Abstract
Background
COVID-19 severity and its late complications continue to be poorly understood. Neutrophil extracellular traps (NETs) form in acute COVID-19, likely contributing to morbidity and mortality.
Objectives
This study evaluated immunothrombosis markers in a comprehensive cohort of acute and recovered COVID-19 patients, including the association of NETs with long COVID.
Methods
One-hundred-seventy-seven patients were recruited from clinical cohorts at 2 Israeli centers: acute COVID-19 (mild/moderate, severe/critical), convalescent COVID-19 (recovered and long COVID), along with 54 non-COVID controls. Plasma was examined for markers of platelet activation, coagulation, and NETs. Ex vivo NETosis induction capability was evaluated after neutrophil incubation with patient plasma.
Results
Soluble P-selectin, factor VIII, von Willebrand factor, and platelet factor 4 were significantly elevated in patients with COVID-19 versus controls. Myeloperoxidase (MPO)-DNA complex levels were increased only in severe COVID-19 and did not differentiate between COVID-19 severities or correlate with thrombotic markers. NETosis induction levels strongly correlated with illness severity/duration, platelet activation markers, and coagulation factors, and were significantly reduced upon dexamethasone treatment and recovery. Patients with long COVID maintained higher NETosis induction, but not NET fragments, compared to recovered convalescent patients.
Conclusions
Increased NETosis induction can be detected in patients with long COVID. NETosis induction appears to be a more sensitive NET measurement than MPO-DNA levels in COVID-19, differentiating between disease severity and patients with long COVID. Ongoing NETosis induction capability in long COVID may provide insights into pathogenesis and serve as a surrogate marker for persistent pathology. This study emphasizes the need to explore neutrophil-targeted therapies in acute and chronic COVID-19.
Keywords: COVID-19, extracellular traps, immunothrombosis, neutrophil, post-acute COVID-19 syndrome
1. INTRODUCTION
Coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is responsible for the death of >6.7 million people worldwide as of January 2023 [1], as well as millions leaving the workforce due to disability from persistent symptoms. Clinical features range from mild symptoms, such as fever, fatigue, dry cough, and pneumonia, to more severe presentations with dyspnea, coagulopathy, venous thromboembolism, acute respiratory distress syndrome, acute cardiac injury, and multi-organ failure in critical illnesses [2,3]. In acute COVID-19, SARS-CoV-2 infection triggers immunothrombosis and endotheliopathy leading to elevated inflammatory markers and increased coagulation parameters (eg, tumor necrosis factor [TNF], interleukin (IL)-1, IL-6, IL-8, RANTES, platelet factor 4 [PF4], factor VIII [FVIII], von Willebrand factor [VWF]) [[3], [4], [5], [6], [7], [8], [9]].
Many studies provide evidence that neutrophil extracellular traps (NETs) contribute to COVID-19-associated thrombosis [[8], [9], [10], [11], [12], [13]]. NETs are extracellular chromatin decorated with specific proteins, including neutrophil elastase (NE) and myeloperoxidase (MPO) [14]. Although potentially helpful for an initial immune response, excessive or dysregulated NETs contribute to a number of acute and chronic pathologies (eg, myocardial infarction, acute kidney injury, pneumonia, rheumatoid arthritis, diabetes, thrombosis, and cancer) [15,16].
The process of NET formation (NETosis) can be measured by a variety of methods that identify neutrophil or NET-specific proteins complexed with external DNA, including MPO-DNA, NE-DNA, and citrullinated histone H3-DNA (H3Cit-DNA). Previous studies have shown elevated levels of NET fragments, although variable in their reliability to reflect ongoing NETosis, consistently correlate with COVID-19 illness severity and outcome [8,[17], [18], [19], [20]]. Neutrophils isolated from patients with COVID-19 demonstrate elevated NET-forming capacity, and plasma and serum from patients with COVID-19 induce robust ex vivo NETosis in neutrophils isolated from healthy subjects [8,10,18,19,21]. A return to baseline levels of circulating NET complexes has been reported in convalescent patients recovered from active COVID-19 [8,20]. No changes in cfDNA or DNase activity were found in a study of patients with COVID-19 who had persistent symptoms [22], a syndrome commonly referred to as long COVID, although no study has measured specific NET biomarkers in this syndrome.
Long COVID has no globally accepted diagnostic criteria but implies symptoms that last for >1 month since recovery from acute COVID-19, with some patients reporting symptoms lasting for >6 months. Establishing internationally recognized guidance to diagnose and manage long COVID continues as more data are gathered. Patients with long COVID experience a wide range of systemic symptoms, including generalized chest and muscle pain, fatigue, shortness of breath, and cognitive dysfunction [23]. It was previously reported that FVIII and VWF levels remain high in patients with long COVID despite reduced acute-phase markers (C-reactive protein [CRP], neutrophil and white cell counts, and IL-6), suggesting a state of persistent endothelial activation and elevated thrombotic risk [22].
Research and clinical efforts continue to target NET-related pathways to find new and improved treatments in the context of COVID-19 [17]. In our study, we investigated clinical cohorts of patients with acute and chronic COVID-19 from 2 Israeli medical centers for markers of increased thrombosis, including platelet activation markers, coagulation factors, and NET-related biomarkers. We utilized a quantitative measurement of ex vivo NETosis induction capacity by COVID-19 plasma that reflects the potential to form NETs (NETosis induction) rather than the quantification of remnant NET fragments in the blood.
2. Methods
2.1. Study design
Plasma samples from patients with acute COVID-19, as well as COVID-19 convalescent patients and non-COVID controls were collected by 2 Israeli medical center biobanks between March 2020 and September 2020: the Shaare Zedek Medical Center (Jerusalem) and the Rambam Health Care Campus (Haifa). The blood samples that were used for plasma preparation were collected into EDTA (226 subjects) or sodium citrate (20 subjects) tubes. Supplementary Table S1 summarizes the sample distribution and clinical information for the cohorts. Demographic information, clinical and laboratory data were obtained from patients’ medical records.
Disease severity classification was performed by the medical centers based on the National Institutes of Health and World Health Organization COVID-19 clinical management guidance [24]. The maximum severity during hospitalization of each patient was used in the analysis. Information about convalescent patients was received from the COVID-19 Recovery Clinic of the Shaare Zedek Medical Center Pulmonary Institute and included documentation regarding post-COVID symptoms. Twenty convalescent patients lacking documented information were followed up by telephone with a questionnaire to collect information about their condition at the time of sampling. For 5 of 66 (7.6%) convalescent patients, this information was not available.
Inclusion criteria for patients with COVID-19 included SARS-CoV-2 positive polymerase chain reaction test and age >18 years. Inclusion criteria for COVID-19 convalescent patients included documentation of previous SARS-CoV-2 infection at least 1 month earlier and age >18. Inclusion criteria for non-COVID control samples included age >18 with no reported COVID-19. Exclusion criteria were human immunodeficiency virus (HIV), hepatitis B virus (HBV), or hepatitis C virus (HCV) infection and pregnancy. Exclusion criteria for non-COVID and convalescent groups were SARS-CoV-2 positive test. The study was approved by the institutional review boards of the Shaare Zedek Medical Center and the Rambam Health Care Campus (0269-20-SZMC and 0237-20-RMB, respectively). All study participants or a legal representative provided informed consent.
2.2. Plasma samples
Plasma samples were stored at -80 °C. For batch analysis, they were thawed at 37 °C for 15 minutes to eliminate cryoprecipitates. Plasma samples from acute COVID-19 patients were taken after a mean of 8 days with a median of 7 days from a positive test result for COVID-19, ranging from -4 to 46 days after swab collection. Plasma samples that showed signs of hemolysis by visual inspection were excluded.
2.3. MPO-DNA complexes
MPO-DNA plasma complex levels were evaluated using a sandwich ELISA-based assay adapted from Kessenbrock et al. [25] with modifications including a calibration curve with in vitro generated MPO-nucleosome-DNA complexes. Complexation of 54 ng reconstituted human MPO standard (from LEGEND MAX Human Myeloperoxidase ELISA Kit, 440007, BioLegend Inc, 200 ng/mL) with excess lambda DNA (1.4 μg from Quant-iT Picogreen dsDNA Assay Kit, P7589, Invitrogen) and excess human native nucleosomes (1.4 μg of 14-1057, Merck) was performed by incubation for 30 minutes at room temperature. Then, 200 μL of PBS were added to prepare a stock standard solution of 100 ng/mL. This was further diluted to generate a standard curve with quantitative estimation of MPO-DNA complex levels ranging from 0.5 to 5 ng/mL MPO component. MaxiSorp 96-well plates (442404, Thermo Scientific) were coated with MPO polyclonal antibody (PA5-16672, Invitrogen) overnight in 2°C to 8 °C. After washing, the plate was blocked with 2.5% BSA in PBS. The plate was washed and incubated with plasma or standard samples for 2 hours at room temperature with agitation at 80 rpm. The plate was washed and incubated with peroxidase-conjugated anti-DNA antibody (from Cell Death Detection ELISAPLUS kit, 11774425001, Roche Diagnostics) for 2 hours. TMB One Component Microwell Substrate (0410-01L, Southern Biotech) was used for ELISA development, with 1M HCl used to stop the reaction. Absorbance measurements at 450 nm with 630 nm reference wavelength were used to plot a calibration curve and evaluate MPO-DNA complex levels. Results were normalized to the average value of samples collected from non-COVID controls, to account for the differences across different assay days needed to measure such a large number of samples. Values below/above the calibration curve range were considered under the detection limit (UDL) or above the detection limit. The UDL values were obtained for 10 non-COVID controls, 16 patients with mild/moderate and 12 with severe/critical COVID-19, and 4 convalescent patients. Data could not be generated for 9 patients (7 with COVID-19 and 2 non-COVID) due to insufficient sample volume.
2.4. NETosis induction by plasma samples
Blood samples were drawn from 13 healthy patients in the Rambam Health Care Campus to perform NETosis induction assays using neutrophils from healthy volunteers. Neutrophils were isolated from the freshly collected blood samples using EasySep Direct Human Neutrophil Isolation Kit (19666, STEMCELL Technologies). Isolated cells were resuspended in RPMI medium (01-100-1A, Biological Industries) supplemented with 0.5% of 70 °C-heat-inactivated fetal bovine serum (04-127-1A, Biological Industries) and 10 mM HEPES buffer (03-025-1C, Biological Industries) to a concentration of 2×106 cells/mL. Cells were seeded (2×105 cells/well) in a poly-L-lysine coated 96-well plate (655930, Greiner Bio-One) and were incubated for one hour to allow cells to settle. All incubations during this assay were performed at 37 °C in 5% CO2/95% air. One IU/mL heparin (289973.01-IL, Teva Medical Marketing) was added to cells to prevent clotting, and then cells were incubated with plasma samples (15% v/v) for 2 hours. Each condition was run in duplicate wells. Next, the plasma-containing supernatants were gently removed from the wells and kept for further analysis. Pre-warmed FastDigest AluI restriction enzyme (FD0014, Thermo Scientific), diluted 1:1250 in RPMI supplemented with 0.5% heat-inactivated fetal bovine serum, was added to each well. After a 20-minute incubation, digested NET supernatants were gently collected and centrifuged (500 g, 5 minutes, 4 °C) to remove the detached cells. To evaluate extracellular DNA in a high throughput fashion, resulting supernatants were mixed 1:1 with 2 μM SYTOX green dye (S7020, Invitrogen) and fluorescence level at an excitation wavelength of 488 nm as well as the emission of 530 nm was measured. To exclude background signal, RPMI supplemented with 0.5% heat-inactivated fetal bovine serum was mixed with SYTOX green dye and quantified for fluorescent signal in the same manner. Equivalent measurements were performed on the plasma-containing supernatants to quantify NETs that were digested and released to the medium during incubation with plasma. NETosis induction level was calculated as the sum of fluorescence measurements of both plasma-containing and digested NET supernatants normalized to the average value of samples drawn from non-COVID controls. Owing to the limited sample volume, data could not be generated for 5 patients with COVID-19. Interassay variation was evaluated for 43 samples and resulted in 14 ± 2% (mean ± SEM).
2.5. Statistical analysis
Nonparametric tests were applied for all data: two-tailed Mann–Whitney test for comparison of 2 unpaired groups, two-tailed Wilcoxon tests for comparison between 2 paired groups, and Kruskal-Wallis test with Dunn’s multiple comparisons tests for 3 or more groups. For correlation analysis, Spearman’s test was used. Comparisons of categorical variables were analyzed using Chi-squared test or Fisher’s exact test. P<.05 was considered statistically significant. R software was used for Fisher’s exact test analysis of sex and blood type distribution. GraphPad Prism 9 software was used for all other statistical analyses and calculations related to ELISA analyses. Bars and error bars in all graphs represent means ± SEM.
3. Results
3.1. Study group characteristics
Study participants were prospectively enrolled in 2 medical centers during the first and second coronavirus pandemic waves in Israel (between March 2020 and September 2020). This study includes COVID-19 cohorts of different severities (“mild/moderate” [n = 52] and “severe/critical patients” [n = 74]), convalescent patients (n = 66, including those with long COVID [n = 46], and non-COVID controls (n = 54). Sixteen patients provided plasma samples during their acute COVID-19 period and after their recovery and were therefore included in more than 1 cohort. Accordingly, all COVID-19 samples were collected during acute disease before recovery (i.e., during the same hospital stay after the initial diagnosis).
Clinical and demographic characteristics of all study cohorts are summarized in the Table , with adjusted P values obtained from multiple comparisons available in Supplementary Table S2. Non-COVID controls and patients with COVID-19 had similar sex distribution. The mean age in the cohort of patients with severe/critical COVID-19 was significantly higher than that of the other cohorts (P<.0001), and BMI was higher than that in the non-COVID cohort (p = .0172) but not higher than that in the other cohorts. For patients with severe/critical COVID-19, hypertension (52%), diabetes (29.3%), non-COVID-19 inflammatory disease (24%), heart disease (21%), and kidney failure (20%) were the most prevalent comorbidities, whereas in mild/moderate COVID-19, hypertension (25.9%) and non-COVID inflammatory disease (20.4%) were the most prevalent. Among the non-COVID cohort, 1 subject had psoriasis, 1 had deep vein thrombosis, and 2 had diabetes. All remaining healthy controls had no inflammatory diseases, autoimmune diseases, cancer, diabetes, or thrombosis. Neither symptomatic SARS-CoV-2 infection nor long COVID symptoms were documented for the non-COVID cohort when the sample was acquired.
Table.
Clinical and demographic characteristics of study subjects.
| Non-COVID | COVID-19 |
Convalescents | P value | ||
|---|---|---|---|---|---|
| Mild/moderate | Severe/critical | ||||
| Study subjects, n | 54 | 52 | 74 | 66 | |
| Age in years, mean ± SEM | 48 ± 1.9 | 52 ± 2.8 | 67.0 ± 1.7 | 52.4 ± 2.2 | <.0001 |
| Sex | |||||
| Men, n | 29 | 28 | 46 | 35 | .5349h |
| Women, n | 25 | 24 | 28 | 31 | |
| Clinical data | |||||
| Survival rate, % | 100% | 74% | |||
| BMI | 27.2 ± 0.6 | 27.2 ± 0.6c | 31.1 ± 1.0e | 29.8 ± 2.1g | .0196 |
| Peak D-dimer, ng/mL, mean ± SEM | 1118 ± 223d | 3007 ± 352f | .0002 | ||
| Peak CRPa, mg/dL, mean ± SEM | 8.3 ± 1.1 | 19.5 ± 1.3 | <.0001 | ||
| Peak WBCsb, 103/uL, mean ± SEM | 8.3 ± 0.5 | 13.2 ± 0.9 | <.0001 | ||
| Peak platelet count, 103/uL, mean ± SEM | 291 ± 22 | 322 ± 19 | .2094 | ||
| Comorbidities, n (%) | |||||
| Non-COVID inflammatory disease | 11 (20.8%) | 21 (28.4%) | 2 (4.1%) | .4086i | |
| Autoimmune disease | 1 | 2 (3.8%) | 1 (1.4%) | 1 (2%) | .5706i |
| Diabetes | 2 | 8 (15.1%) | 23 (31.1%) | 6 (12.2%) | .0584i |
| Cancer (active and history) | 2 (3.8%) | 6 (8.1%) | 1 (2%) | .4671i | |
| Thrombosis | 1 | 4 (7.5%) | 3 (4.1%) | 0 (0%) | .4499i |
| Kidney failure | NA | 1 (1.9%) | 14 (18.9%) | 1 (2%) | .0039i |
| Hypertension | NA | 14 (26.4%) | 40 (54.1%) | 6 (12.2%) | .0021i |
| Lung disease | NA | 2 (3.8%) | 10 (13.5%) | 2 (4.1%) | .0734i |
| Heart disease | NA | 7 (13.2%) | 17 (23%) | 2 (4.1%) | .2501i |
| GI tract disease | NA | 3 (5.7%) | 6 (8.1%) | 2 (4.1%) | .7338i |
| Blood type, n (%) | |||||
| A | 17 (32%) | 23 (44%) | 35 (47%) | 23 (35%) | .0415j |
| B | 13 (24%) | 17 (33%) | 11 (15%) | 20 (30%) | |
| O | 18 (33%) | 9 (17%) | 15 (20%) | 17 (26%) | |
| AB | 6 (11%) | 3 (6%) | 13 (18%) | 6 (9%) | |
%, Percentage subjects in the same cohort; NA, data were not available.
Data were not available for some study subjects.
C-reactive protein.
White blood cells.
3.
19 NA, 1 > 5000.
15.
9 NA, 4 > 5000.
17.
Fisher’s exact test was used to evaluate the differences in sex distribution between non-COVID, mild/moderate, and severe/critical.
Fisher’s exact tests were used to evaluate differences between mild/moderate and severe/critical.
Fisher’s exact tests were used to evaluate the differences in blood type distribution between non-COVID, mild/moderate, and severe/critical.
Blood type was determined for all study subjects, as the ABO blood group is a reported risk factor for severe disease [27] and also impacts the circulating VWF levels [27]. Blood type distributions of all cohorts were similar to the general population ABO distribution in Israel (Fisher’s exact test, non-COVID p = .7321; mild/moderate p = .07101; severe/critical p = .0525; convalescent p = .3301) [28]. However, when comparing the subgroups, we detected a significant difference (p = .0415); severe/critical COVID-19 patients vs. mild/moderate patients contained half the prevalence of blood type B (15% vs. 33%, respectively) and three-fold higher prevalence of blood type AB (18% vs. 6%, respectively).
3.2. Coagulation factors and markers of platelet and endothelial activation are elevated in this COVID-19 cohort
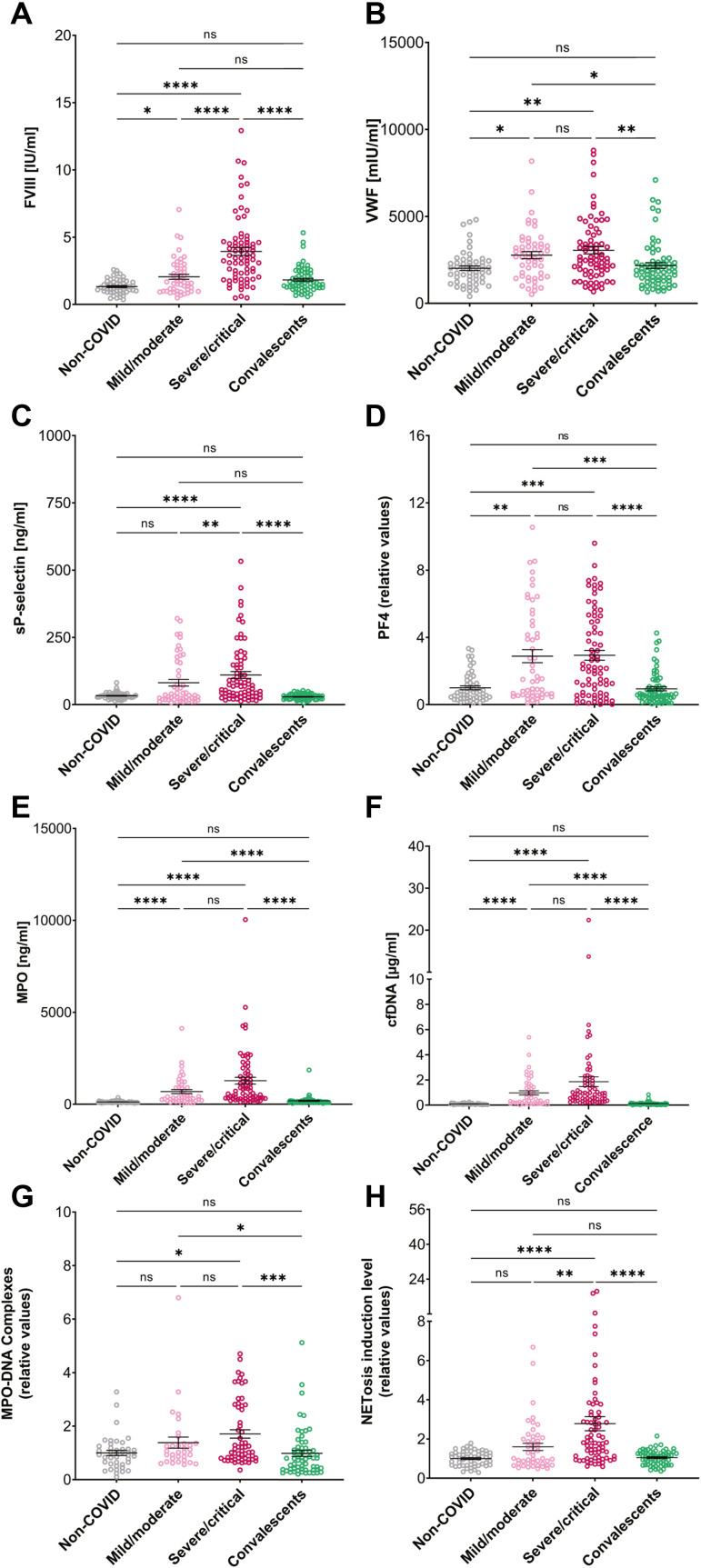
Coagulation factors and platelet/endothelial activation markers (VWF, PF4, sP-selectin, and FVIII) were measured in all plasma samples (Figures 1 A–D). Although these factor levels were previously shown to correlate with COVID-19 illness and in some cases NET formation [20], in this study we were able to explore further trends given the different cohorts it included. The severe/critical COVID-19 cohort had significantly elevated levels of all measured factors compared with the non-COVID and convalescent cohort levels. The mild/moderate COVID-19 cohort had significantly higher FVIII, PF4, and VWF levels than the non-COVID controls. The patients with mild/moderate COVID-19 had significantly lower FVIII and sP-selectin levels than those comprising the severe/critical cohort (Figure 1A–D). Significantly elevated levels of FVIII in convalescent patients were seen compared with the non-COVID controls (Table S3, p = .0012, Mann–Whitney test). Patients with severe/critical COVID-19 had significantly higher levels of D-dimer, CRP, and WBC, compared with those with mild/moderate COVID-19. Both patients with mild/moderate and severe/critical COVID-19 had elevated D-dimer and CRP, whereas WBC counts in patients with mild/moderate form were in the normal range, similar to other reports [29]. D-dimer levels were significantly positively correlated with FVIII, VWF, and sP-selectin (Supplementary Figure S1). In addition, platelet levels were normal among these groups, consistent with reports from other studies [30,31]. Because D-dimer, CRP, and WBC are involved in inflammatory processes and correlate with increased NETosis [9], these findings could indicate a systemic milieu in which initial or progressed NETosis occurs in severe/critical COVID-19 patients but is no longer present in the convalescent period.
Figure 1.
Platelet activation markers and prothrombotic factors of the study cohorts. (A) FVIII, (B) VWF, (C) sP-selectin, and (D) PF4 levels of non-COVID (n = 54, in FVIII graph n = 53), mild/moderate COVID-19 (n = 52), severe/critical COVID-19 (n = 74), convalescents (n = 66) subjects. (E) MPO levels in non-COVID (n = 52), mild/moderate COVID-19 (n = 47), severe/critical COVID-19 (n = 71), convalescent cohorts (n = 65). (F) cfDNA levels in non-COVID (n = 50), mild/moderate COVID-19 (n = 47), severe/critical COVID-19 (n = 70), convalescent cohorts (n = 65). (G) MPO-DNA complex levels in non-COVID (n = 42), mild/moderate COVID-19 (n = 32), severe/critical COVID-19 (n = 59), and convalescents (n = 61). (H) NETosis induction levels in non-COVID (n = 54), mild/moderate COVID-19 (n = 48), severe/critical COVID-19 (n = 73), convalescents (n = 66).
3.3. NETosis induction levels, but not MPO-DNA complexes, correlate with COVID-19 severity
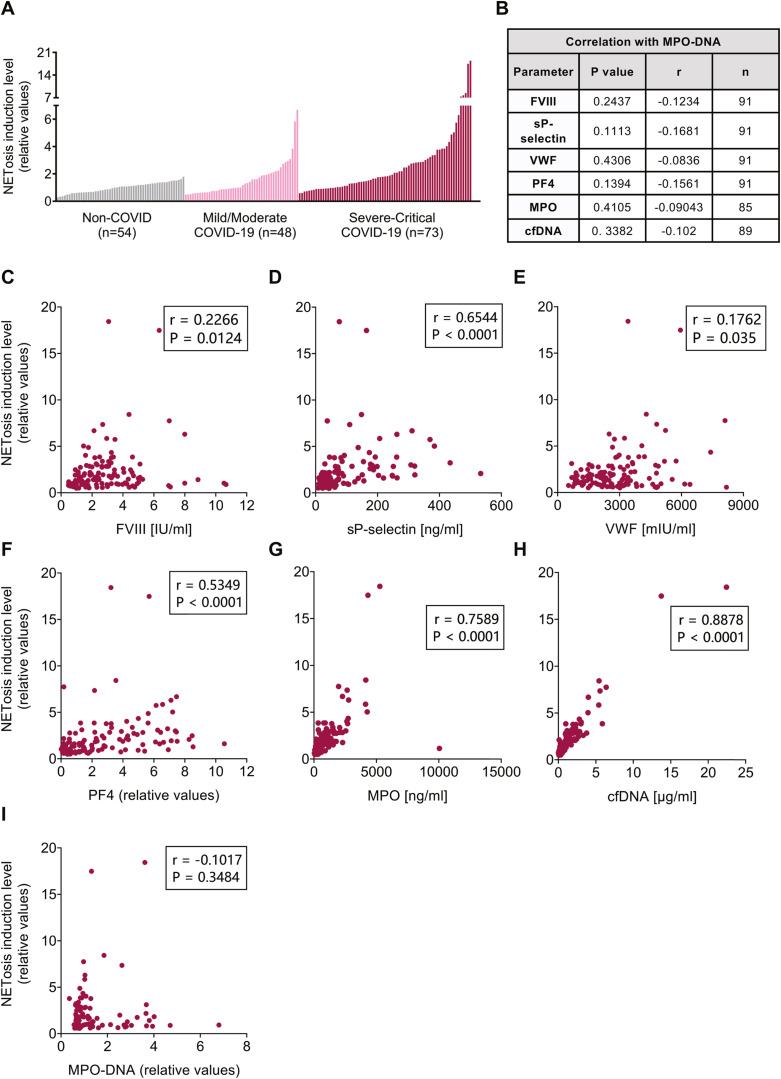
To evaluate NET involvement and confirm their use as a biomarker for COVID-19 severity, plasma samples were analyzed for MPO-DNA complex levels, along with MPO and cfDNA individually (Figure 1E–G) (Supplementary Table S3). These levels were significantly higher in patients with severe/critical COVID-19 than in non-COVID controls. Patients with mild/moderate COVID-19 had higher MPO and cfDNA levels than non-COVID controls. In addition, both the COVID-19 patient cohorts had higher MPO, cfDNA, and MPO-DNA levels than convalescents. Surprisingly, no differences were observed between the 2 COVID-19 patient cohorts with different disease severities. No correlation was found between MPO-DNA complex levels and platelet activation markers and coagulation factors, MPO and cfDNA measured in this study (Figure 2 B) (Supplementary Figure S2).
Figure 2.
Correlations between platelet activation markers and coagulation factors, and NETosis induction level in COVID-19 patient cohorts. (A) NETosis induction levels of plasma samples of non-COVID, mild/moderate and severe/critical COVID-19 subjects. (B) P values and correlation coefficient (r) values of Spearman correlation analysis of MPO-DNA complex levels and platelets and coagulation factors of COVID-19 patient cohorts. The graphs present correlations of (C) FVIII (n = 121), (D) sP-selectin (n = 121), (E) VWF (n = 121), (F) PF4 (n = 121), (G) MPO (n = 118), (H) cfDNA (n = 117) and (I) MPO-DNA (n = 87) with NETosis induction levels.
As a secondary assay to evaluate NET levels, we measured the total amount of extracellular DNA released from healthy human neutrophils after incubation with patients’ plasma, reflecting the NETosis induction level of each plasma sample on a per patient basis. First, this assay was optimized for both cell concentration and digesting enzyme AluI concentration using ionomycin as strong NET inducer to enable optimal NET quantification in a high throughput fashion (Supplementary Figures S3A–B and S4). In addition, occurrence of NETs induced by healthy human neutrophils was confirmed by the extracellular nature of DNA staining and immunofluorescence staining for citrullinated histone H3 (citrulline R2 + R8 + R17) (Supplementary Figure S5). The quantification of NETs in this assay was confirmed using an MPO-DNA ELISA (Supplementary Figure S3C–D).
Figure 1H and Supplementary Table S3 demonstrate that NETosis induction levels of plasma from patients with severe/critical COVID-19 were significantly higher than those in all other cohorts, including plasma from patients with mild/moderate COVID-19. No significant differences in NETosis induction levels between non-COVID and convalescent cohorts were found, similar to previously reported data with other NET-related markers [20].
In contrast to MPO-DNA levels, NETosis induction levels were significantly positively correlated with platelet activation markers and coagulation factors, MPO and cfDNA (Figure 2). D-dimer levels of COVID-19 cohorts were also significantly positively correlated with MPO, cfDNA, and NETosis induction. However, MPO-DNA complex levels did not significantly correlate with NETosis induction level nor with platelet activation markers and coagulation factors, MPO, cfDNA, or D-dimer (Figure 2I) (Supplementary Figures S1 and S2). These data suggest that NETosis induction levels correlate better with COVID-19 severity than MPO-DNA complex levels and could offer further insight into the state of NETosis in individual patients compared with circulating NET fragment measurements. A possible explanation is the presence of a specific combination of NETosis-promoted biomarkers in plasma samples of acute COVID-19. It might be that their individual quantification does not provide enough sensitivity to evaluate the disease severity. This concept should be investigated in future studies and can provide better understanding of NET-related diseases.
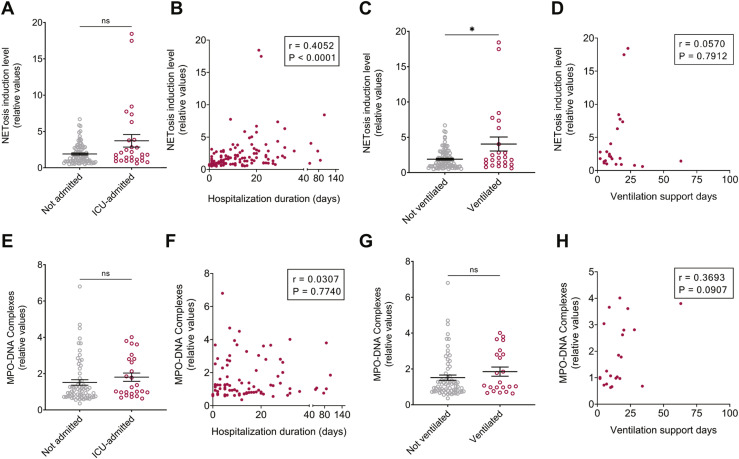
To link NETosis markers and COVID-19 management during hospitalization, MPO-DNA complex and NETosis induction levels in patients were sub-analyzed based on intensive care unit (ICU) admission and need for mechanical ventilation (Figure 3 ). All ICU-admitted COVID-19 subjects (n = 28) were classified as severe/critical patients, and most (n = 22, 79%) were mechanically ventilated during their hospitalization. NETosis induction and MPO-DNA complexes were not significantly different between ICU-admitted patients and patients admitted to the ward (Figure 3A, 3E). However, NETosis induction levels were higher for mechanically ventilated patients versus nonventilated patients and a positive correlation was found between hospitalization duration of ICU patients and NETosis induction level of their plasma (Figures 3B–C). No such significant difference or correlation was found for MPO-DNA complex levels (Figures 3F–G). In addition, no significant correlation was found with ventilation support days either for NETosis induction or for MPO-DNA (Figures 3D, H).
Figure 3.
NETosis induction and MPO-DNA complex levels according to hospitalization duration and ICU support. (A) NETosis induction levels of patients not admitted (n = 91) and admitted (n = 28) to the ICU, p = .0614. (B) Correlations between NETosis induction level and length of hospitalization (n = 119). (C) NETosis induction levels in patients who were either ventilated (n = 24) or not (n = 95), p = .0368. (D) Correlations between NETosis induction level and duration on ventilation support (n = 24). (E) MPO-DNA complex values in patients not admitted (n = 65) or admitted (n = 25) to the ICU, p = .1361. (F) Correlations between MPO-DNA complexes and hospitalization duration (n = 90). (G) MPO-DNA complexes of patients who were ventilated (n = 22) or not (n = 68), p = .2178. For 2 ventilated subjects, MPO-DNA values were under detection limit (UDL). (H) Correlations between MPO-DNA levels and duration on ventilation support (n = 22). Statistical analysis of A, C, E, G was performed using two-tailed Mann–Whitney tests. Statistical analyses of B, D, F, H comprise Spearman correlations.
3.4. Lower NETosis induction levels by plasma of patients with COVID-19 treated with dexamethasone
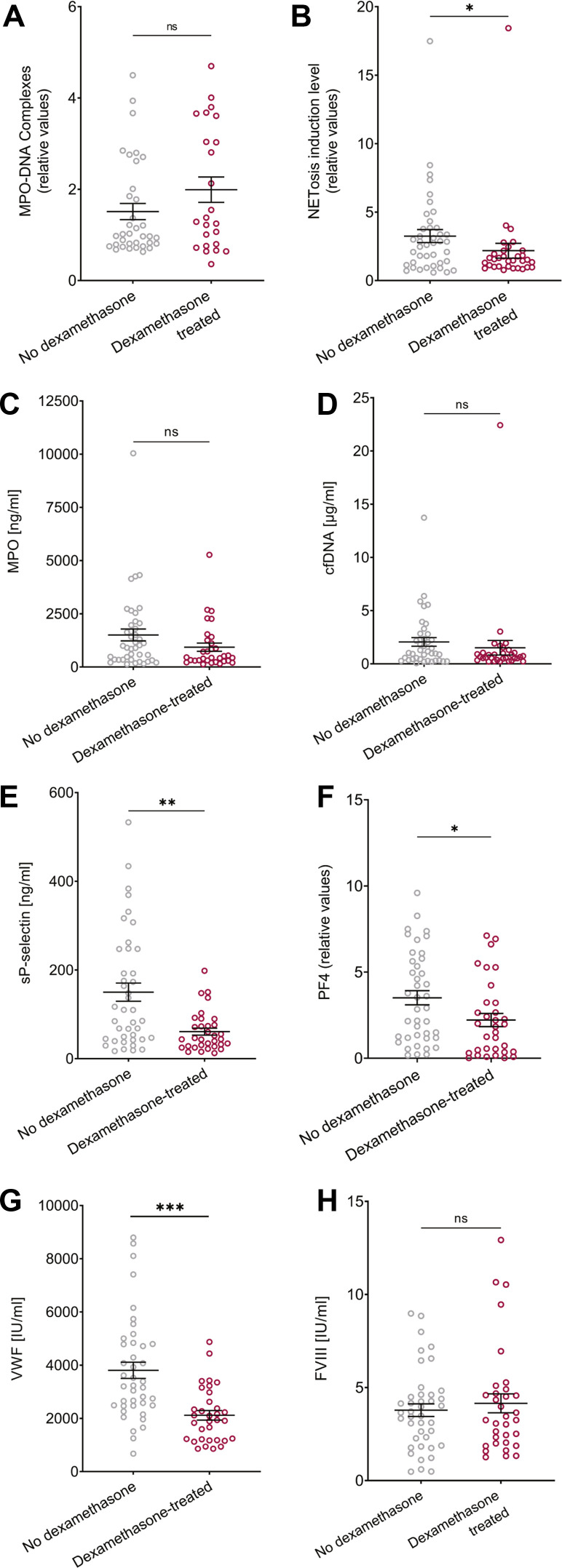
Dexamethasone corticosteroid treatment is one of the few treatments shown in randomized clinical trials to have a protective effect in COVID-19 [32]; we therefore divided our samples by dexamethasone treatment to see whether dexamethasone impacted NET-related measurements in plasma samples of patients with severe/critical COVID-19 (Figure 4 A-D). In our patient cohorts, dexamethasone was administered 0 to 14 days before plasma sample collection, with a mean of 2.4 days (median of 1 day). The levels of MPO-DNA complexes, MPO, and cfDNA were significantly higher stratified by dexamethasone treatment, whereas the NETosis induction levels were significantly lower. Similar to NETosis induction measurements, PF4, VWF, and sP-selectin levels were lower after dexamethasone treatment (Figure 4E–H). No differences were observed for NETosis induction and MPO-DNA complex levels between ventilated and not ventilated patients regardless of dexamethasone treatment (Supplementary Figure S6). These data demonstrate detection differences between MPO-DNA complex levels vs. NETosis induction.
Figure 4.
Subgroup analysis of severe/critical COVID-19 subjects with or without dexamethasone treatment. (A) MPO-DNA (not treated n = 35, treated n = 24, p = .2203). (B) NETosis induction level (not treated n = 41, treated n = 32, p = .0174). (C) MPO (not treated n = 41, treated n = 32, p = .0689). (D) cfDNA (not treated n = 40, treated n = 32, p = .0784). (E) sP-selectin, p = .0014. (F) PF4, p = .0213. (G) VWF, p < .0001. (H) FVIII, p = .8989. For (E)-(H), not treated n = 41, treated n = 33.
3.5. Recovery and long COVID
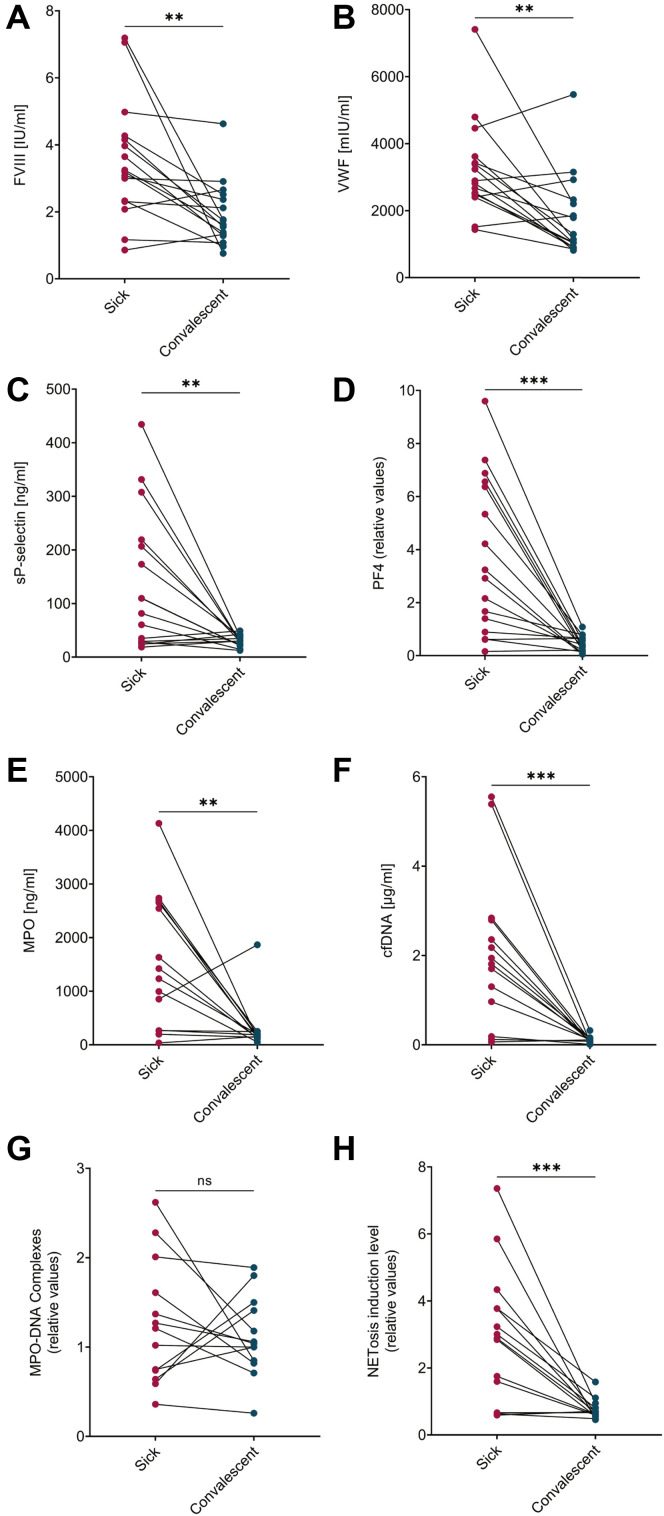
To analyze coagulation and inflammation processes in patients after COVID-19 recovery, plasma levels of platelet activation markers, coagulation factors, and NET-related measurements were compared among 16 individual patients at the time of illness and after their recovery (Figure 5 ). All analyzed measurements, except for MPO-DNA, showed a significant reduction from acute disease into convalescence. Several parameters including VWF, FVIII, and NET induction level, although significantly reduced compared to the peak of disease severity, remained higher than expected in several patients, indicating potential ongoing disease in these patients.
Figure 5.
Paired comparisons between samples taken during acute COVID-19 and in convalescence. (A) FVIII (n = 16), p = .0016. (B) VWF (n = 16), p = .0021. (C) sP-selectin (n = 16), p = .0052. (D) PF4 (n = 16), p = .0002. (E) MPO (n = 14), p = .0052. (F) cfDNA (n = 14), p = .0004, (G) MPO-DNA (n = 13), p = .4548, (H) NETosis induction level (n = 15), p = .0009. Data is not presented for samples with insufficient volume for analysis, 2 COVID-19 samples with under detection limit and 1 with above detection limit MPO-DNA complex levels.
Patients who reported at least one symptom unrelated to their baseline health that continued >1 month postrecovery were defined as having long COVID or as “long haulers” in this study. Reported symptoms and incidence among the long hauler cohort (Table S4) are similar to other studies [23,[33], [34], [35]]. According to our long COVID definition, 46 of 66 (70%) convalescent plasma samples were obtained from patients with postacute COVID-19 syndrome, out of which 34 (74%) were initially hospitalized during their illness. The most prevalent symptoms documented in our long COVID cohort included fatigue, dyspnea, chest pressure, cough, and lung damage (Supplementary Table S4).
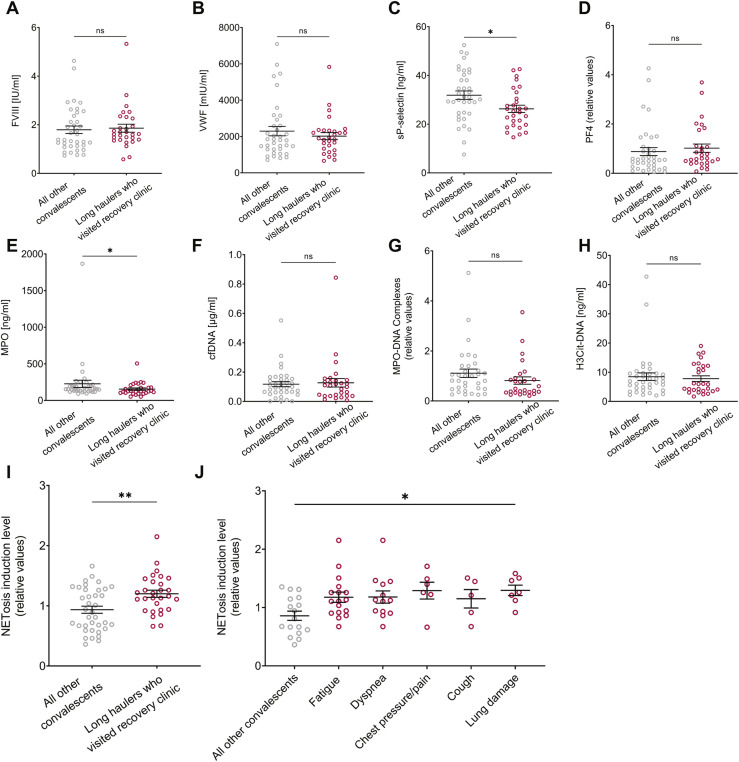
To explore possible predictors for patients with long COVID, we evaluated FVIII, sP-selectin, VWF, PF4, and NET-related measurements in the convalescent subgroups. The convalescent cohort analysis included H3Cit-DNA plasma level quantification, as an additional specific NET measurement [26]. When comparing NETosis induction levels of long haulers who experienced the most common long COVID symptoms vs. those who fully recovered (non-long COVID), no significant differences were found (Supplementary Figure S7A). No significant differences could be found between patients with long COVID and non–long-COVID patients in all biomarker measurements (except for lower sP-selectin levels) (Supplementary Figure S7). We next assessed the same markers in patients who sought medical care through a COVID-19 recovery clinic. Pathological findings of lung damage were documented only for 7 patients, and most of their coagulation factor values and/or of MPO-DNA were not significantly different from the other convalescent patients (Figure 6 ). Interestingly, long haulers who attended the COVID-19 recovery clinic in the Shaare Zedek Medical Center had significantly higher levels of NETosis induction than the long haulers who did not attend the clinic (Figure 6I), and a significant difference in NETosis induction was maintained for patients in the COVID-19 recovery clinic with the most common symptoms (Figure 6J). In summary, patients with symptoms severe enough to seek post-COVID medical care strikingly maintained a systemic plasma environment conducive to NET formation.
Figure 6.
Platelet activation markers, coagulation factors, and NET-related measurements in long COVID. Comparisons between long haulers who visited a recovery clinic (nlh) and all other convalescents (nc) were analyzed for (A) FVIII (nc = 37, nlh = 29), p = .4275, (B) VWF (nc = 37, nlh = 29), p = .9770, (C) sP-selectin (nc = 37, nlh = 29), p = .0178, (D) PF4 (nc = 37, nlh = 29), p = .2325, (E) MPO (nc = 36, nlh = 29), p = .0447, (F) cfDNA (nc = 36, nlh = 29), p = .7957, (G) MPO-DNA complexes (nc = 34, nlh = 27), p = .0860, (H) H3Cit-DNA (nc = 33, nlh = 28), p = .0755, (I) NETosis induction levels (nc = 37, nlh = 29), p = .0088. Statistical analysis was performed using two-tailed Mann–Whitney tests. (J) NETosis induction levels of recovery clinic long haulers who experienced the most common symptoms and of all other convalescents (n = 17). Fatigue (n = 25), shortness of breath (n = 18), chest pressure/pain (n = 8), cough (n = 7), and lung damage (n = 7). A significant difference was found between the presented groups according to the Kruskal-Wallis test (p = .0306).
4. Discussion
NETosis is well-documented as a key player in COVID-19-associated immunothrombosis [8,11,36,37]. After recovery from illness, many patients with COVID-19 have persistent symptoms contributing to postacute COVID syndrome (long COVID), especially those who initially experienced symptomatic COVID-19 [38]. We demonstrate for the first time that this pathology may be exacerbated by ongoing propensity for NETosis.
SARS-CoV-2-induced inflammation involves endothelial cell and platelet activation strongly associated with disease progression and severity [39]. Activated platelets and endothelial cells stimulate NETosis through soluble factors and/or direct contact between the neutrophil and affected cells, including markers quantified in this study [[40], [41], [42]]. These acute-phase reactants have been described at very high levels in the most severe forms of COVID-19 [8,43]. The acute COVID-19 cohorts in our study have risk factors and comorbidities commonly reported by others [[44], [45], [46], [47], [48]] and demonstrated extremely elevated acute-phase reactants related to platelet activation and coagulation, such as D-dimer, CRP, PF4, sP-selectin, VWF, and FVIII [20,49,50]. Similar to other studies, these coagulopathy and endotheliopathy related factors were especially pronounced in the cohort of patients with severe/critical COVID-19 and may contribute to the overall morbidity associated with COVID-19 given their contribution to increased thrombosis [51].
NET-related complexes such as H3Cit-DNA, NE-DNA, and MPO-DNA detection assays provide a specific estimation of NET fragment plasma levels. Previous studies of NETosis in COVID-19 used a combination of these assays. For example, multiple circulating components of NETs (nucleosomal H3Cit, cfDNA, and NE) were found to be higher in patients with COVID-19 than in healthy individuals and diminished after recovery [20]; plasma NET levels assessed by MPO-DNA correlated with COVID-19 severity, including intubation, PaO2/fraction of inspired oxygen, and Sequential Organ Failure Assessment scores [8]; measurement of MPO, MPO-DNA, and H3Cit revealed differences between non-COVID patients and patients with COVID-19; however, H3Cit measurement did not vary between patients with mild-moderate and critical COVID-19 [52].
Some measurements are reported as NET surrogates based on the quantification of a single component of NET structure. This includes cfDNA, NE, or MPO concentrations measured in plasma. Although these single-component assays provide quantitative data about the concentration in plasma reflecting general neutrophil activation or cell death, they do not fully reflect the NET complex structure and therefore should not be used alone as surrogate markers for NETs. Efforts are being made to standardize NET plasma evaluation in human samples by the Vascular Biology Scientific and Standardization Committee, a permanent committee of the International Society on Thrombosis and Haemostasis (ISTH) [53].
To further explore ongoing NETosis in COVID-19 severity and disease classification, we applied multiple NET-related assays on plasma samples from the patients with COVID-19 in two different Israeli health care centers, including convalescent patients. Our study suggests that not all NET-related measurements provide enough sensitivity to differentiate between disease stages. No significant differences were observed between cfDNA, MPO, or MPO-DNA levels in mild/moderate and severe/critical COVID-19 disease in affected patients. Moreover, MPO-DNA did not correlate with thrombotic markers in the COVID-19 group, in contrast to what has been reported in other studies [8,11,54]. The MPO-DNA assay resulted in many samples with undetectable levels, providing another limitation. Accordingly, direct measurements of NET fragments in plasma may not be sufficient for prognosis of a specific subject. The variability of individual levels of endogenous DNase within plasma samples released at different stages of disease may lead to differences in the final measurement of NET complexes. It is important to recognize, this may make comparisons between individuals challenging even within the same disease or clinical cohort.
NETosis induction has previously been reported in healthy neutrophils after incubation with a limited number of selected COVID-19 plasma and serum samples [8,18,19]. We now show at a large individual sample level that NETosis induction correlates with platelet activation and coagulation markers and distinguishes between patients in different stages of disease progression, whereas MPO-DNA levels could not detect these differences. Moreover, for 16 patients with COVID-19, paired analyses of samples taken during acute illness and convalescence demonstrated significantly lower values of NETosis induction levels after patient recovery. Lower levels of coagulation and platelet activation factors but not of MPO-DNA complexes were demonstrated in the same patients.
These findings suggest that although the MPO-DNA ELISA assay can detect NET fragments in plasma, it might not be suitable to prognose COVID-19 illness. Furthermore, it fails to detect ongoing pathological processes in long COVID. Nonspecific signals have also been reported in a different assay format for measuring MPO-DNA complexes [55]. These results further highlight NETosis induction level as an additional promising readout for COVID-19 illness severity. Such measurements of NETosis induction could provide biological insight as to why a state of exacerbated NETosis might occur in sicker patients with acute and chronic disease. This approach has been reported with plasma from autoimmune disease [56], antiphospholipid syndrome [57], and sepsis [58]. Using our NETosis induction assay, we observed several important clinical associations. In our study cohort, MPO-DNA could not detect significant differences in plasma NET levels related to mechanical ventilation nor to ICU admission (as previously reported [59]). However, the NETosis induction assay demonstrated significantly higher levels in the sicker, ventilated population of patients. NETosis induction by COVID-19 serum or plasma has been previously documented using healthy neutrophils, but in smaller sample sizes without detailed clinical correlation [8,10,18]. Our study is the first to our knowledge to quantitatively investigate NETosis induction capability as a marker of disease severity in acute and chronic COVID-19.
Dexamethasone is recommended as an immunomodulator for patients hospitalized due to COVID-19 who require oxygen support by the World Health Organization and Centers for Disease Control and Prevention, and its use is implemented in Israel [24,60,61]. We observed that patients with severe/critical COVID-19 treated with dexamethasone had significantly low NETosis induction levels, whereas their MPO-DNA levels were similar to the nontreated patients. These contradictory findings could be explained by the fact that MPO-DNA may reflect existing NET levels in plasma at a specific timepoint and these highly complex levels may already have been present before sample collection (in fact contributing to the need for dexamethasone treatment). Dexamethasone administration can inhibit cytokine production (such as IL-6, IL-8, TNFα) [62,63], which is important for neutrophil activation. This can explain partially the lower ex vivo NET formation by isolated neutrophils after incubation with the plasma of dexamethasone treated patients [64,65]. Because NETosis induction levels likely reflect the functional improvement in patient condition after dexamethasone treatment and reduced inflammatory cytokines, it may provide more time-sensitive and current information regarding NET-related status.
Postacute COVID-19 syndrome is characterized by symptoms experienced >4 weeks after recovery from infection. Although this condition is reported globally, no agreed definition has been established yet and no unifying mechanism exists for long COVID [23,35,[66], [67], [68]]. Because we demonstrated NETosis induction level as a potential predictor for disease prognosis, we used the same assay to evaluate long COVID plasma samples. The incidence of postacute COVID-19 syndrome among analyzed patients depends on the way the cohort is defined in individual studies; the symptoms and study methodology for how outcome was evaluated can affect the calculated occurrence (voluntary questioner, blood tests, and physical examination). Studies from Italy and France report up to 87% incidence for long COVID in previously hospitalized patients, including 30%–43% with dyspnea and 40%–53% with fatigue [34,69]. Long COVID symptoms are also found in patients who were not hospitalized, with a study in Switzerland reporting at least 32% of nonhospitalized patients with COVID-19 experiencing symptoms 30 to 45 days after diagnosis [33]. In the current study, we defined long COVID or “long haulers” as any patient who reported at least one symptom not related to their normal state of health that persisted for ≥1 month after acute disease recovery. According to this definition, which enables the most inclusive approach for postacute COVID-19 syndrome, nearly 70% of our convalescent cohort experienced long COVID symptoms and almost three-quarters of these patients with long COVID were hospitalized during their active disease. This is consistent with previous reports linking long COVID to hospitalization and disease severity [35,38]. Important to note, only 3 patients in our long COVID cohort were in the ICU which supports the diagnosis of long COVID and not post-ICU syndrome in our study cohort.
In our study, patients with long COVID who proactively attended a Recovery Clinic (presumably for symptom management) had significantly higher NETosis induction levels than the remaining convalescent patients, whereas other NET-related measurements such as cfDNA and MPO-DNA did not demonstrate this trend. Furthermore, significantly higher NETosis induction levels were grouped by the most common symptoms. These data provide the first evidence that NETosis induction levels may reflect the underlying pathophysiology of long COVID due to previous and possibly still increased NETosis with immunothrombosis and end-organ damage.
This study includes several limitations due to its retrospective design. The acute COVID-19 cohorts include only hospitalized patients. During the first pandemic wave in Israel (March-May 2020)[70], all people infected with SARS-CoV-2 were hospitalized. As the pandemic progressed, only the patients with COVID-19 who required inpatient medical care were admitted. Not all plasma samples were collected at the same time point after the patient first tested positive for COVID-19, preventing a comprehensive longitudinal assessment based on the severity at similar timepoints after infection. Another limitation is the lack of a globally accepted definition for long COVID. Accordingly, separate analyses that reflect the varied definitions for long COVID are presented.
In summary, this study provides a comprehensive investigation of COVID-19 and convalescent patients in Israel, with an emphasis on persistent NETosis capability as a key contributor to disease manifestation. Our sample size enabled us to uncover nuances in NET detection and expands existing knowledge about NETosis in COVID-19, including the first description of increased NETosis induction in long COVID. Continued efforts that degrade or block NET formation (e.g., anti-inflammatories [71], DNase administration [72,73], NET inhibitors [8,74,75]) may prove an effective strategy in COVID-19. Measurement of NETosis induction level capability is suggested as a clinical biomarker for ongoing pathology in acute and chronic COVID-19.
Acknowledgments
We thank Trent Fowler and Moran Bleiwis for operational support.
Author contributions
N.K., S.S., J.D.S., M.G., and K.M. wrote the manuscript. N.K., S.S., S.N., A.D., A.S., H.P., M.G., and K.M. designed and performed experiments, analyzed, and interpreted data. E.P. designed experiments and acquired data. S.P, A.R.P., I.L., S.I.C.M., R.A., N.C., R.K., A.J., A.R., and E.B. acquired samples and data. A.F.C., E.B., A.R.P., A.S., and C.C.Y. critically reviewed the manuscript.
Declaration of competing interests
N.K., S.S., S.N., A.D., A.S., H.P., E.P., A.F.C., J.D.S., and M.G. are current or former employees of Peel Therapeutics and hold share options in the company. K.M. and A.S. received consulting fees for scientific advice to Peel Therapeutics and hold stock options in the company. C.C.Y. authors a US patent (patent no. 10,232,023 B2) held by the University of Utah for the use of NET-inhibitory peptides for the "treatment of and prophylaxis against inflammatory disorders," for which Peel Therapeutics, Inc. holds the exclusive license. C.C.Y. and J.D.S. are co-inventors on patent application WO2021226111A1 for NET-inhibitory peptides to treat and prevent immunothrombosis in COVID-19 acute respiratory distress syndrome. S.P., A.R.P, I.L., S.I.C.M., R.A., N.C., R.K., A.J., A.R. and E.B. have no conflicts of interest to declare.
Footnotes
Funding information This work was sponsored by Peel Therapeutics, Inc., and supported in part by the US NIH (R01HD093826 to C.C.Y. - NICHD; Peel Therapeutics, Inc. (Sponsored Research Agreement to C.C.Y.), and the University of Utah Department of Pediatrics, Division of Neonatology.
Manuscript handled by: Patricia Liaw
Final decision: Patricia Liaw, 13 February 2023
Joshua D. Schiffman, Mor Goldfeder, and Kimberly Martinod contributed equally as senior co-authors.
The online version contains supplementary material available at https://doi.org/10.1016/j.jtha.2023.02.033
Supplementary Material
References
- 1.WHO Coronavirus (COVID-19) Dashboard. World Health Organization. Available online: https://covid19.who.int/. Last updated: 13 September 2022. In: World Health Organization.
- 2.Hu B., Guo H., Zhou P., Shi Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 4.Bouck E.G., Denorme F., Holle L.A., Middelton E.A., Blair A.M., de Laat B., Schiffman J.D., Yost C.C., Rondina M.T., Wolberg A.S., Campbell R.A. COVID-19 and sepsis are associated with different abnormalities in plasma procoagulant and fibrinolytic activity. Arterioscler Thromb Vasc Biol. 2021;41:401–414. doi: 10.1161/ATVBAHA.120.315338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mizurini D.M., Hottz E.D., Bozza P.T., Monteiro R.Q. Fundamentals in Covid-19-associated thrombosis: molecular and cellular aspects. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.785738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li S., Jiang L., Li X., Lin F., Wang Y., Li B., Jiang T., An W., Liu S., Liu H., Xu P., Zhao L., Zhang L., Mu J., Wang H., Kang J., Li Y., Huang L., Zhu C., Zhao S., Lu J., Ji J., Zhao J. Clinical and pathological investigation of patients with severe COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McElvaney O.J., McEvoy N.L., McElvaney O.F., Carroll T.P., Murphy M.P., Dunlea D.M., Ní Choileáin O., Clarke J., O'Connor E., Hogan G., Ryan D., Sulaiman I., Gunaratnam C., Branagan P., O'Brien M.E., Morgan R.K., Costello R.W., Hurley K., Walsh S., de Barra E., et al. Characterization of the inflammatory response to severe Covid-19 illness. Am J Respir Crit Care Med. 2020;202:812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Middleton E.A., He X.Y., Denorme F., Campbell R.A., Ng D., Salvatore S.P., Mostyka M., Baxter-Stoltzfus A., Borczuk A.C., Loda M., Cody M.J., Manne B.K., Portier I., Harris E.S., Petrey A.C., Beswick E.J., Caulin A.F., Iovino A., Abegglen L.M., Weyrich A.S., et al. Neutrophil extracellular traps contribute to immunothrombosis in COVID-19 acute respiratory distress syndrome. Blood. 2020;136:1169–1179. doi: 10.1182/blood.2020007008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonaventura A., Vecchié A., Dagna L., Martinod K., Dixon D.L., Van Tassell B.W., Dentali F., Montecucco F., Massberg S., Levi M., Abbate A. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21:319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nicolai L., Leunig A., Brambs S., Kaiser R., Weinberger T., Weigand M., Muenchhoff M., Hellmuth J.C., Ledderose S., Schulz H., Scherer C., Rudelius M., Zoller M., Höchter D., Keppler O., Teupser D., Zwißler B., von Bergwelt-Baildon M., Kääb S., Massberg S., et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142:1176–1189. doi: 10.1161/CIRCULATIONAHA.120.048488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leppkes M., Knopf J., Naschberger E., Lindemann A., Singh J., Herrmann I., Stürzl M., Staats L., Mahajan A., Schauer C., Kremer A.N., Völkl S., Amann K., Evert K., Falkeis C., Wehrfritz A., Rieker R.J., Hartmann A., Kremer A.E., Neurath M.F., et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58 doi: 10.1016/j.ebiom.2020.102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ventura-Santana E., Ninan J.R., Snyder C.M., Okeke E.B. Neutrophil extracellular traps, sepsis and COVID-19 – a tripod stand. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.902206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conway E.M., Mackman N., Warren R.Q., Wolberg A.S., Mosnier L.O., Campbell R.A., Gralinski L.E., Rondina M.T., van de Veerdonk F.L., Hoffmeister K.M., Griffin J.H., Nugent D., Moon K., Morrissey J.H. Understanding COVID-19-associated coagulopathy. Nat Rev Immunol. 2022;22:639–649. doi: 10.1038/s41577-022-00762-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S., Weinrauch Y., Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 15.Jorch S.K., Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. 2017;23:279–287. doi: 10.1038/nm.4294. [DOI] [PubMed] [Google Scholar]
- 16.Mutua V., Gershwin L.J. A review of neutrophil extracellular traps (NETs) in disease: potential anti-NETs therapeutics. Clin Rev Allergy Immunol. 2021;61:194–211. doi: 10.1007/s12016-020-08804-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blanch-Ruiz M.A., Ortega-Luna R., Gómez-García G., Martínez-Cuesta M.Á., Álvarez Á. Role of neutrophil extracellular traps in COVID-19 progression: an insight for effective treatment. Biomedicines. 2022;10:31. doi: 10.3390/biomedicines10010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., Blair C., Weber A., Barnes B.J., Egeblad M., Woods R.J., Kanthi Y., Knight J.S. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skendros P., Mitsios A., Chrysanthopoulou A., Mastellos D.C., Metallidis S., Rafailidis P., Ntinopoulou M., Sertaridou E., Tsironidou V., Tsigalou C., Tektonidou M., Konstantinidis T., Papagoras C., Mitroulis I., Germanidis G., Lambris J.D., Ritis K. Complement and tissue factor-enriched neutrophil extracellular traps are key drivers in COVID-19 immunothrombosis. J Clin Invest. 2020;130:6151–6157. doi: 10.1172/JCI141374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ng H., Havervall S., Rosell A., Aguilera K., Parv K., von Meijenfeldt F.A., Lisman T., Mackman N., Thalin C., Phillipson M. Circulating markers of neutrophil extracellular traps are of prognostic value in patients with COVID-19. Arterioscler Thromb Vasc Biol. 2021;41:988–994. doi: 10.1161/ATVBAHA.120.315267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arcanjo A., Logullo J., Menezes C.C.B., de Souza Carvalho Giangiarulo T.C., Dos Reis M.C., de Castro G.M.M., da Silva Fontes Y., Todeschini A.R., Freire-de-Lima L., Decoté-Ricardo D., Ferreira-Pereira A., Freire-de-Lima C.G., Barroso S.P.C., Takiya C., Conceição-Silva F., Savino W., Morrot A. The emerging role of neutrophil extracellular traps in severe acute respiratory syndrome coronavirus 2 (COVID-19) Sci Rep. 2020;10 doi: 10.1038/s41598-020-76781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fogarty H., Townsend L., Morrin H., Ahmad A., Comerford C., Karampini E., Englert H., Byrne M., Bergin C., O'Sullivan J.M., Martin-Loeches I., Nadarajan P., Bannan C., Mallon P.W., Curley G.F., Preston R.J.S., Rehill A.M., McGonagle D., Ni Cheallaigh C., Baker R.I., et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost. 2021;19:2546–2553. doi: 10.1111/jth.15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rajan S, Khunti K, Alwan N, Steves C, MacDermott N, Morsella A, Angulo E, Winkelmann J, Bryndová L, Fronteira I, et al. European Observatory Policy Briefs. In: In the wake of the pandemic: Preparing for Long COVID. Copenhagen (Denmark): European Observatory on Health Systems and Policies© World Health Organization 2021 (acting as the host organization for, and secretariat of, the European Observatory on Health Systems and Policies). 2021. [PubMed]
- 24.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/ [accessed November 29, 2021]. [PubMed]
- 25.Kessenbrock K., Krumbholz M., Schonermarck U., Back W., Gross W.L., Werb Z., Grone H.J., Brinkmann V., Jenne D.E. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15:623–625. doi: 10.1038/nm.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thålin C., Aguilera K., Hall N.W., Marunde M.R., Burg J.M., Rosell A., Daleskog M., Månsson M., Hisada Y., Meiners M.J., Sun Z.W., Whelihan M.F., Cheek M.A., Howard S.A., Saxena-Beem S., Noubouossie D.F., Key N.S., Sheikh S.Z., Keogh M.C., Cowles M.W., et al. Quantification of citrullinated histones: Development of an improved assay to reliably quantify nucleosomal H3Cit in human plasma. J Thromb Haemost. 2020;18:2732–2743. doi: 10.1111/jth.15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J., Wang X., Chen J., Cai Y., Deng A., Yang M. Association between ABO blood groups and risk of SARS-CoV-2 pneumonia. Br J Haematol. 2020;190:24–27. doi: 10.1111/bjh.16797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blood type distribution in Israel population. MAGEN DAVID ADOM IN ISRAEL (MDA) https://web.archive.org/web/20101126002311/http://mdais.org/362 [accessed December 26, 2021].
- 29.Ghahramani S., Tabrizi R., Lankarani K.B., Kashani S.M.A., Rezaei S., Zeidi N., Akbari M., Heydari S.T., Akbari H., Nowrouzi-Sohrabi P., Ahmadizar F. Laboratory features of severe vs. non-severe COVID-19 patients in Asian populations: a systematic review and meta-analysis. Eur J Med Res. 2020;25:30. doi: 10.1186/s40001-020-00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manne B.K., Denorme F., Middleton E.A., Portier I., Rowley J.W., Stubben C., Petrey A.C., Tolley N.D., Guo L., Cody M., Weyrich A.S., Yost C.C., Rondina M.T., Campbell R.A. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136:1317–1329. doi: 10.1182/blood.2020007214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Comer S.P., Cullivan S., Szklanna P.B., Weiss L., Cullen S., Kelliher S., Smolenski A., Murphy C., Altaie H., Curran J., O'Reilly K., Cotter A.G., Marsh B., Gaine S., Mallon P., McCullagh B., Moran N., Ní Áinle F., Kevane B., Maguire P.B., et al. COVID-19 induces a hyperactive phenotype in circulating platelets. PLoS Biol. 2021;19 doi: 10.1371/journal.pbio.3001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., Prudon B., Green C., Felton T., Chadwick D., Rege K., Fegan C., Chappell L.C., Faust S.N., Jaki T., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nehme M., Braillard O., Alcoba G., Aebischer Perone S., Courvoisier D., Chappuis F., Guessous I., COVICARE TEAM COVID-19 Symptoms: longitudinal evolution and persistence in outpatient settings. Ann Intern Med. 2021;174:723–725. doi: 10.7326/M20-5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carfi A., Bernabei R., Landi F. Gemelli Against C-P-ACSG. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Seres D.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radermecker C., Detrembleur N., Guiot J., Cavalier E., Henket M., d'Emal C., Vanwinge C., Cataldo D., Oury C., Delvenne P., Marichal T. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J Exp Med. 2020;217 doi: 10.1084/jem.20201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petito E., Falcinelli E., Paliani U., Cesari E., Vaudo G., Sebastiano M., Cerotto V., Guglielmini G., Gori F., Malvestiti M., Becattini C., Paciullo F., De Robertis E., Bury L., Lazzarini T., Gresele P. COVIR study investigators. Association of neutrophil activation, more than platelet activation, with thrombotic complications in coronavirus disease 2019. J Infect Dis. 2021;223:933–944. doi: 10.1093/infdis/jiaa756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sudre C.H., Murray B., Varsavsky T., Graham M.S., Penfold R.S., Bowyer R.C., Pujol J.C., Klaser K., Antonelli M., Canas L.S., Molteni E., Modat M., Jorge Cardoso M., May A., Ganesh S., Davies R., Nguyen L.H., Drew D.A., Astley C.M., Joshi A.D., et al. Attributes and predictors of long COVID. Nat Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dupont A., Rauch A., Staessens S., Moussa M., Rosa M., Corseaux D., Jeanpierre E., Goutay J., Caplan M., Varlet P., Lefevre G., Lassalle F., Bauters A., Faure K., Lambert M., Duhamel A., Labreuche J., Garrigue D., De Meyer S.F., Staels B., et al. Vascular endothelial damage in the pathogenesis of organ injury in severe COVID-19. Arterioscler Thromb Vasc Biol. 2021;41:1760–1773. doi: 10.1161/ATVBAHA.120.315595. [DOI] [PubMed] [Google Scholar]
- 40.Wagner D.D., Frenette P.S. The vessel wall and its interactions. Blood. 2008;111:5271–5281. doi: 10.1182/blood-2008-01-078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gollomp K., Kim M., Johnston I., Hayes V., Welsh J., Arepally G.M., Kahn M., Lambert M.P., Cuker A., Cines D.B., Rauova L., Kowalska M.A., Poncz M. Neutrophil accumulation and NET release contribute to thrombosis in HIT. JCI Insight. 2018;3 doi: 10.1172/jci.insight.99445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenkins P.V., Rawley O., Smith O.P., O'Donnell J.S. Elevated factor VIII levels and risk of venous thrombosis. Br J Haematol. 2012;157:653–663. doi: 10.1111/j.1365-2141.2012.09134.x. [DOI] [PubMed] [Google Scholar]
- 43.Escher R., Breakey N., Lammle B. Severe COVID-19 infection associated with endothelial activation. Thromb Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., Cockburn J., McDonald H.I., MacKenna B., Tomlinson L., Douglas I.J., Rentsch C.T., Mathur R., Wong A.Y.S., Grieve R., Harrison D., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammad R., Eldosoky M., Fouad S.H., Elgendy A., Tawfeik A.M., Alboraie M., Abdelmaksoud M.F. Circulating cell-free DNA, peripheral lymphocyte subsets alterations and neutrophil lymphocyte ratio in assessment of COVID-19 severity. Innate Immun. 2021;27:240–250. doi: 10.1177/1753425921995577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu H., Chen S., Liu M., Nie H., Lu H. Comorbid chronic diseases are strongly correlated with disease severity among COVID-19 patients: a systematic review and meta-analysis. Aging Dis. 2020;11:668–678. doi: 10.14336/AD.2020.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., Hosein Z., Padda I., Mangat J., Altaf M. Comorbidity and its impact on patients with covid-19. SN Compr Clin Med. 2020;2:1069–1076. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biswas M., Rahaman S., Biswas T.K., Haque Z., Ibrahim B. Association of sex, age, and comorbidities with mortality in COVID-19 patients: a systematic review and meta-analysis. Intervirology. 2020:1–12. doi: 10.1159/000512592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yatim N., Boussier J., Chocron R., Hadjadj J., Philippe A., Gendron N., Barnabei L., Charbit B., Szwebel T.A., Carlier N., Pène F., Azoulay C., Khider L., Mirault T., Diehl J.L., Guerin C.L., Rieux-Laucat F., Duffy D., Kernéis S., Smadja D.M., et al. Platelet activation in critically ill COVID-19 patients. Ann Intensive Care. 2021;11:113. doi: 10.1186/s13613-021-00899-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ladikou E.E., Sivaloganathan H., Milne K.M., Arter W.E., Ramasamy R., Saad R., Stoneham S.M., Philips B., Eziefula A.C., Chevassut T. Von Willebrand factor (vWF): marker of endothelial damage and thrombotic risk in COVID-19? Clin Med (Lond) 2020;20:e178–e182. doi: 10.7861/clinmed.2020-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goshua G., Pine A.B., Meizlish M.L., Chang C.H., Zhang H., Bahel P., Baluha A., Bar N., Bona R.D., Burns A.J., Dela Cruz C.S., Dumont A., Halene S., Hwa J., Koff J., Menninger H., Neparidze N., Price C., Siner J.M., Tormey C., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanderbeke L., Van Mol P., Van Herck Y., De Smet F., Humblet-Baron S., Martinod K., et al. Monocyte-driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID-19 disease severity. Nat Commun. 2021;12:1–15. doi: 10.1038/s41467-021-24360-w. 4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Towards standardization of Neutrophil Extracellular Trap (NET) measurements in patient samples, Vascular Biology Subcommittee, International Society on Thrombosis and Haemostasis (ISTH), 2019. https://cdn.ymaws.com/www.isth.org/resource/resmgr/ssc/ssc_subcommittee_project_mar.pdf
- 54.Zuo Y., Zuo M., Yalavarthi S., Gockman K., Madison J.A., Shi H., Woodard W., Lezak S.P., Lugogo N.L., Knight J.S., Kanthi Y. Neutrophil extracellular traps and thrombosis in COVID-19. J Thromb Thrombolysis. 2021;51:446–453. doi: 10.1007/s11239-020-02324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayden H., Ibrahim N., Klopf J., Zagrapan B., Mauracher L.M., Hell L., Hofbauer T.M., Ondracek A.S., Schoergenhofer C., Jilma B., Lang I.M., Pabinger I., Eilenberg W., Neumayer C., Brostjan C. ELISA detection of MPO-DNA complexes in human plasma is error-prone and yields limited information on neutrophil extracellular traps formed in vivo. PLoS One. 2021;16 doi: 10.1371/journal.pone.0250265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sur Chowdhury C., Giaglis S., Walker U.A., Buser A., Hahn S., Hasler P. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res Ther. 2014;16:R122. doi: 10.1186/ar4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yalavarthi S., Gould T.J., Rao A.N., Mazza L.F., Morris A.E., Núñez-Álvarez C., Hernández-Ramírez D., Bockenstedt P.L., Liaw P.C., Cabral A.R., Knight J.S. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol. 2015;67:2990–3003. doi: 10.1002/art.39247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abrams S.T., Morton B., Alhamdi Y., Alsabani M., Lane S., Welters I.D., Wang G., Toh C.H. A novel assay for neutrophil extracellular trap formation independently predicts disseminated intravascular coagulation and mortality in critically ill patients. Am J Respir Crit Care Med. 2019;200:869–880. doi: 10.1164/rccm.201811-2111OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mikacenic C., Moore R., Dmyterko V., West T.E., Altemeier W.A., Liles W.C., Lood C. Neutrophil extracellular traps (NETs) are increased in the alveolar spaces of patients with ventilator-associated pneumonia. Crit Care. 2018;22:358. doi: 10.1186/s13054-018-2290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Monitoring and treatment recommendations for COVID-19 patients. Israel ministry of health. The Israeli government website. https://www.gov.il/BlobFolder/reports/report-guide-treating-corona-patients/he/files_publications_corona_%D7%94%D7%9E%D7%9C%D7%A6%D7%95%D7%AA%20%D7%9C%D7%A0%D7%99%D7%98%D7%95%D7%A8%20%D7%95%D7%98%D7%99%D7%A4%D7%95%D7%9C%20%D7%91%D7%97%D7%95%D7%9C%D7%99%20COVID-19.pdf Updated 10 September 2020. [accessed September 23, 2022].
- 61.Assouline O., Ben-Chetrit E., Helviz Y., Kurd R., Leone M., Einav S. Experimental and compassionate drug use during the first wave of the COVID-19 pandemic: a retrospective single-center study. Adv Ther. 2021;38:5165–5177. doi: 10.1007/s12325-021-01890-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Brabander J., Michels E.H.A., van Linge C.C.A., Chouchane O., Douma R.A., Reijnders T.D.Y., Schuurman A.R., van Engelen T.S.R. Amsterdam UMC COVID-19 biobank study group; Wiersinga WJ, van der Poll T. Association between dexamethasone treatment and the host response in COVID-19 patients admitted to the general ward. Respir Res. 2022;23:145. doi: 10.1186/s12931-022-02060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahmed M.H., Hassan A. Dexamethasone for the treatment of coronavirus disease (COVID-19): a review. SN Compr Clin Med. 2020;2:2637–2646. doi: 10.1007/s42399-020-00610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gillot C., Favresse J., Mullier F., Lecompte T., Dogne J.M., Douxfils J. NETosis and the immune system in COVID-19: mechanisms and potential treatments. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.708302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keshari R.S., Jyoti A., Dubey M., Kothari N., Kohli M., Bogra J., Barthwal M.K., Dikshit M. Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PLoS One. 2012;7 doi: 10.1371/journal.pone.0048111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crook H., Raza S., Nowell J., Young M., Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374:n1648. doi: 10.1136/bmj.n1648. [DOI] [PubMed] [Google Scholar]
- 67.Rajan S, Khunti K, Alwan N, Steves C, MacDermott N, Morsella A, Angulo E, Winkelmann J, Bryndová L, Fronteira I, Gandré C, Or Z, Gerkens S, Sagan A, Simões J, Ricciardi W, de Belvis AG, Silenzi A, Bernal-Delgado E, Estupiñán-Romero F, et al. In the wake of the pandemic: Preparing for Long COVID [Internet]. Copenhagen (Denmark): European Observatory on Health Systems and Policies; 2021. [PubMed]
- 68.Groff D., Sun A., Ssentongo A.E., Ba D.M., Parsons N., Poudel G.R., Lekoubou A., Oh J.S., Ericson J.E., Ssentongo P., Chinchilli V.M. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carvalho-Schneider C., Laurent E., Lemaignen A., Beaufils E., Bourbao-Tournois C., Laribi S., Flament T., Ferreira-Maldent N., Bruyère F., Stefic K., Gaudy-Graffin C., Grammatico-Guillon L., Bernard L. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27:258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Israel COVID-19 data tracker, Israel Ministry of Health. In: Israel Ministry of Health [accessed October 13, 2021].
- 71.Caricchio R., Abbate A., Gordeev I., Meng J., Hsue P.Y., Neogi T., Arduino R., Fomina D., Bogdanov R., Stepanenko T., Ruiz-Seco P., Gónzalez-García A., Chen Y., Li Y., Whelan S., Noviello S., CAN-COVID Investigators Effect of canakinumab vs placebo on survival without invasive mechanical ventilation in patients hospitalized with severe COVID-19: a randomized clinical trial. JAMA. 2021;326:230–239. doi: 10.1001/jama.2021.9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okur H.K., Yalcin K., Tastan C., Demir S., Yurtsever B., Karakus G.S., Kancagi D.D., Abanuz S., Seyis U., Zengin R., Hemsinlioglu C., Kara M., Yildiz M.E., Deliceo E., Birgen N., Pelit N.B., Cuhadaroglu C., Kocagoz A.S., Ovali E. Preliminary report of in vitro and in vivo effectiveness of dornase alfa on SARS-CoV-2 infection. New Microbes New Infect. 2020;37 doi: 10.1016/j.nmni.2020.100756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weber A.G., Chau A.S., Egeblad M., Barnes B.J., Janowitz T. Nebulized in-line endotracheal dornase alfa and albuterol administered to mechanically ventilated COVID-19 patients: a case series. Mol Med. 2020;26:91. doi: 10.1186/s10020-020-00215-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Denorme F., Portier I., Rustad J.L., Cody M.J., de Araujo C.V., Hoki C., Alexander M.D., Grandhi R., Dyer M.R., Neal M.D., Majersik J.J., Yost C.C., Campbell R.A. Neutrophil extracellular traps regulate ischemic stroke brain injury. J Clin Invest. 2022;132 doi: 10.1172/JCI154225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Denorme F., Rustad J.L., Portier I., Crandell J.L., de Araujo C.V., Cody M.J., Campbell R.A., Yost C.C. Neutrophil extracellular trap inhibition improves survival in neonatal mouse infectious peritonitis. Pediatr Res. 2023;93:862–869. doi: 10.1038/s41390-022-02219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.