Abstract
Introduction
Infections are a major cause of morbidity and mortality in systemic lupus erythematosus (SLE). We assessed the incidence and risk factors for major infections in SLE in India.
Methods
A retrospective review of a cohort of 1354 patients of adult SLE (ACR 1997 criteria) seen between 2000 and 2021 at a single center was conducted. Serious infections (need for hospitalisation, prolonged intravenous antibiotics, disability, or death) were recorded. Cox regression was used to determine factors associated with serious infection and the effects of serious infection on survival and damage.
Results
Among the 1354 patients (1258 females, mean age of 30.3 years, follow-up of 7127.89 person-years), there were 439 serious infections in 339 patients (61.6 per 1000 person-years follow-up). Bacterial infections (N = 226) were the most common infection followed by mycobacterial infections (n = 81), viral (n = 35), and then invasive fungal infections (N = 13). Mycobacterium tuberculosis was the single most common microbiologically confirmed organism with incidence of 1136.4/100,000 person-years with 72.8% of them being extrapulmonary. Infection free survival at 1 year and 5 years was 82.9% and 73.8%. There were 119 deaths with infection attributable mortality in 65 (54.6%). On multivariable Cox regression analysis, higher baseline activity (HR 1.02, 1.01–1.05), gastrointestinal involvement (HR 2.75, 1.65–4.69), current steroid dose (HR 1.65, 1.55–1.76), and average cumulative steroid dose per year (HR 1.007, 1.005–1.009) were associated with serious infection and higher albumin (HR 0.65, 0.56–0.76) was protective. Serious infections led to greater damage accrual (median SLICC damage index of 1 vs. 0) and mortality (HR was 18.2, 32.7 and 81.6 for the first, second, and third infections).
Conclusion
Serious infections remain a major cause of mortality and damage accrual in SLE and higher disease activity, gastrointestinal involvement, hypoalbuminemia, current steroid dose, and cumulative steroid dose are the risk factors for it.
|
Key Points • Tuberculosis is still a major cause of morbidity in South Asian patients of SLE. • Serious infections are associated with higher damage accrual and mortality in patients with SLE. • Higher disease activity, gastrointestinal involvement, hypoalbuminemia, and current steroid dose were associated with increased risk of infection. • This study provides data to enable specific recommendations for infection prevention in SLE for the South Asian region. |
Supplementary information
The online version contains supplementary material available at 10.1007/s10067-023-06592-x.
Keywords: Infection, Staphylococcus aureus, Streptococcus pneumoniae, Systemic lupus erythematosus, Tuberculosis
Introduction
Systemic lupus erythematosus (SLE) is the prototypic autoimmune disease with chronic multisystem involvement. SLE predominantly affects young females and leads to significant morbidity and mortality. In the first decade of the disease, infections and disease activity are the leading causes of mortality in patients of SLE [1].
The increased risk of infections in SLE arises from the underlying immune defects themselves like complement deficiency, impaired phagocyte function, T lymphocyte and B lymphocyte dysfunction, and immunoglobulin deficiency. In addition, immunosuppressive treatment given for the control of disease activity can lead to lymphopenia, leucopenia, neutrophil dysfunction, and other immune abnormalities [2–4].
Prevalence of serious infections in SLE ranges from 15.4 to 40% across different studies depending on the population studied and location of the study [5–8]. In a Canadian study, patients with SLE had an 82% higher risk of severe infection and had on average twice (rate ratio 2.07) the number of severe infections as compared to the general population [9].
Although overall mortality in SLE has improved over the last few decades with advances in immunosuppressive therapy, early mortality due to infections still persists with serious infection conferring a 22 times higher odds of mortality in the first year of disease [10]. Infection attributable mortality of 25% has been previously reported from a European cohort [5]. We have previously found serious infections to be a major cause of mortality in adult and juvenile onset SLE in the north Indian population [11, 12].
The profile of infections is expected to vary across different geographic regions as per the local epidemiology. Previous cohorts from Spain, Canada, and China have reported high prevalence of acute bacterial infections of the lungs and gastrointestinal and urinary tracts followed by viral infections whereas a multiethnic cohort form South Africa also reported an increased risk of tuberculosis (TB) in addition to bacterial infections [13–16]. Similarly, previous data from our center had TB as one of the important infections [12]. This implies that the preventive strategies need to be tailored according to the profile of infections prevalent in each region. The Asia Pacific League against Rheumatism (APLAR) has recently constituted a special interest group to formulate guidelines for infection prevention in the Asia Pacific region, and they found that there is paucity of data on the profile of major infections in South Asian patients of SLE [17].
Thus, this study was designed to determine the incidence as well as spectrum of serious infections in South Asian patients of SLE in North India and identify predictors of serious infection as well as their impact on patient outcomes.
Methods
We conducted a retrospective review of patients seen between 2000 and 2021 at a single rheumatology center in North India. Adult onset SLE patients with age of onset beyond 18 years, who fulfilled ACR 1997 classification criteria were included in the study. All patients were of South Asian ethnicity. Data were retrieved from hospital clinic files and the electronic health information system. Most patients in this center have follow-up visits every 3 months and at each visit clinical details, laboratory parameters (hemogram, renal function, liver functions, and urine analysis), and clinical SLEDAI scores are recorded.
In addition, detailed baseline data including demographics, organ involvement and disease activity (SLEDAI-2 K) was collected. During follow-up serious infections, corticosteroid use, background immunosuppression, complications, and disease outcomes were also recorded.
Outcomes
Serious infections
Any infection requiring hospitalization, inpatient IV antibiotic therapy, or resulting in disability of death was deemed to be serious [15]. Infections were identified based on the opinion of the treating rheumatologist with supportive evidence of microbiological confirmation by culture from the affected tissue, evidence of recent serological response to a pathogen, isolation of pathogen by polymerase chain reaction (PCR), or radiological evidence of infection. Due to the pandemic nature of the disease, COVID infections and its sequelae were excluded from the study.
Opportunistic infections
Any infection with Mycobacterium tuberculosis, non-tuberculous mycobacteria, viral infections (Herpes simplex, Herpes zoster, and Cytomegalovirus), and invasive fungal infections (Candida species, Aspergillus, Mucormycosis, Histoplasma, and Pneumocystic jiroveci).
Mortality
All inpatient deaths were recorded from the hospital information system. Data on outpatient deaths were available as communicated by the patient’s relatives in the hospital clinic files.
Morbidity
The SLICC damage index was calculated at the last follow-up visit for all patients.
Ethics
The study was approved by the institutional ethics committee. Due to the retrospective nature, the ethics committee approved a waiver of informed consent (IEC code no. 2021–30-DM-EXP-36).
Statistics
Baseline data were expressed as median (IQR) for numerical variables and number (percentage) for categorical data. Data were analyzed using parametric student’s T test for continuous variables. The Chi-squared test or Fischer’s exact test was used for categorical variables. Factors predicting serious infection as determined from the univariate analysis and other biologically plausible variables were used to fit Cox proportional hazards models for multivariate analysis. If missing data was less than 10% of the total, we did not impute the missing values and used the data as is for multivariate analysis. Three separate models were fit. The first was a Cox proportional hazards model with time to first serious infection as the dependent variable. As there were a number of patients who had recurrent serious infections, a second model was fit as per the Anderson–Gill modification of Cox regression for recurrent timed events which accounted for all serious infections in the cohort [18]. A third multistate Cox regression model was fit with time to mortality as the dependent variable and the transition from baseline to 1st infection, 1st to 2nd infection, and 2nd to 3rd infection as the dependent variable. Hazard ratios were obtained for each transition [19]. Kaplan–Meier survival analysis was used to plot serious infection-free survival. Poisson regression with non-lupus-related admissions as the dependent variable and duration of follow-up as the offset was used to determine the rate ratio for admissions in those with serious infections compared to those without.
Results
Demographics and clinical characteristics
A total of 1354 patients were included in the study of whom 1258 were females (female: male = 13: 1). The mean age at presentation was 30.32 ± 9.42 years with a median follow up duration of 3.41 (1.02–8.01) years and a total follow-up of 7127.89 person-years. Briefly, at presentation clinical manifestations were as follows: fever (1004, 74.2%), mucocutaneous (941, 69.5%), arthritis (831, 62.9%), myositis (120, 8.9%), hematological (578, 42.7%), serositis (218, 16.1%), nephritis (656, 48.4%), respiratory (34, 2.5%), cardiovascular (42, 3.1%), neuropsychiatric (142, 10.5%), gastrointestinal (30, 2.2%), vasculitis (65, 4.8%), and secondary anti-phospholipid antibody syndrome (APS) (114, 8.4%). The median SLEDAI-2 K score at presentation was 11 (8–16). Four percent of patients were diabetic.
Profile of infections
A total of 439 major infections occurred in 339 (25.03% of the cohort) individuals at a rate of 61.6 serious infections per 1000 person-years of follow-up. Recurrent infections occurred in 86 (6.35%) patients. Of the recurrent cases, 70 had two infections, 15 had 3 infections, and a single patient had 4 serious infections. There was no microbiological diagnosis in 88 (20.04%) cases. The probability of developing a second serious infection was 20.64%, and the probability of developing a third serious infection in those with previous serious infections was 21.42%. The most common infections were bacterial (308, 70.15%) followed by viral (35, 7.97%) and then fungal (13, 2.96%) with many patients developing more than 1 microbiologically proven serious infection (Tables 1 and 2).
Table 1.
Profile of serious bacterial infections
| Organism and clinical syndrome | N | Incidence rate (per 100,000 person-years) |
|---|---|---|
| Bacterial infections: | ||
| Mycobacterium tuberculosis | 81 | 1136.4 |
| Lung* (22), disseminated (11), central nervous system (10), lymph node (9), abdomen (8), joint (8), spine (3), pleural effusion (2), pericarditis (2), hepatitis (2), skin (1), abscess (1), osteomyelitis (1), tenosynovitis (1) | ||
| Mycobacterium abscessus | 1 | |
| Eschericia coli | 51 | 715.5 |
| Sepsis (20), pyelonephritis (14), cystitisθ (7), pneumonia (6), spontaneous bacterial peritonitis (2), abscess (2) | ||
| Staphylococcus aureus | 65 | 911.9 |
| Abscess (30), pneumonia (12), sepsis (11), cellulitis (7), meningitis (2), brain abscess (1), muscle (1), osteomyelitis (1) | ||
|
Methicillin-resistant Staphylococcus aureus Abscess (4), sepsis (2), lung (1) |
7 | 98.2 |
|
Streptococcus pneumoniae Sepsis (8), pneumonia (7), urinary tract infection (2), meningitis (1), brain abscess (1), abscess (1) |
20 | 280.6 |
|
Klebsiella pneumoniae Lungδ (19), urinary tract infection (5), sepsisδ (3), pyelonephritis (1), abscess (1) |
29 | 406.9 |
|
Pseudomonas aeruginosa Lung (12), sepsis (9), abscess (4), urinary tract infection (4) |
29 | 406.9 |
|
Enterococcus faecalis Urinary tract infection (4), sepsis (2), abscess (1) |
7 | 98.2 |
| Salmonella Typhi/Paratyphi | 3 | 42.1 |
|
Acinetobacter baumannii Pneumonia (4), urinary tract infection (3), abscess (1), apontaneous bacterial peritonitis (1) |
9 | 126.2 |
|
Proteus mirabilis Abscess (1) |
1 | 14 |
|
Listeria monocytogenes Meningitis (1) |
1 | 14 |
|
Nocardia Brain abscess (2), disseminated (1), lung abscess (1) |
4 | 56.1 |
|
No microbiological confirmation: Pneumonia (62), sepsis (21), CSOM (3), ASOM (1), liver abscess (1) |
88 | |
*1 multidrug-resistant tuberculosis
θ2 extended spectrum beta lactamase (ESBL) strains
δ2 and 1 ESBL strains, respectively
Infections are represented here as a rate per 100,000 person-years and are not mutually exclusive: there were patients with more than 1 serious infection
Table 2.
Profile of serious non bacterial infections
| Organism and clinical syndrome | N | Incidence rate (per 100,000 person-years) |
|---|---|---|
| Viral infections: | ||
| Cytomegalovirus | 11 | 154.3 |
| Gastrointestinal tract (4), hepatitis (2), disseminated (2), lung (2), encephalitis (1) | ||
| Herpes zoster | 10 | 140.3 |
| Multidermatomal (9), encephalitis (1) | ||
| Herpes simplex | 1 | 14 |
| Esophagitis (1) | ||
| Hepatitis C | 5 | 70.15 |
| Hepatitis B | 2 | 28.1 |
| Dengue | 2 | 28.1 |
| H1N1 influenza | 3 | 42.1 |
| Parvovirus | 1 | 14 |
| Fungal infections: | ||
| Candida | 5 | 70.1 |
| Oesophagitis (3), sepsis (1), pyelonephritis (1) | ||
| Cryptococcus neoformans | 5 | 70.1 |
| Meningitis (5) | ||
| Histoplasma | 2 | 28.1 |
| Disseminated (2) | ||
| Aspergillus flavus | 1 | 14 |
| Lung (2) | ||
| Parasitic infections: | 4 | 14 each |
| Toxoplasma (1), neurocysticercus (1), Giardia lambia (1), Plasmodium falciparum (1) | ||
Infections are represented here as a rate per 100,000 person-years and are not mutually exclusive: there were patients with more than 1 serious infection
Opportunistic infections
Amongst the serious infections, Mycobacterium tuberculosis was the most common and was seen in 81 patients. The incidence rate of TB was 1136.4 per 100,000 person-years. Most cases (59, 72.8%) were extrapulmonary. One patient had non-tubercular mycobacterial infection-associated skin infection (Table 1). There were 40 more opportunistic infections. Among these, reactivation of CMV (11, 27.5%) was the most common followed by multidermatomal Herpes zoster (HZ) reactivation and HZ encephalitis (10, 25%), invasive candidiasis (5, 12.5%), cryptococcal meningitis (5, 12.5%), disseminated histoplasmosis (2, 5%), and 1 (2.5%) each of Aspergillus flavus pneumonia, central nervous system toxoplasmosis, and chronic diarrhoea due to Giardia lamblia. Eight (20%) of these patients died: 2 patients with cryptococcal meningitis, 1 each with Aspergillus pneumonia, Candidial sepsis, Herpes simplex oesophagitism and chronic giardiasis, and 2 patients with complications arising out of disseminated zoster reactivation.
Univariate analysis of predictors of infections
Serious infections were significantly associated with male gender, higher SLEDAI-2 K score at baseline, fever, myositis, nephritis, gastrointestinal involvement, and neuropsychiatric lupus (NPSLE) as initial disease manifestation (Table 3). Baseline laboratory parameters associated with serious infections were low albumin, low absolute lymphocyte count (ALC), low platelets, high anti-dsDNA antibody levels, and low C3 and C4 levels. They were also on higher daily steroid (prednisolone) doses, had cyclophosphamide as background immunosuppression, and had received higher cumulative steroid doses per year until the time of infection.
Table 3.
Univariate analysis of predictors of serious infection
| Covariate | Infection (n = 339) | No infection (n = 1015) | Effect size (RR, 95% CI) |
|---|---|---|---|
| Demographics: | |||
| Age at onset (yrs) | 29 (23–36) | 28 (23–35) | - |
| Male gender | 36 (10.6%) | 60 (5.9%) | 1.21 (1.04–1.42) |
| Baseline features: | |||
| SLEDAI-2 K* | 14 (10–18) | 10 (6–15.75) | - |
| Fever | 279 (82.3%) | 725 (71.42%) | 1.15 (1.08–1.22) |
| Myositis | 41 (12.09%) | 70 (68.9%) | 1.15 (1.01–1.32) |
| Nephritis | 209 (61.65%) | 447 (43.23%) | 1.19 (1.12–1.27) |
| GI | 16 (4.72%) | 14 (13.8%) | 1.62 (1.10–2.38) |
| NPSLE | 48 (14.16%) | 949.26 | 1.15 (1.02–1.30) |
| Laboratory parameters: | |||
| Albumin* (mg/dl) | 3 (2.4–3.6) | 3.5 (2.9–4) | - |
| Diabetes | 19 (5.6%) | 36 (3.55%) | 1.15 (0.95–1.40) |
| DsDNA* (IU) | 180 (60.3–300) | 142.2 (33–272.2) | - |
| Complement 3* (mg/dl) | 50.9 (30–80.25) | 65.05 (39–101.75) | - |
| Complement 4* (mg/dl) | 10 (6–19) | 12 (6.2–21) | - |
| ALC* (/cumm) | 1298 (850–1891) | 1484 (1012–2088) | - |
| Platelets* (× 105/cumm) | 1.62 (1.03–2.4) | 1.83 (1.27–2.54) | - |
| Treatment: | |||
| Daily steroid (prednisolone) dose* (mg) | 15 (7.5–40) | 5 (0–7.5) | - |
| Average cumulative steroid (prednisolone) dose* (g/year) | 4.07 (1.89–14.31) | 1.82 (1.01–2.66) | - |
| Cyclophosphamide use | 64 (18.88%) | 56 (5.52%) | 1.66 (1.37–2.02) |
| Total admissions | 3 (2–6) | 1 (0–2) | - |
ALC, absolute lymphocyte count
*Significant by the Wilcoxon signed rank test
Multivariate analysis of predictors of infection
On fitting a Cox proportional hazards regression model for time to the first serious infection, higher baseline SLEDAI-2 K score (HR 1.03, 1.01–1.05), gastrointestinal involvement (HR 2.75, 1.65–4.99), daily steroid dose per 10 mg increment (HR 1.65, 1.55–1.76), and average cumulative steroid dose per year (HR 1.007, 1.005–1.009) predicted serious infections whereas higher albumin levels (HR 0.65, 0.56–0.76) were protective (Model 1, Table 4). Using the Anderson–Gill modified Cox regression model for time to serious infection and taking into account recurrent serious infections, the same parameters were found to be associated (Model 2, Table 4). Absolute lymphocyte count, dsDNA, C3, and C4 were not included in the final models as they are a part of the SLEDAI-2 K scores.
Table 4.
Cox proportional hazards models for the predictors of major infection (models 1 and 2) and for risk of death from major infection (model 3)
| Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Covariates | HR | 95% CI | Covariate | HR | 95%CI | Covariates | HR | 95% CI |
| Male sex | 1.2 | 0.82–1.75 | Male sex | 1.34 | 0.93–1.95 | Infection 1 | 18.2 | 11.8–28.1 |
| Fever | 1.04 | 0.78–1.4 | Fever | 1.03 | 0.80–1.33 | Infection 1: 2 | 32.7 | 16.9–63.01 |
| Myositis | 1.05 | 0.74–1.51 | Myositis | 1.05 | 0.77–1.43 | Infection 2: 3 | 81.6 | 29.9–222.7 |
| Nephritis | 0.98 | 0.74–1.3 | Nephritis | 1.002 | 0.78–1.3 | |||
| GI* | 2.75 | 1.65–4.59 | GI* | 2.07 | 1.09–3.96 | |||
| NPSLE | 1.07 | 0.78–1.5 | NPSLE | 1.22 | 0.9–1.67 | |||
| SLEDAI* | 1.02 | 1.01–1.05 | SLEDAI | 1.03 | 1.01–1.05 | |||
| Daily steroid dose* (/10 mg) | 1.65 | 1.55–1.76 | Daily steroid dose* (/10 mg) | 1.43 | 1.36–1.51 | |||
| Average cumulative steroid dose* | 1.007 | 1.005–1.009 | Average cumulative steroid dose* | 1.002 | 1.001–1.004 | |||
| Albumin* | 0.65 | 0.56–0.76 | Albumin* | 0.83 | 0.71–0.96 | |||
| Cyclophosphamide use | 1.16 | 0.85–1.6 | Cyclophosphamide use | 1.21 | 0.92–1.61 | |||
*Significantly associated with risk of serious infection on follow up
Model 1, Cox proportional hazard model for 1st serious infection; Model 2, Anderson Gill modified Cox proportional hazard model for recurring serious infections; Model 3, multistate Cox proportional hazard model for mortality with every infection; GI, gastrointestinal manifestations
Time trend of serious infections
The average number of infections for the entire cohort increased from 9.9 per year for the decade from 2000 to 2009 to 23.7 per year for the decade from 2010 to 2019. However, the incidence density per person-year follow-up remained stable across the study period (Supplementary Fig. 1).
Health care utilization
Median total admissions were higher in those with serious infection (3, IQR 2–6) compared to those without (1, IQR 0–2) (Table 3).
Excluding admissions related to lupus disease activity, those with a major infection were more likely to require recurrent admissions with a rate ratio of 2.34 (95% CI 2.01–2.68) compared to those without. The rate of infection-specific hospitalization was 52.3 per 1000 person-years.
Outcomes
A serious infection ever in the disease course was associated with greater damage. The median SLICC damage index was 1 (IQR 0–2) in those with an infection and 0 (IQR 0–0) in those without. This difference was statistically significant.
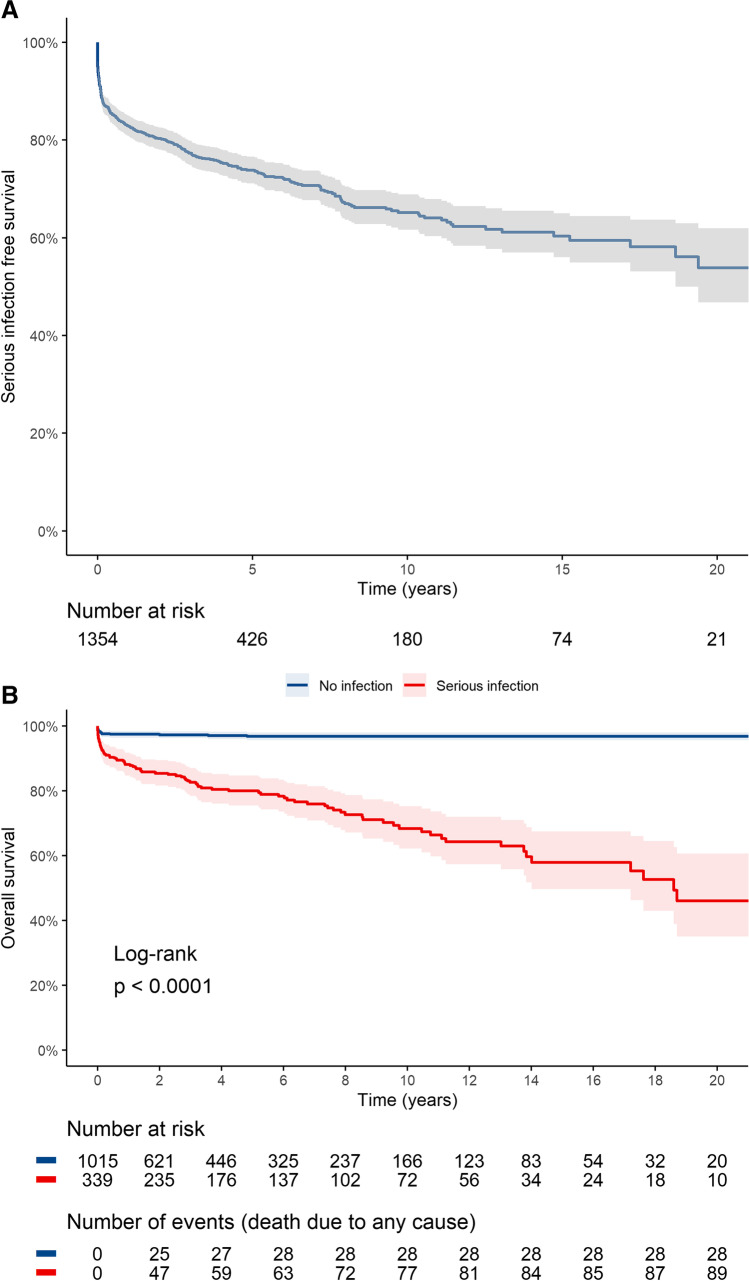
The survival free from serious infections at 1 year, 5 years, and 10 years was 82.9% (95% CI 80.8–85), 73.8% (95% CI 71.1–76.6), and 65.2% (95% CI 61.7–68.9), respectively (Fig. 1a). Any serious infection ever in the disease course predisposed to higher mortality (Fig. 1b).
Fig. 1.
A The Kaplan–Meier survival curve depicts time to first serious infection with the numbers at risk. B The Kaplan–Meier survival curve shows the overall survival difference between those with any serious infection ever compared to those with no serious infection in the disease course
One hundred and nineteen patients succumbed to illness. The hazard ratio for mortality increased progressively with each serious infection and was 18.2, 32.7, and 81.6 for the first, second, and third serious infections, respectively (Model 3, Table 4). Infection attributable mortality occurred in 65 (54.6%) patients.
Discussion
The most common infections in this single-center study were bacterial followed by viral and fungal. Mycobacterial infections were the most common opportunistic infection, and a significant number were extrapulmonary. Higher disease activity at baseline, presence of gastrointestinal disease, lower baseline albumin levels, higher daily steroid dose, and cumulative steroid dose predicted serious infections on follow-up. Serious infection led to more damage and mortality increased with each episode of infection.
Gram-negative bacterial infections cumulatively exceeded Gram-positive infections. However, Staphylococcus aureus was the most common bacterial infection in our cohort and a large majority of these were skin infections. This is similar to previous cohorts from South Africa and Australia (Table 4) [16, 20]. The prevalence of Staphylococcus aureus colonization has been reported to be 50% in patients with active cutaneous lupus [21]. This may explain the high rates of skin infections in our cohort. Staphylococcus aureus has been hypothesized to have a causal role in the induction of autoimmunity as well as systemic flares of glomerulonephritis in lupus-prone mice by activating the IL17 family cytokines and Th17 cells [22]. Streptococcus pneumoniae was the other predominant Gram-positive organism isolated. The incidence of Streptococcus pneumoniae in our cohort was similar to previous reports from Europe (280/100,000 person-years versus 201–236/100000 person-years) [23, 24]. Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa were the other commonly isolated Gram-negative bacteria. This is similar to data from China and Mexico (Table 1) [10, 25].
Mycobacterium tuberculosis was the most common infection in our cohort with a predominance of extrapulmonary forms. Although this is partly expected due to the endemic nature of tuberculosis in India, it is still much higher than the background incidence of tuberculosis of 188 (95% CI 129–257) per 100,000 person-years in the Indian population last reported in 2021 [26]. The proportion of extrapulmonary cases in this study was much higher at 72.8% as compared to 12.5–67% across multiple studies [27–34]. Studies from Southeast Asia report higher prevalence as compared to studies from other regions with Hong Kong reporting a prevalence of 67% for extrapulmonary tuberculosis [30]. Of the autoimmune rheumatic diseases receiving long term steroids, SLE was associated with the highest rate of reactivation of tuberculosis in a prior study (2.22 per 100 recruited cases/year) [35]. Though BCG vaccination is thought to reduce extrapulmonary TB especially meningitis but in patients with SLE the underlying immune deficiency may result in disseminated and extrapulmonary disease.
Recently, antibodies to interferon alpha have been associated with and excess risk of developing tuberculosis over other infections in SLE [36]. This could be a useful screening tool to identify those suited for latent TB prophylaxis. In a seminal study from West India, all patients of SLE at diagnosis expected to be on steroid therapy were initiated on isoniazid (INH) prophylaxis for 1 year and further followed up for 1 year [37]. Tuberculosis developed in 1 patient in the first month of therapy and another in the 2nd year in the follow-up period. Compared to historic controls from the same institute, the incidence of tuberculosis declined from 11 to 2% in newly diagnosed SLE patients. A randomized controlled trial from China found no incident cases of tuberculosis in the group assigned to TB prophylaxis with either rifampicin alone or rifampicin with ethambutol for 1 year compared to 11% in those treated with standard of care immunosuppression alone [38]. Thus, TB prophylaxis, with newer regimens merits, is further studied in a clinical trial especially in TB endemic countries of Asia.
Cytomegalovirus (CMV) reactivation was the most common viral infection in our patients followed by Herpes zoster. CMV infection has not been frequently reported in previous cohorts of lupus. A multicenter study from Japan found a prevalence of 2.04% (151 cases out of 7377 hospitalized patients) in autoimmune diseases [39]. The highest prevalence was in SLE and CMV reactivation led to mortality in about a third of patients. A high index of suspicion is warranted for diagnosis as presentation may often mimic SLE disease flare. Cryptococcal meningitis was the most common invasive fungal infection in our cohort. Cryptococcal meningitis has been reported to be the cause in 25% all cases of invasive fungal infections in SLE [40]. Higher disease activity and higher corticosteroid dose predispose to this often fatal complication [41].
Higher disease activity at baseline as measured by SLEDAI-2 K, hypoalbuminemia, gastrointestinal involvement at baseline, cumulative steroid dose prior to infection, and daily steroid dose at the time of infection were associated with increased risk of serious infection in our cohort. Lower albumin could reflect greater inflammation at baseline or the effect of poor nutrition. Gastrointestinal involvement at baseline was also a significant predictor of mortality in our cohort [12]. Till date, a single prediction model developed from the Spanish multicenter RELESSER cohort has undergone external validation for prediction of serious infection with mixed results (predictive accuracy at baseline 63% and at the time of infection 79%) [15, 42]. This model included age at diagnosis (≥ 46 years), male sex, Latin–American ancestry, current steroid dose (≥ 10 mg), previous hospitalization, and previous serious infection. Most of these factors also predicted infection in our cohort. Other factors associated with serious infection across studies include serositis, renal involvement, anemia, thrombocytopenia, hypoproteinemia, hypoalbuminemia, low C3, and the presence of diabetes (Table 5) [5, 10, 15, 16, 20, 25, 43]. Increasing daily corticosteroid dose and immunosuppressants such as cyclophosphamide, mycophenolate mofetil, and rituximab were also predictors of infection whereas cumulative use of hydroxychloroquine was protective [15, 44, 45]. Our models found a 65% increase in serious infections for every 10-mg increase in corticosteroid dose whereas adjusted for the steroid dose, the use of cyclophosphamide no longer conferred any additional risk. A recent study from Japan showed a higher risk of infection (hazard ratio of 6.8) with doses as low as 5–7.5 mg prednisone per day [46]. Higher daily corticosteroid use is a consistent factor across all studies, and thus, attempt should be made to use minimum dose for a minimum period of time [47, 48].
Table 5.
Comparison of previous studies on serious infections from different regions of the world
| Country | Design | Number of patients | Median follow-up | Serious infections, n (%) | Most common infections | Predictors | |
|---|---|---|---|---|---|---|---|
| Goldblatt et al. [5] | UK | Cohort | 104 | 5 years | 16 (15.4%) |
Pneumonia (no organism isolated) (10) S. pneumoniae (2) S. aureus (2) E. coli (2) |
Recent change in immunosuppression |
| Dubula et al. [16] | South Africa | Cohort | 167 | 1.75 years | 84 (50.3%) |
S. aureus (15) E. coli (12) M. tuberculosis (8) K. pneumoniae (8) P. aeruginosa (5) NTM (3) |
Serositis Seizures |
| Merayo-Chalico et al [25] | Mexico | Case control | 167 | 7.8 years | 89 |
E. coli (16%) E. faecalis (11%) S. pneumoniae (6%) S. aureus (6%) P. aeruginosa (6%) K. pneumoniae (4%) M. tuberculosis (4%) |
Lymphopenia Prednisolone use Low C3 |
| Rua-figueroa et al. [15] | Spain | Cohort | 3658 | 10 years | 705 (19.3%) |
Bacterial (51.9%) Unknown (30.4%) Viral (11.9%) Mycobacterial (3.5%) Fungal (2.3%) |
Age at diagnosis Male sex Hispanic ethnicity Steroids > 10 mg/day Hospitalization Severity Katz index Previous infection |
| Wang et al. [10] | China | Cohort | 494 | 2.8 years | 69 (14%) |
K. pneumoniae (8) P. aeruginosa (7) S. pneumoniae (5) |
SLEDAI > 10 Lymphocyte count < 800/cumm Creatinine > 1.18 mg/dl |
| Ko et al. [20] | Australia | Cohort | 346 | 6.6 years | 86 (24.8%) |
S. aureus (10) E. coli (9) E. faecalis (4) Streptococcus (3) Varicella (3) |
SLEDAI High disease activity state SLICC damage index Cyclophosphamide |
In our cohort, patients with serious infections were likely to have excess hospitalization of 2.34 times compared to those without. SLE is a chronic disease that requires lifelong therapy. Hospitalization significantly adds to the economic burden of the disease and accounts for the largest component of direct cost varying from 25 to 30% across studies [49, 50]. In addition to the direct costs of hospitalization, significant economic burden also arises out of loss of work and economic loss with caregivers reporting 12.8% reduction in paid work time in the USA [51]. In a developing low middle-income country like India, with greater vocational uncertainty, this impact is expected to be greater though there is a lack of data in this regard.
The SLICC damage index at last follow-up was higher in patients with infections in our cohort, and this is consistent with prior studies [15, 52]. Serious infections also predisposed to death in our cohort with each successive infection resulting in a further increase in mortality risk. Infection attributable mortality accounted for more than half of the deaths that occurred. This is similar to the long term outcomes from a UK cohort with infections as the leading cause of death at 31.7% [48]. Infection attributable mortality ranged from 23 to 26% in a Spanish cohort which also corresponded to a threefold increase over the general population (25% versus 8%) [53]. Infections also conferred a hazard ratio for mortality of 2.79 on long-term follow-up of the Hopkins lupus cohort from the USA [54]. Infection-related mortality is the leading cause of excess mortality in SLE as per analysis of a large insurance database in Hungary [55]. In a recent meta-analysis, infection attributable standardized mortality ratio (SMR) in SLE was 4.98 compared to all cause SMR of 3.14 [56]. Nearly a century on from the discovery of the first antibiotic, the prevention and treatment of infections remain an unmet need of care in SLE.
The strength of the present study is its large numbers, data of incidence, and spectrum of infections in SLE from a South Asian cohort which has been lacking in the past. The limitations include the retrospective nature of the study and a survivorship bias in the cohort as evidenced by the greater loss to follow-up seen in the group without serious infection. The other major limitation is patients lost to follow-up cases have been censored in the survival analysis as we could not contact all patients to determine present status.
We also did not have data on damage prior to serious infection and this could be a potential confounder for the higher damage scores at the end of follow-up in those with serious infections.
Conclusion
Serious infections remain a major cause of mortality and damage accrual in SLE. The profile of infections is different in South Asian patients with a predominance of tuberculosis especially extrapulmonary forms. Higher disease activity at baseline, gastrointestinal involvement, hypoalbuminemia, higher current steroid dose, and cumulative steroid dose increase the chances of serious infections.
Supplementary information
Below is the link to the electronic supplementary material.
Data availability
The original data will be available on request sent by email to the corresponding author.
Declarations
Disclosures
None.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study was previously presented as an oral abstract in the American College of Rheumatology annual meeting (ACR convergence 2022) and was subsequently published as an abstract with the following bibliographic information: Chatterjee R, Pattanaik S, Misra D, Agarwal V, Lawrence A, Aggarwal A. Serious Infections in SLE- Incidence, Associated Factors, Impact and Trends over Two Decades (abstract). Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/serious-infections-in-sle-incidence-associated-factors-impact-and-trends-over-two-decades/. Accessed December 11, 2022.
References
- 1.Urowitz MB, Bookman AA, Koehler BE, Gordon DA, Smythe HA, Ogryzlo MA. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med. 1976;60(2):221–225. doi: 10.1016/0002-9343(76)90431-9. [DOI] [PubMed] [Google Scholar]
- 2.Biswas D, Mathias A, Dayal R, Aggarwal A, Misra R, Naik S. Presence of antibodies to SSB/La is associated with decreased phagocytic efficiency of neutrophils in patients with systemic lupus erythematosus. Clin Rheumatol. 2008;27(6):717–722. doi: 10.1007/s10067-007-0776-x. [DOI] [PubMed] [Google Scholar]
- 3.Cuchacovich R, Gedalia A. Pathophysiology and clinical spectrum of infections in systemic lupus erythematosus. Rheum Dis Clin North Am. 2009;35(1):75–93. doi: 10.1016/j.rdc.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Rúa-Figueroa I, Nóvoa J, García-Laorden MI, Erausquin C, García-Bello M, de Castro FR, Herrera-Ramos E, Ojeda S, Quevedo JC, Francisco F, Naranjo A, Rodríguez-Lozano C, Rodríguez-Gallego C. Clinical and immunogenetic factors associated with pneumonia in patients with systemic lupus erythematosus: a case-control study. J Rheumatol. 2014;41(9):1801–1807. doi: 10.3899/jrheum.131470. [DOI] [PubMed] [Google Scholar]
- 5.Goldblatt F, Chambers S, Rahman A, Isenberg DA. Serious infections in British patients with systemic lupus erythematosus: hospitalisations and mortality. Lupus. 2009;18(8):682–689. doi: 10.1177/0961203308101019. [DOI] [PubMed] [Google Scholar]
- 6.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, Mejía JC, Aydintug AO, Chwalinska-Sadowska H, de Ramón E, Fernández-Nebro A, Galeazzi M, Valen M, Mathieu A, Houssiau F, Caro N, Alba P, Ramos-Casals M, Ingelmo M, European Working Party on Systemic Lupus Erythematosus (2003) Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine 82(5):299–308. 10.1097/01.md.0000091181.93122.55 [DOI] [PubMed]
- 7.Noël V, Lortholary O, Casassus P, Cohen P, Généreau T, André MH, Mouthon L, Guillevin L. Risk factors and prognostic influence of infection in a single cohort of 87 adults with systemic lupus erythematosus. Ann Rheum Dis. 2001;60(12):1141–1144. doi: 10.1136/ard.60.12.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobsen S, Petersen J, Ullman S, Junker P, Voss A, Rasmussen JM, Tarp U, Poulsen LH, van Overeem Hansen G, Skaarup B, Hansen TM, Pødenphant J, Halberg P. A multicentre study of 513 Danish patients with systemic lupus erythematosus. II. Disease mortality and clinical factors of prognostic value. Clin Rheumatol. 1998;17(6):478–484. doi: 10.1007/BF01451283. [DOI] [PubMed] [Google Scholar]
- 9.Zhao K, Xie H, Li L, Esdaile JM, Aviña-Zubieta JA. Increased risk of severe infections and mortality in patients with newly diagnosed systemic lupus erythematosus: a population-based study. Rheumatology (Oxford) 2021;60(11):5300–5309. doi: 10.1093/rheumatology/keab219. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Zhou Y, Yu L, Wu W, Zhao L, Geng S, Sun F, Zhang D, Shen N, Chen Y, Ye S. Major infections in newly diagnosed systemic lupus erythematosus: an inception cohort study. Lupus Sci Med. 2022;9(1):e000725. doi: 10.1136/lupus-2022-000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aggarwal A, Phatak S, Srivastava P, Lawrence A, Agarwal V, Misra R. Outcomes in juvenile onset lupus: single center cohort from a developing country. Lupus. 2018;27(11):1867–1875. doi: 10.1177/0961203318791046. [DOI] [PubMed] [Google Scholar]
- 12.Pattanaik SS, Muhammed H, Chatterjee R, Naveen R, Lawrence A, Agarwal V, Misra DP, Gupta L, Misra R, Aggarwal A. In-hospital mortality and its predictors in a cohort of SLE from Northern India. Lupus. 2020;29(14):1971–1977. doi: 10.1177/0961203320961474. [DOI] [PubMed] [Google Scholar]
- 13.Gladman DD, Hussain F, Ibañez D, Urowitz MB. The nature and outcome of infection in systemic lupus erythematosus. Lupus. 2002;11(4):234–239. doi: 10.1191/0961203302lu170oa. [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Xie J, Chen H, Yang Y, Zhan Z, Liang L, Yang X. Infection in southern Chinese patients with systemic lupus erythematosus: spectrum, drug resistance, outcomes, and risk factors. J Rheumatol. 2016;43(9):1650–1656. doi: 10.3899/jrheum.151523. [DOI] [PubMed] [Google Scholar]
- 15.Rúa-Figueroa Í, López-Longo J, Galindo-Izquierdo M, Calvo-Alén J, Del Campo V, Olivé-Marqués A, Pérez-Vicente S, Fernández-Nebro A, Andrés M, Erausquin C, Tomero E, Horcada L, Uriarte E, Freire M, Montilla C, Sánchez-Atrio A, Santos G, Boteanu A, Díez-Álvarez E, Pego-Reigosa JM. Incidence, associated factors and clinical impact of severe infections in a large, multicentric cohort of patients with systemic lupus erythematosus. Semin Arthritis Rheum. 2017;47(1):38–45. doi: 10.1016/j.semarthrit.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Dubula T, Mody GM. Spectrum of infections and outcome among hospitalized South Africans with systemic lupus erythematosus. Clin Rheumatol. 2015;34(3):479–488. doi: 10.1007/s10067-014-2847-0. [DOI] [PubMed] [Google Scholar]
- 17.Oku K, Hamijoyo L, Kasitanon N, Li MT, Navarra S, Morand E, Tanaka Y, Mok CC. Prevention of infective complications in systemic lupus erythematosus: a systematic literature review for the APLAR consensus statements. Int J Rheum Dis. 2021;24(7):880–895. doi: 10.1111/1756-185X.14125. [DOI] [PubMed] [Google Scholar]
- 18.Amorim LDAF, Cai J. Modelling recurrent events: A tutorial for analysis in epidemiology. Int J Epidemiol. 2015;44(1):324–333. doi: 10.1093/ije/dyu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen PK, Keiding N. Multi-state models for event history analysis. Stat Methods Med Res. 2002;11(2):91–115. doi: 10.1191/0962280202SM276ra. [DOI] [PubMed] [Google Scholar]
- 20.Ko T, Koelmeyer R, Li N, Yap K, Yeo AL, Kent J, Pellicano R, Golder V, Kitching AR, Morand E, Hoi A. Predictors of infection requiring hospitalization in patients with systemic lupus erythematosus: a time-to-event analysis. Sem Arthritis Rheum. 2022;57:152099. doi: 10.1016/j.semarthrit.2022.152099. [DOI] [PubMed] [Google Scholar]
- 21.Sirobhushanam S, Parsa N, Reed TJ, Berthier CC, Sarkar MK, Hile GA, Tsoi LC, Banfield J, Dobry C, Horswill AR, Gudjonsson JE, Kahlenberg JM. Staphylococcus aureus colonization is increased on lupus skin lesions and is promoted by IFN-mediated barrier disruption. J Invest Dermatol. 2020;140(5):1066–1074.e4. doi: 10.1016/j.jid.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terui H, Yamasaki K, Wada-Irimada M, Onodera-Amagai M, Hatchome N, Mizuashi M, Yamashita R, Kawabe T, Ishii N, Abe T, Asano Y, Aiba S. Staphylococcus aureus skin colonization promotes SLE-like autoimmune inflammation via neutrophil activation and the IL-23/IL-17 axis. Sci Immunol. 2022;7(76):eabm9811. doi: 10.1126/sciimmunol.abm9811. [DOI] [PubMed] [Google Scholar]
- 23.Luijten RKMAC, Cuppen BVJ, Bijlsma JWJ, Derksen RHWM. Serious infections in systemic lupus erythematosus with a focus on pneumococcal infections. Lupus. 2014;23(14):1512–1516. doi: 10.1177/0961203314543918. [DOI] [PubMed] [Google Scholar]
- 24.Schurder J, Goulenok T, Jouenne R, Dossier A, Van Gysel D, Papo T, Sacre K. Pneumococcal infection in patients with systemic lupus erythematosus. Joint Bone Spine. 2018;85(3):333–336. doi: 10.1016/j.jbspin.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Merayo-Chalico J, Gómez-Martín D, Piñeirúa-Menéndez A, Santana-De Anda K, Alcocer-Varela J. Lymphopenia as risk factor for development of severe infections in patients with systemic lupus erythematosus: a case-control study. QJM: Mon J Assoc Physicians. 2013;106(5):451–457. doi: 10.1093/qjmed/hct046. [DOI] [PubMed] [Google Scholar]
- 26.Global tuberculosis report 2021 (2021) Geneva: World Health Organization. https://www.who.int/publications/i/item/9789240037021. Accessed 29 Jan 2023
- 27.Victorio-Navarra ST, Dy EE, Arroyo CG, Torralba TP. Tuberculosis among Filipino patients with systemic lupus erythematosus. Semin Arthritis Rheum. 1996;26(3):628–634. doi: 10.1016/s0049-0172(96)80013-8. [DOI] [PubMed] [Google Scholar]
- 28.Feng PH, Tan TH. Tuberculosis in patients with systemic lupus erythematosus. Ann Rheum Dis. 1982;41(1):11–14. doi: 10.1136/ard.41.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shyam C, Malaviya AN. Infection-related morbidity in systemic lupus erythematosus: a clinico-epidemiological study from northern India. Rheumatol Int. 1996;16(1):1–3. doi: 10.1007/BF01419946. [DOI] [PubMed] [Google Scholar]
- 30.Tam L-S, Li EK, Wong S-M, Szeto C-C. Risk factors and clinical features for tuberculosis among patients with systemic lupus erythematosus in Hong Kong. Scand J Rheumatol. 2002;31(5):296–300. doi: 10.1080/030097402760375205. [DOI] [PubMed] [Google Scholar]
- 31.Sayarlioglu M, Inanc M, Kamali S, Cefle A, Karaman O, Gul A, Ocal L, Aral O, Konice M. Tuberculosis in Turkish patients with systemic lupus erythematosus: increased frequency of extrapulmonary localization. Lupus. 2004;13(4):274–278. doi: 10.1191/0961203303lu529xx. [DOI] [PubMed] [Google Scholar]
- 32.Hou C-L, Tsai Y-C, Chen L-C, Huang J-L. Tuberculosis infection in patients with systemic lupus erythematosus: pulmonary and extra-pulmonary infection compared. Clin Rheumatol. 2008;27(5):557–563. doi: 10.1007/s10067-007-0741-8. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Thumboo J, Tan BH, Tan TT, Fong CHJ, Ng HS, Fong KY. The risk of tuberculosis in SLE patients from an Asian tertiary hospital. Rheumatol Int. 2017;37(6):1027–1033. doi: 10.1007/s00296-017-3696-3. [DOI] [PubMed] [Google Scholar]
- 34.Lao M, Chen D, Wu X, Chen H, Qiu Q, Yang X, Zhan Z. Active tuberculosis in patients with systemic lupus erythematosus from Southern China: a retrospective study. Clin Rheumatol. 2019;38(2):535–543. doi: 10.1007/s10067-018-4303-z. [DOI] [PubMed] [Google Scholar]
- 35.Long W, Cai F, Wang X, Zheng N, Wu R. High risk of activation of latent tuberculosis infection in rheumatic disease patients. Infect Dis (London, England) 2020;52(2):80–86. doi: 10.1080/23744235.2019.1682187. [DOI] [PubMed] [Google Scholar]
- 36.Beydon M, Nicaise-Roland P, Mageau A, Farkh C, Daugas E, Descamps V, Dieude P, Dossier A, Goulenok T, Farhi F, Mutuon P, Timsit J-F, Papo T, Sacre K. Autoantibodies against IFNα in patients with systemic lupus erythematosus and susceptibility for infection: a retrospective case-control study. Sci Rep. 2022;12(1):11244. doi: 10.1038/s41598-022-15508-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaitonde S. Efficacy of isoniazid prophylaxis in patients with systemic lupus erythematosus receiving long term steroid treatment. Ann Rheum Dis. 2002;61(3):251–253. doi: 10.1136/ard.61.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma L, Lin B, Wang L, Wang D, Li G, Wang G. Preventive therapy for iatrogenic active tuberculosis in systemic lupus erythematosus patients. Zhonghua Yi Xue Za Zhi. 2014;94(45):3579–3582. [PubMed] [Google Scholar]
- 39.Takizawa Y, Inokuma S, Tanaka Y, Saito K, Atsumi T, Hirakata M, Kameda H, Hirohata S, Kondo H, Kumagai S, Tanaka Y. Clinical characteristics of cytomegalovirus infection in rheumatic diseases: multicentre survey in a large patient population. Rheumatology (Oxford) 2008;47(9):1373–1378. doi: 10.1093/rheumatology/ken231. [DOI] [PubMed] [Google Scholar]
- 40.Wang LR, Barber CE, Johnson AS, Barnabe C. Invasive fungal disease in systemic lupus erythematosus: a systematic review of disease characteristics, risk factors, and prognosis. Semin Arthritis Rheum. 2014;44(3):325–330. doi: 10.1016/j.semarthrit.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Molooghi K, Sheybani F, Naderi H, Mirfeizi Z, Morovatdar N, Baradaran A. Central nervous system infections in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Lupus Sci Med. 2022;9(1):e000560. doi: 10.1136/lupus-2021-000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tejera Segura B, Rua-Figueroa I, Pego-Reigosa JM, Del Campo V, Wincup C, Isenberg D, Rahman A. Can we validate a clinical score to predict the risk of severe infection in patients with systemic lupus erythematosus? A longitudinal retrospective study in a British Cohort. BMJ Open. 2019;9(6):e028697. doi: 10.1136/bmjopen-2018-028697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yuan Q, Xing X, Lu Z, Li X. Clinical characteristics and risk factors of infection in patients with systemic lupus erythematosus: a systematic review and meta-analysis of observational studies. Semin Arthritis Rheum. 2020;50(5):1022–1039. doi: 10.1016/j.semarthrit.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Prata AR, Luís M, Assunção H, da Silva JAP, Inês LS. Antimalarial treatment and minimizing prednisolone are associated with lower risk of infection in SLE: a 24-month prospective cohort study. Clin Rheumatol. 2022;41(4):1069–1078. doi: 10.1007/s10067-021-05988-x. [DOI] [PubMed] [Google Scholar]
- 45.Simard JF, Rossides M, Gunnarsson I, Svenungsson E, Arkema EV. Infection hospitalisation in systemic lupus in Sweden. Lupus Sci Med. 2021;8(1):e000510. doi: 10.1136/lupus-2021-000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abe K, Ishikawa Y, Kita Y, Yajima N, Inoue E, Sada K, Miyawaki Y, Yoshimi R, Shimojima Y, Ohno S, Kajiyama H, Ichinose K, Sato S, Fujiwara M. Association of low-dose glucocorticoid use and infection occurrence in systemic lupus erythematosus patients: a prospective cohort study. Arthritis Res Ther. 2022;24(1):179. doi: 10.1186/s13075-022-02869-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh JA, Cleveland JD. Hospitalized infections in lupus: a nationwide study of types of infections, time trends, health care utilization, and in-hospital mortality. Arthritis Rheumatol (Hoboken, N.J.) 2021;73(4):617–630. doi: 10.1002/art.41577. [DOI] [PubMed] [Google Scholar]
- 48.Lorenzo-Vizcaya A, Isenberg D. Analysis of trends and causes of death in SLE patients over a 40-years period in a cohort of patients in the United Kingdom. Lupus. 2021;30(5):702–706. doi: 10.1177/0961203320988607. [DOI] [PubMed] [Google Scholar]
- 49.Sutcliffe N, Clarke AE, Taylor R, Frost C, Isenberg DA. Total costs and predictors of costs in patients with systemic lupus erythematosus. Rheumatology (Oxford) 2001;40(1):37–47. doi: 10.1093/rheumatology/40.1.37. [DOI] [PubMed] [Google Scholar]
- 50.Jönsen A, Bengtsson AA, Hjalte F, Petersson IF, Willim M, Nived O. Total cost and cost predictors in systemic lupus erythematosus-8-years follow-up of a Swedish inception cohort. Lupus. 2015;24(12):1248–1256. doi: 10.1177/0961203315584812. [DOI] [PubMed] [Google Scholar]
- 51.Al Sawah S, Daly RP, Foster SA, Naegeli AN, Benjamin K, Doll H, Bond G, Moshkovich O, Alarcón GS. The caregiver burden in lupus: findings from UNVEIL, a national online lupus survey in the United States. Lupus. 2017;26(1):54–61. doi: 10.1177/0961203316651743. [DOI] [PubMed] [Google Scholar]
- 52.Costa-Reis P, Nativ S, Isgro J, Rodrigues T, Yildirim-Toruner C, Starr A, Saiman L, Imundo L, Eichenfield A. Major infections in a cohort of 120 patients with juvenile-onset systemic lupus erythematosus. Clin Immunol (Orlando, Fla.) 2013;149(3):442–449. doi: 10.1016/j.clim.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Moreno-Torres V, Martínez-Urbistondo M, Gutiérrez-Rojas A, Castejón R, Sánchez E, Calderón-Parra J, Durán-del Campo P, Tutor P, Mellor-Pita S, Vázquez-Comendador J, Vargas-Núñez JA, Ruiz-Irastorza G. Impact of severe infections in SLE: an observational study from the Spanish national registry. Lupus Sci Med. 2022;9(1):e000711. doi: 10.1136/lupus-2022-000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watson P, Brennan A, Birch H, Fang H, Petri M. An integrated extrapolation of long-term outcomes in systemic lupus erythematosus: analysis and simulation of the Hopkins lupus cohort. Rheumatology (Oxford) 2015;54(4):623–632. doi: 10.1093/rheumatology/keu375. [DOI] [PubMed] [Google Scholar]
- 55.Kedves M, Kósa F, Kunovszki P, Takács P, Szabó MZ, Karyekar C, Lofland JH, Nagy G. Large-scale mortality gap between SLE and control population is associated with increased infection-related mortality in lupus. Rheumatology (Oxford) 2020;59(11):3443–3451. doi: 10.1093/rheumatology/keaa188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee YH, Choi SJ, Ji JD, Song GG. Overall and cause-specific mortality in systemic lupus erythematosus: an updated meta-analysis. Lupus. 2016;25(7):727–734. doi: 10.1177/0961203315627202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original data will be available on request sent by email to the corresponding author.