Abstract
Shortwave infrared (SWIR) dyes are characterized by their ability to absorb light from 900 to 1400 nm, which is ideal for deep tissue imaging owing to minimized light scattering and interference from endogenous pigments. An approach to access such molecules is to tune the photophysical properties of known near-infrared dyes. Herein, we report the development of a series of easily accessible (three steps) SWIR xanthene dyes based on a dibenzazepine donor conjugated to thiophene (SCR-1), thienothiophene (SCR-2), or bithiophene (SCR-3). We leverage the fact that SCR-1 undergoes a bathochromic shift when aggregated for in vivo studies by developing a ratiometric nanoparticle for NO (rNP-NO), which we employed to successfully visualize pathological levels of nitric oxide in a drug-induced liver injury model via deep tissue SWIR photoacoustic (PA) imaging. Our work demonstrates how easily this dye series can be utilized as a component in nanosensor designs for imaging studies.
Keywords: D-A-D xanthene dyes, SWIR, Photoacoustic imaging, Nitric oxide, Drug-induced liver injury
Graphical Abstract
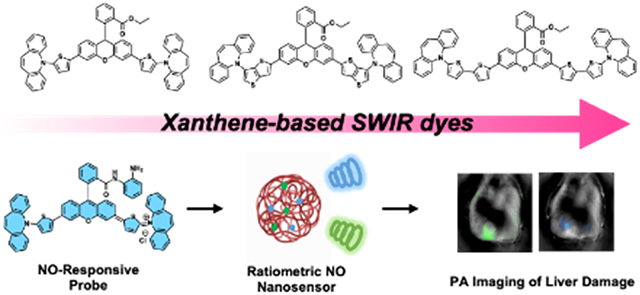
A panel of new SWIR dyes based on the xanthene core was synthesized using C-H activation chemistry. We showcased the biomedical utility of these imaging agents by selecting one example for the development of a ratiometric nanosensor, which we employed to detect elevated levels of nitric oxide in a murine model of drug-induced liver injury via SWIR PA imaging.
Introduction
The “biological window” is traditionally considered to be in the near-infrared (NIR) from 650 to 900 nm, a spectral region where there is reduced absorption attenuation and scattering disturbance caused by endogenous chromophores in comparison to UV and visible light.[1] The unique properties of NIR light have enabled a variety of applications including photodynamic therapy,[2] activation of chemotherapeutics,[3] and photothermal ablation.[4] In vivo molecular imaging via fluorescence[5] and photoacoustic (PA)[6] modes are additional applications that have attracted significant interest owing to their ability to detect biomarkers via activity-based sensing.[7] However, a paradigm shift is currently underway where the preferred incident light is situated at even longer wavelengths. The so-called shortwave infrared (SWIR) window includes light from 900 to 1400 nm, which results in reduced autofluorescence from endogenous pigments like hemoglobin found in blood or melanin found in skin. Moreover, the maximum permission exposure of SWIR light is higher than that of its NIR counterpart.[8] In the context of PA imaging, the use of SWIR imaging agents enables depths (~10 cm) several times greater than comparable NIR systems.[9] Briefly, PA imaging is a modality that relies on the use of light to stimulate the production of acoustic waves (known as the PA effect). After a chromophore is excited by a pulsed laser, a portion of the absorbed energy is released as heat, which causes rapid thermoelastic expansion within the sample being imaged. This increase in temperature leads to pressure fluctuations that generate a detectable ultrasound signal, which can be reconstructed to create high-resolution 3D images of the region of interest.[10] The reduced autofluorescence and tissue scattering as a result of utilizing SWIR light results in higher resolution photoacoustic images in deep tissue. This improvement is necessary for biomedical applications such as the assessment of liver injury from drug overdose. However, most small-molecule dyes operate in the far red to NIR window[11] with only a handful of known SWIR dyes,[12] presumably because they are difficult to prepare, exhibit low chemostability, and are difficult to formulate (poor solubility or hypsochromic shift) for in vivo studies.
Of the existing SWIR-absorbing dyes, most are based on the polymethine structure (e.g., commercially available IR-1061);[13] however, examples from the aza-BODIPY,[14] benzo[1,2-c:4,5- c]bis([1,2,5]-thiadiazole) (BBTD) core,[15] and xanthene families[16] are also known. Shifting the wavelength of maximum absorbance of xanthenes into the SWIR window is an interesting proposition because visible (e.g., fluorescein, rhodamine) and NIR versions are simple to make, can be readily modified, and exhibit remarkable photophysical properties. Initial attempts have resulted in various NIR examples. For example, replacement of the bridging oxygen atom in the core structure with silicon,[17] phosphorus,[18] and sulfone[19] have led to NIR analogs. Likewise, extending the conjugation of the xanthene backbone represents a complementary strategy.[20] Alternatively, our group and others have used a donor-acceptor-donor (D-A-D) approach to develop NIR xanthene dyes.[21] Depending on the strength of the donors, the absorption wavelength can be tuned to span the NIR (and in some instances, extend into the SWIR region). For example, we have reported pyrrole, indole,[22] thienylpiperidine,[23] and indolizine donors with absorption values ranging from ~700 to 920 nm.[24] We hypothesized that further expanding this strategy to include strong donors such as thiophene-dibenzazepine would result in dyes optimized for PA imaging that feature high molar absorptivity, low quantum yields, and SWIR absorbance.
To this end, we report the development of a series of three xanthene-based SWIR dyes using the D-A-D design. We demonstrate their tremendous utility for PA imaging by strategically selecting one example (SCR-1) for a remodeling campaign to yield a nitric oxide (NO)-responsive probe. Furthermore, we co-encapsulate this probe together with a second SWIR dye (non-responsive reference) within a biocompatible polymer matrix to generate a robust nanosensor, which we used to detect NO in deep tissue using a drug-induced liver injury model via ratiometric, SWIR PA imaging.
Results and Discussion
Rational Design of SCR Dyes
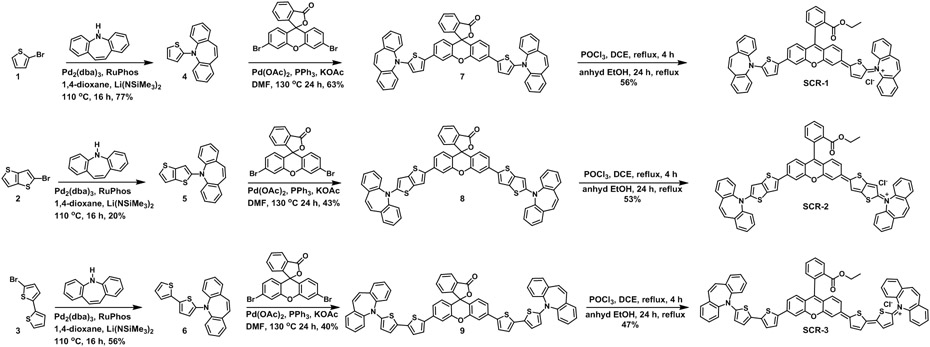
Major considerations for the design of SWIR xanthene dyes include the extension of the dye conjugation,[21] donor strength,[25] a) and the rigidity of the donor moiety.[26] Recent work of extending the xanthene dye wavelength yielded absorption at 1028 nm and emission at 1180 nm.[27] To develop this new series, we anticipated that a dibenzazepine donor moiety would increase the donor strength of the thiophene while also imparting rigidity. Thiophene (SCR-1), thienothiophene (SCR-2), and bithiophene (SCR-3) were incorporated into our dye structures (Scheme 1) to further increase the length of the conjugated system. The synthesis of the dyes begins with the C-N cross-coupling of the dibenzazepine core with the corresponding bromothiophene derivatives (Scheme 1, compounds 1–3), followed by a direct diarylation reaction with 3’,6’-dibromofluoran. From here, the dyes are trapped into the opened form by converting them to the corresponding ethyl ester, giving rise to SCR-1, SCR-2, and SCR-3. It is noteworthy that the synthesis of the dyes occurs in three steps from the bromothiophene derivatives.
Scheme 1.
Chemical synthesis of SCR-1, SCR-2, and SCR-3 SWIR PA dyes starting from the corresponding thiophene starting materials (Compounds 1-3).
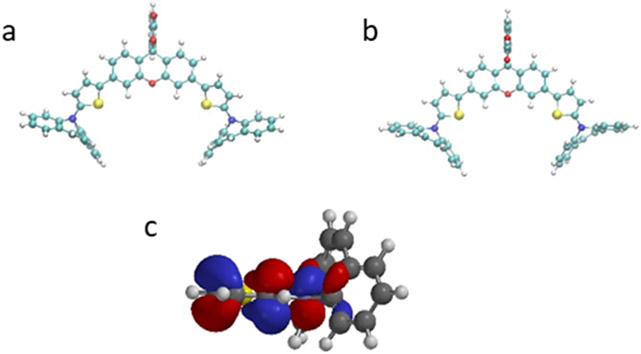
Density-functional theory (DFT) studies of compound 7 and compound 7-COOH (opened form of compound 7) were performed to investigate the donating ability of the dibenzazepine (Figure 1a and b). We found that the thiophene and the rings of the dibenzazepine are nearly perpendicular to each other. This is because the nitrogen of the azepine adopts a sp2 conformation to allow the electron density of the sigma system on the benzenes of the dibenzazepine to donate into the pi system of the thiophene-nitrogen (Figure 1c). Thus, the dibenzazepine increases the electron density in the pi system of the xanthene-thiophene core. At the same time, the rigidity of the dibenzazepine ring leads to very little geometry change between the closed and opened forms. The 3-D shape of the molecule is expected to prevent H-aggregation when encapsulated, ensuring SWIR absorbance in the dye. An assessment of the photophysical properties of SCR-1, SCR-2, and SCR-3 in dichloromethane (DCM) revealed the absorption and emission bands of all three dyes extended well into the NIR to SWIR region, thus fulfilling our primary design objective (Figure S1 and S2, Table 1). The quantum yield (QY) of the dyes decrease as the absorption wavelength increases, which is common to these dyes. However, it is worth noting that the Stokes shift also increases with the conjugation length.
Figure 1.
DFT structures of a) Compound 7, b) Compound 7-COOH, and c) sigma to pi donation
Table 1.
Photophysical properties of SCR-1, SCR-2, and SCR-3
| Dyes | λabs (nm) |
λem (nm) |
QY (%) |
Stokes Shift |
ε (M−1cm−1) x 105 |
|---|---|---|---|---|---|
| SCR-1 | 840 | 950 | 0.31 | 110 | 1.20 |
| SCR-2 | 950 | 1100 | 0.036 | 150 | 1.24 |
| SCR-3 | 1040 | 1260 | 0.016 | 220 | 0.80 |
Development of SCR-NO and Design of Nanosensor (rNP-NO)
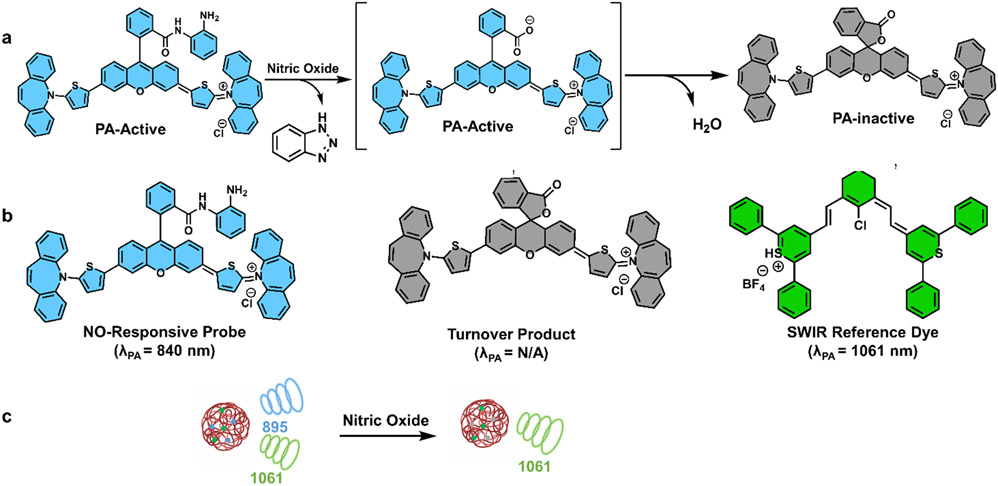
With these dyes in hand, we sought to create an NO-responsive molecule (SCR-NO) that utilized the SCR scaffold. We hypothesized that this could be achieved by capping the carboxylate group of an SCR dye with an NO-responsive unit (i.e., o-phenylenediamine trigger), thus locking the molecule in its ‘open’ PA-active form (Scheme S1).[28] The reaction with NO (via the active species N2O3) would then generate an acyl triazole intermediate that can undergo spontaneous rate-limiting hydrolysis to yield the free dye.[29] After this cleavage event, we anticipated that the dye would immediately close to the PA- inactive lactone form (see Figure 2a). It is noteworthy that the product of the NO reaction with the dye prefers to be in the closed form under the reaction conditions; consequently, we began the experiment with the opened form of the NO probe to determine the success of the reaction using optical measurements. Interestingly, while the probe derived from SCR-1 (herein named SCR-NO) remained opened as we predicted, the corresponding molecule derived from SCR-2 and SCR-3 preferred to adopt a closed spiro-lactam configuration. We ascribe this behavior to the potent electrophilic nature of the extended xanthene core that facilitates intramolecular ring-closing. Having developed SCR-NO, we sought to select a second SWIR-absorbing dye that does not respond to NO to serve as an internal reference. It was essential to select a reference dye on the basis of its stability against NO, as well as other biologically relevant analytes once encapsulated. Another criterion we prioritized was that the reference dye must exhibit minimal spectral overlap with SCR-NO to enable ratiometric calibration. After surveying various SWIR dyes, we chose to employ IR-1061 because it does not react with NO or other biologically relevant analytes such as peroxynitrite and is spectrally resolved from SCR-NO (Figure 2b and 2c).
Figure 2.
a) Proposed reaction mechanism between NO (via N2O3) and SCR-NO. SCR-NO and the free carboxylate shown in blue are PA-active; however, after hydrolysis to the corresponding carboxylic acid product, spontaneous spiro-lactonization yields the closed PA-inactive product shown in grey. b) Chemical structural of SCR-NO (blue), PA-inactive turnover product form of SCR-NO (grey), and IR-1061 (green). c) Cartoon schematic showing the production of two SWIR signals before reaction with NO and one SWIR signal (from the reference) after reaction with NO.
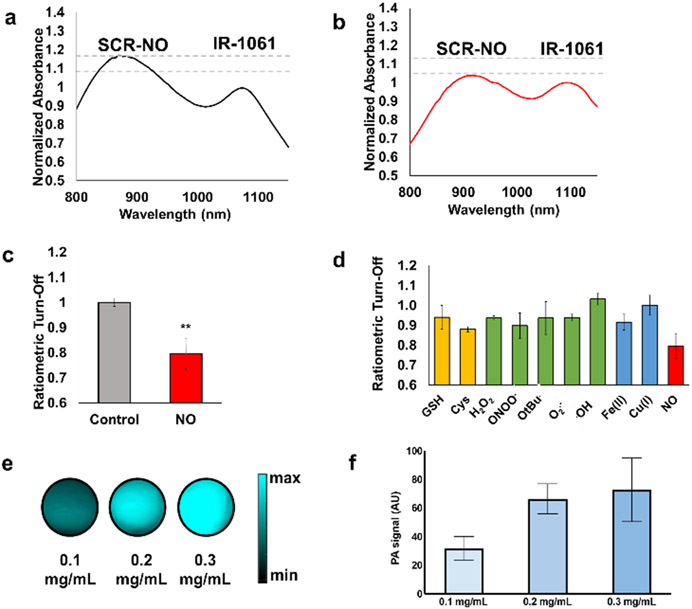
To ensure the relative ratio of the probe and reference remains constant, we turned our attention to identifying a nanoparticle (NP) system that demonstrates minimal leaching of the encapsulated dye components. Moreover, we deemed that it would be essential for the NP to display excellent biocompatibility, display sufficient permeability to allow NO to diffuse into the core to access the encapsulated probe, and exhibit intrinsic liver targeting. Based on unpublished results from our group, we began by individually encapsulating SCR-NO and IR-1061 using DSPE-PEG (a common phospholipids-polymer conjugate) to give NP-SCR-NO and NP-IR-1061, respectively. Both sets of NPs showed the predicted absorbance properties, where the spectra in water were similar to the un-encapsulated parent molecules in organic solvent (Figure S3). However, when the components were co-encapsulated (rNP-NO, ratiometric NP for NO), we observed a favorable ~100 nm red-shift of the SCR-NO λabs into the SWIR region (Figure 3a). The corresponding change for IR-1061 was minimal (1 nm). This key finding likely results from the dyes stacking in a manner that leads to J-aggregate formation. Next, we sought to establish baseline stability of the NPs over a period of several days. Each solution was found to be stable, as no change in the absorbance intensity was observed compared to the initial time point. However, when rNP-NO was treated with NO, the signal corresponding to the probe decreased while the signal from the reference remained unchanged (Figure 3b). The ratiometric turn-off, defined as ratio of λSCR-NO/λIR-1061, changed from 1.00 ± 0.02 to 0.79 ± 0.06 after reaction with NO (Figure 3c). Together, our results indicate the chosen DSPE-PEG matrix imparts sufficient NP stability and is permeable to NO.
Figure 3.
Normalized absorbance spectra of rNP-NO a) before and b) after addition of NO (100 μM). c) Normalized ratiometric turn-off (λSCR-NO/λIR-1061) of rNP-NO after addition of NO (100 μM) and its vehicle control. d) Ratiometric turn-off (λSCR-NO/λIR-1061) of rNP-NO after treatment with various biological analytes for 1 hour. Concentrations were at 100 μM, except for Cys (200 μM), GSH (5 mM), and hydroxyl radical (1 μM). e) PA images of rNP-NO (0.1, 0.2, or 0.3 g/mL) embedded in a tissue-mimicking phantom that was 3 cm thick. Image is compiled from multiple experiments recorded using the same imaging conditions. f) Quantified data from e). n = 3 for all experiments. Error bars = SEM. Statistical analysis was performed using a two-tailed t-test (α = 0.05, ** P < 0.05).
Beyond NO treatment, we also subjected rNP-NO to a panel of biologically relevant analytes found in the liver (Figure 3d). For example, glutathione is present at concentrations in the mM range where it functions as an antioxidant to help the body remove toxins. When rNP-NO was treated with GSH at a concentration of 5 mM, no significant change in the λSCR-NO/λIR-1061 was observed. The same results were found when cysteine, another thiol containing amino acid, was examined at 100 μM. Next, we incubated rNP-NO with various reactive oxygen species (ROS) including hydrogen peroxide, peroxynitrite, tert-butyl hydrogen peroxide, superoxide, and hydroxyl radical. No evidence of probe decomposition was observed. This is impressive since many dye platforms are prone to oxidative decomposition, especially in the presence of highly reactive molecules like hydroxyl radical. Lastly, we evaluated the stability of rNP-NO against iron and copper, which are transition metal ion species that can coordinate to the trigger. The λSCR-NO/λIR-1061 ratio was again unaffected. Together, these results demonstrate that besides reactivity with NO, rNP-NO would be stable to conditions found in the liver when administered to live animals.
In Vitro Assessment of PA Properties
One of the most important determinants of a strong PA signal is the magnitude of the extinction coefficient (EC) of a molecule. This often supersedes the quantum yield (QY) since the former of these two terms dominates the PA brightness value (EC × (1 – QY)). With a calculated value of 8.28 x 104 M−1cm−1 for SCR-1, we anticipated the production of strong ultrasound waves upon irradiation of rNP-NO. To test this, we formulated dense tissue- mimicking phantoms comprised of milk and agar. We then inserted individual samples containing each of the NPs to perform PA imaging. In the case of NP-SCR-NO, a PA signal was observed only when excited at the probe wavelength. Similarly, a PA signal originating from IR-1061 irradiation was the only ultrasound source from NP-IR-1061. However, we recorded PA signals for both the probe and reference when we subjected rNP-NO to the same in vitro test. As can be seen in Figure 3e and 3f, there is a dose-dependent increase in the PA intensity as the NP concentration increases; however, the ratio of λSCR-NO/λIR-1061 remains unchanged.
Evaluation of rNP-NO in a Murine Model of Drug-induced Liver Injury
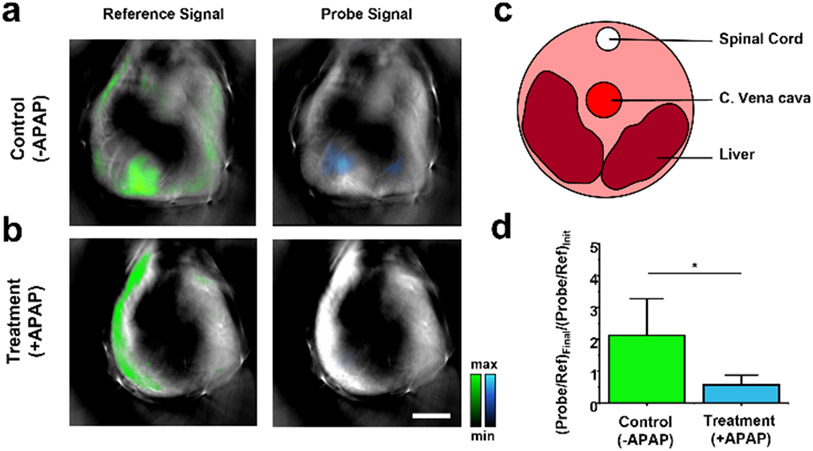
After demonstrating the requisite response to NO and optimal properties for SWIR PA imaging, we turned our attention to in vivo testing of rNP-NO in live mice. For these studies we employed an advanced MSOT (multispectral optoacoustic (photoacoustic) tomography) system to track rNP-NO. PA imaging using the MSOT is essential because: 1) it can be used to visualize the entire animal (including the liver) and present the processed PA images in an easy-to-interpret cross-sectional view; 2) signals from the probe (SCR-NO) and reference dye (IR-1061) can be readily isolated from each other, as well as from interfering endogenous PA-active pigments via spectral unmixing; and 3) it can rapidly switch between the two SWIR wavelengths to facilitate ratiometric calibration. It is noteworthy that the ratiometric feature of rNP-NO is superior to systems where monitoring occurs at a single wavelength. First, a cohort of BALB/c mice (female 6-8-weeks old) were treated with a solution of rNP-NO (6 mg/mL) via systemic administration (intraperitoneal (i.p.) injection). We then determined the biodistribution profile by tracking rNP-NO circulation at two wavelengths. Within 15 minutes, rNP-NO had predominately localized to the liver. Next, we turned our attention to establishing the utility of our nanosensor in a drug-induced liver injury (DILI) model. DILI is responsible for 60% of acute liver failure cases in the United States and is notoriously difficult to diagnose.[30] Visualizing molecular level changes in the liver during DILI is crucial for early intervention and is an important step toward deciphering the mechanistic underpinnings of the disease. For example, NO is believed to contribute to the progression of DILI due to the upregulation of inducible nitric oxide synthases in hepatocytes.[31] Moreover, it is one of the earliest indicators of liver failure as the immune response is rapidly and aggressively activated. We reasoned that by injecting BALB/c mice with acetaminophen (APAP), a drug known to cause DILI when given at high doses, we could reliably monitor the overproduction of NO using rNP-NO and SWIR PA imaging. Furthermore, a recent report by Bai et al. demonstrated an effective NO-responsive ratiometric fluorescent nanoprobe based on modifying IR-1061 with a NO-responsive molecule that showed good ratiometric detection of NO in an APAP-induced DILI model.[32] Subsequently, mice selected to generate the DILI model were i.p. injected with a solution (200 μL) of APAP at a dose of 300 mg/kg. In contrast, mice belonging to the control group were injected with the same volume of 0.9% saline. We then waited for 16 hours (arbitrarily selected) to allow the mice to succumb to DILI before rNP-NO was administered for SWIR PA imaging. Interestingly, the SWIR PA signal from the reference dye was localized to the liver for both groups and the overall signal intensity (color coded in green) was nearly identical (Figure 4a and 4b). However, the SWIR PA signal from the probe could only be seen in the liver of the control animals and was clearly absent in the mice treated with APAP (Figure 4c). Specifically, the (λSCR-NO/λIR-1061)Final/(λSCR-NO/λIR-1061)Init ratio for the control group was determined to be 2.13 ± 1.15 , whereas the corresponding ratio from the APAP treated cohort decreased to 0.56 ± 0.31 (Figure 4d). This result indicates that NO production was indeed upregulated in response to DILI and our probe exhibited sufficient sensitivity to detect this change. Additionally, we confirmed that we induced DILI by submitting blood samples collected ex vivo for liver function analysis (Table S5) and liver samples for H&E staining (Figure S7). It should be noted that we do not anticipate interference from NO in the blood because the concentration is exceedingly low (<0.012 nM) and falls below the detection limit of our nanoprobe.[33] Moreover, while the o-phenylenediamine trigger may react with NO in a pH-dependent manner, the arterial pH of the liver does not change during organ failure.[34]
Figure 4.
Representative cross-sectional SWIR PA images of the liver from mice treated with a) saline (control, n = 3) or b) APAP (treatment group, n = 4). Signals from IR-1061 and SCR-NO are shown in green and blue, respectively. c) Cartoon schematic identifying the liver in cross-sectional view. d) Quantified ratiometric data from a) and b). Scale bar represents 5 mm. Statistical analysis was performed using a two-tailed t-test (α = 0.05, * P < 0.05).
Conclusion
The primary objectives of this study were to employ C-H activation chemistry to develop a panel of synthetically accessible SWIR dyes and to highlight their utility for in vivo activity-based sensing by developing a SWIR PA nanosensor. We were able to successfully show that by incorporating a dibenzazepine donor conjugated to thiophene (SCR-1), thienothiophene (SCR-2), and bithiophene (SCR-3) moiety, the absorbance band of the resulting xanthene dyes bathochromically shifted into the SWIR region. Although we show a more pronounced effect when thienothiophene (SCR-2) and bithiophene (SCR-3) donors were incorporated, the resulting dyes were more prone to ring-closure into the PA-inactive forms. In contrast, strategic capping of the pendant carboxylate group of SCR-1 with an NO-responsive unit resulted in SCR-NO, which remained open prior to encountering NO. We leveraged the ability of this probe to stay “on” or turn “off” to design rNP-NO. When encapsulated, the SCR-NO dye resulted in a bathochromic shift to the SWIR region. We found that co-encapsulation of SCR-NO with IR-1061 as the internal reference dye allowed for a large dynamic range and reliable ratiometric confirmation of NO sensing. This is highlighted in the series of in vitro studies we performed as well as in an in vivo DILI model, where rNP-NO was able to differentiate between mice primed for liver damage using APAP versus a saline control. However, it is notable that a limitation of probes utilizing the o-phenylenediamine trigger is that it is not possible quantify the amount of NO present. While other studies have reported the development of NO probes for this application, our work represents the first example where SWIR is used to monitor the probe and internal reference. Beyond activity-based sensing, the SCR panel of SWIR dyes can be readily transformed into contrast agents by capping carboxylate with an alcohol to yield stable ester products. We envision these SWIR molecules will find utility in cancer treatment (e.g., SWIR PA surgical guidance). Lastly, the insights reported here regarding the importance of the dibenzazepine donor moiety will likely guide future synthetic efforts towards other SWIR xanthene dyes.
Supplementary Material
Acknowledgements
This work was supported by the National Science Foundation for award OIA-1757220 and under the Center for Chemical Innovation in Selective C─H Functionalization (CHE-1700982), and the National Institutes of Health (R35GM133581 to JC). SH acknowledges the Alfred P. Sloan Foundation for support. MYL was supported by the Pines Graduate Fellowship. AKE acknowledges the Beckman Institute for Advanced Science for a graduate fellowship. JC thanks the Helen Corley Petit Scholar Program and the Camille and Henry Dreyfus Foundation. We also acknowledge the Molecular Imaging Laboratory at the Beckman Institute for use of the Nanozoomer, the veterinary histology, and diagnostic laboratories for performing H&E staining and liver function tests. Major funding for the 500 MHz Bruker CryoProbe was provided by the Roy J. Carver Charitable Trust (Muscatine, Iowa; Grant No. 15-4521) to the School of Chemical Sciences NMR Lab. The Q-Tof Ultima mass spectrometer was purchased in part with a grant from the National Science Foundation, Division of Biological Infrastructure (DBI-0100085). The authors thank the Beckman Institute for Advance Sciences for partial support to upgrade the Quantamaster system for probe characterization. The authors also thank Mr. Zhenxiang Zhao for technical assistance for ex vivo experiments.
References
- [1].a) Weissleder R, Nat. Biotechnol 2001, 19, 316–317; [DOI] [PubMed] [Google Scholar]; b) Frangioni JV, Curr. Opin. Chem. Biol 2003, 7, 626–634. [DOI] [PubMed] [Google Scholar]
- [2].Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M, Moan J, Mroz P, Nowis D, Piette J, Wilson BC, Golab J, CA: Cancer J Clin 2011, 61, 250–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kang X, Cai Y, Wang Q, Wang C, Chen W, Yang W, Suryawanshi A, Zhou G, Chen P, Li F, Int J Pharm 2021, 607, 120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Huang X, El-Sayed IH, Qian W, El-Sayed MA, J. Am. Chem. Soc 2006, 128, 2115–2120. [DOI] [PubMed] [Google Scholar]
- [5].Guo Z, Park S, Yoon J, Shin I, Chem. Soc. Rev 2014, 43, 16–29. [DOI] [PubMed] [Google Scholar]
- [6].a) Knox HJ, Chan J, Acc. Chem. Res 2018, 51, 2897–2905; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhao Z, Swartchick CB, Chan J, Chem. Soc. Rev 2022, 51, 829–868; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Yadav AK, Hernandez S, Su S, Chan J, Curr. Opin. Chem. Biol 57, 2020, 114–121. [DOI] [PubMed] [Google Scholar]
- [7].a) Chan J, Dodani SC, Chang CJ, Nat. Chem 2012, 4, 973–984; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) East AK, Lucero MY, Chem. Sci 2021,12, 3393–3405; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Bruemmer KJ, Crossley SWM, Chang CJ, Angew. Chem. Int. Ed 2020, 59, 13734–13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Smith M, Fork RL, Cole S, Opt Express 2001, 8, 537–546. [DOI] [PubMed] [Google Scholar]
- [9].Pu K, Shuhendler AJ, Jokerst JV, Mei J, Gambhir SS, Bao Z, Rao J, Nat Nanotechnol 2014, 9, 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yadav AK, Hernandez S, Su S, Chan J, Curr. Opin. Chem. Biol 2020, 57, 114–121. [DOI] [PubMed] [Google Scholar]
- [11].Xiang H, Cheng J, Ma X, Zhou X, Chruma JJ, Chem. Soc. Rev 2013, 42, 6128–6185. [DOI] [PubMed] [Google Scholar]
- [12].a) Zhao J, Zhong D, Zhou S, J. Mater. Chem. B 2018, 6, 349–365; [DOI] [PubMed] [Google Scholar]; b) Li B, Zhao M, Zhang F, ACS Mater. Lett 2020, 8, 905–917. [Google Scholar]
- [13].a) Cosco ED, Caram JR, Bruns OT, Franke D, Day RA, Farr EP, Bawendi MG, Sletten EM, Angew. Chem. Int. Ed 2017, 56, 13126–13129; [DOI] [PubMed] [Google Scholar]; b) Lucero MY, East AK, Reinhardt CJ, Sedgwick AC, Su S, Lee MC, Chan J, J. Am. Chem. Soc 2021, 143, 7196–7202; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Tian R, Zeng Q, Zhu S, Lau J, Chandra S, Ertsey R, Hettie KS, Teraphongphom T, Hu Z, Niu G, Kiesewetter DO, Sun H, Zhang X, Antaris AL, Brooks BR, Chen X, Sci. Adv 2019, 5, eaaw0672; [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Lei Z, Sun C, Pei P, Wang S, Li D, Zhang X, Zhang F, Angew. Chem. Int. Ed 2019, 58, 8166–8171; [DOI] [PubMed] [Google Scholar]; e) Shi Z, Han X, Hu W, Bai H, Peng B, Ji L, Fan Q, Li L, Huang W, Chem. Soc. Rev 2020, 49, 7533–7567; [DOI] [PubMed] [Google Scholar]; f) Swamy MMM, Murai Y, Monde K, Tsuboi S, Jin T, Bioconjugate Chem. 2021, 8, 905–917; [DOI] [PubMed] [Google Scholar]; g) Li B, Zhao M, Feng L, Dou C, Ding S, Zhou G, Lu L, Zhang H, Chen F, Li X, Li G, Zhao S, Jiang C, Wang Y, Zhao D, Cheng Y, Zhang F, Nat. Commun 2020, 11, 3102; [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Hannah EDC Friedman C, Atallah TL, Jia S, Sletten EM, Caram JR, Chem 2021, 7, 3359–3376.; [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Du Y, Liu X, Zhu S, Front. Chem 2021, 9, 718709; [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Sun C, Li B, Zhao M, Wang S, Lei Z, Lu L, Zhang H, Feng L, Dou C, Yin D, Xu H, Cheng Y, Zhang F, J. Am. Chem. Soc 2019, 141, 19221–19225. [DOI] [PubMed] [Google Scholar]
- [14].Bai L, Sun P, Liu Y, Zhang H, Hu W, Zhang W, Liu Z, Fan Q, Li L, Huang W, Chem. Commun (Cambridge) 2019, 55, 10920–10923. [DOI] [PubMed] [Google Scholar]
- [15].a) Fang Y, Shang J, Liu D, Shi W, Li X, Ma H, J. Am. Chem. Soc 2020, 142, 15271–15275; [DOI] [PubMed] [Google Scholar]; b) Zhang X-D, Wang H, Antaris AL, Li L, Diao S, Ma R, Nguyen A, Hong G, Ma Z, Wang J, Zhu S, Castellano JM, Wyss-Coray T, Liang Y, Luo J, Dai H, Adv. Mater 2016, 28, 6872–6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Liu C, Scott CN, Dyes and Pigments 2021, 196, 109792. [Google Scholar]
- [17].a) Fu M, Xiao Y, Qian X, Zhao D, Xu Y, Chem. Commun 2008, 1780–1782; [DOI] [PubMed] [Google Scholar]; b) Best QA, Sattenapally N, Dyer DJ, Scott CN, McCarroll ME, J. Am. Chem. Soc 2013, 135, 13365–13370; [DOI] [PubMed] [Google Scholar]; c) Kushida Y, Nagano T, Hanaoka K, Analyst 2015, 140, 685–695; [DOI] [PubMed] [Google Scholar]; d Butkevich AN, Mitronova GY, Sidenstein SC, Klocke JL, Kamin D, Meineke DNH, D'Este E, Kraemer P-T, Danzl JG, Belov VN, Hell SW, Angew. Chem. Int. Ed 2016, 55, 3290–3294; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Li J, Dong Y, Wei R, Jiang G, Yao C, Lv M, Wu Y, Gardner SH, Zhang F, Lucero MY, Huang J, Chen H, Ge G, Chan J, Chen J, Sun H, Luo X, Qian X, Yang Y, J. Am. Chem. Soc 2022, 144, 14351–14362; [DOI] [PubMed] [Google Scholar]; f) East A, Lee M, Smaga L, Jiang C, Chan J, ChemRxiv, 2022. [Google Scholar]
- [18].Fukazawa A, Suda S, Taki M, Yamaguchi E, Grzybowski M, Sato Y, Higashiyama T, Yamaguchi S, Chem. Commun 2016, 52, 1120–1123. [DOI] [PubMed] [Google Scholar]
- [19].Liu J, Sun Y-Q, Zhang H, Shi H, Shi Y, Guo W, ACS Appl. Mater. Interfaces 2016, 8, 22953–22962. [DOI] [PubMed] [Google Scholar]
- [20].a) Yuan L, Lin W, Yang Y, Chen H, J. Am. Chem. Soc 2012, 134, 1200–1211; [DOI] [PubMed] [Google Scholar]; b) Gong Y-J, Zhang X-B, Mao G-J, Su L, Meng H-M, Tan W, Feng S, Zhang G, Chem. Sci 2016, 7, 2275–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shi Y, Yuan W, Liu Q, Kong M, Li Z, Feng W, Hu K, Li F, ACS Mater. Lett 2019, 1, 418–424. [Google Scholar]
- [22].Rajapaksha I, Chang H, Xiong Y, Marder S, Gwaltney SR, Scott CN, J. Org. Chem 2020, 85, 12108–12116. [DOI] [PubMed] [Google Scholar]
- [23].Rathnamalala CSL, Pino NW, Herring BS, Hooper M, Gwaltney SR, Chan J, Scott CN, Org. Lett 2021, 23, 7640–7644. [DOI] [PubMed] [Google Scholar]
- [24].Rathnamalala CSL, Gayton JN, Dorris AL, Autry SA, Meador W, Hammer NI, Delcamp JH, Scott CN, J. Org. Chem 2019, 84, 13186–13193. [DOI] [PubMed] [Google Scholar]
- [25].a) Kinnibrugh TL, Salman S, Getmanenko YA, Coropceanu V, Porter WW, Timofeeva TV, Matzger AJ, Brédas J-L, Marder SR, Barlow S, Organometallics 2009, 28, 1350–1357; [DOI] [PMC free article] [PubMed] [Google Scholar]; b Qian G, Wang ZY, Chemistry – An Asian Journal 2010, 5, 1006–1029. [DOI] [PubMed] [Google Scholar]
- [26].Grimm JB, Muthusamy AK, Liang Y, Brown TA, Lemon WC, Patel R, Lu R, Macklin JJ, Keller PJ, Ji N, Lavis LD, Nat. Methods 2017, 14, 987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu D, He Z, Zhao Y, Yang Y, Shi W, Li X, Ma H, J. Am. Chem. Soc 2021, 143, 17136–17143. [DOI] [PubMed] [Google Scholar]
- [28].a) Yadav AK, Lee MC, Lucero MY, Su S, Reinhardt CJ, Chan J, ACS Cent Sci. 2022, 8, 461–472; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Reinhardt CJ, Xu R, Chan J, Chem. Sci 2020, 11, 1587–1592; [Google Scholar]; c Reinhardt CJ, Chan J, Biochemistry 2018, 57, 194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zheng H, Shang GQ, Yang SY, Gao X, Xu JG, Org. Lett 2008, 10, 2357–2360. [DOI] [PubMed] [Google Scholar]
- [30].McGill MR, Jaeschke H, Biochim Biophys Acta Mol Basis Dis 2019, 1865, 1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gardner CR, Heck DE, Yang CS, Thomas PE, Zhang X-J, DeGeorge GL, Laskin JD, Laskin DL, Hepatol. 1998, 27, 748–754. [DOI] [PubMed] [Google Scholar]
- [32].Bai F, Du W, Liu X, Su L, Li Z, Chen T, Ge X, Li Q, Yang H, Song J, Anal. Chem 2021, 93, 15279–15287. [DOI] [PubMed] [Google Scholar]
- [33].Liu X, Yan Q, Baskerville KL, Zweier JL, J. Biol. Chem 2007, 282, 8831–8836. [DOI] [PubMed] [Google Scholar]
- [34].Park R, Leach WJ, Arieff AI, Am J Physiol 1979, 236, F240–245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.