Abstract
Modifications to Fried’s frailty phenotype (FFP) are common. We evaluated a self-reported modified frailty phenotype (Mod-FP) used among people with HIV (PWH). Among 522 PWH engaged in two longitudinal studies, we assessed validity of the four-item Mod-FP compared with the five-item FFP. We compared the phenotypes via receiver operator characteristic curves, agreement in classifying frailty, and criterion validity via association with having experienced falls. Mod-FP classified 8% of PWH as frail, whereas FFP classified 9%. The area under the receiver operator characteristic curve for Mod-FP classifying frailty was 0.93 (95% CI = 0.91–0.96). We observed kappa ranging from 0.64 (unweighted) to 0.75 (weighted) for categorizing frailty status. Both definitions found frailty associated with a greater odds of experiencing a fall; FFP estimated a slightly greater magnitude (i.e., OR) for the association than Mod-FP. The Mod-FP has good performance in measuring frailty among PWH and is reasonable to use when the gold standards of observed assessments (i.e., weakness and slowness) are not feasible.
Keywords: frailty phenotype, feasibility, HIV and aging, people with HIV, validity
Frailty captures vulnerability to health stressors and is associated with mortality and other negative health outcomes, including falls and hospitalization, among aging adults (Morley et al., 2013; Vermeiren et al., 2016). Due to advancements in antiretroviral therapy, HIV has become a chronic condition and people with HIV (PWH), although living longer, are experiencing a growing burden of morbidity and aging-related conditions, including frailty (Desquilbet et al., 2007; Levett et al., 2016; Piggott et al., 2016; Thurn & Gustafson, 2017; Wing, 2016). Reliable, self-reported, low-burden frailty assessments may facilitate greater frailty ascertainment in HIV care and other clinical and research settings, which could enhance our understanding and promotion of successful aging among PWH.
Fried’s frailty phenotype (FFP) is commonly used to measure frailty (Bouillon et al., 2013; Fried et al., 2001; Piggott et al., 2016) and includes five components of physical health and functional status, including unintentional weight loss, low physical activity, exhaustion measured by self-report, and observed weakness and slowness (Fried et al., 2001). Fried et al. (2001) conducted many analyses evaluating the FFP, and the accumulation of their results supports its validity among older adults. However, it can be difficult to collect components of FFP that require specialized equipment in busy or low-resourced care settings. Specifically, slowness (measured by gait speed test of a timed walk over a designated distance) and weakness (measured by grip strength with an instrument such as a dynamometer) require more time and resources to collect and may not be feasible in all clinical settings. As a result, several modified versions of the FFP have proposed substitutions replacing objectively assessed measures with self-report (Op Het Veld et al., 2018; Theou et al., 2015) or excluding the objectively assessed measures (Desquilbet et al., 2007; Theou et al., 2015). There is limited validation work among clinical care cohorts specifically evaluating modifications to FFP (Akgun et al., 2014; Bouillon et al., 2013; McMillan et al., 2021), especially among PWH (Aprahamian et al., 2017; Jung et al., 2021; Kim et al., 2020; Van der Elst et al., 2020), which is important to understand measurement properties within particular settings of use.
Investigators within the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS), a large U.S.-based cohort of PWH (Kitahata et al., 2008), developed a modified Fried phenotype (Mod-FP) based on four self-reported components: unintentional weight loss, low physical activity, fatigue, and poor mobility (Crane et al., 2022). The Mod-FP is similar to other modified phenotypes used among PWH, with the use of self-reported components and exclusion of a weakness/grip strength measure that is not collected at routine appointments in CNICS (Akgun et al., 2014; Desquilbet et al., 2007). Grip strength and gait speed have been observed as strong standalone predictors of frailty among the general aging population (Syddall et al., 2003; Wu et al., 2018). Thus, it is important to evaluate the impact of excluding or modifying these components for the Mod-FP, which has not been published for similar modified phenotypes among PWH (Desquilbet et al., 2007).
The Impact of Physical Activity Routines and Dietary Intake on the Longitudinal Symptom Experience of people living with HIV (PROSPER-HIV) study is an ongoing study collecting in-depth measurements of physical activity and functional status among a subset of PWH in CNICS (Webel et al., 2020), including grip strength and gait speed. Using these measures from PROSPER-HIV in conjunction with the self-reported components from CNICS, we created an FFP with all 5 original components and used it as a gold standard comparator to evaluate the validity and reliability of the Mod-FP among PWH.
Methods
Study Setting and Participants
This study was conducted among PWH enrolled in the PROSPER-HIV study (Webel et al., 2020) nested within the CNICS cohort (Kitahata et al., 2008). CNICS is a large, longitudinal cohort of adult PWH engaged in HIV care at eight academic clinical sites across the United States. PROSPER-HIV is an ongoing longitudinal nested study at four CNICS sites focused on understanding the impact of nutrition, exercise, and functional status on outcomes and symptoms in PWH. PROSPER-HIV administers assessments of physical activity and dietary intake (Webel et al., 2020) to complement comprehensive clinical information (e.g., laboratory values, medications, diagnoses) collected within CNICS.
Most PWH complete the CNICS patient-reported outcomes (PRO) clinical assessment at the beginning of routine HIV primary care appointments (Fredericksen et al., 2012). The CNICS PRO assessment is a tablet-based questionnaire that includes various instruments covering clinically relevant health domains, such as symptoms (HIV Symptom Index; Justice et al., 2001) and physical activity (Lipid Research Clinics Questionnaire; Ainsworth et al., 1993).
Eligibility criteria for PROSPER-HIV include PWH who are active CNICS participants with a completed PRO assessment within the past 12 months; prescribed antiretroviral therapy; have an undetectable HIV viral load (<200 copies/mL); not pregnant, breastfeeding, or planning to become pregnant; English speaking; and have reliable access to a telephone. PWH (n = 522) who completed their Year 1 PROSPER-HIV assessment were included in this study. The PROSPER-HIV–enhanced assessments include physical function measures, including handgrip strength and gait speed, as well as self-reported falls (described below). Data from both the PROSPER-HIV assessment and the CNICS PRO assessments were combined to evaluate the Mod-FP. Data were collected from January 2019 through September 2021. During the COVID-19 pandemic, many clinic visits were conducted via telehealth; at these visits, PWH were able to complete their CNICS PRO remotely. Occasionally, PROSPER-HIV visits were unable to be scheduled in-person to collect the objectively assessed measures (e.g., grip strength and gait speed), so several PWH (<10) did not complete these assessments, thus were unable to be included in this study, but represent a very small proportion of PROSPER-HIV participants. Institutional review boards at participating sites approved CNICS and PROSPER-HIV protocols (Protocol 27674-D at the University of Washington for CNICS and STUDY20180761 at Case Western Reserve University for PROSPER-HIV), and participants completed written informed consent before entry into CNICS and PROSPER-HIV.
It was preferred that PWH complete their first PROSPER-HIV assessment on the same day as their PRO assessment; however, because of limitations in clinical space, some assessments (n=200, 38%) occurred on different days, whereas the majority (n = 322, 62%) occurred on the same day. We further assessed the difference in time between assessments among PWH who had a study visit before the COVID-19 pandemic compared with those who had a study visit during the pandemic. Overall, the mean time between assessments was 36 days (median = 0, interquartile range [IQR] = 0–9) and 77% of PWH took both assessments within 30 days. Among PWH who had their first PROSPER-HIV visit before March 1, 2020 (indication of the start of the COVID-19 pandemic), the mean time between visits was 14 days (median = 0, IQR = 0–6), whereas among PWH who had their first visit after March 1, 2020, the mean time between visits was 101 days (median = 0, IQR = 0–66).
Instrumentation
Fatigue.
Fatigue was collected via the single fatigue item on the HIV Symptom Index where PWH report symptom burden in the preceding 4 weeks with Likert scale response options, including “I do not have this symptom”, “I have this symptom and it doesn’t bother me”, “I have this symptom and it bothers me a little”, “I have this symptom and it bothers me”, “I have this symptom and it bothers me a lot” (Justice et al., 2001). If PWH responded, “I have this symptom and it bothers me” or “I have this symptom and it bothers me a lot,” they were categorized as having the fatigue component.
Unintentional weight loss.
Wasting and unintentional weight loss was also collected using a single self-reported item in the HIV Symptom Index (Justice et al., 2001). With the same Likert scale response options as fatigue, PWH were categorized as having the unintentional weight loss component if they responded, “I have this symptom and it bothers me a little”, “I have this symptom and it bothers me”, or “I have this symptom and it bothers me a lot”. The dichotomization of PRO items based on Likert scales were decided based on clinical knowledge and empirical investigation of the distribution to ensure cut-points were robust.
Low physical activity
Physical activity was collected with the four-item Lipid Research Clinics questionnaire and categorized based on the original scoring scheme for physical activity level, which includes self-report of strenuous exercise and overall activity level (Ainsworth et al., 1993). We categorized PWH as having the low physical activity component if they were in the “very low active” classification, which means they reported not engaging in strenuous exercise and being less active than their peers (compared with people of the same age and gender).
Poor mobility.
Mobility was assessed in the EuroQOL Health-Related Quality of Life questionnaire (EQ-5D-3L), where PWH were asked to report using a single item how they are with doing activities today (Rabin & de Charro, 2001). For mobility, the response options include, “I have no problems in walking about”, “I have some problems walking about”, and “I am unable to walk”. PWH were considered as having poor mobility if they reported either “I have some problems walking about” or “I am unable to walk.”
Slowness.
Slowness, captured via gait speed, was measured by a 4-m timed walk, repeated twice, and the faster of the two trials was recorded as the result. PWH were considered to have slow gait speed if their walk time exceeded 5.0 s total (slower than 0.8 m/s; Abellan van Kan et al., 2009; Cruz-Jentoft et al., 2010).
Weakness.
Weakness was captured by grip strength, measured via Jamar hand-held dynamometer (Sammons Preston, Rolyan, Bolingbrook, IL, USA), with two trials conducted using the self-reported dominant hand after noting historical hand injuries and surgeries. The maximum strength (measured in kg) of the attempts was recorded as the result. A global standard for frailty using hand grip strength has not been established. Per recommendations of the European Working Group on Sarcopenia in Older People, PWH were considered to have low grip strength if their maximum strength was <16 kg for women or <27 kg for men (Blanquet et al., 2022; Cruz-Jentoft et al., 2010).
Phenotypes.
We calculated two frailty phenotypes from our data (Figure 1). First, Mod-FP consisted of the four items collected within the PROs (i.e., fatigue, unintentional weight loss, low physical activity, and poor mobility). The Mod-FP was scored from 0 to 4 based on the presence of each component. Second, we used a combination of three PRO (i.e., fatigue, unintentional weight loss, and low physical activity) and two PROSPER-HIV functional status (i.e., grip strength and gait speed) items to create the FFP. FFP was scored from 0 to 5 based on present components. We categorized the phenotypes by not frail (0 components), prefrail (1–2 components), or frail (≥3 components), as in Fried’s original definition (Fried et al., 2001).
Figure 1.
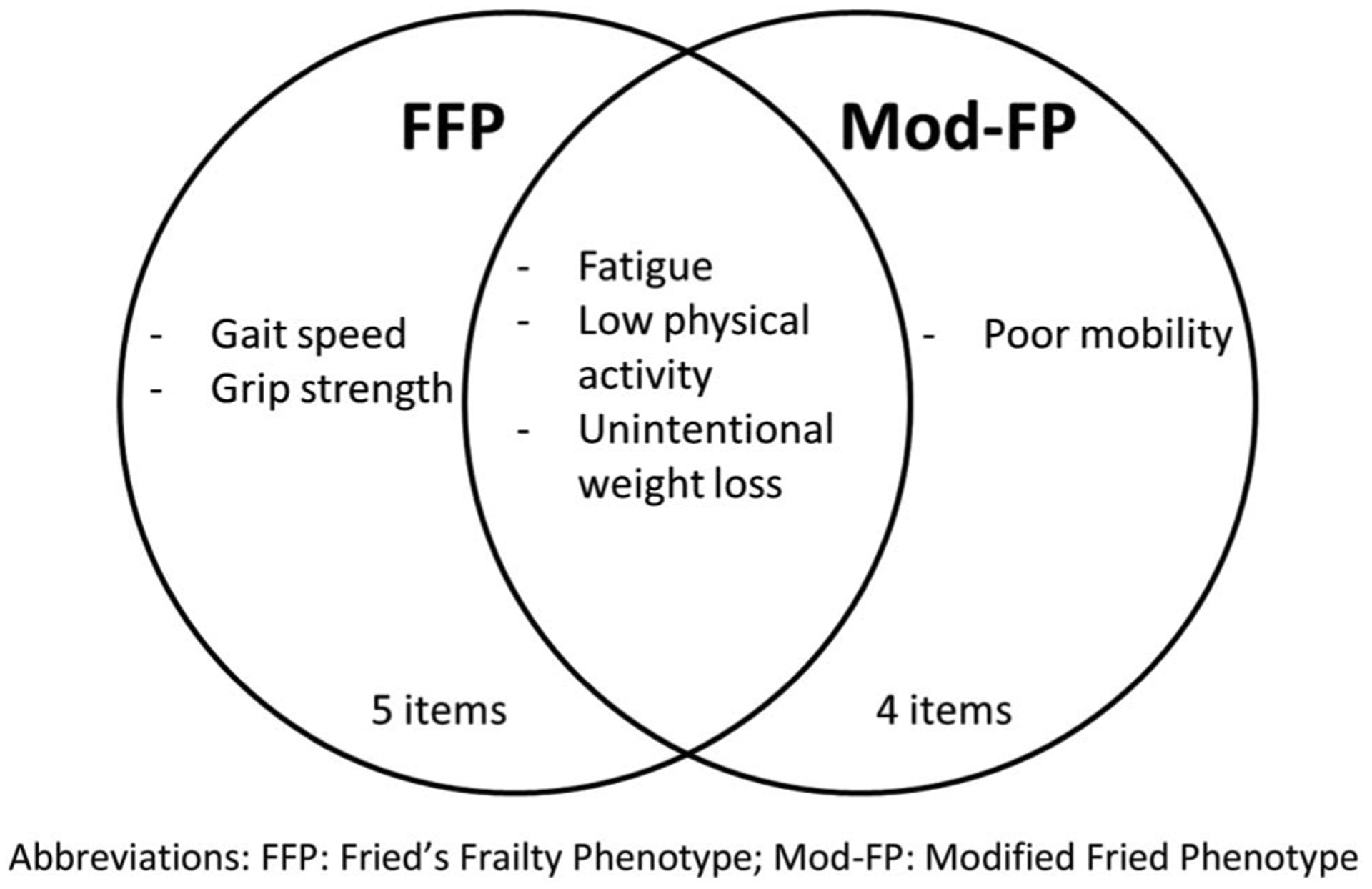
Components included in each frailty phenotype definition. Fried’s frailty phenotype (FFP) includes 5 components, with three overlapping with the modified Fried phenotype (Mod-FP), which includes four components in total. FFP = Fried’s frailty phenotype; Mod-FP = modified Fried phenotype.
Falls
We analyzed cross-sectional associations between frailty and falls to further evaluate the Mod-FP. Frailty and falls are associated in the general population (Cheng & Chang, 2017), and there is limited but compelling evidence of this association among PWH (Erlandson et al., 2012; Piggott et al., 2016; Sharma et al., 2019). We sought to leverage this association and compared the estimates between the Mod-FP and FFP to evaluate criterion validity of the Mod-FP. PWH self-reported the incidence and frequency of falls occurring in the past 12 months during their PROSPER-HIV visit assessment. Response options included “none”, “1 fall”, “2 falls”, and “3 or more”. We dichotomized PWH in two ways for having experienced falls in the past 12 months: (a) any fall (one, two, or three falls vs. none) and (b) recurrent falls (two or three falls vs. none, excluding PWH who self-reported exactly one fall). This question was added to the PROSPER-HIV assessment after study initiation, so falls data are only available for the portion (n = 253) of PWH who had their visit after this question was implemented.
Statistical Analysis
We assessed criterion validity of the Mod-FP via Spearman correlation coefficient between the Mod-FP and FFP. For this comparison, a high correlation would suggest the two phenotype definitions are measuring a similar trait (Mokkink et al., 2010; Rockwood, 2005). We also compared the subjective report of mobility (mobility PRO item) with the objective measure of gait speed to evaluate the degree of overlap between these two items we are substituting to represent the same general trait in the Mod-FP and FFP.
To assess the association of the Mod-FP with the FFP, we estimated receiver operator characteristic (ROC) curves. FFP was used as a gold standard measure with the three-level categorization of not frail, prefrail, and frail. We evaluated the sensitivity, specificity, and classification of the Mod-FP at various cut-offs to determine the optimal points to categorize PWH as not frail, prefrail, and frail. We repeated these analyses among subgroups of PWH by age (≥55 vs. <55 years [55 years selected based on 54 years being the median age]), gender, and race (Black/White only, due to sample size) to confirm the consistency of classification among subgroups and highlight the generalizability of the Mod-FP. We also conducted a sensitivity analysis among PWH based on whether their two visits were within or beyond 30 days apart to evaluate the impact of the time lag on classification of frailty and prefrailty. Using the identified cut-points, we also categorized the Mod-FP into not frail, prefrail, and frail. We then evaluated the agreement of classifying PWH as not frail, prefrail, and frail between the two phenotypes with Cohen’s kappa. We estimated unweighted and weighted (linear and quadratic) kappa.
Finally, we assessed construct validity via hypothesis testing of the Mod-FP by estimating the association between frailty at that study visit and the report of falls in the previous year (any and recurrent) with logistic regression (Mokkink et al., 2010). We compared the estimated associations (i.e., odds ratios) between frailty and falls. We hypothesized that the Mod-FP would distinguish between PWH who reported falls versus no falls. Regression models were adjusted for age and gender assigned at birth. We also stratified by age (and only adjusted for gender in these models) to examine the validity within age strata for the “any fall” models. The results for these models represent the odds ratio of experiencing a fall associated with each additional component on either phenotype. Statistical significance was evaluated at the 95% confidence level. All analyses were performed using Stata version 16.1 (StataCorp, College Station, TX).
Results
Among 522 PWH included in this study, the mean age was 52 years (median: 54 years [interquartile range: 44–61 years]) and 112 (21%) were female (Table 1). Over half (268, 51%) self-reported Black race, whereas 217 (42%) self-reported White race. The prevalence of individual frailty components ranged from 12% for low grip strength to 25% for low physical activity (Table 1). Among the 253 PWH who responded to the falls question, which was implemented after study information thus only among a portion of PWH, 48 (19%) reported at least one fall and 22 (9%) reported recurrent falls in the past 12 months.
Table 1.
Descriptive Statistics of the Analytic Cohort (N = 522)
| Variable | n (%) unless noted |
|---|---|
| Gender | |
| Male | 410 (79) |
| Female | 112 (21) |
| Age (years), mean (SD) | 52 (11) |
| Age (years), median (IQR) | 54 (44–61) |
| <55 | 276 (53) |
| ≥55 | 246 (47) |
| Race/ethnicity | |
| Black | 268 (51) |
| Hispanic | 26 (5) |
| Other | 11 (2) |
| White | 217 (42) |
| Frailty components | |
| Unintentional weight loss | 78 (15) |
| Exhaustion/fatigue | 110 (21) |
| Low physical activity | 129 (25) |
| Poor mobility | 111 (21) |
| Walk time | 85 (16) |
| Grip strength | 65 (12) |
Note. IQR = interquartile range.
The Spearman correlation coefficient between the Mod-FP and FFP was 0.81 (p < .001), suggesting good overlap in their measurement of frailty. We observed that PWH who reported mobility difficulty (i.e., presenting with the low mobility component) had slower average gait speed times than PWH reporting no mobility difficulty, with mean gait speed of 4.3 versus 3.8 s, respectively (Figure 2).
Figure 2.
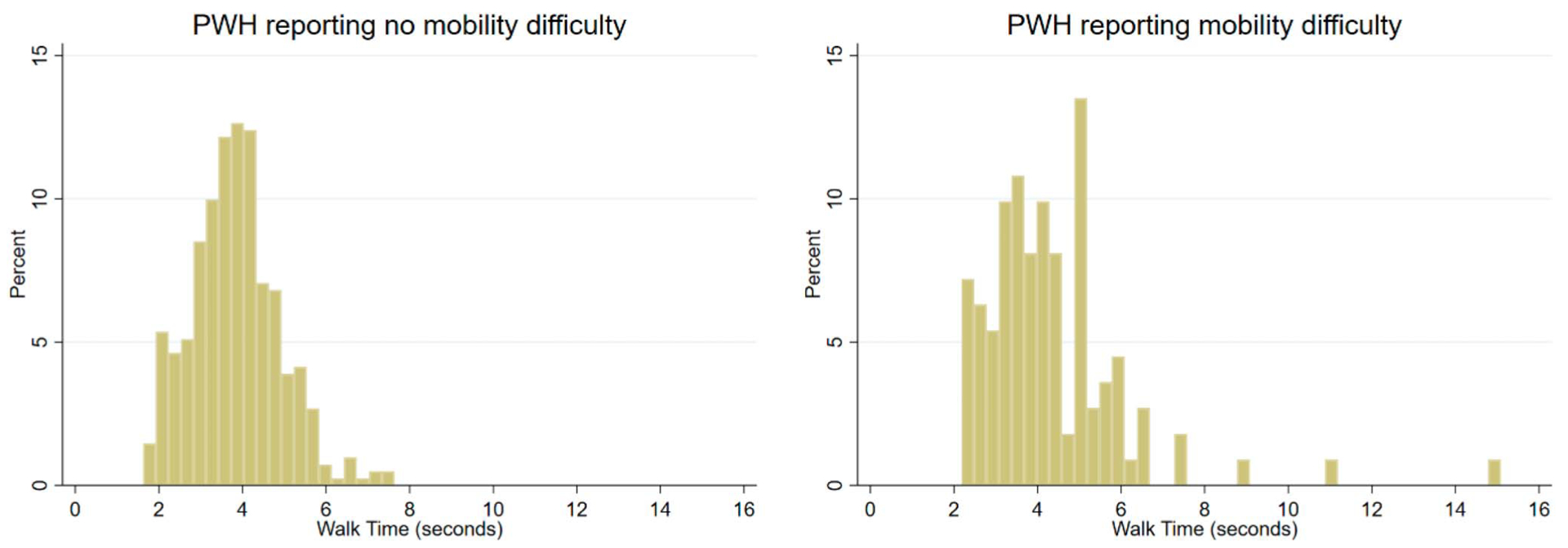
Histograms of walk time (gait speed) among people with HIV (PWH) without versus with mobility difficulty. We graphed histograms of walk time (measured in seconds) based on self-reported mobility. Mobility was dichotomized between PWH reporting mobility difficulty and PWH reporting no mobility difficulty. Walk times among PWH reporting mobility difficulty were skewed toward being slower than times among PWH reporting no difficulty.
Sensitivity, specificity, and ROC area under the curve (AUC) are presented in Table 2. At a cut-point of 3 (i.e., where a Mod-FP score ≥3 was classified as frail), the Mod-FP had a sensitivity of 62%, specificity of 97%, and 94% of PWH correctly classified as frail or not frail based on their status in FFP. There was an AUC of 0.93 (95% CI = 0.91–0.96) for this analysis. In analyses stratified by age, sex, and race, we observed similar results, suggesting robust diagnostic accuracy persisting within the subgroups. We also evaluated the Mod-FP for classification of prefrail PWH and observed a sensitivity of 77% and specificity of 92% at a cut-point of 1 and AUC of 0.86 (95% CI: 0.83–0.89; Table 2). These results suggest that the original cut-points used in the FFP (where a score of 0 is not frail, 1–2 is prefrail, and ≥3 is frail) are appropriate for the Mod-FP as well. In the sensitivity analysis based on time between visits (i.e., PROSPER-HIV and CNICS PRO assessments), we observed comparable ROC AUC values for frailty between the two groups (0.94 for PWH with visits within 30 days vs. 0.94 for beyond 30 days) and worse, but still acceptable, ROC AUC for prefrailty among the PWH with visits beyond 30 days (0.88 for PWH with visits within 30 days vs. 0.77 for beyond 30 days), suggesting the prefrailty stage in particular may be a dynamic state that fluctuates over time, especially because it can be defined with a single component (Table 3).
Table 2.
Receiver Operator Characteristic (ROC) Results for the Classification of Prefrailty and Frailty on the Mod-FP Compared With the FFP
| Sample | ROC AUC(95% CI) | Cut point (Mod-FP Score) | Sensitivity | Specificity | Correctly Classified |
|---|---|---|---|---|---|
| Everyone | 0.934 (0.905−0.963) | ≥1 | 100% | 57% | 60% |
| ≥2 | 89% | 83% | 84% | ||
| ≥3 | 62% | 97% | 94% | ||
| ≥4 | 27% | 100% | 94% | ||
| PWH <55 years | 0.962 (0.935–0.990) | ≥1 | 100% | 56% | 60% |
| ≥2 | 96% | 84% | 85% | ||
| ≥3 | 69% | 99% | 96% | ||
| ≥4 | 31% | 100% | 93% | ||
| PWH ≥55 years | 0.898 (0.843–0.954) | ≥1 | 100% | 58% | 61% |
| ≥2 | 79% | 82% | 82% | ||
| ≥3 | 54% | 95% | 91% | ||
| ≥4 | 21% | 100% | 94% | ||
| Men | 0.929 (0.894–0.963) | ≥1 | 100% | 59% | 62% |
| ≥2 | 85% | 85% | 85% | ||
| ≥3 | 59% | 97% | 94% | ||
| ≥4 | 18% | 100% | 93% | ||
| Women | 0.959 (0.918–0.999) | ≥1 | 100% | 48% | 53% |
| ≥2 | 100% | 76% | 79% | ||
| ≥3 | 73% | 96% | 94% | ||
| ≥4 | 55% | 100% | 96% | ||
| Black PWH | 0.943 (0.906–0.981) | ≥1 | 100% | 58% | 62% |
| ≥2 | 91% | 83% | 84% | ||
| ≥3 | 61% | 98% | 95% | ||
| ≥4 | 35% | 100% | 94% | ||
| White PWH | 0.920 (0.867–0.972) | ≥1 | 100% | 55% | 59% |
| ≥2 | 84% | 83% | 83% | ||
| ≥3 | 63% | 96% | 93% | ||
| ≥4 | 16% | 100% | 93% | ||
| Everyone—prefrail classification | 0.858 (0.828–0.888) | ≥1 | 77% | 92% | 84% |
| ≥2 | 33% | 100% | 65% | ||
| ≥3 | 6% | 100% | 52% |
Note. AUC = area under curve; FFP = Fried’s frailty phenotype; Mod-FP = modified Fried phenotype; PWH = people with HIV.
Table 3.
Receiver Operator Characteristic (ROC) Results for the Classification of Prefrailty and Frailty on the Mod-FP Compared With the FFP, Based on Time Between Visits (Within 30 days and Beyond 30 days)
| Sample | ROC AUC (95% CI) | Cut point (Mod-FP Score) | Sensitivity | Specificity | Correctly Classified |
|---|---|---|---|---|---|
| Visits within 30 days—Frail classification | 0.935 (0.899–0.971) | ≥1 | 100% | 54% | 58% |
| ≥2 | 90% | 82% | 83% | ||
| ≥3 | 63% | 97% | 95% | ||
| ≥4 | 27% | 100% | 95% | ||
| Visits within 30 days—Prefrail classification | 0.883 (0.852–0.914) | ≥1 | 82% | 92% | 87% |
| ≥2 | 35% | 100% | 66% | ||
| ≥3 | 6% | 100% | 52% | ||
| Visits beyond 30 days—Frail classification | 0.936 (0.887–0.985) | ≥1 | 100% | 64% | 69% |
| ≥2 | 87% | 86% | 86% | ||
| ≥3 | 60% | 96% | 92% | ||
| ≥4 | 27% | 100% | 91% | ||
| Visits beyond 30 days—Prefrail classification | 0.770 (0.697–0.844) | ≥1 | 60% | 92% | 75% |
| ≥2 | 27% | 100% | 62% | ||
| ≥3 | 7% | 100% | 51% |
Note. AUC = area under curve; FFP = Fried’s frailty phenotype; Mod-FP = modified Fried phenotype; PWH = people with HIV.
Using these cut-points for categorization of frailty stages, the Mod-FP categorized 43 (8%) PWH as frail and 209 (40%) as prefrail, whereas the FFP categorized 45 (9%) PWH as frail and 246 (47%) as prefrail (Table 4). Agreement between the frailty definitions, measured by Cohen’s kappa, conferred substantial agreement (0.64 unweighted, 0.75 quadratic weighted; Table 4). The unweighted observed agreement was 80%.
Table 4.
Categorization (n [%]) and Agreement of Classification of Frailty Stages by Mod-FP and FFP
| FFP—not frail | FFP—prefrail | FFP—frail | Total | |
|---|---|---|---|---|
| Categorization into frailty stages | ||||
| Mod-FP—not frail | 213 (92) | 57 (23) | 0 (0) | 270 |
| Mod-FP—prefrail | 18 (8) | 174 (71) | 17 (38) | 209 |
| Mod-FP—frail | 0 (0) | 15 (6) | 28 (62) | 43 |
| Total | 231 | 246 | 45 | 522 |
| Weighting Scheme | Agreement | Expected Agreement | κ | Interpretation |
| Agreement between Mod-FP and FFP | ||||
| Unweighted | 79.5% | 42.5% | 0.64 | Substantial |
| Weighted linear | 89.8% | 67.2% | 0.69 | Substantial |
| Weighted quadratic | 94.9% | 79.5% | 0.75 | Substantial |
Note. Not frail (0 components), prefrail (1–2 components), and frail (≥3 components).
Note. FFP = Fried’s frailty phenotype; Mod-FP = modified Fried phenotype.
Finally, in logistic regression models summarizing associations with having experienced a fall, we observed significant associations between additional frailty components and falls, with smaller magnitude of point estimates for the Mod-FP (odds ratio [OR] = 1.36, 95% CI = 1.02–1.81, p = .04) compared with the FFP (OR = 1.63, 95% CI = 1.22–2.18, p < .01), although the confidence intervals overlapped (Figure 3 and Table 5). In age-stratified analyses, these associations and trends persisted among PWH ≥55 years (Mod-FP: OR = 1.76, 95% CI = 1.15–2.68, p = .01; FFP: OR = 2.07, 95% CI = 1.26–3.41, p < .01) and were attenuated among PWH <55 years (Mod-FP: OR = 1.09, 95% CI = 0.72–1.63, p=.69; FFP: OR = 1.41, 95% CI = 0.99–1.99, p = .053; Figure 3). We also estimated associations between frailty and recurrent falls and observed similar results (Mod-FP: OR: 1.56, 95% CI = 1.07–2.28, p = .02; FFP: OR: 1.76, 95% CI = 1.23–2.51, p < .01; Figure 3). We did not stratify these models due to the small number of recurrent falls.
Figure 3.
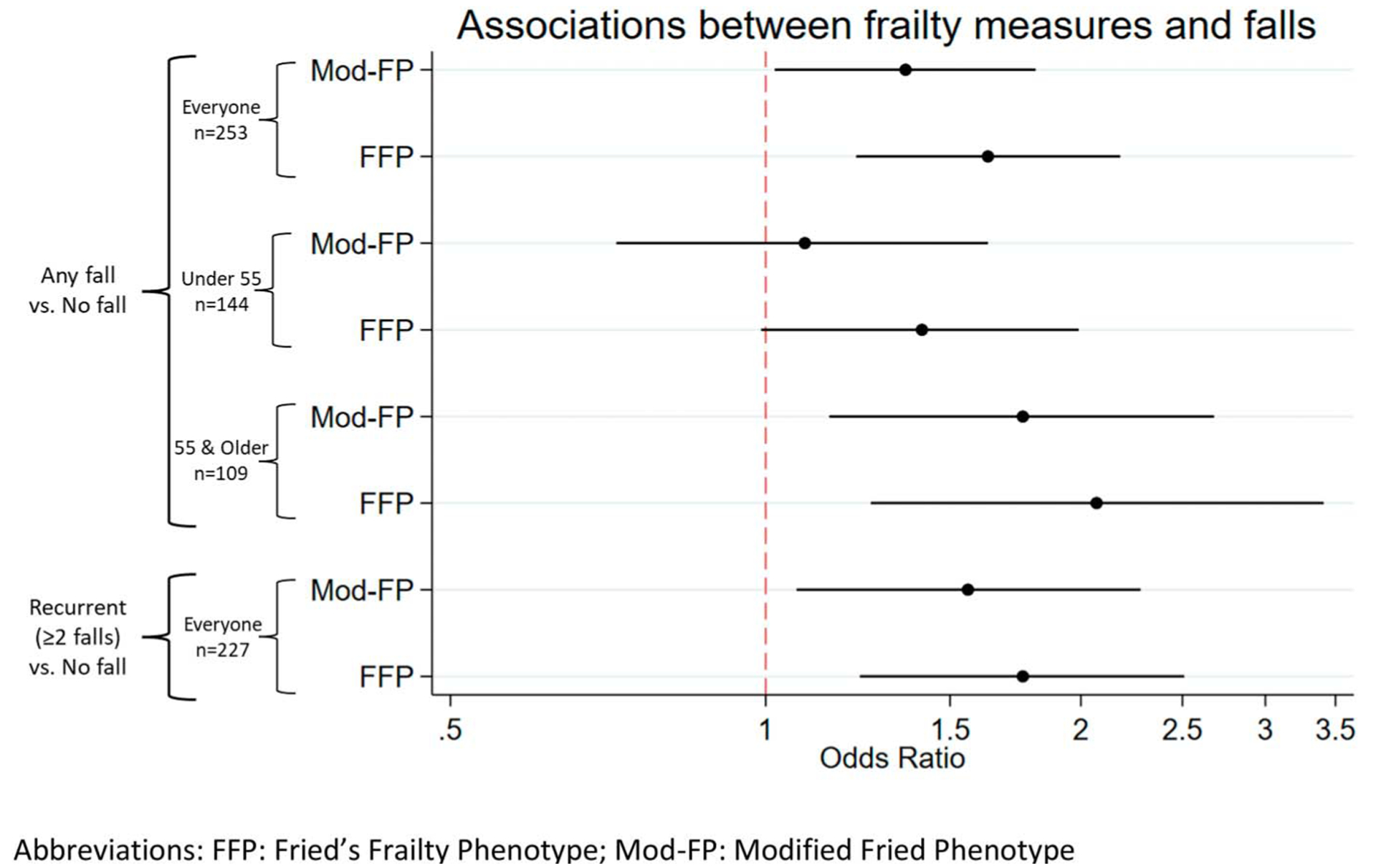
Forest plot comparison of logistic regression estimated associations between frailty measures and falls. We used logistic regression to estimate the association between frailty and self-reported falls in the past 12 months. Falls were dichotomized as any versus none and recurrent versus none. We found both phenotype definitions to be associated with any fall and recurrent falls, with stronger associations with the FFP. We stratified the model for any fall by age (at 55 years) and observed stronger associations among older people with HIV (PWH), whereas the associations were attenuated among younger PWH. FFP = Fried’s frailty phenotype.
Table 5.
Comparison of Logistic Regression Estimated Associations Between Frailty Measures (Mod-FP or FFP) and Falls
| Phenotype | Fall versus No Fall | Recurrent (≥2 Falls) versus No Fall |
|---|---|---|
| OR (95% CI), p-Value | OR (95% CI), p-Value | |
| Everyone,a n = 253 | ||
| Mod-FP score | 1.36 (1.02, 1.81), 0.04 | 1.56 (1.07, 2.28), 0.02 |
| FFP score | 1.63 (1.22,2.18), <0.01 | 1.76 (1.23,2.51), <0.01 |
| <55 years,b n = 144 | ||
| Mod-FP score | 1.09 (0.72, 1.63), 0.69 | |
| FFP score | 1.41 (0.99, 1.99), 0.053 | |
| ≥55 years,b n = 109 | ||
| Mod-FP score | 1.76 (1.15, 2.68), 0.01 | |
| FFP score | 2.07 (1.26,3.41), <0.01 | |
Note. 95% CI = 95% confidence interval; FFP = Fried’s frailty phenotype; Mod-FP = modified Fried phenotype; OR = odds ratio.
Adjusted for age and gender at birth.
Adjusted for gender at birth.
Discussion
We evaluated the utility of Mod-FP compared with FFP in discriminating not frail, prefrail, and frail PWH and observed good measurement properties, including excellent discrimination of frailty and substantial agreement with FFP in classifying PWH by their frailty status. Identical cut-points were used for categorization into the three levels often used to classify frailty stages (not frail, prefrail, and frail), and Mod-FP produced comparable, albeit weaker, association estimates for the relationship between frailty and falls. Our results also support the utility of the observed functional status measures (i.e., grip strength and gait speed) as important components to collect when possible and when heightened sensitivity is required based on research questions and clinic resources because they add additional context beyond the self-reported components. Overall, the Mod-FP has excellent feasibility; it is a low-burden and an easily collected self-report measure of frailty. Mod-FP can be systematically collected in most settings, with specific utility in HIV care settings and large research studies, given the increasing rates of frailty in this aging population, the impact of frailty on long-term outcomes, and growing interest in HIV and aging research specifically focused on frailty.
Mod-FP had excellent discrimination for not frail PWH (specificity of 97%), but had lower discrimination for frail PWH (sensitivity of 62%) at a cutoff of three components. Although low sensitivity is less desirable, the sensitivity for detecting frailty at a cutoff of two components is higher (89%). Therefore, some frail PWH may be misclassified as prefrail but would likely remain within consideration for frailty interventions for slowing or managing the progression. For prefrailty diagnostics, the Mod-FP was also better at discriminating nonprefrail (specificity of 92%) than prefrail (sensitivity of 77%) PWH at a cutoff of one component. These results are consistent with a study by Van der Elst et al. (2020) evaluating the substitution of the functional status measures with self-reported questions. Van der Elst and colleagues hypothesized that these differences in sensitivity and specificity may be indicators of people overestimating their physical health/status in self-report compared with their performance-based assessment results, which may also be the case in our cohort.
Furthermore, our results suggest that cut-points for categorizing not frail, prefrail, and frail PWH could be the same as for the FFP, even though the Mod-FFP has one fewer component: not frail, score of 0; prefrail, score of 1–2; and frail, score of ≥3 (Fried et al., 2001). These classification values and cut-points observed in the full cohort also performed well in stratified analyses in older/younger PWH, men/women, and Black/White PWH. This consistency is important to confirm because future studies may focus on specific population subgroups for certain research questions. This is also a new approach that has not been done in other studies evaluating phenotype validity but is an important feature of the measure (Kim et al., 2020; McMillan et al., 2021; Van der Elst et al., 2020).
We also observed very good agreement between the Mod-FP and FFP in this cohort: 8 and 9% of PWH were classified as frail, respectively, with observed agreement of 80%. The agreement between the phenotypes was substantial in all weighting schemes (Cohen’s kappa ranged from 0.64 to 0.75). Our results were similar to the study by Van der Elst et al. (2020), although they included replacement questions for both weakness and slowness, whereas we replaced slowness and excluded weakness from the Mod-FP. We also confirmed that the substitution of gait speed with the mobility PRO is reasonable.
The prevalence of frailty among the 522 participants in this study was lower than in all of CNICS—8% on the Mod-FP in this study versus 13% as measured by the Mod-FP across the whole CNICS cohort (Crane et al., 2022). This difference may be due to enrolling PWH who are less likely to have frailty perhaps being more likely to volunteer to participate in the PROSPER-HIV study, which is focused on nutrition and physical activity assessments. This particular subset of PWH in CNICS may have better overall health. Also, all PROSPER-HIV participants must be prescribed ART and have an undetectable HIV viral load, which confers better health. Misrepresentation of health in self-report has been hypothesized as a reason for modified phenotypes mis-classifying frail individuals (exemplified by the lower sensitivity values; Van der Elst et al., 2020). Our study sample, PWH in CNICS who were independently assessed at PROSPER-HIV visits and not selected for inclusion based on frailty status, highlights a strength of this analysis in the context of evaluating the Mod-FP.
Both the Mod-FP and the FFP were associated with falls in the previous year, especially for recurrent falls, consistent with the general older adult population (Cheng & Chang, 2017). The observed association (i.e., higher odds of having experienced a fall associated with higher frailty scores) persisted in both phenotype definitions among older PWH when we stratified by age, but was attenuated for younger PWH in the FFP and null for the Mod-FP. Overall, these results show similar estimation of associations for the definitions; however, the observed functional status measures provide additional important information especially for younger PWH, consistent with evidence from other studies (Beanland et al., 2021; Cesari et al., 2009). Of note, this study was not designed to fully evaluate falls among PWH, but we did observe a higher risk of falls associated with higher frailty scores, warranting future studies evaluating the epidemiology of frailty and falls in this population, including risk factors, potential causes, and best strategies for prevention.
The attenuation of associations between frailty and falls among younger PWH requires some additional investigation. Although PWH experience frailty at younger ages than the general population (Desquilbet et al., 2007; Levett et al., 2016; Piggott et al., 2016; Thurn & Gustafson, 2017), less is known about how well the commonly used instruments measure frailty among younger PWH. Our results align with this. Sensitivity and specificity were similar, and the AUC of the ROC was higher among younger PWH than older, suggesting the Mod-FP is comparable with the FFP in identifying younger frail PWH. However, the lower magnitude of the point estimates in the falls analyses highlights the need for further investigation regarding the predictive ability of frailty phenotypes among younger PWH. Frailty assessment among younger adults is not often done in the general population and understanding the limitations of frailty measurement poses clinical importance among PWH. Ultimately, our study provides clear evidence as to the ability of the Mod-FP to identify frailty and prefrailty, which is an essential first step to manage and follow its progression in care.
Our results support the use of the Mod-FP within CNICS; however, it is important to highlight that evaluating the validity of a new measure is a lengthy process involving a large body of research with considerations of different settings and subgroups that the measure may be used among (Hughes, 2018). Further, the “validity” of a measure should be viewed as a dynamic property that can fluctuate along a spectrum (not an “all or nothing” property) and is the sum of its components, including but not limited to the forms of validity evidence presented here, rather than a conclusion from a single analysis (Hughes, 2018; Mokkink et al., 2010). In this study, we collected and analyzed evidence to understand some of these properties in the setting of PWH engaged in clinical care in the United States. We evaluated the performance of the Mod-FP specifically in subgroups to gain a more comprehensive view of these properties. Overall, evaluating the validity of a measure should be an ongoing process, including updating data and results when possible to continue accumulating evidence for these measurement properties (Hughes, 2018).
Strengths of this study include the demographic diversity and size of the cohort, which allowed for stratification and subgroup analyses. Our results are also consistent with other studies that evaluated modifications to the FFP with substitutions and/or exclusions of components, and we were able to expand this work to include the subgroup analyses among PWH (Beanland et al., 2021; Van der Elst et al., 2020).
Notable limitations include limited generalizability due to self-selection into the PROSPER-HIV study. The prevalence of frailty in the PROSPER-HIV cohort was slightly lower than in the overall CNICS cohort, so participants in PROSPER-HIV may be healthier than the larger CNICS cohort. Additionally, we were limited by sample size to robustly assess recurrent falls within different strata; however, future work aims to thoroughly evaluate the epidemiology and risk factors for the relationship between frailty and falls among PWH. Furthermore, the cross-sectional design of this study limits our interpretation of causality or directionality of these associations. The cross-sectional nature also precluded any analyses of the progression of frailty, which could highlight further questions regarding differences in frailty measurements over time and could vary in age strata due to differential likelihood of transitions (both deterioration and recovery) in frailty associated with age (Piggott et al., 2020). Finally, there was a gap for some PWH between the dates of completing their two frailty assessments, particularly among those whose PROSPER-HIV visit occurred during the COVID-19 pandemic; however, most PWH (77%) completed both within 30 days. We assessed this time lag in sensitivity analyses and observed generally comparable ROC values (0.94 vs. 0.94 for frailty and 0.88 vs. 0.77 for prefrailty, Table 3), and the results suggest that prefrailty is a dynamic state.
Conclusion
The Mod-FP has good measurement properties for frailty with highly feasible (e.g., low burden, fast, and does not require care provider administration) and widespread collection throughout CNICS that could be applied in other time- or resource-constrained settings among PWH as well. Nevertheless, validity properties should be reassessed in other settings. We found frailty stages for the Mod-FP (not frail, prefrail, and frail) should be defined as in FFP. Finally, functional status measures (e.g., grip strength and gait speed) provide valuable additional information when available.
Key Considerations.
Ascertainment of frailty status among people with HIV is important to understand risk factors and interventions to slow progression.
Self-reported modified Fried’s frailty phenotypes can accurately and reliably measure frailty status with a low resource burden in HIV clinics across the United States, allowing for widespread collection and characterization of frailty to promote healthy aging among people with HIV.
Functional status measures of grip strength and gait speed provide additional information as to overall physical frailty and could be used for extra measurement after self-reported assessments.
Continued collection of validity evidence for modified frailty phenotypes for use in other settings and populations is warranted.
Acknowledgments
The authors acknowledge all CNICS and PROSPER-HIV participants and study personnel for their essential contributions to this work. This work was supported by AHRQ grant U18HS026154 (recipient: H. M. Crane), NIA grant R33AG067069 (recipient: H. M. Crane), NIAID grant R24AI067039 (recipient: M. S. Saag), NIAAA grant U01AA020793 (recipient: H. M. Crane), NIDA grant R01DA047045 (recipient: H. M. Crane), and NINR grant R01NR018391 (recipient: A. R. Webel).
Contributor Information
Stephanie A. Ruderman, Department of Epidemiology, University of Washington, Seattle, Washington, USA..
Allison R. Webel, School of Nursing, University of Washington, Seattle, Washington, USA..
Amanda L. Willig, School of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA..
Lydia N. Drumright, School of Nursing, University of Washington, Seattle, Washington, USA..
Annette L. Fitzpatrick, Department of Epidemiology, University of Washington, Seattle, Washington, USA..
Michelle C. Odden, Department of Epidemiology and Population Health, School of Medicine, Stanford University, Stanford, California, USA..
John D. Cleveland, School of Public Health, University of Alabama at Birmingham, Birmingham, Alabama, USA..
Greer Burkholder, School of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA..
Christine H. Davey, School of Nursing, Case Western Reserve University, Cleveland, Ohio, USA..
Julia Fleming, Harvard Medical School, Fenway Institute, Boston, Massachusetts, USA..
Thomas W. Buford, School of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA and Birmingham/Atlanta Geriatric Research, Education, and Clinical Center, Birmingham VA Medical Center, Birmingham, Alabama, USA..
Raymond Jones, School of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA..
Robin M. Nance, School of Medicine, University of Washington, Seattle, Washington, USA..
Bridget M. Whitney, School of Medicine, University of Washington, Seattle, Washington, USA..
L. Sarah Mixson, School of Medicine, University of Washington, Seattle, Washington, USA..
Andrew W. Hahn, School of Medicine, University of Washington, Seattle, Washington, USA..
Kenneth H. Mayer, Harvard Medical School, Fenway Institute, Boston, Massachusetts, USA..
Meredith Greene, Department of Medicine, University of California San Francisco, San Francisco, California, USA..
Michael S. Saag, Department of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA..
Charles Kamen, Department of Surgery, University of Rochester, Rochester, New York, USA..
Chintan Pandya, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, USA..
William B. Lober, School of Nursing, University of Washington, Seattle,Washington, USA..
Mari M. Kitahata, School of Medicine, University of Washington, Seattle, Washington, USA..
Paul K. Crane, School of Medicine, University of Washington, Seattle, Washington, USA..
Heidi M. Crane, School of Medicine, University of Washington, Seattle, Washington, USA..
Joseph A. C. Delaney, College of Pharmacy, University of Manitoba, Winnipeg, Manitoba, California, USA..
References
- Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M, & Vellas B (2009, Dec). Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. The Journal of Nutrition, Health & Aging, 13(10), 881–889. 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- Ainsworth BE, Jacobs DR Jr., & Leon AS (1993, Jan). Validity and reliability of self-reported physical activity status: the Lipid Research Clinics questionnaire. Medicine and Science in Sports and Exercise, 25(1), 92–98. 10.1249/00005768-199301000-00013. [DOI] [PubMed] [Google Scholar]
- Akgun KM, Tate JP, Crothers K, Crystal S, Leaf DA, Womack J, Brown TT, Justice AC, & Oursler KK (2014). An adapted frailty-related phenotype and the VACS index as predictors of hospitalization and mortality in HIV-infected and uninfected individuals. J Acquir Immune Defic Syndr, 67(4), 397–404. 10.1097/QAI.0000000000000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprahamian I, Cezar NOC, Izbicki R, Lin SM, Paulo DLV, Fattori A, Biella MM, Jacob Filho W, & Yassuda MS (2017). Screening for Frailty With the FRAIL Scale: A Comparison With the Phenotype Criteria. Journal of the American Medical Directors Association, 18(7), 592–596. 10.1016/j.jamda.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Beanland A, Alagaratnam J, Goffe C, Bailey A, Dosekun O, Petersen C, Ayap W, Garvey LJ, Walsh J, Mackie NE, & Winston A (2021). Objective and subjective rapid frailty screening tools in people with HIV. HIV Med, 22(2), 146–150. 10.1111/hiv.12988. [DOI] [PubMed] [Google Scholar]
- Blanquet M, Ducher G, Sauvage A, Dadet S, Guiyedi V, Farigon N, Guiguet-Auclair C, Berland P, Bohatier J, Boirie Y, & Gerbaud L (2022). Handgrip strength as a valid practical tool to screen early-onset sarcopenia in acute care wards: a first evaluation. European Journal of Clinical Nutrition, 76(1), 56–64. 10.1038/s41430-021-00906-5. [DOI] [PubMed] [Google Scholar]
- Bouillon K, Kivimaki M, Hamer M, Sabia S, Fransson EI, Singh-Manoux A, Gale CR, & Batty GD (2013). Measures of frailty in population-based studies: an overview. BMC Geriatrics, 13, 64. 10.1186/1471-2318-13-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Kritchevsky SB, Newman AB, Simonsick EM, Harris TB, Penninx BW, Brach JS, Tylavsky FA, Satterfield S, Bauer DC, Rubin SM, Visser M, Pahor M, Health A, & Body Composition S (2009, Feb). Added value of physical performance measures in predicting adverse health-related events: results from the Health, Aging And Body Composition Study. Journal of the American Geriatrics Society, 57(2), 251–259. 10.1111/j.1532-5415.2008.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng MH, & Chang SF (2017, Sep). Frailty as a Risk Factor for Falls Among Community Dwelling People: Evidence From a Meta-Analysis. J Nurs Scholarsh, 49(5), 529–536. 10.1111/jnu.12322. [DOI] [PubMed] [Google Scholar]
- Crane HM, Ruderman SA, Whitney BM, Nance RM, Drumright LN, Webel AR, Willig AL, Saag MS, Christopoulos K, Greene M, Hahn AW, Eron JJ, Napravnik S, Mathews WC, Chander G, McCaul ME, Cachay ER, Mayer KH, Landay A, & Austad S (2022). Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) Cohort StudyAssociations between drug and alcohol use, smoking, and frailty among people with HIV across the United States in the current era of antiretroviral treatment. Drug and Alcohol Dependence, 240. 10.1016/j.drugalcdep.2022.109649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, & Zamboni M (2010). European Working Group on Sarcopenia in Older, PSarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and Ageing, 39(4), 412–423. 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, Margolick JB, & Multicenter ACS (2007, Nov). HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med. Sci, 62(11), 1279–1286. 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- Erlandson KM, Allshouse AA, Jankowski CM, Duong S, MaWhinney S, Kohrt WM, & Campbell TB (2012). Risk factors for falls in HIV-infected persons. J Acquir Immune Defic Syndr, 61(4), 484–489. 10.1097/QAI.0b013e3182716e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredericksen R, Crane PK, Tufano J, Ralston J, Schmidt S, Brown T, Layman D, Harrington RD, Dhanireddy S, Stone T, Lober W, Kitahata MM, & Crane HM (2012, Feb). Integrating a web-based, patient-administered assessment into primary care for HIV-infected adults. Australian Health Review: a Publication of the Australian Hospital Association, 4(2), 47–55. 10.5897/jahr11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, & Cardiovascular Health Study Collaborative Research, G. (2001). Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med. Sci, 56(3), M146–M156. 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- Hughes DJ (2018) The Wiley handbook of psychometric testing: A multidisciplinary reference on survey, scale and test development. Psychometric validity: Establishing the accuracy and appropriateness of psychometric measures. (Vols. 1–2, pp. 751–779): Wiley Blackwell. 10.1002/9781118489772.ch24. [DOI] [Google Scholar]
- Jung HW, Kim S, & Won CW (2021, Mar). Validation of the Korean Frailty Index in community-dwelling older adults in a nationwide Korean Frailty and Aging Cohort study. The Korean Journal of Internal Medicine, 36(2), 456–466. 10.3904/kjim.2019.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, Holmes W, Gifford AL, Rabeneck L, Zackin R, Sinclair G, Weissman S, Neidig J, Marcus C, Chesney M, Cohn SE, Wu AW, & Adult ACTUOC (2001, Dec). Development and validation of a self-completed HIV symptom index. J Clin Epidemiol, 54(Suppl. 1), S77–S90. 10.1016/s0895-4356(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Kim S, Kim M, Jung HW, & Won CW (2020, May). Development of a Frailty Phenotype Questionnaire for Use in Screening Community-Dwelling Older Adults. Journal of the American Medical Directors Association, 21(5), 660–664. 10.1016/j.jamda.2019.08.028. [DOI] [PubMed] [Google Scholar]
- Kitahata MM, Rodriguez B, Haubrich R, Boswell S, Mathews WC, Lederman MM, Lober WB, Van Rompaey SE, Crane HM, Moore RD, Bertram M, Kahn JO, & Saag MS (2008, Oct). Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. International Journal of Epidemiology, 37(5), 948–955. 10.1093/ije/dym231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levett TJ, Cresswell FV, Malik MA, Fisher M, & Wright J (2016, May). Systematic Review of Prevalence and Predictors of Frailty in Individuals with Human Immunodeficiency Virus. Journal of the American Geriatrics Society, 64(5), 1006–1014. 10.1111/jgs.14101. [DOI] [PubMed] [Google Scholar]
- McMillan JM, Gill MJ, Power C, Fujiwara E, Hogan DB, & Rubin LH (2021). Sep 1). Construct and Criterion-Related Validity of the Clinical Frailty Scale in Persons With HIV. J Acquir Immune Defic Syndr, 88(1), 110–116. 10.1097/QAI.0000000000002736. [DOI] [PubMed] [Google Scholar]
- Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, Bouter LM, & de Vet HC (2010, Jul). The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol, 63(7), 737–745. 10.1016/j.jclinepi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, Cesari M, Chumlea WC, Doehner W, Evans J, Fried LP, Guralnik JM, Katz PR, Malmstrom TK, McCarter RJ, Gutierrez Robledo LM, Rockwood K, von Haehling S, Vandewoude MF, & Walston J (2013, Jun). Frailty consensus: a call to action. Journal of the American Medical Directors Association, 14(6), 392–397. 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op Het Veld LPM, de Vet HCW, van Rossum E, Kempen G, van Kuijk SMJ, & Beurskens A (2018). Substitution of Fried’s performance-based physical frailty criteria with self-report questions. Archives of Gerontology and Geriatrics, 75, 91–95. 10.1016/j.archger.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Piggott DA, Bandeen-Roche K, Mehta SH, Brown TT, Yang H, Walston JD, Leng SX, & Kirk GD (2020). Jul 1)Frailty transitions, inflammation, and mortality among persons aging with HIV infection and injection drug use. AIDS, 34(8), 1217–1225. 10.1097/QAD.0000000000002527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott DA, Erlandson KM, & Yarasheski KE (2016). Frailty in HIV: Epidemiology, Biology, Measurement, Interventions, and Research Needs. Curr HIV/AIDS Rep, 13(6), 340–348. 10.1007/s11904-016-0334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin R, & de Charro F (2001, Jul). EQ-5D: a measure of health status from the EuroQol Group. Ann Med, 33(5), 337–343. 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- Rockwood K (2005, Sep). What would make a definition of frailty successful?. Age and Ageing, 34(5), 432–434. 10.1093/ageing/afi146. [DOI] [PubMed] [Google Scholar]
- Sharma A, Hoover DR, Shi Q, Gustafson DR, Plankey MW, Tien PC, Weber KM, & Yin MT (2019). Frailty as a predictor of falls in HIV-infected and uninfected women. Antiviral Therapy, 24(1), 51–61. 10.3851/IMP3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syddall H, Cooper C, Martin F, Briggs R, & Aihie Sayer A (2003, Nov). Is grip strength a useful single marker of frailty?. Age and Ageing, 32(6), 650–656. 10.1093/ageing/afg111. [DOI] [PubMed] [Google Scholar]
- Theou O, Cann L, Blodgett J, Wallace LM, Brothers TD, & Rockwood K (2015, May). Modifications to the frailty phenotype criteria: Systematic review of the current literature and investigation of 262 frailty phenotypes in the Survey of Health, Ageing, and Retirement in Europe. Ageing Research Reviews, 21, 78–94. 10.1016/j.arr.2015.04.001. [DOI] [PubMed] [Google Scholar]
- Thurn M, & Gustafson DR (2017, Feb). Faces of Frailty in Aging with HIV Infection. Curr HIV/AIDS Rep, 14(1), 31–37. 10.1007/s11904-017-0348-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Elst MCJ, Schoenmakers B, Op Het Veld LPM, De Roeck EE, Van der Vorst A, Schols J, De Lepeleire J, Kempen G, & Consortium DS (2020, Oct). Validation of replacement questions for slowness and weakness to assess the Fried Phenotype: a cross-sectional study. Eur Geriatr Med, 11(5), 793–801. 10.1007/s41999-020-00337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeiren S, Vella-Azzopardi R, Beckwee D, Habbig AK, Scafoglieri A, Jansen B, Bautmans I, & Gerontopole Brussels Study, g. (2016). Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. Journal of the American Medical Directors Association, 17(12), 1163–1163 e1117. 10.1016/j.jamda.2016.09.010 [DOI] [PubMed] [Google Scholar]
- Webel AR, Long D, Rodriguez B, Davey CH, Buford TW, Crane HM, Mayer K, Saag MS, & Willig AL (2020). The PROSPER-HIV Study: A Research Protocol to Examine Relationships Among Physical Activity, Diet Intake, and Symptoms in Adults Living With HIV. The Journal of the Association of Nurses in AIDS Care: JANAC, 31(3), 346–352. 10.1097/JNC.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing EJ (2016, Dec). HIV and aging. Int J Infect Dis, 53, 61–68. 10.1016/j.ijid.2016.10.004. [DOI] [PubMed] [Google Scholar]
- Wu C, Geldhof GJ, Xue QL, Kim DH, Newman AB, & Odden MC (2018). Development, Construct Validity, and Predictive Validity of a Continuous Frailty Scale: Results From 2 Large US Cohorts. American Journal of Epidemiology, 187(8), 1752–1762. 10.1093/aje/kwy041. [DOI] [PMC free article] [PubMed] [Google Scholar]


