Abstract
Background:
There has been a significant increase in methamphetamine use among persons who use drugs in Vietnam in the last 5–10 years. We examined the degree to which adherence to antiretroviral therapy (ART) mediates the relationship between recent methamphetamine use and unsuppressed HIV viral load among people who inject drugs (PWID) in Hai Phong, Vietnam.
Methods:
We recruited PWID from October 2016-October 2018 and enrolled HIV positive PWID into a cohort, with up to three years of total follow-up. We assessed relationships among recent methamphetamine use frequency, ART adherence and unsuppressed HIV viral load. Mediation analysis was used to estimate the total and natural direct effects of recent methamphetamine use on unsuppressed HIV viral load and the indirect effect proportion.
Results:
We enrolled 792 HIV seropositive PWID into the cohort; approximately 75.9% reported high/perfect ART adherence at baseline and 81.3% were virally suppressed. In mediation analysis, the total effect for the association between methamphetamine use and unsuppressed HIV viral load (1000 copies/mL) was 3.94 (95% CI: 1.95, 7.96); the natural direct effect was 2.14 (95% CI: 1.29, 3.55); the proportion mediated by self-reported ART adherence was 0.444. Similar results were found when examining lower unsuppressed HIV viral load cutpoints of 250 copies/mL and 500 copies/mL.
Conclusions:
Methamphetamine use is associated with unsuppressed HIV viral load among PWID despite high levels of ART adherence. Further research is needed to better understand these relationships, with emphasis on potential biological pathways that may interact with ART.
Keywords: Asia, antiretroviral therapy, Vietnam
Introduction
The World Health Organization (WHO)1 lists nine interventions that are effective in reducing HIV transmission among persons who inject drugs (PWID); antiretroviral therapy (ART) is one of the four most effective.2,3 There are several benefits to ART including the ability to restore immune function, delay the progression to AIDS4-6 improve quality of life,7,8 reduce inflammation,9 and reduce HIV viral load to decrease HIV transmission (undetectable = untransmissible).10 In 2005, Vietnam began to distribute ART to all people living with HIV and by 2019, 70% of the estimated 230,000 people living with HIV/AIDS in Vietnam were receiving ART,11 with the aim of reducing HIV viral loads to undetectable levels.
Vietnam has seen a significant increase in methamphetamine use among PWID; by 2016, over 70% had reported using the drug, with nearly 50% using in the last six months; this increased use has been associated with increased sexual risk behaviors12,13 and reduced ART adherence.14
There have been several studies examining associations between the use of psychostimulants such as methamphetamine and ART adherence, but there is a lack of mediation studies to evaluate how methamphetamine directly affects HIV viral loads when considering the indirect effect of ART adherence,15,16 In this study, we estimate the extent to which the relationship between recent methamphetamine use and unsuppressed HIV viral load is mediated by ART adherence in a cohort of PWID recruited in Hai Phong Vietnam. We estimate the total and natural direct effects of recent methamphetamine use on unsuppressed HIV viral load and the indirect proportion of ART adherence.
Methods
Study participants
The participants included were part of the DRIVE study17 which between 2016–2018 recruited a cohort of PWID in Hai Phong Vietnam using respondent driven sampling. We enrolled all HIV positive and a sample of HIV negative PWID into a cohort to monitor HIV incidence, associated risk behaviors, and use of intervention and support services.
Eligible participants had to be injecting drugs, at least 18 years of age, and capable of giving informed consent. Injection drug use was verified through the examination of injection marks and urinalysis for heroin and/or methamphetamine use.
Each participant received counseling and testing for HIV. HIV antibody testing was conducted using Bioline HIV1/2 3.0 rapid test (Standard Diagnostics Inc. Gyeonggi-do, Republic of Korea). Confirmation testing was conducted using Determine™ HIV-1/2 (Alere™, Waltham, MA, USA) plus the VIKIA® HIV1/2 (Marcy l’Étoile, Lyon, France). HIV viral load was measured at the national reference laboratory (NIHE, Hanoi) using the COBAS Taqman HIV-1 test v2.0 (Roche diagnostics, Hanoi, Vietnam).
We restricted our sample to visits where PWID reported receiving ART, had non missing self-report methamphetamine use and ART adherence scores and HIV viral load measurements from laboratory tests.
Methamphetamine use
Methamphetamine use was based on the number of days used in the last 30 days (this was asked at baseline for each participant and at each cohort follow-up visit and was verified through urine drug screening at each visit). We categorized methamphetamine use frequency in the last 30 days into three categories; no use, intermediate use (defined as using 1–19 days), and heavy use (defined as 20 or more days) based on review of previous studies examining methamphetamine use frequency.18
HIV viral load
HIV viral load was evaluated at baseline as well as every six months after the initial visit with up to 36 months of followup. We categorized HIV viral load into two categories: suppressed HIV viral load (less than 1000 copies/mL, the WHO definition of viral suppression); and unsuppressed HIV viral load (greater than or equal to 1000 copies/mL). We additionally examined unsuppressed HIV viral load cut points of 500 copies/mL and 250 copies/mL.
ART adherence
Participants were asked about the use and adherence to ART at baseline and every six months during cohort follow-up (each participant was asked “How would you rate your level of adherence in the last six months with 1 indicating little to no medication adherence, and 10 indicating high/perfect adherence to ART?”). We used a scale ranging from 1–10, with 1 indicating very low adherence, and perfect/high adherence (i.e. taking all or nearly all ART as scheduled) was defined by scores of 10. We then divided adherence scores into two categories; imperfect adherence (defined as scores 1–8) and perfect/high adherence (defined as scores 9–10), similar to previous studies categorization of HIV viral load, including the BRAVO trial conducted in Vietnam.19 ART adherence measurements were based on self-report (note that previous studies have highlighted the reliability of self-reported ART adherence measurements,20 and our community based organization members (CBOs) worked with our participants to ensure accurate ART adherence reporting).
Confounders
We adjusted for a robust set of demographic and other risk behaviors which have been shown to be associated with the exposure, mediator and outcome in previous literature21-26 including: age (as a continuous measure), education (coded as high school vs. less than high school), gender (male/female), health insurance in last six months (yes or no), income in last year (greater than or equal to versus less than 6 million Vietnamese Dong, the median income level in the survey questionnaire), marital status (married versus not married), hazardous alcohol use in the last 30 days (yes or no, utilizing the AUDIT-C screening tool for alcohol use disorder27), depression (measured using the CES-D scale28 based on symptoms reported in the previous week, with a score of 6 or greater indicating positive depression), a composite variable of any anxiety/worrying (yes or no) in the previous two weeks based on the PHQ-4 questionnaire,29 and street methadone use in the last six months (yes or no).
Statistical analysis
We first conducted unadjusted and adjusted mixed effects logistic regression analysis examining the association between methamphetamine and ART, and the association between ART and HIV viral load; confounders described above were used in the adjusted analysis.
We then conducted mediation analysis for the relationships between recent methamphetamine use and unsuppressed HIV viral load with ART adherence assessed as a mediator.30 We calculated a total effect, the natural direct effect, and the proportion mediated by ART adherence (indirect effect) to estimate the role of distinct pathways to unsuppressed HIV viral load (i.e. the natural direct path from methamphetamine to HIV viral load vs. the indirect path through ART adherence).
We examined interactions between recent methamphetamine use and ART adherence independent of HIV viral load (i.e. exposure-mediator interaction) and found little evidence of interaction (p = 0.869 for interaction term). We examined linearity in the log odds of unsuppressed HIV viral load by ordinal methamphetamine exposure, including ART adherence in the model, verifying linearity was upheld.
We compared multivariable mixed effects logistic regression models with general structural equation modeling (GSEM) in STATA,31 which allowed us to fit a single model to estimate the natural direct effect, total effect and proportion mediated (i.e. indirect effect). It also allowed us to take into account the multiple measures of methamphetamine use, ART adherence, and HIV viral load for each cohort participant, along with the list of confounding variables described above. We calculated total effects, natural direct effects and the proportion mediated by ART adherence (i.e. the indirect effect) from the GSEM results using the nlcom post estimation command (with individual proportion mediated for each level of methamphetamine use). The same methodology was used to examine relationships between methamphetamine, ART adherence and unsuppressed HIV viral load at the lower HIV viral load thresholds: 500 copies/mL and 250 copies/mL to assess consistency in the mediation analysis results.
The study was approved by the Hai Phong Medical University and Pharmacy and New York University School of Medicine Institutional Review Boards. STATA 16 was used for statistical analysis.32,33
Results
Demographics, risk behaviors, and methamphetamine use at baseline (cohort entry)
There were three cohort entry points for those who entered the cohort after the RDS survey each year. A total of 792 PWID were included in the final analysis after removing 18 participants who were not present at the end of cohort follow-up at 36 months. The cohort sample was predominately male (94.6%) and had a mean age of 39.5 (SD 8.9). Approximately 10.3% reported depressive symptoms in the last week and 32.4% reported anxiety/nervousness symptoms in the last two weeks. Approximately 85.5% reported injecting 20 + days per month in last 30 days Table 1 provides overall demographic and other factors for the PWID in the analysis at their baseline visit and provides a bivariate analysis by HIV viral load (at 1000 copies/mL threshold).
Table 1.
Demographic characteristics of HIV positive cohort participants in the DRIVE at enrollment Study (N = 792).
| N (%) | VL <1000 copies/mL | VL 1000 copies/mL or higher | p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (mean, SD.) | 39.5 (8.9) | 40.5 (6.2) | 39.4 (7.6) | 0.060 |
| Gender | ||||
| Female | 43 (5.4) | 30 (4.9) | 13 (7.5) | 0.181 |
| Male | 748 (94.6) | 583 (95.1) | 160 (92.5) | |
| Marital status | ||||
| Married | 276 (34.9) | 222 (36.2) | 53 (30.6) | 0.179 |
| Not married | 516 (65.1) | 392 (63.8) | 120 (69.4) | |
| Income | ||||
| Less than/equal to 6 million Vietnamese dong/Year | 527 (66.5) | 417 (67.9) | 108 (62.4) | 0.176 |
| Greater than 6 million Vietnamese dong/Year | 265 (33.5) | 197 (32.1) | 65 (37.6) | |
| Education | ||||
| High school | 163 (20.6) | 132 (21.5) | 31 (17.9) | 0.305 |
| Less than high school | 629 (79.4) | 482 (78.5) | 142 (82.1) | |
| Has current health insurance card | ||||
| Yes | 359 (45.3) | 310 (50.5) | 47 (27.2) | 0.001 |
| No | 433 (54.5) | 304 (49.5) | 126 (72.8) | |
| History of incarceration | ||||
| Yes | 41 (7.8) | 25 (5.9) | 16 (15.8) | 0.001 |
| No | 485 (92.2) | 400 (94.1) | 85 (84.2) | |
| Substance use | ||||
| Days injecting heroin (last 30 days) | ||||
| 1–10 days per month | 41 (5.2) | 35 (5.7) | 6 (2.9) | 0.287 |
| 11–20 days per month | 74 (9.4) | 30 (4.9) | 7 (4.1) | |
| 21–30 days per month | 676 (85.5) | 549 (89.4) | 161 (93.0) | |
| Street methadone use | ||||
| Yes | 265 (33.5) | 215 (35.1) | 49 (28.3) | 0.100 |
| No | 527 (66.5) | 399 (64.9) | 124 (71.7) | |
| Cannabis use | ||||
| Yes | 127 (16.1) | 106 (17.3) | 21 (12.1) | 0.106 |
| No | 660 (83.8) | 508 (82.7) | 152 (87.9) | |
| Methamphetamine use (last 30 days) | ||||
| 0 days per month | 515 (65.4) | 414 (67.4) | 101 (58.4) | 0.049 |
| 1–19 days per month | 238 (30.3) | 177 (28.8) | 61 (35.3) | |
| 20–30 days per month | 34 (4.3) | 23 (3.8) | 11 (6.4) | |
| Alcohol use disorder (AUDIT-C) | ||||
| Yes | 236 (29.8) | 187 (30.5) | 47 (27.2) | 0.396 |
| No | 555 (70.2) | 426 (69.5) | 126 (72.8) | |
| Mental health | ||||
| CES-D score greater than or equal to 6 (in last week) | ||||
| Yes | 82 (10.3) | 62 (10.1) | 19 (10.9) | 0.735 |
| No | 711 (89.7) | 552 (89.9) | 154 (89.0) | |
| PHQ-4 nervousness/Anxiety symptoms (in last two weeks) | ||||
| Yes | 255 (32.4) | 190 (30.9) | 65 (37.6) | 0.100 |
| No | 532 (67.6) | 424 (69.1) | 108 (62.4) |
Methamphetamine use
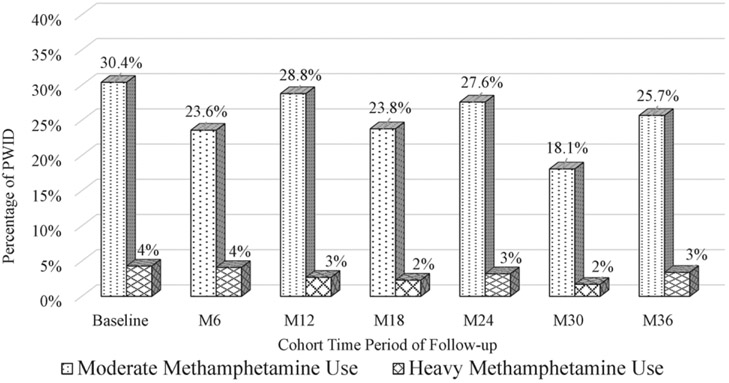
At baseline, methamphetamine use in the last 30 days was as follows: 65.4% reported no use, 30.3% reported intermediate use (defined as 1–19 days in the last 30 days), and 4.3% reported heavy use (defined as 20 + days in the last 30 days). By the end of the follow-up period, 71% reported no use, 25.7% reported intermediate use, and 3.4% reported heavy use. Figure 1 provides methamphetamine frequency by time period for PWID in the cohort.
Figure 1.
Methamphetamine frequency by time period among PWID in Hai Phong Vietnam (N = 792).
ART adherence
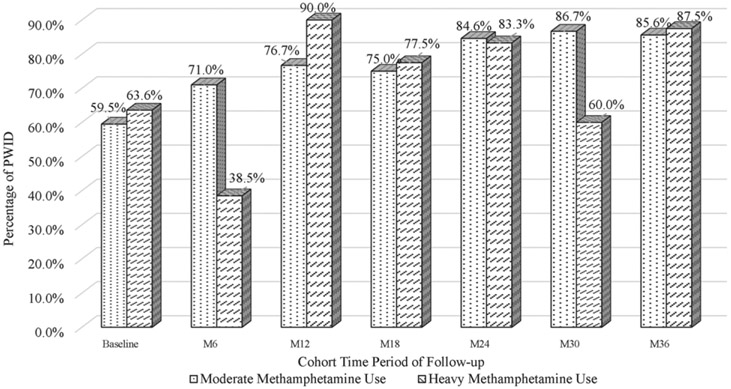
Antiretroviral therapy adherence increased over the cohort study period; PWID reporting high/perfect ART adherence at cohort entry (i.e. at baseline visit in 2016, 2017, 2018) was 69.6% among those with no recent methamphetamine use, 59.5% for those reporting moderate recent methamphetamine use and 63.6% for those reporting heavy methamphetamine use. Among cohort participants present at the final cohort follow-up visit in 2019, perfect/high ART adherence increased to 95.2% among those with no recent methamphetamine use, 85.6% for those reporting moderate recent methamphetamine use and 87.5% for those reporting heavy methamphetamine use. Figure 2 provides ART adherence by time period and by level of methamphetamine use (i.e. moderate and heavy use) for PWID in the cohort.
Figure 2.
High/Perfect ART adherence by time period and by level of methamphetamine use among PWID in Hai Phong Vietnam (N = 792).
HIV viral load
At baseline 81.3% of the participants were virally suppressed. Suppressed HIV viral load increased to approximately 94.2% at the end of follow-up (p < 0.001).
PWID who were virally suppressed at cohort entry (i.e. at baseline visit in 2016, 2017, 2018) was 85% among those with no recent methamphetamine use, 76.4% for those reporting moderate recent methamphetamine use and 66.7% for those reporting heavy methamphetamine use. Among participants present at the final 36 months follow-up visit in 2019, viral suppression increased to 95.5% among those with no recent methamphetamine use, 89.5% for those reporting moderate recent methamphetamine use and 88.9% for those reporting heavy methamphetamine use.
The percentage with perfect/high ART adherence who were virally suppressed increased slightly, from 93.4% at baseline to 96.9% at the end of follow-up (p = 0.157).
Regression and mediation analysis to assess natural direct, indirect and total effects
The unadjusted and adjusted associations between recent methamphetamine use and ART adherence are presented in Table 2. In unadjusted analysis, we found a positive association between recent methamphetamine use and low adherence to ART (OR: 1.81, 95% CI: 1.47, 2.22). Results were similar in multivariable analysis (aOR: 1.92, 95% CI: 1.46, 2.52).
Table 2.
Associations between methamphetamine and ART adherence, ART adherence and viral suppression among HIV positive cohort participants in the DRIVE study (N = 792).
| Exposure outcome model | Odds ratio (95% CI) | Adjusted odds ratio (95% CI) |
|---|---|---|
| Recent methamphetamine and low ART adherence | 1.81 (1.47, 2.22) | 1.92 (1.46, 2.52) |
| High ART adherence and viral suppression | ||
| 1000 copies/mL | 2.72 (2.66, 4.48) | 3.13 (1.75, 5.60) |
| 500 copies/mL | 2.42 (1.50, 3.89) | 2.78 (1.61, 4.81) |
| 250 copies/mL | 2.35 (1.49, 3.71) | 2.83 (1.68, 4.77) |
The unadjusted and adjusted associations between high ART adherence and suppressed HIV viral load are presented in Table 2. In unadjusted analysis, we found a positive association between high adherence to ART and suppressed HIV viral load using <1000 copies/mL cut point (OR: 2.72, 95% CI: 2.66, 4.48). Results were similar in multivariable analysis (adjusted odds ratio (aOR): 3.13, 95% CI: 1.75, 5.60). Similar results were obtained in multivariable analysis examining lower suppressed HIV viral load cut points (<500 copies/mL: aOR: 2.78, 95% CI: 1.61, 4.81; <250 copies/mL: aOR: 2.83, 95% CI: 1.68, 4.77)
The results of the mediation analysis are presented in Table 3. When examining the relationship between methamphetamine and unsuppressed HIV viral load at >1000 copies/mL, the aOR for the association between increasing frequency of methamphetamine use and unsuppressed HIV viral load was 3.94 (95% CI: 1.95, 7.96); the natural direct effect was 2.14 (95% CI: 1.29, 3.55). The proportion mediated by ART was 0.444 (the indirect effect).
Table 3.
Total, direct effect and proportion mediated proportion: multivariable models examining relationship between recent methamphetamine use, ART and HIV viral load among DRIVE cohort participants, 2016–2018 (N = 792).
| Adjusted odds ratio (95% CI) | |
|---|---|
| Viral load measurement level 1000 copies/mL | |
| Total effect | 3.94 (95% CI: 1.95, 7.96) |
| Direct effect | 2.14 (95% CI: 1.29, 3.55) |
| Proportion mediated by ART | 0.444 |
| Viral load measurement level 500 copies/mL | |
| Total effect | 3.32 (95% CI: 1.73, 6.38) |
| Direct effect | 1.92 (95% CI: 1.19, 3.09) |
| Proportion mediated by ART | 0.457 |
| Viral load measurement level 250 copies/mL | |
| Total effect | 3.23 (95% CI: 1.73, 6.02) |
| Direct effect | 1.85 (95% CI: 1.18, 2.91) |
| Proportion mediated by ART | 0.473 |
We also examined the relationship when unsuppressed HIV viral load was defined as >250 copies/mL and >500 copies/mL. The results are also provided in Table 3. For HIV viral load of 500 copies/mL, the aOR for the association between recent methamphetamine use and unsuppressed HIV viral load was 3.32 (95% CI: 1.73, 6.38); the direct effect was 1.92, (95% CI: 1.19, 3.09). The proportion mediated by ART was 0.457.
When unsuppressed HIV viral load was defined as HIV viral load of >250 copies/mL, the aOR for the association between recent methamphetamine use and unsuppressed HIV viral load was 3.23 (95% CI: 1.73, 6.02); the direct effect was 1.79 (95% CI: 1.18, 2.91). The proportion mediated by ART was 0.473. For all three HIV viral load cut points, the associations and proportion mediated remained relatively stable.
Discussion
In this study, we assessed the relationship between recent methamphetamine use and unsuppressed HIV viral load among a cohort of PWID in Hai Phong and consider how self-report ART adherence mediates this direct relationship. Despite increased ART adherence over time, there remained a strong direct relationship between methamphetamine use and unsuppressed HIV viral load, even after examining lower level unsuppressed HIV thresholds. This indicates that while ART attenuates the effect of methamphetamine on HIV viral loads, the use of ART does not eliminate the risk of having an unsuppressed HIV viral load, regardless of frequency of methamphetamine use.
Several previous research studies have highlighted how methamphetamine use can biologically interfere with ART effectiveness and lead to a higher HIV viral load among HIV positive individuals compared to those without recent methamphetamine use.34-37 Studies have also supported models showing that methamphetamine use is associated not only with ART failure38 but also greater proliferation, activation, and exhaustion of CD4+ and CD8+ T-cells.34 While our results are observational, they further advance the possible link between methamphetamine use and ART effectiveness.
The results of this study are concerning given previous campaigns promoting the benefits of ART use and its effectiveness at keeping HIV positive persons virally suppressed, which has been shown to eliminate the risk of transmitting the virus to others (undetectable = untransmittable).10 In cases where individuals use methamphetamine, careful monitoring of HIV viral load and adherence to ART is critical. This can, however, pose a challenge in locations such as Vietnam where PWID may not have the ability to regularly attend clinics for viral load measurements and adjustments to their ART.
Although we observed slight decreases in overall methamphetamine use during follow-up, it should be noted that worsening HIV disease often persists among methamphetamine users, even after discontinuation of drug use. We observed moderate levels of mental health symptoms among our participants; careful monitoring of mental health is therefore crucial to the success of HIV treatment, particularly among those with a history of methamphetamine use.39
There was a slight increase in high/perfect ART adherence among PWID in the DRIVE cohort. However, those with moderate and heavy methamphetamine use consistently scored lower in adherence compared to those reporting no methamphetamine, which is consistent with other studies.39,40 Because lower adherence can be associated with ART resistance,41 it is important that clinicians consider ART regimens that require less frequent doses during the day along with education on the importance of high adherence, to help decrease the chance of developing ART resistance.42
The results of this study are consistent with several related studies examining interactions between methamphetamine use and unsuppressed HIV viral load. A Vancouver study found that methamphetamine use was negatively associated with suppressed HIV viral load among PWID who had recently initiated ART (but did not report specific ART adherence).43 A study among methamphetamine users in Los Angeles recruited through RDS found negative associations with suppressed HIV viral load, after examining several other risk factors including ART use.44 Although these studies did not take place in Vietnam and did not involve mediation analysis to consider ART use, the results provide further evidence to suggest that those who use methamphetamine are likely to have poor outcomes with respect to HIV disease progression compared to those not using methamphetamine.
There are several implications from the results of this study. Interventions aimed at reducing and eliminating methamphetamine use among PWID are critical; pilot studies have shown some moderate success with contingency management approaches,45 but are recognized as labor intensive and unlikely to be successful if methamphetamine use continues to increase among PWID. For those that are successful at discontinuing methamphetamine use, careful monitoring of HIV care is suggested, particularly for those with a history of polysubstance use or psychiatric comorbidities.
Limitations
There are several limitations; ART adherence was based on self-report; there may have been social desirability or recall bias associated with reporting adherence. We tested a small sample of PWID for 3TC to detect ART use; however, we would note that 3TC presence would not necessarily confirm ART adherence for longer recall periods. Our CBOs also worked closely with participants to ensure high levels of ART initiation and adherence, so it would be unlikely that PWID significantly misreported ART adherence. Other studies have highlighted sustained high ART adherence among HIV positive individuals who are monitored by CBOs.46,47 We did have a small number lost to follow-up, which may have biased the overall results.
As we recruited PWID for the cohort study, the results of this analysis may not be generalizable to other populations particularly those without injection drug use histories. Finally, although we did adjust for a robust set of demographic and other risk factors, we cannot rule out residual confounding in the direct and indirect relationships observed between methamphetamine use, ART adherence, and unsuppressed HIV viral load.
Conclusion
In this study we utilized data from a cohort of PWID in Hai Phong Vietnam to assess direct, indirect, and total relationships between methamphetamine, ART adherence, and unsuppressed HIV viral load. The results show that methamphetamine use is an important risk factor for unsuppressed HIV viral load after considering ART adherence. Further research is needed to better understand and consider the biological pathways involved in methamphetamine use on unsuppressed HIV viral load.
Acknowledgements
We would like to thank the community-based organization members in Hai Phong who were vital in the recruitment and retention of participants in the cohort, administering questionnaires to participants in the study, and promoting harm reduction activities and ART initiation and retention for our participants living with HIV in the cohort.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article. Support: This work was supported by grants from NIDA (US) 1R01DA041978 and ANRS (France) 12299. The funding agencies had no role in designing the research, data analyses and preparation of the report.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. The author(s) declare that there is no conflict of interest’.
References
- 1.WHO and UNAIDS. Technical guide for countries to set targets for universal access to HIV prevention, treamtent, and care of injection drug users. Geneva, Switzerland: World Health Organization, 2009. [Google Scholar]
- 2.Ehrenkranz P, Rosen S, Boulle A, et al. The revolving door of HIV care: revising the service delivery cascade to achieve the UNAIDS 95-95-95 goals. PLOS Med 2021; 18(5): e1003651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS. Understanding fast track: accelarating action to end the AIDS epidemic 2030. Geneva Switzerland: Joint United Nations Programme on HIV/AIDS, 2014. [Google Scholar]
- 4.De Cock KM, Crowley SP, Lo Y-R, et al. Preventing HIV transmission with antiretrovirals. Geneva, Switzerland. Bulletin of the World Health Organization, 2009, 87, p. 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu H, Su Y, Zhu L, et al. Effectiveness of ART and condom use for prevention of sexual HIV transmission in sero-discordant couples: a systematic review and meta-analysis. PLoS One 2014; 9(11): e111175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zolopa AR, Andersen J, Komarow L, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One 2009; 4(5): e5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beard J, Feeley F and Rosen S. Economic and quality of life outcomes of antiretroviral therapy for HIV/AIDS in developing countries: a systematic literature review. AIDS Care 2009; 21(11): 1343–1356. [DOI] [PubMed] [Google Scholar]
- 8.Dutra BS, Lédo AP, Lins-Kusterer L, et al. Changes health-related quality of life in HIV-infected patients following initiation of antiretroviral therapy: a longitudinal study. Braz J Infect Dis 2019; 23: 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillo-Mancilla JR, Morrow M, Yap B, et al. Higher ART adherence is associated with lower systemic inflammation in treatment-naive Ugandans who achieve virologic suppression. J Acquired Immune Deficiency Syndromes (1999) 2018; 77(5):507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisinger RW, Dieffenbach CW and Fauci AS. HIV viral load and transmissibility of HIV infection: undetectable equals untransmittable. JAMA 2019; 321(5): 451–452. [DOI] [PubMed] [Google Scholar]
- 11.Ministry of Health Vietnam. Guidelines for HIV/AIDS diagnosis and treatment. Hanoi, Vietnam. Ha Noi: Ministry of Health, 2009. [Google Scholar]
- 12.Feelemyer J, Duong Thi H, Khue Pham M, et al. Increased methamphetamine use among persons who inject drugs in Hai Phong, Vietnam, and the association with injection and sexual risk behaviors. J Psychoactive Drugs 2018; 50(5): 382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Des Jarlais DC, Feelemyer J, Arasteh K, et al. The methamphetamine epidemic among persons who inject heroin in Hai Phong, Vietnam. J Substance Abuse Treat 2021; 126: 108320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai H-H, Kuo Y-C, Kuo C-J, et al. Methamphetamine use associated with non-adherence to antiretroviral treatment in men who have sex with men. Scientific Rep 2020; 10(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carrico AW, Johnson MO, Colfax GN, et al. Affective correlates of stimulant use and adherence to anti-retroviral therapy among HIV-positive methamphetamine users. AIDS Behav 2010; 14(4): 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosen M, Black A, Arnsten J, et al. Association between use of specific drugs and antiretroviral adherence: findings from MACH 14. AIDS Behav 2013; 17(1): 142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Des Jarlais DC. NCT03526939: DRug use and infections in hai phong Vietnam among persons who inject drugs (DRIVE). Bethesda Maryland: National Library of Medicine (NLM), 2018. [Google Scholar]
- 18.McKetin R, Hickey K, Devlin K, et al. The risk of psychotic symptoms associated with recreational methamphetamine use. Drug Alcohol Rev 2010; 29(4): 358–363. [DOI] [PubMed] [Google Scholar]
- 19.King C, Bart G, Kunkel L, et al. HIV care continuum characteristics among people with opioid use disorder and HIV in Vietnam: baseline results from the BRAVO study. BMC Public Health 2020; 20(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simoni JM, Kurth AE, Pearson CR, et al. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS Behav 2006; 10(3): 227–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glasner-Edwards S, Marinelli-Casey P, Hillhouse M, et al. Depression among methamphetamine users: association with outcomes from the methamphetamine treatment project at 3 year follow-up. J Nervous Ment Dis 2009; 197(4): 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uthman OA, Magidson JF, Safren SA, et al. Depression and adherence to antiretroviral therapy in low-, middle-and high-income countries: a systematic review and meta-analysis. Curr HIV/AIDS Rep 2014; 11(3): 291–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartzell JD, Janke IE and Weintrob AC. Impact of depression on HIV outcomes in the HAART era. J Antimicrob Chemother 2008; 62(2): 246–255. [DOI] [PubMed] [Google Scholar]
- 24.Lohse N, Hansen A-BE, Jensen-Fangel S, et al. Demographics of HIV-1 infection in denmark: results from the danish HIV cohort study. Scand J Infect Dis 2005; 37(5): 338–343. [DOI] [PubMed] [Google Scholar]
- 25.Wu ES, Metzger DS, Lynch KG, et al. Association between alcohol use and HIV viral load. J Acquired Immune Deficiency Syndromes 1999 2011; 56(5): e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morojele NK, Kekwaletswe CT and Nkosi S. Associations between alcohol use, other psychosocial factors, structural factors and antiretroviral therapy (ART) adherence among South African ART recipients. AIDS Behav 2014; 18(3): 519–524. [DOI] [PubMed] [Google Scholar]
- 27.Saunders JB, Aasland OG, Babor TF, et al. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction 1993; 88(6): 791–804. [DOI] [PubMed] [Google Scholar]
- 28.Zauszniewski JA and Graham GC. Comparison of short scales to measure depressive symptoms in elders with diabetes. West J Nurs Res 2009; 31(2): 219–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Renovanz M, Soebianto S, Tsakmaklis H, et al. Evaluation of the psychological burden during the early disease trajectory in patients with intracranial tumors by the ultra-brief Patient Health Questionnaire for Depression and Anxiety (PHQ-4). Supportive care in cancer. Official Journal Multinational Assoc Support Care Cancer 2019; 27(12): 4469–4477. [DOI] [PubMed] [Google Scholar]
- 30.VanderWeele TJ. Mediation analysis: a practitioner’s guide. Annu Rev Public Health 2016; 37: 17–32. [DOI] [PubMed] [Google Scholar]
- 31.Stata StataCorp. Manual [SEM] example 42g—one and two-level mediation models (Multilevel): two-level model with GSEM. College Station, Texas: StataCorp, 2017. [Google Scholar]
- 32.Dil E and Karasoy D. GSEM: a Stata command for parametric joint modelling of longitudinal and accelerated failure time models. Comp Methods Programs Biomed 2020; 196: 105612. [DOI] [PubMed] [Google Scholar]
- 33.StataCorp. Stata 16. College Station, TX: STATA Corp, 2019. [Google Scholar]
- 34.Massanella M, Gianella S, Schrier R, et al. Methamphetamine use in HIV-infected individuals affects T-cell function and viral outcome during suppressive antiretroviral therapy. Scientific Rep 2015; 5: 13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lane HC. Pathogenesis of HIV infection: total CD4+ T-cell pool, immune activation, and inflammation. Top HIV Med 2010; 18(1): 2–6. [PubMed] [Google Scholar]
- 36.Mantri CK, Mantri JV, Pandhare J, et al. Methamphetamine inhibits HIV-1 replication in CD4+ T cells by modulating anti-HIV-1 miRNA expression. Am J Pathol 2014; 184(1): 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X and Ho WZ. Drugs of abuse and HIV infection/replication: implications for mother-fetus transmission. Life Sci 2011; 88(21–22): 972–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar S, Rao PS, Earla R, et al. Drug-drug interactions between anti-retroviral therapies and drugs of abuse in HIV systems. Expert Opin Drug Metab Toxicol 2015; 11(3): 343–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore DJ, Blackstone K, Woods SP, et al. Methamphetamine use and neuropsychiatric factors are associated with anti-retroviral non-adherence. AIDS Care 2012; 24(12): 1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai H-H, Kuo Y-C, Kuo C-J, et al. Methamphetamine use associated with non-adherence to antiretroviral treatment in men who have sex with men. Scientific Rep 2020; 10(1): 7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sethi AK, Celentano DD, Gange SJ, et al. Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clin Infect Dis 2003; 37(8): 1112–1118. [DOI] [PubMed] [Google Scholar]
- 42.Stone VE, Hogan JW, Schuman P, et al. Antiretroviral regimen complexity, self-reported adherence, and HIV patients’ understanding of their regimens: survey of women in the her study. JAIDS J Acquired Immune Deficiency Syndromes 2001; 28(2): 124–131. [DOI] [PubMed] [Google Scholar]
- 43.Fairbairn N, Kerr T, Milloy M-J, et al. Crystal methamphetamine injection predicts slower HIV RNA suppression among injection drug users. Addict Behaviors 2011; 36(7): 762–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodman-Meza D, Shoptaw S, Weiss RE, et al. Methamphetamine use drives decreases in viral suppression for people living with HIV released from a large municipal jail: results of the LINK LA clinical trial. Drug and Alcohol Dependence 2019; 202: 178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen TT, Shoptaw S and Giang LM, (eds). Feasibility and outcomes of evidence-based interventions to reduce methamphetamine use among patients on MMT in Hanoi. San Antonio, TX: Vietnam. CPDD, 2019. [Google Scholar]
- 46.Mukherjee JS, Barry D, Weatherford RD, et al. Community-based ART programs: sustaining adherence and follow-up. Curr HIV/AIDS Rep 2016; 13(6): 359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tun W, Apicella L, Casalini C, et al. Community-based antiretroviral therapy (ART) delivery for female sex workers in Tanzania: 6 month ART initiation and adherence. AIDS Behav 2019; 23(2): 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]