As we brace for the imminent impact of the coronavirus disease 2019 (COVID-19) pandemic, we are faced with a controversy on how to best minimize the risk of lethal disease among the most vulnerable of us. Preliminary epidemiological data show an uneven-handed impact on the population, with an exponential increase in disease severity and mortality in those beyond the sixth decade of life with cardiovascular disease (CVD) and diabetes. Given that angiotensin-converting enzyme 2 (ACE2), an enzyme coopted by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to enter epithelial cells, is upregulated in patients with CVD and diabetes treated with angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), it was proposed that this increase in ACE2 expression underpins the greater COVID-19 severity in this population. This has created substantial controversy regarding the approach to patients taking ACEIs/ARBs in preparation for the pandemic, with some advocating for discontinuing these medications while expert opinions recommended against discontinuation, given the lack of strong evidence.1
To begin to unravel this complex dilemma, we must consider the role of ACE2 not only in COVID-19 pathogenesis but also as a component of renin-angiotensin system (RAS) signaling throughout the body. First, one must recognize the scarcity of data on the topic, particularly in humans. Nonetheless, the urgency of the situation makes it imperative to use inductive reasoning to guide our next steps toward protecting our patients. It is well established now that while ACE2 is targeted by SARS-CoV-2 to gain entrance into cells, it plays a major anti-inflammatory role in RAS signaling by converting angiotensin II, the quintessential perpetrator of inflammation,2 to angiotensin 1–7, which carries anti-inflammatory properties.3
What has been missing in discussions of the aforementioned dilemma is the age-associated decline in ACE2 expression, as observed in the lungs of rats,4 which is in line with a constellation of major proinflammatory changes perpetrated by an age-associated increase in RAS signaling throughout the body.5 Exaggerated forms of this proinflammatory profile are also salient pathophysiologic features of hypertension and diabetes, which are highly prevalent at older ages.5 The upregulation of ACE2 in individuals with diabetes and hypertension treated with ACEIs/ARBs is, in a way, restorative of physiological function. Hence, these observations raise an apparent paradox: given ACE2 itself is the gateway of SARS-CoV-2 entry into cells, how can the reduction in ACE2 levels in older persons and those with CVD predispose for greater COVID-19 severity?
This apparent paradox becomes clear if we distinguish the role of ACE2 as a gateway for SARS-CoV-2 facilitating the infection from its pivotal anti-inflammatory function in RAS signaling that is compromised in individuals with COVID-19, contributing to its severity (Figure).3 Indeed, data on the severe acute respiratory syndrome epidemic of 2003 demonstrates this divergence in factors predisposing to disease occurrence and its severity; in the former epidemic, although younger individuals in their third and fourth decades of life accounted for most of those infected,6 these younger patients had lower disease severity and risk of mortality compared with older people with preexisting conditions.
Figure.
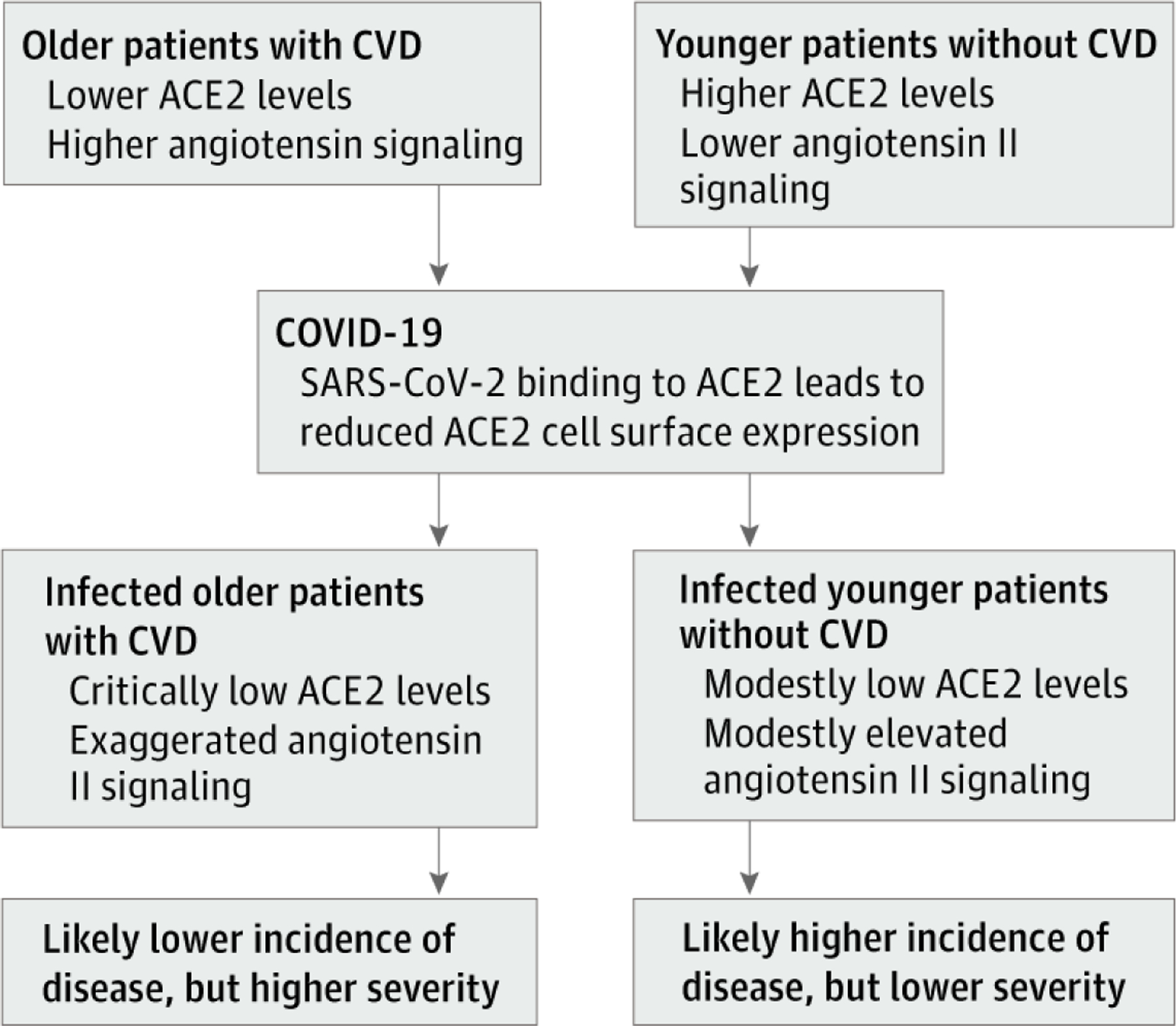
Schematic of Inflammatory Profile Before and After Coronavirus Disease 2019 (COVID-19) Infection
Simplified schematic of the preinfection inflammatory profile among predisposed older individuals vs their younger counterparts. ACE2 indicates angiotensin-converting enzyme 2; CVD, cardiovascular disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Similarly, in the COVID-19 pandemic, it is plausible that greater expression of ACE2 leads to higher predisposition to incur the disease; preliminary epidemiologic data from South Korea, where the most population-wide testing has taken place, show that most cases are among younger adults7 who are expected to have higher levels of ACE2.4 However, when it comes to COVID-19 severity, reduction in ACE2 levels with aging and CVD and its associated upregulation of angiotensin II proinflammatory pathway8 likely predispose older individuals with cardiovascular comorbidities to severe forms of COVID-19, as has been observed in Italy.7 This predisposition is exploited with SARS-CoV-2 binding to ACE2 itself, further reducing ACE2 cell surface expression, upregulating angiotensin II signaling in the lungs, and yielding acute lung injury.3 Hence, compared with young individuals, older persons with CVD who already have reduced ACE2 levels will be expected to be more predisposed to exaggerated inflammation with further reduction in ACE2 expression in the context of COVID-19, manifesting with greater disease severity.
In summary, older individuals, especially those with hypertension and diabetes, have reduced ACE2 expression and upregulation of angiotensin II proinflammatory signaling; the increase in ACE2 levels with ACEI/ARB treatment is more likely to be corrective to these changes. We hypothesize that with superimposed COVID-19 disease, SARS-CoV-2 binding to ACE2 acutely exaggerates this proinflammatory background, predisposing these sub-populations to greater COVID-19 disease severity and mortality (Figure). This hypothesis is in line with the evidence of a protective role of angiotensin II antagonism against sepsis-associated acute lung injury9,10 and supports continuing therapy with ACEIs/ARBs and, more so, urgently calls for expanding ongoing trials treating patients with severe COVID-19 with RAS interventions to examine the role of these interventions in preventing lethal lung complications of COVID-19 as cases surge around the world.
Footnotes
ARTICLE INFORMATION
Conflict of Interest Disclosures: None reported.
Contributor Information
Majd AlGhatrif, Laboratory of Cardiovascular Science, National Institute on Aging, National Institutes of Health, Baltimore, Maryland; and Divisions of Cardiology and Hospital Medicine, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland..
Oscar Cingolani, Division of Cardiology, Department of Medicine, Johns Hopkins School of Medicine, Baltimore, Maryland..
Edward G. Lakatta, Laboratory of Cardiovascular Science, National Institute on Aging, National Institutes of Health, Baltimore, Maryland..
REFERENCES
- 1.Bozkurt B, Kovacs R, Harrington B. HFSA/ACC/AHA statement addresses concerns re: using RAAS antagonists in COVID-19 Accessed March 22, 2020. https://professional.heart.org/professional/ScienceNews/UCM_505836_ HFSAACCAHA-statement-addresses-concerns-re-using-RAAS-antagonists-in-COVID-19.jsp?utm_campaign=sciencenews19-20&utm_source= science-news&utm_medium=email&utm_content= phd03-17-20 [DOI] [PMC free article] [PubMed]
- 2.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation 2003;107(2):346–354. doi: 10.1161/01.CIR.0000048893.62841.F7 [DOI] [PubMed] [Google Scholar]
- 3.Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005;11 (8):875–879. doi: 10.1038/nm1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie X, Chen J, Wang X, Zhang F, Liu Y. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci 2006;78(19):2166–2171. Published correction appears in Life Sci. 2006;79(26):2499. doi: 10.1016/j.lfs.2005.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakatta EG. The reality of getting old. Nat Rev Cardiol 2018;15(9):499–500. doi: 10.1038/s41569-018-0068-y [DOI] [PubMed] [Google Scholar]
- 6.Liang W, Zhu Z, Guo J, et al. ; Beijing Joint SARS Expert Group. Severe acute respiratory syndrome, Beijing, 2003. Emerg Infect Dis 2004;10(1):25–31. doi: 10.3201/eid1001.030553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Backhaus A Coronavirus: why it’s so deadly in Italy Accessed March 22, 2020. https://medium.com/@andreasbackhausab/coronavirus-why-its-so-deadly-in-italy-c4200a15a7bf
- 8.Rodrigues Prestes TR, Rocha NP, Miranda AS, Teixeira AL, Simoes-E-Silva AC. The anti-inflammatory potential of ACE2/angiotensin-(1–7)/Mas receptor axis: evidence from basic and clinical research. Curr Drug Targets 2017;18(11): 1301–1313. doi: 10.2174/1389450117666160727142401 [DOI] [PubMed] [Google Scholar]
- 9.Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005;436(7047):112–116. doi: 10.1038/nature03712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D, Chai XQ, Magnussen CG, et al. Renin-angiotensin-system, a potential pharmacological candidate, in acute respiratory distress syndrome during mechanical ventilation. Pulm Pharmacol Ther 2019;58:101833. doi: 10.1016/j.pupt.2019.101833 [DOI] [PMC free article] [PubMed] [Google Scholar]


