Abstract
Based on initially identified needs for further telemedicine (TM) and chronic wound management research, the objective of this article is twofold: to conduct a systematic review of existing knowledge on TM interventions in chronic wound management—including barriers and opportunities—across the specialist and primary care sectors, and to incorporate the review findings into a system framework that can be further developed and validated through empirical data. We conclude that there is a pressing need for broader and more comprehensive empirical explorations into quality improvement and integration of TM in chronic wound management, including using system frameworks that can capture cross‐sector system perspectives and associated implications. Of practical consideration, we suggest that the design and execution of TM improvement interventions and associated research projects should be conducted in close cooperation with managers and practitioners knowledgeable about barriers and opportunities that can influence the implementation of important interventions within chronic wound management.
Keywords: chronic wound management, system framework, systematic review, telemedicine
1. INTRODUCTION
The objective of this article is twofold; (i) to conduct a systematic review of existing knowledge on TM interventions in chronic wound management—including barriers and opportunities—across the specialist and primary care sectors, and (ii) to incorporate the review findings into a system framework that accounts for dimensions, factors, and dynamics extending beyond clinical effects, which can be further developed in future empirical research. The rationale for this study is based on limited current knowledge and identified research needs for telemedicine (TM) in the chronic wound management domain.
TM is the use of electronic information and communication technology (ICT) to exchange healthcare information between sites. TM aims at providing and supporting health care when distance separates the participants, to improve healthcare delivery and outcomes including patients' health status. 1 , 2 , 3 , 4 , 5 , 6 Totten et al 7 emphasised that TM and research into its effectiveness should target clinical applications that demonstrated rapid expansion during the ongoing COVID‐19 pandemic. TM can replace much face‐to‐face care and thereby reduce exposure to infections including during pandemics. Totten et al 8 created an evidence map of 58 systematic reviews that assessed the impact of TM improvement interventions on clinical outcomes; improvement interventions are “purposeful efforts to secure positive change [that] have become an increasingly important focus of activity within healthcare”. 9 (p325) They found that the most consistent benefit of TM relates to communication and counselling or remote monitoring in chronic conditions, with improvements in outcomes such as mortality and quality of life, and reductions in hospital admissions. Snoswell et al 10 also highlighted the clinical effectiveness potential of TM.
In line with Totten et al 8 and Snoswell et al, 10 Chanussot and Contreras Ruiz 11 concluded that TM can benefit chronic wound management. Specifically, TM reduces the need for patients to travel long distances to the hospital or to consult with a physician and decreases costs, in addition to improving the quality of life for patients with chronic wounds. 11 TM's cost‐reducing and quality of life‐improving effects resonate with Le Goff‐Pronovost et al, 12 who found that TM reduces travel costs and results in a shorter healing time of chronic wounds than traditional follow‐up care. Chen et al 13 found that TM was more effective than traditional care and decreased the risk of amputation in chronic wound patients. On the other hand, Timpel and Harst 14 identified the need for research into TM implementation strategies and intervention studies with adequate length of follow‐up care. According to Kahn, 15 researchers must demonstrate that TM improves patient‐centred outcomes efficiently and that TM can be integrated “[…] into the existing care system in ways that do not detract from the interpersonal and interprofessional relationships that we all recognize are essential to effective patient‐centered care” (p. 1685).
Other knowledge gaps have been identified in the chronic wound management domain. Olsson et al 16 urgently called for the development and implementation of wound management strategies that focus on increasing health‐related quality of life and effectively reduce costs for patients with chronic wounds and poor quality of life, including pain, distress, social isolation, anxiety, extended hospital stay, chronic morbidity or even mortality; many of these consequences are preventable. 17 , 18 , 19 Moore et al 20 found that teams of health care professionals with a shared focus on the patient can enhance clinical outcomes across the wound spectrum, clinical care settings, and geographical locations. However, they suggested that research is needed to demonstrate the effect of the team approach to wound management both in terms of clinical and financial outcomes. Specifically, the growing prevalence of non‐healing wounds and chronic diseases is a health problem that has devastating impacts on patients and costs for healthcare systems and societies. 21 , 22 , 23 From a general TM in wound care perspective, Kostovich et al 24 identified a lack of evidence regarding the use of TM in wound care including inconsistent documentation of outcomes, though the authors suggested that TM is not inferior to in‐person care.
In summary, TM interventions in chronic wound management must adopt a longitudinal and broader system perspective across primary and specialist health care services that accounts for clinical applications, team approaches, and strategies for wound management and implementation. The system perspective must also factor in the quality of life, existing care system, and cost aspects. These research needs are reflected in our article objective as well as the new wound management model explored in the TELE‐AMBUS project, as described next. Through the systematic review in this article (Sections 4 and 5), we gain further insight into system barriers and opportunities to TM in chronic wound management.
2. CONTEXT
TM provides a direct line of communication between primary care and specialist health services and the patient. This facilitates development of an effective and goal‐oriented wound treatment plan that can capture potential complications early in the healing process and be adjusted accordingly. Chronic wound management including TM interventions aim to improve chronic wound diagnosis and treatment of patients across all age groups, in any location, and ranging from patients with mobility challenges to those who require frequent follow‐up of changes in the wound condition.
This article is part of the TELE‐AMBUS research project. With the objective of exploring the possibility of a chronic wound management‐oriented project, in 2019 a team from the Norwegian Research Centre NORCE engaged with health care professionals and management at Norway's first Wound Diagnostic Centre (WDC) located at the Department of Dermatology, Stavanger University Hospital (SUH). In this dialogue, wound specialist nurses at the center expressed a years‐long desire to explore a specific type of chronic wound management model. This model comprises an ambulatory wound care team comprised of specialised nurses who in consultation with the relevant hospital‐physician specialists (dermatologist, vascular surgeon, and general surgeon) provide ambulatory wound care diagnosis and treatment to elderly and vulnerable chronic wound patients located in remote municipalities and inhibited from travelling to the hospital independently. The main rationale for developing and testing the model is the desire to improve competency levels in municipalities (through bed‐side teaching and counselling), to achieve more efficient wound management and communication (aided by TM solution), and consequently to improve patient quality of life and strengthen service quality across specialist and primary care sectors. The TELE‐AMBUS project specifically targets patients with hard‐to‐heal wounds.
3. METHODS
In this section, we present our two‐step systematic review process. We first applied a systematic mapping review approach, comprising a broad sweep of national and international literature on TM interventions in chronic wound management. We then conducted an expert‐informed purposive sampling strategy using the systematic literature review methodology STARLITE (Standards for Reporting Literature searches) by Booth. 25
3.1. Systematic mapping review with protocol
A systematic mapping review does not address a specific research question but rather collates and describes insight on a topic or concept of interest. 25 (p3) This type of review applies broad/open‐framed question formulations, for example, to explore what interventions have been undertaken and studied in a particular field such as chronic wound management.
Figure 1 illustrates the protocol for a systematic mapping review, comprised of six stages and beginning with the steps laid out in Stage 1. Specifically, the main review team (Authors 1–3) was established as part of the TELE‐AMBUS project organisation. The remaining authors of this article are project partners and stakeholders that contributed to the conceptualization of the project, including the systematic review process and associated research questions applied in work package 1 (WP1) of the project. All project partners and stakeholders also contributed to the actual literature review process through regular meeting activities (advisory boards and working meetings) and constructive manuscript input. The scope of the mapping review, informed by project description and stakeholders, is focused on cross‐sector TM interventions in chronic wound management within and outside of Norway.
FIGURE 1.
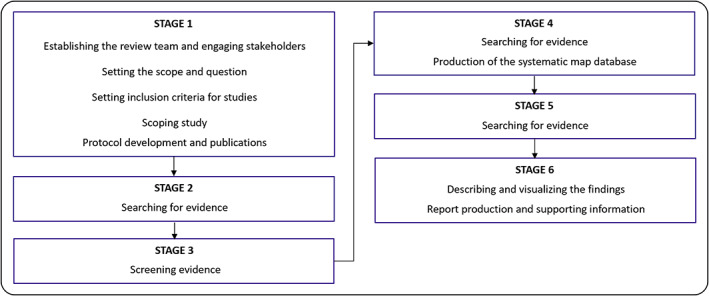
Stages in the systematic mapping review process, as developed by James et al. 26 Note that the figure has been adjusted/laid out horizontally from its original presentation in James et al 26
The broad research question for the mapping review is: What types of TM interventions in chronic wound management have been undertaken across specialist and primary health care sectors and services in a Norwegian context as well as internationally? The inclusion criteria, informed by the project description and stakeholder involvement, include a broad timeframe (no limit specified), study designs, and types of research including studies published in English or Norwegian, thus aligning with the broad nature of mapping review. We then conducted a scoping study, understood as a trial run of a complete search in academic databases; this included a recording of results, screening of a subset of results to assess proportional relevance at title, abstract, and full‐text levels as well as determining sensitivity‐specificity balance issues. Because we wanted to capture both healthcare‐specific literature (nursing and medicine) and literature more broadly related to TM and chronic wound management interventions, we selected the electronic databases Scopus, PubMed, Google Scholar, and CINAHL. Three reviewers (Authors 1–3) were involved, each assigned to review a subset of results in 1–2 databases (Author 1, Google Scholar; Author 2, Scopus and Cinahl; Author 3, PubMed). Two search strings were applied for all databases: “Telemedicine” AND “chronic wound management” AND “interventions” (search String #1); “Ambulatory team” AND “chronic wound” AND “interventions” (search String #2). The inclusion of the search word “ambulatory team” was based on the research need for exploring team approaches (Section 1), which is also reflected in the TELE‐AMBUS project's focus on an ambulatory wound care team.
Due to several review issues encountered during Stage 1 of the systematic mapping review, we changed the procedure to a systematic literature review using STARLITE and specifically a purposive sampling strategy (for short, a purposive systematic literature review). This approach is in line with James et al, 26 who suggested that “sometimes the scoping stage may help identify whether a systematic map or a full systematic review is the most appropriate method to address a question […] influenced by the amount and type of evidence found during the scoping stage” (p. 7). The review issues included a low number of studies (n = 7) identified on broader or more comprehensive TM perspectives related to quality improvement and integration of chronic wound management across sectors (see Section 4), in combination with less productive and non‐productive (in the case of CINAHL) searches (see Figure 2). CINHAL searches consistently returning zero hits may indicate search engine limitations, unsuitability of our specific search strings to the engine, and/or weaknesses in the precision of the search terms themselves. Specifically, it is possible that using the search word telemedicine (TM) instead of telehealth affected the search results. However, one of the main studies 27 identified in our systematic review is on telehealth, suggesting that TM searches are also returning similar/overlapping concepts. This is confirmed by multiple hits on telehealth studies resulting from our original three search strings in the electronic databases. We also identified several studies outside of the project scope in Scopus, PubMed, and Google Scholar. It should be noted that search String 2, which included the search term “ambulatory team,” proved most fruitless. This result could be caused by the search term being too specific or that ambulatory team interventions are rare in the field of chronic wound management, the latter of which would constitute a significant finding.
FIGURE 2.
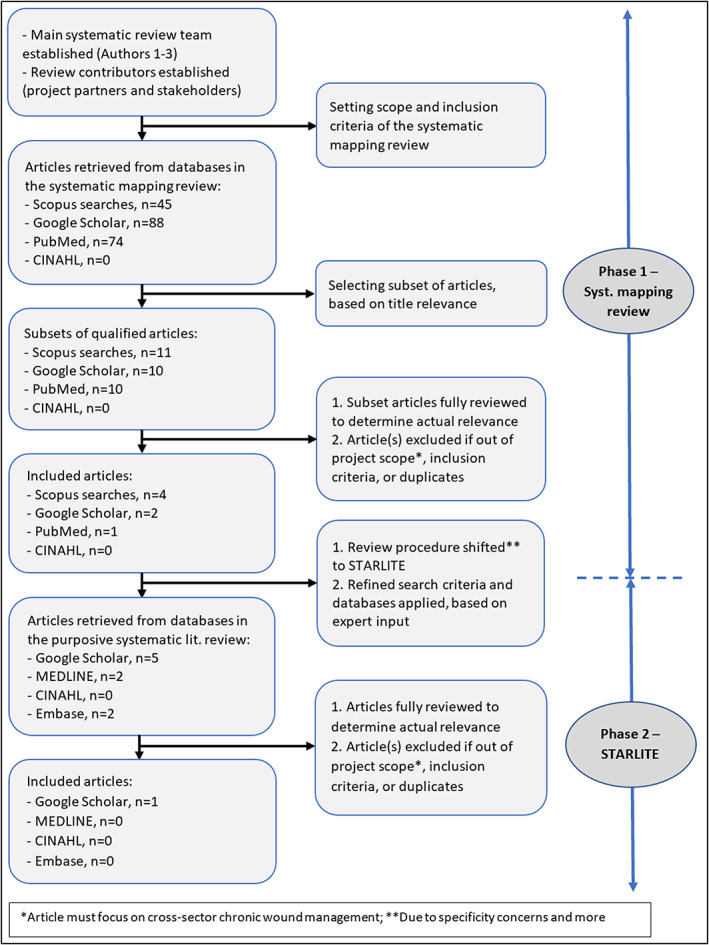
A flowchart of the two‐phased systematic literature review process, comprising phase 1/systematic mapping review and phase 2/STARLITE
3.2. STARLITE systematic literature review with protocol
Authors 1–3 presented the protocol and outcome (as detailed in Section 4.1) of the systematic mapping review to experts on wound management and TM in the project's scientific advisory board (MF, NI, PC, MG, and ER; see the list of authors). The experts provided input on refined/specific search terms, which align with the purposive sampling strategy of the STARLITE review methodology. 25 They suggested focusing on (i) studies of digital apps and the associated database Embase, and (ii) studies within the dermatology discipline (see the corresponding “inclusions and exclusions” section in Table 1 below).
TABLE 1.
The STARLITE systematic review approach developed by Booth, 25 (p424)
| Elements of the STARLITE mnemonic including application in the TELE‐AMBUS project | |
|---|---|
| S: Sampling strategy |
|
| T: Type of studies |
|
| A: Approaches |
|
| R: Range of years (start‐end date) |
|
| L: Limits |
|
| I: Inclusions and exclusions |
|
| T: Terms used |
|
| E: Electronic sources |
|
Note: The table also includes a report of the literature search for the TELE‐AMBUS project, structured according to STARLITE and presented in bold text.
Table 1 outlines the main elements of the STARLITE framework 25 as well as our application or operationalization of the framework for the TELE‐AMBUS project (in bold text). Regarding “terms used” in Table 1, we applied the following search strings for all electronic databases, which included Google Scholar, MEDLINE, CINAHL, and Embase: “Chronic wound management” AND “dermatology” AND “digital applications” (String 1); “Chronic wound management” AND “dermatology” AND “mobile applications” (String 2); “Chronic wound management” AND “dermatology” AND “digital tools” (String 3). In addition, we conducted MEDLINE, CINAHL, and Embase searches using the shorter versions “digital” and “application” as a replacement for the “digital application,” “mobile applications,” and “digital tools” keywords.
Figure 2 visualises the two‐phase systematic review process: (1) a systematic mapping review and (2) a purposive systematic literature review.
3.3. Ethical considerations
The TELE‐AMBUS research project was accepted by the ethical committee REC West in Norway (application id. 375 986) and approved by NSD Norwegian Centre for Research Data (reference no. 236558). The project has also been agreed by the internal data protection representative at Stavanger University Hospital SUH (protocol nr. 2847–2847).
4. RESULTS
4.1. Systematic mapping review—scoping study results
We structured the outcome of the scoping study according to the databases, excluding the CINAHL database that did not return any search results.
4.1.1. Scopus searches
The searches identified several publications that describe cross‐sector TM interventions in chronic wound management, including healing parameters, TM as a patient decision aid, community nurses' knowledge, hospital visits/hospitalisation/length of stay, and cost‐effectiveness aspects such as reduced number of TM visits. 28 , 29 , 30 , 31 , 32 However, few publications apply broader or more comprehensive TM perspectives on quality improvement and integration of chronic wound management across sectors. A Norwegian study by Irgens et al 33 focused on pressure ulcers (PUs) following spinal cord injuries (SCI) and the overall goal to «identify advantages as well as challenges in the management of PU in different follow‐up settings» (p. 1). They described a TM intervention in comparing outpatient follow‐up in a hospital versus the patient's home, focusing on multiple outcome factors including healing, quality of life, cost‐benefits, patient satisfaction, and interactions (changes in collaboration, communication, competence) between health care professionals and the patients and next of kin. Another Norwegian study identified several factors relevant to implementing and improving TM in chronic wound management, including obstacles of organisational (resources and consultation time), technological (need for updated equipment), and individual/professional (patient's confidence in the competence and professional skills of health professional) nature. 34 , 35 Adding to the organisational perspective, Rasmussen et al 36 identified a focus on management, wound training (of nurses), economy, and work absence as key influences on TM implementation.
4.1.2. Google Scholar searches
These searches revealed several cross‐sector publications that address wound healing and reduction (in size), risk of amputation, the accuracy of pressure injury evaluation, quality‐adjusted life‐year resulting from TM interventions or applications, and cost‐effectiveness perspectives. 13 , 37 , 38 , 39 , 40 , 41 We found broader or more comprehensive TM perspectives on quality improvement and integration of chronic wound management across sectors—extending beyond wound healing, risk reduction, and cost‐effectiveness—only in studies by Mills et al 42 and Barrett et al. 27 These studies documented several TM‐related systemic opportunities and barriers to holistic (inpatient and outpatient) service quality improvement. Barrett et al 27 identified barriers in terms of delays in installing the software and workforce shortages, where only about half of those who trained in using the software were still employed at the end of the study. Mills et al 42 identified the benefits of health care provider, caregiver, and patient satisfaction with TM, the potential for technological advancement via TM, and the potential for virtual care/TM to improve health personnel safety and reduce virus spread (such as COVID‐19).
4.1.3. PubMed searches
Comparable to the Scopus and Google Scholar searches, studies identified in the PubMed searches addressed how TM contributed to chronic wound healing and reduction (in size), reduced risk of amputation, reduction in adverse events, and improvements in patient quality of life including from a cost‐effectiveness perspective. 12 , 43 , 44 , 45 , 46 , 47 , 48 One study by Zhang et al 49 focused on technical aspects of developing and validating a tool (mHealth) for home‐based chronic wound self‐monitoring. We found broader or more comprehensive TM perspectives on quality improvement and integration of chronic wound management across sectors, beyond the measuring‐oriented wound healing, risk reductions, and cost‐effectiveness parameters, only in the study by Hofmann‐Wellenhof et al. 50 They identified high teledermatology acceptance by patients, home‐care nurses, and wound experts, which resonates with Mills et al's 42 finding (Section 4.1.2) that health care professionals, caregivers, and patients expressed satisfaction with TM.
4.2. STARLITE systematic literature review—results from purposive sampling
Amongst the nine articles retrieved in the STARLITE searches, two were duplicates appearing in both the Embase and MEDLINE databases and two were master theses outside the inclusion criteria (see Table 1). Amongst the remaining five articles, only two studies fell within the review scope of focusing on cross‐sector TM interventions within the dermatology and digital apps domains: one of them was a duplicate hit 49 identified during the systematic mapping review. Specifically, Kong et al 51 described an innovative remote care strategy comprised of a patient‐facing wound care app (the Swift Patient Connect App) designed to monitor and manage wounds by a patient with diabetes and foot ulcers. They reported that patients found the technology “educational and empowering,” with increased self‐examination and engagement in preventive behaviour such as monitoring for trauma and early signs of infection. This finding aligns with our systematic mapping review (Section 4.1). Both Hofmann‐Wellenhof et al 50 and Mills et al 42 found high acceptance of TM amongst patients, home‐care nurses/caregivers, and wound experts. Mills et al 42 also identified how technological advancements via TM can improve cross‐sector chronic wound management.
4.3. A summary of the two‐phase systematic review
Overall, the searches revealed several studies on cross‐sector TM interventions in chronic wound management, with the majority of them targeting wound healing, risk reductions, patient's quality of life, and cost‐effectiveness parameters. Only eight studies (see Table 2) explore broader or more comprehensive TM perspectives on quality improvement and integration of chronic wound management across sectors. 27 , 33 , 34 , 35 , 36 , 42 , 50 , 51 Findings in three of these studies 42 , 50 , 51 are supported across databases and review methodologies applied in this article.
TABLE 2.
An overview of identified studies on cross‐sector chronic wound improvement perspectives and associated connections to dimensions of the Systems Engineering Initiative for Patient Safety (SEIPS) framework discussed in Section 5
| Identified cross‐sector studies and SEIPS framework connections | ||
|---|---|---|
| Authors, year, and title | Findings related to SEIPS | SEIPS dimension(s) identified |
| Barrett, M. et al. (2009). Challenges faced in implementation of a telehealth enabled chronic wound care system |
|
|
| Hofmann‐Wellenhof, R. et al. (2006). Feasibility and acceptance of telemedicine for wound care in patients with chronic leg ulcers |
|
|
| Irgens et al. (2019). Hospital based care at home; study protocol for a mixed epidemiological and randomised controlled trial |
|
|
| Kong, L. Y. et al. (2021). A 57‐year‐old man with type 1 diabetes mellitus and a chronic foot ulcer successfully managed with a remote patient‐facing wound care smartphone application |
|
|
| Kolltveit, B.H. et al. (2018). Telemedicine follow‐up facilitates more comprehensive diabetes foot ulcer care: A qualitative study in home‐based and specialist health care |
|
|
| Mills, E.C. et al. (2020). Telemedicine and the COVID‐19 Pandemic: Are we ready to go live? |
|
|
| Rasmussen, B.S. et al. (2015). A qualitative study of the key factors in implementing telemedical monitoring of diabetic foot ulcer patients |
|
|
| Smith‐Strøm et al. (2016). An integrated wound‐care pathway, supported by telemedicine, and competent wound management ‐ Essential in follow‐up care of adults with diabetic foot ulcers |
|
|
Note: For full publication details, see the “references” section.
5. DISCUSSION
The objective of this article was two‐fold: (1) to conduct a systematic review of existing knowledge on cross‐sector TM interventions in chronic wound management including barriers and opportunities, and (2) to incorporate the review findings into a system framework that can be further developed and validated through empirical research. This section outlines the system framework, comprised of several dimensions informed by the literature review. The framework is embedded in the TELE‐AMBUS project with its focus on the ambulatory wound team and TM intervention.
Informed by developments within sociotechnical system theories (STS) and the Systems Engineering Initiative for Patient Safety (SEIPS) framework, 52 , 53 , 54 , 55 Figure 3 visualises a process‐embedded cross‐sector work system for the TELE‐AMBUS project. In this work system, various dimensions (represented as oval figures) affect the work system flow‐process (middle‐part of the framework) and associated patient and system outcomes (right‐part of the framework). The system framework extends the focus of current research beyond documenting clinical effects, and emphasises the organisational dimension and associated factors, dynamics between work system dimensions and work processes, the influence of external structures, decisions and context (see Table 2). The current list of work system dimensions is based on insights from our systematic review; we will continue building and enriching the list through empirical data gathered in the TELE‐AMBUS project.
FIGURE 3.
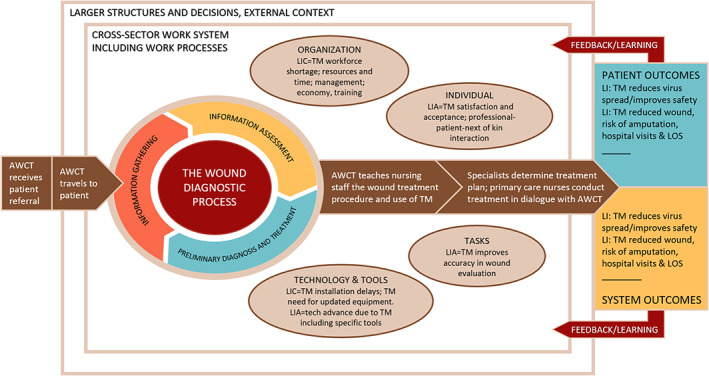
A literature‐informed cross‐sector and process‐embedded SEIPS framework of the TM and ambulatory team intervention. Figure based on Carayon et al 54 , 55 and Balogh et al. 56 AWCT, Ambulatory wound care team; LI, Literature‐informed; LIA, Literature‐informed advantages (opportunities); LIC, Literature‐informed challenges (barriers)
The intervention explored in the TELE‐AMBUS project is comprised of an ambulatory wound care team (AWCT) and TM operating across primary and specialist health care services; it is represented as a work system flow‐process in the middle part of the framework. The flow‐process logic is as follows: the AWCT receives a patient referral from a general practitioner (GP) in the municipality, considers (in this case) the referral to fall within the inclusion criteria of the TELE‐AMBUS project, and then travels to the patient that can be located at home, nursing home, or rehabilitation center (patients with alcohol dependence or substance use disorders). At the patient's location, the AWCT conducts the wound diagnostic process/evaluates the wound, transfers images to the Department of Dermatology via TM, and teaches and guides the nursing staff in both the wound treatment procedure and the use of TM. Following this, the AWCT returns to the hospital and enters patient data into the TM application, enabling the primary care professionals to transfer pictures and written reports on wound development and request additional support. A multidisciplinary medical team consisting of a dermatologist, a vascular surgeon, and a general surgeon decides on a wound diagnosis and makes a treatment plan based on the wound nurses' evaluation and findings. The treatment plan is then communicated via the TM application to the primary care nursing staff with follow‐up correspondence. The work system's flow‐process in the middle part of the framework produces system and patient outcomes that are desirable to a larger or lesser degree, which in turn provides feedback to improve the current work system (indicated by the red arrows in Figure 3).
Our systematic review suggests that the “organisation” and “technology and tools” dimensions are key barriers to TM in chronic wound management, and consequently to the flow‐process of the work system (see Figure 3). Specifically, the technology itself (TM) is the unifying obstacle across the “organisation” and “technology and tools” dimensions, as expressed through TM‐related shortages in the workforce, resources and time, management, economy, training, installation delays, and need for updated equipment. These findings correspond with Carayon et al, 54 who identified several technological barriers to coordinating care for chronically ill patients, including inadequate access to the technological tools, inadequate information in the systems, limited usefulness, poor usability, challenges due to multiple technologies, and technical problems.
The current literature review also revealed several opportunities for TM in chronic wound management, related specifically to the “tasks,” “individual” and “technology and tools” dimensions. This includes the emphasis on technological evolution such as TM improving wound evaluation accuracy and advancing current technology levels through specific tools (e.g., Swift Patient Connect App in Kong et al 51 and mHealth in Zhang et al 49 ). In addition, individuals express TM satisfaction and acceptance via professional‐patient‐next of kin interaction. The current literature review indicates positive outcomes that are attributable to both organisation and patient, namely that TM reduces virus spread (in the case of COVID‐19), improves safety, improves chronic wound healing, and reduces the risk of amputation, hospital visits, and lengths of stay.
It must be noted that Figure 3 is missing the SEIPS dimension “physical environment,” understood as aspects of the physical layout and environment that support or do not support clinical tasks to various degrees. 57 As can be seen in Table 2, this dimension was not present in the studies identified in our review, and we can only speculate that this has to do with the studies lacking awareness and/or understanding of the dimension compared to the more immediate dimensions of organisation and technology. Given that the TELE‐AMBUS project's fieldwork takes place in the physical locations of both the health care professionals and patients, empirical data are likely to shed light on the physical environment dimension. This insight will be incorporated in a revised version of Figure 3 in later publications.
Table 2 shows how the various work system dimensions of the SEIPS framework connect with our systematic literature review and specifically each of the eight studies identified as having broader and more comprehensive cross‐sector wound improvement perspectives.
5.1. Strengths and weaknesses of the systematic review process and framework
In our initial systematic mapping review, the CINAHL searches proved to be less fruitful, which we attributed to search engine limitations, unsuitability of our specific search strings to the engine, and/or weaknesses in the precision of the search term themselves. We found search String 2 particularly challenging, which included the search term “ambulatory team,” and initially attributed this challenge to the search term either being too specific or—as a significant finding—that ambulatory team interventions are rare in the field of chronic wound management. We attempted to counter the review concerns through the STARLITE methodology, with search words informed by experts in the fields of wound management and TM, though the searches returned few hits across all explored databases. We attributed this outcome to the potential presence of weaknesses in search parameter precision, as discussed in Section 3.1.
When selecting the specific systematic literature approach for our study, the STARLITE methodology was chosen above PRISMA given that the former accounts for demands or elements needed to adequately report both quantitative and qualitative systematic reviews. This consideration aligns with the broad inclusion criteria of our study (see “type of studies” [T] in Table 1). A specific qualitative reporting demand is “sampling strategy” (S), as outlined in the STARLITE mnemonic and documented for our study (see Table 1). Thus, STARLITE constitutes the most appropriate methodology for the systematic literature review undertaken in our article.
In this article, we utilised a two‐step systematic review process for the TELE‐AMBUS project, combining a broad sweep of literature using a systematic mapping review, followed by an expert‐informed purposive sampling strategy using the STARLITE methodology. The two‐step review process constitutes a methodological triangulation approach, which strengthens the validity of the literature review process. 58 , 59 We also used analytical triangulation via a designated review team (Authors 1–3) and the project's scientific advisory board, which further improves the validity of our review process. By outlining explicit details of the steps undertaken to conduct the two‐step systematic review, as well as applying triangulation techniques to improve validity of the process, our study can be replicated to further inform cross‐sector TM interventions within the chronic wound management domain.
The SEIPS framework proposed in Figure 3 is currently limited to literature‐based findings. We are incorporating empirical insights from the TELE‐AMBUS project to improve the validity of the framework. We are presently conducting fieldwork to explore the combination of an ambulatory wound team and TM (intervention) operating across the primary and specialist health sectors. We encourage broader exploration of the framework in different regions and contexts, to improve the validity of the framework even further.
6. CONCLUSION
Any improvement intervention is a complex process that can span multiple organisations and sectors (e.g., primary and specialist health care) as well as actors/stakeholders (e.g., regulatory bodies, managers at multiple levels, and practitioners/professions). As we have demonstrated in this article, intervention complexity can be captured with the SEIPS framework. The SEIPS framework is able to show the “dynamic” and mutually influencing nature of (1) work systems dimensions (that can be cross‐organisational and cross‐sectoral), (2) associated work processes and larger external context, and (3) outcome factors (output) of the system and processes. A focus on intervention complexity resonates with Kahn's 15 suggestion that “researchers should explore the crucial issue of context, studying not only whether telemedicine works but also how, when, and where it works best, to provide a roadmap for more effective implementation” (p. 1684).
The main outcome and conclusion of our review are that there is a pressing need for further empirical explorations of TM interventions in chronic wound management, including using the SEIPS framework. This need for exploration specifically concerns broader or more comprehensive cross‐sector TM perspectives, where our review revealed only eight studies. Based on our combined experience with the TELE‐AMBUS project and the review outcome of this article, we suggest that the design and execution of improvement interventions and associated research projects should be conducted in close cooperation with managers and practitioners knowledgeable about current barriers and opportunities—including those from the patient's perspective. Intervention requirements need to be clearly understood, especially by clinicians and patients, this is particularly the case when developing new models of care. 60 , 61
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
The TELE‐AMBUS project was funded by the Research Council of Norway (RCN), project nr. 326 574.
Høyland SA, Holte KA, Islam K, et al. A cross‐sector systematic review and synthesis of knowledge on telemedicine interventions in chronic wound management—Implications from a system perspective. Int Wound J. 2023;20(5):1712‐1724. doi: 10.1111/iwj.13986
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Paul DL, McDaniel RR. Facilitating telemedicine project sustainability in medically underserved areas: a healthcare provider participant perspective. BMC Health Serv Res. 2016;16:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Graves BA. Telehealth for communities: toward eliminating rural health disparities. Online J Rural Nurs Health Care. 2012;10(1):4‐6. [Google Scholar]
- 3. Institute of Medicine . The Role of Telehealth in an Evolving Health Care Environment: Workshop Summary. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- 4. Palen T, Bodily M. PS3‐28: telemedicine specialty consultation in a medically underserved community. Clin Med Res. 2010;8(3–4):200‐201. [Google Scholar]
- 5. Mechanic D. Rethinking medical professionalism: the role of information technology and practice innovations. Milbank Q. 2008;86(2):327‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Institute of Medicine . Telemedicine: a guide to assessing telecommunications in health care. In: Field MJ, ed. Institute of Medicine (US) Committee on Evaluating Clinical Applications of Telemedicine. Washington, DC: National Academies Press (US); 1996. [PubMed] [Google Scholar]
- 7. Totten AM, McDonagh MS, Wagner JH. The Evidence Base for Telehealth: Reassurance in the Face of Rapid Expansion during the COVID‐19 Pandemic. Report no. 20‐EHC015. Agency for Healthcare Research and Quality: Rockville, MD; 2020. [PubMed] [Google Scholar]
- 8. Totten AM, Hansen RN, Wagner J, et al. Telehealth for acute and chronic care consultations. Agen Healthcare Res Quality Compar Effect Rev. 2019;216:1‐159. [PubMed] [Google Scholar]
- 9. Portela MC, Pronovost PJ, Woodcock T, Carter P, Dixon‐Woods M. How to study improvement interventions: a brief overview of possible study types. BMJ Qual Saf. 2015;24(25):325‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Snoswell CL, Chelberg G, De Guzman KR, et al. The clinical effectiveness of telehealth: a systematic review of meta‐analyses from 2010 to 2019. J Telemed Telecare. 2021;0(0). [DOI] [PubMed] [Google Scholar]
- 11. Chanussot C, Contreras RJ. Telemedicine in wound care: a review. Adv Skin Wound Care. 2013;26(2):78‐82. [DOI] [PubMed] [Google Scholar]
- 12. Le Goff‐Pronost M, Mourgeon B, Blanchère JP, Teot L, Benateau H, Dompmartin A. Real‐world clinical evaluation and costs of telemedicine for chronic wound management. Int J Technol Assess Health Care. 2018;34(6):567‐575. [DOI] [PubMed] [Google Scholar]
- 13. Chen L, Cheng L, Gao W, Chen D, Wang C, Ran X. Telemedicine in chronic wound management: systematic review and meta‐analysis. JMIR Mhealth Uhealth. 2020;8(6):e15574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Timpel P, Harst L. Research implications for future telemedicine studies and innovations in diabetes and hypertension – a mixed methods study. Nutrients. 2020;12(5):1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kahn JM. Virtual visits ‐ confronting the challenges of telemedicine. N Engl J Med. 2015;372(18):1684‐1685. [DOI] [PubMed] [Google Scholar]
- 16. Olsson M, Järbrink K, Divakar U, et al. The humanistic and economic burden of chronic wounds: a systematic review. Wound Repair Regen. 2019;27(1):114‐125. [DOI] [PubMed] [Google Scholar]
- 17. Posnett J, Gottrup F, Lundgren H, Saal G. The resource impact of wounds on health‐care providers in Europe. J Wound Care. 2009;18(4):154‐161. [DOI] [PubMed] [Google Scholar]
- 18. Chen YT, Chang CC, Shen JH, Lin WN, Chen MY. Demonstrating a conceptual framework to provide efficient wound management service for a wound care center in a tertiary hospital. Medicine. 2015;94(44):e1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lindholm C, Searle R. Wound management for the 21st century: combining effectiveness and efficiency. Int Wound J. 2016;13(suppl. 2):5‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moore Z, Butcher G, Corbett LQ, McGuiness W, Snyder RJ, van Acker K. Exploring the concept of a team approach to wound care: managing wounds as a team. J Wound Care. 2014;23(Suppl. 5b):S1‐S38. [DOI] [PubMed] [Google Scholar]
- 21. Phillips CJ, Humphreys I, Thayer D, et al. Cost of managing patients with venous leg ulcers. Int Wound J. 2020;17(4):1074‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Phillips CJ, Humphreys I, Fletcher J, Harding K, Chamberlain G, Macey S. Estimating the costs associated with the management of patients with chronic wounds using linked routine data. Int Wound J. 2016;13(6):1193‐1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guest JF, Ayoub N, McIlwraith T, et al. Health economic burden that wounds impose on the National Health Service in the UK. BMJ Open. 2015;5(12):e009283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kostovich CT, Etingen B, Wirth M, et al. Outcomes of telehealth for wound care: a scoping review. Adv Skin Wound Care. 2022;35(7):394‐403. [DOI] [PubMed] [Google Scholar]
- 25. Booth A. “Brimful of STARLITE”: toward standards for reporting literature searches. J Med Libr Assoc. 2006;94(4):421‐429. [PMC free article] [PubMed] [Google Scholar]
- 26. James KL, Randall NP, Haddaway NR. A methodology for systematic mapping in environmental sciences. Environ Evid. 2016;5:7. [Google Scholar]
- 27. Barrett M, Larson A, Carville K, Ellis I. Challenges faced in implementation of a telehealth enabled chronic wound care system. Rural Remote Health. 2009;9(3):1154. [PubMed] [Google Scholar]
- 28. Gamus A, Kaufman H, Chodick G. Remote care of lower extremities ulcers: an observational pilot study. Israel Med Assoc J. 2019;21(4):265‐268. [PubMed] [Google Scholar]
- 29. Terry M, Halstead LS, O'Hare P, et al. Feasibility study of home care wound management using telemedicine. Adv Skin Wound Care. 2009;22(8):358‐364. [DOI] [PubMed] [Google Scholar]
- 30. Dobke MK, Bhavsar D, Gosman A, De Neve J, De Neve B. Pilot trial of telemedicine as a decision aid for patients with chronic wounds. Telemed J E Health. 2008;14(3):245‐249. [DOI] [PubMed] [Google Scholar]
- 31. Rees RS, Bashshur N. The effects of TeleWound management on use of service and financial outcomes. Telemed J E Health. 2007;13(6):663‐674. [DOI] [PubMed] [Google Scholar]
- 32. Ameen J, Coll AM, Peters M. Impact of tele‐advice on community nurses' knowledge of venous leg ulcer care. J Adv Nurs. 2005;50(6):583‐594. [DOI] [PubMed] [Google Scholar]
- 33. Irgens I, Hoff JM, Sørli H, Haugland H, Stanghelle JK, Rekand T. Hospital based care at home; study protocol for a mixed epidemiological and randomized controlled trial. Trials. 2019;20(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kolltveit BH, Thorne S, Graue M, Gjengedal E, Iversen MM, Kirkevold M. Telemedicine follow‐up facilitates more comprehensive diabetes foot ulcer care: a qualitative study in home‐based and specialist health care. J Clin Nurs. 2018;27(5–6):e1134‐e1145. [DOI] [PubMed] [Google Scholar]
- 35. Smith‐Strøm H, Iversen MM, Graue M, Skeie S, Kirkevold M. An integrated wound‐care pathway, supported by telemedicine, and competent wound management ‐ essential in follow‐up care of adults with diabetic foot ulcers. Int J Med Inform. 2016;94:59‐66. [DOI] [PubMed] [Google Scholar]
- 36. Rasmussen BS, Jensen LK, Froekjaer J, Kidholm K, Kensing F, Yderstraede KB. A qualitative study of the key factors in implementing telemedical monitoring of diabetic foot ulcer patients. Int J Med Inform. 2015;84(10):799‐807. [DOI] [PubMed] [Google Scholar]
- 37. Brain D, Tulleners R, Lee X, Cheng Q, Graves N, Pacella R. Cost‐effectiveness analysis of an innovative model of care for chronic wounds patients. PLoS One. 2019;14(3):e0212366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pak C, In Jeon J, Kim H, et al. A smartphone‐based teleconsultation system for the management of chronic pressure injuries. Wound Rep Reg. 2018;26(Suppl. 1):S19‐S26. [DOI] [PubMed] [Google Scholar]
- 39. Arora M, Harvey LA, Glinsky JV, et al. Cost‐effectiveness analysis of telephone‐based support for the management of pressure ulcers in people with spinal cord injury in India and Bangladesh. Spinal Cord. 2017;55(12):1071‐1078. [DOI] [PubMed] [Google Scholar]
- 40. Stern A, Mitsakakis N, Paulden M. Pressure ulcer multidisciplinary teams via telemedicine: a pragmatic cluster randomized stepped wedge trial in long term care. BMC Health Serv Res. 2014;14:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Trubiani G, Stern A, Pham Ba', Carcone S, Krahn M. Specialized Multidisciplinary Community‐Based Care for Chronic Wounds: a Field Evaluation. University of Toronto: Toronto Health Economics and Technology Assessment Collaborative. Research report. 2011.
- 42. Mills EC, Savage E, Lieder J, Chiu ES. Telemedicine and the COVID‐19 pandemic: are we ready to go live? Adv Skin Wound Care. 2020;33(8):410‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Huang Z, Wu S, Yu T, Hu A. Efficacy of telemedicine for patients with chronic wounds: a meta‐analysis of randomized controlled trials. Adv Wound Care. 2021;10(2):103‐112. [DOI] [PubMed] [Google Scholar]
- 44. Kruse CS, Lee K, Watson JB, Lobo LG, Stoppelmoor AG, Oyibo SE. Measures of effectiveness, efficiency, and quality of telemedicine in the management of alcohol abuse, addiction, and rehabilitation: systematic review. J Med Internet Res. 2020;22(1):e13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hrynyschyn R, Dockweiler C, Iltner J, Hornberg C. Telekonsultation bei vaskulär und diabetisch bedingten chronischen Wunden: eine systematische Übersicht der gesundheitlichen und ökonomischen Implikationen [teleconsultation for vascular‐ and diabetes‐associated chronic wounds: a systematic review of health‐related and economic implications]. Hautarzt. 2020;71(2):114‐123. [DOI] [PubMed] [Google Scholar]
- 46. Michaud TL, Zhou J, McCarthy MA, Siahpush M, Su D. Costs of home‐based telemedicine programs: a systematic review. Int J Technol Assess Health Care. 2018;34(4):410‐418. [DOI] [PubMed] [Google Scholar]
- 47. Flodgren G, Rachas A, Farmer AJ, Inzitari M, Shepperd S. Interactive telemedicine: effects on professional practice and health care outcomes. Cochrane Database Syst Rev. 2015;9:CD002098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chanussot‐Deprez C, Contreras‐Ruiz J. Telemedicine in wound care. Int Wound J. 2008;5(5):651‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang J, Mihai C, Tüshaus L, Scebba G, Distler O, Karlen W. Wound image quality from a mobile health tool for home‐based chronic wound management with real‐time quality feedback: randomized feasibility study. JMIR Mhealth Uhealth. 2021;9(7):e26149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hofmann‐Wellenhof R, Salmhofer W, Binder B, Okcu A, Kerl H, Soyer HP. Feasibility and acceptance of telemedicine for wound care in patients with chronic leg ulcers. J Telemed Telecare. 2006;12(Suppl. 1):15‐17. [DOI] [PubMed] [Google Scholar]
- 51. Kong LY, Ramirez‐GarciaLuna JL, Fraser R, Wang SC. A 57‐year‐old man with type 1 diabetes mellitus and a chronic foot ulcer successfully managed with a remote patient‐facing wound care smartphone application. Am J Case Rep. 2021;22:e933879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hendrick HW, Kleiner BM. Macroergonomics: Theory, Methods, and Applications. Mahwah, NJ: Lawrence Erlbaum Associates; 2002. [Google Scholar]
- 53. Carayon P, Hundt AS, Karsh BT, et al. Work system design for patient safety: the SEIPS model. BMJ Qual Saf. 2006;15(Suppl 1):i50‐i58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Carayon P, Wooldridge A, Hoonakker P, Hundt AS, Kelly MM. SEIPS 3.0: human‐centered design of the patient journey for patient safety. Appl Ergon. 2020;84:103033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Balogh EP, Miller BT, Ball JR. Improving Diagnosis in Health Care. Washington, DC: National Academies Press; 2015. [PubMed] [Google Scholar]
- 56. Carayon P, Hundt AS, Hoonakker P. Technology barriers and strategies in coordinating care for chronically ill patients. Appl Ergon. 2019;78:240‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xie A, Carayon P. A systematic review of Human Factors and Ergonomics (HFE)‐based healthcare system redesign for quality of care and patient safety. Ergonomics. 2015;58:33‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Denzin NK. Sociological Methods. New York: McGraw‐Hill; 1978. [Google Scholar]
- 59. Patton M. Qualitative Evaluation and Research Methods. California: Sage Publications; 1990. [Google Scholar]
- 60. Armfield NR, Edirippulige SK, Bradford N, Smith AC. Telemedicine ‐ is the cart being put before the horse? Med J Aust. 2014;200(9):530‐533. [DOI] [PubMed] [Google Scholar]
- 61. Thomas EE, Haydon HM, Mehrotra A, et al. Building on the momentum: sustaining telehealth beyond COVID‐19. J Telemed Telecare. 2022;28(4):301‐308. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.


