. doi: 10.1097/QCO.0b013e32833df718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bisoffi Z, Buonfrate D, Sequi M, Mejia R, Cimino RO, Krolewiecki AJ, et al. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis. 2014;8:1. doi: 10.1371/journal.pntd.0002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paula FM, Malta FM, Corral MA, Marques PD, Gottardi M, Meisel DM, et al. Diagnosis of Strongyloides stercoralis infection in immunocompromised patients by serological and molecular methods. Rev Inst Med Trop Sao Paulo. 2016 doi: 10.1590/S1678-9946201658063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puthiyakunnon S, Boddu S, Li Y, Zhou X, Wang C, Li J, et al. Strongyloidiasis–an insight into its global prevalence and management. PLoS Negl Trop Dis. 2014;8:8. doi: 10.1371/journal.pntd.0003018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luvira V, Trakulhun K, Mungthin M, Naaglor T, Chantawat N, Pakdee W, et al. Comparative diagnosis of strongyloidiasis in immunocompromised patients. Am J Trop Med Hyg. 2016;95:2. doi: 10.4269/ajtmh.16-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamarozzi F, Longoni SS, Mazzi C, Pettene S, Montresor A, Mahanty S, et al. Diagnostic accuracy of a novel enzyme-linked immunoassay for the detection of IgG and IgG4 against Strongyloides stercoralis based on the recombinant antigens NIE/SsIR. Parasit Vectors. 2021;14:1. doi: 10.1186/s13071-021-04916-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Autier B, Boukthir S, Degeilh B, Belaz S, Dupuis A, Chevrier S, et al. Clinical value of serology for the diagnosis of strongyloidiasis in travelers and migrants: a 4-year retrospective study using the Bordier IVD(®) Strongyloides ratti ELISA assay. Parasite. 2021 doi: 10.1051/parasite/2021075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramanathan R, Burbelo PD, Groot S, Iadarola MJ, Neva FA, Nutman TB. A luciferase immunoprecipitation systems assay enhances the sensitivity and specificity of diagnosis of Strongyloides stercoralis infection. J Infect Dis. 2008;198:3. doi: 10.1086/589718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sears WJ, Nutman TB. Strongy detect: preliminary validation of a prototype recombinant Ss-NIE/Ss-IR based ELISA to detect Strongyloides stercoralis infection. PLoS Negl Trop Dis. 2022;16:1. doi: 10.1371/journal.pntd.0010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sadaow L, Sanpool O, Rodpai R, Boonroumkaew P, Maleewong W, Intapan PM. Development of immunochromatographic device as a point-of-care tool for serodiagnosis of human strongyloidiasis cases. Eur J Clin Microbiol Infect Dis. 2020;39:3. doi: 10.1007/s10096-019-03745-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yunus MH, Arifin N, Balachandra D, Anuar NS, Noordin R. Lateral flow dipstick test for serodiagnosis of strongyloidiasis. Am J Trop Med Hyg. 2019;101:2. doi: 10.4269/ajtmh.19-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verweij JJ, Canales M, Polman K, Ziem J, Brienen EA, Polderman AM, et al. Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans R Soc Trop Med Hyg. 2009;103:4. doi: 10.1016/j.trstmh.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Saugar JM, Merino FJ, Martín-Rabadán P, Fernández-Soto P, Ortega S, Gárate T, et al. Application of real-time PCR for the detection of Strongyloides spp. in clinical samples in a reference center in Spain. Acta Trop. 2015 doi: 10.1016/j.actatropica.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Paula FM, Malta Fde M, Marques PD, Sitta RB, Pinho JR, Gryschek RC, et al. Molecular diagnosis of strongyloidiasis in tropical areas: a comparison of conventional and real-time polymerase chain reaction with parasitological methods. Mem Inst Oswaldo Cruz. 2015;110:2. doi: 10.1590/0074-02760140371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janwan P, Intapan PM, Thanchomnang T, Lulitanond V, Anamnart W, Maleewong W. Rapid detection of Opisthorchis viverrini and Strongyloides stercoralis in human fecal samples using a duplex real-time PCR and melting curve analysis. Parasitol Res. 2011;109:6. doi: 10.1007/s00436-011-2419-z. [DOI] [PubMed] [Google Scholar]
- 38.Iamrod K, Chaidee A, Rucksaken R, Kopolrat KY, Worasith C, Wongphutorn P, et al. Development and efficacy of droplet digital PCR for detection of Strongyloides stercoralis in stool. Am J Trop Med Hyg. 2021;106:1. doi: 10.4269/ajtmh.21-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hailu T, Amor A, Nibret E, Munshea A, Anegagrie M, Flores-Chavez MD, et al. Evaluation of five diagnostic methods for Strongyloides stercoralis infection in Amhara National Regional State, northwest Ethiopia. BMC Infect Dis. 2022;22:1. doi: 10.1186/s12879-022-07299-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasegawa H, Hayashida S, Ikeda Y, Sato H. Hyper-variable regions in 18S rDNA of Strongyloides spp as markers for species-specific diagnosis. Parasitol Res. 2009;104:4. doi: 10.1007/s00436-008-1269-9. [DOI] [PubMed] [Google Scholar]
- 41.Repetto SA, Braghini JQ, Risso MG, Argüello LB, Batalla EI, Stecher DR, et al. Molecular typing of Strongyloides stercoralis in Latin America, the clinical connection. Parasitology. 2022;149:1. doi: 10.1017/S0031182021001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanpool O, Intapan PM, Rodpai R, Laoraksawong P, Sadaow L, Tourtip S, et al. Dogs are reservoir hosts for possible transmission of human strongyloidiasis in Thailand: molecular identification and genetic diversity of causative parasite species. J Helminthol. 2019 doi: 10.1017/S0022149X1900107X. [DOI] [PubMed] [Google Scholar]
- 43.Aupalee K, Wijit A, Singphai K, Rödelsperger C, Zhou S, Saeung A, et al. Genomic studies on Strongyloides stercoralis in northern and western Thailand. Parasit Vectors. 2020;13:1. doi: 10.1186/s13071-020-04115-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zueter AM, Mohamed Z, Abdullah AD, Mohamad N, Arifin N, Othman N, et al. Detection of Strongyloides stercoralis infection among cancer patients in a major hospital in Kelantan. Malaysia Singapore Med J. 2014;55:7. doi: 10.11622/smedj.2014088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barda B, Wampfler R, Sayasone S, Phongluxa K, Xayavong S, Keoduangsy K, et al. Evaluation of two DNA extraction methods for detection of Strongyloides stercoralis infection. J Clin Microbiol. 2018;56:4. doi: 10.1128/JCM.01941-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lodh N, Caro R, Sofer S, Scott A, Krolewiecki A, Shiff C. Diagnosis of Strongyloides stercoralis: detection of parasite-derived DNA in urine. Acta Trop. 2016 doi: 10.1016/j.actatropica.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorgani-Firouzjaee T, Kalantari N, Javanian M, Ghaffari S. Strongyloides stercoralis: detection of parasite-derived DNA in serum samples obtained from immunosuppressed patients. Parasitol Res. 2018;117:9. doi: 10.1007/s00436-018-5985-5. [DOI] [PubMed] [Google Scholar]
- 48.Lian QY, Chen A, Zhang JH, Guan WJ, Xu X, Wei B, et al. High-throughput next-generation sequencing for identifying pathogens during early-stage post-lung transplantation. BMC Pulm Med. 2021;21:1. doi: 10.1186/s12890-021-01723-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ju CR, Lian QY, Guan WJ, Chen A, Zhang JH, Xu X, et al. Metagenomic next-generation sequencing for diagnosing infections in lung transplant recipients: a retrospective study. Transpl Int. 2022 doi: 10.3389/ti.2022.10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qu J, Zong Z. Strongyloidiasis in a patient diagnosed by metagenomic next-generation sequencing: a case report. Front Med. 2022 doi: 10.3389/fmed.2022.835252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan AHE, Saralamba N, Saralamba S, Ruangsittichai J, Chaisiri K, Limpanont Y, et al. Sensitive and accurate DNA metabarcoding of parasitic helminth mock communities using the mitochondrial rRNA genes. Sci Rep. 2022;12:1. doi: 10.1038/s41598-022-14176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fernández-Soto P, Celis-Giraldo CT, Collar-Fernández C, Gorgojo Ó, Camargo M, Muñoz J, et al. Strong-LAMP assay based on a Strongyloides spp.-derived partial sequence in the 18S rRNA as potential biomarker for Strongyloidiasis diagnosis in human urine samples. Dis Markers. 2020 doi: 10.1155/2020/5265198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knot IE, Zouganelis GD, Weedall GD, Wich SA, Rae R. DNA barcoding of nematodes using the MinION. Front Ecol Evol. 2020;8:100. doi: 10.3389/fevo.2020.00100. [DOI] [Google Scholar]
- 54.Kim JM, Sivasubramanian G. Strongyloides hyperinfection syndrome among COVID-19 patients treated with corticosteroids. Emerg Infect Dis. 2022;28:7. doi: 10.3201/eid2807.220198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Singh S, Singh US. Coinfection with Strongyloides and Ascaris in a COVID-19-positive male presenting with acute abdomen: a case report. Future Microbiol. 2022;17:1099. doi: 10.2217/fmb-2022-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gautam D, Gupta A, Meher A, Siddiqui F, Singhai A. Corticosteroids in Covid-19 pandemic have the potential to unearth hidden burden of strongyloidiasis. IDCases. 2021 doi: 10.1016/j.idcr.2021.e01192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pereira CVM, Mastandrea GRA, Medeiros A, Gryschek RCB, Paula FM, Corral MA. COVID-19 and strongyloidiasis: what to expect from this coinfection? Clinics (Sao Paulo) 2021 doi: 10.6061/clinics/2021/e3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ortiz-Martínez S, Ramos-Rincón JM, Vásquez-Chasnamote ME, Alarcón-Baldeón JJ, Parraguez-de-la-Cruz J, Gamboa-Paredes ON, et al. A cross-sectional study of seroprevalence of strongyloidiasis in pregnant women (Peruvian amazon basin) Pathogens. 2020;9:5. doi: 10.3390/pathogens9050348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Norsyahida A, Riazi M, Sadjjadi SM, Muhammad Hafiznur Y, Low HC, Zeehaida M, et al. Laboratory detection of strongyloidiasis: IgG-, IgG4—and IgE-ELISAs and cross-reactivity with lymphatic filariasis. Parasite Immunol. 2013 doi: 10.1111/pim.12029. [DOI] [PubMed] [Google Scholar]
- 60.Ming DK, Armstrong M, Lowe P, Chiodini PL, Doherty JF, Whitty CJM, et al. Clinical and diagnostic features of 413 patients treated for imported strongyloidiasis at the hospital for tropical diseases. London Am J Trop Med Hyg. 2019;101:2. doi: 10.4269/ajtmh.19-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fradejas I, Herrero-Martínez JM, Lizasoaín M, de Las Rodríguez, Parras E, Pérez-Ayala A. Comparative study of two commercial tests for Strongyloides stercoralis serologic diagnosis. Trans R Soc Trop Med Hyg. 2018;112:12. doi: 10.1093/trstmh/try101. [DOI] [PubMed] [Google Scholar]
- 62.Anderson NW, Klein DM, Dornink SM, Jespersen DJ, Kubofcik J, Nutman TB, et al. Comparison of three immunoassays for detection of antibodies to Strongyloides stercoralis. Clin Vaccine Immunol. 2014;21:5. doi: 10.1128/CVI.00041-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruantip S, Eamudomkarn C, Kopolrat KY, Sithithaworn J, Laha T, Sithithaworn P. Analysis of daily variation for 3 and for 30 Days of parasite-specific IgG in urine for diagnosis of strongyloidiasis by enzyme-linked immunosorbent assay. Acta Trop. 2021 doi: 10.1016/j.actatropica.2021.105896. [DOI] [PubMed] [Google Scholar]
- 64.Eamudomkarn C, Sithithaworn P, Sithithaworn J, Kaewkes S, Sripa B, Itoh M. Comparative evaluation of Strongyloides ratti and S. stercoralis larval antigen for diagnosis of strongyloidiasis in an endemic area of opisthorchiasis. Parasitol Res. 2015;114:7. doi: 10.1007/s00436-015-4458-3. [DOI] [PubMed] [Google Scholar]
- 65.Gomes BB, Gonzales WHR, Meisel DMC, Gryschek RCB, Paula FM. Evaluation of larval surface antigens from infective larvae of Strongyloides venezuelensis for the serodiagnosis of human strongyloidiasis. Rev Inst Med Trop Sao Paulo. 2023 doi: 10.1590/s1678-9946202365001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huaman MC, Sato Y, Aguilar JL, Terashima A, Guerra H, Gotuzzo E, et al. Gelatin particle indirect agglutination and enzyme-linked immunosorbent assay for diagnosis of strongyloidiasis using Strongyloides venezuelensis antigen. Trans R Soc Trop Med Hyg. 2003;97:5. doi: 10.1016/S0035-9203(03)80017-2. [DOI] [PubMed] [Google Scholar]
- 67.Sithithaworn J, Sithithaworn P, Janrungsopa T, Suvatanadecha K, Ando K, Haswell-Elkins MR. Comparative assessment of the gelatin particle agglutination test and an enzyme-linked immunosorbent assay for diagnosis of strongyloidiasis. J Clin Microbiol. 2005;43:7. doi: 10.1128/JCM.43.7.3278-3282.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schaffel R, Nucci M, Carvalho E, Braga M, Almeida L, Portugal R, et al. The value of an immunoenzymatic test (enzyme-linked immunosorbent assay) for the diagnosis of strongyloidiasis in patients immunosuppressed by hematologic malignancies. Am J Trop Med Hyg. 2001;65:4. doi: 10.4269/ajtmh.2001.65.346. [DOI] [PubMed] [Google Scholar]
- 69.Ahmed SA, El-Moselhy A, El-Moammaly A, El-Shewy K. Strongyloides stercoralis in patients on corticosteroids therapy using enzyme-linked immunosorbent assay and gelatin particles indirect agglutination tests: a diagnostic approach. Acta Parasitol. 2019;64:2. doi: 10.2478/s11686-019-00060-w. [DOI] [PubMed] [Google Scholar]
- 70.Krolewiecki AJ, Ramanathan R, Fink V, McAuliffe I, Cajal SP, Won K, et al. Improved diagnosis of Strongyloides stercoralis using recombinant antigen-based serologies in a community-wide study in northern Argentina. Clin Vaccine Immunol. 2010;17:10. doi: 10.1128/CVI.00259-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loutfy MR, Wilson M, Keystone JS, Kain KC. Serology and eosinophil count in the diagnosis and management of strongyloidiasis in a non-endemic area. Am J Trop Med Hyg. 2002;66:6. doi: 10.4269/ajtmh.2002.66.749. [DOI] [PubMed] [Google Scholar]
- 72.Sudarshi S, Stümpfle R, Armstrong M, Ellman T, Parton S, Krishnan P, et al. Clinical presentation and diagnostic sensitivity of laboratory tests for Strongyloides stercoralis in travellers compared with immigrants in a non-endemic country. Trop Med Int Health. 2003;8:8. doi: 10.1046/j.1365-3156.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 73.Ahmad H, Balachandra D, Arifin N, Nolan TJ, Lok JB, Hayat Khan A, et al. Diagnostic potential of an IgE-ELISA in detecting strongyloidiasis. Am J Trop Med Hyg. 2020;103:6. doi: 10.4269/ajtmh.20-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Faria LS, de Souza DLN, Ribeiro RP, de Sousa JEN, Borges IP, Ávila VMR, et al. Highly specific and sensitive anti-Strongyloides venezuelensis IgY antibodies applied to the human strongyloidiasis immunodiagnosis. Parasitol Int. 2019 doi: 10.1016/j.parint.2019.101933. [DOI] [PubMed] [Google Scholar]
- 75.Feliciano ND, Ribeiro VS, Gonzaga HT, Santos FA, Fujimura PT, Goulart LR, et al. Short epitope-based synthetic peptides for serodiagnosis of human strongyloidiasis. Immunol Lett. 2016 doi: 10.1016/j.imlet.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 76.Arifin N, Yunus MH, Nolan TJ, Lok JB, Noordin R. Identification and preliminary evaluation of a novel recombinant protein for serodiagnosis of strongyloidiasis. Am J Trop Med Hyg. 2018;98:4. doi: 10.4269/ajtmh.17-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Balachandra D, Rahumatullah A, Lim TS, Mustafa FH, Ahmad H, Anuar NS, et al. A new antigen detection ELISA for the diagnosis of Strongyloides infection. Acta Trop. 2021 doi: 10.1016/j.actatropica.2021.105986. [DOI] [PubMed] [Google Scholar]
- 78.Masoori L, Falak R, Mokhtarian K, Bandehpour M, Razmjou E, Jalallou N, et al. Production of recombinant 14-3-3 protein and determination of its immunogenicity for application in serodiagnosis of strongyloidiasis. Trans R Soc Trop Med Hyg. 2019;113:6. doi: 10.1093/trstmh/trz006. [DOI] [PubMed] [Google Scholar]
- 79.Krolewiecki AJ, Koukounari A, Romano M, Caro RN, Scott AL, Fleitas P, et al. Transrenal DNA-based diagnosis of Strongyloides stercoralis (Grassi, 1879) infection: Bayesian latent class modeling of test accuracy. PLoS Negl Trop Dis. 2018;12:6. doi: 10.1371/journal.pntd.0006550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mounsey K, Kearns T, Rampton M, Llewellyn S, King M, Holt D, et al. Use of dried blood spots to define antibody response to the Strongyloides stercoralis recombinant antigen NIE. Acta Trop. 2014 doi: 10.1016/j.actatropica.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pak BJ, Vasquez-Camargo F, Kalinichenko E, Chiodini PL, Nutman TB, Tanowitz HB, et al. Development of a rapid serological assay for the diagnosis of strongyloidiasis using a novel diffraction-based biosensor technology. PLoS Negl Trop Dis. 2014;8:8. doi: 10.1371/journal.pntd.0003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Noordin R, Anuar NS, Juri NM, Wongphutorn P, Ruantip S, Kopolrat KY, et al. Evaluation of a rapid IgG4 lateral flow dipstick test to detect Strongyloides stercoralis infection in northeast Thailand. Am J Trop Med Hyg. 2021;105:3. doi: 10.4269/ajtmh.21-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Becker SL, Piraisoody N, Kramme S, Marti H, Silué KD, Panning M, et al. Real-time PCR for detection of Strongyloides stercoralis in human stool samples from Côte d'Ivoire: diagnostic accuracy, inter-laboratory comparison and patterns of hookworm co-infection. Acta Trop. 2015 doi: 10.1016/j.actatropica.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 84.Sultana Y, Jeoffreys N, Watts MR, Gilbert GL, Lee R. Real-time polymerase chain reaction for detection of Strongyloides stercoralis in stool. Am J Trop Med Hyg. 2013;88:6. doi: 10.4269/ajtmh.12-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Swan C, Phan T, McKew G. Clinical performance of real-time polymerase chain reaction for Strongyloides stercoralis compared with serology in a nonendemic setting. Am J Trop Med Hyg. 2022;107:2. doi: 10.4269/ajtmh.21-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schär F, Odermatt P, Khieu V, Panning M, Duong S, Muth S, et al. Evaluation of real-time PCR for Strongyloides stercoralis and hookworm as diagnostic tool in asymptomatic schoolchildren in Cambodia. Acta Trop. 2013;126:2. doi: 10.1016/j.actatropica.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 87.Sitta RB, Malta FM, Pinho JR, Chieffi PP, Gryschek RC, Paula FM. Conventional PCR for molecular diagnosis of human strongyloidiasis. Parasitology. 2014;141:5. doi: 10.1017/S0031182013002035. [DOI] [PubMed] [Google Scholar]
- 88.Bosqui LR, Marques PD, de Melo GB, Gonçalves-Pires M, Malta FM, Pavanelli WR, et al. Molecular and immnune diagnosis: further testing for human strongyloidiasis. Mol Diagn Ther. 2018;22:4. doi: 10.1007/s40291-018-0340-1. [DOI] [PubMed] [Google Scholar]
- 89.Moghaddassani H, Mirhendi H, Hosseini M, Rokni M, Mowlavi G, Kia E. Molecular diagnosis of Strongyloides stercoralis infection by PCR detection of specific DNA in human stool samples. Iran J Parasitol. 2011;6:2. [PMC free article] [PubMed] [Google Scholar]
- 90.Sharifdini M, Mirhendi H, Ashrafi K, Hosseini M, Mohebali M, Khodadadi H, et al. Comparison of nested polymerase chain reaction and real-time polymerase chain reaction with parasitological methods for detection of Strongyloides stercoralis in human fecal samples. Am J Trop Med Hyg. 2015;93:6. doi: 10.4269/ajtmh.15-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kristanti H, Meyanti F, Wijayanti MA, Mahendradhata Y, Polman K, Chappuis F, et al. Diagnostic comparison of Baermann funnel, Koga agar plate culture and polymerase chain reaction for detection of human Strongyloides stercoralis infection in Maluku. Indonesia Parasitol Res. 2018;117:10. doi: 10.1007/s00436-018-6021-5. [DOI] [PubMed] [Google Scholar]
- 92.Ghasemikhah R, Tabatabaiefar MA, Shariatzadeh SA, Shahbazi A, Hazratian T. A PCR-based molecular detection of Strongyloides stercoralis in human stool samples from Tabriz City. Iran Sci Pharm. 2017;85:2. doi: 10.3390/scipharm85020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Holt DC, Shield J, Harris TM, Mounsey KE, Aland K, McCarthy JS, et al. Soil-transmitted helminths in children in a remote aboriginal community in the northern territory: Hookworm is rare but Strongyloides stercoralis and Trichuris trichiura persist. Trop Med Infect Dis. 2017;2:4. doi: 10.3390/tropicalmed2040051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Al-Mekhlafi HM, Nasr NA, Lim YAL, Elyana FN, Sady H, Atroosh WM, et al. Prevalence and risk factors of Strongyloides stercoralis infection among Orang Asli schoolchildren: new insights into the epidemiology, transmission and diagnosis of strongyloidiasis in Malaysia. Parasitology. 2019;146:12. doi: 10.1017/S0031182019000945. [DOI] [PubMed] [Google Scholar]
- 95.Repetto SA, Ruybal P, Batalla E, López C, Fridman V, Sierra M, et al. Strongyloidiasis outside endemic areas: Long-term parasitological and clinical follow-up after ivermectin treatment. Clin Infect Dis. 2018;66:10. doi: 10.1093/cid/cix1069. [DOI] [PubMed] [Google Scholar]
- 96.Mazzaro MC, Santos ÉAD, Melo GB, Marques PD, Souza LV, Elias-Oliveira J, et al. Importance of detection of Strongyloides stercoralis DNA in fecal samples from patients with type 2 diabetes mellitus. Clinics (Sao Paulo) 2022 doi: 10.1016/j.clinsp.2022.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Won EJ, Jeon J, Koh YI, Ryang DW. Strongyloidiasis in a diabetic patient accompanied by gastrointestinal stromal tumor: cause of eosinophilia unresponsive to steroid therapy. Korean J Parasitol. 2015;53:2. doi: 10.3347/kjp.2015.53.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Javanian M, Gorgani-Firouzjaee T, Kalantrai N. Comparison of ELISA and PCR of the 18S rRNA gene for detection of human strongyloidiasis using serum sample. Infect Dis (Lond) 2019;51:5. doi: 10.1080/23744235.2019.1575978. [DOI] [PubMed] [Google Scholar]
- 99.Wang LF, Xu L, Luo SQ, Xie H, Chen W, Wu ZD, et al. Diagnosis of Strongyloides stercoralis by morphological characteristics combine with molecular biological methods. Parasitol Res. 2017;116:4. doi: 10.1007/s00436-017-5389-y. [DOI] [PubMed] [Google Scholar]
- 100.Hasegawa H, Sato H, Fujita S, Nguema PP, Nobusue K, Miyagi K, et al. Molecular identification of the causative agent of human strongyloidiasis acquired in Tanzania: dispersal and diversity of Strongyloides spp. and their hosts. Parasitol Int. 2010;59:3. doi: 10.1016/j.parint.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 101.Nagayasu E, Aung MPPTHH, Hortiwakul T, Hino A, Tanaka T, Higashiarakawa M, et al. A possible origin population of pathogenic intestinal nematodes, Strongyloides stercoralis, unveiled by molecular phylogeny. Sci Reports. 2017;7:1. doi: 10.1038/s41598-017-05049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thanchomnang T, Intapan PM, Sanpool O, Rodpai R, Tourtip S, Yahom S, et al. First molecular identification and genetic diversity of Strongyloides stercoralis and Strongyloides fuelleborni in human communities having contact with long-tailed macaques in Thailand. Parasitol Res. 2017;116:7. doi: 10.1007/s00436-017-5469-z. [DOI] [PubMed] [Google Scholar]
- 103.Pakdee W, Thaenkham U, Dekumyoy P, Sa-Nguankiat S, Maipanich W, Pubampen S. Genetic differentiation of Strongyloides stercoralis from two different climate zones revealed by 18S ribosomal DNA sequence comparison. Southeast Asian J Trop Med Public Health. 2012;43:6. [PubMed] [Google Scholar]
- 104.Schär F, Guo L, Streit A, Khieu V, Muth S, Marti H, et al. Strongyloides stercoralis genotypes in humans in Cambodia. Parasitol Int. 2014;63:3. doi: 10.1016/j.parint.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 105.Basso W, Grandt LM, Magnenat AL, Gottstein B, Campos M. Strongyloides stercoralis infection in imported and local dogs in Switzerland: from clinics to molecular genetics. Parasitol Res. 2019;118:1. doi: 10.1007/s00436-018-6173-3. [DOI] [PubMed] [Google Scholar]
- 106.Jaleta TG, Zhou S, Bemm FM, Schär F, Khieu V, Muth S, et al. Different but overlapping populations of Strongyloides stercoralis in dogs and humans-Dogs as a possible source for zoonotic strongyloidiasis. PLoS Negl Trop Dis. 2017;11:8. doi: 10.1371/journal.pntd.0005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Laymanivong S, Hangvanthong B, Insisiengmay B, Vanisaveth V, Laxachack P, Jongthawin J, et al. First molecular identification and report of genetic diversity of Strongyloides stercoralis, a current major soil-transmitted helminth in humans from Lao People's Democratic Republic. Parasitol Res. 2016;115:8. doi: 10.1007/s00436-016-5052-z. [DOI] [PubMed] [Google Scholar]
- 108.Sanprasert V, Kerdkaew R, Srirungruang S, Charuchaibovorn S, Phadungsaksawasdi K, Nuchprayoon S. Development of conventional multiplex PCR: a rapid technique for simultaneous detection of soil-transmitted helminths. Pathogens. 2019;8:3. doi: 10.3390/pathogens8030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kridaningsih TN, Sukmana DJ, Mufidah H, Diptyanusa A, Kusumasari RA, Burdam FH, et al. Epidemiology and risk factors of Strongyloides stercoralis infection in Papua, Indonesia: a molecular diagnostic study. Acta Trop. 2020 doi: 10.1016/j.actatropica.2020.105575. [DOI] [PubMed] [Google Scholar]
- 110.Aramendia AA, Anegagrie M, Zewdie D, Dacal E, Saugar JM, Herrador Z, et al. Epidemiology of intestinal helminthiases in a rural community of Ethiopia: is it time to expand control programs to include Strongyloides stercoralis and the entire community? PLoS Negl Trop Dis. 2020;14:6. doi: 10.1371/journal.pntd.0008315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Amor A, Rodriguez E, Saugar JM, Arroyo A, López-Quintana B, Abera B, et al. High prevalence of Strongyloides stercoralis in school-aged children in a rural highland of north-western Ethiopia: the role of intensive diagnostic work-up. Parasit Vectors. 2016;9:1. doi: 10.1186/s13071-016-1912-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beknazarova M, Whiley H, Traub R, Ross K. Opportunistic mapping of Strongyloides stercoralis and hookworm in dogs in remote Australian communities. Pathogens. 2020;9:5. doi: 10.3390/pathogens9050398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mationg MLS, Gordon CA, Tallo VL, Olveda RM, Alday PP, Reñosa MDC, et al. Status of soil-transmitted helminth infections in schoolchildren in Laguna Province, the Philippines: determined by parasitological and molecular diagnostic techniques. PLoS Negl Trop Dis. 2017;11:11. doi: 10.1371/journal.pntd.0006022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Basuni M, Muhi J, Othman N, Verweij JJ, Ahmad M, Miswan N, et al. A pentaplex real-time polymerase chain reaction assay for detection of four species of soil-transmitted helminths. Am J Trop Med Hyg. 2011;84:2. doi: 10.4269/ajtmh.2011.10-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Barratt JLN, Lane M, Talundzic E, Richins T, Robertson G, Formenti F, et al. A global genotyping survey of Strongyloides stercoralis and Strongyloides fuelleborni using deep amplicon sequencing. PLoS Negl Trop Dis. 2019;13:9. doi: 10.1371/journal.pntd.0007609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Beknazarova M, Barratt JLN, Bradbury RS, Lane M, Whiley H, Ross K. Detection of classic and cryptic Strongyloides genotypes by deep amplicon sequencing: a preliminary survey of dog and human specimens collected from remote Australian communities. PLoS Negl Trop Dis. 2019;13:8. doi: 10.1371/journal.pntd.0007241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kramme S, Nissen N, Soblik H, Erttmann K, Tannich E, Fleischer B, et al. Novel real-time PCR for the universal detection of Strongyloides species. J Med Microbiol. 2011;60:4. doi: 10.1099/jmm.0.025338-0. [DOI] [PubMed] [Google Scholar]
- 118.Watts MR, James G, Sultana Y, Ginn AN, Outhred AC, Kong F, et al. A loop-mediated isothermal amplification (LAMP) assay for Strongyloides stercoralis in stool that uses a visual detection method with SYTO-82 fluorescent dye. Am J Trop Med Hyg. 2014;90:2. doi: 10.4269/ajtmh.13-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ahmad AF, Hadip F, Ngui R, Lim YA, Mahmud R. Serological and molecular detection of Strongyloides stercoralis infection among an Orang Asli community in Malaysia. Parasitol Res. 2013;112:8. doi: 10.1007/s00436-013-3450-z. [DOI] [PubMed] [Google Scholar]
- 120.Van De N, Minh PN, Van Duyet L, Mas-Coma S. Strongyloidiasis in northern Vietnam: epidemiology, clinical characteristics and molecular diagnosis of the causal agent. Parasit Vectors. 2019;12:1. doi: 10.1186/s13071-019-3776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gunathilaka N, Chandrasena N, Wijerathna T, Fuji Y, Gunasekara D, Gunatilaka RP, et al. Descriptive investigation of strongyloidiasis infection and characterization of Strongyloides stercoralis using morphological and molecular-based methods. Case Rep Infect Dis. 2020 doi: 10.1155/2020/5431491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fadaei Tehrani M, Sharifdini M, Zahabiun F, Latifi R, Kia EB. Molecular characterization of human isolates of Strongyloides stercoralis and Rhabditis spp based on mitochondrial cytochrome c oxidase subunit 1 (cox1) BMC Infect Dis. 2019;19:1. doi: 10.1186/s12879-019-4407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hunt VL, Tsai IJ, Coghlan A, Reid AJ, Holroyd N, Foth BJ, et al. The genomic basis of parasitism in the Strongyloides clade of nematodes. Nat Genet. 2016;48:3. doi: 10.1038/ng.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kikuchi T, Hino A, Tanaka T, Aung MP, Afrin T, Nagayasu E, et al. Genome-wide analyses of individual Strongyloides stercoralis (Nematoda: Rhabditoidea) provide insights into population structure and reproductive life cycles. PLoS Negl Trop Dis. 2016;10:12. doi: 10.1371/journal.pntd.0005253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.