Abstract
Inflammatory markers have a wide range of predictive values in the prognosis of non-small lung cancer (NSCLC). Poor nutritional status usually means a poor prognosis in patients with NSCLC, which is widely recognized by oncologists and nutritionists. Serum albumin has a certain value in evaluating the prognosis of patients. Several inflammatory albumin-related markers have been proposed, but they have not been widely used in predicting the prognosis of NSCLC in clinical practice. We aim to systematically review the published clinical evidence of albumin-related inflammatory markers in predicting the prognosis of NSCLC and to describe their progress and value. The results showed that the markers included in the review could be prognostic indicators in patients with NSCLC. However, we found that the cut-off value of albumin-related inflammatory markers with quantitative nature was very chaotic and needed to be defined by recognized standards. We summarized and compared the advantages and disadvantages of these markers, but a prospective cohort study with long-term follow-up after adjustment for important confounders is still necessary. Whether the results and conclusions could be directly applied in clinical practice needs to be identified and evaluated. There is an urgent need to classify and standardize the albumin-related inflammatory markers that play an important role in the prognosis of NSCLC, which is the key to ensuring the transformation from clinical study to clinical application.
Keywords: Non-small lung cancer, inflammation, albumin, markers, prognosis
Key Messages
Albumin-related inflammatory markers could be prognostic indicators in non-small cell lung cancer.
The classification and standardization of albumin-related inflammatory markers guarantee the transformation from clinical study to clinical application.
Future prospective studies of albumin-related inflammatory markers excluding confounding factors are very necessary.
Introduction
At present, lung cancer remains the leading cause of cancer-related death in humans worldwide, despite the increasing diversity of treatment therapy. Non-small lung cancer (NSCLC) accounts for about 80% of all cases [1]. We previously reported the role of the inflammatory microenvironment in lung cancer [2]. Inflammation has a broad impact on the formation and progress of NSCLC [3], including proliferation and survival of cancer cells, angiogenesis, tumour metastasis, and tumour response to chemotherapeutic drugs and hormones [4–7].
With an in-depth understanding of the relationship between the molecular mechanism of lung cancer and the inflammatory microenvironment, we found that clinical monitoring of inflammatory markers in patients with NSCLC had important clinical value for prevention and developmental control [8]. There are various laboratory markers of systemic inflammation including C-reactive protein (CRP), neutrophil (NEU), lymphocyte (LYM), and so on. CRP plays an important role in host defence mechanisms and inflammatory responses to infectious agents, mainly produced by hepatocytes in response to stimulation by interleukin-6 (IL-6), tumour necrosis factor-α (TNF-α), and interleukin-1β (IL-1β), which in turn can reactivate leukocytes and platelets, creating a cycle of action [9]. However, one single inflammatory index has limitations in independently predicting survival in patients with NSCLC. Therefore, further studies on composite prognostic indicators are very necessary. Exploring more, newer, and better composite inflammatory markers will guide improving the prognosis of patients with NSCLC in the future.
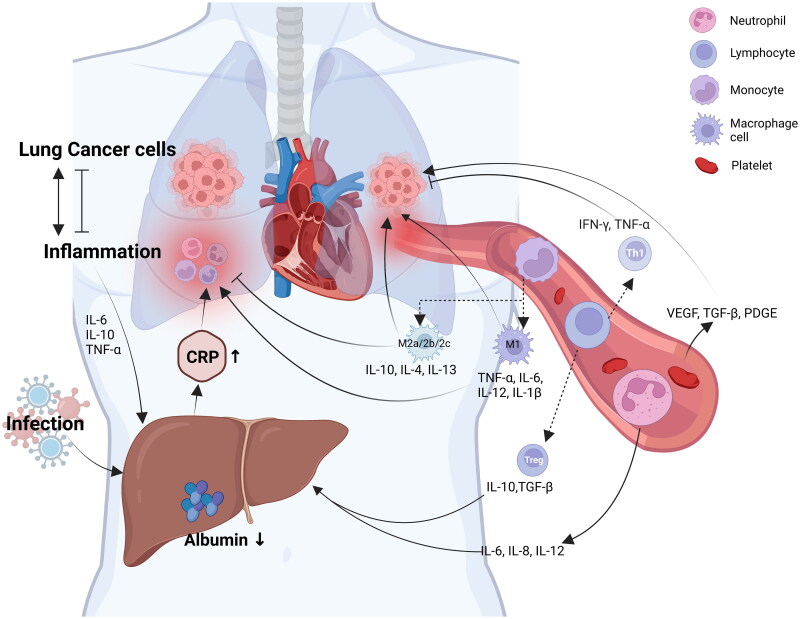
It is well-known that nutritional status affects the prognosis of NSCLC [10]. In patients with NSCLC, there is a complex interaction between Alb, CRP, and peripheral blood cells (Figure 1). As a biomarker, serum albumin (Alb) can not only reflect the nutritional status of the body but also remove pro-inflammatory stimulating factors in the body and relieve inflammatory reactions, indicating the level of systemic inflammatory status to a certain extent, which has a certain value in evaluating the prognosis of patients with NSCLC. And even studies have shown that its level can differentiate benign pulmonary nodules at the early stage of NSCLC [11]. Stares et al. [12] found that Alb < 35 g/L generally represented a poor prognosis for patients with metastatic non-small cell lung cancer (mNSCLC). The level of Alb can also predict the occurrence of adverse reactions in lung cancer patients after treatment [13]. A clinical study by Kazuki et al. showed that Alb was an independent predictor in NSCLC patients treated with programmed cell death protein‑1 (PD-1) inhibitors [14]. Gradually, it was realized that Alb was combined with some inflammatory markers, and a new composite marker was composed after a simple operation to evaluate the prognosis of patients with NSCLC. We call them ‘albumin-related inflammatory markers’. Clinical studies on the role of albumin-related inflammatory markers in the prognosis of NSCLC are in full swing. However, the current clinical application of these markers is not as enthusiastic as in the research. Clinicians do not fully understand the value of these markers and how they should be applied.
Figure 1.
Pro-inflammatory activity of C-reactive protein and peripheral blood cells in non-small cell lung cancer patients with involvement of albumin. Figure created with BioRender.com. IL: interleukin; TNF: tumour necrosis factor; TGF: transforming growth factor; IFN: interferon; VEGF: vascular endothelial growth factor; PDGF: platelet-derived growth factor.
To systematically describe the role of these albumin-associated inflammatory markers in predicting the prognosis of NSCLC, this review synthesized the published clinical evidence on albumin-associated inflammatory markers in patients with NSCLC, reviewed their applications and cut-off values, and summarized their advantages and disadvantages. The findings may provide a useful reference for physicians on how to use the most appropriate prognostic tool in patients with NSCLC.
We explored the literature databases PubMed, EMBASE, Web of Science, and Cochrane Library for studies that may meet the criteria until January 2022. The search terms were set to ‘albumin’ and ‘inflammatory’ and ‘adenocarcinoma*’ or ‘Non-small cell lung cancer’ or ‘NSCLC’ or ‘LAD’ or ‘ADC’ and ‘Prognosis’. The population is limited to patients with a confirmed diagnosis of NSCLC histopathologically. A simple bibliometric visual analysis of the literature retrieved by PubMed was performed. For quantitative markers included in the review, we included them in the table of extracted information only when the literature accurately described its cut-off value and grouped them according to the high or low cut-off value. In this review, we analysed the prognostic value of albumin-related inflammatory markers based on CRP, such as CAR, GPS, MGPS, etc., and albumin-related inflammatory markers based on peripheral blood cells, such as PNI, ALI, etc.
Bibliometric analysis of albumin and non-small cell lung cancer (2000–2021)
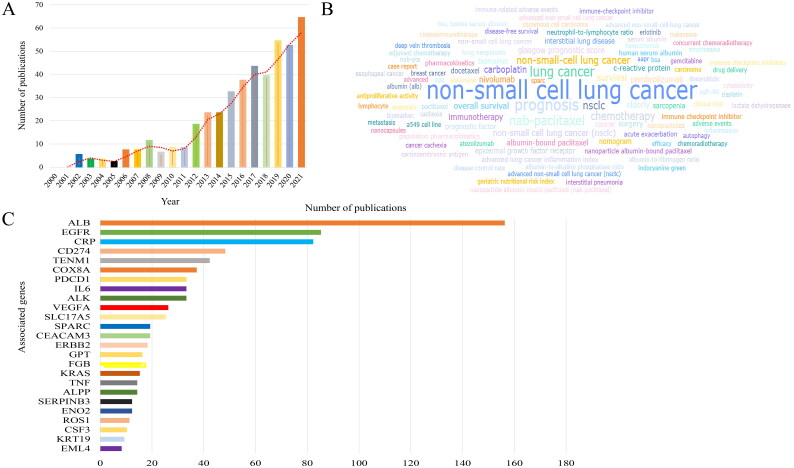
A total of 454 articles from 2000-01 to 2022-01 retrieved by PubMed were visualized by searching ‘albumin’ and ‘non-small cell lung cancer’ as keywords, and the average annual number of articles issued was 23. 2021 reached the peak of 64 annual documents, while the fastest growth rate of 250% was in 2006, suggesting that research in this field developed rapidly and was in a rapidly rising stage (Figure 2(A)). The keywords of the paper are to highly condense and summarize the research purpose, research object, and research method. Keyword-based analysis can reflect the trend of theme evolution and research hotspots within a certain period in a certain research field. As shown in Figure 2(B), the keywords in the top 5 occurrence frequencies were: ‘non-small cell lung cancer’, ‘prognosis’, ‘lung cancer’, ‘nab-paclitaxel’, and ‘NSCLC’. In the part of association gene analysis, we found that NSCLC was very closely related to ALB (Figure 2(C)).
Figure 2.
Bibliometric analysis of albumin and non-small cell lung cancer. (A) Number and trend of annual publications about albumin and non-small cell lung cancer. (B) Keyword frequency analysis of albumin and non-small cell lung cancer. (C) Analysis of associated genes of albumin and non-small cell lung cancer.
Albumin-related inflammation markers based on CRP
CRP is a typical acute-phase reaction protein. Its level rapidly increases during inflammation. It is considered to be one of the most widely used indicators of systemic inflammatory response. The C-reactive protein albumin ratio (CAR) was first proposed by Fairclough and colleagues [15]. Clinical studies in predicting the prognosis of lung cancer were first reported on small cell lung cancer (SCLC), and then gradually proved to have prognostic value in NSCLC [16,17]. In the beginning, ‘cumulative prognostic scores’ including CRP with Alb was proposed by Forest et al. [18]. The results showed that this score was more convenient for prediction in patients with NSCLC. In the following year, the Glasgow prognostic score (GPS) was first proposed and defined by Forest et al. which can also predict the prognosis [19]. Hypoalbuminaemia was not significantly associated with cancer-specific survival in the absence of elevated CRP concentrations. Therefore, patients with elevated CRP were assigned to a modified Glasgow prognostic score (mGPS) of 1 or 2 based on whether they had hypoproteinaemia [20,21]. These three markers are all based on a cut-off value of 10 mg/L for CRP and 35 g/L for Alb [22]. High-sensitivity modified Glasgow prognostic score (Hs-mGPS) was first applied to the study of NSCLC by Osugi et al. based on 3 mg/L for CRP [23]. Unlike Hs-mGPS, adjusted Glasgow prognostic score (a-GPS) raises the threshold for Alb (39 g/L) [24]. Albumin-related inflammatory markers based on CRP mainly include CAR, GPS, mGPS, Hs-mGPS, and a-GPS. Their definitions are detailed in Table 1.
Table 1.
Description of the CAR, GPS, mGPS, Hs-mGPS, and a-GPS.
| Full name | Abbreviation | Calculation formula | Reference |
|---|---|---|---|
| C-reactive protein albumin ratio | CAR | C-reactive protein/Albumin | [15] |
| Glasgow prognostic score | GPS | CRP ≤10 mg/L and albumin ≥35 g/L, score 0; CRP >10 mg/L or albumin <35 g/L, score 1; CRP >10 mg/L and albumin <35 g/L, score 2 | [19] |
| Modified Glasgow prognostic score | mGPS | CRP ≤10 mg/L and albumin ≥35 g/L, score 0; CRP >10 mg/L and albumin ≥35 g/L, score 1; CRP >10 mg/L and albumin <35 g/L, score 2 | [21] |
| High-sensitivity modified Glasgow prognostic score | Hs-mGPS | CRP ≤3 mg/L and albumin ≥35 g/L, score 0; CRP >3 mg/L and albumin ≥35 g/L, score 1; CRP >3 mg/L and albumin <35 g/L, score 2 | [23] |
| Adjusted Glasgow prognostic score | a-GPS | CRP ≤3 mg/L and albumin ≥39 g/L, score 0; CRP >3 mg/L or albumin ≥39 g/L, score 1; CRP >3 mg/L and albumin <39 g/L, score 2 | [24] |
CAR
The CRP/albumin ratio was proposed by Fairclough and his colleagues. But it was not defined as the abbreviation ‘CAR’ [15]. And its cut-off value was also vague. The first evidence-based medical report on NSCLC was performed by Miyazaki et al. [17]. In the article, they applied the abbreviation of ‘CAR’ and confirmed that preoperative CAR is a simple and objective indicator for predicting the prognosis of elderly patients with operable NSCLC. Unlike GPS, mGPS, and Hs-mGPS, CAR is quantitative. Therefore, we summarized the cut-off value of CAR (Table 2). From the table, it could be seen that there was a relatively confusing situation in the cut-off value of CAR, which may be the reason for the delay in the promotion. But it is undeniable that CAR, as a composite marker combining nutrition and inflammation, can be used as an indicator of whether surgical patients need to enhance nutrition and improve inflammation before surgery to a certain extent, with a good prospect of clinical application. In addition, it may also be helpful to recognize the possibility of early recurrence [4]. Matsubara et al. found that CAR could be used as the most valuable prognostic indicator of postoperative immunonutrition in patients with NSCLC [25].
Table 2.
Summary of the characteristics of CAR in clinical studies.
| Parameter | Participants’ conditions | cut-off value | Low/ High(N) | Outcome | AUC | Clinical findings | Reference |
|---|---|---|---|---|---|---|---|
| CAR | Elderly patients with operable NSCLC | 0.28 | 59/49 | OS | 0.59 | CAR is a prognostic marker, but GPS is not. | [17] |
| Patients in pN2-stage IIIA with LADC | 0.6 | 122/25 | RFS | – | CAR is a prognostic marker, better than GPS, mGPS, Hs-mGPS, and PNI. | [30] | |
| Patients with operable NSCLC | 0.424 | 492/125 | DFS, OS | – | CAR is a prognostic marker. | [31] | |
| Patients with advanced NSCLC | 0.2357 | 287/149 | OS | 0.700 | CAR is a prognostic marker, better than GPS, mGPS, NLR, PLR and MLR. | [32] | |
| Chinese patients with NSCLC | 0.14/0.22 | 148/110/129 | OS | – | CAR is a prognostic marker, better than CRP, Alb. | [33] | |
| Patients with advanced NSCLC | 0.35 | 38/39 | OS | – | CAR may be a cheap, easy, and effective tool for predicting the death and its time of hospitalized NSCLC patients better than CRP. | [34] | |
| Patients with NSCLC underwent surgery | 0.4 | 320/172 | RFS | – | CAR and GPS may be independent risk factors for early recurrence. | [4] | |
| Patients with NSCLC underwent surgical resection | 0.156 | 116/480 | RFS, OS | 0.587 | CAR is a prognostic marker, better than GPS, mGPS. | [35] | |
| Patients with NSCLC treated with nivolumab | 0.83 | 74/39 | PFS, OS | – | CAR may be predictive of therapeutic response to nivolumab and long-term survival in NSCLC patients better than GPS, and NLR. | [36] |
NSCLC: non-small cell lung cancer; LADC: lung adenocarcinoma; NLR: neutrophil-to-lymphocyte ratio; OS: overall survival; RFS: relapse-free survival; DFS: disease-free survival; PFS: progression-free survival; N: number of patients.
GPS, mGPS, Hs-mGPS, and a-GPS
Studies on CAR let us realize that combining Alb with CRP may predict the prognosis of NSCLC, but it is calculated in the form of a ratio, which limits its application. Their ratios may not be very different when both Alb and CRP levels are high or low. Therefore, CAR as the ratio of CRP to Alb is not as objective as GPS and MGPS to some extent. When reviewing past clinical studies, the grouping of GPS is highly controversial, some are divided into 0 and 1 or 2 [26], and some are divided into 0, 1, and 2 [27]. Clinical studies on GPS have covered patients with stage I-IV NSCLC, and all of them have shown a good ability to predict prognosis. In our previous analysis, three groups of 0, 1, and 2 were performed according to the GPS. It was found that the GPS is an independent prognostic marker for patients with NSCLC regardless of the comparison between the two groups [28]. McMillan et al. found that the increase of CRP was often accompanied by a decrease of Alb [29]. The mGPS is then further improved based on a greater focus on the relationship between hypoproteinemia and cancer prognosis. Although only a few studies have made tentative adjustments to the cut-off value of CRP and Alb, along with the new prognostic markers of Hs-mGPS and a-GPS, it reminds us that more attention should be paid to the cut-off value of CRP and Alb [23,24].
Albumin-related inflammation markers based on peripheral blood cells
Inflammation of the tumour microenvironment (TME) is characterized by the presence of host leukocytes in both stroma and tumour sites [37]. White blood cells include neutrophils and lymphocytes, eosinophils, basophils, and monocytes, with neutrophils and lymphocytes being the most strongly associated with inflammation [38]. Current studies have shown that neutrophils play a key role at different stages of tumour development. TME can influence the emergence of distinct neutrophil phenotypes that give rise to several key mediators associated with tumour growth and aggressiveness. The neutrophil-to-lymphocyte ratio (NLR) is a commonly used marker of systemic inflammation. NLR >5 is generally considered to indicate ongoing systemic inflammation [39]. NLR can be used to predict the prognosis of patients with stage IIIB-IV NSCLC treated with PD-1 inhibitors [40]. LMR has also been used as one of the markers of systemic inflammation [41,42]. In recent years, the role of platelet count in inflammation has also been gradually appreciated [43,44]. The emergence of the prognostic nutritional index (PNI) threatens the status of NLR to some extent [45,46]. Thus we describe albumin-related inflammatory markers based on peripheral blood cells, mainly including PNI, advanced lung cancer inflammation index (ALI), Alb concentration combined with NLR (COA-NLR), NLR × D-dimer count/albumin (NLDA), albumin and neutrophil combined prognostic grade (ANPG) and HALP. Their definitions were detailed in Table 3.
Table 3.
Description of the PNI, ALI, COA-NLR, NLDA, ANPG, and HALP.
| Full name | Abbreviation | Calculation formula | Cut-off value | Reference |
|---|---|---|---|---|
| Prognostic nutritional index | PNI | 10 × albumin (g/dL) + 0.005 × absolute lymphocyte count (/μL) | 48 | [46] |
| Advanced lung cancer inflammation Index | ALI | (BMI × albumin) / NLR | 18 | [47] |
| Alb concentration combined with NLR | COA-NLR | NLR >2.5 or Alb <35 g/L, score 0; NLR >2.5 or Alb <35 g/L, score 1; NLR >2.5 and Alb <35 g/L, score 2 | – | [48] |
| NLR × D-dimer count/Albumin | NLDA | NLR × D-dimer count/albumin | 0.15 | [49] |
| Albumin and neutrophil combined prognostic grade | ANPG | Grade 1 = elevated albumin and low neutrophil; Grade 2 = low albumin and low neutrophil, as well as elevated albumin and elevated neutrophil; Grade 3 = low albumin and elevated neutrophil. | albumin: 42.55 g/L; neutrophil: 2.895 × 109/L | [50] |
| Haemoglobin, albumin, lymphocyte, and platelet score | HALP | haemoglobin (g/L) × albumin (g/L) × lymphocyte (/L)/platelet (/L) | 48 | [51] |
NLR: neutrophil-to-lymphocyte ratio; BMI: body mass index.
PNI
Kos et al. reported for the first time that the prognostic nutrition index (PNI) was applied to patients with NSCLC in clinical studies. It was found that PNI was a prognostic marker independent of other risk factors, with better ability than NLR in predicting mNSCLC [45]. As shown in Table 4, subsequent multiple studies obtained the predictive value of PNI in patients with completely resected NSCLC [52–58]. It also has prognostic value in elderly patients older than 75 years with NSCLC [53]. The acceptance of adjuvant chemotherapy, platinum-based chemotherapy, targeted therapy, immunotherapy, radiotherapy, and other different treatment modalities have good prognostic value in patients with NSCLC [59–68]. Through the difference between preoperative and postoperative values, the prognostic value of PNI in the perioperative period of NSCLC was confirmed [69,70]. Xu et al. reported for the first time that in patients with bone mNSCLC, higher PNI indicated a better prognosis [71]. Postoperative and advanced non-small cell lung cancer (aNSCLC) patients accounted for the majority of all included studies, which may mean that PNI has a higher prognostic value in these patients [72–76].
Table 4.
Summary of the characteristics of PNI, ALI, NLDA, and HALP in clinical studies.
| Parameter | Participants’ conditions | Cut-off value | Low/ High(N) | Outcome | AUC | Clinical findings | Reference |
|---|---|---|---|---|---|---|---|
| PNI | Patients with NSCLC | 49.5 | 69/69 | OS | – | PNI is an independent prognostic marker. | [45] |
| Patients with completely resected NSCLC | 50 | 149/241 | OS | 0.63 | PNI is an independent prognostic marker. | [52] | |
| Elderly (aged > 75 years) patients with completely resected NSCLC | 49.6 | 146/126 | OS | 0.532 | PNI is an independent prognostic marker. | [53] | |
| Patients with NSCLC underwent radical surgery | 52 | 912/504 | OS | – | Higher PNI in NSCLC patients suggests a favourable prognosis. | [54] | |
| Patients with completely resected NSCLC | 48 | 46/202 | OS, RFS | – | PNI is an independent prognostic marker. | [55] | |
| Patients with completely resected NSCLC | 45/50 | 57/134/324 | OS | – | PNI is an independent prognostic marker. | [56] | |
| Patients with NSCLC underwent open thoracotomy for curative resection | 50 | 285/726 | OS, RFS | 0.727 | PNI was associated with postoperative pulmonary complications and long-term outcomes. | [57] | |
| Patients with NSCLC underwent surgical pulmonary resection and receive neoadjuvant therapy | 46.810 | 37/73 | OS, RFS | 0.628 | PNI is an independent prognostic marker. | [58] | |
| Patients with NSCLC received adjuvant chemotherapy | 50 | 54/52 | RFS | – | PNI is an independent prognostic marker. | [59] | |
| Patients with NSCLC treated with EGFR TKI | 45 | 177/453 | OS, PFS | – | Pre-treatment nutritional status is a prognostic marker. | [60] | |
| Patients with NSCLC treated with ICIs | 45.5 | 52/50 | OS, PFS | 0.694 | PNI is an independent prognostic marker. | [62] | |
| Patients with NSCLC underwent curative radiotherapy | 45.45 | – | OS | 0.666 | PNI is an independent prognostic marker. | [63] | |
| Patients with aNSCLC treated with PD-1 inhibitors | 45 | 47/55 | OS, PFS | – | PNI may be a useful predictive marker of clinical outcomes and irAEs. | [64] | |
| Patients with advanced NSCLC treated with platinum-based chemotherapeutics | 52.525 | 54/45 | OS, PFS | – | PNI is an independent prognostic marker. | [65] | |
| Patients with recurrence NSCLC after complete pulmonary resection who received ICI monotherapy during the therapeutic course | 50 | – | PFS | – | PNI is an independent prognostic marker. | [66] | |
| Patients with aNSCLC treated with PD-1 inhibitors | 46.05 | 53/70 | OS, PFS | 0.780 | PNI was an independent predictor of early progression and survival outcomes. | [67] | |
| Patients with metastatic or recurrent ALK-positive NSCLC received first-line alectinib | 40 | 11/31 | PFS | – | PNI is important in predicting, which reflect the nutritional status of the host | [68] | |
| Patients in stage IA-IIIB with NSCLC underwent chest surgery | 47 | 193/282 | OS, RFS | 0.62 | PNI has high predictive values for postoperative complications and survival. | [70] | |
| Patients with bone mNSCLC without any anti-tumor therapy | 54.5 | 154/105 | OS | – | PNI is an independent prognostic marker. | [71] | |
| patients in stage IIIB/IV with NSCLC | 50 | 179/136 | OS | – | PNI is an independent prognostic marker. | [72] | |
| Patients with aNSCLC | 46.1 | 67/116 | OS | 0.55 | Nutritional status is an important prognostic factor. | [73] | |
| Patients with mNSCLC treated with first-line chemotherapy | 46.7 | 184/149 | OS, PFS | 0.617 | PNI is an independent prognostic marker. | [74] | |
| Patients in stage IIIB with NSCLC | 40.5 | 190/168 | OS, PFS, LPFS | 0.841 | PNI is an independent prognostic marker. | [75] | |
| Patients with aNSCLC | 38.4 | 52/108 | OS, PFS | 0.69 | PNI is an independent prognostic marker. | [76] | |
| ALI | Patients with mNSCLC | 18 | 83/90 | OS, PFS | 0.67 | ALI is an independent prognostic marker. | [47] |
| Patients with mNSCLC | 23.2 | 21/20 | OS, PFS | – | ALI is an independent prognostic marker. | [78] | |
| Patients in stage IV with NSCLC | 18 | 38/74 | OS | – | ALI is an independent prognostic marker. | [79] | |
| Patients with aNSCLC initiated nivolumab treatment | 18 | 69/128 | PFS | – | ALI is an independent prognostic marker. | [80] | |
| Patients with mNSCLC received complete first-line treatment with chemotherapy | 11 | 19/90 | OS, PFS | 0.52 | ALI is associated with survival. | [81] | |
| Patients with NSCLC received complete resection | 37.66 | 121/220 | OS | 0.681 | ALI is an independent prognostic marker. | [82] | |
| Patients with NSCLC underwent VATS | 41.20 | 125/214 | OS | 0.324 | ALI is an independent prognostic marker. | [83] | |
| Patients in stage IA with NSCLC underwent lung resection | 22.2 | 18/148 | OS, RFS | 0.610 | ALI is an independent prognostic marker. | [85] | |
| Patients with early-stage NSCLC received VATS pulmonary resection as their only therapy | 50 | 155/137 | OS, DFS | – | ALI is an independent prognostic marker. | [86] | |
| Patients with NSCLC treated with nivolumab | 18 | – | PFS | – | A high ALI was predictive of better PFS in patients with poor performance status. | [87] | |
| Patients with mNSCLC | 32.6 | 191/127 | OS | – | ALI is an independent prognostic marker. | [88] | |
| NLDA | IV stage NSCLC patients | 0.15 | 32/12 | OS | 0.7 | NLDA is an independent prognostic marker. | [49] |
| HALP | Patients with NSCLC underwent radical lung cancer resection | 48 | 99/139 | OS | 0.666 | HALP is an independent prognostic marker. | [51] |
NSCLC: non-small cell lung cancer; aNSCLC: advanced non-small cell lung cancer; mNSCLC: metastatic non-small cell lung cancer; LA-NSCLC: locally advanced non-small cell lung cancer; TKI: tyrosine kinase inhibitor; PD-1: programmed cell death protein‑1; ICI: immune checkpoint inhibitor; irAE: immune-related adverse event; NLR: neutrophil-to-lymphocyte ratio; OS: overall survival; PFS: progression-free survival; LPFS: local progression-free survival; DFS: disease-free survival; RFS: relapse-free survival; VATS: video-assisted thoracic surgery; N: number of patients.
ALI
The application of ALI was first reported in a clinical study of metastatic non-small cell lung cancer by Jafri et al. and the cut-off value was determined as 18 [47]. Alb and body mass index (BMI) are always used to determine nutritional status. According to the World Health Organization, patients are classified as underweight when their BMI is < 18.5 kg/m2. Therefore, ALI is a quantitative marker based on the numerical embodiment of ALB and BMI bilayer nutrition. We summarized the cut-off value of ALI (Table 4). Although the prognostic value of ALI was not found in the clinical studies by Kobayashi et al. and Watanabe et al. [4,77], multiple studies subsequently confirmed that lower ALI tended to predict poor prognosis in patients with NSCLC [78,79]. Whether receiving targeted treatment [80], 1st line chemotherapy [81], radical surgery [82,83], or chemotherapy combined with targeted treatment [84], ALI shows prognostic value. Even in patients at the early stage of NSCLC, ALI also has a prognostic value [85,86]. In lung cancer patients with poor performance status, ALI has a more prominent prognostic value [87,88]. Palomar-Abril et al. noted that the prognostic role of ALI in NSCLC was not affected by age [89]. Furthermore, it is exciting that Mountzios et al. confirmed that ALI lost its predictive ability when chemotherapy was added to immunotherapy by dividing patients into Cohort ‘A’ (PD-L1 inhibitors in any treatment line alone), cohort ‘B’ (first-line chemo-immunotherapy), and cohort ‘C’ (platinum-based first-line chemotherapy). ALI is a powerful prognostic and predictive marker for the efficacy of immunotherapy when immune checkpoint inhibitors (ICIs) are used as a single therapy rather than in combination with chemotherapy. This also illustrates that for patients with PD-L1-high, an ALI >18 may help select patients who do not require additional chemotherapy [90]. Tomita et al. pointed out that the combined detection of ALI and CRP was a useful indicator for predicting overall survival, and could be used as a simple prognostic tool to help identify patients with operable NSCLC [91].
Others
In addition to PNI, and ALI, two widely studied albumin-related inflammatory markers based on peripheral blood cells, there are other less-studied markers. Weng and colleagues’ clinical studies proposed a new effective biomarker for prognosis in NSCLC patients treated with resection value. Preoperative COA-NLR can effectively stratify prognosis in NSCLC patients by classifying patients into three independent groups [48]. An innovative attempt was made by Sun et al. which put forward a new prognostic marker–NLDA [49]. In terms of this marker, the role of the D-dimer count was also added, and coagulation factors were considered. A retrospective study of 272 patients with stage IV NSCLC showed that NLDA was an independent adverse prognostic factor [49]. The albumin and neutrophil combined prognostic grade (ANPG) was proposed by Sun et al. It was confirmed that higher ANPG independently predicted OS and PFS in patients with NSCLC [50]. HALP is a more comprehensive score that reflects the nutritional status of patients through haemoglobin and albumin and the inflammatory status of patients through LMR. The results of clinical studies confirmed that HALP was an independent prognostic marker [51].
Discussion
Both systemic inflammatory response and poor nutritional status are widely recognized as risk factors for poor prognosis in patients with NSCLC. The combination of the two may be able to make up for their limitations. From the results of bibliometric analysis, literature related to albumin and non-small cell lung cancer show an increasing trend. The heat of keywords such as ‘prognosis’ and ‘CRP’ enlightens us that the inflammation index related to albumin, as a kind of low-cost and less invasive predictive test method, has shown great research value in recent years. Albumin-related inflammation markers are increasingly being evaluated to enhance the prediction of prognosis in NSCLC. More importantly, Alb, CRP, and peripheral blood cells are standardized tests that are easily performed and obtained in daily work. We summarized and compared the advantages, disadvantages, and range of cut-off value of these markers. At the same time, their suitable patients are compared (Table 5). However, most of them only appear in clinical research rather than clinical practice. The main reason for this is that there is no comprehensive assessment of its prognostic role which is specific and sensitive. Its cut-off value is uncertain, and there is little validation of stable prediction models. Therefore, for the identification of predictive markers, improvement and appropriate use are of great interest. Predicting the development of NSCLC and survival after treatment will enable us to better select patients and improve the utilization of expensive resources. And it will be the key to saving healthcare resources.
Table 5.
Comparison of advantages and disadvantages of albumin-related inflammatory markers, its suitable patients, and range of cut-off value.
| Albumin-related inflammatory markers | Advantages | Disadvantages | Suitable patients | Range of cut-off value |
|---|---|---|---|---|
| CAR | An effective tool for predicting the survival of NSCLC patients. | The cut-off value is controversial. | Operable elderly patients with NSCLC. | 0.14–0.83 |
| GPS, mGPS, Hs-mGPS, a-GPS |
More objective than CAR. | Inflexible. Lack of research on prognostic value in NSCLC patients after being treated with immunotherapy. | NSCLC patients, whose CRP and Alb values do not fluctuate around normal values. | – |
| PNI | A sensitive tool for predicting the survival of NSCLC patients. | Poor specificity. | Postoperative or advanced NSCLC patients. | 38.4–52.525 |
| ALI | The prognostic value is not affected by age. | Poor sensitivity and specificity. | Patients with aNSCLC or treated with immunotherapy. | 11–50 |
| COA-NLR | Preoperative COA-NLR can effectively stratify the prognosis of patients. | Inflexible. There are few related studies. | Patients with NSCLC underwent lung resection. | – |
| NLDA | Coagulation factors are considered. | There are few related studies. | Patients with NSCLC in the IV stage. | 0.15 |
| HALP | It is less likely to change under the influence of one factor. | There are few related studies. | Patients with NSCLC underwent lung resection. | 48 |
NSCLC: non-small cell lung cancer; aNSCLC: advanced non-small cell lung cancer; CAR: C-reactive protein albumin ratio; GPS: Glasgow prognostic score; mGPS: modified Glasgow prognostic score; Hs-mGPS: high-sensitivity modified Glasgow prognostic score; a-GPS: adjusted Glasgow prognostic score; PNI: prognostic nutritional index; ALI: advanced lung cancer inflammation Index; COA-NLR: Alb concentration combined with NLR; NLDA: NLR × D-dimer count/Albumin; ANPG: albumin and neutrophil combined prognostic grade; HALP: haemoglobin, albumin, lymphocyte and platelet score.
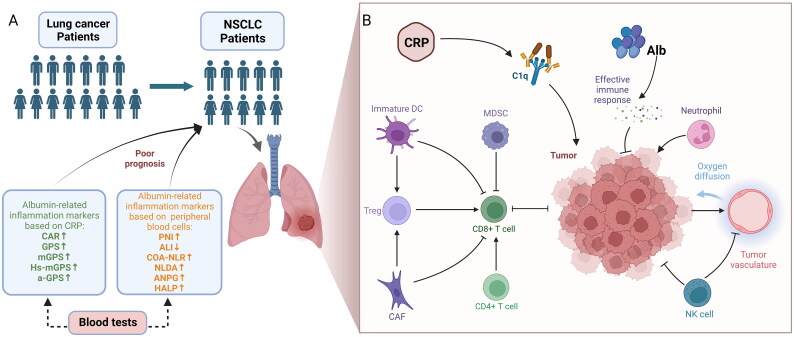
To the best of our knowledge, this is the first review of current albumin-related inflammation markers. In this review, albumin-related inflammation markers based on CRP or peripheral blood cells were introduced, including CAR, GPS, mGPS, HS-mGPS, a-GPS, PNI, ALI, COA-NLR, NLDA, ANPG, and HALP, to predict the prognosis of patients with NSCLC (Figure 3(A)). The key to the role that these markers can play in predicting the prognosis of non-small cell lung cancer may be due to the role of Alb, CRP, and peripheral blood cells in the immune microenvironment of non-small cell lung cancer. The main reason for low Alb in oncology patients is the specific inhibition of Alb gene transcription by TNF [92], which impairs the antitumor immune response activated by Alb. The different roles played by each CRP isoform at sites of local inflammation and infection [93], but what is certain is that CRP is an immune regulator, not just a marker of inflammation or infection, activating C1q to promote lung cancer progression. Similarly, peripheral blood cells play an important role in the NSCLC microenvironment. Neutrophils secrete IL-6, IL-8, and IL-12 to promote the tumour inflammatory microenvironment, and the proliferating tumour cells in turn stimulate neutrophils, forming a vicious circle [94,95]. Lymphocytes are the main immune cells in the body and play a key role in immune surveillance by inhibiting the proliferation, invasion and migration of tumour cells. Monocytes are precursors of macrophages and are also tropic for tumours and their inflammatory microenvironment, promoting tumour invasion [96]. Platelets secrete transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF) and platelet derived growth factor(PDGF), which play a role in tumour progression and metastasis [97] (Figure 3(B)). Obviously, there is more room for optimization of albumin-related inflammation markers. Significantly, the optimal cut-off value of quantitative markers is confusing. This confusion is mainly due to differences in statistical analysis and different clinicopathological characteristics of patients with NSCLC. However, this still suggests that in subsequent clinical studies, attention should be paid to the cut-off value of quantitative markers such as CAR, PNI, ALI, etc., and if the receiver operating characteristic curve (ROC) is applied, the area under curve (AUC) should be reported. In addition, we believe that there is a need for clarity regarding the time points at which these markers are monitored. For example, values monitored before treatment, values monitored after treatment, or the difference between before and after treatment are worth being compared.
Figure 3.
Conceptual framework for albumin-related inflammatory markers in the prognosis and immune microenvironment of non-small cell lung cancer. (A) Increased or decreased albumin-related inflammatory markers predict poor prognosis in patients with non-small cell lung cancer. (B) Albumin, C-reactive protein, and peripheral blood cells play a role in the immune microenvironment of NSCLC. Figure created with BioRender.com. CAR: C-reactive protein albumin ratio; GPS: Glasgow prognostic score; mGPS: modified Glasgow prognostic score; Hs-mGPS: high-sensitivity modified Glasgow prognostic score; a-GPS: adjusted Glasgow prognostic score; PNI: Prognostic nutritional index; ALI: Advanced lung cancer inflammation Index; COA-NLR: Alb concentration combined with NLR; NLDA: NLR × D-dimer count/albumin; ANPG: albumin and neutrophil combined prognostic grade; HALP: haemoglobin: albumin: lymphocyte and platelet score; DC: dendritic cell; MDSC: myeloid-derived suppressor cell; CAF: cancer-associated fibroblast; NK: natural killer.
Anti-PD-1 monotherapy reduces T-cell apoptosis and improves neutrophil and monocyte function. It has shown promising results in NSCLC treatment [98]. The increase in immunotherapy has increased the 5-year survival rate of NSCLC patients from 5% to 26% [99]. Along with the use of immunotherapy, markers to predict prognostic risk and drug response in NSCLC patients receiving immunotherapy have been sought. Tumor mutational load (TMB) [100], epidermal growth factor receptor (EGFR) mutations [101], and soluble programmed cell death ligand-1 (sPD-L1) [98] can predict response to immunotherapy. However, their detection is cumbersome and expensive. We observed that higher CAR in patients with NSCLC treated with nivolumab predicted poorer treatment response [36]. PNI can predict OS and PFS in patients receiving immunotherapy [62,64,66]. ALI has prognostic value in predicting survival in patients with NSCLC treated with nivolumab [87]. Other than that, other markers have not been seen in immunotherapy studies. The prognostic value of these markers in terms of efficacy response to immunotherapy is an area that deserves deeper investigation.
It has to be admitted that the nutritional status reflected by albumin has a limited effect on the prognosis of patients with NSCLC. As a result, the clinical value of albumin-related inflammation markers becomes limited. The Controlling nutritional status (CONUT) based on the serum levels of albumin, cholesterol, and lymphocyte count has been shown to predict the efficacy and prognosis of NSCLC patients treated with pembrolizumab [102]. With the addition of cholesterol, it may be more valuable than the albumin-related inflammation markers described in the article. In addition, it is not difficult to find that some of the ‘participants’ conditions’ we have included have experienced surgery. Therefore, these albumin-related inflammation markers may show greater clinical value in predicting the prognosis of patients with NSCLC undergoing surgery. But at the same time, we must note that patients with NSCLC who can undergo surgery generally have no dietary restrictions, and supplemental nutrition alone is not expected to dramatically improve nutritional indices, let al.one improve the prognosis of patients. And for those patients with poor nutritional indicators but in the early stage of NSCLC, there may still be a good prognosis, so it is necessary to adjust important confounding factors and conduct a long-term follow-up prospective cohort study. Before the cut-off value is determined, it is difficult for these markers to achieve the transformation from research to clinical application. In addition, finding people who are suitable for predicting prognosis, personalizing and accurate, and developing more intelligent inflammation prediction or diagnostic markers related to albumin may be a necessary step to realize them from clinical study to clinical application.
Our review also had some limitations. In the literature we included, single-centre retrospective studies accounted for the majority. And in our review of the included studies, we focused on the value of albumin-related inflammatory markers and the main findings of the study, without too much consideration of the methods of these studies and their limitations. There is also a bias that negative results are not published and cannot be included in the study. Moreover, in addition to albumin-related inflammatory markers, there are many other combined inflammatory markers and scores that can predict the prognosis of NSCLC. Reports on the prognostic value of SIS have never stopped [103]. The predictive value of scores or markers combining CRP with BMI, NLR, and LYM in patients with non-small cell lung cancer has also been reported [104–106]. Recently, a novel tumour marker and inflammation index (TMII) based on serum CEA and CRP has been reported. High preoperative TMII predicted a poor prognosis in patients with NSCLC [107]. But they have not been systematically reviewed in our article.
Conclusion and perspectives
There is no clinical study comparing the current albumin-related inflammatory markers. The calculation of these markers is similar and has a similar predictive effect in previous studies. This review provided in-depth thinking on how to better study and use these markers. It is necessary to adjust important confounding factors and conduct a long-term follow-up prospective cohort study to further clarify their cut-off value and respective application advantages. In addition, the specific mechanism of how these markers affect the prognosis is not clear, which also encourages us to further study it.
Acknowledgment
The authors are grateful for the helpful reviewer comments on this paper.
Funding Statement
The study was supported by a grant from Scientific and Technological Innovation Project of China Academy of Chinese Medical Sciences [grant number CI2021A01805], [grant number CI2021B009]; Science and Technology Commission Capital Clinical Diagnosis and Treatment Technology Research and Demonstration Application Project of Beijing [grant number Z191100006619022]; and Natural Science Foundation of Beijing [grant number 7222296].
Author contributions
Conception and design: CZ, MG, BP, and YC. Acquisition and interpretation of data: CZ, MG, XJ, XP, XZ, YL, and QS. Drafting of the paper: CZ, MG, XJ, XZ, YL, and QS. Revising paper critically for intellectual content: BP, XP, and YC. All authors reviewed and approved the submitted final version of the paper and agree to be held accountable for all aspects of the work.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
All data were included in the manuscript and there was no restriction on availability.
References
- 1.Siegel RL, Miller KD, Fuchs HE, et al. . Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2.Tan Z, Xue H, Sun Y, et al. . The role of tumor inflammatory microenvironment in lung cancer. Front Pharmacol. 2021;12:688625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zitvogel L, Pietrocola F, Kroemer G.. Nutrition, inflammation and cancer. Nat Immunol. 2017;18(8):843–850. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi S, Karube Y, Matsumura Y, et al. . Inflammatory risk factors for early recurrence of Non-Small cell lung cancer within one year following curative resection. World J Surg. 2020;44(10):3510–3521. [DOI] [PubMed] [Google Scholar]
- 5.Brown D, Zingone A, Yu Y, et al. . Relationship between circulating inflammation proteins and lung cancer diagnosis in the national lung screening trial. Cancer Epidemiol Biomarkers Prev. 2019;28(1):110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomes M, Teixeira AL, Coelho A, et al. . The role of inflammation in lung cancer. Adv Exp Med Biol. 2014;816:1–23. [DOI] [PubMed] [Google Scholar]
- 7.Conway EM, Pikor LA, Kung SH, et al. . Macrophages, inflammation, and lung cancer. Am J Respir Crit Care Med. 2016;193(2):116–130. [DOI] [PubMed] [Google Scholar]
- 8.Lagiou P, Trichopoulos D.. Inflammatory biomarkers and risk of lung cancer. J Natl Cancer Inst. 2011;103(14):1073–1075. [DOI] [PubMed] [Google Scholar]
- 9.Pope JE, Choy EH.. C-reactive protein and implications in rheumatoid arthritis and associated comorbidities. Semin Arthritis Rheum. 2021;51(1):219–229. [DOI] [PubMed] [Google Scholar]
- 10.Polanski J, Chabowski M, Swiatoniowska-Lonc N, et al. . Relationship between nutritional status and clinical outcome in patients treated for lung cancer. Nutrients. 2021;13(10):3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei Q, Fang W, Chen X, et al. . Establishment and validation of a mathematical diagnosis model to distinguish benign pulmonary nodules from early non-small cell lung cancer in chinese people. Transl Lung Cancer Res. 2020;9(5):1843–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stares M, Swan A, Cumming K, et al. . Hypoalbuminaemia as a prognostic biomarker of First-Line treatment resistance in metastatic non-small cell lung cancer. Front Nutr. 2021;8:734735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaymak Cerkesli ZA, Ozkan EE, Ozseven A.. The esophageal dose-volume parameters for predicting grade I-II acute esophagitis correlated with weight loss and serum albumin decrease in lung cancer radiotherapy. J Cancer Res Ther. 2021;17(1):94–98. [DOI] [PubMed] [Google Scholar]
- 14.Takada K, Takamori S, Yoneshima Y, et al. . Serum markers associated with treatment response and survival in non-small cell lung cancer patients treated with anti-PD-1 therapy. Lung Cancer. 2020;145:18–26. [DOI] [PubMed] [Google Scholar]
- 15.Fairclough E, Cairns E, Hamilton J, et al. . Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin Med (Lond). 2009;9(1):30–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou T, Zhan J, Hong S, et al. . Ratio of C-Reactive protein/albumin is an inflammatory prognostic score for predicting overall survival of patients with small-cell lung cancer. Sci Rep. 2015;5:10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyazaki T, Yamasaki N, Tsuchiya T, et al. . Ratio of C-reactive protein to albumin is a prognostic factor for operable non-small-cell lung cancer in elderly patients. Surg Today. 2017;47(7):836–843. [DOI] [PubMed] [Google Scholar]
- 18.Forrest LM, McMillan DC, McArdle CS, et al. . Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Br J Cancer. 2003;89(6):1028–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forrest LM, McMillan DC, McArdle CS, et al. . Comparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancer. Br J Cancer. 2004;90(9):1704–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minami S, Ihara S, Kim SH, et al. . Lymphocyte to monocyte ratio and modified glasgow prognostic score predict prognosis of lung adenocarcinoma without driver mutation. World J Oncol. 2018;9(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McMillan DC, Crozier JE, Canna K, et al. . Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for Colon and rectal cancer. Int J Colorectal Dis. 2007;22(8):881–886. [DOI] [PubMed] [Google Scholar]
- 22.O'Gorman P, McMillan DC, McArdle CS.. Prognostic factors in advanced gastrointestinal cancer patients with weight loss. Nutr Cancer. 2000;37(1):36–40. [DOI] [PubMed] [Google Scholar]
- 23.Osugi J, Muto S, Matsumura Y, et al. . Prognostic impact of the high-sensitivity modified glasgow prognostic score in patients with resectable non-small cell lung cancer. J Cancer Res Ther. 2016;12(2):945–951. [DOI] [PubMed] [Google Scholar]
- 24.Kawashima M, Murakawa T, Shinozaki T, et al. . Significance of the glasgow prognostic score as a prognostic indicator for lung cancer surgery. Interact Cardiovasc Thorac Surg. 2015;21(5):637–643. [DOI] [PubMed] [Google Scholar]
- 25.Matsubara T, Okamoto T.. ASO author reflections: the C-Reactive protein (CRP)-albumin ratio may be useful as the most prognostic index among the immuno-nutritional parameters using CRP and albumin for resected NSCLC. Ann Surg Oncol. 2021;28(6):3055–3056. [DOI] [PubMed] [Google Scholar]
- 26.Tomita M, Ayabe T, Chosa E, et al. . Prognostic significance of pre- and postoperative glasgow prognostic score for patients with non-small cell lung cancer. Anticancer Res. 2014;34(6):3137–3140. [PubMed] [Google Scholar]
- 27.Jiang AG, Lu HY.. The glasgow prognostic score as a prognostic factor in patients with advanced non-small cell lung cancer treated with cisplatin-based first-line chemotherapy. J Chemother. 2015;27(1):35–39. [DOI] [PubMed] [Google Scholar]
- 28.Zhang CL, Fan K, Gao MQ, et al. . Prognostic value of glasgow prognostic score in non-small cell lung cancer: a systematic review and Meta-Analysis. Pathol Oncol Res. 2022;28:1610109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McMillan DC, Watson WS, O'Gorman P, et al. . Albumin concentrations are primarily determined by the body cell mass and the systemic inflammatory response in cancer patients with weight loss. Nutr Cancer. 2001;39(2):210–213. [DOI] [PubMed] [Google Scholar]
- 30.Yamauchi Y, Safi S, Muley T, et al. . C-reactive protein-albumin ratio is an independent prognostic predictor of tumor recurrence in stage IIIA-N2 lung adenocarcinoma patients. Lung Cancer. 2017;114:62–67. [DOI] [PubMed] [Google Scholar]
- 31.Zhang F, Ying L, Jin J, et al. . The C-reactive protein/albumin ratio predicts long-term outcomes of patients with operable non-small cell lung cancer. Oncotarget. 2017;8(5):8835–8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ni XF, Wu J, Ji M, et al. . Effect of C-reactive protein/albumin ratio on prognosis in advanced non-small-cell lung cancer. Asia Pac J Clin Oncol. 2018;14(6):402–409. [DOI] [PubMed] [Google Scholar]
- 33.Yang JR, Xu JY, Chen GC, et al. . Post-diagnostic C-reactive protein and albumin predict survival in chinese patients with non-small cell lung cancer: a prospective cohort study. Sci Rep. 2019;9(1):8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karahan I, Yalcin S.. Is C-Reactive protein/albumin ratio of Advanced-Stage non-small cell lung cancer patients able to predict mortality in the admission for palliative care? Indian J Palliat Care. 2020;26(3):365–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsubara T, Takamori S, Haratake N, et al. . Identification of the best prognostic marker among immunonutritional parameters using serum C-Reactive protein and albumin in Non-Small cell lung cancer. Ann Surg Oncol. 2021;28(6):3046-3054. [DOI] [PubMed] [Google Scholar]
- 36.Araki T, Tateishi K, Sonehara K, et al. . Clinical utility of the C-reactive protein:albumin ratio in non-small cell lung cancer patients treated with nivolumab. Thorac Cancer. 2021; 12(5):603-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balkwill F, Mantovani A.. Inflammation and cancer: back to virchow? Lancet. 2001;357(9255):539–545. [DOI] [PubMed] [Google Scholar]
- 38.Dupre A, Malik HZ.. Inflammation and cancer: what a surgical oncologist should know. Eur J Surg Oncol. 2018;44(5):566–570. [DOI] [PubMed] [Google Scholar]
- 39.Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5–14. [PubMed] [Google Scholar]
- 40.Sacdalan DB, Lucero JA, Sacdalan DL.. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther. 2018;11:955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong YF, Chen ZH, Wei L, et al. . Identification of the prognostic value of lymphocyte-to-monocyte ratio in patients with HBV-associated advanced hepatocellular carcinoma. Oncol Lett. 2017;14(2):2089–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Y, Wang W, Zhang X, et al. . Prognostic significance of combined preoperative platelet-to-lymphocyte ratio and lymphocyte-to-monocyte ratio in patients undergoing surgery with stage IB non-small-cell lung cancer. Cancer Manag Res. 2018;10:5411–5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jenne CN, Kubes P.. Platelets in inflammation and infection. Platelets. 2015;26(4):286–292. [DOI] [PubMed] [Google Scholar]
- 44.Rossaint J, Margraf A, Zarbock A.. Role of platelets in leukocyte recruitment and resolution of inflammation. Front Immunol. 2018;9:2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kos FT, Hocazade C, Kos M, et al. . Assessment of prognostic value of "neutrophil to lymphocyte ratio" and "prognostic nutritional index" as a sytemic inflammatory marker in non-small cell lung cancer. Asian Pac J Cancer Prev. 2015;16(9):3997–4002. [DOI] [PubMed] [Google Scholar]
- 46.Migita K, Takayama T, Saeki K, et al. . The prognostic nutritional index predicts long-term outcomes of gastric cancer patients independent of tumor stage. Ann Surg Oncol. 2013;20(8):2647–2654. [DOI] [PubMed] [Google Scholar]
- 47.Jafri SH, Shi R, Mills G.. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. 2013;13:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weng J, Huang J, Yu W, et al. . Combination of albumin concentration and neutrophil-to-lymphocyte ratio for predicting overall survival of patients with non-small cell lung cancer. J Thorac Dis. 2021;13(9):5508–5516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun WW, Hu JA, Niu WQ, et al. . Significance of NLDA, the commixed index of inflammation, immune responses, hemostasis, and nutrition, for predicting metastatic non-small cell lung cancer prognosis and metastases. Oncotarget. 2017;8(47):81978–81993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun H, Hu P, Shen H, et al. . Albumin and neutrophil combined prognostic grade as a new prognostic factor in Non-Small cell lung cancer: results from a large consecutive cohort. PLoS One. 2015;10(12):e0144663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhai B, Chen J, Wu J, et al. . Predictive value of the hemoglobin, albumin, lymphocyte, and platelet (HALP) score and lymphocyte-to-monocyte ratio (LMR) in patients with non-small cell lung cancer after radical lung cancer surgery. Ann Transl Med. 2021;9(12):976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mori S, Usami N, Fukumoto K, et al. . The significance of the prognostic nutritional index in patients with completely resected Non-Small cell lung cancer. PLoS One. 2015;10(9):e0136897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shoji F, Miura N, Matsubara T, et al. . Prognostic significance of immune-nutritional parameters for surgically resected elderly lung cancer patients: a multicentre retrospective study. Interact Cardiovasc Thorac Surg. 2018;26(3):389–394. [DOI] [PubMed] [Google Scholar]
- 54.Qiu C, Qu X, Shen H, et al. . Evaluation of prognostic nutritional index in patients undergoing radical surgery with nonsmall cell lung cancer. Nutr Cancer. 2015;67(5):741–747. [DOI] [PubMed] [Google Scholar]
- 55.Okada S, Shimada J, Kato D, et al. . Clinical significance of prognostic nutritional index after surgical treatment in lung cancer. Ann Thorac Surg. 2017;104(1):296–302. [DOI] [PubMed] [Google Scholar]
- 56.Okada S, Shimada J, Teramukai S, et al. . Risk stratification according to the prognostic nutritional index for predicting postoperative complications after lung cancer surgery. Ann Surg Oncol. 2018;25(5):1254–1261. [DOI] [PubMed] [Google Scholar]
- 57.Park S, Ahn HJ, Yang M, et al. . The prognostic nutritional index and postoperative complications after curative lung cancer resection: a retrospective cohort study. J Thorac Cardiovasc Surg. 2020;160(1):276–285 e1. [DOI] [PubMed] [Google Scholar]
- 58.Matsubara T, Hirai F, Yamaguchi M, et al. . Immunonutritional indices in non-small-cell lung cancer patients receiving adjuvant platinum-based chemotherapy. Anticancer Res. 2021;41(10):5157–5163. [DOI] [PubMed] [Google Scholar]
- 59.Shimizu K, Okita R, Saisho S, et al. . Prognostic nutritional index before adjuvant chemotherapy predicts chemotherapy compliance and survival among patients with non-small-cell lung cancer. Ther Clin Risk Manag. 2015;11:1555–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park S, Park S, Lee SH, et al. . Nutritional status in the era of target therapy: poor nutrition is a prognostic factor in non-small cell lung cancer with activating epidermal growth factor receptor mutations. Korean J Intern Med. 2016;31(6):1140–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nakao M, Muramatsu H, Kagawa Y, et al. . Immunological status may predict response to nivolumab in non-small cell lung cancer without driver mutations. Anticancer Res. 2017;37(7):3781–3786. [DOI] [PubMed] [Google Scholar]
- 62.Shoji F, Takeoka H, Kozuma Y, et al. . Pretreatment prognostic nutritional index as a novel biomarker in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Lung Cancer. 2019;136:45–51. [DOI] [PubMed] [Google Scholar]
- 63.Ozkan EE, Kaymak Cerkesli ZA, Erdogan M.. Predictive value of immune-inflammation indices in metabolic response and outcome after curative radiotherapy in patients with non-small cell lung cancer. Clin Respir J. 2020;14(9):849–856. [DOI] [PubMed] [Google Scholar]
- 64.Peng L, Wang Y, Liu F, et al. . Peripheral blood markers predictive of outcome and immune-related adverse events in advanced non-small cell lung cancer treated with PD-1 inhibitors. Cancer Immunol Immunother. 2020;69(9):1813–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J, Liu Y, Mi X, et al. . The prognostic value of prognostic nutritional index (PNI) and neutrophil to lymphocyte ratio (NLR) for advanced non-small cell lung cancer treated with platinum-based chemotherapeutics. Ann Palliat Med. 2020;9(3):967–978. [DOI] [PubMed] [Google Scholar]
- 66.Kuroda H, Takahashi Y, Shirai S, et al. . Survival benefit of immune checkpoint inhibitor monotherapy in patients with non-small cell lung cancer recurrence after completely pulmonary resection. Ann Transl Med. 2021;9(15):1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu N, Jiang A, Zheng X, et al. . Prognostic nutritional index identifies risk of early progression and survival outcomes in advanced non-small cell lung cancer patients treated with PD-1 inhibitors. J Cancer. 2021;12(10):2960–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takeda T, Yamada T, Tanimura K, et al. . Prognostic markers of survival among japanese patients with anaplastic lymphoma Kinase-Positive Non-Small-Cell lung cancer receiving First-Line alectinib. Diagnostics (Basel). 2021;11(12):2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hayasaka K, Shiono S, Suzuki K, et al. . Postoperative prognostic nutritional index as a prognostic factor after non-small cell lung cancer surgery. Gen Thorac Cardiovasc Surg. 2020;68(10):1163–1171. [DOI] [PubMed] [Google Scholar]
- 70.Takahashi M, Sowa T, Tokumasu H, et al. . Comparison of three nutritional scoring systems for outcomes after complete resection of non-small cell lung cancer. J Thorac Cardiovasc Surg. 2021;162(4):1257–1268 e3. [DOI] [PubMed] [Google Scholar]
- 71.Xu S, Cao S, Geng J, et al. . High prognostic nutritional index (PNI) as a positive prognostic indicator for non-small cell lung cancer patients with bone metastasis. Clin Respir J. 2021;15(2):225–231. [DOI] [PubMed] [Google Scholar]
- 72.Li XL, Yao ZH, Wan YY, et al. . Prognostic impact of prognostic nutritional index in advanced (stage IIIB/IV) non-small cell lung cancer patients. Neoplasma. 2019;66(6):971–977. [DOI] [PubMed] [Google Scholar]
- 73.Seo Y, Eo W, Kim S, et al. . Can nutritional status predict overall survival in patients with advanced Non-Small cell lung cancer? Nutr Cancer. 2019;71(7):1108–1117. [DOI] [PubMed] [Google Scholar]
- 74.Bozkaya Y, Kostek O, Sakin A, et al. . Is the prognostic nutritional index a prognostic and predictive factor in metastatic non-small cell lung cancer patients treated with first-line chemotherapy? Support Care Cancer. 2020;28(5):2273–2282. [DOI] [PubMed] [Google Scholar]
- 75.Ozdemir Y, Topkan E, Mertsoylu H, et al. . Low prognostic nutritional index predicts poor clinical outcomes in patients with stage IIIB non-small-cell lung carcinoma undergoing chemoradiotherapy. Cancer Manag Res. 2020;12:1959–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matsuura S, Morikawa K, Ito Y, et al. . The geriatric nutritional risk index and prognostic nutritional index predict the overall survival of advanced Non-Small cell lung cancer patients. Nutr Cancer. 2022;74(5):1606–1613. [DOI] [PubMed] [Google Scholar]
- 77.Watanabe K, Noma D, Masuda H, et al. . Preoperative inflammation-based scores predict early recurrence after lung cancer resection. J Thorac Dis. 2021;13(5):2812–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bacha S, Sghaier A, Habibech S, et al. . Advanced lung cancer inflammation index: a prognostic score in patients with metastatic non-small cell lung cancer. Tunis Med. 2017;95(11):976–981. [PubMed] [Google Scholar]
- 79.Ozyurek BA, Ozdemirel TS, Ozden SB, et al. . Does advanced lung inflammation index (ALI) have prognostic significance in metastatic non-small cell lung cancer? Clin Respir J. 2018;12(6):2013–2019. [DOI] [PubMed] [Google Scholar]
- 80.Shiroyama T, Suzuki H, Tamiya M, et al. . Pretreatment advanced lung cancer inflammation index (ALI) for predicting early progression in nivolumab-treated patients with advanced non-small cell lung cancer. Cancer Med. 2018;7(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chantharakhit C, Sujaritvanichpong N.. Prognostic impact of the advanced lung cancer inflammation index (ALI) in metastatic Non-Small cell lung cancer treated with first line chemotherapy. Asian Pac J Cancer Prev. 2021;22(4):1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tomita M, Ayabe T, Maeda R, et al. . Comparison of Inflammation-Based prognostic scores in patients undergoing curative resection for non-small cell lung cancer. World J Oncol. 2018;9(3):85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Han Z, Hu Z, Zhao Q, et al. . The advanced lung cancer inflammation index predicts outcomes of patients with non-small cell lung cancer following video-assisted thoracic surgery. J Int Med Res. 2021;49(12):3000605211062442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mandaliya H, Jones M, Oldmeadow C, et al. . Prognostic biomarkers in stage IV non-small cell lung cancer (NSCLC): neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to lymphocyte ratio (PLR) and advanced lung cancer inflammation index (ALI). Transl Lung Cancer Res. 2019;8(6):886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kobayashi S, Karube Y, Inoue T, et al. . Advanced lung cancer inflammation index predicts outcomes of patients with pathological stage IA lung adenocarcinoma following surgical resection. Ann Thorac Cardiovasc Surg. 2019;25(2):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y, Lin L, Ji Y, et al. . Prognostic value of the advanced lung cancer inflammation index in early-stage non-small cell lung cancer patients undergoing video-assisted thoracoscopic pulmonary resection. Ann Palliat Med. 2020;9(3):721–729. [DOI] [PubMed] [Google Scholar]
- 87.Adachi Y, Tamiya A, Taniguchi Y, et al. . Predictive factors for progression-free survival in non-small cell lung cancer patients receiving nivolumab based on performance status. Cancer Med. 2020;9(4):1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu P, Ma Y, Kai J, et al. . A low advanced lung cancer inflammation index predicts a poor prognosis in patients with metastatic Non-Small cell lung cancer. Front Mol Biosci. 2021;8:784667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Palomar-Abril V, Soria-Comes T, Tarazona Campos S, et al. . Impact of age on Inflammation-Based scores among patients diagnosed with stage III Non-Small cell lung cancer. Oncology. 2020;98(8):528–533. [DOI] [PubMed] [Google Scholar]
- 90.Mountzios G, Samantas E, Senghas K, et al. . Association of the advanced lung cancer inflammation index (ALI) with immune checkpoint inhibitor efficacy in patients with advanced non-small-cell lung cancer. ESMO Open. 2021;6(5):100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tomita M, Ayabe T, Maeda R, et al. . Combination of advanced lung cancer inflammation index and C-Reactive protein is a prognostic factor in patients with operable Non-Small cell lung cancer. World J Oncol. 2017;8(6):175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oster HS, Dolev Y, Kehat O, et al. . Serum hypoalbuminemia is a Long-Term prognostic marker in medical hospitalized patients, irrespective of the underlying disease. JCM. 2022;11(5):1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sproston NR, Ashworth JJ.. Role of C-Reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coffelt SB, Wellenstein MD, de Visser KE.. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16(7):431–446. [DOI] [PubMed] [Google Scholar]
- 95.Lee HM, Lee HJ, Chang JE.. Inflammatory cytokine: an attractive target for cancer treatment. Biomedicines. 2022;10(9):2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sedighzadeh SS, Khoshbin AP, Razi S, et al. . A narrative review of tumor-associated macrophages in lung cancer: regulation of macrophage polarization and therapeutic implications. Transl Lung Cancer Res. 2021;10(4):1889–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Goubran HA, Stakiw J, Radosevic M, et al. . Platelets effects on tumor growth. Semin Oncol. 2014;41(3):359–369. [DOI] [PubMed] [Google Scholar]
- 98.Khairil Anwar NA, Mohd Nazri MN, Murtadha AH, et al. . Prognostic prospect of soluble programmed cell death ligand-1 in cancer management. Acta Biochim Biophys Sin (Shanghai). 2021;53(8):961–978. [DOI] [PubMed] [Google Scholar]
- 99.Espana S, Guasch I, Carcereny E.. Immunotherapy rechallenge in patients with non-small-cell lung cancer. Pulmonology. 2020;26(4):252–254. [DOI] [PubMed] [Google Scholar]
- 100.Choucair K, Morand S, Stanbery L, et al. . TMB: a promising immune-response biomarker, and potential spearhead in advancing targeted therapy trials. Cancer Gene Ther. 2020;27(12):841–853. [DOI] [PubMed] [Google Scholar]
- 101.Yucel S, Bilgin B.. The prognostic values of systemic immune-inflammation index and derived neutrophil-lymphocyte ratio in EGFR-mutant advanced non-small cell lung cancer. J Oncol Pharm Pract. 2021;27(1):71–77. [DOI] [PubMed] [Google Scholar]
- 102.Ohba T, Takamori S, Toyozawa R, et al. . Prognostic impact of the controlling nutritional status score in patients with non-small cell lung cancer treated with pembrolizumab. J Thorac Dis. 2019;11(9):3757–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zaitsu J, Yamashita Y, Ishikawa A, et al. . Systemic inflammatory score predicts response and prognosis in patients with lung cancer treated with immunotherapy. Anticancer Res. 2021;41(7):3673–3682. [DOI] [PubMed] [Google Scholar]
- 104.Bacha S, Sghaier A, Habibech S, et al. . Combined C-reactive protein and neutrophil to lymphocyte ratio use predict survival innon-small-cell lung cancer. Tunis Med. 2017;95(12):229–235. [PubMed] [Google Scholar]
- 105.Mitsuyoshi T, Matsuo Y, Itou H, et al. . Evaluation of a prognostic scoring system based on the systemic inflammatory and nutritional status of patients with locally advanced non-small-cell lung cancer treated with chemoradiotherapy. J Radiat Res. 2018;59(1):50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.He Y, Gong R, Peng KW, et al. . Lymphocyte-to-C-reactive protein ratio is a potential new prognostic biomarker for patients with lung cancer. Biomark Med. 2020;14(9):717–726. [DOI] [PubMed] [Google Scholar]
- 107.Tomita M, Maeda R, Ayabe T, et al. . Prognostic impact of a novel tumor marker and inflammation index for patients with non-small-cell lung cancer. Anticancer Res. 2020;40(7):4023–4027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data were included in the manuscript and there was no restriction on availability.