Abstract
The platform wound device (PWD) is a wound coverage system that is designed to decrease wound infection rates by allowing for direct delivery of topical antibiotics and antimicrobials while creating a sealed, protective barrier around the area of injury. This study evaluated the safety and efficacy of the PWD as a protective dressing and a delivery system for topical antibiotics compared to the current standard of care (SoC). This was a multi‐center, prospective, randomised, controlled clinical trial. The wounds were treated with the PWD with gentamicin cream or SoC dressings. The wounds were evaluated before the start of treatment and after 48–96 hours via clinical assessment, photographs, and qualitative bacterial swabs for bacterial analysis. The delivery of gentamicin via the PWD was safe and did not cause any adverse effects. The treatment decreased both inflammation and bacterial growth during the study period. No significant differences in the SoC were observed. The PWD is a transparent and impermeable polyurethane chamber that encloses and protects the injured area. The delivery of topical gentamicin via the PWD was safe and effective. Clinical assessment for infection found the PWD to be non‐inferior to the current SoC treatment options.
Keywords: gentamicin cream, platform wound device, topical treatment, wound healing, wound infection
1. INTRODUCTION
Intact skin is a strong, protective barrier that serves as the first line defence against infectious microorganisms. When there is a breakdown of this barrier, microbes have a clear path of entrance, and the ability to cause infection. 1 Wound infections are highly variable and the choice of treatment after diagnosis of infection is dependent on the type of infecting microorganisms, wound, depth, location of the wound systemic symptoms, any underlying causes, and patient comorbidities. 2 Debridement of necrotic tissue and/or drainage of deeper infections is integral to infection control. 3 Wound culture/biopsy and identification of bacteria with traditional cultures or DNA techniques and sensitivities are important for antibiotic determination. 4 Immediate, broad‐spectrum, empiric therapy is often necessary with antimicrobial narrowing after the microorganism(s) have been identified. Wound infections can usually be treated with outpatient oral or topical antibiotics, or they may require inpatient, intensive treatment with IV antibiotics. Ideally, the prevention of wound infection is the best form of treatment. 5
This study evaluated a new wound coverage device that aims to decrease wound infection rates by allowing for direct delivery of topical antibiotics while creating a sealed, protective barrier enclosing the wound. The Platform Wound Device (PWD) (Applied Tissue Technologies LLC, Hingham, MA) is a transparent and impermeable polyurethane chamber that encloses and protects the entire injured area. The PWD seals to the intact skin surrounding the wound and the flexibility of the membrane allows for conformation of the device to anatomic contours helping to maintain its protective barrier function. 6 It acts as both a protective dressing and a delivery system for topical application of drugs such as antibiotics and analgesics to prevent infection, reduce pain, and promote wound healing. The PWD is embossed on the skin‐facing side with a pattern of small pyramids that promote even distribution of liquid or hydrogel‐formulated medications. This allows for the precise delivery of topical medications directly to the wound. 7
The PWD is applied after initial wound irrigation and debridement similar to current standard of care (SoC) treatments such as negative pressure wound therapy (NPWT) and wet‐to‐dry dressings. The device weighs only a few ounces and has a shelf life of many years after sterilisation, making it portable and cost‐effective. The PWD is available in multiple shapes/sizes, including full extremity devices, allowing for coverage of almost any wound encountered. The transparent membrane enables continued visualisation and evaluation of the wound without removing the device. The ability to leave the device in place for up to 7 days decreases the number of interventions and cost of treatment. 8
The purpose of this prospective, randomised, controlled study was to investigate the safety, efficacy, and feasibility of the use of the PWD with a topical gentamicin cream for the treatment of infected wounds. The treatment was compared to current SoC. This was a multi‐center, clinical study conducted across 4 hospitals in the San Antonio, TX area. The wounds were treated with the PWD with gentamicin cream or a physician‐specified SoC treatment. Infection was evaluated clinically, based on physical exam findings, and with a qualitative scoring system, based on wound swab cultures before and after treatment. Follow‐up ranged from 48 to 96 hours dependent on clinical status. Changes in exam findings and bacterial culture score after treatment were used for analysis.
2. MATERIAL AND METHODS
2.1. Patient selection
This study was approved by the IntegReview Institutional Review Board (IRB No. PWD‐01), the Office of Human Research Protection (HRPO) (A‐20446.b), and was registered at clinicaltrials.gov (NCT04753723). All procedures were performed in accordance with the relevant guidelines and regulations of these centers and all patients enrolled in the study gave written informed consent prior to enrollment. Forty‐seven patients, 18–85 years of age with open wounds admitted to Baptist Medical Center, Northeast Baptist, North Central Baptist, and Mission Trail Baptist in San Antonio, TX, were recruited for participation in this study. The inclusion and exclusion criteria for patient participation are presented in Table 1.
TABLE 1.
Exclusion/inclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
All subjects enrolled must meet all the following criteria:
|
Subjects who meet any of the following criteria will be excluded from the study:
|
2.2. Study design
The participants were randomised, using a computer‐generated randomization schedule, to receive either the study treatment or SoC specified by the wound care provider. The study dressing, PWD (Applied Tissue Technologies LLC, Hingham, MA), is a sterile transparent polyurethane enclosure that was used as a protective dressing and for topical delivery of 0.1% gentamicin sulfate cream (Perrigo, Dublin, Ireland). Three types of PWDs were utilised in the study. A single application of the gentamicin cream was applied to the wounds and the appropriate‐sized PWD was placed over the wound (Figure 1). Subsequently, the PWD was left in place for at least 48 and up to 96 hours. The SoC treatment included but was not limited to, Manuka honey, acetic acid, xeroform, microcin, NPWT as determined by the wound care provider (Table 2). SoC dressings were changed per wound care providers' recommendations.
FIGURE 1.
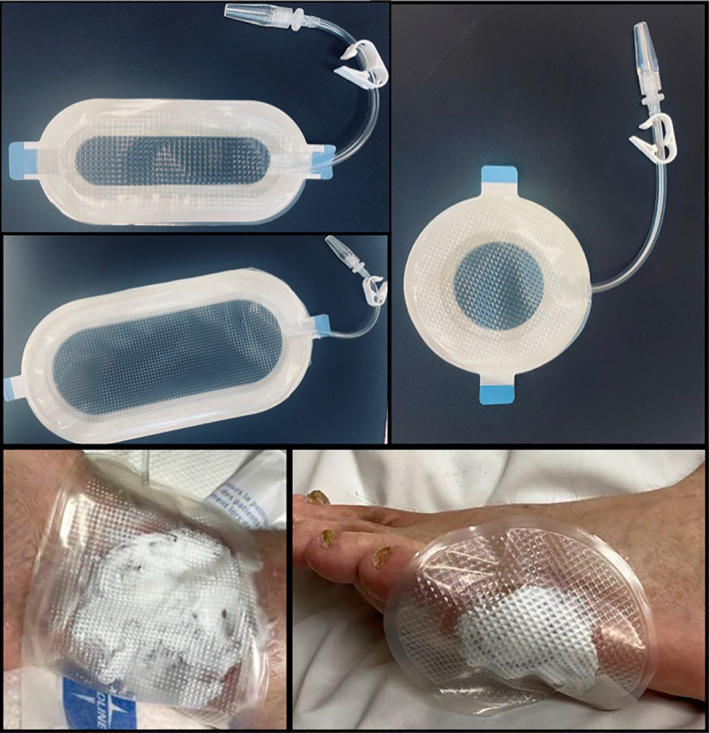
Different shapes of PWD, including circular, oblong, and large oblong. Lower images show PWD covering wounds during the treatment
TABLE 2.
Patient demographics
| Subject # | Age | Gender | Race/Ethnicity | Treatment | MOI |
|---|---|---|---|---|---|
| 1 | 53 | M | Hispanic | SoC (Dakins) | Pressure |
| 2 | 57 | M | Hispanic | SoC (NPWT) | Pressure |
| 3 | 55 | M | Hispanic | PWD | Soft tissue infection, Cellulitis, Necrotic Fasciitis |
| 4 | 54 | M | Caucasian | PWD | Diabetic foot ulcer |
| 5 | 61 | M | Hispanic | SoC (Dakins) | Pressure |
| 6 | 31 | M | Hispanic | PWD | Old gunshot wound reopened |
| 7 | 35 | M | Caucasian | SoC (Manuka Honey) | Unknown |
| 8 | 48 | M | Pacific Islander | SoC (Wet to dry) | Pressure |
| 9 | 68 | M | Caucasian | PWD | Pressure |
| 10 | 74 | M | Caucasian | PWD | Dehisced incision |
| 11 | 74 | M | Caucasian | SoC (NPWT) | Abscess, infection then pressure |
| 12 | 82 | M | Hispanic | PWD | Diabetic foot ulcer |
| 13 | 75 | M | Caucasian | SoC (Wet to dry) | Abscess |
| 14 | 41 | M | Hispanic | SoC (Vashe, Wet to dry) | Spider bite, gangrene |
| 15 | 61 | M | Hispanic | PWD | Non‐healing ulcer |
| 16 | 61 | M | Hispanic | SoC (Microcyn, Wet to dry) | Non‐healing ulcer |
| 17 | 25 | M | Black | PWD | Pressure |
| 18 | 25 | M | Black | SoC (Xeroform) | Pressure |
| 19 | 61 | F | Hispanic | SoC (Wet to dry) | IV infiltrate |
| 20 | 38 | F | Hispanic | PWD | Pressure |
| 21 | 68 | F | Hispanic | PWD | Non‐healing surgical wound with joint infection |
| 22 | 70 | M | Hispanic | SoC (NPWT) | Pressure |
| 23 | 66 | F | Hispanic | PWD | Pressure |
| 24 | 68 | F | Caucasian | SoC (NPWT) | Pressure |
| 25 | 68 | F | Caucasian | PWD | Pressure |
| 26 | 68 | F | Caucasian | PWD | Pressure |
| 27 | 68 | F | Caucasian | PWD | Pressure |
| 28 | 68 | F | Caucasian | PWD | Peg tube wound |
| 29 | 74 | F | Caucasian | SoC (Wet to dry) | Pressure |
| 30 | 74 | F | Caucasian | SoC (Wet to dry) | Pressure |
| 31 | 45 | M | Hispanic | SoC (NPWT) | Gunshot wound infection |
| 32 | 52 | M | Hispanic | SoC (Xeroform) | Burn |
| 33 | 52 | M | Hispanic | SoC (Microcyn) | Diabetic Ulcer |
| 34 | 64 | M | Hispanic | SoC (NPWT) | Pressure |
| 35 | 59 | F | Hispanic | PWD | Abscess |
| 36 | 52 | M | Caucasian | SoC (Dakins) | Pyoderma gangrenosum |
| 37 | 52 | M | Caucasian | SoC (Dakins) | Pyoderma gangrenosum |
| 38 | 52 | M | Caucasian | PWD | Pyoderma gangrenosum |
| 39 | 52 | M | Caucasian | PWD | Pyoderma gangrenosum |
| 40 | 52 | M | Caucasian | PWD | Pyoderma gangrenosum |
| 41 | 40 | F | Caucasian | SoC (Wet to dry) | Infected incision site |
| 42 | 70 | F | Caucasian | SoC (NPWT) | Recurrent infected incision |
| 43 | 70 | M | Black | SoC (Acetic acid) | Cellulitis |
| 44 | 70 | M | Black | SoC (Acetic acid) | Cellulitis |
| 45 | 67 | M | Hispanic | PWD | Rubbing from prosthetic |
| 46 | 56 | M | Caucasian | PWD | Pressure |
| 47 | 79 | F | Caucasian | SoC (NPWT) | Infected surgical incision |
| 48 | 54 | M | Caucasian | SoC | Radiation |
On day 0, prior to treatment with either the PWD + gentamicin cream or the SoC, the wounds were photographed and assessed macroscopically. In addition, wound swabs were collected for bacteriological analysis. The dressings were removed on days 2–4 based on clinical assessment and plan of care as determined by the wound care providers. After the dressing removal the wounds were again photographed assessed macroscopically and swabs were collected for bacteriological analysis.
2.3. Assessments
2.3.1. Digital photography
Standard digital photographs (Nikon Corp., Tokyo, Japan) to document wound progression were obtained before, during and after initiation of the treatment.
2.3.2. Macroscopical assessments of wound inflammatory characteristics
Clinical evaluations of the following wound characteristics were performed by the research nurse at baseline and follow‐up for each subject: erythema, swelling, warmth, discharge, and odour. The average change in each wound characteristic from baseline to follow‐up was calculated and the status of the wound at baseline and at follow‐up was compared. If the status was improved (Baseline YES; Follow‐up NO), it was given a score 1, whereas if no improvement (Baseline YES; Follow‐up YES) was noticed the wound was given a score 0.
2.3.3. Bacteriological analysis of wound swabs
Wound culture swabs were taken prior to initial dressing application on day 0 (baseline), and immediately after dressing removal 2–4 days later (follow‐up) for bacterial growth analysis. The samples were scored for bacterial growth for each bacterial species found in the swab according to the following scale: 0 = No growth, 1 = Very light growth, 2 = Light growth, 3 = Moderate growth, and 4 = Heavy growth.
For each wound, the sum of all the scores for each bacterial species found in the swab culture was calculated before dressing application (baseline score) and after dressing removal (follow‐up score). The difference between these two totals was calculated to obtain the total change in bacterial growth from baseline to follow‐up for each wound (change in bacterial growth = baseline total − follow‐up total). The wounds without baseline or follow‐up swab culture data were excluded from the analysis. The average of all the baseline totals, follow‐up totals, and the average change in bacterial growth was then calculated. Subsequently, the baseline total bacterial scores, the follow‐up total bacterial scores, and the change in bacterial growth were compared between the PWD group and the SoC group. A sub‐analysis comparing the PWD group to a subgroup of the SoC patients treated with NPWT was also performed using the same calculations as described above.
The change in growth of individual bacterial species was also examined. The bacteria included in this sub‐analysis were corynebacterium spp., staphylococcus non‐aureus spp., VRE faecalis, and MRSA. Due to limited subjects, no calculations of significance were able to be conducted.
2.3.4. Statistical analysis
Statistical analysis was carried out using GraphPad Prism (Graph Pad Software, Inc. La Jolla, CA). For the bacterial growth analysis, a two‐tailed Wilcoxon signed‐rank test at a significance level of 0.05 was performed to evaluate for a significant change from baseline bacterial growth scores to follow‐up bacterial growth scores for the PWD group. This was also performed for the SoC group. A two‐tailed Wilcoxon rank‐sum test at a significance level of 0.05 was performed comparing the PWD group and the SoC group to evaluate for a significant difference in the average change in bacterial growth from baseline to follow‐up. This was also used for the sub‐analysis comparing the PWD group to the NPWT subgroup. For the macroscopical wound assessments, the statistical significance was determined using unpaired t tests and an α level of 0.05 was used for all statistical tests. All data is presented as mean ± SEM.
3. RESULTS
3.1. Patient Demographics
A total of 48 patients were enrolled between March 2021 and November 2021. Of these patients, 45 patients completed the study (93%). The majority of patients were male (33)/female (15) (70%). Median patient age at time of enrollment was 61 years old. The most common mechanisms of injury were pressure ulcer (38%) and post‐operative incision infection (14%) respectively. Patient demographics are presented in Table 2.
3.2. Wound inflammatory characteristics
Figure 2A‐E shows wound inflammatory characteristics (erythema, swelling, warmth, discharge, or odour) assessment comparison before and after the treatment. Improvement was seen in all characteristics after treatment with both SoC and PWD. However, only one statistically significant difference was observed in the SoC‐treated wounds in terms of discharge (P < 0.05). The results also demonstrated that 15% of the PWD treated and 27% of the SoC‐treated wounds had an improved status in erythema, 30% and 42% in swelling, 100% and 100% in warmth, and 41% and 38% in discharge (Figure 2).
FIGURE 2.
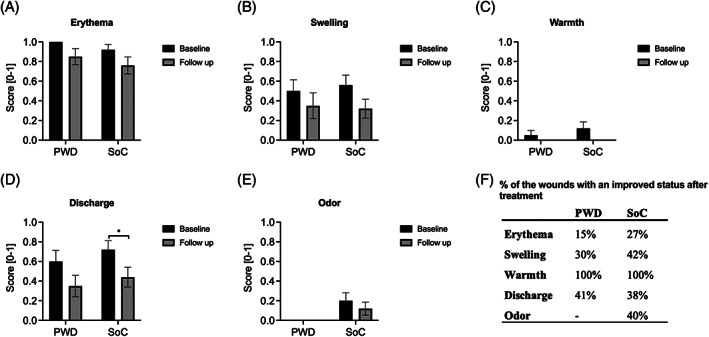
Wound inflammatory characteristics. A‐E, Comparing the average presence of wound inflammation characteristics from baseline to follow‐up. F, Comparing the percentage of improvement in each characteristic after treatment in SOC and PWD groups
3.3. Bacteriological analysis
The average bacterial growth scores for the PWD group and the SoC group before (average baseline score) and after (average follow‐up score) treatment are shown in Figure 3A. A decrease was found between baseline bacterial growth scores and follow‐up bacterial growth scores for the PWD group (P = 0.09). Whereas, the average bacterial growth score for the SoC‐treated wounds increased slightly (3.4 → 3.9) over the treatment period.
FIGURE 3.
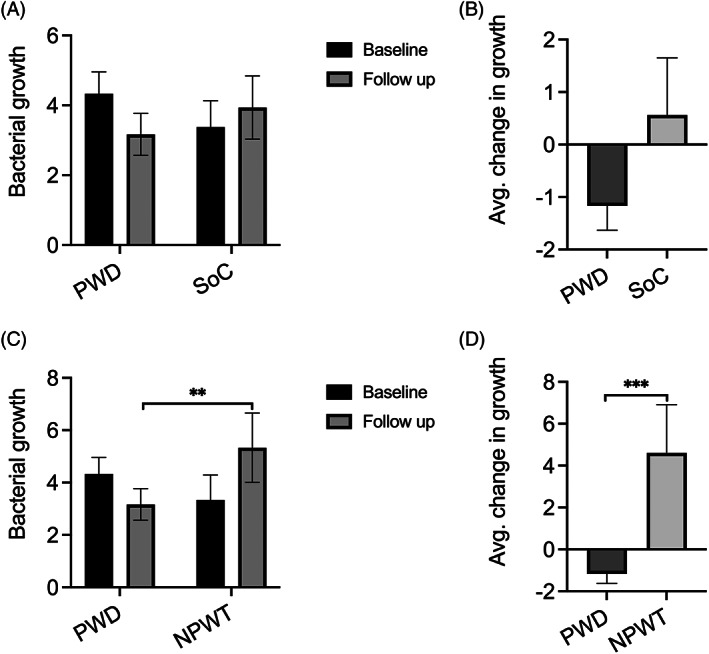
A, Comparing average bacterial growth scores at baseline and at follow‐up for the PWD group versus the SoC group. B, Comparing the average change in bacterial growth scores from baseline to follow‐up for the PWD group versus the SoC group. C, Sub‐analysis comparing average bacterial growth scores at baseline and at follow‐up for the PWD group versus the NPWT subgroup. D, Sub‐analysis comparing the average change in bacterial growth scores from baseline to follow‐up for PWD group versus NPWT subgroup
The average change in bacterial growth from baseline to follow‐up was also calculated for the PWD group and the SoC group (Figure 3B). The comparison of the two is shown in Figure 3B below. The results showed that on average bacterial growth score in the PWD‐treated wounds decreased by 1.2 points and in the SoC‐treated wounds it increased by 0.6. Comparison of the average change in bacterial growth from baseline to follow‐up between the PWD group and the SoC group showed no significant difference (P = 0.14).
In addition, the average bacterial growth scores before and after treatment and the average change in bacterial growth from baseline to follow‐up for the NPWT‐treated wounds were determined. Comparison of the NPWT subgroup to the PWD group is shown in Figure 3C, D respectively. A significant difference between the two groups in the average growth scores was observed at follow‐up showing that the PWD + gentamicin decreased bacterial growth more efficiently (P = 0.004). The results also showed that the average bacterial growth score in the PWD‐treated wounds decreased 1.2 points and in the SoC‐treated wounds it increased by 4.2 (P = 0.0007) (Figure 3D).
3.3.1. Bacterial species sub‐analysis
Change in corynebacterium spp. was evaluated between baseline and follow‐up cultures for PWD and SoC. For the 2 subjects treated with SoC that initially grew corynebacterium spp. there was no change in bacterial growth seen in follow‐up cultures. For all 6 subjects treated with PWD that initially grew corynebacterium spp. complete eradication was seen in all 6 follow‐up cultures. For the 5 subjects treated with SoC that grew staphylococcus non‐aureus spp. in the initial cultures, 2 follow‐up cultures showed no change in bacterial growth, 1 follow‐up culture showed increased growth, and the final two showed decreased growth. For the 5 subjects treated with PWD that grew staphylococcus non‐aureus spp. in the initial cultures, 4 follow‐up cultures showed complete eradication, and the final culture showed decreased growth. No SoC subjects grew VRE faecalis or MRSA on initial culture. For the 2 subjects treated with PWD that grew VRE faecalis and the 3 subjects treated with PWD that grew MRSA, no change was seen in the follow‐up cultures for both VRE faecalis and MRSA.
3.4. Usability and adverse events
The PWD was found to be easy‐to‐use. The transparency of the material allowed wound observation without dressing removal. No adverse events were reported when used in combination with the gentamicin cream.
4. DISCUSSION
Wound infections are difficult and expensive to treat. They increase patient morbidity and mortality, lead to numerous complications, and prolong hospital length of stay. 9 The impact of a wound infection depends greatly on the type of wound and individual patient factors. One study attributed a relative risk of death of 2.2 to surgical site infections when compared to matched surgical patients without infection. 10 In burn wounds, infection is the most common cause of morbidity and mortality, with death rates secondary to infection approaching 61%. 11 , 12 The current SoC in treating infected wounds involves cleansing, wound bed preparation, and intervention with topical antimicrobials and/or intravenous (IV) antibiotics. Without prompt and effective treatment, many wounds will develop an invasive infection that, in the case of extremities, increases the risk of amputation. 13 , 14 Therefore, novel and effective treatment modalities are needed to deal with wound infections.
Management of wound infection locally is attractive. When drugs are administered topically the effective drug concentration in the infected wound bed is much higher than when treating with systemic antibiotics. Furthermore, topical application of antimicrobial agents enables the administration of high concentrations of the drug without the risk of systemic side effects. 15 , 16 , 17 Consequently, several wound dressing manufacturers have incorporated antimicrobial agents into their dressings to prevent and treat infection by reducing the number of bacteria on the wound surface and within the dressing. In addition to antibiotics, the most commonly used antimicrobial agents include silver, iodine, and honey. 18
Here, we introduce a novel treatment platform for local wound management. The purpose of this clinical trial was to evaluate the safety and efficacy of the PWD together with gentamicin cream in the treatment of infected skin wounds. The PWD is a treatment system that encloses the wound allowing for topical delivery of drugs such as antibiotics. This prospective, controlled, randomised, clinical study demonstrated that the combined use of the PWD and the FDA‐approved 0.1% gentamicin cream was safe and efficient when used in the treatment of various infected wounds. A mixture of wound types was included in the study to demonstrate that this platform technology can be used in the treatment of any kind of wounds. Importantly, no adverse effects were reported and the PWD was found convenient to use by the wound care providers. Our results showed that the treatment reduced both wound inflammation characteristics and infection after 48 to 96 hours of treatment. The results also showed that single application of the gentamicin cream with the PWD decreased bacterial growth during the treatment period. However, the decrease was not statistically significant compared to the SoC treatment. In addition, our results showed that when compared to NPWT, the PWD + gentamicin treatment reduced bacterial growth statistically significantly. The biggest limitation of this study was that instead of quantitative bacteriological analysis (Colony forming unit [CFU] counts), a semi quantitative method was used to analyse bacterial growth in the wounds.
Previously, in multiple preclinical models, we have shown that topical antimicrobial treatment with the PWD is safe and efficient and reduces bacterial load more efficiently than treatment with intravenous antibiotics. 6 , 7 , 8 , 19 , 20 The gentamicin sulfate cream was chosen as the topical antibiotic for this study because it is an FDA‐approved broad‐spectrum antibiotic that is commonly used to prevent and treat a wide variety of bacterial infections. A recent systematic review, meta‐analysis found that topical gentamicin cream application for wound infection prophylaxis and treatment significantly increased clinical efficacy and decreased the duration of wound healing. 21 In addition, we have previously demonstrated both in preclinical models as well as in clinical case studies that topical application of gentamicin with the PWD is efficient and safe. 6 In a porcine study, ultrahigh concentrations (1000×MIC) of gentamicin delivered via PWD were used to treat P. aeruginosa‐infected deep partial‐thickness burn wounds. The results showed that the treatment with topical gentamicin significantly reduced bacterial counts in comparison to IV antibiotic treatment. Also, importantly it was demonstrated that the topical application of 1000×MIC did not cause any systemic toxicity. 6 In a clinical study, Vranckx et al (2002) used the PWD to treat infected wounds topically with high concentrations of gentamicin and vancomycin. Their results showed that 48 hours after application 20% of the antibiotic was still present in the PWD. Their study concluded that the PWD provided a safe and powerful tool for the treatment of infected wounds. 22 In another clinical case study, a 66‐year‐old patient with an abdominal wound that had failed to heal was treated topically with the PWD and antibiotics. The wound was sealed with the PWD and high concentrations (up to 2500×MIC) of gentamicin were delivered through the device. After 10 weeks of treatment, the wound was closed. 23
Besides being a delivery platform for drugs, the PWD can be utilised as a platform for tissue transplantation 24 , 25 and as an NPWT system without foam or gauze. 26 , 27 The PWD has been cleared through the FDA 510 k pathway as a class II device to be used in wound care as delivery platform and an NPWT device. Previously, in a clinical case study, we studied the negative pressure capabilities of the PWD in the treatment of closed surgical incisions. Our results indicated that the subjects tolerated the negative pressure PWD on closed surgical incisions well and that all incisions were intact without evidence of inflammation or infection after 2 weeks of follow‐up. 28
In conclusion, this study introduces a novel device for the treatment of wounds, with the capability of delivering topical medications directly to the wound bed. The PWD is a lightweight, sterile, transparent enclosure that can be applied immediately after injury as a protective dressing and a tool for precise topical delivery of analgesics and antibiotics. Our results demonstrated that delivery of topical gentamicin via the PWD is safe and effective at reducing bacterial load in the wound. Clinical assessment for infection found the PWD to be comparable to the current SoC treatment options.
CONFLICT OF INTEREST
Dr. Eriksson is the founder and chief medical officer of Applied Tissue Technologies LLC (ATT). He has not participated in the evaluation or recording of the results or the formulation of the conclusions or summary. Dr. Nuutila used to be an employee of the ATT. All the other authors have no conflict of interest to declare in relation to the content of this article.
ACKNOWLEDGEMENTS
This material is based upon work supported by the U.S. Army Medical Research and Development Command under Contract No W81XWH‐18‐2‐0002. The views, opinions and/or findings contained in this report are those of the author(s) and should not be construed as an official Department of the Army position, policy or decision unless so designated by other documentation.
Cooley J, Obaidi N, Diaz V, et al. Delivery of topical gentamicin cream via platform wound device to reduce wound infection—A prospective, controlled, randomised, clinical study. Int Wound J. 2023;20(5):1426‐1435. doi: 10.1111/iwj.13998
Jennifer Cooley and Noor Obaidi contributed equally to this study.
Contributor Information
Rodney K. Chan, Email: rodneykchan@gmail.com.
Kristo Nuutila, Email: kristo.nuutila@gmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006;19(2):403‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wound infection in clinical practice. An international consensus. Int Wound J 2008;5 Suppl 3(Suppl 3):iii‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hakkarainen TW, Kopari NM, Pham TN, Evans HL. Necrotizing soft tissue infections: review and current concepts in treatment, systems of care, and outcomes. Curr Probl Surg. 2014;51(8):344‐362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bowler PG. The 10(5) bacterial growth guideline: reassessing its clinical relevance in wound healing. Ostomy Wound Manage. 2003;49(1):44‐53. [PubMed] [Google Scholar]
- 5. Negut I, Grumezescu V, Grumezescu AM. Treatment strategies for infected wounds. Molecules. 2018;23(9):2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nuutila K, Grolman J, Yang L, et al. Immediate treatment of burn wounds with high concentrations of topical antibiotics in an alginate hydrogel using a platform wound device. Adv Wound Care (New Rochelle). 2020;9(2):48‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nuutila K, Yang L, Broomhead M, Proppe K, Eriksson E. PWD: treatment platform for both prolonged field care and definitive treatment of burn‐injured warfighters. Mil Med. 2019;184(5–6):e373‐e380. [DOI] [PubMed] [Google Scholar]
- 8. Yang L, Broomhead M, Nuutila K, Proppe K, Eriksson E. Topically delivered minocycline penetrates a full‐thickness burn eschar and reduces tissue bacterial counts. J Burn Care Res. 2018;39(5):790‐797. [DOI] [PubMed] [Google Scholar]
- 9. Haque M, Sartelli M, McKimm J, Abu BM. Health care‐associated infections—an overview. Infect Drug Resist. 2018;11:2321‐2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical‐site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infect Control Hosp Epidemiol. 1999;11:725‐730. [DOI] [PubMed] [Google Scholar]
- 11. Gomez R, Murray CK, Hospenthal DR, et al. Causes of mortality by autopsy findings of combat casualties and civilian patients admitted to a burn unit. J Am Coll Surg. 2009;208(3):348‐354. [DOI] [PubMed] [Google Scholar]
- 12. Norbury W, Herndon DN, Tanksley J, Jeschke MG, Finnerty CC. Infection in burns. Surg Infect (Larchmt). 2016;17(2):250‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cefalu JE, Barrier KM, Davis AH. Wound infections in critical care. Crit Care Nurs Clin North Am. 2017;29:81‐96. [DOI] [PubMed] [Google Scholar]
- 14. Doughty D. Dressings and more: guidelines for topical wound management. Nurs Clin North Am. 2005;40:217‐231. [DOI] [PubMed] [Google Scholar]
- 15. Beele H, Meuleneire F, Nahuys M, Percival SL. A prospective randomised open label study to evaluate the potential of a new silver alginate/carboxymethylcellulose antimicrobial wound dressing to promote wound healing. Int Wound J. 2010;7:262‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Percival SL, Bowler MPhil P, Woods EJ. Assessing the effect of an antimicrobial wound dressing on biofilms. Wound Repair Regen. 2008;16:52‐57. [DOI] [PubMed] [Google Scholar]
- 17. Heal CF, Banks JL, Lepper PD, Kontopantelis E, van Driel ML. Topical antibiotics for preventing surgical site infection in wounds healing by primary intention. Cochrane Database Syst Rev. 2016;11(11):CD011426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nuutila K, Eriksson E. Moist wound healing with commonly available dressings. Adv Wound Care (New Rochelle). 2021;10(12):685‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grolman JM, Singh M, Mooney DJ, Eriksson E, Nuutila K. Antibiotic‐containing agarose hydrogel for wound and burn care. J Burn Care Res. 2019;40(6):900‐906. [DOI] [PubMed] [Google Scholar]
- 20. Daly LT, Tsai DM, Singh M, et al. Topical minocycline effectively decontaminates and reduces inflammation in infected porcine wounds. Plast Reconstr Surg. 2016;138(5):856 e‐868 e. [DOI] [PubMed] [Google Scholar]
- 21. Wang P, Long Z, Yu Z, et al. The efficacy of topical gentamycin application on prophylaxis and treatment of wound infection: a systematic review and meta‐analysis. Int J Clin Pract. 2019;73(5):e13334. [DOI] [PubMed] [Google Scholar]
- 22. Vranckx JJ, Slama J, Preuss S, et al. Wet wound healing. Plast Reconstr Surg. 2002;110:1680. [DOI] [PubMed] [Google Scholar]
- 23. Eriksson E, Perez N, Slama J, Page CP, Andree C, Maguire JH. Treatment of chronic, nonhealing abdominal wound in a liquid envi‐ ronment. Ann Plast Surg. 1996;36:80‐83. [DOI] [PubMed] [Google Scholar]
- 24. Singh M, Nuutila K, Kruse C, Dermietzel A, Caterson EJ, Eriksson E. Pixel grafting: an evolution of mincing for transplantation of full‐thickness wounds. Plast Reconstr Surg. 2016;137(1):92 e‐99 e. [DOI] [PubMed] [Google Scholar]
- 25. Hackl F, Bergmann J, Granter SR, et al. Epidermal regeneration by micrograft transplantation with immediate 100‐fold expansion. Plast Reconstr Surg. 2012;129(3):443 e‐452 e. [DOI] [PubMed] [Google Scholar]
- 26. Nuutila K, Yang L, Broomhead M, Proppe K, Eriksson E. Novel negative pressure wound therapy device without foam or gauze is effective at −50 mmHg. Wound Repair Regen. 2019;27(2):162‐169. [DOI] [PubMed] [Google Scholar]
- 27. Nuutila K, Broomhead M, Proppe K, Eriksson E. Study comparing platform wound dressing, a negative‐pressure device without a filler, with three conventional negative‐pressure wound therapy systems in the treatment of excisional and incisional wounds. Plast Reconstr Surg. 2021;147(1):76‐86. [DOI] [PubMed] [Google Scholar]
- 28. Cooper LE, O'Toole MC, Fields KL, Eriksson EK, Chan RK. Utilization of a novel negative pressure platform wound dressing on surgical incisions: a case series. Plast Reconstr Surg Glob Open. 2021;9(3):e3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


