Abstract
This article describes the October 2020 proceedings of the Million Hearts Cardiac Rehabilitation Think Tank: Accelerating New Care Models, convened with representatives from professional organizations, cardiac rehabilitation (CR) programs, academic institutions, federal agencies, payers, and patient representative groups. As CR delivery evolves, terminology is evolving to reflect not where activities occur (eg, center, home) but how CR is delivered: in-person synchronous, synchronous with real-time audiovisual communication (virtual), or asynchronous (remote). Patients and CR staff may interact through ≥1 delivery modes. Though new models may change how CR is delivered and who can access CR, new models should not change what is delivered—a multidisciplinary program addressing CR core components. During the coronavirus disease 2019 (COVID-19) public health emergency, Medicare issued waivers to allow virtual CR; it is unclear whether these waivers will become permanent policy post-public health emergency. Given CR underuse and disparities in delivery, new models must equitably address patient and health system contributors to disparities. Strategies for implementing new CR care models address safety, exercise prescription, monitoring, and education. The available evidence supports the efficacy and safety of new CR care models. Still, additional research should study diverse populations, impact on patient-centered outcomes, effect on long-term outcomes and health care utilization, and implementation in diverse settings. CR is evolving to include in-person synchronous, virtual, and remote modes of delivery; there is significant enthusiasm for implementing new care models and learning how new care models can broaden access to CR, improve patient outcomes, and address health inequities.
Keywords: cardiac rehabilitation, delivery of health care, patient-centered care, patient advocacy, public health
Million Hearts 2022 is a national initiative, co-led by the Centers for Disease Control and Prevention and the Centers for Medicare and Medicaid Services (CMS), with the goal of preventing 1 million heart attacks, strokes, and other acute cardiovascular events in a 5-year period. Included in the strategies supported by the initiative is achieving a target of 70% participation in cardiac rehabilitation (CR) by eligible patients.1 CR is an evidence-based, class I guideline-recommended secondary prevention strategy for many cardiovascular conditions.2–6 Despite strong evidence of its benefits, CR is extremely underutilized; only 24% of Medicare fee-for-service beneficiaries eligible in 2016 participated in CR through 2017.7 In addition, disparities in participation exist related to age, sex, race/ethnicity, qualifying diagnosis, geography, comorbidities, health care system, and socioeconomic status (SES).7–14
Many barriers impede participation in the standard program of 36 center-based sessions, including transportation, parking, financial concerns, limited program hours, and competing responsibilities (eg, work or caregiving).15 Even if these barriers did not exist, studies suggest that if all center-based CR programs were at 110% capacity, existing programs could only accommodate ≈45% of eligible US patients.16 Moreover, many parts of the United States are CR deserts; 14% of adults live in counties where there are no CR centers, and 74% live in counties where there is <1 CR center per 100000 adults.17
The coronavirus disease 2019 (COVID-19) pandemic added an additional challenge to participation in center-based CR. To mitigate viral spread, many center-based CR programs closed or limited in-person services. As a result, to continue providing essential risk-reduction services, many programs pivoted to new delivery methods.
Million Hearts and the American Association of Cardiac and Pulmonary Rehabilitation (AACVPR) partnered to hold a think tank to advance new care models for CR. Evidence behind new care models has been growing, but many questions remain about implementation in the United States including standards, terminology, payment/reimbursement, scalability and spread, and approaches to address health equity. In October 2020, the Million Hearts Cardiac Rehabilitation Think Tank: Accelerating New Care Models convened, including representatives from professional organizations, CR programs, academic institutions, federal agencies, payers, and patient representative groups (see the Data Supplement for attendees). In this article, we describe the think tank proceedings and recommendations for accelerating new care models.
MODELS AND TERMINOLOGY
The absence of standardized terminology has contributed to confusion about new CR delivery models. Therefore, we propose the following framework to clarify terminology. Historically, CR delivery models have referred to the location where the patient participates in CR (eg, center, home). Recently, hybrid CR models have emerged, with some activities in the center and some in the home or community.18 Flexible delivery models are evolving to better meet patient needs. Future delivery of CR may involve CR activities in ≥1 locations, such as hospitals, physician offices, long-term care facilities, homes, gyms, workplaces, or other community locations. Patient and CR clinician interaction may occur through ≥1 modes of communication, such as in-person synchronous, synchronized real-time 2-way audiovisual communication, telephone, or asynchronous data exchange or messaging.
Many terms were discussed, including center, home, hybrid, hyflex (hybrid and flexible), innovative delivery model, remote, virtual, and patient-centered. Ultimately, it was recognized that terminology for CR delivery is evolving to reflect not where activities occur but how CR is delivered. We propose a new model for describing CR delivery (Figure 1). Virtual CR refers to synchronous CR delivered with real-time audiovisual communication technology to facilitate patient and clinician interaction during an exercise session. Remote CR refers to CR delivered with asynchronous activities without real-time communication between patients and clinicians at the time of an exercise session. An individual program may offer ≥1 modes of delivery; an individual patient may participate through ≥1 modes of delivery.
Figure 1. New models of cardiac rehabilitation (CR) delivery.
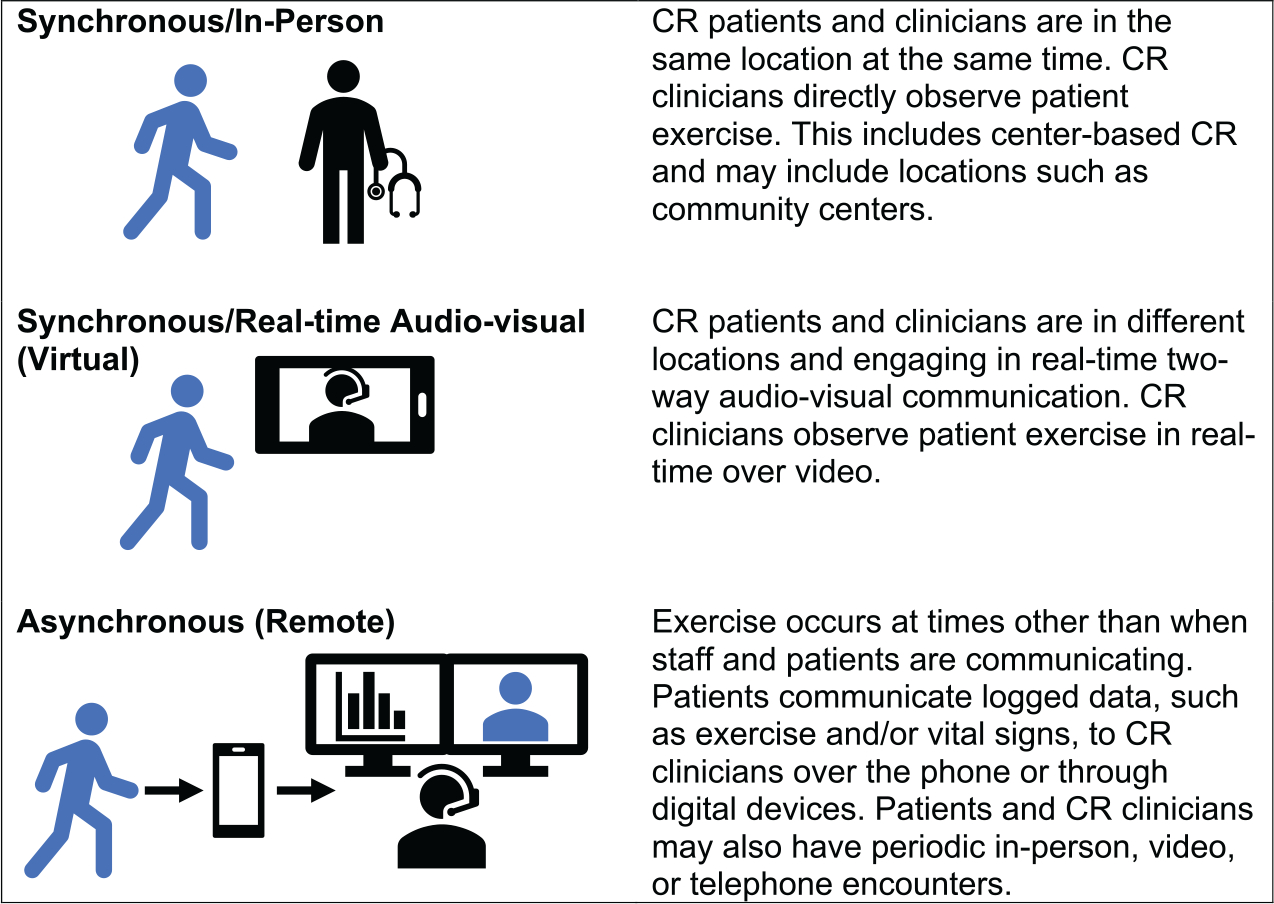
CR PROGRAM CORE COMPONENTS
There was a strong think tank consensus that regardless of the delivery mode, the multidisciplinary components of CR must be preserved. Although exercise training is a vital component of CR programs, it is not the only component. Programs must consider not only how they will provide exercise training but also how they will deliver the full range of CR services in new care models, including disease management (eg, medication adherence) and behavioral education and counseling (eg, healthy dietary patterns, psychosocial wellness, and tobacco cessation).
The structure of a CR program was defined in the Social Security Act (Table 1).19 According to this statute, CR programs can be located in a hospital (on or off the primary hospital campus) or in a physician’s office. In addition, programs must be supervised by a physician who must be immediately available to provide consultation or handle medical emergencies at all times CR services are being provided. Each patient must have an individualized treatment plan, signed by a physician and updated every 30 days. There are additional requirements for qualifying diagnoses, session duration, and intensive CR programs. These requirements apply specifically to patients with Medicare insurance. Nongovernmental payers tend to mirror Medicare but often have varying requirements for CR programs. Of note, nonphysician clinicians will be allowed to provide supervision for CR sessions in lieu of a physician beginning in 2024.
Table 1.
Cardiac Rehabilitation Program Requirements19
| Location |
| Hospital (on or off campus) or physician office |
| Physician supervision |
| Individualized treatment plan |
| Signed by a physician and updated every 30 d |
| Qualifying diagnoses |
| Acute myocardial infarction in the last 12 mo |
| Coronary artery bypass graft surgery |
| Coronary artery angioplasty or stenting |
| Heart valve repair or replacement |
| Heart or heart-lung transplantation |
| Stable angina |
| Stable chronic heart failure* |
| Requirements for session duration |
| Additional requirements for intensive cardiac rehabilitation programs |
| Required components (Table 2) |
Defined as left ventricular ejection fraction <35% after 6 wk of optimal medical therapy.
In addition to program structure, the Social Security Act also defines CR services.19 Based on these requirements, the AACVPR and the American Heart Association defined the core components of CR and secondary prevention programs (Table 2).20 CR is a comprehensive, multidisciplinary, secondary prevention program including exercise training, patient education on the core components of CR, nutritional counseling, cardiovascular disease risk factor modification, and psychosocial management. CR programs consist of a multidisciplinary team of clinicians including physicians, nurses, clinical exercise physiologists, behavioral health experts, dietitians, physical and respiratory therapists, and others who collaborate to deliver CR services. Any new delivery models must include the multidisciplinary team and these core components for successful delivery of CR.
Table 2.
American Association of Cardiovascular and Pulmonary Rehabilitation/American Heart Association Core Components of Cardiac Rehabilitation and Secondary Prevention Programs20
| Patient assessment |
| Nutritional counseling |
| Weight management |
| Blood pressure management |
| Lipid management |
| Diabetes management |
| Tobacco cessation |
| Psychosocial management |
| Physical activity counseling |
| Exercise training |
In addition to requirements for the structure and components of CR programs, professional societies have published quality and performance measures to promote high-quality care.21,22 The American College of Cardiology and American Heart Association performance measures address referral and enrollment of eligible patients from inpatient and outpatient settings, as well as quality measures of time to enrollment, adherence, and communication with referring clinicians.21 The National Quality Forum endorses referral from the inpatient and outpatient settings as quality measures. Healthcare Effectiveness Data and Information Set includes measures for the percentage of eligible members who attend ≥2, ≥12, ≥24, and ≥36 sessions within various time frames. Among programs that meet guidelines, AACVPR provides a recognition and certification program. Recently, AACVPR published additional performance measures including improving functional capacity, blood pressure control, reducing depressive symptoms, and tobacco cessation interventions.22 Consideration of evaluation and program certification pathways for new delivery models are needed to provide external validation of the quality of the services provided.
POLICY
Before the COVID-19 pandemic, CR was provided almost exclusively in-person. US examples of virtual or remote CR existed in the Henry Ford, Kaiser Permanente, and the Veterans Administration health systems.23–26 These delivery models were reimbursed by nongovernmental payers or conducted in a managed-care fashion. Medicare and most nongovernmental payers have historically only reimbursed CR sessions delivered in-person. Thus, in-person synchronous delivery of CR services has overwhelmingly been the most common delivery method in the United States.
As a result of the COVID-19 pandemic, CMS provided waivers to expand the use of audiovisual communications technology to deliver health care services, including CR. Because the overwhelming majority of CR is provided in a hospital outpatient setting, rather than a physician office setting, the remainder of this section focuses on hospital waivers. Similar opportunities, with some caveats, exist for physician office programs. These waivers allow CR delivery in a virtual, synchronous manner using real-time audiovisual communication technology. Hospitals wishing to obtain reimbursement from Medicare for these services must complete a number of administrative tasks such as registering the patient’s home as an outpatient department of the hospital and attaching applicable billing modifiers to indicate virtual delivery.27 CR professionals are encouraged to work with the relevant departments at their institutions regarding reimbursement. It is important to note that these waivers allow for the virtual, synchronous delivery of CR services but not remote, asynchronous delivery. Physicians still must be available for supervision and consultation for CR services, but they can use real-time audiovisual communications technology when needed for virtual sessions.
Though these services are being delivered virtually, all requirements and components of a CR program (Tables 1 and 2) must still be met. These waivers change how CR services can be delivered but do not change what must be delivered. Programs must still deliver the same comprehensive, multidisciplinary intervention of CR via any delivery model.
The future of virtual CR delivery after the public health emergency is unknown at this time (June 2021). There is great enthusiasm to make permanent many of the CMS waivers that have expanded the use of audiovisual communications technology for health care services. To what extent CR services will be included in these initiatives is unknown at present. If included, it may be that only virtual, synchronous delivery of CR services using real-time audiovisual communications technology would be reimbursed by CMS. As of June 2021, there is still no mechanism for CMS reimbursement of remote, asynchronous delivery of CR. In addition, whether nongovernmental payers will reimburse for virtual or remote delivery of CR services during or after the COVID-19 pandemic is unknown at present. Regardless, opportunities may exist to pilot reimbursement opportunities for virtual and remote delivery of CR services as part of the Medicare Advantage (MA) Value-Based Insurance Design Model28,29 or through model tests from the Centers for Medicare and Medicaid Innovation.
EQUITY
A plethora of studies have documented persistent disparities in CR utilization, with lower rates of participation among women, racially or ethnically underrepresented groups, older adults (≥85 years), rural populations, people living in low SES ZIP codes, and people with limited educational attainment.7,8,11,30–32 Individuals of these diverse and underserved populations have a disproportionate burden of cardiovascular disease and worse outcomes, in part, related to untreated risk factors and poor access to care. The disparities in CR utilization begin at referral and continue to manifest in low rates of participation, adherence, and completion of CR.7,13,14 CR attendance is poor in the Medicaid enrolled population.32 Patients with limited educational attainment are less likely to be referred or participate in CR.14 There is geographic variation in referral and participation with lower participation in rural areas and low SES ZIP codes.9,11,31
Both patient and health system factors can contribute to disparities in CR delivery (Table 3). Unfortunately, strategies to combat these factors are less well understood or tested, especially among patient populations with disproportionately low CR participation.
Table 3.
| Patient factors | Health system factors |
|---|---|
| Low educational attainment, low health and digital literacy, digital broadband access | Clinician factors related to CR training or competing priorities limiting clinician endorsement of CR |
| Cultural values, beliefs, and practices in understanding cardiovascular disease management | Clinician lack of systematic CR referral for all eligible patients |
| Language barriers and interpreter availability | Clinicians’ implicit bias or prejudice regarding underserved populations |
| Competing family and work obligations (childcare, eldercare, etc) | Lack of workforce diversity or cultural competency training, which impacts clinician and patient trust relationship |
| Medical comorbidities, psychosocial challenges, and low self-efficacy | Program availability and characteristics Limited facilitation of enrollment after referral Lack of programs that serve specific geographic areas, including rural areas and low-income communities Hours of operation Parking and public transportation access |
| Limited social network and support | |
| Transportation issues: both urban and rural regarding the distance of CR program from the patient’s home | |
| Geographic: low density of CR programs for patients to access | |
| Financial insecurity, insurance out of pocket costs (co-pays), and poor/no insurance coverage |
CR indicates cardiac rehabilitation.
Evolving CR delivery models may help address some of these factors, such as transportation issues or CR deserts. New CR delivery models must also be developed and delivered with attention to avoiding exacerbating disparities due to the digital divide. Technology-facilitated solutions should take into consideration access to technology, digital literacy, usability, and the need for broadband access. Still, the impact new care models have on addressing disparities in CR uptake will not be realized until action is taken to address the larger patient and health system factors at play. It is for this reason that the think tank partnered with the Million Hearts Cardiac Rehabilitation Collaborative—an open forum of >400 individuals committed to achieving the 70% CR participation target, to establish the Cardiac Rehabilitation Collaborative Health Equity Workgroup. This multidisciplinary workgroup is charged to work with the larger Cardiac Rehabilitation Collaborative to develop and implement sustainable and patient-centric strategies that address both the patient and health system factors that contribute to the disparate uptake of CR in the United States. Patient and community members will be included in all workgroups emerging from the think tank to embrace diversity and inclusion throughout the spectrum of CR delivery models. The need to ensure equitable access to new CR delivery models was echoed throughout the think tank, and the following potential solutions were brought forward.
Leveraging Community Resources for Delivery of CR to Underserved Populations
Knowing what potential partners and resources are available in the community is key to ensure patients have what they need to complete their CR program and maintain a healthy lifestyle beyond graduation from a CR program (see the Data Supplement for community resources). Community-based organizations can facilitate access to reliable internet and internet-enabled devices, exercise equipment and healthy lifestyle programs, healthy foods for food insecure patients and their families, translation services, and peer support. Leveraging public programs may also help patients secure health insurance, access the transportation they need to get to medical appointments, and secure housing. Additional physical activity programs and tobacco cessation programs may supplement a patient’s individualized treatment plan or help maintain a heart-healthy lifestyle after CR.
Medicaid Coverage for New CR Models
Medicaid coverage of CR is suboptimal. A 2018 review of state laws identified 21 states with state statutes for CR coverage in their fee-for-service Medicaid programs.34 Of the states that offer coverage, many policies are administratively burdensome with prior authorization requirements, limitations on the number of billable sessions, the duration of coverage, or the qualifying diagnoses and procedures. State Medicaid programs are afforded the flexibility to reimburse for services provided via telehealth without needing a state plan amendment.35 State Medicaid telehealth policies vary based on the services eligible for delivery via telehealth (all telehealth services versus a select list), the mode of delivery (synchronous versus asynchronous), the location of the patient, and other applicable state policies.36 Medicaid programs can also explore or test opportunities to cover new CR models by using Medicaid managed-care authorities (including the use of Performance Improvement Projects), applying section 1115 demonstrations, seeking support from a Medicaid Innovation Accelerator Program, using options through the Medicaid state plan, and joining state-based multipayer initiatives.37
Reducing Financial Barriers
The financial investment to participate in the program is cited as a leading barrier to participation for people with low SES.14,33 For CR, out-of-pocket expenses come in the form of copayments, coinsurance payments, deductibles, direct payments, or transportation costs. In addition, CR becomes more costly as the patient progresses through the 36-session program due to the accumulation of copayments, creating a disincentive for them to continue. A recent study demonstrated a clear dose response such that an increase in cost-sharing was associated with lower CR attendance.38
To circumvent this issue, payers are encouraged to cover CR, no matter the mode of delivery, with zero cost-sharing. CMS issued a final rule that promoted the opportunity for qualified health plans to offer value-based plans that cover CR and 7 other high-value services with zero cost-sharing in exchange for increasing cost-sharing for low-value services.39 The MA Value-Based Insurance Design Model offers MA Organizations the opportunity to eliminate cost-sharing for CR, cover alternative CR delivery models, and provide beneficiaries with cash or monetary rebates for their participation in CR.29 MA plans offered by Blue Cross Blue Shield Michigan have eliminated cost-sharing for CR. Hospitals may also be part of the solution by using a pool of philanthropic funds to supplement the cost of CR for their uninsured or underinsured beneficiaries.40
IMPLEMENTATION
The implementation of a CR program with virtual or remote delivery involves numerous considerations, including patient eligibility and safety, exercise regimen and prescription, patient monitoring, and patient education (see the Data Supplement for resources and patient selection considerations).
Patient Eligibility and Safety
Since CR began in the 1970s, patients have been encouraged to exercise on their own on days when not attending CR. Therefore, patients with cardiovascular disease exercising on their own at home is not a new paradigm. What is relatively new is monitoring and progressing these patients outside the center in a manner that targets the core components of CR.
Though exercise at home requires caution for some patients, such as those receiving continuous inotropic support, having recently received a mechanical support device, or who are symptomatic at low workloads (≤2 metabolic equivalents [METs]), most stable patients can exercise on their own at a relatively low risk of complication. For example, the HF-ACTION trial (Heart Failure: a Controlled Trial Investigating Outcomes of Exercise Training) assessed the safety of exercise training (initially center based, then remote) in 2331 patients with chronic systolic heart failure and found no significant difference between the exercise and usual care groups for the risk of hospitalization (1.9% versus 3.2%, respectively) or death (0.4% versus 0.4%, respectively) within 3 hours of exercise and no significant difference in ICD shocks between groups.41,42 Meta-analyses and other controlled trials investigating hybrid, virtual, or remote CR also report favorable safety data.43–48
Strategies to further promote safety include having the patient (1) compete a symptom-limited exercise test before or soon after starting CR to quantify exercise tolerance and screen for significant arrhythmia or ischemia by ECG, (2) attend ≥1 ECG-monitored sessions in center-based CR before transitioning to virtual or remote, and (3) periodically return for an in-person session to observe symptom and blood pressure responses with exercise training. Initial in-person contact can also orient the patient to the program, its staff, and any equipment that may be used. Finally, before a patient starts a virtual or remote CR program, a mutually agreed upon emergency plan should be in place and have the patient explain it back. At the beginning of each virtual CR session, staff should confirm the patient’s emergency contact information and location in case emergency services are needed.
Exercise Regimen and Exercise Prescription
Regardless of whether a synchronous, asynchronous, or combined model is used, exercise volume should progress patients to 150 minutes per week of moderate-intensity exercise.49 The type of activity should be whole body and rhythmic such as, but not limited to, stationary cycling, walking, or seated rowing. Resistance training using home equipment or bands is encouraged to minimize sarcopenia and dynapenia.50 All patients should start with continuous exercise and only progress to moderate or higher intensity interval training if desired and tolerated. Initially, exercise intensity should be set at a moderate-vigorous level (ie, rating of perceived exertion of 11–14 on 6–20 scale or 55%–80% of heart rate reserve). For higher intensity interval training, the work interval is typically set between 85% to 95% of heart rate reserve or a rating of perceived exertion of 13 to 16.21,51,52
Patient Monitoring
For virtual delivery, real-time audiovisual communication and monitoring of the patient can occur using a commercial video conferencing system or through video systems linked through a patient portal in the electronic health record.23,43,53 For remote delivery, patient-generated data can be transmitted to clinicians for asynchronous review. Remote data can include data manually logged into an electronic platform by the patient or data that are collected by wearable devices.25,48,54 Remote models with paper and pencil logs reported to CR staff by telephone have also been implemented.26,44,55 Although it is uncommon for ECG monitoring to change patient care, even in center-based CR,56 some studies of new delivery models have included ECG monitoring44 but most have not.25,41,53,54 Many virtual and remote delivery models use a chest strap or wrist-worn tracking device for heart rate and exercise intensity monitoring.25,41,43,53–55
Patient Education
As with center-based CR, virtual and remote CR should also have education addressing CR core components and health behaviors including physical activity, healthy dietary pattern, mental wellness, medication adherence, and tobacco cessation.21 A variety of educational models have been deployed in virtual and remote models21,23,25,26,55,56; future research is needed to determine the most effective approach.
RESEARCH
Meta-analysis of randomized clinical trials demonstrated that home-based CR has similar safety and efficacy to center-based CR.46 The American College of Cardiology/American Heart Association/AACVPR Scientific Statement concluded “previous randomized trials have generated low- to moderate-strength evidence that home-based CR and center-based CR can achieve similar improvements in 3- to 12-month clinical outcomes. Although home-based CR appears to hold promise in expanding the use of CR to eligible patients, additional research and demonstration projects are needed to clarify, strengthen, and extend the home-based CR evidence base for key subgroups, including older adults, women, underrepresented minority groups, and other higher-risk and understudied groups.”21
The think tank identified 4 priority research areas for new CR models: (1) use among specific populations including women, racially and ethnically underrepresented groups, and individuals with low SES, (2) impact on patient-centered outcomes, (3) effect on long-term (>1 year) outcomes and health care utilization, and (4) implementation in diverse health care settings, including optimization of delivery models and staffing considerations.
Ongoing Studies
Several ongoing studies will provide evidence about new care models (see PreThink Tank Webinar Recordings in the Data Supplement).57 iATTEND (Improving ATTENDANCE to Cardiac Rehabilitation Trial) is randomizing diverse participants to center-based CR or hybrid CR (using a virtual synchronous model) and examining outcomes including completed CR sessions, improvement in exercise capacity, and patient-centered outcomes (https://www.clinicaltrials.gov; unique identifier: NCT03646760).53 The MACRO study (Modified Application of Cardiac Rehabilitation for Older Adults) is randomizing older adults to standard care versus flexible delivery of personally tailored CR and examining the impact on physical function and patient-centered outcomes (https://www.clinicaltrials.gov; unique identifier: NCT03922529). The Enhancing Cardiac Rehabilitation Through Behavioral Nudges study is comparing center-based CR to a choice of center-based CR or mobile application-assisted home-based CR with or without behavioral nudges and examining the impact on adherence to CR and patient-centered outcomes, including long-term outcomes and health care utilization (https://www.clinicaltrials.gov; unique identifier: NCT03834155). HeartHome: A Nurse-Driven, Home-Based Cardiac Rehabilitation Program study is combining nurse home visits with telephone and electronic supports for participants in a home-based CR program compared with center-based CR (https://www.clinicaltrials.gov; unique identifier: NCT04131816). Though these studies will provide additional evidence about new care models, gaps will remain; more data will be needed on effectiveness of implementation in diverse patients and health care settings.
Research Network
We propose the development of a research network to address knowledge gaps related to new delivery models. This research network will bring together researchers and stakeholders to identify important patient-centered outcomes, plan cooperative studies to understand the long-term effects of new CR delivery models, and attract funding. Outcomes highlighted as important to study include CR referral, CR enrollment, timeliness of enrollment, CR participation, exercise capacity, blood pressure, obesity, hemoglobin A1c, blood lipids, medication adherence, diet quality, mental health, cognition, physical function, tobacco cessation, quality of life, self-efficacy, patient and clinician satisfaction, cardiovascular events, health care utilization, and disparities reduction. Furthermore, research should address and help mitigate impact of the social determinants of health and be inclusive of diverse populations.
Registries and Surveillance
To better understand use of new care models, existing data systems will need to be modified. For example, the AACVPR quality improvement registry will need to be expanded to include identifying how CR was delivered to enable comparisons among delivery models within and across programs.
Referral to and enrollment in CR have been monitored using data from quality improvement registries, Medicare, and VA.9,30,58 Recently, collaborators from the Million Hearts Cardiac Rehabilitation Collaborative conducted surveillance on CR participation among Medicare fee-for-service part B enrollees.7 Additional approaches to surveillance with Medicaid, MA, and nongovernmental payer data can complement Medicare fee-for-service data to capture participation among younger adults and others.59,60 To monitor use of new delivery models, standard codes and modifiers will need to be used and analyzed. These surveillance efforts should seek to understand the population eligible for CR, CR referral, CR enrollment, CR delivery through traditional and new models, and the impact of CR on outcomes and health care utilization. Finally, these efforts should seek to understand the role of social determinants of health, as well as disparities in CR access, participation, and outcomes to guide interventions to promote health equity.
CONCLUSIONS
CR is evolving to include in-person synchronous, virtual synchronous, and remote asynchronous modes of delivery (Figure 2). Though new models may change how CR is delivered, new models should not change what is delivered—a multidisciplinary program that addresses CR core components. Long-term policy changes will be needed for broad uptake of new CR care models. Existing evidence supports the efficacy and safety of virtual and remote CR and many programs provide examples of successful implementation, but questions remain about effects on patient-centered and long-term outcomes in diverse populations and settings. There is significant enthusiasm for efforts to implement new CR care models and learn how new care models can broaden access to CR, improve patient outcomes, and address health inequities.
Figure 2. Key points from the Million Hearts Cardiac Rehabilitation Think Tank.
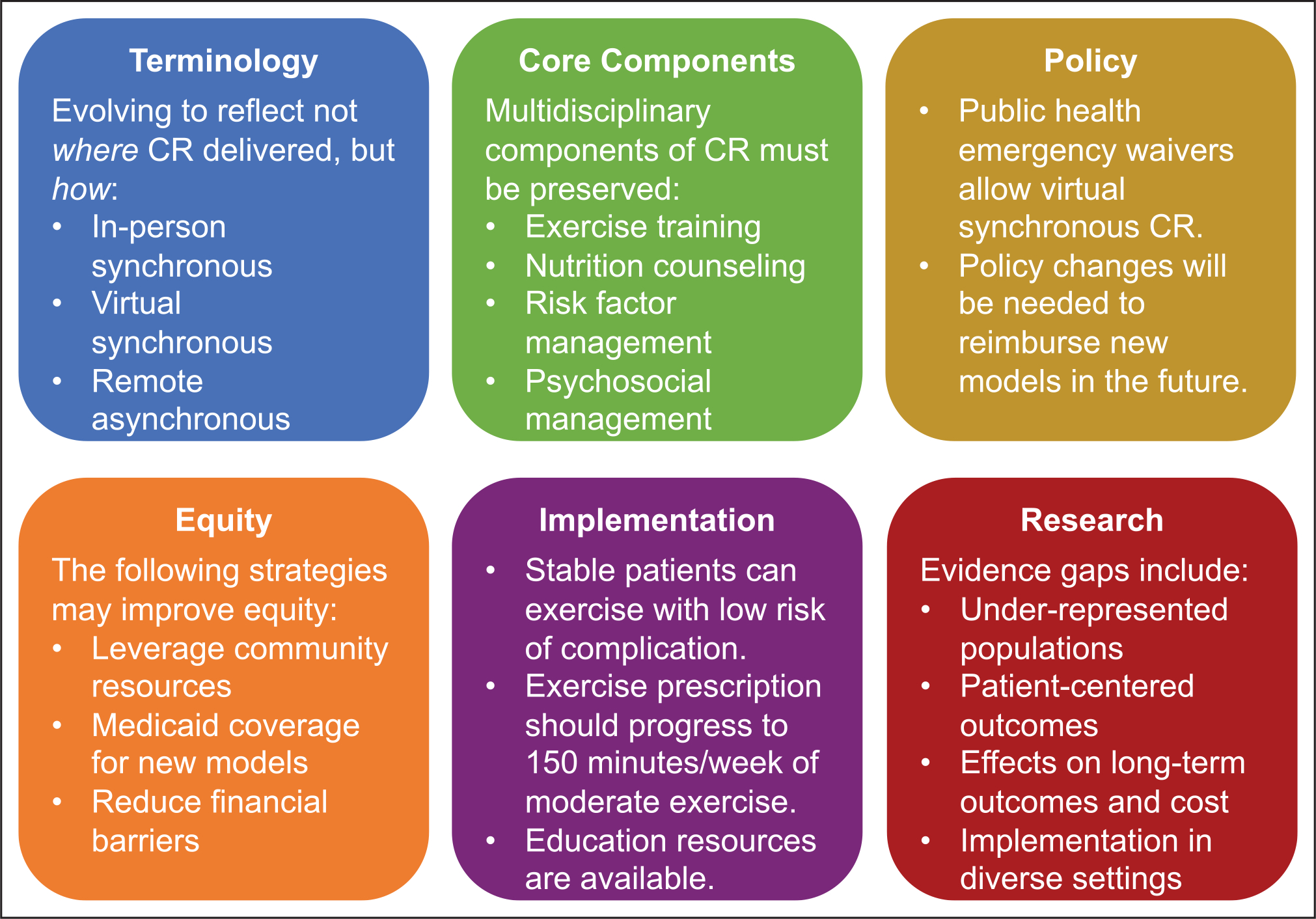
CR indicates cardiac rehabilitation.
Supplementary Material
Acknowledgments
We thank Susan Svencer, MPH, and Lisa Erck, MPH, from the National Association of Chronic Disease Directors for their contribution the planning, execution, and follow-up from the Million Hearts Cardiac Rehabilitation Think Tank.
Nonstandard Abbreviations and Acronyms
- AACVPR
American Association of Cardiovascular and Pulmonary Rehabilitation
- CMS
Centers for Medicare and Medicaid Services
- COVID-19
coronavirus disease 2019
- CR
cardiac rehabilitation
- HF-ACTION
Heart Failure: a Controlled Trial Investigating Outcomes of Exercise Training
- iATTEND
Improving ATTENDANCE to Cardiac Rehabilitation Trial
- MA
Medicare Advantage
- MACRO
Modified Application of Cardiac Rehabilitation for Older Adults
- SES
socioeconomic status
Footnotes
Disclosures
Dr Beatty was formerly employed by and holds stock in Apple, Inc. Dr Keteyian receives research grant support from the National Institutes of Health (HL 143099). The other authors report no conflicts.
The Data Supplement is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCOUTCOMES.121.008215.
Contributor Information
Alexis L. Beatty, Department of Epidemiology and Biostatistics, Medicine, UCSF, San Francisco, CA.
Todd M. Brown, Department of Medicine, University of Alabama, Birmingham.
Mollie Corbett, American Association of Cardiovascular and Pulmonary Rehabilitation, Chicago, IL.
Dean Diersing, Physical Medicine and Rehabilitation, UMC Health System, Lubbock, TX.
Steven J. Keteyian, Division of Cardiovascular Medicine, Henry Ford Medical Group, Detroit, MI.
Ana Mola, Department of Rehabilitation Medicine, NYU Langone Health, New York, NY.
Haley Stolp, IHRC, Inc, Atlanta, GA; CDC, Atlanta, GA.
Hilary K. Wall, CDC, Atlanta, GA.
Laurence S. Sperling, CDC, Atlanta, GA; Emory Center for Heart Disease Prevention, Atlanta, GA.
REFERENCES
- 1.Ades PA, Keteyian SJ, Wright JS, Hamm LF, Lui K, Newlin K, Shepard DS, Thomas RJ. Increasing cardiac rehabilitation participation from 20% to 70%: a road map from the million hearts cardiac rehabilitation collaborative. Mayo Clin Proc. 2017;92:234–242. doi: 10.1016/j.mayocp.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. ; ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:e344–e426. doi: 10.1161/CIR.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 3.O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. ; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. doi: 10.1161/CIR.0b013e3182742cf6 [DOI] [PubMed] [Google Scholar]
- 4.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, Disesa VJ, Hiratzka LF, Hutter AM Jr, et al. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e652–e735. doi: 10.1161/CIR.0b013e31823c074e [DOI] [PubMed] [Google Scholar]
- 5.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, et al. ; American College of Cardiology Foundation/American Heart Association Task Force. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126:e354–e471. doi: 10.1161/CIR.0b013e318277d6a0 [DOI] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure. Circulation. 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 7.Ritchey MD, Maresh S, McNeely J, Shaffer T, Jackson SL, Keteyian SJ, Brawner CA, Whooley MA, Chang T, Stolp H, et al. Tracking cardiac rehabilitation participation and completion among medicare beneficiaries to inform the efforts of a national initiative. Circ Cardiovasc Qual Outcomes. 2020;13:e005902. doi: 10.1161/CIRCOUTCOMES.119.005902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116:1653–1662. doi: 10.1161/CIRCULATIONAHA.107.701466 [DOI] [PubMed] [Google Scholar]
- 9.Beatty AL, Truong M, Schopfer DW, Shen H, Bachmann JM, Whooley MA. Geographic variation in cardiac rehabilitation participation in medicare and veterans affairs populations: opportunity for improvement. Circulation. 2018;137:1899–1908. doi: 10.1161/CIRCULATIONAHA.117.029471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park LG, Schopfer DW, Zhang N, Shen H, Whooley MA. Participation in cardiac rehabilitation among patients with heart failure. J Card Fail. 2017;23:427–431. doi: 10.1016/j.cardfail.2017.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachmann JM, Huang S, Gupta DK, Lipworth L, Mumma MT, Blot WJ, Akwo EA, Kripalani S, Whooley MA, Wang TJ, et al. Association of neighborhood socioeconomic context with participation in cardiac rehabilitation. J Am Heart Assoc. 2017;6:e006260. doi: 10.1161/JAHA.117.006260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang J, Ayala C, Luncheon C, Ritchey M, Loustalot F. Use of outpatient cardiac rehabilitation among heart attack survivors - 20 states and the district of Columbia, 2013 and Four States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66:869–873. doi: 10.15585/mmwr.mm6633a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun EY, Jadotte YT, Halperin W. Disparities in cardiac rehabilitation participation in the United States: a systematic review and meta-analysis. J Cardiopulm Rehabil Prev. 2017;37:2–10. doi: 10.1097/HCR.0000000000000203 [DOI] [PubMed] [Google Scholar]
- 14.Castellanos LR, Viramontes O, Bains NK, Zepeda IA. Disparities in cardiac rehabilitation among individuals from racial and ethnic groups and rural communities-a systematic review. J Racial Ethn Health Disparities. 2019;6:1–11. doi: 10.1007/s40615-018-0478-x [DOI] [PubMed] [Google Scholar]
- 15.Shanmugasegaram S, Oh P, Reid RD, McCumber T, Grace SL. A comparison of barriers to use of home- versus site-based cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2013;33:297–302. doi: 10.1097/HCR.0b013e31829b6e81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pack QR, Squires RW, Lopez-Jimenez F, Lichtman SW, Rodriguez-Escudero JP, Zysek VN, Thomas RJ. The current and potential capacity for cardiac rehabilitation utilization in the United States. J Cardiopulm Rehabil Prev. 2014;34:318–326. doi: 10.1097/HCR.0000000000000076 [DOI] [PubMed] [Google Scholar]
- 17.Wall HK, Stolp H, Wright JS, Ritchey MD, Thomas RJ, Ades PA, Sperling LS. The million hearts initiative: catalyzing utilization of cardiac rehabilitation and accelerating implementation of new care models. J Cardiopulm Rehabil Prev. 2020;40:290–293. doi: 10.1097/HCR.0000000000000547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas RJ, Beatty AL, Beckie TM, Brewer LC, Brown TM, Forman DE, Franklin BA, Keteyian SJ, Kitzman DW, Regensteiner JG, et al. Home-based cardiac rehabilitation: a scientific statement from the American Association of Cardiovascular and Pulmonary Rehabilitation, the American Heart Association, and the American College of Cardiology. Circulation. 2019;140:e69–e89. doi: 10.1161/CIR.0000000000000663 [DOI] [PubMed] [Google Scholar]
- 19.Social Security Administration. Definitions of services, institutions, et al. Accessed March 1, 2021. https://www.ssa.gov/OP_Home/ssact/title18/1861.htm. [Google Scholar]
- 20.Balady GJ, Williams MA, Ades PA, Bittner V, Comoss P, Foody JM, Franklin B, Sanderson B, Southard D; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Nutrition, Physical Activity, and Metabolism; American Association of Cardiovascular and Pulmonary Rehabilitation. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2007;115:2675–2682. doi: 10.1161/CIRCULATIONAHA.106.180945 [DOI] [PubMed] [Google Scholar]
- 21.Thomas RJ, Balady G, Banka G, Beckie TM, Chiu J, Gokak S, Ho PM, Keteyian SJ, King M, Lui K, et al. 2018 ACC/AHA clinical performance and quality measures for cardiac rehabilitation: a report of the American College of Cardiology/American Heart Association Task Force on performance measures. Circ Cardiovasc Qual Outcomes. 2018;11:e000037. doi: 10.1161/HCQ.0000000000000037 [DOI] [PubMed] [Google Scholar]
- 22.Pack QR, Bauldoff G, Lichtman SW, Buckley M, Eichenauer K, Gavic A, Garvey C, King ML; American Association of Cardiovascular and Pulmonary Rehabilitation Quality of Care Committee. Prioritization, development, and validation of american association of cardiovascular and pulmonary rehabilitation performance measures. J Cardiopulm Rehabil Prev. 2018;38:208–214. doi: 10.1097/HCR.0000000000000358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry R, Brawner CA, Kipa SG, Stevens C, Bloom C, Keteyian SJ. Telemedicine home-based cardiac rehabilitation: a CASE SERIES. J Cardiopulm Rehabil Prev. 2020;40:245–248. doi: 10.1097/HCR.0000000000000504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ratchford AM, Hamman RF, Regensteiner JG, Magid DJ, Gallagher SB, Merenich JA. Attendance and graduation patterns in a group-model health maintenance organization alternative cardiac rehabilitation program. J Cardiopulm Rehabil. 2004;24:150–156. doi: 10.1097/00008483-200405000-00003 [DOI] [PubMed] [Google Scholar]
- 25.Funahashi T, Borgo L, Joshi N. Saving Lives with Virtual Cardiac Rehabilitation. Catal Carryover. Accessed February 11, 2020. 10.1056/CAT.19.0624. [DOI] [Google Scholar]
- 26.Wakefield BJ, Drwal K, Paez M, Grover S, Franciscus C, Reisinger HS, Kaboli PJ, El Accaoui R. Creating and disseminating a home-based cardiac rehabilitation program: experience from the Veterans Health Administration. BMC Cardiovasc Disord. 2019;19:242. doi: 10.1186/s12872-019-1224-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medicare Basic Health Program, and Exchanges; Additional Policy and Regulatory Revisions in Response to the COVID– 19 Public Health Emergency and Delay of Certain Reporting Requirements for the Skilled Nursing Facility Quality Reporting Program. Centers for Medicare & Medicaid Services. Accessed March 1, 2021. https://www.govinfo.gov/content/pkg/FR-2020-05-08/pdf/2020-09608.pdf#page=79. [Google Scholar]
- 28.Chernew ME, Fendrick AM, Buxbaum J, Budros M. V-BID X: Creating a Value-Based Insurance Design Plan for the Exchange MarkeT Accessed June 10, 2021. http://vbidcenter.org/wp-content/uploads/2019/09/VBID-X-White-Paper-92019.pdf. [Google Scholar]
- 29.Medicare Advantage Value-Based Insurance Design Model | CMS Innovation Center. Accessed March 1, 2021. https://innovation.cms.gov/innovation-models/vbid. [Google Scholar]
- 30.Brown TM, Hernandez AF, Bittner V, Cannon CP, Ellrodt G, Liang L, Peterson ED, Piña IL, Safford MM, Fonarow GC; American Heart Association Get With The Guidelines Investigators. Predictors of cardiac rehabilitation referral in coronary artery disease patients: findings from the American Heart Association’s Get With The Guidelines Program. J Am Coll Cardiol. 2009;54:515–521. doi: 10.1016/j.jacc.2009.02.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Balady GJ, Ades PA, Bittner VA, Franklin BA, Gordon NF, Thomas RJ, Tomaselli GF, Yancy CW; American Heart Association Science Advisory and Coordinating Committee. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation. 2011;124:2951–2960. doi: 10.1161/CIR.0b013e31823b21e2 [DOI] [PubMed] [Google Scholar]
- 32.Gaalema DE, Higgins ST, Shepard DS, Suaya JA, Savage PD, Ades PA. State-by-state variations in cardiac rehabilitation participation are associated with educational attainment, income, and program availability. J Cardiopulm Rehabil Prev. 2014;34:248–254. doi: 10.1097/HCR.0000000000000059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valencia HE, Savage PD, Ades PA. Cardiac rehabilitation participation in underserved populations. Minorities, low socioeconomic, and rural residents. J Cardiopulm Rehabil Prev. 2011;31:203–210. doi: 10.1097/HCR.0b013e318220a7da [DOI] [PubMed] [Google Scholar]
- 34.Gilchrist S State Law Summaries in Effect as of October 3, 2018: Cardiac Rehabilitation. Centers for Disease Control and Prevention; 2018. [Google Scholar]
- 35.Centers for Medicare & Medicaid Services. State Medicaid & CHIP Telehealth Toolkit: Policy Considerations for States Expanding Use of Telehealth. Centers for Medicare & Medicaid Services. 2020. Accessed Match 1, 2021. https://www.medicaid.gov/sites/default/files/2020-04/medicaid-chip-telehealth-toolkit.pdf. [Google Scholar]
- 36.Current State Laws and Reimbursement Policies | CCHP Website Accessed March 1, 2021. https://www.cchpca.org/telehealth-policy/current-state-laws-and-reimbursement-policies. [Google Scholar]
- 37.Costello AM, Smith B. Value-Based Care Opportunities in Medicaid [Internet]. Department of Health and Human Services. Centers for Medicare & Medicaid Services. 2020. Cited March 1, 2021. https://www.medicaid.gov/sites/default/files/2020-09/smd20004.pdf. [Google Scholar]
- 38.Farah M, Abdallah M, Szalai H, Berry R, Lagu T, Lindenauer PK, Pack QR. Association between patient cost sharing and cardiac rehabilitation adherence. Mayo Clin Proc. 2019;94:2390–2398. doi: 10.1016/j.mayocp.2019.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Notice of Benefit and Payment Parameters for 2021; Notice Requirement for Non-Federal Governmental Plans, in 45 CFR Parts 146, 149, 155, 156 and 158. Centers for Medicare & Medicaid Services; 2020. [Google Scholar]
- 40.American Association of Cardiovascular and Pulmonary Rehabilitation. Cardiac Rehabilitation Enrollment Strategy: Establish a Philanthropic Fund:Spotlight on Henry Ford Health System. Accessed March 1, 2021. https://www.aacvpr.org/Portals/0/Million%20Hearts%20Change%20Package/4.18.2018%20Files/EP-25-CRCP-Turnkey-Establish%20Philanthropic%20Fund.pdf?timestamp=1524152752072. [Google Scholar]
- 41.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, et al. ; HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piccini JP, Hellkamp AS, Whellan DJ, Ellis SJ, Keteyian SJ, Kraus WE, Hernandez AF, Daubert JP, Piña IL, O’Connor CM; HF-ACTION Investigators. Exercise training and implantable cardioverter-defibrillator shocks in patients with heart failure: results from HF-ACTION (Heart Failure and a Controlled Trial Investigating Outcomes of Exercise Training). JACC Heart Fail. 2013;1:142–148. doi: 10.1016/j.jchf.2013.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hwang R, Bruning J, Morris NR, Mandrusiak A, Russell T. Home-based telerehabilitation is not inferior to a centre-based program in patients with chronic heart failure: a randomised trial. J Physiother. 2017;63:101–107. doi: 10.1016/j.jphys.2017.02.017 [DOI] [PubMed] [Google Scholar]
- 44.Piotrowicz E, Pencina MJ, Opolski G, Zareba W, Banach M, Kowalik I, Orzechowski P, Szalewska D, Pluta S, Glówczynska R, et al. Effects of a 9-week hybrid comprehensive telerehabilitation program on long-term outcomes in patients with heart failure: the telerehabilitation in Heart Failure Patients (TELEREH-HF) Randomized Clinical Trial. JAMA Cardiol. 2020;5:300–308. doi: 10.1001/jamacardio.2019.5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cowie A, Thow MK, Granat MH, Mitchell SL. Effects of home versus hospital-based exercise training in chronic heart failure. Int J Cardiol. 2012;158:296–298. doi: 10.1016/j.ijcard.2012.04.117 [DOI] [PubMed] [Google Scholar]
- 46.Anderson L, Sharp GA, Norton RJ, Dalal H, Dean SG, Jolly K, Cowie A, Zawada A, Taylor RS. Home-based versus centre-based cardiac rehabilitation. Cochrane Database Syst Rev. 2017;6:CD007130. doi: 10.1002/14651858.CD007130.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maddison R, Rawstorn JC, Stewart RAH, Benatar J, Whittaker R, Rolleston A, Jiang Y, Gao L, Moodie M, Warren I, et al. Effects and costs of real-time cardiac telerehabilitation: randomised controlled non-inferiority trial. Heart. 2019;105:122–129. doi: 10.1136/heartjnl-2018-313189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Varnfield M, Karunanithi M, Lee CK, Honeyman E, Arnold D, Ding H, Smith C, Walters DL. Smartphone-based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart. 2014;100:1770–1779. doi: 10.1136/heartjnl-2014-305783 [DOI] [PubMed] [Google Scholar]
- 49.US Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd edition. US Department of Health and Human Services; 2018. [Google Scholar]
- 50.American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Lippincott Williams & Wilkins; 2018. [Google Scholar]
- 51.Aamot IL, Forbord SH, Gustad K, Løckra V, Stensen A, Berg AT, Dalen H, Karlsen T, Støylen A. Home-based versus hospital-based high-intensity interval training in cardiac rehabilitation: a randomized study. Eur J Prev Cardiol. 2014;21:1070–1078. doi: 10.1177/2047487313488299 [DOI] [PubMed] [Google Scholar]
- 52.Moholdt T, Bekken Vold M, Grimsmo J, Slørdahl SA, Wisløff U. Home-based aerobic interval training improves peak oxygen uptake equal to residential cardiac rehabilitation: a randomized, controlled trial. PLoS One. 2012;7:e41199. doi: 10.1371/journal.pone.0041199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Keteyian SJ, Grimshaw C, Brawner CA, Kerrigan DJ, Reasons L, Berry R, Peterson EL, Ehrman JK. A comparison of exercise intensity in hybrid versus standard phase two cardiac rehabilitation. J Cardiopulm Rehabil Prev. 2021;41:19–22. doi: 10.1097/HCR.0000000000000569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Snoek JA, Prescott EI, van der Velde AE, Eijsvogels TMH, Mikkelsen N, Prins LF, Bruins W, Meindersma E, González-Juanatey JR, Peña-Gil C, et al. Effectiveness of home-based mobile guided cardiac rehabilitation as alternative strategy for nonparticipation in clinic-based cardiac rehabilitation among elderly patients in Europe: a randomized clinical trial. JAMA Cardiol. 2021;6:463–468. doi: 10.1001/jamacardio.2020.5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rohrbach G, Schopfer DW, Krishnamurthi N, Pabst M, Bettencourt M, Loomis J, Whooley MA. The design and implementation of a home-based cardiac rehabilitation program. Fed Pract. 2017;34:34–39. [PMC free article] [PubMed] [Google Scholar]
- 56.Keteyian SJ, Mellett PA, Fedel FJ, McGowan CM, Stein PD. Electrocardiographic monitoring during cardiac rehabilitation. Chest. 1995;107:1242–1246. doi: 10.1378/chest.107.5.1242 [DOI] [PubMed] [Google Scholar]
- 57.Fleg JL, Keteyian SJ, Peterson PN, Benzo R, Finkelstein J, Forman DE, Gaalema DE, Cooper LS, Punturieri A, Joseph L, et al. Increasing use of cardiac and pulmonary rehabilitation in traditional and community settings: opportunities to reduce health care disparities. J Cardiopulm Rehabil Prev. 2020;40:350–355. doi: 10.1097/HCR.0000000000000527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beatty AL, Li S, Thomas L, Amsterdam EA, Alexander KP, Whooley MA. Trends in referral to cardiac rehabilitation after myocardial infarction: data from the National Cardiovascular Data Registry 2007 to 2012. J Am Coll Cardiol. 2014;63:2582–2583. doi: 10.1016/j.jacc.2014.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sukul D, Seth M, Barnes GD, Dupree JM, Syrjamaki JD, Dixon SR, Madder RD, Lee D, Gurm HS. Cardiac rehabilitation use after percutaneous coronary intervention. J Am Coll Cardiol. 2019;73:3148–3152. doi: 10.1016/j.jacc.2019.03.515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson MP, Yaser JM, Hou H, Syrjamaki JD, DeLucia A III, Likosky DS, Keteyian SJ, Prager RL, Gurm HS, Sukul D. Determinants of hospital variation in cardiac rehabilitation enrollment during coronary artery disease episodes of care. Circ Cardiovasc Qual Outcomes. 2021;14:e007144. doi: 10.1161/CIRCOUTCOMES.120.007144 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


