As the COVID-19 pandemic took hold in early 2020, most countries were poorly equipped to deal with it. Despite the entreaties of the World Health Organization to “test, test, test,”1 few countries had the infrastructure, test equipment, laboratories, or resources to implement wide-scale testing. With the growing realization that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection could be transmitted by those with mild symptoms and genuinely asymptomatic individuals,2 it became clear that relying on testing only symptomatic people who went to health services (and were able to get a test) would fail to interrupt a substantial proportion of transmission and would give a biased view of the pandemic.3 Accurate data on the extent of infection in the community and defining who was most at risk (and where) were essential to plan for demands on health services and to guide public health action in the absence, at that time, of vaccines and effective treatments.
Against this background, some countries invested early in community-wide testing of random members of the population to identify people with current infection. They used swabs and RT–PCR (reverse transcription–polymerase chain reaction) to determine current infection or past infection by testing for SARS-CoV-2 antibodies. The aims were to monitor trends in SARS-CoV-2 infection at a population level; gain new epidemiologic knowledge of who was at risk, when, and where; and (later, through viral sequencing of positive swabs) provide early warning of new variants appearing in the population. Antibody prevalence could also inform estimates of cumulative community infection rates, particularly important in early waves, when there was limited capacity for testing those with mild cases or their contacts.
The Seroepidemiological Survey of SARS-CoV-2 Virus Infection in Spain (Estudio Nacional de Sero-Epidemiología de la Infección por SARS-CoV-2 en España; ENE-COVID; see Pastor-Barriuso et al. [p. 525] in this issue of AJPH), conducted from April 27 to June 22, 2020, during lockdown, demonstrated regional heterogeneity in prevalence, which was higher in central regions of Spain. Even in areas with a high burden from the first wave of the pandemic, only around 10% of participants had antibodies,4 indicating that large numbers of people were still vulnerable to infection.
A subsequent round of data collection was added in November 2020 during the second wave of infections. This high-quality study addressed many of the elements required to provide reliable data on symptom reporting and antibody prevalence at a community level. At the time, policymakers were largely in the dark about the proportion of the population that had been infected with SARS-CoV-2 during the first and second waves. Data on symptoms, comorbidities, and other risk factors and antibody levels were obtained from a stratified random cross-section of the noninstitutionalized population of Spain. The aim was to provide reliable estimates of prevalence by key demographics, including information at the province level.
The first wave of the study included 68 287 participants (69.1% of eligible people) who received a point-of-care test (lateral flow immunoassay [LFIA] device) for SARS-CoV-2 IgG (immunoglobulin G) antibodies, with a more accurate laboratory immunoassay also being done. The investigators used study weights to adjust for any bias introduced by the sample design (including oversampling in relatively less-populated areas) and variable response rates in different subsections of the population. Local primary health care teams obtained data nationally using a common protocol to ensure comparability across areas. Sampling was done by household, with allowance made in the statistical analysis for clustering at the household level. Importantly, the very high response rate ensured that the prevalence estimates provided an accurate representation of the (cumulative) community prevalence of infection. Moreover, the large size and reach of the study, both regionally and by age (from infants to the elderly), meant that relatively precise estimates of prevalence for different demographic groups could be fed back to policymakers.
Meanwhile, in the United Kingdom, two large-scale studies with complementary designs were initiated to measure the prevalence of virus and of antibodies in random samples of the population: the REal-time Assessment of Community Transmission (REACT) Study in England5 and the Office for National Statistics (ONS) COVID-19 Infection Survey (CIS) across the United Kingdom.6 REACT included REACT-1, which tested for the virus by RT–PCR from self-administered throat and nose swabs, and REACT-2, which tested for antibodies using a self-taken LFIA test. Both were designed to be representative of the population of England as a whole; had wide coverage by age, sex, ethnicity, and small geographic area and region; used sample weighting to produce population estimates of prevalence; and aimed to provide rapid, unbiased, and authoritative information to the government, the scientific community, and the public.
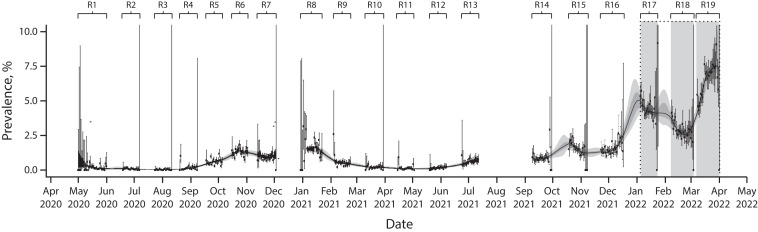
The REACT-1 study ran for approximately two to three weeks every month over 19 distinct rounds from May 1, 2020 to March 31, 2022, giving a detailed and dynamic picture of the pandemic in England as it unfolded (Figure 1).7 Overall, more than 2.5 million people aged 5 years and older took part. The REACT-2 study included more than 900 000 adults over six rounds from June 2020 to May 2021. The ongoing ONS–CIS survey used a random household design and tested both for virus through RT–PCR and antibodies using a laboratory ELISA (enzyme-linked immunosorbent assay) test following a blood draw.8
FIGURE 1—
Timeline and Weighted Prevalence of SARS-CoV-2 Infection: REACT-1 Study, England, May 1, 2020–March 31, 2022
Note. REACT-1 = REal-time Assessment of Community Transmission-1. The figure shows the weighted prevalence of infection (black dots) and 95% credible intervals (vertical bars). P-spline model (black line) and 50% and 95% posterior credible intervals (dark and light gray shading) are fit to the data. Vertical gray–shaded areas represent “twin peaks” of Omicron BA.1 and Omicron BA.2 infections January–March 2022.
Source. Adapted from Elliott et al.7
These examples from Spain and the United Kingdom were some of the earliest (and largest) to use community-based samples, but similar initiatives were developed in other national, regional, and local areas. In mid-2020, Serotracker was established, collating data on antibody prevalence to an interactive dashboard that monitors and synthesizes data from studies across the world.9
What have we learned from these studies and what are the take home messages for monitoring future outbreaks of severe respiratory infections?
-
1.
Antibody prevalence following the first wave of the pandemic in the United Kingdom10 and Spain4 was approximately 6% to 10%, so the capacity for large subsequent waves was high.
-
2.
Patterns of infection in the community were substantially different from patterns of cases or hospitalizations, and these differences were important for policy (e.g., children were key to the pandemic at certain times).
-
3.
There were marked social inequalities in risk of infection (and hence hospitalizations and mortality) during the first wave,3,10 with important implications for planning pandemic response to minimize such inequalities in the future.
-
4.
Relying on the results of routine testing of symptomatic people is biased both by the unavailability of tests (at least early in the pandemic) and by varying test-seeking behaviors in the community. Such data greatly underestimate the true infection rates because of the lack of comprehensive testing (in most countries) and the substantial numbers of asymptomatic infections.
-
5.
ENE-COVID and REACT could make meaningful estimates of population case fatality rates, as they were able to include asymptomatic and mild infections in the calculations.10,11
-
6.
Viral transmission did not occur equally everywhere but varied by place (and demographic groups) at different times,3 with implications for public health measures to control infections.
-
7.
Perhaps most importantly, most countries were massively ill prepared for the pandemic, and population testing and monitoring procedures (e.g., in the United Kingdom and Spain) had to be set up from scratch on an emergency footing.
The REACT and ENE-COVID studies showed that home-based self-sampling and testing is an efficient and effective way of carrying out mass testing at scale even during a lockdown. We now have a very clear picture of the requirements for situational awareness during a respiratory virus pandemic. Surely if we have learned one lesson, it is that we must invest, plan, and be much better prepared for future similar events.
ACKNOWLEDGMENTS
The authors acknowledge funding from the Department of Health and Social Care in England for the REal-time Assessment of Community Transmission-1 (REACT-1) and REACT-2 studies.
CONFLICTS OF INTEREST
P. Elliott (director and principal investigator), S. Riley, and H. Ward (coinvestigators), with other team members, established the REACT-1 and REACT-2 studies of SARS-CoV-2 infection in the population of England. S. Riley is Director General of Data, Analytics and Surveillance at the UK Health Security Agency.
REFERENCES
- 1.World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/331509/WHO-COVID-19-lab_testing-2020.1-eng.pdf
- 2.Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020 Euro Surveill. 202025102000180. [Erratum in: Euro Surveill. 2020;25(22)]. 10.2807/1560-7917.ES.2020.25.10.2000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riley S, Ainslie KEC, Eales O, et al. Resurgence of SARS-CoV-2 in England: detection by community antigen surveillance. Science. 2021;372(6545):990–995. doi: 10.1126/science.abf0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riley S, Atchison C, Ashby D, et al. REal-time Assessment of Community Transmission (REACT) of SARS-CoV-2 virus: study protocol. Wellcome Open Res. 2021;5:200. doi: 10.12688/wellcomeopenres.16228.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ISRCTN Registry. 2023. https://www.isrctn.com/ISRCTN21086382 [DOI]
- 7.Elliott P, Eales O, Steyn N, et al. Twin peaks: the omicron SARS-CoV-2 BA.1 and BA.2 epidemics in England. Science. 2022;376(6600):eabq4411. doi: 10.1126/science.abq4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Office for National Statistics. Coronavirus (COVID-19) infection survey, UK statistical bulletins. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/previousReleases2022
- 9.Arora RK, Joseph A, Van Wyk J, et al. SeroTracker: a global SARS-CoV-2 seroprevalence dashboard. Lancet Infect Dis. 2021;21(4):e75–e76. doi: 10.1016/S1473-3099(20)30631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward H, Atchison C, Whitaker M, et al. SARS-CoV-2 antibody prevalence in England following the first peak of the pandemic. Nat Commun. 2021;12(1):905. doi: 10.1038/s41467-021-21237-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pastor-Barriuso R, Pérez-Gómez B, Hernán MA, et al. Infection fatality risk for SARS-CoV-2 in community dwelling population of Spain: nationwide seroepidemiological study. BMJ. 2020;371:m4509. doi: 10.1136/bmj.m4509. [DOI] [PMC free article] [PubMed] [Google Scholar]