ABSTRACT
Gastric cancer (GC) is one of the most common malignancies. Immunotherapy becomes an indispensable part of GC. This study conducts bibliometric analysis of immunotherapy for GC to clarify the research status and identify potential new research directions. VOS viewer and CiteSpace visualization software were used to demonstrate collaborations and correlations. A total of 1141 English publications from 2012 to 2022 were included. The number of publications increased year by year. The publications were mainly from China (n = 579, 50.70%), followed by the United States. Fudan University published the most publications (n = 48, 4.21%). Frontiers in Oncology and Journal of Clinical Oncology ranked first in cited and co-cited journals, respectively. Kim Kyoung-Mee published the most publications on immunotherapy for GC (n = 14). The clustering of timeline view and co-cited references show the hotspot transformation on immunotherapy for GC. Initially, the hot topic was “cytokine-induced killer cells” and “myeloid-derived suppressor cells.” In recent years, the focus has turned to “targeted therapy.” “CAR-T” has become the hottest topic, and GC has entered precision therapy phase. Screening patients who can benefit from immunotherapy is key to improving prognosis. The combination of immunotherapy with other treatment options, such as chemotherapy and targeted therapy, is currently the focus of research. Chimeric antigen receptor T cell will be further studied in the future.
KEYWORDS: Gastric cancer, immunotherapy, bibliometric analysis, citeSpace, VOS viewer, chimeric antigen receptor T cell, targeted therapy
Research Highlights
Bibliometrics is one of the main tools in the current research and hot spots. Immunotherapy becomes an indispensable part of gastric cancer. Chimeric antigen receptor T cells will play an important role for gastric cancer.
Introduction
Gastric cancer (GC) is one of the most common cancers worldwide.1,2 At present, the treatment options for GC include systemic chemotherapy, surgery, radiotherapy and immunotherapy.3 Most GC patients are diagnosed as advanced disease.4 Surgery or chemotherapy alone often yields poor results, and new therapy methods are urgently needed.
For metastatic or advanced GC, immunotherapy has become a research hotspot with the development of individualized precision therapy.5,6 In recent years, the breakthrough achievements of immune checkpoint inhibitors (ICIs), represented by cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) and programmed cell death protein-1 (PD-1) antibody, have promoted the mode transformation of cancer treatment.7–9 In addition, chimeric antigen receptor T cell (CAR-T) and cytokine-induced killer cells (CIKs) also provide new approaches.10,11 Immunotherapy prolongs survival by long-lasting immune response.5,12 Therefore, it is necessary to systematically explore the publications and hotspots of immunotherapy for GC. The global database is updated daily to provide a basis for improving the prognosis of cancer patients.
The number of publications related to immunotherapy for GC has been rising over the past few decades.13 It is essential to assess the field from a scientific point of view. Bibliometrics is a method or tool to evaluate status and provide reference for clinical medical research.14 The characteristics of quality and quantity of publications can be provided for researchers by analyzing the measurement indexes. In addition, it is helpful to discover the research hotspot in a certain field.15,16 At present, the research in the field of immunotherapy for GC is generally limited to the summary of literature and clinical experience.17 To the best of our knowledge, no publication has conducted a bibliometric analysis of immunotherapy for GC so far.
Therefore, this study systematically searched the publications related to immunotherapy for GC in the past 10 years to analyze the development of this field. In addition, this study also sorted out the research hotspots, aiming to provide an overview for researchers.
Materials and methods
Data retrieval
As a high-quality digital publications resource database, the Web of Science (WOS) has been widely accepted by researchers for bibliometric analysis.18,19 We searched the WOS core database on October 31, 2022 for all publications related to immunotherapy for GC from January 1, 2012 to January 1, 2022. The search formula was as follows: TS= (“gastric cancer” OR “gastric carcinoma” OR “stomach cancer” OR “stomach neoplasms” OR “stomach carcinoma” OR “gastric adenocarcinoma” OR “gastric neoplasms” OR “gastric tumor”) AND TS= (“immunotherapy” OR “immune therapy” OR “immunization therapy” OR “immunotherapies” OR “immunity treatment” OR “immunotherapeutic”). In the included articles, the title, abstract and keywords should be related to immunotherapy for GC.
Screening process
The inclusion criteria are follows as: (1) The subject focused on immunotherapy for GC, and the full text is available; (2) Written in English; (3) The publication type is limited to “article” and “review;” (4) The publications came from Sciences Citation Index Expanded (SCI-E) and Social Sciences Citation Index (SSCI) database of WOS; (5) Time span was between 2012 and 2022. Exclusion criteria are as follows: (1) Topics not related to immunotherapy for GC. (2) The types of publication are conference abstracts, graduation thesis, reports, etc. The two authors independently evaluated the full text of all publications. Any disagreements were resolved by negotiation (between the two authors YZ-C and T-Z). If disagreements still cannot be resolved, decisions are made under the guidance of the corresponding author. Data is exported as a plain text format.
Variables and analysis
Use bibliometric methods to collect research cutting-edge knowledge and trends.20 For the included publications, variables of the following data were extracted: articles published annually; open access articles; top 10 authors, countries, institutions and journals; top 10 keywords and burst keywords. In this study, the VOS viewer (version 1.6.18)21 and Citespace (version 6.1.R3 Basic)22,23 were used for visualizing data to analyze countries, keywords, coauthors, etc. They combine the three functions of data integration analysis, visualization methods and bibliometrics.24
Use CiteSpace to analyze and visualize burst keywords to predict trends on immunotherapy for GC.25,26 The higher the burst intensity means the higher the frequency of the keyword. Each node in the figure represents an observation result, including keywords, institutions, countries, authors, and co-cited references. Node size indicates frequency of citation or occurrence. Connections between nodes represent collaboration, and co-cited relationships.27 Nodes with different colors represent different years. Both the 2021 edition of the Journal Citation Report (JCR) and the Impact Factor (IF) were included in the analysis as key indicators of the scientific value of the research.
Results
Annual growth trend of publications on immunotherapy for GC
After careful screening by two authors according to the inclusion criteria (Figure 1), from January 1, 2012 to January 1, 2022, a total of 1141 publications related to immunotherapy for GC were published on WOS, including 806 articles (70.6%) and 335 reviews (29.4%). Publications cover 59 countries or regions. A total of 229 institutions have published 3 or more publications on immunotherapy for GC. The number of publications on immunotherapy for GC from 2012 to 2022 is shown in Figure 2. Before 2016, the research trend line was flat. After 2016, the number of annual publications began to rise rapidly. The fastest growth is between 2019 and 2022. In particular, the number of publications on immunotherapy for GC in 2021 is the most in the past decade.
Figure 1.
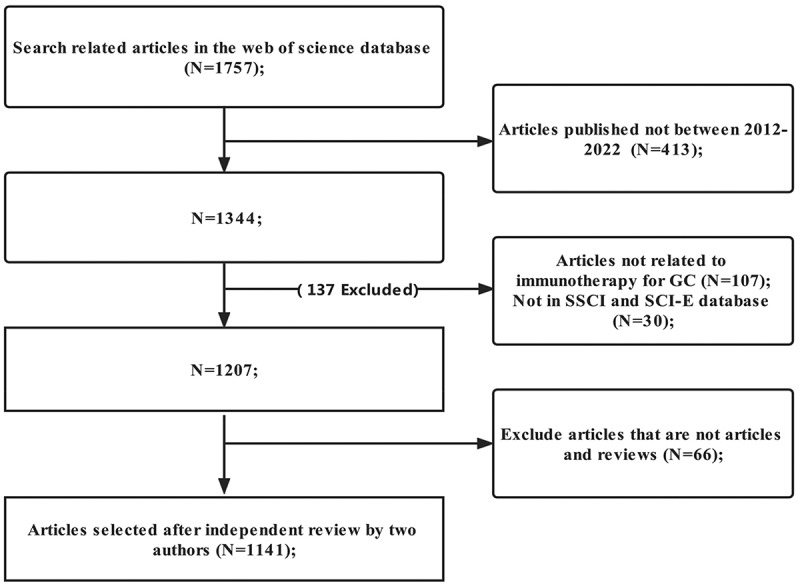
Flowchart of article selection.
SSCI: Social Sciences Citation Index; SCI-E: Citation Index Expanded; GC: gastric cancer
Figure 2.
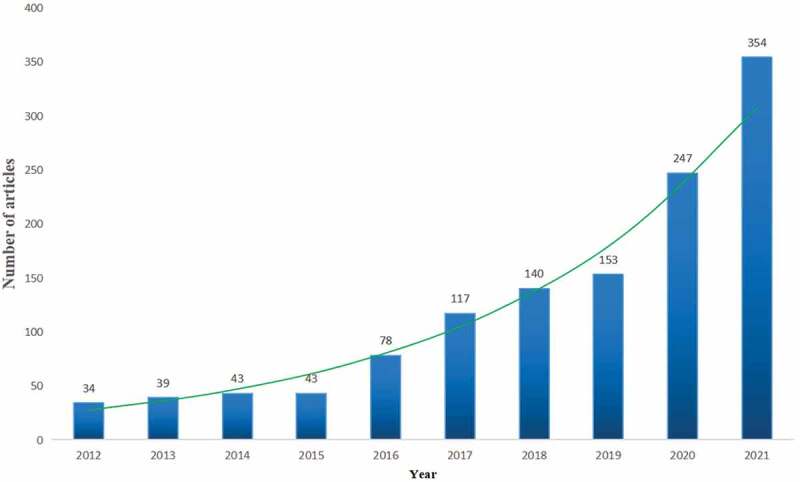
Annual trends of articles published in immunotherapy for GC from 2012 to 2022.
GC: gastric cancer.
Analysis of countries/regions and institutions
China is the most published country with 579 publications (50.7% of 1141 publications), followed by the United States (18.0% of 1141 publications), Japan (8.4% of 1141 publications), Germany (6.7% of 1141 publications), and Italy (6.2% of 1141 publications) (Table 1). China alone contributed half of the publications. In addition, as can be seen from the color of the circles, China and the United States, the two countries with the most published publications, have been contributing to immunotherapy for GC from 2012 to 2022 (Figure 3a). The above two countries have close cooperation (Supplementary Figure S1). Likewise, among the top 10 most published countries or regions, China has 11,654 citations, far exceeding all other countries (Figure 3b). But citation/publication ratio (20.13) of China ranks second to last among the top 10 countries (Table 1). Notably, France had the most citation/publication ratio (33.96). Despite the number of publications is relatively small, this also reflects the high quality of publications.
Table 1.
The top 10 most published countries/regions related to immunotherapy for GC from 2012–2022.
| Rank | Country/Region | Article counts | Citation | Citation per articles |
|---|---|---|---|---|
| 1 | China | 579 | 11654 | 20.13 |
| 2 | United States | 205 | 6418 | 31.31 |
| 3 | Japan | 96 | 2938 | 30.60 |
| 4 | Germany | 76 | 2319 | 30.51 |
| 5 | Italy | 71 | 2008 | 28.28 |
| 6 | South korea | 65 | 1264 | 19.45 |
| 7 | England | 60 | 1829 | 30.48 |
| 8 | France | 28 | 951 | 33.96 |
| 9 | Spain | 26 | 631 | 24.27 |
| 10 | Sngapore | 24 | 804 | 33.50 |
GC: gastric cancer.
Figure 3.
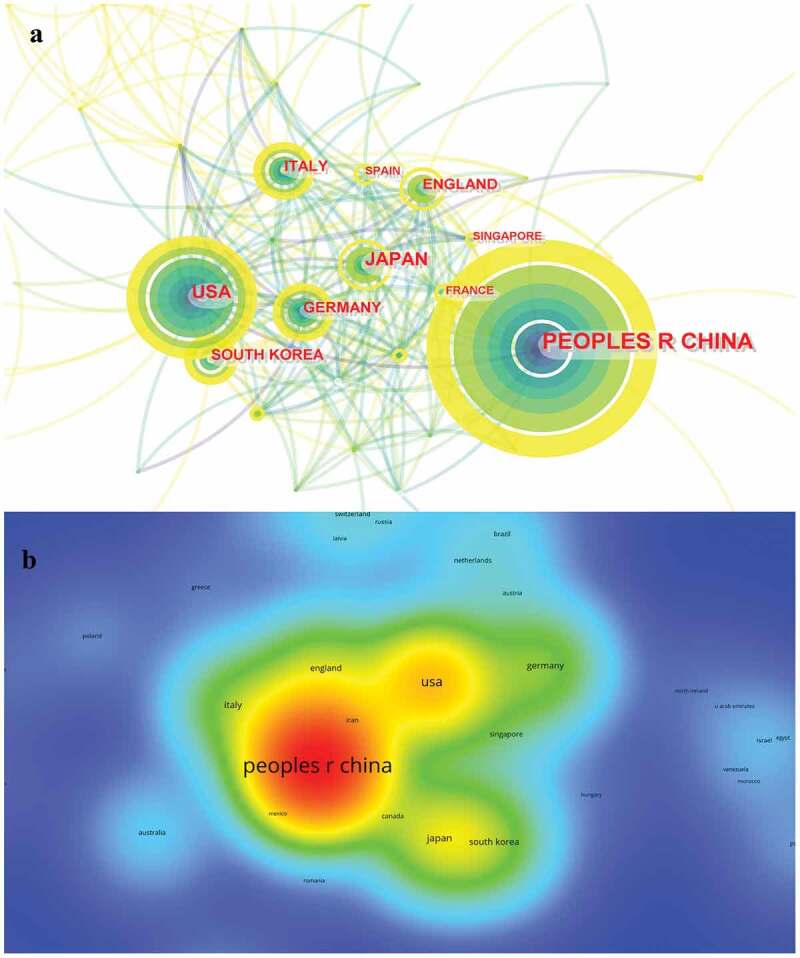
A visualization of countries related to immunotherapy for GC.
a: Cooperative network of publications between countries. Countries are represented by nodes. Partnerships are represented by lines. The node area increases with the number of publications. The colors represent different years. The color represents different years. From 2012 to 2022, it changes from dark blue to yellow; b: Number of citations by country. The size and brightness of the nodes indicate the frequency of citations. GC: gastric cancer.
Eight of the top ten institutions with the most publications are from China (Table 2). It can be seen that Chinese institutions are very concerned about immunotherapy for GC. The top 10 institutions published 290 publications, accounting for 25.4% of total publications. Fudan University published 48 publications, followed by Shanghai Jiaotong University (37 publications) and Nanjing Medical University (36 publications) (Figure 4a). Among the top ten institutions, Fudan University has the most citations, reaching 1213 times (Figure 4b). However, the most citation/publication ratio was Zhejiang University (39.17).
Table 2.
The top 10 most published institutions related to immunotherapy for GC from 2012–2022.
| Rank | Institution | Article counts | Citation | Citation per articles |
|---|---|---|---|---|
| 1 | Fudan University | 48 | 1213 | 25.27 |
| 2 | Shanghai Jiao Tong University | 37 | 997 | 26.95 |
| 3 | Nanjing Medical University | 36 | 715 | 19.86 |
| 4 | Sun Yat-sen University | 31 | 1106 | 35.68 |
| 5 | China Medical University | 25 | 688 | 27.52 |
| 6 | Zhengzhou University | 25 | 621 | 24.84 |
| 7 | Zhejiang University | 24 | 940 | 39.17 |
| 8 | Sungkyunkwan University | 23 | 532 | 23.13 |
| 9 | Peking University | 21 | 813 | 38.71 |
| 10 | National University of Singapore | 20 | 705 | 35.25 |
GC: gastric cancer.
Figure 4.
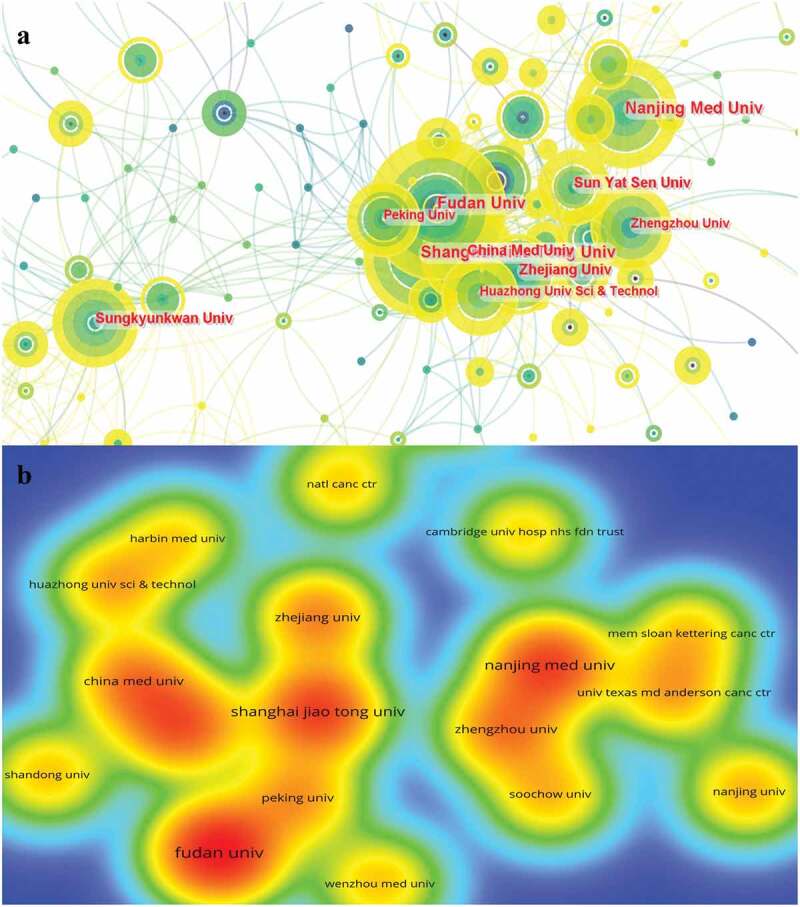
A visualization of institutions related to immunotherapy for GC.
a: Cooperative network of publications between institutions. Institutions are represented by nodes. Partnerships are represented by lines. The node area increases with the number of publications. The colors represent different years. The color represents different years. From 2012 to 2022, it changes from dark blue to yellow; b: Number of citations by institution. The size and brightness of the nodes indicate the frequency of citations. GC: gastric cancer.
Analysis of journals
The journal with the most publications in the field of immunotherapy for GC is Frontiers in Oncology (45 publications, 3.94%), followed by Cancers (37 publications, 3.24%), Oncoimmunology (26 publications, 2.28%), Frontiers in Immunology (20 articles, 1.75%) and Oncotargets and Therapy (20 publications, 1.75%). Among the top 10 most published journals, Frontiers series publishers account for 3 seats (Table 3). Among the top 10 journals, Frontiers in Immunology has the highest IF with 8.786, followed by Oncoimmunology (7.723). Of the top 5 most published journals, 4 journals are open access, a model that is now getting more attention from researchers. Of the top 10 most published journals, 40% were from Switzerland, followed by United States (30%), England (10%), Greece (10%) and Japan (10%). Of the top 10 most published journals, 60% are classified as Q1, 30% are classified as Q2, and the remaining one are classified as Q3. The most cited journal is Oncoimmunology (Figure 5a). Journal with the most citation/publication rate was Oncotargets and Therapy (Table 3).
Table 3.
The top 10 most published journals related to immunotherapy for GC from 2012–2022.
| Rank | Journal | Article counts | Citation | Citation per articles | JCR partition | IF |
|---|---|---|---|---|---|---|
| 1 | Frontiers in Oncology | 45 | 398 | 8.84 | Q2 | 5.738 |
| 2 | Cancers | 37 | 296 | 8.00 | Q1 | 6.575 |
| 3 | Oncoimmunology | 26 | 610 | 23.46 | Q1 | 7.723 |
| 4 | Frontiers in Immunology | 20 | 367 | 18.35 | Q1 | 8.786 |
| 5 | Oncotargets and Therapy | 20 | 560 | 28.00 | Q2 | 4.345 |
| 6 | Oncology Letters | 19 | 171 | 9.00 | Q3 | 3.111 |
| 7 | Gastric Cancer | 18 | 281 | 15.61 | Q1 | 7.701 |
| 8 | Cancer Immunology Immunotherapy | 17 | 331 | 19.47 | Q1 | 6.630 |
| 9 | World Journal of Gastroenterology | 17 | 465 | 27.35 | Q2 | 5.374 |
| 10 | Frontiers in Cell and Developmental Biology | 16 | 131 | 8.19 | Q1 | 6.081 |
GC: gastric cancer; JCR: Journal Citation Reports; IF: Impact Factor.
Figure 5.
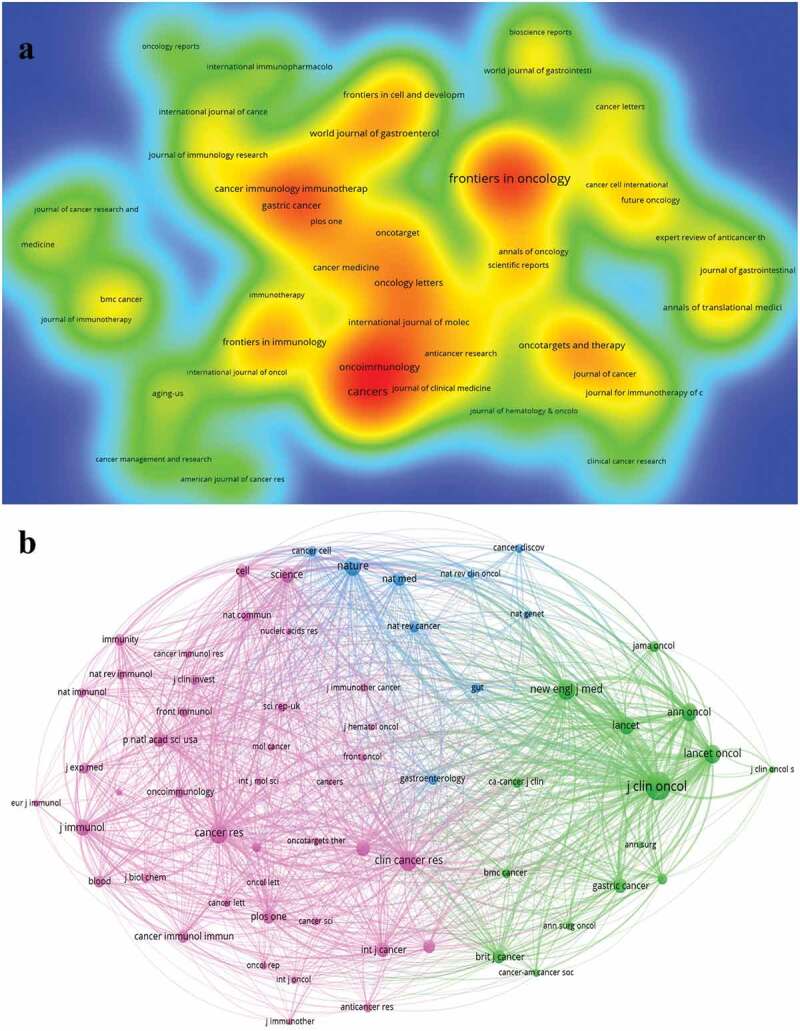
A visualization of journals related to immunotherapy for GC.
a: Number of citations by journals. The size and brightness of the nodes indicate the frequency of citations. b: A network visualization of co-cited journals. The size of the circle represents the number of co-cited. GC: gastric cancer.
The most co-cited journal was Journal of Clinical Oncology (3370 citations), followed by Cancer Research (1751 citations), and New England Journal of Medicine (1750 citations) (Figure 5b). The top ten co-cited journals were all Q1. And Lancet has the highest IF (202.731). A dual-map overlay of the journals describe the subject distribution of academic journals (Figure 6). Color lines represent reference paths.28 These labels identify the disciplines that journals represent. The map is colored to indicate the two main citation pathways. The orange citation path indicates that research from Molecular/Biology/Genetics is frequently cited by Molecular/Biology/Immunology journal. Green paths indicate that research from Molecular/Biology/Genetics is frequently cited by Medicine/Medical/Clinical journal.
Figure 6.
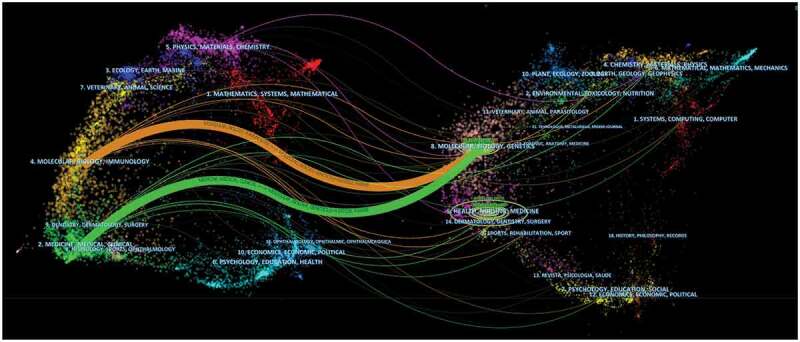
A dual-map overlay of journals related to immunotherapy for GC.
Citing journals on the left and the cited journals on the right, with colored paths indicating citation relationships. GC: gastric cancer.
Analysis of authors
Over the past decade, more than 6,000 researchers have been involved in research related to immunotherapy for GC (Table 4). Kim Kyoung-mee (14 publications) published the most publications, followed by Lordick Florian (13 publications), Liu Baorui (12 publications) and Liu Hao (12 publications). However, the most citation/publication was Shen Lin (34.73), indicating that the publications were recognized by researchers. As shown in Figure 7, the largest node is the author with the most co-cited, including Bang YJ (506 citations), Fuchs CS (442 citations) and Le DT (294 citations). In addition, high-producing authors tend to co-occur more frequently with other authors.
Table 4.
The top 10 most published authors related to immunotherapy for GC from 2012–2022.
| Rank | Author | Article counts | Citation | Citation per articles |
|---|---|---|---|---|
| 1 | Kim Kyoung-mee | 14 | 272 | 19.43 |
| 2 | Lordick Florian | 13 | 259 | 19.92 |
| 3 | Liu Baorui | 12 | 160 | 13.33 |
| 4 | Liu Hao | 12 | 307 | 25.58 |
| 5 | Lee Jeeyun | 11 | 191 | 17.36 |
| 6 | Li He | 11 | 126 | 11.45 |
| 7 | Shen Lin | 11 | 382 | 34.73 |
| 8 | Zhang Heng | 11 | 104 | 9.45 |
| 9 | He Hongyong | 10 | 74 | 7.40 |
| 10 | Lin Chao | 10 | 74 | 7.40 |
GC: gastric cancer.
Figure 7.
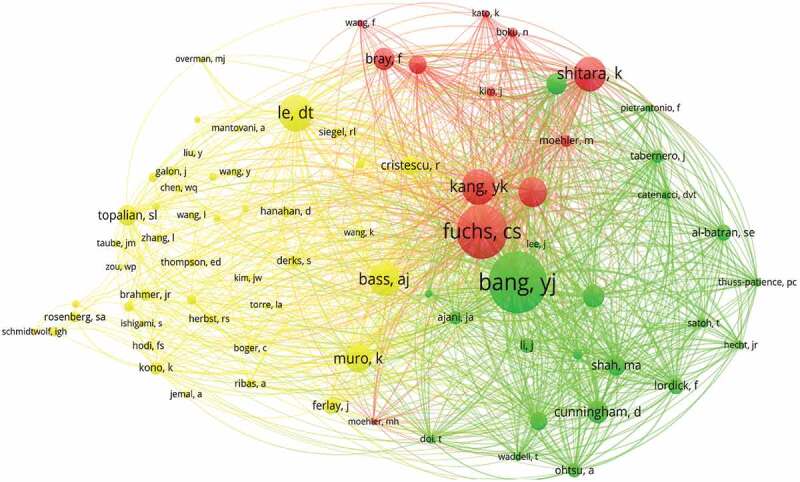
A visualization of co-cited authors related to immunotherapy for GC.
The co-citation relationship is represented by the lines between nodes. The node area increases with the increase of co-citation number. GC: gastric cancer.
Analysis of co-cited references and timeline
It can be seen that the co-cited references are basically divided into 10 clusters (Figure 8a). Co-cited references refer to those that are frequently cited with other publications, which are considered the basis of research in a field. The smaller the number, the more keywords the cluster contains. “0# Targeted therapy” has the most keywords, indicating that it has been widely studied on immunotherapy for GC. Modularity (Q) >0.3 means significant clustering structure. Weighted mean Silhouette (S) > 0.5 is considered a reasonable value for clustering. In addition, S>0.7 means that clustering is convincing. Two data are valid in this article (Q=0.64 and S=0.88). Clusters “#0 targeted therapy,” “#1 Epstein-Barr virus,” “#7 car-t” and “#4 chemotherapy” are closely related (Figure 8a). “Comprehensive molecular characterization of gastric adenocarcinoma” (272 citations) published in Nature (IF=113.914) by Bass Aj et al is the most co-cited publication (Table 5).29 It is worth noting that 3 of the top 5 co-cited references are from the Lancet journal and its sub-journals. In addition, top-ranked co-cited references tend to be more collaborative (Supplementary Figure S2).
Figure 8.
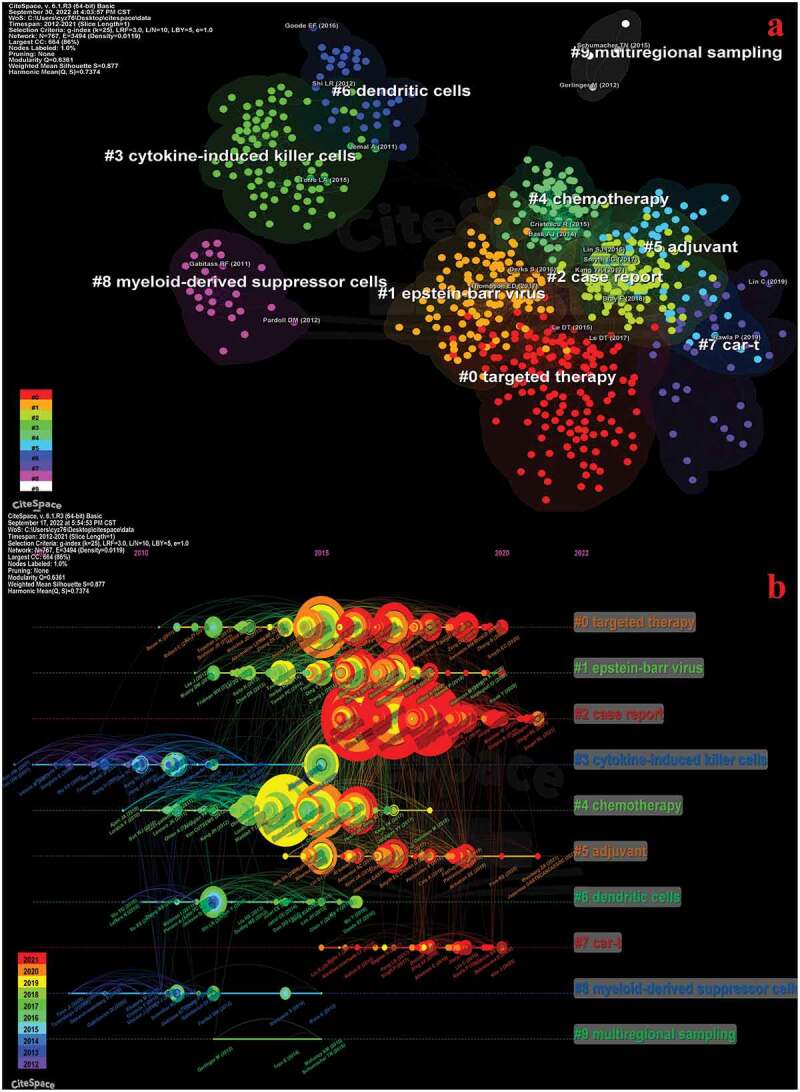
Visualization of co-cited references related to immunotherapy for GC.
a: Knowledge map of co-cited references. Different colors represent different clusters. b: Timeline view of co-cited references related to immunotherapy for GC. The position of the node on the horizontal axis represents the time when the reference first appeared. The size of the node is related to the number of co-cited of the reference. Lines between nodes represent co-cited relationships. Redder the color means closer to 2022. GC: gastric cancer.
Table 5.
The top 5 co-cited references related to immunotherapy for GC from 2012–2022.
| Rank | Title | Count | IF (2021) | First Author |
|---|---|---|---|---|
| 1 | Comprehensive molecular characterization of gastric adenocarcinoma | 272 | 113.914 | Bass Aj |
| 2 | Nivolumab in patients with advanced gastric or gastro-esophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomized, double-blind, placebo-controlled, phase 3 trial | 213 | 202.731 | Kang Yk |
| 3 | Safety and Efficacy of Pembrolizumab Monotherapy in Patients with Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer Phase 2 Clinical KEYNOTE-059 Trial | 181 | 33.005 | Fuchs Cs |
| 4 | Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-esophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial | 175 | 202.731 | Bang Yj |
| 5 | Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial | 166 | 54.431 | Muro K |
GC: gastric cancer.
Timeline view is a data visualization method that combines clustering and time slice techniques. In addition to illustrating the distribution of topics in the field, this method can also show the trends and interrelationships of research topics over time.30 Hence, we draw the timeline view of the co-cited references (Figure 8b). Different colors represent different years. The left side indicates older references. The right represents the newer reference. We found that “# 3 cytokine-induced killer cells” and “# 8 myeloid-derived suppressor cells” were a relatively early hotspot. “#7 CAR-T” has become a new hot spot-on immunotherapy for GC since 2015. Currently, it is still the focus of many researchers. The focus of mid-term period from 2015 to 2018 was on the search for biomarkers, the development of tumor vaccines and the application of immunotherapy in chemotherapy, such as “#1 Epstein – Barr virus,” “#4 chemotherapy,” and “#6 dendritic cells.” Current research focuses on “#0 targeted therapy” and “#5 adjuvant chemotherapy.”
Analysis of keyword co-occurrence clustering and timeline view
After removing the meaningless keywords through the VOS viewer, we found that the keywords with the most frequency were “Expression,” “Prognosis,” “Survival,” “T-cells,” “Microsatellite instability” and “Nivolumab,” etc. (Table 6). The development of keywords over time can reflect the evolution of cutting-edge knowledge. This plays an important role in guiding the future research direction in this field. We designed a timeline view using CiteSpace to clearly show the keywords (Supplementary Figure S3). It can be seen that it is roughly divided into 6 clusters. “#0 Microsatellite instability” and “#3 Targeted therapy” reappear. “#0 Microsatellite instability” has not increased or decreased significantly in the last 10 years. The research related to “# 3 Targeted therapy” and “#2 Cytokine induced killer cells” has decreased since 2016. “#4 Immune checkpoint blockade” has been a hot topic for 10 years.
Table 6.
The top 10 most frequent keywords related to immunotherapy for GC from 2012–2022.
| Rank | Keyword | Occurrences |
|---|---|---|
| 1 | Expression | 233 |
| 2 | Prognosis | 116 |
| 3 | Survival | 107 |
| 4 | T-cells | 105 |
| 5 | Microsatellite instability | 96 |
| 6 | Nivolumab | 89 |
| 7 | Cells | 87 |
| 8 | Blockade | 85 |
| 9 | Therapy | 84 |
| 10 | Pd-L1 | 80 |
GC: gastric cancer.
Keyword burst analysis
Detect bursts keywords to identify research fronts. Burst keywords indicates that a certain keyword has been widely cited over a period of time. We focus on the top ten keywords with the highest burst intensity from January 2017 to January 2022 (Figure 9), including “lymphocyte” (Strength=4.43), “adoptive immunotherapy” (Strength=4.1) and “growth factor receptor” (Strength=3.79), etc.
Figure 9.
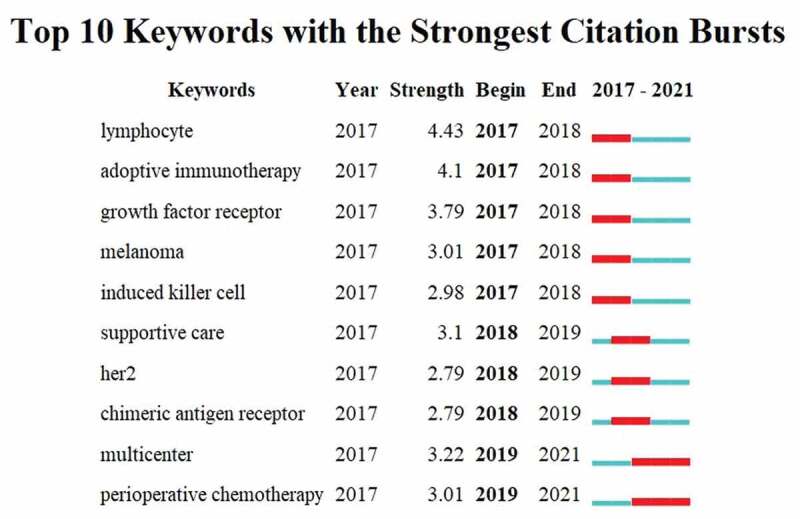
Keywords with the strongest citation bursts related to immunotherapy for GC from 2017 to 2022.
The blue line represents the time axis, with the red part indicating the start year, end year, and duration of the burst. GC: gastric cancer.
Discussion
Perspective of country
The publications were statistically and visually analyzed to further obtain the research status and hotspots on immunotherapy for GC.31 There were 1141 publications on immunotherapy for GC in the past 10 years (2012–2022), from 1579 institutions and 59 countries/regions. The number of publications related to immunotherapy for GC is increasing every year. This field has received a lot of attention in recent years. On current trends, the total number of publications in 2022 is set to surpass 2021 and continue to grow rapidly. In terms of the number of total citation frequency and publications, China has made the greatest contribution to immunotherapy for GC in the past decade. In addition to the large population, China also has a high incidence of GC.32,33 China and the United States, two countries with close cooperation, have made indispensable contributions in the field of immunotherapy for GC. In addition to China, developed countries occupy a mainstream position in the field of immunotherapy for GC. This is related to the large amount of funds and high-tech support needed for research. In the future, the close cooperation between countries is needed to reduce the waste of funds and resources. Of course, this needs to be achieved through the cooperation of institutions and journals.
Perspective of institution
The distribution of institutional rankings is often consistent with countries. China alone accounted for 80% of the top ten institutions that published the most publications. This shows that China has made a great contribution to the academic development in this field. Surprisingly, as the institution with the most number of publications, Fudan University ranked last in citation/publication. The Citation/publication of Zhejiang University is ranked first in the top 10 institutions. It can be seen that the publications published by Zhejiang University in this field are high quality. In addition to the country, close cooperation between institutions has also played a role in promoting immunotherapy for GC. Medical research often involves a variety of complex issues, and in-depth cooperation among institutions can better solve these problems.
Perspective of journal
Frontiers in Oncology (45 publications), Cancers (37 publications), Oncoimmunology (26 publications), Frontiers in Immunology (20 publications), and Oncotargets and Therapy (20 publications) published more than 20 publications. The most published and high-co-cited journals are Q1 and Q2 (JCR partition). It can be concluded that the above journals are particularly interested in immunotherapy for GC. These will help researchers choose journals when submitting relevant manuscripts. In addition, researchers can follow these journals to get updates on relevant research. It is worth noting that the top ten most published journals do not appear in the top ten co-cited journals. Most of the top ten most published journals are related to clinical oncology and immunology. And the top ten co-cited journals are related to oncology and biology. Dual-map overlay is consistent with this analysis (Figure 6). Genetics/Molecular Biology research cited by Biology/Molecular/Immunology research and Medical/Medicine/Clinical research. It shows the research of immunotherapy for GC has gradually transformed from basic research to clinical research. This is the current concern of researchers. In addition, journals need to pay more attention to the transformation of basic achievements into clinical drugs.
Perspective of author
Each of the top ten authors who published the most publications have at least ten publications. The top ten most published authors are from China, America, South Korea and Japan. This proves once again that both these countries have made important contributions to the field of immunotherapy for GC. The authors with the most publications, Kim Kyoung-mee, from South Korea, has made significant contributions to immune checkpoints, molecular mechanisms and PD-1 antibodies.34,35 Kim Kyoung-mee et al explored molecular characteristics associated with the response to pembrolizumab for metastatic GC.36 The study provided biomarkers relevant to the selection of patients who may benefit from PD-1 inhibition.36 This article was also one of the most co-cited references. Bang, Yj (506 citations) ranked first among the co-cited authors. Bang, Yj, also from South Korea, published “Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-esophageal junction cancer (ToGA): a phase 3, open-label, randomized controlled trial” in Lancet (IF=202.73), which was also one of the most co-cited references in this study.37 Chemotherapy combined with trastuzumab was considered a new standard option for advanced GC patients with human epidermal growth factor receptor-2 (HER2)-positive.37 Shen Lin, a researcher from China, had the most citation/publication. Shen Lin was interested in individualized treatment of immunotherapy for GC, mainly explored the efficacy of immunotherapy in Epstein-Barr virus-associated GC,38 multi-dimensional analyses of tumor immune microenvironment to predict the efficacy of immunotherapy in GC,39 and plasma extracellular vesicle-derived protein profile to predict the immunotherapeutic outcomes of GC.40 This suggests that screening suitable patients is one of the priorities of current research. Of course, authors from various countries, especially China and the United States, should strengthen cooperation to promote the development of this field. Researchers focusing on immunotherapy for GC can communicate with the authors who have published the most publications to obtain more resources and experience for research development.
Perspective of reference
The co-cited relationship is defined as two publications being cited jointly by a third publication.41 The most co-cited references can be regarded as the main focus in the certain field. The top five most co-cited references were composed of four clinical studies and one basic trial, all from top journals. The reference with the most co-cited proposed a molecular classification method.29 The study divided GC patients into four subtypes: extreme DNA hypermethylation, and amplification of JAK2, CD274 (PD-L1) and PDCD1LG2 (PD-L2); tumors positive for Epstein – Barr virus; microsatellite unstable tumors; and tumors with chromosomal instability.29 Identification of these subtypes provided guidance for stratification and targeted therapy. Three references with the most co-cited were related to anti-PD-1 antibody.42–44 This means that anti-PD-1 antibody is indispensable on immunotherapy for GC. ICIs represented by anti-PD-1 antibody played an important role in GC.45 Although anti-PD-L1 and anti-PD-1 antibodies have clinical activity in GC, only show prognostic advantage in some selected patients.45–47 This shows that it is important to screen suitable patients according to appropriate molecular markers for immunotherapy.48 The latest research has shown that nivolumab is the first PD-1 inhibitor with superior overall survival (OS) and progression-free survival (PFS) for previously untreated, unresectable, non-HER2-positive gastric adenocarcinoma patients.49 In addition, nivolumab combined with chemotherapy had acceptable safety.49 This adds to the evidence of combined use of anti-PD-L1 antibodies for GC patients. Interestingly, the four most co-cited references focused on clinical trials in advanced GC.37–42–44 How to improve the survival of these patients is the focus of many clinicians and researchers. It can also be concluded that nivolumab and pembrolizumab, the representative drugs of immunotherapy for GC, have played an important role in clinical treatment. These co-cited references have showed the development and treatment hotspots of immunotherapy for GC. Researchers focusing on immunotherapy for GC can obtain meaningful information from these articles. The clinical application of these treatments is a hope for advanced GC patients.
In this study, the timeline view of the co-cited references was divided into three periods. The early period is 2012–2014, the middle period is 2015–2018, and the current period is 2019–2022 (Figure 8b). The early period focused on “#3 cytokine-induced killer” and “#8 myeloid-derived suppressor cells.” Current study suggested that compared to other immune cells, CIKs have stronger antitumor activity, broader antitumor spectrum and higher proliferation rate.50 The latest meta-analysis suggested that CIKs or combined dendritic cell (DC)-CIKs has advantages in enhancing immune function, alleviating adverse events and prolonging the survival in GC patients.51 The early period also focused on myeloid-derived suppressor cells (MDSCs). MDSCs are an important component of Tumor Microenvironment (TME). Exploring TME is crucial for establishing new cancer therapies.52 The clinicopathological significance of TME in predicting outcomes and therapeutic effects has been demonstrated.53 Therefore, exploring the characteristics of TME may help explain the efficacy gap of immunotherapy in different GC populations. And it provides new strategies for treatment, also suggests the importance of molecular markers. It is worth noting that these fields in the early period were rarely studied after 2016 (CIKs and MDSCs). These drugs are more likely to be part of combined chemotherapy in the future.
The middle period focused on “#1 Epstein-Barr virus,” “#4 chemotherapy” and “#6 Dendritic cells.” Epstein-Barr virus (EBV), as a middle period hotspot, indicated that researchers are actively exploring potential markers to screen patients who respond well to ICIs. The infection status of EBV affects the effect of immunotherapy.1,17,36,54 Of course, exploring how the EBV genome is involved in the development of GC and characterizing the molecular and clinicopathological features of EBV-associated GC are also critical for treatment.55,56 Thus, no matter in the early or middle stage, screening suitable patients is an indispensable part of precision therapy. “Chemotherapy” in the middle period and “adjuvant chemotherapy” in the current period indicate that the role of immunotherapy in chemotherapy and adjuvant chemotherapy is actively explored. It is clear that the clinical efficacy of immune agents alone is quite limited. At present, multiple clinical trials are exploring combined therapy. A phase II nonrandomized Keynote-059 study in 2017 showed that for advanced GC, chemotherapy combined with pembrolizumab had a higher objective response rate (ORR) than pembrolizumab monotherapy.57 But the prognosis needs to be further verified. A global multicenter study also showed that chemotherapy combined with pembrolizumab had better efficacy than chemotherapy alone in GC patients.49 Many clinical trials have demonstrated that the addition of immune agents to chemotherapy or adjuvant chemotherapy achieves better clinical efficacy.58,59 Chemotherapy combined with immunotherapy is becoming the standard treatment for GC. Which combination of immune drugs and chemotherapy drugs can achieve better prognosis is the focus of future research. In addition, it is also noteworthy whether the side effects between drugs will be superimposed. Tumor vaccines represented by dendritic cells (DC) have been rarely studied on immunotherapy for GC.17 Among 9 advanced GC patients, one patient achieved partial clinical response after HER2/DC vaccination.17 Recently, clinical trials are being conducted to verify the efficacy of DC tumor vaccine.60 More data are expected to demonstrate the role of DC vaccines in GC patients. With the development of technology and in-depth research on immune vaccines, vaccines and drug regimens with different mechanisms may receive more attention in the future.
The current period focuses on “#0 Targeted Therapy” and “#5 Adjuvant.” Anti-HER2 antibodies and Vascular Endothelial growth factor (VEGF) inhibitors are the two main options for targeted therapy combined immunotherapy for GC.17 Trastuzumab is the first monoclonal antibody drug to block HER2 in GC standard treatment protocol. The results of a 2010 phase III randomized controlled trial suggested that trastuzumab in combination with chemotherapy for HER2-positive GC patients had significantly better OS than chemotherapy alone.37 This publication is also the most cited in this study. The prognostic advantage of combined trastuzumab in GC has also been validated in several prospective trials.61–63 Several global guidelines (CSCO guidelines of China, NCCN guidelines of United States, and Japanese guidelines) recommend chemotherapy plus trastuzumab as first-line treatment for HER2-positive advanced GC.1,64,65 Trastuzumab has changed the treatment mode for HER2-positive GC. Immunotherapy combined with targeted therapy is a hot topic in GC research. The prognostic benefits of immunologic agents combined with targeted agents have been demonstrated by various protocols, such as pembrolizumab plus trastuzumab plus chemotherapy, trastuzumab plus pembrolizumab plus capecitabine plus oxaliplatin and pembrolizumab plus trastuzumab plus fluoropyrimidine, etc.66–68 Targeted drugs have become a critical component of immunotherapy for GC. However, drug resistance hinders the further use of targeted agents,69,70 which remains a major barrier to clinical treatment. In the future, combination therapy or multi-target antitumor drugs may be the main direction to improve the therapeutic effect and reduce the occurrence of drug resistance. It can be seen from each period that how to combine multiple drugs to improve the prognosis of GC patients is the current and future research trend. Researchers and clinicians can pay more attention to the topic of combination therapy.
The hottest topic is “#7 CAR-T,” a new paradigm in cellular immunotherapy. CAR-T is a new revolutionary pillar in cancer treatment.71 Currently, CAR-T therapy for GC is in early stages. Animal studies have proved that CAR-T has anti-GC efficacy and sustained activity in various targets, such as HER2,72,73 Folate receptor 1 (FOLR1),74 Claudin18.2 (CLDN18.2),75 etc. There are only a few data from phase I clinical trials.17 It must be noted that although CAR-T cell therapy has shown clinical efficacy in hematological malignancies, there is no convincing data in GC.76 The latest open-label, single-arm phase I clinical trial in 2022 reported that CLDN18.2 targeted CAR-T cells (CT041) was used for previously treated CLDN18.2-positive digestive system cancer patients.77 The ORR, disease control rate (DCR) and the 6-month OS rate of GC patients were 57.1%, 75.0% and 81.2%, respectively. These preliminary data indicate that CT041 has good therapeutic efficacy and acceptable safety in CLDN18.2-positive digestive system cancer patients, particularly in GC patients.77 In addition to focusing on the final results of this trial, there may be more CAR-T clinical trials of other targets in the future. With the discovery of new targeting sites, the better understanding of the tumor microenvironment, and advances in technology, the potential of CAR-T therapy for GC is foreseeable in the near future.
Perspective of keyword
The timeline view of the keywords again proves that it is crucial to screen the GC patients that benefits from immunotherapy (#0 Microsatellite instability). This is essential for personalized treatment. The status of Microsatellite instability (MSI) determines the effect of immunotherapy.78 Previous studies have confirmed that anti-PD-1 antibodies has shown good efficacy in high-MSI GC patients.79,80 In addition, significant up-regulation of PD-1 and PD-L1 was also found in MSI-positive GC patients.81 However, MSI is only detected in a minority of GC patients.29,82 Therefore, in addition to the development of new therapeutic markers, the combination of multiple markers to screen the beneficiary patients may be a model in the future.
The burst keywords can reflect the research hotspots in the academic field.83 To highlight the latest hot spots, we drawn the burst keywords of the last five years (2017–2022). From the perspective of the evolution of burst keywords in the past 5 years, different treatment methods are constantly innovating on immunotherapy for GC (Figure 9). Of the all burst keywords, “lymphocyte” has the greatest intensity. This is not surprising, since most immunotherapy for GC relies on lymphocytes and various immune cells to work. For example, a streptococcal preparation (OK-432) enhances the activity of natural killer cells and T lymphocytes.84 In addition, “adoptive cellular immunotherapy” (ACI) uses various immune cells to induce effective immunity and eliminate cancer cells. Such as, CIK,85 Tumor-infiltrating lymphocytes (TILs),86 and Lymphokine Activated Killer cells (LAK),87 etc. It can be seen that how to better use of immune cells such as lymphocytes is the basis of immunotherapy. ACI will play an important role on immunotherapy for GC in the future due to its unique treatment mode. In addition, the combined use of ACI in chemotherapy also deserves attention.
ACI has proven to be an effective strategy for advanced GC88,which is also the burst keywords. CAR-T, which appears all in the burst keywords and timeline view of co-cited references, is one of the hottest treatment modalities of ACI in recent years. The specific efficacy of CAR-T in GC has been preliminarily verified.77 More clinical data supporting CAR-T for GC is expected in the near future. “HER2” also appeared again in the burst keywords, indicating that targeted therapy is indispensable on immunotherapy for GC. Immunotherapy combined with targeted therapy is also a hot topic at present. The “growth factor receptor” in burst keywords is the common therapeutic targets in GC therapy, such as HER2, Endothelial Growth Factor Receptor (EGFR). The combination of EGFR inhibitors with immunotherapy for GC has also shown preliminary clinical activity.89,90 Adding VEGF inhibitors to immunotherapy appears to be an effective treatment for advanced GC in the future. It also shows once again that multi-drug immunotherapy is the focus of GC treatment. “Melanoma” appears surprisingly in the burst keywords. In addition to melanoma and GC showing many similar targets in immunotherapy,91–93 melanoma associated antigen A3 peptide (MAGE-A3) have showed anti-tumor effects for advanced GC patients.94 To sum up, the burst keywords prove the researchers’ concern about CAR-T, targeted therapy and combination therapy in recent years. These can help researchers to select the latest hot spots in the study of immunotherapy for GC.
Limitation
This study inevitably has some limitations. First of all, the publications we included may be missing: ①This study is from a single database; ②The included publications are only in English; ③Publications not related to immunotherapy for GC were manually deleted. Therefore, these publications may not fully represent all studies on immunotherapy for GC. But more than 1,000 publications in this study may address the offset caused by such small deviations.
Conclusion
This study analyzed global academic research on immunotherapy for GC. Through systematic literature search, we found that this study is the first bibliometric analysis on immunotherapy for GC. The rapid growth of publications indicates that immunotherapy for GC is attracting more attention from researchers worldwide. Immunotherapy has become the focus of GC research. Authors and institutions of China and United States should strengthen cooperation and continue to contribute in this field. Frontiers in Oncology and Journal of Clinical Oncology ranked first in cited and co-cited journals, respectively. The investigator may publish the immunotherapy for GC article in the above journals. Follow these journals to better obtain the research progress in this field.
In general, the treatment of GC has entered the stage of personalized precision treatment. At present, there are many drugs and combination modes on immunotherapy for GC. Selecting appropriate biomarkers for screening suitable patients and monitoring therapeutic efficacy is the first step for the successful of immunotherapy. The combined application of immune drugs and targeted drugs in chemotherapy and adjuvant chemotherapy is the current strategy and the future research direction of immunotherapy for GC. The effect of anti-PD-1 antibody has been verified in immunotherapy for GC. In addition to the prognostic benefits of immune drugs, attention should also be paid to the problems of resistance and side effects caused by these drugs. Researchers also have a strong interest in CAR-T therapy for GC, which is currently a hot spot in this field and will be further studied in the future. The future of immunotherapy for GC may be multi-drug combination and multi-target mode.
Supplementary Material
Funding Statement
The author(s) reported there is no funding associated with the work featured in this article.
Disclosure statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Authors’ contributions
All authors agree to be accountable for all aspects of the work, and the final approval of the version to be published. C-YZ, Z-T, C-YF: material preparation, search and data collection. T-S, L-SL, Z-YY: figure preparation. C-YZ, M-YL: write original draft. L-ST, Y-CS and L-WH: supervision and conceptualization. L-WH: modify the draft.
Data availability statement
The datasets used and analyzed during the current article are available from the corresponding author on reasonable request.
Supplementary data
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2023.2186684
References
- 1.Ajani JA, D’amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20(2):167–16. doi: 10.6004/jnccn.2022.0008. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fuchs HE, Jemal A.. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Cai H, Li M, Deng R, Wang M, Shi Y.. Advances in molecular biomarkers research and clinical application progress for gastric cancer immunotherapy. Biomarker Res. 2022;10(1):67. doi: 10.1186/s40364-022-00413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet (London, England). 2020;396(10251):635–48. doi: 10.1016/s0140-6736(20)31288-5. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Xu J, Xie J, Yang W. Research progress in targeted therapy and immunotherapy for gastric cancer. Chin Med J. 2022;135(11):1299–313. doi: 10.1097/cm9.0000000000002185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonotto M, Garattini SK, Basile D, Ongaro E, Fanotto V, Cattaneo M, Cortiula F, Iacono D, Cardellino GG, Pella N, et al. Immunotherapy for gastric cancers: emerging role and future perspectives. Expert Rev Clin Pharmacol. 2017;10(6):609–19. doi: 10.1080/17512433.2017.1313113. [DOI] [PubMed] [Google Scholar]
- 7.Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–14. doi: 10.1016/j.cell.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma P, Siddiqui BA, Anandhan S, Yadav SS, Subudhi SK, Gao J, Goswami S, Allison JP. The next decade of immune checkpoint therapy. Cancer Discov. 2021;11(4):838–57. doi: 10.1158/2159-8290.Cd-20-1680. [DOI] [PubMed] [Google Scholar]
- 9.Veillette A, Chen J. SIRPα-CD47 immune checkpoint blockade in anticancer therapy. Trends Immunol. 2018;39(3):173–84. doi: 10.1016/j.it.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Lv J, Zhao R, Wu D, Zheng D, Wu Z, Shi J, Wei X, Wu Q, Long Y, Lin S, et al. Mesothelin is a target of chimeric antigen receptor T cells for treating gastric cancer. J Hematol Oncol. 2019;12(1):18. doi: 10.1186/s13045-019-0704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiao G, Wang X, Zhou L, Zhou X, Song Y, Wang S, Zhao L, Morse MA, Hobeika A, Song J, et al. Autologous dendritic cell-cytokine induced killer cell immunotherapy combined with S-1 plus cisplatin in patients with advanced gastric cancer: a prospective study. Clin Cancer Res. 2019;25(5):1494–504. doi: 10.1158/1078-0432.Ccr-18-2360. [DOI] [PubMed] [Google Scholar]
- 12.Kono K, Nakajima S, Mimura K. Current status of immune checkpoint inhibitors for gastric cancer. Gastric Cancer. 2020;23(4):565–78. doi: 10.1007/s10120-020-01090-4. [DOI] [PubMed] [Google Scholar]
- 13.Wang DK, Zuo Q, He QY, Li B. Targeted immunotherapies in gastrointestinal cancer: from molecular mechanisms to implications. Front Immunol. 2021;12:705999. doi: 10.3389/fimmu.2021.705999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal A, Baskaran S, Panner Selvam MK, Barbăroșie C, Master K. Unraveling the footsteps of proteomics in male reproductive research: a scientometric approach. Antioxid Redox Signaling. 2020;32(8):536–49. doi: 10.1089/ars.2019.7945. [DOI] [PubMed] [Google Scholar]
- 15.Yang DW, Wang XP, Wang ZC, Yang ZH, Bian XF. A scientometric analysis on hepatocellular carcinoma magnetic resonance imaging research from 2008 to 2017. Quant Imaging Med Surg. 2019;9(3):465–76. doi: 10.21037/qims.2019.02.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Powell AG, Hughes DL, Wheat JR, Lewis WG. The 100 most influential manuscripts in gastric cancer: a bibliometric analysis. Int J Surg (London, England). 2016;28:83–90. doi: 10.1016/j.ijsu.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 17.Li K, Zhang A, Li X, Zhang H, Zhao L. Advances in clinical immunotherapy for gastric cancer. Biochimica et Biophysica Acta Rev Cancer. 2021;1876(2):188615. doi: 10.1016/j.bbcan.2021.188615. [DOI] [PubMed] [Google Scholar]
- 18.Brandt JS, Hadaya O, Schuster M, Rosen T, Sauer MV, Ananth CV. A bibliometric analysis of top-cited journal articles in obstetrics and gynecology. JAMA Netw Open. 2019;2(12):e1918007. doi: 10.1001/jamanetworkopen.2019.18007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rogers M, Bethel A, Briscoe S. Resources for forwards citation searching for implementation studies in dementia care: a case study comparing web of science and scopus. Res Synth Methods. 2020;11(3):379–86. doi: 10.1002/jrsm.1400. [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Sun YP, Gao XL, Sui Y. Knowledge domain and emerging trends in alzheimer’s disease: a scientometric review based on CiteSpace analysis. Neural Regener Res. 2019;14(9):1643–50. doi: 10.4103/1673-5374.255995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu YX, Yang Y, Le KJ, Zhang ZL, Cui M, Zhong H, Gu ZC. Antimicrobial stewardship in surgery: a literature bibliometric analysis. Front Public Health. 2022;10:847420. doi: 10.3389/fpubh.2022.847420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao L, Hui L, Yang Z, Chen X, Xiao A. Freshwater microplastics pollution: detecting and visualizing emerging trends based on citespace II. Chemosphere. 2020;245:125627. doi: 10.1016/j.chemosphere.2019.125627. [DOI] [PubMed] [Google Scholar]
- 23.Zhong D, Luo S, Zheng L, Zhang Y, Jin R. Epilepsy occurrence and circadian rhythm: a bibliometrics study and visualization analysis via CiteSpace. Front Neurol. 2020;11:984. doi: 10.3389/fneur.2020.00984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu H, Cheng K, Tong L, Wang Y, Yang W, Sun Z. Knowledge structure and emerging trends on osteonecrosis of the femoral head: a bibliometric and visualized study. J Orthop Surg Res. 2022;17(1):194. doi: 10.1186/s13018-022-03068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghasemi A, Yun S, Li X. Fractal structures arising from interfacial instabilities in bio-oil atomization. Sci Rep. 2021;11(1):411. doi: 10.1038/s41598-020-80059-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu MQ, Wu DQ, Hu CP, Iao LS. Studies on children with developmental coordination disorder in the past 20 years: a bibliometric analysis via CiteSpace. Front Psychiatry. 2021;12:776883. doi: 10.3389/fpsyt.2021.776883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, Wang Y, Yan B. The hotspots of life cycle assessment for bioenergy: a review by social network analysis. Sci Total Environ. 2018;625:1301–08. doi: 10.1016/j.scitotenv.2018.01.030. [DOI] [PubMed] [Google Scholar]
- 28.Shen J, Shen H, Ke L, Chen J, Dang X, Liu B, Hua Y. Knowledge mapping of immunotherapy for hepatocellular carcinoma: a bibliometric study. Front Immunol. 2022;13:815575. doi: 10.3389/fimmu.2022.815575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513(7517):202–09. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan X, Chang C, Chen X, Li K. Emerging trends and focus of human gastrointestinal microbiome research from 2010-2021: a visualized study. J Transl Med. 2021;19(1):327. doi: 10.1186/s12967-021-03009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson M, Sampson M, Barrowman N, Doja A. Bibliometric analysis of neurology articles published in general medicine journals. JAMA Netw Open. 2021;4(4):e215840. doi: 10.1001/jamanetworkopen.2021.5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J. 2021;134(7):783–91. doi: 10.1097/cm9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao M, Li H, Sun D, Chen W. Cancer burden of major cancers in China: a need for sustainable actions. Cancer Commun (London, England). 2020;40(5):205–10. doi: 10.1002/cac2.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon M, An M, Klempner SJ, Lee H, Kim KM, Sa JK, Cho HJ, Hong JY, Lee T, Min YW, et al. Determinants of response and intrinsic resistance to PD-1 blockade in microsatellite instability-high gastric cancer. Cancer Discov. 2021;11(9):2168–85. doi: 10.1158/2159-8290.Cd-21-0219. [DOI] [PubMed] [Google Scholar]
- 35.Zeng D, Wu J, Luo H, Li Y, Xiao J, Peng J, Ye Z, Zhou R, Yu Y, Wang G, et al. Tumor microenvironment evaluation promotes precise checkpoint immunotherapy of advanced gastric cancer. J Immunoth Cancer. 2021;9(8):e002467. doi: 10.1136/jitc-2021-002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, Liu XQ, Sher X, Jung H, Lee M, et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24(9):1449–58. doi: 10.1038/s41591-018-0101-z. [DOI] [PubMed] [Google Scholar]
- 37.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet (London, England). 2010;376(9742):687–97. doi: 10.1016/s0140-6736(10)61121-x. [DOI] [PubMed] [Google Scholar]
- 38.Bai Y, Xie T, Wang Z, Tong S, Zhao X, Zhao F, Cai J, Wei X, Peng Z, Shen L. Efficacy and predictive biomarkers of immunotherapy in Epstein-Barr virus-associated gastric cancer. J Immunoth Cancer. 2022;10(3):e004080. doi: 10.1136/jitc-2021-004080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen Y, Jia K, Sun Y, Zhang C, Li Y, Zhang L, Chen Z, Zhang J, Hu Y, Yuan J, et al. Predicting response to immunotherapy in gastric cancer via multi-dimensional analyses of the tumour immune microenvironment. Nat Commun. 2022;13(1):4851. doi: 10.1038/s41467-022-32570-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang C, Chong X, Jiang F, Gao J, Chen Y, Jia K, Fan M, Liu X, An J, Li J, et al. Plasma extracellular vesicle derived protein profile predicting and monitoring immunotherapeutic outcomes of gastric cancer. J Extracell Vesicles. 2022;11(4):e12209. doi: 10.1002/jev2.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma D, Yang B, Guan B, Song L, Liu Q, Fan Y, Zhao L, Wang T, Zhang Z, Gao Z, et al. A bibliometric analysis of pyroptosis from 2001 to 2021. Front Immunol. 2021;12:731933. doi: 10.3389/fimmu.2021.731933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17(6):717–26. doi: 10.1016/s1470-2045(16)00175-3. [DOI] [PubMed] [Google Scholar]
- 43.Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England). 2017;390(10111):2461–71. doi: 10.1016/s0140-6736(17)31827-5. [DOI] [PubMed] [Google Scholar]
- 44.Fuchs CS, Doi T, Jang RW, Muro K, Satoh T, Machado M, Sun W, Jalal SI, Shah MA, Metges JP, et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018;4(5):e180013. doi: 10.1001/jamaoncol.2018.0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moehler M, Dvorkin M, Boku N, Özgüroğlu M, Ryu MH, Muntean AS, Lonardi S, Nechaeva M, Bragagnoli AC, Coşkun HS, et al. Phase III trial of avelumab maintenance after first-line induction chemotherapy versus continuation of chemotherapy in patients with gastric cancers: results from JAVELIN gastric 100. J Clin Oncol. 2021;39(9):966–77. doi: 10.1200/jco.20.00892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shitara K, Özgüroğlu M, Bang YJ, Di Bartolomeo M, Mandalà M, Ryu MH, Fornaro L, Olesiński T, Caglevic C, Chung HC, et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet (London, England). 2018;392(10142):123–33. doi: 10.1016/s0140-6736(18)31257-1. [DOI] [PubMed] [Google Scholar]
- 47.Boku N, Satoh T, Ryu MH, Chao Y, Kato K, Chung HC, Chen JS, Muro K, Kang WK, Yeh KH, et al. Nivolumab in previously treated advanced gastric cancer (ATTRACTION-2): 3-year update and outcome of treatment beyond progression with nivolumab. Gastric Cancer. 2021;24(4):946–58. doi: 10.1007/s10120-021-01173-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang F, Wei XL, Wang FH, Xu N, Shen L, Dai GH, Yuan XL, Chen Y, Yang SJ, Shi JH, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Annals Oncol. 2019;30(9):1479–86. doi: 10.1093/annonc/mdz197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Campos Bragagnoli A, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet (London, England). 2021;398(10294):27–40. doi: 10.1016/s0140-6736(21)00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W, Wang X, Yang W, Zhong K, He N, Li X, Pang Y, Lu Z, Liu A, Lu X. A CTLA-4 blocking strategy based on nanobody in dendritic cell-stimulated cytokine-induced killer cells enhances their anti-tumor effects. BMC Cancer. 2021;21(1):1029. doi: 10.1186/s12885-021-08732-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mu Y, Zhou CH, Chen SF, Ding J, Zhang YX, Yang YP, Wang WH. Effectiveness and safety of chemotherapy combined with cytokine-induced killer cell/dendritic cell-cytokine-induced killer cell therapy for treatment of gastric cancer in China: a systematic review and meta-analysis. Cytotherapy. 2016;18(9):1162–77. doi: 10.1016/j.jcyt.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 52.Oya Y, Hayakawa Y, Koike K. Tumor microenvironment in gastric cancers. Cancer Sci. 2020;111(8):2696–707. doi: 10.1111/cas.14521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng D, Li M, Zhou R, Zhang J, Sun H, Shi M, Bin J, Liao Y, Rao J, Liao W. Tumor microenvironment characterization in gastric cancer identifies prognostic and immunotherapeutically relevant gene signatures. Cancer Immunol Res. 2019;7(5):737–50. doi: 10.1158/2326-6066.Cir-18-0436. [DOI] [PubMed] [Google Scholar]
- 54.Panda A, Mehnert JM, Hirshfield KM, Riedlinger G, Damare S, Saunders T, Kane M, Sokol L, Stein MN, Poplin E, et al. Immune activation and benefit from avelumab in EBV-Positive gastric cancer. J Natl Cancer Inst. 2018;110(3):316–20. doi: 10.1093/jnci/djx213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang J, Liu Z, Zeng B, Hu G, Gan R. Epstein-Barr virus-associated gastric cancer: a distinct subtype. Cancer Lett. 2020;495:191–99. doi: 10.1016/j.canlet.2020.09.019. [DOI] [PubMed] [Google Scholar]
- 56.Okabe A, Huang KK, Matsusaka K, Fukuyo M, Xing M, Ong X, Hoshii T, Usui G, Seki M, Mano Y, et al. Cross-species chromatin interactions drive transcriptional rewiring in Epstein-Barr virus-positive gastric adenocarcinoma. Nat Genet. 2020;52(9):919–30. doi: 10.1038/s41588-020-0665-7. [DOI] [PubMed] [Google Scholar]
- 57.Bang YJ, Kang YK, Catenacci DV, Muro K, Fuchs CS, Geva R, Hara H, Golan T, Garrido M, Jalal SI, et al. Pembrolizumab alone or in combination with chemotherapy as first-line therapy for patients with advanced gastric or gastroesophageal junction adenocarcinoma: results from the phase II nonrandomized KEYNOTE-059 study. Gastric Cancer. 2019;22(4):828–37. doi: 10.1007/s10120-018-00909-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang H, Zheng Y, Qian J, Mao C, Xu X, Li N, Xiao C, Wang H, Teng L, Zhou H, et al. Safety and efficacy of sintilimab combined with oxaliplatin/capecitabine as first-line treatment in patients with locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma in a phase Ib clinical trial. BMC Cancer. 2020;20(1):760. doi: 10.1186/s12885-020-07251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang H, Yu X, Li N, Kong M, Ma Z, Zhou D, Wang W, Wang H, Wang H, He K, et al. Efficacy and safety of neoadjuvant sintilimab, oxaliplatin and capecitabine in patients with locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma: early results of a phase 2 study. J Immunoth Cancer. 2022;10(3):e003635. doi: 10.1136/jitc-2021-003635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faghfuri E, Shadbad MA, Faghfouri AH, Soozangar N. Cellular immunotherapy in gastric cancer: adoptive cell therapy and dendritic cell-based vaccination. Immunotherapy. 2022;14(6):475–88. doi: 10.2217/imt-2021-0285. [DOI] [PubMed] [Google Scholar]
- 61.Gong J, Liu T, Fan Q, Bai L, Bi F, Qin S, Wang J, Xu N, Cheng Y, Bai Y, et al. Optimal regimen of trastuzumab in combination with oxaliplatin/capecitabine in first-line treatment of HER2-positive advanced gastric cancer (CGOG1001): a multicenter, phase II trial. BMC Cancer. 2016;16(1):68. doi: 10.1186/s12885-016-2092-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kurokawa Y, Sugimoto N, Miwa H, Tsuda M, Nishina S, Okuda H, Imamura H, Gamoh M, Sakai D, Shimokawa T, et al. Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1). Br J Cancer. 2014;110(5):1163–68. doi: 10.1038/bjc.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Franchi M, Tritto R, Torroni L, Reno C, La Vecchia C, Corrao G. Effectiveness and healthcare cost of adding trastuzumab to standard chemotherapy for first-line treatment of metastatic gastric cancer: a population-based cohort study. Cancers. 2020;12(6):1691. doi: 10.3390/cancers12061691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang FH, Zhang XT, Li YF, Tang L, Qu XJ, Ying JE, Zhang J, Sun LY, Lin RB, Qiu H, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (London, England). 2021;41(8):747–95. doi: 10.1002/cac2.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu D, Wang YY, Yan XL, Li J, Wang K, Xing BC. Development of a model to predict pathologic response to chemotherapy in patients with colorectal liver metastases. J Gastrointest Oncol. 2021;12(4):1498–508. doi: 10.21037/jgo-21-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Janjigian YY, Maron SB, Chatila WK, Millang B, Chavan SS, Alterman C, Chou JF, Segal MF, Simmons MZ, Momtaz P, et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2020;21(6):821–31. doi: 10.1016/s1470-2045(20)30169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chung HC, Bang YJ, Sf C, Qin SK, Satoh T, Shitara K, Tabernero J, Van Cutsem E, Alsina M, Cao ZA, et al. First-line pembrolizumab/placebo plus trastuzumab and chemotherapy in HER2-positive advanced gastric cancer: kEYNOTE-811. Future Oncol (London, England). 2021;17(5):491–501. doi: 10.2217/fon-2020-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, Barajas O, Bai Y, Shen L, Tang Y, et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600(7890):727–30. doi: 10.1038/s41586-021-04161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang T, Song C, Zheng L, Xia L, Li Y, Zhou Y. The roles of extracellular vesicles in gastric cancer development, microenvironment, anti-cancer drug resistance, and therapy. Mol Cancer. 2019;18(1):62. doi: 10.1186/s12943-019-0967-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wei L, Sun J, Zhang N, Zheng Y, Wang X, Lv L, Liu J, Xu Y, Shen Y, Yang M. Noncoding RNAs in gastric cancer: implications for drug resistance. Mol Cancer. 2020;19(1):62. doi: 10.1186/s12943-020-01185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han Y, Liu C, Li G, Li J, Lv X, Shi H, Liu J, Liu S, Yan P, Wang S, et al. Antitumor effects and persistence of a novel HER2 CAR T cells directed to gastric cancer in preclinical models. Am J Cancer Res. 2018;8(1):106–19. [PMC free article] [PubMed] [Google Scholar]
- 73.Luo F, Qian J, Yang J, Deng Y, Zheng X, Liu J, Chu Y. Bifunctional αHER2/CD3 RNA-engineered CART-like human T cells specifically eliminate HER2(+) gastric cancer. Cell Res. 2016;26(7):850–53. doi: 10.1038/cr.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim M, Pyo S, Kang CH, Lee CO, Lee HK, Choi SU, Park CH. Folate receptor 1 (FOLR1) targeted chimeric antigen receptor (CAR) T cells for the treatment of gastric cancer. PloS One. 2018;13(6):e0198347. doi: 10.1371/journal.pone.0198347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang H, Shi Z, Wang P, Wang C, Yang L, Du G, Zhang H, Shi B, Jia J, Li Q, et al. Claudin18.2-specific chimeric antigen receptor engineered t cells for the treatment of gastric cancer. J Natl Cancer Inst. 2019;111(4):409–18. doi: 10.1093/jnci/djy134. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Q, Liu G, Liu J, Yang M, Fu J, Liu G, Li D, Gu Z, Zhang L, Pan Y, et al. The antitumor capacity of mesothelin-CAR-T cells in targeting solid tumors in mice. Mol Ther Oncolytics. 2021;20:556–68. doi: 10.1016/j.omto.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qi C, Gong J, Li J, Liu D, Qin Y, Ge S, Zhang M, Peng Z, Zhou J, Cao Y, et al. Claudin18.2-specific CAR T cells in gastrointestinal cancers: phase 1 trial interim results. Nat Med. 2022;28(6):1189–98. doi: 10.1038/s41591-022-01800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kather JN, Pearson AT, Halama N, Jäger D, Krause J, Loosen SH, Marx A, Boor P, Tacke F, Neumann UP, et al. Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer. Nat Med. 2019;25(7):1054–56. doi: 10.1038/s41591-019-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chao J, Fuchs CS, Shitara K, Tabernero J, Muro K, Van Cutsem E, Bang YJ, De Vita F, Landers G, Yen CJ, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials. JAMA Oncol. 2021;7(6):895–902. doi: 10.1001/jamaoncol.2021.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Llosa NJ, Cruise M, Tam A, Wicks EC, Hechenbleikner EM, Taube JM, Blosser RL, Fan H, Wang H, Luber BS, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5(1):43–51. doi: 10.1158/2159-8290.Cd-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suh YS, Na D, Lee JS, Chae J, Kim E, Jang G, Lee J, Min J, Ock CY, Kong SH, et al. Comprehensive molecular characterization of adenocarcinoma of the gastroesophageal junction between esophageal and gastric adenocarcinomas. Ann Surg. 2022;275(4):706–17. doi: 10.1097/sla.0000000000004303. [DOI] [PubMed] [Google Scholar]
- 83.Xiao F, Li C, Sun J, Zhang L. Knowledge domain and emerging trends in organic photovoltaic technology: a scientometric review based on citespace analysis. Front Chem. 2017;5:67. doi: 10.3389/fchem.2017.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oba MS, Teramukai S, Ohashi Y, Ogawa K, Maehara Y, Sakamoto J. The efficacy of adjuvant immunochemotherapy with OK-432 after curative resection of gastric cancer: an individual patient data meta-analysis of randomized controlled trials. Gastric Cancer. 2016;19(2):616–24. doi: 10.1007/s10120-015-0489-9. [DOI] [PubMed] [Google Scholar]
- 85.Peng Z, Liang W, Li Z, Xu Y, Chen L. Interleukin-15-transferred cytokine-induced killer cells elevated anti-tumor activity in a gastric tumor-bearing nude mice model. Cell Biol Int. 2016;40(2):204–13. doi: 10.1002/cbin.10553. [DOI] [PubMed] [Google Scholar]
- 86.Kang BW, Kim JG, Lee IH, Bae HI, Seo AN. Clinical significance of tumor-infiltrating lymphocytes for gastric cancer in the era of immunology. World J Gastrointest Oncol. 2017;9(7):293–99. doi: 10.4251/wjgo.v9.i7.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang GQ, Zhao H, Wu JY, Li JY, Yan X, Wang G, Wu LL, Zhang XG, Shao Y, Wang Y, et al. Prolonged overall survival in gastric cancer patients after adoptive immunotherapy. World J Gastroenterol. 2015;21(9):2777–85. doi: 10.3748/wjg.v21.i9.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jin X, Liu Z, Yang D, Yin K, Chang X. Recent progress and future perspectives of immunotherapy in advanced gastric cancer. Front Immunol. 2022;13:948647. doi: 10.3389/fimmu.2022.948647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nakajima TE, Kadowaki S, Minashi K, Nishina T, Yamanaka T, Hayashi Y, Izawa N, Muro K, Hironaka S, Kajiwara T, et al. Multicenter phase I/II study of nivolumab combined with paclitaxel plus ramucirumab as second-line treatment in patients with advanced gastric cancer. Clin Cancer Res. 2021;27(4):1029–36. doi: 10.1158/1078-0432.Ccr-20-3559. [DOI] [PubMed] [Google Scholar]
- 90.Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, et al. Regorafenib plus nivolumab in patients with advanced gastric or colorectal cancer: an open-label, dose-escalation, and dose-expansion phase ib trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38(18):2053–61. doi: 10.1200/jco.19.03296. [DOI] [PubMed] [Google Scholar]
- 91.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–57. doi: 10.1158/1078-0432.Ccr-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jahn L, Hombrink P, Hagedoorn RS, Kester MG, van der Steen DM, Rodriguez T, Pentcheva-Hoang T, de Ru AH, Schoonakker MP, Meeuwsen MH, et al. TCR-based therapy for multiple myeloma and other B-cell malignancies targeting intracellular transcription factor BOB1. Blood. 2017;129(10):1284–95. doi: 10.1182/blood-2016-09-737536. [DOI] [PubMed] [Google Scholar]
- 93.Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Löwer M, Bukur V, Tadmor AD, Luxemburger U, Schrörs B, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–26. doi: 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
- 94.Zhang K, Peng Z, Huang X, Qiao Z, Wang X, Wang N, Xi H, Cui J, Gao Y, Huang X, et al. Phase II trial of adjuvant immunotherapy with autologous tumor-derived Gp96 vaccination in patients with gastric cancer. J Cancer. 2017;8(10):1826–32. doi: 10.7150/jca.18946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed during the current article are available from the corresponding author on reasonable request.


