Abstract
Autism with co-occurring exceptional cognitive ability is often accompanied by severe internalizing symptoms and feelings of inadequacy. Whether cognitive ability also translates into greater risk for suicidal ideation is unclear. To investigate this urgent question, we examined two samples of high-ability autistic individuals for factors that were predictive of suicidal ideation. In the first sample (N=1,074 individuals seen at a clinic specializing in gifted/talented youth), we observed a striking excess of parent-reported suicidal ideation in autistic individuals with IQ≥120 (Odds Ratio=5.9, p = 0.0007). In a separate sample of SPARK participants, we confirmed higher rates of suicidal thoughts compared to non-autistic children from the ABCD cohort (combined N=16,049, Odds Ratio=6.8, p < 2.2e – 16 ) and further that autistic children with suicidal thoughts had significantly higher cognitive ability (p < 2.2e − 16) than those without. Elevated polygenic scores (PGS) for cognitive performance were associated with increased suicidal thoughts (N=1,983, Z = 2.16, p = 0.03), with PGS for educational attainment trending in the same direction (Z = 1.4, p = 0.17). Notably, similar results were found in parents of these autistic youth, where higher PGS for educational attainment was associated with increasing thoughts of suicide (N=736, Z = 2.28, p = 0.02). Taken together, these results suggest that on a phenotypic and genetic level, increasing cognitive ability is an unexpected risk factor for suicidal ideation in individuals diagnosed with, or at risk for autism.
Keywords: Twice-exceptional, Autism, Suicide, Intelligence, Cognition, Mental health, Psychiatry, Genetics, Polygenic scores
Introduction
Autism is a highly genetic neurodevelopmental condition (heritability > 80% (Bai et al. 2019)) affecting an estimated 1 in 44 children in the U.S. every year (Maenner et al. 2021). Research into the biology underlying autism has primarily focused on the core symptoms defined by the DSM (Association 2013): social communication challenges and restricted or repetitive behaviors. However, less is known about the genetic factors influencing other mental health comorbidities in autism, the most alarming being suicidal ideation, self-harm, and death by suicide.
Previous studies of autism found profoundly increased rates of suicide and depression (Hirvikoski et al. 2016), (Kato et al. 2013), (Hedley et al. 2018), (Hudson, Hall, and Harkness 2019). The rate of death by suicide has been estimated to be 7.5 times higher in autistic people than those without an autism diagnosis (Hirvikoski et al. 2016). While rates of suicide death in autistic individuals have varied across samples (0.17 – 0.4% (Kirby et al. 2019; Kõlves et al. 2021)) they are consistently higher than rates observed in non-autistic individuals. The increased suicide rate in autism may be partially attributable to a broad increase in depressive symptoms, as autistic people have been shown to have a 4-fold increase in lifetime rates of depression (Hudson, Hall, and Harkness 2019). Known protective factors against suicide death, like education, age, and marriage, are not protective in autistic samples (Kõlves et al. 2021). Furthermore, this mental illness burden may be exacerbated by exceptional cognitive ability: in other work, we found that children with a exceptional IQ (≥ 120) and autism have greater feelings of inadequacy and internalizing problems compared to autistic individuals with average IQ (Michaelson et al. 2021). These findings contrast with findings in non-autistic cohorts, where large population studies have found high IQ is a protective factor against suicide death (Batty et al. 2010; Wallin et al. 2018), suggesting that the relationship between intelligence and suicide-related traits may vary across diagnostic boundaries.
There is evidence that suicide risk is partly genetic in nature, with heritability estimates ranging from 17–55% (Vaquero-Lorenzo and Vasquez 2020). Some evidence for potential mechanistic overlap between the biology of suicide and of autism comes from a study that identified mutations in a well-known autism risk gene, NRXN1, as increasing risk for suicide (William et al. 2021). In a general population study (which included autistic individuals) increased polygenic risk for autism was found to be positively associated with suicidal thoughts. Furthermore, autistic children with suicidal thoughts inherit more genetic risk factors for self-harm ideation than expected (Warrier and Baron-Cohen 2021), lending further evidence to potential shared biological mechanisms between suicide and autism. Although connected to autism through these modes of genetic risk, it is unknown what other factors might also contribute to the excess burden of suicidality seen in autism and how these compare to age matched controls.
This previous work points to several key questions, which we aim to address in this study: do autistic children show increased signs of suicidal ideation or self-harm compared to peers? Are suicidal thoughts related to elevated cognitive ability in autistic children? What modes of genetic risk are associated with depressive and suicidal traits in autistic children? In seeking to answer these questions, we assembled evidence from multiple samples: a clinical sample of high academic achievers with neurodevelopmental conditions (Michaelson et al. 2021), the Adolescent Brain Cognitive Development study (ABCD, (Lisdahl et al. 2018)), a general population sample, and SPARK - a nationwide study of autism (Feliciano et al. 2018). Answering these questions will offer new insight into autism-relevant risk factors and the interplay and trade-offs between intelligence, neurodiversity, and mental illness, while also drawing attention to clinically meaningful subgroups most at risk for suicide. To our knowledge, this is the first study to examine the relationship between autism, exceptional cognitive ability, suicidal thoughts, and genetics across multiple large samples of children.
Language choices
Many autistic self-advocates prefer identity-first language (i.e., autistic individuals), and some autistic individuals and their families prefer person-first language (i.e., individuals with autism). We recognize the validity of the arguments behind both of these preferences, and have chosen to use identity-first language for this paper.
Methods
Clinical sample
Over a ten-year period (2009–2019), data from 1,254 evaluations from a university-based clinic that specializes in the assessment and counseling of gifted and twice-exceptional individuals were recorded (Michaelson et al. 2021). The majority of people seen at the clinic are students from local elementary, middle, and high schools. Students referred to this clinic typically have exceptional cognitive abilities (standardized test scores 90th percentile, or a full-scale IQ of ≥ 120), and many of these students have co-occurring behavioral challenges impacting their academic performance. Individuals are evaluated at this clinic to develop individualized learning plans based on in-depth diagnostic and cognitive assessments. Twice-exceptional individuals were defined as having an IQ test score of ≥ 120 (at least 90th percentile) and an autism diagnosis. For individuals who were evaluated more than once, only data from their first evaluation was analyzed leading to a final N=1074. Full Scale IQs from the Wechsler family of IQ tests (Wechsler 2014) in this sample ranged as high as 158, and small handful of the clients evaluated at the clinic had very low IQs (e.g., 55). The mean IQ for the sample was 116.9 (SD of 14.5), median IQ was 117. All participants provided informed consent for their data to be used in research studies, and this study was approved by the University of Iowa IRB (IRB 202002251). Sample demographics are shown in Table 1.
Table 1.
Cohort demographics.
| Sample | Max sample size | Mean age (SD) | Male (%) | White (%) | Multiracial <%) | Hispanic (%) | Black (%) | Asian or Pacific Islander (%) | Native American (%) | Other race reported (%) | No race reported (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| clinical | 1054 | 10.2 (3.7) | 68.4 | 79.6 | 3.3 | 2.1 | 0.4 | 3.3 | 0.1 | NA | 11.2 |
| SPARK | 6766 | 11.4 (3.2) | 78.2 | 70.0 | 8.0 | 15.0 | 3.9 | 1.5 | 0.1 | 0.8 | 0.7 |
| ABCD | 11878 | 10.9 (0.7) | 52.5 | 52.0 | 9.4 | 20.2 | 15.0 | 2.0 | 0.3 | 0.6 | 0.5 |
Data for suicidal ideation from this sample was taken from the clinical intake forms completed by the parents of the children, then merged with data about diagnosis and clinical IQ test scores completed by the licensed psychologist who assessed the child. This data was used for the analyses depicted in Figure 1. A.
Fig. 1. Suicidal thoughts are increased in autistic children.
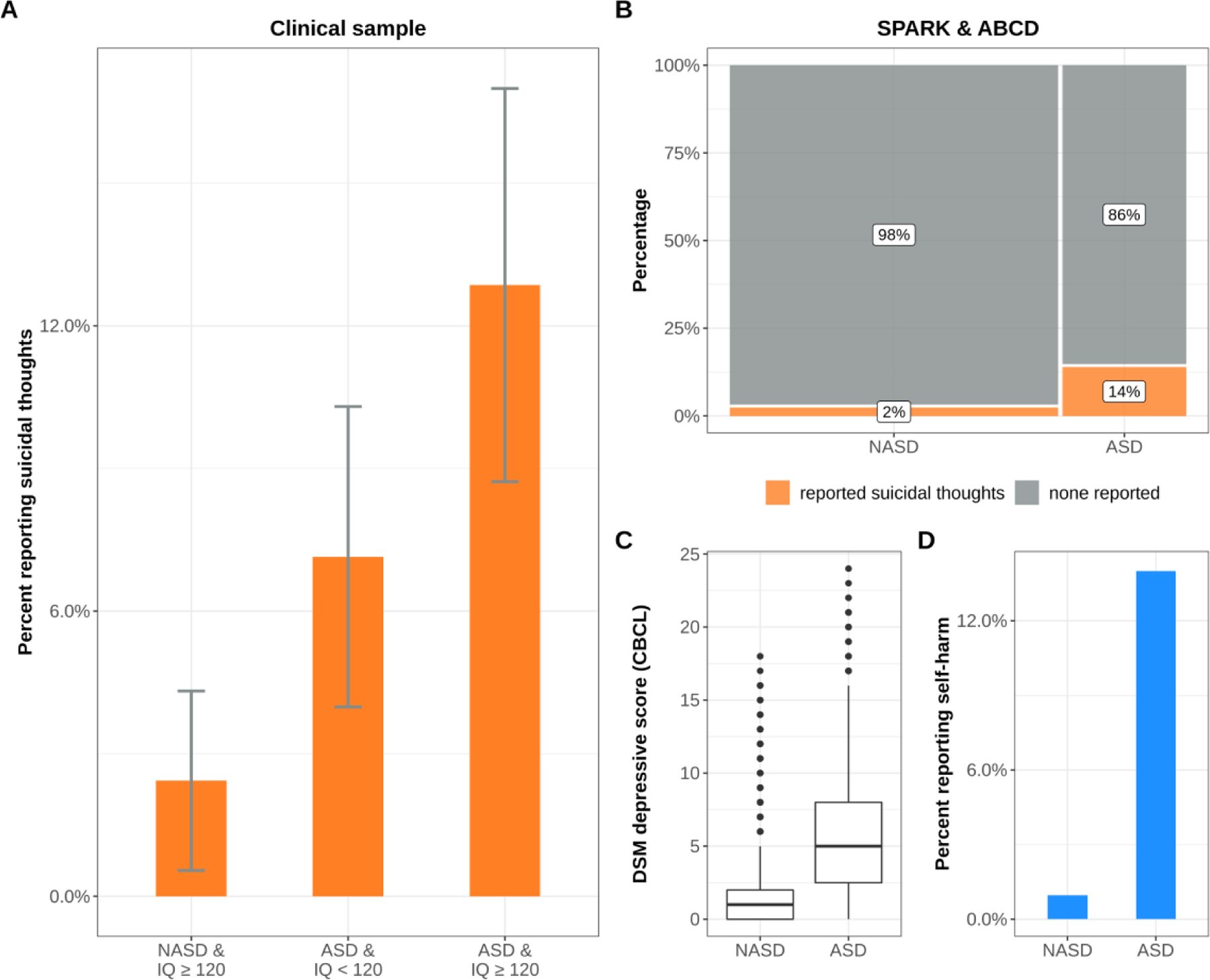
ASD = autistic children. NASD = non-autistic children.
A. Rate of parent-reported suicidal thoughts in children seen at a clinic specializing in clinical assessment of children with exceptional cognitive ability ± 95% CI from 10,000 bootstrap samples.
B. Rate of suicidal thoughts (CBCL item 91) in children in larger population samples.
C. Comparison of scores on the CBCL’s DSM depressive scale.
D. Reported self-harm (CBCL item 18) in children with and without ASD.
SPARK cohort
SPARK (Feliciano et al. 2018) is an ongoing autism study of nearly 300,000 individuals in the United States. Parents of autistic children participating in SPARK were invited to complete the Child Behavior Checklist (CBCL (Achenbach 2011)) online. Data was pooled together from SPARK phenotype V7 release (data released as of December 1st 2021) and a research match study where additional SPARK participants completed the CBCL. The research match study was approved by the University of Iowa IRB (IRB 201812788) and SPARK is approved by the Western IRB (IRB 20151664). All participants provided informed consent. Sample demographics are shown in Table 1.
SPARK participants were included as autism cases in analyses shown in Figures 1. B-D (SPARK N = 4,171), Figure 2 (SPARK N=1,409), Figure 3 (SPARK N=1,409), Figure 4 (SPARK N =1,983), and Supplemental Figure 1 (SPARK N=2,162), and Supplemental Figure 2 (SPARK parents N=736). In order to make SPARK and ABCD cohorts more comparable, only children aged between 8 and 15 years old from SPARK with complete data were included in the statistical analysis reported in Figures 1 and 2.
Fig. 2. ASD and IQ interact to increase suicidal thoughts. (SPARK & ABCD).
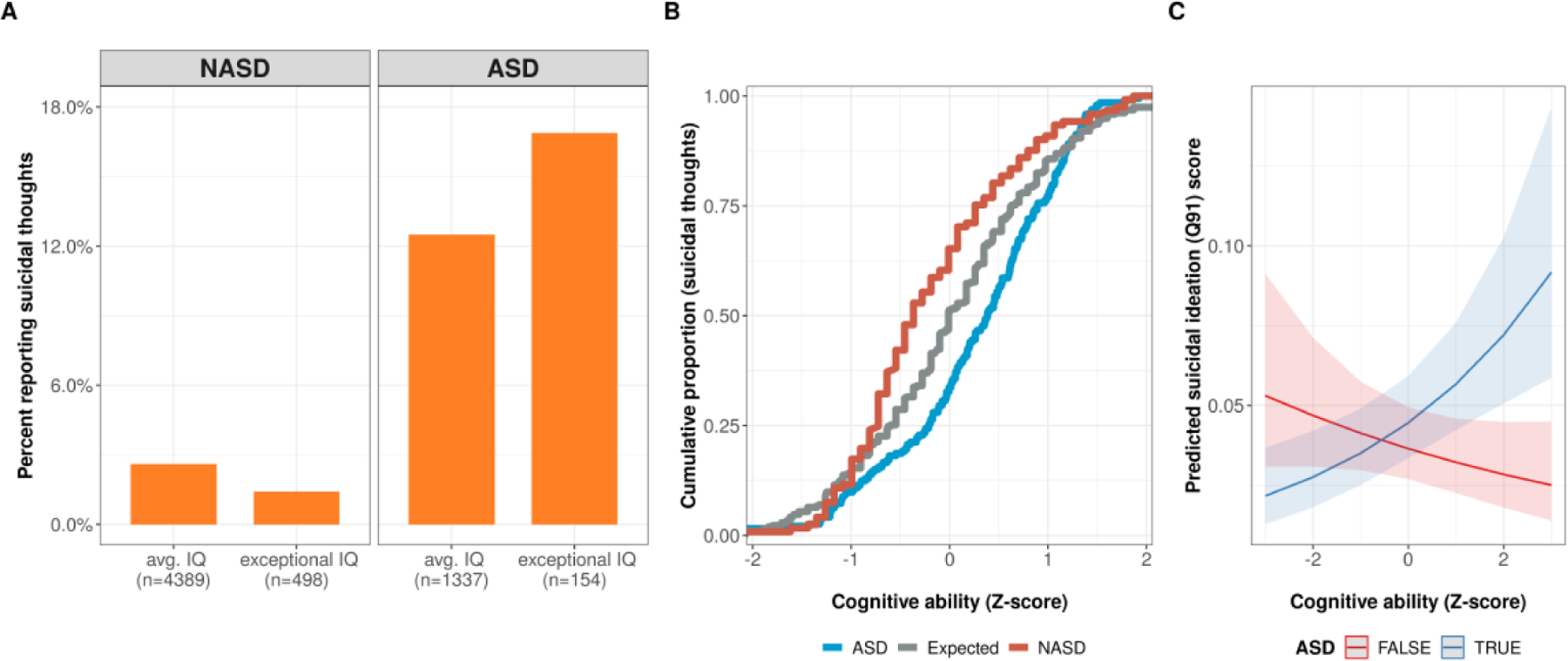
A. Suicidal ideation in children from SPARK and ABCD, grouped by ASD status and whether their IQ proxy scores are in the exceptional range (top decile).
B. Cumulative proportion of suicidal thoughts and their relation to Z-scaled cognitive ability scores for: ASD, NASD, and the expected rate based permutations of the complete data set.
C. Predictions of suicidal thoughts from a generalized linear model trained on the SPARK and ABCD data.
Fig. 3. Imputed full-scale IQ scores are related to depressive phenotypes.
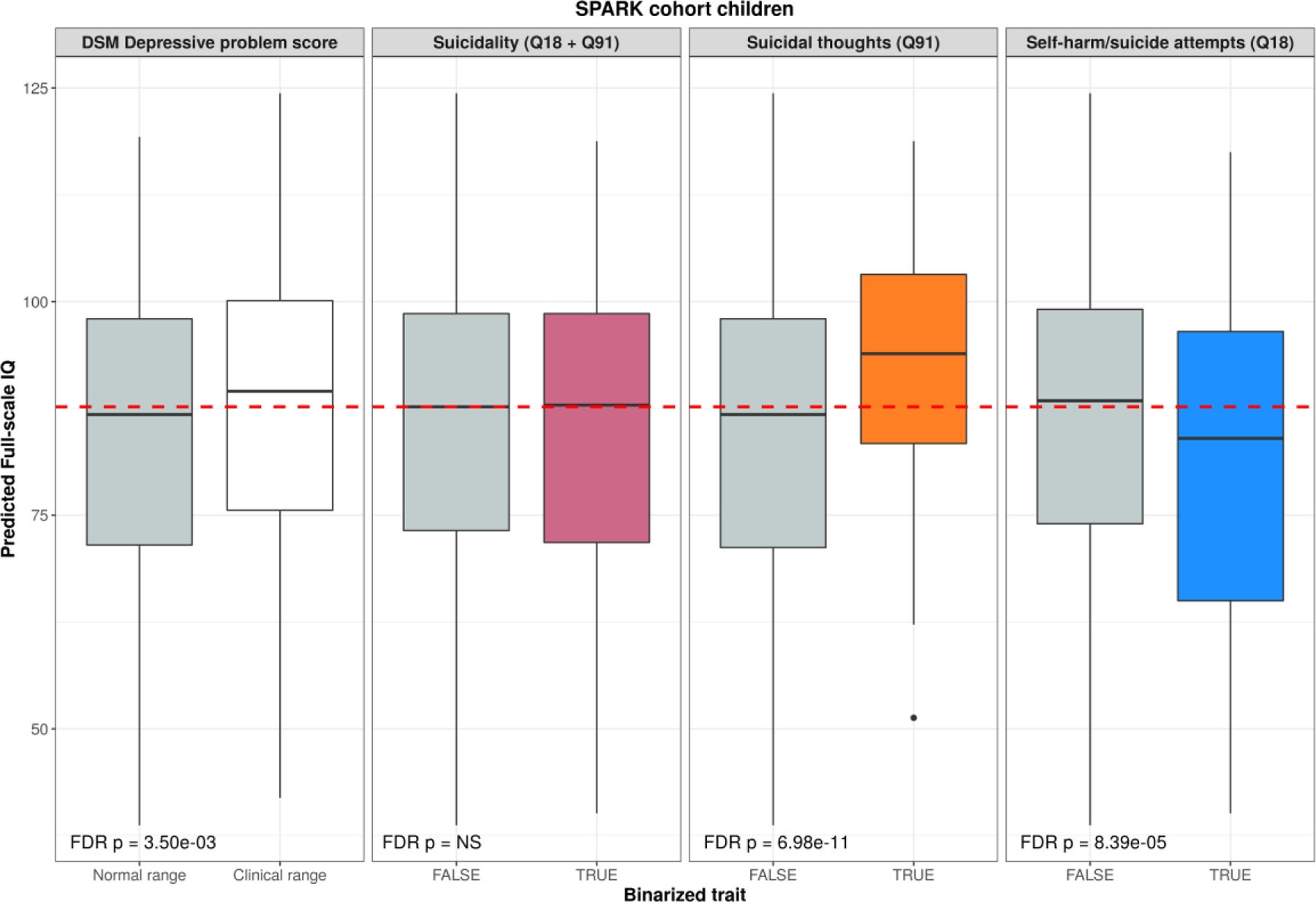
Distributions of behaviorally-predicted IQ score split by depressive phenotypes in autistic children from SPARK. The red dashed line shows the median predicted IQ scores for this sample. FDR-corrected p-values from Wilcoxon tests are reported in the bottom of each boxplot.
Fig. 4. Polygenic scores for cognitive ability and depression are associated with increased suicidal thoughts.
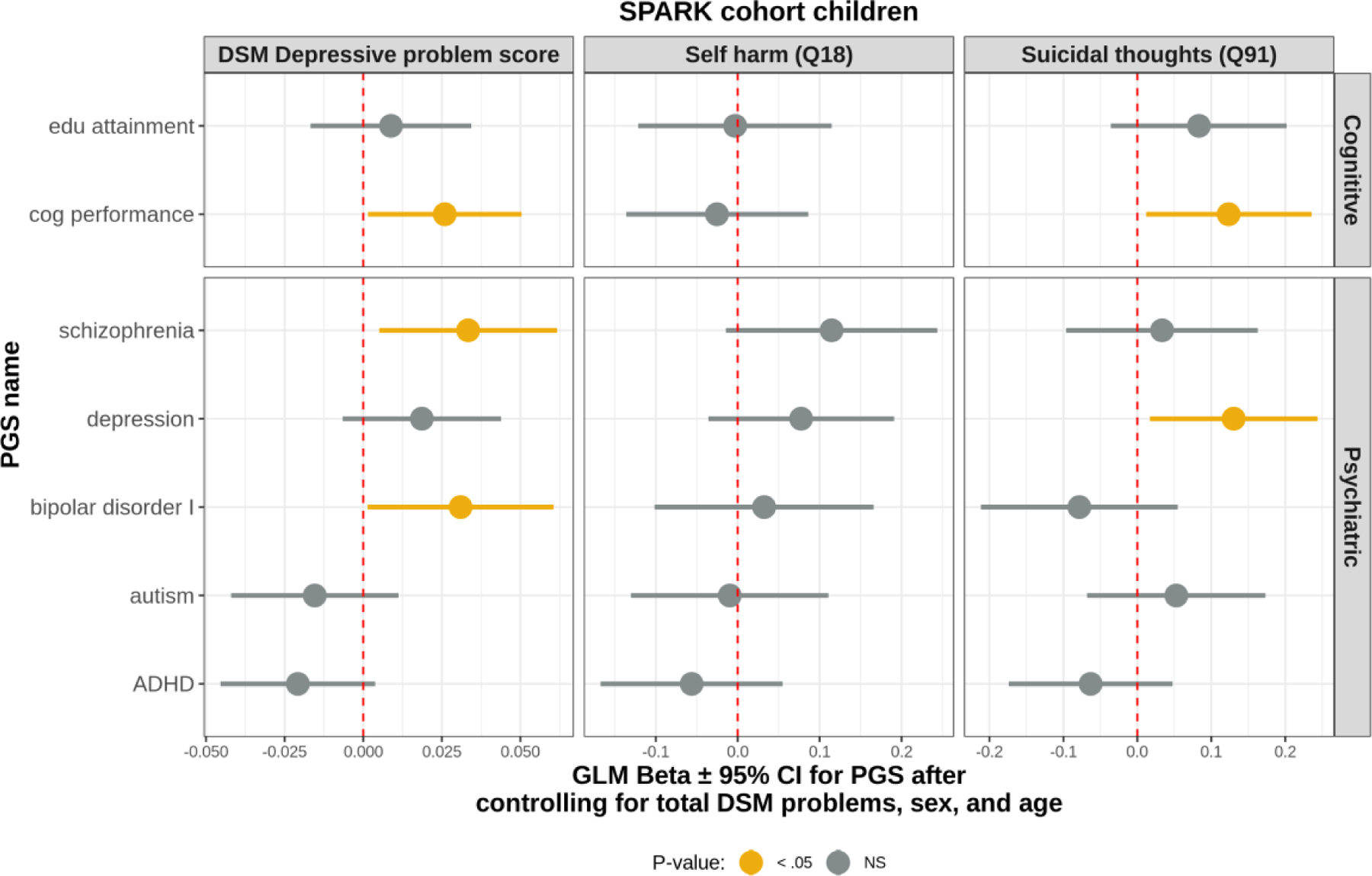
Results from generalized linear models predicting the depressive phenotype of interest. The coefficient for the PGS model term ± 95% confidence interval are indicated. Total DSM problems (except DSM depressive problems), binary sex, and age in months were included as covariates in all models.
ABCD cohort
The ABCD cohort (Lisdahl et al. 2018) is an ongoing longitudinal study of 10,000 typically-developing children in the United States of America. The ABCD cohort is meant to represent a more general population sample than case/control studies. Since this is a longitudinal study only data from one year, “Release 3” (data released in August 2020), were analyzed. CBCL data was available on 11,878 children in ABCD. Fully-corrected Z-scaled NIH toolbox full composite scores(Lisdahl et al. 2018) were the proxy used for IQ in this cohort, and these scores were available for 4,969 children who also had CBCL data. These scores are corrected for demographic factors like age and sex by the ABCD consortium. Briefly, the NIH Toolbox is standardized set of cognitive assessments given in English valid for children and adults (ages 3–85)(Weintraub et al. 2013). The NIH Toolbox assesses processing speed, episodic memory, working memory, attention, language, and executive functioning(Weintraub et al. 2013). NIH toolbox standard scores for this sample ranged from 32.5–258, had a mean of 95.7, standard deviation of 16.8, and a median of 94. ABCD sample demographics are shown in Table 1. ABCD participants were utilized in analyses depicted in Figures 1. B-D (ABCD N=11,878) and Figure 2 (ABCD N=4,969). A total of 200 children from ABCD reported an ASD diagnosis during enrollment, these individuals were grouped with SPARK ASD cases in analyses using both ABCD and SPARK.
Measures of depression and suicidality in SPARK and ABCD
Data for depressive symptoms and suicidal ideation for children in the SPARK and ABCD cohorts came from the CBCL (Achenbach 2011). The CBCL (Achenbach 2011), a parent-report tool for assessing emotional and behavioral problems in children ages 6–18, is a well-validated questionnaire consisting of nearly 200 items. CBCL items can be combined to yield Diagnostic and Statistical Manual of Mental Disorders (DSM) subscales of clinical relevance. The DSM depressive problem scale was used as a quantitative measure of depression and clinical threshold cutoff was set as scoring ≥ the 95th percentile (Achenbach 2011). Suicidal ideation scores came from item #91 from the CBCL, “Talks about killing self”. Additionally, we examined self-harm behavior using item #18 from the CBCL, “Deliberately harms self or attempts suicide”. Due to the ambiguity of item #18 it was not possible to parse out suicide attempts from self-injurious behaviors common in autism. The sum of scores from items #18 and #91 was used as an overall “suicidality” score. A subset of SPARK cohort parents filled out an adult-oriented questionnaire about themselves, called the Adult Self-Report (ASR), which is very similar to the CBCL. Scores from the DSM depressive problems, self-harm (question #18), and suicidal thoughts (question #91) items were used in the analysis shown in Supplemental Figure 2 (N=736).
Behaviorally predicted IQ in SPARK
An imputed IQ score (Shu et al. 2022) was used for the SPARK cohort due to very few individuals with clinically-derived IQ scores, CBCL, and genotype data. A total of 1,983 SPARK participants had complete imputed IQ scores, CBCL, and genotype data. These predicted full-scale IQ scores(Shu et al. 2022), obtained from the SPARK phenotype v7 release, were used for the analyses depicted in Fig. 3. Briefly, these imputed IQ scores were predicted using a number of behavioral and autism diagnostic variables (e.g. language level and repetitive behavior scores), internal validation showed these scores to be strongly correlated with clinically measured IQ in SPARK with clinical and predicted IQ scores, note this subsample had little overlap with the samples in this paper due to missing clinical IQ scores in our SPARK sample (Supplemental Figure 1, N = 2,162, Pearson’s r = .76, p < 2.2e – 16). Imputed IQ scores for this sample ranged from 39–124, had a mean of 85.2, standard deviation of 17.3, and a median of 88.
Genotype quality control and imputation
Genotype data from SPARK Version 3 Freeze (2019) and Version 4 (2020) were merged using PLINK ((Purcell et al. 2007)). Merged genotypes were then lifted from hg38 to hg19 using the LiftOver tool (Kuhn, Haussler, and Kent 2013). The merged genotypes included 43,209 individuals and 616,321 variants and were then quality controlled using the “BIGwas” quality control pipeline (Kässens, Wienbrandt, and Ellinghaus 2021). The default parameters were used, except for skipping Hardy-Weinberg tests due to SPARK not a general population sample. The pre-QC annotation step removed 21 variants (N = 616,299 variants remaining). The SNP QC step removed 101,600 variants due to missingness at a threshold of 0.02 (N = 514,699 variants remaining). The sample QC step removed 1,114 individuals due to missingness, 67 individuals due to heterozygosity, and 176 due to duplicates (monozygotic twins). An additional 9,533 individuals were removed due to genetic ancestry from principal component projections (N = 32,422 individuals remaining). The QC’d set of N = 514,699 variants and N = 32,422 individuals were then imputed to the TopMed (Taliun et al. 2021) reference panel using the Michigan Imputation Server (Das et al. 2016) with the phasing and quality control steps included and to output variants with imputation quality r2 > 0.3. After the genotype imputation, the variants were filtered to only the HapMap SNPs (N = 1,054,330 variants) with imputation quality r2 ≥ 0.8 using bcftools (Danecek et al. 2021). Next, they were lifted over from hg38 to hg19 using the VCF-liftover tool (https://github.com/hmgu-itg/VCF-liftover) and the alleles normalized to the hg19 reference genome. Finally, the files were converted to PLINK binary files (Purcell et al. 2007) with N = 1,018,200 final variants, these files were used for the PGS calculations in the SPARK samples overlapping with our cohort.
PGS calculations
Polygenic scores were calculated using LDpred2 (Privé, Arbel, and Vilhjálmsson 2020) and the “bigsnpr” package (Privé et al. 2018)) in R (Team 2013)). Because SPARK is family-based, an external LD reference based on 362,320 European individuals of the UK Biobank (provided by the developers of LDpred2) was used to calculate the genetic correlation matrix, estimate heritability, and calculate the infinitesimal beta weights. Polygenic scores were calculated from the following genome-wide association studies performed by the Psychiatric Genomics Consortium: ADHD (2019) (Demontis et al. 2019), autism (2019) (Grove et al. 2019), and major depression (2019) (Howard et al. 2019), bipolar disorder I (2021) (Mullins et al. 2021), and schizophrenia (2021) (Psychiatric Genomics Consortium et al. 2020). Polygenic scores for the cognitive ability traits: cognitive performance and educational attainment were calculated using GWAS summary statistics provided by the Social Science Genetic Association Consortium (Lee et al. 2018).
Statistical analysis
All statistical tests were conducted in R 4.02 (Team 2013).
Depressive phenotypes in autism vs. controls
In order to estimate rates of suicidal ideation in autistic children compared to controls we counted the number of autism cases with reported suicidal ideation compared to controls, and conducted Fisher’s exact tests to determine statistical significance (Figures 1A and 1B). Next, we stratified by diagnostic group to understand how cognitive ability is associated with suicidal thoughts in autistic children versus controls. For this interaction analysis of cognitive ability and autism diagnostic status, NIH toolbox scores were used to identify who scored in the top decile in ABCD. Similarly, in SPARK imputed IQ scores were used to identify individuals scoring the top decile of cognitive ability. A regression model was fit predicting suicidal ideation scores in children from the main effects and interaction between whether their cognitive ability scores was in the top decile and autism diagnostic status (Figure 2C). Next, to determine the extent to which cognitive ability is associated with depressive symptoms in autism, autistic children from SPARK were grouped by binarized depressive and suicidal phenotypes (e.g., suicidal thoughts vs. none reported), then tested for group differences with Wilcoxon tests of the imputed IQ scores(Shu et al. 2022) (Figure 3).
PGS regression
To determine potential biological relationships between cognition/psychiatric traits and phenotypes, generalized linear models were fit (Figure 4 and Supplemental Figure 2). Polygenic score main effects were obtained by linear modeling using PGS and covariates to predict 4 different depressive and suicide-related phenotypes from the CBCL. Covariates included were: age in months, designated sex at birth, and total DSM problems from the CBCL minus the DSM depressive problems. Generalized linear models with the ‘quasipoisson’ error distribution were used to more appropriately model the skewed count phenotype data. The glm function in R (Team 2013) was used for all linear modeling. An example regression equation is shown below, where Yi is the phenotype being tested, e.g., score count of DSM depressive problems from the CBCL.
Results
Suicidal thoughts are associated with autism and higher intelligence
Twice-exceptional children, i.e., autistic children and at least one IQ subscale score ≥ the 90th percentile, showed the highest rate of suicidal thoughts, at 12.9%, compared to only 2.4% in IQ matched controls - demonstrated in Figure 1A (odds ratio = 5.9, odds ratio 95% CI=1.9–18.3, p = 0.0007). These results were consistent with those obtained from the Child Behavior Checklist (CBCL) in independent large cohorts (SPARK + ABCD), where nearly 14% of the 4,371 children with an autism diagnosis were reported to express thoughts of suicide, compared to 2% out of the 11,678 non-autistic children from ABCD (Fig. 1B, odds ratio=6.8, odds ratio 95% CI=5.8–7.9, p < 2.2e − 16). Related problems, like depressive symptoms (Fig. 1C, median sample difference = 4, 95% CI = 3.99–4, p < 2.2e − 16) and self-harm were also significantly increased in autism (Fig. 1D, odds ratio=16.9, odds ratio 95% CI=13.7–20.9, p < 2.2e − 16). There was a significant positive interaction between ASD and IQ, suggesting the relationship between IQ and suicidal thoughts is dependent on whether a child has autism or not (Fig. 2, Z = 3.47, FDR p = 0.002).
Taken together, these results from multiple large samples point toward elevated rates of suicidal thoughts in autism overall, with the highest rates being found in “twice-exceptional” or 2e individuals, i.e., those who were both autistic and had high cognitive ability.
Cognitive ability is associated with depressive phenotypes in autism
Predicted IQ (Shu et al. 2022) was most significantly related to suicidal thoughts in autism: children with reported suicidal thoughts (CBCL item 91), had a mean predicted IQ score 8 points above those without reported suicidal thoughts (median sample difference = 7.2, 95% CI = 5.1–9.4, FDR p = 7e − 11). Conversely, autistic children who were coded as engaging in self-harm (CBCL item 18, which may be sensitive to a wide range of self-harm behaviors, some not associated with suicidal ideation) had significantly lower predicted IQ scores, by an average of 5 points (median sample difference = −4.9, 95% CI = −2.6 – −7.2, FDR p = 8e − 5). Notably, the binarized suicidal ideation trait (i.e., the sum of CBCL items 18 and 91) did not show any significant differences in predicted IQ, presumably due to the opposing effects of each constituent item canceling the other out. Together, these observations warrant caution when combining items 18 and 91 of the CBCL into a “suicide score” because they may give misleading results, especially when applied within a neurodevelopmental sample. These findings are also our justification for considering items 18 and 91 separately throughout our analyses. Children who scored at or above the 95th percentile for DSM depressive problems scale (meeting the clinical problem cutoff) on the Child Behavior Checklist (CBCL) had significantly higher predicted full-scale IQ by an average of 2.5 points (median sample difference = 2.4, 95% CI = 0.8–4, FDR p = 0.0035). These results are in agreement with the other results relating to suicidal thoughts (Figure 1A), and together demonstrate that increasing cognitive ability is linked to a broader depressive phenotype in the context of autism. In sum, across multiple samples we find higher cognitive ability in autism to be associated with increased suicidal thoughts (CBCL item 91), increased rates of clinically-relevant depression (CBCL DSM depressive scale), and less self-harm behavior (CBCL item 18).
Polygenic estimators of cognitive ability predict suicidal thoughts and depressive symptoms
The final analysis aimed to determine if the observed relationship between cognitive ability and suicidal thoughts had support at the biological level. Model coefficients for the PGS ± 95% confidence intervals are depicted in Figure 4.
PGS for cognitive performance was positively associated with DSM depressive problems. Higher PGS for cognitive performance (Lee et al. 2018) was associated with increased depressive symptoms in children with ASD (Z = 2.26, p = 0.02). Similarly, elevated cognitive performance PGS was positively associated with suicidal thoughts (CBCL item 91; Z = 2.16, p = 0.03). PGS for major depression was also significantly associated with suicidal thoughts (Z = 2.25, p = 0.02). Notably, similar results were found in the parents of autistic children when examining items from the Adult Self Report (ASR) (Supplemental Fig. 2). PGS for educational attainment (Lee et al. 2018) was significantly associated with suicidal thoughts in parents of autistic children (ASR item 91; Z = 2.28,p = 0.02). Together, these results suggest that genetic propensity for higher cognitive ability is associated with suicidal thoughts in both autistic probands and their parents.
Discussion
The main contribution of this study is in drawing attention to the significantly increased risk of suicidal ideation faced by children with exceptional cognitive ability and autism. Our analyses demonstrate that increasing IQ switches from a protective factor in non-autistic individuals to a risk factor for suicidal ideation in autistic youth (Fig. 2). With multiple large cohorts and through a series of complementary analyses, we repeatedly demonstrated a robust link between higher cognitive ability and signs of suicidal ideation, specifically in an autistic context. There is evidence that this is at least partly genetic in nature: polygenic scores for cognitive performance and educational attainment were positively associated with thoughts of suicide in autistic youth and their parents, respectively.
Our analyses included multiple estimates of cognitive ability: the clinical sample included IQ as measured by the WISC (Wechsler 2014), a gold standard measure, while the SPARK sample used an estimate of IQ based on a variety of behavioral items and that was calibrated against clinically-obtained IQ measures. Our genetically-informed sample in SPARK included polygenic estimators of cognitive performance and educational attainment. Regardless of how cognitive ability was estimated, we found that autistic individuals with suicidal thoughts generally have higher estimated cognitive ability than autistic peers without reported suicidal thoughts. We also found that increased cognitive ability was broadly related to increased depressive problems in autism.
Our finding that intelligence is a risk factor for suicidal ideation contrasts with previous work on non-autistic populations, which has shown men with low cognitive ability are more at risk for suicide death than men with high ability (Batty et al. 2010; Andersson et al. 2008). However, our findings are congruent with previous studies of mortality in autism that showed that higher-ability autistic adults are much more likely to die of suicide than lower-ability autistic adults (Hirvikoski et al. 2016). Further, the rate of suicidal ideation in the SPARK sample (14%), is remarkably consistent with a previous, smaller study of suicidal ideation in autism, which found 14.5% of autistic children had suicidal thoughts (Mayes et al. 2013). When taken together, these studies reiterate our observation of an interaction effect between being autistic and cognitive ability on suicidal ideation. Suicide risk factors appear to be dependent on autism diagnostic status, and autism status needs to be considered when evaluating mental health.
To our knowledge, this is the largest genetically-informed study of risk factors for suicidal ideation and related depressive symptoms in autistic youth. It is also the first to show a biological (i.e., genetic) relationship between propensity for high cognitive ability and suicidal thoughts. We were able to directly compare suicidal ideation rates in clinical samples of autism with exceptional ability to IQ-matched non-autistic patients, showing that the presence of autism is a critical factor in the relationship between cognitive ability and suicidal thoughts. This was brought into focus by the significance (p < 0.01) of the autism:IQ interaction term in the GLM explaining suicidal thoughts from the CBCL (see Figure 2). In non-autistic youth from the combined SPARK+ABCD sample, exceptional cognitive ability (estimated IQ at or above the 90th percentile) was shown to be a protective factor against thoughts of suicide reported via the CBCL. Strikingly, the trend was the opposite in autistic youth: those with exceptional ability were at increased risk for suicidal ideation (Fig. 2). Together, these results suggest that the effects of autism and increased cognitive are not additive when predicting suicidal thoughts as an outcome. Instead, these factors interact in a profound way that flips the effect of IQ on risk of suicidal ideation.
Another unexpected finding that emerged from our analyses related to the summing of items 18 and 91 from the CBCL into a “suicide score”. We found that this summed score did not demonstrate the expected relationships with other variables of interest. Our observation that endorsement of item 18 (self harm behavior) was associated with lower indices of cognitive ability raises the possibility that this item is sensitive to compulsive and repetitive self-harm behaviors sometimes seen in individuals with an intellectual disability, and that are usually not indicative of suicidal ideation. Conversely, endorsement of item 91 (talk of suicide) is associated with higher estimated IQ. The sum of items 18 and 91 has the apparent effect of canceling out the overall association with estimated IQ. This observation would suggest that care is warranted when interpreting the meaning of item 18 with respect to suicidal intent, especially in the context of neurodevelopmental samples like SPARK. This result is perhaps intuitive for clinicians and researchers who work in neurodevelopmental disabilites, and are familiar with self-harm behaviors such as banging the head against the wall or furniture, that are associated with intellectual disabilities (ID), repetitive behaviors, and other behaviors that are unlikely to be related to suicidal intent(Minshawi et al. 2014). The way CBCL item 18 is worded, “deliberately harms self or attempts suicide”, likely makes it sensitive to endorsement based on this kind of compulsive self-harm, in addition to self-harm that is more likely to be related to suicidal ideation.
A number of mechanisms might explain the observation of exceptional cognitive ability transforming from a protective to a risk factor in the context of autism. A review in this special issue has described several viable neurobiological avenues for future study of twice-exceptionality, like disrupted neural circuits and altered neuroplasticity (Kelvington, Nickl-Jockschat, and Abel 2022). Our other work showed a strong autism-dependent decrease in self-worth with increasing IQ(Michaelson et al. 2021). Low self-worth has been linked to dysruption of the hypothalamic–pituitary–adrenal (HPA) axis(Zilioli et al. 2016), a neuroendocrine pathway responsible for releasing the stress hormone cortisol. Autism is strongly linked to elevated HPA axis activity and increased cortisol response(Spratt et al. 2012). Dysfunction of the HPA axis has also been robustly linked to suicidal behavior(O’Connor et al. 2016), providing a possible biological explanation for the elevated rates of suicidal ideation seen in autism. In addition to excessive baseline cortisol linked to autism, it is likely that demonstration of exceptional cognitive ability raises parent, teacher, and peer expectations. Any inability to meet these elevated standards could result in further activation of the HPA axis, potentially explaining the higher rates of suicidal thoughts we observed in 2e children compared to other autistic children. Future work should examine the relationship between cortisol and cognitive ability in children with autism, which could be a key factor mediating suicidal behavior in twice-exceptional children.
A second potential mechanism may be found in the relationship between high cognitive ability and “camouflaging”(Livingston et al. 2018), where individuals suppress less socially accepted behavior to fit in. Camouflaging is a universal behavior(Jorgenson et al. 2020) that has been studied with particular interest in autistic individuals, who engage in this behavior more regularly(Cook et al. 2021). In studies of autism, camouflaging has been associated with higher rates of anxiety and depression (Hull et al. 2021), perhaps due to less support resulting from successful camouflaging, which would tend to delay diagnosis (McQuaid, Lee, and Wallace 2021). Consequently, camouflaging in autism is correlated with increased suicidal behavior (Cassidy et al. 2018). Increased cognitive ability may therefore be linked to maladaptive camouflaging that further increases risk for mental health issues in autistic people.
Limitations
Despite strongly convergent evidence that increased cognitive ability is positively associated with reported suicidal thoughts in autistic children, there are a number of limitations that need to be acknowledged. All data on depression and suicidal ideation in children came from parent reports, and there are likely children in our sample with suicidal thoughts who were reported as not having any. Recent work has shown that parents of autistic children underreport their child’s suicidal ideation (O’Halloran, Coey, and Wilson 2022). It is likely that in the context of internalizing and depressive symptoms, including thoughts of suicide, the first person perspective provides a better signal to noise ratio, and future work should address suicidal ideation from that perspective. The current sample has several sex biases in the data: the autistic children in our samples were predominately male, while the data for parents was predominately from mothers. Previous work has shown higher-ability autistic women are at the greatest risk for suicide death (Hirvikoski et al. 2016), so a future sample where autistic females are better represented would yield key insights.
Additionally, the cognitive ability scores for each sample were derived from different measures given in different settings. This makes it difficult to make one-to-one comparisons between scores in each cohort, though the study of multiple large samples suggest this is a robust finding. Sampling bias should also be considered, as the distribution on cognitive ability scores are not the same in each sample and may not generalize to all samples of autistic children (e.g., the clinical sample has higher than average IQ scores). Additional work should be done to determine whether the increased likelihood of suicidal ideation is mitigated in autistic children with high cognitive ability if they are diagnosed earlier and receive additional resources. Finally, we also do not know how often early suicidal thoughts translate into greater risk for suicide attempts later in life. In the current samples, we have no data on actual suicide attempts or suicide completion. With the current single time point sample, we are unable to develop insight into the temporal and developmental effects that govern suicide risk from childhood through adolescence and adulthood.
Conclusions
Across multiple estimates of intellectual capacity (clinical, behaviorally predicted, and polygenic propensity), we found that higher cognitive ability is significantly related to more suicidal thoughts in autistic children. Specifically, autism diagnosis and cognitive ability were shown to have interacting effects on suicidal ideation, making twice-exceptional youth the group at highest risk for suicidal ideation. These findings build upon previous work that found suicidal ideation is significantly higher in autistic populations and offers clarity into additional mental health risk factors within autism. Future work should focus on 1) longitudinal assessment of suicide risk in autistic youth 2) self-report of risk factors among autistic youth and 3) increasing representation of autistic females in study samples.
Supplementary Material
Acknowledgements
We are grateful to all of the individuals and families in SPARK, the SPARK clinical sites, and SPARK staff. We appreciate obtaining access to genetic and phenotypic data for SPARK data on SFARI Base. We are also appreciative of the individuals and families in ABCD. We are thankful to everyone involved in the clinical sample.
This work was supported by a grant from the Simons Foundation (SFARI Explorer 594788 to JJM). This work was also supported by the University of Iowa Hawkeye Intellectual and Developmental Disabilities Research Center P50 HD103556 (TA and Lane Strathearn, multi-PIs). Additional funding for this work came from the Department of Psychiatry at the University of Iowa and in part by National Institutes of Health through a Predoctoral Training Grant (T32GM008629 to LC and TT) and Research Grant DC014489 and HG012697 to JJM.
Footnotes
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Achenbach Thomas M. 2011. Child Behavior Checklist. Encyclopedia of Clinical Neuropsychology. Springer; New York. 10.1007/978-0-387-79948-3_1529. [DOI] [Google Scholar]
- Andersson L, Allebeck P, Gustafsson J-E, and Gunnell D. 2008. “Association of IQ Scores and School Achievement with Suicide in a 40-Year Follow-up of a Swedish Cohort.” Acta Psychiatrica Scandinavica 118 (August): 99–105. 10.1111/j.1600-0447.2008.01171.x. [DOI] [PubMed] [Google Scholar]
- Association, American Psychiatric. 2013. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. Autor. [Google Scholar]
- Bai Dan, Benjamin Hon Kei Yip, Windham Gayle C., Sourander Andre, Francis Richard, Yoffe Rinat, Glasson Emma, et al. 2019. “Association of Genetic and Environmental Factors with Autism in a 5-Country Cohort.” JAMA Psychiatry 76 (October): 1035. 10.1001/jamapsychiatry.2019.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty GD, Whitley E, Deary IJ, Gale CR, Tynelius P, and Rasmussen F. 2010. “Psychosis Alters Association Between IQ and Future Risk of Attempted Suicide: Cohort Study of 1 109 475 Swedish Men.” BMJ 340 (June): c2506–6. 10.1136/bmj.c2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy Sarah, Bradley Louise, Shaw Rebecca, and Baron-Cohen Simon. 2018. “Risk Markers for Suicidality in Autistic Adults.” Molecular Autism 9. 10.1186/s13229-018-0226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook Julia, Hull Laura, Crane Laura, and Mandy William. 2021. “Camouflaging in Autism: A Systematic Review.” Clin. Psychol. Rev 89 (102080): 102080. [DOI] [PubMed] [Google Scholar]
- Danecek Petr, Bonfield James K, Liddle Jennifer, Marshall John, Ohan Valeriu, Pollard Martin O, Whitwham Andrew, et al. 2021. “Twelve Years of SAMtools and BCFtools.” Gigascience 10: giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das Sayantan, Forer Lukas, Schönherr Sebastian, Sidore Carlo, Locke Adam E, Kwong Alan, Scott I Vrieze, et al. 2016. “Next-Generation Genotype Imputation Service and Methods.” Nature Genetics 48: 1284–87. 10.1038/ng.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demontis Ditte, Walters Raymond K, Martin Joanna, Mattheisen Manuel, Als Thomas D, Agerbo Esben, GBaldursson, et al. 2019. “Discovery of the First Genome-Wide Significant Risk Loci for Attention Deficit/Hyperactivity Disorder.” Nature Genetics 51: 63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciano Pamela, Daniels Amy M, Snyder LeeAnne Green, Beaumont Amy, Camba Alexies, Esler Amy, Gulsrud Amanda G, et al. 2018. “SPARK: A US Cohort of 50,000 Families to Accelerate Autism Research.” Neuron 97: 488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove Jakob, Ripke Stephan, Als Thomas D, Mattheisen Manuel, Walters Raymond K, Won Hyejung, Pallesen Jonatan, et al. 2019. “Identification of Common Genetic Risk Variants for Autism Spectrum Disorder.” Nature Genetics 51: 431–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley Darren, Uljarević Mirko, Foley Kitty Rose, Richdale Amanda, and Trollor Julian. 2018. “Risk and Protective Factors Underlying Depression and Suicidal Ideation in Autism Spectrum Disorder.” Depression and Anxiety 35. 10.1002/da.22759. [DOI] [PubMed] [Google Scholar]
- Hirvikoski Tatja, Mittendorfer-Rutz Ellenor, Boman Marcus, Larsson Henrik, Lichtenstein Paul, and Bölte Sven. 2016. “Premature Mortality in Autism Spectrum Disorder.” British Journal of Psychiatry 208. 10.1192/bjp.bp.114.160192. [DOI] [PubMed] [Google Scholar]
- Howard, David M, Adams Mark J, Clarke Toni-Kim, Hafferty Jonathan D, Gibson Jude, Shirali Masoud, Coleman Jonathan R I, et al. 2019. “Genome-Wide Meta-Analysis of Depression Identifies 102 Independent Variants and Highlights the Importance of the Prefrontal Brain Regions.” Nature Neuroscience 22: 343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson Chloe C., Hall Layla, and Harkness Kate L.. 2019. “Prevalence of Depressive Disorders in Individuals with Autism Spectrum Disorder: A Meta-Analysis.” Journal of Abnormal Child Psychology 47. 10.1007/s10802-018-0402-1. [DOI] [PubMed] [Google Scholar]
- Hull Laura, Levy Lily, Lai Meng-Chuan, Petrides KV, Baron-Cohen Simon, Allison Carrie, Smith Paula, and Mandy Will. 2021. “Is Social Camouflaging Associated with Anxiety and Depression in Autistic Adults?” Mol Autism 12 (1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgenson Courtney, Lewis Timothy, Rose Chad, and Kanne Stephen. 2020. “Social Camouflaging in Autistic and Neurotypical Adolescents: A Pilot Study of Differences by Sex and Diagnosis.” J. Autism Dev. Disord 50 (12): 4344–55. [DOI] [PubMed] [Google Scholar]
- Kato Koji, Mikami Katsunaka, Akama Fumiaki, Yamada Keigo, Maehara Mizuki, Kimoto Keitaro, Kimoto Kousuke, et al. 2013. “Clinical Features of Suicide Attempts in Adults with Autism Spectrum Disorders.” General Hospital Psychiatry 35. 10.1016/j.genhosppsych.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Kässens Jan Christian, Wienbrandt Lars, and Ellinghaus David. 2021. “BIGwas: Single-Command Quality Control and Association Testing for Multi-Cohort and Biobank-Scale GWAS/PheWAS Data.” GigaScience 10 (June). 10.1093/gigascience/giab047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelvington Benjamin A, Thomas Nickl-Jockschat, and Ted Abel. 2022. “Neurobiological Insights into Twice Exceptionality: Circuits, Cells, and Molecules.” Neurobiol. Learn. Mem 195 (107684): 107684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby Anne V, Amanda V Bakian, Yue Zhang, Deborah A Bilder, Brooks R Keeshin, and Hilary Coon. 2019. “A 20-Year Study of Suicide Death in a Statewide Autism Population.” Autism Res 12 (4): 658–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kõlves Kairi, Fitzgerald Cecilie, Nordentoft Merete, Wood Stephen James, and Erlangsen Annette. 2021. “Assessment of Suicidal Behaviors Among Individuals with Autism Spectrum Disorder in Denmark.” JAMA Netw Open 4 (1): e2033565. [DOI] [PubMed] [Google Scholar]
- Kuhn Robert M, David Haussler, and Kent W James. 2013. “The UCSC Genome Browser and Associated Tools.” Briefings in Bioinformatics 14 (March): 144–61. 10.1093/bib/bbs038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee James J, Robbee Wedow, Aysu Okbay, Edward Kong, Omeed Maghzian, Meghan Zacher, Nguyen-Viet Tuan Anh, et al. 2018. “Gene Discovery and Polygenic Prediction from a Genome-Wide Association Study of Educational Attainment in 1.1 Million Individuals.” Nature Genetics 50: 1112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl Krista M, Sher Kenneth J, Conway Kevin P, Gonzalez Raul, Feldstein Ewing Sarah W, Nixon Sara Jo, Tapert Susan, Bartsch Hauke, Goldstein Rita Z, and Heitzeg Mary. 2018. “Adolescent Brain Cognitive Development (ABCD) Study: Overview of Substance Use Assessment Methods.” Developmental Cognitive Neuroscience 32: 80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston Lucy Anne, Colvert Emma, Social Relationships Study Team, Bolton Patrick, and Happé Francesca. 2018. “Good Social Skills Despite Poor Theory of Mind: Exploring Compensation in Autism Spectrum Disorder.” J Child Psychol Psychiatry 60 (1): 102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenner Matthew J., Shaw Kelly A., Bakian Amanda V., Bilder Deborah A., Durkin Maureen S., Esler Amy, Furnier Sarah M., et al. 2021. “Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2018.” MMWR. Surveillance Summaries 70 (December): 1–16. 10.15585/mmwr.ss7011a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayes Susan Dickerson, Gorman Angela A., Hillwig-Garcia Jolene, and Syed Ehsan. 2013. “Suicide Ideation and Attempts in Children with Autism.” Research in Autism Spectrum Disorder 7. 10.1016/j.rasd.2012.07.009. [DOI] [Google Scholar]
- McQuaid Goldie A, Nancy Raitano Lee, and Wallace Gregory L. 2021. “Camouflaging in Autism Spectrum Disorder: Examining the Roles of Sex, Gender Identity, and Diagnostic Timing.” Autism 26 (2): 552–59. [DOI] [PubMed] [Google Scholar]
- Michaelson Jacob J., Doobay Alissa, Casten Lucas, Megan Foley-Nicpon Thomas Nickl-Jockschat, Abel Ted, and Assouline Susan. 2021. “A Discrepancy Between Processing Speed and Verbal Ability in Gifted Youth Is Genetically and Diagnostically Associated with Autism.” medRxiv. 10.1101/2021.11.02.21265802. [DOI] [Google Scholar]
- Minshawi Noha F, Sarah Hurwitz, Fodstad Jill C, Biebl Sara, Morriss Danielle H, and McDougle Christopher J. 2014. “The Association Between Self-Injurious Behaviors and Autism Spectrum Disorders.” Psychol. Res. Behav. Manag 7 (April): 125–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins Niamh, Forstner Andreas J, O’Connell Kevin S, Coombes Brandon, Coleman Jonathan R I, Qiao Zhen, Als Thomas D, et al. 2021. “Genome-Wide Association Study of More Than 40,000 Bipolar Disorder Cases Provides New Insights into the Underlying Biology.” Nature Genetics 53: 817–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor Daryl B, Ferguson Eamonn, Green Jessica A, O’Carroll Ronan E, and O’Connor Rory C. 2016. “Cortisol Levels and Suicidal Behavior: A Meta-Analysis.” Psychoneuroendocrinology 63 (January): 370–79. [DOI] [PubMed] [Google Scholar]
- O’Halloran L, Coey P, and Wilson C. 2022. “Suicidality in Autistic Youth: A Systematic Review and Meta-Analysis.” Clin. Psychol. Rev 93 (102144): 102144. [DOI] [PubMed] [Google Scholar]
- Privé Florian, Arbel Julyan, and Vilhjálmsson Bjarni J. 2020. “LDpred2: Better, Faster, Stronger.” Bioinformatics 36: 5424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privé Florian, Aschard Hugues, Ziyatdinov Andrey, and Michael G B Blum. 2018. “Efficient Analysis of Large-Scale Genome-Wide Data with Two r Packages: Bigstatsr and Bigsnpr.” Bioinformatics 34: 2781–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychiatric Genomics Consortium, The Schizophrenia Working Group of the, Ripke Stephan, Walters James TR, and O’Donovan Michael C. 2020. “Mapping Genomic Loci Prioritises Genes and Implicates Synaptic Biology in Schizophrenia.” medRxiv. 10.1101/2020.09.12.20192922. [DOI] [Google Scholar]
- Purcell Shaun, Neale Benjamin, Todd-Brown Kathe, Thomas Lori, Ferreira Manuel A R, Bender David, Maller Julian, et al. 2007. “PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses.” The American Journal of Human Genetics 81 (September): 559–75. 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Chang, LeeAnne Green Snyder, Yufeng Shen, and Chung Wendy K.. 2022. “Imputing Cognitive Impairment in SPARK, a Large Autism Cohort.” Autism Research 15 (January): 156–70. 10.1002/aur.2622. [DOI] [PubMed] [Google Scholar]
- Spratt Eve G, Nicholas Joyce S, Brady Kathleen T, Carpenter Laura A, Hatcher Charles R, Meekins Kirk A, Furlanetto Richard W, and Charles Jane M. 2012. “Enhanced Cortisol Response to Stress in Children in Autism.” J. Autism Dev. Disord 42 (1): 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliun Daniel, Daniel N Harris, Michael D Kessler, Jedidiah Carlson, Zachary A Szpiech, Torres Raul, Taliun Sarah A, et al. 2021. “Sequencing of 53,831 Diverse Genomes from the NHLBI TOPMed Program.” Nature 590: 290–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team, R Core. 2013. “R: A Language and Environment for Statistical Computing.” http://www.R-project.org/.
- Vaquero-Lorenzo Concepcion, and Vasquez Manuel A.. 2020. Suicide: Genetics and Heritability. 10.1007/7854_2020_161. [DOI] [PubMed] [Google Scholar]
- Wallin A. Sörberg, Zeebari Z, Lager A, Gunnell D, Allebeck P, and Falkstedt D. 2018. “Suicide Attempt Predicted by Academic Performance and Childhood IQ: A Cohort Study of 26 000 Children.” Acta Psychiatrica Scandinavica 137 (April): 277–86. 10.1111/acps.12817. [DOI] [PubMed] [Google Scholar]
- Warrier Varun, and Baron-Cohen Simon. 2021. “Childhood Trauma, Life-Time Self-Harm, and Suicidal Behaviour and Ideation Are Associated with Polygenic Scores for Autism.” Molecular Psychiatry 26. 10.1038/s41380-019-0550-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D 2014. Wechsler Intelligence Scale for Children–Fifth Edition. NCS Pearson. [Google Scholar]
- Weintraub Sandra, Dikmen Sureyya S, Heaton Robert K, Tulsky David S, Zelazo Philip D, Bauer Patricia J, Carlozzi Noelle E, et al. 2013. “Cognition Assessment Using the NIH Toolbox.” Neurology 80 (11 Suppl 3): S54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- William Nancy, Reissner Carsten, Sargent Robert, Darlington Todd M., DiBlasi Emily, Li Qingqin S., Keeshin Brooks, et al. 2021. “Neurexin 1 Variants as Risk Factors for Suicide Death.” Molecular Psychiatry. 10.1038/s41380-021-01190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilioli Samuele, Slatcher Richard B, Chi Peilian, Li Xiaoming, Zhao Junfeng, and Zhao Guoxiang. 2016. “Childhood Adversity, Self-Esteem, and Diurnal Cortisol Profiles Across the Life Span.” Psychol. Sci 27 (9): 1249–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


