Abstract
Summary
In the Diabetes Prevention Program Outcome Study (DPPOS), a cohort at high risk of diabetes, randomization to intensive lifestyle intervention or metformin, both associated with weight loss, did not have long-term negative effects on BMD compared with the placebo group. Potential positive effects of metformin on bone warrant further investigation.
Introduction
Randomization to lifestyle intervention (ILS) or metformin in the Diabetes Prevention Program (DPP) resulted in weight loss and reduced progression to diabetes. Weight loss is associated with reduced bone mineral density (BMD), but the long-term effects of these interventions on BMD are unknown. In the DPP Outcome Study (DPPOS), we determined if randomization to ILS or metformin, compared with placebo, was associated with differences in BMD approximately 16 years later.
Methods
Of 3234 DPP participants, 2779 continued in DPPOS and were offered ILS in group format. Those randomized to metformin were offered unmasked metformin. At DPPOS year 12, 1367 participants had dual-energy X-ray absorptiometry scans. BMD in metformin and ILS groups was compared to placebo using sex-specific linear regression models, adjusted for age, race/ethnicity, and weight and weight-bearing activity at DPP baseline.
Results
At DPPOS year 12, mean age was 66.5 (±9.5) years. Femoral neck BMD was similar in the ILS and placebo groups in men (difference = −0.021 g/cm2, 95%CI (−0.063, 0.021)) and in women (+0.014 g/cm2, 95%CI (−0.014, 0.042)). Femoral neck BMD was higher in the metformin compared to placebo group although not statistically different in men (+0.017 g/cm2, 95% CI (−0.023, 0.058)) and in women (+0.019 g/cm2, 95% CI (−0.009, 0.047)). Prevalence of osteoporosis was low and similar across treatment groups in men (0.9%; p=0.745) and women (2.4%; p=0.466).
Conclusion
In a cohort at high risk of diabetes, lifestyle intervention or metformin did not appear to have long-term negative effects on BMD. Potential positive effects of metformin on bone warrant further research.
Keywords: Bone mineral density, Metformin, Prediabetes, Weight loss
Introduction
Randomization to lifestyle intervention (ILS) or metformin in the Diabetes Prevention Program (DPP) reduced progression to diabetes. Both interventions also caused weight loss, more markedly in the group assigned to ILS [1]. By design, ILS was intended to achieve a loss of 7% of body weight as well as an increase in physical activity. Modest weight loss was also observed in those assigned to metformin, consistent with its known effects as a treatment for diabetes [2]. Weight loss is associated with bone loss in broader populations of older adults [3, 4]. In the Look AHEAD trial, conducted among those with type 2 diabetes, a lifestyle intervention with the goals of weight loss and increased physical activity resulted in greater bone loss compared with controls, assessed at 1 and 4 years post-randomization [5, 6]. Pre-clinical studies suggest that metformin may have positive effects on bone which might counter the effects of weight loss [7]. Reduced bone mineral density (BMD) is a risk factor for fracture, an important concern, particularly in older adults. However, the long-term net effects of either ILS or metformin on BMD, in those at high risk of diabetes, are not known.
Because of the success of both the lifestyle and metformin interventions in preventing diabetes and their potential widespread implementation, it is important to understand each of their skeletal effects over the long-term. Because weight loss has such a strong effect on bone density, we hypothesized that the ILS group and, to a lesser degree, the metformin group would experience reductions in BMD compared with the placebo group. We used data from the DPP Outcome Study (DPPOS) to determine if ILS or metformin, compared with placebo, was associated with differences in bone density over about 16 years.
Methods
As previously described, during DPP, 3234 participants at high risk for diabetes were randomized to ILS, metformin, or placebo [1]; 2775 (85.8%) continued in DPPOS and were offered the ILS intervention in group format [8]. The lifestyle intervention was designed to achieve and maintain 7% weight loss. For physical activity, the lifestyle intervention aimed to achieve and maintain a weekly minimum of 150 min of moderate to vigorous exercise, similar in intensity to brisk walking [1]. During DPP, progress with the lifestyle intervention was monitored on an individual basis with case managers; during DPPOS, group classes were offered to participants every 3 months with educational materials to reinforce the weight loss and physical activity goals [8]. During DPPOS, the metformin group received unmasked metformin, as tolerated, unless discontinued for safety reasons or if a participant developed diabetes requiring management by their own provider.
At DPPOS year 12, 2213 (79.7%) of 2775 participants originally continuing in DPPOS completed a clinic visit. At 16 of the 25 clinical sites, 1513 participants were offered the option of participating in a study of bone density using dual-energy X-ray absorptiometry (DXA). Of those, 1384 (91.5%) participants enrolled in the DXA study, and 1367 successfully completed a DXA scan between July 2013 and March 2014 (Fig. 1). Institutional review boards at all sites approved the DPP and DPPOS protocols and informed consent procedures. Participants provided written consent for participation in the studies.
Fig. 1.
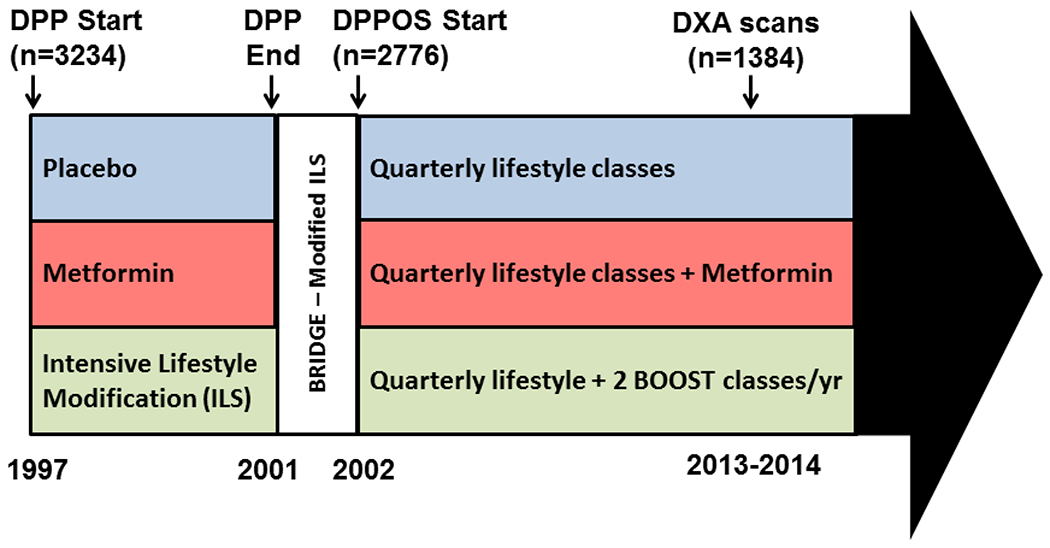
Timeline for the Diabetes Prevention Program (DPP) and DPP Observational Study (DPPOS), including DXA visits
DXA hip and spine scans
DXA scans of the hip and spine were acquired on Hologic or GE Lunar scanners at 16 clinics using 15 scanners. Scans were reviewed for quality and analyzed centrally at the University of California San Francisco (UCSF) using Hologic v13.4 or GE Lunar v14.0 software. Local DXA operators were certified by UCSF. A local spine phantom was scanned regularly throughout the study on each scanner; all scanners were within acceptable limits. GE Lunar BMD values were standardized to Hologic values using published equations for hip and spine [9, 10]. BMD T-scores were calculated using values for young female Caucasians as the reference [11]. Osteoporosis was defined based on femoral neck BMD as T-score ≤−2.5 and low bone density (osteopenia) as −2.5<T-score<−1.
Other measurements
Participants were queried at DPP baseline regarding demographic information. At the DPP baseline and subsequent biannual visits in DPP and DPPOS, participants were weighed while wearing light clothing without shoes. Levels of leisure physical activity during the previous year were assessed annually in DPP and DPPOS, using the Modifiable Activity Questionnaire (MAQ) which has been previously validated [1, 12]. Results are expressed as MET hours/week. For these analyses, activities were identified as weight-bearing and non-weight-bearing prior to analyses, and activity levels summed separately.
Development of diabetes was the primary outcome in DPP and DPPOS. Diagnosis was based on elevated fasting glucose (7.0 mmol/L or higher), assessed every 6 months, or elevated 2-h oral glucose tolerance test (11.1 mmol/L or higher), assessed annually; an initial positive test required confirmation with a repeat test for diagnosis [1]. Participants were queried annually regarding current use of prescription medications, including medications for treatment of diabetes.
Statistical methods
Results were analyzed using an intention-to-treat approach, testing for the effects of randomization to DPP treatments on BMD about 16 years post-randomization. Because weight loss differences across treatment groups were sustained among men but not women in DPPOS and because osteoporosis is more prevalent in women, the results are presented separately by sex. Prevalence of osteopenia and osteoporosis were compared across the 3 treatment groups using Pearson’s chi-squared test. The equality of the average BMD across the treatment groups was tested with ANOVA. Linear regression models adjusted for age, race/ethnicity, DPP baseline weight, and DPP baseline weight-bearing physical activity were used to assess effects of treatment on BMD. Possible effect modification by sex was examined as well as by age using three pre-specified age groups (40–54, 55–69, 70 and older). All analyses were performed in SAS 9.3.
Results
Characteristics of the participants by the original randomization assignment are provided in Table 1. Mean age at Y12 was 66.5 (±9.5) years. Mean time since DPP baseline was 15.6 (±0.7) years. Weight and weight-bearing physical activity were similar across treatment groups at DPP baseline in both men and women. A majority (59%) of participants developed diabetes during the follow-up period. DPPOS participants who completed the DXA study included a higher proportion of white participants (men and women), higher weight at DPP baseline (men), and less weight-bearing physical activity at DPP baseline (women) compared to those who completed a DPPOS Y12 visit but not a DXA visit (Supplementary Table 1).
Table 1.
Characteristics by randomization group
| ILS | MET | PLB | p | |
|---|---|---|---|---|
| MEN | N=144 | N=159 | N=144 | |
| Age at DXA scan (DPPOS Y12) | 70.4 (10.8) | 69.5 ( 9.3) | 67.6 ( 9.6) | 0.030 |
| 40–54 years | 12 (8.3) | 10 (6.3) | 12 (8.3) | |
| 55–69 years | 56 (38.9) | 75 (47.2) | 77 (53.5) | |
| 70+ years | 76 (52.8) | 74 (46.5) | 55 (38.2) | |
| White, non-Hispanic | 89 (61.8) | 102 (64.2) | 81 (56.3) | 0.593 |
| Weight at DPP baseline (kg) | 98.3 (19.3) | 97.3 (15.9) | 99.7 (20.0) | 0.680 |
| Weight-bearing physical activity at DPP baseline (met-hour/week) | 13.0 (17.6) | 13.4 (15.0) | 15.7 (22.4) | 0.429 |
| Diabetes and related parameters at DXA visit | ||||
| Diabetes | 82 (56.9) | 90 (56.6) | 93 (64.6) | 0.290 |
| HbA1c (in DM) (%) | 6.0 (0.6) | 6.0 (0.6) | 6.0 (0.5) | 0.658 |
| Duration of diabetes (year) | 8.8 (4.4) | 9.2 (4.7) | 10.5 (4.3) | 0.031 |
| Any diabetes medication (in DM) | 46 (32) | 49 (31) | 65 (45) | 0.018 |
| Insulin | 8 (5.6) | 9 (5.7) | 12 (8.4) | 0.556 |
| Metformin (includes study drug) | 36 (25) | 105 (66) | 59 (41) | < .001 |
| Sulfonylurea | 12 (8.4) | 11 (7.0) | 17 (11.9) | 0.316 |
| Incretin-based | 9 (6.2) | 14 (8.8) | 12 (8.3) | 0.685 |
| TZD | 2 (1.4) | 3 (1.9) | 4 (2.8) | 0.696 |
| WOMEN | N=307 | N=310 | N=320 | |
| Age at DXA scan (DPPOS Y12) | 64.8 (9.4) | 65.3 (9.2) | 64.4 (8.9) | 0.391 |
| 40–54 years | 48 (15.6) | 38 (12.3) | 43 (13.4) | |
| 55–69 years | 162 (52.8) | 181 (58.4) | 197 (61.6) | |
| 70+ years | 97 (31.6) | 91 (29.4) | 80 (25.0) | |
| White, non-Hispanic | 154 (50.2) | 175 (56.5) | 188 (58.8) | 0.249 |
| Weight at DPP baseline (kg) | 90.0 (18.6) | 90.6 (18.6) | 92.8 (19.6) | 0.160 |
| Weight-bearing physical activity at DPP baseline (met-hour/week) | 7.5 (12.2) | 9.1 (11.5) | 8.0 (14.1) | 0.261 |
| Diabetes and related parameters at DXA visit | ||||
| Diabetes | 170 (55.4) | 175 (56.4) | 201 (62.8) | 0.123 |
| HbA1c (in DM only) (%) | 6.0 (0.5) | 6.0 (0.5) | 5.9 (0.5) | 0.576 |
| Duration of diabetes (year) | 9.0 (4.1) | 9.5 (4.5) | 10.1 (4.3) | 0.051 |
| Any diabetes medication (in DM) | 105 (34.2) | 81 (26.1) | 127 (39.7) | 0.001 |
| Insulin | 17 (5.5) | 22 (7.1) | 19 (5.9) | 0.726 |
| Metformin (includes study drug) | 95 (30.9) | 219 (70.6) | 106 (33.1) | < .001 |
| Sulfonylurea | 28 (9.1) | 23 (7.4) | 27 (8.4) | 0.757 |
| Incretin-based | 24 (7.8) | 25 (8.1) | 26 (8.1) | 0.989 |
| TZD | 1 (0.3) | 5 (1.6) | 3 (0.9) | 0.261 |
Data are mean (SD) or N (%)
Participants in the ILS group experienced substantial weight loss in the first year of the DPP, while those in the metformin group had more modest losses (Fig. 2). At DPPOS Y12 (16 years after baseline) in men, the ILS group still had greater net weight loss from baseline compared to placebo (p=0.0003) but net weight loss was similar in the metformin and placebo groups (p=0.158). At DPPOS Y12 in women, net weight loss from DPP baseline did not differ across treatment groups in women (p=0.891).
Fig. 2.
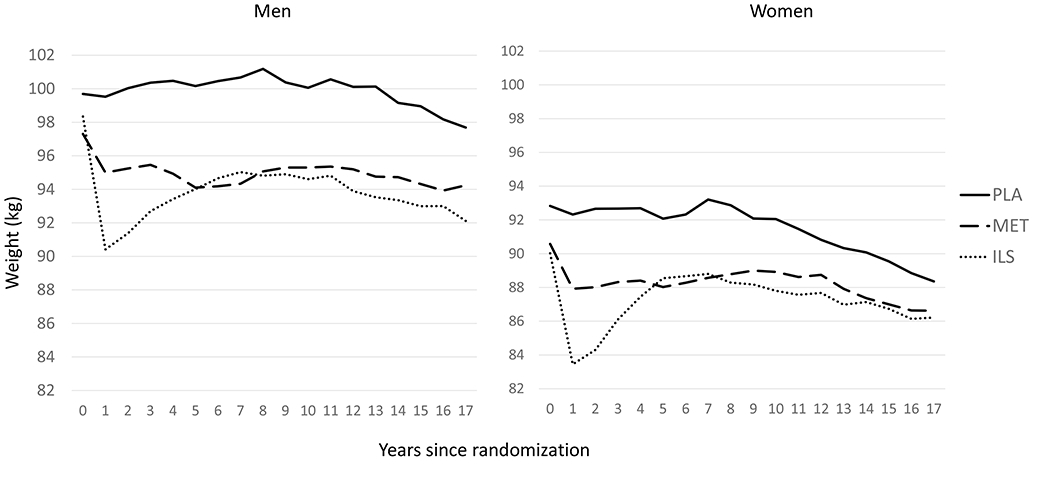
Mean weight at each annual visit in DPP and DPPOS for men and women who completed DXA visit. Mean weight loss from DPP baseline to DPPOS Y12 (about 16 years later) differed across treatment groups in men (−5.9 kg ILS, −3.2 kg metformin, −1.7 kg placebo; p=0.002), but not in women (−3.7 kg for all groups, p=0.891)
Participants in the ILS also experienced an increase during the DPP trial in physical activity levels [13] and more specifically in weight-bearing physical activity (Fig. 3). At DPPOS Y12, weight-bearing physical activity had decreased from DPP baseline in the metformin and placebo, but not the ILS, groups in men and women. However, differences across treatment groups were only statistically significant in women (p=0.036).
Fig. 3.
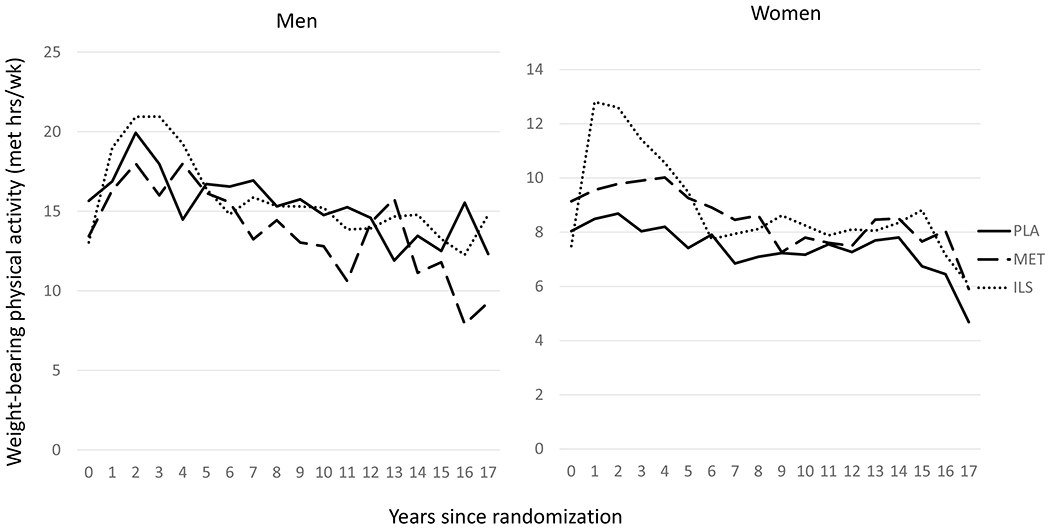
Mean weight-bearing physical activity at each annual visit in DPP and DPPOS for men and women who completed DXA visit. Mean change in weight-bearing physical activity from DPP baseline to DPPOS Y12 (about 16 years) was not statistically different in men (+0.6 met-h/week ILS; −2.3 met-h/week metformin; −2.4 met-h/week placebo; p=0.458) but was different across treatment groups in women (+0.7 met-h/week ILS; −1.9 met-h/week metformin; −2.0 met-h/week placebo; p= 0.036)
In unadjusted comparisons in men, BMD at DPPOS Y12 was lowest in the ILS group but differences between treatment groups were not statistically significant (Table 2). In women, BMD was similar across treatment groups. Prevalence of osteoporosis was low in both men (0.9%) and women (2.4%), and the prevalence was not statistically different across groups (Table 2).
Table 2.
Bone mineral density and prevalence of osteoporosis/osteopenia at DPPOS Y12
| ILS | MET | PLB | p | |
|---|---|---|---|---|
| Men | ||||
| Lumbar spine BMD (g/cm2) | 1.23 (0.22) | 1.27 (0.22) | 1.25 (0.22) | 0.163 |
| Total hip BMD (g/cm2) | 1.04 (0.16) | 1.07 (0.17) | 1.06 (0.15) | 0.186 |
| Femoral neck BMD (g/cm2) | 0.88 (0.18) | 0.92 (0.19) | 0.91 (0.18) | 0.181 |
| Osteoporosisa n (%) | 2 (1.4) | 1 (0.6) | 1 (0.7) | 0.745 |
| Osteopeniab n (%) | 37 (26.1) | 30 (19.2) | 31 (21.7) | 0.361 |
| Women | ||||
| Lumbar spine BMD (g/cm2) | 1.14 (0.19) | 1.14 (0.20) | 1.14 (0.21) | 0.885 |
| Total hip BMD (g/cm2) | 0.98 (0.15) | 0.98 (0.16) | 0.97 (0.16) | 0.739 |
| Femoral neck BMD (g/cm2) | 0.86 (0.16) | 0.87 (0.19) | 0.85 (0.17) | 0.361 |
| Osteoporosisa n (%) | 5 (1.7) | 7 (2.3) | 10 (3.2) | 0.466 |
| Osteopeniab n (%) | 111 (36.6) | 106 (34.6) | 109 (34.4) | 0.816 |
Mean (SD) or n (%).
Femoral neck BMD T-score ≤ −2.5;
−1<Femoral neck BMD T-score<−2.5
In linear regression models adjusted for age, race/ethnicity, baseline weight, and baseline weight-bearing physical activity, ILS was not associated with statistically significant differences in BMD compared with placebo at all three skeletal sites for men or women (Table 3). In women and men, metformin, compared with placebo, was associated with higher BMD, but the results were not statistically significant. Tests for interaction with sex did not find evidence of interaction for total hip (p=0.320), femoral neck (p=0.570), or lumbar spine (p=0.408). In models combining men and women, femoral neck BMD was higher in metformin compared with placebo group, and the difference was statistically significant (+0.027 g/cm2, 95% CI: (0.007, 0.047); p= 0.009).
Table 3.
Difference in BMD (g/cm2) comparing intensive lifestyle (ILS) and metformin (MET) interventions to placebo at DPPOS year 12
| Unadjusted |
Adjusteda |
|||||
|---|---|---|---|---|---|---|
| Difference | 95% CI | Pr > |t| | Difference | 95% CI | Pr > |t| | |
| Men | ||||||
| Total hip | ||||||
| ILS | −0.021 | (−0.059, 0.016) | 0.265 | −0.005 | (−0.039, 0.030) | 0.796 |
| MET | 0.013 | (−0.024, 0.050) | 0.491 | 0.029 | (−0.005, 0.062) | 0.101 |
| Placebo | ref | ref | ||||
| Femoral neck | ||||||
| ILS | −0.021 | (−0.063, 0.021) | 0.321 | −0.001 | (−0.040, 0.037) | 0.943 |
| MET | 0.017 | (−0.023, 0.058) | 0.402 | 0.036 | (−0.001, 0.073) | 0.058 |
| Placebo | ref | ref | ||||
| Lumbar spine | ||||||
| ILS | −0.024 | (−0.076, 0.028) | 0.365 | −0.028 | (−0.022, 0.078) | 0.264 |
| MET | 0.017 | (−0.034, 0.067) | 0.521 | 0.020 | (−0.028, 0.068) | 0.411 |
| Placebo | ref | ref | ||||
| Women | ||||||
| Total hip | ||||||
| ILS | 0.009 | (−0.016, 0.033) | 0.473 | 0.010 | (−0.011, 0.031) | 0.340 |
| MET | 0.006 | (−0.018, 0.031) | 0.623 | 0.012 | (−0.009, 0.033) | 0.258 |
| Placebo | ref | ref | ||||
| Femoral neck | ||||||
| ILS | 0.014 | (−0.014, 0.042) | 0.329 | 0.010 | (−0.014, 0.034) | 0.419 |
| MET | 0.019 | (−0.009, 0.047) | 0.176 | 0.022 | (−0.002, 0.046) | 0.073 |
| Placebo | ref | ref | ||||
| Lumbar spine | ||||||
| ILS | −0.007 | (−0.038, 0.025) | 0.670 | −0.006 | (−0.034, 0.022) | 0.683 |
| MET | 0.000 | (−0.031, 0.032) | 0.990 | 0.003 | (−0.025, 0.031) | 0.863 |
| Placebo | ref | ref | ||||
Adjusted for age, race, baseline weight, baseline weight-bearing physical activity
In men, there was evidence of effect modification by age for the association between metformin treatment, compared to placebo, and total hip BMD (p for interaction = 0.040) and between metformin and femoral neck BMD (p for interaction = 0.046) (Supplemental Table 2). Metformin appeared to have a larger positive effect on BMD in the 40–54 year age group. In men, age also modified the association between ILS and total hip BMD (p for interaction = 0.0496) as well as femoral neck BMD (p for interaction = 0.0304). ILS was associated with higher total hip and femoral neck BMD in the youngest age group (40–54 years) and lower BMD in the oldest age group (70+ years) compared with placebo. In women, none of the interactions with age was statistically significant (p>0.05).
Discussion
This is the first report of the effects of interventions designed to prevent diabetes in a high risk cohort on the longer term outcome of bone density. Lifestyle intervention and metformin were both effective in reducing progression to diabetes and both resulted in weight loss during the DPP. Weight loss, whether intentional or unintentional, is associated with increased bone loss [14]. However, about 16 years after DPP randomization, there was no evidence of reduced BMD in men or women in the lifestyle intervention or metformin groups compared with the placebo group. Interestingly, there was evidence of higher BMD in the metformin group compared with placebo, in spite of greater weight loss with metformin.
The lifestyle intervention was designed to achieve a weight loss of at least 7% of body weight and a physical activity level of at least 150 min of moderate intensity activity a week. By DPP end (average duration 3.2 years), men and women both experienced greater weight loss and physical activity levels in the ILS group compared to placebo [1]. After the initial weight loss due to ILS, participants regained weight on average. At the time of the DXA visit, about 16 years after DPP baseline, the degree of weight loss was similar across the 3 treatment groups in women but remained statistically different in men. The reason for the persistence of a weight loss difference in men but not women is not clear. In part, the difference is due to greater weight loss in the placebo group in women compared with men, possibly due to better attendance at the lifestyle intervention classes offered after the DPP trial concluded [15]. In any case, the persistence of greater weight loss in the ILS group in men did not result in greater bone loss compared with placebo. For both men and women, despite differences in the pattern of weight loss across treatment groups, with large initial weight loss in the ILS group, we did not find evidence of greater bone loss in the ILS group.
One possible explanation is that the increased weight-bearing physical activity in the ILS group helped to ameliorate bone loss due to weight loss. Exercise training [16] and physical activity [17] have modest positive effects on BMD in older adults. There is also evidence that exercise training can preserve bone in the setting of calorie restriction [18]. In a randomized trial among obese older adults, a diet and exercise intervention achieved weight loss of about 8.5% over 6 months. Assignment to resistance training, but not aerobic exercise, prevented bone loss at the hip [19].
Another possible explanation is that weight regain in the ILS group after the initial weight loss may have blunted any long-term impact on BMD. In contrast to our findings, studies that have assessed BMD within a few years of a weight loss intervention have found greater bone loss at the hip. A meta-analysis of weight loss interventions in overweight and obese patients reported an increased loss of 0.012 g/cm2 (95% CI, – 0.024 to 0.000 g/cm2) at the total hip compared with placebo after 24 months [14]. Bone loss at the spine did not differ compared with placebo. In the Look AHEAD trial, a lifestyle intervention similar to the ILS in DPP aimed for weight loss of 10% or more and weekly physical activity of 175 min in older adults with type 2 diabetes [20]. Compared with the diabetes support and education (DSE) group, the lifestyle intervention resulted in greater hip, but not spine, bone loss at 1 and 4 years after baseline [5, 6]. At 4 years, men in Look AHEAD had 1.6% greater bone loss at the total hip in the lifestyle intervention compared with the DSE group [6]. The lifestyle intervention group in Look AHEAD also experienced more fragility fractures compared with the control group [21]. Bone loss has not been assessed after longer follow-up in the Look AHEAD trial, limiting the ability to compare these results with our findings in DPPOS. Our findings provide reassurance that intensive lifestyle intervention for diabetes prevention does not increase long-term risk of bone loss. However, as DXA scans were not obtained earlier for DPP participants, it is not known whether there were more immediate effects of the intervention on BMD changes. Larger studies of incident fracture are needed to clarify whether the net effect of weight loss with increased weight-bearing activity during DPP had any measurable impact on fracture risk.
The metformin group also experienced greater weight loss during the DPP trial compared to placebo although the degree of weight loss was less than the ILS group [1]. However, by the time of the DXA visit, there was no longer a statistically significant difference in weight change in men or women, comparing the metformin and placebo groups. The metformin group did not have increased physical activity compared with the placebo group during DPP or DPPOS. In spite of this earlier weight loss in the metformin group, we found that the metformin group experienced less bone loss at the femoral neck than the placebo group. Rodent and in vitro models suggest positive effects of metformin on bone formation and bone density [22, 23]. Some observational studies have reported lower risk of fractures with metformin in patients with diabetes [24]. However, in the ADOPT trial, there was no evidence of differences in fracture risk comparing metformin with a sulfonylurea [25]. The DPPOS results suggest that metformin may have a positive effect on bone. Further studies, directly focused on this question, are warranted to determine if metformin might protect against bone loss, and ultimately fracture, in patients with pre-diabetes or diabetes.
In men, we found evidence of effect modification by age in the results for DPP treatment assignment and BMD at the total hip and femoral neck. In the oldest age group (70+ years), ILS was associated with lower hip BMD while metformin had little positive effect. While these interactions may be due to chance, the impact of ILS and metformin on the oldest age group warrants further research as this is the population at highest risk of bone loss and fracture.
Diabetes was more prevalent in the placebo group, although not statistically different, by the time of the DXA visit. Reports from several cohorts of older adults have identified higher BMD in those with diabetes compared with prediabetes [26–28]. However, we did not find higher BMD in the placebo group, compared with either ILS or metformin. The thiazolidinediones (TZDs), used to treat diabetes, have a negative effect on BMD [29], but few participants (< 3%) reported current TZD use in any of the groups. Other diabetes medications appear to be neutral with respect to bone [30, 31].
The strengths of this study include a randomized design, large population, and centrally controlled BMD measurements. A limitation of the study is the lack of a baseline BMD measurement at the time of randomization, although the process of randomization likely insured similar baseline BMD levels in the original three groups. In addition, participants who were lost to follow-up during the 16 years from randomization to the DXA visit may have differed in their BMD compared with those who remained in the study, possibly introducing bias in the observed associations between treatment assignment and BMD. Finally, we cannot determine shorter-term effects of the interventions on BMD.
In conclusion, the intensive lifestyle and metformin interventions that successfully reduced diabetes incidence in the DPP trial did not have long-term negative consequences for bone mineral density. Metformin may protect against bone loss but our results were inconclusive, and further studies are needed. Our results suggest that the use of an intensive lifestyle intervention or metformin for diabetes prevention is safe with regard to skeletal health.
Supplementary Material
Acknowledgements
The Research Group gratefully acknowledges the commitment and dedication of the participants of the DPP and DPPOS.
Funding
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) under Award Number U01 DK048489, by providing funding during DPP and DPPOS to the clinical centers and the Coordinating Center for the design and conduct of the study, and collection, management, analysis, and interpretation of the data. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The Southwestern American Indian Centers were supported directly by the NIDDK, including its Intramural Research Program, and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, and the Department of Veterans Affairs supported data collection at many of the clinical centers. Funding was also provided by the National Institute of Child Health and Human Development, the National Institute on Aging, the National Eye Institute, the National Heart Lung and Blood Institute, the National Cancer Institute, the Office of Research on Women’s Health, the National Institute on Minority Health and Health Disparities, the Centers for Disease Control and Prevention, and the American Diabetes Association. Merck KGaA provides medication for DPPOS. DPP/DPPOS have also received donated materials from Bristol-Myers Squibb, Parke-Davis, and LifeScan Inc. LifeScan Inc., Health O Meter, Hoechst Marion Roussel, Inc., Merck-Medco Managed Care, Inc., Merck and Co., Nike Sports Marketing, Slim Fast Foods Co., and Quaker Oats Co. donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices Corp., Matthews Media Group, Inc., and the Henry M. Jackson Foundation provided support services under subcontract with the Coordinating Center. The sponsor of this study was represented on the Steering Committee and played a part in study design, how the study was done, and publication. The opinions expressed are those of the study group and do not necessarily reflect the views of the funding agencies.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00198-021-05989-1.
Ethics approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Institutional review boards at all sites approved the DPP and DPPOS protocols and informed consent procedures.
Consent to participate Written informed consent was obtained from all individual participants included in the study.
Conflicts of interest None.
Availability of data and material
Some or all data generated or analyzed during this study are available in the NIDDK data repository.
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Group DPPR (2012) Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care 35:731–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ensrud KE, Fullman RL, Barrett-Connor E, Cauley JA, Stefanick ML, Fink HA, Lewis CE, Orwoll E (2005) Voluntary weight reduction in older men increases hip bone loss: the osteoporotic fractures in men study. J Clin Endocrinol Metab 90:1998–2004 [DOI] [PubMed] [Google Scholar]
- 4.Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PW, Kiel DP (2000) Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 15:710–720 [DOI] [PubMed] [Google Scholar]
- 5.Schwartz AV, Johnson KC, Kahn SE, Shepherd JA, Nevitt MC, Peters AL, Walkup MP, Hodges A, Williams CC, Bray GA (2012) Effect of one year of an intentional weight loss intervention on bone mineral density in type 2 diabetes: results from the Look AHEAD randomized trial. J Bone Miner Res 27:619–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipkin EW, Schwartz AV, Anderson AM, Davis C, Johnson KC, Gregg EW, Bray GA, Berkowitz R, Peters AL, Hodges A, Lewis C, Kahn SE, the Look AHEAD Research Group (2014) The Look AHEAD Trial: bone loss at 4-year follow-up in type 2 diabetes. Diabetes Care 37:2822–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarthy AD, Cortizo AM, Sedlinsky C (2016) Metformin revisited: does this regulator of AMP-activated protein kinase secondarily affect bone metabolism and prevent diabetic osteopathy. World J Diabetes 7:122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, Brenneman AT, Brown-Friday JO, Goldberg R, Venditti E, Nathan DM (2009) 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 374:1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hui SL, Gao S, Zhou XH, Johnston CC Jr, Lu Y, Gluer CC, Grampp S, Genant H (1997) Universal standardization of bone density measurements: a method with optimal properties for calibration among several instruments. J Bone Miner Res 12:1463–1470 [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, Fuerst T, Hui S, Genant HK (2001) Standardization of bone mineral density at femoral neck, trochanter and Ward’s triangle. Osteoporos Int 12:438–444 [DOI] [PubMed] [Google Scholar]
- 11.Kanis JA, Bianchi G, Bilezikian JP, Kaufman JM, Khosla S, Orwoll E, Seeman E (2011) Towards a diagnostic and therapeutic consensus in male osteoporosis. Osteoporos Int 22:2789–2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kriska AM, Knowler WC, LaPorte RE, Drash AL, Wing RR, Blair SN, Bennett PH, Kuller LH (1990) Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care 13:401–411 [DOI] [PubMed] [Google Scholar]
- 13.Kriska AM, Rockette-Wagner B, Edelstein SL, Bray GA, Delahanty LM, Hoskin MA, Horton ES, Venditti EM, Knowler WC, DPP Research Group (2021) The impact of physical activity on the prevention of type 2 diabetes: evidence and lessons learned from the diabetes prevention program, a long-standing clinical trial incorporating subjective and objective activity measures. Diabetes Care 44:43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zibellini J, Seimon RV, Lee CM, Gibson AA, Hsu MS, Shapses SA, Nguyen TV, Sainsbury A (2015) Does diet-induced weight loss lead to bone loss in overweight or obese adults? A systematic review and meta-analysis of clinical trials. J Bone Miner Res 30:2168–2178 [DOI] [PubMed] [Google Scholar]
- 15.Venditti EM, Bray GA, Carrion-Petersen ML, Delahanty LM, Edelstein SL, Hamman RF, Hoskin MA, Knowler WC, Ma Y, Diabetes Prevention Program Research Group (2008) First versus repeat treatment with a lifestyle intervention program: attendance and weight loss outcomes. Int J Obes 32:1537–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shojaa M, Von Stengel S, Schoene D et al. (2020) Effect of exercise training on bone mineral density in post-menopausal women: a systematic review and meta-analysis of intervention studies. Front Physiol 11:652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinheiro MB, Oliveira J, Bauman A, Fairhall N, Kwok W, Sherrington C (2020) Evidence on physical activity and osteoporosis prevention for people aged 65+ years: a systematic review to inform the WHO guidelines on physical activity and sedentary behaviour. Int J Behav Nutr Phys Act 17:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yarizadeh H, Asadi S, Baharlooi H, Setayesh L, Kakavandi NR, Hambly C, Djafarian K, Mirzaei K (2021) Beneficial impact of exercise on bone mass in individuals under calorie restriction: a systematic review and Meta-analysis of randomized clinical trials. Crit Rev Food Sci Nutr 61:553–565 [DOI] [PubMed] [Google Scholar]
- 19.Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E, Armamento-Villareal R, Qualls C (2017) Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med 376:1943–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryan DH, Espeland MA, Foster GD, Haffner SM, Hubbard VS, Johnson KC, Kahn SE, Knowler WC, Yanovski SZ (2003) Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 24:610–628 [DOI] [PubMed] [Google Scholar]
- 21.Johnson KCLC, Womack C, Garcia KR, Wagenknecht LE, Pownall HJ, Horton ES, Pi-Sunyer X, Gregg E, Schwartz AV (2016) The effect of intentional weight loss on fracture risk in diabetics: results from the look AHEAD Clinical Trial. J Bone Miner Res 65:LB–80 [Google Scholar]
- 22.Wang C, Li H, Chen SG, He JW, Sheng CJ, Cheng XY, Qu S, Wang KS, Lu ML, Yu YC (2012) The skeletal effects of thiazolidinedione and metformin on insulin-resistant mice. J Bone Miner Metab 30:630–637 [DOI] [PubMed] [Google Scholar]
- 23.Lecka-Czernik B (2017) Diabetes, bone and glucose lowering agents – Basic biology. Diabetologia 60:1163–1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starup-Linde J, Gregersen S, Frost M, Vestergaard P (2016) Use of glucose-lowering drugs and risk of fracture in patients with type 2 diabetes. Bone 95:136–142 [DOI] [PubMed] [Google Scholar]
- 25.Kahn SE, Zinman B, Lachin JM, Haffner SM, Herman WH, Holman RR, Kravitz BG, Yu D, Heise MA, Aftring RP, Viberti G, for the A Diabetes Outcome Progression Trial (ADOPT) Study Group (2008) Rosiglitazone associated fractures in type 2 diabetes: an analysis from ADOPT. Diabetes Care 31:845–851 [DOI] [PubMed] [Google Scholar]
- 26.de Liefde II, van der Klift M, de Laet CE, van Daele PL, Hofman A, Pols HA (2005) Bone mineral density and fracture risk in type-2 diabetes mellitus: the Rotterdam Study. Osteoporos Int 16:1713–1720 [DOI] [PubMed] [Google Scholar]
- 27.Napoli N, Strotmeyer ES, Ensrud KE, Sellmeyer DE, Bauer DC, Hoffman AR, Dam TTL, Barrett-Connor E, Palermo L, Orwoll ES, Cummings SR, Black DM, Schwartz AV (2014) Fracture risk in diabetic elderly men: the MrOS study. Diabetologia 57:2057–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Napoli N, Conte C, Eastell R, Ewing SK, Bauer DC, Strotmeyer ES, Black DM, Samelson EJ, Vittinghoff E, Schwartz AV (2020) Bone turnover markers do not predict fracture risk in type 2 diabetes. J Bone Miner Res 35:2363–2371 [DOI] [PubMed] [Google Scholar]
- 29.Billington EO, Grey A, Bolland MJ (2015) The effect of thiazolidinediones on bone mineral density and bone turnover: systematic review and meta-analysis. Diabetologia 58:2238–2246 [DOI] [PubMed] [Google Scholar]
- 30.Meier C, Schwartz AV, Egger A, Lecka-Czemik B (2016) Effects of diabetes drugs on the skeleton. Bone 82:93–100 [DOI] [PubMed] [Google Scholar]
- 31.Mabilleau G, Bouvard B (2020) Update on: effects of anti-diabetic drugs on bone metabolism. Expert Rev Endocrinol Metab 15:415–43 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Some or all data generated or analyzed during this study are available in the NIDDK data repository.


