Abstract
Background
Anemia is one of the common complications of diabetes and is associated with mortality. Phase angle (PhA), ratio of extracellular water to total body water (ECW/TBW) and skeletal muscle mass index (SMI) estimated by bioelectrical impedance analysis (BIA) have been used as prognostic indicators for various chronic diseases and frailty. We aimed to clarify the clinical significance of PhA, ECW/TBW and SMI for anemia in patients with diabetes.
Materials and methods
The values of PhA, ECW/TBW and SMI were estimated by a portable BIA device and blood samples were collected in 371 Japanese patients with diabetes. The relationships of PhA, ECW/TBW and SMI with hemoglobin (Hgb) and hematocrit (Hct) were statistically evaluated.
Results
In simple linear regression analysis, PhA and SMI were positively correlated with Hgb and Hct levels in total subjects, male subjects and female subjects. In contrast, ECW/TBW was negatively correlated with Hgb and Hct levels regardless of sex. Multivariate regression analysis showed that both PhA and ECW/TBW but not SMI independently contributed to Hgb and Hct levels after adjustment of clinical confounding factors in both males and females.
Conclusions
PhA and ECW/TBW but not SMI were associated with levels of Hgb and Hct in patients with diabetes. Therefore, aberrant values of PhA and ECW/TBW suggest a risk of anemia in diabetic patients.
Keywords: Phase angle, Extracellular water-to-total body water ratio, Skeletal muscle mass index, Bioelectrical impedance analysis, Hemoglobin, Hematocrit
1. Introduction
Patients with diabetes often suffer the consequences of renal dysfunction earlier in the course of disease than do non-diabetic patients [1]. It has been reported that erythropoietin (EPO) deficiency occurs early in diabetic nephropathy, and anemia is one of the common complications of diabetes and is mainly caused by diabetic kidney disease (DKD). In addition, a high prevalence of mild anemia in patients with diabetes has recently been recognized [2] and it has been shown that anemia in patients with type 2 diabetes (T2D) is associated with an increased risk of death [3]. Therefore, risk assessment of anemia in the early stage is an important clinical issue in patients with diabetes mellitus (DM).
In recent years, bioelectrical impedance analysis (BIA) has been used for estimating body composition. BIA has also been used to estimate phase angle (PhA), which is an angular shift (phase difference) between electrical voltage and current sinusoidal waveforms indicating cell mass, cellular integrity and healthiness of cell membranes [4,5]. PhA has been reported to be useful as a prognostic indicator in patients with advanced cancer [[6], [7], [8]] and has also been shown to be associated with an increased relative risk of death in patients undergoing hemodialysis [9,10]. In addition, PhA decreases not only with illness but also with aging, and elderly people with low PhA have a high risk of death [11,12]. Therefore, PhA can be expected to be an index of disease prognosis and physical health with aging. BIA can also be used for estimation of the ratio of extracellular water (ECW) to total body water (TBW) as an indicator of cellular fluid volume balance. The clinical utility of ECW/TBW has been studied in patients with renal diseases [[13], [14], [15]] and patients with chronic liver diseases [16].
Examination of PhA is a noninvasive tool for monitoring the catabolic state and progression of disease in patients with DM [17,18], and ECW/TBW has been reported to be associated with DKD progression in T2DM patients [19].
Anemia, congestive heart failure (CHF) and chronic kidney disease have been recognized to interact as a vicious circle so as to cause or worsen each other, the so-called cardio-renal anemia syndrome [20], and both PhA and ECW/TBW have been demonstrated to be associated with the severity of CHF [21,22]. In addition, blood conductivity is directly influenced by the cellular composition of blood [23]. Taken together, we speculate that PhA and ECW/TBW are key factors that interact with anemia and DKD. However, associations of PhA and ECW/TBW with anemia in patients with diabetes except hemodialysis patients, including patients who have been treated with erythropoiesis-stimulating agents (ESAs) [24,25], have not been reported. Therefore, in this study, we aimed to clarify the clinical significance of PhA, ECW/TBW and skeletal muscle mass index (SMI) estimated by BIA for levels of hemoglobin (Hgb) and hematocrit (Hct) in diabetic patients with preserved renal function.
2. Materials and Methods
2.1. Study design, subjects and ethics statement
This study was a cross-sectional study. Diabetic patients were defined as individuals who were receiving hypoglycemic agents or individuals with a glycosylated hemoglobin A1c (HbA1c) level of ≥6.5%. We consecutively recruited 371 Japanese individuals (209 males and 162 females) who were outpatients or inpatients with type 1 diabetes (T1D) or T2D. All of the subjects were older than 20 years of age and were recruited consecutively from the Department of Internal Medicine at Anan Medical Center (Anan, Tokushima, Japan) between May 2020 and December 2021. The exclusion criteria for the patients with diabetes were as follows: 1) patients with advanced cancer, 2) patients with secondary diabetes such as steroid-induced diabetes or pancreatic diabetes, 3) patients who were pregnant, 4) patients with advanced renal disease with a serum creatinine (Cr) level of >2.0 mg/dL, 5) patients with liver cirrhosis and malnutrition (serum albumin (Alb) level of <3.0 g/dL), and 6) patients who were treated with hematinic drugs including iron, folic acid, mecobalamin, ESAs and hypoxia-inducible factor prolyl hydroxylase inhibitors.
All of the subjects enrolled in this study underwent a standardized interview and a physical examination. Current smokers were defined as subjects who had smoked within the past 2 years. Body mass index (BMI) was calculated as an index of obesity. Blood pressure (BP) was measured twice in each participant in a sitting position using an automatic sphygmomanometer, and the BP values were averaged. Hypertensive patients were defined as those with systolic BP (SBP) of ≥140 mm Hg and/or diastolic BP of ≥90 mm Hg or those receiving antihypertensive agents. Patients with dyslipidemia were defined as those with a low-density lipoprotein cholesterol (LDL-C) level of ≥140 mg/dL, a triglyceride (TG) level of ≥150 mg/dL, a high-density lipoprotein cholesterol (HDL-C) level of <40 mg/dL, or those receiving lipid-lowering agents.
Our study followed the institutional guidelines of Anan Medical Center and was approved by the Institutional Review Board of Anan Medical Center (approval ID R2-2). Prior informed consent was obtained from all patients according to the Declaration of Helsinki.
2.2. Bioelectrical impedance analysis
BIA was conducted with reference to a previous report [26]. Water volume analysis and estimation of PhA were performed using a portable direct segmental multifrequency bioimpedance analysis (DSM-BIA) device (InBody S10®, InBody Japan, Tokyo, Japan). DSM-BIA was conducted using an 8-point tactile electrode system with 30 impedance examinations taken by using 6 frequencies (1, 5, 50, 250, 500, 1000 kHz) at each of 5 segments (bilateral thumbs, third fingers and ankles). Patient characteristics including information on age, sex, body weight, and height were entered into the DSM-BIA device. Electrical currents of 1, 5, 50, 250, 500, and 1000 kHz were applied through the electrodes in the standing position. Although this body composition analyzer did not directly measure fluid volume in each cellular component, total body water (TBW), intracellular water (ICW), extracellular water (ECW), ratio of ECW to TBW (ECW/TBW), protein, mineral, body fat mass, and skeletal muscle mass index (SMI) were calculated using formulas in the inner software based on the height, weight, and impedance examined. Whole body resistance and reactance were evaluated with an electrical current of 50 kHz. PhA can be calculated as the arctangent of the ratio of reactance to resistance. Estimation of those data was conducted at a single examination in each subject. This device has been assessed in normal populations, obese individuals, and chronic renal failure patients, and estimations using the device have been shown to be closely correlated with gold standard measurements by D2O and NaBr dilution [27]. Also, this tool is not based on statistical data of any specific population. It is therefore capable of accurately assessing people with very different physical types including obese, elderly and athletic individuals [28]. In addition, body composition data obtained by using the DSM-BIA device were highly correlated with data obtained by dual energy X-ray absorptiometry (DXA) studies [[29], [30], [31], [32], [33]]. In fact, Potter et al. showed that the InBody device provided high precision examinations including %body fat (%BF) and fat-free mass (FFM; kg) based on a high linear correlation (%BF R2 = 0.8429 and FFM R2 = 0.9556) compared with DXA assessment for a large number of physically fit individuals (480 men and 315 women) tested without special instructions about hydration or fasting [32]. Additionally, Buch et al. demonstrated that assessment of appendicular SMI did not differ between the InBody device and DXA (7.43 vs. 7.47 kg/m2; p = 0.84; Intraclass Correlation Coefficient (ICC) = 0.965, p < 0.0001; mean difference = −0.068, p = 0.595) in patients with T2DM [33].
Therefore, the Japan Pharmaceutical and Medical Devices Agency (PMDA: an incorporated administrative agency that evaluates the quality, efficacy and safety of drugs and medical devices), U.S. Food & Drug Administration (FDA) and European CE marking certificated InBody S10 as a medical equipment for body composition analysis.
2.3. Blood cell counts and biochemical analyses
Blood sampling was performed as casual sampling except within 2 h after each meal. Blood samples were collected and used for determination of blood cell counts (white blood cells (WBCs), red blood cells (RBCs), Hgb, Hct, mean corpuscular volume (MCV), platelets), plasma glucose (PG), HbA1c, and serum biochemical parameters including LDL-C, TG, HDL-C, uric acid (UA), Alb and Cr. A complete blood cell count examination and measurements of Hgb and Hct were performed on Sysmex XE-5000 (Kobe, Japan). PG and serum levels of LDL-C, TG, HDL-C, UA and Cr were measured by enzymatic methods. HbA1c was assayed by latex agglutination. eGFR was calculated according to the following formula of the Japanese Society of Nephrology: eGFR (mL/min/1.73 m2) = 194 × serum and creatinine level−1.094 × age−0.287 ( × 0.739 if female).
2.4. Statistical analysis
Normally distributed continuous data were presented as mean ± standard deviation (SD). Skewed continuous data were presented as median and interquartile range (IQR). Categorical variables were compared by performing the χ2 test or Fisher's exact test. For comparisons between two groups, we performed the Mann-Whitney U test or Student's t-test for numeric variables depending on the variable's distribution. The degrees of associations of Hgb and Hct levels with each variable including sex, age, BMI, SBP, serum lipid parameters, UA, Cr, HbA1c, duration of DM, history of current smoking, hypertension, dyslipidemia, PhA, ECW/TBW and SMI were determined by simple linear regression analysis. In addition, we performed multiple linear regression analysis with significant variables determined by the simple linear regression analysis. Then multicollinearity in each multiple regression analysis was also evaluated. These analyses were performed by using Excel (Microsoft Office Excel 2019; Microsoft, Richmond, CA) and GraphPad Prism8 (GraphPad Software, San Diego, CA). Statistical significance was set at p < 0.05. In addition, to evaluate the statistical power of the sample size in the present study, we used G*Power 3.1.9.6 for Mac OSX, a free software program, for performing the power analysis.
3. Results
3.1. Clinical characteristics of subjects
Physical and laboratory characteristics of subjects enrolled in this study are shown in Table 1. On average, females had higher levels of platelets, higher ratios of ECW/TBW and higher serum levels of HDL-C than males did. Male patients showed higher levels of RBCs, Hgb, Hct, MCV, TG, casual PG, UA, Cr, PhA and SMI than those in female patients. There were no significant gender differences in age, BMI, SBP, LDL-C, Alb, HbA1c, duration of DM, eGFR and WBCs. The percentage of male patients who were current smokers and the percentage of male patients who were antiplatelets users was significantly higher than those of female patients.
Table 1.
Clinical characteristics of the subjects enrolled in the cross-sectional study.
| Variables | Total Subjects | Males | Females | p value (M vs F) |
|---|---|---|---|---|
| Number of Subjects | 371 | 209 | 162 | |
| Type 1 DM (n, (%)) | 14 (3.8) | 5 (2.4) | 9 (5.6) | 0.113 |
| Age (years) | 68 ± 11 | 68 ± 11 | 67 ± 11 | 0.646 |
| Height (m) | 1.604 ± 0.090 | 1.660 ± 0.066 | 1.533 ± 0.064 | <0.001 |
| Body Weight (kg) | 63.4 (55.0–72.0) | 67.0 (59.0–74.0) | 57.0 (49.0–67.6) | <0.001 |
| BMI (kg/m2) | 24.3 (22.0–27.0) | 24.4 (22.0–26.6) | 24.1 (21.6–28.2) | 0.892 |
| SBP (mmHg) | 132 ± 17 | 131 ± 16 | 133 ± 17 | 0.381 |
| LDL-C (mg/dL) | 104 ± 30 | 102 ± 30 | 106 ± 31 | 0.242 |
| HDL-C (mg/dL) | 52 (44–61) | 49 (41–58) | 57 (47–67) | <0.001 |
| TG (mg/dL) | 112 (78–163) | 122 (81–171) | 103 (77–154) | 0.048 |
| Alb (g/dL) | 4.2 (4.0–4.4) | 4.2 (4.0–4.4) | 4.2 (4.0–4.4) | 0.426 |
| UA (mg/dL) | 5.0 ± 1.4 | 5.5 ± 1.2 | 4.5 ± 1.4 | <0.001 |
| Casual PG (mg/dL) | 135 (114–177) | 139 (120–187) | 126 (108–159) | 0.191 |
| HbA1c (%) | 6.8 (6.4–7.4) | 6.7 (6.3–7.3) | 6.9 (6.5–7.5) | 0.491 |
| Cr (mg/dL) | 0.74 (0.63–0.92) | 0.85 (0.73–1.01) | 0.63 (0.51–0.72) | <0.001 |
| eGFR (ml/min/1.73m2) | 72.2 ± 21.0 | 70.4 ± 20.0 | 74.5 ± 22.2 | 0.062 |
| WBC ( × 103/μL) | 6.15 (5.13–7.31) | 6.23 (5.31–7.48) | 5.96 (4.98–7.21) | 0.220 |
| RBC ( × 106/μL) | 4.68 ± 0.55 | 4.77 ± 0.57 | 4.56 ± 0.49 | <0.001 |
| Hgb (g/dL) | 14.2 ± 1.7 | 14.7 ± 1.6 | 13.6 ± 1.5 | <0.001 |
| Hct (%) | 41.9 ± 4.7 | 42.9 ± 4.8 | 40.6 ± 4.2 | <0.001 |
| MCV (fL) | 90.4 (87.4–93.4) | 91.0 (87.6–94.3) | 89.5 (86.7–91.8) | 0.125 |
| Plt ( × 103/μL) | 221 ± 57 | 211 ± 54 | 233 ± 57 | <0.001 |
| Current Smoking (n, (%)) | 67 (18.1) | 58 (27.8) | 9 (4.3) | <0.001 |
| Hypertension (n, (%)) | 237 (63.9) | 125 (59.8) | 112 (53.6) | 0.064 |
| Dyslipidemia (n, (%)) | 272 (73.3) | 152 (72.7) | 120 (57.4) | 0.771 |
| Duration of DM (years) | 10 (3–18) | 10 (4–18) | 10 (3–19) | 0.645 |
| PhA (°) | 5.4 ± 0.8 | 5.6 ± 0.8 | 5.1 ± 0.6 | <0.001 |
| ECW (L) | 12.9 ± 2.5 | 14.3 ± 2.1 | 11.0 ± 1.5 | <0.001 |
| ICW (L) | 20.7 ± 4.2 | 23.1 ± 3.7 | 17.6 ± 2.6 | <0.001 |
| ECW/TBW | 0.384 ± 0.009 | 0.383 ± 0.009 | 0.385 ± 0.008 | 0.002 |
| Limbs Muscle Mass (kg) | 18.8 ± 4.5 | 21.4 ± 3.7 | 15.4 ± 2.8 | <0.001 |
| SMI (kg/m2) | 7.2 (6.5–7.8) | 7.6 (7.1–8.3) | 6.5 (5.9–7.1) | <0.001 |
| <Medications Used> | ||||
| ARB or ACEi (n, (%)) | 149 (40.2) | 78 (37.3) | 71 (43.8) | 0.205 |
| CCB (n, (%)) | 137 (36.9) | 76 (36.4) | 61 (37.7) | 0.798 |
| β blocker (n, (%)) | 15 (4.0) | 10 (4.8) | 5 (3.1) | 0.410 |
| MR antagonist (n, (%)) | 3 (0.8) | 2 (1.0) | 1 (0.6) | 0.717 |
| Statin (n, (%)) | 172 (46.4) | 88 (42.1) | 84 (51.9) | 0.062 |
| Ezetimibe (n, (%)) | 24 (6.5) | 13 (6.2) | 11 (6.8) | 0.825 |
| Other lipid-lowering drugs (n, (%)) | 9 (2.4) | 5 (2.4) | 4 (2.5) | 0.962 |
| Antiplatelet (n, (%)) | 39 (10.5) | 30 (14.4) | 9 (5.6) | 0.006 |
| SU or Glinide (n, (%)) | 74 (19.9) | 47 (22.5) | 27 (16.7) | 0.164 |
| Metformin (n, (%)) | 188 (50.7) | 105 (50.2) | 83 (51.2) | 0.849 |
| DPP-4i (n, (%)) | 219 (59.0) | 125 (59.8) | 94 (58.0) | 0.729 |
| SGLT2i (n, (%)) | 156 (42.0) | 91 (43.5) | 65 (40.1) | 0.508 |
| αGI (n, (%)) | 51 (13.7) | 27 (12.9) | 24 (14.8) | 0.599 |
| Pioglitazone (n, (%)) | 12 (3.2) | 6 (2.9) | 6 (3.7) | 0.653 |
| Insulin (n, (%)) | 85 (22.9) | 44 (21.1) | 41 (25.3) | 0.333 |
| GLP-1RA (n, (%)) | 40 (10.8) | 20 (9.6) | 20 (12.3) | 0.392 |
Normally distributed continuous data were presented as mean ± standard deviation (SD). Skewed continuous data were presented as median and interquartile range (IQR).
Abbreviations: BMI: body mass index; SBP: systolic blood pressure; LDL-C: low-density lipoprotein cholesterol; TG: triglyceride; HDL-C: high-density lipoprotein cholesterol; PG: plasma glucose; HbA1c: hemoglobin A1c; UA: uric acid; Alb: albumin; Cr: creatinine; eGFR: estimated glomerular filtration rate; WBC: white blood cells; RBC: red blood cells; Hgb: hemoglobin; Hct: hematocrit; MCV: mean corpuscular volume; Plt: platelets; DM: diabetes mellitus; PhA: phase angle; ECW: extracellular water; ICW: intracellular water; ECW/TBW: extracellular water-to-total body water ratio; SMI: skeletal muscle mass index; ARB: angiotensin II receptor blocker; ACEi: angiotensin-converting enzyme inhibitor; CCB; calcium channel blocker; MR: mineral corticoid receptor; SU: sulfonylurea; DPP4i: dipeptidyl peptidase 4 inhibitor; SGLT2i: sodium glucose cotransporter 2 inhibitor; αGI: alpha-glucosidase inhibitor; GLP-1RA: glucagon-like peptide-1 receptor agonist.
3.2. Associations among BIA-associated parameters (PhA, ECW/TBW and SMI)
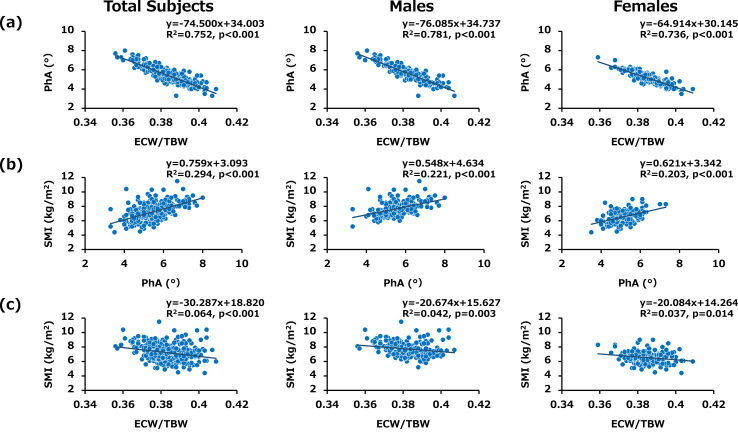
Since it has been reported that low PhA and high ECW/TBW ratio can indicate malnutrition in critically ill patients [34] and since those two parameters were calculated on the basis of identical impedance data examined by InBody S10, they have been thought to have a close association. We therefore evaluated the relationship between PhA and ECW/TBW and found that the level of PhA was closely and inversely correlated with the ratio of ECW/TBW in total subjects (R2 = 0.752, p < 0.001), in male subjects (R2 = 0.781, p < 0.001) and in female subjects (R2 = 0.736, p < 0.001) (Fig. 1(a)). In addition, statistically significant associations were found between SMI and PhA in total subjects (R2 = 0.294, p < 0.001), in male subjects (R2 = 0.221, p < 0.001) and in female subjects (R2 = 0.203, p < 0.001) (Fig. 1(b)) and between SMI and ratio of ECW/TBW in total subjects (R2 = 0.064, p < 0.001), in male subjects (R2 = 0.042, p = 0.003) and in female subjects (R2 = 0.037, p = 0.014) (Fig. 1(c)). Thus, close associations were found among the three BIA-associated parameters, PhA, ECW/TBW and SMI. Therefore, we thought that each parameter needs to be divided into an independent linear regression analysis for the increments of Hgb and Hct.
Fig. 1.
Associations between BIA-associated parameters in total subjects, male subjects and female subjects (PhA, ECW/TBW and SMI) Scatter plots showing relationships between (a) PhA and ECW/TBW, (b) SMI and PhA and (c) SMI and ECW/TBW. Inverse relationships were found between PhA and ECW/TBW and between SMI and ECW/TBW in each group. A positive relationship was found between SMI and PhA in each group. Data for all 209 males and 162 females enrolled in this study are shown.
3.3. Associations of PhA with Hgb and Hct levels
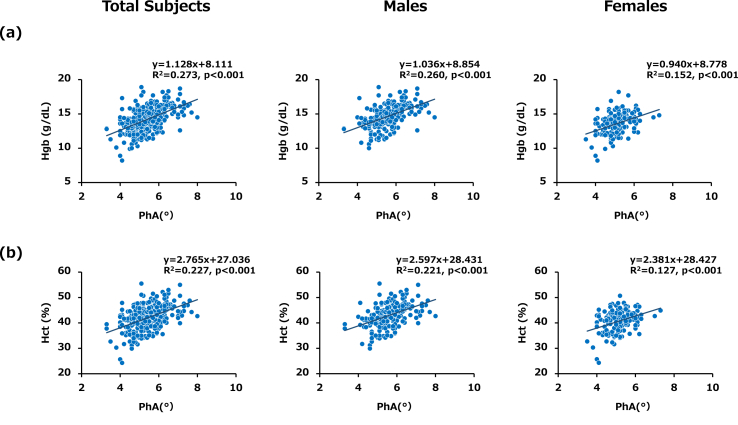
Simple linear regression analysis for the entire series of subjects showed that PhA was positively correlated with Hgb and Hct levels in total subjects (R2 = 0.273, p < 0.001 and R2 = 0.227, p < 0.001, respectively), in male subjects (R2 = 0.260, p < 0.001 and R2 = 0.221, p < 0.001, respectively) and in female subjects (R2 = 0.152, p < 0.001 and R2 = 0.127, p < 0.001, respectively) (Table 2 and Fig. 2 (a) and (b)). The common clinical factors identified for increments of Hgb and Hct levels in total subjects were age, male gender, BMI, Alb, current smoking, PhA, and treatment with a sodium-glucose cotransporter 2 inhibitor (SGLT2i) (Table 2). Conversely, Cr and treatment with an angiotensin II receptor blocker (ARB)/angiotensin converting enzyme inhibitor (ACEi) were inverse clinical factors against increments of Hgb and Hct levels in total subjects (Table 2).
Table 2.
Simple linear regression analysis for determinants of Hgb and Hct levels in the cross-sectional study.
| Total Subjects (n = 371) |
Males (n = 209) |
Females (n = 162) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hgb | Hct | Hgb | Hct | Hgb | Hct | |||||||
| variables | coefficient | p value | coefficient | p value | coefficient | p value | coefficient | p value | coefficient | p value | coefficient | p value |
| Male | 1.095 | <0.001 | 2.297 | <0.001 | ||||||||
| Age | −0.057 | <0.001 | −0.125 | <0.001 | −0.062 | <0.001 | −0.119 | <0.001 | −0.052 | <0.001 | −0.141 | <0.001 |
| BMI | 0.092 | <0.001 | 0.226 | <0.001 | 0.107 | <0.001 | 0.223 | 0.004 | 0.078 | 0.002 | 0.233 | 0.001 |
| SBP | −0.005 | 0.336 | −0.005 | 0.729 | −0.007 | 0.330 | −0.006 | 0.784 | 0.000 | 0.969 | 0.002 | 0.904 |
| LDL-C | 0.007 | 0.009 | 0.015 | 0.066 | 0.011 | 0.005 | 0.021 | 0.057 | 0.006 | 0.117 | 0.012 | 0.257 |
| HDL-C | −0.002 | 0.766 | 0.005 | 0.753 | 0.014 | 0.100 | 0.046 | 0.070 | 0.005 | 0.581 | 0.013 | 0.579 |
| TG | 0.002 | 0.024 | 0.003 | 0.339 | 0.002 | 0.067 | 0.002 | 0.666 | 0.001 | 0.758 | 0.001 | 0.805 |
| Alb | 1.487 | <0.001 | 4.020 | <0.001 | 1.493 | <0.001 | 4.115 | <0.001 | 1.316 | <0.001 | 3.513 | <0.001 |
| UA | 0.132 | 0.040 | 0.164 | 0.361 | 0.104 | 0.280 | 0.172 | 0.541 | −0.132 | 0.130 | −0.489 | 0.043 |
| Casual PG | 0.003 | 0.009 | 0.004 | 0.245 | 0.005 | 0.007 | 0.007 | 0.200 | 0.001 | 0.490 | 0.000 | 0.994 |
| HbA1c | 0.134 | 0.005 | 0.210 | 0.118 | 0.118 | 0.059 | 0.074 | 0.689 | 0.180 | 0.005 | 0.437 | 0.015 |
| Cr | −0.661 | 0.005 | −2.231 | 0.001 | −0.916 | 0.001 | −2.420 | 0.004 | −2.287 | <0.001 | −6.934 | <0.001 |
| Hypertension | −0.255 | 0.160 | −0.600 | 0.236 | −0.189 | 0.417 | −0.321 | 0.638 | −0.086 | 0.737 | −0.454 | 0.523 |
| Current Smoking | 0.992 | 0.000 | 1.915 | 0.002 | 0.518 | 0.041 | 0.840 | 0.260 | 1.241 | 0.016 | 2.752 | 0.054 |
| Dyslipidemia | 0.077 | 0.694 | 0.183 | 0.740 | 0.159 | 0.535 | 0.269 | 0.720 | 0.012 | 0.965 | 0.158 | 0.833 |
| Duration of DM | −0.020 | 0.015 | −0.026 | 0.275 | −0.029 | 0.008 | −0.049 | 0.130 | −0.012 | 0.302 | −0.002 | 0.960 |
| PhA | 1.128 | <0.001 | 2.587 | <0.001 | 1.036 | <0.001 | 2.347 | <0.001 | 0.939 | <0.001 | 2.381 | <0.001 |
| ECW/TBW | −93.337 | <0.001 | −220.179 | <0.001 | −95.590 | <0.001 | −222.136 | <0.001 | −69.844 | <0.001 | −177.214 | <0.001 |
| SMI | 0.605 | <0.001 | 1.336 | <0.001 | 0.431 | <0.001 | 0.777 | 0.028 | 0.535 | <0.001 | 1.568 | <0.001 |
| ARB or ACEi | −0.512 | 0.004 | −1.389 | 0.005 | −0.266 | 0.260 | −0.571 | 0.410 | −0.657 | 0.005 | −2.064 | 0.002 |
| CCB | −0.495 | 0.006 | −1.212 | 0.016 | −0.614 | 0.009 | −1.502 | 0.030 | −0.309 | 0.206 | −0.772 | 0.254 |
| β blocker | −0.276 | 0.533 | −0.717 | 0.562 | −0.441 | 0.410 | −0.873 | 0.578 | −0.304 | 0.658 | −1.149 | 0.545 |
| MR antagonist | −0.885 | 0.362 | −2.313 | 0.395 | −0.848 | 0.470 | −1.293 | 0.707 | −1.302 | 0.389 | −5.065 | 0.227 |
| Statin | −0.136 | 0.430 | 0.065 | 0.893 | 0.004 | 0.985 | 0.505 | 0.448 | −0.082 | 0.729 | −0.004 | 0.995 |
| Ezetimibe | −0.155 | 0.662 | −0.172 | 0.862 | 0.126 | 0.790 | 0.188 | 0.815 | −0.433 | 0.358 | −0.943 | 0.470 |
| Other lipid-lowering drugs | −0.672 | 0.235 | −2.158 | 0.173 | −0.809 | 0.279 | −2.040 | 0.352 | −0.481 | 0.529 | −2.265 | 0.285 |
| Antiplatelet | −0.440 | 0.121 | −0.868 | 0.274 | −0.786 | 0.015 | −1.651 | 0.083 | −0.465 | 0.369 | −0.742 | 0.605 |
| SU or Glinide | −0.050 | 0.820 | 0.132 | 0.828 | −0.085 | 0.755 | 0.188 | 0.815 | −0.251 | 0.430 | −0.499 | 0.572 |
| Metformin | 0.330 | 0.058 | 0.766 | 0.115 | 0.454 | 0.046 | 0.861 | 0.198 | 0.194 | 0.413 | 0.696 | 0.289 |
| DPP4i | −0.143 | 0.418 | −0.507 | 0.306 | −0.179 | 0.443 | −0.729 | 0.285 | −0.144 | 0.549 | −0.319 | 0.632 |
| SGLT2i | 0.884 | <0.001 | 2.889 | <0.001 | 0.910 | <0.001 | 2.653 | <0.001 | 0.765 | 0.001 | 3.024 | <0.001 |
| αGI | −0.311 | 0.219 | −0.750 | 0.289 | −0.064 | 0.851 | 0.021 | 0.983 | −0.502 | 0.131 | −1.444 | 0.118 |
| Pioglitazone | 0.480 | 0.329 | 2.152 | 0.118 | 0.768 | 0.261 | 2.461 | 0.219 | 0.335 | 0.594 | 2.146 | 0.217 |
| Insulin | −0.358 | 0.083 | −1.245 | 0.031 | −0.254 | 0.363 | −1.332 | 0.104 | −0.339 | 0.214 | −0.862 | 0.254 |
| GLP-1RA | 0.074 | 0.792 | 0.257 | 0.743 | −0.392 | 0.312 | −0.952 | 0.403 | 0.714 | 0.047 | 1.838 | 0.065 |
Fig. 2.
Associations of PhA with Hgb and Hct levels in total subjects, male subjects and female subjects (a) Scatter plot showing the relationship between PhA and Hgb. A positive relationship was found between PhA and Hgb in each group. (b) Scatter plot showing the relationship between PhA and Hct. A positive relationship was found between PhA and Hct in each group. Data for all 209 males and 162 females enrolled in this study are shown.
We next performed multiple regression analysis with PhA and those identified variables. Conclusively, the common clinical factors confirmed for increments of Hgb and Hct levels were male gender, BMI, Alb, current smoking, SGLT2i medication and PhA and the common clinical factors for decrements of Hgb and Hct levels were Cr and ARB/ACEi medication in total subjects (Table 3). Significant positive associations of PhA with Hgb and Hct were also found regardless of sex (Table 3). Power analysis in each multiple regression analysis proved that the sample sizes in total analysis, male analysis and female analysis were valid. Because each value of variance inflation factor (VIF) was less than 2.0 (Supplemental data 1), all multiple regression analyses including PhA were appropriate. In addition, the t values in multiple regression analysis indicated significant clinical contributions of PhA for increments of both Hgb and Hct in total subjects, male subjects and female subjects.
Table 3.
Multiple linear regression analysis with variables (including PhA) identified by simple linear regression analysis for determinants of Hgb and Hct levels in the cross-sectional study.
| Total Subjects (n = 371) |
Males (n = 209) |
Females (n = 162) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hgb (R2 = 0.455) | Hct (R2 = 0.455) | Hgb (R2 = 0.394) | Hct (R2 = 0.385) | Hgb (R2 = 0.445) | Hct (R2 = 0.513) | |||||||
| variables | t value | p value | t value | p value | t value | p value | t value | p value | t value | p value | t value | p value |
| Male | 5.486 | <0.001 | 4.475 | <0.001 | ||||||||
| Age | −2.288 | 0.023 | −1.975 | 0.049 | −2.322 | 0.021 | −1.964 | 0.051 | 0.112 | 0.911 | 0.196 | 0.845 |
| BMI | 2.714 | 0.007 | 2.747 | 0.006 | 1.462 | 0.145 | 1.151 | 0.251 | 2.243 | 0.026 | 2.658 | 0.009 |
| Alb | 2.987 | 0.003 | 2.315 | 0.021 | 2.381 | 0.018 | 1.658 | 0.099 | 2.195 | 0.030 | 2.154 | 0.033 |
| Cr | −3.997 | <0.001 | −4.559 | <0.001 | −2.027 | 0.044 | −2.235 | 0.027 | −4.780 | <0.001 | −5.557 | <0.001 |
| Current Smoking | 2.078 | 0.038 | 2.031 | 0.043 | 1.307 | 0.193 | 1.377 | 0.170 | 2.644 | 0.009 | 2.327 | 0.021 |
| ARB or ACEi | −2.260 | 0.024 | −2.841 | 0.005 | −0.652 | 0.515 | −0.981 | 0.328 | −2.739 | 0.007 | −3.346 | 0.001 |
| SGLT2i | 5.587 | <0.001 | 7.685 | <0.001 | 4.060 | <0.001 | 5.190 | <0.001 | 3.701 | <0.001 | 5.637 | <0.001 |
| PhA | 4.930 | <0.001 | 4.819 | <0.001 | 3.496 | <0.001 | 3.532 | <0.001 | 3.701 | <0.001 | 3.693 | <0.001 |
3.4. Associations of ECW/TBW with Hgb and Hct levels
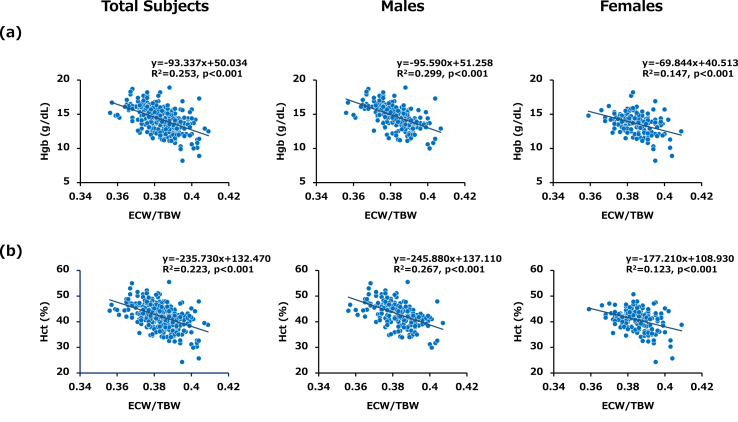
Simple linear regression analysis for the entire series of subjects showed that the ratio of ECW/TBW was negatively correlated with Hgb and Hct levels in total subjects (R2 = 0.253, p < 0.001 and R2 = 0.223, p < 0.001, respectively), in male subjects (R2 = 0.299, p < 0.001 and R2 = 0.267, p < 0.001, respectively) and in female subjects (R2 = 0.147, p < 0.001 and R2 = 0.123, p < 0.001, respectively) (Fig. 3 (a) and (b)). We next performed multiple regression analysis with ECW/TBW and the confirmed variables as in PhA analysis. Consequently, independently inverse associations of ECW/TBW with Hgb and Hct were observed in the subjects regardless of sex (Table 4). All values of VIF were below 2.0 (Supplemental data 1). The t values in multiple regression analysis showed considerable clinical contributions of ECW/TBW for decrements of both Hgb and Hct in total subjects, male subjects and female subjects. Moreover, not only ECW/TBW but also ECW/ICW was a negative contributor for levels of both Hgb and Hct in total subjects, male subjects and female subjects (Supplemental data 2).
Fig. 3.
Associations of ECW/TBW with Hgb and Hct levels in total subjects, male subjects and female subjects (a) Scatter plot showing the relationship between ECW/TBW and Hgb. An inverse relationship was found between ECW/TBW and Hgb in each group. (b) Scatter plot showing the relationship between ECW/TBW and Hct. An inverse relationship was found between ECW/TBW and Hct in each group. Data for all 209 males and 162 females enrolled in this study are shown.
Table 4.
Multiple linear regression analysis with variables (including ECW/TBW) identified by simple linear regression analysis for determinants of Hgb and Hct levels in the cross-sectional study.
| Total Subjects (n = 371) |
Males (n = 209) |
Females (n = 162) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hgb (R2 = 0.468) | Hct (R2 = 0.471) | Hgb (R2 = 0.423) | Hct (R2 = 0.420) | Hgb (R2 = 0.435) | Hct (R2 = 0.504) | |||||||
| variables | t value | p value | t value | p value | t value | p value | t value | p value | t value | p value | t value | p value |
| Male | 6.691 | <0.001 | 5.576 | <0.001 | ||||||||
| Age | −2.068 | 0.039 | −1.665 | 0.097 | −1.931 | 0.055 | −1.500 | 0.135 | −0.177 | 0.860 | −0.107 | 0.915 |
| BMI | 3.569 | <0.001 | 3.601 | <0.001 | 2.121 | 0.035 | 1.811 | 0.072 | 2.690 | 0.008 | 3.096 | 0.002 |
| Alb | 2.626 | 0.009 | 1.886 | 0.060 | 2.033 | 0.043 | 1.242 | 0.216 | 2.060 | 0.041 | 2.032 | 0.044 |
| Cr | −3.802 | <0.001 | −4.385 | <0.001 | −1.874 | 0.062 | −2.094 | 0.038 | −4.564 | <0.001 | −5.332 | <0.001 |
| Current Smoking | 2.112 | 0.035 | 2.060 | 0.040 | 1.287 | 0.199 | 1.351 | 0.178 | 2.736 | 0.009 | 2.421 | 0.017 |
| ARB or ACEi | −2.053 | 0.041 | −2.663 | 0.008 | −0.586 | 0.558 | −0.931 | 0.353 | −2.739 | 0.007 | −2.919 | 0.004 |
| SGLT2i | 5.654 | <0.001 | 7.794 | <0.001 | 3.930 | <0.001 | 5.076 | <0.001 | 3.661 | <0.001 | 5.569 | <0.001 |
| ECW/TBW | −5.832 | <0.001 | −5.940 | <0.001 | −4.793 | <0.001 | −5.017 | <0.001 | −3.294 | <0.001 | −3.254 | 0.001 |
3.5. Associations of SMI with Hgb and Hct levels
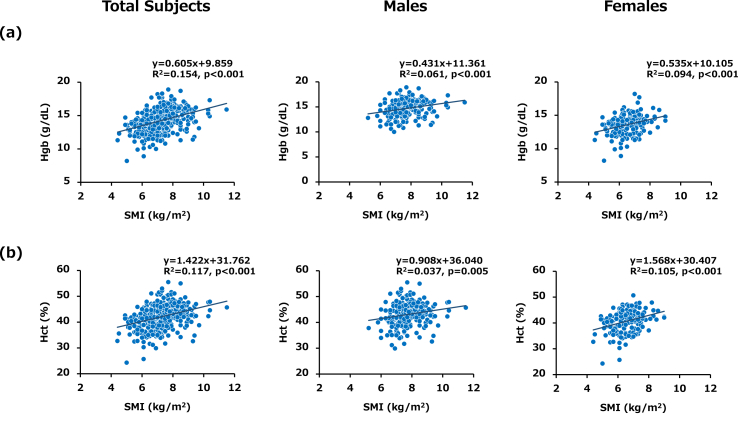
Simple linear regression analysis for the entire series of subjects showed that SMI was positively correlated with Hgb and Hct levels in total subjects (R2 = 0.154, p < 0.001 and R2 = 0.117, p < 0.001, respectively), in male subjects (R2 = 0.061, p < 0.001 and R2 = 0.037, p = 0.005, respectively) and in female subjects (R2 = 0.094, p < 0.001 and R2 = 0.105, p < 0.001, respectively) (Fig. 4 (a) and (b)). As in the other analyses, we performed multiple regression analysis with SMI and the confirmed variables. Unexpectedly, neither Hgb nor Hct was significantly associated with SMI in total subjects and in male subjects (Table 5). Because multiple regression analysis for determinants of SMI showed that age and BMI were colsely associated with SMI (Supplemental data 3), the significant relationships found in simple linear regression analysis between SMI and Hgb and between SMI and Hct were feigned associations.
Fig. 4.
Associations of SMI with Hgb and Hct levels in total subjects, male subjects and female subjects (a) Scatter plot showing the relationship between SMI and Hgb. A positive relationship was found between SMI and Hgb in each group. (b) Scatter plot showing the relationship between SMI and Hct. A positive relationship was found between SMI and Hct in each group. Data for all 209 males and 162 females enrolled in this study are shown.
Table 5.
Multiple linear regression analysis with variables (including SMI) identified by simple linear regression analysis for determinants of Hgb and Hct levels in the cross-sectional study.
| Total Subjects (n = 371) |
Males (n = 209) |
Females (n = 162) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hgb (R2 = 0.420) | Hct (R2 = 0.421) | Hgb (R2 = 0.357) | Hct (R2 = 0.347) | Hgb (R2 = 0.409) | Hct (R2 = 0.487) | |||||||
| variables | t value | p value | t value | p value | t value | p value | t value | p value | t value | p value | t value | p value |
| Male | 4.465 | <0.001 | 3.823 | <0.001 | ||||||||
| Age | −4.015 | <0.001 | −3.726 | <0.001 | −3.653 | <0.001 | −3.479 | <0.001 | −1.107 | 0.270 | −0.899 | 0.370 |
| BMI | 1.507 | 0.133 | 1.637 | 0.102 | 1.617 | 0.108 | 1.640 | 0.102 | 0.077 | 0.939 | 0.067 | 0.947 |
| Alb | 4.801 | <0.001 | 4.068 | <0.001 | 3.548 | <0.001 | 2.782 | 0.006 | 3.530 | <0.001 | 3.503 | <0.001 |
| Cr | −3.850 | <0.001 | −4.378 | <0.001 | −1.930 | 0.550 | −2.086 | 0.038 | −4.689 | <0.001 | −5.497 | <0.001 |
| Current Smoking | 2.260 | 0.024 | 2.213 | 0.027 | 1.466 | 0.144 | 1.537 | 0.126 | 2.645 | 0.009 | 2.331 | 0.021 |
| ARB or ACEi | −1.929 | 0.054 | −2.478 | 0.014 | −0.548 | 0.585 | −0.809 | 0.419 | −2.273 | 0.024 | −2.921 | 0.004 |
| SGLT2i | 5.616 | <0.001 | 7.651 | <0.001 | 4.451 | <0.001 | 5.532 | <0.001 | 3.024 | 0.003 | 4.915 | <0.001 |
| SMI | 1.041 | 0.299 | 0.881 | 0.379 | −0.137 | 0.891 | −0.506 | 0.614 | 1.861 | 0.065 | 2.215 | 0.028 |
3.6. Influence of diabetic conditions on PhA, ECW/TBW and SMI
To clarify the influence of diabetic conditions on the BIA-related parameters, we performed multiple liner regression analysis including sex, age, HbA1c, duration of DM and use of hypogycemic agents for each index. Conclusively, both PhA and ECW/TBW were significantly associated with duration of DM but not with HbA1c. On the other hand, SMI was not associated with either duration of DM or HbA1c (Supplemental data 4).
4. Discussion
In the present study, we showed that PhA was positively associated with Hgb and Hct levels and that ECW/TBW was inversely associated with Hgb and Hct levels in patients with diabetes. Since PhA reflects the overall nutritional status in the body and since ECW/TBW ratio is an index of systemic fluid retention, a close relationship between those BIA-associated parameters was found in this study.
4.1. Associations of PhA with Hgb and Hct levels
Several studies have shown a relationship between bioimpedance of RBCs and RBC-associated disorders. Varlet-Marie et al. reported that aggregability and deformability of RBCs were associated with whole body impedance at 50 kHz [35] and Tran et al. reported that the cytoplasm released following RBC hemolysis is closely associated with the change in electrical characteristics of blood cells [36]. In addition, Ringaitiene et al. reported that lower PhA before cardiac surgery is one of the risk factors for RBC transfusion after surgery [37]. The results of those studies suggest that impedance of RBCs is associated with the lifespan of RBCs by reflecting the membrane stability and deformability of RBCs.
Vallentino et al. demonstrated that subjects with PhA <4 presented an increased risk for sarcopenia in cancer patients after hydration status and suggested that adequate hydration and evaluation of fluid status via BIA are very important issues [38]. Moreover, Gomes et al. emphasized the importance of measuring PhA to assess intracellular and extracellular hydration in cancer patients [39]. Since it has been reported that renal anemia is associated with hydration status in patients with CKD [40], PhA may be associated with Hgb and Hct levels via influence of the hydration status in individuals.
Moreover, chronic inflammation plays a pivotal role in the development of anemia [41] and because it has been reported that PhA was inversely associated with high-sensitivity C-reactive protein (hs-CRP) levels in both sexes regardless of body weight [42], the inverse associations of PhA with Hgb and Hct levels found in our study suggest that PhA reflects levels of chronic inflammation.
Taken together, our results showing positive associations of PhA with Hgb and Hct levels raised the possibilities that high PhA indicates preserved RBC lifespan with sufficient membrane stability and deformability of the cells, adequate hydration status and low level of inflammation in the human body.
4.2. Associations of ECW/TBW with Hgb and Hct levels
It has been reported that there is a relationship between fluid retention and anemia in patients with CKD [43] and patients with heart failure [44,45], and hemodilutional anemia is often found in patients with volume-overloaded heart failure. No patients with volume-overloaded heart failure were included in the present study; however, we speculate that the inverse associations found between ECW/TBW and Hgb and between ECW/TBW and Hct were reflected by subclinical edema. In addition, Hara et al. demonstrated that a high ECW/TBW ratio was positively associated with EPO hyporesponsiveness represented by the EPO resistance index in patients on continuous ambulatory peritoneal dialysis [46]. Their study suggested that fluid retention invalidates erythropoiesis by EPO stimulation. As in the case of PhA, it has also been demonstrated that ECW/TBW ratio was positively correlated with hs-CRP levels in peritoneal dialysis patients [47].
Taken together, our results showing negative associations of ECW/TBW ratio with Hgb and Hct levels provided the possibility that high ECW/TBW indicates overhydration status and excessive inflammation in individuals.
4.3. Other clinical variables for determinants of Hgb and Hct levels
Multiple regression analysis revealed that male gender and BMI were positively associated with Hgb and Hct levels. Because severe hypogonadism induced by medical or surgical castration significantly reduces Hgb, testosterone has been recognized to stimulate erythropoiesis [48,49]. Therefore, the anabolic effect of an androgen is plausible reasoning for higher levels of Hgb and Hct in males than in females.
Increased BMI has been shown to be associated with a high prevalence of obstructive sleep apnea syndrome (OSAS) and it has been demonstrated that the severity of OSAS is significantly associated with increased Hct, even after controlling for possible confounding variables [50]. Therefore, increased BMI might elevate levels of Hgb and Hct via acceleration of an OSAS-induced hypoxic condition in the body. In this study, current smoking was positively and independently associated with Hgb and Hct levels. It is well known that tissue hypoxia induced by carbon monoxide in tobacco smoke causes secondary polycythemia [[51], [52], [53]].
Malnutrition and chronic inflammation can cause hypoalbuminemia leading to reduced erythropoiesis and hemodilutional anemia [54]. Since it has been shown that serum Alb concentration is a pivotal predictor of both Hgb level and EPO sensitivity in chronic hemodialysis patients, the positive associations found between serum Alb level and values of Hgb and Hct in our study are consistent with previous reports [[55], [56], [57], [58]].
As for medications used in this study, ARB and ACEi medications were inversely associated with Hgb and Hct levels. A meta-analysis showed that use of renin angiotensin system (RAS) inhibitors was significantly and positively associated with incidence of anemia [59], and previous studies showed that RAS inhibitors reduce circulating levels of insulin-like growth factor 1 and EPO [[60], [61], [62]]. Therefore, our results regarding the negative influence of ARB/ACEi on Hgb and Hct levels are not inconsistent with those reports. Furthermore, SGLT2i medication was found to be a positive determinant for increments of Hgb and Hct levels. Previous studies showed that Hct increases during SGLT2i administration [63,64], and an improvement in erythropoiesis via promotion of EPO production is thought to be one of the reasons [65]. Additionally, Ghanim et al. showed that dapagliflozin treatment significantly reduced circulating hepcidin and ferritin concentrations while causing an increase of plasma erythroferrone, a hepcidin inhibitor, and they showed that dapagliflozin increased plasma transferrin levels [66]. Taken together, SGLT2i promotes erythropoiesis via improving EPO production and ferrokinetics.
Since a meta-analysis of randomized controlled trials has shown that the addition of SGLT2i to conventional diuretic therapy in patients with heart failure (HF) was associated with lower rates of all-cause death, readmissions for HF, and the composite of cardiovascular death [67] and since SGLT2i administration has shown beneficial effects in renal complications via lowering diabetic glomerular hyperfiltration, BP, body weight, UA concentrations, lipid peroxidation and inflammation [68], SGLT2i administration seems to be a pivotal pharmacological intervention for prevention of cardio-renal anemia syndrome.
4.4. Study limitations
The results of the present study cannot be extended to the general population because (i) we enrolled only patients with DM and (ii) the physiological roles of PhA and ECW/TBW in erythropoiesis may differ in patients with T1DM and patients with T2DM. The number of patients with T1DM who were enrolled in this study was not sufficient to clarify this issue, and a large-scale investigation is required to assess and clarify the prognostic value of the BIA-associated biomarkers in the general population as well as differences between patients with T1DM and patients with T2DM. Second, since ferrokinetics-associated markers and EPO levels were not analyzed in the present study, the associations between those markers and PhA or ECW/TBW remained unclear. Thus, a large-scale investigation and further assessment of the influence of ferrokinetics-associated markers and EPO levels are required to clarify the physiological roles of PhA and ECW/TBW in levels of Hgb and Hct in the general population. Third, although BIA examinations are recommended after at least a 4-h fasting condition, BIA testing were performed after at least a 2-h fasting condition in our study. Therefore, there is a possibility that the shorter fasting time influenced the BIA-estimated values. Fourth, since this study was a cross-sectional study, causal relationships between values of PhA or ECW/TBW and RBC content including Hgb and Hct remained unclear. A large-scale prospective study is also needed to clarify these issues. Fifth, because the BIA-estimated parameters in this study reflect the whole body cell condition and because RBCs are a very small fraction of the whole body cell mass contributing to PhA and ECW/TBW, further examination of specificity for estimation of erythropoiesis and RBC life span is required.
5. Conclusion
In conclusion, the present study demonstrated that PhA and ECW/TBW were associated with levels of Hgb and Hct in patients with diabetes regardless of sex and confounding factors. Since both PhA and ECW/TBW have been shown to be associated with the development of CHF and CKD, those indices may be key parameters for risk assessment of cardio-renal anemia syndrome. Thus, a large-scale longitudinal study is needed to clarify whether examinations of PhA and ECW/TBW enable prediction of the development of anemia in patients with diabetes.
Author contribution statement
Taiki Hori, Shingen Nakamura and Ken-ichi Aihara: conceived and designed the experiments, performed the experiments, analyzed and interpreted the data and wrote the paper.
Hiroki Yamagami, Saya Yasui, Minae Hosoki, Tomoyo Hara, Yukari Mitsui, Shiho Masuda, Kiyoe Kurahashi, Sumiko Yoshida and Takeshi Harada: performed the experiments and contributed the analysis tools or data.
Akio Kuroda, Toshiki Otoda, Tomoyuki Yuasa, Itsuro Endo, Munehide Matsuhisa and Masahiro Abe: analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are very grateful to Professor Kokichi Arisawa (Department of Preventive Medicine, Tokushima University Graduate School of Biomedical Sciences) for statistical advice. In addition, we would like to thank S.E.S Translation and Proofreading Services for English language editing.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e14724.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Bosman D.R., Winkler A.S., Marsden J.T., Macdougall I.C., Watkins P.J. Anemia with erythropoietin deficiency occurs early in diabetic nephropathy. Diabetes Care. 2001;24:495–499. doi: 10.2337/diacare.24.3.495. [DOI] [PubMed] [Google Scholar]
- 2.Thomas M.C., MacIsaac R.J., Tsalamandris C., Power D., Jerums G. Unrecognized anemia in patients with diabetes: a cross-sectional survey. Diabetes Care. 2003;26:1164–1169. doi: 10.2337/diacare.26.4.1164. [DOI] [PubMed] [Google Scholar]
- 3.Gauci R., Hunter M., Bruce D.G., Davis W.A., Davis T.M.E. Anemia complicating type 2 diabetes: prevalence, risk factors and prognosis. J. Diabet. Complicat. 2017;31:1169–1174. doi: 10.1016/j.jdiacomp.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner R.N., Chumlea W.C., Roche A.F. Bioelectric impedance phase angle and body composition. Am. J. Clin. Nutr. 1988;48:16–23. doi: 10.1093/ajcn/48.1.16. [DOI] [PubMed] [Google Scholar]
- 5.Selberg O., Selberg D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur. J. Appl. Physiol. 2002;86:509–516. doi: 10.1007/s00421-001-0570-4. [DOI] [PubMed] [Google Scholar]
- 6.Hui D., Bansal S., Morgado M., Dev R., Chisholm G., Bruera E. Phase angle for prognostication of survival in patients with advanced cancer: preliminary findings. Cancer. 2014;120:2207–2214. doi: 10.1002/cncr.28624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S.Y., Lee Y.J., Yang J.H., Kim C.M., Choi W.S. The association between phase angle of bioelectrical impedance analysis and survival time in advanced cancer patients: preliminary study. Korean J Fam Med. 2014;35:251–256. doi: 10.4082/kjfm.2014.35.5.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Władysiuk M.S., Mlak R., Morshed K., Surtel W., Brzozowska A., Małecka-Massalska T. Bioelectrical impedance phase angle as a prognostic indicator of survival in head-and-neck cancer. Curr. Oncol. 2016;23:e481–e487. doi: 10.3747/co.23.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maggiore Q., Nigrelli S., Ciccarelli C., Grimaldi C., Rossi G.A., Michelassi C. Nutritional and prognostic correlates of bioimpedance indexes in hemodialysis patients. Kidney Int. 1996;50:2103–2108. doi: 10.1038/ki.1996.535. [DOI] [PubMed] [Google Scholar]
- 10.Chertow G.M., Jacobs D.O., Lazarus J.M., Lew N.L., Lowrie E.G. Phase angle predicts survival in hemodialysis patients. J. Ren. Nutr. 1997;7:204–207. [Google Scholar]
- 11.Wilhelm-Leen E.R., Hall Y.N., Horwitz R.I., Chertow G.M. Phase angle, frailty and mortality in older adults. J. Gen. Intern. Med. 2014;29:147–154. doi: 10.1007/s11606-013-2585-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kilic M.K., Kizilarslanoglu M.C., Arik G., Bolayir B., Kara O., Dogan Varan H., et al. Association of bioelectrical impedance analysis-derived phase angle and sarcopenia in older adults. Nutr. Clin. Pract. 2017;32:103–109. doi: 10.1177/0884533616664503. [DOI] [PubMed] [Google Scholar]
- 13.Ryu H., Park H.C., Kim H., Heo J., Kang E., Hwang Y.H., et al. Bioelectrical impedance analysis as a nutritional assessment tool in autosomal dominant polycystic kidney disease. PLoS One. 2019;14 doi: 10.1371/journal.pone.0214912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J.H., Jo Y.I., Lee J.H. Clinical usefulness of bioimpedance analysis for assessing volume status in patients receiving maintenance dialysis. Korean J. Intern. Med. (Korean Ed.) 2018;33:660–669. doi: 10.3904/kjim.2018.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim C.R., Shin J.H., Hwang J.H., Kim S.H. Monitoring volume status using bioelectrical impedance analysis in chronic hemodialysis patients. Asaio j. 2018;64:245–252. doi: 10.1097/MAT.0000000000000619. [DOI] [PubMed] [Google Scholar]
- 16.Kishino K., Enomoto H., Shimono Y., Moriwaki E.I., Nishikawa H., Nishimura T., et al. Association of an overhydrated state with the liver fibrosis and prognosis of cirrhotic patients. In Vivo. 2020;34:1347–1353. doi: 10.21873/invivo.11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dittmar M., Reber H., Kahaly G.J. Bioimpedance phase angle indicates catabolism in Type 2 diabetes. Diabet. Med. 2015;32:1177–1185. doi: 10.1111/dme.12710. [DOI] [PubMed] [Google Scholar]
- 18.Jun M.H., Ku B., Kim J., Kim K.H., Kim J.U. Mediation effect of the duration of diabetes mellitus on the decrease in bioimpedance phase angles in ethnically Korean people: a multicenter clinical study. J Diabetes Investig. 2020:790–802. doi: 10.1111/jdi.13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Low S., Pek S., Liu Y.L., Moh A., Ang K., Tang W.E., et al. Higher extracellular water to total body water ratio was associated with chronic kidney disease progression in type 2 diabetes. J. Diabet. Complicat. 2021;35 doi: 10.1016/j.jdiacomp.2021.107930. [DOI] [PubMed] [Google Scholar]
- 20.Silverberg D.S., Wexler D., Iaina A., Steinbruch S., Wollman Y., Schwartz D. Anemia, chronic renal disease and congestive heart failure--the cardio renal anemia syndrome: the need for cooperation between cardiologists and nephrologists. Int. Urol. Nephrol. 2006;38:295–310. doi: 10.1007/s11255-006-0064-8. [DOI] [PubMed] [Google Scholar]
- 21.Scicchitano P., Massari F. The role of bioelectrical phase angle in patients with heart failure. Rev. Endocr. Metab. Disord. 2022:1–13. doi: 10.1007/s11154-022-09757-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miranda Alatriste P.V., Ramirez E.C., Carsi X.A., Cruz-Rivera C., Espinosa-Cuevas A. Hydration status according to impedance vectors and its association with clinical and biochemical outcomes and mortality in patients with chronic kidney disease. Nutr. Hosp. 2022;39:1037–1046. doi: 10.20960/nh.03970. [DOI] [PubMed] [Google Scholar]
- 23.Valentinuzzi M.E., Morucci J.P., Felice C.J. Bioelectrical impedance techniques in medicine. Part II: monitoring of physiological events by impedance. Crit. Rev. Biomed. Eng. 1996;24:353–466. [PubMed] [Google Scholar]
- 24.Vannini F.D., Antunes A.A., Caramori J.C., Martin L.C., Barretti P. Associations between nutritional markers and inflammation in hemodialysis patients. Int. Urol. Nephrol. 2009;41:1003–1009. doi: 10.1007/s11255-009-9563-8. [DOI] [PubMed] [Google Scholar]
- 25.Kim D.H., Oh D.J. Phase angle values, a good indicator of nutritional status, are associated with median value of hemoglobin rather than hemoglobin variability in hemodialysis patients. Ren. Fail. 2021;43:327–334. doi: 10.1080/0886022X.2020.1870137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brantlov S., Jodal L., Lange A., Rittig S., Ward L.C. Standardisation of bioelectrical impedance analysis for the estimation of body composition in healthy paediatric populations: a systematic review. J. Med. Eng. Technol. 2017;41:460–479. doi: 10.1080/03091902.2017.1333165. [DOI] [PubMed] [Google Scholar]
- 27.Sartorio A., Malavolti M., Agosti F., Marinone P.G., Caiti O., Battistini N., et al. Body water distribution in severe obesity and its assessment from eight-polar bioelectrical impedance analysis. Eur. J. Clin. Nutr. 2005;59:155–160. doi: 10.1038/sj.ejcn.1602049. [DOI] [PubMed] [Google Scholar]
- 28.Cha K., Chertow G.M., Gonzalez J., Lazarus J.M., Wilmore D.W. Multifrequency bioelectrical impedance estimates the distribution of body water. J. Appl. Physiol. 1995;79:1316–1319. doi: 10.1152/jappl.1995.79.4.1316. [DOI] [PubMed] [Google Scholar]
- 29.Yanishi M., Kinoshita H., Tsukaguchi H., Kimura Y., Koito Y., Jino E., et al. Dual energy X-ray absorptiometry and bioimpedance analysis are clinically useful for measuring muscle mass in kidney transplant recipients with sarcopenia. Transplant. Proc. 2018;50:150–154. doi: 10.1016/j.transproceed.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan P.A., Still C.D., Jamieson S.T., Dixon C.B., Irving B.A., Andreacci J.L. Evaluation of multi-frequency bioelectrical impedance analysis for the assessment of body composition in individuals with obesity. Obes Sci Pract. 2019;5:141–147. doi: 10.1002/osp4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fang W.H., Yang J.R., Lin C.Y., Hsiao P.J., Tu M.Y., Chen C.F., et al. Accuracy augmentation of body composition measurement by bioelectrical impedance analyzer in elderly population. Medicine (Baltim.) 2020;99 doi: 10.1097/MD.0000000000019103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Potter A.W., Nindl L.J., Soto L.D., Pazmino A., Looney D.P., Tharion W.J., et al. High precision but systematic offset in a standing bioelectrical impedance analysis (BIA) compared with dual-energy X-ray absorptiometry (DXA) BMJ Nutr Prev Health. 2022;5:254–262. doi: 10.1136/bmjnph-2022-000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buch A., Ben-Yehuda A., Rouach V., Maier A.B., Greenman Y., Izkhakov E., et al. Validation of a multi-frequency bioelectrical impedance analysis device for the assessment of body composition in older adults with type 2 diabetes. Nutr. Diabetes. 2022;12:45. doi: 10.1038/s41387-022-00223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee Y., Kwon O., Shin C.S., Lee S.M. Use of bioelectrical impedance analysis for the assessment of nutritional status in critically ill patients. Clin Nutr Res. 2015;4:32–40. doi: 10.7762/cnr.2015.4.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Varlet-Marie E., Vachoud L., Marion B., Roques C., Fidani T., Chevalier C., et al. Leg electrical resistance predicts venous blood viscosity and hematocrit. Clin. Hemorheol. Microcirc. 2019;71:397–402. doi: 10.3233/CH-199003. [DOI] [PubMed] [Google Scholar]
- 36.Tran A.K., Sapkota A., Wen J., Li J., Takei M. Linear relationship between cytoplasm resistance and hemoglobin in red blood cell hemolysis by electrical impedance spectroscopy & eight-parameter equivalent circuit. Biosens. Bioelectron. 2018;119:103–109. doi: 10.1016/j.bios.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Ringaitiene D., Puodziukaite L., Vicka V., Gineityte D., Serpytis M., Sipylaite J. Bioelectrical impedance phase angle-predictor of blood transfusion in cardiac surgery. J. Cardiothorac. Vasc. Anesth. 2019;33:969–975. doi: 10.1053/j.jvca.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 38.Valentino N.P., Gomes T.L.N., Barreto C.S., Borges T.C., Soares J.D.P., Pichard C., et al. Low phase angle is associated with the risk for sarcopenia in unselected patients with cancer: effects of hydration. Nutrition. 2021;84 doi: 10.1016/j.nut.2020.111122. [DOI] [PubMed] [Google Scholar]
- 39.Gomes T.L.N., Soares J.D.P., Borges T.C., Pichard C., Pimentel G.D. Phase angle is not associated with fatigue in cancer patients: the hydration impact. Eur. J. Clin. Nutr. 2020;74:1369–1373. doi: 10.1038/s41430-020-0597-4. [DOI] [PubMed] [Google Scholar]
- 40.Hildegard Stancu S., Stanciu A., Lipan M., Capusa C. Renal anemia and hydration status in non-dialysis chronic kidney disease: is there a link? J Med Life. 2018;11:293–298. doi: 10.25122/jml-2019-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiss G., Ganz T., Goodnough L.T. Anemia of inflammation. Blood. 2019;133:40–50. doi: 10.1182/blood-2018-06-856500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barrea L., Muscogiuri G., Pugliese G., Laudisio D., de Alteriis G., Graziadio C., et al. Phase angle as an easy diagnostic tool of meta-inflammation for the nutritionist. Nutrients. 2021;13 doi: 10.3390/nu13051446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hung S.C., Kuo K.L., Peng C.H., Wu C.H., Wang Y.C., Tarng D.C. Association of fluid retention with anemia and clinical outcomes among patients with chronic kidney disease. J. Am. Heart Assoc. 2015;4 doi: 10.1161/JAHA.114.001480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colín-Ramírez E., Castillo-Martínez L., Orea-Tejeda A., Asensio Lafuente E., Torres Villanueva F., Rebollar González V., et al. Body composition and echocardiographic abnormalities associated to anemia and volume overload in heart failure patients. Clin. Nutr. 2006;25:746–757. doi: 10.1016/j.clnu.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 45.Androne A.S., Katz S.D., Lund L., LaManca J., Hudaihed A., Hryniewicz K., et al. Hemodilution is common in patients with advanced heart failure. Circulation. 2003;107:226–229. doi: 10.1161/01.cir.0000052623.16194.80. [DOI] [PubMed] [Google Scholar]
- 46.Hara T., Mukai H., Nakashima T., Sagara R., Furusho M., Miura S., et al. Factors contributing to erythropoietin hyporesponsiveness in patients on long-term continuous ambulatory peritoneal dialysis: a cross-sectional study. Nephron Extra. 2015;5:79–86. doi: 10.1159/000441154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Unal A., Kavuncuoglu F., Duran M., Oguz F., Kocyigit I., Sipahioglu M.H., et al. Inflammation is associated to volume status in peritoneal dialysis patients. Ren. Fail. 2015;37:935–940. doi: 10.3109/0886022X.2015.1040337. [DOI] [PubMed] [Google Scholar]
- 48.Eisenberger M.A., Blumenstein B.A., Crawford E.D., Miller G., McLeod D.G., Loehrer P.J., et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N. Engl. J. Med. 1998;339:1036–1042. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 49.Beer T.M., Tangen C.M., Bland L.B., Hussain M., Goldman B.H., DeLoughery T.G., et al. The prognostic value of hemoglobin change after initiating androgen-deprivation therapy for newly diagnosed metastatic prostate cancer: a multivariate analysis of Southwest Oncology Group Study 8894. Cancer. 2006;107:489–496. doi: 10.1002/cncr.22029. [DOI] [PubMed] [Google Scholar]
- 50.Choi J.B., Loredo J.S., Norman D., Mills P.J., Ancoli-Israel S., Ziegler M.G., et al. Does obstructive sleep apnea increase hematocrit? Sleep Breath. 2006;10:155–160. doi: 10.1007/s11325-006-0064-z. [DOI] [PubMed] [Google Scholar]
- 51.Malenica M., Prnjavorac B., Bego T., Dujic T., Semiz S., Skrbo S., et al. Effect of cigarette smoking on haematological parameters in healthy population. Med. Arch. 2017;71:132–136. doi: 10.5455/medarh.2017.71.132-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pankaj J., Reena J., Mal K., Ketan M. Effect of cigarette smoking on haematological parameters: comparison between male smokers and non-smokers. IJSN. 2014;5:740–743. [Google Scholar]
- 53.Verma R., Patel C. Effect of smoking on haematological parameters in human beings. J. Cell and Tissue Res. 2015;5:337. [Google Scholar]
- 54.The economic cost of ESRD and Medicare spending for alternative modalities of treatment. United States Renal Data System. Am. J. Kidney Dis. 1998;32:S118–S131. doi: 10.1053/ajkd.1998.v32.pm9713414. [DOI] [PubMed] [Google Scholar]
- 55.Gunnell J., Yeun J.Y., Depner T.A., Kaysen G.A. Acute-phase response predicts erythropoietin resistance in hemodialysis and peritoneal dialysis patients. Am. J. Kidney Dis. 1999;33:63–72. doi: 10.1016/s0272-6386(99)70259-3. [DOI] [PubMed] [Google Scholar]
- 56.Locatelli F., Andrulli S., Memoli B., Maffei C., Del Vecchio L., Aterini S., et al. Nutritional-inflammation status and resistance to erythropoietin therapy in haemodialysis patients. Nephrol. Dial. Transplant. 2006;21:991–998. doi: 10.1093/ndt/gfk011. [DOI] [PubMed] [Google Scholar]
- 57.Portolés J., López-Gómez J.M., Aljama P. Anemia management and treatment response in patients on hemodialysis: the MAR study. J. Nephrol. 2006;19:352–360. [PubMed] [Google Scholar]
- 58.Agarwal R., Davis J.L., Smith L. Serum albumin is strongly associated with erythropoietin sensitivity in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2008;3:98–104. doi: 10.2215/CJN.03330807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cheungpasitporn W., Thongprayoon C., Chiasakul T., Korpaisarn S., Erickson S.B. Renin-angiotensin system inhibitors linked to anemia: a systematic review and meta-analysis. QJM: Int. J. Med. 2015;108:879–884. doi: 10.1093/qjmed/hcv049. [DOI] [PubMed] [Google Scholar]
- 60.Pratt M.C., Lewis-Barned N.J., Walker R.J., Bailey R.R., Shand B.I., Livesey J. Effect of angiotensin converting enzyme inhibitors on erythropoietin concentrations in healthy volunteers. Br. J. Clin. Pharmacol. 1992;34:363–365. doi: 10.1111/j.1365-2125.1992.tb05644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kamper A.L., Nielsen O.J. Effect of enalapril on haemoglobin and serum erythropoietin in patients with chronic nephropathy. Scand. J. Clin. Lab. Invest. 1990;50:611–618. doi: 10.3109/00365519009089178. [DOI] [PubMed] [Google Scholar]
- 62.Morrone L.F., Di Paolo S., Logoluso F., Schena A., Stallone G., Giorgino F., et al. Interference of angiotensin-converting enzyme inhibitors on erythropoiesis in kidney transplant recipients: role of growth factors and cytokines. Transplantation. 1997;64:913–918. doi: 10.1097/00007890-199709270-00021. [DOI] [PubMed] [Google Scholar]
- 63.Packer M. Lessons learned from the DAPA-HF trial concerning the mechanisms of benefit of SGLT2 inhibitors on heart failure events in the context of other large-scale trials nearing completion. Cardiovasc. Diabetol. 2019;18:129. doi: 10.1186/s12933-019-0938-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sano M., Takei M., Shiraishi Y., Suzuki Y. Increased hematocrit during sodium-glucose cotransporter 2 inhibitor therapy indicates recovery of tubulointerstitial function in diabetic kidneys. J. Clin. Med. Res. 2016;8:844–847. doi: 10.14740/jocmr2760w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maruyama T., Takashima H., Oguma H., Nakamura Y., Ohno M., Utsunomiya K., et al. Canagliflozin improves erythropoiesis in diabetes patients with anemia of chronic kidney disease. Diabetes Technol. Therapeut. 2019;21:713–720. doi: 10.1089/dia.2019.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghanim H., Abuaysheh S., Hejna J., Green K., Batra M., Makdissi A., et al. Dapagliflozin suppresses hepcidin and increases erythropoiesis. J. Clin. Endocrinol. Metab. 2020;105 doi: 10.1210/clinem/dgaa057. [DOI] [PubMed] [Google Scholar]
- 67.Carvalho P.E.P., Veiga T.M.A., Simoes E.S.A.C., Gewehr D.M., Dagostin C.S., Fernandes A., et al. Cardiovascular and renal effects of SGLT2 inhibitor initiation in acute heart failure: a meta-analysis of randomized controlled trials. Clin. Res. Cardiol. 2023:1–12. doi: 10.1007/s00392-022-02148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nazari S., Mirkhani H. Cardiorenal protections of SGLT2 inhibitors in the treatment of type 2 diabetes. Curr. Diabetes Rev. 2022 doi: 10.2174/1573399819666221222160035. Online ahead of print. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.