Abstract
Background:
Cardiac arrest guidelines recommend epinephrine every 3–5 minutes during cardiac arrest resuscitation. However, it is unclear if multiple epinephrine doses are associated with improved outcomes. The objective of this study was to determine if a single-dose epinephrine protocol was associated with improved survival compared to traditional multidose protocols.
Methods:
We conducted a pre-post study across five North Carolina EMS agencies from 11/1/2016 to 10/29/2019. Patients ≥ 18 years old with attempted resuscitation for non-traumatic prehospital cardiac arrest were included. Data were collected 1 year before and after implementation of the single-dose epinephrine protocol. Prior to implementation, all agencies used a multidose epinephrine protocol. The Cardiac Arrest Registry to Enhance Survival was used to obtain patient outcomes. Study outcomes were survival to hospital discharge (primary) and return of spontaneous circulation (ROSC). Analysis was by intention to treat. Outcomes were compared pre- vs. post-implementation using generalized estimating equations to account for clustering within EMS agencies. Adjusted analyses included age, sex, race, shockable vs. non-shockable rhythm, witnessed arrest, automatic external defibrillator availability, EMS response interval, and bystander cardiopulmonary resuscitation.
Results:
During the study period there were 1,690 encounters (899 pre- and 791 post-implementation). The population was 74.7% white, 61.1% male, and had a median age of 65 (IQR 53–76) years. Survival to hospital discharge was similar pre- vs. post-implementation [13.6% (122/899) vs. 15.4% (122/791); OR 1.19, 95%CI 0.89–1.59]. However, ROSC was more common post-implementation [42.3% (380/899) vs. 32.5% (257/791); OR 0.66, 95%CI 0.54–0.81]. After adjusting for covariates, the single-dose protocol was associated with similar survival to discharge rates (aOR 0.88, 95%CI 0.77–1.29), but with decreased ROSC rates (aOR 0.58, 95%CI 0.47–0.72).
Conclusion:
A prehospital single-dose epinephrine protocol was associated with similar survival to hospital discharge, but decreased ROSC rates compared to the traditional multidose epinephrine protocol.
Keywords: Out-of-hospital, prehospital, emergency medical services (EMS), cardiac arrest, epinephrine, cardiopulmonary resuscitation (CPR)
INTRODUCTION
Each year approximately 350,000 adults experience out-of-hospital cardiac arrest (OHCA) and receive cardiopulmonary resuscitation (CPR) by emergency medical services (EMS) in the United States (US).(1) The American Heart Association Advanced Cardiac Life Support and European Resuscitation Council’s cardiac arrest resuscitation guidelines recommend epinephrine administration every 3–5 minutes.(2,3) This potent catecholamine increases chronotropy and inotropy,(4,5) which in theory improve myocardial blood flow and may increase the rate of return of spontaneous circulation (ROSC).(6,7) However, there are possible harms from epinephrine, including lethal dysrhythmia, increased myocardial oxygen demand, thrombosis, and cerebral ischemia.(1,5,7–9)
Despite decades of OHCA guidelines with emphasis on multidose epinephrine administration, survival to hospital discharge rates in the US have remained low, at 6–11% since 1980.(10,11) Previous trials suggest that while epinephrine may improve ROSC rates, it may not improve patient-centered outcomes, such as survival to hospital discharge or favorable neurologic outcome rates.(12–16) No trial has examined the effect of using a single dose of epinephrine in OHCA resuscitation on patient-centered outcomes. Therefore, it remains unclear if a single dose of epinephrine is associated with improved outcomes, such as survival to hospital discharge or ROSC, compared to guideline-based epinephrine administration every 3–5 minutes.
To address this evidence gap, we conducted a pre-post study comparing adult OHCA patients receiving a single dose of epinephrine to patients receiving epinephrine every 3–5 minutes. We hypothesized that patients receiving a single dose of epinephrine would have increased survival to hospital discharge rates despite having lower ROSC rates compared to patients receiving epinephrine every 3–5 minutes. Therefore, the objective of this study was to compare survival to hospital discharge and ROSC rates among patients with resuscitation guided by a single-dose epinephrine protocol compared to those receiving care with a protocol for epinephrine every 3–5 minutes. In addition, this study aimed to explore whether the single-dose epinephrine protocol was associated with improved favorable neurologic outcomes.
METHODS
Study Design
We conducted a pre-post study among five North Carolina (NC) EMS agencies from 11/1/2016 to 10/31/2019. The Wake Forest University Health Sciences Institutional Review Board approved the study protocol and granted a waiver of informed consent. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines helped direct the research and reporting process.(17) The data will not be made publicly available.
Study Setting and Population
The study was conducted in a diverse area of NC among urban, suburban, and rural communities in five counties with a total population nearing 850,000 people (Supplemental Table 1). Each county operated its own third service, single-tier advanced life support (ALS) EMS agency and received medical direction from emergency physicians with subspeciality board certification in EMS. A consecutive sample of patients ≥ 18 years old with attempted resuscitation for non-traumatic OHCA were included. While prehospital cardiac arrest management was guided by the NC College of Emergency Physician’s cardiac arrest protocol, EMS medical directors can amend the protocol with the approval of the state EMS medical director.(18) Given the clinical equipoise surrounding epinephrine in OHCA, changing the local cardiac arrest protocols to a single dose of epinephrine was considered reasonable. Prior to implementation of the new single-dose epinephrine protocol, agencies followed traditional guideline-based recommendations and provided 1 mg of 1:10,000 intravenous (IV) or intraosseous (IO) epinephrine every 3–5 minutes. In the unified, new single-dose epinephrine protocol adopted by the five participating EMS agencies, each patient was given a single 1:10,000 dose of 1 mg of IV/IO epinephrine. No additional doses of epinephrine were allowed per protocol. No additional protocol changes occurred. Supplemental Appendix 1 provides the multidose and single-dose epinephrine protocols.
Data Collection and Variables
Data were collected 1 year before and after implementation of the single-dose epinephrine protocol. Description of each participating county and the protocol switch date is in Supplemental Table 1. Each EMS agency’s electronic patient care report was electronically queried for patient demographics, vital signs, initial heart rhythm, interventions provided, and disposition information. In addition, EMS response interval, defined as the interval from EMS being dispatched to arriving on-scene, was determined from the patient care report, as longer response intervals are associated with worse patient outcomes.(19) For each case, the Cardiac Arrest Registry to Enhance Survival (CARES) was used to determine if bystander CPR occurred, the arrest was witnessed, an automatic external defibrillator (AED) was available, the initial rhythm was shockable, ROSC occurred, the patient survived to hospital discharge and, if so, what the patient’s neurologic status was at the time of discharge, as well as the presumed cardiac arrest etiology (cardiac, trauma, etc.).
Outcomes
Consistent with prior studies and CARES definitions, survival to hospital discharge was defined as leaving the hospital alive regardless of neurologic status and ROSC as a pulse being present for ≥ 20 minutes without requiring additional chest compressions.(20,21) The rate of discharge with a favorable neurologic outcome, defined as a Cerebral Performance Category (CPC) of “good” or “moderate” (CPC category 1 or 2 out of 4), was considered an exploratory outcome.(20,22,23) Survival to hospital discharge and favorable neurologic outcome were considered patient-centered end-points, as these outcomes are more important to patients and caregivers than ROSC alone.(20,24,25) Per the CARES definitions, CPC 1 corresponds to being “conscious, able to work and lead a normal life” and CPC 2 suggests being “conscious and able to function independently but with hemiplegia, seizures, or permanent memory or mental changes.” CPC 3 indicates that the patient is “dependent on others because of impaired brain function” and CPC 4 indicates that the patient “is not conscious or aware.”
Statistical Analysis
Based on previous data from the participating counties, we anticipated that at least 750 patients would be included in both the pre- and post-implementation cohorts. With this sample size, and assuming a two-sided test with an alpha of 0.05, there is at least 80% power to detect an improvement in the rate of survival to hospital discharge from 7.6% (national average) to 11.9%.(10)
We used descriptive statistics to describe the study population, including counts and percentages for categorical variables and median and interquartile ranges (IQR) for continuous variables. The OHCA encounter was the unit of analysis. Analysis was by intention to treat. A per protocol analysis was not possible because the CARES registry did not collect the number of doses of epinephrine administered and this information could not be abstracted from the electronic patient care records. Generalized estimating equations (GEE) with a logit link were used to compare survival to hospital discharge, ROSC, and favorable neurologic outcome rates (overall and among just survivors) pre- vs. post-implementation while accounting for clustering within EMS agencies. In multivariable analysis, models also included age, sex, race, initial cardiac rhythm (shockable vs. non-shockable), witnessed arrest, bystander CPR, AED availability, and EMS response interval. These covariates were selected a priori based on existing OHCA resuscitation research.(3,10,19,22,26–28) For modeling purposes, race was treated as a two-level variable, White and non-White. Unadjusted and adjusted odds ratios (aOR) with corresponding 95% confidence intervals (95%CI) were calculated. A prespecified subgroup analysis was conducted among patients who experienced OHCA from presumed primary cardiac etiologies.
RESULTS
During the study period, there were 1,690 OHCA encounters, with 899 occurring pre-implementation and 791 post-implementation. Figure 1 presents the study flow diagram. The cohort was 74.7% (1,262/1,690) White, 61.1% (1,033/1,690) male, and had a median age of 65 (IQR 53–76) years. Table 1 presents baseline characteristics by cohort.
Figure 1.
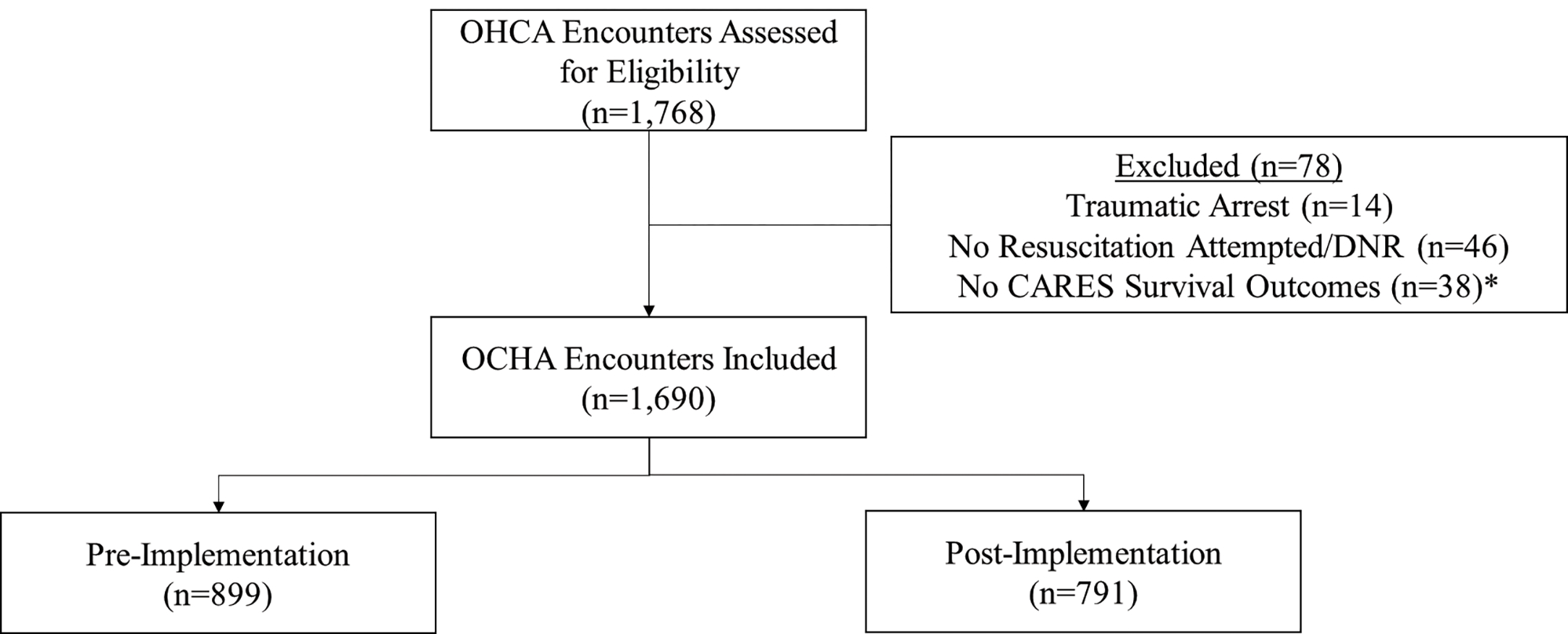
The study flow diagram.
OHCA – out-of-hospital cardiac arrest, DNR – do not resuscitate; CARES – Cardiac Arrest Registry to Enhance Survival
†: While 38 encounters did not have CARES outcomes regarding survival, only 18 additional encounters were excluded once encounters for traumatic arrest and those with no resuscitation attempted/DNR were excluded
Table 1.
Cohort characteristics.
| Pre-Implementation (n=899), n (%) | Post-Implementation (n=791), n (%) | |
|---|---|---|
| Age (median, IQR) (years) | 65 (51–76) | 66 (54–77) |
| Sex | ||
| Female | 364 (40.5) | 293 (37.0) |
| Race | ||
| White | 673 (74.9) | 589 (74.5) |
| Black | 213 (23.7) | 179 (22.6) |
| Other | 13 (1.5) | 23 (2.9) |
| Ethnicity | ||
| Hispanic/Latino | 5 (0.6) | 14 (1.8) |
| County | ||
| Forsyth | 461 (51.3) | 371 (46.9) |
| Iredell | 133 (14.8) | 135 (17.1) |
| Randolph | 179 (19.9) | 178 (22.5) |
| Stanly | 28 (3.1) | 34 (4.3) |
| Surry | 98 (10.9) | 73 (9.2) |
| Presumed cardiac arrest etiology | ||
| Cardiac | 711 (79.1) | 650 (82.2) |
| Non-cardiac | 188 (20.9) | 141 (17.8) |
| Respiratory | 103 (11.5) | 97 (12.3) |
| Overdose | 74 (8.2) | 37 (4.7) |
| Drowning | 2 (0.2) | 0 (0) |
| Electrocution | 1 (0.1) | 2 (0.3) |
| Other | 8 (0.9) | 5 (0.6) |
| Initial cardiac rhythm | ||
| Shockable | 144 (16.0) | 180 (22.8) |
| Ventricular fibrillation | 96 (10.7) | 113 (14.3) |
| Ventricular tachycardia | 11 (1.2) | 6 (0.8) |
| Other shockable rhythm | 37 (4.1) | 61 (7.7) |
| Non-shockable | 755 (84.0) | 610 (77.2) |
| Asystole | 482 (53.6) | 365 (46.1) |
| Pulseless electrical activity | 182 (20.2) | 171 (21.6) |
| Other non-shockable rhythm | 91 (10.1) | 74 (9.4) |
| Witnessed cardiac arrest | 517 (57.5) | 467 (59.0) |
| Bystander CPR | 366 (40.7) | 292 (36.9) |
| AED available | 86 (9.6) | 104 (13.2) |
| Response interval (median, IQR) (minutes) | 8.2 (5.9–10.8) | 7.8 (5.5–10.5) |
IQR – interquartile range, CPR – cardiopulmonary resuscitation, AED – automatic external defibrillator. All rows show n, % unless otherwise indicated.
The survival to hospital discharge rate was similar pre- vs. post-implementation (OR 1.19, 95%CI 0.89–1.59). ROSC rates were higher in the pre-implementation cohort (OR 0.66, 95%CI 0.54–0.81). Rates of favorable neurologic outcomes were similar in the multidose and single-dose epinephrine cohorts among all patients (OR 0.97, 95%CI 0.72–1.31) and among those surviving to hospital discharge (OR 0.44, 95%CI 0.18–1.06). Table 2 displays the unadjusted results. Supplemental Tables 2 and 3 further describe neurologic outcomes by CPC status.
Table 2.
Pre- vs. post-implementation outcomes among all patients.
| Pre-Implementation (n=899), n (%) | Post-Implementation (n=791), n (%) | Odds Ratio (95%CI) | ||
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| Survival to hospital discharge | 122 (13.6) | 122 (15.4) | 1.19 (0.89–1.59) | 1.00 (0.77–1.29) |
| Favorable neurologic outcome among survivors | 99/122 (81.2) | 85/122 (69.7) | 0.44 (0.18–1.06) | 0.57 (0.26–1.22)1 |
| Favorable neurologic outcome (all) | 99 (11.0) | 85 (10.8) | 0.97 (0.72–1.31) | 0.83 (0.64–1.07) |
| Return of spontaneous circulation | 380 (42.3) | 257 (32.5) | 0.66 (0.54–0.81) | 0.58 (0.47–0.73) |
Bold font indicates a statistically significant result
Adjusted only for age, shockable vs. non-shockable rhythm, and response interval due to the small number of events
In the adjusted model, survival to hospital discharge rates were similar post-implementation (aOR 1.00, 95%CI 0.77–1.29). The single-dose epinephrine protocol remained associated with decreased ROSC rates (aOR 0.58, 95%CI 0.47–0.73). Rates of favorable neurologic survival were similar pre- vs. post-implementation among all patients (aOR 0.83, 95%CI 0.64–1.07) and among just survivors (aOR 0.57, 95%CI 0.26–1.22). Table 2 and Figure 2 show the aORs for each outcome.
Figure 2.
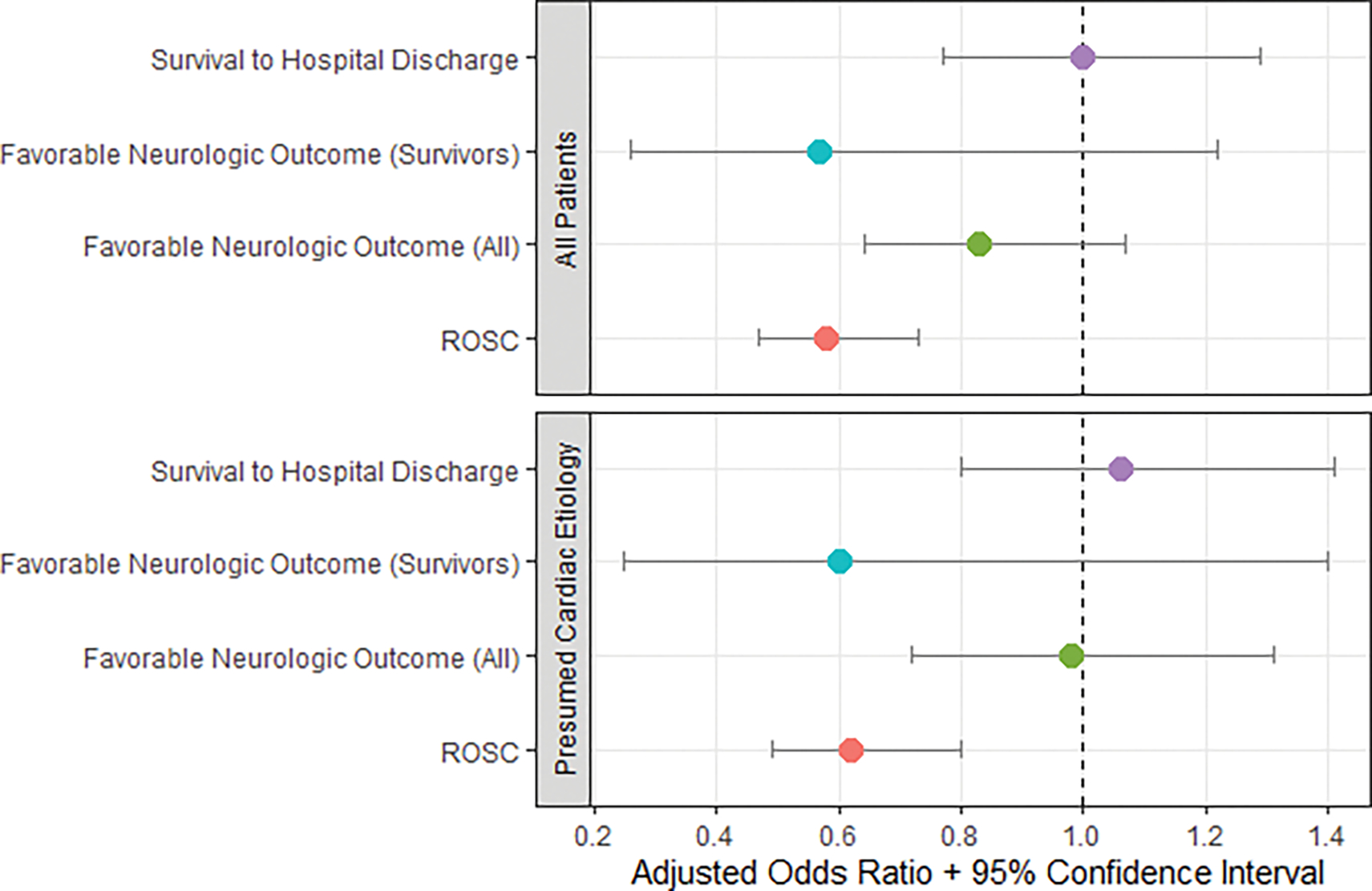
Pre- vs. post-implementation adjusted odds ratios for study outcomes among all patients and among patients with presumed arrest from cardiac etiologies.
ROSC – return of spontaneous circulation.
Note: In the presumed cardiac etiology subgroup, favorable neurologic outcome (survivors) is unadjusted due to the small number of events
Among patients who experienced cardiac arrest from presumed cardiac etiologies, survival to hospital discharge rates were similar pre- vs. post-implementation (OR 1.38, 95%CI 0.99–1.91). ROSC rates were higher in the pre-implementation group (OR 0.72, 95%CI 0.57–0.91). Favorable neurologic outcome rates were similar pre- vs. post-implementation among all patients (OR 1.22, 95%CI 0.83–1.79) and among just survivors (OR 0.60, 95%CI 0.25–1.40). After adjusting for covariates in this subgroup, survival to hospital discharge rates (aOR 1.06, 95%CI 0.80–1.41) and favorable neurologic outcomes among all patients (aOR 0.98, 95%CI 0.72–1.31) were similar while ROSC rates (aOR 0.62, 95%CI 0.49–0.80) were lower in the single dose cohort. Table 3 and Figure 2 summarize these results. Supplemental Tables 4 and 5 further describe neurologic outcomes by CPC status.
Table 3.
Pre- vs. post-implementation outcomes among patients presumed to have arrested from primary cardiac etiologies.
| Pre-Implementation (n=711), n (%) | Post-Implementation (n=650), n (%) | Odds Ratio (95%CI) | ||
|---|---|---|---|---|
| Unadjusted | Adjusted | |||
| Survival to hospital discharge | 70 (9.9) | 86 (13.2) | 1.38 (0.99–1.91) | 1.06 (0.80–1.41) |
| Favorable neurologic outcome among survivors | 54/70 (77.1) | 59/86 (68.6) | 0.60 (0.25–1.40) | NA1 |
| Favorable neurologic outcome (all) | 54 (7.6) | 59 (9.1) | 1.22 (0.83–1.79) | 0.98 (0.72–1.31)2 |
| Return of spontaneous circulation | 272 (38.3) | 201 (30.9) | 0.72 (0.57–0.91) | 0.62 (0.49–0.80) |
Bold font indicates a significantly significant result
Unable to adjust due to the small number of events
Race, sex, and automated external defibrillator availability were excluded due to the small number of events
DISCUSSION
Our team hypothesized that a single-dose epinephrine protocol would improve survival to hospital discharge and favorable neurologic outcome rates despite having lower ROSC rates. Existing prehospital resuscitation research supports minimizing interruptions to chest compressions and decreasing peri-shock pause times.(26,27) We reasoned deemphasizing repeat epinephrine doses would allow EMS crews to focus on other tasks, such as chest compressions and defibrillation. However, this study found that patient-centered outcomes remained poor, regardless of the epinephrine protocol used. Even among patients who died from suspected primary cardiac etiologies, the single-dose epinephrine protocol was associated with decreased ROSC rates while showing no association with survival to hospital discharge or favorable neurologic outcome rates. However, among this key subgroup, there was a near 3.3% increase in the rate of survival to hospital discharge with the single-dose epinephrine protocol. While not statistically significant, the study was likely underpowered to detect this difference.
To the best of our knowledge, this is the first prehospital resuscitation study to compare the performance of a single dose of epinephrine to multiple doses of epinephrine. However, our results are similar to those of studies assessing other epinephrine dosing strategies and those comparing epinephrine to placebo. A systematic review of the cardiac arrest epinephrine literature concluded that epinephrine improves ROSC but not survival to discharge or favorable neurologic outcome rates.(16) The recent randomized, double-blind PARAMEDIC-2 trial in the United Kingdom found that compared to placebo, epinephrine every 3–5 minutes increased ROSC and 30-day survival rates but did not affect neurologic outcomes.(15) Another large randomized prehospital trial in Australia also found that epinephrine increased ROSC rates but had similar survival to discharge and favorable neurologic outcome rates compared to placebo.(14) A low-dose epinephrine trial (0.5 mg vs. 1.0 mg) found similar survival to hospital discharge and favorable neurologic outcome rates between groups.(29)
Neurologic outcomes are reported among all patients and just survivors. This distinction allows complete data reporting while promoting patient-centeredness by emphasizing the outcome most important to patients and families: survival to discharge with a favorable neurologic outcome.(20,24,25) This study did not detect a statistically significant difference in favorable neurologic outcome rates. However, this study was underpowered for detecting a meaningful difference in favorable neurologic outcomes among survivors, making this an exploratory objective. Despite this limitation, the findings of this study are consistent with existing randomized clinical trial and systematic review evidence, which suggest that epinephrine does not improve favorable neurologic outcomes for patients with OHCA.(14–16)
Although the single-dose epinephrine protocol was associated with similar survival to hospital discharge rates as the multidose epinephrine protocol, it was associated with decreased ROSC rates. These results in context of the existing epinephrine literature should give clinicians pause when interpreting and applying resuscitation guidelines that prioritize epinephrine for cardiac arrest.(2,3) The American Heart Association gives epinephrine administration the highest possible recommendation. This Class 1 recommendation indicates that epinephrine “is recommended,” “should be administered,” and that it “is indicated/useful/beneficial.”(3) However, our study and contemporary resuscitation literature do not support this level of recommendation. It is not clear that multiple doses of epinephrine are associated with improved outcomes, especially patient-centered outcomes. Furthermore, achieving ROSC in a neurologically devastated patient or in a patient who does not survive to discharge is expensive, contributes to hospital overcrowding, and is not cost-effective.(30) Therefore, it may be reasonable for EMS medical directors to modify their resuscitation protocols to a single-dose epinephrine protocol and to emphasize interventions such as team-focused CPR, high-quality chest compressions, and defibrillation, which are known to improve patient-centered outcomes.(22) However, medical directors must also weigh other unintended consequences of the single-dose epinephrine protocol, such as the possible effect on organ donation.(30–32)
LIMITATIONS
This study has limitations. Patients were not randomized to a single-dose vs. multidose epinephrine protocol, thereby opening the study to unknown confounders and maturation effects. Although this study occurred in a diverse area with rural, urban, and suburban communities with a variety of ALS EMS systems in central NC, generalizability to other regions and non-ALS EMS systems may be limited. Furthermore, the survival to hospital discharge rates were higher in the single-dose and multidose epinephrine cohorts than the national average.(10,11) This is likely reflective of each participating EMS agency being a regional leader in OHCA resuscitation, with each focusing on team-focused CPR, early defibrillation, and minimizing peri-shock pause times. Therefore, the high survival to hospital discharge rates seen with these EMS agencies may not be reflective of national care patterns. Additionally, the receiving hospitals varied, with some being tertiary-care centers and others being community hospitals. The outcomes provided by CARES were not adjudicated, thus risking misclassification bias. Due to the nature of the study and data collection procedures, a per protocol analysis was not possible. The study was not powered to detect a difference in neurologic outcomes among survivors given that only 244 patients survived. Baseline neurologic status was also unknown, further limiting inferences regarding epinephrine dosing and neurologic outcomes.
CONCLUSION
Our findings indicate that among adult OHCA patients, a single-dose epinephrine protocol is associated with similar rates of survival to hospital discharge and favorable neurologic outcomes despite having lower ROSC rates compared to traditional guideline-based epinephrine dosing every 3–5 minutes. Given that the single-dose epinephrine and multidose epinephrine protocols were both associated with similar rates of favorable patient-centered outcomes, it may be reasonable for EMS medical directors to consider a single-dose epinephrine protocol and focus on outcome-oriented interventions, such as team-focused CPR, early defibrillation, and minimizing peri-shock pause time.
Supplementary Material
Supplemental Table 1. Description of each participating county and the protocol switch date.
Supplemental Table 2. Neurologic outcomes pre- vs. post-implementation among all patients.
Supplemental Table 3. Neurologic outcomes pre- vs. post-implementation among survivors.
Supplemental Table 4. Neurologic outcomes pre- vs. post-implementation among all patients presumed to have arrested from primary cardiac etiologies.
Supplemental Table 5. Neurologic outcomes pre- vs. post-implementation among survivors presumed to have arrested from primary cardiac etiologies.
Supplemental Appendix 1. Traditional guideline-based multidose epinephrine protocol and the single dose epinephrine protocol.
Acknowledgements:
We appreciate the EMS agencies in Forsyth, Iredell, Randolph, Stanly, and Surry counties for participating in this work. We also thank Clark Tyson from CARES for his assistance. We also appreciate Amanda Treadway from Iredell EMS for offering her time and support.
Footnotes
Declaration of Interest
Dr. Ashburn receives funding from NHLBI (T32HL076132).
Dr. Snavely receives funding from Abbott and HRSA (1H2ARH399760100).
Dr. Stopyra receives research funding from NCATS/NIH (KL2TR001421), HRSA (1H2ARH399760100), Roche Diagnostics, Abbott Laboratories, Pathfast, Genetesis, Cytovale, Forest Devices, Vifor Pharma, and Chiesi Farmaceutici.
Dr. Mahler receives funding/support from Roche Diagnostics, Abbott Laboratories, Ortho Clinical Diagnostics, Siemens, Grifols, Pathfast, Quidel, Genetesis, Cytovale, and HRSA (1H2ARH399760100). He is a consultant for Roche, Quidel, Abbott, Genetesis, Inflammatix, Radiometer, and Amgen and the Chief Medical Officer for Impathiq Inc.
The other authors have no disclosures to report.
REFERENCES
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation [Internet]. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 2.Soar J, Böttiger BW, Carli P, Couper K, Deakin CD, Djärv T, Lott C, Olasveengen T, Paal P, Pellis T, et al. European Resuscitation Council Guidelines 2021: Adult advanced life support. Resuscitation [Internet]. 2021;161:115–151. doi: 10.1016/j.resuscitation.2021.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Panchal AR, Bartos JA, Cabañas JG, Donnino MW, Drennan IR, Hirsch KG, Kudenchuk PJ, Kurz MC, Lavonas EJ, Morley PT, et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation [Internet]. 2020;142:S366–S468. doi: 10.1161/CIR.0000000000000916. [DOI] [PubMed] [Google Scholar]
- 4.Drug Result Page - MICROMEDEX [Internet]. [cited 2022 Feb 1]. Available from: https://www.micromedexsolutions.com/micromedex2/librarian/PFDefaultActionId/evidencexpert.DoIntegratedSearch?navitem=topHome&isToolPage=true.
- 5.Overgaard CB, Dzavík V. Inotropes and vasopressors: review of physiology and clinical use in cardiovascular disease. Circulation [Internet]. 2008;118:1047–1056. doi: 10.1161/CIRCULATIONAHA.107.728840. [DOI] [PubMed] [Google Scholar]
- 6.Michael JR, Guerci AD, Koehler RC, Shi AY, Tsitlik J, Chandra N, Niedermeyer E, Rogers MC, Traystman RJ, Weisfeldt ML. Mechanisms by which epinephrine augments cerebral and myocardial perfusion during cardiopulmonary resuscitation in dogs. Circulation [Internet]. 1984;69:822–835. doi: 10.1161/01.cir.69.4.822. [DOI] [PubMed] [Google Scholar]
- 7.Callaway CW. Epinephrine for cardiac arrest. Curr Opin Cardiol [Internet]. 2013;28:36–42. doi: 10.1097/HCO.0b013e32835b0979. [DOI] [PubMed] [Google Scholar]
- 8.Ristagno G, Tang W, Huang L, Fymat A, Chang Y-T, Sun S, Castillo C, Weil MH. Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Crit Care Med [Internet]. 2009;37:1408–1415. doi: 10.1097/CCM.0b013e31819cedc9. [DOI] [PubMed] [Google Scholar]
- 9.Larsson PT, Hjemdahl P, Olsson G, Egberg N, Hornstra G. Altered platelet function during mental stress and adrenaline infusion in humans: evidence for an increased aggregability in vivo as measured by filtragometry. Clin Sci [Internet]. 1989;76:369–376. doi: 10.1042/cs0760369. [DOI] [PubMed] [Google Scholar]
- 10.Sasson C, Rogers MAM, Dahl J, Kellermann AL. Predictors of survival from out-of-hospital cardiac arrest: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes [Internet]. 2010;3:63–81. doi: 10.1161/CIRCOUTCOMES.109.889576. [DOI] [PubMed] [Google Scholar]
- 11.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation [Internet]. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 12.Ng KT, Teoh WY. The Effect of Prehospital Epinephrine in Out-of-Hospital Cardiac Arrest: A Systematic Review and Meta-Analysis. Prehosp Disaster Med [Internet]. 2019;34:532–539. doi: 10.1017/S1049023X19004758. [DOI] [PubMed] [Google Scholar]
- 13.Hagihara A, Hasegawa M, Abe T, Nagata T, Wakata Y, Miyazaki S. Prehospital epinephrine use and survival among patients with out-of-hospital cardiac arrest. JAMA [Internet]. 2012;307:1161–1168. doi: 10.1001/jama.2012.294. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs IG, Finn JC, Jelinek GA, Oxer HF, Thompson PL. Effect of adrenaline on survival in out-of-hospital cardiac arrest: A randomised double-blind placebo-controlled trial. Resuscitation [Internet]. 2011;82:1138–1143. doi: 10.1016/j.resuscitation.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 15.Perkins GD, Ji C, Deakin CD, Quinn T, Nolan JP, Scomparin C, Regan S, Long J, Slowther A, Pocock H, et al. A Randomized Trial of Epinephrine in Out-of-Hospital Cardiac Arrest. N Engl J Med [Internet]. 2018;379:711–721. doi: 10.1056/NEJMoa1806842. [DOI] [PubMed] [Google Scholar]
- 16.Lin S, Callaway CW, Shah PS, Wagner JD, Beyene J, Ziegler CP, Morrison LJ. Adrenaline for out-of-hospital cardiac arrest resuscitation: a systematic review and meta-analysis of randomized controlled trials. Resuscitation [Internet]. 2014;85:732–740. doi: 10.1016/j.resuscitation.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 17.The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies | The EQUATOR Network; [Internet]. Available from: http://www.equator-network.org/reporting-guidelines/strobe/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.North Carolina Office of EMS Protocols [Internet]. North Carolina Office of EMS. [cited2022 Feb 1]. Available from: https://www.ncmhtd.com/owncloud/index.php/s/p3bD1gMHWSoQf4Y. [Google Scholar]
- 19.Stoesser CE, Boutilier JJ, Sun CLF, Brooks SC, Cheskes S, Dainty KN, Feldman M, KoDT, Lin S, Morrison LJ, et al. Moderating effects of out-of-hospital cardiac arrest characteristics on the association between EMS response time and survival. Resuscitation [Internet]. 2021;169:31–38. doi: 10.1016/j.resuscitation.2021.10.014. [DOI] [PubMed] [Google Scholar]
- 20.CARES Data Dictionary [Internet]. 2021. Available from: https://mycares.net/sitepages/uploads/2020/Data%20Dictionary%20(2021).pdf.
- 21.Chan PS, Girotra S, Tang Y, Al-Araji R, Nallamothu BK, McNally B. Outcomes for Out-of-Hospital Cardiac Arrest in the United States During the Coronavirus Disease 2019 Pandemic. JAMA Cardiol [Internet]. 2021;6:296–303. doi: 10.1001/jamacardio.2020.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearson DA, Darrell Nelson R, Monk L, Tyson C, Jollis JG, Granger CB, Corbett C, Garvey L, Runyon MS. Comparison of team-focused CPR vs standard CPR in resuscitation from out-of-hospital cardiac arrest: Results from a statewide quality improvement initiative. Resuscitation [Internet]. 2016;105:165–172. doi: 10.1016/j.resuscitation.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Jennett B, Bond M. Assessment of Outcome After Severe Brain Damage: A Practical Scale. Lancet [Internet]. 1975;305:480–484. doi: 10.1016/S0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 24.Frank L, Basch E, Selby JV, Patient-Centered Outcomes Research Institute. The PCORI perspective on patient-centered outcomes research. JAMA [Internet]. 2014;312:1513–1514. doi: 10.1001/jama.2014.11100. [DOI] [PubMed] [Google Scholar]
- 25.Bardes CL. Defining “patient-centered medicine.” N Engl J Med [Internet]. 2012;366:782–783. doi: 10.1056/NEJMp1200070. [DOI] [PubMed] [Google Scholar]
- 26.Valenzuela TD, Kern KB, Clark LL, Berg RA, Berg MD, Berg DD, Hilwig RW, Otto CW, Newburn D, Ewy GA. Interruptions of chest compressions during emergency medical systems resuscitation. Circulation [Internet]. 2005;112:1259–1265. doi: 10.1161/CIRCULATIONAHA.105.537282. [DOI] [PubMed] [Google Scholar]
- 27.Cheskes S, Schmicker RH, Christenson J, Salcido DD, Rea T, Powell J, Edelson DP, Sell R,May S, Menegazzi JJ, et al. Perishock pause: an independent predictor of survival from out-of-hospital shockable cardiac arrest. Circulation [Internet]. 2011;124:58–66. doi: 10.1161/CIRCULATIONAHA.110.010736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stankovic N, Holmberg MJ, Høybye M, Granfeldt A, Andersen LW. Age and sex differences in outcomes after in-hospital cardiac arrest. Resuscitation [Internet]. 2021;165:58–65. doi: 10.1016/j.resuscitation.2021.05.017. [DOI] [PubMed] [Google Scholar]
- 29.Fisk CA, Olsufka M, Yin L, McCoy AM, Latimer AJ, Maynard C, Nichol G, Larsen J, Cobb LA, Sayre MR. Lower-dose epinephrine administration and out-of-hospital cardiac arrest outcomes. Resuscitation [Internet]. 2018;124:43–48. doi: 10.1016/j.resuscitation.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Achana F, Petrou S, Madan J, Khan K, Ji C, Hossain A, Lall R, Slowther A-M, Deakin CD, Quinn T, et al. Cost-effectiveness of adrenaline for out-of-hospital cardiac arrest. Crit Care [Internet]. 2020;24:579. doi: 10.1186/s13054-020-03271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandroni C, D’Arrigo S, Callaway CW, Cariou A, Dragancea I, Taccone FS, Antonelli M. The rate of brain death and organ donation in patients resuscitated from cardiac arrest: a systematic review and meta-analysis. Intensive Care Med [Internet]. 2016;42:1661–1671. doi: 10.1007/s00134-016-4549-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faucher A, Savary D, Jund J, Dorez D, Debaty G, Gaillard A, Atchabahian A, Tazarourte K. Out-of-hospital traumatic cardiac arrest: an underrecognized source of organ donors. Transpl Int [Internet]. 2014;27:42–48. doi: 10.1111/tri.12196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Description of each participating county and the protocol switch date.
Supplemental Table 2. Neurologic outcomes pre- vs. post-implementation among all patients.
Supplemental Table 3. Neurologic outcomes pre- vs. post-implementation among survivors.
Supplemental Table 4. Neurologic outcomes pre- vs. post-implementation among all patients presumed to have arrested from primary cardiac etiologies.
Supplemental Table 5. Neurologic outcomes pre- vs. post-implementation among survivors presumed to have arrested from primary cardiac etiologies.
Supplemental Appendix 1. Traditional guideline-based multidose epinephrine protocol and the single dose epinephrine protocol.


