Abstract
We designed, developed, built, and utilised a robotic system of a leg with two venous leg ulcers for testing the fluid handling performance of three wound dressing types. The results showed that a foam‐based dressing technology is inferior in fluid handling performance when applied to an exuding venous leg ulcer, such that the dressing needs to manage the exudate in a vertical configuration with respect to the ground, that is, so that gravity pulls the exudate to concentrate in a small region at the bottom of the dressing. Moreover, wound dressings containing superabsorbent polymers do not necessarily function equally in fluid handling for venous leg ulcer scenarios, as the extreme requirements from the dressing (to manage the viscous fluid of a vertical and typically highly‐exuding wound) appear to distinguish between optimal and suboptimal product performances despite that the tested products contain a superabsorbent, theoretically lumping them together to belong to a so‐called ‘superabsorbent dressing category’. In other words, it is a false premise to categorise products from different manufacturers into families based on material contents, and then assume that their laboratory or clinical performance is equal, so that from this point they can be judged solely on the basis of price.
Keywords: compression bandaging, chronic wound model, exudate management, laboratory testing methods, wound care
Abbreviations
- ANOVA
analyses of variance
- FBD
foam‐based dressing
- MPD
multipurpose dressing
- SAD
superabsorbent dressing
- SWF
simulated wound fluid
- VLUs
venous leg ulcers
1. INTRODUCTION
Venous leg ulcers (VLUs) caused by chronic venous insufficiency are a common wound type accounting for approximately 70% of all chronic leg ulcers, and resulting in significant and prolonged disability, poor quality of life and substantial socioeconomic burden. 1 , 2 , 3 The mainstay of VLU treatment includes the use of compression therapy to apply external pressure to the legs, with the aim of improving venous function, in combination with advanced wound dressings for exudate management. Multilayer compression therapy, incorporating padding under elastic bandages, is currently considered the gold standard for treating VLUs. 4 , 5 , 6 A dressing applied to treat a VLU under the compression bandaging must provide effective fluid handling to maintain the wound‐bed moist but not wet at all times; poor absorbency and retention performance of the dressing and/or exudate leakage may cause maceration of wound and peri‐wound tissues, which delays the wound healing or even deteriorates the wound condition. 7 , 8 As VLUs may be heavily draining on the one hand, and the dressing is compressed and squeezed under the compression bandaging so cannot necessarily use its entire exudate storage capacity on the other hand, selection of an appropriate dressing technology and product is critical for achieving positive clinical outcomes for VLUs. Multiple dressing choices exist, but even for those dressings recommended for use on VLUs under compression bandaging, no clinically relevant laboratory test data were reported. Recently, a series of robotic wound simulator test systems, replicating different chronic wound aetiologies, was developed by the research group of the senior author to serve as advanced, clinically relevant testing systems for wound care products. 9 , 10 , 11 , 12 , 13 , 14 , 15 This work features our latest robotic wound system, of VLUs, which we previously suggested that is the most challenging configuration for dressings in a fluid handling aspect. 10 This is because, in addition to the high exudation of typically viscous wound fluids, and the sustained compression of the dressing, the VLU and dressing are vertical to the ground during upright or sitting postures, resulting in pulling of the wound fluid downwards by gravity. The exudate therefore concentrates in the lower dressing part, that is, characteristically, for VLUs, the fluid retention capacity of the dressing is only partially utilised.
We therefore used our novel VLU robotic system to evaluate the fluid handling performance of different dressing materials and structures, to understand which dressing technologies better suit VLU treatment with compression therapy. We consider the current method and metrics to be of vital importance towards development of better testing standards for wound dressings indicated for treating VLUs in combination with compression bandaging given the clinical relevance of the current innovative ‘robotic VLU’ study approach.
2. METHODS
2.1. Robotic phantom system of VLUs for automated testing of wound dressings
We designed, developed, built, and utilised a versatile robotic phantom system of a leg with one medial and one lateral VLUs; these robotic leg systems were produced in a triplicate (Figure 1). The VLU simulator units included in each robotic leg (Figure 1) were all identical, and simulated highly exuding, elliptically shaped wounds, at a maximum depth of 1.5 mm (other wound and VLU unit dimensions are detailed in Figure 1B).
FIGURE 1.
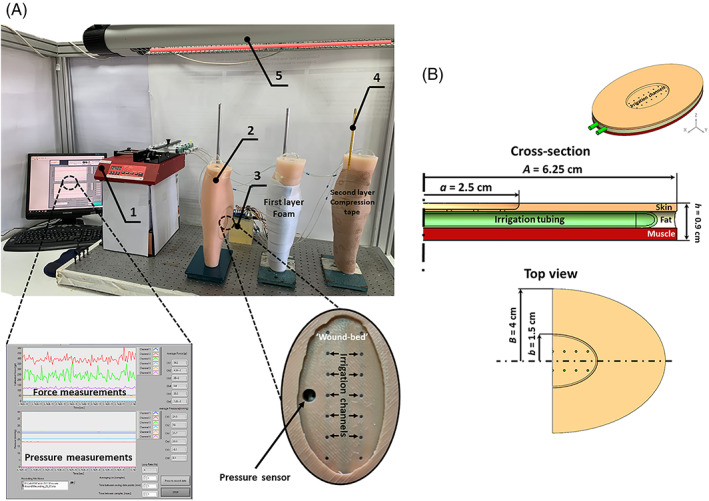
The robotic phantom system of venous leg ulcers (VLUs) (A): Experiments were conducted in three identical leg units, each including one lateral and one medial VLU at identical locations, shapes and sizes per anatomical position (upper frame). The setup consisted of: (1) Computer‐controlled syringe pump (the control panel of the LabView code developed for these robotic wound systems, showing the sensor readings in real‐time, is magnified in the bottom left frame); (2) Robotic wound systems (three identical leg units). (3) VLU simulator (magnified in the bottom right frame); (4) Thermometer; and (5) Infrared heating lamp for temperature control. The geometry of the VLU simulator (B) with its soft tissue simulant components. Dimensions A and B are halves of the length and width of the whole elliptically shaped VLU simulator unit, respectively, and likewise, dimensions a and b are halves of the length and width of the elliptically shaped wound‐bed part of the VLU simulator, respectively
Each VLU unit contained three layers of synthetic soft tissue simulants (Figure 1B), as follows. Externally, the peri‐wound was represented by a 2 mm‐thick skin simulant layer (SSL) made of commercial silicone that is commonly accepted for representing skin in medical and cosmetic applications (Dragon Skin 20, Smooth‐On, Inc., Macungie, Pennsylvania). Subdermal fat was represented by an adipose simulant layer (ASL), similarly made of a 4 mm‐thick layer of a Dragon Skin 20 mixed with Slacker (Smooth‐On, Inc.) which lowers the Shore hardness of the silicone to approximate that of native adipose tissue, as recommended by the manufacturer. The innermost layer was a skeletal muscle simulant layer (MSL), made of the same silicone type of the SSL, but at a thickness of 3 mm. According to the manufacturer's technical datasheets, the tensile strength and tangent modulus at 100% strain of the Dragon Skin 20 silicone material (following the relevant American Society for Testing and Materials (ASTM) international testing standard ASTM D412‐06 16 ) are 3.8 MPa and 338 kPa, respectively, which are characteristic to human skin and skeletal muscle tissues subjected to large deformations. 17 , 18 , 19 The addition of the Slacker to the silicone reduces both the strength and stiffness of the treated silicone by an order of magnitude, yielding a material suitable for representing adipose tissue with properties similar to silicone gel or paraffin gel. 15 , 20 , 21 The assembly forming each VLU simulator unit was formed by moulding the ASL onto the SSL, and then similarly layering the MSL onto the ASL, which altogether provided the ‘look and feel’ of a real‐world VLU.
To simulate continuous exudate secretion from the VLU simulator units, a perforated irrigation tube was incorporated in each wound unit, and each such irrigation tube was connected to a multi‐channel electromechanical syringe pump system (NE‐1600, New Era Pump Systems Inc., Farmingdale, New York) (Figure 1A), to provide precision control over the flow volumes and release rates of an exudate‐substitute fluid. The effective wet surface of each VLU simulator unit (i.e., the area of the ‘wound‐bed’ from which the exudate‐like fluid was released) was 10.5 cm2. A xanthan gum‐based simulant wound fluid (SWF) described in detail in our published work, 9 , 10 , 11 , 12 , 13 , 15 with density of 1.03 g/cc and viscosity of 0.71 Pa × s was used with the robotic leg systems throughout all the experiments reported here.
2.2. Simulated treatments of the robotic wounds by means of dressings
Three types of wound dressings with sizes of 10 × 20 cm, each produced by a different manufacturer, were applied to the robotic legs with VLU simulators for systematically testing and quantitatively comparing their fluid handling performance. Specifically, the Curea P1 (Curea medical GmbH, Steinfurt, Germany) multipurpose dressing (MPD) was compared against a market‐popular superabsorbent dressing (SAD) indicated specifically for use on moderate to highly‐exuding VLUs and under compression therapy; and also, against a foam‐based dressing (FBD) type indicated for application on moderately exuding wounds. Of note, use of FBDs for treating highly‐exuding VLUs in clinical practice is generally debated in the literature, with a recent trend to prefer a superabsorbent technology (which locks‐in the wound fluids under compression therapy) for this particular wound aetiology. 22 , 23 , 24 However, for scientific completeness, and given that many clinicians still use FBDs on VLUs under compression bandaging (as indicated in the aforementioned 2021‐2022 citations), we decided to include an FBD here to compare and contrast its fluid handling performance against those of the other, superabsorbent‐polymer‐based dressing types in this specific VLU treatment scenario.
All dressing products were first weighed in their ‘out‐of‐the‐package’ dry state, and then applied onto the VLU simulator units as per the product‐specific manufacturer's instructions for use (IFU). Next, a two‐layer compression therapy kit (CoFlex TLC LS, Milliken Healthcare Products, LLC, Ladson, South Carolina), designed to induce standard compression pressure of 30 to 40 mm Hg, was applied on the legs with VLUs, again according to the corresponding IFU provided by the manufacturer. The robotic legs were then placed in an upright standing position (Figure 1A) for the entire testing duration, which was 12, 18 or 24 hours. The surface temperature of the robotic legs was controlled and maintained within the 31°C to 33°C interval throughout all the experiments, corresponding to the real‐world VLU temperature range. 25 The compression pressure applied de facto by the compression bandage was measured using thin and flexible resistive force sensors (Force Sensing Resistor model UX 402, Interlink Electronics, Inc., California) placed on the ‘peri‐wound skin’ 2.5 cm from the edge of the VLU ‘wound‐bed’, and connected to a microcontroller board (Arduino‐mega 2560, Ivrea, Italy). In addition, the pressure applied by a dressing on the ‘wound‐bed’ of the VLU, as the dressing gradually swells whilst absorbing the SWF, was sampled and recorded every 5 minutes using a pressure sensor (model XGZP6847A, CFSensor, Wuhu, China) installed within each ‘wound‐bed’ of a VLU simulator unit (Figure 1A; bottom right frame).
2.3. Absorbency and retention studies
At the end of each simulated use session, the dressings were removed and reweighed, and the net fluid mass present in each dressing was calculated. Any SWF which remained in the VLU simulator cavities, named herein the residual fluid, was collected and weighed; likewise, any spillover fluid (if such existed) was carefully collected from a bottom tray and weighed as well. Next, these measured fluid masses were converted to volumes, by dividing the absorbed, residual and spillover SWF masses by the fluid density. The total SWF volume delivered to each VLU simulator unit over the time course of each test was then calculated, as the sum of the retained fluid volume in the dressing plus the residual and spillover fluid volumes (if any). Then, the percentage retention of SWF in each dressing specimen was calculated, based on the ratio of the fluid volume that was retained in the tested dressing over the total fluid volume that was delivered to the corresponding VLU simulator unit throughout the duration of the test (separately for each VLU simulator unit and test condition). The percentages of the residual and spillover SWF volumes were similarly calculated. Last, the percentage of the evaporated SWF was calculated, as 100% minus the sum of the fluid percentage retained in the dressing, and the residual and the spillover (if occurred) SWF shares.
2.4. Data and statistical analyses of the fluid handling outcome measures
All experiments for a given dressing type were conducted in the leg triplicate system and descriptive statistics were calculated for the percentage retained, residual, spillover and evaporated SWF volume shares per each simulated use duration (i.e., 12, 18 and 24 hours). To identify potential statistically significant differences in these fluid handling metrics across the tested dressing types, analyses of variance (ANOVA) followed by post hoc Tukey‐Kramer multiple pairwise comparisons were conducted, and a P‐value lower than .05 was considered statistically significant. In addition, the measured ‘wound‐bed’ pressures were plotted over time, separately for the medial and lateral VLU simulator units.
3. RESULTS
The MPD exhibited the highest shares of retained SWF (which were steady and approximately 80%) amongst the tested dressing types, for both the medial and lateral VLUs, and for each trial duration (Figure 2). For example, following the longest, 24 hours simulated use trials, the MPD retained 1.3‐times and 1.1‐times more SWF than the SAD, and 1.4‐times and 1.5‐times more than the FBD, for the medial and lateral simulated VLUs, respectively (Figure 2). The SAD and FBD dressings lost approximately twice the amount of SWF through evaporation to the environment with respect to the MPD per each trial duration. Use of the FBD was also associated with increased cavity fluid shares, which became more profound with the duration of the trials (Figure 2). Specifically, after 24 hours, the cavity fluid was 5‐fold greater, on average, for the FBD than for the MPD and SAD (for which the share of cavity fluid was similar and under 2%). Importantly, for both the medial and lateral VLUs, the MPD exhibited the most consistent and stable retention across the 12, 18 and 24 hour time point (Figure 2). The SAD demonstrated more variability but overall, the share of the retained SWF tended to increase (as demonstrated in Figure 2B), whereas the FBD clearly (and statistically significantly; Figure 2A) lost fluid retention capacity over time (Figure 2). The FBD also failed to handle the SWF at the lateral VLU, leading to a spillover fluid share of 4% of the total delivered SWF volume at the 24 hour time point (Figure 2B). Statistically significant differences in fluid share distributions existed between the MPD and SAD dressings at the 18 and 24 hour time point, indicating that these dressing types are not equivalent in their fluid handling performance (as detailed above) despite that both types contain a superabsorbent polymer (Figure 2).
FIGURE 2.
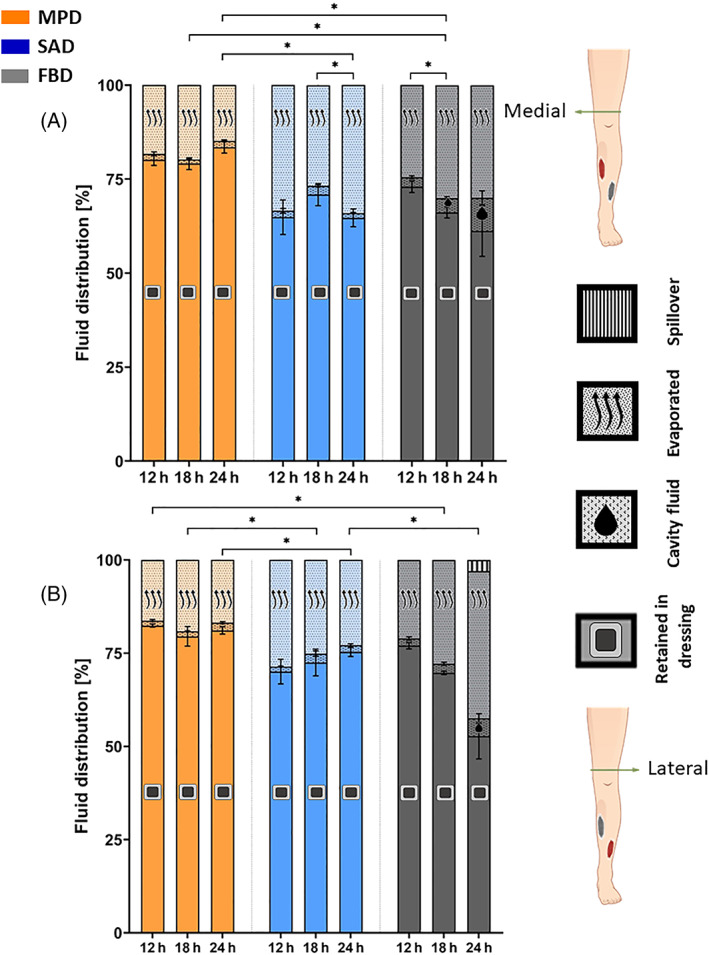
Retention performance of the tested wound dressing types for the medial (A) and lateral (B) anatomical positions of the venous leg ulcer (VLU) simulators, after 12, 18 and 24 hours of simulated use. The error bars are the SDs from the mean values of three test repetitions per VLU simulator location and test configuration, and an asterisk indicates a statistically significant difference in the relevant outcome measure (P < .01). FBD, foam‐based dressing; MPD, multipurpose dressing; SAD, superabsorbent dressing
The pressure sensor data consistently demonstrated that the FBD swells the least, and accordingly, applied the lowest pressure on the peri‐wound, between 5 and 10 mm Hg, whereas the MPD and SAD dressings, which swell more (due to the superabsorbent polymer contents), applied 10 to 20 mm Hg on the ‘peri‐wound skin’ (Figure 3). These pressures tended to rise more rapidly until approximately 6 to 9 hours from the time of application of the dressings, and then stabilise, hence, the pressure rise behaviour at the ‘peri‐wound’ is non‐linear with respect to time (Figure 3). The pressure growth curves of the MPD and SAD were generally more variable than those of the FBD (Figure 3).
FIGURE 3.
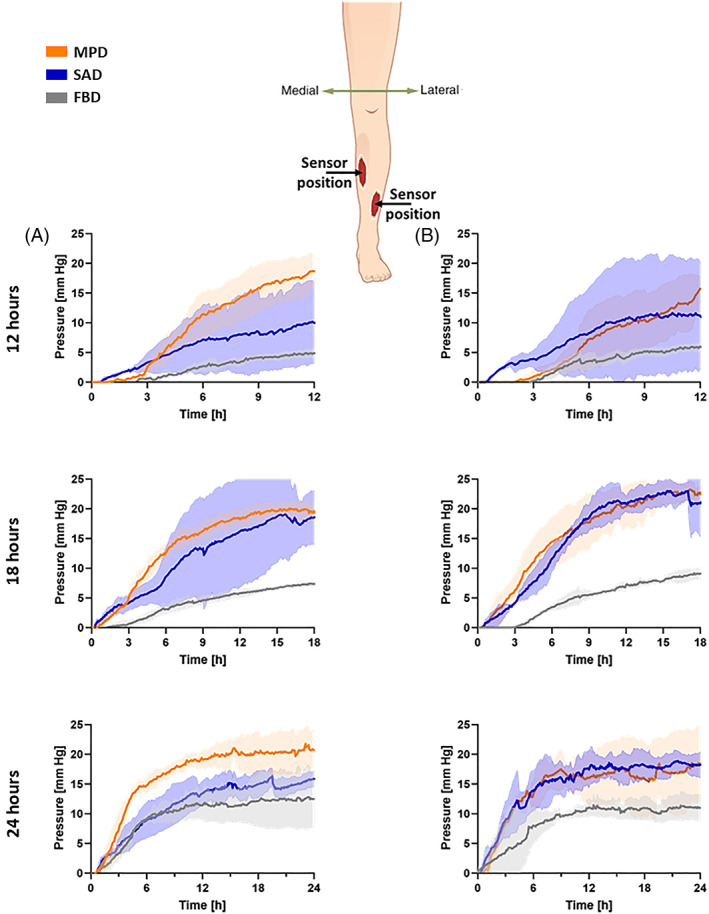
Pressure sensor data measured in the simulated wound‐bed region (shown in Figure 1; bottom right frame) for the tested wound dressings at the medial (A) and lateral (B) anatomical positions of the venous leg ulcer (VLU) simulators, after 12, 18 and 24 hours of simulated use. The shades are the SDs from the mean values of the three test repetitions per VLU simulator location and test configuration. FBD, foam‐based dressing; MPD, multipurpose dressing; SAD, superabsorbent dressing
4. DISCUSSION
It is well established that excess wound exudate not appropriately managed by a dressing can deteriorate a wound and cause maceration of the peri‐wound skin, 26 , 27 , 28 , 29 hence, exudate beyond the level required to keep a wound moist should be continuously absorbed and retained by the applied dressing, and exudate spillover episodes should not occur. 9 , 10 , 11 , 12 , 13 , 15 Amongst the common chronic wound aetiologies, VLUs are likely the most challenging from a wound dressing fluid handling performance perspective, because, as recently explained by Orlov and Gefen, 10 the wound surface is approximately vertical to the ground, and so is the applied dressing, such that gravity pulls the wound fluid downwards to the lower part of the dressing, where it accumulates, as opposed to a horizontally oriented wound and dressing where the fluid is spread on the entire wound pad surface of the dressing. In addition, VLUs are known to often exude heavily and the exudate may be highly viscous, and the underlying chronic venous insufficiency is treated by compression therapy to increase the venous and lymphatic return (which deforms the dressing in sustained compression), all of which challenge the dressing capacity to handle the fluids even further. In view of the above, we developed the current robotic phantom system of a leg with VLUs to test wound dressings in an objective, standardised, but clinically relevant manner, and in a form that allows acquisition of quantitative performance metrics, specifically, the retained, residual, evaporated and (potentially) spillover fluid shares, as function of the simulated use time. This laboratory testing system goes above and beyond any existing testing standards for wound dressings in terms of the clinical relevance of the test. The specific characteristics of the challenge, as explained above, makes this test method sensitive for identifying differences between product performances (as indeed demonstrated here) whilst providing the robustness and reproducibility of an advanced bioengineering laboratory approach, as further indicated in the relatively small variabilities of the measured test outcomes for a certain product and given test conditions (Figure 2).
Amongst the dressing technologies which were investigated here, the MPD demonstrated the most effective and consistent fluid handling performance for the studied VLU clinical scenario. Regardless of the specific dressing location (medial or lateral) and simulated use time (12‐24 hours), the retained SWF was always the greatest in the MPDs and no spillover events were recorded for this dressing type (Figure 2). The SAD and FBD demonstrated greater evaporation of the SWF to the environment with respect to the MPD, but less retention, particularly for the 18 and 24 hour durations, and for the trials lasting 24 hours, there was spillover from the FBD. Of note, the SAD dressing tested here is indicated specifically for use on moderate to highly‐exuding VLUs and under compression therapy, whereas the FBD is indicated for application on moderately exuding wounds, and there is no manufacturer recommendation to use it on VLUs. With that said, as clinicians may consider an FBD for the treatment of VLUs, it was important to include this dressing type in the testing protocol, for completeness and comprehensiveness. Overall, the differences in fluid handling performance specified in Figure 2, and in particular, between the MPD and SAD, must relate to the product‐specific material composition and structural differences between the studied dressing types, as both the MPD and SAD contain superabsorbent polymers. 10
As with regards to any experimental laboratory system, our current test configuration and trials had limitations which should be discussed here. For example, we included two (medial and lateral) relatively localised VLUs in each robotic leg, each at a size of approximately 12 cm2, but so‐called ‘complex’ VLUs may have sizes exceeding 100 cm2 and such wounds sometime occupy most of the lower leg surface. 1 Our robotic system likewise does not represent leg ulcers with mixed aetiology, legs with partial amputations, or legs of paediatric patients with VLUs which is a less frequent but existing pathology, associated with, for example, congenital or genetic syndromes, sickle cell disease, prolidase deficiency, scleroderma, or vasculopathies, to mention a few relevant conditions. 30 The SWF used in this study can also be improved further and be made more specific to representing VLUs, for instance, as related to the fluid surface tension if relevant experimental data become available for human VLU exudates. Motion of the robotic leg can be further included, as opposed to simulating only static standing, by incorporating a moving or vibrating surface under the foot of the robotic leg in future work. Moreover, specific activity profiles can be simulated to represent the daily behaviour of patients, such as alternating between static standing, motion and a horizontal position, for example, as seen when leg elevation is implemented. Further modifications of the robotic legs can include the thigh and pelvis, as frequently, when these patients elevate their legs to a resting position there is hip flexion that may impede the lymphatic return. 31 Last, infected VLUs can be simulated by culturing colour‐coded unharmful (e.g., yogurt) bacteria in the SWF, and then quantifying their presence in the tested dressings and surroundings of the VLU simulator units. Another potential limitation is that in the robotic leg system, the SWF is delivered from an external fluid reservoir, and hence, fluids are added to the leg‐dressing two‐compartment system, whereas in real life, the fluids originate from the oedematous wound/peri‐wound tissues and are absorbed and retained in the dressing, that is, transferred (internally) from the leg to the dressing compartments. Accordingly, it would be interesting to conduct studies incorporating soft, flexible pressure sensors at the peri‐wound skin‐dressing interface of patients with VLUs, to determine whether the same extent of skin pressure increase under the dressings as reported in Figure 3 occurs in the real‐world, and what is its potential effect (if any) on the perfusion of the peri‐wound.
To conclude, we found that a FBD technology is inferior in fluid handling performance when applied to an exuding VLU under compression therapy, such that the (compressed) dressing needs to manage the exudate in a vertical configuration with respect to the ground (i.e., so that the gravity vector pulls the wound fluids to concentrate in a small region at the bottom of the vertically‐oriented dressing). Moreover, different wound dressing brands containing superabsorbent polymers do not necessarily function equally in fluid handling for VLU scenarios, as the extreme requirements from the dressing (to manage the viscous fluid of a vertical and typically highly‐exuding wound) appear to distinguish between optimal and suboptimal performance of different dressing products, despite that the tested products contain superabsorbent elements, theoretically lumping them together to belong to a so‐called ‘superabsorbent dressing category’. In other words, it is a false premise to categorise products from different manufacturers into families that are based, for example, on material contents, and then assume that their laboratory (or clinical) performance is equal, so that from this point they can be judged solely on the basis of price. This underpins the responsibility of clinicians and other decision‐makers in health care systems to request product‐specific, clinically relevant performance evaluations from wound dressing manufacturers, and ensure that the information received represent the specific clinical scenarios for the intended use of the dressing product under consideration, in their facility.
ACKNOWLEDGEMENTS
Ms Orel Belo, the Laboratory Engineer at the research group of Professor Amit Gefen is thanked for her help with conducting the experiments reported in this work. This project has received funding from the European Union's Horizon 2020 Research and Innovation Programme under the Marie Skłodowska‐Curie Grant Agreement No. 811965; project STINTS (Skin Tissue Integrity under Shear). This work was also partially supported by the Israeli Ministry of Science & Technology (Medical Devices Program Grant no. 3‐17421, awarded to Professor Amit Gefen in 2020) and by curea medical GmbH (Steinfurt, Germany).
Orlov A, Gefen A. Fluid handling performance of wound dressings tested in a robotic venous leg ulcer system under compression therapy. Int Wound J. 2023;20(5):1384‐1392. doi: 10.1111/iwj.13985
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Harding K, Dowsett C, Fias L, et al. Simplifying venous leg ulcer management: consensus recomendations. Wounds Int. 2015;Special Edition:1‐25. [Google Scholar]
- 2. Kolluri R, Lugli M, Villalba L, et al. An estimate of the economic burden of venous leg ulcers associated with deep venous disease. Vasc Med. 2022;27:63‐72. doi: 10.1177/1358863X211028298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nelson EA, Adderley U. Venous leg ulcers. BMJ Clin Evid. 2016;2016:1902. [PMC free article] [PubMed] [Google Scholar]
- 4. Cullum N, Nelson EA, Fletcher AW, Sheldon TA. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2000;3:CD000265. doi: 10.1002/14651858.CD000265 [DOI] [PubMed] [Google Scholar]
- 5. Cullum N, Nelson E, Fletcher A, Sheldon T. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2001;2:1‐27. doi: 10.1002/14651858.CD000265 [DOI] [PubMed] [Google Scholar]
- 6. Sackheim K, De Araujo TS, Kirsner RS. Compression modalities and dressings: their use in venous ulcers. Dermatol Ther. 2006;19:338‐347. doi: 10.1111/J.1529-8019.2006.00092.X [DOI] [PubMed] [Google Scholar]
- 7. Gefen A, Ousey K. Safe and effective wound care during the COVID‐19 pandemic. J Wound Care. 2020;29:622‐623. 10.12968/jowc.2020.29.11.622 [DOI] [PubMed] [Google Scholar]
- 8. Gefen A, Alves P, Beeckman D, et al. How should clinical wound care and management translate to effective engineering standard testing requirements from foam dressings? Mapping the existing gaps and needs. Adv Wound Care. 2022;1‐19. doi: 10.1089/wound.2021.0173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lustig A, Gefen A. The performance of gelling fibre wound dressings under clinically relevant robotic laboratory tests. Int Wound J. 2022;19:1‐19. doi: 10.1111/IWJ.13761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Orlov A, Gefen A. The fluid handling performance of the curea P1 multipurpose dressing against superabsorbent and foam dressing technologies. Int Wound J. 2022;19:945‐956. doi: 10.1111/IWJ.13774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lustig A, Alves P, Call E, Santamaria N, Gefen A. The sorptivity and durability of gelling fibre dressings tested in a simulated sacral pressure ulcer system. Int Wound J. 2020;18:194‐208. doi: 10.1111/iwj.13515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lustig A, Gefen A. Three‐dimensional shape‐conformation performances of wound dressings tested in a robotic sacral pressure ulcer phantom. Int Wound J. 2021;18:670‐680. doi: 10.1111/iwj.13569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lustig A, Gefen A. Fluid management and strength post‐simulated use of primary and secondary dressings for treating diabetic foot ulcers: robotic phantom studies. Int Wound J. 2021;19:305‐315. doi: 10.1111/iwj.13631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gefen A, Timmons J, Carlsson E, Wendel M, Hamberg K, Rook S. Exufiber® and Exufiber® Ag+: a review of the scientific and clinical evidence. Wounds Int. 2021;Special Edition:1‐25. [Google Scholar]
- 15. Orlov A, Lustig A, Grigatt A, Gefen A. Fluid handling dynamics and durability of silver‐containing gelling fiber dressings tested in a robotic wound system. Adv Skin Wound Care. 2021;35:326‐334. doi: 10.1097/01.ASW.0000823972.16446.ff [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. ASTM D412–06 . Standard Test Methods for Vulcanized Rubber and Thermoplastic Elastomers‐Tension. West Conshohocken, PA: ASTM International; 2006, www.astm.org. doi: 10.1520/D0412-06 [DOI] [Google Scholar]
- 17. Xu F, Lu TJ. Chapter 3 skin biothermomechanics: Modeling and experimental characterization. Adv Appl Mech. 2009;43:147‐248. doi: 10.1016/S0065-2156(09)43003-5 [DOI] [Google Scholar]
- 18. Kuthe CD, Uddanwadiker RV. Investigation of effect of fiber orientation on mechanical behavior of skeletal muscle. J Appl Biomater Funct Mater. 2016;14:e154‐e162. doi: 10.5301/jabfm.5000275 [DOI] [PubMed] [Google Scholar]
- 19. Graham HK, McConnell JC, Limbert G, Sherratt MJ. How stiff is skin? Exp Dermatol. 2019;28:4‐9. doi: 10.1111/exd.13826 [DOI] [PubMed] [Google Scholar]
- 20. Gefen A, Haberman E. Viscoelastic properties of ovine adipose tissue covering the gluteus muscles. J Biomech Eng. 2007;129:924‐930. doi: 10.1115/1.2800830 [DOI] [PubMed] [Google Scholar]
- 21. Vieira SL, Pavan TZ, Junior JE, Carneiro AAO. Paraffin‐gel tissue‐mimicking material for ultrasound‐guided needle biopsy phantom. Ultrasound Med Biol. 2013;39:2477‐2484. doi: 10.1016/j.ultrasmedbio.2013.06.008 [DOI] [PubMed] [Google Scholar]
- 22. Omeara S, Martyn‐St JM. Foam dressings for venous leg ulcers. Cochrane Database Syst Rev. 2013;5:CD009907. doi: 10.1002/14651858.CD009907.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones J, Hampton S. Use of a superabsorbent dressing in the management of exudate in hard‐to‐heal wounds. Br J Community Nurs. 2021;26:S20‐S29. 10.12968/BJCN.2021.26.SUP3.S20 [DOI] [PubMed] [Google Scholar]
- 24. Veličković VM, Prieto PA, Krga M, Jorge AM. Superabsorbent wound dressings versus foams dressings for the management of moderate‐to‐highly exuding venous leg ulcers in French settings: an early stage model‐based economic evaluation. J Tissue Viability. 2022;31:523‐530. doi: 10.1016/J.JTV.2022.04.005 [DOI] [PubMed] [Google Scholar]
- 25. Gethin G, Ivory JD, Sezgin D, Muller H, O'Connor G, Vellinga A. What is the “normal” wound bed temperature? A scoping review and new hypothesis. Wound Repair Regen. 2021;29:843‐847. doi: 10.1111/WRR.12930 [DOI] [PubMed] [Google Scholar]
- 26. Junker JPE, Kamel RA, Caterson EJ, Eriksson E. Clinical impact upon wound healing and inflammation in moist, wet, and dry environments. Adv Wound Care. 2013;2:348‐356. doi: 10.1089/WOUND.2012.0412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Okan D, Woo K, Ayello EA, Sibbald G. The role of moisture balance in wound healing. Adv Skin Wound Care. 2007;20:39‐53. doi: 10.1097/00129334-200701000-00013 [DOI] [PubMed] [Google Scholar]
- 28. Rodgers A, Watret L. Maceration and its effect on periwound margins. Wounds Int. 2003;6:2‐5. [Google Scholar]
- 29. Rippon MG, Ousey K, Rogers AA, Atkin L. Wound hydration versus maceration: understanding the differences. Wounds UK. 2016;12:62‐68. [Google Scholar]
- 30. Say M, Tella E, Boccara O, et al. Leg ulcers in childhood: a multicenter study in France. Ann Dermatol Venereol. 2022;149:51‐55. doi: 10.1016/J.ANNDER.2021.05.004 [DOI] [PubMed] [Google Scholar]
- 31. Havas E, Parviainen T, Vuorela J, Toivanen J, Nikula T, Vihko V. Lymph flow dynamics in exercising human skeletal muscle as detected by scintography. J Physiol. 1997;504(Pt 1):233‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


