ABSTRACT
Real-world evidence on the effectiveness of COVID-19 vaccines marketed in China against the Omicron BA.2.2 variant remains scarce. A case–control study was conducted to estimate the vaccine effectiveness (VE) of COVID-19 vaccines marketed in China (inactivated vaccines, an Ad5-nCoV vaccine, and a recombinant protein vaccine). There were 414 cases infected with SARS-CoV-2 and 828 close contacts whose test results were consecutively negative as controls during the outbreak of the Omicron variant in Lu’an City, Anhui Province, China, in April 2022. The overall adjusted VE against Omicron BA.2.2 variant infection in the vaccinated group with any COVID-19 vaccine was 35.0% (95% CI: −9.1–61.3%), whereas the adjusted VE for booster vaccination was 51.6% (95% CI: 15.2–72.4%). Subgroup analysis showed that the overall adjusted VE of the Ad5-nCoV vaccine (65.8%, 95% CI: 12.8–86.6%) during the outbreak while any dose of inactivated vaccines and recombinant protein vaccine offered no protection. The adjusted VE of three-dose inactivated vaccines was 48.0% (95% CI: 8.0–70.6%), and the two-dose Ad5-nCoV vaccine was 62.9% (95% CI: 1.8–86%). There is no protection from a three-dose recombinant protein vaccine. COVID-19 vaccines offered 46.8% (95% CI: 9.5–68.7%) protection from infection within six months. There were statistically significant differences between the VEs of heterologous booster (VE = 76.4%, 95% CI: 14.3–93.5%) and homologous booster vaccination (VE = 51.8%, 95% CI: 9.6–74.3%) (P = .036). Booster vaccination of COVID-19 vaccines offered more protection than full vaccination. A booster vaccination campaign for a booster dose after three doses of a recombinant protein vaccine must be urgently conducted.
KEYWORDS: COVID-19 vaccines, Omicron, vaccine effectiveness, inactivated vaccine, Ad5-nCoV vaccine, recombinant protein vaccine
Introduction
Since the first coronavirus disease 2019 (COVID-19) case was reported in Wuhan in December 2019 and resulted in many deaths and serious cases worldwide, several new variants of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus have been identified.1,2 Subsequently, SARS-CoV-2-related mutant variants have emerged. The SARS-CoV-2 B.1.617.2 (Delta) variant was first identified in October 2020 in Maharashtra, India, which was approximately 50–60% more transmissible than the original variant.3 Thereafter, the B.1.1.529 (Omicron) variant was detected in South Africa in November 2021, and this variant has fueled more daily cases across many countries from the time it was discovered until today than any other variants.1,4,5 Moreover, an increasing number of studies have consistently reported that the Omicron variant has increased transmissibility, severity, and immune escape abilities, which resulted in lower vaccine effectiveness (VE) against SARS-CoV-2 infection compared with previous variants.1–2-5–8 The two-dose (mRNA and inactivated vaccines) VEs were reported to be 50.5–74.6% against the Delta variant infection and 0–44% against the Omicron variant infection.9–15 Limited data showed that the three-dose (mRNA vaccines) VE was 86.0–93.7% against the Delta variant infection and 7–47.4% against the Omicron variant infection.15–17 These findings confirmed the substantially decreased protection from the booster dose against the Omicron variant infection. However, its effectiveness against severe illness and death related to the Omicron variant remained much better.18
A total of three types of COVID-19 vaccines were authorized in China, including inactivated, Ad5-nCoV, and recombinant protein vaccines, among which inactivated vaccines were used most frequently.19,20 There are few real-world studies on the VE of COVID-19 vaccines, especially recombinant protein vaccines against SARS-COV-2 in China, and most were limited to the Alpha or Delta variant.11,16,19,21 Besides, unlike the vaccination of adults≥18 years old, the entire population is now recommended for COVID-19 vaccines except for special cases. Most previous studies in China have been limited to adult study subjects. The adjusted VEs of the inactivated vaccines and Ad5-nCoV vaccine were 51.8% (95% CI: 20.3–83.2%) and 61.5% (95% CI: 9.5–83.6%) against the Delta variant, respectively. In contrast, the adjusted VEs of the inactivated vaccines and Ad5-nCoV were 16.3% (15.4–17.2%) and 13.2% (10.9–15.5%) against the Omicron variant, respectively.22 As China has undertaken a strategy entitled the “dynamic zero-COVID” beginning in August 2021, there were much far fewer large-scale local outbreaks after the first epidemic wave in 2020 than in other countries.16,23 Therefore, there were few studies on the effectiveness of the COVID-19 vaccines in China after this policy came out, throwing a monkey wrench into the prevention and control of outbreaks. Recently, Huang, et al.22 reported that high and durable two- and three-dose inactivated VE against Omicron that was associated with preventing severe or critical illness and death across all age groups but provided lower effectiveness against the Omicron variant infection. However, further data are needed to support the VE of recombinant vaccines to date.
The SARS-CoV-2 outbreak was reported in Lu’an City in April 2022, providing an opportunity to estimate the VE of three types of COVID-19 vaccines used in China and globally (inactivated vaccines, recombinant protein vaccine, and Ad5-nCoV vaccine) in actual use. The outbreak occurred after China advocated COVID-19 vaccines for the whole population except for special cases. Thus, the results of this study will be applicable to the whole population regardless of age. Moreover, recombinant protein vaccines have been less used in other provinces except for Anhui Province in China. As a result, Lu’an city is in a unique position to evaluate the VE of a recombinant protein vaccine. We aim to adjust the policies to keep up-to-date with mutated variants, duration of vaccine-induced protection, implementation of booster doses, including fourth doses, and reduction of future mortality from SARS-COV-2.
Materials and methods
Outbreak description
On April 3, 2022, a 46-year-old female who worked in a garment factory in the south of Lu’an City was diagnosed with COVID-19. There were 187 workers in the garment factory. Over the next few days, some other workers had illness onset and were also SARS-CoV-2-positive. There are several schools near the garment factory where the children of positive workers studied, resulting in two garment factories and 24 schools occurring COVID-19 clusters. From April 3 to April 22, a total of 419 cases were reported, including five individuals who did not live in Lu’an. As of April 22, 2022, 33 secondary genome sequences were obtained from this outbreak and compared with the nucleotide sequences in the GenBank and GISAID databases. Finally, we found that all those sequences were confirmed to be Omicron BA.2.2 variant. A total of 358 individuals had been vaccinated with at least one of the COVID-19 vaccines, and 66 individuals have never received any of the COVID-19 vaccines.
Definitions
A case was defined as an individual who tested positive in a medical institution regardless of the presence of symptoms and had polymerase chain reaction (PCR)-confirmed COVID-19 testing performed in the Lu’an Center for Disease Control and Prevention (LACDC), in which all testing organizations followed the same diagnostic criteria. An asymptomatic infection was defined as a PCR-confirmed individual who did not meet any of the following clinical criteria: fever, cough, sore throat, or other self-perceived or clinically identifiable symptoms or signs, and had no radiographic evidence of pneumonia. An infected patient was defined as an individual who tested positive no matter whether he or she was symptomatic. Close contact was defined as an individual without effective protection who was in contact with someone with COVID-19 within four days of the time before the infected person developed symptoms or the sampling date if he or she did not have any symptoms. A core close contact was defined as an individual who was in frequent close contacts (such as living, eating, or working in the same room, sharing meals and enclosed hallways, taking the elevator together, or attending social activities without wearing a mask, or maintaining a high risk of exposure) with an infected patient without effective protection within two days of the onset of symptoms, or within two days prior to testing of an asymptomatic patient.
Study design and population
A case–control study was conducted to explore the effectiveness of COVID-19 vaccines against the SARS-CoV-2 Omicron variant outbreak in Lu’an City, which has the highest total area of 15,451.2 square kilometers in Anhui Province and about 4.39 million permanent residents.24 Cases included 414 SARS-CoV-2 infected patients who were diagnosed by PCR testing between April 4 and April 20, 2022 and lived in Lu’an. Controls were selected from individuals with negative SARS-CoV-2 PCR test result who were in core close contact with cases and either lived in the same household or ate together or took the same enclosed stairways or elevators. We coded 1016 core close contacts with numbers 1 to 1016 in excel, without any prior grouping stratification. From the 1016 codes, we sampled two controls matched to each case based on a random sampling method, all with an equal chance to obtain the final 828 controls.
Data collection
Once one case was confirmed by laboratory testing, the epidemiological and clinical criteria and medical records were submitted and accepted by LACDC immediately. After the receipt of the submitted reports, the LACDC performed an epidemiological investigation within 24 h, including verification of the activity trajectory, close contact determination, and tracing. General information (such as name, sex, age, ID number, last exposure time, frequency of exposure, mode of exposure, and location of exposure) of confirmed and asymptomatic infections between April 4, 2022 and April 20, 2022 in Lu’an was extracted from the epidemiological survey reports and National Notifiable Diseases Registry System (NNDRS), which is a web-based computerized reporting system and has been in operation since 2004 in China.25 Clinical information was obtained from epidemiological survey reports. We obtained the following information from the AIIMS through the ID numbers and names of the individuals: vaccine type, vaccination dose, vaccination site, vaccine manufacturer, vaccination date, vaccination status, and all vaccination records. We considered vaccinations to be valid only if documented in either the national or the provincial immunization information system.
Vaccination data
Three types of vaccines, including inactivated, Ad5-nCoV, and recombinant protein vaccines against SARS-CoV-2, were authorized and widely used in China.26 Vaccination was considered incomplete if the time from the last dose of vaccination to the last exposure time was less than 14 d. We divided all participants into four groups: unvaccinated (zero doses or first dose<14 d from the time of last exposure), partial vaccination (one-dose inactivated vaccine or two-dose recombinant protein vaccine, with the last dose of vaccination>14 d from the time of last exposure), full vaccination (one-dose Ad5-nCoV vaccine or two-dose inactivated vaccine or three-dose recombinant protein vaccine, with the last dose of vaccination>14 d from the time of last exposure), and booster vaccination (three-dose of any COVID-19 vaccine, or two-dose Ad5-CoV vaccine, or two-dose any inactivated vaccine with a one-dose Ad5-CoV vaccine/recombinant protein vaccine, with the last dose of vaccination> 14 d from the time of last exposure).27,28 Additionally, homogenous booster vaccination (same as the primary vaccine) and heterologous booster vaccination (third dose with an Ad5-nCoV or recombinant protein vaccine) following a two-dose regimen of inactivated vaccines were included in our study.24,29
Data analysis
Data analysis was performed using excel 2021 and statistical software SPSS 26. Symptoms were presented as percentages of the group total. Continuous variables while non-normally distributed were expressed as medians [interquartile ranges (IQR)] and analyzed by the independent samples Mann – Whitney U test. Categorical variables were analyzed by the chi-square test, with p < .05 considered statistically significant. We assessed the VEs against SARS-CoV-2 Omicron BA.2.2 variant infection of partial vaccinations, full vaccinations, and booster vaccinations compared with no vaccination, using binary logistic regression models to estimate the odds ratio (OR) of vaccination among case and control groups, adjusting by prespecified covariables considered to be potential confounders: gender, age, and time since the last dose of vaccination, expressed by OR and 95% confidence interval (95% CI). Furthermore, we performed subgroup analyses on gender, age group, vaccine type, time since the last dose of vaccination (months), and the type of booster vaccination.
The unadjusted OR was only used to calculate the crude VE, and the adjusted VE was estimated from the adjusted OR. The formula was used as follows: crude VE =(1 − crude OR)× 100%; adjusted VE = (1 – adjusted OR) × 100%.
Sensitivity analysis
We performed a sensitivity analysis. Both univariable and multivariable (adjusted for age, gender, and time since the last dose of vaccination) regression analyses were conducted for every subgroup in which intervals (days) were defined as the number of days between the positive test date and the date of the last dose of vaccination minus 7 d.
Results
Study participants
A total of 419 cases were confirmed inside Lu’an City from April 4 to April 20, 2022. There were 4817 close contacts, among which 1025 were core close contacts. We chose 828 core close contacts as controls based on a random sampling method. However, five study participants were excluded because they were not residents of Lu’an. Consequently, 414 test-positive cases and 828 test-negative controls were included in the final analysis. The flowchart of case and control selection is shown in Figure 1.
Figure 1.
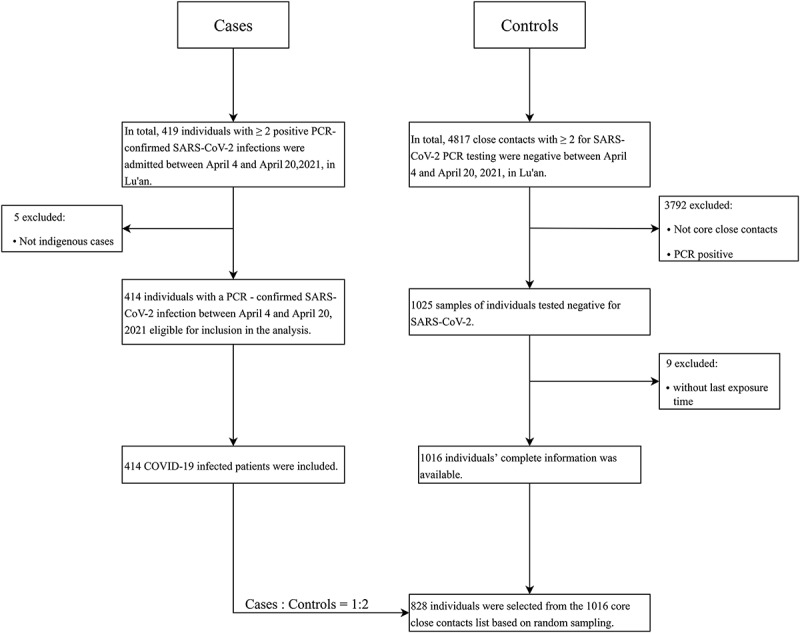
Flowchart shows recruitment of the cases and controls.
Characteristics of study participants
Of the positive cases, 239 (57.7%) were asymptomatic patients, and the remaining 42.3% were symptomatic. Among the symptomatic patients, symptoms reportedly included fever (n = 76, 43.4%), cough (n = 66, 37.7%), pharyngitis (n = 45, 25.7%), rhinitis (n = 21, 12%), fatigue (n = 16, 9.1%), diarrhea (n = 2, 1.1%), and chest discomfort (n = 1, 0.6%) (Table 1). Among the 414 cases and 828 controls, 255 (61.6%) and 394 (47.6%) individuals were female, respectively. The “time since the last dose of vaccination” was non-normally distributed (Z-score = 1.217/0.143 = 8.51 > 1.96). The mean “time since the last dose of vaccination” was 6.8 months (3.0–8.0 months) for cases and 3.8 months (2.9–7.5 months) for controls. There was a significant difference between the two groups in terms of “gender” and “time since the last dose of vaccination” (p < .001, p = .01). The median age was 38 y (18–55 y) in the case group and 40 y (25–56 y) in the control group, and no statistical difference was observed (p = .353) and was non-normally distributed (Z-score = −0.927/0.139=–6.6 < 1.96) (Table 2).
Table 1.
Clinical characteristics during an outbreak of SARS-CoV-2 Omicron BA.2 variant in Lu’an, China, 2022.
| Symptoms | Number of cases (N = 414) | Percentage (%) |
|---|---|---|
| Asymptomatic patients | 239 | 57.7 |
| Symptomatic patients | 175 | 42.3 |
| Fever | 76 | 43.4 |
| Cough | 66 | 37.7 |
| Pharyngitis | 45 | 25.7 |
| Rhinitis | 21 | 12.0 |
| Fatigue | 16 | 9.1 |
| Diarrhea | 2 | 1.1 |
| Chest discomfort | 1 | 0.6 |
Table 2.
Baseline characteristics of the study participants.
| Variables | Cases (N = 414) |
Controls (N = 828) |
P value |
|---|---|---|---|
| Gender, n (%) | < .001a | ||
| Male | 159 (38.4) | 434 (52.4) | |
| Female | 255 (61.6) | 394 (47.6) | |
| Age (years), median (IQR) | 38 (18–55) | 40 (25–56) | .353b |
| Time since last vaccination dose (months), median (IQR) | 6.8 (3.0–8.0) | 3.8 (2.9–7.5) | .01b |
| Vaccination status, n (%) | .006a | ||
| Unvaccinated | 29 (7.0) | 37 (4.5) | |
| Partial vaccination | 10 (2.4) | 10 (1.2) | |
| Full vaccination | 243 (58.7) | 445 (53.7) | |
| Booster vaccination | 132 (31.9) | 336 (40.6) |
aχ2 test.
bIndependent samples Mann – Whitney U test.
Vaccination status
The vaccination status of all participants is shown in Table 2. A greater proportion of unvaccinated (7.0%) individuals was in the case group than in the control group (4.5%). Among the case group, 2.4% completed a partial vaccination, and 58.7% completed a full vaccination, whereas, among the control group, the corresponding proportions were 1.2% and 53.7%, respectively. A total of 132 (31.9%) individuals had completed a booster vaccination in the case group, and 336 (40.6%) in the control group before the outbreak in April 2022. There was a significant difference in vaccination status between the case and control groups (p = .006).
Vaccine effectiveness
The VE of any vaccine in preventing SARS-CoV-2 Omicron BA.2.2 variant infection is shown in Table 3 and Figure 2. The overall adjusted VE was 35.0% (95% CI: −9.1–61.3%) against infection with any vaccine. Partial and full vaccination provided no protection. Adjusted VE was found to be 51.6% (95% CI: 15.2–72.4%) in the booster vaccination group for preventing Omicron variant infection. Compared with the unvaccinated group, Ad5-nCoV vaccines offered 65.8% (95% CI: 12.8–86.6%) protection for infection, whereas inactivated vaccines and a recombinant protein vaccine offered no protection. Adjusted VE was 48.0% (95% CI: 8.0–70.6%) in the inactivated vaccines booster vaccination group (Table S1) and 62.9% (95% CI: 1.8–86%) in the Ad5-nCoV vaccine booster vaccination group (Table S3). Overall (any vaccine), there was no protection for males, but the adjusted VE was 55.1% (95 CI: 9.6–77.7%) for females. The adjusted VE was not significantly different between the two age groups. Compared to the time since the last dose of vaccination, 48.1% of the case group and 38.3% of the control group were more than six months from the last dose of vaccination to exposure. The adjusted VEs were 14.2% (95% CI: −46.9–49.9%) if the time since the last vaccination dose was more than six months, and 44.8% (95% CI: 6.1–67.6%) if within six months. The heterologous booster vaccination adjusted VE (76%, 95% CI: 15–93%) was much better than that of the homologous booster vaccination (52%, 95% CI: 10–74%).
Table 3.
VE of any vaccine in preventing SARS-CoV-2 Omicron BA.2 variant infection.
| Variables | Cases (N = 414) |
Controls (N = 828) |
Crude OR (95% CI) |
Adjusted ORa (95% CI) |
Adjusted VE, % (95% CI) |
||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| Unvaccinated | 29 | 7.0 | 37 | 4.5 | |||
| With any vaccine | 385 | 93.0 | 791 | 95.5 | 0.621 (0.376–1.025) | 0.650 (0.387–1.091) | 35.0 (−9.1–61.3) |
| Partial vaccination | 10 | 2.4 | 10 | 1.2 | 1.276 (0.468–3.476) | 1.311 (0.476–3.611) | −31.1 (−261.1–52.4) |
| Full vaccination | 243 | 58.7 | 445 | 53.7 | 0.697 (0.418–1.161) | 0.689 (0.408–1.161) | 31.1 (−16.1–59.2) |
| Booster vaccination | 132 | 31.9 | 336 | 40.6 | 0.501 (0.296–0.848) | 0.484 (0.276–0.848) | 51.6 (15.2–72.4) |
| Vaccine typeb | |||||||
| Inactivated vaccine | 303 | 73.2 | 626 | 75.6 | 0.618 (0.373–1.023) | 0.645 (0.383–1.086) | 35.5 (−8.6–61.7) |
| Recombinant protein vaccine | 68 | 16.4 | 106 | 12.8 | 0.818 (0.461–1.453) | 0.870 (0.430–1.761) | 13.0 (−76.1–57.0) |
| Ad5-nCoV vaccine | 10 | 2.4 | 39 | 4.7 | 0.327 (0.140–0.764) | 0.342 (0.134–0.872) | 65.8 (12.8–86.6) |
| Genderc | |||||||
| Male | 151 | 36.5 | 412 | 49.8 | 1.008 (0.439–2.312) | 1.075 (0.461–2.507) | −7.5 (−150.7–53.9) |
| Female | 234 | 56.5 | 379 | 45.8 | 0.441 (0.223–0.873) | 0.449 (0.223–0.904) | 55.1 (9.6–77.7) |
| Age group (years)d | |||||||
| 0–59 | 318 | 76.8 | 659 | 79.6 | 0.612 (0.360–1.042) | 0.615 (0.359–1.053) | 38.5 (−5.3–64.1) |
| ≥60 | 67 | 16.2 | 132 | 15.9 | 0.677 (0.147–3.112) | 0.670 (0.145–3.093) | 33.0 (−209–85.5) |
| Time since last dose vaccination (months) | |||||||
| 0–6 | 186 | 44.9 | 474 | 57.2 | 0.501 (0.299–0.838) | 0.532 (0.313–0.904) | 46.8 (9.6–68.7) |
| >6 | 199 | 48.1 | 317 | 38.3 | 0.801 (0.501–1.469) | 0.858 (0.501–1.469) | 14.2 (−46.9–49.9) |
| Type of booster vaccination | |||||||
| Homologous booster vaccination | 128 | 30.9 | 316 | 38.2 | 0.517 (0.305–0.876) | 0.236 (0.065–0.857) | 51.8 (9.6–74.3) |
| Heterologous booster vaccination | 4 | 1.0 | 20 | 2.4 | 0.255 (0.079–0.829) | 0.482 (0.257–0.904) | 76.4 (14.3–93.5) |
aORs were from univariate conditional logistic regression and were adjusted by gender and age.
bIndividuals who had received a heterologous booster vaccination were not included.
cORs were adjusted by age only. The reference groups for male and female subgroups were the corresponding populations in the unvaccinated group.
dORs were adjusted by gender only. The reference groups for 0–59 and ≥60-year-old subgroups were the corresponding populations in the unvaccinated group.
Figure 2.
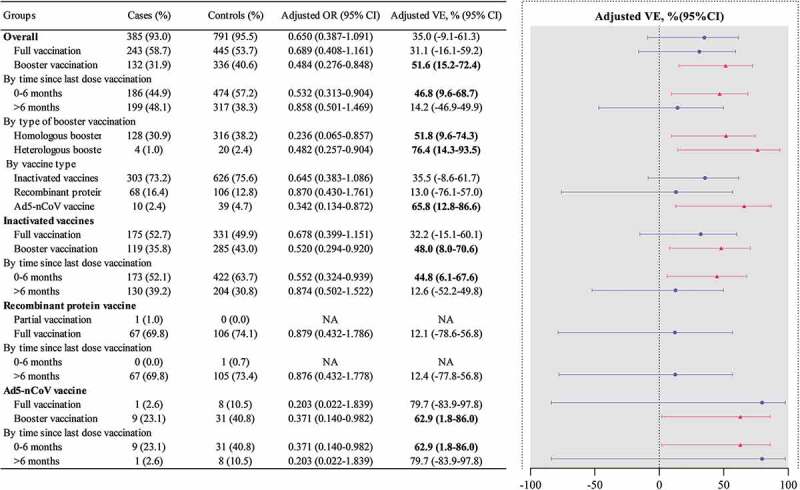
ORs and VE of COVID-19 vaccines in the case–control study during an outbreak of SARS-CoV-2 Omicron BA.2 variant.
Discussion
In this case–control study conducted from April 4, 2022 to April 20, 2022 in Lu’an City, we examined the real-world effectiveness of COVID-19 vaccines against the Omicron BA.2.2 variant among the population. In total, the overall VE against the Omicron variant infection was 35.0% (95% CI: −9.1–61.3%) for any COVID-19 vaccine. Compared with the unvaccinated group, with any dose of inactivated vaccines and recombinant protein vaccine offered no protection, while Ad5-nCoV vaccine offered 65.8% (95% CI: 12.8–86.6%) protection against the Omicron BA2.2 variant infection. There were statistically significant differences between heterologous booster vaccination (VE = 76.4%, 95% CI: 14.3–93.5%) and homologous booster vaccination (VE = 51.8%, 95% CI: 9.6–74.3%) VEs in the booster groups (P = .036).
Cases included symptomatic or asymptomatic patients, and asymptomatic cases were 57.7% of all cases. A growing number of reports indicated that the Omicron-associated COVID-19 disease was less severe than the Delta-associated COVID-19 disease, resulting in more asymptomatic patients.30,31 A study from India showed that the majority of patients infected with Omicron were asymptomatic (56.7%),32 consistent with our study. The Faroe Islands study reported that in over half the cases, patients assessed their illness as asymptomatic or mild.33 Infected patients showing no or mild symptoms indicate that the Omicron variant has increased immune escape ability, making it difficult to prevent. Among all cases, fever (43.4%), cough (37.7%), pharyngitis (25.7%), and rhinitis (12%) were the most prevalent symptoms, which was consistent with a study conducted in India, which revealed that the predominant symptoms reported included fever (43.2%), and the rest of the infected patients had milder symptoms.34 In this study, we found that COVID-19 vaccines offered 46.8% (95% CI: 9.5–68.7%) protection from infection within six months. In contrast, no protection was provided after six months. One study from Qatar showed that the effectiveness of the last dose of vaccination persisted at an approximately relatively high level for about six months, then it dramatically declined after six months.35 Another study from Mexico showed that the VE against illness declined from 48% after 14–60 d following full vaccination to 20% after 61–120 d.36 The median months since the last dose of vaccination in the case group (6.8, 3.0–8.0) were longer than in the control group (3.8, 2.9–7.5), indicating that the VE of COVID-19 vaccines had declined in the case group. These findings highlight the importance of booster vaccination in persons >6 months after the full vaccination.
Vaccination status was statistically different between the cases and control groups (p = .006), and there were more unvaccinated individuals in the case group compared to the control group. Our results revealed that the overall protection of partial and full vaccination was negligible but showed 51.6% (95% CI: 15.2–72.4%) in the booster vaccination group, with the same results for each type of COVID-19 vaccine. The results were in line with previous studies. A study conducted in Hong Kong found statistically significant protection against the Omicron variant among individuals who received three doses of a COVID-19-inactivated vaccine, and the adjusted VE was 52%.37 A previous study in Hong Kong estimated that the third-dose VE for CoronaVac against BA.2.2 was 51.0%.38 Similar results were reported in England, Italy, Hong Kong, Japan, and Israel.37,39–42 Unexpectedly low effectiveness against Omicron in vaccinated individuals could reflect the gradual waning of vaccine protection, strongly suggesting that booster shots for eligible people, on schedule, are critically necessary. Our study design was consistent with previous real-world VE studies and expanded upon their findings as most of the other studies only assessed the VEs of inactivated vaccines and Ad5-nCoV vaccines, while we also considered a recombinant protein vaccine. Three doses of inactivated vaccines provided 48.0% (95% CI: 8.0–70.6%) protection in our study. One study from Shanghai showed that inactivated vaccines were 16.3% effective overall against Omicron variant infection.22 Considering the large number of people who had been infected in that outbreak in Shanghai, the risk of population exposure was greatly increased. Surprisingly, the Ad5-nCoV vaccine showed good protection (VE = 62.9%, 95%CI: 1.8–86%). The Ad5-nCoV vaccine is a replication-defective vaccine that expresses the spike glycoprotein of SARS-CoV-2, which induced significant immune responses after a single vaccination with 5 × 1010 viral particles.43,44 Nevertheless, only 3.9% of all subjects had received the Ad5-nCoV vaccine because of the low supply of the Ad5-nCoV vaccine in Lu’an City, and further observations are needed. The recombinant protein vaccine (ZF2001) has received conditional marketing authorization in China and was only used in several provinces of China at the beginning of its launch.45 Frustratingly, our findings indicated that recombinant protein vaccine offered no protection even with a third dose. In our study, 99.4% of individuals vaccinated with the recombinant protein vaccine had received the last dose of vaccination more than six months prior to last exposure, both in the cases and control groups, and none of them received a booster dose due to Chinese immunization strategies. Given the aforementioned, the VE of the recombinant protein vaccine decreased precipitously, and boosters are urgently needed for those who have already received three doses of the recombinant protein vaccine.
Our findings showed that heterologous booster vaccination offered more protection than homologous booster vaccination compared with the unvaccinated group. Jin, et al.43 reported that heterologous booster vaccination seemed to be more effective in minimizing the negative effect of the response to the vectors. Similarly, the results of a clinical trial conducted in China suggested that heterologous booster vaccination with the Ad5-nCoV vaccine was safe and more immunogenic than homologous booster vaccination.46 Promisingly, the results of previous clinical studies revealed that the incidence of adverse reactions in the heterologous booster vaccination group was significantly less than in the homologous booster vaccination group.47 Pain and fever were the most frequently reported injection site adverse reactions and systemic adverse reactions, respectively, and none of the serious adverse events were related to vaccination.48 As heterologous booster vaccination was implemented for the population shortly before the outbreak in Lu’an, only 24 individuals (1.9%) had completed heterologous booster vaccination in this study. Despite this, heterologous booster vaccination provided significantly better protection than homologous booster vaccination.
Strengths and limitations
Our study has some strengths. First, our study is somewhat unique in that we investigated recombinant protein vaccine against Omicron BA.2.2 variant infection in the real world. Previous studies conducted in China concentrated on the VE of inactivated vaccines and the Ad5-nCoV vaccine, and most of the studies were clinical studies. Second, all age groups of participants were included in our study compared to previous studies in which most considered participants were over 18 years old. Third, we performed a sensitivity analysis, potential confounding factors (age, gender, and time since the last dose of vaccination) were adjusted, and the final results indicated that the adjusted VEs were robust.
Our findings are also subject to the following limitations. First, the sample size is small, as indicated by the very wide confidence intervals. However, this outbreak in Lu’an had a limited number of positive individuals. Second, we did not estimate the VE of the heterologous booster vaccination with the Ad5-CoV vaccine and recombinant protein vaccine separately, though there may be a possibility for differences in the VE, which was due to the small number of individuals in the heterologous booster vaccination group in the minority. Third, although all subjects in the control group were core close contacts, the frequency of exposure was ultimately different for each individual and may have had a slight effect on the final results. We reviewed the epidemiological survey reports of each positive individual and searched for testing results of negative controls with a high frequency of exposure, making every effort to ensure that the frequency of exposure was consistent across controls.
Conclusion
This case–control study confirmed that full or booster vaccination of three types of vaccines was able to provide limited protection against Omicron variant infection, although the VE of each type of vaccine showed slightly different results. There was substantially increased protection from the booster vaccination (homologous and heterologous booster vaccinations) against the Omicron variant infection compared with full vaccination. Moreover, heterologous booster vaccination conferred better protective immunity against the Omicron variant than homogenous booster vaccination. Even three doses of recombinant protein vaccine haven’t provided satisfactory protection against the Omicron BA.2.2 variant infection. A vaccination campaign for a booster dose after three doses of recombinant protein vaccine urgently needs to be conducted and requires further observation.
Supplementary Material
Funding Statement
This study was funded by the Scientific Research Project of the Health Commission of Anhui Provinceby grant number AHWJ2022c040 and the Special Funds for Expanded Program on Immunization in 2022 of the Lu’an municipal government.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Authors’ contributions
Xiaqing Zhang designed and conceptualized this study, and drafted the manuscript. Yao Wang and Wei Qin participated in the data collection and analysis. Yong Lyu and Wei Qin critically reviewed and supervised the development of the paper. Chengyang Hu, Pengpeng Xu, Liguo Ma, Lei Liu, Jie Sun, Yang Liu, Hui Yang, Fan Pan, Jieying Hu, Chengsong Cao, Kai Cheng, and Dawei Gao participated in the case investigation and the quality control of the epidemiological survey reports of all subjects. All authors have read and agreed to the published version of the manuscript.
Ethical considerations
An outbreak investigation was a part of the public health response, and ethical clearance was not required. The authors assert that all procedures contributing to this investigation comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration. Furthermore, the data were confidentially maintained by all authors and participants.
Supplemental data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2194189.
References
- 1.Sun S, Wu J, Chen R, Levitt M.. SARS-CoV-2 Omicron variant: viral spread dynamics, disease burden, and vaccine effectiveness. Curr Med (Cham). 2022;1(1):14. doi: 10.1007/s44194-022-00014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kiss Z, Wittmann I, Polivka L, Surján G, Surján O, Barcza Z, Molnár GA, Nagy D, Müller V, Bogos K, et al. Nationwide effectiveness of first and second SARS-CoV2 booster vaccines during the delta and omicron pandemic waves in hungary (HUN-VE 2 study). Front Immunol. 2022;13:905585. doi: 10.3389/fimmu.2022.905585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li D, Li AE, Li ZQ, Bao Y, Liu T, Qin X-R, Yu X-J.. SARS-CoV-2 delta variant in Jingmen City, Hubei Province, China, 2021: children susceptible and vaccination breakthrough infection. Front Microbiol. 2022;13:856757. doi: 10.3389/fmicb.2022.856757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan SHX, Cook AR, Heng D, Ong B, Lye DC, Tan KB. Effectiveness of BNT162b2 vaccine against Omicron in children 5 to 11 years of age. N Engl J Med. 2022;387(6):525–9. doi: 10.1056/NEJMoa2203209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, Zhang H, He X. SARS-CoV-2 Omicron: a new challenge for pandemic and vaccine. Signal Transduct Target Ther. 2022;7(1):211. doi: 10.1038/s41392-022-01088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andreano E, Paciello I, Marchese S, Donnici L, Pierleoni G, Piccini G, Manganaro N, Pantano E, Abbiento V, Pileri P, et al. Anatomy of Omicron BA.1 and BA.2 neutralizing antibodies in COVID-19 mRNA vaccinees. Nat Commun. 2022;13(1):3375. doi: 10.1038/s41467-022-31115-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li J, Song R, Yuan Z, Xu Z, Suo L, Wang Q, Li Y, Gao Y, Li X, Chen X, et al. Protective effect of inactivated COVID-19 vaccines against progression of SARS-CoV-2 Omicron and delta variant infections to pneumonia in Beijing, China, in 2022. Vaccines (Basel). 2022;10(8):1215. doi: 10.3390/vaccines10081215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu H, Krishnan P, Ng DYM, Chang LDJ, Liu GYZ, Cheng SSM, Hui MMY, Fan MCY, Wan JHL, Lau LHK, et al. Probable transmission of SARS-CoV-2 Omicron variant in quarantine hotel, Hong Kong, China, November 2021. Emerg Infect Dis. 2022;28(2):460–2. doi: 10.3201/eid2802.212422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hua Q, Zheng D, Yu B, Tan X, Chen Q, Wang L, Zhang J, Liu Y, Weng H, Cai Y, et al. Effectiveness of inactivated COVID-19 vaccines against COVID-19 caused by the SARS-CoV-2 delta and Omicron variants: a retrospective cohort study. Vaccines (Basel). 2022;10(10):1753. doi: 10.3390/vaccines10101753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu D, Zhang Y, Tang L, Wang F, Ye Y, Ma C, Zheng H, Yu W, Cao L, Song Y, et al. Effectiveness of inactivated COVID-19 vaccines against symptomatic, pneumonia, and severe disease caused by the delta variant: real world study and evidence — China, 2021. China CDC Wkly. 2022;4(4):57–65. doi: 10.46234/ccdcw2022.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma C, Sun W, Tang T, Jia M, Liu Y, Wan Y, Han J, Rodewald L, Li J, Song Y, et al. Effectiveness of adenovirus type 5 vectored and inactivated COVID-19 vaccines against symptomatic COVID-19, COVID-19 pneumonia, and severe COVID-19 caused by the B.1.617.2 (Delta) variant: evidence from an outbreak in Yunnan, China, 2021. Vaccine. 2022;40(20):2869–74. doi: 10.1016/j.vaccine.2022.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu B, Gidding H, Stepien S, Cretikos M, Macartney K. Relative effectiveness of COVID-19 vaccination with 3 compared to 2 doses against SARS-CoV-2 B.1.1.529 (Omicron) among an Australian population with low prior rates of SARS-CoV-2 infection. Vaccine. 2022;40(43):6288–94. doi: 10.1016/j.vaccine.2022.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nittayasoot N, Thammawijaya P, Tharmaphornpilas P, Sansilapin C, Jiraphongsa C, Suphanchaimat R. Rapid method through routine data to evaluate real-world vaccine effectiveness against coronavirus disease 2019 (COVID-19) infection: lessons from Thailand. Health Res Policy Syst. 2022;20(1):29. doi: 10.1186/s12961-022-00821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tseng HF, Ackerson BK, Luo Y, Sy LS, Talarico CA, Tian Y, Bruxvoort KJ, Tubert JE, Florea A, Ku JH, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med. 2022;28(5):1063–71. doi: 10.1038/s41591-022-01753-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang P, Hasan MR, Chemaitelly H, Yassine HM, Benslimane FM, Al Khatib HA, AlMukdad S, Coyle P, Ayoub HH, Al Kanaani Z, et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 delta variant in Qatar. Nat Med. 2021;27(12):2136–43. doi: 10.1038/s41591-021-01583-4. [DOI] [PubMed] [Google Scholar]
- 16.Hu Z, Tao B, Li Z, Song Y, Yi C, Li J, Zhu M, Yi Y, Huang P, Wang J. Effectiveness of inactivated COVID-19 vaccines against severe illness in B.1.617.2 (Delta) variant–infected patients in Jiangsu, China. Int J Infect Dis. 2022;116:204–9. doi: 10.1016/j.ijid.2022.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara M, Furue T, Fukuoka M, Iwanaga K, Matsuishi E, Miike T, Sakamoto Y, Mukai N, Kinugasa Y, Shigyo M, et al. Real-world effectiveness of the mRNA COVID-19 vaccines in Japan: a case–Control study. Vaccines (Basel). 2022;10(5):779. doi: 10.3390/vaccines10050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kherabi Y, Launay O, Luong Nguyen LB. COVID-19 vaccines against Omicron variant: real-world data on effectiveness. Viruses. 2022;14(10):2086. doi: 10.3390/v14102086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang M, Yi Y, Li Y, Sun L, Deng A, Hu T, Zhang J, Liu J, Cheng M, Xie S, et al. Effectiveness of inactivated COVID-19 vaccines against illness caused by the B.1.617.2 (delta) variant during an outbreak in Guangdong, China: a cohort study. Ann Intern Med. 2022;175(4):533–40. doi: 10.7326/M21-3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu Y, Zhao J, Wei X, Han P, Yang L, Ren T, Zhan S, Li L. Effectiveness and cost-effectiveness of inactivated vaccine to address COVID-19 pandemic in China: evidence from randomized control trials and real-world studies. Front Public Health. 2022;10:917732. doi: 10.3389/fpubh.2022.917732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li XN, Huang Y, Wang W, Jing Q-L, Zhang C-H, Qin P-Z, Guan W-J, Gan L, Li Y-L, Liu W-H, et al. Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: a test-negative case–control real-world study. Emerg Microbes Infect. 2021;10(1):1751–9. doi: 10.1080/22221751.2021.1969291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Z, Xu S, Liu J, Wu L, Qiu J, Wang N, Ren J, Li Z, Guo X, Tao F, et al. Effectiveness of inactivated and Ad5-nCoV COVID-19 vaccines against SARS-CoV-2 Omicron BA. 2 variant infection, severe illness, and death. BMC Med. 2022;20(1):400. doi: 10.1186/s12916-022-02606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bai W, Sha S, Cheung T, Su Z, Jackson T, Xiang Y-T. Optimizing the dynamic zero-COVID policy in China. Int J Biol Sci. 2022;18(14):5314–6. doi: 10.7150/ijbs.75699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin W, Zhang X, Wang Y, Pan F, Cheng K, Huang F, Song J, Su H. The acceptance to heterologous booster vaccination of COVID-19 vaccine among HCWs and targeted population: a cross-sectional study in central China. Front Public Health. 2022;10:943876. doi: 10.3389/fpubh.2022.943876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin Z, Wen T, Fang Q, Zheng C, Gong X, Li J, Wang S, Xiang Z. Assessment of mumps-containing vaccine effectiveness by dose during 2006 to 2020 in Quzhou, China. Hum Vaccin Immunother. 2022;18(5):2086774. doi: 10.1080/21645515.2022.2086774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin Y, Lin J, Yuan S, Tong S, Chen E, Zheng J, Wang W. A booster shot of vaccine against SARS-CoV-2 should be rigorously promoted and implemented in China. J Infect. 2023;86(2):e49–50. doi: 10.1016/j.jinf.2022.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Belayachi J, Yang Y, Fu Q, Rodewald L, Li H, Yan B, Wang Y, Shen Y, Yang Q, et al. Real-world study of the effectiveness of BBIBP-CorV (Sinopharm) COVID-19 vaccine in the Kingdom of Morocco. BMC Public Health. 2022;22(1):1584. doi: 10.1186/s12889-022-14016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adams K, Rhoads JP, Surie D, Gaglani M, AA Ginde, McNeal T, HK Talbot, JD Casey, Zepeski A, NI Shapiro, et al. Vaccine effectiveness of primary series and booster doses against omicron variant COVID-19-associated hospitalization in the United States medRxiv [Preprint]. 2022. Jun 14. 2022.06.09.22276228.
- 29.Kong W, Zhong Q, Chen M, Yu P, Xu R, Zhang L, Lai C, Deng M, Zhou Q, Xiong S, et al. Ad5-nCoV booster and Omicron variant breakthrough infection following two doses of inactivated vaccine elicit comparable antibody levels against Omicron variants. J Med Virol. 2023;95(1):e28163. doi: 10.1002/jmv.28163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolter N, Jassat W, Walaza S, Welch R, Moultrie H, Groome M, Amoako DG, Everatt J, Bhiman JN, Scheepers C, et al. Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: a data linkage study. Lancet. 2022;399(10323):437–46. doi: 10.1016/S0140-6736(22)00017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garrett N, Tapley A, Andriesen J, Seocharan I, Fisher LH, Bunts L, Espy N, Wallis CL, Randhawa AK, Miner MD, et al. High asymptomatic carriage with the Omicron variant in South Africa. Clin Infect Dis. 2022;75(1):e289–92. doi: 10.1093/cid/ciac237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma RP, Gautam S, Sharma P, Singh R, Sharma H, Parsoya D, Deeba F, Bhomia N, Pal N, Potdar V, et al. Genomic profile of SARS-CoV-2 Omicron variant and its correlation with disease severity in Rajasthan. Front Med (Lausanne). 2022;9:888408. doi: 10.3389/fmed.2022.888408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen MS, K SÍ, Eliasen EH, Larsen S, Hansen JL, Vest N, Dahl MM, Christiansen DH, Møller LF, Kristiansen MF. Clinical characteristics of the Omicron variant - results from a nationwide symptoms survey in the Faroe Islands. Int J Infect Dis. 2022;122:636–43. doi: 10.1016/j.ijid.2022.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raju MK, Vivian Thangaraj JW, Selvavinayagam TS, Somasundaram A, Parthipan K, Sivadoss R, Sabarinathan R, Subramaniam S, Rozario A, Rani S, et al. Clinical profile of patients infected with suspected SARS-CoV-2 Omicron variant of concern, Tamil Nadu, India, December 2021-January 2022. Indian J Med Res. 2022;155(1):165–70. doi: 10.4103/ijmr.ijmr_312_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chemaitelly H, Tang P, Hasan MR, AlMukdad S, Yassine HM, Benslimane FM, Al Khatib HA, Coyle P, Ayoub HH, Al Kanaani Z, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385(24):e83. doi: 10.1056/NEJMoa2114114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richardson VL, Camacho Franco MA, Bautista Márquez A, Martínez Valdez L, Castro Ceronio LE, Cruz Cruz V, Gharpure R, Lafond KE, Yau TS, Azziz-Baumgartner E, et al. Vaccine effectiveness of CanSino (Adv5-nCoV) Coronavirus Disease 2019 (COVID-19) Vaccine Among Childcare Workers—Mexico, March–December 2021. Clin Infect Dis. 2022;75(Supplement_2):S167–S173. doi: 10.1093/cid/ciac488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang B, Wong IOL, Xiao J, Tsang TK, Liao Q, Cowling BJ. Effectiveness of CoronaVac and BNT162b2 vaccines against severe acute respiratory syndrome coronavirus 2 Omicron BA.2 infections in Hong Kong. J Infect Dis. 2022;226(8):1382–4. doi: 10.1093/infdis/jiac360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mcmenamin ME, Nealon J, Lin Y, Wong JY, Cheung JK, Lau EHY, Wu P, Leung GM, Cowling BJ. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis. 2022;22(10):1435–43. doi: 10.1016/S1473-3099(22)00345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brosh-Nissimov T, Hussein K, Wiener-Well Y, Orenbuch-Harroch E, Elbaz M, Lipman-Arens S, Maor Y, Yagel Y, Chazan B, Hershman-Sarafov M, et al. Hospitalized patients with severe COVID-19 during the Omicron wave in Israel - benefits of a fourth vaccine dose. Clin Infect Dis. 2023;76(3):e234–9. doi: 10.1093/cid/ciac501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Andrews N, Stowe J, Kirsebom F, Toffa S, Sachdeva R, Gower C, Ramsay M, Lopez Bernal J. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat Med. 2022;28(4):831–7. doi: 10.1038/s41591-022-01699-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Acuti Martellucci C, Flacco ME, Soldato G, Di Martino G, Carota R, Caponetti A, Manzoli L. Effectiveness of COVID-19 vaccines in the general population of an Italian region before and during the Omicron wave. Vaccines (Basel). 2022;10(5):662. doi: 10.3390/vaccines10050662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyauchi S, Hiyama T, Nakano Y, Yoshida M, Yoshino A, Miyake Y, Okamoto Y. Real-world effectiveness of a booster dose of the COVID-19 vaccines among Japanese university students. Vaccines (Basel). 2022;10(8):1283. doi: 10.3390/vaccines10081283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin P, Guo X, Chen W, Ma S, Pan H, Dai L, Du P, Wang L, Jin L, Chen Y, et al. Safety and immunogenicity of heterologous boost immunization with an adenovirus type-5-vectored and protein-subunit-based COVID-19 vaccine (Convidecia/ZF2001): a randomized, observer-blinded, placebo-controlled trial. PLoS Med. 2022;19(5):e1003953. doi: 10.1371/journal.pmed.1003953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu FC, Guan XH, Li YH, Huang J-Y, Jiang T, Hou L-H, Li J-X, Yang B-F, Wang L, Wang W-J, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2020;396(10249):479–88. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu K, Fan C, Han Y, Dai L, Gao GF. Immunogenicity, efficacy and safety of COVID-19 vaccines: an update of data published by 31 December 2021. Int Immunol. 2022;34(12):595–607. doi: 10.1093/intimm/dxac031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Hou L, Guo X, Jin P, Wu S, Zhu J, Pan H, Wang X, Song Z, Wan J, et al. Heterologous AD5-nCOV plus CoronaVac versus homologous CoronaVac vaccination: a randomized phase 4 trial. Nat Med. 2022;28(2):401–9. doi: 10.1038/s41591-021-01677-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li JX, Wu SP, Guo XL, Tang R, Huang B-Y, Chen X-Q, Chen Y, Hou L-H, Liu J-X, Zhong J, et al. Safety and immunogenicity of heterologous boost immunisation with an orally administered aerosolised Ad5-nCoV after two-dose priming with an inactivated SARS-CoV-2 vaccine in Chinese adults: a randomised, open-label, single-centre trial. Lancet Respir Med. 2022;10(8):739–48. doi: 10.1016/S2213-2600(22)00087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li ZP, Shi YF, Hou LH, Jin P-F, Ma S-H, Pan H-X, Zhang J-L, Shan Y-M, Huang H-T, Wu S-P, et al. Batch-to-batch consistency trial of an adenovirus type-5 vector-based COVID-19 vaccine in adults aged 18 years and above. Expert Rev Vaccines. 2022;21(12):1843–9. doi: 10.1080/14760584.2022.2119133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


