Abstract
Juvenile spondyloarthropathies (JSpA) are defined as a heterogeneous group of diseases that start before the age of 16, which is associated with peripheral joint (especially large joints of the lower limbs) and axial skeletal (spine and sacroiliac joint) involvement, enthesitis, and human leukocyte antigen (HLA) B27 positivity. Juvenile spondyloarthropathies mainly cover juvenile ankylosing spondylitis (JAS), psoriatic arthritis, reactive arthritis, inflammatory bowel disease-associated arthritis, seronegative enthesopathy, arthropathy syndrome (SEA), and enthesitis-associated arthritis. Symptoms associated with spondyloarthropathies are enthesitis, inflammatory low back pain, dactylitis, nail changes, psoriasis, acute anterior uveitis, and inflammatory bowel disease-related symptoms. In JSpA, axial involvement is rarely seen in the early stages of the disease, in contrast to adult patients with ankylosing spondylitis (AS). The disease usually begins as asymmetric oligoarthritis of lower extremities in children, and axial skeletal involvement can occur in the course of the disease. Although the debate on the classification of juvenile spondyloarthropathies continues due to its initial nonspecific findings and the heterogeneity of the disease phenotype, the International League of Associations Rheumatology (ILAR) classification criteria are the most commonly used pediatric criteria. In that set of criteria, patients with JSpA are mainly classified under enthesitis-related arthritis or psoriatic arthritis group. Since juvenile spondyloarthropathies can cause severe loss of function and long-term sequelae, the main goal in treatment should be suppression of inflammation as early as possible and prevent sequelae.
Keywords: Juvenile, spondyloarthropaty, enthesitis-related arthritis, psoriatic arthritis
Introduction
Juvenile spondyloarthropathies (JSpA) is defined as a heterogeneous group of diseases that start before the age of 16, which is associated with peripheral joint (especially large joints of the lower limbs) and axial skeletal (spine and sacroiliac joint) involvement, enthesitis, and human leukocyte antigen (HLA) B27 positivity.1 In JSpA, axial joint involvement is rarely seen in the early stages of the disease, in contrast to adult patients with ankylosing spondylitis (AS). The disease usually begins as asymmetric oligoarthritis in children, and enthesitis and axial skeletal involvement can occur in the course of the disease.2 Although “spondyloarthropathies” in adult patients is well defined, the terminology and classification of JSpA is still an important controversy in childhood.3 Although JSpA is distinguished from other causes of chronic arthritis in childhood by basic features such as being seronegative, involvement of the spine and sacroiliac joint, and presence of extraarticular manifestations such as psoriatic skin rash and symptoms of inflammatory bowel disease, they are classified under juvenile idiopathic arthritis (JIA).4
In this review, the definition, etiopathogenesis, genetic background, classification criteria, clinical findings, and treatment of the disease will be discussed in the light of current literature.
Definition
The term of spondyloarthropathies is an umbrella definition that includes heterogeneous inflammatory diseases associated with HLA B27, in which axial skeletal and sacroiliac joint involvement can be seen.5 Symptoms associated with spondyloarthropathies are enthesitis, inflammatory low back pain, dactylitis, nail changes, psoriasis, acute anterior uveitis, and symptoms of inflammatory bowel disease.5 Ankylosing spondylitis (AS), the well-defined and widely known form of spondyloarthropathies, is characterized by inflammatory low back pain and progressive involvement of axial skeleton. It has been reported that 10-20% of the patients diagnosed with ankylosing spondylitis developed their initial symptoms before 16 years of age.5
Juvenile spondyloarthropathies include a group of seronegative diseases associated with HLA B27, which are more common in males. There are differentiated and undifferentiated forms of the disease. While differentiated forms are juvenile ankylosing spondylitis (JAS), psoriatic arthritis, reactive arthritis, inflammatory bowel disease-associated arthritis, the undifferentiated types are seronegative enthesopathy and arthropathy syndrome (SEA) and enthesitis-associated arthritis.6 The signs and symptoms associated with spondylitis and the forms of JSpA are summarized in Table 1.
Table 1.
The signs and symptoms associated with spondylitis and the forms of juvenile spondyloarthropathies.
| Clinical phenotypes of juvenile spondyloarthropathies | Clinical findings |
|---|---|
| • Juvenile ankylosing spondylitis | • Enthesis |
| • Juvenile psoriatic arthritis | • Inflammatory low back pain |
| • Reactive arthritis | • Psoriatic lesions |
| • Inflammatory bowel diseases-related arthritis | • Inflammatory bowel diseases-related symptoms |
| • Enthesitis related arthritis | • Dactylitis |
| • Seronegative enthesopathy and arthropathy syndrome | • Nail changes • Acute anterior uveitis |
Classification Criteria
Several diagnostic and/or classification criteria have been proposed for both juvenile-onset and adult-onset spondyloarthropathies. Modified New York criteria which is most widely used criteria for ankylosing spondylitis were proposed in 1984.7 In order to classify adult patients as ankylosing spondylitis according to these criteria; sacroiliitis should be demonstrated radiologically and it should be accompanied by at least one of the clinical findings related to axial skeleton involvement, such as inflammatory back pain, limitation of spine mobility, and chest expansion.7 The absence of sacroiliac joint involvement in the early stages of juvenile cases and the rarity of inflammatory low back pain in the majority of patients with JSpA have limited the applicability of the modified New York criteria for pediatric cases.
The concept of “undifferentiated spondylitis” has been brought to the agenda because most adult patients with spondylitis-related symptoms summarized in Table 1 are not classified as spondylitis according to the modified New York criteria due to the absence of axial skeleton and sacroiliac joint involvement. In the following years, European Spondyloarthropathy Study Group criteria (ESSG) and Amor criteria were proposed, covering undifferentiated cases.8,9 The absence of any lower age limit in both of these criteria and the fact that criteria related to axial joint involvement were not mandatory enabled these criteria to be used for pediatric cases as well. Kasapçopur et al.10 reported that the sensitivity and specificity for ESSG criteria in pediatric cases were 83.9% and 87.5%, and for Amor criteria were 82.3% and 95.3%, respectively.
Main Points
The term of spondyloarthropathies is an umbrella definition that includes heterogeneous inflammatory diseases associated with HLA B27, in which axial skeletal and sacroiliac joint involvement can be seen.
Juvenile spondyloarthropathies differ from spondyloarthropathies in adult patients not only by their early onset but also by the distribution and course of clinical findings.
The disease usually begins as asymmetric oligoarthritis in children and axial skeletal involvement can occur in the course of the disease.
The main clinical findings of the disease are arthritis of the lower extremities or hip, enthesitis, inflammatory back pain, psoriatic lesions, dactylitis, and uveitis.
Spondyloarthropathies should be suspected in the presence of lower extremity arthritis and enthesitis or inflammatory back pain, especially in a male adolescent.
In later studies, it was shown that when tumor necrosis alpha antagonists were initiated after the patients met the modified New York criteria, radiological progression could not be prevented. Further observations showed that the majority of “undifferentiated SpA” cases classified according to ESSG or Amor criteria could not meet the diagnostic criteria for AS even after many years and undifferentiated SpA cases are not a definite early form for AS. These new findings revealed the need for new criteria to detect early radiological axial disease.5 From this point of view, two new sets of criteria, axial SpA and peripheral SpA, were proposed by ASAS (Assessment of Spondyloarthritis International Society) in 2009.11,12 Since the most important findings suggested by these criteria for detecting preradiological disease are inflammatory low back pain and sacroiliitis on magnetic resonance imaging which are rarely seen in JSpA, the use of also these criteria in children seems to be limited.13
The debate on the classification of juvenile spondyloarthropathies continues due to its nonspecific initial findings and the heterogeneity of the disease phenotype. The main classification criteria proposed for JSpA are summarized in Table 2.4,14-18 In pediatric cases, the sensitivity and specificity were reported as 62.9% and 92.2% for seronegative enthesopathy and arthropathy (SEA) criteria; 95.2% and 78.1% for Garmisch–Partenkirschen criteria; and 61.3% and 98.4% for atypical spondyloarthropathy criteria, respectively.6,10,14,15 The International League of Associations Rheumatology (ILAR) JIA classification criteria are the most commonly used pediatric criteria.4 In these classification criteria, patients with JSpA are mainly classified as enthesitis-associated arthritis or psoriatic arthritis. Although these criteria are the most frequently used classification criteria by pediatric rheumatologists, their adequacy in the classification of juvenile spondyloarthropathies is still a matter of debate. The most important criticism about these criteria is that it does not include inflammatory bowel disease-related arthritis, juvenile ankylosing spondylitis, and Reiter's syndrome, which are well-defined forms of JSpA. Another important criticism is that many patients with JSpA have to be classified under the undifferentiated arthritis group due to the exclusion criteria of enthesitis-related arthritis (for example, first-degree relative with psoriasis) or meeting more than one classification criteria of JIA subtypes.19
Table 2.
Classification criteria recommended for juvenile spondyloarthropathies.
| Seronegative enthesopathy and arthropathy (SEA) criteria14 | |
|---|---|
| • Beginning of musculoskeletal symptoms before the age of 17 | |
| • Rheumatoid factor and antinuclear antibody negativity | |
| • Enthesopathy | |
| • Arthralgia or arthritis | |
| Garmisch–Partenkirschen criteria15 | |
| • Major: Asymmetric oligoarthritis involving the hip, knee and ankle; enthesopathy, lumbar spine or sacroiliac pain; acute iridocyclitis | |
| • Minor: ≥ 5 peripheral joint involvement, male gender, onset of symptoms after age 6, HLA-B27 positivity, family history of spondyloarthropathy (or suspicion) | |
| 2 major or 1 major + 2 minor criteria are required for diagnosis. | |
| Atypical spondyloarthropathy criteria in children16 | |
| • Major: Family history for spondylitis, enthesopathy, arthritis of the finger joints, sacroiliitis, HLA-B27 positivity, frequent development of arthritis or arthralgia | |
| • Minor: The onset of the disease after the age of 10, male gender, lower extremity involvement, acute iridocyclitis or conjunctivitis, hip joint involvement, development after enteritis | |
| Four major or three major + three minor criteria are required for diagnosis. | |
| International League of Associations Rheumatology (ILAR) classification criteria4 | |
| Arthritis of unknown etiology | |
| Disease onset before the age of 16 | |
| Continuation of symptoms from ≥6 weeks | |
| Enthesitis-related arthritis | Psoriatic arthritis |
| • Arthritis and enthesitis or | • Arthritis and psoriasis |
| • With arthritis or enthesitis at least two of the following: | • With arthritis, at least two of them: |
| • Sacroiliac joint tenderness and / or inflammatory spinal pain | ○ Dactylitis, |
| • HLA B27 positivity | ○ Thimble nail or onycholysis, |
| • HLA B27-associated disease in 1st degree relatives | ○ Presence of psoriasis in a first degree relative |
| • Arthritis, uveitis in men ≥6 years old (Exclusion criteria: Psoriasis, RF positivity, presence of systemic arthritis in a first degree relative, arthritis conforming to other subtypes of JIA) | (Exclusion criteria: HLA-B27-positive arthritis in male after 6 years of age, ankylosing spondylitis in 1st degree relative, enthesitis-related arthritis, inflammatory bowel disease with sacroiliitis, presence or history of reactive arthritis or acute anterior uveitis, rheumatoid factor positivity, presence of systemic arthritis, arthritis that fits other subtypes of JIA) |
| Cerrahpas ¸ a 2016 criteria17 | |
| • Major criteria: Oligoarthritis, enthesopathy, disease onset after 6 years of age, inflammatory lumbar pain | |
| • Minor criteria: Hip arthritis, tarsometatarsal arthritis, male sex, NSAID response, sacroiliitis (in MR or radiography), HLA B27 positivity, limitation in Schober test (<4 cm), family history of SpA group of disease, dactylitis, psoriasis, or presence of IBD | |
| There should be three major or two major and three minor criteria for diagnosis. | |
| PRINTO (Pediatric Rheumatology International Trials Organization) criteria (first edition)18 | |
| Arthritis of unknown etiology | |
| The onset of illness before the age of 18 | |
| Continuation of symptoms from ≥6 weeks | |
| • Peripheral arthritis and enthesitis or | |
| • Inflammatory back pain lasting more than 3 months with arthritis or enthesitis and sacroiliitis detected on imaging or | |
| ○ With arthritis or enthesitis, either two of the following: | |
| ○ 1) Sacroiliac joint tenderness | |
| ○ 2) Inflammatory back pain | |
| ○ 3) HLA-B27 positivity | |
| ○ 4) Acute anterior uveitis | |
| ○ 5) History of spondyloarthropathy in 10 relatives | |
In 2014, our group has proposed a new set of classification criteria for JSpA.20 In this set of criteria, while oligoarthritis, enthesopathy, disease onset after six years of age, and inflammatory lumbar pain are considered as major criteria, hip arthritis, tarsometatarsal arthritis, male sex, good response to nonsteroidal anti-inflammatory drugs, sacroiliitis (in MR or radiography), HLA B27 positivity, limitation in Schober test (<4 cm), family history of SpA group of disease, dactylitis, psoriasis, or presence of IBD are accepted as the minor criteria. According to these criteria, three major or two major and three minor criteria are needed for the diagnosis. Adrovic et al.17 reported that the sensitivity and specificity of these criteria were 90.3% and 90.7%. In another study, it is shown that this set of criteria is applicable in the classification of spondyloarthropathies in children with familial Mediterranean fever (FMF).21
Pediatric Rheumatology International Trials Organization (PRINTO) proposed new classification criteria for JIA in 2019.18 These criteria differ from the ILAR classification criteria on some important points. One of the important differences is that the JIA upper age limit, which is 16 years in ILAR criteria, is increased to 18 years in PRINTO criteria. In addition, psoriatic arthritis is not included in PRINTO criteria as a separate group. Moreover, the group called enthesitis-related arthritis (ERA) in ILAR criteria was changed as enthesitis/spondylitis-related arthritis (ESRA) in PRINTO criteria, and sacroiliitis detected on imaging, which was not present in the previous criteria, was also accepted as a criterion.18 It should be noted that these criteria are still in the development phase.
Epidemiology
The epidemiological data about JSpA are unclear, as the prevalence of the disease varies between geographical regions and there is heterogeneity in the classification criteria used between studies. In a systemic review conducted in 2013, the incidence and prevalence of JSpA (excluding psoriatic form) in Europe were reported as 2 (1.8-2.1)/100,000 and 4.9 (4.4-5.6)/100,000, respectively.22 In another study, the estimated prevalence of juvenile spondylitis (enthesitis-associated arthritis and psoriatic arthritis combined) worldwide was reported as 0.28-88/100,000.5 While oligoarticular JIA is the most common JIA subtype in Europe and North America, in some Asian countries it has been reported that the most common JIA subtype is enthesitis-related arthritis.23-25 The frequency of ERA among patients with juvenile idiopathic arthritis is stated as 13.4% in Canada and Poland, 18.9-21.7% in Turkey, 24.8% in Thailand, and 37.4% in Taiwan.26,27 These reports suggest that the disease is more common in developing countries.
It has been shown that 10%–20% of adult AS patients have juvenile onset.5,28 In the study of Saurenmann et al.,29 the mean age of onset was 11.7 years for ERA and 8.9 years for psoriatic arthritis.30 Similarly, Adrovic et al.1 reported the mean age of disease onset as 11.4 years and the mean age at diagnosis as 12.4 years in their JSpA cohorts.
Etiopathogenesis and Genetic Background
Although the etiopathogenesis of spondyloarthropathies has not been fully elucidated yet, with current knowledge, it is thought that the inclusion of some environmental variables in the presence of genetic predisposing factors triggers to the development of the disease.
The most important environmental factor suggested to be associated with the disease is infection. Studies showing that spondylitis develops in HLA B27-positive transgenic mice only in the presence of intestinal bacteria indicate the role of the microbial environment in the etiopathogenesis of the disease.30-32 It was also reported that HLA B27-positive patients may have defects in clearance of intracellular microorganisms.33,34 Ekman et al.33 reported that salmonella species were killed more difficultly by HLA B27-positive individuals. In addition, affected fecal bacterial microbiota and decreased bacterial diversity have been shown in patients with enthesitis-related arthritis.30,35,36 In another study, decreased number of Faecalibacterium prausnitzii and Bacteroides fragilis species which have anti-inflammatory activity was reported in fecal microbiota of patients with enthesitis-related arthritis.37
The most important genetic factor associated with spondylitis is HLA B27 positivity, a member of the HLA I class. HLA B27 positivity varies between 60 and 90% in juvenile spondyloarthropathies and varies according to the clinical phenotypes of the disease.38,39 HLA B27 positivity was reported as 90% in juvenile ankylosing spondylitis, 72% in SEA, 15% in psoriatic arthritis, and 15% in the remaining JIA except spondyloarthropathies and 5-10% in a healthy population.38,40 In a study conducted in Turkey, HLA-B27 positivity was found to be 7% in healthy subjects.1 The fact that only a small proportion (approximately 5%) of HLA B27-positive healthy individuals develops ankylosing spondylitis suggests that different genes may also play role in the development of the disease.41 Many genes have been identified in genome-wide association studies (GWAS) performed in adult patients with ankylosing spondylitis. ERAP-1, IL23R, TNFRSF1A, IL1A, CARD, and ANTXR2 are the most frequently studied genes related spondyloarthropathies.41 It is known that ERAP-1 has a role in the regulation and folding of antigens to be presented to T cells by MHC I alleles. It has been shown that there is an epistatic interaction between ERAP-1 and HLA B27, and it has been shown that misfolding in HLA B27 causes stress in peroxisomes, leading to an increase in IL23/IL17 levels, which play a role in disease pathogenesis.42 The fact that the IL23R gene also poses a risk for psoriasis and inflammatory bowel disease indicates the common genetic background of the diseases under the spondyloarthropathy group.41
In addition to aforementioned genes, Gülhan et al.43 showed that the Mediterranean fever MEFV gene mutations may predispose for enthesitis related arthritis especially in the eastern Mediterranean. Similarly, Tufan et al.44 found increased frequency of enthesopathy in adult patients with FMF carrying M694V mutation. In another study, it has been reported that the frequency of MEFV mutations among patients with SpA is higher than in healthy controls (32% vs 15%).45 Ozer et al.21 reported that 10.2% (33/323) of their patients with FMF have also JSpA. In a prospective meta-analysis, the association between interleukin 1 gene complex polymorphisms and ankylosing spondylitis has been highlighted.46 Since most of the MEFV mutations cause the increased IL-1 levels, the relationship between MEFV mutations and JSpA/AS may be related to IL-1-associated pathways.43 It should be noted that the relationship between FMF and JSpA has not been enlightened yet and further studies are apparently needed for making solid conclusion.
Main Differences between Juvenile-Onset and Adult-Onset Spondyloarthropathies
Juvenile spondyloarthropathies differ from spondyloarthropathies in adult patients not only by their early onset, but also by the distribution and course of clinical findings.47 Unlike adult patients, in the early stages of JSpA, while inflammatory back pain, axial skeleton, and sacroiliac joint involvement are rare, hip and/or peripheral joint involvement and enthesitis are more common. In addition, pediatric cases may experience difficulties in expressing symptoms, which may delay the diagnosis. Furthermore, Weiss et al.48 showed in their study that no physical examination maneuver was effective in detecting sacroiliitis in pediatric patients. Although magnetic resonance imaging has been shown to be effective in detecting sacroiliitis, physiological bone marrow changes in pediatric cases may be mistakenly evaluated as sacroiliitis.49 In addition, cardiac and valvular findings, which are frequently reported in adult patients, are reported much less frequently in juvenile cases. In a recently published study, subclinical increased aortic stiffness was reported in patients with JSpA with advanced cardiological methods.5,38,50,51
Clinical Findings
Juvenile spondyloarthropathies have usually insidious onset and present with various combinations of clinical findings like arthritis of lower extremities or hip, enthesitis, inflammatory back pain, psoriatic lesions, dactylitis, and uveitis. Spondyloarthropathies should be suspected in the presence of lower extremity arthritis and enthesitis or inflammatory back pain, especially in a male adolescent.30 However, it should be kept in mind that inflammatory low back pain is less common in children than in adults.30,52 In a study assessing the sensitivity and specificity of various clinical and laboratory findings for juvenile spondyloarthropathies, the most sensitive clinical findings were reported as asymmetric arthritis or lower extremity arthritis, enthesitis, HLA B27 positivity, and rapid response to nonsteroidal drugs (NSAIDs). The same study also stated that inflammatory lumbosacral pain, which is the most sensitive clinical finding for adult patients with spondylitis, has lower sensitivity in children.38 Another critical point to note is that psoriatic plaques or inflammatory bowel disease-related symptoms may develop later than arthritis, and exact clinical classification may not be possible in the early stages of the disease, especially in children.
Articular findings
Unlike the higher frequency of axial skeleton involvement in adult patients with ankylosing spondylitis, JSpA is most commonly presented by asymmetric involvement of the lower extremity joints and hip. Among lower extremity joints, knee, tibiotalar joint, and metatarsal joints are commonly effected ones.30 Tarsitis which is characterized by inflammation of the intertarsal joints, surrounding ligaments and soft tissue, is an unique finding of JSpA (Figure 1).6,53 Adrovic et al.1 reported that 77.6% of their patients with JSpA were initially presented with lower extremity arthritis and 58.9% of them had hip arthritis at the time of diagnosis. Dactylitis, inflammation of interphalangeal joints and surrounding ligaments and soft tissue of a finger, is a hallmark clinical finding of psoriatic arthritis.1
Figure 1.
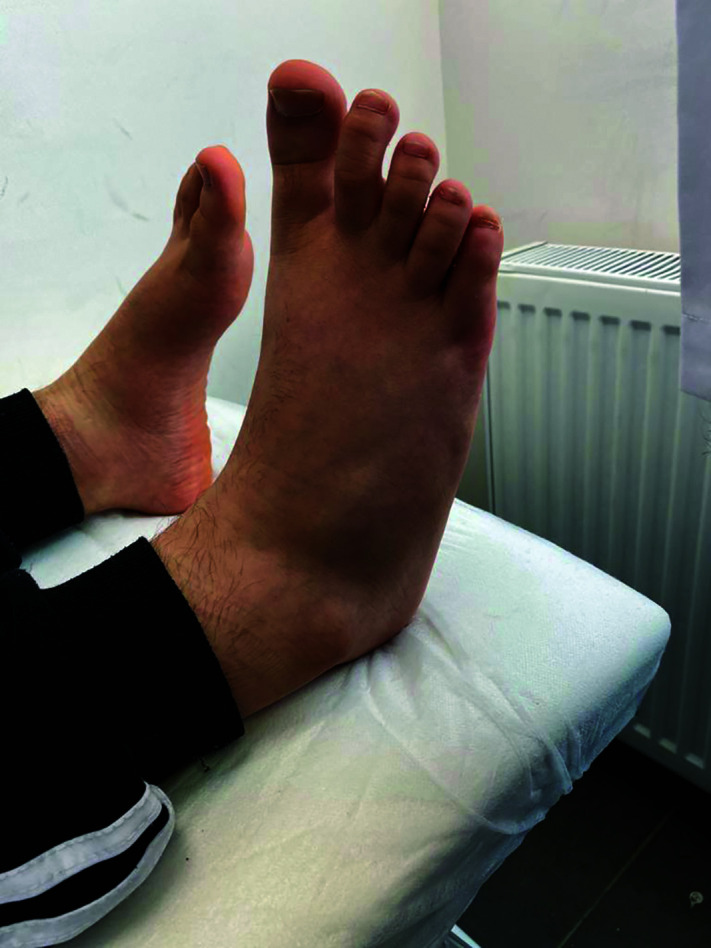
Tarsitis detected in an HLA B27-positive patient.
The frequency of axial skeleton involvement has been reported to be 28-48.6% in children with JSpA.1,30 Sacroiliitis should be considered in patients with chronic inflammatory back and low back pain, hip pain, morning stiffness, high CRP with HLA B27 positivity, sacroiliac joint tenderness on palpation, and positive flexion, abduction, and external rotation (FABER) test.30
Enthesitis is the inflammation at sites where tendons and ligaments attach to the bones (Figure 2). Although it can be seen in all JIA subtypes, it is observed much more frequently in JSpA and is accepted as one of the most important findings of JSpA. In juvenile spondyloarthropathies, enthesitis tends to be symmetrical, persistent, and affects multiple ligaments.30 In a study conducted by Rumsey et al.,54 it was reported that enthesitis was detected in 16% of their patients with JIA, and only two-third of them met the ERA criteria. The frequency of enthesitis in patients with JSpA has been reported to be 66-75%.1,30,54,55
Figure 2.
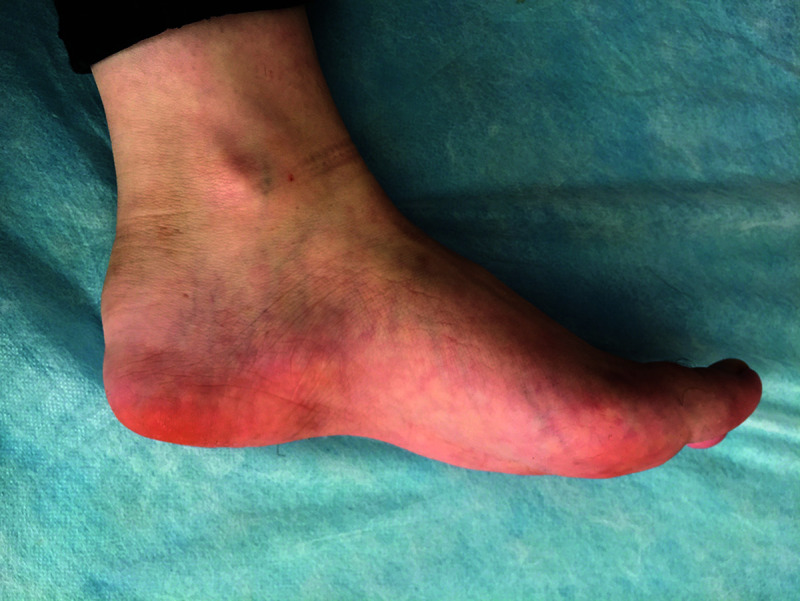
Swelling and edema consistent with enthesitis in the adhesion area of the Achilles tendon to the calcaneus.
Extra-Articular Findings
Extra-articular findings of juvenile spondyloarthropathies can be mentioned under the headings of uveitis, skin findings, gastrointestinal, and cardiovascular system findings.
Uveitis is one of the most important extra-articular findings of JSpA. Apart from uveitis detected in other JIA subtypes, uveitis in JSpA is acute-onset and symptomatic. The frequency of uveitis in JSpA is reported to be 4.5-10.1%.1,55
Skin findings are another important extra-articular finding of the disease. Erythematous, plaque-like and scaling psoriatic rash is the most prominent skin finding in patients with psoriatic arthritis. In addition, onycholysis and pitting on nails can be detected in patients with psoriasis (Figure 3).1 Erythema nodosum and more rarely pyoderma gangrenosum can be seen in juvenile spondyloarthropathies associated with inflammatory bowel diseases.30
Figure 3.
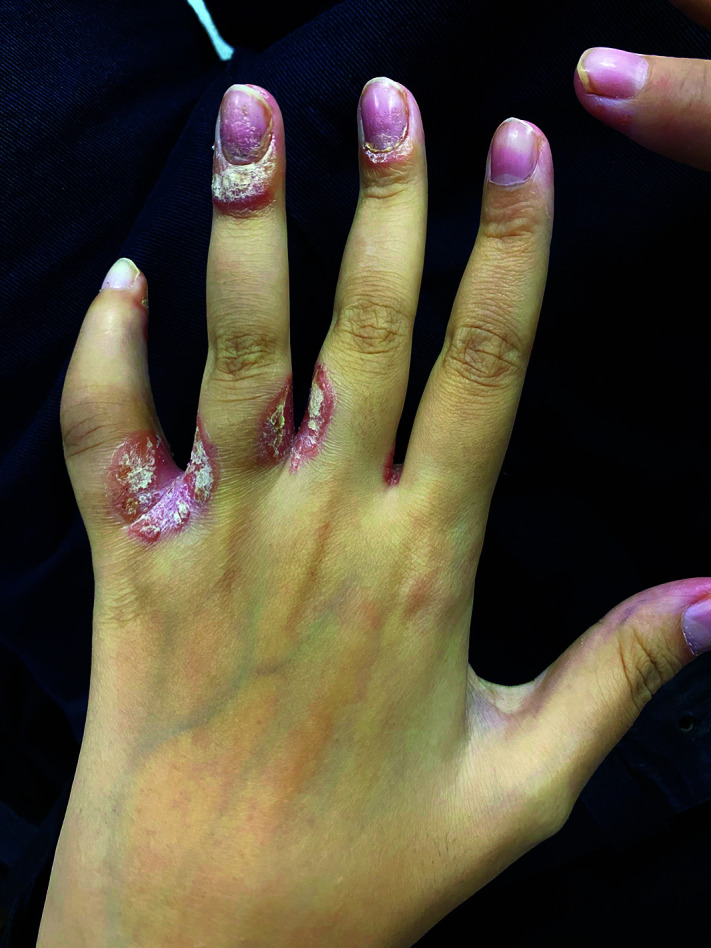
Combination of psoriatic rash, dactylitis, and nail changes in a patient with psoriatic arthritis.
Gastrointestinal system involvement may be symptomatic or asymptomatic.6 Clinical findings suggestive of intestinal inflammation are weight loss, growth retardation, abdominal pain, persistent diarrhea, and bloody stools.30
Valvular diseases reported in adult spondylitis are not expected findings in JSpA. There are rare studies reporting subclinical increased aortic stiffness in juvenile spondylitis.51
Treatment
Since juvenile spondyloarthropathies can cause serious loss of function and long-term sequelae, the main goal in treatment should be suppression of inflammation as early as possible and prevent sequelae. As with all other forms of juvenile idiopathic arthritis, JSpA should be managed with a multidisciplinary approach with the active participation of pediatric rheumatologists, physiotherapists, ophthalmologists, orthopedists, child psychiatrists, patients, and their legal representatives.
The first step in the treatment of juvenile spondyloarthropathies is nonsteroidal anti-inflammatory drugs (NSAIDs).56 NSAIDs are useful in suppressing inflammation and relieving symptoms. The most preferred NSAIDs for pediatric cases are ibuprofen, indomethacin, and naproxen sodium.1 In the American College of Rheumatology (ACR) JIA treatment guideline published in 2019, it is recommended to start treatment with NSAIDs as monotherapy in patients with enthesitis or sacroiliitis.56 Although nonsteroidal anti-inflammatory drugs are quite effective in relieving symptoms in most patients with JSpA, some authors recommend their use as bridge therapy or complementary therapy rather than as long-term monotherapy due to concern that they would not prevent the long-term damage of the disease.57
Although corticosteroids have very strong anti-inflammatory effects, their systemic and long-term use should be avoided as much as possible due to their severe side effects. Systemic steroid therapy is recommended to be used as bridge therapy until the effects of disease-modifying drugs (DMARDs) begin. Intraarticular steroid injections are also recommended as complementary therapy, especially in patients with oligoarticular pattern. In the ACR JIA treatment guideline, the use of triamcinolone hexacetonide for intraarticular injections is recommended.56
The main conventional DMARDs (cDMARDs) used in the management of juvenile spondyloarthropathies are methotrexate and sulfasalazine.1 Although methotrexate is the most commonly used agent in JIA treatment, its effectiveness in axial SpA is controversial.57,58 In an analysis from Cochrane investigating the efficacy of methotrexate in ankylosing spondylitis, it was reported that there is no evidence of methotrexate efficacy in adult patients with AS.59 Therefore, methotrexate is mainly recommended for patients with peripheral joint involvement without axial joint involvement in JSpA.58 In ACR JIA treatment guideline, the authors strongly recommended against using methotrexate as monotherapy in patients with sacroiliitis.56 Sulfasalazine is another conventional DMARD that can be used in the treatment of JSpA.58 It can be used as an alternative to methotrexate in cases with JSpA with peripheral arthritis.57 In the case of persistent sacroiliitis despite NSAIDs, sulfasalazine is conditionally recommended for patients with contraindications for anti-TNF agents or who have failed two or more anti-TNF agents. It has also been reported that it can be used as an alternative to methotrexate in cases with JSpA with mild enthesitis and active peripheral polyarthritis despite NSAIDs.56
Antitumor necrosis factor agents have become one of the most important parts of chronic arthritis treatment. The most commonly used anti-TNF agents in children are etanercept (TNF receptor p75 fusion protein), adalimumab, and infliximab (monoclonal antibody).57 It has been shown that antitumor necrosis factor agents are effective in the treatment of JSpA in peripheral arthritis, enthesitis, and axial skeletal involvement.58,60 While these three agents were reported not to superior to each other in terms of articular findings in adult studies, it was shown that monoclonal antibodies were superior for uveitis and inflammatory bowel disease.57 Antitumor necrosis factor agents are recommended in patients with resistant peripheral joint involvement despite NSAIDs and cDMARDs, or in patients with persistent sacroiliitis and/or enthesitis despite NSAIDs.56 Although some adult studies report that methotrexate does not provide an additive effect in the combined treatment with anti-TNF agents, there are no pediatric data on this issue.61 Yet, it should be noted that it has also been reported that the combined treatment with anti-TNF agents and methotrexate reduces the development of antidrug antibodies.57
In recent years, it has been shown that IL17 and IL23 blockade may be effective in the treatment of patients with spondylitis who have failed anti-TNF agents. Secukinumab, anti-IL 17 agent, has been shown to be effective in the treatment of adult psoriatic arthritis patients.57 It is stated that secukinumab can be used in children with peripheral or axial spondylitis unresponsive to anti-TNF agents.57 Anti-IL 17 agents are recommended to be avoided in patients with IBD due to the crucial role of IL 17 for intestinal wall integrity. On the other hand, ustekinumab, an IL12/23 human monoclonal antibody, is shown to be effective in adult patients with psoriatic arthritis.57 It has been suggested that ustekinumab can be used especially in peripheral JSpA associated with inflammatory bowel disease.57
Although the efficacy of abatacept, apremilast, and tofacitinib has been demonstrated in adult studies, there is not enough experience and data in children.
Conclusion
Juvenile spondyloarthropathies are a group of HLA B27-related diseases that differ from adult-onset spondyloarthropathies not only by their early onset, but also by the distribution and course of the clinical findings. Due to the nonspecific initial findings of these groups of diseases, it is hard to diagnose in early stages. A new classification criterion covering all of the phenotypes of the disease and focusing the differences between JSpA and the other causes of chronic arthritis in childhood is apparently needed.
Footnotes
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - M.Y., F.H., Ö.K.; Design - M.Y., F.H., A.A., S.Ş.; Supervision - A.A., S.Ş., K.B., Ö.K.; Data collection and/or processing - M.Y., F.H., A.A., S.Ş.; Analysis and/or interpretation - M.Y., F.H., A.A., S.Ş.; Literature search - M.Y., F.H., A.A., S.Ş., K.B.; Writing manuscript - M.Y., F.H., A.A.; Critical review - A.A., S.Ş., K.B., Ö.K.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Adrovic A, Barut K, Sahin S, Kasapcopur O. Juvenile spondyloarthropathies. Curr Rheumatol Rep. 2016;18(8):55. [DOI] [PubMed] [Google Scholar]
- 2.Maniscalco V, Marrani E, Lamot L, Lionetti P, Simonini G. The conundrum of juvenile spondyloarthritis classification: Many names for a single diseaseϿ. Lesson learned from an instructive clinical case. Int J Rheum Dis. 2020;23(9):1248-51. [DOI] [PubMed] [Google Scholar]
- 3.Yildiz M, Haslak F, Adrovic A, Sahin S, Barut K, Kasapcopur O. Comment on: the conundrum of juvenile spondyloarthritis classification: Many names for a single disease? Lesson learned from an instructive clinical case. Int J Rheum Dis. 2020;23:1430-1. [DOI] [PubMed] [Google Scholar]
- 4.Petty RE, Southwood TR, Manners P, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision. J Rheumatol. 2004;31(2):390-2. [PubMed] [Google Scholar]
- 5.Colbert RA. Classification of juvenile spondyloarthritis: Enthesitis-related arthritis and beyond. Nat Rev Rheumatol. 2010;6(8):477-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tse SM, Laxer RM. New advances in juvenile spondyloarthritis. Nat Rev Rheumatol. 2012; 8(5):269-79. [DOI] [PubMed] [Google Scholar]
- 7.van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4):361-8. [DOI] [PubMed] [Google Scholar]
- 8.Dougados M, van der Linden S, Juhlin R, et al. The European spondylarthropathy study group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34(10): 1218-27. [DOI] [PubMed] [Google Scholar]
- 9.Amor B, Dougados M, Mijiyawa M. Criteria of the classification of spondylarthropathies. Rev Rhum Mal Osteoartic. 1990;57(2):85-9. [PubMed] [Google Scholar]
- 10.Kasapçopur O, Demirli N, Ozdoğan H, et al. Evaluation of classification criteria for juvenile-onset spondyloarthropathies. Rheumatol Int. 2005;25(6):414-8. [DOI] [PubMed] [Google Scholar]
- 11.Rudwaleit M, van der Heijde D, Landewé R, et al. The assessment of SpondyloArthritis international society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70(1):25-31. [DOI] [PubMed] [Google Scholar]
- 12.Rudwaleit M, van der Heijde D, Landewé R, et al. The development of assessment of SpondyloArthritis international society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68(6):777-83. [DOI] [PubMed] [Google Scholar]
- 13.Burgos-Vargas R. The assessment of the spondyloarthritis international society concept and criteria for the classification of axial spondyloarthritis and peripheral spondyloarthritis: a critical appraisal for the pediatric rheumatologist. Pediatr Rheumatol. 2012;10(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg AM, Petty RE. A syndrome of seronegative enthesopathy and arthropathy in children. Arthritis Rheum. 1982;25(9):1041-7. [DOI] [PubMed] [Google Scholar]
- 15.Häfner R. [Juvenile spondarthritis. Retrospective study of 71 patients]. Monatsschrift Kinderheilkunde: Organ der Deutschen Gesellschaft fur Kinderheilkunde. 1987;135(1):41-6. [PubMed] [Google Scholar]
- 16.Hussein A, Abdul-Khaliq H, von der Hardt H. Atypical spondyloarthritis in children: proposed diagnostic criteria. Eur J Pediatr. 1989; 148(6):513-7. [DOI] [PubMed] [Google Scholar]
- 17.Adrovic A, Sezen M, Barut K, et al. The performance of classification criteria for juvenile spondyloarthropathies. Rheumatol Int. 2017;37(12):2013-8. [DOI] [PubMed] [Google Scholar]
- 18.Martini A, Ravelli A, Avcin T, et al. Toward new classification criteria for juvenile idiopathic arthritis: first steps, pediatric rheumatology international trials organization international consensus. J Rheumatol. 2019;46(2):190-7. [DOI] [PubMed] [Google Scholar]
- 19.Burgos-Vargas R, Rudwaleit M, Sieper J. The place of juvenile onset spondyloarthropathies in the Durban 1997 ILAR classification criteria of juvenile idiopathic arthritis. International league of associations for rheumatology. J Rheumatol. 2002;29(5):869-74. [PubMed] [Google Scholar]
- 20.Sezen M, Barut K, Açıkel C, Kasapcopur O. The new proposal classification criteria for juvenile spondyloarthropathies. Pediatr Rheumatol. 2014;12(1):P45. [DOI] [PubMed] [Google Scholar]
- 21.Ozer E, Seker D, Taner E, et al. The frequency of juvenile spondyloarthropathies in childhood familial Mediterranean fever. Clin Exp Rheumatol. 2018;36(6 Suppl 115):141-145. [PubMed] [Google Scholar]
- 22.Thierry S, Fautrel B, Lemelle I, Guillemin F. Prevalence and incidence of juvenile idiopathic arthritis: a systematic review. Joint, Bone, Spine Revue rhumat. 2014;81(2):112-7. [DOI] [PubMed] [Google Scholar]
- 23.Shih YJ, Yang YH, Lin CY, Chang CL, Chiang BL. Enthesitis-related arthritis is the most common category of juvenile idiopathic arthritis in Taiwan and presents persistent active disease. Pediatr Rheumatol. 2019;17(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srivastava R, Phatak S, Yadav A, Bajpai P, Aggarwal A. HLA B27 typing in 511 children with juvenile idiopathic arthritis from India. Rheumatol Int. 2016;36(10):1407-11. [DOI] [PubMed] [Google Scholar]
- 25.Oen K, Tucker L, Huber AM, et al. Predictors of early inactive disease in a juvenile idiopathic arthritis cohort: results of a Canadian multicenter, prospective inception cohort study. Arthritis Rheum. 2009;61(8):1077-86. [DOI] [PubMed] [Google Scholar]
- 26.Mistry RR, Patro P, Agarwal V, Misra DP. Enthesitis-related arthritis: current perspectives. OARRR. 2019;11:19-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasapçopur O, Yologlu N, Ozyazgan Y, et al. Uveitis and antinuclear antibody positivity in children with juvenile idiopathic arthritis. Indian Pediatr. 2004;41(10):1035-39. [PubMed] [Google Scholar]
- 28.Gomez KS, Raza K, Jones SD, Kennedy LG, Calin A. Juvenile onset ankylosing spondylitis–more girls than we thought? J Rheumatol. 1997;24(4):735-7. [PubMed] [Google Scholar]
- 29.Saurenmann RK, Rose JB, Tyrrell P, et al. Epidemiology of juvenile idiopathic arthritis in a multiethnic cohort: ethnicity as a risk factor. Arthritis Rheum. 2007;56(6):1974-84. [DOI] [PubMed] [Google Scholar]
- 30.Weiss PF, Colbert RA. Juvenile spondyloarthritis: a distinct form of juvenile arthritis. Pediatric clinics of North America. 2018;65(4):675-90. [DOI] [PubMed] [Google Scholar]
- 31.Taurog JD, Richardson JA, Croft JT, et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180(6): 2359-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rath HC, Herfarth HH, Ikeda JS, et al. Normal luminal bacteria, especially bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest. 1996;98(4):945-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ekman P, Saarinen M, He Q, et al. HLA-B27-transfected (salmonella permissive) and HLA-A2-transfected (salmonella nonpermissive) human monocytic U937 cells differ in their production of cytokines. IAI. 2002;70(3):1609-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reveille JD. Major histocompatibility genes and ankylosing spondylitis. Best Pract Res Clin Rheumatol. 2006;20(3):601-9. [DOI] [PubMed] [Google Scholar]
- 35.Stoll ML, Kumar R, Morrow CD, et al. Altered microbiota associated with abnormal humoral immune responses to commensal organisms in enthesitis-related arthritis. Arthritis Res Ther. 2014;16(6):486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costello ME, Ciccia F, Willner D, et al. Brief report: intestinal dysbiosis in ankylosing spondylitis. Arthritis Rheumatol. 2015;67(3):686-91. [DOI] [PubMed] [Google Scholar]
- 37.Stoll ML, Weiss PF, Weiss JE, et al. Age and fecal microbial strain-specific differences in patients with spondyloarthritis. Arthritis Res Ther. 2018;20(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hofer M. Spondylarthropathies in children–are they different from those in adults? Best Pract Res Clin Rheumatol. 2006;20(2):315-28. [DOI] [PubMed] [Google Scholar]
- 39.Burgos-Vargas R, Pacheco-Tena C, Vázquez-Mellado J. Juvenile-onset spondyloarthropathies. Rheumat Dis Clin North America. 1997; 23(3):569-98. [DOI] [PubMed] [Google Scholar]
- 40.Cabral DA, Malleson PN, Petty RE. Spondyloarthropathies of childhood. Pediat Clin North America. 1995;42(5):1051-70. [DOI] [PubMed] [Google Scholar]
- 41.Dougados M, Baeten D. Spondyloarthritis. Lancet (London, England). 2011;377(9783):2127-37. [DOI] [PubMed] [Google Scholar]
- 42.Vitulano C, Tedeschi V, Paladini F, Sorrentino R, Fiorillo MT. The interplay between HLA-B27 and ERAP1/ERAP2 aminopeptidases: from anti-viral protection to spondyloarthritis. Clin Exp Immunol. 2017;190(3):281-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gülhan B, Akkuş A, Ozçakar L, Beşbaş N, Ozen S. Are MEFV mutations susceptibility factors in enthesitis-related arthritis patients in the Eastern Mediterranean? Clin Exp Rheumatol. 2014;32(4 Suppl 84):S160-4. [PubMed] [Google Scholar]
- 44.Tufan A, Mercan R, Tezcan ME, et al. Enthesopathy in patients with familial Mediterranean fever: increased prevalence in M694 V variant. Rheumatol Int. 2013;33(8):1933-7. [DOI] [PubMed] [Google Scholar]
- 45.Yigit S, Inanir A, Karakus N, Kesici E, Bozkurt N. Common Mediterranean fever (MEFV) gene mutations associated with ankylosing spondylitis in Turkish population. Dis Markers. 2012; 33(3):113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sims AM, Timms AE, Bruges-Armas J, et al. Prospective meta-analysis of interleukin 1 gene complex polymorphisms confirms associations with ankylosing spondylitis. Ann Rheum Dis. 2007;67(9):1305-9. [DOI] [PubMed] [Google Scholar]
- 47.Weiss PF, Roth J. Juvenile-versus adult-onset spondyloarthritis: similar, but different. Rheumat Dis Clin North America. 2020;46(2):241-57. [DOI] [PubMed] [Google Scholar]
- 48.Weiss PF, Xiao R, Biko DM, et al. Assessment of sacroiliitis at diagnosis of juvenile spondyloarthritis by radiography, magnetic resonance imaging, and clinical examination. Arthritis Care Res. 2016; 68(2):187-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akdeniz B, Akyel N, Yildiz M, et al. Comparison of the efficacy of physical examination and radiological imaging in detecting sacroiliitis in patients with juvenile spondyloarthropathies Clin Exp Rheumatol. 2020;38(5):1021-8. [PubMed] [Google Scholar]
- 50.Jadon DR, Ramanan AV, Sengupta R. Juvenile versus adult-onset ankylosing spondylitis – clinical, radiographic, and social outcomes. A systematic review. J Rheumatol. 2013;40(11):1797-805. [DOI] [PubMed] [Google Scholar]
- 51.Dedeoglu R, Yildiz M, Karagozlu F, et al. Unexpected increase of aortic stiffness in juvenile spondyloarthropathies. Cardiol Young. 2020;30:1806-09. [DOI] [PubMed] [Google Scholar]
- 52.Riley MJ, Ansell BM, Bywaters EG. Radiological manifestations of ankylosing spondylitis according to age at onset. Ann Rheum Dis. 1971;30(2):138-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alvarez-Madrid C, Merino R, De Inocencio J, García-Consuegra J. Tarsitis as an initial manifestation of juvenile spondyloarthropathy. Clin Exp Rheumatol. 2009;27(4):691-94. [PubMed] [Google Scholar]
- 54.Rumsey DG, Guzman J, Rosenberg AM, et al. Characteristics and course of enthesitis in a juvenile idiopathic arthritis inception cohort. Arthritis Care Res. 2018;70(2):303-8. [DOI] [PubMed] [Google Scholar]
- 55.Gmuca S, Xiao R, Brandon TG, et al. Multicenter inception cohort of enthesitis-related arthritis: variation in disease characteristics and treatment approaches. Arthritis Res Ther. 2017; 19(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ringold S, Angeles-Han ST, Beukelman T, et al. 2019 American college of rheumatology/arthritis foundation guideline for the treatment of juvenile idiopathic arthritis: therapeutic approaches for Non-Systemic polyarthritis, sacroiliitis, and enthesitis. Arthritis Rheumatol. 2019;71(6):846-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bridges JM, Stoll ML. Treatment of juvenile spondyloarthritis: where we stand. Paediatr Drugs. 2020;22(6):603-615. [DOI] [PubMed] [Google Scholar]
- 58.Gmuca S, Weiss PF. Evaluation and treatment of childhood Enthesitis-Related arthritis. Curr Treat Options Rheum. 2015;1(4):350-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen J, Veras MMS, Liu C, Lin J. Methotrexate for ankylosing spondylitis. Cochrane Database Syst Rev. 2013; 2; CD004524. [DOI] [PubMed] [Google Scholar]
- 60.Hugle B, Burgos-Vargas R, Inman RD, et al. Long-term outcome of anti-tumor necrosis factor alpha blockade in the treatment of juvenile spondyloarthritis. Clin Exp Rheumatol. 2014;32(3):424-31. [PubMed] [Google Scholar]
- 61.Combe B, Behrens F, McHugh N, et al. Comparison of etanercept monotherapy and combination therapy with methotrexate in psoriatic arthritis: Results from 2 clinical trials. J Rheumatol. 2016;43(6):1063-7. [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a